Abstract
Chronic obstructive pulmonary disease-associated pulmonary hypertension (COPD-PH) is an increasingly recognised condition which contributes to worsening dyspnoea and poor survival in COPD. It is uncertain whether specific treatment of COPD-PH, including use of medications approved for pulmonary arterial hypertension (PAH), improves clinical outcomes. This systematic review and meta-analysis assesses potential benefits and risks of therapeutic options for COPD-PH.
We searched Medline and Embase for relevant publications until September 2020. Articles were screened for studies on treatment of COPD-PH for at least 4 weeks in 10 or more patients. Screening, data extraction, and risk of bias assessment were performed independently in duplicate. When possible, relevant results were pooled using the random effects model.
Supplemental long-term oxygen therapy (LTOT) mildly reduced mean pulmonary artery pressure (PAP), slowed progression of PH, and reduced mortality, but other clinical or functional benefits were not assessed. Phosphodiesterase type 5 inhibitors significantly improved systolic PAP (pooled treatment effect −5.9 mmHg; 95% CI −10.3, −1.6), but had inconsistent clinical benefits. Calcium channel blockers and endothelin receptor antagonists had limited haemodynamic, clinical, or survival benefits. Statins had limited clinical benefits despite significantly lowering systolic PAP (pooled treatment effect −4.6 mmHg; 95% CI −6.3, −2.9).
This review supports guideline recommendations for LTOT in hypoxaemic COPD-PH patients as well as recommendations against treatment with PAH-targeted medications. Effective treatment of COPD-PH depends upon research into the pathobiology and future high-quality studies comprehensively assessing clinically relevant outcomes are needed.
Short abstract
The presence of PH in COPD patients is associated with worsening morbidity and mortality. These findings support guideline recommendations for LTOT in hypoxaemic COPD-PH patients as well as recommendations against treatment using PAH-targeted medications. https://bit.ly/3Al4rLb
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive and incurable disease that represents one of the five leading causes of death worldwide [1, 2]. COPD is characterised by exertional dyspnoea, functional limitation, poor health-related quality of life (HRQoL), recurrent exacerbations and hospitalisations, as well as shortened survival [1, 2]. The presence of pulmonary hypertension (PH) in patients with COPD is increasingly recognised as an important contributing factor to its clinical manifestations and adverse clinical outcomes including increased mortality [3, 4]. For example, severe PH and resulting right ventricular (RV) failure are associated with more severe dyspnoea and limited exercise capacity [5, 6]. Indeed, the presence of PH has a stronger association with mortality in COPD than forced expiratory volume in 1 s (FEV1) or gas exchange variables [7, 8]. Moreover, enlarged pulmonary artery diameter on computed tomography scan is independently associated with a higher risk of acute COPD exacerbations and related hospitalisations [8, 9].
Estimates of the prevalence of PH in COPD (COPD-PH) vary widely (20–91%) [5, 10, 11], with increasing prevalence with greater severity of COPD [4]. For example, the most severe Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage IV COPD is associated with mild-moderate PH in up to 90% of patients [5]. PH in a patient with COPD could be due to a broad range of underlying conditions, such as left-heart disease [12], concomitant interstitial lung diseases or sleep disordered-breathing, or chronic thromboembolic PH. Management of associated cardiac and respiratory conditions can improve the clinical status and outcomes in COPD-PH patients [4, 13].
Specific medical treatment of COPD-PH may also offer clinical benefits, including improved dyspnoea, functional capacity, and long-term outcomes. Thus, we conducted a systematic review and meta-analysis for benefits and risks of treatment options for COPD-PH.
Methods
Search strategy and eligibility criteria
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we searched MEDLINE and Embase databases from 1947 to 30 September 2020, using the search terms “pulmonary hypertension” AND “chronic obstructive airway disease or chronic obstructive pulmonary disease or COPD” AND “treatment or management”.
We also reviewed bibliographies, identifying additional relevant studies. Titles and abstracts were screened, and full-text articles were reviewed independently and in duplicate (R. Arif and S. Mehta) in order to identify studies meeting the predefined inclusion and exclusion criteria (supplementary table S1): studies of 10 or more patients reporting the effects of at least 4 weeks of treatment on pulmonary haemodynamics, survival, or other clinical outcomes in patients with COPD-PH. Risk of bias was assessed using the Newcastle Ottawa Scale for observational studies and the Cochrane Collaboration tool for randomised controlled trials (RCTs). Disagreements were resolved by consensus.
Data collection
Data collection was performed independently by at least two authors (R. Arif, A. Pandey and Y. Zhao). The data extracted included: study characteristics, patients demographics and comorbidities, method of PH diagnosis, intervention type, dosage and frequency, duration of and loss to follow-up, as well as outcomes, including clinical outcomes (e.g. survival), cardiopulmonary haemodynamics (e.g. mean pulmonary artery pressure (mPAP)), pulmonary vascular resistance (PVR), cardiac output (CO), and others) as listed in supplementary table S1.
Data analysis
Subgroups based on the method of PH diagnosis were defined a priori and a sensitivity analysis performed; patients diagnosed using right-heart catheterisation (RHC)-determined mPAP versus those diagnosed using non-invasive echocardiography by estimating systolic PAP (sPAP) or calculating mPAP. During data analysis, another subset of COPD-PH patients was identified; those with more severe PH and RV failure, often in the setting of only mild to moderate COPD without resting hypoxaemia. This subgroup was analysed separately. Details of statistical analysis are given in the supplementary material.
Results
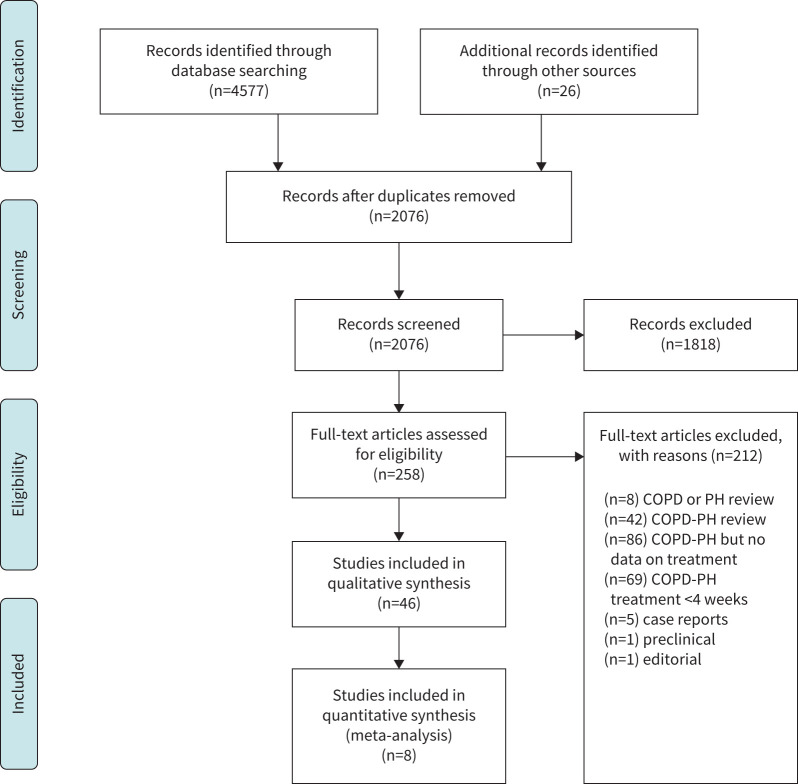
We retrieved and screened 4577 reports, and an additional 26 records were identified through other sources (figure 1). 4557 studies were excluded, leaving 46 studies reporting treatment of COPD-PH, including 23 RCTs (1159 patients) and 23 non-RCTs (1187 patients). Patients ranged from 35–85 years in age and were predominantly male in the majority of studies (range 32–100%). Lung function varied widely (FEV1 13–94% predicted), but most patients had moderate to severe COPD, many with hypoxaemia at rest.
FIGURE 1.
PRISMA flow diagram of identification of relevant articles for inclusion in systematic review and quantitative analysis. PH: pulmonary hypertension.
We identified five categories of COPD-PH therapies, including supplemental oxygen (table 1), calcium-channel blockers (supplementary table S2), pulmonary arterial hypertension (PAH)-targeted therapy (table 2), statins (supplementary table S3), and miscellaneous therapies (supplementary table S4).
TABLE 1.
