Abstract
Background:
Propolis, a natural antibiotic, which is in high demand in dentistry is a resinous substance. The main ingredient of propolis that is required for antibiotic effect is flavonoids and phenolic acids. Although propolis is a promising option for the control of oral microbes with lower related hazards and a good immunomodulator effect, its composition differs considerably depending on its botanical origin, the site and the season of collection. This original research aims to find the chemical composition and minimum inhibitory concentration (MIC) of propolis procured from different places of Karnataka state. The results would help the dentist and the pharmacist to select the best propolis to use as antibiotics in treating oral disease.
Materials and Methods:
Propolis sample from 5 different locations of Karnataka was procured from single apiary in Bangalore. Extraction of propolis using two different extracting solvents was carried out. The total phenolic content, total flavonoid content and MIC of each sample were analyzed.
Results:
Water extract propolis of Sullia and Hubli was highly active against tested organism with the MIC <0.312; alcohol extract of Sullia, Hubli and Chitradurga was moderately active with the MIC between 0.312 and 5 mg/ml. Vijayapura and Bagalkot were least active with the MIC >5 mg/ml at tested concentration.
Conclusion:
Propolis procured from different locations of Karnataka can be used as an antimicrobial agent with varying concentrations. However, when propolis is procured for therapeutic purpose, then it needs to be tested for its chemical composition before being utilized.
Keywords: Actinobacillus actinomycetemcomitans, Candida albicans, flavonoid content, phenolic content, propolis, Staphylococcus aureus, Streptococcus mutans
INTRODUCTION
Research has proven that natural products, mainly of the plant origin, are an cardinal source of medicinal products.[1,2,3,4] These products may be in the form of phytochemicals or the secondary metabolites.[5] The secondary metabolites act as defense agent by infringing with the molecular targets of the invaders and microbes and hence protect the host.[6,7,8,9]
Propolis (bee glue) is a natural product with CAS (chemical abstract service) registry number 9009-62-5.[10] The propolis resin is the amalgamation of materials secreted by plants that are collected by bees[11,12] along with components derived from bee saliva.
Because of the presence of these chemical components with least toxicity,[13] propolis is considered as one of the health-enhancing agents. The principle components or the bioactive components that are reported for antimicrobial effect are the phenolic compounds.[8,9,10,11,12,13] A major constituent of phenolic compound is flavonoids amounting about 50%.[14] As these bioactive materials essential for propolis activity are foraged from the vegetation, their composition depends on the location and the season of collection from the hive.
Bankova has carried out a vast number of studies on antimicrobial activity of propolis from Brazil. He has reported that standardized chemical components of propolis will allow scientists to formulate effective products for human use.[15] At present, research on Indian propolis is limited. Only few studies related to Indian propolis are available [Table 1].[16,17,18,19,20]
Table 1.
Studies on Indian propolis
| Activity of propolis tested | Geographic region | References |
|---|---|---|
| Antimicrobial | Madhya Pradesh (single place) | Bhadauria[16] |
| Hepatoprotective and antimicrobial | Maharashtra (single place) | Wagh et al.[17] |
| Antioxidant | West Bengal (single place) | Laskar et al.[18] |
| Antioxidant, antimicrobial | Tamil Nadu (single place - Javadi hills) | Kumar et al.[19] |
| Antibacterial | Karnataka (single place - Bangalore) | Selvan et al.[20] |
In this endeavor as a step to document national wide information and to provide best propolis medication in dentistry, different locations in Karnataka have been selected as procurement points. Studying the Karnataka propolis for its total phenolic content, total flavonoid (TF) content and minimum inhibitory concentration (MIC) against four oral microorganisms is the novelty of this research work.
MATERIALS AND METHODS
The study was conducted in the Department of Prosthodontics, Crown and Bridge, department of Pharmacognosy and department of Microbiology.
Materials
Sodium carbonate (Na3CO3), Folin Ciocalteau's (FC's) phenol reagent, aluminum trichloride (AlCl3), DPPH (3, 3-diphenyl-1-picrylhydrazyl), gallic acid and ascorbic acid – all chemical and instruments were of analytical grade and available commercially. The UV-visible spectrophotometric values were recorded in UV-500 Spectrophotometer.
Study sample
Inclusion criteria
The sample collected in spring from certified single apiary by manual scraping technique of extracting from wooden hive was selected.
