Abstract
Accumulating evidence points toward a very high prevalence of prolonged neurological symptoms among coronavirus disease 2019 (COVID-19) survivors. To date, there are no solidified criteria for ‘long-COVID’ diagnosis. Nevertheless, ‘long-COVID’ is conceptualized as a multi-organ disorder with a wide spectrum of clinical manifestations that may be indicative of underlying pulmonary, cardiovascular, endocrine, hematologic, renal, gastrointestinal, dermatologic, immunological, psychiatric, or neurological disease. Involvement of the central or peripheral nervous system is noted in more than one-third of patients with antecedent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, while an approximately threefold higher incidence of neurological symptoms is recorded in observational studies including patient-reported data. The most frequent neurological manifestations of ‘long-COVID’ encompass fatigue; ‘brain fog’; headache; cognitive impairment; sleep, mood, smell, or taste disorders; myalgias; sensorimotor deficits; and dysautonomia. Although very limited evidence exists to date on the pathophysiological mechanisms implicated in the manifestation of ‘long-COVID’, neuroinflammatory and oxidative stress processes are thought to prevail in propagating neurological ‘long-COVID’ sequelae. In this narrative review, we sought to present a comprehensive overview of our current understanding of clinical features, risk factors, and pathophysiological processes of neurological ‘long-COVID’ sequelae. Moreover, we propose diagnostic and therapeutic algorithms that may aid in the prompt recognition and management of underlying causes of neurological symptoms that persist beyond the resolution of acute COVID-19. Furthermore, as causal treatments for ‘long-COVID’ are currently unavailable, we propose therapeutic approaches for symptom-oriented management of neurological ‘long-COVID’ symptoms. In addition, we emphasize that collaborative research initiatives are urgently needed to expedite the development of preventive and therapeutic strategies for neurological ‘long-COVID’ sequelae.
Keywords: COVID-19, long-COVID, long-haul, neurological manifestations, post-acute sequelae of SARS-CoV-2, SARS-CoV-2
Introduction
Since the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, it has become evident that the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) would have immense repercussions on healthcare systems and socioeconomic structures worldwide. 1 Besides the exorbitant death toll of the pandemic across the globe, with the rising number of COVID-19 survivors, increasing attention has been drawn to the prolonged or late-onset sequelae of SARS-CoV-2 infection, which are colloquially referred to as ‘long-COVID’ syndrome.2,3 The term ‘long-COVID’ was, in fact, first coined and disseminated through social media by patients, who, already during the first months of the pandemic, acknowledged a more complex disease course than laid out in early reports from Wuhan.4,5 Subsequently, cognate terms were introduced,6–8 including ‘long-haul’,9,10 ‘post-COVID’,11,12 ‘post-acute COVID syndrome (PACS)’, 13 and ‘post-acute sequelae of SARS-CoV-2 (PASC)’, 14 to refer to persistent symptoms and/or delayed or long-term complications beyond acute COVID-19 (Table 1). 13 Currently, despite the emergence of new variants, following the implementation of mass vaccination campaigns, many countries have witnessed significant decreases in the number of new COVID-19 cases and hospitalizations.15,16 Nonetheless, although cautious optimism has been expressed for the beginning of the end of the pandemic, concerns have been raised that ‘long-COVID’ could comprise a ‘next public health disaster in the making’. 9
Table 1.
Alternative definitions of ‘long-COVID’ syndrome.
| Nomenclature | Description |
|---|---|
| Long COVID4,7 | Long-term COVID-19 symptoms characterized by cyclical, progressive, or multiphasic course. |
| Long COVID 2 | Symptoms persisting for >2 months |
| Long COVID 3 | Signs and symptoms that persist for >4 weeks and can be attributed to COVID-19 infection. |
| Long-haul COVID
10
Long-tail COVID |
Symptoms persisting for >100 days |
| Long post-COVID symptoms 6 | Symptoms lasting for 12–24 weeks |
| Post-acute COVID syndrome (PACS) 13 | Persistent symptoms and/or delayed or long-term complications beyond 4 weeks from symptom onset |
| Post-acute sequelae of SARS-CoV-2 (PASC)14,17 | Symptoms persisting for >1 month |
| Post-COVID-1912 | Symptoms persisting for >2 months |
| Persistent post-COVID symptoms 6 | Symptoms lasting for >24 weeks |
| Post-COVID-19 syndrome8,18 | Signs and symptoms that develop during or after an infection consistent with COVID-19, present for >12 weeks and are not attributable to alternative diagnoses. |
Consequently, prevention of ‘long-COVID’ ranks very high on the public health agenda. Currently, however, there is only scant understanding of the underlying processes implicated in the pathogenesis of ‘long-COVID’ syndrome, a fact that is also reflected in the tentative definitions and preliminary classification schemes proposed by national and international health organizations.13,17,19 To enable better documentation and characterization of ‘long-COVID’, the World Health Organization (WHO) has recently assigned an emergency use International Classification of Diseases, Tenth Revision (ICD-10) code (U09.9) referring to ‘Post-COVID conditions, unspecified’. 20 In addition, a clinical case definition was proposed by the WHO based on Delphi consensus, suggesting that ‘post COVID-19 occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis’. According to this definition,
Common symptoms include fatigue, shortness of breath, cognitive dysfunction but also others, and generally have an impact on everyday functioning. Symptoms may be of new onset following initial recovery from an acute COVID-19 episode or persist from the initial illness, may fluctuate or relapse over time. 18
Several limitations of this definition have been acknowledged, so far, including difficulties in approaching the diagnostic ‘time-windows‘ (especially in asymptomatic patients), the lack of robust clinical data to define clinical ‘cut-offs’, and the low specificity of proposed diagnostic criteria. Nevertheless, as more refined diagnostic criteria for ‘long-COVID’ are still underway, there is current consensus that the exclusion of acute COVID-19 should be regarded as a prerequisite for ‘long-COVID’ diagnosis. 13
Neurological manifestations comprise one of the many facets of ‘long-COVID’ syndrome. 21 In accord with the aforementioned definitions of ‘long-COVID’, the long-term or late-onset neurological sequelae of COVID-19 should be distinguished from the well-characterized acute neurological manifestations of SARS-CoV-2 infection5,22–24 Moreover, as ‘long-COVID’ is conceptualized as a multi-organ disease, central nervous system (CNS) and/or peripheral nervous system (PNS) involvement may present alone or in conjunction with pulmonary, cardiovascular, psychiatric, endocrine, hematologic, renal, gastrointestinal, dermatologic, or immunological symptoms.13,25 Similar to WHO, the National Institutes of Health (NIH) has linked ‘long-COVID’ to symptoms such as ‘fatigue, shortness of breath, “brain fog”, sleep disorders, fever, gastrointestinal symptoms, anxiety, and depression’, thereby acknowledging neurological symptoms as core aspects of ‘long-COVID’. 26 Moreover, recent reports indicate an extremely high prevalence of long-term neurological manifestations among COVID-19 survivors, with nearly one-third of patients being diagnosed with neurological or psychiatric illnesses in the first 6 months following acute COVID-19. 27
At present, neurologists are daily confronted with an increased demand for ‘long-COVID’ patient care. Yet, as scarce evidence has been consolidated so far, diagnosing and managing neurological complications of ‘long-COVID’ calls for navigation in ‘uncharted waters’. The aim of the present narrative review is thus to provide a comprehensive overview of our current understanding of the long-term neurological sequelae of COVID-19, along with a methodological framework for a systematic diagnostic approach and management of patients with neurological manifestations of ‘long-COVID’ syndrome.
