Abstract
Study design
Narrative review.
Objectives
To provide an overview of contemporary therapies for the James Lind Alliance priority setting partnership for degenerative cervical myelopathy (DCM) question: ‘Can novel therapies, including stem-cell, gene, pharmacological and neuroprotective therapies, be identified to improve the health and wellbeing of people living with DCM and slow down disease progression?’
Methods
A review of the literature was conducted to outline the pathophysiology of DCM and present contemporary therapies that may hold therapeutic value in 3 broad categories of neuroprotection, neuroregeneration, and neuromodulation.
Results
Chronic spinal cord compression leads to ischaemia, neuroinflammation, demyelination, and neuronal loss. Surgical intervention may halt progression and improve symptoms, though the majority do not make a full recovery leading to lifelong disability. Neuroprotective agents disrupt deleterious secondary injury pathways, and one agent, Riluzole, has undergone Phase-III investigation in DCM. Although it did not show efficacy on the primary outcome modified Japanese Orthopaedic Association scale, it showed promising results in pain reduction. Regenerative approaches are in the early stage, with one agent, Ibudilast, currently in a phase-III investigation. Neuromodulation approaches aim to therapeutically alter the state of spinal cord excitation by electrical stimulation with a variety of approaches. Case studies using electrical neuromuscular and spinal cord stimulation have shown positive therapeutic utility.
Conclusion
There is limited research into interventions in the 3 broad areas of neuroprotection, neuroregeneration, and neuromodulation for DCM. Contemporary and novel therapies for DCM are now a top 10 priority, and whilst research in these areas is limited in DCM, it is hoped that this review will encourage research into this priority.
Keywords: degenerative cervical myelopathy, neuroprotection, neuromodulation, neuroregeneration, inflammation, demyelination, electrical stimulation, spinal cord stimulation, neuromuscular electrical stimulation, functional electrical stimulation
Introduction
Individuals diagnosed with Degenerative Cervical Myelopathy (DCM) may require surgical and/or non-surgical interventions depending on the severity of the disease and clinical manifestations. Surgical intervention in the form of decompressive surgery with or without fusion is the mainstay of treatment for moderate to severe disease as assessed by modified Japanese Orthopaedic Association (mJOA) scale.1,2 The optimal management of mild DCM, early in its course, is unknown, as studies have not pointed towards a significant benefit for prophylactic surgical intervention.3,4 However, this opens a window for the possible application of non-surgical interventions such as disease modifying therapeutics physical therapy, cervical traction, collars and spinal injection.4,5
Despite the success of surgical decompression in halting neurological decline and providing some recovery, myelopathy symptoms may reoccur, 6 and full recovery is rarely seen, with estimates suggesting less than 5% make a full recovery,7,8 with lower limb and sphincter function demonstrating the slowest recovery responses. 9 Ongoing myelopathy may affect domains such as mobility, weakness, manual dexterity, pain and bladder/bowel dysfunction resulting in a considerable impact and decline in quality of life.10,11 Therefore, halting neurological decline and improving rates of functional recovery remain major priorities of patients and surgeons dealing with DCM. 12 The importance of this unmet need is further compounded by the fact that DCM is one of the commonest causes of spinal cord dysfunction worldwide, and the incidence is expected to increase with an ageing population.1,13
This uncertainty was highlighted in the recent James Lind Alliance 12 (JLA) priority setting partnership, with one of the top priorities and questions agreed upon being, ‘Can novel therapies, including stem-cell, gene, pharmacological and neuroprotective therapies, be identified to improve the health and wellbeing of people living with DCM and slow down disease progression?’. The objective of this narrative review is to provide an overview of potential contemporary therapies that may enhance recovery in DCM. We will briefly outline the pathophysiology of DCM and then present contemporary therapies in 3 main areas, namely, neuroprotection, neuroregeneration and neuromodulation, which may in the future have a therapeutic role in DCM.
Pathophysiology of DCM
Degenerative cervical myelopathy is an umbrella term encompassing a number of degenerative processes and responses in the cervical spine, which leads to progressive chronic spinal cord compression.14,15 Osseous, ligamentous, and intervertebral disc tissue undergo degenerative changes as part of normal ageing and/or repetitive stress leading to structural changes such as spondyloses, hypertrophy/ossification/calcification of ligaments (e.g Ossification of the posterior longitudinal ligament) and disc herniation/prolapse.1,16 Whilst these changes lead to static compression and direct injury to the spinal cord, they are it compounded by the mobility of the cervical spine, causing further compression due to physiological or pathological (ie spondylolisthesis) movements.1,16 Mechanical compression leads to direct primary injury but also initiates a complex sequence of secondary injury processes in the spinal cord. 17 These include macro- and microvascular compromise causing hypoxia and ischaemia18,19 and neuroinflammation.17,20 The consequence of these processes include loss of neurons, oligodendrocytes, 20 demyelination 21 and axonal degeneration. 17
Demyelination can act as a functional conduction block on axons contributing to the sensorimotor deficits seen in DCM. Post-mortem studies have found extensive thin myelinated fibres in the spinal cord white matter suggestive of focal demyelination and remyelination. 22 Studies have also found an association between reduced myelin content on myelin water imaging on MRI and impaired somatosensory evoked potential in DCM, which demonstrates the impact of demyelination seen on long tract axonal function in DCM. 23 Therefore, strategies to mitigate demyelination and stimulate endogenous mechanisms of myelin repair may provide a viable therapeutic strategy.
The neuroinflammatory cascade resulting from chronic compression is of interest as it can be both protective and damaging. An increase in activated microglia and macrophages has been observed at the site of chronic spinal cord compression and are a source of pro-inflammatory cytokines and can lead to further cell death by necrosis and apoptosis. 24 Alternatively, Immune cells can also exhibit neuroprotective effects such as releasing neuroprotective cytokines and growth factors. 25 This has created interest in immunomodulatory strategies to alter this balance as a therapeutic strategy.