Exclusion criteria
Less than 50 g of sample which is inadequate quantity to carry out test and mixed between collection dates samples were rejected.
Sample collection
Propolis samples were obtained from apiary authenticated by Karnataka Government situated at Bangalore (GSTIN: 29NUPG0440JIZT). The samples were collected from the six different locations of Karnataka as per the inclusion criteria.
The six different locations of Karnataka which followed the inclusion criteria were Bagalkot (A), Bijapur (B), Sullia (C), Chitradurga (D), Hubballi (E) and Bangalore (F). The sample from Bangalore was not sufficient to carry out the procedure and efforts made to procure the 2nd batch were futile hence it was eliminated. Procured sample was cut into pieces, grounded, divided into two equal parts, placed in the zip lock freezer bags and labeled.
Extraction of propolis
Raw form of propolis needs to be purified by extraction with solvents before its use as medicament. The extraction techniques should not destroy the principal compounds, especially flavonoids and phenolic. Previous studies revealed that maceration and refluxing techniques will not alter the chemical structures of propolis and the techniques are very simple, affordable and can be used as a routine extraction procedure.[21,22,23,24,25,26,27,28] Therefore, these techniques were followed in this research. Commonly used solvents for the extraction of propolis are water and ethanol in different percentages. Hence, in this study, both water extract propolis (WEP) and 70% ethanol extract propolis (AEP) were used. Obtained filtrates were concentrated and dried on boiling water bath. From the concentrated sample, 1 mg/ml stock solution was prepared [Figure 1]. This stock solution was used for analyzing the total phenolic content by FC colorimetric method, TF content by aluminum chloride colorimetric method which was modified from the procedure reported by Woisky and Salatino[22] and antimicrobial activity, i.e., MIC. Antimicrobial activity of propolis procured from different parts of Karnataka was analyzed against following oral microorganisms.
Figure 1.

Stock solution
Staphylococcus aureus (S. aureus) (ATCC No12598)
Streptococcus mutans (S. mutans) (ATCC No 25175)
Actinobacillus actinomycetemcomitans (Aa) (ATCC No 43718)
Candida albicans (C. albicans) (ATCC No 2091).
Estimation of total phenolic content
To estimate phenolic content of extracts of propolis, FC reagent was used with gallic acid as standard. The reaction mixture consists of 1 ml of FC phenol reagent diluted with water 1:10, v/v and 1 ml of extract of propolis with a concentration of 100 μg and 200 μg was treated to the mix and shaken well. 5 min later, 1 ml sodium carbonate of 8% was put to the mixture. It was incubated for 30 min at room temperature. UV spectrophotometer at 765 nm the optical density was used for standard and test was determined against reagent blank. Milligram gallic acid equivalents (GAEs) per gram of extract (mg GAE/g extract) expressed the total phenolic content. All samples were analyzed in triplicates.
Estimation of total flavonoid content
Woisky and Salatino method was modified and the aluminum chloride colorimetric procedure was carried on. Standard calibration curve was prepared using quercetin. 10 mg of quercetin was dissolved in 80% ethanol, different aliquots of quercetin (0–100 μg) and 100 ul of extracts were taken and was made up to 1 ml with 80% ethanol. To this 10% of aluminum chloride, 0.1 ml of potassium acetate and 3.8 ml of distilled water were added and allowed to stand for 30 min at room temperature. The absorbance was measured at 415 nm using UV-spectrophotometer. The TF content was calculated according to the standard quercetin calibration curve. The mean of three readings was used and expressed as mg of quercetin equivalents (QE)/100 g of propolis.
Antimicrobial activity
The MIC of the propolis extract was carried out with broth dilution technique. The MIC was determined according to NCCLS guidelines. Inoculum suspension was prepared from 24 h broth cultures. Bacterial culture was adjusted to 0.5 McFarland turbidity standards (1.5 × 10 8 CFU/mL), and 10 μL of diluted suspensions of bacterial culture was added to 50 μL of various concentrations of given extract into the well. Chlorhexidine gluconate was used as a commercial standard. 50% DMSO and water in the ratio of 1:1 was used as vehicle control and highest concentration of the sample without bacterial suspension were served as a sample blank. Plates were incubated at 37°C for 24 h. After incubation OD was taken at 600 nm to analyze the bacterial inhibition [Figure 2].
