Abstract
Crohn’s disease is a chronic inflammatory, relapsing-remitting, and progressive gastrointestinal disorder with an often-negative impact on the physical, emotional, and psychological well-being. Over the past two decades, the medical compendium for the treatment of Crohn’s disease has increased significantly, enabling treatment beyond symptoms. Indeed, early and timely use of effective medical therapy has been reflected by improved outcomes with reduction in surgery and ability to achieve clinical and endoscopic remission, reduce corticosteroid dependance, and prevent long-term complications in more patients. In this review, we discuss the key milestones in the medical management of Crohn’s disease.
Keywords: Crohn’s disease, inflammatory bowel disease, ulcerative colitis
Introduction
Crohn’s disease (CD) is a chronic inflammatory, relapsing-remitting, and progressive gastrointestinal disorder with variable disease location and behaviour. 1 It was first recognised as a separate entity from ulcerative colitis (UC) in the landmark publication by Crohn et al. in 1952, 2 although there have been previous reports describing ‘regional ileitis’ or ‘regional enteritis’. 3 Currently, it is believed that CD is caused by the interaction between the environment we live in, our immune system, genetics, and the microbiome 4 .
Over a course of 20 years, the actuarial rate of developing inflammatory, structuring, and penetrating disease is 12%, 18%, and 70%, respectively. 5 These complications will likely result in multiple surgeries and eventual disability, affecting patients’ physical and psychosocial functioning. 6 Encouragingly, recent evidence indicates that surgery rates are declining in CD, which may partly be associated with the early and timely use of medical therapy. 7
Over the last five decades, rapid strides in our understanding of the immuno-pathogenesis of CD and consequent improvements in pharmacological therapies has enabled clinicians to realise what can be achieved through abrogation of the immuno-inflammatory pathway. Our paradigms have evolved from symptom management to clinical and endoscopic remission, with the aim of reducing the long-term use of corticosteroids and preventing long-term complications and disability. In this review article, we discuss the key milestones in the medical management of CD, including current therapies (See Figure 1), a look at the immediate future with promising therapies on the horizon.
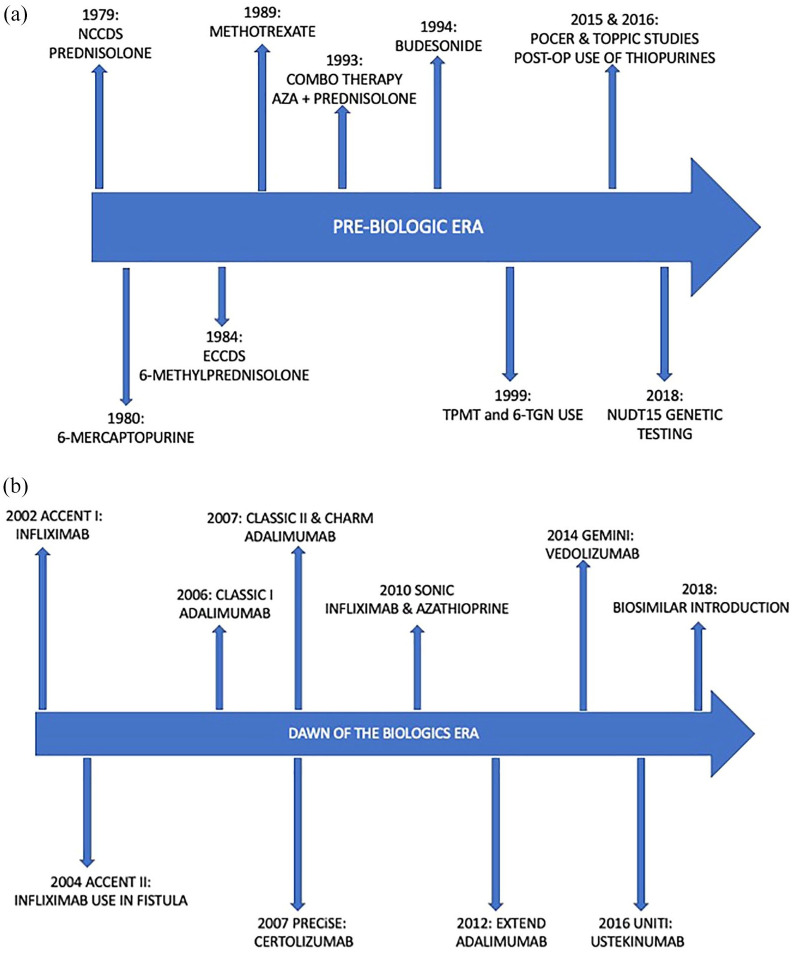
Figure 1.
(a) The timeline of the introduction of immunosuppressive medication prior to the use of biologics. (b) The timelines of the milestone studies that introduced the biological medication used today for CD.
Corticosteroids
Truelove and Witts first demonstrated the efficacy of corticosteroid treatment in acute severe UC in 1955. 8 In 1979 and 1984, two landmark studies, the National Cooperative Crohn’s Disease Study (NCCDS) and the European Cooperative Crohn’s Disease Study (ECCDS) established the efficacy of prednisolone and 6-methylprednisolone, respectively, in inducing remission in patients with active moderate to severe CD.9,10 When evaluating 192 patients with CD over a 2-year period, 6-methylprednisolone was shown to be the most effective drug overall compared with 6-methylprednisolone and sulfasalazine combination, sulfasalazine alone, or placebo. The superiority of this drug was shown in overall comparison of all patients (p < 0.001), isolated small bowel disease (p < 0.05), and in those with small and large bowel disease (p < 0.05). 10
Corticosteroids, however, have numerous unwanted side effects, such as metabolic (steroid-induced diabetes, cushingoid appearance, and hepatic steatosis), central nervous system (psychosis, insomnia, and emotional disturbances), gastrointestinal (dyspepsia and peptic ulcer), musculoskeletal (osteonecrosis of the jaw and hip, osteoporosis, and growth failure), skin (easy bruising, skin thinning, weight gain, acne, hirsutism, striae, and purpura), and ocular effects (glaucoma and cataracts).11,12 Long-term use can also increase the risk of infection, lead to impaired wound healing, and can result in steroid dependence. Furthermore, patients may suffer with glucocorticoid withdrawal syndrome when attempting to stop or wean this medication. More recently, prolonged corticosteroid therapy has shown to be associated with an increase in mortality in patients with CD. 13 In a prospective study, the TREAT registry 14 found that prednisolone was linked to increased mortality risk (hazard ratio (HR): 2.14, 95% confidence interval (CI): 1.55–2.95; p < 0.001) with similar findings from the European ENCORE registry 15 (HR: 3.58, 95% CI: 1.49–8.61).
In 1994, a newer glucocorticoid formulation, budesonide, was shown to have equal efficacy to prednisolone, 16 with a 15 times greater affinity for glucocorticoid receptors, such that 5 mg of budesonide is equivalent to 12 mg of prednisolone. 17 Budesonide has an added advantage of a high first pass liver metabolism and rapid elimination, resulting in minimal systemic absorption and thereby reducing the risk of steroid-induced side effects. 18 While budesonide 9 mg once daily has been shown to be superior than placebo in inducing remission for patients with mild to moderate ileocaecal CD (OR: 2.92, 95% CI: 1.52–5.39),6,19 budesonide was inferior to prednisolone for the induction of clinical remission in severe ileocaecal CD or in colonic CD (relative risk (RR): 0.52, 95% CI: 0.28–0.95). 19
Corticosteroids remained the mainstay for induction therapy until the late 1990s 12 when evidence began showing that they only induce complete clinical remission in 48% and partial clinical remission in 32% of patients with active CD. 20 However, 20% of patients were found to be resistant from the onset, and at 1-year follow-up, 45% of the patients who responded initially became steroid-dependent with only 32% of patients maintaining a prolonged clinical response. 21 It became rapidly apparent that corticosteroids were ineffective at maintaining remission, reducing flares, or disease recurrence.9,22 –24 The French GETAID study provided further proof that corticosteroids were not disease-modifying agents with limited evidence in their ability to achieve endoscopic mucosal healing or preventing endoscopic relapse. 25 In this study, patients were given prednisolone 1 mg/kg for 7 weeks, and only 29% achieved endoscopic and clinical remission, with 71% showing active endoscopic lesions. In fact, 9% of patients had worsening endoscopic lesions despite symptomatic improvement. 25 All of the above limitations associated with prolonged corticosteroid use make a compelling case for using newer safer therapies that maintain remission without exposing patients to unwanted side effects.