Methods and data synthesis
We performed a comprehensive literature search in SCOPUS, Embase, Google Scholar, and LitCOVID 28 (that includes all COVID-19-related articles published in PubMed and Medline) up to 10 December 2021, using the following terms in combination: ‘long COVID’, ‘post COVID’, ‘long haul’, ‘chronic COVID’, ‘post-acute sequelae of SARS-CoV-2’, ‘PASC’, ‘post-acute COVID syndrome’, ‘PACS’, ‘persisting COVID’, ‘COVID complications’, ‘SARS-CoV-2 complications’, or ‘lingering COVID’. We also searched manually the reference lists of all relevant articles. Both English and foreign language articles were reviewed. The initial literature search was performed by three independent authors (M-IS, LP, and EB). Duplicate publications were excluded from further evaluation (including duplicates from databases with overlapping records). Given the lack of standardized definitions for ‘long-COVID’ syndrome at the time of publication of most retrieved articles, all studies reporting new, recurrent, or persistent neurological symptoms following acute COVID-19 were considered eligible for inclusion. We included peer-reviewed, case-control, population-based, cohort studies or clinical trials in humans, as well as systematic reviews and guidelines, while thematically irrelevant studies, animal studies, editorials, commentaries, case reports, and preprints were excluded. Screening was performed based on title or abstract from three reviewers (M-IS, LP, and EB) and any disagreements were resolved via consensus or discussion with the corresponding author (GT). Structured reports of identified studies were extracted to include author names, title, abstract, study type, year or publication, and journal. To evaluate the relevance and strength of the evidence provided by identified studies and their potential contribution to the present narrative review, an expert panel was formed, consisting of (a) senior neurologists (M-IS, LP, EB, MP, GPP, NG, CK, and SG), with expertise in neurological complications of COVID-19, including neurovascular (CK, GT, NG, and SG), PNS (MP), and neurodegenerative sequelae (GPP); (b) senior psychiatrists with expertise in cognitive impairment and psychiatric complications of COVID-19 (NS and ER); (c) experts in internal and respiratory medicine (EB and MG); and (d) public or population health researchers and accredited experts in COVID-19 and ‘long-COVID’ syndrome (EB, ST, and MG). Evidence synthesis was based on expert recommendation to include articles from the generated databases, while studies were evaluated based on reporting of relevant primary data, validity of the methods used, and novelty and clarity of the results. Given the nonsystematic nature of the present review, panel members were allowed to adjudicate on quality and relevance of recommended studies in their domains of expertise, prioritizing research that was considered of high quality, most pertinent, and insightful. The generated database following duplicate removal included 38,402 publications, which were assessed based on extracted data by the expert panel. Of these, 147 full publications were selected for inclusion in the present narrative review.
Neurological manifestations of ‘long-COVID’ syndrome
A wide array of neurological manifestations, involving both the CNS and PNS, have been described in the context of ‘long-COVID’ syndrome (Table 2). It is important to emphasize, however, that neurological symptoms are often inextricable from ‘long-COVID’ manifestations that involve other organ systems, while nonspecific symptoms, including fatigue, ‘brain fog’, postexertional malaise, and sleep disorders, may comprise epiphenomena of underlying respiratory, cardiovascular, endocrine, renal, hematologic, autoimmune, or psychiatric diseases.8,25,29 As novel evidence continues to emerge, the spectrum of clinical characteristics of ‘long-COVID’ continues to widen. With respect to neurological manifestations, however, there is only scarce epidemiological evidence to date on the incidence of neurological disorders in populations with antecedent SARS-CoV-2 infection.30,31 Conversely, there are abundant data from observational studies, including evidence from patient-led research,32,33 reporting highly variable prevalence estimates of diverse neurological symptoms among ‘long-COVID’ patients.34–38 Due to inherent methodological caveats of most so-far published studies, including selection and reporting biases, lack of standardized neurological assessment, and lack of adjustment for comorbidities or concomitant ‘long-COVID’ manifestations in other organ systems, particular caution is warranted when attempting to characterize neurological sequelae of ‘long-COVID’.
Table 2.
Neurological manifestations of ‘long-COVID’ syndrome, according to the localization in the nervous system.
| Localization in the nervous system | Neurological symptoms |
|---|---|
| Central nervous system | Fatigue |
| ‘Brain fog’ | |
| Headache | |
| Sleep disorders | |
| Cognitive impairment | |
| Emotional/mood disorders | |
| Dizziness | |
| Dysautonomia | |
| Peripheral nervous system | Muscle weakness |
| Myalgias | |
| Hyposmia | |
| Hypogeusia | |
| Hearing loss/tinnitus | |
| Sensorimotor deficits (hypoesthesia, dysesthesia, tremor) |
Taking the foregoing considerations into account, a number of observational studies have made a strong claim that involvement of both CNS and PNS may occur in patients with ‘long-COVID’ syndrome.34,35,39 For example, in a large longitudinal analysis of 1733 consecutive patients with laboratory-confirmed COVID-19, 76% of patients reported at 6 months following acute SARS-CoV-2 infection at least one of the following symptoms: fatigue or muscle weakness (63%), sleep disturbances (26%), smell or taste impairment (11% and 7%, respectively), myalgias (2%), and headache (2%). Notably in this study, 23% and 27% of patients reported concomitant symptoms of anxiety/depression or pain/discomfort, respectively. 35 Similar results were obtained by a longitudinal analysis of 2433 patients with antecedent SARS-CoV-2 infection; at 1-year follow-up, the reported neurological symptoms included fatigue (30%), myalgia (8%), dizziness (3%), headache (2%), and taste/smell disorders (1%). 5 In an online international survey of 3762 participants with antecedent COVID-19, however, a much higher prevalence of neurological manifestations affecting the CNS and PNS was reported. 32 In the first 6 months after acute disease, the most frequently reported symptoms encompassed sensorimotor deficits (91%), cognitive dysfunction (85%), emotional/mood disorders (88%), sleep disturbances (79%), headache (77%), memory impairment (73%), and smell/taste disorders (58%). Crucially, 65% of the patients reported persistence of neurological symptoms beyond 6 months, with the most frequent being fatigue (80%), postexertional malaise (73%) and cognitive dysfunction (58%).
In line with the previous findings, an observational study including 165 COVID-19 survivors reported that at 6-month follow-up after hospitalization, the most commonly reported symptoms were fatigue, memory/attention deficits, sleep disorders, and myalgias, with each symptom affecting one-third of patients with antecedent SARS-CoV-2 infection. Further reported symptoms included depression/anxiety (27%), dyspnea (21%), visual disturbances (20%), numbness/tingling (19%), hyposmia/hypogeusia (16%), urinary dysfunction (14%), confusion/dizziness (13%), headache (10%), postural instability (9%), and swallowing difficulties (6%). A key finding of this study was that 40% of patients presented objectifiable abnormalities on neurological examination, with the most common being hyposmia (18%), cognitive deficits (18%), postural tremor (14%), and motor/sensory deficits (8%). 40 These results thus indicate that objectifiable correlates of CNS or PNS involvement are observed in more than one-third of ‘long-COVID’ patients.
While evidence from observational studies continues to accrue, a recently published systematic review and meta-analysis, including data from 47,910 patients, provided prevalence estimates for long-term COVID-19 effects. 41 This meta-analysis revealed that 80% of patients infected with SARS-CoV-2 developed one or more long-lasting symptoms, with the most frequent being fatigue (58%), headache (44%), and attention disorder (27%). In addition, the following prevalence estimates for neurological manifestations were reported: ageusia (23%), anosmia (21%), memory loss (16%), hearing loss or tinnitus (15%), chills (7%), dizziness (3%), and stroke (3%). Crucially, psychiatric symptoms, including anxiety and depression, were observed in 13% and 12% of patients, respectively, while a lower prevalence was recorded for mood disorders, dysphoria, obsessive-compulsive disorder (OCD), and posttraumatic stress disorder (PTSD), each affecting 2% of patients. 41
Perhaps the most robust estimates of incidence rates of neurological and psychiatric diagnoses in the 6 months following COVID-19 have been so far provided by an electronic health records analysis including 236,379 patients. 27 This study disclosed an incidence of neurological or psychiatric diagnoses of 34% in the 6 months following SARS-CoV-2 infection, with 13% of patients receiving their first such diagnosis within this period. Particularly with respect to neurological sequelae of COVID-19, in terms of predefined major neurological outcomes occurring within 6 months after acute SARS-CoV-2 infection, the authors found that the highest incidence was observed for nerve/nerve root/plexus disorders (2.9%) and insomnia (2.7%), followed by ischemic stroke (0.8%), dementia (0.7%), neuromuscular junction or muscle disease (0.5%), intracranial hemorrhage (0.3%), parkinsonism (0.1%), encephalitis (0.1%), and Guillain–Barré syndrome (0.1%). Moreover, when the researchers compared the probability of major neurological outcomes in patients previously diagnosed with COVID-19 with matched controls diagnosed with influenza or with other respiratory tract infections during the same study period, they showed a significantly higher risk for all the previous neurological outcomes – except for parkinsonism and Guillain–Barré syndrome – in patients who have had COVID-19 compared to those with influenza, and significantly greater risk for all outcomes in patients who have had COVID-19 compared to patients with respiratory tract infections. 27 Nevertheless, as only a small subset of neurological diagnoses was included in this electronic health records analysis and significant discrepancies between the incidence estimates of this study and previous results from observational research are noted, further epidemiological data are urgently needed to enable better characterization of neurological manifestations in the context of ‘long-COVID’ syndrome.
