Highlights
-
•
Chondroitin sulfate (CS) is a glycosaminoglycan with a growing variety of applications.
-
•
CS can be produced from microbial fermentation of native or engineered strains.
-
•
Synthetic biology tools are being used to improve CS yields in different hosts.
-
•
Integrated polymerization and sulfation can generate cost-effective CS.
Keywords: Chondroitin, Glycosaminoglycans, Biosynthetic pathway, Metabolic engineering, Microbial fermentation, Heterologous production
Abstract
Chondroitin sulfate (CS) is a glycosaminoglycan with a broad range of applications being a popular dietary supplement for osteoarthritis. Usually, CS is extracted from animal sources. However, the known risks of animal products use have been driving the search for alternative methods and sources to obtain this compound. Several pathogenic bacteria naturally produce chondroitin-like polysaccharides through well-known pathways and, therefore, have been the basis for numerous studies that aim to produce chondroitin using non-pathogenic hosts. However, the yields obtained are not enough to meet the high demand for this glycosaminoglycan. Metabolic engineering strategies have been used to construct improved heterologous hosts. The identification of metabolic bottlenecks and regulation points, and the screening for efficient enzymes are key points for constructing microbial cell factories with improved chondroitin yields to achieve industrial CS production. The recent advances on enzymatic and microbial strategies to produce non-animal chondroitin are herein reviewed. Challenges and prospects for future research are also discussed.
1. Introduction
Glycosaminoglycans (GAGs) are naturally occurring heteropolysaccharides with polyanionic character. They consist of repeating disaccharide units composed of an N-acetylated or N-sulfated hexosamine (glucosamine, GlcN or galactosamine, GalN) and either a uronic acid (glucuronic acid, GlcA or iduronic acid, IdoA) or galactose (Gal). There are four main types of GAGs that vary in their monomeric composition, glycosidic bonds and sulfation pattern: hyaluronic acid (hyaluronate, hyaluronan, HA); keratan sulfate (KS); chondroitin sulfate/dermatan sulfate (CS/DS, stereoisomers); and heparin/heparan sulfate (HP/HS) [1]. The chemical structures of the repeating disaccharide units of different GAGs are represented in Fig. 1. While HA lacks sulfate groups, the remaining GAGs can contain sulfates at various positions. DS is distinguished from CS by the presence of IdoA. HS is the only glycosaminoglycan that contains a N-sulfated hexosamine and differs from HP by possessing less sulfated units and less content of epimerized entities into IdoA. Heparosan is the non-sulfated precursor to HS and HP. Keratan sulfate lacks uronic acids and instead contains sulfated Gal residues.
Fig. 1.
Structures of the main glycosaminoglycans (GAGs) a) hyaluronic acid, b) keratan sulfate, c) chondroitin and chondroitin sulfate, d) dermatan sulfate, and e) heparosan, heparan sulfate and heparin. Monomers of the disaccharide building blocks are abbreviated as GlcA - d-glucuronic acid, GlcNAc – N-acetyl-d-glucosamine, Gal – d-galactose, GalNAc – N-acetyl-d-galactosamine, IdoA – l-iduronic acid, GlcN, d-glucosamine. Hyaluronic acid (a) does not go under post-polymerization modifications. Keratan sulfate (b) has di-sulfated, mono-sulfated and non-sulfated disaccharide units (each R6 = H or SO3H) due to O-sulfotransferases action. Chondroitin (c) is the simple non-sulfated backbone (R2, R3, R4 and R6 = H) which can be modified by different tissue-specific O-sulfotransferases to form chondroitin sulfate (each R2, R3, R4 and R6 = H or SO3H). Dermatan sulfate (d) is formed from chondroitin through epimerization of GlcA into IdoA by tissue-specific epimerases followed by O-sulfotransferases (each R2, R4 and R6 = H or SO3H). Heparosan (e) has non-modified sugar moieties, that can be further modified through actions of tissue-specific N-sulfotransferases, C5 epimerases and O-sulfotransferases to generate the sulfated forms heparan sulfate and heparin (R2 from uronic acid = H or SO3H; when the hexosamine unit is GlcN, R2 in that unit = SO3H, while R2 = Ac when the unit is GlcNAc; other groups R3, R6 = H or SO3H). Heparin has more sulfate groups and IdoA content than heparan sulfate. Depending on the GAG type and source the molecular size can generally vary between 4 and 200 mer (n = 4 – 200). Exceptionally, the highest size can be found for hyaluronic acid that can achieve 20,000 repeating units.
In animals, GAGs usually exist as long chains covalently bound to a protein core, as part of proteoglycans [2]. Depending on their molecular structure, GAGs have a wide distribution through tissues and perform different physiologic functions including in structural support, cell recognition and signaling, matrix organization, inflammation, cell division, and tissue repair and development [3, 4], being particularly common in connective tissues such as the skin, bone, cartilage, and the intervertebral discs [5]. Some bacterial pathogens produce GAG-like polysaccharides as part of their capsule composition, which contribute to their pathogenicity [[6], [7], [8]].
The wide applications of GAGs in medicine, veterinary, cosmetics, and pharmaceutics have been extensively reviewed [5, 9, 10]. The most famous GAG clinical application is the use of HP as an anticoagulant drug. Furthermore, GAGs also exist as nutraceuticals in human and veterinary supplements, serve as cosmetic or pharmaceutical ingredients, or act as materials for several biomedical applications such as tissue engineering.
The industrial production of GAGs can be classified in 4 types: (i) extraction from animal sources; (ii) chemical synthesis; (iii) enzymatic or chemoenzymatic production or (iv) microbial or animal cells fermentation. Regarding microbial fermentation, the industrial production of HA is currently the most well established due to its simple structure that does not undergo sulfation or epimerization contrarily to the other GAGs. However, sulfated GAGs such as CS, HS, HP and KS are usually chemically extracted from animal sources, through a laborious process that requires a large amount of environmentally hazardous compounds [11, 12]. Also, the use of animal sources brings concerns on contamination with other animal products, viruses, and prions. In addition, religious motivations and vegetarianism trends have also led to the search for non-animal sources [13].
This review is focused on the recent advances of production processes for animal-free chondroitin and CS production, including enzymatic and microbial strategies, which are areas with great advances in the last few years. With the increasing knowledge on biosynthesis, regulation, transportation, bottlenecks, metabolic engineering strategies, bioprocess optimization, and with the discovery of new enzymes, a sustainable process for the microbial production of value-added natural and artificial chondroitin derivatives is closer to becoming a reality.
2. Occurrence and biological functions of chondroitin and its derivatives
Chondroitin is the unsulfated precursor of CS, the most abundant GAG in the human body. It comprises a repeated disaccharide structurally composed by a glucuronic acid (GIcA) residue and a N-acetyl-d-galactosamine (GaINAc) residue linked by β(1–3) and β(1–4) bonds, respectively (Fig. 1, Table 1). According to its sulfation pattern, CS is mainly classified into CS-A, CS-C, CS-D, CS-E and CS-O (unsulfated chondroitin) [2, 14]. However, novel CS disaccharide units have been discovered in natural sources (CS-F, CS-G, CS-K, CS-L, CS-M, CS-S and CS-T) [[15], [16], [17]] or chemically synthesized (CS-R, CS-U and CS-V) [18]. Considered by some authors as a type of CS, DS (CS-B) is distinguished by the presence of at least one IdoA residue, resulting from the epimerization of GlcA. In higher animals, CS exists anchored to proteins as part of proteoglycans, being the predominant types CS-A and CS-C, and is present in connective tissues, for example, cartilage, cornea, bone, skin, arterial walls [19]. Examples of natural sources of CS and DS are presented in Table 1. CS has biological roles in inflammation prevention, immune modulation, maintenance of the structure, elasticity and shock-absorbing properties of cartilage, regulation of cell adhesion to the extracellular matrix, facilitation of nutrient and oxygen diffusion, mediation of tumor growth and metastasis, pathogen adhesion, angiogenesis, osteogenic differentiation and in brain development, plasticity and regeneration [20, 21 – 23, 24]. Interestingly, CS biological functions depend on the chain length, sulfation pattern and percentage of the structural units, which vary upon animal age and tissue. DS is predominant in skin, heart valve, tendons, and blood vessels [25, 26, 27]. The physiologic roles of DS include regulation of transforming growth factor-β activity, cell proliferation, cell development, cell adhesion, homeostasis, collagen organization, anticoagulant activity, tumorigenesis, infection, wound repair, fibrosis and stabilization of the basement membrane [25, 28, 29, 30, 31, 32]. Particularly, the well-studied anticoagulant activity of DS occurs by its binding to HP cofactor II. The resulting complex can inhibit the procoagulant effect of thrombin, while not affecting the clotting cascade (factor X) or the platelet function. DS selective inhibition of thrombin makes it an interesting alternative to HP [33, 34].
Table 1.
Examples of natural sources of different types of chondroitin, chondroitin sulfate (CS), and dermatan sulfate (DS) and their biological functions in humans. In higher animals, different types of GAGs can occur in different proportions and sizes depending for example on the animal, tissue, age or diet.
| GAG type | Disaccharide repeat | Natural sources | Biological functions | References |
|---|---|---|---|---|
| CS-A | GlcA(β1–3)GalNAc(4S)(β1–4) | Dogfish, shark and whale cartilage; human, bovine, porcine and chicken cartilaginous tissues | mediates malaria-infected erythrocytes adhesion; negatively regulates axonal guidance and growth; activates metastatic cascate | [13, 22, 38, 39, 40] |
| DS (CS-B) | GlcA/IdoA(2S)(β/α1–3)GalNAc(4S)(β1–4) | Animal skin/hide, cornea, cartilage, heart valve, tendons, blood vessels, and bone | regulates growth factors activity; has anticoagulant activity; promotes proliferation of serveral cell lines; mediates homeostasis, tumorigenesis, infection, wound repair, collagen organization, fibrosis and stabilization of the basement membrane | [25, 26, 27, 28, 29, 30, 31, 32] |
| CS-C | GlcA(β1–3)GalNAc(6S)(β1–4) | Dogfish and shark cartilage; human, bovine, porcine and chicken cartilaginous tissues | may promote progression of epilepsy; neuroprotective properties in Alzheimer's disease | [13, 38, 41, 42, 43] |
| CS-D | GlcA(2S)(β1–3)GalNAc(6S)(β1–4) | Shark cartilage; animal brain | promotes neuron growth; interacts with humoral factors | [22, 44, 45, 46] |
| CS-E | GlcA(β1–3)GalNAc(4, 6diS)(β1–4) | Squid cartilage; animal lung | mediates angiogenesis; acts as cell surface receptor for herpes virus; modulates humoral factors; stimulates neurite outgrowth; promotes neural stem cells proliferation; mediates osteogenic differentiation | [22, 24, 32, 46, 47, 48] |
| Fucosylated CS (fCS, CS-F) | different types of CS with l-fucosyl branches usually attached to the O-3 of GlcA unit | Sea cucumbers | – | [49, 50, 51, 52, 53, 54] |
| CS-G | different types of CS with glucose attached to the O-6 of GalNAc unit | Squid cartilage | – | [55] |
| CS-H (highly sulfated DS) | IdoA(α1–3)GalNAc(4S, 6S)(β1–4) | Hagfish notochord | – | [56] |
| CS-K | GlcA(3S)(β1−3)GalNAc(4S)(β1–4) | Squid, king crab and octopus | – | [57, 58, 59, 60] |
| CS-L | GlcA(3S)(β1−3)GalNAc(6S)(β1–4) | Squid | – | [60] |
| CS-M | GlcA(3S)(β1−3)GalNAc(4, 6diS)(β1–4) | Squid | – | [60] |
| Unsulfated chondrotin (CS-O) | GlcA(β1–3)GalNAc(β1–4) | Pasteurella multocida Type F; Avibacterium paragallinarium genotype I | – | [35, 36] |
| Fructosylated chondroitin | GlcA(3Fru)(β1–3) GalNAc(β1–4) | E. coli K4 | – | [37, 61, 62, 63, 64, 65] |
| N-glycolyl chondroitin (Gc-CS) | GlcA(β1–3)GalNGc(β1–4) | Serum of humans who eat red meat (beef, lamb, and pork) | – | [66] |
In bacteria, unsulfated chondroitin backbone is produced as capsular polysaccharide by pathogens Pasteurella multocida Type F and Avibacterium paragallinarium genotype I [35, 36], while a chondroitin decorated with fructose residues is produced by Escherichia coli K4 [37]. Bacteria use these capsular polysaccharides to mask their infection and thus increase their pathogenicity [6].
Commercial CS is usually provided as a mixture of CS-A and CS-C, the most common CS in animal cartilage [67], which is obtained through chemical extraction and purification from animal cartilaginous tissues, mainly from bovine, porcine, shark and chicken. The structural composition, molecular weight and yields of CS obtained from extraction vary not only with the extraction method but also with the animal and tissue used. Some of the reported CS yields include: extraction from shark fin resulted in 150.5 mg/g dry cartilage [68]; from blue shark head wastes 120.8 mg/g dry cartilage was obtained; crocodile hyoid, rib, sternum, trachea cartilages generated 91 – 274 mg/g of dry cartilage [68]; 75 mg/g of dry weight has been achieved by extraction from ray [68]; buffalo nasal, joint and tracheal cartilages generated 60 to 62 mg/g of dry cartilage [69]; using chicken keel, 24.8 mg/g of wet cartilage were obtained [70]; from different sea cucumbers, fucosylated CS (fCS) yields varied from 63 to 110 mg/g weight [54]; and from fish by-products, yields from 19 to 137 mg/g dry cartilage have been achieved [71]. In addition to the relatively low yields, the shortage of such materials and the concerns on intraspecies contamination led to studies on artificial synthesis of the chondroitin backbone, either using chemical synthesis, enzymatic, chemo-enzymatic and microbial fermentation strategies.
3. Clinical applications of CS
Given the growing potential applications of CS, and the rising prevalence of osteoarthritis due to the increase in average life expectancy among the population, a boost in its demand and market volume has been registered. The global CS market size was valued as USD 1.17 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 3.0% from 2020 to 2028 [72]. In 2018, China was the biggest producer accounting for 83.7% of CS produced globally [73].
Depending on their application, different systems have been implemented for the administration of chondroitin and its derivatives, including oral administration as food additives, in pharmaceutical preparations, nutraceuticals or veterinary supplements [74, 75, 76, 77]; incorporation in intra-articular or intravenous injectables [78]; integration in skin dermatology/cosmeceutical and ophthalmic products [79, 80, 81]; as part of medical devices [82, 83, 84, 85]; or as biomaterials for regenerative medicine [9, 86].
The current main application of CS is as nutraceutical to treat osteoarthritis symptoms and retard cartilage degradation [77, 87]. These supplements have been widely prescribed for humans and animals and are usually formulated with glucosamine. Although the results on the efficacy of chondroitin or its combination with glucosamine in joint repair and pain relief compared with placebo have not always been concurrent [88, 89, 90, 91], CS and glucosamine have been recommended in guidelines, prescribed by general practitioners and rheumatologists as over-the-counter drugs and suggested by the European League Against Rheumatism (EULAR) to patients with knee and hand osteoarthritis [92]. The variable efficacy of CS is in part attributed to its highly variable composition. Since they are over-the-counter supplements, they are not tightly controlled and can differ in the biological source, purification method and ingredient amount and type, thus resulting in heterogenous CS mixtures with different sulfation patterns or even containing other GAGs as contaminants. Condrosulf (fish CS, IBSA Institut Biochimique SA, Lugano, Switzerland), Condrosan (bovine CS, Bioiberica S.A.U, Barcelona, Spain), and Structum (avian CS, Laboratoires Pierre Fabre, Paris, France) are examples of commercialized pharmaceutical-grade CS products that have shown benefits for osteoarthritis in clinical trials [93, 94, 95, 96, 97, 98, 99, 100]. In these studies, CS has shown to be safe and, generally, exhibited slight to moderate efficacy over placebo in pain relief and function improvement in osteoarthritis patients. Also, CS is included in eye drop solutions for dry eyes [79, 80]. Preservative-free ophthalmic solutions combining xanthan gum and CS or combining sodium HA and CS showed similar efficacy compared to polyethylene glycol and propylene glycol solutions in the treatment of dry eye disease. The CS oral supplementation in combination with glucosamine hydrochloride has also shown improved therapeutic benefits in reducing systemic inflammation in overweighted individuals, over placebo [101]. For interstitial cystitis, the intravesical treatment with CS achieved improved efficiency in pain reduction and nocturia and had superior tolerability over DMSO, a standard approved therapy [84]. CS is also found in solutions for preserving corneas [102], or in injectable devices to protect the eye during cataract surgery (phacoemulsification) [82, 83]. The ophthalmic viscosurgical devices containing HA and CS (DisCoVisc, Viscoat and Duovisc) showed greater efficiency during phacoemulsification, and improved protection of corneal endothelium, compared to hydroxypropylmethylcellulose [82] and to HA and lidocaine hydrochloride (Visthesia) injectables [83].