Effects of supplemental oxygen therapy including long-term oxygen therapy (LTOT) and nocturnal oxygen therapy (NOT) in patients with COPD-associated pulmonary hypertension (PH)
| Study | Design | Population | Intervention | Significant outcomes |
| Stark 1972 [19] | Randomised parallel-group | n=11: LTOT of different duration Age: 39–67 years Gender: 100% male FEV1: 0.80 L PaO2: 49 mmHg RHC mPAP: 41.9 mmHg |
LTOT (2 L·min−1) for 18 h·day−1 (n=4), 15 h·day−1 (n=4), or 12 h·day−1 (n=3) for 3–7 weeks | Outcomes with LTOT: Decreased mPAP (18 h·day−1: 51 to 31 mmHg, p<0.05; 12 h·day−1: 37 to 30 mmHg, p<0.05) Decreased PVR (18 h·day−1: 10 to 5.6 WU, p<0.05; 15 h·day−1: 6.2 to 5 WU, p<0.05) |
| Nocturnal Oxygen Therapy Trial 1980 [20] | Randomised parallel-group | n=203: LTOT (n=101) versus NOT (n=102) Inclusion criteria: PaO2 ≤55 or PaO2 ≤59 mmHg and either oedema or haematocrit ≥55% or ECG p pulmonale Age: 65 years Gender: 78.8% male FEV1: 29.7% PaO2: 51.2 mmHg RHC mPAP: 29.5 mmHg |
LTOT (17.7±4.8 (sd) h·day−1) versus NOT (12±2.5 h·day−1) to target PaO2 60 to 80 mmHg for >1 year (mean 19.3 months) |
Outcomes in LTOT (n=87/101) versus NOT (n=80/102): Decreased PVR (11.1%; n=52) versus increased (6.5%; n=49) at 6 months (p=0.04) Lower 12 months mortality (11.9±3.2 versus 20.6±4.0%; n=87 versus 80, respectively; p=0.01) Lower 24 months mortality (22.4±4.6 versus 40.8±5.5%; n=37 versus 29, respectively; p=0.01) Survival benefit of LTOT in patient s with baseline mPAP <27 (p=0.03), PVR <3.5 WU (p=0.03) |
| Medical Research Council 1981 [14] | RCT | n=87: LTOT (n=42) versus control (n=45) Inclusion criteria: PaO2 40–60 mmHg Age: 58.2 years Gender: 75.9% male FEV1: males 0.70 versus females 0.61 L PaO2: 51 mmHg RHC mPAP: 34.4 (male) versus 32.7 (female) mmHg |
LTOT (>15 h·day−1) to target PaO2 >60 mmHg versus control (room air) for 5 years | Outcomes in LTOT versus control: Change in mPAP (−0.06 versus +2.79 mmHg·year−1; n=21 men surviving >500 days; “significant” but p-value not specified) Change in TPR (0 versus +1.4 WU·year−1) Decreased mortality in females (p<0.05) Decreased mortality in males only after 500 days (n=19 versus 30; 12%/year versus 29%/year, p=0.04) |
| Gluskowski 1983 [15] | Case series | n=16 Inclusion criteria: PaO2 <60 mmHg or haematocrit >60% Age: 50.4 years Gender: 81.3% male FEV1: 0.84±0.33 L (sd) PaO2: 51.8±8.8 mmHg RHC mPAP: 42.5±13.3 mmHg |
LTOT (17 h·day−1) on 28% facemask for 6 weeks | Outcomes with LTOT: Decreased mPAP (42.5±13.3 to 38.1±10.4 mmHg; p<0.001) Increased FEV1 (0.84±0.33 to 1.06±0.55 L; p<0.05) No change in cardiac index or PaO2 |
| Weitzenblum 1985 [16] | Case series | n=16 Inclusion criteria: PaO2 <60 mmHg, and RHC mPAP >20 mmHg or history of right heart failure or ECG RVH Age: 58.1±7.9 years (sd) Gender: 93.8% male FEV1: 0.89±0.28 L PaO2: 50.2±6.6 mmHg RHC mPAP: 28.0±7.4 mmHg |
LTOT (>15 h·day−1) to target PaO2 ≥65 mmHg (mean 31±19 months) |
Outcomes with LTOT: Decreased mPAP (28.0±7.4 to 23.9±6.6 mmHg; annual change −2.2±4.4 mmHg·year−1, p<0.05) versus increased mPAP pre-LTOT (23.3±6.8 to 28.0±7.4 mmHg; +1.5±2.3 mmHg·year−1, p<0.005) |
| Timms 1985 [21] | Randomised parallel-group | n=118/203 with RHC at baseline and 6 months: LTOT (n=61) versus NOT (n=57) Inclusion criteria: PaO2 ≤55 mmHg or PaO2 ≤59 mmHg with signs of right heart failure or erythrocytosis Age: 65.6±7.7 years (sd) Gender: 83.1% male FEV1: 32.7±14.1% (n=114) PaO2: 51.9±4.9 mmHg (n=117) RHC mPAP: 29±10 mmHg (n=178) |
LTOT versus NOT (12 h·day−1) for mean 32 months | Outcomes in LTOT versus NOT: Decreased resting mPAP 3±11 mmHg (p=0.02) and PVR 0.85±2.2 WU (p=0.007) versus no change; differences between LTOT and NOT were not significant Decreased exercise mPAP (p=0.005) and exercise PVR (p=0.001), increased exercise SVI (p=0.004) versus no change Changes in mPAP during first 6 months associated with subsequent survival after adjustment for baseline values (p<0.01) in both LTOT and NOT |
| Cooper 1987 [17] | Case series | n=72 Inclusion: FEV1 <50% and PaO2 <60 mmHg and ≥1 episode of peripheral oedema Age: 60.5±7.5 years (sd) Gender: 73.6% male FEV1: 29±10% PaO2: 45.8±7.5 mmHg RHC mPAP: 28.3±10.2 mmHg (n=45) PVR: 5.0±2.2 WU (n=45) |
LTOT (1.5–2.5 L·min−1) to target PaO2 ≥60 mmHg 15 h·day−1 for 5 years | No difference in mPAP at 1-year versus baseline (n=40) No association of survival with PAP or PVR |
| Fletcher 1992 [22] | RCT | n=16/38: NOT (n=7) versus sham (n=9) Inclusion criteria: daytime PaO2 ≥60 mmHg, episodic desaturation in REM sleep Age: 61.6±2.2 years (sem) Gender: not specified FEV1: 1.42±0.20 versus 1.42±0.14 L PaO2: 73.7±2.6 versus 76.7±3.4 mmHg RHC mPAP: 26.7±2.2 versus 22.5±1.8 mmHg |
NOT (3 L·min−1) versus sham for 3 years |
Outcomes in NOT versus sham: Change in mPAP −3.7 versus +3.9 mmHg/3 years (p<0.02) No difference in PVR, CO, mortality |
| Zielinski 1998 [18] | Case series | n=73/95 who survived at least 2 years Inclusion criteria: PaO2 ≤55 mmHg or PaO2 56–65 mmHg with cor pulmonale (ECG RVH, PH on chest radiograph) or haematocrit ≥55% Age: 58±9 years (sd) Gender: 72% male FEV1: 0.84±0.31 L PaO2: 55±6 mmHg RHC mPAP: 28±11 mmHg |
LTOT (mean 13.5–14.7 h·day−1) for 2–6 years |
Outcomes at 2 year (n=39), 4 year (n=20), and 6 year (n=12): No change in mPAP, PVR or CO at any timepoint versus baseline Increased mPAP at 4 year (p<0.05) and 6 year (p<0.01) versus 2 year (n=12) Decreased PaO2 at 2 year (p<0.05), 4 year (p<0.05), and 6 year (p<0.001) versus baseline |
| Chaouat 1999 [23] | RCT | n=76: NOT (n=41) versus control (n=35) Inclusion: PaO2 56–69 mmHg and nocturnal desaturation (SaO2 <90% for ≥30% of time in bed) Exclusion: OSA with AHI ≥10 events·h−1 Age: 63.5±7.1 years (sd) Gender: not specified FEV1: 1.1±0.5 versus 1.0±0.3 L PaO2: 62.6±3 versus 62.8±3 mmHg RHC mPAP: 19.7±5.3 versus 19.5±5.3 mmHg PH at baseline (mPAP ≥20 mmHg): n=36/76 |
NOT: 8.9±1.9 h·night−1) to target SpO2 >90% for 2 years (mean 35.1±14.3 months) |
Outcomes in NOT versus control at 2 years: No difference in mPAP or CO at rest or with exercise (n=24 versus 22) No difference in mPAP in patients with PH at baseline (n=9 versus 10) No difference in mortality (n=9 versus 7) |
Data are mean±sem, unless otherwise specified. AHI: apnoea-hypopnoea index; CO: cardiac output; FEV1: forced expiratory volume in 1 s; mPAP: mean pulmonary artery pressure; OSA: obstructive sleep apnoea; PaO2: partial pressure of oxygen in arterial blood; PVR: pulmonary vascular resistance; RCT: randomised controlled trial; REM: rapid eye movement; RHC: right heart catheterisation; RVH: right ventricular hypertrophy; SaO2: arterial oxygen saturation (%); SpO2: transcutaneous pulse oximetry oxygen saturation (%); SVI: stroke volume index; TPR: total pulmonary resistance, WU: Wood unit (mmHg·L−1·min−1).
TABLE 2.
