Abstract
Mucormycosis is almost always confined to the patients with altered host defenses amongst which diabetes is considered as the strongest risk factor. COVID-19 only been seen in severe cases but also in mild and moderate cases of SARS-CoV-2 infections. After preliminary clinical and radiological diagnosis, surgical management in the form of endoscopic sinus surgery, debridement, and orbital exenteration (8) was performed. Medical management in the form of antifungal therapy (amphotericin-B, posaconazole, and isavuconazole) was initiated. In this case series, 79 proven cases of COVID-19 associated rhino-orbital-cerebral mucormycosis were analyzed retrospectively from mid-April 2021 to mid-September 2021. 67 patients were known diabetics, whereas rest 12 had new onset diabetes mellitus. Of these 79 cases, 27 cases had the disease limited to sinuses (rhino-mucormycosis), 43 had orbital involvement also (rhino–orbital mucormycosis), and 9 had cerebral involvement as well (rhino–orbital–cerebral mucormycosis). During this time-period, a total of 14 mortalities occurred. Most of the patients were discharged after completion of amphotericin-B therapy and rest stayed little longer till their general condition improved. COVID-19 causes dysregulation and alteration of immune response in the body which predispose to invasive fungal infections. In addition, uncontrolled diabetes mellitus and corticosteroid treatment increase the risk of mucormycosis by many folds.
Keywords: COVID-19, rhino-orbital-cerebral mucormycosis, diabetes, debridement, orbital exenteration
Introduction
India is recognized as the diabetes and mucormycosis “capital” of the world.1 Diabetes is the fastest growing non-communicable disease in India. The second wave of Coronavirus disease 2019 (COVID-19) in India has presented in a catastrophic way as compared to the first wave and startled the medical fraternity. Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) was found to be associated with systemic immune alterations causing a wide range of manifestations including various bacterial and fungal infections.2 During the second wave, India faced the major morbidities and mortalities in the form of post-COVID-19 sepsis and the abrupt spike of COVID-19 associated mucormycosis (CAM). In the previous 3 years (January 2018 to March 2021), we encountered only 5 cases of rhinomaxillary or rhino–orbital–cerebral mucormycosis, accounting institutional incidence of less than 2 cases per year. COVID-19 infection has led to worst consequences in the patients with uncontrolled diabetes, immunocompromised, immunosuppressed (post-organ transplant), and those who were managed with long periods or high doses of systemic corticosteroids.3
Invasive fungal infection or mucormycosis is almost always confined to the patients with altered host defenses such as in transplant recipients, diabetics, patients with malignancies, and patients on corticosteroid therapy (autoimmune disorders). Hyperglycemia or uncontrolled diabetes, particularly diabetic acidosis, is considered as the strongest and very well known risk factor for mucormycosis. It has spread rapidly amongst the active COVID-19 and post-COVID-19 diabetic patients. Many studies across the world have established the definitive severity of SARS-CoV-2 infection amongst diabetic patients.4
Mucormycosis is classified based on the site of involvement as rhino–orbital–cerebral, pulmonary, cutaneous, gastrointestinal, and disseminated form.5 In the literature, only a limited number of post-COVID-19 mucormycosis case series have been published, the comparative description of which has been tabulated in discussion.6-10 This paper discusses the institutional experience of 79 cases of rhino–orbital–cerebral mucormycosis related to COVID-19 infection, which to our best knowledge is one of the biggest institutional case series.
Methods
In this case series, we are retrospectively analyzing the 79 proven cases of COVID-19 related rhino–orbital–cerebral mucormycosis. Throughout the second wave of COVID-19 infection in India, we operated 96 suspected cases of mucormycosis between mid-April 2021 and mid-September 2021 which also included 7 patients operated elsewhere and referred for further management. All patients received systemic steroids in the form of oral methylprednisolone, intravenous dexamethasone, or intravenous methylprednisolone as a part of management of COVID-19 infection.
In all suspected patients, a diagnostic nasal endoscopy (DNE) with a preliminary nasal swab was sent for KOH (potassium hydroxide) mount and a magnetic resonance imaging (MRI) of paranasal sinuses, orbit, and brain was performed to look for extent of the disease if present. In COVID-19 positive patients, swab (with or without biopsy) was taken bedside under full PPE cover. Based on preliminary KOH mount report and MRI findings, surgery was planned according to the extent of disease. Systemic antifungals were started empirically after the opinion of our infectious disease specialist. Sinusitis was seen in the form of varied level of mucosal thickening of sinuses in all patients. Predominantly unilateral ethmoidal and maxillary sinusitis was seen.
Surgical treatment in the form of endoscopic sinus surgery and debridement, total or partial maxillectomy, orbital clearance, or exenteration was performed. Orbital exenteration was performed by combination of external eyelid sparing and endoscopic power-assisted technique. All the debrided tissue was sent for histopathology and some tissue with pus (if present) was sent for bacterial and fungal culture (Figure 1). Thorough local application of amphotericin B .1% ointment was done after debridement and nasal cavities were packed. On the basis of clinical and radiological suspicion of invasive fungal infection, amphotericin B was started in all 96 patients with doses monitored and modified after definitive diagnosis by the infectious disease specialist.