In addition, the potential applications of exogenous CS are wide-ranging since CS exhibits anti-inflammatory activity [50, 103, 104, 105, 106], anticoagulant properties [53, 54, 107], promotes the regeneration of different tissues [108, 109, 110], has antiviral activity [111 – 116], and can be used in cancer treatment [47, 105]. Specifically, CS-E has been considered a powerful antiviral agent against flavivirus dengue, herpes viruses and T-cell leukemia virus type I [111, 112, 114], tobacco mosaic virus is inhibited by CS-A and CS-C [113], fCS showed inhibitory activity against human immunodeficiency virus type-1 [115] and against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [116]. CS has also potential applications in engineering scaffolds for regenerative medicine and tissue engineering, by combination with other biopolymers such as HA and collagen [117, 118].
DS exhibits several reported therapeutic applications. Drug formulations of DS have been marketed for the prevention of venous thromboembolism under the commercial names Mistral (Mediolanum Farmaceutici, Milan, Italy) [119] and Venorix (Laboratório Medinfar, Amadora, Portugal). It is also a component of sulodexide, a more widely-used antithrombotic agent [120].
Table 2 lists clinical trials with recently published results where CS or DS are used as treatment for different conditions, either as single GAG in the formulation or when formulated with other GAGs.
Table 2.
Main applications of chondroitin sulfate and dermatan sulfate under clinical trials, either as the single glycosaminoglycan (GAG) of the formulation or in combination with other GAGs.
| Glycosaminoglycan | Condition / Potential application | Clinical trials ID |
|---|---|---|
| Chondroitin sulfate | Osteoarthritis (knee, hand) |
NCT00291499 [93] NCT00604539 [95] NCT01354145 [96, 97] NCT00955552 [121] NCT01425853 [122] NCT01893905 [123] NCT02830919 [124] NCT01271218 [125] NCT00513422 [126, 127] |
| Inflammation reduction and prevention | NCT01682694 [101] | |
| Interstitial cystitis | NCT04268810 [84] | |
| Dry eye | NCT01657253 [79] | |
| Hemostasis in surgeries | NCT03725098 [128] | |
| Corneal storage medium | NCT01657500 [102] | |
| Chondroitin sulfate and hyaluronic acid | Knee osteoarthritis | NCT04352322 [129] |
| Recurrent urinary tract infections | NCT02016118 [85] | |
| Adjuvant during phacoemulsification |
NCT01387620 [82] NCT02304861 [83] |
|
| Post-surgical rehabilitation | NCT03355651 [130] | |
| Dermatan sulfate and heparan sulfate (mesoglycan) | Post-operative thrombosis | NCT04481698 [131] |
| Dermatan sulfate and heparin (sulodexide) | Anticoagulant | NCT04257487 [132] |
| Diabetic retinopathy | NCT01295775 [133] | |
| Diabetic kidney disease (albuminuria, nephropathy) |
NCT00130312 [134] NCT00130208 [135] |
4. Enzymatic and chemoenzymatic CS production
Enzymes can be used to produce CS oligosaccharides by degrading CS polymers or by synthesizing CS oligosaccharides/polymers through polymerization. The main advantages of using enzymes over chemical approaches is that enzymes catalyze stereoselective and regioselective reactions, potentially resulting in homogeneous CS in an eco-friendlier and faster manner [136, 137]. However, these enzymatic steps are often combined with chemical synthesis or modifications (chemoenzymatic strategies) to either provide synthetic precursors or to perform the sulfation step. The depolymerizing/ degrading enzymes and the polysaccharide substrates used are relatively inexpensive [136]. However, the commercial polysaccharides are usually from animal sources which raises the concerns already mentioned. The degrading enzymes will be briefly discussed since they can give insights on structural analysis of CS, and because they can perform the contrary polymerization reaction under certain conditions. Enzymatic synthesis of CS from natural and unnatural precursors is the alternative for the preparation of homogeneous CS that most resembles the biological process. However, the high cost of such precursors generally limits the industrial implementation of these enzymatic methods.
4.1. Enzymatic depolymerization of CS polysaccharides
CS depolymerizing enzymes able to produce CS oligosaccharides are CS lyases [138, 139, 140] and CS hydrolases [141]. While polysaccharide hydrolases are found in almost every organism, polysaccharide lyases do not occur in vertebrates. According to their substrate specificity, the polysaccharide lyases acting on CS can be classified into chondroitinases ABC, AC, B, and C. They perform the depolymerization of CS/DS polymers via endolytic or exolytic β-elimination resulting mainly in unsaturated oligosaccharides [138, 140]. Proteus vulgaris, Flavobacterium heparinum (Pedobacter heparinus), Sphingomonas paucimobilis, Bacteroides stercoris, Pseudopedobacter saltans (Pedobacter saltans), and Bacteroides thetaiotaomicron are some examples of different CS lyase producers [138, 140, 142, 143]. Differently from CS lyases, CS hydrolases usually depolymerize CS with no unsaturated bonds formed, which make them more attractive for the enzymatic production of CS [1]. Some animal HA hydrolases, hyaluronidases, show depolymerizing activity against CS and some isoforms can only accept CS as substrate [144, 145]. Therefore, these hyaluronidases have been used to depolymerize CS polysaccharides to obtain CS oligosaccharides (Fig. 2a) up to tetrasaccharides. The substrate specificity of CS lyases and CS hydrolases can retrieve functional characterization of GAGs.
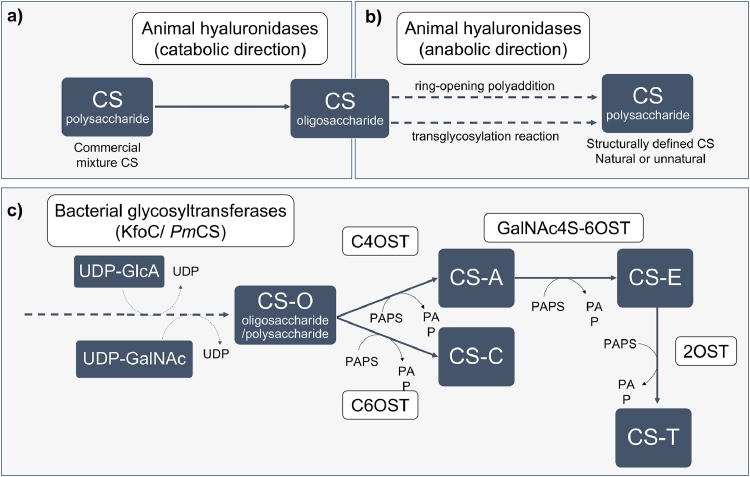
Fig. 2.
Enzymatic synthesis of chondroitin sulfate (CS). a) Depolymerizing enzymes, such as animal hyaluronidases, can be used to obtain CS oligosaccharides from CS polysaccharides; b) the same type of enzymes is able to, under different conditions, polymerize the CS oligosaccharides through chemoenzymatic approaches. c) Bacterial glycosyltransferases (such as chondroitin synthase from Escherichia coli K4, KfoC, or from Pasteurella multocida type F, PmCS) act by transferring alternate residues of glucuronic acid (GlcA) and acetylgalactosamine (GalNAc), using uridine diphosphate (UDP)-GlcA and UDP-GalNAc as donors, to the nonreducing end of a chondroitin chain acceptor to elongate the chondroitin oligosaccharide/ polysaccharide backbones. Sulfotransferases such as 4-O-sulfotransferase (C4OST), 6-O-sulfotransferase (C6OST), N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase (GalNAc4S-6OST), and 2-O-sulfotransferase (2OST) that require the presence of 3′-phosphoadenosine-5′-phosphosulfate (PAPS) as sulfate donor, convert the unsulfated backbone (CS-O) in CS with different sulfation patterns such as CS-A, CS-C, CS-E and CS-T. Only CSs with a homogenous defined sulfation pattern are shown although a CS chain may have different CS units if a combination of sulfotransferases is used. Dashed arrows represent polymerization steps.
4.2. Enzymatic synthesis of CS oligosaccharides and polysaccharides
For synthesis of CS oligosaccharides and polysaccharides, degrading enzymes that in the reverse direction synthesize polymers (such as hyaluronidases, Fig. 2b), or synthases (such as glycosyltransferases, Fig. 2c) can be used. Further post-polymerization modifications of the chondroitin backbone are required to achieve the CS oligosaccharides or polysaccharides. Specialized CS sulfotransferases, including 4-O-sulfotransferase (C4OST), 6-O-sulfotransferase (C6OST), 2-O-sulfotransferase (2OST) and N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase (GalNAc4S-6OST), sulfate the chondroitin composing units in the presence of the co-factor 3′-phosphoadenosine-5′-phosphosulfate (PAPS) to generate CS with different sulfation patterns and consequently different biological activities.
Chemoenzymatic methods using HA or CS degrading enzymes in the anabolic direction for the production of CS and its derivatives have been established, by either ring-opening polyaddition [146, 147] or by transglycosylation reaction [148]. The works of Kobayashi et al. [146] and Fujikawa et al. [147] used testicular hyaluronidases from ovine or bovine to catalyze the synthesis of structurally defined CS (natural and unnatural) from chemically synthesized disaccharide oxazoline precursors which acted as transition state analogues. Kakizaki et al. [148] used immobilized bovine testicular hyaluronidase to produce hybrid polysaccharides composed by both HA and CS units. First, the hyaluronidase was used to prepare oligosaccharides by digestion of commercial CS and HA polysaccharides. The oligosaccharides were fluorolabeled (to facilitate analytical identification) generating pyridylaminated acceptors which underwent transglycosylation catalyzed by immobilized hyaluronidase using HA and CS donor polysaccharides. These methods enabled to control the molecular weight of the resulting CS and the construction of unnatural CS derivatives. However, the use of expensive sugar precursors and dedicated reactors, as well as the low product yields are drawbacks that limited large-scale application [149].
The discovery of microbial counterparts of glycosyltransferases in pathogenic bacteria further boosted the development of enzymatic strategies to produce chondroitin and CS. Bacterial glycosyltransferases used for chondroitin synthesis, also named chondroitin synthases or chondroitin polymerases, include PmCS from Pasteurella multocida and KfoC from E. coli K4 strains. These enzymes act by transferring alternate residues of GlcA and GalNAc, using uridine diphosphate (UDP)-GlcA and UDP-GalNAc as donors, to the nonreducing end of a chondroitin chain acceptor to form chondroitin oligosaccharide backbones (Fig. 2c). DeAngelis et al. [150] identified for the first time a microbial gene encoding the chondroitin synthase PmCS and cloned it in E. coli. The same authors further used this recombinant glycosyltransferase for in vitro production of chondroitin from the substrates UDP-GlcA and UDP-GalNAc [151]. Other microbial glycosyltransferase KfoC was used by Sugiura et al. [152] to produce chondroitin oligosaccharides (CS-O) with controlled size from 7-mer to 16-mer. The authors first digested a commercial unsulfated chondroitin polymer with hyaluronidase to achieve even-numbered oligosaccharides. Further digestion with β-glucuronidase allowed to obtain odd-numbered oligosaccharides. The oligosaccharides were subjected to pyridylamination to facilitate their analysis. Then, two mutated recombinant KfoC enzymes were expressed each being selective for one of the substrates (UDP-GlcA or UDP-GalNAc) and the engineered enzymes, immobilized in beads, were used to elongate the chondroitin chain. The same group employed chemoenzymatic strategies for the preparation of CS oligosaccharides and polysaccharides with different sulfation patterns using the CS polymerase KfoC and recombinant sulfotransferases C4OST, C6OST, GalNAc4S-6OST and 2OST [153, 154, 155]. The chondroitin backbone was obtained by desulfating commercial CS from different sources and HEK293T cells were used to express the human sulfotransferases. However, the resulting products were heterogeneous, low amounts were achieved, and the process required laborious techniques for the separation of oligosaccharides with different sizes. Afterwards, the enzymatic production of 15 structurally diversified and homogeneous CS-A and CS-C oligosaccharides was achieved using KfoC expressed in E. coli BL21 [156]. Amounts of 4 –30 mg of CS oligosaccharides were obtained. The authors also synthesized the expensive substrate UDP-GalNAc using Bifidobacterium longum N-acetylhexosamine kinase and human GalNAc pyrophosphorylase both expressed in E. coli BL21, which significantly reduced the process cost. Human C4OST and mouse C6OST were afterwards used for sulfation of chondroitin backbones. Sf9 insect cells were used to express C4OST and C6OST. The authors were unsuccessful expressing C6OST in E. coli which they rationalized to be due to the glycosylation being required for the sulfotransferase activity as shown in Yusa et al. [157]. Accordingly, attempts to express soluble mouse C4OST in E. coli were also unsuccessful, and C4OST and C6OST were effectively expressed and secreted by the Pichia pastoris [158]. However, active human C4OST has been expressed in E. coli BL21 and P. pastoris [159]. Those authors showed that the glycosylated and non-glycosylated forms of the enzyme had similar activities. More recently, the soluble expression of human C4OST enzyme was also achieved in E. coli K4 and in E. coli K-12 MG1655 [160].
In addition to the most common CS types, CS-A and CS-C, efforts to enzymatically produce alternative CS structures have resulted in the synthesis of CS-E. Despite the enzymatic synthesis of structurally heterogeneous CS-E polysaccharides has been primarily reported [161], the enzymatic production of homogenous CS-E oligosaccharides was more recently achieved [106]. The process included a serial elongation of p-nitrophenyl glucuronide (GlcA-pNP) with UDP-GalNAc using KfoC followed by the sulfotransferase modifications on the unsulfated backbone by C4OST and GalNAc4S-6OST. Mouse GalNAc4S-6OST was expressed in Sf9 cells, the glycotransferase KfoC and C4OST were expressed as in Li et al. [156] and the structural units UDP-GlcA, UDP-GalNAc, and the co-factor PAPS were synthesized by enzymatic approaches.
Another recently reported chemoenzymatic strategy for the synthesis of homogenous chondroitin polymers combined stepwise oligosaccharides synthesis from GalNAc and GlcA units with further enzymatic polymerization by PmCS [162]. The authors showed that PmCS needs a suitable oligosaccharide (at least a chondroitin trisaccharide) as the acceptor to trigger the in vitro chondroitin chain polymerization. The polymers were further chemically sulfated to form CS.
Even when the CS backbone is not obtained through enzymatic polymerization, enzymatic in vitro reactions can be used to participate in the post-polymerization modification of the chondroitin backbone. Rat aryl sulfotransferase IV (ASST IV) expressed in E. coli and mouse C4OST and C6OST sulfotransferases expressed in P. pastoris were used to modify a chondroitin backbone produced in vivo [158]. Instead of using sulfotransferases to add sulfate groups to the chondroitin, an alternative way to produce structurally defined CS was performed by employing a chemoenzymatic strategy for regioselective desulfation using a recombinant 4-O-endo-sulfatase from B. thetaiotaomicron [163].
Regarding the sulfate donor for sulfotransferases, PAPS, a chemoenzymatic synthesis [164], enzymatic [165] or in vivo production using engineered E. coli [166] are recent attractable strategies to supply this co-factor for the synthesis of CS. These strategies have been applied for CS-A synthesis in vitro [166] and in vivo [160].
Drawbacks of the enzymatic CS synthesis include difficulty in controlling glycosyltransferase and sulfotransferase activities for a homogenous compound with a defined sulfation pattern [136]. The chemoenzymatic CS synthesis can provide the flexibility of chemical synthesis along with the efficiency and selectivity offered by enzymes to produce more defined CS oligosaccharides [167]. Nevertheless, the poor availability of substrates and co-factors, the poor enzymes activity and consequent low CS yields are still major limitations for the implementation of enzymatic and chemoenzymatic methods at industrial scale [168].
5. Microbial production of GAGs
Biotechnological processes using microorganisms have been developed for the production of GAGs and its oligosaccharides, based on the ability of certain pathogenic microbes to produce them. This strategy offers several advantages over animal extraction or chemical synthesis, such as avoiding using expensive substrates and environment-hazard chemicals, avoiding interspecies infection or contaminations, improving homogeneity, and precisely controlling the degree of sulfation and molecular weight [1]. The main benefit of producing GAGs in vivo over the enzymatic methods is taking advantage of biological machinery to avoid the use of expensive substrates and co-factors (and instead use cheap simple carbon sources as substrates). However, the industrial biotechnological production of GAGs, and specially of sulfated GAGs such as CS, is limited due to safety concerns for culturing pathogenic microorganisms and low yields [169]. Therefore, research efforts have been focused on the improvement of the production process, as well as on the design of better microbial cell factories for production of GAGs. An overview on the metabolic pathways from native microbial GAG producers will be provided, which can give insights on the design of biosynthetic pathways for chondroitin and CS production. Then it is discussed the current state of the biotechnological production of chondroitin and CS. In Table 3, advantages and disadvantages of the available chondroitin production methods are summarized.
Table 3.