Effects of pulmonary arterial hypertension-targeted therapies in patients with COPD-associated pulmonary hypertension (COPD-PH)
| Study | Design | Population | Intervention | Significant outcomes | Adverse effects |
| Stolz 2008 [35] | RCT | n=30: bosentan (n=20) versus placebo (n=10) Inclusion criteria: COPD GOLD stage III–IV Age: 68±8.5 years (sd) Gender: 60% male FEV1: 39±13.3% 6MWD: 336.3±92.6 m SpO2: 92.6±3.3% Echo sPAP: 32 (median; IQR 29–38) versus 37 mmHg (20–42) in (n=14) Bosentan versus (n=6) placebo |
Bosentan 62.5 mg PO BID for 2 weeks then 125 mg PO BID for 12 weeks Note: (n=8 on LTOT) in Bosentan versus (n=3) placebo |
Outcomes in Bosentan (n=14/20) versus placebo (n=9/10): No change in echo PVR versus increase (p=0.006) No difference in sPAP or cardiac index Improved SF-36 total and physical domain scores Decreased 6MWD 339±81 to 329±94 m (p=0.04) versus no change No change in BDI Decreased PaO2 (p=0.029) |
Bosentan (n=6) versus placebo (n=1) withdrew (p=0.37) Bosentan (n=2) dose reduction (elevated liver enzymes) |
| Valerio 2009 [34] | RCT (not blinded) | n=40: Bosentan (n=20) versus placebo (n= 20) Inclusion criteria: RHC mPAP >20 mmHg and PAWP <15 mmHg Age: 65.5±9.5 years Gender: 78.1% male (n=32/40) FEV1: 38±18% 6MWD: 257±118 versus 270±150 m PaO2: 57±10 versus 58±9 mmHg mPAP: 37±5 mmHg (variance not defined) |
Bosentan 125 mg PO BID versus placebo for 18 months Note: 40% of each group were on LTOT |
Outcomes in Bosentan (n=16/20) versus placebo (n=16/20): Decreased mPAP (37±5 to 31±6 mmHg; p=0.002) versus no change Decreased PVR (5.5±2.4 to 4.9±2.3 WU; p=0.012) versus no change Increased 6MWD (257±118 to 321±122 m; p=0.003) versus no change Decreased mNYHA FC (3.2±0.8 to 2.8±1.2; p=0.05) versus no change Decreased BODE index (6.6±2.8 to 5.5±3; p=0.002) versus no change No change in PaO2 |
n=8 withdrew (noncompliance, other health problems) |
| Rao 2011 [28] | RCT | n=37: sildenafil (n=17) versus placebo (n=20) Inclusion criteria: echo sPAP >40 mmHg Age: 62.3±7.5 years (sd) Gender: not specified FEV1: 32.5±11.1 versus 28.5±7.5% 6MWD: 268.9±139.9 versus 323.1±165.6 m Echo sPAP: 52.7±11.9 versus 47.8±13.4 mmHg |
Sildenafil 20 mg PO TID versus placebo for 12 weeks Note: none of the study participants used LTOT |
Outcomes in sildenafil (n=15/17) versus placebo (n=18/20): At 4 weeks: Increased mean 6MWD 150±123 versus 24±117 m (p<0.05) At 12 weeks: Increased mean 6MWD 191±127 versus 39±87 m (p<0.025) Decreased echo sPAP 53±12 to 41±8 mmHg (p<0.05) versus no change |
Sildenafil n=2 (epigastric pain and lost to follow up) versus placebo n=2 (acute exacerbation and lost to follow up) |
| Badesch 2012 [36] | Case series | n=24 (11%) with COPD-PH of n=224 with PH Inclusion criteria: FEV1 ≥50% RHC mPAP >35 mmHg and PVR > 3.5 mmHg·L−1·min−1 6MWD (150–450 m) Age: 68±11 years (sd) Gender: 71% male 6MWD:241±84 m BNP: 243±245 ng·L−1 mPAP: 45±10 mmHg |
Ambrisentan 5 mg PO daily for 24 weeks Note: 52.2% receiving background PH therapy |
Outcomes in COPD-PH: No change in 6MWD (−5 m; 95% CI −34, 24) Change in BNP (−38%; 95% CI −54, −17) Outcomes in all PH: 181 patients (81%) contributed to the primary endpoint, 34 patients (15%) discontinued prior to the week 24 visit Six patients died during the 24-week treatment period Improved mNYHA FC in 23%, worse in 7% (p<0.001). Change in BDI (−0.5; 95% CI −0.8, −0.3) |
No specific data in COPD-PH |
| Hurdman 2013 [39] | Retrospective non-randomised cohort analysis of prospective registry (ASPIRE) |
n=59: PH-targeted therapy (n=43) versus no therapy (n=16) Inclusion criteria: Post-bronchodilator FEV1 ≤0.7 RHC mPAP ≥40 mmHg (“severe”) Age: 70±9 years (sd) Gender: 47% male (n=28/59) FEV1: 65±23% ISWD: 40 (Median:IQR 18–100) m PaO2: 45.8±11.3 mmHg mPAP: 49±8 mmHg |
PDE-5i (n=31), ERA (n=10), SC treprostinil (n=1), nebulised iloprost (n=1), Treatment for ≥3 months or until death Note: 85% received LTOT |
Outcomes with PH-targeted therapy versus no therapy: No change in survival 72% versus 63%/1 year (p=0.67) PH-targeted therapy responder subgroup: N=8 (19%) objective response to PH therapy based on improved mNYHA FC or >20% fall in PVR Better survival versus non-responder (p<0.05) |
No difference in SpO2 between groups; no discontinuation of medication (median 178 days) |
| Blanco 2013 [29] | RCT | n=63: Sildenafil per-protocol (n=32) versus placebo (n=31) Inclusion criteria: echo sPAP >34 or RHC mPAP ≥25 mmHg Age 65.5±8 years (n=60) Gender: 90% male (n=60) FEV1: 32±12% (sd) 6MWD: 392±81 versus 379±100 m (n=60) Echo sPAP: 42±10 or RHC mPAP mean 31±5 mmHg in 22% of patients |
Sildenafil 20 mg TID versus placebo for 3 months All patients underwent pulmonary rehabilitation 3 times per week for 3 months Note: n=18 on LTOT |
Primary outcome in sildenafil-treated (n=29/32) versus placebo (n=31) per protocol: No significant difference in improvement in cycle endurance time (p=0.77) Outcomes in sildenafil-treated (n=24/29) versus placebo (n=27/ 31) who completed study: No statistically significant differences in incremental exercise test, 6MWD, HRQoL |
COPD exacerbation which occurred in about third of patients lead to 10% d/c and 8% hospitalisation with no difference between groups |
| Goudie 2014 [33] | RCT | n=120: Tadalafil (n=60) versus placebo (n=60) Inclusion criteria: Age 35–85 years Post-bronchodilator FEV1 <80% and FEV1/FVC <70% Echo sPAP >30 mmHg or PAAT ≤120 ms Age: 69±7.5 years (sd) Gender: 68.5% male FEV1: 40.5±16% 6MWD: 347.5±104.5 m SpO2: 95.4±2.9% Echo sPAP: 42±9.5 mmHg |
Tadalafil 10 mg daily versus placebo for 12 weeks n=13 (11%) of patients were on LTOT |
Outcomes in tadalafil (n=56/60) versus placebo (n=57/60): Primary end point: no difference in 6MWD (p=0.94) No significant changes in HRQoL (SF-36, SGRQ, MLHFQ), BNP Mean placebo-corrected decreased sPAP from baseline (12.3 mmHg; p=0.007; n=12 tadalafil versus n=13 placebo) Mean placebo-corrected decreased calculated mPAP from baseline (3.5 mmHg; p=0.025) |
Expected Tadalafil side-effects (e.g. dyspepsia, headache) more common than placebo No difference in SpO2 between groups |
| Fossati 2014 [41] | Retrospective cohort | n=27/48 with COPD-PH, of n=463 attending PH clinic Inclusion criteria: FEV1/FVC <0.7 RHC mPAP ≥25 mmHg and PAWP ≤ 15 mmHg PH-targeted therapy for at least 3 months Age: 70 (Median:IQR 60–76) years Gender: 74% male FEV1: 60 (46–78) % 6MWD: 373 (236–452) m SpO2: 92 (86–94) % NT-proBNP: 653 (159–1194) ng·L−1 mPAP: 39 (32–44) mmHg |
Sequential combination therapy; final: ERA (n=15), PDE-5i (n=25), Prostanoids: inhaled (n=10), s/c (n=2), iv (n=3) Note: 60% of patients used supplemental oxygen at least during nights |
Median follow-up 5.9 years: mNYHA FC improved at 3 and 6 months (p=0.02 and p=0.008, respectively), not significant at 1 and 2 years 6MWD increased significantly at 3, 6, and 12 months (p<0.01 at each timepoint), not significant at 2 years No change in NT-proBNP and resting SpO2 Peak exercise SpO2 during 6MWD decreased at 3, 6, and 24 months (p<0.05 at each timepoint) No difference in transplant-free survival between PH-targeted therapies |
None reported |
| Lange 2014 [42] | Retrospective, non-randomised cohort | n=29 COPD-PH of n=72 WHO Group 3 PH Inclusion criteria: FEV1/FVC <0.7 FEV1 <70% or more than mild CT emphysema RHC mPAP >25 mmHg and PAWP ≤15 mmHg (N=72) Age: 67±9 years (sd) Gender: 68% male FEV1: 70±24% 6MWD: 300±100 m mPAP: 37.3±9.1 mmHg (n=12 severe mPAP ≥35 mmHg) |