Pathophysiological mechanisms underlying neurological manifestations of ‘long-COVID’
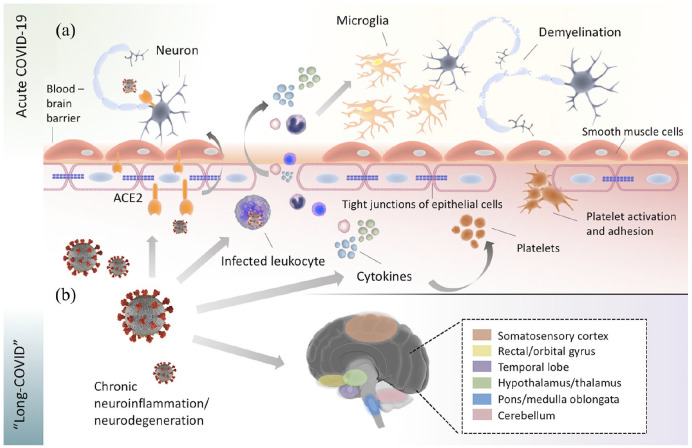
The pathophysiological processes implicated in CNS and PNS manifestations of acute COVID-19 have been extensively studied and reviewed in the scientific literature.22,23,42,43 Besides severe affection of the respiratory system, cardiovascular, renal, and gastrointestinal manifestations, including liver and pancreatic dysfunction, are well-characterized complications of acute COVID-19. 44 In brief, several overlapping pathogenetic mechanisms of neurological manifestations of acute COVID-19 have been established, including viral neuroinvasion accompanied by aberrant neuroimmunological responses, endotheliopathy associated with blood–brain barrier dysfunction, coagulopathies that precipitate hypoxic–ischemic neuronal injury, metabolic imbalances, oxidative stress cascades, and cellular apoptosis (Figure 1).2,45–49 In contrast to acute neurological manifestations of COVID-19, however, the biological underpinnings of neurological ‘long-COVID’ sequelae remain today poorly characterized. In the absence of diagnostic markers and robust neuropathological data, most published articles have so far proposed putative pathophysiological mechanisms for neurological ‘long-COVID’ sequelae while drawing parallels with the pathophysiology of acute COVID-19.
Figure 1.
Potential pathophysiological mechanisms implicated in the manifestation of acute and ‘long-COVID’ manifestations in the central nervous system (CNS). (a) In acute COVID-19, SARS-CoV-2 cell entry within the CNS is facilitated by hematogenous or direct transsynaptic pathways via engagement of the angiotensin-converting enzyme 2 (ACE2) receptor, which is located on the surface of diverse cell types, including neurons, endothelial cells, and smooth muscle cells of cerebral blood vessels. SARS-CoV-2-induced cytokine storm may cause disruption of the tight junctions at the endothelial lining of the blood–brain barrier, which leads to increased blood–brain barrier permeability and allows the transmigration of virus-infected leukocytes into the CNS. In addition, the cytokine release precipitates platelet activation and adhesion, which causes further endothelial impairment and has been linked to the increased thrombotic risk noted in acute COVID-19. Once cytokines and leukocytes have crossed the blood–brain barrier, they activate microglial cells, which in turn trigger apoptotic cascades and demyelination. (b) In ‘long-COVID’, chronic inflammatory and secondary degenerative processes are thought to prevail in propagating neurological ‘long-term’ sequelae. In ‘ACE2-rich’ brain areas, including areas of the somatosensory cortex, rectal/orbital gyrus, temporal lobe, hypothalamus/thalamus, brainstem, and cerebellum, 18 F-FDG brain PET studies in ‘long-COVID’ patients have revealed prominent hypometabolism. The reduced glucose metabolism observed in these areas may be further explained by oxidative stress processes, mitochondrial dysfunction, or impaired cerebral autoregulation, which are secondary to SARS-CoV-2 infection.
Direct neurotropic effects of SARS-CoV-2 are currently assumed to play a subordinate role in the pathogenesis of ‘long-COVID’, analogous to the circumscribed role of direct neuroinvasion in acute COVID-19.50,51 Notably, SARS-CoV-2 cell entry within the CNS and PNS is facilitated by hematogenous or transsynaptic pathways via engagement of the angiotensin-converting enzyme 2 (ACE2) receptor, 47 which is located on the surface of diverse cell types, including neurons, astrocytes, endothelial and smooth muscle cells of cerebral blood vessels, as well as skeletal muscle cells. 46 Even in patients with severe neurological involvement in the setting of acute SARS-CoV-2 infection, however, SARS-CoV-2 RNA is infrequently detected in cerebrospinal fluid (CSF) analyses, or in brain and skeletal muscle biopsies of symptomatic patients.48,52–54 As CSF or neuropathological evidence from ‘long-COVID’ patients is currently missing, the question of whether cellular SARS-CoV-2 reservoirs could perpetuate a chronic SARS-CoV-2 infection within the CNS or PNS remains unanswered. 55 In analogy to neuropathological correlates of acute COVID-19,48,53 however, virus-induced neuroinflammatory, prothrombotic, hypoxic, metabolic, and apoptotic cascades are thought to prevail in propagating neurological symptoms in the context of ‘long-COVID’.7,50,56
In accordance with the spatial distribution of ACE2 receptors in the CNS, which are predominantly expressed in the olfactory bulb, amygdala, hippocampus, middle temporal gyrus, posterior cingulate cortex, and the brainstem,57,58 a multitude of neurological symptoms encountered in ‘long-COVID’ patients, including hyposmia, mood disorders, cognitive impairment, sleep disorders, and dysautonomia, have been linked to dysfunction of ‘ACE2-rich’ brain areas. 46 Intriguingly, a similar pattern of hypometabolism has been recently revealed in neuroimaging studies using 18F-FDG (fluorodeoxyglucose) brain positron emission tomography (PET) in ‘long-COVID’ patients that exhibited hypometabolic areas extending from the rectal/orbital gyrus, including the olfactory gyrus, to the temporal lobe, including the amygdala and the hippocampus, to the hypothalamus and thalamus, and further to the brainstem and the cerebellum.59,60 With respect to the latter, the imaging evidence of brainstem and cerebellum involvement is crucial as it indicates that involvement of these brain regions may underlie several neurological manifestations of ‘long-COVID’ that bear similarities with the myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS), and the postural orthostatic tachycardia syndrome (POTS).9,61,62 Accordingly, it has been suggested that the reduced glucose metabolism observed in these areas may be attributed to pathophysiological processes, such as oxidative stress, mitochondrial dysfunction, neuronal degeneration, and impaired cerebral autoregulation, that are secondary to SARS-CoV-2 infection.63–65
The hypothesis of a predominant autonomic dysfunction in ‘long-COVID’ has been recently bolstered by findings of impaired regulation of heart rate variability (HRV) in ‘long-COVID’ patients. 66 In a case-control study that included 27 patients with antecedent SARS-CoV-2 infection and 12 healthy controls, HRV dysregulation was significantly associated with foregoing COVID-19, as well as with the presence of fatigue in ‘long-COVID’ patients. In conjunction with the aforementioned evidence of brainstem involvement from neuroimaging studies, these data suggest that afferent and efferent pathways of the vagus and glossopharyngeal nerves, which are also implicated in acute SARS-CoV-2 infection,67,68 could be involved in demyelinating or Wallerian degeneration processes secondary to viral invasion. 45 Although further research is needed to evaluate these hypotheses, neuronal atrophy and degeneration of other cranial nerves, including the olfactory nerve and the adjacent olfactory bulb, have been previously described in patients with persisting hyposmia or anosmia following the resolution of acute COVID-19.42,69 Important insights into the role of neuroinflammation in ‘long-COVID’ have recently been provided by a series of studies, which have signified that abnormal humoral and cellular immune responses,70,71 systemic inflammatory markers such as interleukin-6 (IL-6),41,65 and autoantibodies targeting cellular receptors 72 may be implicated in systemic and neurological ‘long-COVID’ sequelae. Particularly with respect to neuroinflammatory processes, a study that appeared recently on a preprint server, including 56 patients with persisting neurological deficits for more than 6 weeks after acute SARS-CoV-2 infection, showed a reduced effector molecule expression in memory T cells, which was significantly associated with the severity of cognitive impairment. 73 In addition, prolonged endothelial dysfunction and vascular inflammation have also been linked to ‘long-COVID’ syndrome,74,75 albeit 18F-FDG PET studies have not revealed areas of brain hypermetabolism, as would be expected in the case of unabating brain inflammation.59,60 Crucially, ongoing blood–brain barrier dysfunction, as well as a prolonged hypercoagulable state that could precipitate cerebrovascular disease and hypoxic–ischemic neuronal injury, may also be implicated in neurological manifestations of ‘long-COVID’. Notably, a recent case-control prospective study reported an independent association between SARS-CoV-2 infection, oxidative stress, and endothelial/vascular dysfunction, which was associated with impaired cardiac performance and persistence of COVID-related symptoms 4 months after COVID-19 infection. 75 To date, however, there are no available data in ‘long-COVID’ patients with neurological symptoms in support of these hypotheses.