Figure 2.
Minimum inhibitory concentration
Data entry and analysis
The data were entered at the end of each procedure and analyzed using the SPSS software (IBM Corp.Released 2016.IBM SPSS Statistics for Window, Version 24.0.Armonk, NY:IBM Corp).
RESULTS
Results of water extracted Propolis and alcohol extracted Propolis from each study place in terms of total phenolic content (TPC), total flavonoid content (TFC) & minimum inhibitory concentration (MIC) are shown in Tables 2 and 3 and Graph 1.
Table 2.
Comparison of chemical composition of propolis procured from different geographic locations in Karnataka in terms of total phenolic compound and flavonoid content
| Place | Total phenolic content (mg of GAE value per g) | Total flavonoid content (mg of QE value per g) |
|---|---|---|
| Bagalkot (A1) | 173.2±0.70 | 36.8±0.43 |
| Bagalkot (A2) | 196.7±0.26 | 11.53±0.73 |
| Vijayapura (B1) | 191.9±1.05 | 46.00±1.71 |
| Vijayapura (B2) | 259.5±1.40 | 08.58±0.51 |
| Sullia (C1) | 180.0±0.60 | 32.32±0.81 |
| Sullia (C2) | 171.2±1.02 | 21.43±1.10 |
| Chitradurga (D1) | 181.0±1.02 | 32.13±1.20 |
| Chitradurga (D2) | 178.0±0.55 | 22.10±1.00 |
| Hubballi (E1) | 192.2±0.33 | 33.08±1.00 |
| Hubballi (E2) | 175.4±0.57 | 31.73±0.85 |
Values are expressed mean±SD for triplicate. SD: Standard deviation, GAE: Gallic acid equivalents, QE: Quercetin equivalents
Table 3.
Comparison of chemical composition (total phenolic content and total flavonoid content) and antimicrobial activity of propolis procured from different geographic locations in Karnataka
| Place | TPC (mg of GAE value per gram) | TFC (mg of QE value per gram) | Ratio of F/P content | Streptococcus mutans (mg/ml) | Staphylococcus aureus (mg/ml) | Aa (mg/ml) | Candida albicans (mg/ml) |
|---|---|---|---|---|---|---|---|
| Bagalkot (A1) | 173.2±0.70 | 36.8±0.43 | 20.8 | 0.12 | 0.50 | 0.12 | 0.50 |
| Bagalkot (A2) | 196.7±0.26 | 11.53±0.73 | 05.8 | 0.06 | 00 | 0.25 | 00 |
| Vijayapura (B1) | 191.9±1.05 | 46.00±1.71 | 23.9 | 0.50 | 0.25 | 0.25 | 0.50 |
| Vijayapura (B2) | 259.5±1.40 | 08.58±0.51 | 03.3 | 0.06 | 00 | 0.25 | 00 |
| Sullia (C1) | 180.0±0.60 | 32.32±0.81 | 17.9 | 0.01 | 0.50 | 0.25 | 0.50 |
| Sullia (C2) | 171.2±1.02 | 21.43±1.10 | 12.5 | 0.06 | 00 | 0.12 | 0.00 |
| Chitradurga (D1) | 181.0±1.02 | 32.13±1.20 | 17.7 | 0.12 | 0.25 | 0.12 | 0.06 |
| Chitradurga (D2) | 178.0±0.55 | 22.10±1.00 | 12.4 | 0.25 | 0.25 | 0.25 | 0.12 |
| Hubballi (E1) | 192.2±0.33 | 33.08±1.00 | 17.1 | 0.12 | 0.25 | 0.12 | 0.01 |
| Hubballi (E2) | 175.4±0.57 | 31.73±0.85 | 18.6 | 0.12 | 0.12 | 0.12 | 0.03 |
TPC: Total phenolic content, TFC: Total flavonoid content, Aa: Actinobacillus actinomycetemcomitans, F/P: Flavonoid to phenolic, GAE: Gallic acid equivalents, QE: Quercetin equivalents
Graph 1.