PDE-5i (n=29), ERA (n=11), nebulised iloprost (n=6) Note: dual therapy (n=8), triple therapy (n=2) for median 25.5 months Note: PH-targeted therapy in 65% of severe PH and 25% of less severe PH |
COPD-PH subgroup Outcomes with PH-targeted therapy (n=12) versus no therapy (n=17): reduced mortality (HR 0.235, p=0.075) Entire WHO group 3 PH cohort Outcomes with PH-targeted therapy (n=34; including n=26 severe PH) versus no therapy (n=38; including 14 severe PH): reduced mortality (HR 0.262, p=0.004) |
Not reported |
| Girard 2015 [ 37 ] | Retrospective non-randomised cohort analysis of prospective registry data | n=26 Inclusion criteria: Post-bronchodilator FEV1/FVC <0.7 Precapillary PH: RHC mPAP ≥25 and PAWP ≤15 mmHg Severe PH: mPAP >35 mmHg and/or cardiac index 2 L·min−1·m−2 Age: 66±11 years (sd) Gender: 96% male FEV1: 57±20% 6MWD: 212±104 m NT-proBNP: 3205±4250 ng·L−1 Nuclear RVEF: 22±6% mPAP: 48±9 mmHg |
PDE-5i (n=11), ERA (n=11), CCB (n=1), prostanoids (n=2), dual therapy (n=3) for median 6±3 months Note all study participants were on optimal COPD treatment including LTOT |
Outcomes with PH-targeted therapy: RHC (3–12 months post-treatment) mPAP decreased 48±9 to 42±10 mmHg (p=0.008) PVR decreased 8.5±3.0 to 6.6±2.0 WU (p=0.001) TD cardiac index improved 2.4±0.4 to 2.7±0.6 L·min−1·m−2 (p=0.015) Nuclear RVEF increased (p=0.03) No significant differences in mNYHA FC, 6MWD, echo parameters, or NT-proBNP levels |
Decreased SpO2% in n=2 (ERA), leading to discontinuation of study treatment |
| Tanabe 2015 [43] | Multicentre, retrospective cohort study | n=18 COPD-PH of n=70 WHO Group 3 PH Inclusion criteria: RHC mPAP ≥35 mmHg and “normal” PAWP Age: 67±9 years (sd) Gender: 94% male FEV1: 58±33% 6MWD: 263±97 m BNP: 397±608 pg·mL−1 PaO2: 52±16 mmHg mPAP: 47±15 |
78% (n=14) treated with PH-targeted therapy: PDE-5i (n=14), ERA (n=8), beraprost (n=7) for mean 1.9±1.7 years Note: 96% (n=67) of study participants used LTOT |
COPD-PH subgroup Cumulative survival 50%/3 years No change in survival with PDE-5i treatment: 53.6% versus 37.5%/ 3 years (p= 0.56) Entire WHO group 3 PH cohort Improved survival with PDE-5i treatment (multivariate analysis, p=0.01) |
Not reported |
| Brewis 2015 [ 40 ] | Retrospective cohort study | n=40: COPD-PH of n=118 WHO Group 3 PH Inclusion criteria: FEV1/FVC <0.7 either FEV1 <60% or emphysema on CT with FEV1 <80% RHC mPAP ≥35 mmHg and PAWP ≤ 15 mmHg Age: 64±10 years (sd) Gender: 55% male FEV1: 56±16% 6MWD: 216±110 m NT-proBNP: 2169 (median; IQR 769–3919) pg·mL−1 PaO2: 57±10.5 mmHg mPAP: 49±10 mmHg |
Initial: ERA (n=10), PDE-5i (n=26), CCB (n=2), Prostanoid (n=2) for ≥
3 months Note: n=5 on LTOT |
COPD-PH subgroup No change in 6MWD No change in mNYHA FC No change in NT-proBNP Entire WHO group 3 PH cohort No change in 6MWD No change in mNYHA FC NT-proBNP improved (p=0.015) No change in PaO2 |
|
| Calcaianu 2016 [38] | Single centre, retrospective cohort study |
n=28/537 Inclusion criteria: FEV1/FVC <70% and FEV1 >50% RHC mPAP ≥35 mmHg PAWP <15 mmHg Age: 71.2±9.4 years (sd) Gender: 79% male FEV1: 69.3±13.8% 6MWD: 259±104 m BNP: 296±389 ng·L−1 PaO2: 49.6±9.5 mmHg mPAP: 44.2±8.7 mmHg |
Initial: ERA (n=23), PDE-5i (n=1), prostanoid (n=1), CCB (n=1), combination therapy (n=2); for 6–12 months (median 3 years) Note: All study participants used LTOT |
Outcomes with PH-targeted therapy at 6–12 months (n=16/28): PVR decreased 8.4±4.2 to 5.0±1.7 WU (p= 0.008) Cardiac index increased 2.5±0.7 to 3.2±0.6 L·min−1·m−2 (p=0.003) No change in mPAP No change in mNYHA FC (3 months) No change in 6MWD, PaO2 Cumulative survival 57.2% /3 years (sequential combination therapy in n=10) |
No side-effects leading to withdrawal of study treatment |
| Alkhayat 2016 [32] | Parallel group cohort study | n=139: Sildenafil (n=69) versus placebo (n=70) Inclusion criteria: COPD diagnosis: unclear criteria. Echo calculated mPAP ≥25 mmHg 6MWD (100–450) m Age: 48±15.5 years (sd) Gender: 76% male 6MWD: 345.5±84.5 m Calculated mPAP: 45±13 versus 56±16 mmHg |
Sildenafil 20 mg PO TID versus placebo for 12 weeks | Outcomes in Sildenafil versus placebo: Mean placebo-corrected increase in 6MWD 51 m from baseline (p<0.001) Decreased mPAP from baseline 2.1 mmHg (-4.3, 0.0) versus increased 0.6 (-0.8, 2.0; p= 0.04) |
Expected Sildenafil side-effects (e.g. flushing, dyspepsia, and diarrhoea) |
| Vitulo 2016 [30] | Multicentre, RCT | n=28: Sildenafil (n=18) versus placebo (n=10) Inclusion criteria: RHC mPAP ≥30 mmHg for FEV1 >30% post-bronchodilator or RHC mPAP ≥35 mmHg if FEV1 <30% post-bronchodilator PAWP ≤15 mmHg LTOT ≤6 L·min−1 PaCO2 ≤55 mmHg No decrease in PaO2 ≤55 mmHg after first dose of blinded study medication Age: 67.9±8.1 years (sd) Gender: 75% male FEV1: 52.3±23.4% 6MWD: 229.2±101.4 versus 308.5±99.6 m PaO2: 74.3±14.5 mmHg mPAP: 39.2±9.35 mmHg |
Sildenafil 20 mg TID versus placebo for 16 weeks | Outcomes in Sildenafil (n=15/18) versus placebo (n=10): PVR decreased 1.4 WU (p=0.04) Cardiac index increased 0.4 L·min−1·m−2 (p=0.004) Secondary end points: BODE index improved 0.40 units (p=0.02) mMRC dyspnoea improved (0.6 units; p=0.03) No change in 6MWD |
Expected Sildenafil side-effects (e.g. (headache, flushing, myalgia) mild-moderate in n=5; no interruption of study treatment; no difference in SpO2 between groups |
| Shrestha 2017 [31] | Non-placebo RCT | n=72: Sildenafil (n=36) versus standard medical therapy (n=36) Inclusion criteria: echo sPAP >36 mmHg Age: 64.2±5 years Gender: not specified FEV1: 46.1±12.8% 6MWD: 183±78 m mPAP: 71.3±14.7 mmHg (variance not defined) |
Sildenafil 25 mg PO TID versus standard medical therapy for 4 weeks Note: LTOT permitted |
Outcomes in Sildenafil (n=30) versus standard medical therapy (n=31): Decreased sPAP 9.9±7.8 versus 5.9±7.4 mmHg (p=0.048) Increased 6MWD 48±26 versus 33±33 m (p=0.047) Secondary outcomes: Decreased mMRC (p=0.037) No difference in mNYHA FC, BDI |
Expected Sildenafil side-effects (e.g. flushing, diarrhoea, syncope), no interruption of study treatment. No difference in SpO2 between groups |
Data are mean±sem unless otherwise specified. 6MWD: 6-minute walk distance (m); BDI: Borg dyspnoea index; BID, twice daily; BNP, brain natriuretic peptide; BODE index: Body mass index, Obstruction by FEV1, Dyspnoea by mMRC grade, and Exercise capacity by 6MWD; CCB, calcium channel blocker; CT, computed tomography; ERA, endothelin receptor antagonist; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR: hazard ratio; HRQoL: health-related quality of life; IQR, interquartile range; ISWD, incremental shuttle walk distance; LTOT: long-term oxygen therapy; mMRC, modified Medical Research Council; mNYHA FC, modified New York Heart Association functional class; mPAP: mean pulmonary artery pressure; NT-proBNP, N-terminal propeptide of brain natriuretic peptide; PAAT, pulmonary artery acceleration time; PaCO2; partial pressure of carbon dioxide in arterial blood; PaO2: partial pressure of oxygen in arterial blood; PAWP, pulmonary arterial wedge pressure; PDE-5i, phosphodiesterase type 5 inhibitors; PH: pulmonary hypertension; PO: by mouth; PVR: pulmonary vascular resistance; RCT: randomised controlled trial; RHC: right heart catheterisation; RVEF: right ventricular ejection fraction; SaO2, arterial oxygen saturation (%); SGRQ, St George's Respiratory Questionnaire; SC, subcutaneous; sPAP, systolic pulmonary arterial pressure (mmHg); SpO2: transcutaneous pulse oximetry oxygen saturation (%); TID, three times a day; WHO: World Health Organization; WU: Wood unit (mmHg·L−1·min−1).
