Abstract
Basaloid squamous cell carcinoma (BSCC) is a rare variant of squamous cell carcinoma characterized by a conglomerate of clinically aggressive course and disparate histopathological features. It is frequently seen in upper aerodigestive tract area. Histopathologically, it is biphasic and composed of two types of tumor cells, namely basaloid and squamous cells. Tumor markers, namely, BerEp4, epithelial membrane antigen and p53 are used in this case to differentiate from similar tumors which impersonate BSCC histologically but differ prognostically. We report a case of BSCC in a 48-year-old female patient, involving the lateral border of the tongue with an exhaustive picture of its histological and immunohistochemical appearance.
Keywords: Basaloid squamous cell carcinoma, BerEp4, epithelial membrane antigen, oral cavity, oropharynx, p53, squamous cell carcinoma
INTRODUCTION
Basaloid squamous cell carcinoma (BSCC) is an aggressive and histologically distinct variant of squamous cell carcinoma, first described by Wain et al. in 1986.[1] It has a predilection for upper aerodigestive tract. It is rarely seen in oral cavity with predilection for base of the tongue.[2] It commonly affects males usually above sixth decade.[3] It is considered to be more aggressive when compared to classical squamous cell carcinoma, due to its frequent metastasis.[4] The presence of ulceration makes it difficult to diagnose histopathologically, as it might mask the origin from the superficial mucosa. In the above scenario, it is prudent to use the immunohistochemistry markers which can help us in supporting the diagnosis. We are present a case of similar situation where three tumor markers are used namely p53, BerEp4 and epithelial membrane antigen (EMA) to establish the diagnosis.[5,6,7]
CASE DETAILS
A 48-year-old female patient came with the chief complaint of difficulty in opening the mouth and painful ulcer in the tongue for the past 6 months. Medical history reveals no significant findings. The patient had no history of tobacco habits and was well built. On extraoral examination, submandibular lymph nodes were palpable on the left side which was firm in consistency. On intraoral examination, an ulceroproliferative lesion was found on the left lateral side of the tongue measuring 3 cm × 2 cm, which was firm in consistency with indurated margin.
Microscopic findings
Overlying epithelium showed dysplastic stratified squamous epithelium infiltrating into the underlying connective tissue [Figure 1a]
Two types of tumor cells, namely squamous cells and basaloid cells, are seen [Figure 1b]
Tumor cells are characterized by sheets of basaloid cells with hyperchromatic nuclei and scanty cytoplasm predominantly arranged in lobular pattern [Figure 1c and d]
Squamous differentiation seen in center of tumor islands [Figure 2a]
Tumor cells show peripheral palisading [Figure 2b], marked mitotic activity [Figure 2c] and skeletal muscle infiltration [Figure 2d].
Figure 1(a-d).
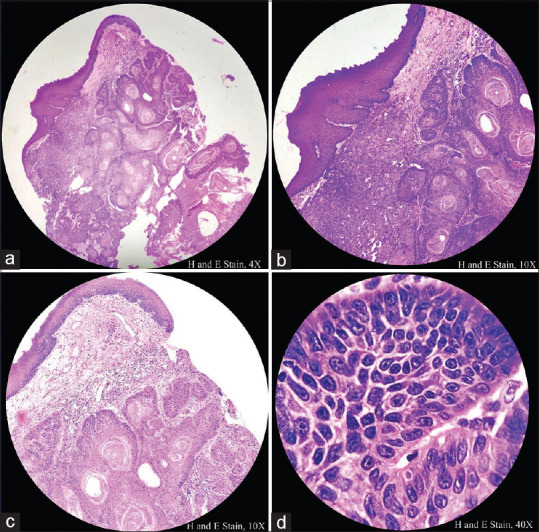
Histopathological image shows overlying dysplastic stratified squamous epithelium infiltrating into the underlying connective in the form of sheets and lobular pattern. Tumor cells show hyperchromatic nuclei, scanty cytoplasm
Figure 2(a-d).
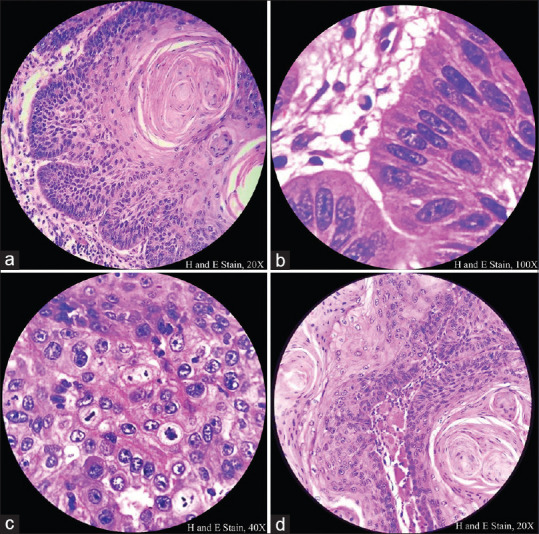
Histopathological image shows central squamous differentiation, peripheral palisading, increased mitotic activity and skeletal muscle infiltration of tumor cells
Immunohistochemistry findings
Tumor cells stained positive for p53, except in the areas of squamous differentiation in the center of tumor island [Figure 3a-d]
Tumor cells stained positive for EMA, except in the areas of squamous differentiation within tumor island, which stained positive for EMA [Figure 5a-d].
