Abstract
Background
Glucagon-like peptide-1 receptor agonists (GLP1RA) augment glucose-dependent insulin release and reduce glucagon secretion and gastric emptying, enabling their successful development for the treatment of type 2 diabetes (T2D). These agents also inhibit food intake and reduce body weight, fostering investigation of GLP1RA for the treatment of obesity.
Scope of review
Here I discuss the physiology of Glucagon-like peptide-1 (GLP-1) action in the control of food intake in animals and humans, highlighting the importance of gut vs. brain-derived GLP-1 for the control of feeding and body weight. The widespread distribution and function of multiple GLP-1 receptor (GLP1R) populations in the central and autonomic nervous system are outlined, and the importance of pathways controlling energy expenditure in preclinical studies vs. reduction of food intake in both animals and humans is highlighted. The relative contributions of vagal afferent pathways vs. GLP1R+ populations in the central nervous system for the physiological reduction of food intake and the anorectic response to GLP1RA are compared and reviewed. Key data enabling the development of two GLP1RA for obesity therapy (liraglutide 3 mg daily and semaglutide 2.4 mg once weekly) are discussed. Finally, emerging data potentially supporting the combination of GLP-1 with additional peptide epitopes in unimolecular multi-agonists, as well as in fixed-dose combination therapies, are highlighted.
Major conclusions
The actions of GLP-1 to reduce food intake and body weight are highly conserved in obese animals and humans, in both adolescents and adults. The well-defined mechanisms of GLP-1 action through a single G protein-coupled receptor, together with the extensive safety database of GLP1RA in people with T2D, provide reassurance surrounding the long-term use of these agents in people with obesity and multiple co-morbidities. GLP1RA may also be effective in conditions associated with obesity, such as cardiovascular disease and non-alcoholic steatohepatitis (NASH). Progressive improvements in the efficacy of GLP1RA suggest that GLP-1-based therapies may soon rival bariatric surgery as viable options for the treatment of obesity and its complications.
Keywords: Weight loss, Hunger, Diabetes, Obesity, Brain, G protein-coupled receptor
Highlights
-
•
Brainstem-derived but not gut-derived GLP-1 contributed to the regulation of food intake.
-
•
GLP-1 actions reduce food intake through a single G protein-coupled receptor (GPCR).
-
•
GLP-1 receptors coupled to food intake are widely distributed in the central nervous system.
-
•
GLP-1-based co-agonists and their combinations may transform the therapy of obesity.
1. Introduction
Glucagon-like peptide-1 (GLP-1) is expressed in the gut, the brainstem, and to a lesser extent, within the endocrine pancreas and exerts its actions, including the control of energy balance, through a single well-characterized GLP-1 receptor (GLP1R) [1]. Multiple studies have sought to identify key GLP1R + brain regions and specific nuclei essential for the physiological control of food intake and the pharmacological responses to GLP1R agonists (GLP1RA). The available data suggest that multiple GLP1R+ neuronal populations contribute to the anorectic actions of GLP1RA. Here we focus on the distinct roles of GLP1Rs in the physiological control of food intake, as well as the specific brain regions important for transduction of the anorectic actions of exogenous GLP1RA. Additionally, we highlight the clinical data supporting the approval of two GLP1RA, liraglutide and semaglutide, for the treatment of people with obesity.
2. Does GLP-1 regulate energy expenditure?
Though overwhelming evidence supports the role of GLP-1 in the reduction of food intake in animals and humans, the effects of GLP-1 on energy expenditure vary across species. Studies examining mice and rats reveal that GLP1RA increases energy expenditure, contributing to weight loss detected in preclinical experiments. These actions induced by GLP-1 are largely mediated by central nervous system (CNS) activation of adrenergic and AMP-activated protein kinase (AMPK) pathways that converge on brown adipose tissue (BAT) activation [2,3]. Conversely, selective knockdown of the GLP1R in the dorsomedial nucleus of the rat hypothalamus reduced adipose tissue uncoupling protein 1 (UCP1) expression, decreased BAT temperature and energy expenditure, and increased body weight and adiposity [4]. Nonetheless, preclinical studies examining how GLP1RA such as semaglutide induces weight loss identify a reduction of food intake without changes in locomotion or energy expenditure, as the predominant mechanism that contributes to the reduction of body weight [5]. While GLP-1-regulated energy expenditure pathways are promising, this mechanism does not appear to meaningfully contribute to weight loss ensuing from the administration of GLP1RA in humans. For example, the administration of exenatide (10 μg twice daily) in people with obesity or type 2 diabetes (T2D) studied for up to 24 weeks revealed a reduction in food intake, while energy expenditure remained constant [6]. Similarly, 12 weeks of continuous exenatide (2 mg once weekly of an extended-release formulation) administration in non-diabetic men increased BAT glucose uptake; however, no increase in resting energy expenditure or substrate oxidation was observed [7]. Twenty-six weeks of once-daily liraglutide administration (1.8 mg daily) in people with T2D and mean body mass index (BMI) of ∼32, reduced body weight (4.3 kg) and decreased resting energy expenditure, without changing the proportion of fat within BAT [8]. Furthermore, administration of semaglutide (1 mg once weekly) to people with obesity for 12 weeks moderately reduced food intake by 24%, associated with a ∼5 kg mean weight loss, reflecting predominant loss of fat mass, without any change in resting energy expenditure [9]. Therefore, though GLP1R-regulated CNS pathways may indirectly augment BAT activity and energy expenditure in mice and rats, the role of these mechanisms in humans remains poorly defined.
3. How important is gut-derived GLP-1 for the control of food intake and body weight?
As gut-derived GLP-1 circulates at very low levels in the systemic circulation, considerable uncertainty surrounds the importance of GLP-1 signals originating from the gut for regulating energy homeostasis. Genetic deletion of the glucagon (GCG) gene from the distal gut using Cdx2-Cre, or from the small and large bowel using Vil1-Cre, produces a partial or near-complete reduction of circulating GLP-1 levels in mice [10]. Nevertheless, the near absence of circulating bioactive GLP-1 is not associated with increased food intake or bodyweight under regular chow diet conditions, nor do these Glp1rGut−/− mice exhibit enhanced weight gain after high-fat diet (HFD) feeding. Hence, gut-derived GLP-1 is not essential for the control of food intake or body weight.
3.1. Mechanisms and cell types contributing to GLP1RA-mediated inhibition of food intake and weight loss
GLP1R localization in the brain has been assessed by in situ hybridization (ISH), ligand binding, including the use of fluorescent GLP-1 analogs for detection of receptor binding, immunocytochemistry, the use of reporter mice, and single-cell RNA-seq. Interpretation of some reports detecting GLP1R immunoreactivity is complicated by the use of non-selective antisera that often do not recognize the authentic GLP1R [11,12]. Glp1r expression was detected by ISH within multiple nuclei in the rat hypothalamus [13], the thalamus, cortex, brainstem, preoptic area, ventral tegmental area, area postrema (AP), dorsal nucleus of the vagus, lateral reticular nucleus, and within the spinal cord [14]. The widespread distribution of CNS Glp1r expression was subsequently confirmed in studies using reverse transcription-polymerase chain reaction (RT-PCR), demonstrating Glp1r expression in the cerebral cortex, cerebellum, hypothalamus, and brainstem in the fetal and adult mouse brain [15].
A broad distribution of GLP-1 binding sites was detected in the rat brain using labeled GLP-1 or exendin-4, including the lateral septum, subfornical organ (SFO), thalamus, hypothalamus, interpeduncular nucleus, posterodorsal tegmental nucleus, AP, inferior olive, and the nucleus of the solitary tract (NTS) [16] (Figure 1). Studies of GLP-1 and the reduction of food intake also identified robust binding of labeled GLP-1 in the rat hypothalamus, central nucleus of the amygdala, and anterodorsal thalamic nucleus, with GLP-1 binding competed by addition of excess exendin (9–39), a GLP1R antagonist [17]. Similar regional patterns of labeled GLP-1 binding were detected in the mouse brain, whereas no GLP-1 binding sites were detected in the CNS of the Glp1r−/- mouse [18]. Subsequent studies confirmed the widespread distribution of GLP1R mRNA transcripts and GLP-1 binding sites within multiple regions of the non-human primate brain, especially in regions of the brainstem and hypothalamus that regulate feeding [19].
Figure 1.
Representative targets for GLP-1 action and sites of GLP1R expression within the nervous system, and consequences of GLP-1 therapy in people with obesity.
Of relevance to the use of GLP1RA for the treatment of obesity, peripheral administration of fluorescently-labeled liraglutide or semaglutide detects binding in multiple populations of GLP1R + neurons, including circumventricular organs, the choroid plexus, the lateral septal nucleus, the septofimbrial nucleus, the arcuate and the paraventricular nucleus, the median preoptic nucleus (MnPO), the NTS, the dorsomedial hypothalamic nucleus, the medial mammillary nucleus, the supraoptic nucleus, the tuberal nucleus, and dorsal motor nucleus of the vagus nerve [5]. In agreement with these findings, peripheral injection of a fluorescent exendin (9–39) derivative in mice detected binding in the median eminence, NTS, AP, arcuate nucleus, and choroid plexus [20]. The mechanisms underlying penetration of GLP1RA beyond the blood–brain barrier are incompletely understood but may involve the interaction of GLP-1 with tanycytes [5].
Multiple GLP1R + neurons within the hindbrain and the hypothalamus are functionally important for transducing signals linking GLP1R activation to reduced food intake and weight loss. Experimental designs utilized for functional interrogation of the GLP-1 system include central and peripheral administration of native GLP-1, GLP1RA, and receptor antagonists, the use of cell type-specific GLP1R knockout mice, as well as an interrogation of GLP1R-regulated neural circuits using chemogenetics. The expression of GLP1R in these regions generally corresponded to cellular sites of c-FOS expression following administration of GLP1RA [21].
Ludwig and colleagues used single-cell RNA-seq to identify populations of GLP1R+ cells within the dorsal vagal complex of obese mice exhibiting transcriptional responses to a 7-day treatment regimen of liraglutide or semaglutide [22]. The detected transcriptional responses showed remarkable similarity in animals treated with liraglutide vs. semaglutide. The Glp1r was co-expressed in some noradrenergic neurons with mRNAs for two receptors mediating anorectic activity, with Gfral, predominantly in the AP and Calcr, mainly in the NTS [22]. Transcriptional responses to semaglutide were detected in neurons, glial cells, astrocytes, tanycytes, vascular and leptomeningeal cells, and ependymal cells. The AP exhibited the largest number of differentially expressed mRNA transcripts, including Bdnf, Ghrh, Pam, Ptprn, Vgf, Cartpt, and Pdyn. Notably, the majority of Glp1r+ and glucose-dependent insulinotropic polypeptide receptor (Gipr)+ cells within the AP did not co-express both receptor mRNAs.