5-Aminosalicylic acid compounds
5-Aminosalicylic acids (5-ASAs) play a fundamental role in inducing and maintaining remission for patients with UC. However, despite their extensive use, multiple studies over many years have demonstrated that there is limited evidence of benefit, if any, in CD (Figure 2). The initial study conducted by Gendre in 1993 demonstrated effectiveness with 5-ASAs in achieving and maintaining remission in CD (45% 5-ASA vs 29% placebo for 2-year remission rate). 26 However, this efficacy shown by Gendre could not be replicated in subsequent studies.27 –29 Systematic literature reviews also have not demonstrated efficacy of oral 5-ASAs compared to placebo in inducing or maintaining clinical remission in CD, with relapse rates at 12 and 24 months to be 53% versus 54% (RR: 0.98, 95% CI: 0.91–1.07) and 54% versus 58% (RR: 0.94, 95% CI: 0.68–1.29), respectively.30,31 A modest benefit in using 5-ASAs to prevent post-operative CD relapse, however, was demonstrated in a recent meta-analysis showing RR of relapse with 5-ASA versus placebo to be 0.86 (95% CI: 0.74–0.99). 32 Despite international guidelines from the British Society of Gastroenterology (BSG), 1 European Crohn’s and Colitis Organisation (ECCO), 33 American Gastroenterological Association (AGA), 34 and the United Arab Emirates (UAE IBD) association 35 advising against their use in CD, 5-ASAs remain widely prescribed. Notably, 5-ASA’s may also be associated with adverse effects including, headaches, nausea, malaise, rash, and the rare side effects such as interstitial nephritis, haemolytic anaemia, hepatitis, pancreatitis, and paradoxical worsening of colitis. 36
Figure 2.
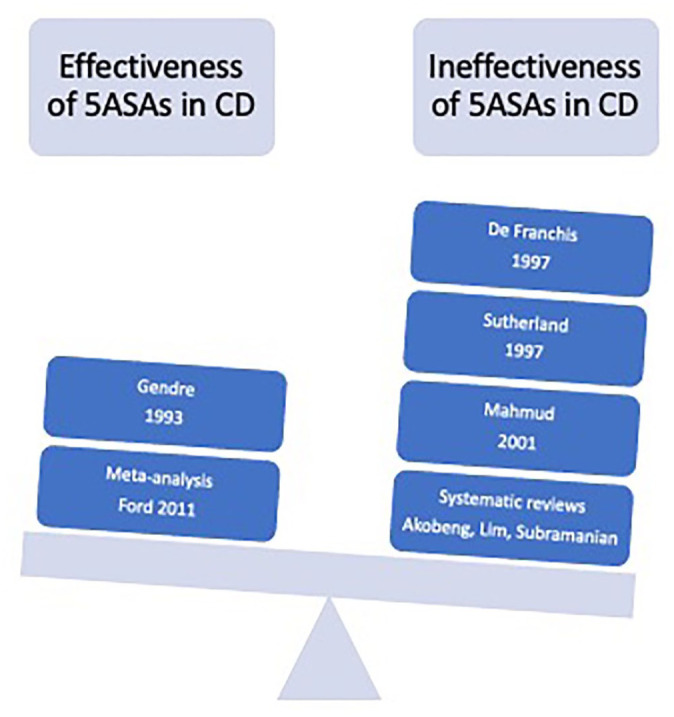
The initial study conducted by Gendre in 1993 showed efficacy with 5ASAs with further support by Ford et al. in their use for post-operative CD. However, there are multiple studies over the years that counteracted these studies and as such, 5ASAs are not currently recommended for use in induction or maintenance of CD.
Antibiotics
It is widely accepted that bacteria play a role in the pathogenesis of certain manifestations of CD, such as abscesses and fistulae. 12 There is also established evidence that bacterial overgrowth secondary to strictures and blind loops may respond well to antibiotic therapy. 12 Although the precise mechanisms of how broad-spectrum antibiotics work are uncertain, it is thought to consist of immunosuppressive activity (i.e. metronidazole), treatment of bacterial overgrowth and suppression of a bacteria-induced antigenic stimulus. 37
Metronidazole
Metronidazole, a nitroimidazole compound, was first reported to be effective in CD in 1975 who reported a response in four out of five patients with large bowel disease after 2 to 4 weeks treatment. 38 Metronidazole has high antimicrobial activity against anaerobes, which are present in high quantities in the neo-terminal ileum. As such, this drug has the greatest effect in patients with ileocolonic disease, which was first illustrated in a placebo-controlled trial of 56 patients. 39 Initial studies also suggested a benefit with metronidazole in treating perineal disease, although 100% of patients had recurrence of disease when discontinuation or dosage reduction was attempted.40,41 A small double-blind placebo-controlled study of 60 CD patients showed that metronidazole reduced the incidence of severe endoscopic recurrence 3 months post-operatively (13% in metronidazole vs 43% in placebo group; p = 0.02). 42 The results were borderline significant at 1 year (4% vs 25%) and was not significant at 2 (26% vs 43%) and 3 years (30% vs 50%) after resection; however, this may be due to the small sample size rather than clear lack of benefit from metronidazole.
The main drawback of metronidazole is the frequency of adverse effects, including gastrointestinal intolerance, metallic taste, and neurotoxicity. 12 It is often poorly tolerated, particularly at the high doses required to treat these patients. Thus, long-term therapy is not recommended.
Others
In a small pilot study of 33 patients, ciprofloxacin did not show much benefit over placebo 43 and trials with the gut-specific antibiotic rifaximin are currently awaited in the post-operative setting. 44 In a randomised placebo-controlled trial lasting 2 years with 213 patients receiving treatment with either clarithromycin, rifabutin, or clofazimine, early benefit of antibiotics was noted. However, there were no significant differences in relapse rates during follow-up. 45 In another phase-III trial, 331 patients with moderate to severe CD were randomised to receive either five capsules of RHB-104 (clarithromycin 95 mg, rifabutin 45 mg, and clofazimine 10 mg) twice daily or placebo. Remission was achieved at 26 weeks in 37% versus 23% on placebo; p = 0.019. A more recent study compared oral budesonide with a combination treatment of ciprofloxacin, doxycycline, and hydroxychloroquine. 46 Results showed that although budesonide had higher induction rates than antibiotics at 10 weeks (25% vs 7.4%), their efficacy reduced at 24 (3.1% vs 7.4%) and 52 weeks (3.1% vs 3.7%), respectively. Thus, this study illustrated that there may be some benefit for antibiotics in long-term remission use. However, further studies are needed before it can be said that antibiotics have a definitive role to play in CD management.
Thiopurines
Thiopurines (6-mercaptopurine (6MP) and azathioprine (AZA)), which act as antimetabolites and immunomodulators, were first discovered in the 1950s and originally used for the treatment of childhood leukaemia. 47 Although the first IBD patient to be treated with thiopurines was in 1962 and for UC, 48 studies in the early 1990s demonstrated the advantages of using thiopurines in CD. 49 Present et al. 49 showed an improvement with 6MP compared to placebo in the treatment of fistulas (31% vs 6%) and in allowing discontinuation of corticosteroids (75% vs 36%) over a 2-year double-blind crossover study of 83 patients (p < 0.001). The advantages of using thiopurines are its steroid-sparing effects; however, its slow onset of action (8–12 weeks) makes them ineffective for short-term induction in active, symptomatic disease.50,51 Regardless, thiopurines have been shown to be more effective than placebo for maintenance of remission in CD, although the quality of evidence for this has been reported as low (number needed to treat (NNT) = 9).
Candy et al. added further weight to the growing evidence of thiopurines in the use of CD. In their double-blind placebo-controlled trial of 63 patients, they were able to demonstrate a clinically significant advantage of using AZA at a dose of 2.5 mg/kg for the maintenance of remission while reducing the dose of prednisolone (42% vs 7% at 15 months, p = 0.001). 52 In the study by Ewe et al., 53 76% of patients on a combined regimen of azathioprine and corticosteroid went into clinical remission earlier (CDAI < 150) and maintained remission longer (12 weeks vs 4 weeks) than in the placebo-treated group (38%); p = 0.03.
As per any medication, thiopurines have their own collection of adverse effects including nausea, infections, allergic reactions, pancreatitis, myelosuppression, hepatotoxicity, and malignancy, particularly lymphoma and nonmelanoma skin cancer.54,55 In 1999, Cuffari et al. 56 first observed the link between leucopenia to thiopurine methyltransferase (TPMT) genetic polymorphism and bone marrow toxicity in adolescent CD. Sandborn 57 and Belaiche et al. 58 then validated the use of 6-thioguanine nucleotide (6-TGN) levels when prescribing thiopurines. 2018 brought about the discovery of NUDT15 gene testing, which if found to be deficient in individuals indicates susceptibility to thiopurine-related toxicity. 59 These studies were important as it allowed clinicians to use drug-level monitoring and genetic testing to personalise prescriptions and determine which patients thiopurines were safe to use in. They not only helped to confirm therapy compliance but also allowed thiopurine dose optimisation in non-responders first before escalating or changing treatment.