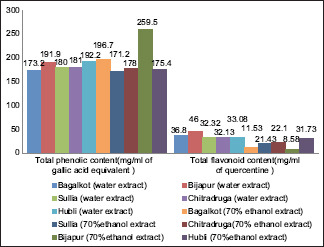
Total phenolic content and the total flavonoid content of all 5 places
DISCUSSION
Propolis cannot be utilized as antibiotic agent directly from the hive, because of its complex structure. It has to be extracted using solvent for human use. The most common extraction solvents are ethanol, chloroform, water, methanol, dichloromethane ether and acetone.[29,30,31,32] Numerous studies have concentrated on the constituents and antioxidant activities of propolis extracts with various extraction solvents. According to Laskar et al.[18] study, the combination of water and ethanol solvents was more effective in extracting propolis chemical constituent than water, and the ethanol extracts alone. Similar results were illustrated in the previous research, reporting that ethanol and water combination solvent is particularly suitable to obtain propolis extracts rich in phenolic components, especially flavonoids with high contents. Another literature data suggested that nonethanolic propolis extracts have better pharmacological activity, as compared to ethanolic extracts.[33] Therefore, in this study, investigation of the chemical composition (total phenolic content [TPC] and total flavonoid content [TFC]) was carried out with two different extraction solvents, i.e., 70% ethanol and distilled water. There are many techniques to extract raw material using these solvents. However, there are no standard protocols for extraction procedures for propolis. The routine extraction procedure that maintains original chemical structure of propolis such as maceration followed by refluxing was done.
In the current research, the total phenolic content (TP) values ranged from 171.2 mg/g to 259 mg/g and TF content ranged from 8.58 mg/g to 46 mg/g [Table 2 and Graph 1]. These results are comparable with the study results obtained from other countries [Table 4].
Table 4.
The total phenolic content and total flavonoid content of propolis from various countries
| Places | TPC (mg/g) | TFC (mg/g) | Reference number |
|---|---|---|---|
| Algeria | 55-279 | 10-69 | Boufadi et al.[33] |
| Argentina | 257-393 | 66-133 | Lima et al.[34] |
| Brazil | 94-149 | 06-21 | Schmidt et al.[35] |
| China | 43-32 | 08-162 | Ahn et al.[36] |
| Greece and Cyprus | 80-338 | 09-183 | Kalogeropoulos et al.[37] |
| Japan | 53-431 | 18-113 | Hamasaka et al.[38] |
| Morocco | 0.74-91.22 | 0.2-34.27 | Miguel et al.[39] |
| Poland | 150-197 | 36-62 | Socha et al.[40] |
| South Korea | 85-283 | 16-135 | Ahn et al.[41] |
TPC: Total phenolic content, TFC: Total flavonoid content
The examined propolis samples possess considerable total phenol and flavonoid contents as compared to studies carried in various regions of the world.[33,34,35,36,37,38,39,40,41] The results of our studies clearly indicate that tested propolis samples procured from different locations of Karnataka with comparable high total phenolic (TP) and TF contents can be selected for commercial propolis products even though the TP and TF vary widely according to geographic location.
In addition, TPC and TFC of propolis from Vijayapura excelled. It means that it had better flavonoid content. The reason for Vijayapura to be better in flavonoid content could be the high temperature climate favorable for hive compared to other tested locations.
The next key detection of the study was that, water extract of propolis from Karnataka fared better in TPC and TFC than the alcohol extract. This is the major advantage to overcome the demerits of alcohol extract which had strong residual flavor and difficultly of its usage in dentistry.
The WEP results in the study were in agreement with study by Nagai et al.[42,43]
Antimicrobial sensitivity test
The MIC results of current study revealed that the antimicrobial activity of propolis irrespective of geographic location ranged from 0.01 mg/ml to >0.5 mg/ml [Table 4]. This is in accordance with the study done by Seidel et al.[44] The author carried out broth microdilution assay and documented that propolis of Europe, South America and North America origin had MIC ranging from 0.1255 mg/ml to >0.55 mg/ml whereas the African ranged from 0.085 mg/ml to >0.5 mg/ml. The present study MIC values and comparing with the previous literature MIC values clearly suggest that the tested propolis extracts have significant antimicrobial against S. aureus, S. mutans, A. actinomycetemcomitans and C. albicans.