Figure 3(a-d).
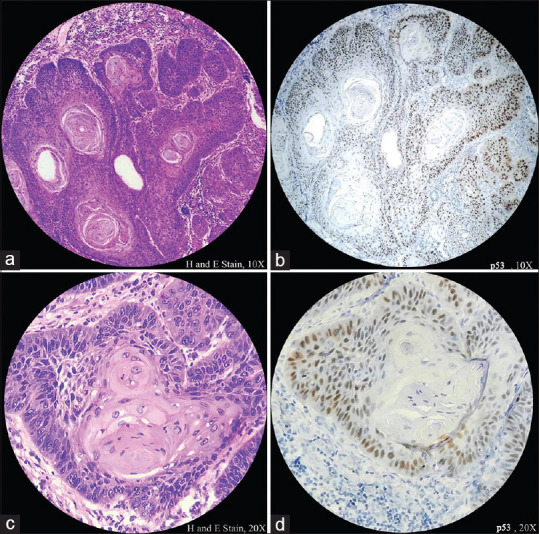
Histopathological image shows tumor cells stained positive for p53 except in the areas of squamous differentiation within tumor island
Figure 4(a-d).
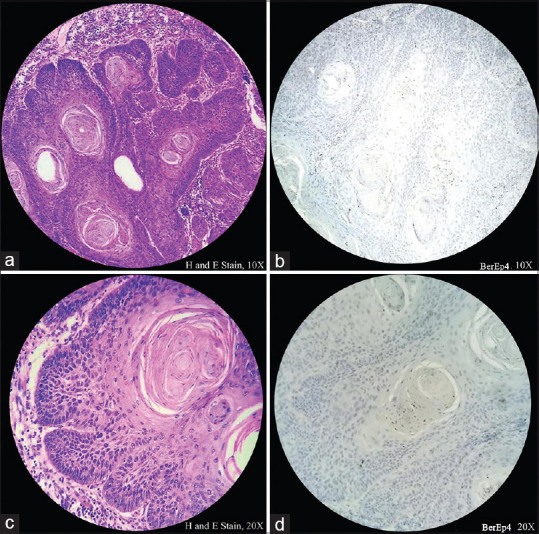
Histopathological image shows tumor cells stained negative for Ber-Ep4
Figure 5(a-d).
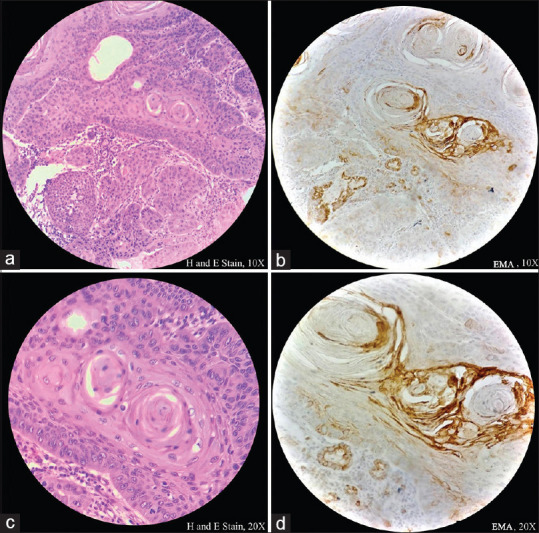
Histopathological image shows tumor cells predominantly stained negative for epithelial membrane antigen. Focal positive stain is seen in the areas of squamous differentiation
Differential diagnosis of BSCC is mentioned in Table 1,[8,9,10,11,12,13,14] Final diagnosis was made BSCC.
Table 1.
