Oral lichen planus (OLP) is an auto immune, common, chronic T-cell-mediated inflammatory disorder of the skin and mucous membranes that occurs in various clinical forms that often poses a diagnostic and therapeutic challenge due to its refractory course and relapsing nature.[1,2] The term Lichen is derived from a Greek word Leichen that refers to moss tree. In some literature, lichens are primitive plants composed of symbiotic algae and fungi. The word Planus is a Latin word which means flat. The term suggests a meaning of flat fungal condition.[3,4] Ferdinand Ritter von Hebra (1816 – 1880), Dermatologist, described skin disease “Oral Ruber Planus” and termed as Lichen Ruber in 1860. Erasmus Wilson (British Dermatologist) first used the term as Lichen Planus in 1869. Thibierge described oral lichens symmetrically in 1893. In 1895 Wickham described the striae. Later, Andreasen described the six clinical forms and Dubreulith described the microscopic picture of OLP. In 1910, Francois Henri Hallopeau reported the first OLP-related carcinoma.[4,5]
DEFINITIONS
OLP is defined as a common chronic immunological mucocutaneous disorder that varies in appearance from keratotic to erythematous and ulcerative (Wilson 1896)[6]
Lichen planus is a relatively common disorder of the stratified squamous epithelia (Duske and Frick, 1982: Skully and El-kom 1985)[7,8]
OLP as a relatively common chronic inflammatory disorder affecting the stratified squamous epithelia[7]
Lichen planus is a common disorder in which auto-cytotoxic T-lymphocytes trigger apoptosis of epithelial cells leading to chronic inflammation. OLP can be a source of severe morbidity and has a small potential to be malignant (Scully et al. 2008).[8]
ETIOPATHOGENESIS
The exact etiology remains to be in controversy. There are numerous potential triggers involved in the pathogenesis of OLP, which probably could be unknown local and systemic factors.[9,10,11]
Factors connected with lichen planus:
Genetic predisposition
Microorganisms
Allergy (food, drug, cosmetics, metals etc.)
Mechanical trauma
Stress, nervous and insomnia
GIT disturbances
Systemic diseases (diabetes mellitus, thyroid and dermatological disease)
Occupation and lifestyle
Mucosal trauma (severe attrition/reduced vertical dimension/sharp teeth)
Nutritional status.
OLP is a T-cell-mediated immunological disease to an unknown antigenic change in the skin or oral mucosa in genetically predisposed patients.[12,13]
Several studies have analyzed those genes that are potentially involved in the pathogenesis and evolution of OLP include JUN, EGFR, FOS, IL2 and ITGB4 and other MHC genes such as HLA B 27, HLA B 51, HLA BW-57, HLA-DR1 and HLA-DR6 also play a role.[14,15,16]
In a genetically predetermined individual, various triggering factors cause unmasking of lichen planus-specific antigen, which is displayed by MHC class 1 molecules. This, in turn, favors the recruitment of CD8+ T-cells (cytotoxic) and it is activated. This aids in the release of tumor necrosis factor (TNF)-α, and various other cytokines and chemokines leading to release of mucous membrane pemphigoid (MMP), eventually causing basement membrane disruption resulting in migration of T-cells into the epithelium thus resulting in keratinocyte apoptosis. Other nonspecific immune responses such as chemokine ligand activation and mast cell degranulation also play a role in basement membrane disruption in the pathogenesis of OLP. Cytokine polymorphisms TH1 and TH2 determine the appearance of lesions in the oral mucosa (interferon - associated) or on the skin (TNF-α associated).[10,13,17]
Stress also plays a major etiological factor. Patients with OLP often report an exacerbation of the lesions during periods of great stress and depression. Previous studies conducted reveal the presence of increased levels of oxidative stress markers (malondialdehyde, Heat Shock Proteins, etc.) in oral mucous cells, serum and saliva of the patients. Thus, psychological stress amplifies an immunological response in a previously established lesion aggravating the clinical signs and symptoms.[9,13,17,18]
Thyroid dysfunction is also said to be in association with the pathogenesis of OLP. Increased levels of thyroid-stimulating hormone and low levels of Free Thyroxine (FT4 levels) are often observed in OLP patients, which might likely suggest that some mechanisms in autoimmune thyroid disease are involved in the disease pathogenesis. Few studies have confirmed that OLP is associated with hepatitis C virus (HCV), which could be also involved in the pathogenesis of lichen planus occurrence.[16,17]
CLINICAL FEATURES
OLP affects 1%–2% of the population of all racial groups. It occurs more commonly in females in middle and younger age groups. The mean age of onset is the fourth decade of life with a female-to-male ratio of 1.4:1. The prevalence rate is about 0.1% to 2.2%. The clinical appearance of OLP is diverse.[19] Andreasen classified clinical patterns into six different forms including reticular, plaque-like, atrophic, papular, erosive and bullous patterns that could be differentiated in OLP.[20,21]
The reticular lichen planus presents with white papules that enlarge and coalesce to form a reticular, annular or plaque-like pattern, the so-called Wickham's striae a fine, lace-like network of white lines referred to as Wickham striae [Figure 1]. Honiton lace was described by Louis Frederic Wickham. The most common location is the buccal mucosa, followed by tongue, gingivae, lower lip and palate. The Wickham striae are the most common variant seen, followed by an erosive pattern.
Figure 1.