Further, WEP of Sullia and Hubballi was highly active with the MIC <0.312 mg/ml [Map 1]. AEP of Sullia, Hubballi and Chitradurga was moderately active with the MIC between 0.3125 and 0.5 mg/ml. Bijapur and Bagalkot were less active with the MIC >0.5 mg/ml. According to Przybyłek and Karpiński[45] et al., the possible antimicrobial action of propolis is by the action of propolis on the cellular membrane permeability of the microorganism, membrane potential disruption, decreasing adenosine triphosphate production, reducing bacterial mobility, increase the host immune system. Further, Havsteen[46] and Oksuz et al.[47] suggested that propolis acts by preventing cell division hence there is inhibition of protein synthesis and reduction of bacterial growth, formation of pseudo-multicellular bacterial forms.
Map 1.
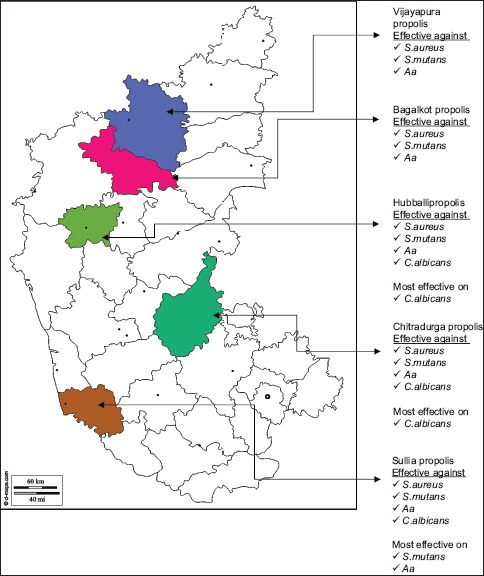
Antimicrobial activity of propolis procured from different locations of Karnataka
Al-Ani et al. in their study results on European propolis reported the bactericidal effects against Gram-positive microorganisms with MIC ranging from 0.04 to 1.2 mg/ml and bacteriostatic effect against Gram-negative microorganisms, with MIC ranging from 0.6 to >5 mg/ml.[48] The results of present research showed MIC range from 0.0165 mg/ml to >0.5 mg/ml against Gram-positive microorganisms and MIC range from 0.125 mg/ml to >0.25 mg/ml against Gram-negative microorganisms. With this MIC inference, it can be assumed that the samples procured from Karnataka have bactericidal effect on Gram-positive and bacteriostatic effect on Gram-negative microorganisms. Along with these, the antimicrobial activity results of present study are in line with the findings that Gram-positive is sensitive to low propolis concentration and Gram-negative microbes are inhibited at little higher concentration.[49,50,51]
Fernandes Júnior et al.[52] stated that propolis has different antibacterial mechanisms, such as cell division inhibition, collapsing microbial cytoplasm cell membranes and cell walls, bacterial motility is inhibited, enzyme responsible for bacterial activity was inactivated, bacteriolysis, inhibition of protein synthesis. Further Orsi et al. described that drug resistance in microorganism can be overcome by using propolis which has multitarget effect by combing with other antibiotics.[53]
Another interesting analysis of this research was that the C. albicans (MIC values 0.125 mg/ml to >0.5 mg/ml) was highly sensitive to propolis sample [Table 4]. Mello et al.[54] in the imaging study with electron microscopy reported the mechanisms of action (MOA) of Brazilian green propolis. According to the study, MOA of propolis on the C. albicans was by rupturing of the cell wall of the fungus. Da Silva et al. in 2008 demonstrated that the propolis can reduce the Candida infection at a higher rate than fluconazole and nystatin.[55]
However, when the profile of individual place of Karnataka was analyzed from the present study, it is interesting to know that out of 5 places selected based on inclusion criteria, the 4 places were from northern part of Karnataka. Even though they were from northern part, not all had the same antimicrobial activity and chemical composition (TPC and TFC). Hubballi propolis showed the best activity whereas Sullia which is known for green belt and good vegetation did not show good antimicrobial activity as compared to Hubballi and Chitradurga.
Hence, as per the observational analysis, the results depicted that propolis procured from different locations of Karnataka has antimicrobial activity against tested oral pathogen but chemical composition (TPC and TFC) does not proportionately reflects antimicrobial activity of the propolis.