Methotrexate
Although thiopurines were the first medication to demonstrate they can maintain remission in CD, there was still concern among clinicians regarding their side effects and potential toxicity. Approximately 15% of patients are intolerant to thiopurines from non-specific nausea and malaise 12 and up to 50% will discontinue treatment within the first 2 years either due to adverse drug events or therapy failure. 60 Methotrexate (MTX) was a medication that attempted to bridge this gap. Although first developed for use in the 1950’s as a chemotherapeutic agent in cancers such as leukaemia, lymphoma and choriocarcinoma, 60 it was subsequently established in treating two chronic inflammatory diseases: rheumatoid arthritis and psoriasis. 61
Kozarek et al. first reported the use of MTX in 1989 in a pilot study of 21 patients with IBD (14 with CD). This study reported a clinical improvement in 79% of patients with CD with a reduction in steroid requirement (p = 0.006). 62 In a larger study of 141 patients, Feagan et al.63,64 further demonstrated that when compared to placebo, MTX was more effective at inducing (19.1% vs 39.4%, p = 0.025) and maintaining remission (39% vs 65%, p = 0.04) in patients with active CD. In addition, despite concerns regarding hepatic toxicity with MTX, no severe adverse events were recorded in these studies, concluding it to be a safe and effective drug.
Thus far, conventional management typically involved the use of broad-spectrum anti-inflammatory agents and immunosuppressants, often sequentially with the aim of relieving symptoms and preventing long-term complications in CD. Although we achieved modest and real benefits with steroid-sparing agents in inducing and maintaining clinical remission in CD, the advent of the biological era would bolster ambitions with treatment goals (Table 1).
Table 1.
Landmark studies that helped define the use of biologics in CD management
| Biologic agent | Study | Recruitment numbers | Treatment groups | Duration of study | Response/remission criteria | Limitations | Milestone achieved |
|---|---|---|---|---|---|---|---|
| Infliximab | Accent I | 580 | Infliximab (5 mg/kg OR 10 mg/kg) vs placebo | 54 weeks | CDAI score of 70 or more from baseline and at least 25% reduction in total score (at week 2 and week 30) | Efficacy measured by clinical response (CDAI and IBDQ scores) | Infliximab can be used as maintenance treatment |
| Accent II | 306 | Infliximab (5 mg/kg OR 10 mg/kg) vs placebo | 54 weeks | 50% reduction from baseline in number of draining fistulas at week 10 and 14 | Fistula closure assessed by physical examination and symptom report rather than objective radiological evidence | Infliximab can be used to treat rectovaginal fistulas | |
| Sonic | 508 | Infliximab (5 mg/kg) and oral placebo, azathioprine (2.5 mg/kg) and intravenous placebo, infliximab (5 mg/kg) and azathioprine (2.5 mg/kg) | 50 weeks | CDAI score < 150 points Mucosal healing defined as absence of mucosal ulceration at week 26 Corticosteroid-free remission: no budesonide of >6 mg/day or systemic steroids for minimum 3 weeks. |
Unable to conclude if combination therapy is disease modifying No cost analysis |
Combination therapy has greatest efficacy in corticosteroid-free remission, followed by infliximab monotherapy | |
| Adalimumab | Classic I | 299 | Adalimumab (40 mg/20 mg, 80 mg/40 mg, 160 mg/80 mg) at week 0 and 2, vs placebo | 4 weeks | Response: CR-70 or CR100 from week 0 in CDAI score Remission: CDAI < 150 |
Short duration. Efficacy measured by clinical response |
High-dose induction of 160 mg/80 mg more efficacious in inducing remission |
| Classic II | 55 | Adalimumab 40 mg every other week or weekly vs placebo | 56 weeks | Small sample size unable to determine statistical significance Efficacy measured by clinical response |
Adalimumab effective in maintaining remission | ||
| Charm | 854 | Adalimumab (40 mg weekly or alternate weeks) vs placebo | 56 weeks | Response: CR-70 at week 4 Remission: CDAI < 150 at weeks 26 and 56 |
Efficacy measured by clinical response | Weekly or alternate weekly dosing was equally effective in maintaining remission Can treat fistulas Effective in patients who are intolerant or lost response to infliximab |
|
| Gain | 325 | Adalimumab (160 mg or 80 mg) at week 0 and 2 vs placebo | 4 weeks | Response: CR-70/CR100 Remission: CDAI < 150, steroid discontinuation and fistula remission (absence of drainage) |
Efficacy measured by clinical response. Short study duration |
Adalimumab can be used as second option if previous anti-TNF treatment failed | |
| Extend | 135 | Adalimumab (40 mg) every other week vs placebo | 52 weeks | Absence of mucosal ulceration at week 12 | Mucosal healing not correlated with histology results | Adalimumab provides early and sustained mucosal healing | |
| Serene | 514 | Adalimumab (160 mg at week 1, 2, and 3) vs adalimumab (160 mg/80 mg at week 0 and 2) followed by 40 mg every other week. | 12 weeks | CDAI < 150 at week 4 Decrease in SES-CD > 50% from baseline at week 12 |
Ongoing study | ||
| Certolizumab pegol | Precise 1 | 662 | Certolizumab (400 mg at weeks 0, 2, and 4) vs placebo followed by every month. | 26 weeks | Response at week 6: CR100 Remission at week 26: CDAI < 150 |
Efficacy measured by clinical response | Certolizumab provides an early improvement in symptoms. |
| Precise 2 | 668 | After initial certolizumab (400 mg weeks 0, 2, and 4): 400 mg certolizumab vs placebo every month. | 26 weeks | Efficacy measured by clinical response | Certolizumab effective in inducing remission. | ||
| Vedolizumab | Gemini 2 | 185 | Vedolizumab 2 mg/kg, 0.5 mg/kg vs placebo at days 1 and 29. | 180 days | Response at week 6: CR100 Remission at week 56: CDAI < 150 |
Efficacy measured by clinical response, small sample size in each arm of study. | Vedolizumab can provide induction and maintenance therapy. |
| Gemini 3 | 315 | Vedolizumab 300 mg vs placebo at weeks 0, 2, and 6. | 10 weeks | Remission at week 6: CDAI < 150 | Short study duration, efficacy measured by clinical response. | Vedolizumab provides a modest benefit in inducing remission in patients who previously failed anti-TNF therapy | |
| Ustekinumab | UNITI-1 | 741 | Ustekinumab 130 mg vs 6 mg/kg vs placebo at week 0. All patients non-responses to anti-TNF therapy. | 8 weeks | CR\100 or CDAI < 150 at week 6 | Efficacy measured by clinical response | Effective induction of remission following previous non-response to anti-TNF therapy. |
| UNITI-2 | 628 | Ustekinumab 130 mg vs 6 mg/kg vs placebo at week 0. All patients failed immunosuppressants or glucocorticoid therapy. | 8 weeks | Efficacy measured by clinical response | Effective induction of remission following previous immunosuppression or glucocorticoid failure. | ||
| IM-UNITI | 397 | Ustekinumab 90 mg every 8 weeks vs 90 mg every 12 weeks vs placebo in patients who responded in UNIT-1/2. | 44 weeks | CDAI < 150 at week 44 | Efficacy measured by clinical response | Ustekinumab is an effective maintenance therapy. |
The millenium and the dawn of a new (biological) era in IBD therapeutics
In 1985, Beutler et al. 65 showed that the pro-inflammatory cytokine, tumour necrosis factor (TNF), played a significant role in endotoxin-mediated shock. Keffer et al. 66 subsequently demonstrated the role of TNF in the pathogenesis of inflammatory arthritis in mice; but it was not until 1990 when MacDonald et al. 67 found an increase in TNF concentration in tissue inflammation in rheumatoid synovial membranes and interestingly, in the mucosa and lamina propria of patients with CD that the prospect of anti-TNF therapy received attention. Elliot et al. 68 conducted the first human trial for rheumatoid arthritis using a TNF-alpha-directed chimeric mouse/human monoclonal antibody, which was followed shortly after by the first human trial successfully using this drug in a small sample size of 10 CD patients. 69 The advent of anti-TNF therapy and its ability to effectively induce and maintain remission while boasting of its corticosteroid-sparing effects, mucosal healing and reduced hospitalisation and surgery rates represent a defining moment in IBD therapeutics.