Advantages and disadvantages of different chondroitin and chondroitin derivatives production methods.
| Animal extraction | Chemical synthesis | Enzymatic production | Microbial production | |
|---|---|---|---|---|
| Substrate cost | Cheap | Expensive | Expensive | Cheap |
| Shortage of materials | Yes | No | No | No |
| Presence of contaminants (prions, viruses, growth factors) | Yes | No | No | No |
| Vegan/ vegetarian | No | Yes | Yes | Yes |
| Requires feeding co-factors | No | No | Yes | No |
| Chemical steps to obtain substrates or sulfation | No | Yes | Most times | Sometimes |
| Stereoselective and regioselective reactions | not applicable | No | Yes | Yes |
| Time-consuming process | Yes | Yes | Yes, for protein purification | No |
| Harsh conditions (pH, temperature, pressure) | Yes | Yes | No | No |
| Scale-up | Limited and expensive | Difficult | Difficult | Easy |
| Control of degree of sulfation and size | No | Yes | Yes | Yes |
| Yields | Highest | Low | Lowest | Low |
| Environmental-friendly process | No | No | Yes | Yes |
| Possibility to obtain unnatural compounds | No | Yes | Yes | Yes |
| Final product purification complexity | Very heterogeneous, polydisperse and usually contaminated with other glycosaminoglycans | Easy | Easy | May require cell lysis and purification. For pathogenic hosts, endotoxins need to be removed |
5.1. Biosynthetic pathways for microbial GAGs production
As already mentioned, native microbial GAG and GAG-like producers are pathogens that use these compounds as constituents of their capsule to camouflage their infection and improve pathogenicity [6]. In these microorganisms, the genes involved in the capsular polysaccharide production from UDP-sugars and its transport are usually expressed in the form of an operon. The pathways for the biosynthesis of microbial GAGs are represented in Fig. 3. Representative native GAG-producers are E. coli K4 for chondroitin, E. coli K5 for heparosan, and P. multocida type A for HA. Capsular polysaccharide synthesis gene cluster includes three functional regions. Region 1 and region 3 are mainly responsible for the modification, transport, and localization of newly synthesized polysaccharide chain. Region 2 is mostly responsible for encoding enzymes related to the synthesis of polysaccharides and their precursors, so it is the most variable region across strains, according to the capsular GAG produced. The polymerization of these capsular polysaccharides occurs entirely on the cytoplasm through the action of glycosyltransferases followed by transportation, known to be adenosine triphosphate (ATP)-binding cassette (ABC) transporter-dependent [4, 170, 171, 172].
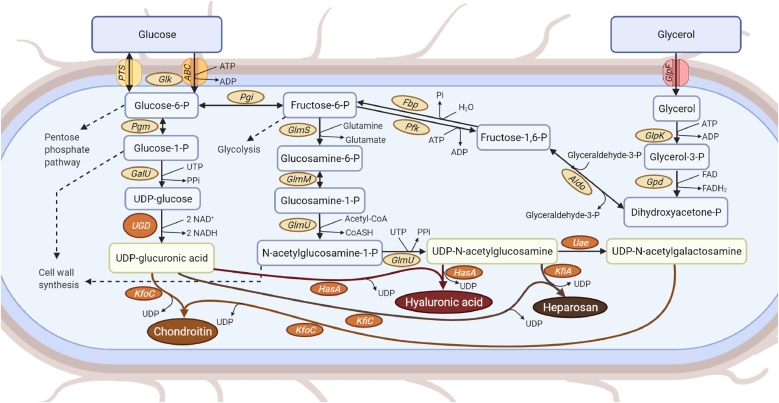
Fig. 3.
Production of glycosaminoglycans in microbes and its possible use in the biosynthesis of microbial chondroitin, hyaluronic acid or heparosan. Depending on the microbial host, the heterologous expression of the enzymes shown in orange boxes might be required for glycosaminoglycans production. Enzyme abbreviations: ABC, adenosine triphosphate (ATP)-binding cassette transporters; Aldo, fructose-6-phosphate aldolase; Fbp, fructose-1, 6-bisphosphatase; GalU, uridine triphosphate:glucose-1-phosphate uridylyltransferase; Glk, glucokinase; GlmM, phosphoglucosamine mutase; GlmS, glucosamine-6-phosphate synthase; GlmU, glucosamine-1-phosphate N-acetyltransferase/N-acetylglucosamine-1-phosphate uridyltransferase; GlpF, Glycerol uptake facilitator protein; GlpK, glycerol kinase; Gpd, glyceraldehyde-3-phosphate dehydrogenase; HasA, hyaluronan synthase; KfiA, β−1, 3-glucuronyltransferase; KfiC, α−1, 4-N-acetylglucosaminyltransferase; KfoC, chondroitin synthase; Pfk, 6-phosphofructokinase; Pgi, glucose-6-phosphate isomerase; Pgm, phosphoglucomutase; PTS, phosphotransferase system; Uae, UDP-N-acetylglucosamine 4-epimerase; UGD, uridine diphosphate (UDP)-glucose 6-dehydrogenase.
5.2. Current state of biotechnological GAGs production using microbes
Organisms producing other GAGs may comprise an important source of genes with relevance for the chondroitin heterologous pathway given that the majority of the steps involved in such production are the same.
HA is naturally produced by Streptococcus group A or C and by P. multocida type A [6, 172, 173, 174, 175, 176]. The genes required for HA biosynthesis are encoded by the HA synthesis (HAS) operon (hasA-E) in Streptococci. Since HA does not require modifications after polymerization, the product from the fermentation can be directly isolated and purified. Commercial HA has been obtained mainly by microbial fermentation of Streptococcus equi and S. zooepidermidis [176, 177].
Industrial production of sulfated GAGs is more challenging because it requires post-polymerization modifications of the microbially-produced polysaccharide backbone.
Biosynthesis of HP from microbial sources requires the microbial production of the precursor heparosan [178, 179, 180] with further chemical and/or enzymatic modifications [19, 181, 182, 183, 184]. E. coli K5, P. multocida type D and Avibacterium paragallinarum genotype II can synthesize heparosan [35, 36, 185, 186]. High titers of heparosan have been achieved using E. coli K5 (up to 15 g/L in bioreactor) [178, 179] which has the heparosan biosynthetic genes encoding glycosyltransferases and dehydrogenases organized in an operon (kfiA-D, Fig. 3). Regarding DS and KS, there have been no reports on their production using microorganisms.
Unsulfated chondroitin, a starting material for CS, is produced by P. multocida type F and A. paragallinarium genotype I [35, 36]. The pathogenic strain E. coli K4 natively synthesizes a capsular polysaccharide that shares a similar structure with the unsulfated chondroitin, but contains a residue of fructose [187]. Table 4 presents recent reported studies on in vivo production of chondroitin and CS, including in native producers and in heterologous hosts.
Table 4.
Last decade studies on microbial production of chondroitin and chondroitin sulfate by natural producer microbial strains and engineered hosts.
| GAG | Host | Substrate(s) | Genetic modification(s) | Process (working volume) | Maximal yield (mg/L) | Reference |
|---|---|---|---|---|---|---|
| Fructosylated chondroitin | E. coli O5:K4:H4 | Glycerol/ glucose | Insertion of multiple copies of the autologous rfaH gene | Shake flask | 280/ 300 | [192] |
| Batch (2.5 L) | 475/ 525 | |||||
| Fed-batch (2.5 L) | 4000/ 5100 | |||||
| Three-phase fermentation (2.5 L): batch-fed batch-in microfiltration regimen | 8400/ 9200 | |||||
| Three-phase fermentation (1000 L) | 9000/(not tested with glucose) | |||||
| E. coli O5:K4:H4 | Glycerol/ glucose | Overexpression of rfaH | Shake-flask (200 mL) | 212/ 283 | [63] | |
| Batch (2 L) | 466 | |||||
| Fed- batch (2 L) | 5300 | |||||
| E. coli O5:K4:H4 | Glycerol | Overexpression of the transcriptional regulator slyA | Shake-flask (70 mL) | 1000 | [194] | |
| Batch (4 L) | 2600 | |||||
| E. coli O5:K4:H4 | Glycerol | IS2 transposon-mediated kfoC overexpression | Shake flasks | 302 (plasmid) | [205] | |
| Batch (2 L) | 425 (integrative) | |||||
| Fed-batch (2.5–22 L) | 3470 (integrative) | |||||
| E. coli O5:K4:H4 | Glycerol | Overexpression of rfaH, pgm and galU | Shake-flask (200 mL) | 391 (with glutamine supplementation) | [65] | |
| Batch (4 mL) | 592 | |||||
| E. coli O5:K4:H4 | Glycerol | pfkA deletion, overexpression of pgm, galU, glmS, glmM and mutated kfoC, and RBS optimization | Fed-batch (30 L) | 8430 | [187] | |
| E. coli O5:K4:H4 | Glucose | Overexpression of kfoA and kfoF | Shake-flask (0.2 L) | ∼1739 (61 mg/OD) | [193] | |
| Fructosylated chondroitin | E. coli O5:K4:H4 | Glycerol | glmM, glmS | Fed-batch (2.5 L) | 3990 | [206] |
| E. coli O5:K4:H4 | Glucose | Overexpression of kfoF Overexpression of pgm, galU and kfoF |
Fed-batch (2 L) | 2000 2090 |
[190] | |
| Overexpression of pgm, galU and kfoF | Fed-batch (22 L) | 2140 | ||||
| E. coli O5:K4:H4 | Glucose/ glycerol | Wild-type | Batch in microfermenter (4 mL) and in bioreactor (1.8 L) | 315 (microfermenter); 300 (bioreactor) |
[195] | |
| Fed-batch microfermenter (3 mL) and in bioreactor (1.6 L) | 1410 (microfermenter); 1570 (bioreactor) | |||||
| Chondroitin | E. coli O10:K5:H4 | Glucose | kfoC and kfoA expression | Shake flask (20 mL) | 52.6 | [197] |
|
Streptococcus equi subsp. zooepidemicus |
Sucrose | kfoA and kfoC expression | Shake-flask (0.6 L) | 90 | [176] | |
| Batch (1.6 L) | 300 | |||||
| E. coli BL21 Star (DE3) | Glucose | kfoC, kfoA and kfoF expression in pseudo-operon gene configuration | Shake flask (25 mL) | 213 | [198] | |
| Fed-batch (1 L) | 2400 | |||||
| Bacillus subtilis 168 | Sucrose | kfoC, kfoA expression and tuaD up-regulation | Shake flask (50 mL) | 2540 | [180] | |
| Fed-batch (1.35 L) | 5220 | |||||
| Corynebacterium glutamicum | Glucose | kfoC, kfoA expression (codon-optimized), ldh deletion, and ugd overexpression | Fed-batch (2 L) | 1910 | [200] | |
| Furyl-terminated chondroitin | E. coli DH1 (K-12 derivative, lacking kps genes) | Glucose-glycerol-lactosides | Expression of kfoC, kfoG, wbpP, kfiD and mouse glcAT | Fed-batch (0.2 L) | ∼2500 | [201] |
| Chondroitin sulfate A | E. coli C2987 | Catabolizable amino acids from LB | Expression of kfoA, kfoC, kfoF (plasmid from [198]) and vgb | Shake flask (25 mL); Chemical sulfation | Not reported | [202] |
| Chondroitin sulfate A and C |
B. subtilis (chondroitin production) E. coli BL21 and Pichia pastoris (STs expression) |
Sucrose | Expression of tuaD, glmU, gtaB, glmM, glmS, and kfoA from B. subtilis E168C [180]; expression of ASST IV, C4OST and C6OST for sulfation | Fed-batch (1.35 L); Enzymatic sulfation | 7150 | [158] |
| Chondroitin sulfate A |
E. coli O5:K4:H4 (chondroitin production) E. coli K-12 MG1655 (cell lysate with PAPS) E. coli BL21 Star (DE3) (ST expression) |
Glucose (supplementation of sodium sulfate) | kfoE deletion in E. coli K4 for chondroitin backbone production; deletion of cysH, overexpression of cysDNCQ in E. coli MG1655 – provided cell lysate with PAPS for in vitro sulfation reaction; expression of C4OST in E. coli BL21 for sulfation | Shake flask (1 L) Enzymatic sulfation (50 µL) |
Not reported for in vivo production In vitro: 8.3 ng chondroitin sulfate A with 0.035% sulfation |
[166] |
| E. coli O5:K4:H4 | Glucose | kfoE deletion, C4OST expression and engineering, cysH deletion | Shake-flask (25 mL) | 0.01076 (extracellular) (126.64 µg/g dry cell weight intracellular) |
[160] | |
| E. coli K-12 MG1655 | Glucose | kfoC, kfoA, kfoF, engineered C4OST expression, cysH deletion | Shake-flask (25 mL) Fed-batch (1 L) |
0 (extracellular; chondroitin sulfate was only produced intracellularly- 13.14 µg/g dry cell weight intracellular) | ||
| Pichia pastoris | Methanol | kfoC, kfoA, tuaD, C4OST expression, overexpression of endogenous genes coding ATPS and APSK | Fed-batch (0.9 L) | 2100 with 4.0% sulfation | [204] | |
| N-glycolyl chondroitin | E. coli O5:K4:H4 | Glucose and N-glycolylglucosamine | kfoE deletion | Shake-flask (100 mL) | ∼300 | [196] |
Genes: APSK, adenosine-5′-phosphosulfate kinase; ASST IV, aryl sulfotransferase IV; ATPS, adenosine-5′-triphosphate sulfurylase; C4OST, chondroitin 4-sulfotransferase; C6OST, chondroitin 6-sulfotransferase; cysC, adenylyl-sulfate kinase; cysDN, adenosine triphosphate sulfurylase; cysH, 3′-phosphoadenosine-5′-phosphosulfate (PAPS) reductase; cysQ, adenosine-3′, 5′-bisphosphate nucleotidase; galU, uridine triphosphate-glucose-1-phosphate uridylyltransferase; glcAT, β−1, 3-glucuronyl transferase; glmM, phosphoglucosamine mutase; glmS, glucosamine-6-phosphate synthase; glmU, glucosamine-1-phosphate N-acetyltransferase/N-acetylglucosamine-1-phosphate uridyltransferase; kfiD, uridine diphosphate-glucose 6-dehydrogenase from E. coli K5; kfoA, uridine diphosphate-acetylglucosamine 4-epimerase from E. coli K4; kfoC, chondroitin synthase from E. coli K4; kfoE, fructosyltransferase; kfoF, uridine diphosphate-glucose 6-dehydrogenase from E. coli K4; kfoG, chondroitin synthase protein helper from E. coli K4; kps, surface polysaccharide synthesis genes; ldh, lactate dehydrogenase; pfkA, adenosine triphosphate-dependent 6-phosphofructokinase; pgm, phosphoglucomutase; rfaH, transcription antitermination protein; slyA, transcriptional regulator; tuaD, uridine diphosphate-glucose 6-dehydrogenase from Bacillus subtilis; ugd, uridine diphosphate-glucose 6-dehydrogenase; vgb, Vitreoscilla hemoglobin; wbpP, uridine diphosphate-acetylglucosamine 4-epimerase from Pseudomonas aeruginosa. Other abbreviations: PAPS, 3′-phosphoadenosine-5′-phosphosulfate; RBS, ribosome binding site; ST, sulfotransferase.
E. coli K4 became the first and most studied microorganism to be used for the biotechnological production of chondroitin [37]. In fact, food grade CS has been industrially produced by microbial fermentation. The native producer E. coli K4 is used to produce microbial K4 polysaccharide followed by its chemical defructosylation and regioselective sulfation into CS sodium. The product is sold under the name of Mythocondro™ (marketed since 2017 by Gnosis S.p.A., which was acquired by Lesaffre in 2018).
The genes required for fructosylated chondroitin production and transportation in E. coli K4 are organized in an operon containing kfoA-G genes. From the seven constituent genes, the most relevant genes in the biosynthesis of fructosylated chondroitin are: kfoA encoding UDP-GlcNAc 4-epimerase that provides UDP-GalNAc; kfoC encoding chondroitin synthase (homolog to PmCS); and kfoF encoding UDP-glucose dehydrogenase that provides UDP-GlcA. Fructosylated chondroitin can undergo a subsequent step of hydrolysis of fructose monomer and chemical sulfation [188, 189, 190]. The identification of the enzyme responsible for inserting the fructose residue (KfoE, fructosyltransferase) and its consequent deletion led to the production of unsulfated chondroitin [191]. Genetic engineering has been applied to E. coli K4 to further improve the polysaccharide yields [61, 63, 187, 190, 192, 193, 194, 195]. In fact, the highest reported yields so far for biotechnological production of chondroitin have been achieved with genetically engineered E. coli K4 through fed-batch fermentations, reaching 8.43 – 9.2 g/L of fructosylated chondroitin [187, 192]. Feeding alternative synthetic precursors to the fermentation medium is a possible strategy to synthesize rare or unnatural chondroitin derivatives in vivo. In the work of Awofiranye et al. [196], N‑glycolyl chondroitin has been produced from chemically synthesized precursors (N-glycolylglucosamine replacing GlcNAc in the pathway) in E. coli K4 lacking kfoE gene.
The first study aiming to produce chondroitin in a heterologous host used the pathogenic bacteria E. coli K5 expressing kfoC and kfoA from E. coli K4 and 52.6 mg/L of chondroitin was produced without the need for defructosylation [197]. Other pathogen, HA producer S. zooepidemicus, was also attempted as an heterologous host for chondroitin using the same genes [176] but it also resulted in low yields (300 mg/L).
