Abstract
Aim: The main of the present study was to investigate the role of insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) in oral squamous cell carcinoma (OSCC) with the overarching of providing new biomarkers or potential therapeutic targets for OSCC.
Methods: We combined datasets downloaded from Gene Expression Omnibus (GEO), The Cancer Genome Atlas (TCGA), and samples collected from the clinic to evaluate the expression of IGF2BP2 in OSCC. IGF2BP2 survival analysis was respectively performed based on TCGA, GEO, and clinical samples. Correlations between IGF2BP2 expression and clinicopathological parameters were then analyzed, and signaling pathways associated with IGF2BP2 expression were identified using gene set enrichment analysis (GSEA 4.1.0). Moreover, an IGF2BP2 co-expressed gene network was constructed, followed by gene ontology (GO) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis on IGF2BP2 co-expressed genes. Finally, TIMER and CIBERSORT were used to analyze the correlations among IGF2BP2, IGF2BP2-coexpressed genes, and tumor-infiltrating immune cells (TICs).
Results: IGF2BP2 was highly expressed in OSCC and significantly correlated with overall survival of OSCC patients (P<0.01). High IGF2BP2 expression correlated with poor overall survival. The GSEA results showed that cell apoptosis-, tumor-, and immune-related pathways were significantly enriched in samples with high IGF2BP2 expression. Furthermore, GO and KEGG enrichment analyses results of IGF2BP2 co-expressed genes indicated that these genes are mainly associated with immunity/inflammation and tumorigenesis. In addition, IGF2BP2 and its co-expressed genes are associated with TICs (P<0.01).
Conclusion: IGF2BP2 may be a potential prognostic biomarker in OSCC and correlates with immune infiltrates.
Keywords: biomarkers, IGF2BP2, immune infiltrates, OSCC, Prognosis
Introduction
Oral squamous cell carcinoma (OSCC) is the eighth most common type of human cancer in the world and often has a poor prognosis. Statistics indicate that it accounts for approximately 90% of all types of oral malignancies, with over 300000 new cases and 145000 deaths every year [1]. In recent decades, the incidence and mortality of OSCC has remained at a relatively high level despite the enormous progress in diagnosis and treatments such as radiotherapy and chemotherapy. Currently, the overall 5-year survival rate of OSCC is below 60% [2]. Although the treatment methods for malignant tumors have been continuously improving from traditional surgical treatment, radiotherapy, and chemotherapy to biologically targeted therapy, the high recurrence rate and metastasis of OSCC are still not sufficiently solved and the prognosis of advanced patients is still unsatisfactory [3]. Similar to other types of tumors, the occurrence of OSCC involves a series of complex interactions between a variety of genes and proteins, which results in a multifactor interaction [4]. Therefore, this calls for elucidation of the mechanisms underlying the occurrence and development of OSCC, and the search for new specific molecular markers of OSCC with the overarching goal of facilitating development of new treatment options.
In recent years, many studies have focused on the tumor microenvironment (TME). As a complex ecosystem, TME is involved in the occurrence and development of many cancers, especially the immune components. However, studies have shown that transforming TME from tumor-friendly to tumor suppressor is a very promising new strategy for cancer treatment [5]. In addition, a previous study confirmed the correlation between immune cell infiltration and the prognosis of patients with head and neck squamous cell carcinoma (HNSC) [6]. Recently, it has been shown that immune cell dysfunction in HNSC-TME can promote immune suppression, thereby promoting the survival and progression of related tumors, and the ICI score is an effective prognostic biomarker and predictive indicator for evaluating immunotherapy response [7]. The results of Chen et al. showed that Th17 cells play a beneficial role in the prognosis of colorectal adenocarcinoma (COAD). Genes such as KRT23, ULBP2, ASRGL1, SERPINA1, and SCIN have also been identified as being related to the prognosis of Th17 cells and COAD [8]. Thus, using multilayer data analysis to identify potential immunotherapy targets and improve the therapeutic effect of OSCC has gradually become a new direction of our research.
Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) is located in chromosome 3q27 [9]. It is a member of the IGF2 mRNA-binding protein family. It is a post-transcriptional regulator of mRNA localization, stability and translation control. It is also a member of M6A methyltransferase-related genes [10,11]. Studies have shown that m6A modification is closely related to immune infiltration in various diseases. For example, METTL3-mediated m6A methylation can promote the activation of dendritic cells (DCs) on one hand [12], but on the other hand, it can destroy the balance between Treg cells and natural T cells, resulting in the destruction of their regulatory role in immune response [13]. CDC25C, FOXM1, MCM3, MCM7 and many other key genes regulated by IGF2BP2-mediated RNA N6-methyladenosine are related to a variety of immune cell infiltration and tumor purity, and play an important role in the prognosis of hepatocellular carcinoma [14]. Previous studies have shown that dysregulation of IGF2BP2 is associated with the growth, migration, adhesion, and energy metabolism of cancer cells, and it modulates the occurrence and development of many human diseases such as diabetes and malignant tumors [15,16]. IGF2BP2 knockout mice experiments also confirmed its promotion of tumor development [17]. Studies have shown that IGF2BP2 is associated with immune cell infiltration in esophageal cancer [18]. In addition, a recent study conducted in Taiwan reported that the genetic polymorphism of IGF2BP2 is associated with less favorable clinical features and prognosis of patients with OSCC [19]. However, there is limited evidence regarding the association between IGF2BP2 and OSCC, and the role of IGF2BP2 in OSCC tumorigenesis has not yet been elucidated.
The present study analyzed the expression of IGF2BP2 in OSCC, and the correlations between IGF2BP2 expression and clinicopathological features, as well as prognosis using public datasets. The results were further confirmed using clinical samples and the possible molecular function of IGF2BP2 was revealed through gene set enrichment analysis (GSEA) using data retrieved from The Cancer Genome Atlas (TCGA) database. In addition, IGF2BP2-related genes were screened out and used to construct gene co-expression network. Finally, the association among IGF2BP2, its co-expressed genes, and tumor-infiltrating immune cells (TICs) was investigated. Results obtained in the present study revealed the potential role of IGF2BP2 in tumor immunology and its prognostic value, which will help in elucidating its possible mechanism in OSCC.
Materials and methods
Resources and description of public datasets
Gene Expression Omnibus (GEO) microarray series (GSE31056 [20], GSE42743 [21], and GSE51010 [22]) containing OSCC tumor and non-tumor samples were obtained from the National Center for Biotechnology Information (NCBI) (GEO, https://www.ncbi.nlm.nih.gov/geo/). All three datasets met the following inclusion criteria: (a) used human oral tissue samples; (b) had a healthy control group; and (c) contained at least 30 samples. Table 1 shows the summarized platforms and samples of GEO series.
Table 1. Details of GEO series included in this analysis.
All the publicly available OSCC RNA-Seq data were downloaded from TCGA’s official website (https://cancergenome.nih.gov/) using the GDC Data Transfer Tool [23]. Notably, the dataset contains survival data with clinical information and mRNA expression counts. After excluding samples with missing information, the RNA-Seq gene expression data and clinical data of 341 patients with OSCC were retained and further analyzed (Supplementary Tables S1 and S2).
IGF2BP2 filtering
Differentially expressed genes (DEGs) were filtered according to the log fold change (|logFC|>1) and adjusted P values (adj. P<0.001). Next, the Online Omicshare3.0 (http://www.omicshare.com/tools) was performed to discover the overlapping genes among different profiles. Finally, IGF2BP2 was selected as the subject of the present study based on the association between the expression of overlapping genes and the prognosis of OSCC (P<0.01).
Expression analysis of IGF2BP2
Raw CEL files of the microarray from each GEO dataset were normalized using the quantile method of Robust Multichip Analysis (RMA) from the R affy package and the normalized gene expression levels were presented as log2-transformed values by RMA [24]. IGF2BP2 gene expression was determined by comparing tumor and non-tumor samples using the R limma package [25]. The edgeR package in R language version 3.6.3 was used to compare the mRNA expression of tumor and non-tumor samples retrieved from TCGA database [26]. Next, studies that had previously compared IGF2BP2 expression between OSCC tumor and non-tumor samples were selected, with a threshold of P-value ≤ 1E-4, fold change ≥ 2, and top 10% gene rank in the Oncomine database (https://www.oncomine.org/) [27].