Concomitant involvement of other organ systems should also be considered when addressing the pathophysiology of neurological ‘long-COVID’ sequelae. While pathophysiological associations between nonspecific neurological symptoms, such as fatigue, ‘brain fog’, and postexertional malaise, and concomitant COVID-19-induced pulmonary or cardiac changes are well characterized,76,77 an involvement of the gastrointestinal tract and the brain–gut axis have also been recently proposed as a possible link to neurological manifestations of ‘long-COVID’.78,79 In particular, prolonged SARS-CoV-2 shedding has been observed in the gastrointestinal tract for up to 3 months postacute infection, with a recent study showing persistence of SARS-CoV-2 nucleic acids and proteins in 50% of patients who underwent intestinal biopsy. 80 Bearing the role of brain–gut axis in the pathogenesis of neurodegenerative disorders in mind, 81 further research is warranted to evaluate whether insidious gastrointestinal SARS-CoV-2 infection may be causally linked to neurological ‘long-COVID’ sequelae.
Finally, as a concluding remark on the pathophysiology of neurological manifestations of ‘long-COVID’, despite some ominous predictions expressed in the literature for perpetuation of neurodegeneration following SARS-CoV-2 infection, a recently published longitudinal study has indicated attenuation of CNS injury, despite the persistence of neurological symptoms at 6 months after acute COVID-19. 82 In this study, the researchers measured biomarkers of astrocytic and neuronal injury, including neurofilament light-chain (NfL), glial fibrillary acidic protein (GFAp), and growth differentiation factor 15 (GDF-15), in 100 patients with antecedent SARS-CoV-2 infection and showed that despite significant elevation of these biomarkers’ concentrations during the acute phase of COVID-19, at 6-month follow-up, a normalization of all biomarkers was noted in all included patients. Nonetheless, one half of patients reported persistent neurological symptoms at 6 months, with the most frequent being fatigue in 40%, followed by ‘brain fog’ and cognitive changes in 29% and 25%, respectively. These data suggest that ongoing CNS injury may not necessarily accompany neurological ‘long-COVID’ sequelae 82 and further indicate the pivotal role of a systematic approach for characterization, differential diagnosis, and management of ‘long-COVID’ symptoms. As acute COVID-19 has been associated with impaired neurotransmission and upper layer cortical circuitry dysfunction, 83 a prolonged recovery of neurotransmission could underlie the prolonged neurological and cognitive deficits noted in ‘long-COVID’ patients.
Diagnostic approach to neurological ‘long-COVID’ sequelae
Taken together, the previous data reveal a very wide spectrum of neurological symptoms among COVID-19 survivors with only partially elucidated underlying pathophysiological mechanisms. There is thus a lot of ground still to be covered before diagnostic criteria for neurological ‘long-COVID’ sequelae can be solidified. In the absence of widely accepted operational definitions for ‘long-COVID’, the presence of symptoms, signs, or abnormal findings that persist beyond the resolution of acute COVID-19 and do not return to a premorbid baseline may be currently considered as long-term effects of the disease.41,84 As a consequence, a pragmatic approach to neurological sequelae of ‘long-COVID’ entails assessment of change from neurological baseline (Figure 2). Notably, premorbid neurological disorders have been shown to confer an increased susceptibility to ‘long-COVID’.85,86 Thus, exclusion of potential exacerbations of preexisting neurological or psychiatric disease due to SARS-CoV-2 is required before ascribing neurological symptoms to ‘long-COVID’. Moreover, as previously noted, ‘long-COVID’ comprises a multisystem disease. It is thus imperative to evaluate potential involvement of other organ systems that could precipitate secondary neurological symptoms. 87 In addition, it should be kept in mind that vigilance is warranted in the differential diagnosis of neurological symptoms that manifest in temporal association with COVID-19. In particular, exclusion of ‘long-COVID’ mimics is required,88,89 including neurological and non-neurological disorders that may occur after COVID-19, but be causally unrelated to antecedent SARS-CoV-2 infection. 90
Figure 2.
Proposed diagnostic and therapeutic algorithm for patients presenting with neurological symptoms compatible with ‘long-COVID’.
ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; POTS, postural orthostatic tachycardia syndrome.
Besides the challenges in distinction of ‘true long-COVID’ from exacerbations or manifestations of new unrelated underlying disorders, some further aspects should be taken into account when approaching ‘long-COVID’ syndrome. First, there is currently lack of consensus on whether confirmed COVID-19 in the patient history or serological evidence of antecedent SARS-CoV-2 infection should be regarded as a prerequisite for ‘long-COVID’ diagnosis, as a substantial proportion of patients infected with SARS-CoV-2 remain asymptomatic or undiagnosed91,92 and highly variable seroprevalence rates have been reported during the post-COVID period,93–95 which are further confounded by serological responses to SARS-CoV-2 vaccines.96,97 Second, there is no consensus on an exact timeframe for defining ‘long-COVID’ in the literature. Some authors have proposed that ‘long-COVID’ may be considered after 2 weeks following acute SARS-CoV-2 infection,41,86 while a cut-off of 4 weeks after acute infection has been proposed by the CDC (Centers for Disease Control and Prevention). 17 Conversely, the NICE (National Institute for Health and Care Excellence) guidelines distinguish between ‘ongoing symptomatic COVID-19’, which can be diagnosed in the period between 4 and 12 weeks postinfection, and ‘post-COVID-19 syndrome’, which may be diagnosed if symptoms persist beyond 12 weeks, with the latter definition also supported by the WHO (Table 1).19,18 While the issue of when to draw a line between acute and long-COVID remains unresolved, the more recent inclusive approach of WHO6,20 may be currently more appropriate, until more evidence has been acquired to enable refinement of operational case definitions. In addition, the maximum time interval that allows ‘long-COVID’ diagnosis remains to be established, with recent evidence pointing toward a duration of ‘long-COVID’ symptoms of more than 7 or 12 months after viral infection,5,32,98 albeit the duration of ‘long-COVID’ may be currently underestimated due to the short follow-up periods of most so-far published observational studies. 41
Another salient issue that merits mention when approaching neurological manifestations of ‘long-COVID’ is the question of whether neurological sequelae of intensive care unit (ICU) COVID-19 survivors may fall under the scope of ‘long-COVID’. Most published studies have focused so far on all hospitalized and/or nonhospitalized patients, analyzing jointly those with severe and mild disease.41,99,100 Nonetheless, criticism has been raised that attributing ‘long-COVID’ diagnosis to formerly critically ill COVID-19 patients may further confound the characterization of the syndrome and even jeopardize patient care.100,101 Notably, acute neurological COVID-19 manifestations, including encephalopathy/encephalitis, delirium, cerebrovascular events, epileptic seizures, hypoxic–ischemic brain injury, and neuromuscular disorders,102–104 differ significantly in incidence and severity between critically ill, hospitalized nonventilated, and nonhospitalized patients.104,105 Moreover, severe non-neurological complications and long-term organ dysfunction, including respiratory and cardiac failure, have been significantly associated with the severity of previous COVID-19.100,106 Consequently, it remains unclear whether, from a pathophysiological perspective, long-term symptomatic ICU survivors and mildly affected COVID-19 patients could share the same common denominator of ‘long-COVID’ syndrome. In fact, a growing body of evidence indicates that long-lasting neurological deficits of ICU COVID-19 survivors display many similarities to postintensive care syndrome (PICS).27,40,101,107 Thus, further research is required to identify potential overlaps and enable distinction between neurological manifestations of ‘long-COVID’ and PICS.