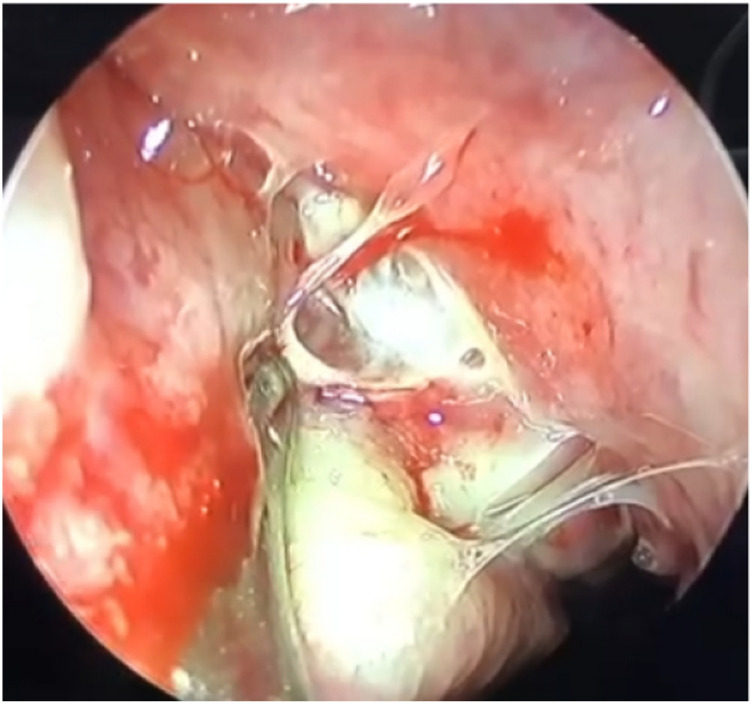
Figure 1.
Endoscopic image showing pus draining from maxillary sinus (positive for fungal culture).
In all patients of mucormycosis, repeat imaging was performed between post-operative days 3 and 7 to see the course of disease and to take measures for further debridement if needed (Figures 2 and 3).
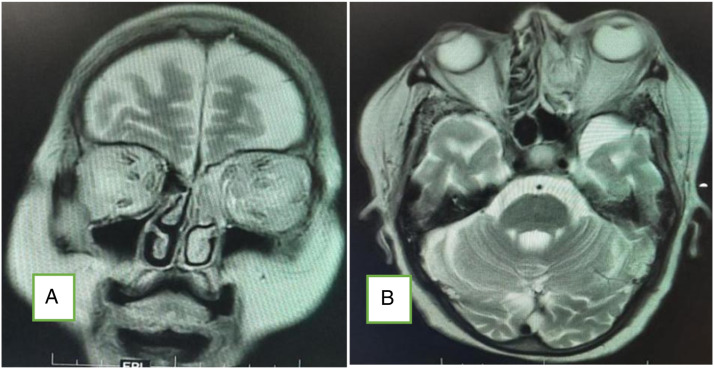
Figure 2.
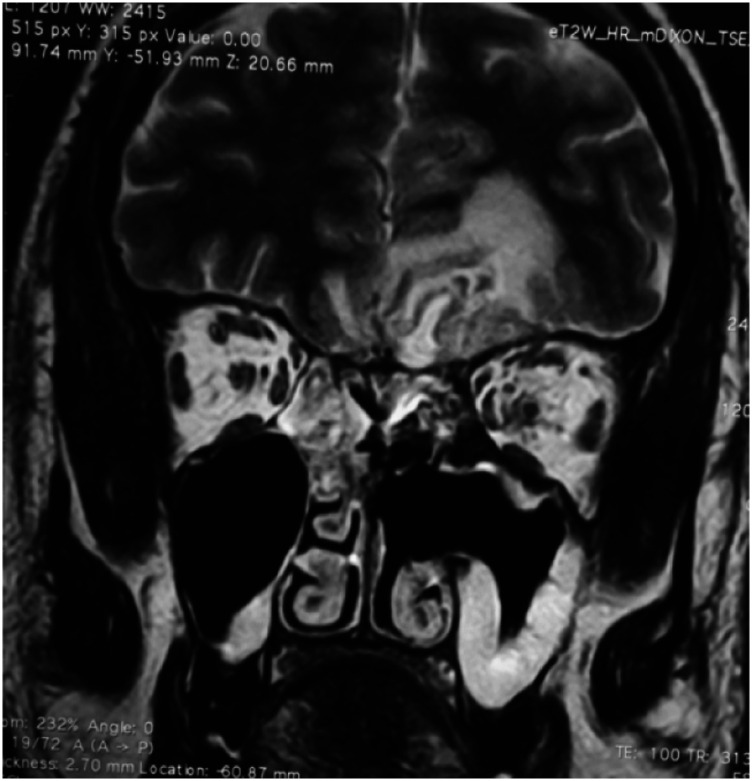
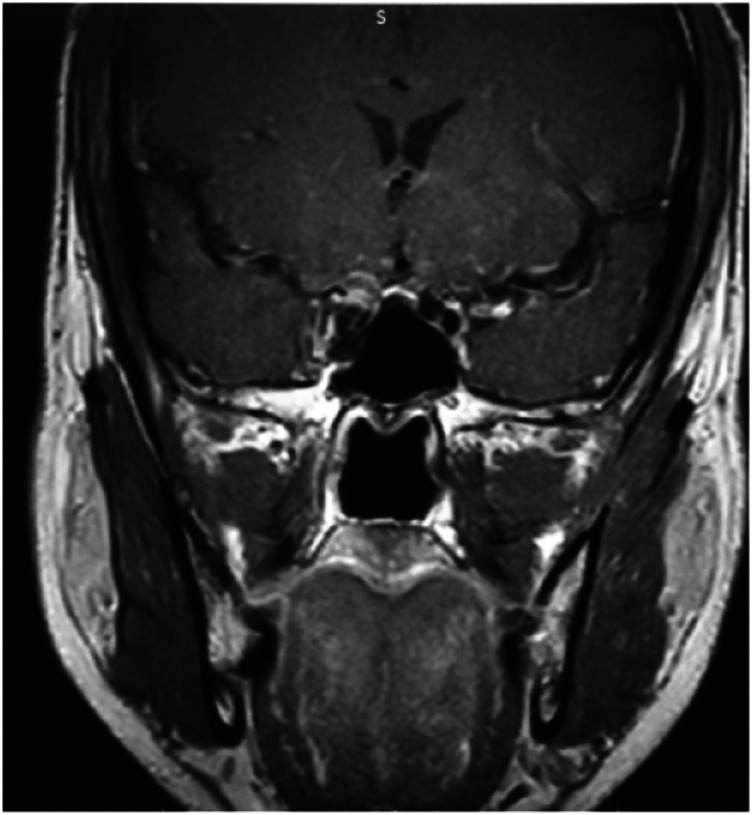
CEMRI (A: coronal; B: axial) of the brain and orbits showing features of fungal sinusitis predominantly involving left side ethmoid and frontal sinuses with extension of the disease in intraorbital region through the medial orbital wall with resultant stranding of the retro-orbital and extraconal fat and thickening and hyperintensity of the extraocular muscles. There is proptosis of the left eye. The fat stranding is extending up to the orbital apex with mild asymmetry of the left cavernous sinus.