Furthermore, 30 pairs of OSCC tissue samples were obtained from Beijing Stomatological Hospital Affiliated to Capital Medical University from September 2019 to March 2021, followed by determination of IGF2BP2 gene expression at the mRNA and protein level. Notably, the patients signed informed consents before the study began and the study was approved by the ethics committee of Beijing Stomatological Hospital Affiliated to Capital Medical University. Total RNA was extracted with TRIzol® reagent (Invitrogen, Carlsbad, CA, U.S.A.) and reverse transcribed to cDNA using Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, U.S.A.) according to the manufacturer’s instructions. Next, quantitative real-time polymerase chain reaction (qRT-PCR) was performed on the Light-Cycler96 Sequence Detection system (Roche Diagnostics, Basel, Switzerland) using SYBR® Premix ExTaq™ (Takara Bio, Inc., Otsu, Japan). Relative mRNA expression was normalized to the expression of GADPH mRNA and calculated using the the 2−ΔΔCt method. The sequences of primers used were as follows: IGF2BP2: forward: 5′-AGTGGAATTGCATGGGAAAATCA-3′, reverse: 5′-GTA CTC TTT GCG GTC GAG CA-3′; and GAPDH: forward: 5′-GGAGCGAGATCCCTCCAAAAT-3′, reverse: 5′-GGCTGTTGTCATACTTCTCATGG-3′. For Western blot analysis, protein samples were isolated using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) containing protease inhibitor cocktail tablet (Roche Applied Science) and quantified using BCA protein assay (Beyotime Biotechnology, Shanghai, China). Next, the proteins were resolved on SDS/PAGE and transferred on to a nitrocellulose membrane. After blocking with Tris-buffered saline containing 5% skimmed milk for 1 h at room temperature, the membrane was incubated with anti-IGF2BP2 (Abclonal Technology, Wuhan, China) and anti-GAPDH primary antibody (Abclonal Technology, Wuhan, China) overnight at 4°C. The membrane was subsequently incubated with a goat anti-mouse/rabbit secondary antibody (Boster, Wuhan, China) for 1 h at room temperature. Finally, enhanced chemiluminescence was used to visualize the protein bands in a Bio-Rad ChemiDoc XRS Imaging System (Supplementary Figures S1 and S2).
Prognostic value of IGF2BP2 in OSCC
Survival analysis was carried out using both survminer and survival packages in R (v.3.6.3). Eligible OSCC samples were screened in accordance with the following criteria: (i) removal of normal samples and (ii) removal of samples with incomplete clinical information. Logistic regression and the KS test were then used to analyze the correlation between the expression level of IGF2BP2 gene and clinicopathological features of OSCC. Moreover, univariate and multivariate Cox regression analyses were performed to determine whether the prognostic significance of IGF2BP2 was independent of the above-mentioned clinicopathological variables in OSCC. Notably, the statistical significance was tested via log-rank with the significant threshold of P-value set as 0.05.
GSEA
A total of 311 OSCC tumor samples retrieved from TCGA database were divided into high and low IGF2BP2 expression groups according to the median expression value of IGF2BP2 [28]. GSEA 4.1.0 software was then used to determine the pathways that were enriched by the top ranked genes in the two groups: C2. CP. KEGG.v7.2 gene sets and Hallmark collections were acquired from Molecular Signatures Database (MSigDB). The number of gene set permutations for each analysis was set to 1000, and significant gene sets were determined using nominal (NOM) P-value <0.05 and false discovery rate (FDR) q-value < 0.25.
Identification of IGF2BP2-related genes and construction of gene co-expression network
OSCC samples were divided into high- and low-expression groups according to the median expression value of IGF2BP2, and the DEGs between the two groups were analyzed using edge R package, with P-value <0.05 and |FC| > 1.5 cutoffs. Next, we calculated the Pearson coefficients of the DEGs and IGF2BP2. It is worth noting that DEGs with a P-value of <0.05 and a correlation coefficient of >0.3 were defined as IGF2BP2-related genes, and were used to construct the gene co-expression network. The top three significant genes that were positively associated with IGF2BP2 were selected for further analysis, and their correlation with IGF2BP2 was verified using GEIPA [29] and TIMER [30], respectively.
Functional analyses
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were conducted using R packages clusterProfiler, enrichplot, and ggplot2 to explore the biological functions and signaling pathways of IGF2BP2-related genes. The significantly enriched terms were determined at P-value <0.05 and q-value <0.05.
Correlation analysis among IGF2BP2, IGF2BP2 co-expressed genes, and immune infiltrating cells
To determine the association among TICs, IGF2BP2, and IGF2BP2 co-expressed genes, CIBERSORT was utilized to approximately evaluate the proportion of TICs profile in the OSCC tumor samples [31]. Furthermore, TIMER was used to verify the association among IGF2BP2, its co-expressed genes, and TICs. Notably, ggplot2, tidy-verse, and reshape2 packages in R version 3.6.3 software were used for analysis and plotting, and follow-up analyses were only conducted for cases with P-value <0.05.
Statistical analysis
All data analyses were conducted using SPSS version 19.0 and R version 3.6.3 software. Measurement data are presented as mean ± SD. Independent-sample t test and paired-sample t test were used to analyze the differential expression levels of IGF2BP2 mRNA between OSCC tumor tissues and non-tumor tissues retrieved from TCGA database, and samples collected at the clinic. Moreover, the association between IGF2BP2 expression and clinicopathological characteristics was evaluated using Logistic regression and the KS test. Univariate and multivariate analyses were based on Cox proportional hazard regression models. P<0.05 was considered to be statistically significant.
Results
The analysis process of the present study
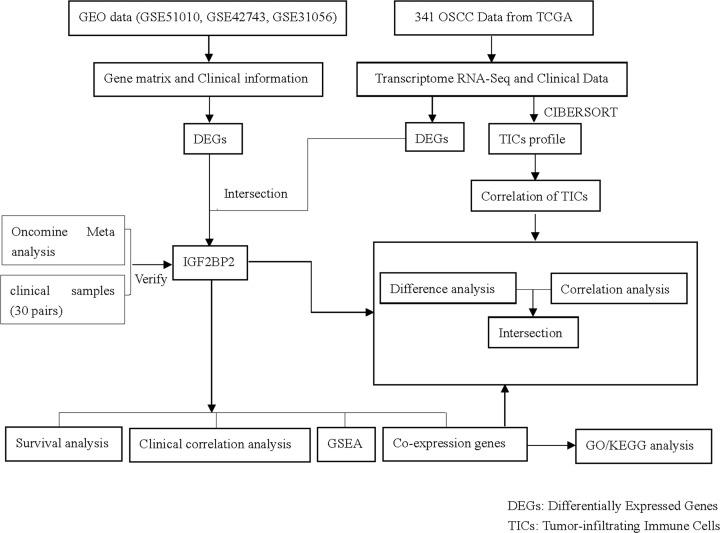
Figure 1 shows the process through which the present study was analyzed. Firstly, the OSCC gene expression datasets and corresponding clinical files were downloaded from the TCGA and GEO databases, respectively. Next, the common DEGs were screened, and IGF2BP2 was obtained after further filtering. Furthermore, the accuracy of this result was verified through Oncomine meta-analysis and collected clinical samples. The present study focused on in-depth analysis of IGF2BP2, including correlation analysis of OS and clinicopathological characteristics, GSEA, co-expressed genes, and correlation with TICs. In addition, GO/KEGG enrichment analysis was performed on the co-expressed genes of IGF2BP2, followed by correlation analysis with TICs.
Figure 1. The analysis workflow of the present study.
Analysis of gene expression profiles and filtering of DEGs
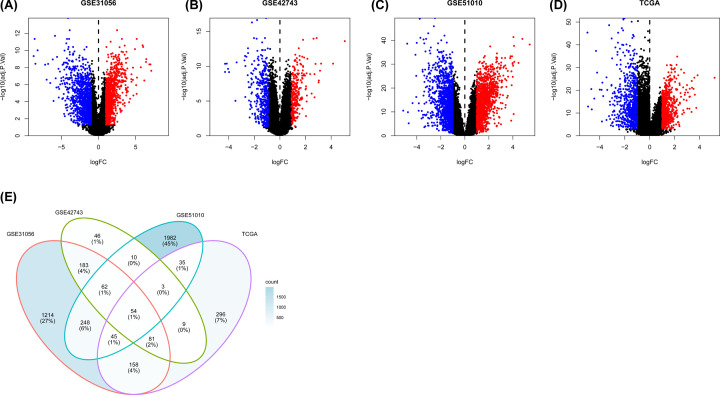
In total, 1494 DEGs, 448 DEGs, 2439 DEGs, and 1309 DEGs were screened from GSE31056, GSE42743, GSE51010, and TCGA databases, respectively (Figure 2A–D). After filtering, 54 four-crossing DEGs were identified among GSE31056, GSE42743, GSE51010, and TCGA databases (Figure 2E). Finally, IGF2BP2 was selected for analysis in the present study after comparing the OSCC prognostic value.
Figure 2. DEGs in public datasets (|logFC| > 1, P<0.001).
(A–C) DEGs in GSE31056, GSE42743, GSE51010. (D) DEGs in TCGA. (E) Intersection of DEGs among GSE31056, GSE42743, GSE51010 and TCGA.
High expression of IGF2BP2 in OSCC
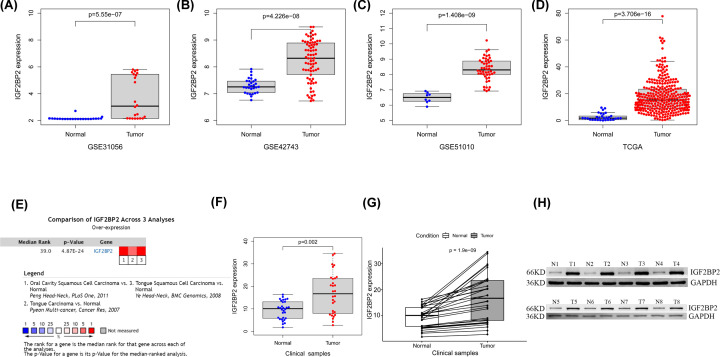
Results showed a significantly higher expression of IGF2BP2 in OSCC tumor samples than in non-tumor samples in GSE31056, GSE42743, GSE51010, and TCGA datasets (Figure 3A–D, P<0.001). To verify the accuracy of the result, the Oncomine database was used to perform a meta-analysis of IGF2BP2 expression in three analyses with the threshold set as P-value ≤1E-4, fold change ≥ 2, and top 10% gene rank. Results indicated that IGF2BP2 was significantly up-regulated in OSCC tumor samples compared with non-tumor tissues (Figure 3E). Similar results were obtained after further verification using 30 pairs of matched clinical samples. Moreover, the results of mRNA and protein expression analysis of clinical samples showed that the expression of IGF2BP2 was significantly higher in OSCC tumor samples than in non-tumor samples (Figure 3F–H).