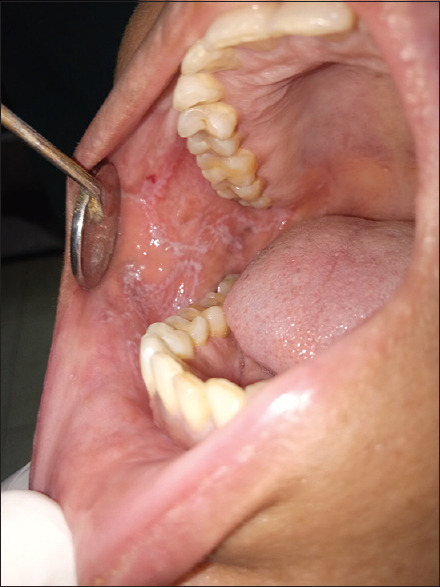
Wickham's striae
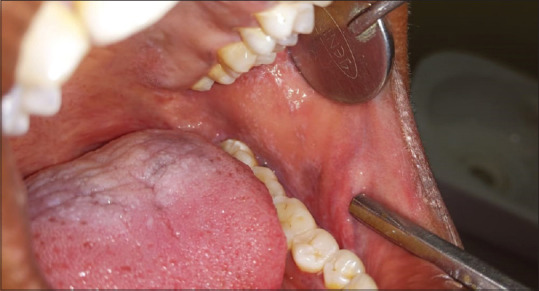
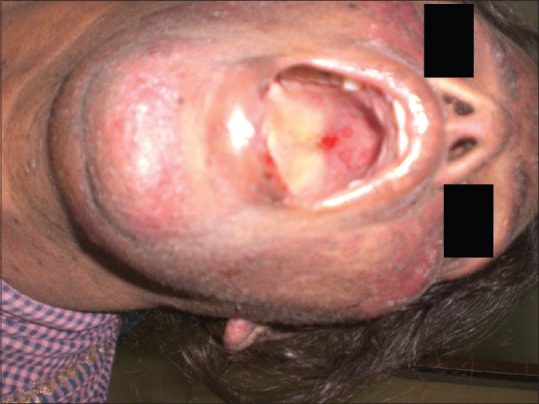
Erosive pattern presents with an erythematous background [Figure 2] and obvious erosions of mucosa of affected areas with finely radiating keratotic striae when erosive OLP involves the gingiva called desquamative gingivitis [Figure 3]. Patients with this form of OLP often present with burning sensation that can interfere with daily routine. The burning pain that can be provoked by friction due to sharp teeth, severe attrition of teeth, chronic cheek bite and or by spicy, sour or hot foods. When the erosive lesions occur in the vulva, vagina and gingiva, it has been termed as “vulvo-vaginal-gingival syndrome.”
Figure 2.
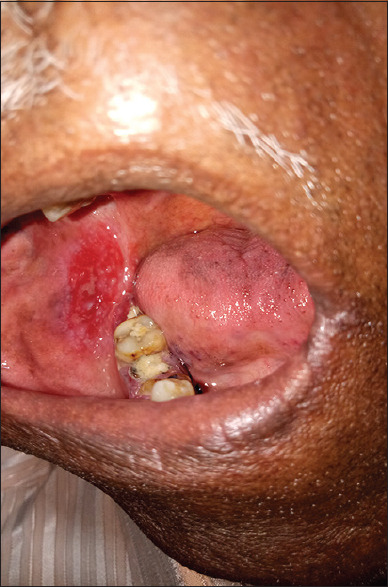
Erosive type of oral lichen planus
Figure 3.
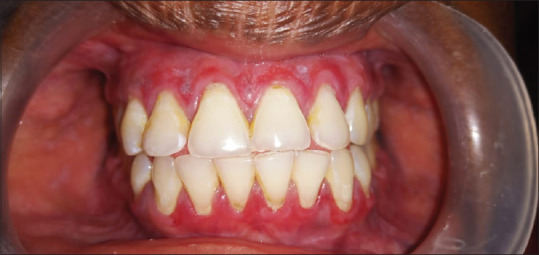
Desquamative gingivitis
The occurrence of malignant transformation potential in OLP is controversial, and patients with longstanding erosive lesions must evaluate for genetic and epigenetic factors involved in malignant transformation.
The other manifestations are the atrophic and bullous patterns that are considered as variants of erosive lichen planus. Atrophic OLP [Figure 4] appears as diffuse, erythematous patches surrounded by fine white striae that make the patient symptomatic. The bullous form [Figure 5] appears frequently on the buccal mucosa and the lateral borders of the tongue and ruptures to become burning and erosive lesion.[20,22]
Figure 4.
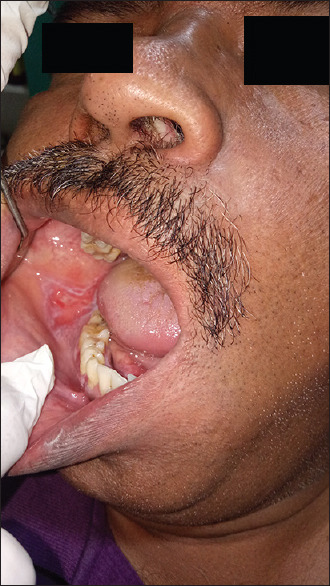
Atrophic type of oral lichen planus
Figure 5.
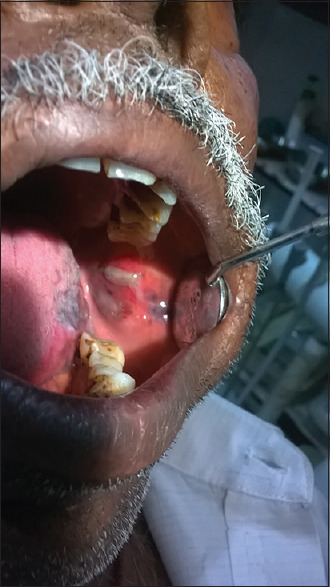
Bullous type of oral lichen planus
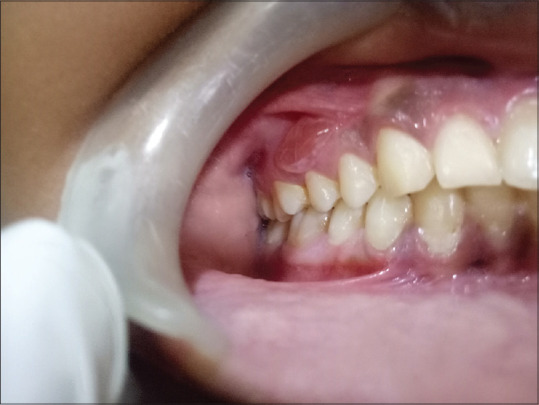
A hypertrophic lesion described as plaque type [Figure 6] is found either in the dorsum or lateral border of the tongue that may or may not be symptomatic. Long-lasting plaque-like lesions, particularly in smokers, may confuse with leukoplakia and dyskeratosis congenita, and therefore, a thorough examination of extraoral site is essential in arriving at a clinical diagnosis.
Figure 6.