Differential diagnosis
| Tumor | Histopathology features | Immunhistochemistry feature | Our case |
|---|---|---|---|
| Basosquamous carcinoma | Basal cell carcinoma component with basaloid cells showing peripheral palisading, coexisting with squamous cell carcinoma component | BerEp4 – Positive EMA – Negative |
BerEp4 – Positive EMA – Negative |
| Adenoid cystic carcinoma | Tumor cells do not show marked nuclear atypia and mitotic figures, while hyaline material is extensively seen. Squamous differentiation within tumor islands and/or overlying dysplastic epithelium is usually absent | EMA – luminal cells shows positivity | |
| Small cell endocrine carcinoma | Small hyperchromatic cells with scanty cytoplasm, absence of nucleoli and presence of necrosis | EMA – Positive | EMA – Negative (except areas of squamous differentiation) |
| Adenosquamous carcinoma | Presence of ductoglandular differentiation and intracellular mucin Absence of basaloid cells with peripheral palisading |
p53 – Positive | p53 – Positive |
| Squamous cell carcinoma | Lacks basaloid cells with increased mitotic activity, peripheral palisading and comedo necrosis | BerEp4 – Negative EMA – Positive |
BerEp4 – Negative EMA – Negative (except areas of squamous differentiation) |
EMA: Epithelial membrane antigen
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: Report of 10 cases. Hum Pathol. 1986;17:1158–66. doi: 10.1016/s0046-8177(86)80422-1. [DOI] [PubMed] [Google Scholar]
- 2.Hirai E, Yamamoto K, Yamamoto N, Yamashita Y, Kounoe T, Kondo Y, et al. Basaloid squamous cell carcinoma of the mandible: Report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e54–8. doi: 10.1016/j.tripleo.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Linskey KR, Gimbel DC, Zukerberg LR, Duncan LM, Sadow PM, Nazarian RM. BerEp4, cytokeratin 14, and cytokeratin 17 immunohistochemical staining aid in differentiation of basaloid squamous cell carcinoma from basal cell carcinoma with squamous metaplasia. Arch Pathol Lab Med. 2013;137:1591–8. doi: 10.5858/arpa.2012-0424-OA. [DOI] [PubMed] [Google Scholar]
- 4.Cardesa A, Zidar N, Ereño C. Basaloid squamous cell carcinoma. In: Barnes L, Eveson JW, Reichart P, editors. WHO Classification Head and Neck Tumours. Pathology & Genetics Head and Neck Tumours. Lyon: IARC Press; 2005. pp. 124–5. [Google Scholar]
- 5.Mane DR, Kale AD, Angadi P, Hallikerimath S. Expression of cytokeratin subtypes: MMP-9, p53, and αSMA to differentiate basaloid squamous cell carcinoma from other basaloid tumors of the oral cavity. Appl Immunohistochem Mol Morphol. 2013;21:431–43. doi: 10.1097/PAI.0b013e31827c00e1. [DOI] [PubMed] [Google Scholar]
- 6.Sramek B, Lisle A, Loy T. Immunohistochemistry in ocular carcinomas. J Cutan Pathol. 2008;35:641–6. doi: 10.1111/j.1600-0560.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 7.Beer TW, Shepherd P, Theaker JM. Ber EP4 and epithelial membrane antigen aid distinction of basal cell, squamous cell and basosquamous carcinomas of the skin. Histopathology. 2000;37:218–23. doi: 10.1046/j.1365-2559.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 8.Ciążyńska M, Sławińska M, Kamińska-Winciorek G, Lange D, Lewandowski B, Reich A, et al. Clinical and epidemiological analysis of basosquamous carcinoma: Results of the multicenter study. Sci Rep. 2020;10:18475. doi: 10.1038/s41598-020-72732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson L. Malignant neoplasm of the larynx, hypopharynx and trachea. In: Thompson L, Bishop J, editors. Head and Neck Pathology. Philadelphia: Elsevier; 2006. pp. 51–88. [Google Scholar]
- 10.Ereño C, Gaafar A, Garmendia M, Etxezarraga C, Bilbao FJ, López JI. Basaloid squamous cell carcinoma of the head and neck: A clinicopathological and follow-up study of 40 cases and review of the literature. Head Neck Pathol. 2008;2:83–91. doi: 10.1007/s12105-008-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JC, Gnepp DR, Bedrossian CW. Adenoid cystic carcinoma of the salivary glands: An immunohistochemical analysis. Oral Surg Oral Med Oral Pathol. 1988;65:316–26. doi: 10.1016/0030-4220(88)90116-8. [DOI] [PubMed] [Google Scholar]
- 12.Robinson RA. Lippincott Williams & Wilkins; 2010. Upper airway squamous dysplasia, early invasive squamous carcinoma, and squamous carcinoma variants. In: Head and Neck Pathology: Atlas for Histologic and Cytologic Diagnosis; pp. 1–24. [Google Scholar]
- 13.Mittal R, Kaza H, Agarwal S, Rath S, Gowrishankar S. Small cell neuroendocrine carcinoma of the orbit presenting as an orbital abscess in a young female. Saudi J Ophthalmol. 2019;33:308–11. doi: 10.1016/j.sjopt.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinno Y, Nagatsuka H, Chong-Huat S, Tsujigiwa H, Tamamura R, Gunduz M, et al. Basaloid squamous cell carcinoma of the tongue in a Japanese male patient: A case report. Oral Oncol Extra. 2005;4:65–9. [Google Scholar]