To avoid the contamination of the final product with virulence factors and toxins and to eliminate the defructosylation step, alternative microorganisms have been engineered with K4 polysaccharide production pathway. The chondroitin production in non-pathogenic heterologous hosts has been achieved in E. coli BL21 (DE3) [198], B. subtilis [158, 180, 199], Corynebacterium glutamicum [200] and E. coli K-12 [160, 201]. Using safer heterologous hosts, the highest chondroitin yield reported (2.4 g/L) was achieved by expressing kfoC, kfoA, kfoF in E. coli BL21 using the high copy number vector pETM6, with a pseudo-operon gene configuration [198].
The chondroitin backbone can be further modified through the action of various sulfotransferases to produce CS, or it can undergo epimerization of GlcA into IdoA and subsequent sulfation to generate DS. Several studies combined the microbial in vivo production of chondroitin with enzymatic or chemical strategies to produce CS [158, 166, 202]. By combining the in vivo production of chondroitin backbone and in vitro enzymatic sulfation, CS-A and CS-C production has been achieved [158]. Bacillus subtilis harboring the kfoA and kfoC genes from E. coli K4 was engineered. E. coli was used to express rat aryl sulfotransferase IV (ASST IV) and P. pastoris was the expression host for mouse C4OST and C6OST sulfotransferases.
In the work of Erenler [202], a non-pathogenic E. coli strain was engineered to express a previously described biosynthetic pathway for chondroitin production [198] along with a Vitreoscilla hemoglobin gene (vgb) whose expression has previously been reported to provide benefits to bacteria growth and recombinant protein expression and improved accumulation of different biopolymers [203]. The authors produced and purified the microbial chondroitin and performed chemical sulfation. Recently, the construction of the complete pathway for in vivo production of sulfated chondroitin in E. coli has been reported [160]. The authors evaluated both E. coli K4 and K-12 (containing K4 kfoCAF chondroitin genes) with expression of C4OST and PAPS reductase (cysH) deletion (to improve the pool of sulfate donor PAPS) for CS production. Only E. coli K4 was able to produce extracellular CS (10.76 µg/L in shaken flasks), and the intracellular yield was also higher than the one found for the engineered E. coli K-12. However, the percentage of sulfation of intracellular CS was 36% higher in the K-12 strain. After improving the activity of C4OST through mutation, optimizing growth and C4OST induction conditions, and inhibiting transport system through clustered regularly interspaced short palindromic repeats interference (CRISPRi), the levels of sulfation (∼55%) reached the same ones achieved in K-12. The authors also optimized the fermentation process in bioreactor using K-12 which resulted in 27 μg/g dry cell weight of intracellular CS-A with a sulfation degree of 96%, a level of 4-O-sulfation similar to the ones found in animals. More recently, P. pastoris has been successfully engineered towards the production of CS-A using methanol as substrate [204]. The expression of kfoC and kfoA from E. coli K4, tuaD from B. subtilis, engineered mouse C4OST and overexpression of endogenous genes coding adenosine-5′-triphosphate sulfurylase (ATPS) and adenosine-5′-phosphosulfate kinase (APSK) to improve PAPS supply and sulfation, resulted in a production of 2.1 g/L CS-A with 4.0% sulfation using a fed-batch fermentation. The integrated approach from these two recent studies [160, 204] provides significant cost reduction to the process. However, the CS yields in E. coli are still low while the CS produced by engineered P. pastoris has a low sulfation degree. Therefore, more efforts are required to genetically improve the host and to optimize the process towards interesting yields and sulfation degrees for biotechnological applications.
5.3. Metabolic engineering to optimize CS production
Despite the recent advances on biotechnological production of CS, the yields are still not sufficient to meet the increasing demand of this widely used nutraceutical. Metabolic engineering of the host microorganisms for improving CS precursors (UDP-GlcA, UDP-GlcNAc and UDP-GalNAc) production pathways should be performed to improve intermediate pools and their subsequent availability.
In order to redirect the metabolic flux towards chondroitin or CS production in microbes, the most obvious modification is the overexpression of chondroitin polymerase/synthase which in E. coli K4 is encoded by kfoC. When kfoC was overexpressed in E. coli K4 using plasmids, the fructosylated chondroitin yield improved 2-fold compared to the wild-type [61]. When the kfoC overexpression was IS2 transposon-mediated, the K4 production was 2.5 times higher than the wild-type [205]. Strategies to improve KfoC enzymatic activity have also been addressed. By using random mutagenesis it was possible to improve KfoC activity (R313Q) that led to 82% improvement on fructosylated chondroitin over the wild-type [207].
Regulatory elements of expression of the bacterial GAG-like polysaccharides have also been targets for genetic engineering. The overexpression of the transcriptional activators rfaH [63] and slyA [194] have enhanced the expression of capsular genes and consequently improved E. coli K4 polysaccharide production by 58% and by a 1.5-fold, respectively, over the wild-type. RfaH acts on capsular gene cluster by preventing the transcript termination of genes related to the modification, transport, and localization of newly synthesized polysaccharide chain (region 3 genes), and consequently improve the expression of genes related to the synthesis of polysaccharides and their precursors (region 2 genes) [208]. SlyA activates the transcription of the whole capsular gene operon [194].
Regarding the precursor availability, balancing UDP-sugars is an established strategy for metabolic engineering of HA [209] and fructosylated chondroitin [187]. The genes pgm, galU and ugd/kfoF (encoding phosphoglucomutase, uridine triphosphate-glucose-1-phosphate uridylyltransferase and UDP-glucose dehydrogenase, respectively) for UDP-GlcA synthesis and glmS, glmM and glmU (encoding glucosamine-6-phosphate synthase, phosphoglucosamine mutase and glucosamine-1-phosphate N-acetyltransferase/N-acetylglucosamine-1-phosphate uridyltransferase, respectively) for UDP-GalNAc production might be interesting targets for overexpression (Fig. 3). Overexpression of these genes have resulted in improved production of K4 and other polysaccharides. Levander and coworkers [210] found that the overexpression of the endogenous galU gene in S. thermophilus LY03 led to a 10-fold increase in galU activity, however exopolysaccharide yield was not affected. Nevertheless, when galU was overexpressed in combination with pgmA (pgm homolog), the exopolysaccharide yield increased. Overexpression of pgm and galU in E. coli AD202 also resulted in increased UDP-galactose derived disaccharides from 2.5 mM to 20 mM by improvement of carbon flux through UDP-glucose synthesis pathway [211]. Engineering E. coli K4 with one copy of endogenous genes pgm and galU also improved capsular polysaccharide production [65]. The authors further increased capsular polysaccharide yields in 45% using glutamine supplementation to boost UDP-GalNAc precursor production [63, 65]. Engineering Saccharomyces cerevisiae with the endogenous genes pgm2 and ugp1 (equivalent to galU) resulted in a 17% improvement of scutellarein 7-O-glucoside production rate by improving UDP-glucose pool [212]. Overexpressing glmM and glmU genes resulted in higher capsular polysaccharide production in E. coli K4 [206]. Despite the step catalyzed by ugd-codified enzyme was considered in previous works as the limiting factor of GAG biosynthesis in homologous and heterologous organisms [61, 180, 213, 214], few examples evaluating alternative genes for this step have been reported, possibly missing interesting catalysts.
On the other hand, the repression of genes from competing pathways can also be beneficial for redirecting the metabolic flux towards the production of GAG precursors. Down-regulating the expression of three genes (glucose-6-phosphate 1-dehydrogenase zwf, adenosine triphosphate-dependent 6-phosphofructokinase pfkA, and glmM) that control the major competing reactions (cell wall recycling pathways) of GlcNAc synthesis by CRISPRi improved GlcNAc titers in B. subtilis [215]. Silencing zwf and pfkA also resulted in improved HA production in B. subtilis [216]. By favoring the production of fructose 6-phosphate, pfkA knock-out resulted in increased chondroitin production in E. coli K4 [187].
Combination of both repression and activation can provide optimized microbial cell flux towards GAGs production. Knock-out of pfkA and zwf genes coupled with overexpression of the genes galU-ugd and glmS-glmM-glmU improved HA production [217] in E. coli K-12 W3110 harboring HA synthase gene (hasA) from S. zooepidemicus, and with transcriptional repressors genes galR and galS deleted.
When the intended biosynthetic pathway has intermediates that are critical for biomass production, as it is the case for chondroitin, redirecting the carbon flux might result in lower yield and growth rate. To assess this question, there have been interesting advances on dynamical control to balance the metabolic flux according to the intermediate concentration. This strategy has been applied for overproduction of GlcNAc in B. subtilis which consisted in engineering the native glucosamine-6-phosphate responsive glmS ribozyme switch to act as a sensor and dynamically control the metabolic flux [218]. This strategy increased about 2-fold the native GlcNAc titer, being a potential strategy to improve chondroitin producing strains.
Finally, computational methods can be useful to aid on the prediction of potential targets for improving CS production in heterologous hosts. The use of stoichiometric or kinetic models of the heterologous host to perform in silico predictions has been shown to be useful to guide engineering strategies towards the improved production of valuable compounds. In fact, a computational approach was already performed to engineer an optimal HA production in C. glutamicum [219].
Sophisticated genetic engineering tools for genome editing, such as CRISPR [220], are shown to be rapid and cost-effective and, therefore, can be implemented on the intended system to generate engineered hosts with improved chondroitin production.
5.4. Alternative hosts
Alternative hosts can provide more suitable platforms for CS production. Besides replacing the native pathogenic bacteria and, consequently, avoiding the presence of virulence factors in the final product, the application of industrially used E. coli, B. subtilis, and Corynebacterium for CS production has the advantages of being fast-growing organisms, with well-characterized genetic and physiological backgrounds, and many available tools for gene expression and genome editing [221].
However, biosynthetic enzymes expressed in bacterial systems usually present low solubility, stability, and activity, which may limit CS synthesis. On the other hand, enzymes expressed in eukaryotes typically show enhanced activity but are expressed at lower amounts [167]. Specially for the expression of eukaryotic genes, in particular sulfotransferases, eukaryotic microbial cells such as the broadly used S. cerevisiae and P. pastoris can provide beneficial conditions regarding codon preference, enzyme folding and glycosylation patterns more similar to the original enzymes. Also, since mammalian CHO cells have shown to be able to produce HP [222], animal cell fermentation can also be a possible, however expensive, alternative strategy for the production of CS.
5.5. Additional optimization strategies
Besides the metabolic engineering strategies to improve intermediate pools, or the use of alternative hosts, other approaches to make biotechnological CS production more cost-effective include screening for more efficient enzymes, modification of the enzymes through protein engineering and immobilization to improve activity and productivity. Also, salvage pathways can be added to improve precursors – for example, UDP-GlcA can also be produced in two different ways directly from GlcA using glucuronokinase, UDP-sugar pyrophosphorylase, and inorganic phosphatase instead of depending on UDP-glucose conversion through ugd step. Increasing energy through ATP supply, and implementing ATP regeneration systems can also benefit the CS in vivo production. The control of CS molecular weight in fermentation is also desirable to achieve a more monodisperse product [190, 223]. In addition, optimization of the fermentation process, media composition, temperature and pH conditions have been shown to be effective for improved GAG yields [178, 224]. Using low-cost media can provide a more competitive process, namely agro-industrial by-products such as sugarcane molasses and corn steep liquor which have already been used for HA production [225, 226, 227], therefore they present potential applicability for CS production. Additionally, engineering of CS transportation can aid to improve CS titers, by even repressing transporters for increased CS intracellular in vivo sulfation [160], or by contrarily strengthening cell CS exportation, which would be more valuable from an industrial point of view but would require the extracellular expression of the sulfotransferases. Finally, optimizing environmental conditions for maximal enzyme activity to perform the post-polymerization modifications of biotechnological CS polysaccharides in vivo or in vitro would aid to obtain a final commercial sulfated product at competitive yields.
6. Conclusions and future perspectives
Compared to animal-derived products, heterologous microbes can provide pathogen-free chondroitin without the use of hazardous chemicals. With the increasing applications of CS, the studies on its microbial production have been growing with much focus on the native GAG producers’ pathway to polymerize the backbone starting material, and on its post-polymerization modifications through enzymatic in vitro reactions. However, there are bottlenecks such as insufficient supply of precursors, low activity and/or stability of the enzymes and difficult and costly sulfotransferase enzymes purification. Strategies that can help solving these issues include genetic engineering for improving the precursors pool and transport; evaluation of alternative hosts for efficient expression of enzymes (since most genes required for sulfated GAGs production are from animals, expression in eukaryotic hosts could provide more soluble and active enzymes); improvement of enzyme activity and solubility through enzyme engineering and environment optimization; and the use of integrated approaches of microbial production and post-polymerization. The knowledge improvement on GAGs enzymes for polymerization, transport and post-polymerization, together with the development of efficient technologies for gene editing, are expected to lead to the establishment of efficient microbial cell factories for the CS production.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020 unit. The authors acknowledge FCT for funding the doctoral grant SFRH/BD/132998/2017 to Couto, M. R.
References
- 1.Kang Z., Zhou Z., Wang Y., Huang H., Du G., Chen J. Bio-based strategies for producing glycosaminoglycans and their oligosaccharides. Trends Biotechnol. 2018;36:806–818. doi: 10.1016/j.tibtech.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Mende M., Bednarek C., Wawryszyn M., Sauter P., Biskup M.B., Schepers U., Bräse S. Chemical synthesis of glycosaminoglycans. Chem. Rev. 2016;116:8193–8255. doi: 10.1021/acs.chemrev.6b00010. [DOI] [PubMed] [Google Scholar]
- 3.Casale J., Crane J.S. StatPearls Publishing; Treasure Island (FL): 2021. Biochemistry, Glycosaminoglycans.http://www.ncbi.nlm.nih.gov/pubmed/31335015 (accessed July 4, 2021) [PubMed] [Google Scholar]
- 4.A. Varki, R.D. Cummings, J.D. Esko, P. Stanley, G.W. Hart, M. Aebi, A.G. Darvill, T. Kinoshita, N.H. Packer, J.H. Prestegard, R.L. Schnaar, P.H. Seeberger, Essentials of Glycobiology, Cold Spring Harb. (2017) 823. https://www.ncbi.nlm.nih.gov/books/NBK310274/ (accessed July 24, 2021).
- 5.Kovensky J., Grand E., Uhrig M.L. In: Ind. Appl. Renew. Biomass Prod. Goyanes S.N., D'Accorso N.B., editors. Springer International Publishing; Cham: 2017. Applications of glycosaminoglycans in the medical, veterinary, pharmaceutical, and cosmetic fields; pp. 135–164. [DOI] [Google Scholar]
- 6.Cress B.F., Englaender J.A., He W., Kasper D., Linhardt R.J., Koffas M.A.G. Masquerading microbial pathogens: capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol. Rev. 2014;38:660–697. doi: 10.1111/1574-6976.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang K.W., Weng S.F., Tseng Y.H. UDP-glucose dehydrogenase gene of Xanthomonas campestris is required for virulence. Biochem. Biophys. Res. Commun. 2001;287:550–555. doi: 10.1006/bbrc.2001.5591. [DOI] [PubMed] [Google Scholar]
- 8.Guan L., Zhang L., Xue Y., Yang J., Zhao Z. Molecular pathogenesis of the hyaluronic acid capsule of Pasteurella multocida. Microb. Pathog. 2020;149 doi: 10.1016/j.micpath.2020.104380. [DOI] [PubMed] [Google Scholar]
- 9.Köwitsch A., Zhou G., Groth T. Medical application of glycosaminoglycans: a review. J. Tissue Eng. Regen. Med. 2018;12:e23–e41. doi: 10.1002/term.2398. [DOI] [PubMed] [Google Scholar]
- 10.Volpi N. Therapeutic Applications of glycosaminoglycans. Curr. Med. Chem. 2006;13:1799–1810. doi: 10.2174/092986706777452470. [DOI] [PubMed] [Google Scholar]
- 11.F. Zhang, Z. Zhang, R.J. Linhardt, Glycosaminoglycans, in: Handbook of Glycomics, 2009: pp. 59–80. 10.1016/B978-0-12-373600-0.00003-2.