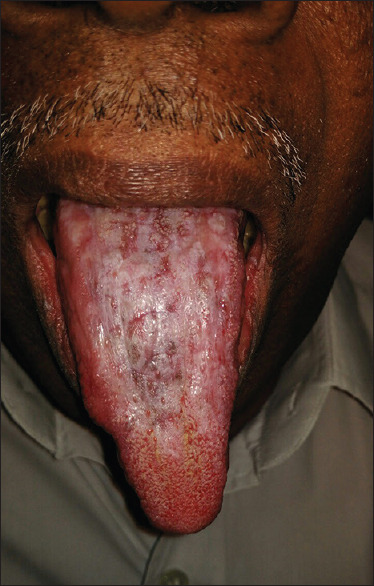
Plaque type of oral lichen planus
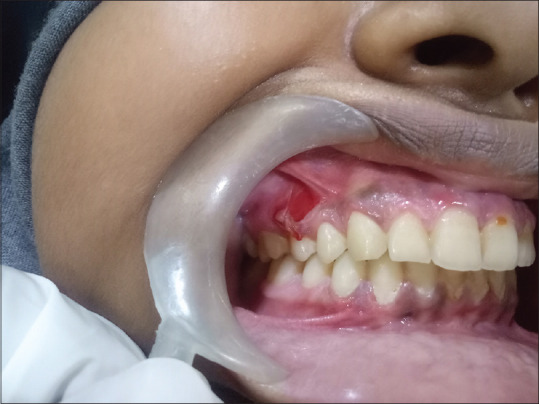
The papular [Figure 7] variants initially present with tiny white papules which, in turn, coalesce later to appear as reticular form.
Figure 7.
Papular type of oral lichen planus
Cutaneous lesion [Figure 8] occurs with clinical variants described as 6 p's planar, purple polygonal, pruritic, papule and plaque.
Figure 8.
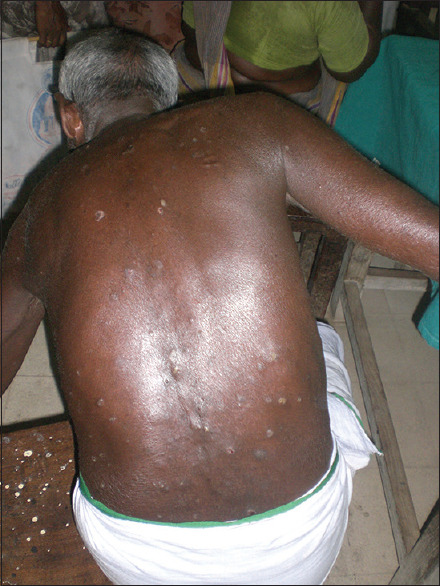
Cutaneous LP
The clinical diagnosis of lichen planus can be made more confidently in the presence of characteristic pathognomonic appearance of Wickham's striae. The differential diagnosis of erosive OLP includes systemic lupus erythematosus [Figure 9], candidiasis, benign MMPs [Figures 10 and 11], pemphigus vulgaris [Figure 12], chronic ulcerative stomatitis [Figure 13], erythema multiforme, frictional keratosis [Figure 14], lichenoid drug reaction [Figure 15] and lichenoid lesions [Figure 16]. The plaque form of reticular OLP can resemble oral leukoplakia.[20,22,23]
Figure 9.
Systemic lupus erythematosus
Figure 10.
Pemphigoid
Figure 11.
Ruptured bullae in pemphigoid
Figure 12.
Pemphigus vulgaris
Figure 13.
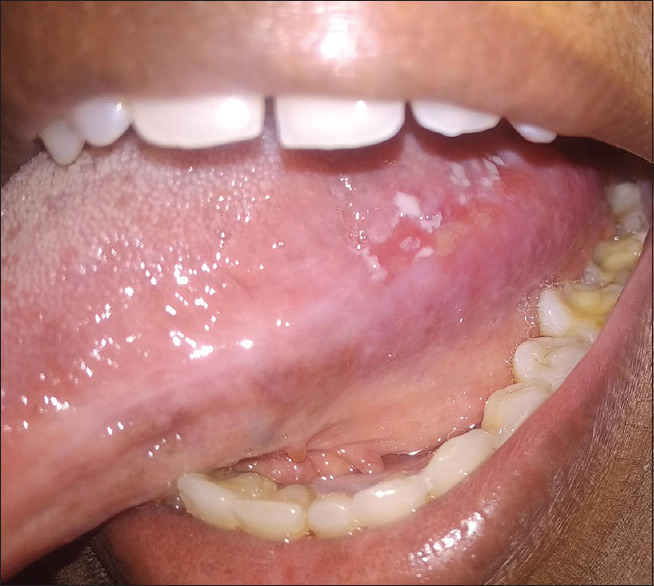
Chronic ulcerative stomatitis
Figure 14.
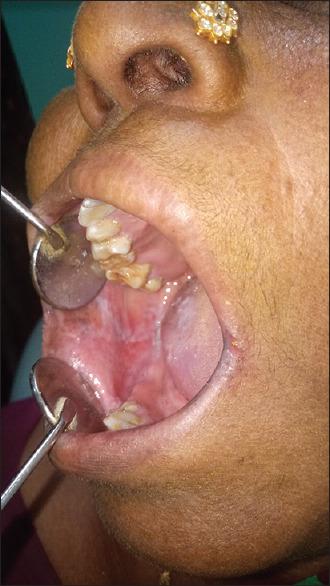
Frictional keratosis
Figure 15.
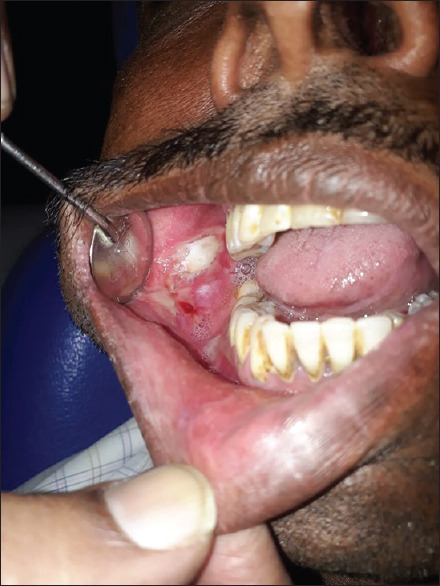
Lichenoid drug reaction
Figure 16.
