Abstract
MLN4924 is a specific small-molecule inhibitor of NEDD8-activating enzyme (NAE) that blocks the neddylation modification cascade. Several I/II/III clinical trials suggested that MLN4924 exerts an antitumor effect against various malignancies. However, recent studies have also found that MLN4924 activates the PI3K/AKT and MAPK/ERK signal pathways, important regulators of tumorigenesis, and drug resistance in human urothelial carcinoma (UC). This study examined the synergistic effect of celecoxib, a cyclooxygenase-2 (COX-2) selective inhibitor, on MLN4924-induced cytotoxicity and epithelial–mesenchymal transition (EMT) inhibition via AKT and ERK pathways in human UC. We performed both in vitro and in vivo experiments. Briefly, a combination of MLN4924 and celecoxib reduced the protein expression of p-AKT(S473) and p-ERK in UC cell lines. Moreover, celecoxib shifted the half-maximal inhibitory concentration (IC50) curve of MLN4924 to the left, and the combinational effect of MLN4924 and celecoxib showed significant synergism in T24 and 5637 cells. Also, celecoxib enhanced the MLN4924 antitumor effects of inhibiting UC cell growth, colony formation, migration, invasion, and inducing apoptosis. In addition, celecoxib potentiated the MLN4924-induced EMT, decreased the expression of N-cadherin and vimentin, and activated the expression of E-cadherin. Celecoxib also increased the expression of pro-apoptosis proteins PARP and BAX and reduced the expression of antiapoptosis protein Bcl2. In vivo study indicated that the combination of MLN4924 and celecoxib synergistically suppressed the tumor growth in a UC xenograft nude-mice model, which was further supported by immunohistochemistry of tumor tissues. To sum up, our study revealed that celecoxib synergistically enhanced MLN4924-induced cytotoxicity and EMT inhibition in UC. It also inhibited the activation of AKT and ERK pathways, which were activated by MLN4924. These discoveries provide a new drug combination strategy for UC treatment.
Keywords: urothelial carcinoma, MLN4924, celecoxib, EMT, neddylation
Introduction
Human urothelial carcinoma (UC) is the most common histologic type of bladder carcinoma and the fourth most common cancer among men in the United States. It has been estimated that more than 83,000 adults (64,280 men and 19,450 women) will be diagnosed with UC in 2021 1 . Chemotherapy followed by radical cystectomy is the most common treatment for UC 2 . Nevertheless, more than 30% of patients with advanced-stage metastatic UC develop chemoresistance. Thus, novel strategies to overcome drug resistance are urgently required.
The ubiquitin-proteasome system (UPS) has a key regulatory role in maintaining cell homeostasis by controlling numerous protein degradation, which regulates cell apoptosis, gene transcription, and cell cycle and is also critical for almost all cellular signaling pathways 3 . Alteration in this process may lead to drug resistance, cancer progression, and metastasis 4 ; therefore, targeting abnormal protein degradation is a valuable way for cancer treatment.
The UPS system is regulated (activated) by a three-step enzymatic process, which includes a ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3). The cullin ring ligases (CRLs) are the largest family of target proteins transferred, recognized, and recruited by E3 ligase 5 . Neddylation is the process of transferring a small ubiquitin-like molecule, NEDD8, to cullins. The reaction involves a three-step enzymatic process of NEDD8-activating enzyme E1 (NAE), NEDD8-conjugating enzyme E2 (Ubc12), and NEDD8-E3 ligase, just like ubiquitination6,7. MLN4924, which is the first-in-class inhibitor of NAE 8 , binds to the active site of NAE to form a covalent NEDD8. MLN4924-adduct inhibits NAE activities, therefore blocking the cullin neddylation 9 . As a result, MLN4924 causes the accumulation of CRL E3 substrates and suppresses cancer cell proliferation by inducing cell autophagy, apoptosis, and senescence10,11. MLN4924 is currently undergoing phase I/II/III clinical trials investigating its treatment either alone or in conjunction with chemotherapeutic drugs for human solid or hematologic malignancy12,13. Yet, antitumor activity of MLN4924 in UC has been reported by only two preclinical studies10,14.
Mechanistically, it is believed that MLN4924 induces the dimerization of epidermal growth factor receptor (EGFR) and triggers EGFR activation and its downstream signals, including PI3K/AKT1/mTOR and RAS/RAF/MEK/ERK signal pathways 15 . Moreover, MLN4924 stimulates the proliferation of stem cells by activating the RAS/MAPK pathway and cooperating with EGF promoting skin wounding healing 15 . AKT and REK pathways have a crucial part in cancer progression and metastasis16,17, and their overexpression has been linked with the progression, antiapoptosis, migration, and invasion of UC18,19,20.
Celecoxib is a nonsteroidal anti-inflammatory drug (NSAID), selective, noncompetitive inhibitor of cyclooxygenase-2 (COX-2) enzyme, which has been used as an anti-inflammatory and antipyretic drug for over 20 years. However, over recent years, numerous studies have reported the antitumor effects of celecoxib in various cancer 21 - 23 . Some studies have reported that celecoxib can depress UC cell growth and metastasis by inducing apoptosis, inhibiting cell cycle and epithelial–mesenchymal transition (EMT)24,25. Moreover, it can depress the AKT and ERK signal pathways in other cancer cells26,27. In this study, we hypothesized that the downregulation of AKT and ERK pathways might enhance the antitumor effects of MLN4924. Thus, in this study, we examined a combination of antitumor effect of celecoxib and MLN4924 on UC in vitro and in vivo.
Materials and Methods
Cell Lines and Chemicals
Two high-grade UC cell lines, T24 and 5637, were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). T24 and 5637 were maintained in dulbecco’s modified eagle medium (DMEM, Gibco, Grand Island, NY, USA) and RPMI-1640 (Gibco) medium, respectively, supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin in a humidified atmosphere containing 5%CO2/95% air at 37°C. MLN4924 (BlpBio, USA) and celecoxib (MCE, Wuhan, Hubei, China) were dissolved in dimethyl sulfoxide (DMSO, MCE); the volume of DMSO < 0.1% of the volume of culture medium.
Cell Viability Survival Assays and Combination Index
T24 (3,000 cells/well) and 5637 (6,000 cells/well) cells were plated in triplicate in 96-well plates and treated with various concentrations of celecoxib (1, 3, 10, 30, 80, 100, 300 μM), MLN4924 (0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, 100 μM), or the combination of MLN4924 and celecoxib (50 μM) for 48 h. Cell viability was evaluated by the Cell Counting Kit-8 (CCK-8; Abcam, USA). We used Compusyn software (http://www.combosyn.com/index.html) to analyze the combined effect and calculate the combination index (CI) 28 . The drug combination was considered as synergistic if CI < 1, and the additive effect was considered if CI = 1, and antagonistic if CI > 1 28 .