GLP1RA activates subsets of glutamatergic and GABAergic GLP1R+ neurons, including populations within the brainstem and hypothalamus of mice and rats [5,23,24]. Selective genetic disruption of the murine Glp1r using either vGAT-Cre or vGlut2-Cre to target the GABAergic or glutamatergic neurons, respectively, generated vGATGlp1r−/− and vGlut2Glp1r−/− mice that exhibited no baseline change in basal food intake or body weight. Liraglutide significantly reduced food intake in vGATGlp1r−/− mice; however, the anorectic actions of liraglutide were partially attenuated and delayed in vGlut2Glp1r−/− mice [25]. Conditioned taste aversion induced by liraglutide was also blunted in vGlut2Glp1r−/− mice. Consistent with these findings, a 2-week course of once-daily liraglutide reduced food intake and body weight in HFD-fed vGATGlp1r−/− not in vGlut2Glp1r−/− mice.
A potential role for cytokine signaling in the transduction of anorectic GLP-1 signals has also been suggested. Co-localization of interleukin-6 (IL-6) and GLP1R+ cells has been detected in the hypothalamus, brainstem, and the lateral parabrachial nucleus, and administration of exendin-4 increased the proportion of IL-6+ cells in the external parabrachial nucleus [26]. Acute intracerebroventricular (ICV) injection of exendin-4 also increased the expression of IL-6 in the brainstem, and of IL-6 and IL-1β in the rat hypothalamus. Pharmacological or genetic reduction of hypothalamic IL-1β and IL-6 activity in rats and mice, respectively, attenuated the anorectic actions of exendin-4 [27].
3.2. Hindbrain PPG GLP-1+ neurons and the control of food intake
Studies using chemogenetics or deletion or knockdown of gut or CNS Gcg expression in mice have implicated CNS-derived GLP-1, rather than gut GLP-1, as the predominant source of endogenous GLP-1 controlling physiological anorectic pathways in the brain. Viral expression of diphtheria toxin to enable ablation of mouse brainstem preproglucagon (PPG) neurons substantially reduces GLP-1 peptide content in the brainstem, hypothalamus, and spinal cord, but did not affect food intake or body weight under regular chow ad libitum feeding conditions [28]. Nevertheless, PPG-ablated mice did increase food intake after a prolonged fast or after administration of a liquid diet preload, findings confirmed independently in separate groups of mice with chemogenetic inhibition of PPG neurons [28]. Moreover, restraint stress-associated reduction of food intake required PPG neurons. Hence, brainstem PPG neurons may serve to limit the extent of food intake when the animal is presented with an excess of ingestable energy or under conditions of stress that are known to induce the brain GLP-1 system.
Although exogenous leptin administration activates PPG neurons in the rat hindbrain [29], hindbrain Gcg mRNA transcripts were reduced in rats treated with liraglutide [30]. Similarly, mice with diphtheria toxin subunit A-mediated ablation of NTS PPG neurons did not exhibit attenuation of the actions of liraglutide or semaglutide to reduce food intake or promote weight loss over 24 h [31]. Chemogenetic activation of NTS PPG neurons together with pharmacological administration of semaglutide (60 μg/kg) produced a greater reduction in food intake than that achieved with either intervention alone [31].
Viral expression of diphtheria toxin in brainstem PPG neurons did not impact circadian rhythms of food intake, energy expenditure, or bodyweight, but was associated with transient hyperphagia during post-fast refeeding in mice [31]. Chemogenetic inhibition of PPG neurons in the NTS increased meal size during re-feeding of large meals but had no effect on ad libitum feeding. In contrast, activation of PPG NTS neurons reduced ad libitum food intake and body weight over 48 h, without impacting changes in behavior. Chemogenetic or optogenetic activation of vagal afferent neurons (VANs) produced a more modest and transient inhibition of food intake and body weight over a 24 h period [31], as per findings associated with the development of conditioned taste aversion. Importantly, activation of GLP1R+ VANs did not induce c-FOS expression within brainstem PPG neurons, whereas a subset of oxytocin receptor+ VANs or peripheral administration of oxytocin communicated anorectic signals reflecting gut distension via mechanisms requiring brainstem PPG neurons.
Chemogenetic activation of GLP1R+ VANs acutely reduced dark phase food intake in both fasted and fed mice, associated with activation of c-FOS expression within the NTS and the AP [32], including subpopulations of neurons that expressed either cholecystokinin (CCK), Dopamine beta-hydroxylase (DBH), or neuropeptide Y (NPY). Stimulation of these GLP1R+ vagal circuits was associated with the activation of calcitonin gene-related peptide (CGRP)+ neurons within the lateral parabrachial nucleus. Acute chemogenetic inhibition of these neurons produced a modest increase in food intake, but no detectable impact on the response to fasting-refeeding. Chemogenetic inhibition of GLP1R+ vagal afferents markedly attenuated the acute anorectic response to lithium chloride and CCK, but had minimal effect on the anorectic response to liraglutide [32].
3.3. GLP-1 action in the hypothalamus reduces food intake and body weight
Early studies of the anorectic actions of GLP-1 were focused on the hypothalamus, demonstrating that ICV administration of GLP-1 inhibited food intake in rats, associated with induction of c-FOS expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala [17]. These actions of GLP-1 were blocked by the GLP1R antagonist exendin (9–39) and were absent in Glp1r−/- mice [18]. Secher et al. detected binding and internalization of fluorescently labeled liraglutide within the arcuate nucleus, but not the hindbrain of mice and rats. Nevertheless, in subsequent studies by the same group, peripheral administration of semaglutide enabled the detection of fluorescent semaglutide within the hypothalamus, brainstem, and septal nucleus [5].
Using a combination of in situ hybridization to detect GLP1R and immunocytochemistry to detect proopiomelanocortin (POMC)/cocaine and amphetamine-regulated transcript (CART), the majority of arcuate nucleus POMC/CART neurons were GLP1R+ [30], supporting a model whereby exogenous GLP-1 directly activates POMC/CART neurons which inhibit the NPY)/Agouti-related peptide (AGRP) pathway via GABAergic interneurons. These actions were localized predominantly to the arcuate, not to the paraventricular nucleus. A functional role for melanocortin (MC) receptors downstream of the GLP1R was inferred from studies in rats, where co-administration of the MC3/4 receptor antagonist SHU9119, administered by stereotactic injection into the rat dorsal vagal complex, blunted the acute anorectic and weight loss actions of liraglutide administered by intraperitoneal injection [33]. Consistent with these findings, studies using wildtype and reporter mice demonstrated that liraglutide directly activated arcuate POMC neurons, indirectly inhibiting NPY/AGRP neurons through post-synaptic GABA receptors, and increasing activity of pre-synaptic GABAergic neurons [34].
Approximately 10% of POMC+ neurons in the arcuate nucleus express both the Glp1r and the leptin receptor (Lepr), with chemogenetic activation of POMC/GLP1R+ neurons leading to acute reduction of food intake preferentially in male mice, without affecting locomotor activity or energy expenditure [35]. Genetic inactivation of murine hypothalamic GLP1Rs using Nkx2-Cre, Pomc-Cre, and Sim1-Cre could not abrogate the anorexic responses to exogenous GLP1RA, emphasizing the importance of redundant pathways beyond the hypothalamus for GLP-1 actions on food intake.
3.4. GLP-1 action in the hindbrain controls food intake and body weight
Considerable evidence supports the importance of hindbrain GLP1Rs as key targets for both exogenous and endogenous GLP-1 in the control of food intake. Administration of exenatide acutely induces c-FOS expression in the AP, NTS, and the dorsal motor nucleus of the vagus [36]. Experiments using chronic supracollicular decerebrate (CD) rats demonstrated that ICV or intraperitoneal injection of GLP-1 or exendin-4 directly into the hindbrain acutely reduced food intake and inhibited gastric emptying [37]. Consistent with these findings, selective ICV 4th ventricle (targeting the hindbrain) administration of the GLP1R antagonist exendin (9–39) increased food intake and blocked the suppression of food intake following acute gastric distension [38]. Conversely, knockdown of NTS Glp1r mRNA in rats using Adeno-associated virus (AAV)-shRNA increased 24-h food intake, not body weight in rats [39].
ICV administration of exendin-4 into the rat hindbrain activated protein kinase A (PKA) and mitogen-activated protein kinase (MAPK) and decreased phosphorylation of 5′ AMP-activated protein kinase (AMPK), in association with reduction of meal number. Conversely, simultaneous co-administration of the PKA antagonist, 3′,5′-cyclic monophosphothioate (Rp=cAMPs), reversed the suppressive effects of exendin-4 on food intake and body weight [40]. Similarly, pretreatment with the MAP kinase/ERK kinase (MEK) inhibitor U0126 attenuated reduction of food intake and body weight observed with ICV exendin-4. Directed administration of exendin-4 or native GLP-1 into the NTS acutely inhibited food intake, preferentially through reduction of palatable food vs. chow [41]. Related experiments showed that hindbrain exendin-4 also reduced AKT phosphorylation, whereas inhibition of hindbrain PI3 kinase activity using LY294002 attenuated the actions of exendin-4 in the hindbrain to suppress food intake and reduce body weight [42]. Subsequent studies identified neuronal populations within the hindbrain essential for transduction of the anorectic actions of GLP1RA. Moreover, a combination of adenoviral knockdown of the GLP1R and chemogenetic experiments revealed an essential role of hindbrain GABAergic neurons in the actions of liraglutide to reduce food intake and body weight in rats over 3 weeks [24].
