Abstract
Aims
To examine the association between rosuvastatin and VTE risk, and whether effects vary in different subpopulations stratified by key demographic, cardiovascular disease (CVD) risk factors, and other risk factors associated with VTE.
Methods and results
An individual participant data meta-analysis was conducted across two randomized controlled trials in 30 507 participants over a mean follow-up of 3.62 years, individuals had no prior history of vascular disease but were at intermediate CV risk. In both trials, participants were randomized to receive rosuvastatin or matching placebo. The primary outcome was VTE during follow-up, defined as either deep vein thrombosis or pulmonary embolism. Associations between rosuvastatin and VTE were examined in the overall pooled cohort, and subpopulations stratified by demographic risk factors (i.e. age and sex), CVD risk factors (i.e. obesity, smoking, lipid levels, blood pressure levels, and C-reactive protein level), and a history of cancer. Mean age was 65.96 (SD 7.19) years of age, and 17 832 (58.45%) were male and 5434 (17.82%) were smokers, median BMI was 27.6 [interquartile range (IQR) 24.7–31.1] kg/m2, and median CRP level was 3.4 (IQR 2.1–6.0) mg/L. There were 139 VTE events. In the pooled cohort, rosuvastatin was associated with a large proportional reduction in the risk of VTE (hazard ratio 0.53, 95% CI 0.37–0.75). No significant interactions were observed between treatment with rosuvastatin and the risk of VTE across subpopulations stratified by demographic, CVD risk factors, or a history of cancer (P-values for interactions >0.05 for all subgroups).
Conclusion
Rosuvastatin is associated with a 47% proportional reduction in the risk of VTE, and its effect is consistent both in the presence or absence of VTE-related clinical risk factors.
Keywords: Statin, Venous thromboembolism
Graphical Abstract
1. Introduction
Venous thromboembolism (VTE) is a common cause of morbidity and mortality. In addition to traditional factors known to provoke VTE (e.g. cancer), several common cardiovascular disease (CVD) risk factors have now been shown to share associations with both arterial atherosclerosis and VTE, including age, sex, smoking, obesity, and inflammation.1–4 While statins are widely used for the prevention of CVD, it was only in 2009 that the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) reported that these agents have similar efficacy in preventing VTE. Meta-analyses of several additional clinical trials suggested an overall 15% RRR in VTE with statin therapy (with effect shown to vary by statin type); and of observational data suggested that effects were larger in populations at higher VTE risk (although this could only be crudely measured in the context of aggregate data meta-analysis).5 Some of the proposed mechanisms by which statins prevent VTE (e.g. direct anti-inflammatory effects, direct anti-thrombotic effects, and secondary effects from lipid reduction) could also result in varying degrees of efficacy based on underlying risk factors. Therefore, while prior studies have demonstrated a reduction in VTE risk with statins, it remains unclear as to whether these effects differ in subpopulations based on the presence or absence of specific VTE clinical risk factors. Understanding this requires analysing effects across a broad range of clinical risk factor profiles associated with VTE, while also controlling for statin type. Results could provide greater insight on how statins can be most effectively used to prevent VTE.
Among studies that have examined the effect of statins on VTE, the JUPITER and Heart Outcomes Prevention Evaluation (HOPE)-3 randomized trials are unique in that they are both large, double-blind, placebo controlled, primary CVD prevention trials that investigated the same statin (rosuvastatin), and documented incident VTE events in a prospective manner during follow-up. Together these cohorts have sufficient variations in common VTE-related risk factors [e.g. degree of systemic inflammation, age, sex, and body mass index (BMI)] to study whether certain at-risk groups derive a larger benefit for preventing VTE with rosuvastatin. In this pooled cohort, we undertook an individual participant data meta-analysis to examine the effect of rosuvastatin on VTE risk in individuals at risk of developing CVD, and across key subgroups according to demographic risk factors, CVD risk factors, and other risk factors for VTE.
