Abstract
Background:
Lichen planus is a chronic mucocutaneous disease of unknown etiology with well-established clinical and microscopic features. Oral lichenoid reactions are a family of lesions triggered by contact with specific agents such as metallic restorative materials, resins and drugs. Oral lichenoid reactions share clinical and histological features of oral lichen planus (OLP) but has varied management options. Hence, the discrimination between these two lesions is a major challenge for clinicians as well as pathologists.
Aim:
Histopathologic categorization of OLP and oral lichenoid reaction by comparing the thickness of basement membrane and the distribution of mast cells using special stains.
Materials and Methods:
The test group consists of formalin-fixed paraffin-embedded blocks of OLP (n = 15), and oral lichenoid reactions (n = 15) obtained from the archives of the Department of Oral Pathology, Amrita School of Dentistry. Three serial sections of 4 μm thickness were cut from each block. The sections were stained with H & E, periodic acid-Schiff (PAS) and toluidine blue, respectively.
Results:
A significant increase in the maximum basement membrane thickness (BMT) was noticed in OLP when compared to oral lichenoid reaction. A definite increase was also noticed in the number of mast cells in OLP and oral lichenoid reaction when compared to normal oral mucosa. There was a statistically significant increase in the number of degranulated mast cells in the deeper connective tissue in oral lichenoid reaction when compared to OLP.
Conclusion:
OLP and oral lichenoid reactions are two different entities showing similar clinical and microscopic presentation. The histochemical analysis of basement membrane and mast cells in these lesions might provide a more authentic method for differentiating these two lesions and might be of utmost value in deciding the treatment options.
Keywords: Basement membrane thickness, mast cells, oral lichen planus, oral lichenoid reaction
INTRODUCTION
Oral mucosa is affected by a number of lichenoid lesions, the etiology of which is attributed to infective, inflammatory, dysplastic and immune-mediated conditions, resulting in distinct disease entities in terms of diagnosis and prognosis.[1,2] Clinically, they present as white lesions associated with erosive areas. The presence of reticular pattern is quite characteristic and is often helpful in the clinical diagnosis of lichenoid reactions. The overlapping of features is due to the similarity in the pathogenic mechanisms involved in the development of these lesions. Lichenoid group of lesions include lichen planus, graft versus host disease, lichenoid reaction (drug/amalgam induced), chronic ulcerative stomatitis and discoid lupus erythematosus.[3,4] As these lesions show similar clinical and microscopic presentation differentiating one lesion from the other is extremely difficult and is often based on proper history, blood investigations and microscopic features.
Oral lichen planus (OLP) and oral lichenoid reactions commonly occurring in oral mucosa often pose a diagnostic challenge for the clinician as well as pathologist. Differentiating these two lesions become important as the management options varies between these two lesions. Oral lichenoid reactions usually result from systemic drug exposure (oral lichenoid drug reaction) or local allergic contact hypersensitivity (oral lichenoid contact reaction). Hence, recognition and removal of the causative agent is enough to manage such lesions.[5,6] Whereas OLP, a cell-mediated autoimmune disease is presently treated with corticosteroids locally or systemically depending on the severity of the lesion and a treatment modality for a complete cure is yet to be attained.
Many studies had been previously done for histopathological categorization of OLP and oral lichenoid reaction, but a definite criteria is not yet defined.[7,8] We took basement membrane as a major criteria because in both the lesions pathological alterations are happening at the basement membrane, and additional criteria as mast cells since they play an important role in the pathogenesis of these lesions. The aim of the study was to differentiate these two lesions by assessing the difference in the basement membrane thickness (BMT) and mast cell distribution using special stains such as PAS and toluidine blue.
MATERIALS AND METHODS
The study sample included formalin-fixed paraffin-embedded blocks obtained from the archives of the Department of Oral Pathology and Microbiology, Amrita School of Dentistry, Kochi. The test group consisted of OLP from 15 participants (n = 15), and oral lichenoid reactions from 15 participants (n = 15). Sample size was calculated based on the findings of a previous study.[4] Three serial sections of 4 μm thickness were cut from each block. The sections were stained with H & E, PAS, and toluidine blue, respectively.
For the assessment of BMT, sections stained with PAS were used. The thickness was measured by using a calibrated graticule. In each slide, six zones were selected, 3 being the maximum BMT1 and the other 3 with minimum BMT2. The measurements were obtained in micrometer and BMT1 and BMT2 in OLP and oral lichenoid reactions were compared.
For the assessment of the number of mast cells, sections stained with toluidine blue were divided into six different zones.