With respect to potential risk factors for neurological ‘long-COVID’ sequelae, there are no reliable data to date to facilitate identification of high-risk patients in clinical practice. 108 Although, as stated previously, preexisting neurological morbidity and the severity of antecedent COVID-19 may portend poor long-term neurological outcome, some studies have reported opposite results that suggest a particularly high prevalence of long-term neurological symptoms among previously healthy individuals, who had not been hospitalized for COVID-19.106,109 Discordant findings are also found in the literature concerning the contribution of demographic factors to ‘long-COVID’. With respect to age, for example, although a number of studies support that increasing age confers an increased susceptibility to long-term sequelae,5,40,109 other studies reported that younger patients may be at higher risk of ‘long-COVID’. 110 Moreover, a striking prevalence of ‘long-COVID’ symptoms of nearly 43% has been reported in children with antecedent SARS-CoV-2 infection, albeit these prevalence estimates remain debatable.111,112 Similarly, highly disparate findings have been published regarding the potential sex predisposition to ‘long-COVID’, with some studies suggesting an association between female sex and neurological ‘long-COVID’ symptoms, some detecting no associations with sex, and some reporting a higher vulnerability of male patients (in analogy to acute COVID-19) to ‘long-COVID’ sequelae.109,110,113,114 Further potential risk factors, including coexisting cerebrovascular disease, 5 constitutional factors and psychosocial stressors, have been described in other studies. A meaningful interpretation of these data is, however, currently not possible due the high heterogeneity of the so-far available observational studies and due to considerable methodological constraints, including mixed patient populations, variable follow-up periods, and unstandardized documentation of risk factors, comorbidities, and clinical symptoms.29,108,109,115
Notwithstanding the aforementioned limitations in defining and approaching ‘long-COVID’, our understanding of long-term neurological sequelae of COVID-19 has begun to steadily expand. Emerging studies have provided promising evidence of improvement or even resolution of neurological symptoms, including hyposmia, 116 anxiety/depression, cognitive impairment, fatigue, and overall functional status,117,118 at longer follow-up periods, up to 1 year following acute SARS-CoV-2 infection. It should be noted that in the upcoming months, the results from international registries and NeuroCOVID-19 task forces are anticipated, which will expectedly shed more light onto characteristics, risk factors and long-term outcomes of ‘long-COVID’ patients. 119 In the meantime, standardized approaches for evaluating and reporting neurological manifestations of ‘long-COVID’ syndrome are warranted to allow for development of operational case definitions, which will eventually enable better characterization and prevention of long-term neurological COVID-19 sequelae.
Therapeutic approach to neurological ‘long-COVID’ sequelae
While focusing on neurological manifestations of ‘long-COVID’ syndrome, it should be highlighted that as ‘long-COVID’ comprises a multisystem disease, comprehensive patient evaluation and interdisciplinary collaboration are fundamental for optimal patient care. As post-COVID-19 clinics continue to expand worldwide,120,121 it has become apparent that the clinical management of ‘long-COVID’ demands both specialized expertise and a whole-patient perspective. 87 Several national and international medical societies have issued diagnostic algorithms and treatment guidelines to facilitate ‘long-COVID’ patient care.19,87,122 Particularly with respect to neurological manifestations of ‘long-COVID’, however, there is a striking gap in the scientific literature as to what concerns treatment recommendations for neurological ‘long-COVID’ sequelae. Here, we propose a practical algorithm for diagnosis and management of patients presenting with neurological symptoms in the context of ‘long-COVID’ syndrome (Figure 2).
As previously discussed, nonspecific neurological symptoms may be causally linked to other persisting organ system dysfunction due to COVID-19, including respiratory, cardiovascular, psychiatric, endocrine, renal, hematologic, or autoimmune disease.8,25,29 Thus, early patient referral to other medical specialties and initiation of appropriate targeted treatments should be promptly considered. 21 It is important to note that the use of standardized clinical, neurological, and functional scales is pivotal for initial patient assessment and follow-up.123–126 Crucially, clinical evaluation should entail a thorough assessment of respiratory and cardiac function, as ‘silent hypoxia’, cardiac arrhythmias, and heart failure are frequently encountered in ‘long COVID’ patients.127,128 Moreover, particularly in patients presenting with fatigue, dyspnea, and postexertional malaise or autonomic dysfunction, assessment of oxygen saturation at rest and post exertion, performance of the 6-Minute Walk Test, and measurement of blood pressure (in lying position and while standing) are recommended.87,122,129 With respect to neurological and neuropsychological scales, assessment of the fatigue severity scale,21,130 depression scale, 131 anxiety, PTSD and apathy scales,132,133 smell identification tests, 134 neurological impairment scales, 123 cognitive assessment, and specific scales depending on the neurological symptoms and signs of ‘long-COVID’ patients may be considered. In addition, a functional status scale has been recently introduced to aid monitoring of functional status in ‘long-COVID’, but its utility in clinical practice awaits validation. 135
After initial clinical assessment, tailored ancillary testing, including blood tests, respiratory and cardiac function tests, neuroimaging, CSF and electrophysiological studies, may complement the neurological examination and should be decided on an individual patient basis. 136 With respect to blood testing, assessment of full blood count, electrolytes, liver and renal function parameters, troponin, C-reactive protein, creatine kinase, D-dimer, brain natriuretic peptides, ferritin, and thyroid hormonal status has been recommended for initial patient screening.19,87 In addition, chest X-ray, electrocardiogram (ECG), and urine tests may be indicated depending on the findings of the clinical examination.87,122 Taken together, these recommendations underline that early identification and treatment of comorbidities comprise a cornerstone for the management of patients with neurological ‘long-COVID’ sequelae. Moreover, tailored neuroimaging and neurophysiological studies may be indicated for the exclusion of serious neurological CNS or PNS disorders, albeit previously published guidelines on ‘long-COVID’ emphasize that overinvestigation should be avoided.87,137
After excluding serious comorbidities or ongoing complications of COVID-19, management of neurological manifestations of ‘long-COVID’ should be pragmatic and symptom-oriented.21,87,137 To date, there is very limited evidence that pharmacological approaches could be effective in the treatment of neurological ‘long-COVID’ sequelae.29,87 Recently, results from a randomized, multicenter, double-blind, placebo-controlled trial that included 200 patients with post-COVID fatigue were published. 138 In this randomized-controlled clinical trial (RCT), supplementation with systemic enzyme complex (ImmunoSEBTM) and probiotic complex (ProbioSEB CSC3TM) resulted in a significantly greater attenuation of physical and mental fatigue scores in treated patients compared with patients allocated to the placebo group. Despite this promising preliminary evidence, however, due to several methodological limitations, these findings warrant replication in future, larger, well-designed RCTs. In addition, some expert recommendations have been recently published arguing that supplementation of vitamins, including vitamin B2, E, and C, and administration of antioxidants, including coenzyme Q10, alpha lipoic acid, L-carnitine, or L-creatine,21,139 may hold some therapeutic potential. Nevertheless, their utility in patients with neurological manifestations of ‘long-COVID’ remains to be verified in the context of prospective RCTs. In addition, as neurological ‘long-COVID’ sequelae have prompted comparisons with ME/CFS, it should be noted that previous RCTs on ME/CFS have failed to detect any consistent benefit from pharmacological treatments (including antidepressants), while highly discordant results have been obtained from nonpharmacological therapies (including cognitive-behavior therapy, graded-exercise-related therapies, rehabilitation, and acupuncture).140,141 Crucially, although the role of antidepressants has been appraised after results from recent RCTs were published, indicating positive effects from the use of fluvoxamine on the course of acute COVID-19,142,143 only small anecdotal, nonrandomized studies have so far provided some preliminary positive evidence from antidepressant use in post-COVID depression. 144 Thus, the question of whether repurposing of antidepressants would be meaningful for patients with neuropsychiatric symptoms post-COVID, including mood disorders and fatigue, remains to be established in prospective, well-designed RCTs.
Hence, pending evidence from ongoing RCTs on neurological ‘long-COVID’ sequelae,119,145 the therapeutic approach of patients with prolonged neurological symptoms following SARS-CoV-2 infection remains today supportive. Specifically with respect to neurorehabilitation, the Stanford Hall consensus statement recommends early initiation of rehabilitation for patients with moderate-to-severe neurological symptoms to maximize functional recovery. 126 Crucially, neurorehabilitation strategies require involvement of multidisciplinary care teams, including neurologists, psychiatrists, psychologists, physiotherapists, and occupational therapists. 136 In addition, involvement of geriatric specialists is of particular strategic importance to mitigate the global burden of ‘long-COVID’, especially in the frail elderly population.146,147 Finally, the implementation of psychological interventions for people experiencing debilitating ‘long-COVID’ symptoms is of paramount importance for the restoration of health and well-being amid the ongoing COVID-19 pandemic. 148
Discussion
In this narrative review, we presented a comprehensive overview of the so-far documented neurological manifestations of ‘long-COVID’ syndrome, along with the currently proposed pathophysiological mechanisms for propagation of neurological ‘long-COVID’ sequelae. In accord with the presented literature, manifestations of ‘long-COVID’ may affect any part of the human nervous system, while the most frequent neurological symptoms among ‘long-COVID’ patients encompass fatigue; ‘brain fog’; headache; cognitive impairment; sleep, mood, smell, or taste disorders; myalgias; sensorimotor deficits; and dysautonomia. Currently, there are insufficient data to allow unequivocal inferences regarding the neuropathological constituents of ‘long-COVID’, albeit neuroimmunological and ongoing oxidative stress processes are thought to prevail in the propagation of neurological symptoms in ‘long-COVID’ patients.7,50,56 Moreover, in analogy to acute COVID-19, while autopsy studies have revealed vascular-related and infection-related secondary inflammatory damage in brain tissue, histopathological studies are urgently needed to assess whether such long-lasting effects could represent the histopathological substrate of ‘long-COVID’ neurological sequelae. 149
Conversely, based on the so-far published epidemiological data several inferences can be drawn. First, neurological ‘long-COVID’ sequelae, with clinically objectifiable correlates of CNS or PNS involvement, seem to affect at least one-third of patients with antecedent SARS-CoV-2 infection.27,40 Nonetheless, a threefold higher incidence is documented in observational studies including self-reported data from COVID-19 survivors.32,34–38,150 Second, there is a substantial overlap between neurological and psychiatric ‘long-COVID’ symptoms. In a significant proportion of ‘long-COVID’ patients, however, concomitant respiratory, cardiovascular, endocrine, renal, hematologic, or autoimmune disease may underlie the manifestation of nonspecific neurological ‘long-COVID’ symptoms.8,25,29 Third, there is preliminary evidence indicating an increased risk of neurological long-term sequelae among patients with antecedent SARS-CoV-2 infection compared with patients infected with other respiratory tract viruses, including influenza but also other coronaviruses such as SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).27,151,152 Thus, further research is required to delineate the pathophysiological underpinnings of long-term SARS-CoV-2 effects in the nervous system, which may ultimately facilitate development of targeted therapies for neurological ‘long-COVID’ sequelae.