Cell Clonogenic Assays
T24 (300 cells/well) and 5637 (600 cells/well) cells were plated in triplicate in six-well plates. T24 cells were exposed to MLN4924 (0, 0.1, 0.3 μM) alone or in combination with celecoxib (40 μM) for 7 days; 5637 cells were exposed to MLN4924 (0, 0.3, 0.5 μM) alone or combined with celecoxib (40 μM) for 12 days; the nontreated control group received the same volume of DMSO. Next, the cells were fixed with 5% glutaraldehyde and stained with 10% Giemsa staining (Solarbio, Beijing, China) for 15 min. The colonies were photographed and counted by ImageJ.
Analysis of Apoptosis by Flow Cytometry
Cells were exposed to increasing concentrations of MLN4924 alone or in combination with celecoxib for 48 h. Then, the cells were stained with PI and Annexin V for 30 min (Trans Gen, Beijing, China). Samples were subjected to flow cytometry using a FACSCanto II (Bio-Rad, SanJose, CA, USA) to assess FITC-Annexin V/PI fluorescence intensity. The apoptosis cell rate was analyzed by FlowJoV10 Software.
Cell Migration Assays
We measured the cell migration ability by performing a wound-healing assay. T24 and 5637 cells were cultured in triplicate in six-well plates and grown up to 90% confluence. Then, a line was drawn using a marker on the bottom of the dish, and a sterile 200-μl pipette tip was used to scratch three separate wounds through the cells, moving perpendicular to the line. The cells were then exposed to MLN4924 (0, 0.5 μM) alone or combined with celecoxib (50 μM) for 24 and 48 h. Images of the scratches were taken using an inverted microscope at 10× magnification.
Cell Invasion Assay
A transwell invasion assay was performed to measure cell invasion ability. T24 and 5637 cells were exposed to MLN4924 (0, 0.3 μM) alone or in combination with celecoxib (50 μM) for 24 h, and then, T24 (2 × 105) cells and 5637 (5 × 105) cells were harvested and seeded in the upper chamber with serum-free medium for 24 and 48 h; the bottom chamber contained a medium with 10% FBS. After each time point, cells were fixed with 5% paraformaldehyde for 15 min, and then stained with 0.1% crystal violet (Solarbio) for 15 min, followed by washing three times with phosphate-buffered saline (PBS). Cells were photographed and counted in five random visions.
Western Blotting
T24 and 5637 cells were cultured in six-well plates and exposed to various concentrations of MLN4924 (0, 0.1, 0.3, 1 μM), with or without celecoxib (60 μM) for 24 h. Then, the cells were harvested at 80% confluency with a cell scraper and lysed in RIPA lysis buffer (Solarbio) with 1-mM phenylmethylsulfonyl fluoride (PMSF) and 1%-mM phosphatase inhibitors (Solarbio) on ice for 30 min. After measuring protein concentration by the Pierce BCA protein Assay kit (Thermo, USA), the samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad). After blocking with 5% skim milk for 1 h (Solarbio), the membranes were incubated with primary antibodies AKT, phospho-AKT (S473), p44/42MAPK (ERK1/2), phospho-p44/42MAPK (ERK1/2), PARP, Bcl2, BAX, N-cadherin, E-cadherin, vimentin, and β-actin (CST, Danvers, MA, USA) at 4°C overnight. Then, the membranes were washed with tris buffered saline tween (TBST, Solarbio) three times (10 min each) and incubated with horseradish peroxidase-conjugated secondary antibodies to IgG (CST) for 1 h at room temperature. The PVDF membrane was quantified using LAS-4000 Mini (Fuji Film, Japan).
Xenograft Assay
Twenty-four male 4-week old BALB/c-nu mice were procured from the SJA laboratory (Hunan, China). All the animals were housed in an environment with a temperature of 22 ± 1°C, relative humidity of 50 ± 1%, and a light/dark cycle of 12/12 h.
Xenografts were built by subcutaneously injecting 5 × 105 T24 cells into the dorsal flanks; the T24 cells were suspended in 200 mL of a 1:1 mixture of Matrigel (Bio-Rad) and serum-free media. When the tumors had grown to approximately 200 mm3, the 48 mice were randomly divided into four groups (n = 6 for each group): MLN4924 group, celecoxib group, MLN4924+celecoxib group, and DMSO (control) group. The drug solutions of MLN4924 and celecoxib were prepared in 10% DMSO, 40% PEG300, 5% Tween-80, and 45% saline. The MLN4924-treated groups received intraperitoneal injections of 25-mg/kg MLN4924 three times weekly, and celecoxib groups received 50-mg/kg celecoxib orally once a day. All the treatments were given for 4 weeks. The nontreated control group was treated with the same volume of DMSO and drug solution, and the combined group received the same doses of both drugs at the same frequency and duration. Tumor size was measured with a standard caliper every 4 days; the size was calculated using the following formula: length × width2 × 0.5. After 4 weeks of treatment, tumors were excised and photographed.
Immunohistochemistry
The tissues were harvested, fixed in 5% paraformaldehyde, and embedded in paraffin. Sections that were 5-μm thick were cut for H&E staining. Then, the sections were incubated with antibodies against Ki67 (1:200, Affinity Biosciences, USA) and cleaved caspase-3 (1:200, Affinity Biosciences). Antibody binding was detected with a biotinylated goat anti-rabbit secondary antibody (1:1000, CST). Sections were developed with 3,3’-diaminobenzidine (DAB) and counterstained with hematoxylin.
Statistical Analysis
All data were represented as mean ± standard deviation from at least three independent experiments, if it was not indicated otherwise. Statistical analyses were performed using paired or unpaired Student’s t test or one-way analysis of variance (ANOVA). Not significant (NS): P > 0.05; *0.01 < P < 0.05; **0.005 < P < 0.01; ***P < 0.005.