Zone 1: Within the epithelium
Zone 2: At the basement membrane
Zone 3: Within the inflammatory infiltrate
Zone 4: At the junction of the infiltrate and the connective tissue.
Zone 5: Around the blood vessels
Zone 6: Deeper connective tissue.
The total number of mast cells was calculated in six different zones by using a calibrated graticule.
Statistical analysis
The BMT and total number of mast cells of lichen planus and lichenoid reactions were compared by using the Student's t-test using IBM Corp. Released 2011.IBM SPSS Statistics for Windows, Version 20.0.Armonk, NY:IBM Corp. The number and type of mast cells in lichen planus and lichenoid reactions as well as within the different zones in the same group were compared using the analysis of variance test.
RESULTS
Assessment of basement membrane thickness
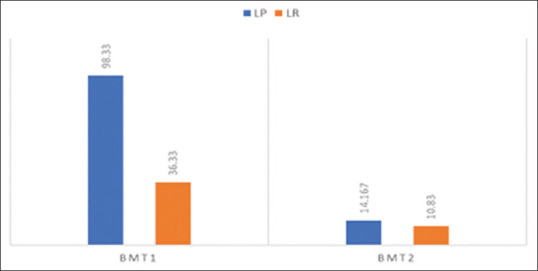
A statistically significant increase (P < 0.05) in the means of maximum BMT1 and minimum BMT2 were noticed in OLP when compared to oral lichenoid reaction [Table 1 and Figure 1].
Table 1.
Comparison of BMT1 in lichen planus and lichenoid reactions
| BMT | Type of lesion | n | Mean | SD | P |
|---|---|---|---|---|---|
| BMT 1 | Lichen planus | 15 | 98.33 | 33.4700 | <0.001 |
| Lichenoid reaction | 15 | 36.33 | 10.55 | ||
| BMT 2 | Lichen planus | 15 | 14.167 | 3.3630 | <0.001 |
| Lichenoid reaction | 15 | 10.833 | 3.6187 |
BMT: Basement membrane thickness, SD: Standard deviation
Figure 1.
Comparison of maximum and minimum basement membrane thickness in oral lichen planus and oral lichenoid reaction
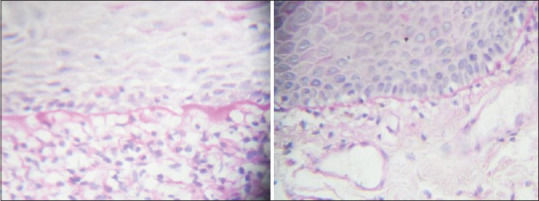
The increase in the thickness of basement membrane in OLP was not uniform and showed areas of focal thickenings. However, in oral lichenoid reactions, the increase in thickness of basement membrane was uniform [Figure 2].
Figure 2.
Maximum basement membrane thickness and minimum basement membrane thickness depicted as basement membrane thickness 1 and basement membrane thickness 2
Assessment of number of mast cells
A definite increase in the number of mast cells was noticed in OLP and oral lichenoid reaction compared to normal oral mucosa. There was no statistically significant difference in the total number of mast cells between two groups of OLP and oral lichenoid reactions [Table 2].
Table 2.
Comparison of total number of mast cells between two groups of oral lichen planus and oral lichenoid reactions
| Type of lesion | n | Mean | SD | P | |
|---|---|---|---|---|---|
| Number of mast cells | Lichen planus | 15 | 21.66 | 10.24 | 0.205 |
| Lichenoid reaction | 15 | 27.40 | 13.97 |
SD: Standard deviation
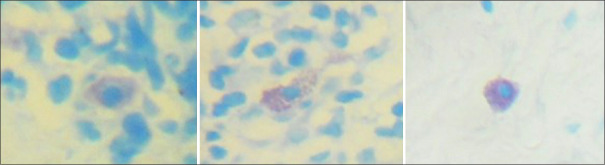
Based on the morphology, three different types of mast cells have been identified [Figure 3]. We have observed a difference between the morphology of mast cells in different zones. A statistically significant increase in the number of degranulated mast cells in deeper connective tissue (P = 0.02) was noticed in oral lichenoid reaction when compared to OLP [Table 3].
Figure 3.
Three morphological variants of mast cells; intact, degranulating, and degranulated
Table 3.