Neuroprotection
Neuroprotective agents prevent further cell death and dysfunction by altering secondary injury pathways. A notable example is Riluzole, a benzothiazole sodium channel-blocker developed in the mid-20th century as a muscle relaxant, 26 with later indications as an anticonvulsant 27 and neuroprotective agent to prolong survival in ALS. 28 In the context of DCM, animal studies have shown RIluzole can mitigate secondary injury mechanisms related to sodium channels and glutamate excitotoxicity thus resulting in functional improvements.29-31 These promising findings have led to a phase III randomized placebo-controlled trial (CSM-PROTECT) to assess the efficacy of Riluzole as an adjunct to surgical decompression in chronic cervical myelopathy to promote neurologic recovery. 32 The study was recently published and reported no significant difference in the primary endpoint of mJOA at 6-month follow-up. However, Riluzole was associated with potentially promising reduction in neck pain at 6- and 12-month follow-up compared to placebo. 33 As pain is a major patient priority, further investigations may be warranted into which subgroups would benefit most. Inflammatory pathways are implicated in the deleterious effects of chronic cord compression and recent work in humans and animal models has implicated microRNA21 (miR21) as a key mediator of the inflammatory/ischemic cell injury in DCM. A prospective cohort study of patients with DCM has identified a positive correlation between miR21, initial symptom severity and poor treatment outcomes, the findings of which were further corroborated in mouse models of DCM. This work suggests the possibility of miR21 as a possible biomarker of disease risk in DCM and a potential therapeutic target for intervention. 34
Anti-inflammatory agents such as corticosteroids are also candidates for potential clinical translation as a neuroprotective adjunct to surgery and have been extensively studied in traumatic SCI.35-37 In a rodent study of DCM, Methylprednisolone, an agent used in acute-onset traumatic spinal cord injury, 38 was used as a perioperative adjunct to surgical decompression. 39 Significant improvement was found in the group treated by methylprednisolone when compared to the control 2-weeks post decompression in forepaw function. However, by 5-weeks, no significant difference was apparent between the groups. 39 Few human clinical studies have been conducted, though one retrospective study of thoracic myelopathy used intraoperative methylprednisolone found better neurological recovery at 2 weeks, though no significant difference in neurological outcomes between methylprednisolone and control group at long-term follow-up. 40 The use of corticosteroids perioperatively in anterior cervical spine surgery has however shown benefit in reducing post-operative complications with significant reduction in airway oedema, pain, hospital stay and improved swallowing in Phase-III randomized controlled trials.41,42 This surgical approach is used in the surgical management of DCM and future studies should look into the potential functional benefits of corticosteroids in these patients.
The perioperative period is a key time point in which neuroprotective agents should be considered due to ischaemia-reperfusion injury, but also due to axonal plasticity, which is thought to emerge after decompression. 43 Whilst no neuroprotective agent has shown efficacy in Phase 3 clinical trials, improved understanding of the pathophysiology of DCM may provide an avenue for the development of more targeted neuroprotective therapies. Of note, neck and arm pain represent potentially important targets if intervention in DCM, as suggested by the recent CSM-Protect trial. 33 However, this will require the design of trials which use more sensitive outcomes to detect changes in neck and arm pain.
Regenerative Medicine
People with DCM often develop and then suffer from lifelong disability, with less than 5% making a full recovery even despite surgical decompression.7,8 This occurs due to failure to recognize the clinical deterioration, delayed medical intervention, the limited regenerative capacity of the spinal cord, loss of local cellular structures and disruption of the spinal cord architecture. Replacing lost tissue and enhancing intrinsic recovery capacity is an area of interest in spinal cord injuries as a means to enhance recovery. Attempts are being made to achieve this through a number of different approaches, including pharmacological and cell-based therapies.44-48
One potential beneficial pharmacological agent is Ibudilast, which is currently licensed in Japan for the treatment of asthma and post-stroke dizziness. 49 The mechanisms by which it exerts its effects for the aforementioned indications have been attributed to its anti-inflammatory, bronchodilatory and vasodilatory effects.50-55 More recently, it has been found to exhibit central anti-inflammatory, neuroprotective and neurotrophic/regenerative effects by its inhibition of phosphodiesterase-4 (PDE-4 and -10) and macrophage migration inhibitory factor, leading to attenuation of activated glial cells and enhancement of neurotrophic factors.49,50,56,57 This has generated interest in its application in a number of neurological conditions. These include early-stage clinical trials in progressive Multiple Sclerosis,58,59 ALS, 60 alcoholism,61,62 drug addiction63,64 and pain.65,66 The combination of anti-inflammatory, neuroprotective and neuro-regenerative properties has led to interest for its use in DCM and is the basis for RECEDE-Myelopathy (NCT04631471), a phase 3, double-blind, randomized controlled trial assessing the efficacy of Ibudilast as an adjuvant treatment to decompressive surgery for DCM on mJOA score and neck pain.
Cell-based therapies have also gained considerable attention over the last few decades. Stem cells possess the ability to differentiate into a variety of cell types creating the possibility of a potential therapeutic tool to repair and/or replace damaged tissue. 67 Challenges for cell-based therapies include the cell type first surviving the transplantation, then migrating appropriately to the site of therapeutic action, differentiating into the correct lineage and finally to behave physiologically in the manner intended. 68 Currently, the most widely applied clinical use of this therapy is haematopoietic stem cell transplantation with research dating back to the 1950s and indicated for conditions such as lymphoma, leukaemia and anaplastic anaemia with curative potential.69,70 Whilst there has been little research for its application in DCM; specifically, there is ongoing research to develop stem cell therapies for traumatic spinal cord injury sharing pathophysiological features.67,71 Several mechanisms by which stem cells can promote recovery in spinal cord injuries can be applicable to DCM. These include replacement of lost tissue (ie neurones 72 /glial precursors/oligodendrocytes 73 ), integration into host neuronal circuits,74,75 release of neurotrophic factors(e.g BDNF, GDNF, NGF and VEGF),76-78 anti-apoptotic,79,80 anti-inflammatory81,82 and immunomodulatory effects. 83 Trials in SCI are typically underpowered for efficacy and lack controls as they are early-stage open-label trials.84,85 In addition, there is considerable heterogeneity between cell-based interventions such as type of cell utilized, source of stem cell (i.e embryonic stem cells, adult stem cells and induced pluripotent stem cells; autologous vs allogeneic), processing (i.e lab purification, amplification, good manufacturing practice adherence and quality control) and delivery of stem cells (i.e Intravenous, intrathecal, direct intraspinal and impregnated tissue engineered materials) highlighting uncertainty in optimal intervention parameters.44,45,85 Further, adjuncts to enhance cell-based therapies are undergoing investigation to overcome some of the challenges faced such as poor cell survival, migration and integration. These include co-administration of growth factors, cell delivery and structural support with biomaterials/scaffolds, guidance of migration and differentiation by electric fields (galvanotaxis), degradation of glial scar and self-assembling peptides to improve extracellular matrix environment.45,71,86
One of the inciting factors for DCM is cervical spondylosis and disc degeneration. Cell-based, growth-factor based and small molecule-based therapies aiming to repair or regenerate the degenerate intervertebral disc may offer an opportunity to halt DCM progression or even reverse its symptoms. This may be particularly important in patients with mild DCM symptoms and in asymptomatic patients with imaging evidence of cord compression. Several human clinical trials using stem cells (autologous or allogenic; bone marrow and adipose-derived mesenchymal, notochordal and chondrocyte-like nucleus pulposus cells) for intervertebral disc degeneration have been or are currently being undertaken. 87 While most studies report significant reduction in pain, increase in disc height, improved patient mobility and quality of life,88-91 concerns have been raised due to the poor design of some studies and their low number of patients and lack appropriate controls.
Due to the nature of DCM, the majority of patients are in an older age category (>55 years), which presents a unique challenge. The central nervous system undergoes structural and functional changes as part of the normal ageing process. 92 This includes reduced neuroplasticity, which could contribute to post-decompression recovery, 43 and strategies to enhance endogenous regenerative processes may be confounded by aging. Regenerative therapies provide prospects for new treatments in DCM; however, it is currently a very young field of research. Research in this field aiming to overcome some of the current challenges and specific to its role in DCM are warranted.