- 12.Abdallah M.M., Fernández N., Matias A.A., do R. Bronze M. Hyaluronic acid and chondroitin sulfate from marine and terrestrial sources: extraction and purification methods. Carbohydr. Polym. 2020;243 doi: 10.1016/j.carbpol.2020.116441. [DOI] [PubMed] [Google Scholar]
- 13.Cimini D., Restaino O.F., Schiraldi C. Microbial production and metabolic engineering of chondroitin and chondroitin sulfate. Emerg. Top. Life Sci. 2018;2:349–361. doi: 10.1042/ETLS20180006. [DOI] [PubMed] [Google Scholar]
- 14.Tian R., Liu Y., Liu L. In: Systems and Synthetic Biotechnology Production of Nutraceuticals. Liu L., Chen J., editors. Springer Singapore; Singapore: 2019. Microbial production of oligosaccharides and polysaccharides; pp. 75–91. [DOI] [Google Scholar]
- 15.Pomin V.H., Vignovich W.P., Gonzales A.V., Vasconcelos A.A., Mulloy B. Galactosaminoglycans: medical applications and drawbacks. Molecules. 2019;24:1–33. doi: 10.3390/molecules24152803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vessella G., Vázquez J.A., Valcárcel J., Lagartera L., Monterrey D.T., Bastida A., García-junceda E., Bedini E., Fernández-mayoralas A., Revuelta J. Deciphering structural determinants in chondroitin sulfate binding to FGF-2: paving the way to enhanced predictability of their biological functions. Polymers (Basel) 2021;13:1–15. doi: 10.3390/polym13020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malavaki C., Mizumoto S., Karamanos N., Sugahara K. Recent advances in the structural study of functional chondroitin sulfate and dermatan sulfate in health and disease. Connect. Tissue Res. 2008;49:133–139. doi: 10.1080/03008200802148546. [DOI] [PubMed] [Google Scholar]
- 18.Vessella G., Traboni S., Cimini D., Iadonisi A., Schiraldi C., Bedini E. Development of semisynthetic, regioselective pathways for accessing the missing sulfation patterns of chondroitin sulfate. Biomacromolecules. 2019;20:3021–3030. doi: 10.1021/acs.biomac.9b00590. [DOI] [PubMed] [Google Scholar]
- 19.P. Datta, R.J. Linhardt, S.T. Sharfstein, Industrial production of glycosaminoglycans, in: Encycl. Microbiol., Elsevier, 2019: pp. 681–690. 10.1016/B978-0-12-809633-8.12224-1.
- 20.Maeda N., Ishii M., Nishimura K., Kamimura K. Functions of chondroitin sulfate and heparan sulfate in the developing brain. Neurochem. Res. 2011;36:1228–1240. doi: 10.1007/s11064-010-0324-y. [DOI] [PubMed] [Google Scholar]
- 21.Kwok J.C.F., Warren P., Fawcett J.W. Chondroitin sulfate: a key molecule in the brain matrix. Int. J. Biochem. Cell Biol. 2012;44:582–586. doi: 10.1016/j.biocel.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Mikami T., Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta - Gen. Subj. 2013;1830:4719–4733. doi: 10.1016/j.bbagen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Dinglasan R.R., Alaganan A., Ghosh A.K., Saito A., Van Kuppevelt T.H., Jacobs-Lorena M. Plasmodium falciparum ookinetes require mosquito midgut chondroitin sulfate proteoglycans for cell invasion. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15882–15887. doi: 10.1073/pnas.0706340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kastana P., Choleva E., Poimenidi E., Karamanos N., Sugahara K., Papadimitriou E. Insight into the role of chondroitin sulfate E in angiogenesis. FEBS J. 2019;286:2921–2936. doi: 10.1111/febs.14830. [DOI] [PubMed] [Google Scholar]
- 25.Trowbridge J.M., Gallo R.L. Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology. 2002;12:117R–125R. doi: 10.1093/glycob/cwf066. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg L.C., Choi H.U., Tang L.H., Johnson T.L., Pal S., Webber C., Reiner A., Poole A.R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J. Biol. Chem. 1985;260:6304–6313. doi: 10.1016/s0021-9258(18)88971-2. [DOI] [PubMed] [Google Scholar]
- 27.Sugahara K., Mikami T., Uyama T., Mizuguchi S., Nomura K., Kitagawa H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003;13:612–620. doi: 10.1016/j.sbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 28.E.H. Song, J. Shang, D.M. Ratner, Polysaccharides, in: Polym. Sci. A Compr. Ref. 10 Vol. Set, Elsevier, 2012: pp. 137–155. 10.1016/B978-0-444-53349-4.00246-6.
- 29.Vallières M., du Souich P. Modulation of inflammation by chondroitin sulfate. Osteoarthr. Cartil. 2010;18:S1–S6. doi: 10.1016/j.joca.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Tykesson E., Maccarana M., Thorsson H., Liu J., Malmström A., Ellervik U., Westergren-Thorsson G. Recombinant dermatan sulfate is a potent activator of heparin cofactor II-dependent inhibition of thrombin. Glycobiology. 2019;29:446–451. doi: 10.1093/glycob/cwz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tovar A.M.F., De Mattos D.A., Stelling M.P., Sarcinelli-Luz B.S.L., Nazareth R.A., Mourão P.A.S. Dermatan sulfate is the predominant antithrombotic glycosaminoglycan in vessel walls: implications for a possible physiological function of heparin cofactor II. Biochim. Biophys. Acta - Mol. Basis Dis. 2005;1740:45–53. doi: 10.1016/j.bbadis.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Ida M., Shuo T., Hirano K., Tokita Y., Nakanishi K., Matsui F., Aono S., Fujita H., Fujiwara Y., Kaji T., Oohira A. Identification and functions of chondroitin sulfate in the milieu of neural stem cells. J. Biol. Chem. 2006;281:5982–5991. doi: 10.1074/jbc.M507130200. [DOI] [PubMed] [Google Scholar]
- 33.M.K. Sarangi, M.E.B. Rao, V. Parcha, D.K. Yi, S.S. Nanda, Marine polysaccharides for drug delivery in tissue engineering, in: Nat. Polysaccharides Drug Deliv. Biomed. Appl., Elsevier, 2019: pp. 513–530. 10.1016/B978-0-12-817055-7.00022-4.
- 34.Liaw P.C.Y., Becker D.L., Stafford A.R., Fredenburgh J.C., Weitz J.I. Molecular basis for the susceptibility of fibrin-bound thrombin to inactivation by heparin cofactor II in the presence of dermatan sulfate but not heparin. J. Biol. Chem. 2001;276:20959–20965. doi: 10.1074/jbc.M010584200. [DOI] [PubMed] [Google Scholar]
- 35.DeAngelis P.L., Gunay N.S., Toida T., Mao W., Linhardt R.J. Identification of the capsular polysaccharides of Type D and F Pasteurella multocida as unmodified heparin and chondroitin, respectively. Carbohydr. Res. 2002;337:1547–1552. doi: 10.1016/S0008-6215(02)00219-7. [DOI] [PubMed] [Google Scholar]
- 36.Wu J.-.R., Chen P.-.Y., Shien J.-.H., Shyu C.-.L., Shieh H.K., Chang F., Chang P.-.C. Analysis of the biosynthesis genes and chemical components of the capsule of Avibacterium paragallinarum. Vet. Microbiol. 2010;145:90–99. doi: 10.1016/j.vetmic.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Cimini D., Restaino O.F., Catapano A., De Rosa M., Schiraldi C. Production of capsular polysaccharide from Escherichia coli K4 for biotechnological applications. Appl. Microbiol. Biotechnol. 2010;85:1779–1787. doi: 10.1007/s00253-009-2261-8. [DOI] [PubMed] [Google Scholar]
- 38.Gargiulo V., Lanzetta R., Parrilli M., De Castro C. Structural analysis of chondroitin sulfate from Scyliorhinus canicula: a useful source of this polysaccharide. Glycobiology. 2009;19:1485–1491. doi: 10.1093/glycob/cwp123. [DOI] [PubMed] [Google Scholar]
- 39.Sugahara K., Masuda M., Harada T., Yamashina I., de Waard P., Vliegenthart J.F.G. Structural studies on sulfated oligosaccharides derived from the carbohydrate-protein linkage region of chondroitin sulfate proteoglycans of whale cartilage. J. Biol. Chem. 1991;202:805–811. doi: 10.1111/j.1432-1033.1991.tb16436.x. [DOI] [PubMed] [Google Scholar]
- 40.Bedini E., Corsaro M.M., Fernández-Mayoralas A., Iadonisi A. Springer; Cham: 2019. Chondroitin, Dermatan, Heparan, and Keratan Sulfate: Structure and Functions. [DOI] [Google Scholar]
- 41.Sugahara K., Ohi Y., Harada T., de Waard P., Vliegenthart J.F.G. Structural studies on sulfated oligosaccharides derived from the carbohydrate-protein linkage region of chondroitin 6-sulfate proteoglycans of shark cartilage. I. Six compounds containing 0 or 1 sulfate and/or phosphate residue. J. Biol. Chem. 1992;267:6027–6035. doi: 10.1016/s0021-9258(18)42658-0. [DOI] [PubMed] [Google Scholar]
- 42.Yutsudo N., Kitagawa H. Involvement of chondroitin 6-sulfation in temporal lobe epilepsy. Exp. Neurol. 2015;274:126–133. doi: 10.1016/j.expneurol.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q., Li J., Liu C., Song C., Li P., Yin F., Xiao Y., Li J., Jiang W., Zong A., Zhang X., Wang F. Protective effects of low molecular weight chondroitin sulfate on amyloid beta (Aβ)-induced damage in vitro and in vivo. Neuroscience. 2015;305:169–182. doi: 10.1016/j.neuroscience.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Nadanaka S., Clement A., Masayama K., Faissner A., Sugahara K. Characteristic hexasaccharide sequences in octasaccharides derived from shark cartilage chondroitin sulfate D with a neurite outgrowth promoting activity. J. Biol. Chem. 1998;273:3296–3307. doi: 10.1074/jbc.273.6.3296. [DOI] [PubMed] [Google Scholar]
- 45.Rauch U., Gao P., Janetzko A., Flaccus A., Hilgenberg L., Tekotte H., Margolis R.K., Margolis R.U. Isolation and characterization of developmentally regulated chondroitin sulfate and chondroitin/keratin sulfate proteoglycans of brain identified with monoclonal antibodies. J. Biol. Chem. 1991;266:14785–14801. doi: 10.1016/s0021-9258(18)98755-7. [DOI] [PubMed] [Google Scholar]
- 46.Zhang B., Chi L. Chondroitin sulfate/dermatan sulfate-protein interactions and their biological functions in human diseases: implications and analytical tools. Front. Cell Dev. Biol. 2021;9:1–13. doi: 10.3389/fcell.2021.693563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng C., Wang Q., Jiao R., Xu Y., Han N., Wang W., Zhu C., Li F. A novel chondroitin sulfate E from Dosidicus gigas cartilage and its antitumor metastatic activity. Carbohydr. Polym. 2021;262 doi: 10.1016/j.carbpol.2021.117971. [DOI] [PubMed] [Google Scholar]
- 48.Stevens R.L., Fox C.C., Lichtenstein L.M., Austen K.F. Identification of chondroitin sulfate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc. Natl. Acad. Sci. U. S. A. 1988;85:2284–2287. doi: 10.1073/pnas.85.7.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ustyuzhanina N.E., Bilan M.I., Dmitrenok A.S., Nifantiev N.E., Usov A.I. Two fucosylated chondroitin sulfates from the sea cucumber Eupentacta fraudatrix. Carbohydr. Polym. 2017;164:8–12. doi: 10.1016/j.carbpol.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 50.Ustyuzhanina N.E., Bilan M.I., Panina E.G., Sanamyan N.P., Dmitrenok A.S., Tsvetkova E.A., Ushakova N.A., Shashkov A.S., Nifantiev N.E., Usov A.I. Structure and anti-inflammatory activity of a new unusual fucosylated chondroitin sulfate from Cucumaria djakonovi. Mar. Drugs. 2018;16:389. doi: 10.3390/md16100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ustyuzhanina N.E., Bilan M.I., Dmitrenok A.S., Borodina E.Y., Stonik V.A., Nifantiev N.E., Usov A.I. A highly regular fucosylated chondroitin sulfate from the sea cucumber Massinium magnum: structure and effects on coagulation. Carbohydr. Polym. 2017;167:20–26. doi: 10.1016/j.carbpol.2017.02.101. [DOI] [PubMed] [Google Scholar]
- 52.Ustyuzhanina N.E., Bilan M.I., Dmitrenok A.S., Shashkov A.S., Nifantiev N.E., Usov A.I. The structure of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria frondosa. Carbohydr. Polym. 2017;165:7–12. doi: 10.1016/j.carbpol.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Mou J., Wang C., Li W., Yang J. Purification, structural characterization and anticoagulant properties of fucosylated chondroitin sulfate isolated from Holothuria mexicana. Int. J. Biol. Macromol. 2017;98:208–215. doi: 10.1016/j.ijbiomac.2017.01.123. [DOI] [PubMed] [Google Scholar]
- 54.Chen S., Xue C., Yin L., Tang Q., Yu G., Chai W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011;83:688–696. doi: 10.1016/j.carbpol.2010.08.040. [DOI] [Google Scholar]
- 55.Kinoshita-Toyoda A., Yamada S., Haslam S.M., Khoo K.H., Sugiura M., Morris H.R., Dell A., Sugahara K. Structural determination of five novel tetrasaccharides containing 3-O-sulfated d-glucuronic acid and two rare oligosaccharides containing a β-d-glucose branch isolated from squid cartilage chondroitin sulfate E. Biochemistry. 2004;43:11063–11074. doi: 10.1021/bi049622d. [DOI] [PubMed] [Google Scholar]
- 56.Ueoka C., Nadanaka S., Seno N., Khoo K.H., Sugahara K. Structural determination of novel tetra- and hexasaccharide sequences isolated from chondroitin sulfate H (oversulfated dermatan sulfate) of hagfish notochord. Glycoconj. J. 1999;16:291–305. doi: 10.1023/A:1007022229813. [DOI] [PubMed] [Google Scholar]
- 57.Higashi K., Okamoto Y., Mukuno A., Wakai J., Hosoyama S., Linhardt R.J., Toida T. Functional chondroitin sulfate from Enteroctopus dofleini containing a 3-O-sulfo glucuronic acid residue. Carbohydr. Polym. 2015;134:557–565. doi: 10.1016/j.carbpol.2015.07.082. [DOI] [PubMed] [Google Scholar]
- 58.Sugahara K., Tanaka Y., Yamada S., Seno N., Kitagawa H., Haslam S.M., Morris H.R., Dell A. Novel sulfated oligosaccharides containing 3-O-sulfated glucuronic acid from king crab cartilage chondroitin sulfate K: unexpected degradation by chondroitinase ABC. J. Biol. Chem. 1996;271:26745–26754. doi: 10.1074/jbc.271.43.26745. [DOI] [PubMed] [Google Scholar]
- 59.Kitagawa H., Tanaka Y., Yamada S., Seno N., Haslam S.M., Morris H.R., Dell A., Sugahara K. A novel pentasaccharide sequence GlcA(3-sulfate)(β1-3)GalNAc(4-sulfate)(β1-4)(Fucα1-3)GlcA(β1-3)GalNAc(4-sulfate) in the oligosaccharides isolated from king crab cartilage chondroitin sulfate K and its differential susceptibility to chondroitinases and hyalurinidase. Biochemistry. 1997;36:3998–4008. doi: 10.1021/bi962740j. [DOI] [PubMed] [Google Scholar]
- 60.Kinoshita A., Yamada S., Haslam S.M., Morris H.R., Dell A., Sugahara K. Isolation and structural determination of novel sulfated hexasaccharides from squid cartilage chondroitin sulfate E that exhibits neuroregulatory activities. Biochemistry. 2001;40:12654–12665. doi: 10.1021/bi015577n. [DOI] [PubMed] [Google Scholar]
- 61.Cimini D., De Rosa M., Viggiani A., Restaino O.F., Carlino E., Schiraldi C. Improved fructosylated chondroitin production by kfoC overexpression in E. coli K4. J. Biotechnol. 2010;150:324–331. doi: 10.1016/j.jbiotec.2010.09.954. [DOI] [PubMed] [Google Scholar]
- 62.Schiraldi C., Alfano A., Cimini D., De Rosa M., Panariello A., Restaino O.F., De Rosa M. Application of a 22L scale membrane bioreactor and cross-flow ultrafiltration to obtain purified chondroitin. Biotechnol. Prog. 2012;28:1012–1018. doi: 10.1002/btpr.1566. [DOI] [PubMed] [Google Scholar]
- 63.Cimini D., De Rosa E.Carlino, Ruggiero A., Schiraldi C. Homologous overexpression of rfaH in E. coli K4 improves the production of chondroitin-like capsular polysaccharide. Microb. Cell Fact. 2013;12:1. doi: 10.1186/1475-2859-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu J., Yang A., Liu J., Ding X., Liu L., Shi Z. KfoE encodes a fructosyltransferase involved in capsular polysaccharide biosynthesis in Escherichia coli K4. Biotechnol. Lett. 2014;36:1469–1477. doi: 10.1007/s10529-014-1502-9. [DOI] [PubMed] [Google Scholar]
- 65.Cimini D., Carlino E., Giovane A., Argenzio O., Dello Iacono I., De Rosa M., Schiraldi C. Engineering a branch of the UDP-precursor biosynthesis pathway enhances the production of capsular polysaccharide in Escherichia coli O5:K4:H4. Biotechnol. J. 2015;10:1307–1315. doi: 10.1002/biot.201400602. [DOI] [PubMed] [Google Scholar]
- 66.Samraj A.N., Pearce O.M.T., Läubli H., Crittenden A.N., Bergfeld A.K., Band K., Gregg C.J., Bingman A.E., Secrest P., Diaz S.L., Varki N.M., Varki A., Kornfeld S.A. A red meat-derived glycan promotes inflammation and cancer progression. Proc. Natl. Acad. Sci. U. S. A. 2015;112:542–547. doi: 10.1073/pnas.1417508112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao T., Zhang J., Liu X., Wang F. Analysis of chondroitin sulfate from different sources of cartilage by electrophoretically mediated microanalysis. RSC Adv. 2015;5:52314–52319. doi: 10.1039/c5ra05576h. [DOI] [Google Scholar]
- 68.Garnjanagoonchorn W., Wongekalak L., Engkagul A. Determination of chondroitin sulfate from different sources of cartilage. Chem. Eng. Process. Process Intensif. 2007;46:465–471. doi: 10.1016/j.cep.2006.05.019. [DOI] [Google Scholar]
- 69.Sundaresan G., Abraham R.J.J., Appa Rao V., Narendra Babu R., Govind V., Meti M.F. Established method of chondroitin sulphate extraction from buffalo (Bubalus bubalis) cartilages and its identification by FTIR. J. Food Sci. Technol. 2018;55:3439–3445. doi: 10.1007/s13197-018-3253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo X.M., Fosmire G.J., Leach R.M. Chicken keel cartilage as a source of chondroitin sulfate. Poult. Sci. 2002;81:1086–1089. doi: 10.1093/ps/81.7.1086. [DOI] [PubMed] [Google Scholar]
- 71.Novoa-Carballal R., Pérez-Martín R., Blanco M., Sotelo C.G., Fassini D., Nunes C., Coimbra M.A., Silva T.H., Reis R.L., Vázquez J.A. By-products of Scyliorhinus canicula, Prionace glauca and Raja clavata: a valuable source of predominantly 6S sulfated chondroitin sulfate. Carbohydr. Polym. 2017;157:31–37. doi: 10.1016/j.carbpol.2016.09.050. [DOI] [PubMed] [Google Scholar]
- 72.Grand View Research, Chondroitin Sulfate Market Size, Share & Trends Analysis Report By Source (Bovine, Swine, Poultry), By Application (Nutraceuticals, Pharmaceuticals, Animal Feed, Personal Care & Cosmetics), and Segment Forecascts, 2021–2028, 2021. https://www.grandviewresearch.com/industry-analysis/chondroitin-sulfate-market#.