Long-term oxygen therapy
In COPD-PH patients, Long-term oxygen therapy (LTOT) may have haemodynamic and clinical benefits. The evidence base consists of eight reports (n=596; 72–100% men), including one RCT [14], two randomised parallel group studies comparing LTOT versus nocturnal oxygen therapy (NOT), and four case series [15–18] (table 1). All patients underwent RHC which documented the presence and severity of baseline PH. Most studies report outcome data over longer than 1 year (range 2–6 years), but two studies were <8 weeks in duration [15, 19]. Most LTOT studies had an unclear or high risk of bias in at least one domain; only one study had a low risk of bias (supplementary tables S5 and S6) [20], which limits our confidence in the effects of LTOT in COPD-PH.
The haemodynamic benefit of LTOT varied, with small reductions (3–5 mmHg) in mPAP in four of eight studies [15, 16, 19, 21], and/or PVR in three [19–21], but no reported change in CO (three studies). Even in the absence of actual improvement in the severity of PH, LTOT may be associated with less progression of PH over time [14, 16]. For example, a progressive increase in mPAP in control patients was completely attenuated in LTOT patients in the Medical Research Council (MRC) trial [14].
No studies assessed clinical or functional patient outcomes other than mortality benefits of LTOT. Survival was assessed in four studies (n=480), of which three (n=408) reported improved survival [14, 20, 21], but one study found no effect [17]. Pulmonary haemodynamic improvement may be associated with greater survival [20, 21], but this was not consistently observed [22].
In summary, in COPD-PH patients with hypoxaemia, LTOT may mildly reduce severity of PH, slow PH progression over time, and reduce mortality, but without any other clinical or functional benefit (table 3). There are limited, conflicting data on NOT, with haemodynamic benefit in only one of two RCTs [22, 23], and no clinical benefits in either.
TABLE 3.
Summary of outcomes in treatment of COPD-associated pulmonary hypertension (PH)
| Treatment | PH outcomes | Clinical outcomes | |||||
| Cardiopulmonary haemodynamic | RV function | Symptoms | Functional capacity | HRQoL | Hospitalisation | Survival | |
| Oxygen (n=4) | |||||||
| LTOT (n=8) | + | NA | NA | NA | NA | NA | + |
| NOT (n=2) | +/− | NA | NA | NA | NA | NA | 0 |
| CCBs (n=4) | |||||||
| Nifedipine (n=3) | 0 | NA | + | NA | NA | NA | 0 |
| Felodipine (n=1) | + | NA | NA | 0 | NA | NA | NA |
| PH-targeted therapy (n=9) | |||||||
| PDE type 5 inhibitors | |||||||
| Sildenafil (n=5) | + | NA | +/− | +/− | +/− | NA | NA |
| Tadalafil (n=1) | + | NA | 0 | 0 | 0 | NA | NA |
| ERA | |||||||
| Bosentan (n=2) | +/− | NA | + | +/− | + | NA | NA |
| Ambrisentan (n=1) | NA | + | +/− | 0 | NA | NA | NA |
| Statins (n=6) | |||||||
| Atorvastatin (n=4) | + | 0 | NA | 0 | NA | NA | NA |
| Rosuvastatin (n=1) | + | 0 | 0 | + | 0 | NA | NA |
| Pravastatin (n=1) | + | NA | + | + | NA | NA | NA |
RV: right ventricular; HRQoL: health-related quality of life; LTOT: long-term oxygen therapy; NOT: nocturnal oxygen therapy; CCB: calcium channel blocker; PDE: phosphodiesterase; ERA: endothelin receptor antagonist. Clinically relevant effects: +: significant; +/−: uncertain; 0: none; NA: not assessed.
Calcium channel blockers
Four studies defining PH using mPAP threshold of 20 mmHg, including two RCTs (n=80) [24, 25] and two case series [26, 27], evaluated effects of calcium channel blockers (CCBs) over at least 8 weeks (supplementary table S2). All studies had an unclear or high risk of bias in at least one domain. Two small studies found no RHC-assessed haemodynamic benefit of nifedipine [25, 26], but felodipine decreased echo-calculated mPAP and total pulmonary resistance (TPR) as well as increased CO in a case-series [27]. Only one study assessed symptoms, reporting decreased dyspnoea scores, but found no difference in survival [24]. Another study reported no change in exercise capacity [27]. Side-effects of CCBs were common and many patients required dose reduction (50%) and/or withdrawal of therapy (7–27%).
In summary, based on limited evidence, CCBs may mildly improve haemodynamics with no evidence to suggest any clinical or survival benefits, and they are generally poorly tolerated (table 3).
PAH-targeted medications
Based on strong benefits in the treatment of PAH, 15 reports describe potential benefits of PAH-targeted therapies, including oral phosphodiesterase type 5 inhibitors (PDE-5i), oral endothelin receptors antagonists (ERAs), and prostanoids, in patients with COPD-PH (table 2).
PDE-5i
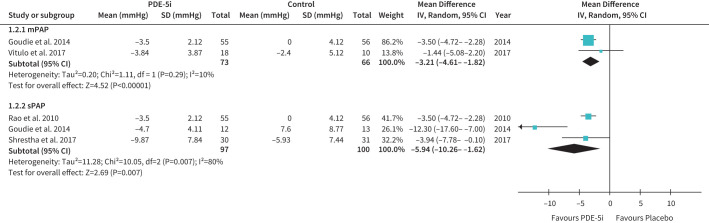
Six studies (n=459) assessed effects of PDE-5i, including sildenafil (5 studies) [28–32] and tadalafil [33]. In five studies, PH was echo-defined using variable thresholds (sPAP >30–40 mmHg) [28, 29, 31, 33], whereas a single study variably defined PH by RHC (mPAP >30–35 mmHg), depending on FEV11% predicted [30]. Three studies had a low risk of bias, one RCT was unclear [28], and two had a high risk of bias [31, 32]. All five studies assessing haemodynamics reported benefits of PDE-5i. Sildenafil improved echo-sPAP [28, 31], echo-calculated mPAP [32], and RHC-mPAP [30], and tadalafil improved both echo-sPAP and calculated mPAP [33]. Pooled analyses showed favourable effects on both sPAP and mPAP (figure 2).
FIGURE 2.
The effect of treatment with phosphodiesterase type 5 inhibitors (PDE-5i) on mean pulmonary artery pressure (mPAP; upper panel) and systolic pulmonary artery pressure (sPAP; lower panel) in COPD-associated pulmonary hypertension. Note: mPAP was measured by right heart catheterisation (Vitulo 2017) or estimated from echo measurement of sPAP (Goudie 2014). IV: inverse variance.
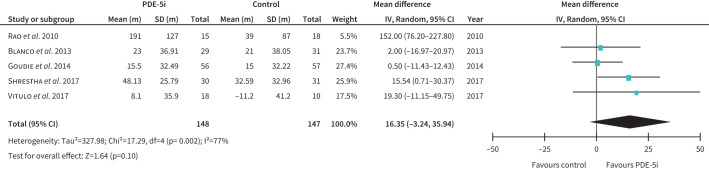
Of six studies assessing functional capacity [28–33], sildenafil improved six-minute walk distance (6 MWD) in two RCTs [28, 31] and one cohort study [32] but had no effect in two other RCTs [29, 30]. The one study of tadalafil showed a similar lack of benefit [33]. The pooled analysis of 6 MWD showed no clear benefit with a trend towards improvement (figure 3). PDE-5i were generally well-tolerated with expected side-effects and did not worsen hypoxaemia.
FIGURE 3.
The effect of treatment with phosphodiesterase type 5 inhibitors (PDE-5i) on 6-min walk distance in patients with COPD-associated pulmonary hypertension (PH). Note: PH was diagnosed either by right heart catheterisation (Vitulo 2017) or by echocardiogram in the other studies. IV: inverse variance.
There were inconsistent benefits in HRQoL in four RCTs using different measurement tools [29–31, 33]. Sildenafil improved mMRC dyspnoea [30, 31], 36-item Short Form survey (SF-36) score, and the multi-parameter COPD BODE index (body mass index, obstruction by FEV1, mMRC dyspnoea, and 6 MWD) [30], but not HRQoL in an unspecified questionnaire [29]. Tadalafil had no effect using different scores (SF-36, SGRQ, MLHFQ) [33].