Figure 3. The expression level of IGF2BP2 is up-regulated in OSCC.
(A–C) IGF2BP2 mRNA levels in OSCC tissues and normal tissues in the GSE31056, GSE42743, and GSE51010 datasets. (D) IGF2BP2 mRNA levels in OSCC tissues and normal tissues in TCGA. (E) Meta-analysis of IGF2BP2 expression across three analyses in the ONCOMINE database. (F) mRNA expression of IGF2BP2 based on 30 pairs of clinical samples. (G) IGF2BP2 mRNA levels in OSCC tumor tissues and matched normal tissues in the 30 pairs of clinical samples. (H) Western blot was performed to determine the protein expression of IGF2BP2 in OSCC tumor samples.
Prognostic value of IGF2BP2 in OSCC
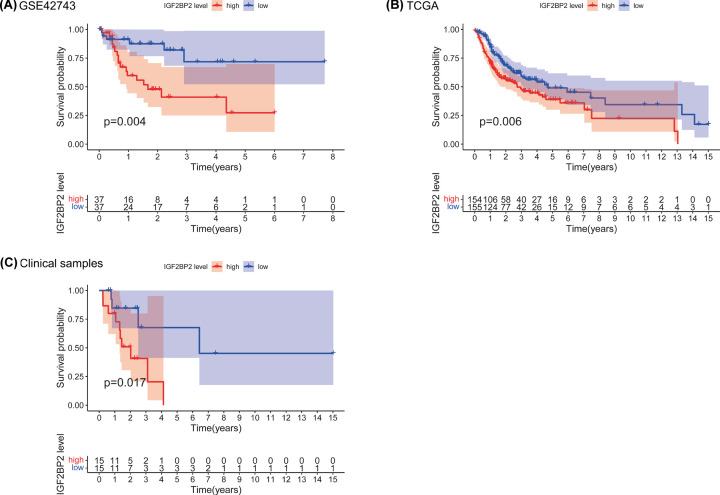
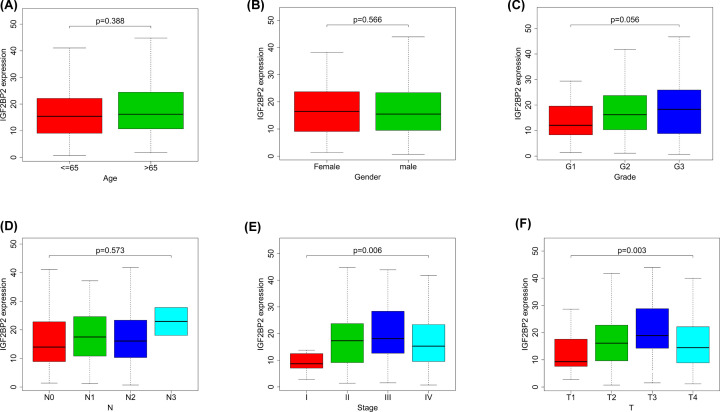
To estimate the prognostic value of IGF2BP2 in OSCC, Kaplan–Meier survival analysis was used to evaluate the correlation between IGF2BP2 expression and overall survival in the TCGA and GEO datasets, and collected clinical samples, respectively. From the results, the survival of patients with high IGF2BP2 expression was relatively poor (all P<0.01, Figure 4A–C). In addition, the results of the correlation analysis showed that the expression of IGF2BP2 was significantly correlated with T stage and clinical stage, but not the N stage, grade, age, or gender (Figure 5A–F and Table 2).
Figure 4. High IGF2BP2 expression is associated with poor survival in OSCC patients.
(A) Overall survival of IGF2BP2high and IGF2BP2low patients analyzed with the dataset, GSE42743. (B) Overall survival of IGF2BP2high and IGF2BP2low OSCC patients analyzed with TCGA (n=311). (C) Overall survival of IGF2BP2high and IGF2BP2low OSCC patients analyzed with clinical samples (n=30).
Figure 5. Correlation between IGF2BP2 expression and clinicopathologic characteristics.
(A) Subgroup analysis of Age (≤65 and >65 years). (B) Subgroup analysis of Gender (female and male). (C) Subgroup analysis of Grade (G1/G2/G3). (D) Subgroup analysis of N stage (N0/N1/N2/N3). (E) Subgroup analysis of clinical stage (I/II/III/IV). (F) Subgroup analysis of T stage (T1/T2/T3/T4). Wilcox test in (A,B), Kruskal test in (C–F). When P<0.05, there was significant difference in the expression level of IGF2BP2 between subgroups with clinicopathological features.
Table 2. Correlation between the clinicopathologic characteristics and IGF2BP2 mRNA expression (logistic regression).
| Clinical characteristics | Total (n) | Odds ratio in IGF2BP2 expression | P-value |
|---|---|---|---|
| Age (≤65 vs. >65) | 310 | 1.027322 (0.6514473–1.620499) | 0.9076 |
| Gender (female vs. male) | 311 | 1.113137 (0.6931055–1.790307) | 0.6574 |
| Grade (G1–2 vs. G3) | 307 | 1.447293 (0.830286–2.547501) | 0.1946 |
| Clinical stage (I vs. II–IV) | 287 | 2.972308 (1.102964–9.407618) | 0.0418* |
| T stage (T1 vs. T2–4) | 291 | 2.398589 (1.081688–5.721332) | 0.0372* |
| N stage (N0 vs. N1–3) | 266 | 1.276364 (0.7865679–2.076311) | 0.3238 |
P<0.05 was considered statistically significant.
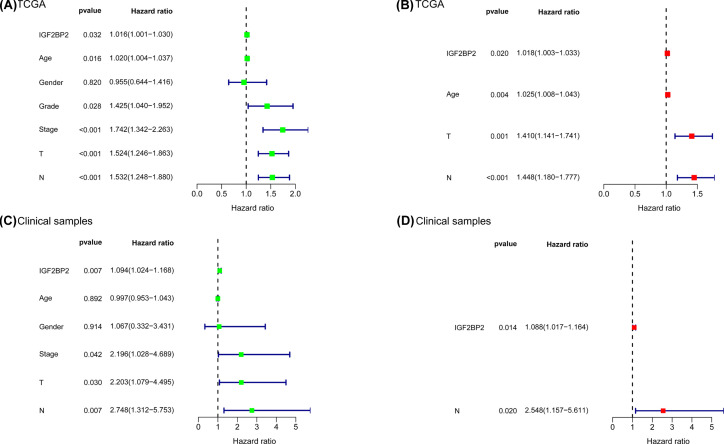
IGF2BP2 was an independent prognostic factor in OSCC
Next, we performed univariate and multivariate cox regression analyses using the TCGA datasets and collected clinical samples, respectively. Univariate Cox regression analysis of TCGA datasets revealed that age, grade classification, clinical stage, T stage, N stage, and IGF2BP2 were important factors for OSCC prognosis (Table 3 and Figure 6A). On the other hand, multivariate Cox regression analysis demonstrated that age, T stage, N stage, and IGF2BP2 were independent prognostic elements for OSCC patients (Table 4 and Figure 6B). Moreover, univariate Cox regression analysis of collected clinical samples revealed that clinical stage, N stage, T stage, and IGF2BP2 were important factors for OSCC prognosis (Supplementary Table S3 and Figure 6C), while multivariate Cox regression analysis demonstrated that N stage and IGF2BP2 were independent prognostic elements for OSCC patients (Supplementary Table S4 and Figure 6D).
Table 3. Univariate Cox regression of overall survival and clinicopathologic characteristics in TCGA OSCC patients.
| Clinical characteristics | Hazard ratio | HR (95% CI) | p-value |
|---|---|---|---|
| Age (≤65 vs. >65) | 1.0201 | 1.0036–1.0368 | 0.01645* |
| Gender (female vs. male) | 0.9552 | 0.6442–1.4163 | 0.81960 |
| Grade (G1/G2/G3) | 1.4248 | 1.0400–1.9518 | 0.02750* |
| Clinical stage (I/II/III/IV) | 1.7425 | 1.3420–2.2625 | 0.00003* |
| T stage (T1/2/3/4) | 1.5236 | 1.2459–1.8631 | 0.00004* |
| N stage (N0/1/2/3) | 1.5317 | 1.2478–1.8802 | 0.00004* |
| IGF2BP2 expression (low/high) | 1.0156 | 1.0013–1.0301 | 0.03151* |
P<0.05 was considered statistically significant.
Figure 6. Analysis of prognostic factors for OSCC.