2. Methods
2.1. Trial designs
We conducted an individual participant data meta-analysis of the JUPITER and HOPE-3 studies. The designs of both studies have been previously described.6,7 JUPITER enrolled men ≥50 and women ≥60 years; without clinical vascular disease but at increased CVD risk based on a high sensitivity C-reactive protein (CRP) level ≥2.0 mg/L; a low-density lipoprotein (LDL) level <3.4 mmol/L; and a triglyceride level <5.6 mmol/L. Participants were randomized to receive rosuvastatin 20 mg daily or matching placebo. HOPE-3 enrolled men ≥55 years and women ≥65 years without clinical vascular disease but at intermediate CV risk based on the presence of at least 1 of the following risk factors (women 60–65 years of age with at least 2 risk factors could be enrolled): elevated waist-to-hip ratio, low high-density lipoprotein (HDL) cholesterol, tobacco use, dysglycaemia, family history of premature coronary disease, or mild renal dysfunction. Participants were randomized in a 2 × 2 factorial design to receive (i) rosuvastatin 10 mg daily or matching placebo, and (ii) candesartan/hydrochlorothiazide 16/12.5 mg or matching placebo. JUPITER excluded participants with active cancer within the prior 5 years (with the exception of basal or squamous cell carcinoma of the skin), but participants with a diagnosis of cancer that occurred beyond 5 years prior to screening could still be enrolled. HOPE-3 excluded participants with other serious medical illness likely to interfere with study participation or completion including a history of cancer that may affect prognosis other than basal or squamous cell carcinoma of the skin. All participants provided written consent to participate in the study. The study was approved by local ethics research boards and conformed to the principles outlined in the Declaration of Helsinki.
2.2 Study VTE endpoints
In both trials, incident VTE were predefined ancillary endpoints, defined as the development of deep vein thrombosis or pulmonary embolism during follow-up. First, follow-up visits were performed at 13 weeks in JUPITER and at 6 weeks in HOPE-3; and thereafter at 6 months intervals. Through standardized case report forms for each trial, investigators were prompted to identify incident VTE events at each follow-up visit. Upon identification of a VTE event, site investigators completed a corresponding VTE case report form, which also documented the method of diagnostic confirmation.
2.3 Statistical analysis
Baseline characteristics were summarized for each study, and for the pooled study population. Our primary method of analyses utilized a one-step approach as outlined by Riley et al.,8 where individual participant-level data from both studies were pooled, and analysed using a Cox frailty model, with clustering by trial included as a random effect. To examine for consistency, we also performed a sensitivity analysis for overall VTE risk with statin therapy using a two-step approach, where trial-specific data were pooled using a random effect model. We then examined for variation in the effect of rosuvastatin across subgroups stratified by the following baseline data: (i) demographic characteristics (consisting of age, sex, and ethnicity), (ii) CVD risk factors (consisting of blood pressure, obesity, high sensitivity CRP level, LDL level, and HDL level), and (iii) a history of cancer. Based on the exclusion criteria for both trials, our definition of a history of cancer was related to diagnoses that occurred beyond the exclusion criteria of the trials, with the exception of basal or squamous cell carcinoma of the skin. We also examined the effect of statins based on baseline anti-platelet use. All analyses in subgroups were performed using Cox frailty models, accounting for clustering within each trial as a random effect. Results are presented as hazard ratios (HRs) with 95% confidence intervals (95% CI). For each defined subgroup, interactions were formally tested between strata by the Wald test. A P-value of <0.05 was considered to be statistically significant. For the overall cohort, we calculated the 5-year NNT to prevent one VTE using the method outlined by Bender et al.9 Analyses were performed using SAS and R.
3. Results
3.1 Participant characteristics
Baseline characteristics for the pooled study population, and for each trial population are summarized in Table 1. Mean age of the pooled study population was 65.96 (SD 7.19) years of age, and 17 832 (58.45%) were male. 15 022 (49.25%) of the study population had a self-reported history of hypertension. Median BMI was 27.6 [interquartile range (IQR) 24.7–31.1] kg/m2, HDL was 46.4 (IQR 38.6–56.8) mg/dL, LDL was 113.0 (IQR 97.0–126.0) mg/dL, and CRP was 3.4 (IQR 2.1–6.0) mg/L. HOPE-3 and JUPITER study populations differed in several characteristics. The JUPITER population was comprised of a higher proportion of males. Median BMI, median CRP, and percentage with hypertension were higher in JUPITER. The HOPE-3 study population had a higher percentage of Asian and Hispanic/Latin American participants, and higher mean LDL cholesterol. In 102 (73.4%) participants, VTE was documented to have supportive diagnostic testing (which included compression or Doppler ultrasound, angiography, computed tomography, ventilation perfusion scan, magnetic resonance imaging, or echocardiography) or by autopsy.