4. Miscellaneous brain regions communicating anorectic actions of GLP1RA
Beyond interrogation of the hypothalamus and brainstem, several other regions in the CNS express GLP1Rs that transduce signals reducing food intake (Figure 1). For example, administration of GLP-1 directly into the nucleus accumbens reduced food intake, whereas exendin (9–39) increases food intake in the rat [43]. Similarly, hindbrain GLP-1+ neurons project to the lateral parabrachial nucleus, and gain- and loss-of-function studies in rats and mice demonstrate that acute exendin-4 administration targeted to the lateral parabrachial nucleus reduced food intake and body weight, whereas blockade of the GLP1R with exendin (9–39) transiently increased food intake in rats [44,45]. GLP1Rs within the hippocampus may also contribute to the control of food intake. Acute administration of exendin-4 directly into the hippocampal formation of rats reduced food intake and body weight over 24 h by reducing meal size, whereas administration of exendin (9–39) transiently increased food intake over the first 6 h [46]. The lateral septum also contains immunoreactive GLP-1 fibers and GLP1Rs and both gain and loss of GLP1R signaling within the lateral septum, targeted by microinjection, inhibits and increases food intake, respectively in rats [47]. Similarly, the GLP1R is expressed in the paraventricular nucleus of the rat thalamus, as well as the lateral dorsal tegmental nucleus, and administration of exendin-4 or exendin (9–39) directly into these two areas inhibited or stimulated acute food intake, respectively, via changes in meal size [48,49]. Furthermore, administration of GLP-1 directly into the bed nucleus of the mouse stria terminalis (BNST), which receives GLP-1+ innervation from the NTS PPG neurons, reduced food intake, whereas exendin (9–39) increased food intake, actions somewhat attenuated in mice fed on HFD [50]. Overall, these results reveal that multiple regions within the CNS transduce pharmacological signals linking GLP1R activation to a reduction in food intake and body weight.
4.1. The physiological importance of endogenous GLP1Rs for control of food intake
Whether GLP1R signaling is essential for the control of food intake and body weight depends on the precise experimental context. Acute ICV administration of exendin (9–39) into the rat brain increases food intake [17]; however, Glp1r−/- mice do not exhibit increased food intake or weight gain on a regular chow diet [18], and exhibit increased locomotor activity and energy expenditure and resistance to weight gain after HFD feeding [51,52]. Moreover, deletion of the GLP1R in ob/ob mice does not impact the extent of hyperphagia or weight gain [53]. Consistent with these findings, broad disruption of the CNS GLP1R system in mice using Nes-Cre did not lead to increased weight gain or food intake. Similarly, mice with the elimination of GLP1Rs within the Wnt1-Cre expression domain, including the brainstem, hypothalamus, and enteric nervous system, exhibit normal food intake and body weight [54]. Moreover, postnatal ablation of brainstem PPG neurons using diphtheria toxin does not impact the control of food intake, meal size, energy expenditure, or body weight [31].
The importance of hypothalamic GLP1Rs for the control of food intake is also highly context- and experiment-dependent. Adenoviral knockdown of GLP1Rs within the lateral hypothalamus of the rat produced a substantial increase in food intake, body weight, and fat mass, evident over 25–30 days and predominantly in male rats [55]. Similarly, daily ICV administration of exendin (9–39) for three days increased food intake and body weight in rats [56], and peripheral administration of a selective acylated GLP1R antagonist daily for one week increased food intake and body weight in HFD-fed mice [57]. Selective lentiviral-mediated knockdown of the Gcg gene within PPG neurons in the rat NTS using RNA interference, or ICV infusion of exendin (9–39) increased food intake and body weight in HFD-fed rats [58]. Moreover, knockdown of GLP1Rs in the rat dorsomedial hypothalamus using RNA interference increased food intake, decreased energy expenditure, and increased body weight over 11 weeks of observation [4]. In contrast, Burmeister et al. used Nkx2-Cre, Pomc-Cre, and Sim1-Cre to broadly or selectively inactivate the GLP1R in the mouse hypothalamus. Remarkably, no consistent increase in food intake or body weight was detected [59]. Similarly, the genetic knockdown of the GLP1R in the ventromedial hypothalamus did not affect food intake or body weight [60]. In contrast with these findings, postnatal knockdown of the Glp1r in the paraventricular nucleus (PVN) using targeted injections of AAV-Cre led to increased food intake and weight gain, without changes in energy expenditure [61]. Hence, the central importance of endogenous brain GLP1Rs for control of food intake and body weight appears highly context- and technique-dependent.
4.2. Role of vagal afferent signals in the GLP-1-dependent control of food intake
Genetic, pharmacological, and surgical approaches have identified the role of GLP1Rs in VANs in the control of food intake. Higher doses of exendin-4 and liraglutide were required to achieve maximal suppression of food intake in acute studies with rats subjected to complete subdiaphragmatic surgical denervation [62]. Transection of the vagus below the diaphragm attenuated the induction of c-FOS expression induced by exendin-4 in the nodose ganglion, the medial NTS, and the PVN of the hypothalamus [63], supporting the vital role for VANs in communicating systemic GLP1R-dependent signals to the nervous system. In contrast, Secher et al. did not detect any difference in reduction of food intake or body weights in rats subjected to subdiaphragmatic vagal afferent vagotomy or sham surgery and treated with liraglutide 200 μg/kg twice daily for 14 days [30]. Furthermore, liraglutide retained its ability to reduce food intake and body weight in rats with surgical ablation of the AP [24,30].
GLP-1 action has also been studied in animal models with the disruption of the nodose ganglion Glp1r. Nodose ganglion Glp1r mRNA transcripts were reduced in rats prone to the development of obesity on an HFD, in association with relative resistance to the anorectic actions of exogenous exendin-4 [64]. Bilateral knockdown of Glp1r mRNA transcripts in the rat nodose ganglia did not change long-term body weight, but increased meal size and enhanced gastric emptying [65]. Although multiple structurally distinct GLP1RA inhibited food intake and produced weight loss in mice with inactivation of the GLP1R within autonomic neurons including VANs, targeted by Phox2b-Cre, the magnitude of weight loss achieved with chronic administration of dulaglutide was diminished in Glp1rPhox2b−/− mice [54]. Moreover, Glp1rPhox2b−/− mice exhibited dysregulated control of insulin, glucagon, and glucose homeostasis, as well as accelerated gastric emptying, in the absence of treatment with GLP1RA. Chemogenetic activation of VANs also reveals transduction of anorexigenic signals to the parabrachial nucleus, enabling meal termination and the control of glucose homeostasis [32]. Similar results were obtained in independent chemogenetic experiments to activate VANs in mice, leading to transient reduction of food intake and body weight. Collectively, these data highlight the importance of basal GLP1R signaling in VANs for metabolic homeostasis and suggest that they convey a subset of signals to the CNS linking systemic GLP1RA to the control of food intake (Figure 1).
5. The actions of GLP1Rs in astrocytes
Beyond neurons, several studies report GLP1R expression in astrocytes, within the arcuate and paraventricular nucleus of the hypothalamus, the hippocampus, and the hindbrain [66,67]. The acute inhibitory actions of exendin-4 on food intake and body weight were attenuated in rats following targeted administration of the astrocyte-selective Krebs cycle inhibitor, fluorocitrate, into the rat brainstem [66]. Conditional postnatal deletion of the GLP1R in astrocytes using GFAP-Cre mice did not perturb basal control of food or water intake, energy expenditure, or body weight [67]. Hence the available data, albeit limited does not invoke important contributions for the astrocyte GLP1R in the physiological control of food intake.
6. GLP-1 reduces hunger, food intake, and body weight in humans
The finding that acute GLP-1 administration rapidly promotes satiety and reduces energy intake in humans [68] has prompted extensive investigation of how GLP-1 communicates with regions of the brain critical for control of appetite and hunger. Multiple studies have examined changes in CNS activity in humans following administration of native GLP-1 or GLP1RAs, often in conjunction with meal ingestion. Infusion of GLP-1 in the fasting state to healthy non-obese volunteers increased the sensation of fullness and reduced the perception of hunger, as revealed by findings correlated with reduced brain activation in the amygdala, caudate, insula, nucleus accumbens, orbitofrontal cortex, and putamen as assessed by Blood Oxygen Level-Dependent (BOLD) functional magnetic resonance imaging (fMRI) [69]. Changes in BOLD fMRI activation seen with food ingestion were directionally similar to those obtained in the same subjects during GLP-1 infusion. Studies of exenatide infusion in people with obesity demonstrated that changes in functional connectivity within the hypothalamus, assessed by fMRI, correlated with the suppression of food intake in responder vs. non-responder individuals [70]. The effect of exenatide infusion on sensations of hunger and the fMRI response to pictures of food was studied in people with obesity, with or without T2D. Exenatide reduced food intake and decreased the extent of brain activation to pictures of food assessed by fMRI [71] in the insula, amygdala, putamen, and orbitofrontal cortex; these actions were markedly attenuated by co-infusion of exendin (9–39). Infusion of exendin (9–39) alone to block endogenous GLP1R activity markedly attenuated meal-induced insula activation in people with T2D [72]. The actions of exenatide to inhibit food-induced brain activation appear to be preserved in people with obesity, yet absent in normal-weight individuals [73]. Complementary studies using positron emission tomography (PET) showed that acute exenatide infusion increased the rates of CNS glucose metabolism in total gray matter, the cortex, the frontal, occipital, temporal, and parietal lobes, limbic system, insula, putamen, and in areas involved in the food reward system such as the orbitofrontal lobe, thalamus, and anterior and posterior cingulate [74]. In contrast, glucose metabolism was decreased in the hypothalamus after exenatide administration. Hence, the available data support a role for both endogenous GLP1R signaling, as well as pharmacological infusion of GLP1RAs, to attenuate hunger and brain activation in the context of meal ingestion, particularly in people with obesity and with heightened sensitivity to food cues.
6.1. Liraglutide and the treatment of obesity
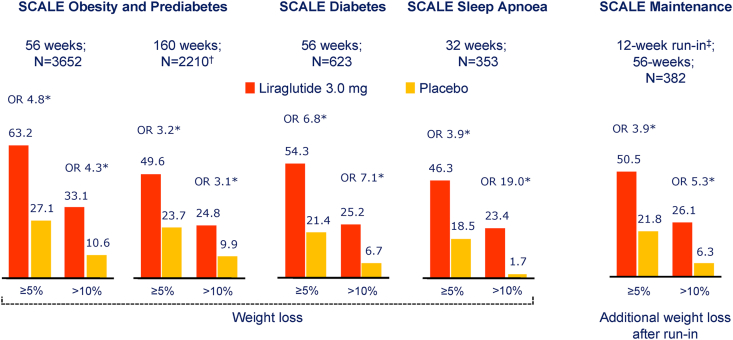
Several decades of preclinical experiments linking GLP1R activation to weight loss, coupled with a reduction in body weight with the use of liraglutide (1.2 and 1.8 mg once daily) in people with T2D, prompted exploration of higher doses of liraglutide for the treatment of overweight and obesity in the Satiety and Clinical Adiposity – Liraglutide Evidence (SCALE) program (Figure 2). A 52-week trial assessed the efficacy of liraglutide 3 mg once daily in people with overweight and BMI >27 and at least one comorbid condition or obesity (BMI >30). The trial population was predominantly female (78.5%), mean age 45.1 years, baseline BMI 38.3. The mean weight loss achieved was 8.4 kg after 56 weeks, with 33.1% of trial participants losing more than 10% of their body weight [75]. Rates of study discontinuation due to adverse events (AEs) were 9.9% vs. 3.8% for liraglutide vs. placebo, respectively, with nausea, vomiting, diarrhea, and gallbladder AEs reported more frequently with liraglutide therapy. The effectiveness of liraglutide was diminished in people with a BMI >40. Fasting lipid levels, HbA1c, blood pressure, high-sensitivity C-reactive protein, plasminogen activator inhibitor-1, adiponectin, and physical and mental health showed improvement in people treated with liraglutide [75].