(A) Univariate Cox of IGF2BP2 and six clinical phenotypes (Age, Gender, Grade, T, N, Stage) in TCGA. (B) Multivariate Cox of age, T, N and IGF2BP2 in TCGA. (C) Univariate Cox of IGF2BP2 and five clinical phenotypes (Age, Gender, T, N, Stage) in clinical samples. (D) Multivariate Cox of N and IGF2BP2 in clinical samples.
Table 4. Multivariate analyses of overall survival and clinicopathologic characteristics in TCGA OSCC patients.
| Clinical characteristics | Hazard ratio | HR (95% CI) | P-value |
|---|---|---|---|
| Age (≤65 vs. >65) | 1.0289 | 1.0098–1.0483 | 0.00284* |
| T stage (T1/2/3/4) | 1.4096 | 1.1412–1.7419 | 0.00144* |
| N stage (N0/1/2/3) | 1.4479 | 1.1798–1.7769 | 0.00039* |
| IGF2BP2 expression (low/high) | 1.0176 | 1.0028–1.0332 | 0.01991* |
P<0.05 was considered statistically significant.
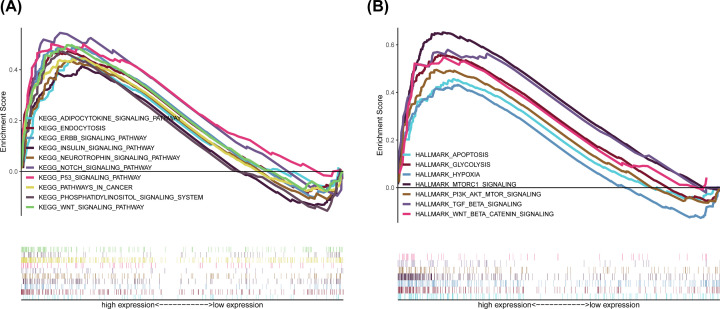
GSEA identified IGF2BP2-related signaling pathways in OSCC
To explore the potential molecular function of IGF2BP2 in OSCC, GSEA was conducted between tumor samples with low and high IGF2BP2 expression in order to predict IGF2BP2-related signaling pathways. Results showed that a total of 128 out of 178 signaling pathways were up-regulated, and 54 signaling pathways were significantly enriched at NOM P<0.05 and FDR q-value <0.25 (Table 5). The significantly up-regulated terms involved in tumorigenesis enriched in the high IGF2BP2 group were ‘WNT signaling pathway’, ‘Notch signaling pathway’, ‘P53 signaling pathway’, ‘ERBB signaling pathway’, and ‘Phosphatidylinositol signaling pathway’, while the associated terms involved in immune and inflammatory responses included ‘endocytosis’, ‘insulin signaling pathway’, and ‘adipocytokine signaling pathway’ (Figure 7A). In addition, multiple immune activities and metabolic functions were respectively enriched in the IGF2BP2 high expression group for HALLMARK gene sets (Figure 7B and Table 6). Collectively, these results suggest that IGF2BP2 may be a promising immune-related indicator of OSCC.
Table 5. GSEA pathways up-regulated due to high expression of IGF2BP2.
| Gene set name | NES | NOM P-val | FDR q-val |
|---|---|---|---|
| KEGG_CELL_CYCLE | 2.144 | 0.000 | 0.011 |
| KEGG_RNA_DEGRADATION | 2.101 | 0.000 | 0.011 |
| KEGG_UBIQUITIN_MEDIATED_PROTEOLYSIS | 2.007 | 0.000 | 0.011 |
| KEGG_OOCYTE_MEIOSIS | 2.070 | 0.002 | 0.006 |
| KEGG_SPLICEOSOME | 2.096 | 0.002 | 0.007 |
| KEGG_NUCLEOTIDE_EXCISION_REPAIR | 2.058 | 0.002 | 0.007 |
| KEGG_BASAL_TRANSCRIPTION_FACTORS | 2.032 | 0.002 | 0.009 |
| KEGG_PYRIMIDINE_METABOLISM | 1.952 | 0.004 | 0.020 |
| KEGG_WNT_SIGNALING_PATHWAY | 1.838 | 0.004 | 0.034 |
| KEGG_HOMOLOGOUS_RECOMBINATION | 1.931 | 0.004 | 0.022 |
| KEGG_PROGESTERONE_MEDIATED_OOCYTE_MATURATION | 1.866 | 0.004 | 0.034 |
| KEGG_ENDOCYTOSIS | 1.846 | 0.004 | 0.034 |
| KEGG_SMALL_CELL_LUNG_CANCER | 1.851 | 0.004 | 0.034 |
| KEGG_RNA_POLYMERASE | 1.831 | 0.006 | 0.034 |
| KEGG_BASE_EXCISION_REPAIR | 1.900 | 0.006 | 0.028 |
| KEGG_PANCREATIC_CANCER | 1.806 | 0.006 | 0.039 |
| KEGG_AMINOACYL_TRNA_BIOSYNTHESIS | 1.799 | 0.008 | 0.039 |
| KEGG_INOSITOL_PHOSPHATE_METABOLISM | 1.866 | 0.008 | 0.037 |
| KEGG_N_GLYCAN_BIOSYNTHESIS | 1.864 | 0.008 | 0.032 |
| KEGG_BLADDER_CANCER | 1.615 | 0.008 | 0.079 |
| KEGG_THYROID_CANCER | 1.766 | 0.008 | 0.042 |
| KEGG_CHRONIC_MYELOID_LEUKEMIA | 1.772 | 0.008 | 0.045 |
| KEGG_PENTOSE_PHOSPHATE_PATHWAY | 1.660 | 0.008 | 0.067 |
| KEGG_PURINE_METABOLISM | 1.754 | 0.010 | 0.043 |
| KEGG_NOTCH_SIGNALING_PATHWAY | 1.769 | 0.010 | 0.042 |
| KEGG_RENAL_CELL_CARCINOMA | 1.788 | 0.010 | 0.040 |
| KEGG_PATHWAYS_IN_CANCER | 1.700 | 0.012 | 0.056 |
| KEGG_REGULATION_OF_ACTIN_CYTOSKELETON | 1.718 | 0.014 | 0.051 |
| KEGG_CYSTEINE_AND_METHIONINE_METABOLISM | 1.745 | 0.014 | 0.042 |
| KEGG_ADHERENS_JUNCTION | 1.811 | 0.016 | 0.039 |
| KEGG_DNA_REPLICATION | 1.797 | 0.016 | 0.038 |
| KEGG_MISMATCH_REPAIR | 1.753 | 0.016 | 0.042 |
| KEGG_FRUCTOSE_AND_MANNOSE_METABOLISM | 1.619 | 0.018 | 0.081 |
| KEGG_CYTOSOLIC_DNA_SENSING_PATHWAY | 1.754 | 0.018 | 0.045 |
| KEGG_ERBB_SIGNALING_PATHWAY | 1.707 | 0.018 | 0.054 |
| KEGG_P53_SIGNALING_PATHWAY | 1.722 | 0.018 | 0.051 |
| KEGG_PROTEASOME | 1.771 | 0.019 | 0.043 |
| KEGG_GLYCOSYLPHOSPHATIDYLINOSITOL_GPI_ANCHOR_BIOSYNTHESIS | 1.695 | 0.020 | 0.056 |
| KEGG_AMINO_SUGAR_AND_NUCLEOTIDE_SUGAR_METABOLISM | 1.623 | 0.021 | 0.081 |
| KEGG_PROTEIN_EXPORT | 1.681 | 0.023 | 0.061 |
| KEGG_PATHOGENIC_ESCHERICHIA_COLI_INFECTION | 1.641 | 0.023 | 0.075 |
| KEGG_GLIOMA | 1.585 | 0.025 | 0.093 |
| KEGG_PHOSPHATIDYLINOSITOL_SIGNALING_SYSTEM | 1.604 | 0.026 | 0.084 |
| KEGG_GLYOXYLATE_AND_DICARBOXYLATE_METABOLISM | 1.619 | 0.030 | 0.079 |
| KEGG_NEUROTROPHIN_SIGNALING_PATHWAY | 1.572 | 0.031 | 0.099 |
| KEGG_ADIPOCYTOKINE_SIGNALING_PATHWAY | 1.534 | 0.032 | 0.108 |
| KEGG_ONE_CARBON_POOL_BY_FOLATE | 1.677 | 0.033 | 0.061 |
| KEGG_LONG_TERM_POTENTIATION | 1.496 | 0.042 | 0.126 |
| KEGG_INSULIN_SIGNALING_PATHWAY | 1.540 | 0.045 | 0.108 |
| KEGG_VASOPRESSIN_REGULATED_WATER_REABSORPTION | 1.514 | 0.045 | 0.118 |
| KEGG_GLYCEROPHOSPHOLIPID_METABOLISM | 1.469 | 0.046 | 0.139 |
| KEGG_EPITHELIAL_CELL_SIGNALING_IN_HELICOBACTER_PYLORI_INFECTION | 1.535 | 0.046 | 0.109 |
| KEGG_ENDOMETRIAL_CANCER | 1.553 | 0.049 | 0.105 |
| KEGG_ALANINE_ASPARTATE_AND_GLUTAMATE_METABOLISM | 1.542 | 0.049 | 0.110 |
Abbreviation: NES, normalized enrichment score. Gene sets with NES > 1, NOM P-value <0.05 and FDR q-value <0.1 are considered as significant.