CONCLUSION
Propolis procured from different locations of Karnataka varies and possesses notable antimicrobial effect against common oral pathogens (S. mutans, S. aureus, Aa and C. albicans). Propolis from Sullia and Hubbali was very active against S. mutans and C.albicans, respectively. This promising antimicrobial effect along with literature evidence of immune modulating action of propolis encourages to utilize Karnataka propolis in controlling oral infection. Further clinical trials are needed to establish relative efficacy of propolis and synthetic drugs. Collectively, the study results benefit dentists, research scholars, microbiologist and pharmacists in selecting the most active propolis which delivers better clinical results than the current synthetic products.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Oral Health WHO Website. [Last accessed on 2018 Sep 24]. Available from: http://www.who.int/newsroom/factsheet/detail/oralhealth .
- 2.Barnett ML. The role of therapeutic antimicrobial mouthrinses in clinical practice: Control of supragingival plaque and gingivitis. J Am Dent Assoc. 2003;134:699–704. doi: 10.14219/jada.archive.2003.0255. [DOI] [PubMed] [Google Scholar]
- 3.Kurek A, Grudniak AM, Kraczkiewicz-Dowjat A, Wolska KI. New antibacterial therapeutics and strategies. Pol J Microbiol. 2011;60:3–12. [PubMed] [Google Scholar]
- 4.Aminov RI. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front Microbiol. 2010;1:134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calixto JB. Twenty-five years of research on medicinal plants in Latin America: A personal view. J Ethnopharmacol. 2005;100:131–4. doi: 10.1016/j.jep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Croteau R, Kutchan TM, Lewis NG. Natural Products (Secondary Metabolites). Natural Products (Secondary Metabolites). Biochemistry and Molecular Biology of Plants. Rock Ville: American Society of Plant Physiologists; 2000. pp. 1250–318. [Google Scholar]
- 7.Erler S, Moritz RF. Pharmacophagy and pharmacophory: Mechanisms of self-medication and disease prevention in the honeybee colony (Apis mellifera) Apidologie. 2016;47:389–411. [Google Scholar]
- 8.Middleton E Jr. Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol. 1998;439:175–82. doi: 10.1007/978-1-4615-5335-9_13. [DOI] [PubMed] [Google Scholar]
- 9.Molan PC. The potential of honey to promote oral wellness. Gen Dent. 2001;49:584–9. [PubMed] [Google Scholar]
- 10.Park YK, Alencar SM, Agujar CL. Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem. 2002;50:2502–6. doi: 10.1021/jf011432b. [DOI] [PubMed] [Google Scholar]
- 11.Silva-Carvalho R, Baltazar F, Almeida-Aguiar C. Propolis: A complex natural product with a plethora of biological activities that can be explored for drug development. Evid Based Complement Alternat Med. 2015;2015:206439. doi: 10.1155/2015/206439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva BB, Rosalen PL, Cury JA, Ikegaki M, Souza VC, Estevez A, et al. Chemical composition and botanical origin of red propolis, a new type of Brazilian propolis. Evid Based Complement Alternat Med. 2008;103:487–92. doi: 10.1093/ecam/nem059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogdanov S. Propolis: Composition, health, medicine: A review. Bee Product science [Doi: 10.1155/2015/206439] Available from: http://www.bee-hexagon.net .