Table 1.
Baseline characteristics of the pooled, HOPE-3, and JUPITER study populations
| Baseline characteristics | Overall | HOPE 3 | JUPITER |
|---|---|---|---|
| Number of subjects randomized | 30 507 (100.0) | 12 705 (100.0) | 17 802 (100.0) |
| Age in years, mean (SD) | 65.96 (7.19) | 65.72 (6.38) | 66.13 (7.72) |
| Male, n (%) | 17 832 (58.45) | 6831 (53.77) | 11 001 (61.80) |
| Ethnicity, n (%) | |||
| Asian | 6524 (21.39) | 6241 (49.12) | 283 (1.59) |
| Caucasian | 15 229 (49.92) | 2546 (20.04) | 12 683 (71.25) |
| Black | 2449 (8.03) | 225 (1.77) | 2224 (12.49) |
| Hispanic or Latin American | 5757 (18.87) | 3496 (27.52) | 2261 (12.70) |
| Other | 546 (1.79) | 197 (1.55) | 349 (1.96) |
| BMI, kg/m2, median(IQR) | 27.6 (24.7–31.1) | 26.7 (24.1–29.7) | 28.4 (25.3–32.0) |
| Baseline systolic blood pressure, mmHg, median(IQR) | 136.0 (126.0–146.5) | 137.5 (128.5–147.5) | 134.0 (124.0–145.0) |
| Baseline diastolic blood pressure, mmHg, median(IQR) | 80.0 (75.0–88.0) | 82.0 (75.5–88.0) | 80.0 (75.0–87.0) |
| HDL, mg/dL, median(IQR) | 46.4 (38.6–56.8) | 43.6 (36.2–52.1) | 49.0 (40.0–60.0) |
| LDL, mg/dL, median(IQR) | 113.0 (97.0–126.0) | 127.0 (103.9–151.0) | 108.0 (94.0–119.0) |
| High sensitivity C-Reactive protein, mg/L, median(IQR) | 3.4 (2.1–6.0) | 2.0 (1.0–4.0) | 4.3 (2.8–7.1) |
| Hypertension, n (%) | 15 022 (49.25) | 4814 (37.89) | 10 208 (57.37) |
| Current smoking, n (%) | 5434 (17.82) | 2614 (20.58) | 2820 (15.85) |
| Anti-platelet therapy, n (%) | 4472 (14.66) | 1393 (10.96) | 3079 (17.30) |
| History of cancer, n (%) | 1065 (3.49) | 153 (1.20) | 912 (5.12) |
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
3.2 VTE occurrence
The mean follow-up period for the pooled study population was 3.62 years; 1.92 years in JUPITER and 5.60 years in HOPE-3. VTE event rates in the pooled study population, and for each individual study are summarized in Table 2. During follow-up, 139 (0.46%) VTE events occurred in the pooled study population, corresponding to an event rate of 0.128 per 100 person years. The number of VTE events and corresponding event rate were larger in JUPITER (94 events, 0.252 events per 100 person years) compared with HOPE-3 (45 events, 0.063 events per 100 person years).
Table 2.