Figure 2.
Summary of categorical weight loss with liraglutide in the SCALE trials. ∗p < 0.001. Data are observed proportions (except SCALE Diabetes, which is estimated proportions) with the last observation carried forward (LOCF) at end of the trial. N = number contributing to the analysis. Data are from [[75], [76], [77], [78], [79]]. †, individuals with prediabetes at trial entry; ‡, low-calorie diet (total energy intake 1200–1400 kcal/day). OR= Odds Ratio.
The SCALE obesity and prediabetes trial evaluated the effect of liraglutide 3 mg daily vs. placebo plus lifestyle intervention in people with a BMI >30 and prediabetes, with evaluations of treatment efficacy at 56 and 160 weeks. Among 2,254 randomized subjects, 50% completed the entire study over 160 weeks. Fewer people were diagnosed with diabetes in the liraglutide arm (2% vs. 6%, liraglutide vs. placebo, respectively) [76].
In the SCALE Diabetes trial, 6% weight loss was achieved over 52 weeks in people with T2D treated with 3 mg liraglutide once daily, with 25.2% of the trial subjects experiencing >10% weight loss [77]. The SCALE sleep apnea trial assessed the effectiveness of liraglutide vs. placebo, in addition to diet and exercise counseling in reduction of the apnea-hypopnea index (AHI) in 359 patients (BMI <30, >70% male) with sleep apnea unable to tolerate continuous positive airway pressure [78]. A mean weight loss of 5.7%, together with a reduction in the AHI (∼6 fewer events per hour), was observed in people treated with liraglutide 3 mg daily, of whom 74% of people completed the trial. The majority of subjects experienced improvements in the AHI within the first 12 weeks of therapy, with greater benefits accruing in individuals with higher baseline severity of obstructive sleep apnea [78]. Not surprisingly, greater weight loss was associated with enhanced improvement in the AHI in subjects randomized to liraglutide or placebo. Weight loss at 160 weeks was 6.1% vs. 1.9% for subjects randomized to liraglutide vs. placebo, respectively.
The SCALE maintenance study assessed the efficacy of liraglutide over 52 weeks in subjects with >5% weight loss following a low-calorie diet for up to 12 weeks. After a mean weight loss of 6% on diet alone, subjects randomized to liraglutide lost an additional 6.2% of body weight while continuing to follow a 500 kcal per day deficit diet [79]. Trial completion rates were 75% and 69% for subjects randomized to liraglutide and placebo, respectively. More liraglutide-treated subjects maintained >5% weight loss (81.4 vs. 48.9%, liraglutide vs. placebo, respectively). Gastrointestinal AEs, predominantly nausea and vomiting, were more common with liraglutide therapy.
Liraglutide has also been approved in several countries for the treatment of adolescents with obesity based on the results of a 56-week-long randomized controlled trial in male and female adolescents, 12–<18 years of age [80]. End of trial weight loss >5% or >10% was observed in 43.3 and 26.1% of liraglutide-treated subjects, respectively, with a mean placebo-subtracted weight loss of 5% of body weight. Approximately 81% of subjects randomized to liraglutide (vs. 79.4% for placebo) completed the trial. Adverse events, especially gastrointestinal complaints were consistent with those defined for the GLP1RA class.
The combination of liraglutide and intensive behavioral therapy (IBT) produced greater weight loss than either intervention alone in subjects (n = 50 per arm) maintained on a portion-controlled diet of 1,000–1,200 calories per day over 24 weeks; however, the benefit of combining these interventions was not sustained over 52 weeks [81]. In a larger cohort, the SCALE IBT trial randomized 282 subjects receiving IBT to liraglutide 3 mg or placebo over 56 weeks [82]. Mean weight loss achieved was 7.5% vs. 4% for liraglutide vs. placebo at 56 weeks, with 30.5% vs. 10.8% of subjects reporting more than 10% weight loss, liraglutide vs. placebo, respectively.
6.2. Semaglutide and weight loss in humans with overweight or obesity
Semaglutide, a degradation-resistant, acylated long-acting GLP1RA, was originally developed for the treatment of T2D at 0.5 and 1 mg doses (and later 2 mg doses) administered subcutaneously once weekly, as well as 7 or 14 mg doses administered once daily in a tablet formulation [83]. Semaglutide was also evaluated for the treatment of obesity in the Semaglutide Treatment Effect in People with Obesity (STEP) program at a dose of 2.4 mg once weekly (Figure 3). STEP 1 enrolled 1,961 people with obesity or individuals with a BMI>27 with at least one weight-related pre-existing condition, and randomized subjects to lifestyle intervention and placebo or 2.4 mg semaglutide once weekly, with a trial duration of 68 weeks [84]. Semaglutide was started at the dose of 0.25 mg once weekly, and dose escalation to the final dose of 2.4 mg was carried out over 16 weeks. Approximately 94% of study subjects completed the trial and 81.1% of subjects adhered to the treatment protocol. The mean starting BMI was ∼38, just >70% of enrolled subjects were female. The mean weight loss in semaglutide-treated individuals was 16.9% vs. 2.4% in the placebo group [84]. Blood pressure, HbA1c, C-reactive protein, and fasting lipid levels were lower in people treated with semaglutide. Reversion of prediabetes evident at baseline was noted in 84.1% vs. 47.8% of subjects treated with semaglutide vs. placebo, respectively. Weight loss of >15% was achieved by 54.8% vs 5% of the semaglutide vs. placebo-treated group. The proportion of lean body mass increased with semaglutide therapy, although absolute lean body mass was reduced by ∼3% [84]. The most common AEs were nausea, vomiting, diarrhea, and constipation, and a slight imbalance in gallbladder events was also observed in the semaglutide arm, consistent with the well-described profile of the GLP1RA class. Discontinuation due to AEs was more common with semaglutide relative to placebo (7.0% vs 3.1%, respectively).
Figure 3.
Weight loss in the STEP trials with semaglutide. Data represent the trial product estimand, assuming the medication has been taken as assigned. ∗Statistically significant vs placebo. BW, body weight; IBT, intensive behavioral therapy. Data are from [[84], [85], [86], [87]].
STEP 2 evaluated weight loss in 1,210 people with T2D not treated with insulin (HbA1c 7–10.5%) and overweight or obesity randomized to placebo, semaglutide 1 mg or semaglutide 2.4 mg weekly, together with lifestyle intervention, over 68 weeks [85]. The mean change in body weight was 9.6% vs. 3.4% for subjects treated with 2.4 vs 1.0 mg of semaglutide, with 49.9% and 28.2% of study subjects achieving greater than 10% and 15% weight loss, respectively, on 2.4 mg of semaglutide. The end of study HbA1c achieved was comparable (6.4 vs. 6.6%) with semaglutide 2.4 vs 1 mg once weekly and ∼10.6% of subjects treated with 2.4 mg semaglutide once weekly discontinued therapy due to adverse events, predominantly gastrointestinal [85].
The STEP 3 trial evaluated the effect of semaglutide on weight loss in individuals assigned to an initial low-calorie diet for 8 weeks and 30 sessions of intensive behavioral therapy plus 200 min of physical activity, over 68 weeks [86]. Approximately 80% of the 611 randomized subjects were women (mean age 46) with a mean starting BMI of 38, and 82.7% of subjects receiving treatment at the trial conclusion. Discontinuation of therapy was reported in 16.7% and 18.6% of subjects randomized to semaglutide vs. placebo, respectively. Mean weight loss on semaglutide was 17.6% vs. 5% in the placebo arm [86]. The proportion of people with >15% weight loss was 55.8% vs. 13.2%, and >20% weight loss was 35.7% vs. 3.7% for semaglutide vs. placebo treatment, respectively. The degree of physical activity was increased, and blood pressure, waist circumference, HbA1c, and plasma lipid profiles were reduced in subjects treated with semaglutide [86]. AEs were predominantly gastrointestinal and more often reported with semaglutide.
The STEP 4 trial examined the consequences of an initial 20 weeks of semaglutide administered 2.4 mg once weekly followed by either continued therapy with semaglutide vs. switch to placebo in 803 subjects with overweight or obesity. Study subjects were simultaneously maintained on a low-calorie diet and increased physical activity while receiving IBT [87]. Approximately 92% of the subjects completed 68 weeks of treatment. Body weight was reduced by 10.6% (initial mean BMI of 38.4) during the initial 20 weeks run-in period, and subjects maintained on semaglutide lost an additional 7.9% of body weight, vs. weight gain of 6.9% in subjects re-randomized to placebo. Semaglutide therapy showed association with greater improvements in physical function scores and reductions in HbA1c, lipids, blood pressure, and weight circumference [87]. The proportion of subjects with >15% and >20% weight loss at 68 weeks was 63.7% vs. 9.2% and 39.6% vs. 4.8% for semaglutide vs. placebo, respectively. Mechanistically, humans with obesity treated with 2.4 mg semaglutide once weekly report reduced hunger, decreased cravings, and reduced energy intake (∼35%), with minimal effects on the control of gastric emptying, assessed after a 20-week-treatment period [88]. The safety and efficacy of semaglutide 2.4 mg once weekly for weight management are also being studied in adolescents with obesity.
7. GLP1RA and cardiovascular disease
The safety of GLP1RA in the treatment of people with T2D at high risk for the development of cardiovascular disease, as well as in individuals with established cardiovascular disease, has been extensively studied [89,90]. The results of these trials, which included a substantial proportion of people with overweight or obesity and co-existing T2D, strongly suggest that the reduction of cardiovascular events seen with long-acting GLP1RA in these studies represents a class effect for highly effective long-acting GLP1RAs. The available preclinical data demonstrate cardioprotective actions of GLP1RA in animals with obesity without diabetes [91,92]. Moreover, several GLP1RA such as albiglutide or efpeglenatide reduce major adverse cardiovascular events (MACE) in people with T2D despite the achievement of only modest weight loss [93,94]. The use of semaglutide for the treatment of T2D has been associated with cardiovascular safety (oral semaglutide) [95], or a reduction in rates of major cardiovascular events with once-weekly injectable semaglutide [96]. Liraglutide (1.8 mg) reduced the rates of MACE events in people with T2D; however, the cardiovascular safety of liraglutide 3 mg daily in people with obesity has not been assessed in a dedicated cardiovascular outcomes trial.