- 14.Opsenica DM, Ristivojević P, Trifković J, Vovk I, Lušić D, Tešić Z. TLC fingerprinting and pattern recognition methods in the assessment of authenticity of poplar-type propolis. J Chromatogr Sci. 2016;54:1077–83. doi: 10.1093/chromsci/bmw024. [DOI] [PubMed] [Google Scholar]
- 15.Bankova V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol. 2005;100:114–7. doi: 10.1016/j.jep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Bhadauria M. Propolis prevents hepatorenal injury induced by chronic exposure to carbon tetrachloride. Evid Based Complement Alternat Med. 2012;2012:235358. doi: 10.1155/2012/235358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagh VD, Borkar RD, Kalaskar MG, Nerkar PP, Surana SJ. “HPLC Method for the Identification and Qualitatively Estimation of Tannic acid and Quercetin in Indian Propolis,” In Proceedings of the National Conference on Pharmaceutical Analysis, Dr. B A. Marathwada University, Aurangabad, India. 2011 October [Google Scholar]
- 18.Laskar RA, Sk I, Roy N, Begum NA. Antioxidant activity of Indian propolis and its chemical constituents. Food Chem. 2010;122:233–7. [Google Scholar]
- 19.Kumar N, Ahmad MK, Dang R, Husain A. Antioxidant and antimicrobial activity of propolis from Tamil Nadu zone. J Med Plants Res. 2008;2:361–4. [Google Scholar]
- 20.Selvan A, Singh R, Prabhu D. Anti-bacteria activity of bee propolis against clinical strains of Streptococcus mutants and synergism with chlorhexidine. Int J Pharm Stud Res. 2011;2:85–90. [Google Scholar]
- 21.Marcucci MC. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. [Google Scholar]
- 22.Woiski RG, Salatino A. Analysis of propolis: Some parameters and procedures for chemical quality control. J Apicult Res. 1998;37:99–105. [Google Scholar]
- 23.Cunha IB, Sawaya AC, Caetano FM, Shimizu MT, Marcucci MC, Drezza FT, et al. Factors that influence the yield and composition of Brazilian propolis extracts. J Braz Chem Soc. 2004;15:964–70. [Google Scholar]
- 24.Trusheva B, Trunkova D, Bankova V. Different extraction methods of biologically active components from propolis: A preliminary study. Chem Cent J. 2007;1:13. doi: 10.1186/1752-153X-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machado BA, Silva RP, de Abreu Barreto G, Costa SS, da Silva DF, Brandão HN, et al. Chemical composition and biological activity of extracts obtained by supercritical extraction and ethanolic extraction of brown, green and red propolis derived from different geographic regions in Brazil. PLoS One. 2016;11:e0145954. doi: 10.1371/journal.pone.0145954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutierrez-Gonçalves ME, Marcucci MC. Antimicrobial and antioxidant activities of propolis from Ceará state. Fitos. 2009;4:81–6. [Google Scholar]
- 27.Margeretha I, Suniarti DF, Herda E, Mas’ud ZA. Optimization and comparative study of different extraction methods of biologically active components of Indonesian propolis Trigona spp. J Nat Prod. 2012;5:233–42. [Google Scholar]
- 28.Pobiega K, Kraśniewska K, Derewiaka D, Gniewosz M. Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. J Food Sci Technol. 2019;56:5386–95. doi: 10.1007/s13197-019-04009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gómez-Caravaca AM, Gómez-Romero M, Arráez-Román D, Segura-Carretero A, Fernández-Gutiérrez A. Advances in the analysis of phenolic compounds in products derived from bees. J Pharm Biomed Anal. 2006;41:1220–34. doi: 10.1016/j.jpba.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Miguel MG, Nunes S, Dandlen SA, Cavaco AM, Antunes MD. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem Toxicol. 2010;48:3418–23. doi: 10.1016/j.fct.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Volpert R, Elstner EF. Biochemical activities of propolis extracts. II. Photodynamic activities. Z Naturforsch C J Biosci. 1993;48:858–62. doi: 10.1515/znc-1993-11-1207. [DOI] [PubMed] [Google Scholar]
- 32.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boufadi YM, Soubhye J, Riazi A, Rousseau A, Vanhaeverbeek M, Nève J, et al. Characterization and antioxidant properties of six Algerian propolis extracts: Ethyl acetate extracts inhibit myeloperoxidase activity. Int J Mol Sci. 2014;15:2327–45. doi: 10.3390/ijms15022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lima B, Tapia A, Luna L, Fabani MP, Schmeda-Hirschmann G, Podio NS, et al. Main flavonoids, DPPH activity and metal content allow determination of the geographical origin of propolis from the province of San Juan (Argentina) J Agric Food Chem. 2009;57:2691–8. doi: 10.1021/jf803866t. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt EM, Stock D, Chada FJ, Finger D, Sawaya AC, Eberlin MN, et al. A comparison between characterization and biological properties of Brazilian fresh and aged propolis. Biomed Res Int. 2014;2014:257617. doi: 10.1155/2014/257617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn MR, Kumazawa S, Usui Y, Nakamura J, Matsuka M, Zhu F, et al. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007;101:1383–92. [Google Scholar]