Rates of VTE in the pooled study population and in individual studies
| Overall |
Statin |
Placebo |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Number of subjects | N (%) | Event rate per 100 person years | Number of subjects | N (%) | Event rate per 100 person years | Number of subjects | N (%) | Event rate per 100 person years |
| HOPE 3 | 12 705 | 45 (0.35) | 0.063 | 6361 | 14 (0.22) | 0.039 | 6344 | 31 (0.49) | 0.088 |
| JUPITER | 17 802 | 94 (0.53) | 0.252 | 8901 | 34 (0.38) | 0.182 | 8901 | 60 (0.67) | 0.321 |
| Overall | 30 507 | 139 (0.46) | 0.128 | 15 262 | 48 (0.31) | 0.088 | 15 245 | 91 (0.60) | 0.169 |
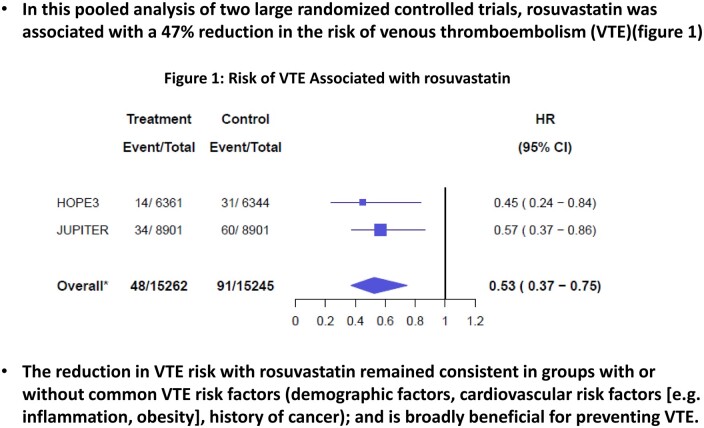
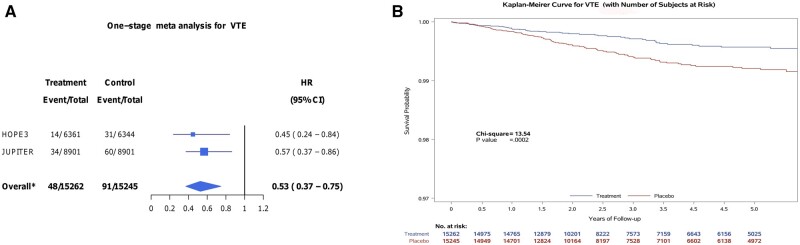
In pooled analysis of individual participant data, the risk of VTE was lower with rosuvastatin compared with placebo (HR 0.53, 95% CI 0.37–0.75) (Figure 1A and B). Results were consistent when aggregate trial level data were pooled using a random effect model [HR 0.53, 95% CI 0.53 (0.37–0.75)]. This corresponded to a 5-year NNT of 276 individuals to avoid a VTE event. Results were consistent when the analysis was restricted to participants with a VTE with documented supportive diagnostic imaging or autopsy data (HR 0.54, 95% CI 0.36–0.82).
Figure 1.
(A and B) Risk of VTE associated with rosuvastatin in pooled analysis. (A) Forest plot and (B) Kaplan–Meyer survival curves for VTE are presented for the comparison of rosuvastatin vs. placebo across both randomized controlled trials. The HOPE-3 follow-up period was longer than in JUPITER, and Kaplan–Meyer curves were truncated to 5 years since beyond this time, effects would almost exclusively be related to the HOPE-3 study population. Analysis (N=30 507 participants) was performed using Cox frailty model. 95% CI, 95% confidence interval; HR, hazard ratio.