Results
Celecoxib Reduces the Activation of AKT and ERK Signal Pathways in UC Cells by MLN4924
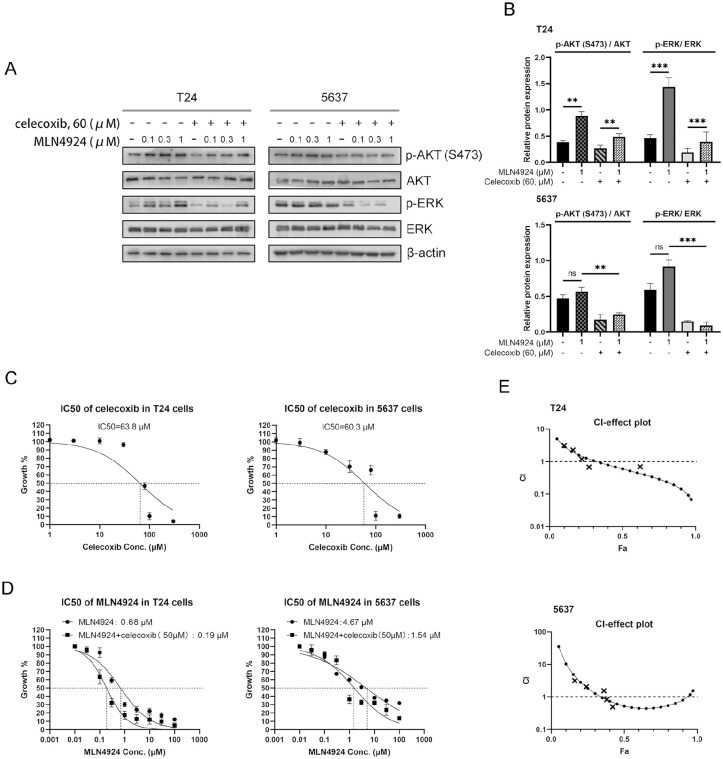
Increasing evidence has shown that the activation of the AKT and ERK signal pathways contribute to cancer cells proliferation, migration, antiapoptosis, and drug resistance16,19. To determine if MLN4924 in UC cells activates the AKT and ERK pathways, we first tested the expression level of phospho-AKT (S473) and phospho-ERK in two high-grade UC cell lines, T24 and 5637, after exposing these cells to various concentrations of MLN4924 (0, 0.1, 0.3, 1 μM) for 24 h. In T24 cells, the AKT and ERK signaling pathway was activated by MLN4924 in a dose-dependent manner, as reflected by increased AKT phosphorylation on the S473 site and ERK phosphorylation. In 5637 cells, MLN4924 did not activate the AKT and ERK signals; however, the expression levels of phospho-AKT (S473) and phospho-ERK were still high (Fig. 1A, B). After the combination of celecoxib, the phosphorylation of AKT and ERK signaling pathways significantly decreased in both T24 and 5637 cells. In view of the abnormal activation of the PI3K/AKT signaling pathway associated with UC drug resistance 20 , we speculated that celecoxib could sensitize UC cells to MLN4924.
Figure 1.
Celecoxib suppresses the activation of AKT and ERK signal pathways which activated by MLN4924, and enhances the suppression of growth in UC cells by MLN4924 (A–B) T24 and 5637 cells were incubated with various concentration of MLN4924 (0, 0.1, 0.3, 1 μM) with or without celecoxib (60 μM) for 24 h, followed by Western blotting using antibodies against p-AKT (S473), AKT, ERK, and p-ERK. β-actin was used as a loading control. (C–D) A total of 4000 T24 cells and 6000 5637 cells were seeded in triplicate in 96-well plates and treated with various concentrations of celecoxib, MLN4924, or the combination of MLN4924 and celecoxib (50 μM) for 48 h, followed by the CCK-8 assay (mean ± SEM, n = 3). (E) CI-fraction affected plot of the MLN4924/celecoxib combination in T24 and 5637 cells assessed with Compusyn Software (Fa, corresponding to the fraction of cell viability, the synergistic effect was considered if CI < 1, and additive if CI = 1, antagonistic if CI > 1). UC: urothelial carcinoma; CCK-8: cell counting kit-8; SEM: standard error of the mean; CI: combination index. **0.005 < P < 0.01, ***P < 0.005.
Celecoxib Synergistically Enhances the Suppression of Growth in UC Cells by MLN4924
Given that celecoxib could downregulate AKT and ERK signal pathways in UC cells, we chose celecoxib to verify our hypothesis, that is, the sensitizing effect of celecoxib to MLN4924. We first tested the half-maximal inhibitory concentration (IC50) of celecoxib in T24 and 5637 cells. Cells were exposed to various concentrations of celecoxib for 48 h, then using a CCK-8 assay to account the cell viability, and the result shows the IC50 of celecoxib is 63.8 μΜ in T24 cells and 60.3 μΜ in 5637 cells (Fig. 1C). To reduce the cytotoxicity of UC cells, we chose the 40- to 60-μΜ concentrations of celecoxib (which lower than IC50 of celecoxib) for the following experiments. T24 and 5637 cells were exposed to various concentrations of MLN4924 alone or in combination with celecoxib (50 μM) for 48 h. The CCK-8 assay showed that MLN4924 inhibited UC cells viability in a dose-dependent manner, and the combination drugs group (MLN4924/celecoxib) significantly decreased the cell viability (Fig. 1D); the IC50 values of MLN4924 were significantly reduced from approximately 0.68 to 0.19 μM after being combined with celecoxib in T24 cells, and approximately 4.67 to 1.54 μM in 5637 cells (Fig. 1D). These results indicated that celecoxib sensitized UC cells to the MLN4924 treatment, and the combination of celecoxib and MLN4924 exhibited synergistic treatment effects in T24 and 5637 cells.
To test the synergistic effect of MLN4924 combined with celecoxib, we used CCK8 assay and CompuSyn software to analyze combined drug effects. MLN4924 and celecoxib were combined at a concentration ratio of 0.7:50 and were subjected to median drug effect and CI analyses; the CI plot is shown in Fig. 1E. The MLN4924/celecoxib combination was antagonistic for Fa lower than approximately 0.3 but synergistic above this value in T24 cells, and antagonistic lower than approximately 0.35 but synergistic above this value in 5637 cells (CI < 1), which means that the combination of both drugs increased the inhibitory effect on UC cell viability 28 .