In summary, PDE-5i significantly improved haemodynamics in COPD-PH patients, but this did not translate to clinical, functional, or HRQoL benefits (table 3).
ERAs
Two placebo-controlled RCTs assessed the effects of bosentan in severe COPD. In a non-blinded study in RHC-diagnosed moderate to severe PH, bosentan had mild haemodynamic benefit associated with improved exercise capacity and limited symptomatic benefit [34]. In contrast, bosentan had inconsistent haemodynamic effects, uncertain clinical benefits (6 MWD fell slightly, HRQoL improved), and reduced PaO2 in mild echo-defined PH [35]. Ambrisentan treatment in a case series (n=24) of RHC-diagnosed severe PH decreased brain natriuretic peptide (BNP) with no change in 6 MWD [36]. Two studies had a high risk of bias and one RCT had a low risk of bias [35].
In summary, ERAs have limited haemodynamic and uncertain clinical benefits in COPD-PH patients.
Studies of multiple PAH-targeted therapies
Four retrospective cohort studies assessed the effects of multiple PAH-targeted therapies individually in RHC-defined COPD-PH [37–40], reporting haemodynamic improvement with no clinical or functional benefits [37, 38], or no effects at all [39, 40]. Three other retrospective cohort studies [41–43] reported no survival benefit of PAH-targeted therapies in various combinations in RHC-defined PH, but one found short-term clinical (improved New York Heart Association (NYHA) functional class) and functional (improved 6 MWD) benefits up to 1 year which were not sustained at 2 years [41]. Three studies suggested greater improvements with PAH-targeted therapy in patients with more severe PH, including greater RHC-measured haemodynamic effects [37, 38, 41], and one showed clinical and functional benefits up to 1 year [41]. Risk of bias was high for six of seven studies of multiple PAH-targeted therapies, and unclear for one study, which limits confidence in the results.
In summary, combination PAH-targeted therapy does not improve survival but may offer some transient clinical and/or functional benefits. Patients with objective “response” to therapy, including improved mNYHA FC or PVR, may have improved survival [39].
Statins
Statins are widely used in COPD due to the prevalence of cardiovascular diseases and were used for treatment of COPD-PH in six studies (n=394; supplementary table S3), including five RCTs using echo-defined PH [44–48] and one RHC-defined PH cohort study [49]. Only one study had low risk of bias [44], but the other five studies had an unclear risk of bias.
Three RCTs showed statins decreased echo sPAP at rest [46–48] or during exercise [44], whereas another RCT showed no change [45]. Clinical and functional outcomes were infrequently assessed, and changes in dyspnoea, HRQoL, and functional capacity are inconsistent [44, 45, 47].
In summary, statins are well-tolerated, significantly reduced sPAP (figure 4) but had no clinical or functional benefits.
FIGURE 4.
The effect of treatment with atorvastatin on systolic pulmonary artery pressure in COPD-associated pulmonary hypertension (PH). Note: PH was diagnosed by echocardiogram in all studies. IV: inverse variance.
Other therapies
Single studies have reported on several miscellaneous, non-traditional potential therapies in patients with COPD-PH (supplementary table S4) [50–52]. Some therapies demonstrated improved pulmonary haemodynamics at rest (e.g. Dipyridamole [53], cicletanine [54], ACE inhibitors [55, 56], inhaled nitric oxide (iNO) [57]) or on exercise (e.g. Waon therapy [58]), reduced dyspnoea (e.g. Waon therapy [58]), and/or improved exercise capacity (e.g. iNO [57]), whereas many other therapies had no reported benefits. Combinations of such therapies may improve multiple parameters; for example, combination of azithromycin, simvastatin, and LTOT decreased RHC sPAP and increased 6 MWD [59].
Discussion
Our systematic review focuses on the effect of various therapeutic options in COPD-PH. We identified studies that focused on treatment of COPD-PH for at least 4 weeks and captured haemodynamics and clinical outcomes including survival. Overall, many treatments improve PH haemodynamics and some may improve survival, but few are associated with improved symptoms, functional capacity, or HRQoL. For example, supplemental LTOT mildly reduces PH haemodynamic severity, may slow PH progression over time, and reduces mortality. However, other clinical and functional benefits of LTOT were not assessed. Similarly, PAH-targeted therapy using sildenafil improved PH haemodynamics, but had uncertain clinical and functional benefits. In contrast, other PAH-targeted medications, such as ERAs, had inconsistent effects, as did other therapies including CCBs and statins.
The presence and severity of PH in COPD patients is a significant contributor to clinical morbidity, including worse dyspnoea, functional capacity, and HRQoL [5, 6, 30], as well as being a prognostic marker for more frequent exacerbations and worse survival. However, there are no specific treatments for COPD-PH, and current guidelines for management of WHO group 3 PH, including COPD-PH, simply suggest LTOT for resting hypoxaemia and optimisation of underlying chronic cardiopulmonary conditions [4, 13].
COPD-PH is believed to be largely the result of hypoxaemia. As such, LTOT could be effective in the treatment of hypoxaemic COPD-PH. The data suggest mild improvements in severity of PH, some evidence for slowing progression of PH, and importantly, improved survival. However, oxygen did not normalise mPAP and there were no other symptomatic or functional clinical benefits reported. As for NOT, the limited available data shows no clear benefits in COPD-PH patients with either daytime or isolated nocturnal hypoxaemia. We did not find studies that assessed the long-term effect of supplemental oxygen in COPD-PH patients with exertional hypoxaemia.
Besides hypoxaemia, COPD-PH may also be driven through other potential mechanisms [60], including pathophysiologic features similar to PAH, including pulmonary micro-vessel rarefaction and endothelial dysfunction, for example decreased expression of endothelial nitric oxide synthetase (eNOS) [3, 4, 60]. Thus, PAH-targeted therapy may have a potential role in COPD-PH management. However, guidelines generally recommend against PAH-targeted therapy for mild to moderate WHO group 3 PH, including COPD-PH [13, 61].
In our systematic review, PAH-targeted therapy in patients with COPD-PH had inconsistent effects, including limited clinical benefits (for example, symptoms, functional capacity, HRQoL) but no assessment of hospitalisation or survival. Overall, our findings are similar to other analyses [4, 62, 63]. Some PAH-targeted medications may offer benefits, as PDE-5i (sildenafil and tadalafil) significantly improved pulmonary haemodynamics, and sildenafil improved mMRC [30, 31], BODE index, and SF-36 [30]. In our pooled analysis, 6 MWD increased slightly but not significantly with PDE-5i treatment (+16 m; figure 3), which was less than the significant pooled effect of sildenafil on 6 MWD (+29 m) in another review of COPD-PH [64]. Differences include our inclusion of a negative trial on tadalafil, possibly due to an ineffective small dose [33], and exclusion of several positive studies from China. Comparatively, there are fewer studies of other PAH-targeted therapies such as ERAs, but similar overall limited clinical benefits despite some haemodynamic effects. Combination PAH-targeted therapy is now standard of care in PAH [13, 61], but there are limited data in COPD-PH to suggest any benefit.
Interestingly, an objective “response” to PAH-targeted therapy (PDE-5i or ERA), as characterised by improved mNYHA FC or PVR (>20% fall), was predictive of better survival [39]. Furthermore, some COPD patients with more severe PH, generally defined as mPAP≥35 mmHg, may respond better to PAH-targeted therapy [37, 38, 41]. A subset of COPD patients with this severe precapillary PH and possibly RV failure, often in the setting of only mild to moderate COPD has been labelled, and may reflect a “vascular” phenotype [65] that may be at particularly high risk of long-term PH-related morbidity and mortality [5, 66]. This group of patients may have a genetic predisposition to PH, similar to heritable PAH, which may become manifest in the context of COPD, either driven by hypoxaemia, cigarette smoke, airway or systemic inflammation [60, 65], or simply due to concurrent COPD and unrelated PAH. This subset of COPD patients merits further study and may benefit clinically from referral to expert PH centres for further assessment and consideration of treatment [4, 13].
Concerns over potential risks of PAH-targeted therapies worsening ventilation/perfusion matching and hypoxaemia because of non-selective widespread pulmonary vasodilation are not supported by any evidence for any adverse effect on oxygenation [29–31, 33, 34]. Expected side-effects of PAH-targeted therapy were observed, for examplec flushing, headache, diarrhoea, but did not lead to high rates of medication discontinuation.
Among other treatment options, CCBs may mildly improve haemodynamics, but there is no evidence to suggest any clinical or survival benefits, and they are generally poorly tolerated. Statins reduced sPAP (mPAP in one study) but had limited clinical benefits. Although the statin effect in PH could be mediated through systemic vascular and/or left-ventricular effects rather than direct pulmonary vascular action, a multiple regression analysis suggested statins reduce mPAP independent of pulmonary artery wedge pressure [49]. Statins may also prevent COPD progression and improve PH by reducing C-reactive protein and other inflammatory factors [67]. Several other therapies (e.g. iNO, Waon, cicletanine) improved pulmonary haemodynamics with minimal clinical benefits.