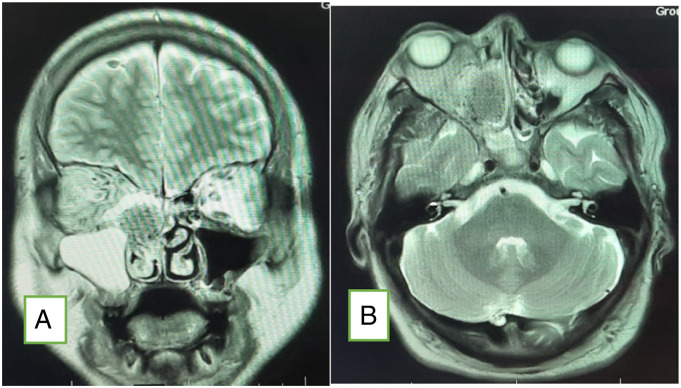
Figure 3.
Plain MRI brain and orbit (A: coronal; B: axial) showing features of fungal sinusitis predominantly involving the right side sinuses. There is proptosis of the right eye with fat stranding up to the orbital apex.
Amongst systemic antifungals, liposomal amphotericin-B (5 mg/kg/day) was started in all operated 96 patients with close monitoring of renal parameters. In all histopathology proven mucormycosis, oral posaconazole was started, as 300 mg twice a day as a loading dose on first day followed by 300 mg once daily. Due to a huge bulk of mucormycosis patients, we had a shortage of liposomal form of amphotericin-B, as a result of which patients were switched to conventional amphotericin-B therapy. Many patients developed acute or acute on chronic renal injury which made us switch to posaconazole or isavuconazole monotherapy. High blood sugar levels were managed by endocrinologist with insulin as per sliding scale. Patients were discharged when they had no evidence of disease with clinical and general physical improvement. All discharged patients were kept in minimum follow-up period of 6 months, many of which have been completed this period.
Results
Out of all 96 operated cases, 79 were histopathologically diagnosed as mucormycosis. These patients presented to us with varied disease load. There were 62 males and 17 females. The youngest patient was 21 years old and eldest one was 72 years old. Fifteen (15) patients were operated during their active COVID-19 infection, whereas rest 64 patients operated during post-COVID-19 status after an initial recovery.
All patients had one or other symptoms or signs of sinusitis including facial pain or numbness, facial swelling, headache, and nasal congestion. Of these 79 patients of mucormycosis, 27 patients had disease restricted to nose and paranasal sinuses (PNS) (rhino-mucormycosis) (Figure 4), 43 showed orbital involvement as well (rhino–orbital mucormycosis), and 9 patients had severe disease involving the brain also (rhino–orbital–cerebral mucormycosis). Hence, a total of 52 patients (43+9) had orbital involvement. All 52 patients who had orbital involvement had lid edema with proptosis, orbital pain with periocular swelling, conjunctival edema, and double vision in varied severity.
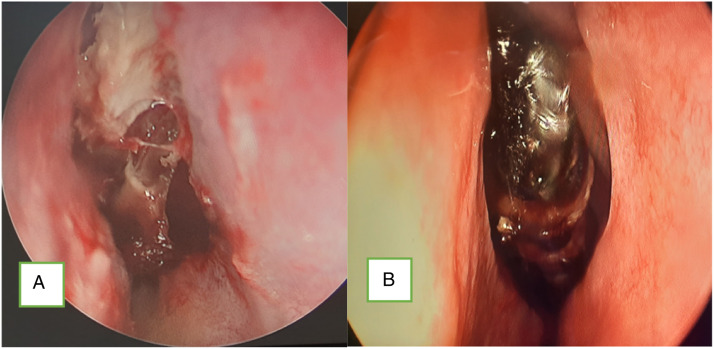
Figure 4.
Endoscopic images of (A) necrotic middle turbinate (mucormycosis) and (B) black turbinate due to rhino-mucormycosis.
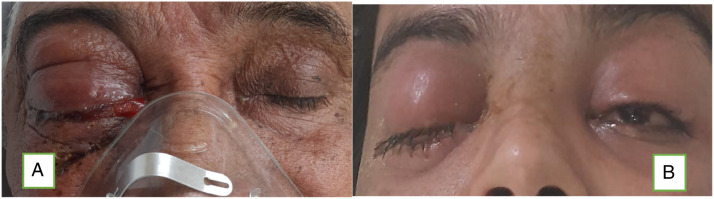
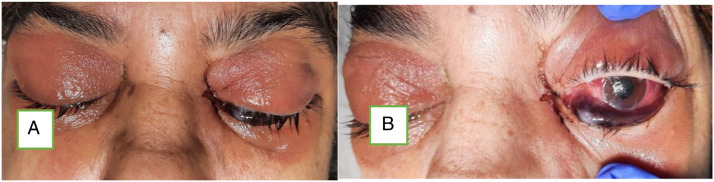
Among 43 patients of rhino–orbital mucormycosis, 11 patients had positive perception of light (PL) only, 6 of 43 patients had no perception of light with dilated non-reactive pupils (total ophthalmoplegia), whereas rest had just diminished vision of variable grade (Figure 5). One patient among 6 patients of total ophthalmoplegia had bilateral involvement and 2 developed pulmonary mucormycosis who died during ICU care (Figure 6).
Figure 5.
Images showing right orbital involvement due to mucormycosis (total ophthalmoplegia).
Figure 6.
Images showing bilateral orbital involvement due to mucormycosis (A) and non-reactive dilated pupil with total ophthalmoplegia (B).