Celecoxib Synergistically Enhances the Suppression of Survival in UC Cells by MLN4924
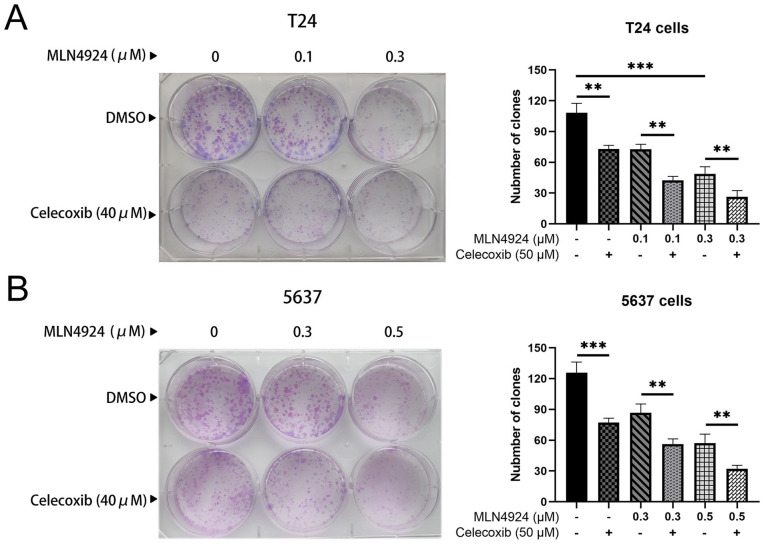
Given that AKT and ERK pathways act as critical regulators of cell survival 19 , we determined whether celecoxib could enhance the suppression of survival in UC cells by MLN4924. T24 cells were exposed to MLN4924 (0, 0.1, 0.3 μM) alone or combined with celecoxib (40 μM) for 7 days, and 5637 cells were exposed to MLN4924 (0, 0.3, 0.5 μM) alone or combined with celecoxib (40 μM) for 12 days. We used a clone formation assay to evaluate cell survival. MLN4924 significantly reduced the number of colonies compared with the control group. Furthermore, the colony formation of the combination group (MLN4924/celecoxib) was remarkably lower than the MLN4924-only group (P < 0.05, Fig. 2A, B). Taken together, celecoxib synergistically suppressed T24 and 5637 cells’ survival with MLN4924.
Figure 2.
Celecoxib enhances the suppression of survival in UC cells by MLN4924. (A–B) A total of 300 T24 cells and 600 5637 cells were seeded in six-well plates and treated with various concentration of MLN4924 alone or in combination with celecoxib (40 μM). T24 and 5637 cell colonies were stained and counted after 7 and 12 days, respectively (>50 cells in a colony). Rate of colony formation (%) = colony number/(300 or 500) × 100% (mean ± SEM, n = 3, *0.01 < P < 0.05, **0.005 < P < 0.01, ***P < 0.005). UC: urothelial carcinoma; SEM: standard error of the mean.
Celecoxib Synergistically Enhances the MLN4924-Induced Apoptosis in UC Cells
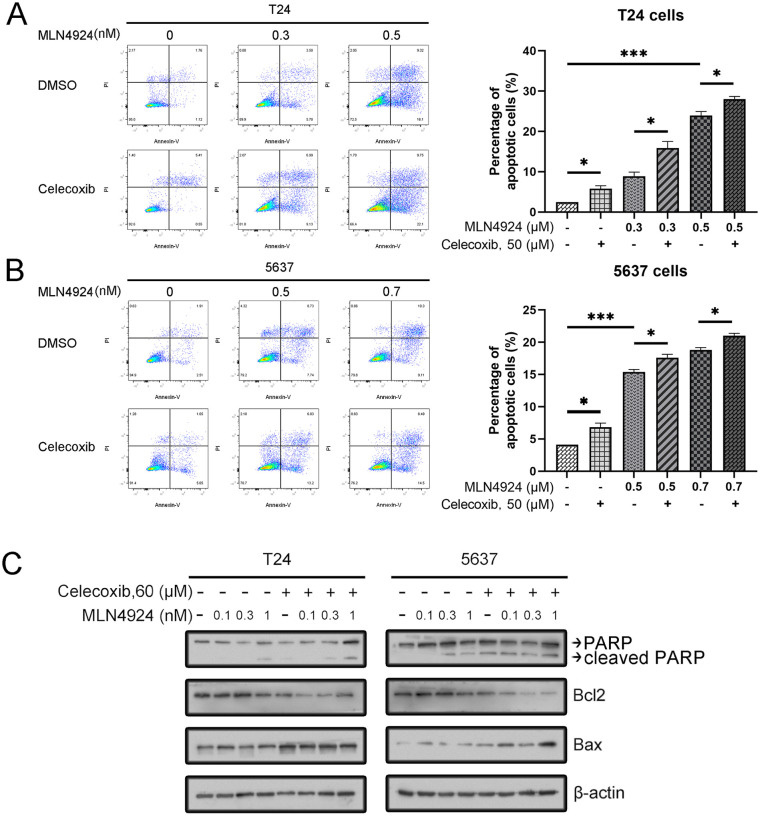
Previous studies have reported that both MLN4924 and celecoxib induced apoptosis in UC cells10,25. Thus, we tested whether celecoxib could synergistically induce cell apoptosis with MLN4924. First, T24 and 5637 cells were exposed to various concentrations of MLN4924 (0, 0.3, 0.5 μM) and MLN4924 (0, 0.3, 0.5 μM), respectively, with or without celecoxib (50 μM) for 48 h. The result of flow cytometry showed the following: (1) MLN4924 induced apoptosis both in T24 and 5637 cell lines in a dose-dependent manner; (2) celecoxib alone induced apoptosis in T24 and 5637 cells; and (3) higher apoptosis was seen in the combination group than in other groups (Fig. 3A, B).
Figure 3.
Celecoxib enhances the induction of apoptosis induced by MLN4924. (A–B) Cells were treated with MLN4924 alone or in combination with celecoxib. After 48 h, the cells were harvested and stained using the FITC-Annexin V/PI apoptosis detection kit. Cells with Annexin V+ and PI + staining located in the right upper and lower quadrants were considered as apoptotic cells (mean ± SEM, n = 3, *0.01 < P < 0.05, **0.005 < P < 0.01, ***P < 0.005). (C) Cells were incubated with various concentrations of MLN4924 (0, 0.1, 0.3, 1 μM), with or without celecoxib (60 μM) for 24 h. Then cells were harvested for Western blotting using indicated antibodies. SEM: standard error of the mean; DMSO: dimethyl sulfoxide.
Next, we tested the expression of apoptosis-related proteins cleaved PARP, BAX, and Bcl2 by using Western blotting (WB). The result showed that MLN4924 increased the expression of cleaved PARP in a dose-dependent manner. Moreover, the expression of apoptosis-related proteins was higher in the combination group than the MLN4924-only group in both T24 and 5637 cells (P < 0.05). Celecoxib also increased the expression of pro-apoptosis proteins BAX and reduced the expression of antiapoptosis protein Bcl2 and in both T24 and 5637 cells (Fig. 3C). To sum up, our results indicated that celecoxib synergistically enhanced the MLN4924-induced apoptosis in UC cell (Fig. 3). Celecoxib enhances the induction of apoptosis induced by MLN4924. (A–B) Cells were treated with MLN4924 alone or in combination with celecoxib. After 48 h, the cells were harvested and stained using the FITC-Annexin V/PI apoptosis detection kit. Cells with Annexin V+ and PI + staining located in the right upper and lower quadrants were considered as apoptotic cells [mean ± standard error of the mean (SEM), n = 3, *0.01 < P < 0.05, **0.005 < P < 0.01, ***P < 0.005]. (C) Cells were incubated with various concentrations of MLN4924 (0, 0.1, 0.3, 1 μM), with or without celecoxib (60 μM) for 24 h. Then, cells were harvested for WB using indicated antibodies.