Gene therapy offers the prospect for sustained and localized production of therapeutics that is particularly attractive for ‘biologics’ that are otherwise complex to deliver. One therapy has been studied extensively for possible direct tissue application in SCI with a lead strategy aimed at neuroplasticity using an enzyme, chondroitinase, that remodels the basal lamina of neuronal nets to enhance neuroplasticity.93,94 In spinal muscular atrophy, a gene therapy (Zolgensma) that restores a critical functional protein (SMN protein, important in motor neurone survival) to motor neurons has been approved for clinical use. 95 Gene therapies such as myostatin inhibition are also under development for muscular dystrophy, 96 which aims to improve muscle function. Whilst gene therapy is in its early stages of development for many conditions, it offers the prospects for sustained production of therapeutic molecules which remains to be explored in DCM.
Neuromodulation
Neuromodulation is broadly defined by the International Neuromodulation Society as ‘the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body’. 97 Electrical neuromodulation is used in the treatment of conditions and symptoms including but not limited to chronic pain,98,99 movement disorders,100-102 epilepsy,102-104 psychiatric disorders,105,106 stroke,107,108 traumatic brain injury109,110 and sensory deficits.111,112 A variety of devices have been developed to deliver stimulation to the target of interest, with some being invasive such as deep brain stimulation and spinal cord stimulation (SCS) and others noninvasive such as transcranial Direct Current Stimulation (tcDCS), repetitive Transcranial Magnetic Stimulation (rTMS) and peripheral surface electrode Neuromuscular Electrical Stimulation (NMES) or Functional Electrical Stimulation (FES). The mechanism of action of these devices in modulating the nervous system and pathways and is not fully understood, but here we will briefly explore their application in related conditions.
Spinal cord stimulation has become an established therapeutic tool in the management of chronic neuropathic and ischaemic pain syndromes, including Failed Back Surgery Syndrome (FBSS),113,114 Complex Regional Pain Syndrome Type 1115,116 and chronic leg ischaemia. 117 Though the exact mechanism of its analgesic effects is elusive, early proposals included the gate control theory of pain, but more recent biochemical hypotheses propose SCS works by enhancing GABAergic systems of dorsal horn cells by stimulating their dendrites.118,119 There is limited evidence for the use of SCS for pain in DCM but one case study of a cervical spinal cord stimulator placed (C3-C6) for significant post-operative pain following posterior decompression (C5-C7), resulting in a significant reduction in pain. 120 Of note, the efficacy of SCS for SCI-induced pain appears to be more limited when compared to the aforementioned indications, such as FBSS, which may be due to the significant damage to underlying neural circuits required for the analgesic effects of SCS.121,122
Although the use in spinal cord–mediated pain has been limited, epidural SCS has shown to be capable in restoring motor and autonomic function in a small number of chronic complete SCI patients.123-125 It is thought that epidural SCS increases the excitability of the spared spinal cord circuitry within the injury site, leading to enhancement of transmission and volitional control. 126 Whether this strategy could be used for paralysis due to severe DCM remains to be investigated.
Transcranial Magnetic Stimulation (TMS) allows painless, noninvasive, cortical stimulation by means of electromagnetic induction from a coil positioned over the scalp. 127 It serves as a useful electrophysiological diagnostic tool by measuring parameters such as central motor conduction time (CMCT), a sensitive measure to detect myelopathy.128-130 Repeated stimulation in the form of rTMS has gained interest as a method of neuromodulation to induce changes in brain activity lasting beyond the duration of stimulation suggestive of neuroplastic changes. 131 In the clinical sphere, it has gained FDA approval as a treatment for major depressive disorder, obsessive-compulsive disorder and migraine,132-135 with ongoing research in a number of other psychiatric and neurological conditions. 136 Early clinical studies in SCI have found potential application of rTMS in the management of SCI-related pain,137-139 spasticity,140,141 motor function141-143 and autonomic function,144-146 albeit the outcomes across studies were not consistent. This may be attributed to the heterogeneity in rTMS protocols used, and ongoing research is warranted to further optimize existing protocols and to investigate their potential application in DCM. 130
Transcranial direct current stimulation modulates neural activity non-invasively by surface electrodes, which are placed over the scalp with low-intensity currents are passed across them. In contrast to TMS, tcDCS does not induce action potentials but modulates the resting membrane potentials to alter excitability. There is ongoing research investigating its role in a number of psychiatric and neurological conditions. 147 A recent meta-analysis found tcDCS improves upper-limb motor performance in healthy adults. 148 Its utility in chronic incomplete cervical SCI has been investigated in early-stage studies and noted significant improvement in hand grasp149,150 which were synergistic when combined with physical training. 151
Neuromuscular electrical stimulation, also known as FES, is a well-established tool in which surface electrodes are placed over muscles and peripheral nerves to deliver electrical stimulation and achieve muscle contraction. It is used routinely for motor retraining in conditions such as SCI and stroke to restore motor functions such as standing or grasping.152,153 Whilst the artificial stimulus leading to muscle contraction can lead to muscle strengthening, effects have also been demonstrated in the central nervous system with modulation of spinal reflex, corticospinal excitability and neurophysiological changes in the cortex. 154 It is proposed that the underlying mechanism stems not only from the propagation of action potentials leading to muscle contraction but also antidromic propagation of action potential across motor axons and sensory afferents, which traverse to the central nervous system.154,155 Despite extensive research for its use for injuries to the CNS such as SCI, only case reports have been published for its use in DCM, which have demonstrated significant improvement in upper limb function 156 and gait. 157 Whilst these case reports are encouraging, it presents low level of evidence and further research is necessary, especially as NMES is a safe and less inexpensive intervention.
An exciting new clinical trial in SCI is the Up-LIFT study (NCT04697472) that employs transcutaneous spinal stimulation over the injury region. In an open-label study, this method of stimulation was been shown to increase arm and hand function in subjects with chronic cervical SCI. 158 Given the similarities between chronic DCM and SCI, an effect of this methodology might be observed.