- 37.Kalogeropoulos N, Konteles SJ, Troullidou E, Mourtzinos I, Karathanos V. Chemical composition, antioxidant activity and antimicrobial properties of propolis extract from Greece and Cyprus. Food Chem. 2009;116:452–61. [Google Scholar]
- 38.Hamasaka T, Kumazawa S, Fujimoto T, Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of Japan. Food Sci Technol Res. 2004;10:86–92. doi: 10.1021/jf048726s. [DOI] [PubMed] [Google Scholar]
- 39.Miguel MG, Doughmi O, Aazza S, Antunes D, Lyoussi B. Antioxidant, anti-inflammatory and acetylcholinesterase inhibitory activities of propolis from different regions of Morocco. Food Sci Biotechnol. 2014;23:313–22. [Google Scholar]
- 40.Socha R, Gałkowska D, Bugaj M, Juszczak L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat Prod Res. 2015;29:416–22. doi: 10.1080/14786419.2014.949705. [DOI] [PubMed] [Google Scholar]
- 41.Ahn MR, Kumazawa S, Hamasaka T, Bang KS, Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J Agric Food Chem. 2004;52:7286–92. doi: 10.1021/jf048726s. [DOI] [PubMed] [Google Scholar]
- 42.Nagai T, Inoue R, Inoue H, Suzuki N. Preparation and antioxidante properties of water extract of própolis. Food Chem. 2003;80:29–33. [Google Scholar]
- 43.Kumazawa S, Hamasaka T, Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004;84:329–39. [Google Scholar]
- 44.Seidel V, Peyfoon E, Watson DG, Fearnley J. Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytother Res. 2008;22:1256–63. doi: 10.1002/ptr.2480. [DOI] [PubMed] [Google Scholar]
- 45.Przybyłek I, Karpiński TM. Antibacterial properties of propolis. Molecules. 2019;24:2047. doi: 10.3390/molecules24112047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32:1141–8. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 47.Oksuz H, Duran N, Tamer C, Cetin M, Silici S. Effect of propolis in the treatment of experimental Staphylococcus aureus keratitis in rabbits. Ophthalmic Res. 2005;37:328–34. doi: 10.1159/000087943. [DOI] [PubMed] [Google Scholar]
- 48.Al-Ani I, Zimmermann S, Reichling J, Wink M. Antimicrobial activities of European propolis collected from various geographic origins alone and in combination with antibiotics. Medicines (Basel) 2018;5:2. doi: 10.3390/medicines5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tosi B, Donini A, Romagnoli C, Bruni A. Antimicrobial activity of some commercial extracts of propolis prepared with different solvents. Phytother Res. 1996;10:335–6. [Google Scholar]
- 50.Stepanović S, Antić N, Dakić I, Svabić-Vlahović M. In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol Res. 2003;158:353–7. doi: 10.1078/0944-5013-00215. [DOI] [PubMed] [Google Scholar]
- 51.Onlen Y, Duran N, Atik E, Savas L, Altug E, Yakan S, et al. Antibacterial activity of propolis against MRSA and synergism with topical mupirocin. J Altern Complement Med. 2007;13:713–8. doi: 10.1089/acm.2007.7021. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes Júnior A, Balestrin EC, Betoni JE, Orsi Rde O, da Cunha Mde L, Montelli AC. Propolis: Anti-Staphylococcus aureus activity and synergism with antimicrobial drugs. Mem Inst Oswaldo Cruz. 2005;100:563–6. doi: 10.1590/s0074-02762005000500018. [DOI] [PubMed] [Google Scholar]
- 53.Orsi RD, Fernandes A, Bankova V, Sforcin JM. The effects of Brazilian and Bulgarian propolis in vitro against Salmonella typhi and their synergism with antibiotics acting on the ribosome. Nat Prod Res. 2012;26:430–7. doi: 10.1080/14786419.2010.498776. [DOI] [PubMed] [Google Scholar]
- 54.Mello AM, Gomes RT, Lara SR, Silva LG, Alves JB, Cortés ME, et al. The effect of Brazilian propolis on the germ tube formation and cell wall of candida. Pharmacologyonline. 2006;3:352–8. [Google Scholar]
- 55.da Silva WJ, Rached RN, Rosalen PL, Del bel Cury AA. Effects of nystatin, fluconazole and propolis on poly (methyl methacrylate) resin surface. Braz Dent J. 2008;19:190–6. doi: 10.1590/s0103-64402008000300003. [DOI] [PubMed] [Google Scholar]