Anti-TNF biologics
Infliximab
Infliximab (IFX), a chimeric anti-TNF-alpha monoclonal antibody was the first anti-TNF agent to gain a licence for CD therapy. This was demonstrated in the landmark ACCENT I study, whose primary aim was to demonstrate that maintenance IFX therapy for CD patients can provide better long-term efficacy than no further treatment after a single-dose infusion. 70 Results showed that patients who received maintenance IFX therapy were two times more likely to maintain clinical remission compared with placebo treatment (OR: 2.7, 95% CI: 1.6–4.6). The median time to loss of response was 46 weeks (interquartile range (IQR): 17 to >54) in the treatment group versus 19 weeks (10–45) in the placebo group. This study was important for many reasons. Steroid dependent and immunomodulatory refractory patients now had a realistic medical option, with evidence that IFX use was safe and well-tolerated. Another important treatment advance with this study was patients on maintenance IFX were able to reduce steroid use, with a third of patients being able to stop steroids completely (29% vs 9% in placebo; OR: 4.2, 95% CI: 1.5–11.5; p = 0.004). Furthermore, the study also supported the concept that combination therapy with AZA or MTX might have an additional or synergistic efficacy with IFX, with 50% of patients who received a concomitant immunosuppressive maintaining a clinical response at week 54 compared with 41% not receiving these drugs. 70
Fistula development in CD is common and, prior to the use of biological therapy, was notoriously difficult to medically manage with surgery usually as the main treatment option. The ACCENT-II study demonstrated that IFX treatment was effective in 72.2% of patients in inducing closure of rectovaginal fistulas when given at 0, 2, and 6 weeks. 71 Furthermore, IFX provided a 3 months longer duration of closure than placebo when IFX induction was followed by a maintenance regimen. However, a limitation to this study was that fistula closure was assessed with only physical examination and symptom report. Optimism around fistula response from initial studies should be tempered by evolving paradigms and objective assessments with examination under anaesthesia (EUA), pelvic magnetic resonance imaging (MRI), or anorectal endoscopic ultrasonography (EUS), which often show persistent fistula tracts even when fistula drainage has ceased. 71
Building on early evidence supporting combination therapy using thiopurines with IFX from ACCENT-I, 70 the SONIC trial investigated the efficacy of IFX, AZA, and a combination of the two drugs for inducing and maintaining corticosteroid-free clinical remission in patients with moderate to severely active CD. 72 The primary aim of this study was to evaluate the rate of corticosteroid-free clinical remission at week 26. Disease severity was evaluated using the CDAI and IBD-Questionnaire (IBDQ) scores, as well as with direct visualisation of mucosal healing with ileocolonoscopy at week 26. Corticosteroid-free remission was achieved greatest with combination therapy (43.9%) although results were significantly better with IFX-based strategies (30.1%) as compared with AZA alone (16.5%). Furthermore, antibodies to IFX were detected at week 30 in only 1 of 116 patients receiving combination therapy versus 15 of 103 patients receiving IFX alone, suggesting that AZA may have a protective effect against IFX antibodies. 72 In a post hoc analysis of the SONIC trial, it was highlighted that those who were on combination therapy (IFX and AZA) had significantly greater composite remission rates (mucosal healing and clinical) than those on monotherapy (IFX) (range: 52.3%–63.6% vs 12.9%–29.0%; p ⩽ 0.013 for all comparisons). Furthermore, this study highlighted that those who achieved this composite endpoint were more likely to have higher IFX trough levels hence the suggestion that AZA may help boost the effectiveness of IFX through increasing IFX trough levels but as yet this remains association rather than causation. 72
Adalimumab
An important limitation with IFX is the development of anti-chimeric antibodies which can occur in 7–10% of patients regularly receiving 4- or 8-weekly maintenance infusions. 73 Antibody formation can lead to infusion reactions, loss of efficacy and delayed hypersensitivity reactions. While IFX was developed as a humanised murine antibody, there was resurgent interest in a molecule of entirely human origin, and therefore less likely to cause adverse allergic reactions. 74 Adalimumab was developed as a subcutaneous injection which can be self-injected by the patient. The CLASSIC-I trial was a short 4-week dose-ranging study evaluating the efficacy of another anti-TNF drug, adalimumab in the Crohn’s cohort. A small number of patients (299) were included who were randomly assigned to receive adalimumab induction treatment doses at weeks 0 and 2 of either 40 mg/20 mg, 80 mg/40 mg, 160 mg/80 mg, or placebo. Compared to placebo, the only induction-loading dose regimen that achieved statistical significance for remission rates was 160 mg/80 mg. Furthermore, only 1 out of 225 patients developed antibodies against adalimumab, although this may have been an underestimation considering the short course of the study duration. 75
While the CLASSIC-I study demonstrated that adalimumab was effective in inducing remission by week 4, the CLASSIC II study went on to show that adalimumab was equally effective in maintaining remission in patients with moderate to severe CD, either at a dose of 40 mg weekly or every other week. At week 56, those on adalimumab were 1.5–2 times more likely to have maintained remission compared to placebo. The CLASSIC-II study was praised for selecting remission as the primary outcome measure as opposed to maintenance of response. However, a significant limitation to this study is the relatively small sample size (<20 patients in each group) such that it was not powered to detect statistical significance. It is also important to mention that patients who were randomised into the study were from a highly selected cohort that had rapidly responded to the drug within 4 weeks of treatment. 76
The CHARM trial supported and extended the findings of the CLASSIC-I and -II studies by confirming with a greater sample size that adalimumab was effective in inducing and maintaining long-term clinical remission in CD patients who initially responded to induction therapy with adalimumab. Specifically, patients given adalimumab 40 mg every other week or weekly had greater remission rates compared to placebo at week 26 (40%, 47% and 17% respectively, p < 0.001) and week 56 (36%, 41%, and 12% respectively, p < 0.001). In contrast to CLASSIC-II, this study demonstrated a statistical difference in lowering disease activity and improving quality of life (QoL) in the adalimumab treatment group compared to the placebo group. The study also demonstrated that adalimumab dosing of either weekly or alternate weeks were equally effective in maintaining remission in patients with CD. Importantly, the efficacy results from this study showed similar response and remission rates to IFX, not only in maintaining corticosteroid-free remission but also in the complete closures of fistulas. Furthermore, this study was crucial in demonstrating that adalimumab was effective in patients who previously lost response or were intolerant to infliximab. However, the patients who were naïve to anti-TNF therapy had numerically greater remission rates at week 26 and week 54 as compared to those with a previous history of anti-TNF use. 77
Following on from the CHARM study, the GAIN study was the first randomised, double-blind placebo-controlled trial in any immune-mediated disease to demonstrate the efficacy of a second TNF antagonist where the first TNF antagonist had failed. Compared to placebo (7%), patients with moderate to severe CD who were given adalimumab (21%) had a superior response in inducing remission in patients who were previously intolerant or lost response to infliximab; p < 0.001. The main limitation to this study was the short 4-week duration, although the efficacy for maintenance treatment was previously demonstrated in the 52-week long CHARM study. 78
Up to now, studies on adalimumab used clinical response with CDAI and IBDQ mean scores to assess remission rates as primary treatment goals for patients with CD. EXTEND was the first study designed to evaluate mucosal healing as the primary end point. Results demonstrated that adalimumab can provide early and sustained mucosal healing in patients with moderate to severe ileocolonic CD. Higher rates of mucosal healing with adalimumab compared with placebo were observed by week 12 (27% vs 13%, p = 0.056) and week 52 (24% vs 0%, p < 0.001). Patients were eligible if they had longer disease duration, in whom conventional therapy had failed, with 52% of patients receiving adalimumab as their second TNF antagonist, and they were allowed to remain on corticosteroids if they were receiving them at baseline. Recruitment of these patients were reflective of the ‘real-world’ and was a main strength of this study. 79
The SERENE-CD trial is currently investigating high (160 mg at week 0, 1, 2, and 3) versus standard (160 mg at week 0 followed by 80 mg at week 2 and 40 mg at week 4) adalimumab induction dosing regimens. Although the study is ongoing, early results have not shown a significant difference in the rate of clinical remission at week 4 or endoscopic remission at week 12 between the high or standard doses. 80