Implementation Strategies
A broad overview of contemporary interventions which may have therapeutic utility in DCM is presented in the 3 broad categories of neuroprotection, neuromodulation and neuroregeneration. Whilst specific interventions within these groups may overlap between these broad categories (i.e interventions with regenerative and neuroprotective properties), these categories also provide useful reference for when interventions could be given in the natural course of the disease. Mild DCM may benefit from neuroprotective agents to slow neurological deterioration. In moderate to severe cases, patients may have received surgical decompression and restorative therapies with neuromodulation or neuroregenrative strategies could be beneficial. The effects of these categories of intervention on the natural history of DCM are presented in Figures 1 and 2. Combinations of interventions may also enhance recovery as they can work on different aspects of the pathological and regenerative process’ to reduce neurological injury and enhance recovery. Combinatory strategies have been gaining traction in SCI research and are useful to consider in DCM. 159
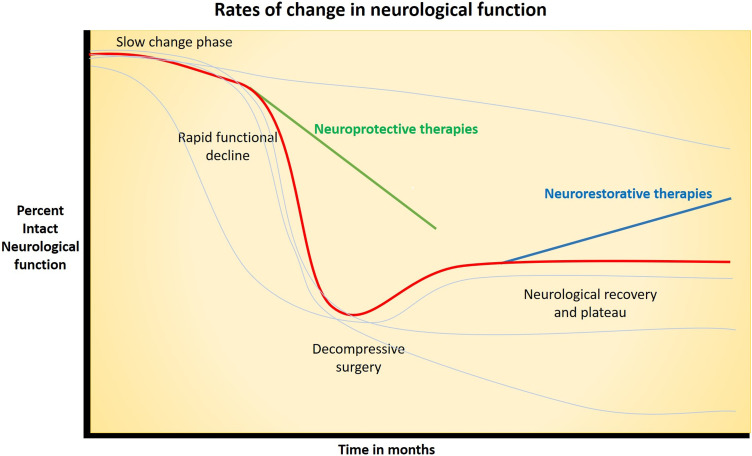
Figure 1.
Graph illustrating simplified natural history of degenerative cervical myelopathy with progressive deterioration of neurological function including slow change phase and rapid functional decline phase with red line. Timepoints in natural history which neuroprotective and neurorestorative intervention can be of therapeutic value in slowing neurological decline and regaining neurological function are highlighted. Paler lines indicate differing natural history which may be experienced by people with degenerative cervical myelopathy, including continuous slow decline with no rapid phase, rapid decline with no slow phase, and after intervention those with no significant improvement in neurology or deterioration.
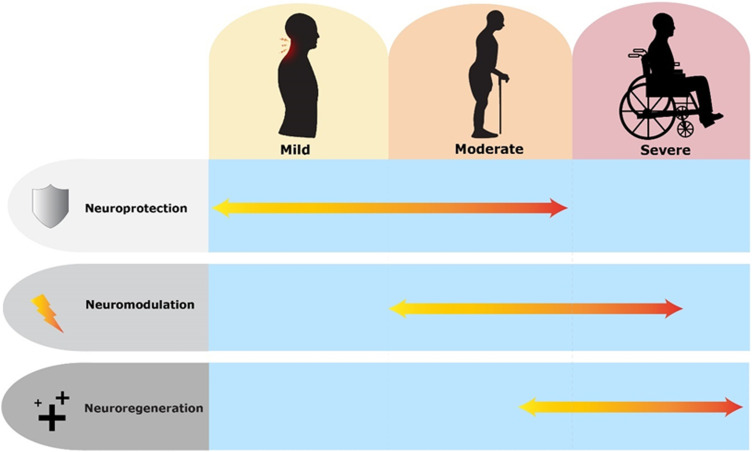
Figure 2.
Timeline of potential application of contemporary interventions (neuroprotection, neuromodulation and neuroregeneration) according to natural history and severity of degenerative cervical myelopathy. In mild-moderate degenerative cervical myelopathy, neuroprotective strategies may prevent/slow down progression by interfering in pathological process. In severe degenerative cervical myelopathy, it is likely that the spinal cord is too damaged for there to be significant improvement gained from neuroprotective strategies. In moderate-severe cases, surgery will remove the focus of compression. At this stage, neuromodulatory strategies may enhance plasticity implicated in the recovery process and neuroregenerative strategies can be considered after the spine has been decompressed if significant neurological damage is present. Graphics produced with support of Myelopathy.org.
The interventions presented are at different stages of development and readiness for clinical testing in adequately powered efficacy studies. Some interventions are quite mature and are currently undergoing phase-III trials (e.g Ibudilast) or completed efficacy trials (e.g Riluzole), whilst other are undergoing early stage pre-clinical investigation (e.g miR21 regulators). Neuroprotective and neuroregenerative trials typically follow the more typical drug development process, whilst neuromodulatory interventions are devices, and are technologically mature and developed, though ongoing research into optimal intervention protocols and utilization in DCM are required. DCM and chronic cervical SCI have considerable pathophysiological overlap, and potential benefit of an intervention in one can be used to gauge potential efficacy in the other, though careful consideration of severity of cord injury and pathophysiological/restorative targets of interventions are required. An example is acute intermittent hypoxia, which has demonstrated efficacy as an adjunct to rehabilitation in improving gait function in subacute incomplete SCI, which may show similar benefit in DCM. 160 Figure 3 demonstrates technological development and readiness of potential interventions for DCM.
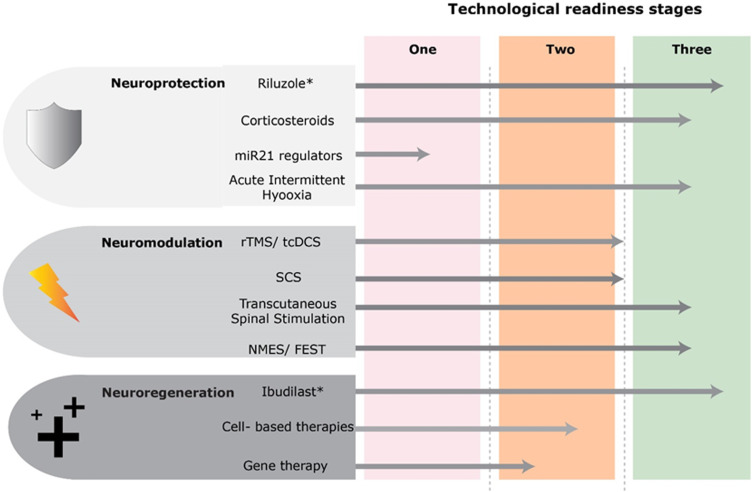
Figure 3.
Technological readiness of a number of contemporary therapies in the 3 broad categories of neuroprotection, neuromodulation and neuroregeneration. Stage 1 indicates therapies that are predominantly in preclinical developmental stage and will require further research at this stage. Stage 2 shows technologies that are developed, though require further early stage testing and exploration of protocols in degenerative cervical myelopathy prior to phase-III efficacy trials. These may have been investigated in chronic cervical SCI population. Stage 3 shows therapies which are mature in development and may be considered for investigation in degenerative cervical myelopathy at an earlier timeframe. Graphics developed with the support of Myelopathy.org. *These therapies have undergone or are currently undergoing phase-III trials.
Conclusion
Therapeutic research in DCM over the last few decades has predominantly focused on the role and timing of surgical decompression. Surgery has shown improved outcomes for DCM patients; however, the majority do not make a full recovery and have a subsequent lifelong disability. The impetus for this article was the newly formed JLA priority setting partnership for DCM, which has determined the research priority and question of whether novel therapies can improve health and wellbeing in people with DCM. We present contemporary therapies in the broad domains of neuroprotection, neuroregeneration and neuromodulation, which may have potential therapeutic utility in DCM. As research in this area has been limited, it is hoped that this review will encourage research into this priority.