3.3 Subgroup analyses by demographic and cardiovascular risk factors
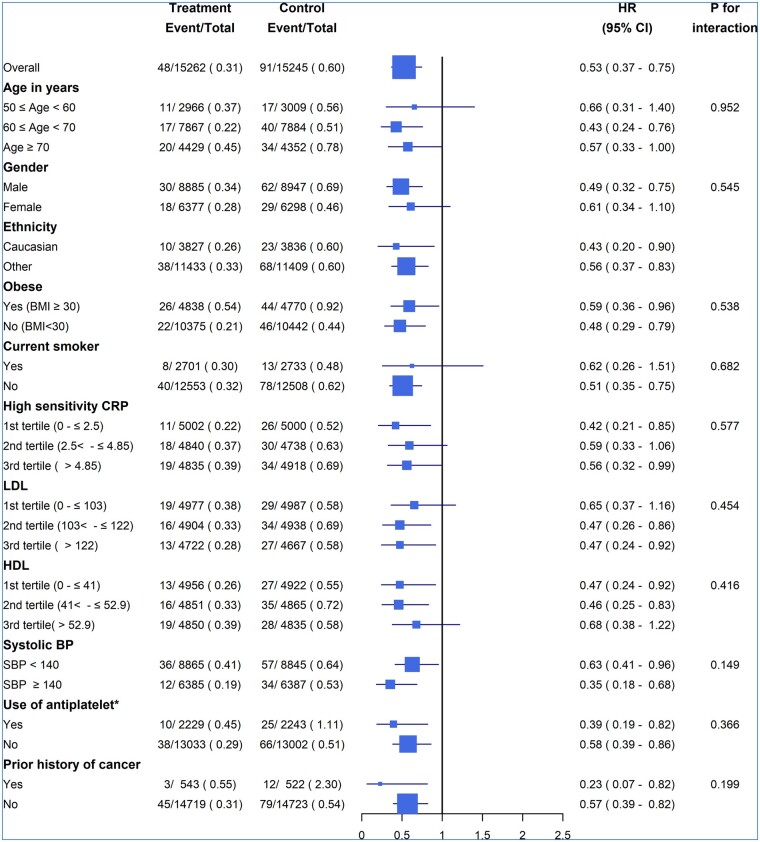
Comparing the rates of VTE in placebo group participants, VTE was more common in higher age groups, males, Caucasians, obese participants, at higher CRP levels, and in those with a prior history of cancer (Figure 2). VTE rates were also higher in individuals using anti-platelet agents, but this is likely confounded by underlying indications for their use. No significant interactions were observed for the HR for VTE with rosuvastatin compared with placebo across subgroups.
Figure 2.
Effect of rosuvastatin on VTE risk by subgroups. *The higher proportion of participants receiving anti-platelet therapy having VTE events may be due to confounding conditions for which anti-platelet therapy was indicated. Analysis (N=30 507 participants) was performed using Cox frailty model. BP, blood pressure; CRP, C-reactive protein; 95% CI, 95% confidence interval; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein.
4. Discussion
In this individual participant data meta-analysis, rosuvastatin reduced the risk of VTE by 47% across a pooled primary CVD prevention population with a broad clinical risk factor profile for VTE. Effects were consistent across the range of VTE-related risk factors studied.
Some mechanisms proposed to explain how statins prevent VTE (i.e. downstream inactivation of inflammatory and coagulation systems by reducing oxidized LDL, decreased expression of plasminogen activator inhibitor-1) suggest that they may only be effective in the presence of certain risk factor profiles.10–14 Furthermore, large differences in the benefit of statin therapy for reducing VTE risk have been previously reported across different clinical trials, but it was unclear as to whether this was related to differences in population characteristics between trials.5 In this regard, combined individual participant data meta-analysis of the JUPITER and HOPE-3 trials had several advantages. Different baseline characteristics of the trial populations allowed us to examine the effects of statins on VTE risk across a range of risk factor profiles to better understand whether certain population characteristics impacted the effect of rosuvastatin on reducing VTE. Interestingly, we observed that the relative effect for reducing VTE risk with rosuvastatin was fairly consistent across risk factor subgroups that were studied. Therefore, while the risk of VTE is higher in certain groups, the benefit of rosuvastatin remains largely consistent even in the absence of these provoking factors. The lack of difference in benefit according to specific risk groups suggests that the mechanism by which statins prevent VTE is not likely to be mediated through a single pathway that is correlated with a specific risk factor (e.g. inflammation). Alternatively, their benefit may occur through a combination of pathways, or through an independent mechanism. Also, by demonstrating similar treatment effects in both trials and across key risk factor profiles, we believe that the large reduction in VTE risk initially observed with rosuvastatin in JUPITER was not likely due to trial-related factors (i.e. characteristics of the population, statin dose used, or its early termination), but rather due to the pharmacological agent itself.