Limitations of this review include paucity of RHC diagnosed PH, as only some studies reported RHC-mPAP, whereas most studies only reported echo-estimated sPAP ± calculated mPAP. A systemic vascular effect of a putative treatment could result in apparent pulmonary haemodynamic benefit as assessed simply by echocardiogram, for example, a decrease in sPAP with statins. Moreover, studies used various thresholds for both RHC and echo measurements to define presence of PH. In addition, study populations exhibited marked heterogeneity, including severity of COPD and presence of hypoxaemia. There was also treatment heterogeneity, as studies used various doses and duration of therapy, and in some studies of combination PAH-targeted therapies, specific combinations were not clearly defined. Most importantly, very few studies provided a comprehensive assessment of the potential benefits of PAH-targeted therapies, including multi-parameter characterisation of haemodynamic, clinical, and functional benefits.
In conclusion, this systematic review identifies the large number of studies assessing multiple treatments for patients with COPD-PH and highlights the limited evidence base. This review supports recent guidelines which recommend LTOT in hypoxaemic COPD-PH patients but do not recommend other treatments for COPD-PH, including PAH-targeted medications. Development of future therapies depends upon new ideas on the pathobiology of COPD-PH, as well as higher-quality studies on more homogeneous populations, including patients with more severe PH or a “vascular” phenotype, using a standardised RHC diagnosis of PH and comprehensive assessment of outcomes.
Footnotes
Provenance: Submitted article, peer reviewed.
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: R. Arif has nothing to disclose.
Conflict of interest: A. Pandey has nothing to disclose.
Conflict of interest: Y. Zhao has nothing to disclose.
Conflict of interest: K. Arsenault-Mehta has nothing to disclose.
Conflict of interest: D. Khoujah has nothing to disclose.
Conflict of interest: S. Mehta reports grants or contracts from Altavant Pharmaceuticals, Eiger Pharmaceuticals, Ikaria Pharmaceuticals, Janssen Pharmaceuticals, Reata Pharmaceuticals, and United Therapeutics; consulting fees from Acceleron Pharmaceuticals, Janssen Pharmaceuticals and Natco Pharmaceuticals; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer Pharmaceuticals, Janssen Pharmaceuticals, Natco Pharmaceuticals and SpecialtyRx Pharmacy; payment for expert testimony from Bergeron Clifford LLP, Lerner Law and St. Lawrence Barristers LLP; support for attending meetings and/or travel from Janssen Pharmaceuticals; participation on a data safety monitoring or advisory board for Ozmosis Research; board directorship for the Pulmonary Hypertension Association of Canada (unpaid position); and receipt of equipment, materials, drugs, medical writing, gifts or other services from Janssen Pharmaceuticals, all outside the submitted work.
References
- 1.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 2.Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet 2017; 389: 1931–1940.doi: 10.1016/S0140-6736(17)31222-9 [DOI] [PubMed] [Google Scholar]
- 3.Gredic M, Blanco I, Kovacs G, et al. Pulmonary hypertension in chronic obstructive pulmonary disease. Br J Pharmacol 2020; 178: 132–151. doi: 10.1111/bph.14979 [DOI] [PubMed] [Google Scholar]
- 4.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1801914.doi: 10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 189–194. doi: 10.1164/rccm.200401-006OC [DOI] [PubMed] [Google Scholar]
- 6.Hilde JM, Skjørten I, Hansteen V, et al. Haemodynamic responses to exercise in patients with COPD. Eur Respir J 2013; 41: 1031–1041. doi: 10.1183/09031936.00085612 [DOI] [PubMed] [Google Scholar]
- 7.Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest 1995; 107: 1193–1198. doi: 10.1378/chest.107.5.1193 [DOI] [PubMed] [Google Scholar]
- 8.Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: Suppl. 25, D109-D116. doi: 10.1016/j.jacc.2013.10.036 [DOI] [PubMed] [Google Scholar]
- 9.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012; 367: 913–921. doi: 10.1056/NEJMoa1203830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 2005; 127: 1531–1536. doi: 10.1378/chest.127.5.1531 [DOI] [PubMed] [Google Scholar]
- 11.Scharf SM, Iqbal M, Keller C, et al. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med 2002; 166: 314–322. doi: 10.1164/rccm.2107027 [DOI] [PubMed] [Google Scholar]
- 12.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirani N, Brunner NW, Kapasi A, et al. Canadian Cardiovascular Society/Canadian Thoracic Society position statement on pulmonary hypertension. Can J Cardiol 2020; 36: 977–992. doi: 10.1016/j.cjca.2019.11.041 [DOI] [PubMed] [Google Scholar]
- 14.Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet 1981; 1: 681–686. [PubMed] [Google Scholar]
- 15.Gluskowski J, Jedrzejewska-Makowska M, Hawryłkiewicz I, et al. Effects of prolonged oxygen therapy on pulmonary hypertension and blood viscosity in patients with advanced cor pulmonale. Respiration 1983; 44: 177–183. doi: 10.1159/000194546 [DOI] [PubMed] [Google Scholar]
- 16.Weitzenblum E, Sautegeau A, Ehrhart M, et al. Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1985; 131: 493–498. doi: 10.1164/arrd.1985.131.4.493 [DOI] [PubMed] [Google Scholar]
- 17.Cooper CB, Waterhouse J, Howard P. Twelve year clinical study of patients with hypoxic cor pulmonale given long term domiciliary oxygen therapy. Thorax 1987; 42: 105–110. doi: 10.1136/thx.42.2.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zieliński J, Tobiasz M, Hawryłkiewicz I, et al. Effects of long-term oxygen therapy on pulmonary hemodynamics in COPD patients: a 6-year prospective study. Chest 1998; 113: 65–70. doi: 10.1378/chest.113.1.65 [DOI] [PubMed] [Google Scholar]
- 19.Stark RD, Finnegan P, Bishop JM. Daily requirement of oxygen to reverse pulmonary hypertension in patients with chronic bronchitis. Br Med J 1972; 3: 724–728. doi: 10.1136/bmj.3.5829.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 1980; 93: 391–398. doi: 10.7326/0003-4819-93-3-391 [DOI] [PubMed] [Google Scholar]
- 21.Timms RM, Khaja FU, Williams GW. Hemodynamic response to oxygen therapy in chronic obstructive pulmonary disease. Ann Intern Med 1985; 102: 29–36. doi: 10.7326/0003-4819-102-1-29 [DOI] [PubMed] [Google Scholar]
- 22.Fletcher EC, Luckett RA, Goodnight-White S, et al. A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mm Hg. Am Rev Respir Dis 1992; 145: 1070–1076. doi: 10.1164/ajrccm/145.5.1070 [DOI] [PubMed] [Google Scholar]
- 23.Chaouat A, Weitzenblum E, Kessler R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J 1999; 14: 1002–1008. doi: 10.1183/09031936.99.14510029 [DOI] [PubMed] [Google Scholar]
- 24.Vestri R, Philip-Joet F, Surpas P, et al. One-year clinical study on nifedipine in the treatment of pulmonary hypertension in chronic obstructive lung disease. Respiration 1988; 54: 139–144. doi: 10.1159/000195514 [DOI] [PubMed] [Google Scholar]
- 25.Saadjian AY, Philip-Joet FF, Vestri R, et al. Long-term treatment of chronic obstructive lung disease by Nifedipine: an 18-month haemodynamic study. Eur Respir J 1988; 1: 716–720. [PubMed] [Google Scholar]
- 26.Agostoni P, Doria E, Galli C, et al. Nifedipine reduces pulmonary pressure and vascular tone during short- but not long-term treatment of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1989; 139: 120–125. doi: 10.1164/ajrccm/139.1.120 [DOI] [PubMed] [Google Scholar]
- 27.Sajkov D, McEvoy RD, Cowie RJ, et al. Felodipine improves pulmonary hemodynamics in chronic obstructive pulmonary disease. Chest 1993; 103: 1354–1361. doi: 10.1378/chest.103.5.1354 [DOI] [PubMed] [Google Scholar]
- 28.Rao RS, Singh S, Sharma BB, et al. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci 2011; 53: 81–85. [PubMed] [Google Scholar]
- 29.Blanco I, Santos S, Gea J, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J 2013; 42: 982–992. doi: 10.1183/09031936.00176312 [DOI] [PubMed] [Google Scholar]
- 30.Vitulo P, Stanziola A, Confalonieri M, et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: A randomized controlled multicenter clinical trial. J Heart Lung Transplant 2017; 36: 166–174. doi: 10.1016/j.healun.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 31.Shrestha SK, Srivastava B, Karki M, et al. Effect of sildenafil citrate on pulmonary arterial systolic pressure and sub-maximal exercise capacity in chronic obstructive pulmonary disease. Kathmandu Univ Med J 2017; 15: 271–278. [PubMed] [Google Scholar]