Comparison of morphological variants of mast cells in different zones between two groups of oral lichen planus and oral lichenoid reactions
| Zones, types | Sum of squares | DF | Mean square | F | P |
|---|---|---|---|---|---|
| Zone 1 type 2 | 0.041 | 1 | 0.041 | 1.161 | 0.291 |
| Zone 1 type 3 | 0.003 | 1 | 0.003 | 0.014 | 0.907 |
| Zone 2 type 2 | 0.041 | 1 | 0.041 | 1.161 | 0.291 |
| Zone 3 type 1 | 8.545 | 1 | 8.545 | 3.409 | 0.076 |
| Zone 3 type 3 | 1.143 | 1 | 1.143 | 0.217 | 0.645 |
| Zone 4 type 2 | 3.289 | 1 | 3.289 | 0.169 | 0.685 |
| Zone 4 type 3 | 2.464 | 1 | 2.464 | 1.051 | 0.315 |
| Zone 5 type 1 | 8.232 | 1 | 8.232 | 3.530 | 0.072 |
| Zone 5 type 2 | 4.807 | 1 | 4.807 | 1.422 | 0.244 |
| Zone 5 type 3 | 2.726 | 1 | 2.726 | 6.056 | 0.021 |
| Zone 6 type 1 | 0.018 | 1 | 0.018 | 0.033 | 0.857 |
| Zone 6 type 2 | 1.172 | 1 | 1.172 | 0.096 | 0.759 |
| Zone 6 type 3 | 5.934 | 1 | 5.934 | 5.334 | 0.029 |
DISCUSSION
In our study we found a significant increase in the maximum BMT1 in OLP when compared to oral lichenoid reaction. However, the difference in the minimum BMT2 between the two groups was not significant. A similar finding has been reported by Juneja et al.[4] who found an increase in thickness of the basement membrane in OLP compared to oral lichenoid reaction. However, contrary to this finding, Janardhanan et al.[5] found that there was no significant difference between the BMT in OLP and oral lichenoid reaction. In our study, the increase in thickness of basement membrane in OLP was not uniform and showed areas of focal thickenings. However, in oral lichenoid reactions, a uniform increase in the thickness of basement membrane was noticed. Zhou et al. and Juneja et al. also noted a focal thickening in the basement membrane of lichen planus and the focal thickening in the basement membrane had been attributed to matrix metalloproteinases secreted by T-cells and macrophages, which was further enhanced by mast cell chymase and tryptase.[6]
An increase in the number of mast cells was noticed in OLP and oral lichenoid reaction when compared to normal oral mucosa. Jahanshahi et al.[7] noted an increase in the number of mast cells in OLP, whereas Reddy et al.[1] noted an increase in the number of mast cells in OLP and oral lichenoid reaction when compared to normal mucosa. We also observed that there was a statistically significant increase in the number of degranulated mast cells in deeper connective tissue of oral lichenoid reaction when compared to OLP. A similar increase in the number of degranulated mast cells in the deeper connective tissue in oral lichenoid reaction had been previously reported.[7] Mast cells are bone marrow derived immune cells containing numerous basophilic granules in the cytoplasm. In oral mucosa, they are commonly found in the lamina propria along the microvascular bed in close approximity with the basement membrane of vascular endothelial cells and nerves. On activation by various stimuli (antigens, drugs, neuropeptides, chemokines etc.,), they undergo degranulation by the release of biologically active mediators like TNF alpha, tryptase, histamine, heparin, serotonin, chymase, and acid hydrolases.[8,9] These mediators play a major role in the extravasation and subepithelial migration of T-lymphocytes through various mechanisms including enhanced vascular endothelial adhesion molecule, degradation of the extracellular matrix by proteinases and through the induction of gelatinases and stromelysins and also through chemotaxis.[10,11] The reason for the increased number of degranulated mast cells in the deeper connective tissue in oral lichenoid reaction when compared to OLP could be due to the fact that oral lichenoid reaction is a type of delayed hypersensitivity reaction.[12,13] Here the presence of an allergen locally or systemically causes degranulation of mast cells and subsequent migration of T-lymphocytes.[14,15] Degranulation of mast cells can be regarded as a primary reaction in early stages of oral lichenoid reactions. However, OLP is an autoimmune disease in which cytotoxic T-cells are activated due to a modified keratinocyte surface antigen.[16,17] Here the degranulation of mast cells occurs after T-cell activation and this occurs in final phases of the event.
Based on our study, it can be inferred that the BMT and the number and distribution of mast cells can be used as a method to differentiate OLP and oral lichenoid reaction. The significant increase in the maximum BMT along with a focal increase in thickness in basement membrane can be considered as a diagnostic feature of OLP, whereas an increase in the number of degranulated mast cells in the deeper connective tissue is more suggestive of oral lichenoid reaction.