Celecoxib Synergistically Enhances the Suppression of Migration and Invasion in UC Cells by MLN4924
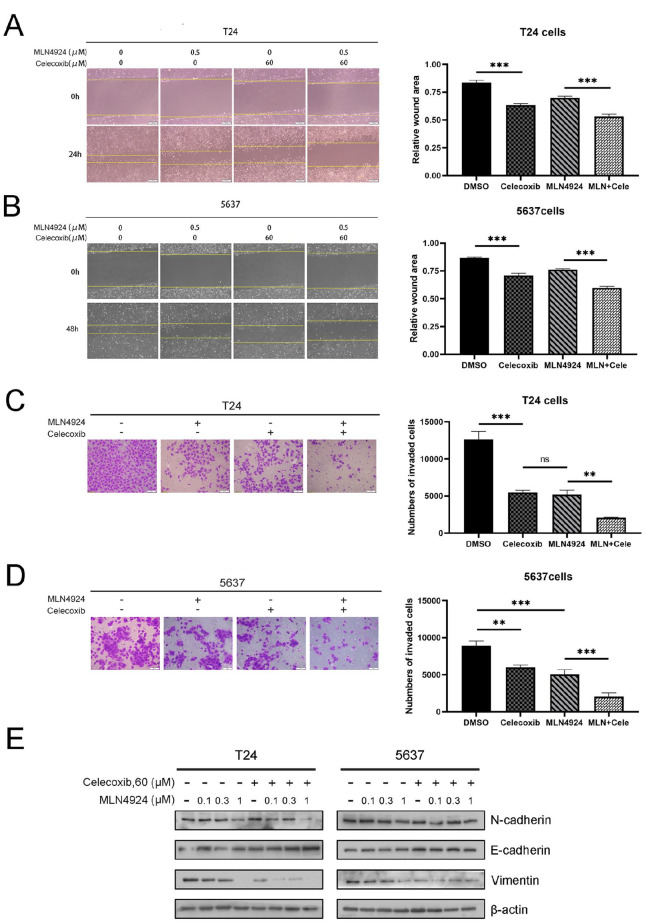
AKT and ERK signal pathways are engaged in much physiological activity, including the EMT of cancer cells 29 . We tested the synergistic effect of celecoxib with MLN4924 on UC cell migration and invasion by a wound-healing assay and a transwell invasion assay (Fig. 4A, B). We discovered the following: (1) MLN4924 and celecoxib alone remarkably inhibited cell migration compared with a control group (P < 0.05) and (2) compared with MLN4924 and celecoxib alone, the combination group significantly inhibited UC cell migration (P < 0.05) (Fig. 4C, D). Next, we explored the protein expression levels of EMT-associated molecule. The expression of epithelial marker E-cadherin was higher in the combination group than in the MLN4924-only group. Also, the expression of mesenchymal markers vimentin and N-cadherin was remarkably decreased in the combination group, compared with those in the MLN4924-only group (Fig. 4E). In summary, the combination of MLN4924 and celecoxib synergistically inhibited cell migration and invasion.
Figure 4.
Celecoxib enhances the suppression of migration and invasion in UC cells by MLN4924. (A) T24 and 5637 cells were seeded in six-well plates and treated with MLN4924 (0.5 μM) with or without celecoxib treatment (50 μM) for 24 h. After serum starvation for 12–18 h, used a pipette tip to scratch across the well. The T24 and 5637 cells were photographed at 24 and 48 h, respectively. Data were shown as the relative wound area normalized to the control. (B) A total 3 × 105 T24 cells and 6 × 105 5637 cells were treated with the indicated agents and then seeded into the upper chamber containing serum-free medium. Then, T24 and 5637 cells were fixed and stained after 24 and 48 h, respectively, followed by photography and counting. (C) Cells were treated with the drugs for 24 h, followed by Western blotting using antibodies against N-cadherin, E-cadherin, and vimentin (mean ± SEM, n = 3, *0.01 < P < 0.05, **0.005 < P < 0.01, ***P < 0.005). UC: urothelial carcinoma; SEM: standard error of the mean; DMSO: dimethyl sulfoxide.
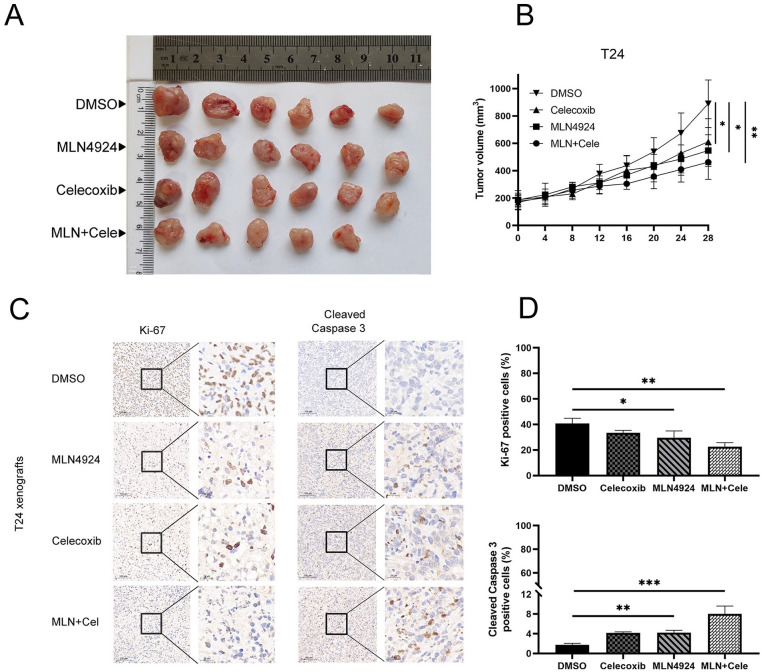
Celecoxib Synergistically Enhances the MLN4924-Induced Antitumor Effect in a Xenograft Mouse Model
We explored the antitumor effects of the combination of MLN4924 and celecoxib in vivo. Briefly, 5 × 105 T24 cells were mixed with Matrigel and subcutaneously injected into homozygous null (BALB/c-nu) mice flanks. After the tumors had reached approximately 200 mm3, the mice were divided into four groups. The tumor sizes in the combination group (MLN4924/ celecoxib) were smaller than those in single drug groups, and the tumor sizes in the control group were biggest among the four groups (all P < 0.05); tumor in the combination group fully disappeared after they received combinational drugs for 1 week. UC xenograft tumors in the control group kept growing and reached the largest size of approximately 900 mm3. Moreover, MLN4924 and celecoxib treatment did not affect the weight of the mice compared with the control group (P > 0.05), indicating the minimal toxicity of MLN4924 and celecoxib. These results suggested that celecoxib enhanced the MLN4924 antitumor effect on a T24 xenograft mice model (Fig. 5A, B).