Switching from infliximab to adalimumab is extremely common in clinical practice and is often a result of convenience or financial burden rather than clinical necessity. The SWITCH trial investigated the impact of electively switching treatments from intravenous infliximab to subcutaneous adalimumab in patients with well-controlled CD. Results demonstrated that this elective switch was associated with loss of tolerance and loss of efficacy within 1 year and as such, led to worse outcomes in the CD patients. Due to the limited number of approved biological agents, this study highlighted the importance to adhere with the first anti-TNF agent unless there is loss of response or tolerance. 81
Immunogenicity
Anti-TNF drugs are highly effective in the management of CD but treatment failure is a common downfall to these medications. The personalised anti-TNF therapy in Crohn’s disease study (PANTS) aimed to identify specific clinical and pharmacokinetic factors that predicted primary non-response. Their multivariate analysis demonstrated that the only factor independently associated with primary non-response was low drug concentration at week 14 for both infliximab and adalimumab. For both drugs, suboptimal drug concentrations at week 14 predicted immunogenicity, with the formation of anti-drug antibodies. 82
Certolizumab pegol
The anti-TNF therapies, IFX and adalimumab, were shown to be effective for induction and maintenance of moderate to severe CD. However, 40–50% of anti-TNF ‘primary responders’ develop either a loss of response and/or develop acute or delayed hypersensitivity reactions within 6–12 months. 83 Certolizumab pegol is a pegylated humanised Fab’ fragment of the anti-TNF monoclonal antibody which has a high affinity for TNF-alpha. Similar to adalimumab, it has the added advantage that it can be administered subcutaneously. The PRECiSE 1 study was a double-blind randomized controlled trial (RCT) that compared the efficacy and safety of certolizumab pegol against placebo in patients with moderate to severe CD. 83 Results showed a modest benefit with statistically more patients displaying >100-point CDAI reduction (CR100) at week 6 (37% certolizumab vs 26% placebo) but not at week 26 (22% certolizumab vs 12% placebo). The PRECiSE-284 study was undertaken in parallel with PRECiSE-1, with similar inclusion criteria but conducted at separate sites. Interestingly, 64% had a response at week 6 and thus continued into the trial with 43% in remission (defined as CDAI score of less than or equal to 150) following induction therapy. By week 26, there was a statistically significant response to certolizumab (48%) as compared to placebo (29%). This study highlighted the superior response of subcutaneous certolizumab; however, there is a clear discrepancy in the response and remission rates at week 6 from the PRECiSE-1 and -2 studies, for reasons that remain unexplained. Thus, although the PRECiSE-1 study indicates a lack of efficacy with certolizumab, the PRECiSE-2 data suggest the opposite in the induction of CD remission. As a result, the European Medicines Agency (EMA) cited concerns about the insufficient evidence of the efficacy of certolizumab and the short study duration of the PRECiSE trials. Thus, it is currently only approved for use in CD in the United States, Switzerland, and Russia. 84
Biosimilars
Due to the recent expiry of infliximab and adalimumab patents, several biosimilars have been approved for use in IBD by the EMA and US Food and Drug Administration (FDA).85,86 These agents are lower priced than the original compounds by up to 70% and by definition, are highly similar to the reference drug such that any molecular and/or structural differences should not affect its quality, safety, or efficacy. 85 The use of biosimilars provides several advantages including easing the economic burden of anti-TNF treatment, increasing access to anti-TNF therapy, thereby allowing earlier access to treatment, and availability of assays for measuring drug concentrations to optimise patient care. 87 This paves the way for reducing complications and functional disability associated with IBD. 86
In 2021, Schreiber et al. conducted an open-label study investigating the use of subcutaneous IFX biosimilar compared to its intravenous route. A total of 131 patients were recruited and results showed comparable clinical remission rates between the two treatment modes. Efficacy, safety, and immunogenicity assessments were also comparable. A subcutaneous option for IFX may have multiple advantages including ease of administration and reduced requirement for medical visits and associated travel, in addition to optimising medical resources and improving treatment options for our IBD patients. 88
In summary, anti-TNF therapies have transformed the care of patients with IBD, re-defining our perceptions around meaningful disease control, moving beyond symptom control to bolder definitions such as mucosal healing, histological and deep remission, and an improvement in QoL.89,90 Even as anti-TNF agents fuelled our ambitions with hitherto unachievable outcomes, it became obvious that they are not universally effective, with 30–50% of patients being primary non-responders and with further attrition from subsequent loss of response (mechanistic escape, immunogenicity, or intolerance). 91 There is also the real risk of infectious complications attributable to non-specific inhibition of TNF-mediated immunologic cascades.91,92
Recently developing biologics
Vedolizumab
Evolution in our understanding of the involvement of T-lymphocyte biology orchestrating gut inflammation has paved the way for the development of several agents directed against trafficking of effector T-lymphocytes towards the gut mucosa. 93 In 2014, vedolizumab was introduced into the biologic armamentarium. Vedolizumab is a highly selective monoclonal antibody that blocks lymphocytic gut migration via antagonism of α4β7 integrin on lymphocytes. 94 This inhibits binding to mucosal addressin cell adhesion molecule-1, which is overexpressed in the intestinal vasculature in IBD, and thus reduces trafficking to the gut. 94 The GEMINI-II study 95 investigated the efficacy of vedolizumab in inducing and maintaining remission in CD patients. The primary endpoint was to assess clinical remission (CDAI score of less than or equal to 150) at week 6. The study showed that of the 368 randomised patients, vedolizumab induction therapy was more likely than placebo to result in remission at week 6 (14.5% vs 6.8%; p = 0.02). However, by week 52, patients who had an initial response to induction therapy had higher rates of clinical remission, CR100 response and glucocorticoid-free remission than placebo (21.6%) when vedolizumab was given 4- or 8-weekly (36.4% and 39% respectively); p = 0.004 and p < 0.001, respectively. The modest effect of vedolizumab induction at week 6 could be attributed to the severity of disease in the study population, where a large proportion of patients had fistulising disease (37%), undergone previous surgery (42%), previous treatment failure with one or more TNF antagonists (50%), or treatment failure with two or more TNF antagonists (30%).
The GEMINI-3 study focused specifically on the efficacy of vedolizumab with previous anti-TNF failure. 96 The results did not show a significant difference between vedolizumab and placebo at week 6 (15.2% vs 12.1% respectively, p = 0.433), but there was a modest benefit at week 10 (26.6% vs 12.1% respectively, p = 0.001). The concern over vedolizumab’s inability to reduce remission at week 6 and the lack of mucosal healing data do not provide compelling evidence for its use in CD but one must consider certain caveats. It is likely that the timing of assessment was the limiting factor as evidenced by the Gemini-3 trial, wherein vedolizumab was superior to placebo for induction at 10 weeks but not at week 6, in patients with prior anti-TNF failure. For maintenance of remission at 52 weeks, vedolizumab demonstrated superiority over placebo with a magnitude of effect generally similar to that seen in UC. 97 Although the induction data appear less compelling, the clearly and clinically meaningful effect after 30 weeks suggests that vedolizumab is an appropriate option for selected patients in whom concomitant use of bridging strategies (such as co-induction with corticosteroids) is possible and where surgery may not be the appropriate option. Also, it may be an appropriate first-line biologic in patients where the focus is safety, such as in the elderly.98,99 Furthermore, several ‘real-world’ studies have gone on to showcase vedolizumab’s safety and efficacy in moderate-severe CD or those who have failed previous conventional therapy.100 –102 These studies demonstrated that vedolizumab may be more beneficial for patients who are biologically naïve and in patients with an inflammatory phenotype, as opposed to a stricturing or penetrating presentation. 103
Vedolizumab is currently licenced via the intravenous route; however, the latest VISIBLE-2 study has demonstrated that the subcutaneous form can also maintain clinical remission with a similar safety profile as per the intravenous route. 104 As stated above, subcutaneous biologics are preferred over the intravenous for both patients and healthcare services.