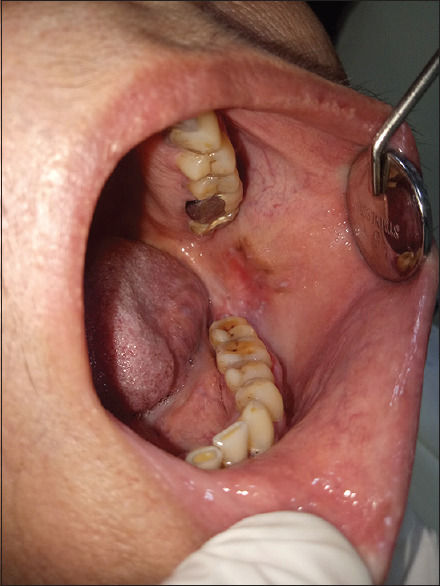
Lichenoid lesion
DIAGNOSIS
Diagnosis of OLP is challenging because of the presence of overlapping clinical and histopathological features. The diagnosis of an OLP may be made on visual examination without any biopsy when the lesion presents with the characteristic Wickham's striae.[24,25] A complete history and clinical assessment by various specialists such as dermatologist, ophthalmic surgeon, general physician and gastroenterologist may be required to investigate the involvement other than oral cavity.
The investigations include (1) cytology, (2) hematology and (3) biopsy (histopathological and immunofluorescence).
Cytology
Smear examination is mandatory in a desquamative lesion and Tzanck test is a simple, fast and inexpensive diagnostic test that can be performed with minimal patient discomfort that is used in erosive lesion, especially on the gingiva to identify the presence of acantholytic cells [Figure 17] and to rule out pemphigus vulgaris. Cytology is also helpful to distinguish the presence or absence of hyphae by doing a direct potassium hydroxide stain and or by culture in Sabouraud dextrose agar media [Figure 18]. Monitoring the development of candidiasis during the treatment while administering the topical steroids may be helpful toward a successful treatment [Figure 19].
Figure 17.
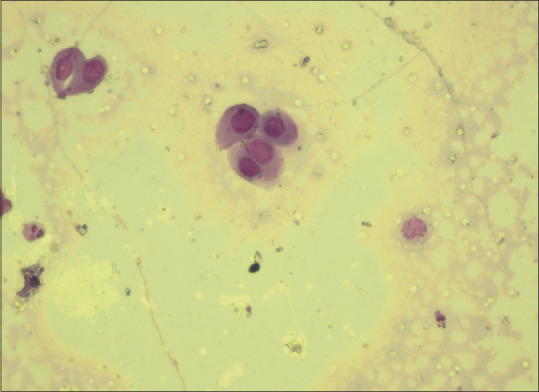
Acantholytic cells
Figure 18.
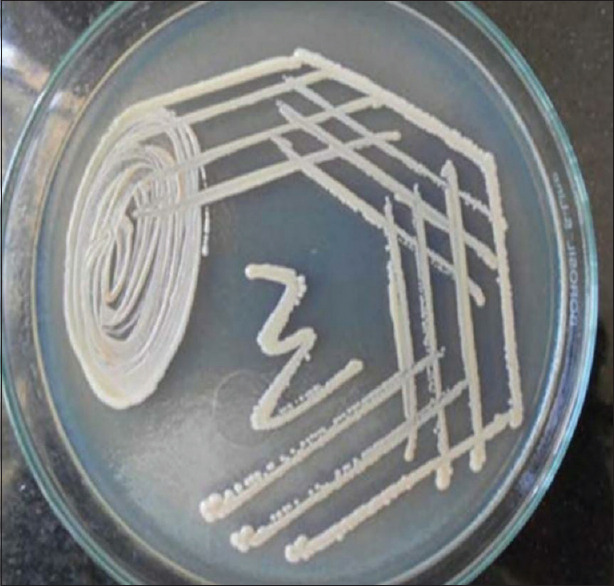
Sabouraud dextrose agar
Figure 19.
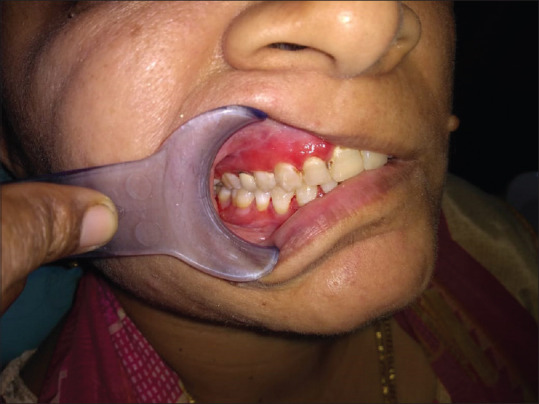
Candidiasis following steroid application
HEMATOLOGICAL INVESTIGATIONS
Total leukocyte count
Differential leukocyte count
Platelets
Erythrocyte sedimentation rate
Hematocrit
Hemoglobin
Total red blood cell
MCHC
MCV
MCH
Platelets
Hb C Ag (hepatitis C virus)
Antibiotic drug sensitivity test
Patch test
Rheumatologic evaluation.
BIOPSY
The histopathology [Figure 20] is the gold standard method used in diagnosing OLP. Previous studies have suggested that the interobserver and intraobserver variations have been found in making a conclusive diagnosis. However, the disease shows a waxing and waning pattern in healing and recurrence that reflects microscopically either in the intensity and or in the type of chronic inflammatory infiltrate seen. Various authors have proposed the histopathological features and criteria to observe in the lesion.[25,26,27]
Figure 20.
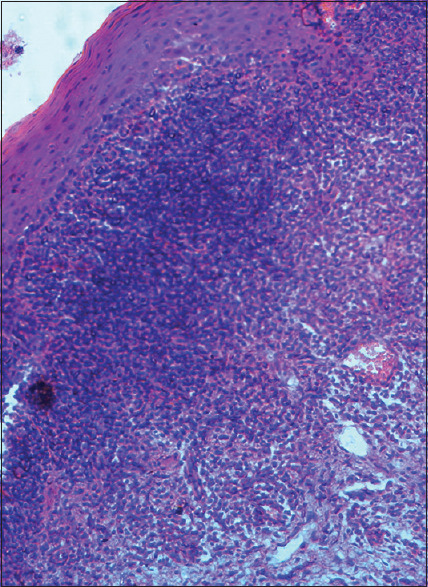
Histopathology of oral lichen planus
It was First described by DUHRENILL in 1906 and later revised by SHKLAR in 1972:[25,27]
Hyperkeratosis of surface epithelium (orthokeratosis or parakeratosis)
Sawtooth rete ridges
Thickening of granular layer
Acanthosis of spinous layer
Intercellular edema in spinous layer
Max Joseph spaces which are histological clefts between basement membrane lamina and propria interface caused due to liquefaction necrosis of basal layer
Juxta-epithelial band of inflammatory cells predominantly T-lymphocytes
Civatte/hyaline/cytoid/colloid bodies which are Degenerating keratinocytes at the epithelial connective tissue interface
An eosinophillic band may be seen just beneath the basement membrane and represent fibrin covering lamina propria.