Several key aspects regarding the diagnostic and therapeutic approach to neurological ‘long-COVID’ sequelae can also be summarized based on the present review. First, there is an urgent need for establishment of operational ‘long-COVID’ case definitions that should be integrated in the design of prospective RCTs. Second, stringent documentation of clinical symptoms/signs, risk factors, foregoing COVID-19 complications, and comorbidities is required for a systematic approach and management of ‘long-COVID’ patients. Third, multidisciplinary collaboration is essential to provide comprehensive and integrative ‘long-COVID’ patient care. 87 Fourth, early neurorehabilitation should be recommended for patients experiencing long-lasting neurological symptoms after the resolution of acute COVID-19. 126 Fifth, it should be emphasized that due to the nonsystematic nature of the present review, despite expert consensus on the included literature, selection bias cannot be excluded and a systematic review of the literature is warranted to broaden our understanding on neurological ‘long COVID’ sequelae. Finally, although there is a current paucity of evidence on nonpharmacological and pharmacological therapies for ‘long-COVID’, preliminary data suggest that supplementation with antioxidants may hold some therapeutic potential in ‘long-COVID’ fatigue.21,139 Future RCTs are thus warranted to evaluate the potential utility of pharmacological and nonpharmacological interventions in ‘long-COVID’ patients.
In conclusion, neurologists are confronted today with an unprecedented need to comprehend and manage neurological ‘long-COVID’ sequelae. These challenging times call for international collaborations between medical societies, swift and transparent communication of research data, and further mandate basic research to gain insight into the pathophysiological correlates of ‘long-COVID’. In addition, considering the increasing societal demand for evidence-based interventions for ‘long-COVID’ patients, it is time for scientists to join forces for the development of preventive and therapeutic strategies for COVID-19 survivors, with the aim to mitigate the community-wide toll of an impending ‘long-COVID’ pandemic.
Footnotes
Author contributions: Maria Ioanna Stefanou: Visualization; Writing – original draft; Writing – review & editing.
Lina Palaiodimou: Supervision; Visualization; Writing – original draft.
Eleni Bakola: Validation; Writing – review & editing.
Nikolaos Smyrnis: Supervision; Writing – review & editing.
Marianna Papadopoulou: Supervision; Writing – review & editing.
George P. Paraskevas: Supervision; Writing – review & editing.
Emmanouil Rizos: Supervision; Writing – review & editing.
Eleni Boutati: Supervision; Validation; Writing – review & editing.
Nikolaos Grigoriadis: Supervision; Validation; Writing – review & editing.
Christos Krogias: Supervision; Writing – review & editing.
Sotirios Giannopoulos: Supervision; Validation.
Sotirios Tsiodras: Resources; Supervision.
Mina Gaga: Supervision; Validation.
Georgios Tsivgoulis: Conceptualization; Methodology; Project administration.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Georgios Tsivgoulis  https://orcid.org/0000-0002-0640-3797
https://orcid.org/0000-0002-0640-3797
Contributor Information
Maria-Ioanna Stefanou, Second Department of Neurology, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Lina Palaiodimou, Second Department of Neurology, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Eleni Bakola, Second Department of Neurology, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Nikolaos Smyrnis, Second Department of Psychiatry, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Marianna Papadopoulou, Second Department of Neurology, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Athens, Greece; Department of Physiotherapy, University of West Attica, Athens, Greece.
George P. Paraskevas, Second Department of Neurology, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Athens, Greece
Emmanouil Rizos, Second Department of Psychiatry, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Eleni Boutati, Second Propaedeutic Department of Internal Medicine and Research Institute, University General Hospital Attikon, National and Kapodistrian University of Athens, Athens, Greece.
Nikolaos Grigoriadis, Second Department of Neurology, ‘AHEPA’ University Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Christos Krogias, Department of Neurology, St. Josef-Hospital Bochum, Ruhr University Bochum, Bochum, Germany.
Sotirios Giannopoulos, Second Department of Neurology, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Sotirios Tsiodras, 4th Department of Internal Medicine, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Mina Gaga, 7th Respiratory Medicine Department and Asthma Center, Athens Chest Hospital ‘Sotiria’, Athens, Greece.
Georgios Tsivgoulis, Second Department of Neurology, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Rimini 1, Chaidari, 12462 Athens, Greece. Department of Neurology, The University of Tennessee Health Science Center, Memphis, TN, USA.
References
- 1. Nicola M, Alsafi Z, Sohrabi C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg 2020; 78: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med 2021; 27: 28–33. [DOI] [PubMed] [Google Scholar]
- 3. Sivan M, Taylor S. NICE guideline on long COVID. BMJ 2020; 371: m4938. [DOI] [PubMed] [Google Scholar]
- 4. Callard F, Perego E. How and why patients made Long Covid. Soc Sci Med 2021; 268: 113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open 2021; 4: e2127403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health 2021; 18: 2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis 2021; 53: 737–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah W, Hillman T, Playford ED, et al. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021; 372: n136. [DOI] [PubMed] [Google Scholar]
- 9. Phillips S, Williams MA. Confronting our next national health disaster – Long-Haul Covid. N Engl J Med 2021; 385: 577–579. [DOI] [PubMed] [Google Scholar]
- 10. Nath A. Long-Haul COVID. Neurology 2020; 95: 559–560. [DOI] [PubMed] [Google Scholar]
- 11. Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021; 6: 100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davido B, Seang S, Tubiana R, et al. Post-COVID-19 chronic symptoms: a postinfectious entity. Clin Microbiol Infect 2020; 26: 1448–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oh ES, Vannorsdall TD, Parker AM. Post-acute sequelae of SARS-CoV-2 infection and subjective memory problems. JAMA Netw Open 2021; 4: e2119335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rossman H, Shilo S, Meir T, et al. COVID-19 dynamics after a national immunization program in Israel. Nat Med 2021; 27: 1055–1061. [DOI] [PubMed] [Google Scholar]
- 16. Pritchard E, Matthews PC, Stoesser N, et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med 2021; 27: 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control Prevention (CDC). Post-COVID conditions: information for healthcare providers, 2021, https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html
- 18. World Health Organization (WHO). A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021, 2021, https://www.who.int/docs/default-source/coronaviruse/corrigendum-2021.1-post-covid-19-clinical-case-definition-2021-10-06-corr-2021-10-06-en.pdf?sfvrsn=1ebb697c_5 [Google Scholar]
- 19. National Institute for Health Care Excellence (NICE). COVID-19 rapid guideline: managing the long-term effects of COVID-19, 2020, https://www.nice.org.uk/guidance/ng188 [PubMed]
- 20. World Health Organization (WHO). Emergency use ICD codes for COVID-19 disease outbreak, 2020, https://www.who.int/standards/classifications/classification-of-diseases/emergency-use-icd-codes-for-covid-19-disease-outbreak
- 21. Moghimi N, Di Napoli M, Biller J, et al. The neurological manifestations of post-acute sequelae of SARS-CoV-2 infection. Curr Neurol Neurosci Rep 2021; 21: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsivgoulis G, Palaiodimou L, Katsanos AH, et al. Neurological manifestations and implications of COVID-19 pandemic. Ther Adv Neurol Disord 2020; 13: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsivgoulis G, Palaiodimou L, Zand R, et al. COVID-19 and cerebrovascular diseases: a comprehensive overview. Ther Adv Neurol Disord 2020; 13: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palaiodimou L, Stefanou MI, Katsanos AH, et al. Prevalence, clinical characteristics and outcomes of Guillain-Barré syndrome spectrum associated with COVID-19: a systematic review and meta-analysis. Eur J Neurol 2021; 28: 3517–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruggeri RM, Campennì A, Deandreis D, et al. SARS-COV-2-related immune-inflammatory thyroid disorders: facts and perspectives. Expert Rev Clin Immunol 2021; 17: 737–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Institutes of Health (NIH). NIH launches new initiative to study ‘Long COVID’, 2021, https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid
- 27. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 2021; 8: 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Q, Allot A, Lu Z. LitCovid: an open database of COVID-19 literature. Nucleic Acids Res 2021; 49: D1534–D1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crook H, Raza S, Nowell J, et al. Long covid – mechanisms, risk factors, and management. BMJ 2021; 374: n1648. [DOI] [PubMed] [Google Scholar]