The safety of semaglutide 2.4 mg once weekly is being evaluated in the Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity (SELECT) trial [97]. This trial will enroll both male and female subjects (n = 17,500), age >45, BMI >27, with established cardiovascular disease, and an HbA1c <6.5%. The trial is event-driven (first report of new MACE event) and is expected to report by 2023.
7.1. GLP1RA and non-alcoholic steatohepatitis (NASH)
The therapeutic potential of GLP1RA such as liraglutide and semaglutide for the treatment of NASH has been studied in humans. The safety and efficacy of liraglutide 1.8 mg daily were studied in people with overweight and non-alcoholic steatosis over 48 weeks [98]. Of the 52 volunteers enrolled in the trial, 39% of liraglutide-treated subjects demonstrated resolution of NASH, vs. 9% randomized to placebo. Progression to fibrosis was also observed in fewer subjects randomized to liraglutide. The efficacy of 3 different doses of semaglutide once daily (0.1, 0.2, and 0.4 mg) vs. placebo was evaluated in a larger group (n = 320) of individuals with NASH, aged 18–75 years, 61% female, 62% with T2D, over 72 weeks [99]. Among volunteers treated with the highest dose of semaglutide, NASH resolution was detected in 59% of people, vs. 17% in the placebo arm. In contrast, improvement in fibrosis was not different between groups, detected in 43% vs. 33% of people taking 0.4 mg of semaglutide vs. placebo, respectively. The combined endpoint of NASH resolution and improvement in fibrosis was observed in 37% vs. 15% of semaglutide vs. placebo-treated subjects and subjects treated with 0.4 mg of semaglutide had a mean weight loss of 13% vs. 1% for those treated with placebo [99]. A phase 3 trial with semaglutide 2.4 mg once weekly for 72 weeks is going on. The extent to which the improvement in NASH with GLP1RA is driven predominantly by weight loss or through weight loss-independent mechanisms requires additional investigation.
8. GLP-1-based co-agonists and weight loss
Based on the results obtained with semaglutide, intense interest has been evinced in exploring whether combining one or more additional peptide epitopes, together with GLP-1, can produce even greater weight loss, while maintaining the safety profile of GLP1RA [100].
8.1. Leptin and GLP-1
Leptin receptors have been detected on and activate brainstem PPG neurons [29], fostering interest in whether PPG neurons mediate a subset of leptin actions, or whether leptin may be useful in combination with GLP1RA for weight loss. Notably, Leptin maintains its anorectic activity in Glp1r−/- mice [101], and chemogenetic activation of LepRbNTS neurons robustly reduces food intake in mice with ablation of PPG expression in the NTS [102]. Moreover, administration of GLP1RA produces weight loss in db/db and ob/ob mice [103]. Acute leptin administration augments the GLP1RA-dependent reduction of food intake in fasted rats [104]. Supporting these findings, additive effects were reported for hindbrain leptin and GLP1RA administration on suppression of food intake in rats, whereas hindbrain GLP1R blockade using exendin (9–39) diminished the inhibitory effects of hindbrain leptin on food intake [105,106]. Moreover, acute leptin administration potentiated the inhibitory effects of liraglutide on POMC activation and food intake in mice [34]. Nevertheless, the potential utility of combining metreleptin and GLP1R in humans with obesity is not being actively explored.
8.2. Glucagon-GLP-1 co-agonists
A unimolecular combination of peptide sequences activating both the GCG and GLP-1 receptors, including the naturally occurring co-agonist oxyntomodulin [107], has produced compelling weight loss in preclinical studies [108,109]. Studies in mice and rats demonstrate that glucagon acutely reduces food intake [110], and activates thermogenic pathways in the liver and adipose tissue to increase energy expenditure [111,112]. Short-term infusion of glucagon also increases energy expenditure in healthy human volunteers without detectable increases in metabolic activity of the supraclavicular fat pads assessed by (18) F-fluorodeoxyglucose uptake using positron emission and computerized tomography scanning [113]. Oxyntomodulin modestly reduces food intake and increases activity-related energy expenditure leading to several kg of weight loss in short-term studies in people with overweight or obesity [114].
The therapeutic limitations of native oxyntomodulin have spurred clinical development of more effective GCG-GLP1R co-agonists, the best of which to date exhibit up to 10% weight loss in people with obesity studied for 26 weeks [115]. However, nausea and vomiting are prominent features associated with the use of glucagon-containing co-agonists, and it remains undefined whether GCG-GLP1R co-agonists are more effective for obesity compared to recently approved therapies such as semaglutide once weekly. Although the majority of GCG-GLP1R co-agonists produce an effective reduction of HbA1c, a single agent, JNJ-64565111, failed to reduce HbA1c in a 12 week clinical trial of people with T2D and obesity [116]. Hence, optimization of GCG-GLP1R co-agonists will be required to maximize weight loss, without disrupting glucose homeostasis, while minimizing gastrointestinal AEs. The actions of glucagon to reduce hepatic lipid synthesis and promote lipid oxidation [117] have fostered the repurposing of GCG-GLP1R co-agonists such as cotadutide for the treatment of non-alcoholic steatohepatitis [118].
8.3. Amylin-GLP-1 co-agonists
Originally developed as medicines for both T1D and T2D, amylin analogs such as pramlintide generate weight loss in human subjects, with or without diabetes. A combination of pramlintide (360 μg twice daily) and metreleptin (5 mg twice daily) produced substantial (∼12% at week 20 in the responder population) weight loss in people with obesity, with some individuals experiencing 15–20% weight loss [119]. Cagrilintide is an acylated long-acting non-selective human amylin receptor agonist [120] that is being evaluated, in combination with semaglutide, for the treatment of obesity. In a 20-week ascending dose-escalation Phase 1B safety and pharmacokinetic analysis, individuals with obesity treated with 4.5 mg of cagrilintide and 2.4 mg of semaglutide once weekly reported a mean weight loss of 17.1%, with gastrointestinal complaints, such as nausea and vomiting being the most common AEs [121]. The efficacy and safety of the cagrilintide-semaglutide combination for the treatment of obesity is under investigation in larger clinical trials.
8.4. The weight-reducing actions of the GIPR-GLP1R co-agonist tirzepatide
Considerable interest is focused on the mechanisms of action of tirzepatide, a highly effective GIPR-GLP1R co-agonist that produces superior reductions in HbA1c and body weight, relative to that achieved with 1 mg once weekly of semaglutide in people with T2D [122]. The GIPR is expressed in multiple regions of the mouse and human brain, in subsets of neurons and glial cells, with some hypothalamic and hindbrain cells exhibiting co-expression of the GIPR and GLP1R [123,124]. Chemogenetic activation of GIPR + cells in the mouse hypothalamus acutely reduced food intake; however, co-administration of exendin-4 did not produce an additive reduction of food intake when compared to either intervention alone [123]. Therapy with tirzepatide, 5–15 mg once weekly produces 8–12% body weight reduction in people with T2D, prompting ongoing development of tirzepatide as a weight loss agent for people with overweight or obesity. The importance of GIP for the weight loss properties of tirzepatide in humans is uncertain; however, tirzepatide failed to reduce body weight in Glp1r−/- mice [125], implicating a dominant role for the GLP1R in the weight loss observed with this agent. Glucose-dependent insulinotropic polypeptide (GIP) has been shown to reduce the extent of aversive responses induced by GLP-1 in mice and rats and decrease nausea and vomiting in the shrew [126]: however, whether these intriguing actions of GIP are conserved in humans remains unclear.
8.5. Structurally distinct GLP1RA produces different degrees of weight loss
The extent of weight loss achieved with different GLP1RA has steadily improved over time, from a rather modest 1–3% weight loss with exenatide, lixisenatide, and albiglutide, to 3–4% weight loss with dulaglutide and liraglutide in people with T2D. The recognition that higher doses of liraglutide were possible without onerous adverse events enabled achievement of 5–10% weight loss with 3 mg liraglutide once daily in people with obesity. It can be posited that structurally larger drugs such as albiglutide, dulaglutide, or efpeglenatide may be less able to penetrate regions within the brain, relative to smaller peptides such as liraglutide, semaglutide, or tirzepatide. What accounts for the even greater weight loss achieved with the GLP1RA semaglutide? While it is tempting to invoke differentially greater brain access to GLP-1Rs controlling hunger with semaglutide, this hypothesis has not yet been convincingly proven in preclinical studies comparing the activities and localization of semaglutide vs. liraglutide in the brain. Semaglutide penetrates regions of the brain not protected by the blood–brain barrier [5]} and the regional localization of exogenously administered semaglutide within the mouse brain is somewhat different, relative to regions accessed by liraglutide [5]. Nevertheless, when administered at doses that produce similar degrees of weight loss, liraglutide and semaglutide produce nearly identical transcriptional responses in multiple regions of the mouse brain [22]. The available data in humans with T2D suggest that pharmacokinetics, namely achievement of high circulating levels of GLP1RA, are highly correlated with the extent of weight loss for most GLP1RA, including semaglutide [127]. However, it is not possible to rule out unique molecule-specific interactions at the GLP-1R, that may also preferentially activate and sustain receptor signaling and enhance the anorectic actions of various GLP1RA.
9. Summary and future directions
The widespread distribution of GLP1Rs within the CNS that reduce food intake in response to exogenous GLP1RA makes it unlikely that targeting one specific population of GLP1R+ neurons will produce a unique therapy that produces greater weight loss than non-targeted agents. Whether differential biased signaling at the GLP1R will allow for more sustained receptor signaling and greater reduction of food intake and body weight remains unknown. The introduction of GLP1RA (twice daily exenatide) into the clinic in 2005 was associated with considerable nausea and vomiting, limiting adherence, and diminishing enthusiasm for these therapies. Preclinical studies suggest the possibility of dissociating adverse events from the reduction of food intake, perhaps leading to enhanced tolerability and persistence. The realization that gradual dose escalation successfully reduces the frequency of these adverse events, followed by the development of more effective GLP1RA allowing achievement of greater weight loss, has set the stage for a new era of obesity therapeutics. From a baseline of ∼15% weight loss with semaglutide, tirzepatide, and semaglutide-cagrilintide combination therapy will likely approach or exceed a mean weight loss of 20%. Although the majority of clinical studies to date have focused on people with non-genetic causes of obesity, emerging evidence also suggests that GLP1RA may be useful for therapy of some individuals with genetic etiologies contributing to weight gain and obesity [128].