Acknowledgements
Further details on this priority, including how it was prioritized, why it was prioritized, and on-going research activity can be found at aospine.org/recode/novel-therapies We would like to thank Myelopathy.org for their time and support in the development of graphics utilized within this article.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research priorities were organized and funded by AO Spine through the AO Spine Knowledge Forum Spinal Cord Injury, a focused group of international Spinal Cord Injury experts. AO Spine is a clinical division of the AO Foundation, which is an independent medically guided not-for-profit organization. Study support was provided directly through the AO Spine Research Department. MRNK is supported by the National Institute for Health Research (NIHR) Brain Injury MedTech Co-operative based at Cambridge University Hospitals NHS Foundation Trust and University of Cambridge, and BMD a NIHR Clinical Doctoral Research Fellowship. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
ORCID iDs
Aref-Ali Gharooni https://orcid.org/0000-0002-6705-1115
Michael G. Fehlings https://orcid.org/0000-0002-5722-6364
Timothy F. Boerger https://orcid.org/0000-0003-1587-3704
Ricardo Rodrigues-Pinto https://orcid.org/0000-0002-6903-348X
Paul Aarne Koljonen https://orcid.org/0000-0002-9250-653X
Jefferson R. Wilson https://orcid.org/0000-0001-5965-0305
Benjamin M. Davies https://orcid.org/0000-0003-0591-5069
James D Guest https://orcid.org/0000-0003-0931-0286
References
- 1.Davies BM, Mowforth OD, Smith EK, Kotter MR. Degenerative cervical myelopathy. BMJ. 2018;360:k186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fehlings MG, Tetreault LA, Riew KD, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J. 2017;7(3 suppl):70s-83s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadaňka Z, Bednařík J, Novotný O, Urbánek I, Dušek L. Cervical spondylotic myelopathy: conservative versus surgical treatment after 10 years. Eur Spine J. 2011;20(9):1533-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee J, Tetreault LA, Chapman JR, et al. Nonoperative versus operative management for the treatment degenerative cervical myelopathy: an updated systematic review. Global Spine J. 2017;7(3 suppl):35S-41S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghobrial GM, Harrop JS. Surgery vs conservative care for cervical spondylotic myelopathy: nonoperative operative management. Neurosurgery. 2015;62(Suppl 1):62-65. [DOI] [PubMed] [Google Scholar]
- 6.Gharooni A-A, Grodzinski B, Davies BM, Kotter MRN. How common is repeat surgery and multi-level treatment in degenerative cervical myelopathy? Findings from a patient perspective survey. J Clin Neurosci. 2020;77:181-184. [DOI] [PubMed] [Google Scholar]
- 7.Fehlings MG, Ibrahim A, Tetreault L, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine. 1976;40(17):1322-1328. [DOI] [PubMed] [Google Scholar]
- 8.Davies B, Mowforth O, Sadler I, et al. Recovery priorities in degenerative cervical myelopathy: a cross-sectional survey of an international, online community of patients. BMJ Open. 2019;9(10):e031486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung WY, Arvinte D, Wong YW, Luk KDK, Cheung KMC. Neurological recovery after surgical decompression in patients with cervical spondylotic myelopathy - a prospective study. Int Orthop. 2008;32(2):273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebl DR, Hughes A, Cammisa FP, Jr., O’Leary PF. Cervical spondylotic myelopathy: pathophysiology, clinical presentation, and treatment. HSS J. 2011;7(2):170-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh T, Lafage R, Lafage V, et al. Comparing quality of life in cervical spondylotic myelopathy with other chronic debilitating diseases using the short form survey 36-health survey. World Neurosurg. 2017;106:699-706. [DOI] [PubMed] [Google Scholar]
- 12.Alliance JL. Degenerative Cervical Myelopathy Top 10 Research Priorities; 2020. https://www.jla.nihr.ac.uk/priority-setting-partnerships/Degenerative-Cervical-Myelopathy/top-10-priorities.htm (Accessed 01/08/2020). [Google Scholar]
- 13.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative Cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine. 2015;40(12):E675-E693. [DOI] [PubMed] [Google Scholar]
- 14.Tetreault L, Goldstein CL, Arnold P, et al. Degenerative cervical myelopathy: a spectrum of related disorders affecting the aging. Neurosurgery. 2015;77(suppl_1):S51-S67. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi S, Mitsuhara T, Abiko M, Takeda M, Kurisu K. Epidemiology and overview of the clinical spectrum of degenerative cervical myelopathy. Neurosurg Clin. 2018;29(1):1-12. [DOI] [PubMed] [Google Scholar]
- 16.Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 suppl):190s-197s. [DOI] [PubMed] [Google Scholar]
- 17.Akter F, Yu X, Qin X, et al. The pathophysiology of degenerative cervical myelopathy and the physiology of recovery following decompression. Front Neurosci. 2020;14(138):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gooding MR, Wilson CB, Hoff JT. Experimental cervical myelopathy: autoradiographic studies of spinal cord blood flow patterns. Surg Neurol. 1976;5(4):233-239. [PubMed] [Google Scholar]
- 19.Gooding MR, Wilson CB, Hoff JT. Experimental cervical myelopathy. J Neurosurg. 1975;43(1):9-17. [DOI] [PubMed] [Google Scholar]
- 20.Yu WR, Liu T, Kiehl TR, Fehlings MG. Human neuropathological and animal model evidence supporting a role for Fas-mediated apoptosis and inflammation in cervical spondylotic myelopathy. Brain. 2011;134(Pt 5):1277-1292. [DOI] [PubMed] [Google Scholar]
- 21.Uchida K, Nakajima H, Watanabe S, et al. Apoptosis of neurons and oligodendrocytes in the spinal cord of spinal hyperostotic mouse (twy/twy): possible pathomechanism of human cervical compressive myelopathy. Eur Spine J. 2012;21(3):490-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito T, Oyanagi K, Takahashi H, Takahashi HE, Ikuta F. Cervical spondylotic myelopathy. Clinicopathologic study on the progression pattern and thin myelinated fibers of the lesions of seven patients examined during complete autopsy. Spine. 1976;21(7):827-833. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, MacMillian EL, Jutzeler CR, et al. Assessing structure and function of myelin in cervical spondylotic myelopathy: evidence of demyelination. Neurology. 2017;89(6):602-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-421. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209(2):378-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domino EF, Unna KR, Kerwin J. Pharmacological properties of Benzazoles I. Relationship between structure and paralyzing action. J Pharmacol Exp Therapeut. 1952;105(4):486-497. [PubMed] [Google Scholar]
- 27.Mizoule J, Meldrum B, Mazadier M, et al. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission--I. Anticonvulsant properties. Neuropharmacology. 1985;24(8):767-773. [DOI] [PubMed] [Google Scholar]