- 73.Beroe Inc, Chondroitin sulfate market intelligence. Price, forecast, cost models, market analysis - market research, Beroe. (2018). https://www.beroeinc.com/category-intelligence/chondroitin-sulfate-market/.
- 74.Bhathal A., Spryszak M., Louizos C., Frankel G. Glucosamine and chondroitin use in canines for osteoarthritis: a review. Open Vet. J. 2017;7:36–49. doi: 10.4314/ovj.v7i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oliviero F., Ramonda R., Hoxha A., Scanu A., Galozzi P., Favero M., Frallonardo P., Punzi L. Effect of an oral preparation containing hyaluronic acid, chondroitin sulfate, hydrolyzed collagen type II and hydrolyzed keratin on synovial fluid features and clinical indices in knee osteoarthritis. A pilot study. Reumatismo. 2020;72:125–130. doi: 10.4081/reumatismo.2020.1272. [DOI] [PubMed] [Google Scholar]
- 76.Santos G.R.C., Piquet A.A., Glauser B.F., Tovar A.M.F., Pereira M.S., Vilanova E., Mourão P.A.S. Systematic analysis of pharmaceutical preparations of chondroitin sulfate combined with glucosamine. Pharmaceuticals. 2017;10 doi: 10.3390/ph10020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bottegoni C., Muzzarelli R.A.A., Giovannini F., Busilacchi A., Gigante A. Oral chondroprotection with nutraceuticals made of chondroitin sulphate plus glucosamine sulphate in osteoarthritis. Carbohydr. Polym. 2014;109:126–138. doi: 10.1016/j.carbpol.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 78.Uebelhart D. Clinical review of chondroitin sulfate in osteoarthritis. Osteoarthr. Cartil. 2008;16:19–21. doi: 10.1016/j.joca.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 79.Pérez-Balbuena A.L., Ochoa-Tabares J.C., Belalcazar-Rey S., Urzúa-Salinas C., Saucedo-Rodríguez L.R., Velasco-Ramos R., Suárez-Sánchez R.G., Rodríguez-Carrizalez A.D., Oregón-Miranda A.A. Efficacy of a fixed combination of 0.09% xanthan gum/0.1% chondroitin sulfate preservative free vs polyethylene glycol/propylene glycol in subjects with dry eye disease: a multicenter randomized controlled trial. BMC Ophthalmol. 2016;16:1–6. doi: 10.1186/s12886-016-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Belalcázar-Rey S., Sánchez Huerta V., Ochoa-Tabares J.C., Altamirano Vallejo S., Soto-Gómez A., Suárez-Velasco R., García-Félix F., Baiza-Durán L., Olvera-Montaño O., Muñoz-Villegas P. Efficacy and safety of sodium hyaluronate/chondroitin sulfate preservative-free ophthalmic solution in the treatment of dry eye: a clinical trial. Curr. Eye Res. 2021;46:919–929. doi: 10.1080/02713683.2020.1849733. [DOI] [PubMed] [Google Scholar]
- 81.Theodosakis J., Grove M.L., Cohen M., Randomized A. Double blind, placebo controlled trial of a topical cream containing glucosamine sulfate, chondroitin sulfate, and camphor for osteoarthritis of the knee. J. Rheumatol. 2004;31:826–827. [PubMed] [Google Scholar]
- 82.Espíndola R.F., Castro E.F.S., Santhiago M.R., Kara N. A clinical comparison between discovisc and 2% hydroxypropylmethylcellulose in phacoemulsification: a fellow eye study. Clinics. 2012;67:1059–1062. doi: 10.6061/clinics/2012(09)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Labiris G., Sideroudi H., Rousopoulos K., Kozobolis V.P. Cohesive versus dispersive-cohesive ophthalmic viscosurgical device in torsional intelligent phaco. J. Cataract Refract. Surg. 2015;41:681–682. doi: 10.1016/j.jcrs.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 84.Tutolo M., Ammirati E., Castagna G., Klockaerts K., Plancke H., Ost D., van der Aa F., Ridder D. A prospective randomized controlled multicentre trial comparing intravesical DMSO and chondroïtin sulphate 2% for painful bladder syndrome/interstitial cystitis. Int. Braz. J. Urol. 2017;43:134–141. doi: 10.1590/S1677-5538.IBJU.2016.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ciani O., Arendsen E., Romancik M., Lunik R., Costantini E., Di Biase M., Morgia G., Fragalà E., Roman T., Bernat M., Guazzoni G., Tarricone R., Lazzeri M. Intravesical administration of combined hyaluronic acid (HA) and chondroitin sulfate (CS) for the treatment of female recurrent urinary tract infections: a European multicentre nested case-control study. BMJ Open. 2016;6:1–8. doi: 10.1136/bmjopen-2015-009669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang J., Shen M., Wen H., Luo Y., Huang R., Rong L., Xie J. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate. Carbohydr. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115650. [DOI] [PubMed] [Google Scholar]
- 87.Volpi N. Chondroitin sulphate for the treatment of osteoarthritis. Curr. Med. Chem. Anti-Inflamm. Anti-Allergy Agents. 2005;4:221–234. doi: 10.2174/1568014054065276. [DOI] [Google Scholar]
- 88.Zhu X., Sang L., Wu D., Rong J., Jiang L. Effectiveness and safety of glucosamine and chondroitin for the treatment of osteoarthritis: a meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2018;13:1–9. doi: 10.1186/s13018-018-0871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vasiliadis H.S., Tsikopoulos K. Glucosamine and chondroitin for the treatment of osteoarthritis. World J. Orthop. 2017;8:1–11. doi: 10.5312/wjo.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wandel S., Jüni P., Tendal B., Nüesch E., Villiger P.M., Welton N.J., Reichenbach S., Trelle S. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:711. doi: 10.1136/bmj.c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh J.A., Noorbaloochi S., Macdonald R., Maxwell L.J. Chondroitin for osteoarthritis. Cochrane Database Syst. Rev. 2015;2017:1–297. doi: 10.1002/14651858.CD005614.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kloppenburg M., Kroon F.P.B., Blanco F.J., Doherty M., Dziedzic K.S., Greibrokk E., Haugen I.K., Herrero-Beaumont G., Jonsson H., Kjeken I., Maheu E., Ramonda R., Ritt M.J.P.F., Smeets W., Smolen J.S., Stamm T.A., Szekanecz Z., Wittoek R., Carmona L. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann. Rheum. Dis. 2019;78:16–24. doi: 10.1136/annrheumdis-2018-213826. [DOI] [PubMed] [Google Scholar]
- 93.Gabay C., Medinger-Sadowski C., Gascon D., Kolo F., Finckh A. Symptomatic effects of chondroitin 4 and chondroitin 6 sulfate on hand osteoarthritis: a randomized, double-blind, placebo-controlled clinical trial at a single center. Arthritis Rheum. 2011;63:3383–3391. doi: 10.1002/art.30574. [DOI] [PubMed] [Google Scholar]
- 94.Möller I., Pérez M., Monfort J., Benito P., Cuevas J., Perna C., Doménech G., Herrero M., Montell E., Vergés J. Effectiveness of chondroitin sulphate in patients with concomitant knee osteoarthritis and psoriasis: a randomized, double-blind, placebo-controlled study. Osteoarthr. Cartil. 2010;18:S32–S40. doi: 10.1016/j.joca.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 95.Wildi L.M., Raynauld J.P., Martel-Pelletier J., Beaulieu A., Bessette L., Morin F., Abram F., Dorais M., Pelletier J.P. Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI. Ann. Rheum. Dis. 2011;70:982–989. doi: 10.1136/ard.2010.140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martel-Pelletier J., Raynauld J.P., Mineau F., Abram F., Paiement P., Delorme P., Pelletier J.P. Levels of serum biomarkers from a two-year multicentre trial are associated with treatment response on knee osteoarthritis cartilage loss as assessed by magnetic resonance imaging: an exploratory study. Arthritis Res. Ther. 2017;19:1–11. doi: 10.1186/s13075-017-1377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pelletier J.P., Raynauld J.P., Beaulieu A.D., Bessette L., Morin F., de Brum-Fernandes A.J., Delorme P., Dorais M., Paiement P., Abram F., Martel-Pelletier J. Chondroitin sulfate efficacy versus celecoxib on knee osteoarthritis structural changes using magnetic resonance imaging: a 2-year multicentre exploratory study. Arthritis Res. Ther. 2016;18:1–12. doi: 10.1186/s13075-016-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mazières B., Hucher M., Zaïm M., Garnero P. Effect of chondroitin sulphate in symptomatic knee osteoarthritis: a multicentre, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2007;66:639–645. doi: 10.1136/ard.2006.059899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Railhac J.-.J., Zaim M., Saurel A.-.S., Vial J., Fournie B. Effect of 12 months treatment with chondroitin sulfate on cartilage volume in knee osteoarthritis patients: a randomized, double-blind, placebo-controlled pilot study using MRI. Clin. Rheumatol. 2012;31:1347–1357. doi: 10.1007/s10067-012-2022-4. [DOI] [PubMed] [Google Scholar]
- 100.Fardellone P., Zaim M., Saurel A.-.S., Maheu E. Comparative efficacy and safety study of two chondroitin sulfate preparations from different origin (avian and bovine) in symptomatic osteoarthritis of the knee. Open Rheumatol. J. 2013;7:1–12. doi: 10.2174/1874312901307010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Navarro S.L., White E., Kantor E.D., Zhang Y., Rho J., Song X., Milne G.L., Lampe P.D., Lampe J.W. Randomized trial of glucosamine and chondroitin supplementation on inflammation and oxidative stress biomarkers and plasma proteomics profiles in healthy humans. PLoS ONE. 2015;10:1–20. doi: 10.1371/journal.pone.0117534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Price M.O., Knight O.J., Benetz B.A., Debanne S.M., Verdier D.D., Rosenwasser G.O., Rosenwasser M., Price F.W., Lass J.H. Randomized, prospective, single-masked clinical trial of endothelial keratoplasty performance with 2donor cornea 4°C storage solutions and associated chambers. Cornea. 2015;34:253–256. doi: 10.1097/ICO.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 103.Volpi N. Anti-inflammatory activity of chondroitin sulphate: new functions from an old natural macromolecule. Inflammopharmacology. 2011;19:299–306. doi: 10.1007/s10787-011-0098-0. [DOI] [PubMed] [Google Scholar]
- 104.Iovu M., Dumais G., du Souich P. Anti-inflammatory activity of chondroitin sulfate. Osteoarthr. Cartil. 2008;16:S14–S18. doi: 10.1016/j.joca.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 105.Borsig L., Wang L., Cavalcante M.C.M., Cardilo-Reis L., Ferreira P.L., Mourão P.A.S., Esko J.D., Pavão M.S.G. Selectin blocking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber: effect on tumor metastasis and neutrophil recruitment. J. Biol. Chem. 2007;282:14984–14991. doi: 10.1074/jbc.M610560200. [DOI] [PubMed] [Google Scholar]
- 106.Li J., Sparkenbaugh E.M., Su G., Zhang F., Xu Y., Xia K., He P., Baytas S., Pechauer S., Padmanabhan A., Linhardt R.J., Pawlinski R., Liu J. Enzymatic synthesis of chondroitin sulfate E to attenuate bacteria lipopolysaccharide-induced organ damage. ACS Cent. Sci. 2020;6:1199–1207. doi: 10.1021/acscentsci.0c00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen S., Li G., Wu N., Guo X., Liao N., Ye X., Liu D., Xue C., Chai W. Sulfation pattern of the fucose branch is important for the anticoagulant and antithrombotic activities of fucosylated chondroitin sulfates. Biochim. Biophys. Acta - Gen. Subj. 2013;1830:3054–3066. doi: 10.1016/j.bbagen.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 108.Muzzarelli R.A.A., Greco F., Busilacchi A., Sollazzo V., Gigante A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: a review. Carbohydr. Polym. 2012;89:723–739. doi: 10.1016/j.carbpol.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 109.Yamada S., Sugahara K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr. Drug Discov. Technol. 2008;5:289–301. doi: 10.2174/157016308786733564. [DOI] [PubMed] [Google Scholar]
- 110.Laabs T., Carulli D., Geller H.M., Fawcett J.W. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr. Opin. Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 111.Bergefall K., Trybala E., Johansson M., Uyama T., Naito S., Yamada S., Kitagawa H., Sugahara K., Bergström T. Chondroitin sulfate characterized by the E-disaccharide unit is a potent inhibitor of herpes simplex virus infectivity and provides the virus binding sites on gro2C cells. J. Biol. Chem. 2005;280:32193–32199. doi: 10.1074/jbc.M503645200. [DOI] [PubMed] [Google Scholar]
- 112.Kato D., Era S., Watanabe I., Arihara M., Sugiura N., Kimata K., Suzuki Y., Morita K., Hidari K.I.P.J., Suzuki T. Antiviral activity of chondroitin sulphate E targeting dengue virus envelope protein. Antiviral Res. 2010;88:236–243. doi: 10.1016/j.antiviral.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 113.Sano Y. Antiviral activity of chondroitin sulfate against infection. Carbohydr. Polym. 1997;33:125–129. doi: 10.1016/S0144-8617(98)00119-2. [DOI] [Google Scholar]
- 114.Jinno-Oue A., Tanaka A., Shimizu N., Mori T., Sugiura N., Kimata K., Isomura H., Hoshino H. Inhibitory effect of chondroitin sulfate type E on the binding step of human T-cell leukemia virus type 1. AIDS Res. Hum. Retroviruses. 2013;29:621–629. doi: 10.1089/aid.2012.0156. [DOI] [PubMed] [Google Scholar]
- 115.Huang N., Wu M.Y., Zheng C.B., Zhu L., Zhao J.H., Zheng Y.T. The depolymerized fucosylated chondroitin sulfate from sea cucumber potently inhibits HIV replication via interfering with virus entry. Carbohydr. Res. 2013;380:64–69. doi: 10.1016/j.carres.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 116.Song S., Peng H., Wang Q., Liu Z., Dong X., Wen C., Ai C., Zhang Y., Wang Z., Zhu B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020;11:7415–7420. doi: 10.1039/d0fo02017f. [DOI] [PubMed] [Google Scholar]
- 117.Zhang L., Li K., Xiao W., Zheng L., Xiao Y., Fan H., Zhang X. Preparation of collagen-chondroitin sulfate-hyaluronic acid hybrid hydrogel scaffolds and cell compatibility in vitro. Carbohydr. Polym. 2011;84:118–125. doi: 10.1016/j.carbpol.2010.11.009. [DOI] [Google Scholar]
- 118.Liu Y., Lv H., Ren L., Xue G., Wang Y. Improving the moisturizing properties of collagen film by surface grafting of chondroitin sulfate for corneal tissue engineering. J. Biomater. Sci. Polym. Ed. 2016;27:758–772. doi: 10.1080/09205063.2016.1160561. [DOI] [PubMed] [Google Scholar]
- 119.Saivin S., Cambus J.-.P., Thalamas C., Lau G., Boneu B., Houin G., Gianese F. Pharmacokinetics and pharmacodynamics of intramuscular dermatan sulfate revisited. Clin. Drug Investig. 2003;23:533–543. doi: 10.2165/00044011-200323080-00006. [DOI] [PubMed] [Google Scholar]
- 120.Coccheri S., Mannello F. Development and use of sulodexide in vascular diseases: implications for treatment. Drug Des. Devel. Ther. 2013;8:49–65. doi: 10.2147/DDDT.S6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lomonte A.B.V., Mendonça J.A., de Castro Brandão G., Castro M.L. Multicenter, randomized, double-blind clinical trial to evaluate efficacy and safety of combined glucosamine sulfate and chondroitin sulfate capsules for treating knee osteoarthritis. Adv. Rheumatol. (London, England) 2018;58 doi: 10.1186/s42358-018-0041-9. [DOI] [PubMed] [Google Scholar]