WHO 1978 – DIAGNOSTIC CRITERIA[27,28]
In 1978, WHO formulated diagnostic criteria for OLP, which included both clinical and histopathological features for consideration.
Clinical criteria
Usually multiple and often symmetric in distribution
White papular-, reticular (lace-like network of slightly raised gray-white lines), annular- or plaque-type lesions
White lines radiating from the papules
Atrophic lesions with or without erosion
Rare appearance of bullae.
Histopathological criteria
Orthokeratosis or parakeratosis of epithelium
Epithelial thickness varies and sawtooth rete ridges may be seen
Presence of Civatte bodies in the basal layer of the epithelium or in the superficial lamina propria
A narrow band of eosinophilic material in the basement membrane
Presence of a well-defined band like zone of cellular infiltration that is confined to the superficial part of the connective tissue, consisting mainly of lymphocyte
Signs of “liquefaction degeneration” in the basal cell layer.
Eisenberg's criteria[25,26,27]
He considered some essential criteria for diagnosis that includes:
Basal cell liquefaction
Band-like lymphocytic infiltrate at the epithelial–stromal junction, with obfuscation of the basal cell region
Presence of normal epithelial maturation pattern.
He suggested some features to be excluded:
Atypical cytomorphologies (suggestive of epithelial dysplasia) – hyperchromasia, prevalent dyskeratosis and increased mitotic figures
Heterogeneous population of inflammatory infiltrate, deeper submucosal extension of infiltrate beyond superficial stroma and perivascular infiltration.
MODIFIED WHO DIAGNOSTIC CRITERIA OF OLP AND ORAL LICHENOID LESIONS (2003)[28,29]
Clinical criteria
Presence of bilateral, more or less symmetrical lesions
Erosive-, atrophic-, bulbous- and plaque-type lesions are only accepted as a subtype in the presence of reticular lesions elsewhere in the oral mucosa
Presence of a lace-like network of slightly raised gray-white lines (reticular pattern)
In all other lesions that resemble OLP but do not complete the aforementioned criteria, the term “clinically compatible with” should be used.
Histopathological criteria
Presence of a well-defined band-like zone of cellular infiltration that is confined to the superficial part of the connective tissue or superficial lamina propria, consisting mainly of lymphocytes
Signs of liquefaction degeneration in the basal cell layer
Absence of epithelial dysplasia
When the histopathological features are less obvious, the term “histopathologically compatible with” should be used.
Final diagnosis oral lichen planus or OLL
To achieve the final diagnosis, clinical as well as histopathologic criteria should be included:
OLP – A diagnosis of OLP requires fulfillment of both clinical and histopathological criteria
-
OLL –The term OLL will be used under the following conditions.
- Clinically typical of OLP but histopathologically only “compatible with” OLP
- Histopathologically typical of OLP but clinically only “compatible with” OLP
- Clinically “compatible with” OLP and histopathologically “compatible with” OLP.
Diagnostic criteria by the American Academy of Oral and Maxillofacial Pathology, 2016.[29]
Clinical criteria
Multifocal Symmetric distribution
-
White and red lesions exhibiting one or more of the following forms:
- Reticular/papular
- Atrophic (erythematous)
- Erosive (ulcerative)
- Plaque
- Bullous
Lesions are not localized
To the sites of smokeless tobacco placement
Adjacent to and in contact with dental restorations.
Lesion onset does not correlate with
The start of a medication
With the use of cinnamon-containing products
Histopathologic criteria
Band-like or patchy, predominately lymphocytic infiltrate in the lamina propria confined to the epithelium–lamina propria interface
Basal cell liquefactive (hydropic) degeneration
Lymphocytic exocytosis
Absence of epithelial dysplasia
Absence of verrucous epithelial architectural change.
HISTOPATHOLOGICAL DIFFERENTIAL DIAGNOSIS[29,30]
The non-erosive type of OLP should be differentiated from frictional keratosis, lichenoid reactions, leukoplakia and discoid lupus erythematosus both clinically and histopathologically.
Similarly, the differential diagnosis for erosive or atrophic OLP will be chronic ulcerative stomatitis, pemphigus vulgaris, MMP, lupus erythematosus and erythematous candidiasis.
Lichenoid drug reactions are generally associated with a history of drug intake and usually present with unilateral distribution. Patch test is one of the consistent methods to diagnose lichenoid drug reactions. Histopathologically, more diffuse lymphocytic infiltrate mixed with plasma cells and eosinophils which extends into the deeper stroma is observed. Although no marked disruption of the basal cell layer is evident, there is liquefaction degeneration of basal cells with increased Civatte bodies and characteristic deep perivascular infiltrate.[29,30]
Oral lesions in graft-versus-host disease are usually found with lesions in widespread involvement in oral mucosa with the presentations of reticulations, plaques and/or erosions. It manifests with diffuse erythematous mucositis (both keratinized and non-keratinized mucosa), loss of filiform papillae and loss of gingival stippling. Acute cases present with subepithelial blister.
microscopic features of chronic GVHD are nonspecific and include hydropic degeneration of basal epithelial cells and a less intense lymphocytic infiltrate (usually sparse and ill defined) mixed with plasma cells and eosinophils in the lamina propria. Marked fibrosis is appreciated in the subepithelial stroma in chronic cases.
Discoid and systemic lupus erythematosus shows elongated thin rete ridges extending into the subepithelial band of lymphoid aggregates with rete hyperplasia showing dyskeratosis and localized appearance of pseudoepitheliomatous hyperplasia. Thickened or degenerated endothelium with perivascular infiltrate is one of the striking features to differentiate.[31,32]
Lesions exhibiting dysplasia show histopathological evidence of cellular and architectural changes, shows mixed inflammatory infiltrate without predominating lymphocytes and lacks basal layer degenerative changes. Clinically, they exhibit isolated lesions, whereas OLP is multifocal and symmetrical in presentation. Lesions with both features of OLP and dysplasia should be properly evaluated and can be considered as lichenoid dysplasia.[29,30,31]
Chronic ulcerative stomatitis has similar histopathologic features, but the epithelium tends to be more atrophic with mixed inflammatory infiltrate and plasma cells. Clinically, most common sites are gingiva, lateral border of tongue and buccal mucosa. Direct immunofluorescence shows deposition of IgG autoantibodies in the nucleus of basal and parabasal epithelial cells in a speckled and/or granular pattern, whereas OLP exhibits fibrinogen reactivity at the basement membrane.