Despite the large relative reduction in VTE risk observed with rosuvastatin, since the incidence of VTE was low, the absolute reduction in risk was small. General use of a statin would require treatment of 276 individuals over a 5-year period to prevent a VTE event in our overall study population. In some populations’ groups at higher risk of developing VTE (e.g. older age groups, obese participants, history of cancer), utilizing statins specifically for VTE prevention may be more favourable from the absolute risk reduction perspective, but due to the small number of VTE events within subgroups, this could not be reliably quantified in our study. Future collaborative individual participant data meta-analyses that include a larger number of statin clinical trials and examine VTE outcomes may be valuable to confirm the effects observed in our study, in addition to quantifying absolute risk reductions in key subgroups. Finally, it is important to consider that irrespective of the presence of specific CVD risk factors, participants in both JUPITER and HOPE-3 benefitted from large reductions in overall CVD events with statin therapy. The effect of statins on VTE prevention is an added benefit to the prevention of arterial vascular events, and so reducing the risk for VTE per se should not be the criterion to define who should receive a statin.15
Some limitations of the current analysis also warrant consideration. We were not able to compare different statins, and therefore observed effect sizes may not be generalizable to other statins. In the future, larger individual participant data meta-analyses across additional clinical trials could provide greater insights into the comparative impact of different statins, and further validate the findings of trial level meta-analyses.5,16 While VTE was not the primary outcome event for either trial, both trials collected clinical VTE events in a systematic and prospective manner. Participants were not routinely screened for VTE events in follow-up (e.g. by routine ultrasound examinations), which occurs in some dedicated VTE trials that are of a short duration (e.g. post-operative studies). Our approach was focused on the identification of clinical VTE events, and the majority of events identified in both trials were confirmed with appropriate diagnostic testing. Moreover, given the double-blind randomized study designs of JUPITER and HOPE-3, it is highly unlikely that the lack of VTE screening would introduce bias in our estimates. Further, routine screening would be impractical and not consistent with clinical practice standards for the general population. Formal event adjudication for VTE did not occur for either study. However, in both studies documenting the diagnostic method used for VTE diagnosis was requested for all events to minimize false positive events. Finally, although we did not observe a difference in treatment effect with rosuvastatin in those with or without a history of cancer, few participants had cancer and the number of VTE events were low in this group. Future studies focusing specifically on cancer patients may provide further insight into the potential value of statins for preventing VTE specific to this population.
In conclusion, rosuvastatin is associated with a 47% reduction in the risk of VTE, and is effective both in the presence or absence of VTE-related clinical risk factors. The benefit of rosuvastatin is overall consistent with previous reports in CV prevention trials using statins, and adds to its benefits above the prevention of arterial vascular events.
Authors’ contributions
P.J., E.L., S.Y., R.G., and P.R. contributed to the conception of the work. P.J. drafted the work. C.R. performed the data analysis. J.E. provided critical revisions for intellectual content. J.M. contributed to the data acquisition and provided critical revisions for intellectual content. P.J. and C.R. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the manuscript.
Conflict of interest: Dr Yusuf reports receiving grant support and honoraria from AstraZeneca. Dr Ridker reports receiving grant support and consulting fees from AstraZeneca. Drs Glynn and Lonn report receiving grant support from AstraZeneca. The rest of the authors do not report any conflicts of interest relevant to this study.
Funding
This work was supported by the following agencies. The JUPITER study was supported primarily by AstraZeneca and also by a grant from the National Institute on Aging (AG031061). HOPE-3 was supported by a grant (IR2-91038) from the Canadian Institutes of Health Research and by AstraZeneca.
Data availability
Data related to this analysis will not be available publically.
Translational perspective
In this individual participant data meta-analysis of two large randomized controlled trials comparing rosuvastatin to placebo, rosuvastatin was associated with a 47% proportional reduction in the risk of VTE. The effect of rosuvastatin was consistent across a broad range of demographic factors, cardiovascular risk factors, and a history of cancer. This study demonstrates that rosuvastatin is broadly affective at reducing the risk of VTE both in the presence or absence of VTE-associated clinical risk factors. Results inform future research on the use of statins for this indication.