In 9 patients with cerebral involvement, all patients had altered sensorium, confusion, or disorientation of variable grade. Three (3) out of 9 had unilateral involvement, whereas 1 had bilateral involvement with dilated and non-reactive pupil and ophthalmoplegia. Rest had diminished or blurred vision of variable grade.
All patients were hyperglycemic during the time of clinical diagnosis or suspicion of mucormycosis. Sixty seven (67) patients had known history of diabetes, whereas 12 patients developed steroid-induced hyperglycemia.
Out of total 79 patients, 18 patients had history of ICU care, whereas rest 61 patients had non-ICU care. Fifty nine (59) patients required oxygen support at some period of COVID-19 infection, whereas 20 patients did not give any history of oxygen support.
Out of all 96 operated cases, mucor fungal hyphae with tissue invasion in histopathology were seen in 53 cases; mixed fungal involvement (Mucor, Aspergillus, and Candida) in 16 cases; isolated Aspergillus was identified in 10 cases, whereas in 17 cases there were no fungal elements identified.
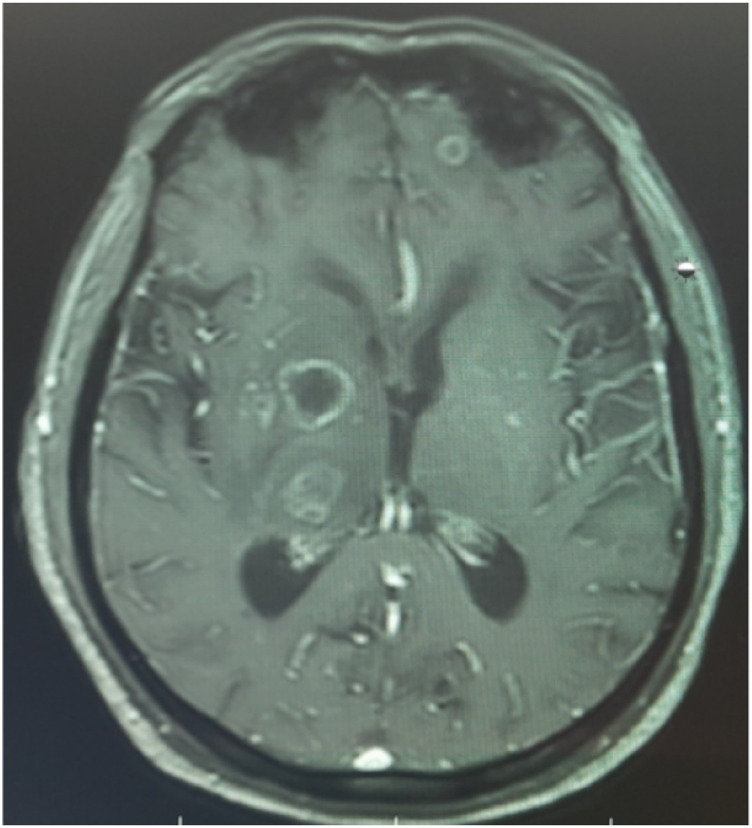
Among 9 patients of cerebral involvement (rhino–orbital–cerebral mucormycosis), 3 showed cavernous sinus involvement, 4 developed cerebral abscess, and 2 patients showed isolated intracranial fungal lesion (likely hematogenous spread) (Figures 7, 8, and 9). All of them landed in ICU during the management, and the general condition of 8 patients deteriorated subsequently during ICU care despite appropriate antifungal therapy and did not survive. The only patient who survived had cavernous sinus involvement and underwent orbital exenteration.
Figure 7.
T2 MRI coronal cut of the brain and orbits showing postoperative sinus changes with intracranial extension of the disease through cribriform plate.
Figure 8.
CEMRI axial cut of the brain showing multiple predominantly rim enhancing thick walled lesions with surrounding edema in left frontal, right thalamus, and right ganglio-capsular regions.
Figure 9.
CEMRI coronal cut of the brain showing mild asymmetry with thickening convexity of lateral walls of right cavernous sinus with hypo-enhancement suggesting early signs of cavernous sinus thrombosis.
In 6 out of 43 patients of rhino–orbital mucormycosis and 4 out of 9 patients of rhino–orbital–cerebral mucormycosis with no visual potential, the decision of orbital exenteration was taken after sub-optimal response to antifungals in 72 to 96 hours. But consent for orbital exenteration was not given by 2 patients and their family. So, total 8 orbital exenterations were done during the second-stage surgery after initial endoscopic sinus debridement. All patients having total ophthalmoplegia PL (−) (10) was due to direct spread or indirect inflammation of the orbital apex. All orbital exenterated patients had extensive necrotic orbital tissue which showed evidence of fungal elements except in 1 specimen where no fungal element was seen in histopathology but sinus specimen in previous tissue showed fungal elements. Out of these 8 patients, 5 patients (4 rhino–orbital mucormycosis and 1 rhino–orbital–cerebral mucormycosis) did not survive but rest 3 patients (2 rhino–orbital mucormycosis and 1 rhino–orbital–cerebral mucormycosis) are doing well on follow-up. Rest 37 out of 43 rhino–orbital mucormycosis patients showed improvement in ptosis, proptosis, congestion, and vision with antifungal therapy. Those 2 patients whose family did not give consent for orbital exenteration having cerebral involvement died after few days of ICU care.
All 27 patients with disease limited to sinuses underwent extended endoscopic sinus surgery and debridement. Four (4) patients showed residual or new enhancements on serial radiological investigation and underwent revision and further debridement.
Condition of 12 patients deteriorated over the next few days after debridement surgery due to acute respiratory distress syndrome and those patients were initially started on non-invasive ventilation. Eleven (11) of 12 patients got intubated and were started on mechanical ventilation with ionotropic support. All 11 patients who were on ventilator support did not survive. Cause of death suspected to be thromboembolic event and eventually cardiac arrest was suspected in all of them. Thirteen (13) patients (excluding those who required exenteration during second surgery) required revision and further debridement of sinonasal tissue, which included 7 patients who were operated at other centers previously.