OLP pemphigoides occur at rare instances in prolonged cases of OLP that depicts with histopathological features of OLP along with sub-basilar epithelial separation. Clinically, it resembles features of OLP with vesicle or bullous eruptions in buccal mucosa and gingiva, palate, vestibule and labial mucosa.[31,32,33]
ROLE OF IMMUNOFLUORESCENCE IN LICHEN PLANUS
The direct immunofluorescence is applied mainly in the erosive lesions, especially desquamative gingivitis to rule out blistering diseases such as pemphigus and pemphigoids. In the lichen planus, shaggy deposition of fibrinogen and complement along the basement membrane zone is observed without the immunoglobulin found other than colloid bodies. The deposition of fibrinogen is not pathognomonic of OLP because OPMD also shares a similar pattern. Indirect immunofluorescence (IIF) is not useful in OLP diagnosis.[29]
ROLE OF IMMUNOHISTOCHEMISTRY IN ORAL LICHEN PLANUS
Immunohistochemistry (IHC) has a limited role in the diagnosis of OLP. However, it has a wide role in understanding the pathogenesis and prediction toward a malignant change in a given lesion. The various markers connected with every step of pathogenesis may be advocated and studied. Thus, the IHC markers have a better role in future research and may be beneficial in the management.
TREATMENT
The first line of treatment is counseling the patient and makes to understand about the nature of the disease, its various factors, diverse manifestations and recurrent nature. Majority of the patients imagine it as a cancer due to the persistence of the lesions for a long duration. The main aim of the treatment of OLP is to alleviate the fear and stress, thereby reducing the symptoms. The elimination of factors associated with the occurrence is an important basic protocol in the management of symptomatic OLP. Elimination of factors that affects the healing and prognosis of the lesions like sharp or fractured teeth, poorly fitting dentures and severe attrition and reduced vertical dimension (which favors candidiasis) is mandatory to achieve a better result.
Maximum attention should be given to stress, restless state and insomnia and under the physician's care, prescribing anti-anxiety drugs such as alprazolam is much beneficial and helps in reducing the stress and promotes early healing and thereby reducing the remission. The diet should be customized (should have both macro and micronutrients) so that it should be nutritionally supportive. A good amount of pre- and probiotic and regular deworming is advised to maintain a healthy intestine. The patient should be educated to have a good oral hygiene, and if necessary, antiseptic mouthwash may be advocated as a reduction in the microbial plaque may have beneficial effects on the lesions and to reduce the severity of the symptoms. A good number of nutritional supplements have to be given to patients with nutritional deficiency.[34,35]
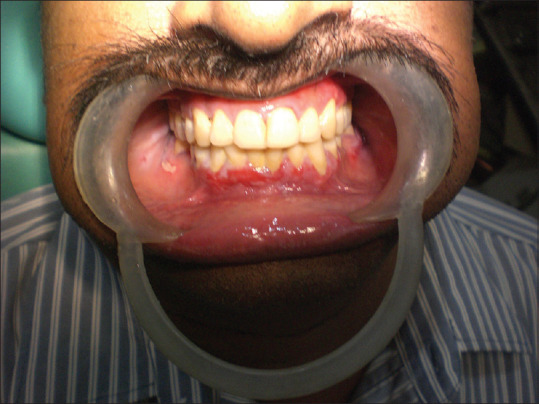
Asymptomatic lesions such as reticular and plaque variants of OLP do not require any treatment but require a regular follow-up. The most accepted treatment protocol for OLP is topical or systemic corticosteroids. Topical corticosteroids (Triamcinalone Acetonide) are advised for the mild-to-moderate lesions that occur for the first time, and give better results [Figures 21-28]. Although there are no many side effects, topical application produces lesser side effects including candidiasis, atrophy of the oral mucosa and telangiectasia. In patients with widespread symptomatic lesions, a minimal dose of systemic steroid (Prednisone 10–20 mg/day) may be advised in the morning. If the systemic administration prolongs for more than 2 weeks, then the tapering module should be followed. An anti-fungal agent (topical or systemic) will be beneficial especially in patients with erythematous candidiasis that developed following the administration of long-term steroids.[35,36]
Figure 21.
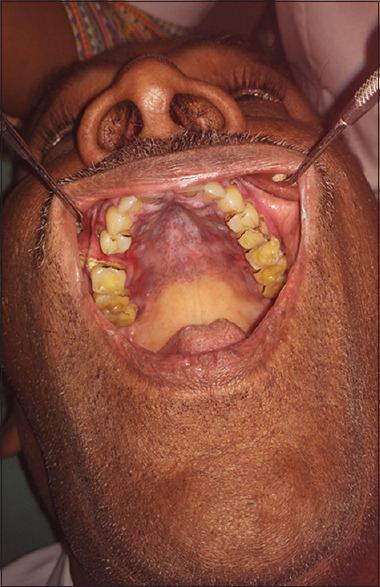
Pretreatment (oral lichen planus palate)
Figure 28.
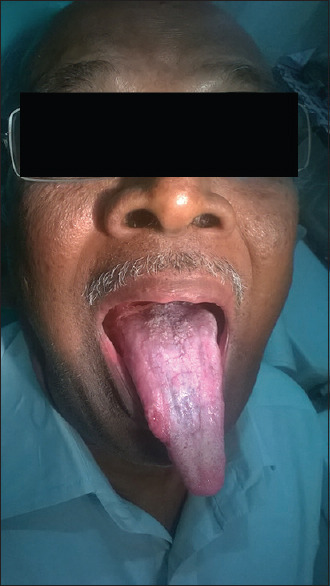
Posttreatment (plaque type of oral lichen planus in tongue)
Figure 22.