Ustekinumab
In 2016, ustekinumab was introduced as another out-of-class biologic option for patients with CD. Ustekinumab is a monoclonal antibody against the p40 subunit of interleukin-12 and interleukin-23, which was first approved for use in patients with psoriasis in 2009 and in 2013 for psoriatic arthritis. 105 To investigate its efficacy in CD, two 8-week placebo-controlled induction trials (UNITI-1 and UNITI-2) and one 44-week maintenance trial (IM-UNITI) were undertaken. 106 Patients who completed either UNITI-1 or UNITI-2 could then enrol in the IM-UNITI maintenance trial. Results of the three trials showed consistent superiority with ustekinumab over placebo in inducing and maintaining remission in patients with moderately to severely active CD. At week 6, patients receiving intravenous ustekinumab at a dose of either 130 mg or 6 mg/kg had significantly higher response rates than placebo (UNITI-1: 34.3% vs 33.7% vs 21.5% respectively; p < 0.003, UNITI-2: 51.7%, 55.5% and 28.7% respectively; p < 0.001). At week 44, patients receiving maintenance doses of ustekinumab every 8 or 12 weeks were more likely to be in remission than placebo (53.1% vs 48.8% vs 35.9%, respectively; p < 0.05). These results were irrespective of previous treatment or response to a TNF antagonist, and its benefit was demonstrated as early as week 3. Moreover, the rate of adverse events was not significantly different from that of placebo. 106 Subsequently in 2016, both the FDA and European Commission approved the use of ustekinumab for CD treatment for whom previous therapies have failed. Since then, several real-world studies have confirmed the efficacy and safety profile of ustekinumab107–109 and its efficacy in perianal disease and fistula healing. 110
The future
For many years, CD was managed inadequately using steroids, 5ASAs, immunomodulators, and antibiotics. The introduction of anti-TNF agents in the late 1990s created a paradigm shift in the management of this chronic incurable disease. Indeed, this was the first medication class that reduced the risk of surgery and hospitalisation, particularly if used early in the disease course. 111 Anti-TNF therapy did not come without its list of problems, including high rates of primary and secondary non-responders, and the long-term risk for complications. With the advent of vedolizumab and ustekinumab, clinicians were able to overcome these issues. However, anti-TNF agents are still first-line treatment for complex patients including fistulizing disease, pregnancy, children, post-op recurrence, and peri-operative safety. 112 Furthermore, despite the development of biologics, there is still a high rate of surgery and post-operative recurrence. Over the last decade, the probability of surgery has been reported to be between 3% and 96% within 15 years of diagnosis, with clinical relapse and reoperation rates of 50–60% and 28–45%, respectively. 7 Subsequently, there are several ongoing studies investigating new biological therapies for the treatment of CD (Table 2), which are likely to provide a greater array of medications in the armamentarium towards helping patients with CD.
Table 2.
Novel therapies for the management of CD that are currently undergoing trials
| Name | Mechanism of action | Route of administration | Trial phase | Trial name | Management | Results |
|---|---|---|---|---|---|---|
| Risankizumab 113 | Anti-IL23 antibody | SC | 3 | ADVANCE MOTIVATE |
Moderate to severe CD | Superior to placebo, study ongoing |
| Mirikizumab 114 | IgG4 monoclonal antibody that binds to P19 subunit of IL23 | IV | 2–3 | SERENITY (Phase-2 trial) VIVID (Phase-3 trial) |
Moderate to severe CD | Ongoing |
| Etrolizumab 115 (RG7413) | Anti-integrin | SC | 3 | BERGAMOT | Moderate to severe CD | Ongoing |
| Ontamalimab 116 (SHP647) | Monoclonal IgG2 antibody against mucosal addressin cell adhesion molecule-1 (MAdCAM-1) | SC | 3 | CARMEN | Moderate to severe CD | Ongoing |
| Filgotinib 117 | Janus kinase 1 (JAK1) selective inhibitor | Oral | 3 | FITZROY | Moderate to severe CD | Ongoing |
| Upadacitinib 118 | JAK1 selective inhibitor | Oral | 2 | CELEST | Moderate to severe refractory CD | Superior to placebo in inducing endoscopic improvement |
| Mesenchymal stem cells 119 | Adult allogeneic bone marrow derived mesenchymal stem cells | Injection | IB/IIA | — | Perianal fistulising CD | Study ongoing |
| Faecal microbial transplant (FMT) 120 | Microbial restoration | Via colonoscopy | 3 | — | CD HBI > 3 | Currently not enough evidence for benefit |
| Stem cell transplant 121 | Autologous hematopoetic Stem Cell Transplantation (HSCT) | Injection | 3 | ASTIC | Refractory CD (treatment with >3 immunosuppressive or biologic agents and steroids) | No significant improvement and associated with significant toxicity. Study terminated prematurely |
Conclusion
CD is a chronic relapsing-remitting disease with a high morbidity rate. Its disease complexity can result in long-lasting physical, emotional, and psychological effects on patients. Over the past two decades, the medical compendium for the treatment of CD has expanded exponentially. Although surgery continues to play a pivotal role in achieving disease control for these patients with aggressive disease, novel mechanistic approaches and deeper insights with existing therapies hold real promise. The prospect of these intellectual efforts being rewarded through meaningful outcomes for individuals living with CD is now more realistic than ever before.
Footnotes
Author contributions: Aditi Kumar: Data curation; Investigation; Writing – original draft; Writing – review & editing.
Alexander Cole: Writing – original draft.
Jonathan Segal: Conceptualisation; Supervision; Writing – review & editing.
Philip Smith: Writing – review & editing.
Jimmy K. Limdi: Conceptualisation; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JKL has served as a speaker for Abbvie, Janssen, Takeda and Tillotts, consultant for Abbvie, Galapagos, Janssen and Pfizer and an advisory board member for Arena Pharma, Galapagos and Janssen and has received research funding from Galapagos and Takeda. PJS has served as a speaker and advisory board member for Janssen, Takeda, Tillotts, and Celltrion. JPS has received speaker fees for Takeda He has received conference funding by Tillots, Abbvie, and Janssen. He has received a research grant from Tillots.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Aditi Kumar  https://orcid.org/0000-0003-1026-3173
https://orcid.org/0000-0003-1026-3173
Jimmy K. Limdi  https://orcid.org/0000-0002-1039-6251
https://orcid.org/0000-0002-1039-6251
Contributor Information
Aditi Kumar, Gastroenterology Department, The Royal Wolverhampton NHS Trust, Wolverhampton WV10 0QP, UK.
Alexander Cole, Imperial College Healthcare NHS Trust, London, UK.
Jonathan Segal, Department of Gastroenterology and Hepatology, St Mary’s Hospital, London, UK.
Philip Smith, Department of Gastroenterology, The Royal Liverpool and Broadgreen University Hospitals, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK; Faculty of Medicine, University of Liverpool, Liverpool, UK.
Jimmy K. Limdi, Department of Gastroenterology, Northern Care Alliance NHS Foundation NHS Trust, Manchester, UK Manchester Academic Health Sciences, University of Manchester, Manchester, UK.
References
- 1. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68: s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis; a pathologic and clinical entity. Am J Med 1952; 13: 583–590. [DOI] [PubMed] [Google Scholar]
- 3. Smith MS, Wakefield AJ. Crohn’s disease: ancient and modern. Postgrad Med J 1994; 70: 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torres J, Mehandru S, Colombel J-F, et al. Crohn’s disease. Lancet 2017; 389: 1741–1755. [DOI] [PubMed] [Google Scholar]
- 5. Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002; 8: 244–250. [DOI] [PubMed] [Google Scholar]
- 6. Moja L, Danese S, Fiorino G, et al. Systematic review with network meta-analysis: comparative efficacy and safety of budesonide and mesalazine (mesalamine) for Crohn’s disease. Aliment Pharmacol Ther 2015; 41: 1055–1065. [DOI] [PubMed] [Google Scholar]
- 7. Golovics PA, Mandel MD, Lovasz BD, et al. Inflammatory bowel disease course in Crohn’s disease: is the natural history changing? World J Gastroenterol 2014; 20: 3198–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955; 2: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Summers RW, Switz DM, Sessions JT, Jr, et al. National Cooperative Crohn’s Disease Study: results of drug treatment. Gastroenterology 1979; 77: 847–869. [PubMed] [Google Scholar]
- 10. Malchow H, Ewe K, Brandes JW, et al. European Cooperative Crohn’s Disease Study (ECCDS): results of drug treatment. Gastroenterology 1984; 86: 249–266. [PubMed] [Google Scholar]
- 11. Dorrington AM, Selinger CP, Parkes GC, et al. The historical role and contemporary use of corticosteroids in inflammatory Bowel disease. J Crohns Colitis 2020; 14: 1316–1329. [DOI] [PubMed] [Google Scholar]
- 12. Rutgeerts PJ. An historical overview of the treatment of Crohn’s disease: why do we need biological therapies? Rev Gastroenterol Disord 2004; 4: S3–S9. [PubMed] [Google Scholar]