- 122.Hochberg M.C., Martel-Pelletier J., Monfort J., Möller I., Castillo J.R., Arden N., Berenbaum F., Blanco F.J., Conaghan P.G., Doménech G., Henrotin Y., Pap T., Richette P., Sawitzke A., Du Souich P., Pelletier J.P. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann. Rheum. Dis. 2016;75:37–44. doi: 10.1136/annrheumdis-2014-206792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roman-Blas J.A., Castañeda S., Sánchez-Pernaute O., Largo R., Herrero-Beaumont G. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 2017;69:77–85. doi: 10.1002/art.39819. [DOI] [PubMed] [Google Scholar]
- 124.Lomonte A.B.V., Gimenez E., da Silva A.C., Radominski S.C., Scheinberg M.A., Ximenes A.C., de Freitas Zerbini C.A. Treatment of knee osteoarthritis with a new formulation of a fixed-dose combination of glucosamine sulfate and bovine chondroitin: a multicenter, randomized, single-blind, non-inferiority clinical trial. Adv. Rheumatol. 2021;61 doi: 10.1186/s42358-021-00165-9. [DOI] [PubMed] [Google Scholar]
- 125.Magrans-Courtney T., Wilborn C., Rasmussen C., Ferreira M., Greenwood L., Campbell B., Kerksick C.M., Nassar E., Li R., Iosia M., Cooke M., Dugan K., Willoughby D., Soliah L.A., Kreider R.B. Effects of diet type and supplementation of glucosamine, chondroitin, and MSM on body composition, functional status, and markers of health in women with knee osteoarthritis initiating a resistance-based exercise and weight loss program. J. Int. Soc. Sports Nutr. 2011;8:1–17. doi: 10.1186/1550-2783-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fransen M., Agaliotis M., Nairn L., Votrubec M., Bridgett L., Su S., Jan S., March L., Edmonds J., Norton R., Woodward M., Day R., Buckland-Wright C., Bridges-Webb C., Heritier S., Jones G., Sambrook P., Speechly C., Vignon E., Jovanovic G., Jeram S., Dowd M., Champion M., Grealy S., Watson D., Vrataric A., Johnson C., White R., O'Connell P., Milwraith F., Kennedy D., Faulkes C., Bulton O., Johnson K. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann. Rheum. Dis. 2015;74:851–858. doi: 10.1136/annrheumdis-2013-203954. [DOI] [PubMed] [Google Scholar]
- 127.Simic M., Harmer A.R., Agaliotis M., Nairn L., Bridgett L., March L., Votrubec M., Edmonds J., Woodward M., Day R., Fransen M. Clinical risk factors associated with radiographic osteoarthritis progression among people with knee pain: a longitudinal study. Arthritis Res. Ther. 2021;23:1–10. doi: 10.1186/s13075-021-02540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dang N.C., Ardehali A., Bruckner B.A., Parrino P.E., Gillen D.L., Hoffman R.W., Spotnitz R., Cavoores S., Shorn I.J., Manson R.J., Spotnitz W.D. Prospective, multicenter, randomized, controlled trial evaluating the performance of a novel combination powder vs hemostatic matrix in cardiothoracic operations. J. Card. Surg. 2020;35:313–319. doi: 10.1111/jocs.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang S.J., Wang Y.H., Huang L.C. The effect of oral low molecular weight liquid hyaluronic acid combination with glucosamine and chondroitin on knee osteoarthritis patients with mild knee pain: an 8-week randomized double-blind placebo-controlled trial. Medicine (Baltimore) 2021;100:e24252. doi: 10.1097/md.0000000000024252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.López-Vidriero E., Olivé-Vilas R., López-Capapé D., Varela-Sende L., López-Vidriero R., Til-Pérez L. Efficacy and tolerability of progen, a nutritional supplement based on innovative plasma proteins. Orthop. J. Sport. Med. 2019;7 doi: 10.1177/2325967119827237. ACL reconstruction: a multicenter randomized controlled trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gallo G., Mistrangelo M., Passera R., Testa V., Pozzo M., Perinotti R., Lanati I., Lazzari I., Tonello P., Ugliono E., De Luca E., Luc A.R., Clerico G., Trompetto M. Efficacy of mesoglycan in pain control after excisional hemorrhoidectomy: a pilot comparative prospective multicenter study. Gastroenterol. Res. Pract. 2018;2018:1–8. doi: 10.1155/2018/6423895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Palareti G., Legnani C., Antonucci E., Zorzi S., Bignamini A.A., Lodigiani C., Tosetto A., Bertù L., Pengo V., Testa S., Ageno W., Prisco D., Prandoni P., Poli D. Design and rationale of a randomized, placebo-controlled trial on the efficacy and safety of sulodexide for extended treatment in elderly patients after a first venous thromboembolism. Intern. Emerg. Med. 2021;16:359–368. doi: 10.1007/s11739-020-02381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song J.H., Chin H.S., Kwon O.W., Lim S.J., Kim H.K. Effect of sulodexide in patients with non-proliferative diabetic retinopathy: diabetic retinopathy sulodexide study (DRESS) Graefe's Arch. Clin. Exp. Ophthalmol. 2015;253:829–837. doi: 10.1007/s00417-014-2746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bidadkosh A., Lambooy S.P.H., Heerspink H.J., Pena M.J., Henning R.H., Buikema H., Deelman L.E. Predictive properties of biomarkers GDF-15, NTproBNP, and hs-TnT for morbidity and mortality in patients with type 2 diabetes with nephropathy. Diabetes Care. 2017;40:784–792. doi: 10.2337/dc16-2175. [DOI] [PubMed] [Google Scholar]
- 135.Lewis E.J., Lewis J.B., Greene T., Hunsicker L.G., Berl T., Pohl M.A., de Zeeuw D., Heerspink H.L., Rohde R.D., Atkins R.C., Reutens A.T., Packham D.K., Raz I. Sulodexide for kidney protection in type 2 diabetes patients with microalbuminuria: a randomized controlled trial. Am. J. Kidney Dis. 2011;58:729–736. doi: 10.1053/j.ajkd.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 136.Ji Y., Zhang S., Qiao M., Jiao R., Li J., Song P., Zhang X., Huang H. Synthesis of structurally defined chondroitin sulfate: paving the way to the structure-activity relationship studies. Carbohydr. Polym. 2020;248 doi: 10.1016/j.carbpol.2020.116796. [DOI] [PubMed] [Google Scholar]
- 137.DeAngelis P.L., Liu J., Linhardt R.J. Chemoenzymatic synthesis of glycosaminoglycans: re-creating, re-modeling and re-designing nature’s longest or most complex carbohydrate chains. Glycobiology. 2013;23:764–777. doi: 10.1093/glycob/cwt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.E. Petit, C. Delattre, D. Papy-Garcia, P. Michaud, Chondroitin sulfate lyases: applications in analysis and glycobiology, in: Adv. Pharmacol., Elsevier, 2006: pp. 167–186. 10.1016/S1054-3589(05)53008-4. [DOI] [PubMed]
- 139.Li Y., Zhou Z., Chen Z. High-level production of ChSase ABC I by co-expressing molecular chaperones in Escherichia coli. Int. J. Biol. Macromol. 2018;119:779–784. doi: 10.1016/j.ijbiomac.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 140.Linhardt R.J., Avci F.Y., Toida T., Kim Y.S., Cygler M. CS lyases: structure, activity, and applications in analysis and the treatment of diseases. Adv. Pharmacol. 2006;53:187–215. doi: 10.1016/S1054-3589(05)53009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.S. Yamada, Chondroitin sulfate-specific novel hydrolase in human, in: P.R. Sudhakaran, A. Surolia (Eds.), Adv. Exp. Med. Biol., Springer New York, 2012: pp. 47–56. 10.1007/978-1-4614-3381-1_4. [DOI] [PubMed]
- 142.Fu J., Jiang Z., Chang J., Han B., Liu W., Peng Y. Purification, characterization of chondroitinase ABC from Sphingomonas paucimobilis and in vitro cardiocytoprotection of the enzymatically degraded CS-A. Int. J. Biol. Macromol. 2018;115:737–745. doi: 10.1016/j.ijbiomac.2018.04.117. [DOI] [PubMed] [Google Scholar]
- 143.Rani A., Goyal A. A new member of family 8 polysaccharide lyase chondroitin AC lyase (PsPL8A) from Pedobacter saltans displays endo- and exo-lytic catalysis. J. Mol. Catal. B Enzym. 2016;134:215–224. doi: 10.1016/j.molcatb.2016.11.001. [DOI] [Google Scholar]
- 144.Gushulak L., Hemming R., Martin D., Seyrantepe V., Pshezhetsky A., Triggs-Raine B. Hyaluronidase 1 and β-hexosaminidase have redundant functions in hyaluronan and chondroitin sulfate degradation. J. Biol. Chem. 2012;287:16689–16697. doi: 10.1074/jbc.M112.350447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaneiwa T., Miyazaki A., Kogawa R., Mizumoto S., Sugahara K., Yamada S. Identification of amino acid residues required for the substrate specificity of human and mouse chondroitin sulfate hydrolase (Conventional hyaluronidase-4) J. Biol. Chem. 2012;287:42119–42128. doi: 10.1074/jbc.M112.360693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kobayashi S., Fujikawa S.I., Ohmae M. Enzymatic synthesis of chondroitin and its derivatives catalyzed by hyaluronidase. J. Am. Chem. Soc. 2003;125:14357–14369. doi: 10.1021/ja036584x. [DOI] [PubMed] [Google Scholar]
- 147.Fujikawa S.I., Ohmae M., Kobayashi S. Enzymatic synthesis of chondroitin 4-sulfate with well-defined structure. Biomacromolecules. 2005;6:2935–2942. doi: 10.1021/bm050364p. [DOI] [PubMed] [Google Scholar]
- 148.Kakizaki I., Suto S., Tatara Y., Nakamura T., Endo M. Hyaluronan-chondroitin hybrid oligosaccharides as new life science research tools. Biochem. Biophys. Res. Commun. 2012;423:344–349. doi: 10.1016/j.bbrc.2012.05.127. [DOI] [PubMed] [Google Scholar]
- 149.Shi Y., Meng Y., Li J., Chen J., Liu Y., Bai X. Chondroitin sulfate: extraction, purification, microbial and chemical synthesis. J. Chem. Technol. Biotechnol. 2014;89:1445–1465. doi: 10.1002/jctb.4454. [DOI] [Google Scholar]
- 150.DeAngelis P.L., Padgett-McCue A.J. Identification and molecular cloning of a chondroitin synthase from Pasteurella multocida type F. J. Biol. Chem. 2000;275:24124–24129. doi: 10.1074/jbc.M003385200. [DOI] [PubMed] [Google Scholar]
- 151.DeAngelis P.L. Chondroitin synthase gene and methods of making and using same. US Patent. 2005;0164984:A1. [Google Scholar]
- 152.Sugiura N., Shimokata S., Minamisawa T., Hirabayashi J., Kimata K., Watanabe H. Sequential synthesis of chondroitin oligosaccharides by immobilized chondroitin polymerase mutants. Glycoconj. J. 2008;25:521–530. doi: 10.1007/s10719-008-9105-0. [DOI] [PubMed] [Google Scholar]
- 153.Sugiura N., Shioiri T., Chiba M., Sato T., Narimatsu H., Kimata K., Watanabe H. Construction of a chondroitin sulfate library with defined structures and analysis of molecular interactions. J. Biol. Chem. 2012;287:43390–43400. doi: 10.1074/jbc.M112.412676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sugiura N., Clausen T.M., Shioiri T., Gustavsson T., Watanabe H., Salanti A. Molecular dissection of placental malaria protein VAR2CSA interaction with a chemo-enzymatically synthesized chondroitin sulfate library. Glycoconj. J. 2016;33:985–994. doi: 10.1007/s10719-016-9685-z. [DOI] [PubMed] [Google Scholar]
- 155.Shioiri T., Tsuchimoto J., Watanabe H., Sugiura N. Sequence determination of synthesized chondroitin sulfate dodecasaccharides. Glycobiology. 2016;26:592–606. doi: 10.1093/glycob/cww008. [DOI] [PubMed] [Google Scholar]
- 156.Li J., Su G., Liu J. Enzymatic synthesis of homogeneous chondroitin sulfate oligosaccharides. Angew. Chemie - Int. Ed. 2017;56:11784–11787. doi: 10.1002/anie.201705638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yusa A., Kitajima K., Habuchi O. N-linked oligosaccharides on chondroitin 6-sulfotransferase-1 are required for production of the active enzyme, golgi localization, and sulfotransferase activity toward keratan sulfate. J. Biol. Chem. 2006;281:20393–20403. doi: 10.1074/jbc.M600140200. [DOI] [PubMed] [Google Scholar]
- 158.Zhou Z., Li Q., Huang H., Wang H., Wang Y., Du G., Chen J., Kang Z. A microbial–enzymatic strategy for producing chondroitin sulfate glycosaminoglycans. Biotechnol. Bioeng. 2018;115:1561–1570. doi: 10.1002/bit.26577. [DOI] [PubMed] [Google Scholar]
- 159.He W., Zhu Y., Shirke A., Sun X., Liu J., Gross R.A., Koffas M.A.G., Linhardt R.J., Li M. Expression of chondroitin-4-O-sulfotransferase in Escherichia coli and Pichia pastoris. Appl. Microbiol. Biotechnol. 2017;101:6919–6928. doi: 10.1007/s00253-017-8411-5. [DOI] [PubMed] [Google Scholar]
- 160.Badri A., Williams A., Awofiranye A., Datta P., Xia K., He W., Fraser K., Dordick J.S., Linhardt R.J., Koffas M.A.G. Complete biosynthesis of a sulfated chondroitin in Escherichia coli. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-21692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Habuchi O., Moroi R., Ohtake S. Enzymatic synthesis of chondroitin sulfate E by N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase purified from squid cartilage. Anal. Biochem. 2002;310:129–136. doi: 10.1016/S0003-2697(02)00277-4. [DOI] [PubMed] [Google Scholar]
- 162.Wang Y., Li S., Xu X., Tan Y., Liu X., Fang J. Chemoenzymatic synthesis of homogeneous chondroitin polymers and its derivatives. Carbohydr. Polym. 2020;232 doi: 10.1016/j.carbpol.2019.115822. [DOI] [PubMed] [Google Scholar]
- 163.Benito-Arenas R., Doncel-Pérez E., Fernández-Gutiérrez M., Garrido L., García-Junceda E., Revuelta J., Bastida A., Fernández-Mayoralas A. A holistic approach to unravelling chondroitin sulfation: correlations between surface charge, structure and binding to growth factors. Carbohydr. Polym. 2018;202:211–218. doi: 10.1016/j.carbpol.2018.08.120. [DOI] [PubMed] [Google Scholar]
- 164.An C., Zhao L., Wei Z., Zhou X. Chemoenzymatic synthesis of 3′-phosphoadenosine-5′-phosphosulfate coupling with an ATP regeneration system. Appl. Microbiol. Biotechnol. 2017;101:7535–7544. doi: 10.1007/s00253-017-8511-2. [DOI] [PubMed] [Google Scholar]
- 165.Datta P., Fu L., He W., Koffas M.A.G., Dordick J.S., Linhardt R.J. Expression of enzymes for 3′-phosphoadenosine-5′-phosphosulfate (PAPS) biosynthesis and their preparation for PAPS synthesis and regeneration. Appl. Microbiol. Biotechnol. 2020;104:7067–7078. doi: 10.1007/s00253-020-10709-6. [DOI] [PubMed] [Google Scholar]
- 166.Badri A., Williams A., Xia K., Linhardt R.J., Koffas M.A.G. Increased 3′-phosphoadenosine-5′-phosphosulfate levels in engineered Escherichia coli cell lysate facilitate the in vitro synthesis of chondroitin sulfate A. Biotechnol. J. 2019;14 doi: 10.1002/biot.201800436. [DOI] [PubMed] [Google Scholar]
- 167.Zhang X., Lin L., Huang H., Linhardt R.J. Chemoenzymatic synthesis of glycosaminoglycans. Acc. Chem. Res. 2020;53:335–346. doi: 10.1021/acs.accounts.9b00420. [DOI] [PubMed] [Google Scholar]
- 168.Lin L., Qiao M., Zhang X., Linhardt R.J. Site-selective reactions for the synthesis of glycoconjugates in polysaccharide vaccine development. Carbohydr. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115643. [DOI] [PubMed] [Google Scholar]
- 169.Schiraldi C., Cimini D., De Rosa M. Production of chondroitin sulfate and chondroitin. Appl. Microbiol. Biotechnol. 2010;87:1209–1220. doi: 10.1007/s00253-010-2677-1. [DOI] [PubMed] [Google Scholar]
- 170.Willis L.M., Whitfield C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr. Res. 2013;378:35–44. doi: 10.1016/j.carres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 171.Whitfield C., Wear S.S., Sande C. Assembly of bacterial capsular polysaccharides and exopolysaccharides. Annu. Rev. Microbiol. 2020;74:521–543. doi: 10.1146/annurev-micro-011420-075607. [DOI] [PubMed] [Google Scholar]