- 28.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2012;3:CD001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz G, Fehlings MG. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: improved behavioral and neuroanatomical recovery with riluzole. J Neurosurg. 2001;94(2 suppl):245-256. [DOI] [PubMed] [Google Scholar]
- 30.Ates O, Cayli SR, Gurses I, et al. Comparative neuroprotective effect of sodium channel blockers after experimental spinal cord injury. J Clin Neurosci. 2007;14(7):658-665. [DOI] [PubMed] [Google Scholar]
- 31.Tetreault LA, Zhu MP, Wilson JR, Karadimas SK, Fehlings MG. The impact of riluzole on neurobehavioral outcomes in preclinical models of traumatic and nontraumatic spinal cord injury: results from a systematic review of the literature. Global Spine J. 2020;10(2):216-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fehlings MG, Wilson JR, Karadimas SK, Arnold PM, Kopjar B. Clinical evaluation of a neuroprotective drug in patients with cervical spondylotic myelopathy undergoing surgical treatment: design and rationale for the CSM-protect trial. Spine. 2013;38(22S):S68-S75. [DOI] [PubMed] [Google Scholar]
- 33.Fehlings MG, Badhiwala JH, Ahn H, et al. Safety and efficacy of riluzole in patients undergoing decompressive surgery for degenerative cervical myelopathy (CSM-Protect): a multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Neurol. 2021;20(2):98-106. [DOI] [PubMed] [Google Scholar]
- 34.Laliberte AM, Karadimas SK, Vidal PM, Satkunendrarajah K, Fehlings MG. Mir21 modulates inflammation and sensorimotor deficits in cervical myelopathy: data from humans and animal models. Brain Communications. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bracken MB, Collins WF, Freeman DF, et al. Efficacy of methylprednisolone in acute spinal cord injury. J Am Med Assoc. 1984;251(1):45-52. [PubMed] [Google Scholar]
- 36.Fehlings MG, Wilson JR, Harrop JS, et al. Efficacy and safety of methylprednisolone sodium succinate in acute spinal cord injury: a systematic review. Global Spine J. 2017;7(3 suppl):116S-137S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. N Engl J Med. 1990;322(20):1405-1411. [DOI] [PubMed] [Google Scholar]
- 38.Fehlings MG, Wilson JR, Tetreault LA, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: recommendations on the use of methylprednisolone sodium succinate. Global Spine J. 2017;7(3 suppl):203s-211s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vidal PM, Ulndreaj A, Badner A, Hong J, Fehlings MG. Methylprednisolone treatment enhances early recovery following surgical decompression for degenerative cervical myelopathy without compromise to the systemic immune system. J Neuroinflammation. 2018;15(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huo X, Zhou J, Liu S, Guo X, Xue Y. Clinical efficacy of single intraoperative 500 mg methylprednisolone management therapy for thoracic myelopathy caused by ossification of the ligamentum flavum. BMC Musculoskelet Disord. 2020;21(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeyamohan SB, Kenning TJ, Petronis KA, Feustel PJ, Drazin D, DiRisio DJ. Effect of steroid use in anterior cervical discectomy and fusion: a randomized controlled trial. J Neurosurg Spine. 2015;23(2):137-143. [DOI] [PubMed] [Google Scholar]
- 42.Cui S, Daffner SD, France JC, Emery SE. The effects of perioperative corticosteroids on dysphagia following surgical procedures involving the anterior cervical spine: a prospective, randomized, controlled, double-blinded clinical trial. J Bone Joint Surg Am. 2019;101(22):2007-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhillon RS, Parker J, Syed YA, et al. Axonal plasticity underpins the functional recovery following surgical decompression in a rat model of cervical spondylotic myelopathy. Acta Neuropathol Commun. 2016;4(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao L, Peng Y, Xu W, et al. Progress in stem cell therapy for spinal cord injury. Stem Cell Int. 2020;2020:2853650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ashammakhi N, Kim H-J, Ehsanipour A, et al. Regenerative therapies for spinal cord injury. Tissue Eng Part B Rev. 2019;25(6):471-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Courtine G, Sofroniew MV. Spinal cord repair: advances in biology and technology. Nat Med. 2019;25(6):898-908. [DOI] [PubMed] [Google Scholar]
- 47.Anderson KD, Guest JD, Dietrich WD, et al. Safety of autologous human schwann cell transplantation in subacute thoracic spinal cord injury. J Neurotrauma. 2017;34(21):2950-2963. [DOI] [PubMed] [Google Scholar]
- 48.Santamaría AJ, Benavides FD, DiFede DL, et al. Clinical and neurophysiological changes after targeted intrathecal injections of bone marrow stem cells in a C3 tetraplegic subject. J Neurotrauma. 2019;36(3):500-516. [DOI] [PubMed] [Google Scholar]
- 49.Rolan P, Gibbons JA, He L, et al. Ibudilast in healthy volunteers: safety, tolerability and pharmacokinetics with single and multiple doses. Br J Clin Pharmacol. 2008;66(6):792-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizuno T, Kurotani T, Komatsu Y, et al. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46(3):404-411. [DOI] [PubMed] [Google Scholar]
- 51.Wakita H, Tomimoto H, Akiguchi I, et al. Ibudilast, a phosphodiesterase inhibitor, protects against white matter damage under chronic cerebral hypoperfusion in the rat. Brain Res. 2003;992(1):53-59. [DOI] [PubMed] [Google Scholar]
- 52.Rolan P, Hutchinson M, Johnson K. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expet Opin Pharmacother. 2009;10(17):2897-2904. [DOI] [PubMed] [Google Scholar]
- 53.Souness JE, Aldous D, Sargent C. Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacology. 2000;47(2):127-162. [DOI] [PubMed] [Google Scholar]
- 54.Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9(1048):1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuyama H, Kimura J, Yarnaguchi S, et al. Pharmacological effects of ibudilast on cerebral circulation: a PET study. Neurol Res. 1993;15(3):169-173. [DOI] [PubMed] [Google Scholar]
- 56.Johnson KW, Matsuda K, Iwaki Y. Ibudilast for the treatment of drug addiction and other neurological conditions. Clin Invest. 2014;4:269-279. [Google Scholar]
- 57.Xu L, Li Y, Sun H, et al. Current developments of macrophage migration inhibitory factor (MIF) inhibitors. Drug Discov Today. 2013;18(11):592-600. [DOI] [PubMed] [Google Scholar]
- 58.Fox RJ, Coffey CS, Conwit R, et al. Phase 2 trial of ibudilast in progressive multiple sclerosis. N Engl J Med. 2018;379(9):846-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naismith RT, Bermel RA, Coffey CS, et al. Effects of ibudilast on MRI measures in the phase 2 SPRINT-MS study. Neurology. 2020;96:e491-e500. doi: 10.1212/WNL.0000000000011314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oskarsson B, Dojillo J, Makhay M, Matsuda K. COMBAT-ALS Phase 2b/3 Trial of MN-166 (Ibudilast) in ALS: study design and trial update (5149). Neurology. 2020;94(15 suppl):5149. [Google Scholar]