- 13. Lewis JD, Scott FI, Brensinger CM, et al. Increased mortality rates with prolonged corticosteroid therapy when compared with antitumor necrosis factor-alpha-directed therapy for inflammatory bowel disease. Am J Gastroenterol 2018; 113: 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lichtenstein GR, Feagen BG, Cohen RH, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol 2012; 107: 1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D’Haens G, Reinisch W, Colombel JF, et al. Five-year safety data from ENCORE, a European Observational Safety Registry for adults with Crohn’s disease treated with infliximab [Remicade(R)] or conventional therapy. J Crohns Colitis 2017; 11: 680–689. [DOI] [PubMed] [Google Scholar]
- 16. Rutgeerts P, Löfberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn’s disease. N Engl J Med 1994; 331: 842–845. [DOI] [PubMed] [Google Scholar]
- 17. Greenberg GR, Feagan BG, Martin F, et al. Oral budesonide for active Crohn’s disease. Canadian inflammatory bowel disease study group. N Engl J Med 1994; 331: 836–841. [DOI] [PubMed] [Google Scholar]
- 18. Edsbacker S, Andersson P, Lindberg C, et al. Liver metabolism of budesonide in rat, mouse, and man. Drug Metab Dispos 1987; 15: 403–411. [PubMed] [Google Scholar]
- 19. Rezaie A, Kuenzig ME, Benchimol EI, et al. Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2015; 6: CD000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munkholm P, Langholz E, Davidsen M, et al. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut 1994; 35: 360–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology 2001; 121: 255–260. [DOI] [PubMed] [Google Scholar]
- 22. Steinhart AH, Ewe K, Griffiths AM, et al. Corticosteroids for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2003; 4: CD000301. [DOI] [PubMed] [Google Scholar]
- 23. Kuenzig ME, Rezaie A, Kaplan GG, et al. Budesonide for the induction and maintenance of remission in Crohn’s disease: systematic review and meta-analysis for the cochrane collaboration. J Can Assoc Gastroenterol 2018; 1: 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuenzig ME, Rezaie A, Seow CH, et al. Budesonide for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2014; 8: CD002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Modigliani R, Mary JY, Simon JF, et al. Clinical, biological, and endoscopic picture of attacks of Crohn’s disease. Evolution on prednisolone. Groupe d’Etude Therapeutique des Affections Inflammatoires Digestives. Gastroenterology 1990; 98: 811–818. [DOI] [PubMed] [Google Scholar]
- 26. Gendre JP, Mary JY, Florent C, et al. [Maintenance treatment of Crohn’s disease using orally administered mesalazine (Pentasa). A controlled multicenter study. The Study Groups on the Treatment of Inflammatory Digestive Disorders]. Ann Gastroenterol Hepatol (Paris) 1993; 29: 251–256. [PubMed] [Google Scholar]
- 27. de Franchis R, Omodei P, Ranzi T, et al. Controlled trial of oral 5-aminosalicylic acid for the prevention of early relapse in Crohn’s disease. Aliment Pharmacol Ther 1997; 11: 845–852. [DOI] [PubMed] [Google Scholar]
- 28. Sutherland LR, Martin F, Bailey RJ, et al. A randomized, placebo-controlled, double-blind trial of mesalamine in the maintenance of remission of Crohn’s disease. The Canadian Mesalamine for Remission of Crohn’s Disease Study Group. Gastroenterology 1997; 112: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 29. Mahmud N, Kamm MA, Dupas L, et al. Olsalazine is not superior to placebo in maintaining remission of inactive Crohn’s colitis and ileocolitis: a double blind, parallel, randomised, multicentre study. Gut 2001; 49: 552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akobeng AK, Zhang D, Gordon M, et al. Oral 5-aminosalicylic acid for maintenance of medically-induced remission in Crohn’s disease. Cochrane Database Syst Rev 2016; 9: CD003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lim WC, Wang Y, MacDonald JK, et al. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev 2016; 7: CD008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ford AC, Khan KJ, Talley NJ, et al. 5-aminosalicylates prevent relapse of Crohn’s disease after surgically induced remission: systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 413–420. [DOI] [PubMed] [Google Scholar]
- 33. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 2019; 14: 4–22. [DOI] [PubMed] [Google Scholar]
- 34. Feuerstein JD, Ho EY, Shmidt E, et al. AGA clinical practice guidelines on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease. Gastroenterology 2021; 160: 2496–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alkhatry M, Al-Rifai A, Annese V, et al. First United Arab Emirates consensus on diagnosis and management of inflammatory bowel diseases: a 2020 Delphi consensus. World J Gastroenterol 2020; 26: 6710–6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stein RB, Hanauer SB. Comparative tolerability of treatments for inflammatory bowel disease. Drug Saf 2000; 23: 429–448. [DOI] [PubMed] [Google Scholar]
- 37. Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 661–673. [DOI] [PubMed] [Google Scholar]
- 38. Ursing B, Kamme C. Metronidazole for Crohn’s disease. Lancet 1975; 1: 775–777. [DOI] [PubMed] [Google Scholar]
- 39. Sutherland L, Singleton J, Sessions J, et al. Double blind, placebo controlled trial of metronidazole in Crohn’s disease. Gut 1991; 32: 1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brandt LJ, Bernstein LH, Boley SJ, et al. Metronidazole therapy for perineal Crohn’s disease: a follow-up study. Gastroenterology 1982; 83: 383–387. [PubMed] [Google Scholar]
- 41. Solomon MJ, McLeod RS, O’Connor BI, et al. Combination of ciprofloxacin and metronidazole in severe perianal Crohn’s disease. Can J Gastroenterol 1993; 7: 610272. [Google Scholar]
- 42. Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology 1995; 108: 1617–1621. [DOI] [PubMed] [Google Scholar]
- 43. Herfarth HH, Katz JA, Hanauer SB, et al. Ciprofloxacin for the prevention of postoperative recurrence in patients with Crohn’s disease: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 2013; 19: 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jigaranu AO, Nedelciuc O, Blaj A, et al. Is rifaximin effective in maintaining remission in Crohn’s disease. Dig Dis 2014; 32: 378–383. [DOI] [PubMed] [Google Scholar]
- 45. Selby W, Pavli P, Crotty B, et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn’s disease. Gastroenterology 2007; 132: 2313–2319. [DOI] [PubMed] [Google Scholar]
- 46. Rhodes JM, Subramanian S, Flanagan PK, et al. Randomized trial of ciprofloxacin doxycycline and hydroxychloroquine versus budesonide in active Crohn’s disease. Dig Dis Sci 2021; 66: 2700–2711. [DOI] [PubMed] [Google Scholar]
- 47. Elion GB. The purine path to chemotherapy. Science 1989; 244: 41–47. [DOI] [PubMed] [Google Scholar]
- 48. Bean RH. The treatment of chronic ulcerative colitis with 6-mercaptopurine. Med J Aust 1962; 49: 592–593. [PubMed] [Google Scholar]
- 49. Present DH, Korelitz BI, Wisch N, et al. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med 1980; 302: 981–987. [DOI] [PubMed] [Google Scholar]
- 50. Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology 2015; 148: 344–354. [DOI] [PubMed] [Google Scholar]
- 51. Chande N, Patton PH, Tsoulis DJ, et al. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2015; 10: CD000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Candy S, Wright J, Gerber M, et al. A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut 1995; 37: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ewe K, Press AG, Singe CC, et al. Azathioprine combined with prednisolone or monotherapy with prednisolone in active Crohn’s disease. Gastroenterology 1993; 105: 367–372. [DOI] [PubMed] [Google Scholar]
- 54. Kappelman MD, Farkas DK, Long MD, et al. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol 2014; 12: 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol 2015; 13: 847–858. [DOI] [PubMed] [Google Scholar]
- 56. Cuffari C, Theoret Y, Latour S, et al. 6-mercaptopurine metabolism in Crohn’s disease: correlation with efficacy and toxicity. Gut 1996; 39: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sandborn WJ. 6-MP metabolite levels: a potential guide to Crohn’s disease therapy. Gastroenterology 1997; 113: 690–692. [DOI] [PubMed] [Google Scholar]
- 58. Belaiche J, Desager JP, Horsmans Y, et al. Therapeutic drug monitoring of azathioprine and 6-mercaptopurine metabolites in Crohn disease. Scand J Gastroenterol 2001; 36: 71–76. [DOI] [PubMed] [Google Scholar]
- 59. Moyer AM. NUDT15: a bench to bedside success story. Clin Biochem 2021; 92: 1–8. [DOI] [PubMed] [Google Scholar]
- 60. HERTZ R, LI MC, SPENCER DB. Effect of methotrexate therapy upon choriocarcinoma and chorioadenoma. Proc Soc Exp Biol Med 1956; 93: 361–366. [DOI] [PubMed] [Google Scholar]
- 61. Nielsen OH, Steenholdt C, Juhl CB, et al. Efficacy and safety of methotrexate in the management of inflammatory bowel disease: a systematic review and meta-analysis of randomized, controlled trials. EClinicalMedicine 2020; 20: 100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kozarek RA, Patterson DJ, Gelfand MD, et al. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med 1989; 110: 353–356. [DOI] [PubMed] [Google Scholar]