- 172.Guan L., Xue Y., Ding W., Zhao Z. Biosynthesis and regulation mechanisms of the Pasteurella multocida capsule. Res. Vet. Sci. 2019;127:82–90. doi: 10.1016/j.rvsc.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 173.de Oliveira J.D., Carvalho L.S., Gomes A.M.V., Queiroz L.R., Magalhães B.S., Parachin N.S. Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microb. Cell Fact. 2016;15:1–19. doi: 10.1186/s12934-016-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zakeri A., Rasaee M.J., Pourzardosht N. Enhanced hyluronic acid production in Streptococcus zooepidemicus by over expressing HasA and molecular weight control with Niscin and glucose. Biotechnol. Reports. 2017;16:65–70. doi: 10.1016/j.btre.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chu X., Han J., Guo D., Fu Z., Liu W., Tao Y. Characterization of UDP-glucose dehydrogenase from Pasteurella multocida CVCC 408 and its application in hyaluronic acid biosynthesis. Enzyme Microb. Technol. 2016;85:64–70. doi: 10.1016/j.enzmictec.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 176.Cimini D., Dello Iacono I., Carlino E., Finamore R., Restaino O.F., Diana P., Bedini E., Schiraldi C. Engineering S. equi subsp. zooepidemicus towards concurrent production of hyaluronic acid and chondroitin biopolymers of biomedical interest. AMB Express. 2017:7. doi: 10.1186/s13568-017-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schiraldi C., La A., De M. Biopolymers. Sciyo; 2010. Biotechnological production and application of hyaluronan. [DOI] [Google Scholar]
- 178.Wang Z., Ly M., Zhang F., Zhong W., Suen A., Hickey A.M., Dordick J.S., Linhardt R.J. E. coli K5 fermentation and the preparation of heparosan, a bioengineered heparin precursor. Biotechnol. Bioeng. 2010;107:964–973. doi: 10.1002/bit.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Z., Dordick J.S., Linhardt R.J. Escherichia coli K5 heparosan fermentation and improvement by genetic engineering. Bioeng. Bugs. 2011;2:63–67. doi: 10.4161/bbug.2.1.14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jin P., Zhang L., Yuan P., Kang Z., Du G., Chen J. Efficient biosynthesis of polysaccharides chondroitin and heparosan by metabolically engineered Bacillus subtilis. Carbohydr. Polym. 2016;140:424–432. doi: 10.1016/j.carbpol.2015.12.065. [DOI] [PubMed] [Google Scholar]
- 181.Wang Z., Li J., Cheong S., Bhaskar U., Akihiro O., Zhang F., Dordick J.S., Linhardt R.J. Response surface optimization of the heparosan N-deacetylation in producing bioengineered heparin. J. Biotechnol. 2011;156:188–196. doi: 10.1016/j.jbiotec.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chavaroche A.A.E., van den Broek L.A.M., Eggink G. Production methods for heparosan, a precursor of heparin and heparan sulfate. Carbohydr. Polym. 2013;93:38–47. doi: 10.1016/j.carbpol.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 183.Peterson S., Frick A., Liu J. Design of biologically active heparan sulfate and heparin using an enzyme-based approach. Nat. Prod. Rep. 2009;26:610–627. doi: 10.1039/b803795g. [DOI] [PubMed] [Google Scholar]
- 184.Bhaskar U., Li G., Fu L., Onishi A., Suflita M., Dordick J.S., Linhardt R.J. Combinatorial one-pot chemoenzymatic synthesis of heparin. Carbohydr. Polym. 2015;122:399–407. doi: 10.1016/j.carbpol.2014.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Restaino O.F., di Lauro I., Di Nuzzo R., De Rosa M., Schiraldi C. New insight into chondroitin and heparosan-like capsular polysaccharide synthesis by profiling of the nucleotide sugar precursors. Biosci. Rep. 2017;37:1–12. doi: 10.1042/BSR20160548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang T.-.T., Zhu C.-.Y., Zheng S., Meng C.-.C., Wang T.-.T., Meng D.-.H., Li Y.-.J., Zhu H.-.M., Wang F.-.S., Sheng J.-.Z. Identification and characterization of a chondroitin synthase from Avibacterium paragallinarum. Appl. Microbiol. Biotechnol. 2018;102:4785–4797. doi: 10.1007/s00253-018-8926-4. [DOI] [PubMed] [Google Scholar]
- 187.Zhang Q., Yao R., Chen X., Liu L., Xu S., Chen J., Wu J. Enhancing fructosylated chondroitin production in Escherichia coli K4 by balancing the UDP-precursors. Metab. Eng. 2018;47:314–322. doi: 10.1016/j.ymben.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 188.Bedini E., Decastro C., Derosa M., Dinola A., Iadonisi A., Restaino O.F., Schiraldi C., Parrilli M. A microbiological-chemical strategy to produce chondroitin sulfate A, C. Angew. Chemie - Int. Ed. 2011;50:6160–6163. doi: 10.1002/anie.201101142. [DOI] [PubMed] [Google Scholar]
- 189.Vázquez J.A., Rodríguez-Amado I., Montemayor M.I., Fraguas J., del P. González M., Murado M.A. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: characteristics, applications and eco-friendly processes: a review. Mar. Drugs. 2013;11:747–774. doi: 10.3390/md11030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.D'ambrosio S., Alfano A., Cassese E., Restaino O.F., Barbuto Ferraiuolo S., Finamore R., Cammarota M., Schiraldi C., Cimini D. Production and purification of higher molecular weight chondroitin by metabolically engineered Escherichia coli K4 strains. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-70027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.A. Trilli, I. Busiello, S. Daly, F. Bagatin, Biotechnological production of chondroitin, WO Patent 2012/004063 A1, 2012.
- 192.M. De Rosa, C. Schiraldi, D. Cimini, Biotechnological production of chondroitin, US Patent 8, 592, 186 B2, 2013.
- 193.Cimini D., Russo R., D'Ambrosio S., Dello Iacono I., Rega C., Carlino E., Argenzio O., Russo L., D'Abrosca B., Chambery A., Schiraldi C. Physiological characterization and quantitative proteomic analyses of metabolically engineered E. coli K4 strains with improved pathways for capsular polysaccharide biosynthesis. Biotechnol. Bioeng. 2018;115:1801–1814. doi: 10.1002/bit.26597. [DOI] [PubMed] [Google Scholar]
- 194.Wu Q., Yang A., Zou W., Duan Z., Liu J., Chen J., Liu L. Transcriptional engineering of Escherichia coli K4 for fructosylated chondroitin production. Biotechnol. Prog. 2013;29:1140–1149. doi: 10.1002/btpr.1777. [DOI] [PubMed] [Google Scholar]
- 195.D'ambrosio S., Ventrone M., Alfano A., Schiraldi C., Cimini D. Microbioreactor (micro-Matrix) potential in aerobic and anaerobic conditions with different industrially relevant microbial strains. Biotechnol. Prog. 2021:1–7. doi: 10.1002/btpr.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Awofiranye A.E., Baytas S.N., Xia K., Badri A., He W., Varki A., Koffas M., Linhardt R.J. N-glycolyl chondroitin synthesis using metabolically engineered E. coli. AMB Express. 2020;10:1–17. doi: 10.1186/s13568-020-01084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Suzuki K., Miyamoto K., Kaseyama H. Chondroitin-producing bacterium and method of producing chondroitin. US Patent. 2012;8:283. 145 B2. [Google Scholar]
- 198.He W., Fu L., Li G., Andrew Jones J., Linhardt R.J., Koffas M. Production of chondroitin in metabolically engineered E. coli. Metab. Eng. 2015;27:92–100. doi: 10.1016/j.ymben.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 199.DeAngelis P.L. Methods of expressing gram-negative glycosaminoglycan synthase genes in gram-positive hosts. US Patent. 2010;7:642. 071 B2. [Google Scholar]
- 200.Cheng F., Luozhong S., Yu H., Guo Z. Biosynthesis of chondroitin in engineered Corynebacterium glutamicum. J. Microbiol. Biotechnol. 2019;29:392–400. doi: 10.4014/jmb.1810.10062. [DOI] [PubMed] [Google Scholar]
- 201.Priem B., Peroux J., Colin-Morel P., Drouillard S., Fort S. Chemo-bacterial synthesis of conjugatable glycosaminoglycans. Carbohydr. Polym. 2017;167:123–128. doi: 10.1016/j.carbpol.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 202.Erenler A.S. Capsular polysaccharide biosynthesis from recombinant E. coli and chondroitin sülfate production. Cell. Mol. Biol. 2019;65:17–21. doi: 10.14715/cmb/2019.65.6.4. [DOI] [PubMed] [Google Scholar]
- 203.X.-.X. Wei, G.-.Q. Chen, Applications of the VHb gene vgb for improved microbial fermentation processes, in: Methods Enzymol., 2008: pp. 273–287. 10.1016/S0076-6879(08)36015-7. [DOI] [PubMed]
- 204.Jin X., Zhang W., Wang Y., Sheng J., Xu R., Li J., Du G., Kang Z. Biosynthesis of non-animal chondroitin sulfate from methanol using genetically engineered Pichia pastoris. Green Chem. 2021;23:4365–4374. doi: 10.1039/d1gc00260k. [DOI] [Google Scholar]
- 205.Cimini D., Fantaccione S., Volpe F., De Rosa M., Restaino O.F., Aquino G., Schiraldi C. IS2-mediated overexpression of kfoC in E. coli K4 increases chondroitin-like capsular polysaccharide production. Appl. Microbiol. Biotechnol. 2014;98:3955–3964. doi: 10.1007/s00253-014-5506-0. [DOI] [PubMed] [Google Scholar]
- 206.Wu J., Zhang Q., Liu L., Chen X., Liu J., Luo Q. Recombinant Escherichia coli for high efficiency production of fructosylated chondroitin and method for making thereof. US Patent. 2019;10:508. 279 B2. [Google Scholar]
- 207.Zanfardino A., Restaino O.F., Notomista E., Cimini D., Schiraldi C., De Rosa M., De Felice M., Varcamonti M. Isolation of an Escherichia coli K4 kfoC mutant over-producing capsular chondroitin. Microb. Cell Fact. 2010;9:1–8. doi: 10.1186/1475-2859-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Stevens M.P., Clarke B.R., Roberts I.S. Regulation of the Escherichia coli K5 capsule gene cluster by transcription antitermination. Mol. Microbiol. 1997;24:1001–1012. doi: 10.1046/j.1365-2958.1997.4241780.x. [DOI] [PubMed] [Google Scholar]
- 209.Badle S.S., Jayaraman G., Ramachandran K.B. Ratio of intracellular precursors concentration and their flux influences hyaluronic acid molecular weight in Streptococcus zooepidemicus and recombinant Lactococcus lactis. Bioresour. Technol. 2014;163:222–227. doi: 10.1016/j.biortech.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 210.Levander F., Svensson M., Rådström P. Enhanced exopolysaccharide production by metabolic engineering of Streptococcus thermophilus. Appl. Environ. Microbiol. 2002;68:784–790. doi: 10.1128/AEM.68.2.784-790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mao Z., Shin H.D., Chen R.R. Engineering the E. coli UDP-glucose synthesis pathway for oligosaccharide synthesis. Biotechnol. Prog. 2006;22:369–374. doi: 10.1021/bp0503181. [DOI] [PubMed] [Google Scholar]
- 212.Wang H., Yang Y., Lin L., Zhou W., Liu M., Cheng K., Wang W. Engineering Saccharomyces cerevisiae with the deletion of endogenous glucosidases for the production of flavonoid glucosides. Microb. Cell Fact. 2016;15:1–12. doi: 10.1186/s12934-016-0535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Widner B., Behr R., Von Dollen S., Tang M., Heu T., Sloma A., Sternberg D., DeAngelis P.L., Weigel P.H., Brown S. Hyaluronic acid production in Bacillus subtilis. Appl. Environ. Microbiol. 2005;71:3747–3752. doi: 10.1128/AEM.71.7.3747-3752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yu H., Stephanopoulos G. Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab. Eng. 2008;10:24–32. doi: 10.1016/j.ymben.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 215.Wu Y., Chen T., Liu Y., Lv X., Li J., Du G., Ledesma-Amaro R., Liu L. CRISPRi allows optimal temporal control of N-acetylglucosamine bioproduction by a dynamic coordination of glucose and xylose metabolism in Bacillus subtilis. Metab. Eng. 2018;49:232–241. doi: 10.1016/j.ymben.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 216.Westbrook A.W., Ren X., Oh J., Moo-Young M., Chou C.P. Metabolic engineering to enhance heterologous production of hyaluronic acid in Bacillus subtilis. Metab. Eng. 2018;47:401–413. doi: 10.1016/j.ymben.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 217.Woo J.E., Seong H.J., Lee S.Y., Jang Y.S. Metabolic engineering of Escherichia coli for the production of hyaluronic acid from glucose and galactose. Front. Bioeng. Biotechnol. 2019;7:1–9. doi: 10.3389/fbioe.2019.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Niu T., Liu Y., Li J., Koffas M., Du G., Alper H.S., Liu L. Engineering a glucosamine-6-phosphate responsive glmS ribozyme switch enables dynamic control of metabolic flux in Bacillus subtilis for overproduction of N-acetylglucosamine. ACS Synth. Biol. 2018;7:2423–2435. doi: 10.1021/acssynbio.8b00196. [DOI] [PubMed] [Google Scholar]
- 219.Cheng F., Yu H., Stephanopoulos G. Engineering Corynebacterium glutamicum for high-titer biosynthesis of hyaluronic acid. Metab. Eng. 2019;55:276–289. doi: 10.1016/j.ymben.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 220.Rainha J., Rodrigues J.L., Rodrigues L.R. CRISPR-Cas9: a powerful tool to efficiently engineer Saccharomyces cerevisiae. Life. 2021;11:1–16. doi: 10.3390/life11010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Williams A., Linhardt R.J., Koffas M.A.G. Metabolic engineering of capsular polysaccharides. Emerg. Top. Life Sci. 2018;2:337–348. doi: 10.1042/ETLS20180003. [DOI] [PubMed] [Google Scholar]
- 222.Baik J.Y., Gasimli L., Yang B., Datta P., Zhang F., Glass C.A., Esko J.D., Linhardt R.J., Sharfstein S.T. Metabolic engineering of Chinese hamster ovary cells: towards a bioengineered heparin. Metab. Eng. 2012;14:81–90. doi: 10.1016/j.ymben.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Restaino O.F., D'ambrosio S., Cassese E., Ferraiuolo S.B., Alfano A., Ventriglia R., Marrazzo A., Schiraldi C., Cimini D. Molecular weight determination of heparosan- and chondroitin-like capsular polysaccharides: figuring out differences between wild -type and engineered Escherichia coli strains. Appl. Microbiol. Biotechnol. 2019;103:6771–6782. doi: 10.1007/s00253-019-09969-8. [DOI] [PubMed] [Google Scholar]
- 224.Restaino O.F., Cimini D., De Rosa M., Catapano A., De Rosa M., Schiraldi C. High cell density cultivation of Escherichia coli K4 in a microfiltration bioreactor: a step towards improvement of chondroitin precursor production. Microb. Cell Fact. 2011;10:1–10. doi: 10.1186/1475-2859-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Amado I.R., Vázquez J.A., Pastrana L., Teixeira J.A. Cheese whey: a cost-effective alternative for hyaluronic acid production by Streptococcus zooepidemicus. Food Chem. 2016;198:54–61. doi: 10.1016/j.foodchem.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 226.Amado I.R., Vázquez J.A., Pastrana L., Teixeira J.A. Microbial production of hyaluronic acid from agro-industrial by-products: molasses and corn steep liquor. Biochem. Eng. J. 2017;117:181–187. doi: 10.1016/j.bej.2016.09.017. [DOI] [Google Scholar]
- 227.Pan N.C., Pereira H.C.B., da Silva M.L.C., Vasconcelos A.F.D., Celligoi M.A.P.C. Improvement production of hyaluronic acid by Streptococcus zooepidemicus in sugarcane molasses. Appl. Biochem. Biotechnol. 2017;182:276–293. doi: 10.1007/s12010-016-2326-y. [DOI] [PubMed] [Google Scholar]