Figure 5.
The xenograft model demonstrates the efficacy of the combination of MLN4924 and celecoxib in vivo. (A–B) Nude mice bearing T24 xenograft tumors were treated with DMSO (as control), MLN4924, celecoxib, or the MLN4924/celecoxib combination for 4 weeks (n = 6 for each group, one tumor has disappeared after given the combinational drugs for 1 week). Tumor volume = longest tumor diameter × (shortest tumor diameter) 2 /2. (C–D) Tumors were fixed in 10% formalin and embedded in paraffin. Each sample was stained for Ki67 and cleaved caspase-3 with representative images shown. Positive cells were counted from three independent tumors in each group (mean ± SEM, n = 3, *0.01 < P < 0.05, **0.005 < P < 0.01, ***P < 0.005). SEM: standard error of the mean; DMSO: dimethyl sulfoxide.
Next, immunohistochemistry staining was performed using cleaved caspase-3 and Ki67 antibodies. Either MLN4924 or celecoxib treatment decreased the proliferation of UC cells and induced apoptosis; the maximal effect was seen in the combinational drugs group of MLN4924 and celecoxib (Fig. 5C, D).
Discussion
Bortezomib is the first Food and Drug Administration (FDA)-approved proteasome inhibitor for multiple myeloma and mantle cell lymphoma treatment. It targets intracellular peptides that are produced by the proteasome 30 . MLN4924 is a selective NAE1 inhibitor identified as a novel anticancer agent by triggering cell apoptosis, senescence, autophagy, and chemosensitization/radiosensitization31,32, which has a similar effect as bortezomib; it selectively blocks the protein degradation regulated by CRL E3 ligases and produces limited cytotoxicity. However, compared with MLN4924, bortezomib blocks all the protein degradation through the 26S proteasome and thus offers higher cytotoxicity 8 . Yet, it has also been found that MLN4924 can activate EGFR and its downstream PI3K/AKT1/mTOR and RAS/MAPK/ERK signals, which lead to accelerated EGF-mediated wound healing, inhibition of ciliogenesis, and promotion of the tumorsphere formation 15 . In UC, AKT and ERK signal pathways have been involved in UC development and metastasis, and the overexpression of those signals contributes to UC cells growth, migration, and antiapoptosis25,33. So, the inhibition of these signals is an attractive way for UC treatment 34 . Yet, previous studies have also suggested that heterozygous mutations of NAEβ subunit in cancer cells may lead to resistance to MLN4924 35 ; thus, the drug combination is a feasible way to reduce the UC cell resistance MLN4924.
Functional activation of AKT phosphorylation requires phosphorylation at two distinct sites, namely serine 473 by mTORC2 and threonine 308 by PDK1 35 . Herein, we tested the expression level of phospho-Akt (Ser473) and phospho-ERK; both signals were highly expressed in T24 and 5637 cell lines. We also found that MLN4924 activates the expression of p-AKT (S473) and p-ERK in a dose-dependent manner in T24 cells but not in 5637 cells. We assume this was due to the concentration of MLN4924 that was not high enough to activate those signals as the IC50 of 5637 was higher than that of T24. However, after treating cells with celecoxib, the levels of p-AKT (S473) and p-ERK were significantly suppressed both in T24 and 5637 cells. This suggested that celecoxib may prominently inhibit the activation of AKT and ERK signal pathways, which could counteract the off-target effects reduced by MLN4924 (Fig. 6). Thus, celecoxib might be a good combinational drug for MLN4924.
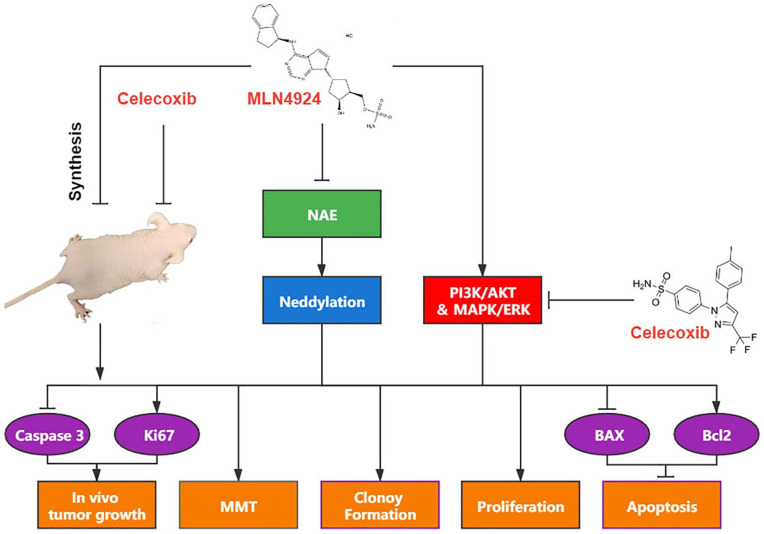
Figure 6.
Mechanism of action. MLN4924 treatment blocks neddylation by inactivation of NAE; yet, it also activates AKT and ERK pathways. Celecoxib downregulates the AKT and ERK, synergistically enhancing MLN4924-induced suppression of growth, survival, and EMT, and inducing apoptosis via Bcl2 signal pathways of UC cells. The combination of MLN4924 with celecoxib in vivo significantly suppresses the growth of UC by increasing the expression of caspase 3 and decreasing the expression of Ki67. NAE: NEDD8-activating enzyme; EMT: epithelial–mesenchymal transition; UC: urothelial carcinoma.
Celecoxib is an NSAID, an inhibitor of the COX-2 enzyme 36 . Metabolism of arachidonic acid controls a mass of physiological activities like inflammatory process, constriction of blood vessels, and cell apoptosis via the COX pathway, with the synthesis of prostaglandins 23 . It is well known that inflammation is closely associated with cancer development. In the tumor microenvironment, the inflammatory cells participate in the oncogenesis process 37 . COX-2 also participates in cancer cell proliferation, survival, and migration 38 , while COX-2 inhibitors can constrain the biological behavior of tumor cells23,39. Celecoxib has antineoplastic properties and has been shown to be effective against various cancers 23 . Celecoxib can inhibit UC cell growth and migration, induce apoptosis26,31, and depress the AKT and ERK signal pathways in other cancer cells26,27. But one study reported the off-target effect of celecoxib could block chemotherapeutic agent-induced apoptosis in hematopoietic cancer cells 40 . In our research, celecoxib suppressed the activation of AKT and ERK signals in T24 and 5637 cells, so we chose celecoxib to combine it with MLN4924 for UC treatment. Based on previous studies24,41, the IC50 of celecoxib in T24 and 5637 cells is approximately 70 μM, which is close to our result (approach to 60 μM). Concentration of celecoxib used in all experiments was lower than 60 μM.