While the risk: benefit ratio for GLP1RA has been established over 16 years, and cardiovascular safety has been demonstrated in large outcome studies in people with T2D, there is currently no information about the long-term safety of GLP-1 combination therapy. Nevertheless, one can envisage randomized controlled trials comparing outcomes for bariatric surgery vs. optimal medical peptide therapy, in people with obesity. The predicted benefits and safety profile of therapy with GLP1RA (Figure 1) will be further validated in the SELECT trial with semaglutide. The results of the SELECT trial may be fundamental for determining the benefits and safety of obesity therapy with semaglutide, potentially overcoming current barriers to treatment and reimbursement. Moreover, the importance and benefits of combining lifestyle interventions such as diet and exercise, together with GLP1RA, should not be neglected in clinical practice [129].
It is important to highlight that successful therapy of obesity extends well beyond weight loss [130]. Important endpoints measuring the quality of life, mobility, arthritis, sleep apnea, reproductive health, and pregnancy outcomes, rates of gestational diabetes and hepatosteatosis, as well as changes in blood pressure, and health care utilization will be important to quantify in outcome studies. Overall, the bench to bedside GLP-1 story [131] illuminates the importance of how understanding mechanisms of peptide hormone action enabled the development of multiple new classes of therapies for people living with metabolic disorders, providing new effective options for individuals with obesity.
Acknowledgments
Daniel Drucker is supported in part by a Banting and Best Diabetes Centre Novo Nordisk Chair in Incretin Biology and the Sinai Health Novo Nordisk Fund in Regulatory peptides. This work was supported in part by CIHR grant 154321 and the Canada–Israel Health Research Initiative, jointly funded by the Canadian Institutes of Health Research, the Israel Science Foundation, International Development Research Centre, and the Azrieli Foundation, project 109150
Conflict of interest
Mt. Sinai Hospital has received funding for DJD from Novo Nordisk for preclinical studies of GLP-1 action. DJD has served as a speaker or consultant for Eli Lilly Inc., Forkhead Biotherapeutics Inc., Kallyope Inc, Merck Inc., Nestle Inc., Novo Nordisk Inc., and Pfizer Inc.
References
- 1.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proceedings of the National Academy of Sciences of the U S A. 1992;89(18):8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockie S.H., Heppner K.M., Chaudhary N., Chabenne J.R., Morgan D.A., Veyrat-Durebex C., et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like Peptide-1 receptor signaling. Diabetes. 2012;61(11):2753–2762. doi: 10.2337/db11-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beiroa D., Imbernon M., Gallego R., Senra A., Herranz D., Villarroya F., et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63(10):3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 4.Lee S.J., Sanchez-Watts G., Krieger J.P., Pignalosa A., Norell P.N., Cortella A., et al. Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT thermogenesis and increases adiposity. Molecular and Metabolism. 2018:1133–1146. doi: 10.1016/j.molmet.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabery S., Salinas C.G., Paulsen S.J., Ahnfelt-Ronne J., Alanentalo T., Baquero A.F., et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6) doi: 10.1172/jci.insight.133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basolo A., Burkholder J., Osgood K., Graham A., Bundrick S., Frankl J., et al. Exenatide has a pronounced effect on energy intake but not energy expenditure in non-diabetic subjects with obesity: a randomized, double-blind, placebo-controlled trial. Metabolism. 2018:85116–85125. doi: 10.1016/j.metabol.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen L.G.M., Nahon K.J., Bracke K.F.M., van den Broek D., Smit R., Sardjoe Mishre A.S.D., et al. Twelve weeks of exenatide treatment increases [(18)F]fluorodeoxyglucose uptake by brown adipose tissue without affecting oxidative resting energy expenditure in nondiabetic males. Metabolism. 2020 doi: 10.1016/j.metabol.2020.154167. 106154167. [DOI] [PubMed] [Google Scholar]
- 8.van Eyk H.J., Paiman E.H.M., Bizino M.B., SL I.J., Kleiburg F., Boers T.G.W., et al. Liraglutide decreases energy expenditure and does not affect the fat fraction of supraclavicular brown adipose tissue in patients with type 2 diabetes. Nutrition, Metabolism, and Cardiovascular Diseases. 2020;30(4):616–624. doi: 10.1016/j.numecd.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Blundell J., Finlayson G., Axelsen M., Flint A., Gibbons C., Kvist T., et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes, Obesity and Metabolism. 2017;19(9):1242–1251. doi: 10.1111/dom.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Y., Koehler J.A., Baggio L.L., Powers A.C., Sandoval D.A., Drucker D.J. Gut-proglucagon-derived peptides are essential for regulating glucose homeostasis in mice. Cell Metabolism. 2019;30(5):976–986 e973. doi: 10.1016/j.cmet.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLean B.A., Wong C.K., Campbell J.E., Hodson D.J., Trapp S., Drucker D.J. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocrine Reviews. 2021;42(2):101–132. doi: 10.1210/endrev/bnaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panjwani N., Mulvihill E.E., Longuet C., Yusta B., Campbell J.E., Brown T.J., et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE-/- mice. Endocrinology. 2013;154(1):127–139. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- 13.Shughrue P.J., Lane M.V., Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology. 1996:1375159–1375162. doi: 10.1210/endo.137.11.8895391. [DOI] [PubMed] [Google Scholar]
- 14.Merchenthaler I., Lane M., Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. The Journal of Comparative Neurology. 1999;403(2):261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Campos R.V., Lee Y.C., Drucker D.J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994:1342156–1342164. doi: 10.1210/endo.134.5.8156917. [DOI] [PubMed] [Google Scholar]
- 16.Goke R., Larsen P.J., Mikkelsen J.D., Sheikh S.P. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. European Journal of Neuroscience. 1995;7(11):2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 17.Turton M.D., O'Shea D., Gunn I., Beak S.A., Edwards C.M.B., Meeran K., et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996:37969–37972. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 18.Scrocchi L.A., Brown T.J., MacLusky N., Brubaker P.L., Auerbach A.B., Joyner A.L., et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide receptor gene. Nature Medicine. 1996:21254–21258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 19.Heppner K.M., Kirigiti M., Secher A., Paulsen S.J., Buckingham R., Pyke C., et al. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology. 2015;156(1):255–267. doi: 10.1210/en.2014-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ast J., Arvaniti A., Fine N.H.F., Nasteska D., Ashford F.B., Stamataki Z., et al. Super-resolution microscopy compatible fluorescent probes reveal endogenous glucagon-like peptide-1 receptor distribution and dynamics. Nature Communications. 2020;11(1):467. doi: 10.1038/s41467-020-14309-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Dijk G., Thiele T.E., Donahey J.C.K., Campfield L.A., Smith F.J., Burn P., et al. Central infusions of leptin and GLP-1-(7-36) amide differentially stimulate c-FLI in the rat brain. American Journal of Physiology. 1996 doi: 10.1152/ajpregu.1996.271.4.R1096. 271R1096-R1100. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig M.Q., Cheng W., Gordian D., Lee J., Paulsen S.J., Hansen S.N., et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nature Metabolism. 2021;3(4):530–545. doi: 10.1038/s42255-021-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mietlicki-Baase E.G., Ortinski P.I., Reiner D.J., Sinon C.G., McCutcheon J.E., Pierce R.C., et al. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. Journal of Neuroscience. 2014;34(20):6985–6992. doi: 10.1523/JNEUROSCI.0115-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fortin S.M., Lipsky R.K., Lhamo R., Chen J., Kim E., Borner T., et al. GABA neurons in the nucleus tractus solitarius express GLP-1 receptors and mediate anorectic effects of liraglutide in rats. Science Translational Medicine. 2020;12(533) doi: 10.1126/scitranslmed.aay8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams J.M., Pei H., Sandoval D.A., Seeley R.J., Chang R.B., Liberles S.D., et al. Liraglutide modulates appetite and body weight through glucagon-like peptide 1 receptor-expressing glutamatergic neurons. Diabetes. 2018;67(8):1538–1548. doi: 10.2337/db17-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anesten F., Mishra D., Dalmau Gasull A., Engstrom-Ruud L., Bellman J., Palsdottir V., et al. Glucagon-like peptide-1-, but not growth and differentiation factor 15-, receptor activation increases the number of interleukin-6-expressing cells in the external lateral parabrachial nucleus. Neuroendocrinology. 2019;109(4):310–321. doi: 10.1159/000499693. [DOI] [PubMed] [Google Scholar]
- 27.Shirazi R., Palsdottir V., Collander J., Anesten F., Vogel H., Langlet F., et al. Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6. Proceedings of the National Academy of Sciences of the U S A. 2013;110(40):16199–16204. doi: 10.1073/pnas.1306799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holt M.K., Richards J.E., Cook D.R., Brierley D.I., Williams D.L., Reimann F., et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68(1):21–33. doi: 10.2337/db18-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elias C.F., Kelly J.F., Lee C.E., Ahima R.S., Drucker D.J., Saper C.B., et al. Chemical characterization of leptin-activated neurons in the rat brain. The Journal of Comparative Neurology. 2000;423(2):261–281. [PubMed] [Google Scholar]