- 30. Baig AM. Counting the neurological cost of COVID-19. Nat Rev Neurol 2021: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chou SH, Beghi E, Helbok R, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19-A report for the GCS-NeuroCOVID consortium and the energy consortium. JAMA Netw Open 2021; 4: e2112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38: 101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Herck M, Goërtz YMJ, Houben-Wilke S, et al. Severe fatigue in Long COVID: web-based quantitative follow-up study in members of online long COVID support groups. J Med Internet Res 2021; 23: e30274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 2021; 4: e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lechien JR, Chiesa-Estomba CM, Beckers E, et al. Prevalence and 6-month recovery of olfactory dysfunction: a multicentre study of 1363 COVID-19 patients. J Intern Med 2021; 290: 451–461. [DOI] [PubMed] [Google Scholar]
- 38. Mandal S, Barnett J, Brill SE, et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021; 76: 396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jarrahi A, Ahluwalia M, Khodadadi H, et al. Neurological consequences of COVID-19: what have we learned and where do we go from here? J Neuroinflammation 2020; 17: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pilotto A, Cristillo V, Cotti Piccinelli S, et al. Long-term neurological manifestations of COVID-19: prevalence and predictive factors. Neurol Sci 2021; 42: 4903–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021; 11: 16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsivgoulis G, Fragkou PC, Lachanis S, et al. Olfactory bulb and mucosa abnormalities in persistent COVID-19-induced anosmia: a magnetic resonance imaging study. Eur J Neurol 2021; 28: e6–e8. [DOI] [PubMed] [Google Scholar]
- 43. Tsivgoulis G, Fragkou PC, Karofylakis E, et al. Hypothyroidism is associated with prolonged COVID-19-induced anosmia: a case-control study. J Neurol Neurosurg Psychiatry 2021; 92: 911–912. [DOI] [PubMed] [Google Scholar]
- 44. Tsatsakis A, Calina D, Falzone L, et al. SARS-CoV-2 pathophysiology and its clinical implications: an integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem Toxicol 2020; 146: 111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bulfamante G, Bocci T, Falleni M, et al. Brainstem neuropathology in two cases of COVID-19: SARS-CoV-2 trafficking between brain and lung. J Neurol 2021; 268: 4486–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zubair AS, McAlpine LS, Gardin T, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol 2020; 77: 1018–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 2020; 181: 894–904.e899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 2020; 19: 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pennisi M, Lanza G, Falzone L, et al. SARS-CoV-2 and the nervous system: from clinical features to molecular mechanisms. Int J Mol Sci 2020; 21: 5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Balcom EF, Nath A, Power C. Acute and chronic neurological disorders in COVID-19: potential mechanisms of disease. Brain 2021; 144: 3576–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Y, Fu L, Gonzales DM, et al. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J Virol 2004; 78: 3398–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lewis A, Frontera J, Placantonakis DG, et al. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J Neurol Sci 2021; 421: 117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med 2020; 383: 989–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aschman T, Schneider J, Greuel S, et al. Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol 2021; 78: 948–960. [DOI] [PubMed] [Google Scholar]
- 55. Ramakrishnan RK, Kashour T, Hamid Q, et al. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front Immunol 2021; 12: 686029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baig AM. Deleterious outcomes in Long-Hauler COVID-19: the effects of SARS-CoV-2 on the CNS in chronic COVID syndrome. ACS Chem Neurosci 2020; 11: 4017–4020. [DOI] [PubMed] [Google Scholar]
- 57. Lukiw WJ, Pogue A, Hill JM. SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol Neurobiol 2020; 42: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen R, Wang K, Yu J, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol 2020; 11: 573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Blazhenets G, Schroeter N, Bormann T, et al. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. J Nucl Med 2021; 62: 910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Guedj E, Campion JY, Dudouet P, et al. (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging 2021; 48: 2823–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Raj SR, Arnold AC, Barboi A, et al. Long-COVID postural tachycardia syndrome: an American Autonomic Society statement. Clin Auton Res 2021; 31: 365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Johansson M, Ståhlberg M, Runold M, et al. Long-Haul Post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep 2021; 3: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tenney JR, Rozhkov L, Horn P, et al. Cerebral glucose hypometabolism is associated with mitochondrial dysfunction in patients with intractable epilepsy and cortical dysplasia. Epilepsia 2014; 55: 1415–1422. [DOI] [PubMed] [Google Scholar]
- 64. Bekar LK, Wei HS, Nedergaard M. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J Cereb Blood Flow Metab 2012; 32: 2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Doykov I, Hällqvist J, Gilmour KC, et al. ‘The long tail of Covid-19’ – The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Res 2020; 99: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barizien N, Le Guen M, Russel S, et al. Clinical characterization of dysautonomia in long COVID-19 patients. Sci Rep 2021; 11: 14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Eshak N, Abdelnabi M, Ball S, et al. Dysautonomia: an overlooked neurological manifestation in a critically ill COVID-19 patient. Am J Med Sci 2020; 360: 427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aoyagi Y, Ohashi M, Funahashi R, et al. Oropharyngeal dysphagia and aspiration pneumonia following coronavirus disease 2019: a case report. Dysphagia 2020; 35: 545–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kandemirli SG, Altundag A, Yildirim D, et al. Olfactory Bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad Radiol 2021; 28: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Peluso MJ, Deitchman AN, Torres L, et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep 2021; 36: 109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Townsend L, Dyer AH, Naughton A, et al. Longitudinal analysis of COVID-19 patients shows age-associated T cell changes independent of ongoing ill-health. Front Immunol 2021; 12: 676932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wallukat G, Hohberger B, Wenzel K, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun 2021; 4: 100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Visvabharathy L, Hanson B, Orban Z, et al. Neuro-COVID long-haulers exhibit broad dysfunction in T cell memory generation and responses to vaccination. medRxiv, 2021, https://www.medrxiv.org/content/10.1101/2021.08.08.21261763v1.full
- 74. Sollini M, Ciccarelli M, Cecconi M, et al. Vasculitis changes in COVID-19 survivors with persistent symptoms: an [(18)F]FDG-PET/CT study. Eur J Nucl Med Mol Imaging 2021; 48: 1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lambadiari V, Mitrakou A, Kountouri A, et al. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur J Heart Fail 2021; 23: 1916–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology 2021; 27: 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Q, Zheng X-S, Shen X-R, et al. Prolonged shedding of severe acute respiratory syndrome coronavirus 2 in patients with COVID-19. Emerg Microb Infect 2020; 9: 2571–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020; 5: 434–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gaebler C, Wang Z, Lorenzi JC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021; 591: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Quigley EMM. Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep 2017; 17: 94. [DOI] [PubMed] [Google Scholar]
- 82. Kanberg N, Simrén J, Edén A, et al. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. Ebiomedicine 2021; 70: 103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yang AC, Kern F, Losada PM, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 2021; 595: 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network – United States, March-June 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kubota T, Kuroda N. Exacerbation of neurological symptoms and COVID-19 severity in patients with preexisting neurological disorders and COVID-19: a systematic review. Clin Neurol Neurosurg 2021; 200: 106349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network – United States, March–June 2020. Morb Mortal Wkly Rep 2020; 69: 993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Greenhalgh T, Knight MA, Court C, et al. Management of post-acute covid-19 in primary care. BMJ 2020; 370: m3026. [DOI] [PubMed] [Google Scholar]
- 88. Hanfi SH, Lalani TK, Saghir A, et al. COVID-19 and its mimics: what the radiologist needs to know. J Thorac Imaging 2021; 36: W1–W10. [DOI] [PubMed] [Google Scholar]
- 89. Nickel CH, Bingisser R. Mimics and chameleons of COVID-19. Swiss Med Wkly 2020; 150: w20231. [DOI] [PubMed] [Google Scholar]