- 61.Ray LA, Bujarski S, Shoptaw S, Roche DJ, Heinzerling K, Miotto K. Development of the neuroimmune modulator ibudilast for the treatment of alcoholism: a randomized, placebo-controlled, human laboratory trial. Neuropsychopharmacology. 2017;42(9):1776-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burnette EM, Baskerville WA, Grodin EN, Ray LA. Ibudilast for alcohol use disorder: study protocol for a phase II randomized clinical trial. Trials. 2020;21:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heinzerling KG, Briones M, Thames AD, et al. Randomized, placebo-controlled trial of targeting neuroinflammation with ibudilast to treat methamphetamine use disorder. J Neuroimmune Pharmacol. 2020;15(2):238-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Metz VE, Jones JD, Manubay J, et al. Effects of ibudilast on the subjective, reinforcing, and analgesic effects of oxycodone in recently detoxified adults with opioid dependence. Neuropsychopharmacology. 2017;42(9):1825-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16(7):935-950. [DOI] [PubMed] [Google Scholar]
- 66.Kwok YH, Swift JE, Gazerani P, Rolan P. A double-blind, randomized, placebo-controlled pilot trial to determine the efficacy and safety of ibudilast, a potential glial attenuator, in chronic migraine. J Pain Res. 2016;9:899-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rowland JW, Hawryluk GWJ, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5):E2. [DOI] [PubMed] [Google Scholar]
- 69.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813-1826. [DOI] [PubMed] [Google Scholar]
- 70.Thomas ED, Lochte HL, Jr., Cannon JH, Sahler OD, Ferrebee JW. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest. 1959;38(10 Pt 1-2):1709-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahuja CS, Mothe A, Khazaei M, et al. The leading edge: emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl Med. 2020;9(12):1509-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riley J, Glass J, Feldman EL, et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I trial, cervical microinjection, and final surgical safety outcomes. Neurosurgery. 2014;74(1):77-87. [DOI] [PubMed] [Google Scholar]
- 73.Manley NC, Priest CA, Denham J, Wirth ED, 3rd, Lebkowski JS. Human embryonic stem cell-derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Transl Med. 2017;6(10):1917-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao J, Sun W, Cho HM, et al. Integration and long distance axonal regeneration in the central nervous system from transplanted primitive neural stem cells. J Biol Chem. 2013;288(1):164-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu P. Chapter 1 - Stem cell transplantation for spinal cord injury repair. In: Dunnett SB, Björklund A, eds Progress in Brain Research. Elsevier; 2017; 231; 1-32. [DOI] [PubMed] [Google Scholar]
- 76.Pandamooz S, Salehi MS, Zibaii MI, Ahmadiani A, Nabiuni M, Dargahi L. Epidermal neural crest stem cell-derived glia enhance neurotrophic elements in an ex vivo model of spinal cord injury. J Cell Biochem. 2018;119(4):3486-3496. [DOI] [PubMed] [Google Scholar]
- 77.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181(2):115-129. [DOI] [PubMed] [Google Scholar]
- 78.Li Z, Guo G-H, Wang G-S, Guan C-X, Yue L. Influence of neural stem cell transplantation on angiogenesis in rats with spinal cord injury. Genet Mol Res. 2014;13(3):6083-6092. [DOI] [PubMed] [Google Scholar]
- 79.Gu C, Li H, Wang C, et al. Bone marrow mesenchymal stem cells decrease CHOP expression and neuronal apoptosis after spinal cord injury. Neurosci Lett. 2017;636:282-289. [DOI] [PubMed] [Google Scholar]
- 80.Nicola Fd. C, Marques MR, Odorcyk F, et al. Neuroprotector effect of stem cells from human exfoliated deciduous teeth transplanted after traumatic spinal cord injury involves inhibition of early neuronal apoptosis. Brain Res. 2017;1663:95-105. [DOI] [PubMed] [Google Scholar]
- 81.Cheng Z, Zhu W, Cao K, et al. Anti-inflammatory mechanism of neural stem cell transplantation in spinal cord injury. Int J Mol Sci. 2016;17(9):1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng Z, Bosco DB, Sun L, et al. Neural stem cell-conditioned medium suppresses inflammation and promotes spinal cord injury recovery. Cell Transplant. 2017;26(3):469-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cofano F, Boido M, Monticelli M, et al. Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci. 2019;20(11):2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Willison AG, Smith S, Davies BM, Kotter MRN, Barnett SC. A scoping review of trials for cell-based therapies in human spinal cord injury. Spinal Cord. 2020;58(8):844-856. [DOI] [PubMed] [Google Scholar]
- 85.Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20(5):637-647. [DOI] [PubMed] [Google Scholar]
- 86.Zweckberger K, Ahuja CS, Liu Y, Wang J, Fehlings MG. Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury. Acta Biomater. 2016;42:77-89. [DOI] [PubMed] [Google Scholar]
- 87.Binch ALA, Fitzgerald JC, Growney EA, Barry F. Cell-based strategies for IVD repair: clinical progress and translational obstacles. Nat Rev Rheumatol. 2021. [DOI] [PubMed] [Google Scholar]
- 88.Bae HW, Amirdelfan K, Coric D, et al. A phase II study demonstrating efficacy and safety of mesenchymal precursor cells in low back pain due to disc degeneration. Spine J. 2014;14(11):S31-S32. [Google Scholar]
- 89.Noriega DC, Ardura F, Hernández-Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101(8):1945-1951. [DOI] [PubMed] [Google Scholar]
- 90.Pettine KA, Suzuki RK, Sand TT, Murphy MB. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int Orthop. 2017;41(10):2097-2103. [DOI] [PubMed] [Google Scholar]
- 91.Elabd C, Centeno CJ, Schultz JR, Lutz G, Ichim T, Silva FJ. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med. 2016;14(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7(1):30-40. [DOI] [PubMed] [Google Scholar]
- 93.Rosenzweig ES, Salegio EA, Liang JJ, et al. Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nat Neurosci. 2019;22(8):1269-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.James ND, Shea J, Muir EM, Verhaagen J, Schneider BL, Bradbury EJ. Chondroitinase gene therapy improves upper limb function following cervical contusion injury. Exp Neurol. 2015;271:131-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Zaidy SA, Kolb SJ, Lowes L, et al. AVXS-101 (Onasemnogene Abeparvovec) for SMA1: comparative study with a prospective natural history cohort. J Neuromuscul Dis. 2019;6(3):307-317. [DOI] [PubMed] [Google Scholar]
- 96.Hagg A, Kharoud S, Goodchild G, et al. TMEPAI/PMEPA1 Is a positive regulator of skeletal muscle mass. Front Physiol. 2020;11(1420):560225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.International Neuromodulation Society . https://www.neuromodulation.com/. https://www.neuromodulation.com/.Accessed 23/01/2021
- 98.Hofmeister M, Memedovich A, Brown S, et al. Effectiveness of neurostimulation technologies for the management of chronic pain: a systematic review. Neuromodulation. 2020;23(2):150-157. [DOI] [PubMed] [Google Scholar]