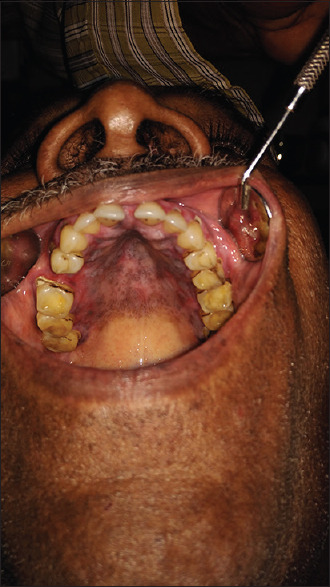
Posttreatment (oral lichen planus)
Figure 23.
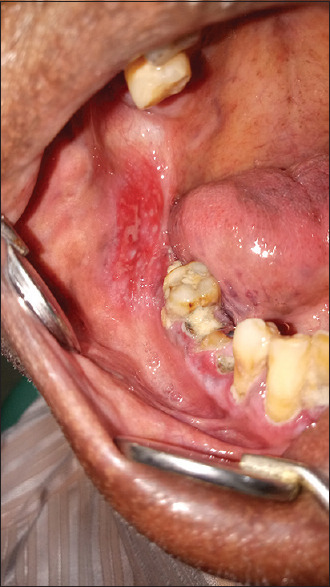
Pretreatment (erosive oral lichen planus in buccal mucosa)
Figure 24.
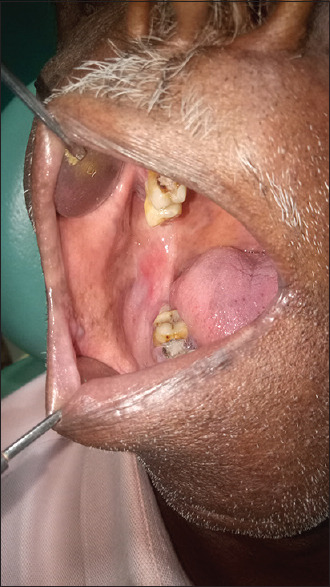
Posttreatment (erosive oral lichen planus in buccal mucosa)
Figure 25.
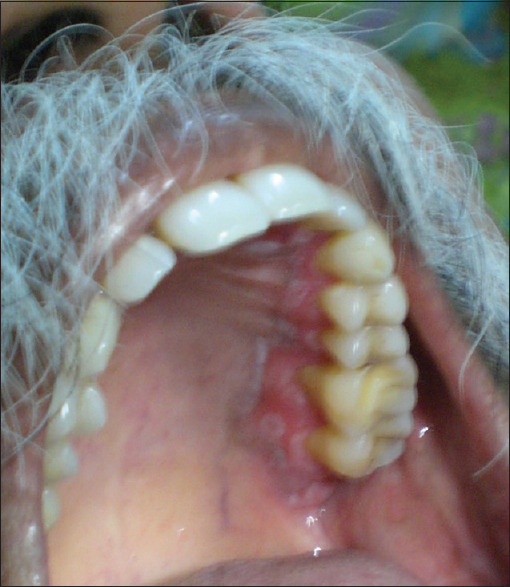
Pretreatment (erosive oral lichen planus in palate)
Figure 26.
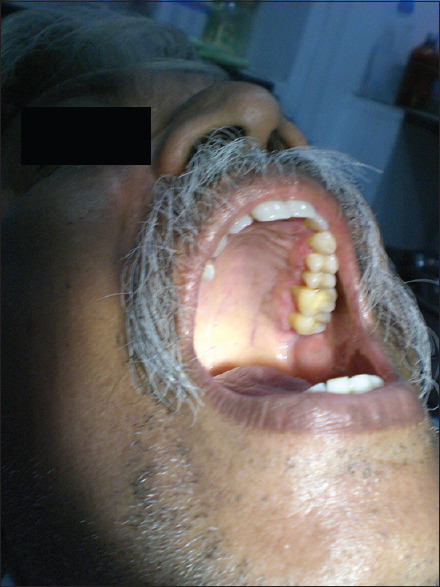
Posttreatment (erosive oral lichen planus in palate)
Figure 27.
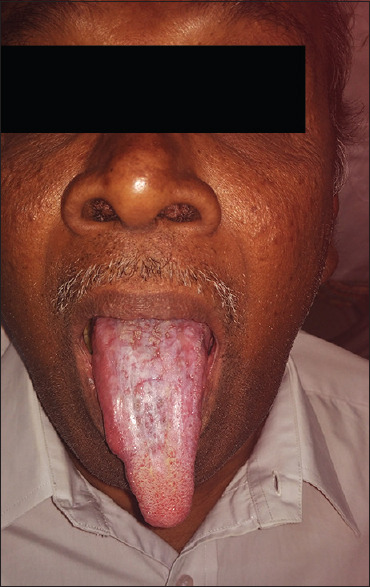
Pretreatment (plaque type of oral lichen planus in tongue)
CONCLUSION
OLP, an immunological disease, requires a thorough evaluation of past medical history, clinical examination including dental status, conclusive histopathological findings and elimination of connected factors, right treatment protocol and a regular follow-up. Recalcitrant and recurrence lesions should be handled with special care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Neville BW, Damm DD, Allen CM, Bouquet JE. Oral and Maxillofacial Pathology. 2nd ed. Philadelphia: W. B Saunders; 2002. pp. 680–7. [Google Scholar]
- 2.Sivapadasundaram B. Shafer's Textbook of Oral Pathology. 9th ed. New Delhi, India: Elsevier; 2012. pp. 557–62. [Google Scholar]
- 3.Edwards PC, Kelsch R. Oral lichen planus: Clinical presentation and management. J Can Dent Assoc. 2002;68:494–9. [PubMed] [Google Scholar]
- 4.Schifter M, Suran L. Fernando Jamma Li. Oral lichen planus. In: Fernand S, editor. Skin Biopsy: Diagnosis and Treatment. IntechOpen: United Kingdom; 2013. p. 14970. [Google Scholar]
- 5.Parashar P. Oral lichen planus. Otolaryngol Clin North Am. 2011;44:89–107. doi: 10.1016/j.otc.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Wilson E. On lichen planus. J Cutan Med Dis Skin. 1869;3:117. [Google Scholar]
- 7.Eisen D, Carrozzo M, Bagan Sebastian JV, Thongprasom K. Number V Oral lichen planus: Clinical features and management. Oral Dis. 2005;11:338–49. doi: 10.1111/j.1601-0825.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- 8.Scully C, Carrozzo M. Oral mucosal disease: Lichen planus. Br J Oral Maxillofac Surg. 2008;46:15–21. doi: 10.1016/j.bjoms.2007.07.199. [DOI] [PubMed] [Google Scholar]
- 9.Andreasen JO. Oral lichen planus. 1. A clinical evaluation of 115 cases. Oral Surg Oral Med Oral Pathol. 1968;25:31–42. doi: 10.1016/0030-4220(68)90194-1. [DOI] [PubMed] [Google Scholar]
- 10.Tyldesley WR. Oral lichen planus. Br J Oral Surg. 1974;11:187–206. doi: 10.1016/0007-117x(74)90101-2. [DOI] [PubMed] [Google Scholar]
- 11.Devi S, Duraisamy R. Prevalence of oral lichen planus and assessment of factors associated with it – A retrospective study. Indian J Forensic Med Toxicol. 2020;14:5938–46. [Google Scholar]
- 12.Gupta S, Jawanda MK. Oral lichen planus: An update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222–9. doi: 10.4103/0019-5154.156315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sameera A, Erugula SR, Rahman MA, Farooq MU, Veldurthi D, Imran S, et al. Oral lichen planus – A review. IOSR JPBS. 2017;12:75–80. [Google Scholar]
- 14.Shklar G. Lichen planus as an oral ulcerative disease. Oral Surg Oral Med Oral Pathol. 1972;33:376–88. doi: 10.1016/0030-4220(72)90467-7. [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg E. Clinicopathologic patterns of oral lichenoid lesions. Oral Maxillofac Surg Clin North Am. 1994;6:445. [Google Scholar]
- 16.Rotaru DI, Sofineti D, Bolboacă SD, Bulboacă AE. Diagnostic criteria of oral lichen planus: A narrative review. Acta Clin Croat. 2020;59:513–22. doi: 10.20471/acc.2020.59.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patil S, Rao RS, Sanketh DS, Sarode SC, Sarode GS. “A universal diagnostic criteria for oral lichen planus: An exigency!,”. Int J Contemp Dent Med Rev. 2014 Article ID 041214,2014;1-4. [Google Scholar]