- 63. Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med 1995; 332: 292–297. [DOI] [PubMed] [Google Scholar]
- 64. Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med 2000; 342: 1627–1632. [DOI] [PubMed] [Google Scholar]
- 65. Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 1985; 229: 869–871. [DOI] [PubMed] [Google Scholar]
- 66. Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J 1991; 10: 4025–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. MacDonald TT, Hutchings P, Choy MY, et al. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol 1990; 81: 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Elliott MJ, Maini RN, Feldmann, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum 1993; 36: 1681–1690. [DOI] [PubMed] [Google Scholar]
- 69. van Dullemen HM, van Deventer SJ, Hommes DW, et al. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology 1995; 109: 129–135. [DOI] [PubMed] [Google Scholar]
- 70. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359: 1541–1549. [DOI] [PubMed] [Google Scholar]
- 71. Sands BE, Blank MA, Patel K, et al. Long-term treatment of rectovaginal fistulas in Crohn’s disease: response to infliximab in the ACCENT II Study. Clin Gastroenterol Hepatol 2004; 2: 912–920. [DOI] [PubMed] [Google Scholar]
- 72. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362: 1383–1395. [DOI] [PubMed] [Google Scholar]
- 73. Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol 2004; 2: 542–553. [DOI] [PubMed] [Google Scholar]
- 74. Stallmach A, Giese T, Schmidt C, et al. Severe anaphylactic reaction to infliximab: successful treatment with adalimumab – report of a case. Eur J Gastroenterol Hepatol 2004; 16: 627–630. [DOI] [PubMed] [Google Scholar]
- 75. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006; 130: 323–333. [DOI] [PubMed] [Google Scholar]
- 76. Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007; 56: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007; 132: 52–65. [DOI] [PubMed] [Google Scholar]
- 78. Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 2007; 146: 829–838. [DOI] [PubMed] [Google Scholar]
- 79. Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012; 142: 1102–1111. [DOI] [PubMed] [Google Scholar]
- 80. D’Haens G, Sandborn W, Loftus EV, et al. High versus standard adalimumab induction dosing regimens in patients with moderately to severely active Crohn’s disease: results from the SERENE-CD induction study [UEG Week abstract LB27]. United Eur Gastroenterol J 2019; 7: 13–15. [Google Scholar]
- 81. Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61: 229–234. [DOI] [PubMed] [Google Scholar]
- 82. Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019; 4: 341–353. [DOI] [PubMed] [Google Scholar]
- 83. Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007; 357: 228–238. [DOI] [PubMed] [Google Scholar]
- 84. Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med 2007; 357: 239–250. [DOI] [PubMed] [Google Scholar]
- 85. Papamichael K, Van Stappen T, Jairath V, et al. Review article: pharmacological aspects of anti-TNF biosimilars in inflammatory bowel diseases. Aliment Pharmacol Ther 2015; 42: 1158–1169. [DOI] [PubMed] [Google Scholar]
- 86. Peyrin-Biroulet L, Danese S, Cummings F, et al. Anti-TNF biosimilars in Crohn’s Disease: a patient-centric interdisciplinary approach. Expert Rev Gastroenterol Hepatol 2019; 13: 731–738. [DOI] [PubMed] [Google Scholar]
- 87. Bhat S, Limdi JK, Cross RK, et al. Does similarity breed contempt? A review of the use of biosimilars in inflammatory bowel disease. Dig Dis Sci 2021; 66: 2513–2532. [DOI] [PubMed] [Google Scholar]
- 88. Schreiber S, Ben-Horin S, Leszczyszyn J, et al. Randomized controlled trial: subcutaneous vs intravenous infliximab CT-P13 maintenance in inflammatory bowel disease. Gastroenterology 2021; 160: 2340–2353. [DOI] [PubMed] [Google Scholar]
- 89. Cholapranee A, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther 2017; 45: 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Feagan BG, Reinisch W, Rutgeerts P, et al. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol 2007; 102: 794–802. [DOI] [PubMed] [Google Scholar]
- 91. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003; 348: 601–608. [DOI] [PubMed] [Google Scholar]
- 92. Bonovas S, Fiorino G, Allocca M, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol 2016; 14: 1385–1397. [DOI] [PubMed] [Google Scholar]
- 93. Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol 2003; 24: 327–334. [DOI] [PubMed] [Google Scholar]
- 94. Lamb CA, O’Byrne S, Keir ME, et al. Gut-selective integrin-targeted therapies for inflammatory bowel disease. J Crohns Colitis 2018; 12: S653–S668. [DOI] [PubMed] [Google Scholar]
- 95. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 96. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014; 147: 618–627. [DOI] [PubMed] [Google Scholar]
- 97. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 98. Cottone M, Kohn A, Daperno M, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol 2011; 9: 30–35. [DOI] [PubMed] [Google Scholar]
- 99. Segal JP, Htet HMT, Limdi J, et al. How to manage IBD in the ‘elderly’. Frontline Gastroenterol 2020; 11: 468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shahidi N, Bressler B, Panaccione R. The role of vedolizumab in patients with moderate-to-severe Crohn’s disease and ulcerative colitis. Therap Adv Gastroenterol 2016; 9: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lenti MV, Levison S, Eliadou E, et al. A real-world, long-term experience on effectiveness and safety of vedolizumab in adult patients with inflammatory bowel disease: the Cross Pennine study. Dig Liver Dis 2018; 50: 1299–1304. [DOI] [PubMed] [Google Scholar]
- 102. Schreiber S, Dignass A, Peyrin-Biroulet L, et al. Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol 2018; 53: 1048–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bressler B. Use of vedolizumab for the treatment of Crohn’s disease. Gastroenterol Hepatol 2019; 15: 204–206. [PMC free article] [PubMed] [Google Scholar]
- 104. Vermeire S, Sandborn W, Baert F, et al. OP23 Efficacy and safety of vedolizumab SC in patients with moderately to severely active Crohn’s disease: results of the VISIBLE 2 study. Eur Crohns Colitis Org 2020; 14: S20–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis 2014; 73: 1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016; 375: 1946–1960. [DOI] [PubMed] [Google Scholar]
- 107. Viola A, Muscianisi M, Macaluso FS, et al. Ustekinumab in Crohn’s disease: real-world outcomes from the Sicilian network for inflammatory bowel diseases. JGH Open 2021; 5: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rutgeerts P, Gasink C, Chan D, et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology 2018; 155: 1045–1058. [DOI] [PubMed] [Google Scholar]
- 109. Macaluso FS, Maida M, Ventimiglia M, et al. Effectiveness and safety of Ustekinumab for the treatment of Crohn’s disease in real-life experiences: a meta-analysis of observational studies. Expert Opin Biol Ther 2020; 20: 193–203. [DOI] [PubMed] [Google Scholar]
- 110. Honap S, Meade S, Ibraheim H, et al. Effectiveness and safety of ustekinumab in inflammatory bowel disease: a systematic review and meta-analysis. Dig Dis Sci. Epub ahead of print 16 March 2021. DOI: 10.1007/s10620-021-06932-4. [DOI] [PubMed] [Google Scholar]
- 111. Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet 2015; 386: 1825–1834. [DOI] [PubMed] [Google Scholar]
- 112. Shim HH, Chan PW, Chuah SW, et al. A review of vedolizumab and ustekinumab for the treatment of inflammatory bowel diseases. JGH Open 2018; 2: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Feagan BG, Panes J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol 2018; 3: 671–680. [DOI] [PubMed] [Google Scholar]
- 114. A study of Mirikizumab (LY3074828) in participants with active Crohn’s disease, https://clinicaltrials.gov/ct2/show/NCT03926130
- 115. Sandborn WJ, Vermeire S, Tyrrell H, et al. Etrolizumab for the treatment of ulcerative colitis and Crohn’s disease: an overview of the phase 3 clinical program. Adv Ther 2020; 37: 3417–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Efficacy and Safety study of Ontamalimab as induction therapy in participants with moderate to severe Crohn’s disease (CARMEN CD 306), https://clinicaltrials.gov/ct2/show/NCT03559517
- 117. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017; 389: 266–275. [DOI] [PubMed] [Google Scholar]
- 118. Sandborn WJ, Feagan BG, Loftus Jr, EV, et al. Efficacy and safety of upadacitinib in a randomized trial of patients with Crohn’s disease. Gastroenterology 2020; 158: 2123–2138.e8. [DOI] [PubMed] [Google Scholar]
- 119. Mesenchymal stem cells for the treatment of perianal fistulizing Crohn’s disease, 2021, https://clinicaltrials.gov/ct2/show/NCT04519671 [DOI] [PubMed]
- 120. Gutin L, Piceno Y, Fadrosh D, et al. Fecal microbiota transplant for Crohn disease: a study evaluating safety, efficacy, and microbiome profile. United European Gastroenterol J 2019; 7: 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hawkey CJ, Allez M, Clark MM, et al. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. JAMA 2015; 314: 2524–2534. [DOI] [PubMed] [Google Scholar]