CONCLUSION
The existing histopathologic criteria to differentiate these lesions are based on subtle microscopic changes and cannot be relied upon completely. The histochemical analysis of basement membrane and mast cells in these lesions might prove to be a more authentic method for differentiating these two lesions and might be of utmost value in deciding the treatment options. Thus, our present observations indicate that the two histopathological parameters, the BMT and the number of degranulated mast cells in the deeper connective tissue can be used as a reliable diagnostic feature to distinguish between OLP and oral lichenoid reactions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Reddy DS, Sivapathasundharam B, Saraswathi TR, SriRam G. Evaluation of mast cells, eosinophils, blood capillaries in oral lichen planus and oral lichenoid mucositis. Indian J Dent Res. 2012;23:695–6. doi: 10.4103/0970-9290.107422. [DOI] [PubMed] [Google Scholar]
- 2.Farthing PM, Speight PM. Problems and pitfalls in oral mucosal pathology. Curr Diag Pathol. 2006;12:66–74. [Google Scholar]
- 3.Gonzalez-Moles MA, Scully C, Gil-Montoya JA. Oral lichen planus: Controversies surrounding malignant transformation. Oral Dis. 2008;14:229–43. doi: 10.1111/j.1601-0825.2008.01441.x. [DOI] [PubMed] [Google Scholar]
- 4.Juneja M, Mahajan S, Rao NN, George T, Boaz K. Histochemical analysis of pathological alterations in oral lichen planus and oral lichenoid lesions. J Oral Sci. 2006;48:185–93. doi: 10.2334/josnusd.48.185. [DOI] [PubMed] [Google Scholar]
- 5.Janardhanan M, Ramesh V. Mast cells in oral lichen planus. Oral Maxillofac Pathol J. 2010;1:49–52. [Google Scholar]
- 6.Schlosser BJ. Lichen planus and lichenoid reactions of the oral mucosa. Dermatol Ther. 2010;23:251–67. doi: 10.1111/j.1529-8019.2010.01322.x. [DOI] [PubMed] [Google Scholar]
- 7.Jahanshahi G, Ghalayani P, Maleki L. Mast cells distribution and variations in epithelium thickness and basement membrane in oral lichen planus lesion and oral lichenoid reaction. Dent Res J (Isfahan) 2012;9:180–4. doi: 10.4103/1735-3327.95233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou XJ, Sugerman PB, Savage NW, Walsh LJ. Matrix metalloproteinases and their inhibitors in oral lichen planus. J Cutan Pathol. 2001;28:72–82. doi: 10.1034/j.1600-0560.2001.280203.x. [DOI] [PubMed] [Google Scholar]
- 9.DeRossi SS, Ciarrocca KN. Lichen planus, lichenoid drug reactions, and lichenoid mucositis. Dent Clin North Am. 2005;49:77–89. doi: 10.1016/j.cden.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Walsh LJ, Savage NW, Ishii T, Seymour GJ. Immunopathogenesis of oral lichen planus. J Oral Pathol Med. 1990;19:389–96. doi: 10.1111/j.1600-0714.1990.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 11.Jose M, Raghu AR, Rao NN. Evaluation of mast cells in oral lichen planus and oral lichenoid reaction. Indian J Dent Res. 2001;12:175–9. [PubMed] [Google Scholar]
- 12.Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 13.Sharma R, Sircar K, Singh S, Rastogi V. Role of mast cells in pathogenesis of oral lichen planus. J Oral Maxillofac Pathol. 2011;15:267–71. doi: 10.4103/0973-029X.86674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laine J, Konttinen YT, Beliaev N, Happonen RP. Immunocompetent cells in amalgam-associated oral lichenoid contact lesions. J Oral Pathol Med. 1999;28:117–21. doi: 10.1111/j.1600-0714.1999.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 15.Thornhill MH, Pemberton MN, Simmons RK, Theaker ED. Amalgam-contact hypersensitivity lesions and oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:291–9. doi: 10.1067/moe.2003.115. [DOI] [PubMed] [Google Scholar]
- 16.Eversole LR. Immunopathology of oral mucosal ulcerative, desquamative, and bullous diseases. Selective review of the literature. Oral Surg Oral Med Oral Pathol. 1994;77:555–71. doi: 10.1016/0030-4220(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 17.Sugerman PB, Savage NW, Xu LJ, Walsh LJ, Seymour GJ. Heat shock protein expression in oral lichen planus. J Oral Pathol Med. 1995;24:1–8. doi: 10.1111/j.1600-0714.1995.tb01121.x. [DOI] [PubMed] [Google Scholar]