- 18.McCartan BE. Psychological factors associated with oral lichen planus. J Oral Pathol Med. 1995;24:273–5. doi: 10.1111/j.1600-0714.1995.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 19.Hiremath S, Kale AD, Hallikerimath S. Clinico-pathological study to evaluate oral lichen planus for the establishment of clinical and histopathological diagnostic criteria. Turk Patoloji Derg. 2015;31:24–9. doi: 10.5146/tjpath.2014.01285. [DOI] [PubMed] [Google Scholar]
- 20.Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–65. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 21.DeAngelis LM, Cirillo N, McCullough MJ. The immunopathogenesis of oral lichen planus – Is there a role for mucosal associated invariant T cells? J Oral Pathol Med. 2019;48:552–9. doi: 10.1111/jop.12898. [DOI] [PubMed] [Google Scholar]
- 22.Sousa FA, Rosa LE. Oral lichen planus: Clinical and histopathological considerations. Braz J Otorhinolaryngol. 2008;74:284–92. doi: 10.1016/S1808-8694(15)31102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivas K, Aravinda K, Ratnakar P, Nigam N, Gupta S. Oral lichen planus – Review on etiopathogenesis. Natl J Maxillofac Surg. 2011;2:15–6. doi: 10.4103/0975-5950.85847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill Y, Griffiths M, et al. Update on oral lichen planus: Etiopathogenesis and management. Crit Rev Oral Biol Med. 1998;9:86–122. doi: 10.1177/10454411980090010501. [DOI] [PubMed] [Google Scholar]
- 25.Haqiqi MA, Pourmoshir N, Bereshneh AH. Clinical and genetic aspects of oral lichen planus. IJBAR. 2016;07:06. [Google Scholar]
- 26.Bermejo-Fenoll A, López-Jornet P. Familial oral lichen planus: Presentation of six families. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e12–5. doi: 10.1016/j.tripleo.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1:173–9. [Google Scholar]
- 28.Carrozzo M, Uboldi de Capei M, Dametto E, Fasano ME, Arduino P, Broccoletti R, et al. Tumor necrosis factor-alpha and interferon-gamma polymorphisms contribute to susceptibility to oral lichen planus. J Invest Dermatol. 2004;122:87–94. doi: 10.1046/j.0022-202X.2003.22108.x. [DOI] [PubMed] [Google Scholar]
- 29.Cheng YS, Gould A, Kurago Z, Fantasia J, Muller S. Diagnosis of oral lichen planus: A position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:332–54. doi: 10.1016/j.oooo.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Mravak-Stipetić M, Lončar-Brzak B, Bakale-Hodak I, Sabol I, Seiwerth S, Majstorović M, et al. Clinicopathologic correlation of oral lichen planus and oral lichenoid lesions: A preliminary study. ScientificWorldJournal. 2014;2014:746874. doi: 10.1155/2014/746874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiremath SK, Kale AD, Charantimath S. Oral lichenoid lesions: Clinico-pathological mimicry and its diagnostic implications. Indian J Dent Res. 2011;22:827–34. doi: 10.4103/0970-9290.94679. [DOI] [PubMed] [Google Scholar]
- 32.Chiang CP, Yu-Fong Chang J, Wang YP, Wu YH, Lu SY, Sun A. Oral lichen planus – Differential diagnoses, serum autoantibodies, hematinic deficiencies, and management. J Formos Med Assoc. 2018;117:756–65. doi: 10.1016/j.jfma.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Odell E, Gale N, Thavaraj S, Nadal A, Zidar N, Douglas R. Gnepp precursor lesions for squamous carcinoma in the upper aerodigestive tract. Gnepp's Diagnostic Surgical Pathology of the Head and Neck. 3rd ed. canada: Elsevier Inc; 2021. p. 9. [Google Scholar]
- 34.Barbosa NG, Silveira ÉJ, Lima EN, Oliveira PT, Soares MS, de Medeiros AM. Factors associated with clinical characteristics and symptoms in a case series of oral lichen planus. Int J Dermatol. 2015;54:e1–6. doi: 10.1111/ijd.12485. [DOI] [PubMed] [Google Scholar]
- 35.Nosratzehi T. Oral lichen planus: An overview of potential risk factors, biomarkers and treatments. Asian Pac J Cancer Prev. 2018;19:1161–7. doi: 10.22034/APJCP.2018.19.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53–60. [PubMed] [Google Scholar]