Figure 7. GSEA for samples with high IGF2BP2 expression.
(A) Enriched gene sets in C2 collection, the KEGG gene sets, by samples of high IGF2BP2 expression. Each line represents one particular gene set with unique color, and up-regulated genes are located on the left which approach the origin of the coordinates. Only gene sets both with NOM P<0.05 and FDR q < 0.25 were considered significant. Only several top gene sets are shown in the plot. (B) The enriched gene sets in HALLMARK collection by samples with high IGF2BP2 expression sample.
Table 6. The enriched gene sets in HALLMARK collection due to high expression of IGF2BP2.
| Gene set name | NES | NOM P-val | FDR q-val |
|---|---|---|---|
| HALLMARK_MITOTIC_SPINDLE | 2.283 | 0.000 | 0.000 |
| HALLMARK_UNFOLDED_PROTEIN_RESPONSE | 2.229 | 0.000 | 0.000 |
| HALLMARK_MTORC1_SIGNALING | 2.180 | 0.000 | 0.000 |
| HALLMARK_MYC_TARGETS_V1 | 2.160 | 0.000 | 0.001 |
| HALLMARK_GLYCOLYSIS | 2.158 | 0.000 | 0.001 |
| HALLMARK_G2M_CHECKPOINT | 2.126 | 0.000 | 0.001 |
| HALLMARK_E2F_TARGETS | 2.036 | 0.000 | 0.004 |
| HALLMARK_PROTEIN_SECRETION | 1.983 | 0.002 | 0.007 |
| HALLMARK_MYC_TARGETS_V2 | 1.977 | 0.002 | 0.006 |
| HALLMARK_DNA_REPAIR | 1.967 | 0.002 | 0.006 |
| HALLMARK_PI3K_AKT_MTOR_SIGNALING | 1.845 | 0.004 | 0.021 |
| HALLMARK_UV_RESPONSE_UP | 1.747 | 0.004 | 0.043 |
| HALLMARK_APOPTOSIS | 1.747 | 0.006 | 0.039 |
| HALLMARK_TGF_BETA_SIGNALING | 1.721 | 0.026 | 0.045 |
| HALLMARK_P53_PATHWAY | 1.711 | 0.008 | 0.045 |
| HALLMARK_WNT_BETA_CATENIN_SIGNALING | 1.661 | 0.016 | 0.062 |
| HALLMARK_APICAL_JUNCTION | 1.642 | 0.018 | 0.066 |
| HALLMARK_HYPOXIA | 1.624 | 0.031 | 0.070 |
| HALLMARK_SPERMATOGENESIS | 1.506 | 0.046 | 0.128 |
| HALLMARK_HEME_METABOLISM | 1.493 | 0.036 | 0.129 |
Abbreviation: NES, normalized enrichment score. Gene sets with NES > 1, NOM P-value <0.05 and FDR q-value <0.1 are considered as significant.
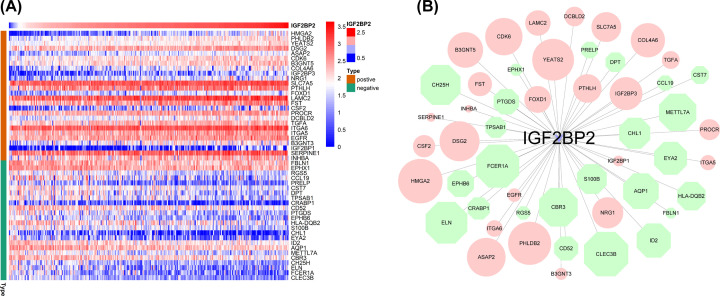
Analysis of genes co-expressed with IGF2BP2 in OSCC
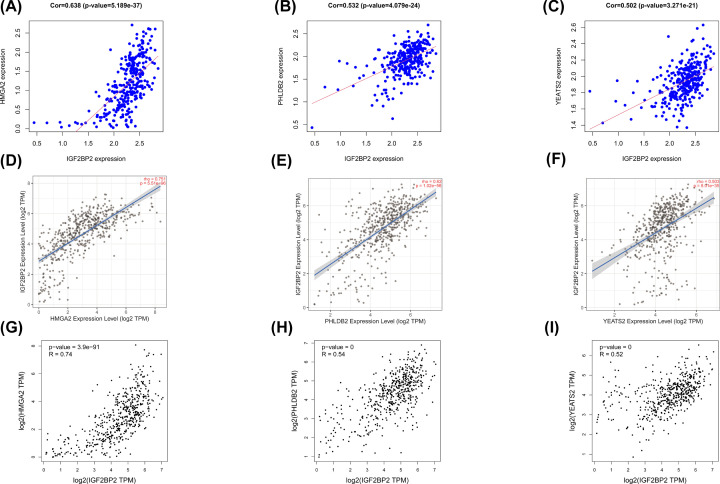
A total of 50 IGF2BP2 significantly related genes were screened from the 181 DEGs of the two groups with high and low expression of IGF2BP2, and used to further investigate the possible effect of IGF2BP2 in OSCC (Figure 8A). Next, the gene co-expression network was constructed using Cytoscape 3.8.1 software (Figure 8B), followed by selection of the top three significant genes that were positively correlated with IGF2BP2 (Figure 9A–C). Furthermore, the correlation between IGF2BP2 and these genes was verified in TIMER and GEIPA, respectively (Figure 9D–I). Results revealed that IGF2BP2 was significantly correlated with HMGA2 (r = 0.638, P=5.189e−37), PHLDB2 (r = 0.532, P=4.079e−24), and YEATS2 (r = 0.502, P=3.27e−21). In addition, results indicated that IGF2BP2-related genes were remarkably up-regulated in OSCC (Figure 10A–F). These results suggest that IGF2BP2 and its co-expressed genes may collectively contribute to OSCC, thereby resulting in poor survival in OSCC patients.
Figure 8. IGF2BP2 gene co-expression network.
(A) Heatmap of IGF2BP2 co-expression genes. The heatmap shows the top 50 genes co-expressed with IGF2BP2, including 26 positive and 24 negative genes. The row name on the right side of the heat map is gene symbol, the type on the left side of the heatmap is green, which indicates IGF2BP2 negative-related gene, and brown represents IGF2BP2 positive-related gene. (B) The IGF2BP2 gene co-expression network constructed by Cytoscape version 3.8.1, red represents IGF2BP2 positive-related genes, green represents IGF2BP2 negative-related genes. The size of the graph drawn is proportional to the correlation of IGF2BP2.
Figure 9. A filter of the top three significant genes that were positively associated with IGF2BP2.
(A–C) The genes positively associated with IGF2BP2 in OSCC (absolute Pearson’s r ≥ 0.5) were assessed with the TCGA database. (D–F) IGF2BP2 was significantly correlated with HMGA2 (cor = 0.751, P=5.51e−96), PHLDB2 (cor = 0.62, P=1.02e−56), YEATS2 (cor = 0.503, P=8.01e−35) in OSCC (via analysis in the TIMER database). (G–I) IGF2BP2 was significantly correlated with HMGA2 (cor = 0.74, P=3.9e−91), PHLDB2 (cor = 0.54, P<0.001), YEATS2 (cor = 0.52, P<0.001) in OSCC (via analysis in the GEIPA database).
Figure 10. The expression of HMGA2, PHLDB2 and YEATS2 in OSCC.
(A–C) HMGA2, PHLDB2, and YEATS2 mRNA levels in OSCC tissues and normal tissues in the GSE31056 dataset. (D–F) HMGA2, PHLDB2, and YEATS2 mRNA levels in OSCC tissues and normal tissues in TCGA.
Functional analyses of IGF2BP2-related genes
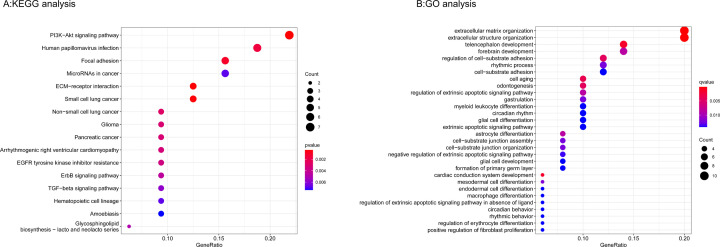
KEGG pathway enrichment of IGF2BP2-related genes showed that ECM–receptor interaction, PI3K–Akt signaling pathway, focal adhesion, microRNAs in cancer, and Human papillomavirus infection were the most enriched pathways (Supplementary Table S5 and Figure 11A). In addition, GO analysis results proved that IGF2BP2-related genes were significantly enriched in regulation of the extrinsic apoptotic signaling pathway, regulation of cell–substrate adhesion, odontogenesis at BP levels; collagen containing extracellular matrix, basement membrane, and basal part of cell at CC levels; and extracellular matrix structural constituent at MF levels (Supplementary Table S6 and Figure 11B).
Figure 11. KEGG and GO biological function enrichment analyses of IGF2BP2-related genes.
(A) KEGG signal pathway enrichment analysis. (B) GO biological function enrichment analyses (when P-value <0.05 and q-value <0.05, the results were statistically significant).