We found that celecoxib combined with MLN4924 significantly decreased cell viability, suppressed the colony formation, increased cell apoptosis, and decreased migration and invasion in both T24 and 5637 cells compared with cells treated with MLN4924 or celecoxib alone. Previous study also proved that MLN4924 or celecoxib alone induced UC cells apoptosis10,25 and that celecoxib alone could induce UC cell autophagy when the concentration was 80 μM14,25, which is consistent with our results. Next, by using Compusyn software, we detected CI plots of the combinational drugs and found that the MLN4924/celecoxib combination had a strong synergistic effect on inhibiting the proliferation of T24 and 5637 cells.
EMT is a critical process for cancer progression, through which epithelial lineage cancer cells lose their epithelial traits and transform to mesenchymal cancer cells, increase cell motility, and reduce intercellular adhesion 42 . The PI3K/AKT/mTOR signal pathway has been demonstrated to have a key role in inducing EMT of UC cells43,44. Moreover, several studies have suggested that celecoxib inhibits EMT in human BC cell lines20,24. We found the combination of celecoxib and MLN4924 remarkably reduced the cell migration and EMT in T24 and 5637 cells, which was observed by a significant reduction of N-cadherin and vimentin, and upregulation of E-cadherin. These data suggest that celecoxib combined with MLN4924 synergistically depressed the migration and EMT of T24 and 5637 cells.
Previous studies reported that MLN4924 alone or in combination with cisplatin might inhibit the growth of UC tumors in vivo10,14. Another study found that celecoxib can depress the xenografted T24 tumor growth in BALB/c-nu mice 41 . In this study, we treated mice bearing UC (T24 cells) tumors with celecoxib with MLN4924 for 4 weeks. We found that compared with celecoxib or MLN4924 alone, celecoxib/MLN4924 could synergistically depress the UC tumor growth in vivo study. The immunohistochemistry staining further confirmed these data.
The main findings of this study are as follows: (1) MLN4924 activates AKT and ERK signal pathways in a T24 cell line, while celecoxib depresses the activation of the two signals both in T24 and 5637; (2) celecoxib combined with MLN4924 synergistically depresses the UC cell growth, colony formation, migration, and invasion and induces apoptosis; and (3) celecoxib combined with MLN4924 synergistically depresses the tumor growth in xenograft mice model. These results suggest that celecoxib might be an effective combinational drug for enhancing antitumor effect of MLN4924 and reducing the dose of MLN4924 used for cancer treatment, thus reducing its toxic and side effects. Our research may provide a new drug combination strategy for the treatment of UC.
Footnotes
Author Contributions: Ju Guo, Shida Xiong, and Xiaoqiang Liu conceived and designed the experiments; Shida Xiong, Xiaoqiang Liu, Yi Ding, Haoxuan Huang, and Ru Zhang performed the experiments; Shida Xiong, Wei Huang, and Qian Chen analyzed the data; Ju Guo and Wei Huang supervised the study; and Shida Xiong wrote the article.
Ethical Approval: All animal studies (including the mice euthanasia procedure) were approved by Nanchang University College of Medicine institutional animal care regulations.
Statement of Human and Animal Rights: All procedures in this study were conducted according to Nanchang University College of Medicine institutional animal care regulations.
Statement of Informed Consent: This article does not contain any studies with human participants.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Natural Science Foundation of China (grant number 81860454) and Jiangxi Provincial Science and Technology Program (grant number20181BAB205053).
ORCID iD: Ju Guo  https://orcid.org/0000-0002-3413-2234
https://orcid.org/0000-0002-3413-2234
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Tan WS, Rodney S, Lamb B, Feneley M, Kelly J. Management of non-muscle invasive bladder cancer: a comprehensive analysis of guidelines from the United States, Europe and Asia. Cancer Treat Rev. 2016;47:22–31. [DOI] [PubMed] [Google Scholar]
- 3. Rajalingam K, Dikic I. SnapShot: expanding the ubiquitin code. Cell. 2016;164(5):1074.e1071. [DOI] [PubMed] [Google Scholar]
- 4. Morrow JK, Lin HK, Sun SC, Zhang S. Targeting ubiquitination for cancer therapies. Future Med Chem. 2015;7(17):2333–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mansour MA. Ubiquitination: friend and foe in cancer. Int J Biochem Cell Biol. 2018;101:80–93. [DOI] [PubMed] [Google Scholar]
- 6. Xirodimas DP. Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochem Soc Trans. 2008;36(Pt 5):802–806. [DOI] [PubMed] [Google Scholar]
- 7. Zhao Y, Morgan M, Sun Y. Targeting Neddylation pathways to inactivate cullin-RING ligases for anticancer therapy. Antioxid Redox Signal. 2014;21(17):2383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458(7239):732–36. [DOI] [PubMed] [Google Scholar]
- 9. Brownell JE, Sintchak MD, Gavin JM, Liao H, Bruzzese FJ, Bump NJ, Soucy TA, Milhollen MA, Yang X, Burkhardt AL, Ma J, et al. Substrate-assisted inhibition of ubiquitin-like protein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol Cell. 2010;37(1):102–11. [DOI] [PubMed] [Google Scholar]
- 10. Ho IL, Kuo KL, Liu SH, Chang HC, Hsieh JT, Wu JT, Chiang CK, Lin WC, Tsai YC, Chou CT, Hsu CH, et al. MLN4924 synergistically enhances cisplatin-induced cytotoxicity via JNK and Bcl-xL pathways in human urothelial carcinoma. Sci Rep. 2015;5:16948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou Q, Sun Y. MLN4924: additional activities beyond neddylation inhibition. Mol Cell Oncol. 2019;6(5): e1618174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou L, Zhang W, Sun Y, Jia L. Protein neddylation and its alterations in human cancers for targeted therapy. Cell Signal. 2018;44:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou L, Jiang Y, Luo Q, Li L, Jia L. Neddylation: a novel modulator of the tumor microenvironment. Mol Cancer. 2019;18(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuo KL, Ho IL, Shi CS, Wu JT, Lin WC, Tsai YC, Chang HC, Chou CT, Hsu CH, Hsieh JT, Chang SC, et al. MLN4924, a novel protein neddylation inhibitor, suppresses proliferation and migration of human urothelial carcinoma: in vitro and in vivo studies. Cancer Lett. 2015;363(2):127–36. [DOI] [PubMed] [Google Scholar]