- 90. Sisó-Almirall A, Brito-Zerón P, Conangla Ferrín L, et al. Long Covid-19: proposed primary care clinical guidelines for diagnosis and disease management. Int J Environ Res Public Health 2021; 18: 4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Byambasuren O, Cardona M, Bell K, et al. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. J Ass Med Microb Infect Dis Canada 2020; 5: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. He J, Guo Y, Mao R, et al. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol 2021; 93: 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fill Malfertheiner S, Brandstetter S, Roth S, et al. Immune response to SARS-CoV-2 in health care workers following a COVID-19 outbreak: a prospective longitudinal study. J Clin Virol 2020; 130: 104575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 2020; 71: 2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 2021; 384: 1372–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stamatatos L, Czartoski J, Wan YH, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021; 372: 1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nehme M, Braillard O, Chappuis F, et al. Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann Intern Med 2021; 174: 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2021; 76: 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Parker AJ, Humbir A, Tiwary P, et al. Recovery after critical illness in COVID-19 ICU survivors. Br J Anaesth 2021; 126: e217–e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Stam HJ, Stucki G, Bickenbach J. Covid-19 and post intensive care syndrome: a call for action. J Rehabil Med 2020; 52: jrm00044. [DOI] [PubMed] [Google Scholar]
- 102. Cabañes-Martínez L, Villadóniga M, González-Rodríguez L, et al. Neuromuscular involvement in COVID-19 critically ill patients. Clin Neurophysiol 2020; 131: 2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fan S, Xiao M, Han F, et al. Neurological manifestations in critically ill patients with COVID-19: a retrospective study. Front Neurol 2020; 11: 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Berlit P, Bösel J, Gahn G, et al. ‘Neurological manifestations of COVID-19’ – guideline of the German society of neurology. Neurol Res Pract 2020; 2: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ding H, Yin S, Cheng Y, et al. Neurologic manifestations of nonhospitalized patients with COVID-19 in Wuhan, China. Medcomm 2020; 1: 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome. ERJ Open Res 2020; 6: 00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Inter Med 2017; 5: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Iqbal FM, Lam K, Sounderajah V, et al. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine 2021; 36: 100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021; 27: 626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Carvalho-Schneider C, Laurent E, Lemaignen A, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 2021; 27: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr 2021; 110: 2208–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Castro VM, Gunning FM, Perlis RH. Persistence of neuropsychiatric symptoms associated with SARS-CoV-2 positivity among a cohort of children and adolescents. medRxiv, 2021, https://www.medrxiv.org/content/10.1101/2021.09.28.21264259v1
- 113. Vahidy FS, Pan AP, Ahnstedt H, et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: cross-sectional analysis from a diverse US metropolitan area. PLoS ONE 2021; 16: e0245556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pivonello R, Auriemma RS, Pivonello C, et al. Sex disparities in COVID-19 severity and outcome: are men weaker or women stronger? Neuroendocrinology 2021; 111: 1066–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Qi T, Hu T, Ge QQ, et al. COVID-19 pandemic related long-term chronic stress on the prevalence of depression and anxiety in the general population. BMC Psychiatry 2021; 21: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Renaud M, Thibault C, Le Normand F, et al. Clinical outcomes for patients with anosmia 1 year after COVID-19 diagnosis. JAMA Netw Open 2021; 4: e2115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021; 398: 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Latronico N, Peli E, Calza S, et al. Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax. Epub ahead of print 29 September 2021. DOI: 10.1136/thoraxjnl-2021-218064. [DOI] [PubMed] [Google Scholar]
- 119. The Lancet Neurology. Long COVID: understanding the neurological effects. Lancet Neurol 2021; 20: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Rovere Querini P, De Lorenzo R, Conte C, et al. Post-COVID-19 follow-up clinic: depicting chronicity of a new disease. Acta Biomed 2020; 91: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pinto M, Gimigliano F, De Simone S, et al. Post-acute COVID-19 rehabilitation network proposal: from intensive to extensive and home-based IT supported services. Int J Environ Res Public Health 2020; 17: 9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. British Thoracic Society. British Thoracic Society guidance on respiratory follow up of patients with a clinico-radiological diagnosis of COVID-19 pneumonia, 2020, https://www.brit-thoracic.org.uk/document-library/quality-improvement/COVID-19/resp-follow-up-guidance-post-COVID-pneumonia
- 123. Turner-Stokes L, Thu A, Williams H, et al. The Neurological Impairment Scale: reliability and validity as a predictor of functional outcome in neurorehabilitation. Disabil Rehabil 2014; 36: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rouby C, Thomas-Danguin T, Vigouroux M, et al. The lyon clinical olfactory test: validation and measurement of hyposmia and anosmia in healthy and diseased populations. Int J Otolaryngol 2011; 2011: 203805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kounti F, Tsolaki M, Kiosseoglou G. Functional Cognitive Assessment Scale (FUCAS): a new scale to assess executive cognitive function in daily life activities in patients with dementia and mild cognitive impairment. Hum Psychopharmacol 2006; 21: 305–311. [DOI] [PubMed] [Google Scholar]
- 126. O’Barker-Davies RM, O’Sullivan O, Senaratne KPP, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med 2020; 54: 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rahman A, Tabassum T, Araf Y, et al. Silent hypoxia in COVID-19: pathomechanism and possible management strategy. Mol Biol Rep 2021; 48: 3863–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Abbasi J. Researchers investigate what COVID-19 does to the heart. JAMA 2021; 325: 808–811. [DOI] [PubMed] [Google Scholar]
- 129. Mantha S, Tripuraneni SL, Roizen MF, et al. Proposed modifications in the 6-minute walk test for potential application in patients with mild COVID-19: a step to optimize triage guidelines. Anesth Analg 2020; 131: 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis Care Res 2011; 63: S263–S286. [DOI] [PubMed] [Google Scholar]
- 131. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 132. Zhuang L, Yang Y, Gao J. Cognitive assessment tools for mild cognitive impairment screening. J Neurol 2021; 268: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 133. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991; 38: 143–162. [DOI] [PubMed] [Google Scholar]
- 134. Joseph T, Auger SD, Peress L, et al. Screening performance of abbreviated versions of the UPSIT smell test. J Neurol 2019; 266: 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Klok FA, Boon G, Barco S, et al. The post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur Respir J 2020; 56: 2001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Korompoki E, Gavriatopoulou M, Hicklen RS, et al. Epidemiology and organ specific sequelae of post-acute COVID19: a narrative review. J Infect 2021; 83: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020; 26: 1017–1032. [DOI] [PubMed] [Google Scholar]
- 138. Rathi A, Jadhav SB, Shah N. A randomized controlled trial of the efficacy of systemic enzymes and probiotics in the resolution of post-COVID fatigue. Medicines 2021; 8: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sahebnasagh A, Saghafi F, Avan R, et al. The prophylaxis and treatment potential of supplements for COVID-19. Eur J Pharmacol 2020; 887: 173530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kim D-Y, Lee J-S, Park S-Y, et al. Systematic review of randomized controlled trials for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med 2020; 18: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fluge Ø, Rekeland IG, Lien K, et al. B-lymphocyte depletion in patients with myalgic encephalomyelitis/chronic fatigue syndrome: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2019; 170: 585–593. [DOI] [PubMed] [Google Scholar]
- 142. Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2022; 10: e42–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lenze EJ, Mattar C, Zorumski CF, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA 2020; 324: 2292–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mazza MG, Zanardi R, Palladini M, et al. Rapid response to selective serotonin reuptake inhibitors in post-COVID depression. Eur Neuropsychopharmacol 2021; 54: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mullard A. Long COVID’s long R&D agenda. Nat Rev Drug Discov 2021; 20: 329–331. [DOI] [PubMed] [Google Scholar]
- 146. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res 2020; 32: 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Dasgupta A, Kalhan A, Kalra S. Long term complications and rehabilitation of COVID-19 patients. J Pak Med Assoc 2020; 70 S131–S135. [DOI] [PubMed] [Google Scholar]
- 148. Duan L, Zhu G. Psychological interventions for people affected by the COVID-19 epidemic. Lancet Psychiatry 2020; 7: 300–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Fisicaro F, Di Napoli M, Liberto A, et al. Neurological sequelae in patients with COVID-19: a histopathological perspective. Int J Environ Res Public Health 2021; 18: 1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Van Herck M, Goërtz YMJ, Houben-Wilke S, et al. Severe fatigue in long COVID: follow-up study in members of online long COVID support groups. J Med Internet Res 2021; 23: e30274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol 2011; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. O’Sullivan O. Long-term sequelae following previous coronavirus epidemics. Clin Med 2021; 21: e68–e70. [DOI] [PMC free article] [PubMed] [Google Scholar]