IGF2BP2 and its co-expressed genes significantly correlate with TICs in OSCC
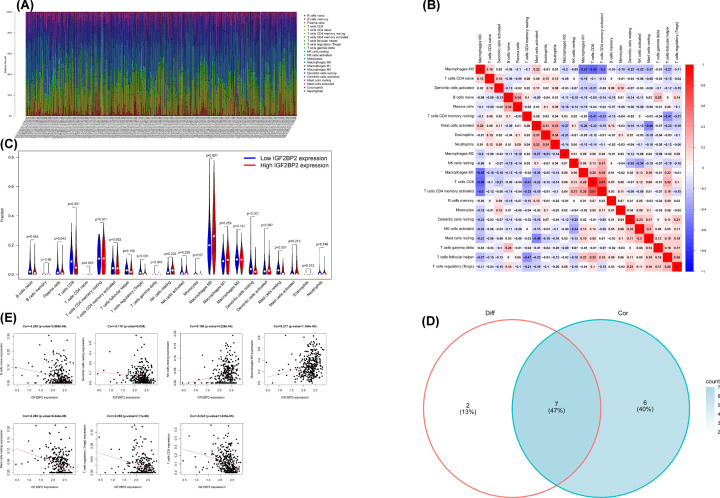
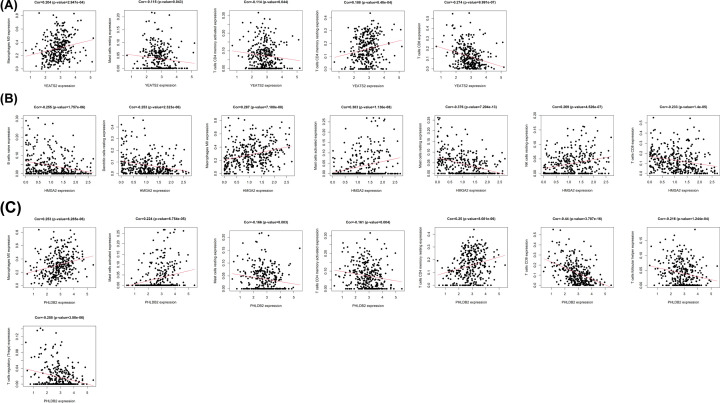
The CIBERSORT method was applied to confirm the association between IGF2BP2 expression and the immune component through constructing 21 types of immune cell profiles in OSCC cases and analyzing the proportion of tumor-infiltrating immune subtypes (Figure 12A–D). A total of seven kinds of TICs were found to have an association with IGF2BP2 expression (P<0.001, Figure 12E). The results revealed that two TICs had a positive relationship with IGF2BP2 expression, including resting NK cells and macrophages M0, while five kinds of TICs had a negative correlation with IGF2BP2 expression, including naïve B cells, resting DCs, resting mast cells, CD8+ T cells, and regulatory T cells. Furthermore, we determined whether the IGF2BP2 co-expressed genes (HMGA2, PHLDB2, and YEATS2) had an association with TICs (Figure 13). The above results suggest that IGF2BP2 and its co-expressed genes may be involved in the immune response in the TME by affecting immune cells.
Figure 12. TICs profile in OSCC samples and correlation analysis, and correlation of TICs proportion with IGF2BP2 expression.
(A) Barplot shows the proportion of 21 types of TICs in OSCC tumor samples. The row name on the right side of the figure is the name of 21 TICs, and the column name at the bottom of the figure is sample ID. (B) Heatmap shows the correlation between 21 kinds of TICs and numeric in each tiny box, indicating the correlation coefficient of the correlation between two cells. The shadow of each tiny color box represented a corresponding correlation value between two cells, and the Pearson’s coefficient was used for the significance test. Red represents the positive correlation between the two cells, and blue represents the negative correlation between the two cells. The darker the color, the more significant the correlation. (C) Violin plot showed the ratio differentiation of 21 types of immune cells between OSCC tumor samples with low or high IGF2BP2 expression relative to the median of IGF2BP2 expression level, and Wilcoxon rank sum was applied for the significance test. (D) Venn plot displayed seven kinds of TICs correlated with IGF2BP2 expression co-determined by difference and correlation tests displayed in the violin and scatter plots, respectively. (E) The Scatter plot showed the correlation of seven kinds of TICs proportion with the IGF2BP2 expression (P<0.05). The red line in each plot was a fitted linear model indicating the proportion tropism of the immune cell along with IGF2BP2 expression, and the Pearson coefficient was used for the correlation test.
Figure 13. IGF2BP2 co-expression genes were significantly correlated with the level of immunofiltration in OSCC.
Correlation of YEATS2 (A), HMGA2 (B), and PHLDB2 (C) expression with TICs in OSCC.
Discussion
Cancer has become one of the most important cause of death among middle-aged and elderly people as a result of the accelerated pace of global aging. As one of the most common malignant tumors of the head and neck, OSCC has seriously threatened human health and welfare due to its high recurrence and metastasis rate. Previous studies reported that factors such as TME, aberrant gene expression, and immune infiltration may be involved in the occurrence of tumors [32–34]. However, the molecular mechanism of OSCC pathogenesis has not yet been elucidated.
IGF2BP2 is a member of the IGF2 mRNA-binding protein family. Many studies have shown that IGF2BP2 is abnormally expressed in pancreatic cancer, liver cancer, thyroid cancer, and other malignant tumors [35]. Overexpression of IGF2BP2 can promote tumor cell proliferation, stimulate migration and invasion, inhibit cell apoptosis, and accelerate tumor progression [36]. The study of Wang et al. showed that IGF2BP2 up-regulated the expression of circ 0000745 through microRNA-3187-3p/ErbB4/PI3K/Akt axis and promoted the aggressiveness and stemness of ovarian cancer cells [37]. Deng et al. confirmed that IGF2PB2 can specifically bind to TP53I11, PKP2, BMP6, CFH and COL1A1, through ECM–receptor interaction, cytokine–cytokine receptor interaction, and TGF-β signaling pathway in Alz play an important regulatory role in Haimer’s disease [38]. Other research indicates that IGF2BP2 plays a regulatory role in the pathological mechanisms of Lung ischemia–reperfusion injury (LIRI), Hemoglobin H-Constant Spring disease (HbH-CS), Autoimmune inflammation, and other diseases [39–41]. So far, there are few studies on IGF2BP2 in OSCC. The study of Chou et al. found that patients with oral cancer and the IGF2BP2 rs11705701 GA+AA, rs4402960 GT+TT, and rs1470579 AC+CC genotypes had higher risk in terms of clinical stage, tumor size, and lymph node metastasis compared with those with the IGF2BP2 rs11705701 GG, rs4402960 GG, and rs1470579 AA genotypes. Studies have confirmed that HOXB-AS3 encodes a protein that directly interacts with IGF2BP2 and promotes the proliferation and viability of OSCC cell lines by stabilizing c-Myc [42]. This study confirmed that the expression of IGF2BP2 was significantly increased in OSCC tumor tissues after combining TCGA and GEO datasets, Oncomine, and clinical samples. The results showed that there is a statistical correlation between IGF2BP2 and the T stage, the clinical stage of OSCC. In addition, aberrant expression of IGF2BP2 is significantly associated with poor prognosis and overall survival rate in OSCC. Collectively, these results suggest that IGF2BP2 may act as an oncogene to promote the occurrence of OSCC, and hopefully become a potential predictor of the prognosis of OSCC patients.
To explore the molecular function and potential mechanism of IGF2BP2 in OSCC, samples were divided into IGF2BP2-high and IGF2BP2-low expression groups according to the expression of IGF2BP2, and further analysis was carried out using the GSEA software (version 4.1.0). For the C2 set defined by MSigDB, results showed that the immune and inflammation-related signaling pathways enriched in the IGF2BP2-high expression group include adipocytokine signaling pathway, insulin signaling pathway, and endocytosis, while tumorigenesis-related signaling pathways include Notch signaling pathway, P53 signaling pathway, WNT signaling pathway, ERBB signaling pathway, and phosphatidylinositol signaling pathway. Notably, adipocytokines is a type of soluble factor produced by adipose tissue, including adiponectin, leptin, resistin, and other components [43]. A previous study reported that the increase in leptin levels can significantly increase the expression of PD-1 and increase the exhaustion of CD8+ T cells in the TME, thereby affecting antitumor immunotherapy [44]. Endocytosis is an energy-dependent process that internalizes cell surface receptors through pinocytosis, phagocytosis, or receptor-mediated endocytosis, and is a very potential mechanism in regulating tumor metastasis. Many endocytic proteins are dysregulated in cancer and regulate tumor metastasis, especially migration and invasion [45]. Insulin is a cancer-related regulatory peptide. Studies have confirmed that IGF1R, one of the receptors associated with the insulin signaling pathway, can significantly promote proliferation of OSCC cells, and affect the occurrence and development of OSCC [46]. The Pi3k-akt signaling pathway, one of the phosphatidylinositol signaling systems, has been deeply studied in a variety of cancers. For example, results have shown that IGF2BP2 can promote the progression of pancreatic cancer by activating this pathway [47]. On the other hand, various immune activities and metabolic functions, including apoptosis, glycolysis, pi3k-akt-mtor signaling, and mtorc1 signaling were enriched in the HALLMARK gene sets of the IGF2BP2-high expression group. It is worth noting that tumor cells favor glycolysis as the main source of energy metabolism due to the Warburg effect. One study reported that overexpression of IGF2BP2 can promote glycolysis and stimulate tumor cell proliferation, thereby affecting the occurrence and development of tumors [48]. mTORC1 is one of the two complexes of mTOR (mammalian target of rapamycin), and is also a regulator of immune cell metabolism. Research has confirmed that IGF2BP2 can regulate the cap-independent translation of IGF2 mRNA through dual phosphorylation with mTOR [49]. Collectively, these results indicate that IGF2BP2 is involved in the tumor and immune-related KEGG pathway. The findings of the present study suggest that high expression of IGF2BP2 can be used to predict poor prognosis and survival rate of OSCC patients. The reason may be that IGF2BP2 modulates OSCC by affecting these signaling pathways, thereby resulting in poor prognosis for OSCC patients.