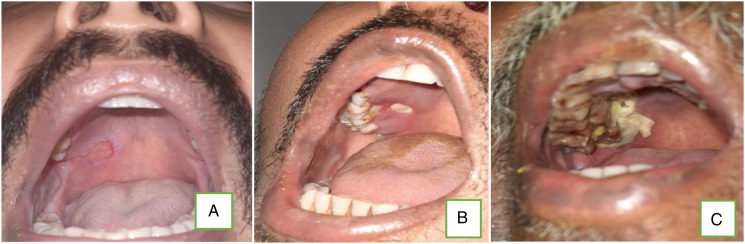
A different pattern of presentation has been encountered amongst the patients who presented during later phase of this post-COVID-19 mucormycosis. These patients had disease restricted to unilateral sinuses and maxilla (palate) (Figures 10, 11, and 12). Seven (7) patients (out of 27 rhino-mucormycosis patients) presented with sinus and floor of maxilla involvement with blackish discoloration within nose or palate, loosening of teeth, and abscess in the gingival tissue. They had history of COVID-19 infection of more than 3 months back; all were diabetics and had history of corticosteroid treatment. All the patients with maxillary involvement had a common clinical presentation of draining sinuses and mobility of teeth, for which routine dental treatment was given. Few had history of multiple consultations of dentists for tooth pain. Hence, the delay in definitive clinical management of mucormycosis. They all showed sequestrum and erosions in maxillary floor and sinusitis on MRI. None had orbital or intracranial involvement. Later, all these patients underwent sequestrectomy and debridement of the infected area along with extraction of involved teeth with the guidance of a maxillo-facial surgeon (Figure 13). Primary closure was achieved in all patients. All the patients with maxillofacial involvement were subjected to amphotericin B and posaconazole therapy with no complications reported and are doing well on follow-up.
Figure 10.
Transoral images showing palatal (floor of right maxilla) involvement due to mucormycosis (in increasing order of severity).
Figure 11.
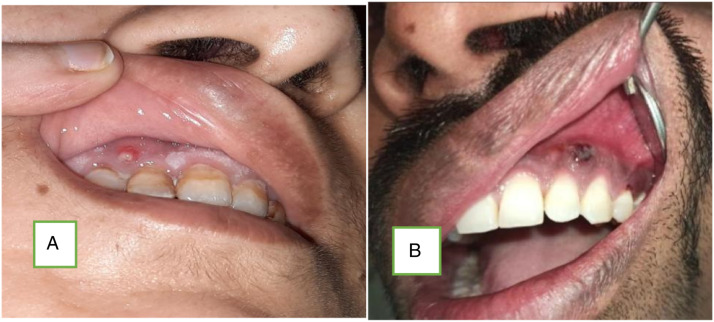
Images showing (A) draining sinus from alveolar process. The patient is having isolated involvement of right maxilla. (B) Showing necrosis of alveolus.
Figure 12.
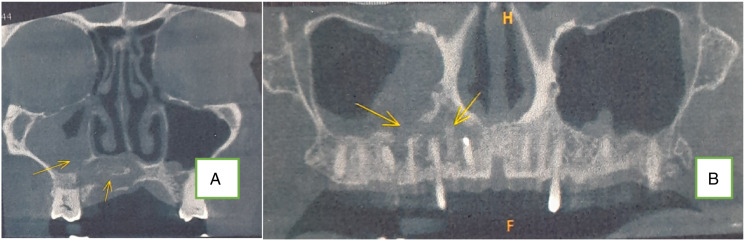
Plain CT scan (coronal) showing erosion of maxilla and alveolus (fungal osteomyelitis).
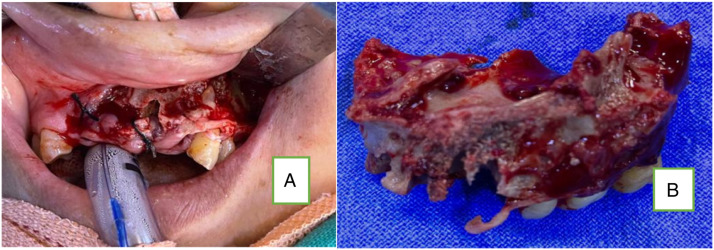
Figure 13.
(A)Showing necrosed and eroded upper alveolus and (B) showing the same after sequestrectomy.
Interestingly, 3 patients (out of this case series) reported with exclusive involvement of mandible with no signs of disease in maxilla. The presentation in mandible was that of mobile teeth and draining sinuses, possibly suggesting hematogenous spread. The clinical impression of mandibular involvement was classically similar to osteomyelitic process, wherein, the superior alveolar bone was necrotic and easily separable from the underlying basal bone. However, the basal bone was also found to be avascular, necessitating the need to make multiple burr holes for neovascularization.
During this time-period, 14 mortalities occurred accounting for 18.18% mortality rate. All 14 patients who died had thromboembolic events, cardiac arrest, and respiratory failure (acute respiratory distress syndrome, ARDS). Most of the patients were discharged after completion of 10 to 14 days of amphotericin-B therapy. They were discharged with oral posaconazole 300 mg once a day for 3 months and were called for regular sino-nasal endoscopic examination (Table 1).
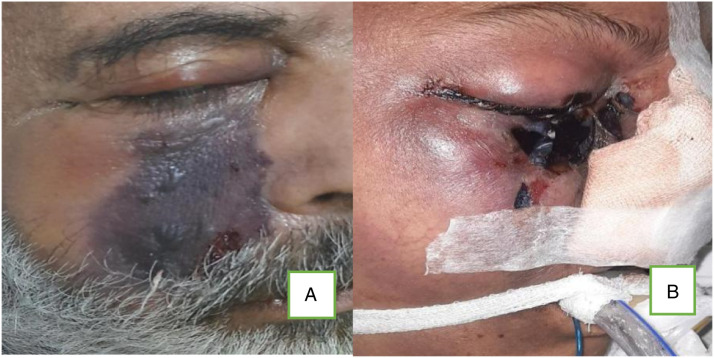
Figure 14.