- 15. Zhou X, Tan M, Nyati MK, Zhao Y, Wang G, Sun Y. Blockage of neddylation modification stimulates tumor sphere formation in vitro and stem cell differentiation and wound healing in vivo. Proc Natl Acad Sci U S A. 2016;113(21):E2935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun Y, Liu W, Liu T, Feng X, Yang N, Zhou H. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600–604. [DOI] [PubMed] [Google Scholar]
- 17. Revathidevi S, Munirajan AK. Akt in cancer: mediator and more. Semin Cancer Biol. 2019;59:80–91. [DOI] [PubMed] [Google Scholar]
- 18. Zhao H, Bo Q, Wang W, Wang R, Li Y, Chen S, Xia Y, Wang W, Wang Y, Zhu K, Liu L, et al. CCL17-CCR4 axis promotes metastasis via ERK/MMP13 pathway in bladder cancer. J Cell Biochem. 2018;120(2):1979–89. [DOI] [PubMed] [Google Scholar]
- 19. Mayer I, Arteaga C. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28. [DOI] [PubMed] [Google Scholar]
- 20. Gao YE, Guan Z, Chen J, Xie H, Yang Z, Fan J, Wang X, Li LEI. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int J Oncol. 2015;47(2):690–700. [DOI] [PubMed] [Google Scholar]
- 21. Gakis G. The role of inflammation in bladder cancer. Adv Exp Med Biol. 2014;816:183–96. [DOI] [PubMed] [Google Scholar]
- 22. Kamali K, Nikbakht J, Ayubi E, Nabizadeh M, Sarhadi S. Comparison of the efficacy of oxybutynin, phenazopyridine, celecoxib, and placebo in the treatment of urinary tract symptoms after BCG therapy in patients with bladder tumors. Urol J. 2020;18:439–44. [DOI] [PubMed] [Google Scholar]
- 23. Tołoczko-Iwaniuk N, Dziemiańczyk-Pakieła D, Nowaszewska BK, Celińska-Janowicz K, Miltyk W. Celecoxib in cancer therapy and prevention—review. Curr Drug Targets. 2019;20(3):302–15. [DOI] [PubMed] [Google Scholar]
- 24. Liu X, Wu Y, Zhou Z, Huang M, Deng W, Wang Y, Zhou X, Chen L, Li Y, Zeng T, Wang G, et al. Celecoxib inhibits the epithelial-to-mesenchymal transition in bladder cancer via the miRNA-145/TGFBR2/Smad3 axis. Int J Mol Med. 2019;44(2):683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang KH, Kuo KL, Ho IL, Chang HC, Chuang YT, Lin WC, Lee PY, Chang SC, Chiang CK, Pu YS, Chou CT, et al. Celecoxib-induced cytotoxic effect is potentiated by inhibition of autophagy in human urothelial carcinoma cells. PLoS ONE. 2013;88(12):e82034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu Z, Zhang C, Zhou J, Hu J, Sheng L, Li X, Chen L, Li X, Deng X, Zheng G. Celecoxib alleviates AKT/c-Met-triggered rapid hepatocarcinogenesis by suppressing a novel COX-2/AKT/FASN cascade. Mol Carcinog. 2019;58(1):31–41. [DOI] [PubMed] [Google Scholar]
- 27. Sun YZ, Cai N, Liu NN. Celecoxib down-regulates the hypoxia-induced expression of HIF-1alpha and VEGF through the PI3K/AKT pathway in retinal pigment epithelial cells. Cell Physiol Biochem. 2017;44(4):1640–50. [DOI] [PubMed] [Google Scholar]
- 28. Ashton J. Drug combination studies and their synergy quantification using the Chou-Talalay method–letter. Cancer Res. 2015;75(11):2400. [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Lin F, Chen Y, Wang R, Liu J, Jin Y, An R. Cryptotanshinone inhibits bladder cancer cell proliferation and promotes apoptosis via the PTEN/PI3K/AKT pathway. J Cancer. 2020;11(2):488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14(6):1649–57. [DOI] [PubMed] [Google Scholar]
- 31. Zhou L, Jia L. Targeting protein neddylation for cancer therapy. Adv Exp Med Biol. 2020;1217:297–315. [DOI] [PubMed] [Google Scholar]
- 32. Zheng S, Tao W. Targeting cullin-RING E3 ligases for radiosensitization: from NEDDylation inhibition to PROTACs. Front Oncol. 2020;10:1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol. 2018;1655:335–50. [DOI] [PubMed] [Google Scholar]
- 34. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Conaghan PG. A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol Int. 2012;32(6):1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coussens L, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tabriz H, Olfati G, Ahmadi S, Yusefnia S. Cyclooxygenase-2 expression in urinary bladder transitional cell carcinoma and its association with clinicopathological characteristics. Asian Pac J Cancer Prev. 2013;14(8):4539–43. [DOI] [PubMed] [Google Scholar]
- 39. Bourn J, Cekanova M. Cyclooxygenase inhibitors potentiate receptor tyrosine kinase therapies in bladder cancer cells in vitro. Drug Des Devel Ther. 2018;12:1727–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cerella C, Sobolewski C, Chateauvieux S, Henry E, Schnekenburger M, Ghelfi J, Dicato M, Diederich M. COX-2 inhibitors block chemotherapeutic agent-induced apoptosis prior to commitment in hematopoietic cancer cells. Biochem Pharmacol. 2011;82(10):1277–90. [DOI] [PubMed] [Google Scholar]
- 41. Mohammed SI, Dhawan D, Abraham S, Snyder PW, Waters DJ, Craig BA, Lu M, Wu L, Zheng R, Stewart J, Knapp DW. Cyclooxygenase inhibitors in urinary bladder cancer: in vitro and in vivo effects. Mol Cancer Ther. 2006;5(2): 329–36. [DOI] [PubMed] [Google Scholar]
- 42. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. [DOI] [PubMed] [Google Scholar]
- 43. Karimi Roshan M, Soltani A, Soleimani A, Rezaie Kahkhaie K, Afshari AR, Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. 2019;165:229–34. [DOI] [PubMed] [Google Scholar]
- 44. Iskender B, Izgi K, Hizar E, Jauch J, Arslanhan A, Yuksek EH, Canatan H. Inhibition of epithelial-mesenchymal transition in bladder cancer cells via modulation of mTOR signalling. Tumour Biol. 2016;37(6):8281–91. [DOI] [PubMed] [Google Scholar]