The results of the GEIPA and TIMER co-expression analyses indicated that IGF2BP2 was correlated with HMGA2, PHLDB2, and YEATS2, which are involved in the inflammation/immune response or tumorigenesis [50–52]. In this study, results indicated that the expression of IGF2BP2 co-expressed genes (HMGA2, PHLDB2, and YEATS2) was significantly increased in OSCC samples, and was correlated with a variety of TICs. Furthermore, CIBERSORT analysis based on the differential expression of IGF2BP2 was used to evaluate the distribution ratio of TICs in OSCC. The TICs were then screened together through correlation and differential analyses. Results indicated that seven TICs were associated with IGF2BP2 expression, seven TICs were associated with HGMA2 expression, eight TICs were associated with PHLDB2 expression, and five TICs were associated with YEATS2 expression. These results suggest that IGF2BP2 and its co-expressed genes may be involved in the immune response during the occurrence of OSCC, thereby leading to a poor prognosis in OSCC patients.
At present, most bioinformatics studies only focus on a gene in a single database, and there are relatively few model analysis of multidatabase joint gene prediction. In addition, due to the limited sample size of a single dataset, the results of differential gene analysis may be biased, resulting in no biological effects. Compared with other single dataset analysis, the present study combined oncomine database, multiple datasets of GEO (GSE31056, GSE42743, GSE51010) and TCGA database for gene prediction model analysis to identify possible biomarkers in OSCC, which laid a more reliable and accurate foundation for our research. At the same time, we combined these data with patient data in our hospital to verify the existence of biomarkers.
Nevertheless, our research still has some limitations: (1) our research is verified on the basis of database data analysis combined with our own clinical samples, but the relatively small clinical sample size is still the limitation of the present study. (2) Bioinformatics analysis is only a prediction tool based on public database. Its operation process is cumbersome, involving the setting and adjustment of a variety of software analysis parameters. A certain technical threshold is required to ensure the accuracy of prediction results. (3) The impact of specific characteristics such as race, smoking history, drinking history and HPV history on prognosis was not analyzed in detail. Therefore, these will also be the focus of our next research.
Conclusion
In summary, the present study has confirmed the high expression of IGF2BP2 in OSCC, the survival and prognosis of patients in the IGF2BP2-high expression group is poor, and that the high expression of IGF2BP2 is associated with some clinicopathological parameters such as T stage and clinical stage. In addition, the study found that IGF2BP2 and its co-expressed genes (HMGA2, PHLDB2, and YEATS2) are all associated with a variety of TICs in OSCC tumor samples. Therefore, the present study has revealed the potential role of IGF2BP2 in tumor immunology and its prognostic value. It is evident that IGF2BP2 has the potential to be a prognostic biomarker and therapeutic target for OSCC. However, further studies should be conducted to elucidate the specific mechanism of the interaction of IGF2BP2 and its co-expressed genes with TICs.
Supplementary Material
Abbreviations
- BP
Biological process
- CC
Cellular component
- COAD
colorectal adenocarcinoma
- DC
dendritic cell
- DEG
differentially expressed gene
- FDR
false discovery rate
- GEIPA
Gene expression profiling interactive analysis
- GEO
Gene Expression Omnibus
- GO
gene ontology
- GSEA
gene set enrichment analysis
- HNSC
head and neck squamous cell carcinoma
- HPV
human papilloma virus
- IGF2BP2
insulin-like growth factor 2 mRNA-binding protein 2
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- KS
Kolmogorov-Smirnov test
- MF
Molecular function
- MSigDB
Molecular Signatures Database
- mTOR
mammalian target of rapamycin
- NCBI
National Center for Biotechnology Information
- NOM
nominal
- OSCC
oral squamous cell carcinoma
- TCGA
The Cancer Genome Atlas
- TIC
tumor-infiltrating immune cell
- TIMER
Tumor Immune estimation resource
- TME
tumor microenvironment
- Treg
Regulatory T cells
Data Availability
The datasets generated and analyzed in the present study are available in the TCGA database (https://portal.gdc.cancer.gov) and the NCBI’s GEO (https://www.ncbi.nlm.nih.gov/geo/).
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special [grant number XMLX201714]; the Beijing Natural Science [grant number 7212046]; the Discipline Construction Fund of Beijing Stomatological Hospital Affiliated to Capital Medical University [grant number 19-09-24]; and the National Natural Science Foundation of China [grant number 81771909].
CRediT Author Contribution
Xiangpu Wang: Writing—original draft, Writing—review & editing. Haoyue Xu: Software. Zuo Zhou: Visualization, Methodology. Siyuan Guo: Data curation, Formal analysis. Renji Chen: Data curation, Writing—review & editing.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J. and Jemal A. (2015) Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Bloebaum M., Poort L., Bockmann R. and Kessler P. (2014) Survival after curative surgical treatment for primary oral squamous cell carcinoma. J. Craniomaxillofac. Surg. 42, 1572–1576 10.1016/j.jcms.2014.01.046 [DOI] [PubMed] [Google Scholar]
- 3.Rowe D.E., Carroll R.J. and Day C.J. (1992) Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J. Am. Acad. Dermatol. 26, 976–990 10.1016/0190-9622(92)70144-5 [DOI] [PubMed] [Google Scholar]
- 4.Chiou S.H., Yu C.C., Huang C.Y., Lin S.C., Liu C.J., Tsai T.H.et al. (2008) Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 14, 4085–4095 10.1158/1078-0432.CCR-07-4404 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z., Bao S., Yan C., Hou P., Zhou M. and Sun J. (2021) Computational principles and practice for decoding immune contexture in the tumor microenvironment. Brief. Bioinform. 22, bbaa075 10.1093/bib/bbaa075 [DOI] [PubMed] [Google Scholar]
- 6.Schneider K., Marbaix E., Bouzin C., Hamoir M., Mahy P., Bol V.et al. (2018) Immune cell infiltration in head and neck squamous cell carcinoma and patient outcome: a retrospective study. Acta Oncol. 57, 1165–1172 10.1080/0284186X.2018.1445287 [DOI] [PubMed] [Google Scholar]
- 7.Zhang X., Shi M., Chen T. and Zhang B. (2020) Characterization of the immune cell infiltration landscape in head and neck squamous cell carcinoma to aid immunotherapy. Mol. Ther. Nucleic Acids 22, 298–309 10.1016/j.omtn.2020.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen K., Zeng Z., Ma C., Dang Y. and Zhang H. (2021) Commentary on: Screening of immunosuppressive cells from colorectal adenocarcinoma and identification of prognostic markers. Biosci. Rep. 41, 34850851 10.1042/BSR20211096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen J., Christiansen J., Lykke-Andersen J., Johnsen A.H., Wewer U.M. and Nielsen F.C. (1999) A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol. Cell. Biol. 19, 1262–1270 10.1128/MCB.19.2.1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christiansen J., Kolte A.M., Hansen T. and Nielsen F.C. (2009) IGF2 mRNA-binding protein 2: biological function and putative role in type 2 diabetes. J. Mol. Endocrinol. 43, 187–195 10.1677/JME-09-0016 [DOI] [PubMed] [Google Scholar]
- 11.Qian B., Wang P., Zhang D. and Wu L. (2021) m6A modification promotes miR-133a repression during cardiac development and hypertrophy via IGF2BP2. Cell Death Discov. 7, 157 10.1038/s41420-021-00552-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H., Hu X., Huang M., Liu J., Gu Y., Ma L.et al. (2019) Mettl3-mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat. Commun. 10, 1898 10.1038/s41467-019-09903-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z., Wang T., She Y., Wu K., Gu S., Li L.et al. (2021) N(6)-methyladenosine-modified circIGF2BP3 inhibits CD8(+) T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol. Cancer 20, 105 10.1186/s12943-021-01398-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Q. (2021) Bioinformatical identification of key genes regulated by IGF2BP2-mediated RNA N6-methyladenosine and prediction of prognosis in hepatocellular carcinoma. J. Gastrointest. Oncol. 