Images showing cutaneous involvement due to mucormycosis. Both patients (A and B) had orbital and cerebral involvement (rhino-orbital-cerebral mucormycosis) and died during ICU care.
Table 1.
Demographic data of the case series.
| Parameter | Value |
|---|---|
| ● Clinically and radiologically suspected cases operated | 96 |
| ● Histopathological diagnosed cases (mucormycosis) | 79 |
| ● Age (range in years) | 21–72 |
| Mean age (in years)(standard deviation) | 52.24 (12.23) |
| ● Male:Female | 3.6:1 |
| ● Patients with active COVID-19 infection | 15 |
| Patients with post-COVID-19 status | 64 |
| ● Clinical category of mucormycosis | |
| 1. Rhino-mucormycosis | 27 |
| With palatal and dental involvement | 7 |
| 2. Rhino–orbital mucormycosis | 43 |
| With PL positive only | 11 |
| With PL negative (total ophthalmoplegia)*, 2 patients had pulmonary mucormycosis also | 6 |
| Vision better than PL positive | 26 |
| 3. Rhino–orbital–cerebral mucormycosis | 9 |
| With PL negative and total ophthalmoplegia* | 4 |
| With cutaneous involvement | 3 |
| Note: Total patients with orbital involvement | 52(43+9) |
| Total patients with ophthalmoplegia and PL(−) | 10 (6+4) |
| ● Known diabetics | 67 |
| Steroid-induced diabetes | 12 |
| ● Post-renal transplant patients (all are with associated diabetes/steroid-induced hyperglycemia) | 5 |
| ● Other comorbidity | 6 |
| Chronic kidney disease (CKD), excluding renal transplant recipients | 1 |
| Chronic liver disease (CLD) | 1 |
| Malignancy | 4 |
| Heart disease*** | 14 |
| Hypertension | |
| ● Patient with history of ICU care and mechanical ventilation | 18 |
| Patients with history of oxygen therapy | 59 |
| Patients with no history of oxygen therapy | 20 |
| ● Isolated mucor infection# | 53 |
| Mixed infection (mucor+aspergillus+/-candida)# | 16 |
| Isolated aspergillus infection# | 10 |
| No fungal elements## (preoperatively suspected cases; not included in series) | 17 |
| ● Patients required revision sinus debridement (including 7 patients referred from other centers) | 13 |
| ● Total orbital exenteration performed | 8 |
| Rhino–orbital mucormycosis (died) | 6(4) |
| Rhino–orbital–cerebral mucormycosis | 2(1) |
| ● Outcome (status till 30th Sep 2021) | 63 |
| Alive | 14 |
| Deceased | (18.18%)@ |
| Loss to follow-up (leave against medical advice; LAMA) | 2 |
*Includes 1 patient with bilateral total ophthalmoplegia**Malignant melanoma***ischemic heart disease, coronary artery disease, post-CABG (coronary artery bypass grafting)#Antifungal therapy continued after definitive diagnosis##Antifungal therapy modified or stopped as per clinical and radiological judgment@Calculated from 77 patients, excluding those 2 patients who went LAMA
Discussion
Epidemiology
Mucorales are the molds found abundantly in the environment, predominantly in hot and humid conditions of tropical countries like India. Rhizopus and Mucor are the two most common species causing mucormycosis belonging to family mucoraceae. The prevalence of mucormycosis in India is attributed around 140 cases per million population, and it is much less in European countries and the United States of America.11 Mucormycosis has emerged as one of the life-threatening complications of COVID-19 infection in India.
Etiopathology
As mucormycosis is considered a “diabetes-defining illness,” a major bulk is seen among diabetic patients and its severity is more prevalent among uncontrolled diabetics. Rhino–orbital–cerebral mucormycosis is a serious invasive fungal disease, the mortality rate of which is very high even with best treatment. It acts by invading blood vessels and mycotic thrombosis causing infarction and ischemic necrosis of host tissue including surrounding bones.12,13 Several factors are considered responsible for this sudden spike in incidence of invasive fungal infection. SARS-CoV-2 infection and post-COVID-19 sepsis results in dysregulated and altered immune response causing cytokine storm, thromboembolic events, and secondary bacterial and fungal infections.14 There are some pathophysiological phenomena like immune dysregulation, reduced CD4+ T and CD8+T cells, and impaired phagocytic immune-cell response that may enhance the risk of invasive fungal infections.15,16 Furthermore, the prolonged corticosteroid treatment in such patients especially with pre-existing conditions such as diabetes mellitus, organ transplant, neutropenia, and high free iron levels made them a susceptible host for invasive fungal infections like mucormycosis, aspergillosis, cryptococcosis, and candidiasis.17 Corticosteroid therapy induces immunosuppression, lymphopenia, and hyperglycemia predisposing to invasive fungal infection.18 Another indirect potential correlation has been established between COVID-19 and mucormycosis in India is the use of contaminated water with fungal spores for oxygen humidifier or use of contaminated and poor quality industrial oxygen during the shortfall of medical oxygen during the second wave of COVID-19 infection.19
Invasive mucormycosis has not only been seen in severe cases but also in mild and moderate cases of SARS-CoV-2 infections. These patients found to have uncontrolled hyperglycemia or steroid-induced hyperglycemia and were on immunosuppresants (renal recipients). Low dose and short duration of corticosteroids have shown benefit in patients of moderate to severe illness. But in second wave of SARS-CoV-2 infection, higher doses and longer duration of corticosteroids have been used in many patients with severe diseases. The invasive fungal infections in such patients alter the natural history of disease resulting in poor prognosis.20,21 Uncontrolled diabetes and persistent hyperglycemia are considered to effect neutrophil function and hence cause phagocytosis. Furthermore, diabetic acidosis impairs binding of iron to transferrin letting high levels of free iron promoting fungal growth.22 These fungi causing invasive infection thrive best in individuals having high serum glucose and acidic condition.23 It is pertinent to note that majority of invasive fungal infections develop during the later stages of COVID-19 infection. Similar pattern was also noted by Song et al in their study.21
The fungal spores reach sinuses through inhalation via nares, depositing in nasal mucosa and reaching orbit through lamina papyracea, ethmoid bone, inferior orbital fissure, or via orbital apex. The brain is involved when fungal infection directly involves cribriform plate, supraorbital fissure, or by perineural invasion and hematogenous spread.24 These patients can present with unilateral or bilateral facial swelling, numbness, proptosis, diminished vision, palatal involvement, and headache. The intracranial extension can be in the form of cavernous sinus thrombosis, cerebral infarction, aneurysm, or abscess formation. These patients can present with confusion and disorientation. Majority of the patients came into medical attention when they developed some orbital signs and symptoms such as orbital swelling, blurred or double vision, visual loss, or ophthalmoplegia.