- 32.Alkhayat K, Eid M. Sildenafil citrate therapy for secondary pulmonary arterial hypertension due to chronic obstructive lung disease. Egyptian J Chest Dis Tuberculosis 2016; 65: 805–809. doi: 10.1016/j.ejcdt.2016.05.005 [DOI] [Google Scholar]
- 33.Goudie AR, Lipworth BJ, Hopkinson PJ, et al. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med 2014; 2: 293–300. doi: 10.1016/S2213-2600(14)70013-X [DOI] [PubMed] [Google Scholar]
- 34.Valerio G, Bracciale P, Grazia DAA. Effect of bosentan upon pulmonary hypertension in chronic obstructive pulmonary disease. Ther Adv Respir Dis 2009; 3: 15–21. doi: 10.1177/1753465808103499 [DOI] [PubMed] [Google Scholar]
- 35.Stolz D, Rasch H, Linka A, et al. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J 2008; 32: 619–628. doi: 10.1183/09031936.00011308 [DOI] [PubMed] [Google Scholar]
- 36.Badesch DB, Feldman J, Keogh A, et al. ARIES-3: ambrisentan therapy in a diverse population of patients with pulmonary hypertension. Cardiovasc Ther 2012; 30: 93–99. doi: 10.1111/j.1755-5922.2011.00279.x [DOI] [PubMed] [Google Scholar]
- 37.Girard A, Jouneau S, Chabanne C, et al. Severe pulmonary hypertension associated with COPD: hemodynamic improvement with specific therapy. Respiration 2015; 90: 220–228. doi: 10.1159/000431380 [DOI] [PubMed] [Google Scholar]
- 38.Calcaianu G, Canuet M, Schuller A, et al. Pulmonary arterial hypertension-specific drug therapy in COPD patients with severe pulmonary hypertension and mild-to-moderate airflow limitation. Respiration 2016; 91: 9–17. doi: 10.1159/000441304 [DOI] [PubMed] [Google Scholar]
- 39.Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J 2013; 41: 1292–1301. doi: 10.1183/09031936.00079512 [DOI] [PubMed] [Google Scholar]
- 40.Brewis MJ, Church AC, Johnson MK, et al. Severe pulmonary hypertension in lung disease: phenotypes and response to treatment. Eur Respir J 2015; 46: 1378–1389. doi: 10.1183/13993003.02307-2014 [DOI] [PubMed] [Google Scholar]
- 41.Fossati L, Müller-Mottet S, Hasler E, et al. Long-term effect of vasodilator therapy in pulmonary hypertension due to COPD: a retrospective analysis. Lung 2014; 192: 987–995. doi: 10.1007/s00408-014-9650-1 [DOI] [PubMed] [Google Scholar]
- 42.Lange TJ, Baron M, Seiler I, et al. Outcome of patients with severe PH due to lung disease with and without targeted therapy. Cardiovasc Ther 2014; 32: 202–208. doi: 10.1111/1755-5922.12084 [DOI] [PubMed] [Google Scholar]
- 43.Tanabe N, Taniguchi H, Tsujino I, et al. Multi-institutional retrospective cohort study of patients with severe pulmonary hypertension associated with respiratory diseases. Respirology 2015; 20: 805–812. doi: 10.1111/resp.12530 [DOI] [PubMed] [Google Scholar]
- 44.Lee TM, Chen CC, Shen HN, et al. Effects of pravastatin on functional capacity in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Clin Sci (Lond) 2009; 116: 497–505. doi: 10.1042/CS20080241 [DOI] [PubMed] [Google Scholar]
- 45.Moosavi SA, Raji H, Faghankhani M, et al. Evaluation of the effects of atorvastatin on the treatment of secondary pulmonary hypertension due to chronic obstructive pulmonary diseases: a randomized controlled trial. Iran Red Crescent Med J 2013; 15: 649–654. doi: 10.5812/ircmj.8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu HF, Qi XW, Ma LL, et al. Atorvastatin improves endothelial progenitor cell function and reduces pulmonary hypertension in patients with chronic pulmonary heart disease. Exp Clin Cardiol 2013; 18: e40–e43. [PMC free article] [PubMed] [Google Scholar]
- 47.Chogtu B, Kuriachan S, Magazine R, et al. A prospective, randomized study: evaluation of the effect of rosuvastatin in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Indian J Pharmacol 2016; 48: 503–508. doi: 10.4103/0253-7613.190721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arian A, Moghadam SG, Kazemi T, et al. The effects of statins on pulmonary artery pressure in patients with chronic obstructive pulmonary disease: a randomized controlled trial. J Res Pharm Pract 2017; 6: 27–30. doi: 10.4103/2279-042X.200985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reed RM, Iacono A, DeFilippis A, et al. Statin therapy is associated with decreased pulmonary vascular pressures in severe COPD. COPD 2011; 8: 96–102. doi: 10.3109/15412555.2011.558545 [DOI] [PubMed] [Google Scholar]
- 50.Schonhofer B, Barchfeld T, Wenzel M, et al. Long term effects of non-invasive mechanical ventilation on pulmonary haemodynamics in patients with chronic respiratory failure. Thorax 2001; 56: 524–528. doi: 10.1136/thx.56.7.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrell NW, Higham MA, Phillips PG, et al. Pilot study of losartan for pulmonary hypertension in chronic obstructive pulmonary disease. Respir Res 2005; 6: 88. doi: 10.1186/1465-9921-6-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fallahi MJ, Ghayumi SM, Moarref AR. Effects of pentoxifylline on oxygenation and exercise tolerance in patients with severe chronic obstructive pulmonary disease. Iran J Med Sci 2013; 38: Suppl. 2, 163–168. [PMC free article] [PubMed] [Google Scholar]
- 53.Nenci GG, Berrettini M, Todisco T, et al. Effects of dipyridamole on the hypoxemic pulmonary hypertension of patients with chronic obstructive pulmonary disease. Respiration 1988; 53: 13–19. doi: 10.1159/000195390 [DOI] [PubMed] [Google Scholar]
- 54.Saadjian A, Philip-Joët F, Paganelli F, et al. Long-term effects of cicletanine on secondary pulmonary hypertension. J Cardiovasc Pharmacol 1998; 31: 364–371. doi: 10.1097/00005344-199803000-00006 [DOI] [PubMed] [Google Scholar]
- 55.Pison CM, Wolf JE, Levy PA, et al. Effects of captopril combined with oxygen therapy at rest and on exercise in patients with chronic bronchitis and pulmonary hypertension. Respiration 1991; 58: 9–14. doi: 10.1159/000195888 [DOI] [PubMed] [Google Scholar]
- 56.Martiniuc C, Braniste A, Braniste T. Angiotensin converting enzyme inhibitors and pulmonary hypertension. Rev Med Chir Soc Med Nat Iasi 2012; 116: 1016–1020. [PubMed] [Google Scholar]
- 57.Vonbank K, Ziesche R, Higenbottam TW, et al. Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax 2003; 58: 289–293. doi: 10.1136/thorax.58.4.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umehara M, Yamaguchi A, Itakura S, et al. Repeated waon therapy improves pulmonary hypertension during exercise in patients with severe chronic obstructive pulmonary disease. J Cardiol 2008; 51: 106–113. doi: 10.1016/j.jjcc.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 59.Wang P, Yang J, Yang Y, et al. Effect of azithromycin in combination with simvastatin in the treatment of chronic obstructive pulmonary disease complicated by pulmonary arterial hypertension. Pak J Med Sci 2017; 33: 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blanco I, Piccari L, Barberà JA. Pulmonary vasculature in COPD: the silent component. Respirology 2016; 21: 984–994. doi: 10.1111/resp.12772 [DOI] [PubMed] [Google Scholar]
- 61.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. doi: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prins KW, Duval S, Markowitz J, et al. Chronic use of PAH-specific therapy in World Health Organization Group III Pulmonary Hypertension: a systematic review and meta-analysis. Pulm Circ 2017; 7: 145–155. doi: 10.1086/690017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X, Tang S, Liu K, et al. Therapy in stable chronic obstructive pulmonary disease patients with pulmonary hypertension: a systematic review and meta-analysis. J Thorac Dis 2015; 7: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hao Y, Zhu Y, Mao Y, et al. Efficacy and safety of Sildenafil treatment in pulmonary hypertension caused by chronic obstructive pulmonary disease: a meta-analysis. Life Sci 2020; 257: 118001.doi: 10.1016/j.lfs.2020.118001 [DOI] [PubMed] [Google Scholar]
- 65.Kovacs G, Agusti A, Barberà JA, et al. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Is there a pulmonary vascular phenotype? Am J Respir Crit Care Med 2018; 198: 1000–1011. doi: 10.1164/rccm.201801-0095PP [DOI] [PubMed] [Google Scholar]
- 66.Boerrigter BG, Bogaard HJ, Trip P, et al. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest 2012; 142: 1166–1174. doi: 10.1378/chest.11-2798 [DOI] [PubMed] [Google Scholar]
- 67.Lu Y, Chang R, Yao J, et al. Effectiveness of long-term using statins in COPD - a network meta-analysis. Respir Res 2019; 20: 17. doi: 10.1186/s12931-019-0984-3 [DOI] [PMC free article] [PubMed] [Google Scholar]