12, 1773–1785 10.21037/jgo-21-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell J.L., Wachter K., Muhleck B., Pazaitis N., Kohn M., Lederer M.et al. (2013) Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell. Mol. Life Sci. 70, 2657–2675 10.1007/s00018-012-1186-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao J., Mu Q. and Huang H. (2018) The roles of insulin-like growth factor 2 mRNA-binding protein 2 in cancer and cancer stem cells. Stem Cells Int. 2018, 4217259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai N., Ji F., Wright J., Minichiello L., Sadreyev R. and Avruch J. (2017) IGF2 mRNA binding protein-2 is a tumor promoter that drives cancer proliferation through its client mRNAs IGF2 and HMGA1. eLife 6, e27155 10.7554/eLife.27155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H., Xu Y., Xie Y., Zhang L., Gao M., Li S.et al. (2021) m6A regulators is differently expressed and correlated with immune response of esophageal cancer. Front. Cell Dev. Biol. 9, 650023 10.3389/fcell.2021.650023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou C.H., Chang C.Y., Lu H.J., Hsin M.C., Chen M.K., Huang H.C.et al. (2020) IGF2BP2 polymorphisms are associated with clinical characteristics and development of oral cancer. Int. J. Mol. Sci. 21, 5662 10.3390/ijms21165662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reis P.P., Waldron L., Perez-Ordonez B., Pintilie M., Galloni N.N., Xuan Y.et al. (2011) A gene signature in histologically normal surgical margins is predictive of oral carcinoma recurrence. BMC Cancer 11, 437 10.1186/1471-2407-11-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohavanichbutr P., Mendez E., Holsinger F.C., Rue T.C., Zhang Y., Houck J.et al. (2013) A 13-gene signature prognostic of HPV-negative OSCC: discovery and external validation. Clin. Cancer Res. 19, 1197–1203 10.1158/1078-0432.CCR-12-2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saeed A.A., Sims A.H., Prime S.S., Paterson I., Murray P.G. and Lopes V.R. (2015) Gene expression profiling reveals biological pathways responsible for phenotypic heterogeneity between UK and Sri Lankan oral squamous cell carcinomas. Oral Oncol. 51, 237–246 10.1016/j.oraloncology.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 23.Li R., Qu H., Wang S., Wei J., Zhang L., Ma R.et al. (2018) GDCRNATools: an R/Bioconductor package for integrative analysis of lncRNA, miRNA, and mRNA data in GDC. Bioinform. 34, 2515–2517 10.1093/bioinformatics/bty124 [DOI] [PubMed] [Google Scholar]
- 24.Gautier L., Cope L., Bolstad B.M. and Irizarry R.A. (2004) affy–analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20, 307–315 10.1093/bioinformatics/btg405 [DOI] [PubMed] [Google Scholar]
- 25.Lu L., Townsend K.A. and Daigle B.J. (2021) GEOlimma: differential expression analysis and feature selection using pre-existing microarray data. BMC Bioinformatics 22, 44 10.1186/s12859-020-03932-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lun A.T. and Smyth G.K. (2016) csaw: a Bioconductor package for differential binding analysis of ChIP-seq data using sliding windows. Nucleic Acids Res. 44, e45 10.1093/nar/gkv1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhodes D.R., Yu J., Shanker K., Deshpande N., Varambally R., Ghosh D.et al. (2004) ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6, 1–6 10.1016/S1476-5586(04)80047-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A.et al. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Z., Li C., Kang B., Gao G., Li C. and Zhang Z. (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45, W98–W102 10.1093/nar/gkx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li T., Fu J., Zeng Z., Cohen D., Li J., Chen Q.et al. (2020) TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 48, W509–W514 10.1093/nar/gkaa407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O.et al. (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, l1 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z., Huang Q., Xu W., Wang H., Yang J. and Zhang L.J. (2020) PRKD3 promotes malignant progression of OSCC by downregulating KLF16 expression. Eur. Rev. Med. Pharmacol. Sci. 24, 12709–12716 [DOI] [PubMed] [Google Scholar]
- 33.Li X., Bu W., Meng L., Liu X., Wang S., Jiang L.et al. (2019) CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp. Cell Res. 378, 131–138 10.1016/j.yexcr.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 34.Sasahira T. and Kirita T. (2018) Hallmarks of cancer-related newly prognostic factors of oral squamous cell carcinoma. Int. J. Mol. Sci. 19, 2413 10.3390/ijms19082413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye M., Dong S., Hou H., Zhang T. and Shen M. (2021) Oncogenic role of long noncoding RNAMALAT1 in thyroid cancer progression through regulation of the miR-204/IGF2BP2/m6A-MYC signaling. Mol. Ther. Nucleic Acids 23, 1–12 10.1016/j.omtn.2020.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pu J., Wang J., Qin Z., Wang A., Zhang Y., Wu X.et al. (2020) IGF2BP2 promotes liver cancer growth through an m6A-FEN1-dependent mechanism. Front. Oncol. 10, 578816 10.3389/fonc.2020.578816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S., Li Z., Zhu G., Hong L., Hu C., Wang K.et al. (2021) RNA-binding protein IGF2BP2 enhances circ_0000745 abundancy and promotes aggressiveness and stemness of ovarian cancer cells via the microRNA-3187-3p/ERBB4/PI3K/AKT axis. J. Ovarian Res. 14, 154 10.1186/s13048-021-00917-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng Y., Zhu H., Xiao L., Liu C., Liu Y.L. and Gao W. (2021) Identification of the function and mechanism of m6A reader IGF2BP2 in Alzheimer’s disease. Aging (Albany N.Y.) 13, 24086–24100 10.18632/aging.203652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao K., Liu P., Yan P., Liu Y., Song L., Liu Y.et al. (2021) N6-methyladenosine reader YTH N6-methyladenosine RNA binding protein 3 or insulin like growth factor 2 mRNA binding protein 2 knockdown protects human bronchial epithelial cells from hypoxia/reoxygenation injury by inactivating p38 MAPK, AKT, ERK1/2, and NF-kappaB pathways. Bioengineered, 10.1080/21655979.2021.1999550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruan H., Yang F., Deng L., Yang D., Zhang X., Li X.et al. (2021) Human m(6)A-mRNA and lncRNA epitranscriptomic microarray reveal function of RNA methylation in hemoglobin H-constant spring disease. Sci. Rep. 11, 20478 10.1038/s41598-021-99867-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bechara R., Amatya N., Bailey R.D., Li Y., Aggor F., Li D.D.et al. (2021) The m(6)A reader IMP2 directs autoimmune inflammation through an IL-17- and TNFalpha-dependent C/EBP transcription factor axis. Sci. Immunol. 6, eabd1287 10.1126/sciimmunol.abd1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leng F., Miu Y.Y., Zhang Y., Luo H., Lu X.L., Cheng H.et al. (2021) A micro-peptide encoded by HOXB-AS3 promotes the proliferation and viability of oral squamous cell carcinoma cell lines by directly binding with IGF2BP2 to stabilize c-Myc. Oncol. Lett. 22, 697 10.3892/ol.2021.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tilg H. and Moschen A.R. (2006) Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 6, 772–783 10.1038/nri1937 [DOI] [PubMed] [Google Scholar]
- 44.Wang Z., Aguilar E.G., Luna J.I., Dunai C., Khuat L.T., Le C.T.et al. (2019) Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 25, 141–151 10.1038/s41591-018-0221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan I. and Steeg P.S. (2021) Endocytosis: a pivotal pathway for regulating metastasis. Br. J. Cancer 124, 66–75 10.1038/s41416-020-01179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du Y., Li Y., Lv H., Zhou S., Sun Z. and Wang M. (2015) miR-98 suppresses tumor cell growth and metastasis by targeting IGF1R in oral squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 8, 12252–12259 [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X., Yu Y., Zong K., Lv P. and Gu Y. (2019) Up-regulation of IGF2BP2 by multiple mechanisms in pancreatic cancer promotes cancer proliferation by activating the PI3K/Akt signaling pathway. J. Exp. Clin. Cancer Res. 38, 497 10.1186/s13046-019-1470-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y., Lu J.H., Wu Q.N., Jin Y., Wang D.S., Chen Y.X.et al. (2019) LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol. Cancer 18, 174 10.1186/s12943-019-1105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai N., Rapley J., Angel M., Yanik M.F., Blower M.D. and Avruch J. (2011) mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ribosomal entry. Genes Dev. 25, 1159–1172 10.1101/gad.2042311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fatalska A., Rusetska N., Bakula-Zalewska E., Kowalik A., Zieba S., Wroblewska A.et al. (2020) Inflammatory proteins HMGA2 and PRTN3 as drivers of vulvar squamous cell carcinoma progression. Cancers (Basel) 13, 27 10.3390/cancers13010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen G., Zhou T., Ma T., Cao T. and Yu Z. (2019) Oncogenic effect of PHLDB2 is associated with epithelial-mesenchymal transition and E-cadherin regulation in colorectal cancer. Cancer Cell Int. 19, 184 10.1186/s12935-019-0903-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mi W., Guan H., Lyu J., Zhao D., Xi Y., Jiang S.et al. (2017) YEATS2 links histone acetylation to tumorigenesis of non-small cell lung cancer. Nat. Commun. 8, 1088 10.1038/s41467-017-01173-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed in the present study are available in the TCGA database (https://portal.gdc.cancer.gov) and the NCBI’s GEO (https://www.ncbi.nlm.nih.gov/geo/).