- 30.Secher A. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. Journal of Clinical Investigation. 2014;124(10):4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brierley D.I., Holt M.K., Singh A., de Araujo A., McDougle M., Vergara M., et al. Central and peripheral GLP-1 systems independently suppress eating. Nature Metabolism. 2021;3(2):258–273. doi: 10.1038/s42255-021-00344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borgmann D., Ciglieri E., Biglari N., Brandt C., Cremer A.L., Backes H., et al. Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metabolism. 2021;33(7):1466–1482 e1467. doi: 10.1016/j.cmet.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fortin S.M., Chen J., Hayes M.R. Hindbrain melanocortin 3/4 receptors modulate the food intake and body weight suppressive effects of the GLP-1 receptor agonist, liraglutide. Physiology & Behavior. 2020 doi: 10.1016/j.physbeh.2020.112870. 220112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He Z., Gao Y., Lieu L., Afrin S., Cao J., Michael N.J., et al. Direct and indirect effects of liraglutide on hypothalamic POMC and NPY/AgRP neurons - implications for energy balance and glucose control. Molecular and Metabolism. 2019:28120–28134. doi: 10.1016/j.molmet.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biglari N., Gaziano I., Schumacher J., Radermacher J., Paeger L., Klemm P., et al. Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nature Neuroscience. 2021;24(7):913–929. doi: 10.1038/s41593-021-00854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Washington M.C., Raboin S.J., Thompson W., Larsen C.J., Sayegh A.I. Exenatide reduces food intake and activates the enteric nervous system of the gastrointestinal tract and the dorsal vagal complex of the hindbrain in the rat by a GLP-1 receptor. Brain Research. 2010:1344124–1344133. doi: 10.1016/j.brainres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Hayes M.R., Skibicka K.P., Grill H.J. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149(8):4059–4068. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes M.R., Bradley L., Grill H.J. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150(6):2654–2659. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alhadeff A.L., Mergler B.D., Zimmer D.J., Turner C.A., Reiner D.J., Schmidt H.D., et al. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology. 2017;42(7):1471–1479. doi: 10.1038/npp.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayes M.R., Leichner T.M., Zhao S., Lee G.S., Chowansky A., Zimmer D., et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metabolism. 2011;13(3):320–330. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richard J.E., Anderberg R.H., Goteson A., Gribble F.M., Reimann F., Skibicka K.P. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rupprecht L.E., Mietlicki-Baase E.G., Zimmer D.J., McGrath L.E., Olivos D.R., Hayes M.R. Hindbrain GLP-1 receptor-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt. American Journal of Physiology. Endocrinology and Metabolism. 2013;305(6):E751–E759. doi: 10.1152/ajpendo.00367.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dossat A.M., Lilly N., Kay K., Williams D.L. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. Journal of Neuroscience. 2011;31(41):14453–14457. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alhadeff A.L., Baird J.P., Swick J.C., Hayes M.R., Grill H.J. Glucagon-like Peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology. 2014;39(9):2233–2243. doi: 10.1038/npp.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richard J.E., Farkas I., Anesten F., Anderberg R.H., Dickson S.L., Gribble F.M., et al. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology. 2014;155(11):4356–4367. doi: 10.1210/en.2014-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu T.M., Hahn J.D., Konanur V.R., Lam A., Kanoski S.E. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology. 2015;40(2):327–337. doi: 10.1038/npp.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terrill S.J., Jackson C.M., Greene H.E., Lilly N., Maske C.B., Vallejo S., et al. Role of lateral septum glucagon-like peptide 1 receptors in food intake. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2016;311(1):R124–R132. doi: 10.1152/ajpregu.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ong Z.Y., Liu J.J., Pang Z.P., Grill H.J. Paraventricular thalamic control of food intake and reward: role of glucagon-like peptide-1 receptor signaling. Neuropsychopharmacology. 2017;42(12):2387–2397. doi: 10.1038/npp.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reiner D.J., Leon R.M., McGrath L.E., Koch-Laskowski K., Hahn J.D., Kanoski S.E., et al. Glucagon-like peptide-1 receptor signaling in the lateral dorsal tegmental nucleus regulates energy balance. Neuropsychopharmacology. 2018;43(3):627–637. doi: 10.1038/npp.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams D.L., Lilly N.A., Edwards I.J., Yao P., Richards J.E., Trapp S. GLP-1 action in the mouse bed nucleus of the stria terminalis. Neuropharmacology. 2018:13183–13195. doi: 10.1016/j.neuropharm.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scrocchi L.A., Drucker D.J. Effects of aging and a high fat diet on body weight and glucose control in GLP-1R-/- mice. Endocrinology. 1998:1393127–1393132. doi: 10.1210/endo.139.7.6092. [DOI] [PubMed] [Google Scholar]
- 52.Hansotia T., Maida A., Flock G., Yamada Y., Tsukiyama K., Seino Y., et al. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. Journal of Clinical Investigation. 2007;117(1):143–152. doi: 10.1172/JCI25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scrocchi L.A., Hill M.E., Saleh J., Perkins B., Drucker D.J. Elimination of GLP-1R signaling does not modify weight gain and islet adaptation in mice with combined disruption of leptin and GLP-1 action. Diabetes. 2000:491552–491560. doi: 10.2337/diabetes.49.9.1552. [DOI] [PubMed] [Google Scholar]
- 54.Varin E.M., Mulvihill E.E., Baggio L.L., Koehler J.A., Cao X., Seeley R.J., et al. Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action. Cell Reports. 2019;27(11):3371–3384. doi: 10.1016/j.celrep.2019.05.055. e3373. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Ferreras L., Richard J.E., Noble E.E., Eerola K., Anderberg R.H., Olandersson K., et al. Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Molecular Psychiatry. 2018;23(5):1157–1168. doi: 10.1038/mp.2017.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meeran K., O'Shea D., Edwards C.M., Turton M.D., Heath M.M., Gunn I., et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology. 1999;140(1):244–250. doi: 10.1210/endo.140.1.6421. [DOI] [PubMed] [Google Scholar]
- 57.Patterson J.T., Ottaway N., Gelfanov V.M., Smiley D.L., Perez-Tilve D., Pfluger P.T., et al. A novel human-based receptor antagonist of sustained action reveals body weight control by endogenous GLP-1. ACS Chemical Biology. 2011;6(2):135–145. doi: 10.1021/cb1002015. [DOI] [PubMed] [Google Scholar]
- 58.Barrera J.G., Jones K.R., Herman J.P., D'Alessio D.A., Woods S.C., Seeley R.J. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. Journal of Neuroscience. 2011;31(10):3904–3913. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burmeister M.A., Ayala J.E., Smouse H., Landivar-Rocha A., Brown J.D., Drucker D.J., et al. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes. 2017;66(2):372–384. doi: 10.2337/db16-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burmeister M.A., Brown J.D., Ayala J.E., Stoffers D.A., Sandoval D.A., Seeley R.J., et al. The glucagon-like peptide-1 receptor in the ventromedial hypothalamus reduces short-term food intake in male mice by regulating nutrient sensor activity. American Journal of Physiology. Endocrinology and Metabolism. 2017;313(6):E651–E662. doi: 10.1152/ajpendo.00113.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J., Conde K., Zhang P., Lilascharoen V., Xu Z., Lim B.K., et al. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron. 2017;96(4):897–909. doi: 10.1016/j.neuron.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanoski S.E., Fortin S.M., Arnold M., Grill H.J., Hayes M.R. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152(8):3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baraboi E.D., St-Pierre D.H., Shooner J., Timofeeva E., Richard D. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011;301(4):R1011–R1024. doi: 10.1152/ajpregu.00424.2010. [DOI] [PubMed] [Google Scholar]
- 64.Duca F.A., Sakar Y., Covasa M. Combination of obesity and high-fat feeding diminishes sensitivity to GLP-1R agonist exendin-4. Diabetes. 2013;62(7):2410–2415. doi: 10.2337/db12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krieger J.P., Arnold M., Pettersen K.G., Lossel P., Langhans W., Lee S.J. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65(1):34–43. doi: 10.2337/db15-0973. [DOI] [PubMed] [Google Scholar]
- 66.Reiner D.J., Mietlicki-Baase E.G., McGrath L.E., Zimmer D.J., Bence K.K., Sousa G.L., et al. Astrocytes regulate GLP-1 receptor-mediated effects on energy balance. Journal of Neuroscience. 2016;36(12):3531–3540. doi: 10.1523/JNEUROSCI.3579-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Timper K., Del Rio-Martin A., Cremer A.L., Bremser S., Alber J., Giavalisco P., et al. GLP-1 receptor signaling in astrocytes regulates fatty acid oxidation, mitochondrial integrity, and function. Cell Metabolism. 2020;31(6):1189–1205. doi: 10.1016/j.cmet.2020.05.001. e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flint A., Raben A., Astrup A., Holst J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. Journal of Clinical Investigation. 1998:101515–101520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Silva A., Salem V., Long C.J., Makwana A., Newbould R.D., Rabiner E.A., et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metabolism. 2011;14(5):700–706. doi: 10.1016/j.cmet.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schlogl H., Kabisch S., Horstmann A., Lohmann G., Muller K., Lepsien J., et al. Exenatide-induced reduction in energy intake is associated with increase in hypothalamic connectivity. Diabetes Care. 2013;36(7):1933–1940. doi: 10.2337/dc12-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Bloemendaal L., RG I.J., Ten Kulve J.S., Barkhof F., Konrad R.J., Drent M.L., et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014 doi: 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- 72.ten Kulve J.S., Veltman D.J., van Bloemendaal L., Barkhof F., Deacon C.F., Holst J.J., et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia. 2015;58(12):2688–2698. doi: 10.1007/s00125-015-3754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eldor R., Daniele G., Huerta C., Al-Atrash M., Adams J., DeFronzo R., et al. Discordance between central (brain) and pancreatic action of exenatide in lean and obese subjects. Diabetes Care. 2016;39(10):1804–1810. doi: 10.2337/dc15-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daniele G., Iozzo P., Molina-Carrion M., Lancaster J., Ciociaro D., Cersosimo E., et al. Exenatide regulates cerebral glucose metabolism in brain areas associated with glucose homeostasis and reward system. Diabetes. 2015;64(10):3406–3412. doi: 10.2337/db14-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpern A., Krempf M., et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New England Journal of Medicine. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 76.le Roux C.W., Astrup A., Fujioka K., Greenway F., Lau D.C.W., Van Gaal L., et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–1409. doi: 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 77.Davies M.J., Bergenstal R., Bode B., Kushner R.F., Lewin A., Skjoth T.V., et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 78.Blackman A., Foster G.D., Zammit G., Rosenberg R., Aronne L., Wadden T., et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. International Journal of Obesity. 2016;40(8):1310–1319. doi: 10.1038/ijo.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wadden T.A., Hollander P., Klein S., Niswender K., Woo V., Hale P.M., et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. International Journal of Obesity. 2013;37(11):1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 80.Kelly A.S., Auerbach P., Barrientos-Perez M., Gies I., Hale P.M., Marcus C., et al. A randomized, controlled trial of liraglutide for adolescents with obesity. New England Journal of Medicine. 2020;382(22):2117–2128. doi: 10.1056/NEJMoa1916038. [DOI] [PubMed] [Google Scholar]
- 81.Chao A.M., Wadden T.A., Walsh O.A., Gruber K.A., Alamuddin N., Berkowitz R.I., et al. Effects of liraglutide and behavioral weight loss on food cravings, eating behaviors, and eating disorder psychopathology. Obesity. 2019;27(12):2005–2010. doi: 10.1002/oby.22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wadden T.A., Tronieri J.S., Sugimoto D., Lund M.T., Auerbach P., Jensen C., et al. Liraglutide 3.0 mg and intensive behavioral therapy (IBT) for obesity in primary care: the SCALE IBT randomized controlled trial. Obesity. 2020;28(3):529–536. doi: 10.1002/oby.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drucker D.J. Advances in oral peptide therapeutics. Nature Reviews Drug Discovery. 2020;19(4):277–289. doi: 10.1038/s41573-019-0053-0. [DOI] [PubMed] [Google Scholar]