References
- 1. Folsom AR, Lutsey PL, Astor BC, Cushman M.. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thromb Haemost 2009;102:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fox EA, Kahn SR.. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost 2005;94:362–365. [DOI] [PubMed] [Google Scholar]
- 3. Gregson J, Kaptoge S, Bolton T, Pennells L, Willeit P, Burgess S, Bell S, Sweeting M, Rimm EB, Kabrhel C, Zöller B, Assmann G, Gudnason V, Folsom AR, Arndt V, Fletcher A, Norman PE, Nordestgaard BG, Kitamura A, Mahmoodi BK, Whincup PH, Knuiman M, Salomaa V, Meisinger C, Koenig W, Kavousi M, Völzke H, Cooper JA, Ninomiya T, Casiglia E, Rodriguez B, Ben-Shlomo Y, Després J-P, Simons L, Barrett-Connor E, Björkelund C, Notdurfter M, Kromhout D, Price J, Sutherland SE, Sundström J, Kauhanen J, Gallacher J, Beulens JWJ, Dankner R, Cooper C, Giampaoli S, Deen JF, Gómez de la Cámara A, Kuller LH, Rosengren A, Svensson PJ, Nagel D, Crespo CJ, Brenner H, Albertorio-Diaz JR, Atkins R, Brunner EJ, Shipley M, Njølstad I, Lawlor DA, van der Schouw YT, Selmer RM, Trevisan M, Verschuren WMM, Greenland P, Wassertheil-Smoller S, Lowe GDO, Wood AM, Butterworth AS, Thompson SG, Danesh J, Di Angelantonio E, Meade T; Emerging Risk Factors Collaboration. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol 2019;4:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roach REJ, Venemans A, Cannegieter SC, Lijfering WM.. Differential risks in men and women for first and recurrent venous thrombosis: the role of genes and environment: reply. J Thromb Haemost 2015;13:886–887. [DOI] [PubMed] [Google Scholar]
- 5. Kunutsor SK, Seidu S, Khunti K.. Statins and primary prevention of venous thromboembolism: a systematic review and meta-analysis. Lancet Haematol 2017;4:e83–e93. [DOI] [PubMed] [Google Scholar]
- 6. Glynn RJ, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Ridker PM.. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009;360:1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López-Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJG, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez-Vallejo G, McKelvie R, Pogue J, Jung H, Gao P, Diaz R, Lonn E.. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
- 8. Riley RD, Lambert PC, Abo-Zaid G.. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340:c221. [DOI] [PubMed] [Google Scholar]
- 9. Bender R, Kromp M, Kiefer C, Sturtz S.. Absolute risks rather than incidence rates should be used to estimate the number needed to treat from time-to-event data. J Clin Epidemiol 2013;66:1038–1044. [DOI] [PubMed] [Google Scholar]
- 10. Obermayer G, Afonyushkin T, Binder CJ.. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J Thromb Haemost 2018;16:418–428. [DOI] [PubMed] [Google Scholar]
- 11. Gaertner S, Cordeanu E-M, Nouri S, Mirea C, Stephan D.. Statins and prevention of venous thromboembolism: myth or reality? Arch Cardiovasc Dis 2016;109:216–222. [DOI] [PubMed] [Google Scholar]
- 12. Rodriguez AL, Wojcik BM, Wrobleski SK, Myers DD, Wakefield TW, Diaz JA.. Statins, inflammation and deep vein thrombosis: a systematic review. J Thromb Thrombolysis 2012;33:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Violi F, Calvieri C, Ferro D, Pignatelli P.. Statins as antithrombotic drugs. Circulation 2013;127:251–257. [DOI] [PubMed] [Google Scholar]
- 14. Biere-Rafi S, Hutten BA, Squizzato A, Ageno W, Souverein PC, de Boer A, Gerdes VEA, Büller HR, Kamphuisen PW.. Statin treatment and the risk of recurrent pulmonary embolism. Eur Heart J 2013;34:1800–1806. [DOI] [PubMed] [Google Scholar]
- 15. Chou R, Dana T, Blazina I, Daeges M, Jeanne TL.. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;316:2008–2024. [DOI] [PubMed] [Google Scholar]
- 16. Rahimi K, Bhala N, Kamphuisen P, Emberson J, Biere-Rafi S, Krane V, Robertson M, Wikstrand J, McMurray J.. Effect of statins on venous thromboembolic events: a meta-analysis of published and unpublished evidence from randomised controlled trials. PLoS Med 2012;9:e1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data related to this analysis will not be available publically.