Management
The nasal endoscopy of suspected patients may show necrosis or discoloration of nasal mucosa with blackish crusting, with or without anesthesia of nasal mucosa. The different diagnostic methods for mucormycosis include KOH fungal mount, biopsy, fungal staining (calcofluor), and culture. KOH is a rapid test which shows the presence of broad aseptate filamentous hyphae with right angled irregular branching. Computed tomography (CT)/magnetic resonance imaging (MRI) of paranasal sinuses, orbits, and brain should be obtained to see the extent of the disease. CT may show bony erosion or soft tissue invasion, but MRI is more sensitive for detecting characteristic paranasal sinus, orbital, and intracranial invasion. MRI may reveal non-enhancing mucosal tissue of sinuses and turbinates, subcutaneous facial tissue infratemporal, and temporal fossa inflammatory infiltration. Orbital involvement can be depicted as thickening of medial rectus, proptosis, preseptal edema or orbital apex infiltration. Culture takes many days to grow fungus, but biopsy of the suspected tissue is the mainstay method of definitive diagnosis. Detection of fungal elements can be enhanced by using special fungal stains such as calcofluor white.
Treatment of mucormycosis includes surgical drainage of paranasal sinuses, debridement of necrotic and unhealthy tissues, intravenous antifungal therapy, and treating underlying predisposing factors. Hyperbaric oxygen therapy can be added to the regimen, but no controlled studies have shown its efficacy. Early surgical debridement of nasal, paranasal sinus, and orbital tissue along with systemic antifungal therapy is the mainstay of treatment of mucormycosis.25
According to few studies, orbital exenteration is a crucial part of management in extensively diseased orbit, preventing further deterioration.26,27 But in a retrospective analysis, a high mortality rate (88.9%) has been seen in patients who underwent orbital exenteration. Deciding the need and timing of orbital exenteration is quite complicated. In limited involvement of orbit with preserved vision, resection of lamina papyracea should be considered. If disease progresses after 72 hours of aggressive antifungal therapy and in patients with non-reactive pupil or no visual perception, orbital exenteration should be done without any further delay.28
Among antifungal agents, amphotericin-B is the standard antifungal drug used against invasive mucormycosis. Lipid complex amphotericin-B is well known for its nephrotoxicity which is the major dose-limiting toxicity of using it. Liposomal amphotericin-B is less or non-nephrotoxic form of amphotericin-B. Its standard dose is 5 to 10 mg/kg/day. Other labeled antifungals against invasive mucormycosis are triazoles like posaconazole and isavuconazole, for at least 6 weeks. Posaconazole and isavuconazole are good options as a salvage therapy in patients of chronic kidney disease (CKD) or in patients who may develop renal failure with uncontrolled diabetes. These can be used as monotherapy, but in refractory cases they are usually given as combined therapy. Posaconazole is given twice a day (300 mg) orally for 1 day followed by 300 mg once a day up to 6 months. Similarly, isavuconazole is given thrice daily (200 mg) orally for 1 day followed by 200 mg once a day up to 6 months. They are used as step-down therapy after initial intravenous amphotericin-B therapy.
Serial radiological investigations (preferably MRI brain, orbit, and PNS) are the part of management and are must to assess the progression or response to therapy. Some studies concluded PET-CT as a useful investigation for postoperative assessment, but cost is the limiting factor.28-30
The quick identification and diagnosis of invasive fungal infection, subsequently early surgical debridement, is the essential management modality in reducing the disease burden and can significantly reduce the morbidity and mortality. Surgical debridement helps in penetration of intravenous antifungal agents more efficiently and limits further spread of disease. Correction of the underlying metabolic and immune derangement is equally important for favorable outcome.
Mucormycosis is a fatal opportunistic invasive fungal infection, the prognosis of which is poor affecting mainly immunocompromised and patients with uncontrolled diabetes. The mortality rate for rhino–orbital–cerebral mucormycosis is about 40 to 80% depending upon the extent of disease.25
Few similar case series have been reported in the literature recently.5-10 Diabetes mellitus is the strongest association found in all COVID-19 associated mucormycosis, and all of them were managed with corticosteroids during the active phase of COVID-19 infection. The comparative analysis of all published series of COVID-19 associated mucormycosis is assessed in Table 2.
Table 2.