- 84.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., et al. Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine. 2021;384(11):989. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 85.Davies M., Faerch L., Jeppesen O.K., Pakseresht A., Pedersen S.D., Perreault L., et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 86.Wadden T.A., Bailey T.S., Billings L.K., Davies M., Frias J.P., Koroleva A., et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–1413. doi: 10.1001/jama.2021.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rubino D., Abrahamsson N., Davies M., Hesse D., Greenway F.L., Jensen C., et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414–1425. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Friedrichsen M., Breitschaft A., Tadayon S., Wizert A., Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes, Obesity and Metabolism. 2021;23(3):754–762. doi: 10.1111/dom.14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kristensen S.L., Rorth R., Jhund P.S., Docherty K.F., Sattar N., Preiss D., et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 90.Sattar N., Lee M.M.Y., Kristensen S.L., Branch K.R.H., Del Prato S., Khurmi N.S., et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 91.Noyan-Ashraf M.H., Shikatani E.A., Schuiki I., Mukovozov I., Wu J., Li R.K., et al. A glucagon-like peptide-1 analogue reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127(1):74–85. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 92.Drucker D.J. The cardiovascular Biology of glucagon-like peptide-1. Cell Metabolism. 2016;24(1):15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 93.Hernandez A.F., Green J.B., Janmohamed S., D'Agostino R.B., Sr, Granger C.B., et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 94.Gerstein H.C., Sattar N., Rosenstock J., Ramasundarahettige C., Pratley R., Lopes R.D., et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. New England Journal of Medicine. 2021;385(10):896–907. doi: 10.1056/NEJMoa2108269. [DOI] [PubMed] [Google Scholar]
- 95.Husain M., Birkenfeld A.L., Donsmark M., Dungan K., Eliaschewitz F.G., Franco D.R., et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New England Journal of Medicine. 2019;381(9):841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 96.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jodar E., Leiter L.A., et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New England Journal of Medicine. 2016:3751834–3751844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 97.Ryan D.H., Lingvay I., Colhoun H.M., Deanfield J., Emerson S.S., Kahn S.E., et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. American Heart Journal. 2020:22961–22969. doi: 10.1016/j.ahj.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 98.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R., et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 99.Newsome P.N., Buchholtz K., Cusi K., Linder M., Okanoue T., Ratziu V., et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. New England Journal of Medicine. 2021;384(12):1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 100.Baggio L.L., Drucker D.J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Molecular and Metabolism. 2021:46101090. doi: 10.1016/j.molmet.2020.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scrocchi L.A., Brown T.J., Drucker D.J. Leptin sensitivity in non-obese GLP-1 receptor-/- mice. Diabetes. 1997 doi: 10.2337/diab.46.12.2029. 462029-2034. [DOI] [PubMed] [Google Scholar]
- 102.Cheng W., Ndoka E., Hutch C., Roelofs K., MacKinnon A., Khoury B., et al. Leptin receptor-expressing nucleus tractus solitarius neurons suppress food intake independently of GLP1 in mice. JCI Insight. 2020;5(7) doi: 10.1172/jci.insight.134359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Young A.A., Gedulin B.R., Bhavsar S., Bodkin N., Jodka C., Hansen B., et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta) Diabetes. 1999;48(5):1026–1034. doi: 10.2337/diabetes.48.5.1026. [DOI] [PubMed] [Google Scholar]
- 104.Williams D.L., Baskin D.G., Schwartz M.W. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55(12):3387–3393. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 105.Zhao S., Kanoski S.E., Yan J., Grill H.J., Hayes M.R. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. International Journal of Obesity. 2012;36(12):1522–1528. doi: 10.1038/ijo.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanoski S.E., Ong Z.Y., Fortin S.M., Schlessinger E.S., Grill H.J. Liraglutide, leptin and their combined effects on feeding: additive intake reduction through common intracellular signalling mechanisms. Diabetes, Obesity and Metabolism. 2015;17(3):285–293. doi: 10.1111/dom.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dakin C.L., Gunn I., Small C.J., Edwards C.M., Hay D.L., Smith D.M., et al. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142(10):4244–4250. doi: 10.1210/endo.142.10.8430. [DOI] [PubMed] [Google Scholar]
- 108.Day J.W., Ottaway N., Patterson J.T., Gelfanov V., Smiley D., Gidda J., et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nature Chemical Biology. 2009;5(10):749–757. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- 109.Pocai A., Carrington P.E., Adams J.R., Wright M., Eiermann G., Zhu L., et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58(10):2258–2266. doi: 10.2337/db09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parker J.A., McCullough K.A., Field B.C., Minnion J.S., Martin N.M., Ghatei M.A., et al. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. International Journal of Obesity. 2013;37(10):1391–1398. doi: 10.1038/ijo.2012.227. [DOI] [PubMed] [Google Scholar]
- 111.Kim T., Nason S., Holleman C., Pepin M., Wilson L., Berryhill T.F., et al. Glucagon receptor signaling regulates energy metabolism via hepatic farnesoid X receptor and fibroblast growth factor 21. Diabetes. 2018;67(9):1773–1782. doi: 10.2337/db17-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beaudry J.L., Kaur K.D., Varin E.M., Baggio L.L., Cao X., Mulvihill E.E., et al. The brown adipose tissue glucagon receptor is functional but not essential for control of energy homeostasis in mice. Molecular and Metabolism. 2019:2237–2248. doi: 10.1016/j.molmet.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salem V., Izzi-Engbeaya C., Coello C., Thomas D.B., Chambers E.S., Comninos A.N., et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes, Obesity and Metabolism. 2016;18(1):72–81. doi: 10.1111/dom.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wynne K., Park A.J., Small C.J., Meeran K., Ghatei M.A., Frost G.S., et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. International Journal of Obesity. 2006;30(12):1729–1736. doi: 10.1038/sj.ijo.0803344. [DOI] [PubMed] [Google Scholar]
- 115.Alba M., Yee J., Frustaci M.E., Samtani M.N., Fleck P. Efficacy and safety of glucagon-like peptide-1/glucagon receptor co-agonist JNJ-64565111 in individuals with obesity without type 2 diabetes mellitus: a randomized dose-ranging study. Clinical Obesity. 2021;11(2) doi: 10.1111/cob.12432. [DOI] [PubMed] [Google Scholar]
- 116.Di Prospero N.A., Yee J., Frustaci M.E., Samtani M.N., Alba M., Fleck P. Efficacy and safety of glucagon-like peptide-1/glucagon receptor co-agonist JNJ-64565111 in individuals with type 2 diabetes mellitus and obesity: a randomized dose-ranging study. Clinical Obesity. 2021;11(2) doi: 10.1111/cob.12433. [DOI] [PubMed] [Google Scholar]
- 117.Longuet C., Sinclair E.M., Maida A., Baggio L.L., Maziarz M., Charron M.J., et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metabolism. 2008;8(5):359–371. doi: 10.1016/j.cmet.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boland M.L., Laker R.C., Mather K., Nawrocki A., Oldham S., Boland B.B., et al. Resolution of NASH and hepatic fibrosis by the GLP-1R/GcgR dual-agonist Cotadutide via modulating mitochondrial function and lipogenesis. Nature Metabolism. 2020;2(5):413–431. doi: 10.1038/s42255-020-0209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ravussin E., Smith S.R., Mitchell J.A., Shringarpure R., Shan K., Maier H., et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity. 2009;17(9):1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kruse T., Hansen J.L., Dahl K., Schaffer L., Sensfuss U., Poulsen C., et al. Development of cagrilintide, a long-acting amylin analogue. Journal of Medicinal Chemistry. 2021;64(15):11183–11194. doi: 10.1021/acs.jmedchem.1c00565. [DOI] [PubMed] [Google Scholar]
- 121.Enebo L.B., Berthelsen K.K., Kankam M., Lund M.T., Rubino D.M., Satylganova A., et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2.4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet. 2021;397(10286):1736–1748. doi: 10.1016/S0140-6736(21)00845-X. [DOI] [PubMed] [Google Scholar]
- 122.Frias J.P., Davies M.J., Rosenstock J., Perez Manghi F.C., Fernandez Lando L., Bergman B.K., et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. New England Journal of Medicine. 2021;385(6):503–515. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 123.Adriaenssens A.E., Biggs E.K., Darwish T., Tadross J., Sukthankar T., Girish M., et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metabolism. 2019;30(5):987–996 e986. doi: 10.1016/j.cmet.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dowsett G.K.C., Lam B.Y.H., Tadross J.A., Cimino I., Rimmington D., Coll A.P., et al. A survey of the mouse hindbrain in the fed and fasted states using single-nucleus RNA sequencing. Molecular and Metabolism. 2021;53101240 doi: 10.1016/j.molmet.2021.101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Samms R.J., Christe M.E., Collins K.A., Pirro V., Droz B.A., Holland A.K., et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. Journal of Clinical Investigation. 2021;131(12) doi: 10.1172/JCI146353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borner T., Geisler C.E., Fortin S.M., Cosgrove R., Alsina-Fernandez J.A., Dogra M., et al. GIP receptor agonism attenuates GLP-1 receptor agonist induced nausea and emesis in preclinical models. Diabetes. 2021 doi: 10.2337/db21-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Overgaard R.V., Navarria A., Ingwersen S.H., Baekdal T.A., Kildemoes R.J. Clinical pharmacokinetics of oral semaglutide: analyses of data from clinical pharmacology trials. Clinical Pharmacokinetics. 2021 doi: 10.1007/s40262-021-01025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Welling M.S., de Groot C.J., Kleinendorst L., van der Voorn B., Burgerhart J.S., van der Valk E.S., et al. Effects of glucagon-like peptide-1 analogue treatment in genetic obesity: a case series. Clinical Obesity. 2021:12481. doi: 10.1111/cob.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lundgren J.R., Janus C., Jensen S.B.K., Juhl C.R., Olsen L.M., Christensen R.M., et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. New England Journal of Medicine. 2021;384(18):1719–1730. doi: 10.1056/NEJMoa2028198. [DOI] [PubMed] [Google Scholar]
- 130.Wharton S., Lau D.C.W., Vallis M., Sharma A.M., Biertho L., Campbell-Scherer D., et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–E891. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Drucker D.J., Habener J.F., Holst J.J. Discovery, characterization, and clinical development of the glucagon-like peptides. Journal of Clinical Investigation. 2017;127(12):4217–4227. doi: 10.1172/JCI97233. [DOI] [PMC free article] [PubMed] [Google Scholar]