Comparative analysis of demographic and clinical data of COVID-19 associated rhino–orbital–cerebral mucormycosis case series6-11.
| Study | Cases | Patients with current or past COVID-19 infection | Patients with no history of COVID-19 infection | Diabetes mellitus (DM) | Cerebral involvement | Number of patients received surgical treatment | Death | Remarks |
|---|---|---|---|---|---|---|---|---|
| 1.Fouad et al (2020)6 | 12 | 6 | 6 | 9 | 8 | 7 | 6 | — |
| 2. Buil et al (2020-2021)7 | 4 | 4 | — | 2 | — | 1 | 3 | |
| 3. Nehara et al (2020)8 | 5 | 5 | 5 | 3 | — | 2 | ||
| 4. John et al (2019-2021)9 | 41 | 38 | 3 | 41* | 14 | 33 | 20 | *8 had new onset DM |
| 5. Sharma et al (2020)10 | 23 | 23 | — | 21 | 2 | 23 | — | |
| 6. Moorthy et al (2020)11 | 18 | 18 | 16 | 9 | 18 | 6 | — | |
| 7. Naruka et al (present study) (2021) | 79 | 79 | 79# | 9 | 79 | 14 | #12 had new onset DM | |
Conclusion
COVID-19 in diabetics and in immunocompromised patients is associated with various manifestations among which mucormycosis is one of the grave association. Further, the use of long-term high dose corticosteroids in COVID-19 treatment exaggerates the probability of developing invasive fungal infection. Delay in treatment even of few days can increase the mortality. Timely diagnosis and intervention in the form of surgical debridement and antifungal therapy are critical aspects in improving clinical outcomes in such patients. The clinicians should now be cautious of possibility of development of invasive fungal infection during the management of COVID-19 patients with risk factors. Continuous vigilance with regular follow-up in recovered patients should be strictly followed.
Acknowledgments
Department of ENT and Head and Neck Surgery, Dental and Maxillofacial Surgery, Pathology, Microbiology, and Radiology, Indraprastha Apollo Hospitals, New Delhi, India.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Nishant Rana https://orcid.org/0000-0003-1588-2117
References
- 1.Soman R, Sunavala A. Post COVID-19 mucormycosis - from the frying pan into the fire. J Assoc Phys India. 2021;69(1):13-14. doi: 10.4103/ijdr.ijdr_594_21. [DOI] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, china: A descriptive study. Lancet. 2020;395(10223):507-513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge: The perfect storm for mucormycosis. J Fungai. 2021;7(4):298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim S, Bae JH, Kwon H-S, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11-30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papadogeorgakis N, Parara E, Petsinis V, Vourlakou C. A case of successfully treated rhinocerebral mucormycosis: Dental implications. Intern J Density. 2010;2010:1-4. doi: 10.1155/2010/273127. Epub2011 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouad YA, Abdelaziz TT, Askoura A, et al. Spike in rhino-orbital-cerebral mucormycosis cases presenting to a tertiary care center during the COVID-19 pandemic. Front Med. 2021;8:645270. doi: 10.3389/fmed.2021.645270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buil JB, van Zanten ARH, Bentvelsen RG, et al. Case series of four secondary mucormycosis infections in COVID-19 patients, the netherlands, december 2020 to may 2021. Euro Surveill. 2021;26(23):2100510. doi: 10.2807/1560-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nehara HR, Puri I, Singhal V, Ih S, Bishnoi BR, Sirohi P. Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India. Indian J Med Microbiol. 2021;39(3):380PMC8153224-383. doi: 10.1016/j.ijmmb.2021.05.009. Epub 2021 May 26. PMID: 34052046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: A deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135(5):442-447. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moorthy A, Gaikwad R, Krishna S, et al. SARS-CoV-2, Uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxill Acial Oral surg. 2021;20(3):418-425. doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungai. 2019;5(1):26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:e5-264. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl_1):S23-S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 14.Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9):e10726. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangneux J-P, Bougnoux M-E, Dannaoui E, Cornet M, Zahar JR. Invasive fungal diseases during COVID-19: We should be prepared. J Mycolog Med. 2020;30(2):100971. doi: 10.1016/j.mycmed.2020.100971. Epub2020 Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: A clinical and diagnostic perspective from china. Mycopathologia. 2020;185:599-606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl_1):S16-S22. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meher R, Wadhwa V, Kumar V, et al. COVID associated mucormycosis: A preliminary study from a dedicated COVID hospital in delhi. Am J Otolaryngol. 2022;43(1):103220. doi: 10.1016/j.amjoto.2021.103220. Epub 2021 Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID‐19‐associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID‐19 pneumonia: An observational study from Pakistan. Mycoses. 2020;63(8):766-770. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartoletti M, Pascale R, PREDICO Study Group , et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients With COVID-19: A prospective study. Clin Infect Dis. 2021;73(11):e3606-e3614. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balai E, Mummadi S, Jolly K, Darr A, Aldeerawi H. Rhinocerebral Mucormycosis: A Ten-Year Single Centre Case Series. Cureus. 2020;12(11):e11776. doi: 10.7759/cureus.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GALE GR, WELCH AM. Studies of opportunistic fungi. Am J Med Sci. 1961;237:604-612. [PubMed] [Google Scholar]
- 24.Honavar S, Sen M, Lahane S, Lahane T, Parekh R. Mucor in a viral land: A tale of two pathogens. Indian J Ophthalmol. 2021;69(2):244-252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the european confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405-e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Songu M, Unlu HH, Gunhan K, Ilker SS, Nese N. Orbital exenteration: A dilemma in mucormycosis presented with orbital apex syndrome. Am J Rhinol. 2008;22(1):98-103. doi: 10.2500/ajr.2008.22.3121. [DOI] [PubMed] [Google Scholar]
- 27.Pelton RW, Peterson EA, Patel BCK, Davis K. Successful treatment of rhino-orbital mucormycosis without exenteration. Ophthalmic Plast Reconstr Surg. 2001;17(1):62-66. doi: 10.1097/00002341-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee B, Raichura N, Alam M. Fungal infections of the orbit. Indian J Ophthalmol. 2016;64(5):337-345. doi: 10.4103/0301-4738.185588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Wu H, Huang F, Fan Z, Xu B. Utility of 18F-FDG PET/CT in diagnosis and management of mucormycosis. Clin Nucl Med. 2013;38(9):e370-e371. doi: 10.1097/RLU.0b013e3182867d13. [DOI] [PubMed] [Google Scholar]
- 30.Altini C, Niccoli Asabella A, Ferrari C, Rubini D, Dicuonzo F, Rubini G. (18)F-FDG PET/CT contribution to diagnosis and treatment response of rhino-orbital-cerebral mucormycosis. Hellenic J Nuclear Medicine. 2015;18(1):68-70. doi: 10.1967/s002449910167. [DOI] [PubMed] [Google Scholar]