- 99.Fishman MA, Antony A, Esposito M, Deer T, Levy R. The evolution of neuromodulation in the treatment of chronic pain: forward-looking perspectives. Pain Med. 2019;20(suppl 1):S58-S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dallapiazza R, McKisic MS, Shah B, Elias WJ. Neuromodulation for movement disorders. Neurosurg Clin N Am. 2014;25(1):47-58. [DOI] [PubMed] [Google Scholar]
- 101.Bledsoe IO, Viser AC, San Luciano M. Treatment of dystonia: medications, neurotoxins, neuromodulation, and rehabilitation. Neurotherapeutics; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pereira EA, Green AL, Nandi D, Aziz TZ. Deep brain stimulation: indications and evidence. Expert Rev Med Devices. 2007;4(5):591-603. [DOI] [PubMed] [Google Scholar]
- 103.Sisterson ND, Kokkinos V. Neuromodulation of epilepsy networks. Neurosurg Clin. 2020;31(3):459-470. [DOI] [PubMed] [Google Scholar]
- 104.Kwon C-S, Ripa V, Al-Awar O, Panov F, Ghatan S, Jetté N. Epilepsy and neuromodulation—randomized controlled trials. Brain Sci. 2018;8(4):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ishii R, Nishida K, Youssef NA, Jann K, Takahashi S. Editorial: neuromodulation in basic, translational and clinical research in psychiatry. Front Hum Neurosci. 2019;13(438):438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sonmez AI, Camsari DD, Nandakumar AL, et al. Accelerated TMS for depression: a systematic review and meta-analysis. Psychiatry Res. 2019;273:770-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiang C-F, Lin M-T, Hsiao M-Y, Yeh Y-C, Liang Y-C, Wang T-G. Comparative efficacy of noninvasive neurostimulation therapies for acute and subacute poststroke dysphagia: a systematic review and network meta-analysis. Arch Phys Med Rehabil. 2019;100(4):739-750. [DOI] [PubMed] [Google Scholar]
- 108.Dukelow S, Kirton A. Enhancing stroke recovery across the life span with noninvasive neurostimulation. J Clin Neurophysiol. 2020;37(2):150-163. [DOI] [PubMed] [Google Scholar]
- 109.Buhagiar F, Fitzgerald M, Bell J, Allanson F, Pestell C. Neuromodulation for mild traumatic brain injury rehabilitation: a systematic review. Front Hum Neurosci. 2020;14(554):598208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Bernabeu M, Tormos JM, Pascual-Leone A. Noninvasive brain stimulation in traumatic brain injury. J Head Trauma Rehabil. 2012;27(4):274-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gall C, Schmidt S, Schittkowski MP, et al. Alternating current stimulation for vision restoration after optic nerve damage: a randomized clinical trial. PLoS One. 2016;11(6):e0156134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hoare DJ, Adjamian P, Sereda M. Electrical stimulation of the ear, head, cranial nerve, or cortex for the treatment of tinnitus: a scoping review. Neural Plast. 2016;2016:5130503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63(4):762-770. discussion 770. [DOI] [PubMed] [Google Scholar]
- 114.Kapural L, Peterson E, Provenzano DA, Staats P. Clinical evidence for spinal cord stimulation for failed back surgery syndrome (FBSS): systematic review. Spine. 1976;42(Suppl 14):S61-S66. [DOI] [PubMed] [Google Scholar]
- 115.Taylor RS, Buyten J-P, Buchser E. Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10(2):91. [DOI] [PubMed] [Google Scholar]
- 116.Moore DM, McCrory C. Spinal cord stimulation. BJA Education. 2016;16(8):258-263. [Google Scholar]
- 117.Ubbink DT, Vermeulen H. Spinal cord stimulation for non‐reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev. 2003;3:CD004001. [DOI] [PubMed] [Google Scholar]
- 118.Jensen MP, Brownstone RM. Mechanisms of spinal cord stimulation for the treatment of pain: still in the dark after 50 years. Eur J Pain. 2019;23(4):652-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971-978. [DOI] [PubMed] [Google Scholar]
- 120.Lawson McLean A, Kalff R, Reichart R. Spinal cord stimulation for acute pain following surgery for cervical myelopathy: a novel treatment strategy. Pain Pract. 2019;19(3):310-315. [DOI] [PubMed] [Google Scholar]
- 121.Huang Q, Duan W, Sivanesan E, et al. Spinal cord stimulation for pain treatment after spinal cord injury. Neurosci Bull. 2019;35(3):527-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chari A, Hentall ID, Papadopoulos MC, Pereira EA. Surgical neurostimulation for spinal cord injury. Brain Sci. 2017;7(2):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harkema S, Gerasimenko Y, Hodes J, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781):1938-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gill ML, Grahn PJ, Calvert JS, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24(11):1677-1682. [DOI] [PubMed] [Google Scholar]
- 125.Grahn PJ, Lavrov IA, Sayenko DG, et al. Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin Proc. 2017;92(4):544-554. [DOI] [PubMed] [Google Scholar]
- 126.Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137(5):1394-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;325(8437):1106-1107. [DOI] [PubMed] [Google Scholar]
- 128.Chen R, Cros D, Curra A, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119(3):504-532. [DOI] [PubMed] [Google Scholar]
- 129.Deftereos SN, Kechagias E, Ioakeimidou C, Georgonikou D. Transcranial magnetic stimulation but not MRI predicts long-term clinical status in cervical spondylosis: a case series. Spinal Cord. 2015;53(1):S16-S18. [DOI] [PubMed] [Google Scholar]
- 130.Gharooni A-A, Khan M, Yang X, Anwar F, Davies B, Kotter M. Therapeutic repetitive transcranial magnetic stimulation (rTMS) for neurological dysfunction in degenerative cervical myelopathy: an unexplored opportunity? Findings from a systematic review. J Clin Neurosci. 2021;90:76-81. [DOI] [PubMed] [Google Scholar]
- 131.Klomjai W, Katz R, Lackmy-Vallée A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Annals of Physical and Rehabilitation Medicine. 2015;58(4):208-213. [DOI] [PubMed] [Google Scholar]
- 132.McClintock SM, Reti IM, Carpenter LL, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1):16cs10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.FDA . FDA Permits Marketing of Transcranial Magnetic Stimulation for Treatment of Obsessive Compulsive Disorder. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-transcranial-magnetic-stimulation-treatment-obsessive-compulsive-disorder. Accessed 23/01/2021. [Google Scholar]
- 134.Trevizol AP, Shiozawa P, Cook IA, et al. Transcranial magnetic stimulation for obsessive-compulsive disorder: an updated systematic review and meta-analysis. J Ect. 2016;32(4):262-266. [DOI] [PubMed] [Google Scholar]
- 135.Lan L, Zhang X, Li X, Rong X, Peng Y. The efficacy of transcranial magnetic stimulation on migraine: a meta-analysis of randomized controlled trails. J Headache Pain. 2017;18(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lefaucheur J-P, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol. 2020;131(2):474-528. [DOI] [PubMed] [Google Scholar]
- 137.Yılmaz B, Kesikburun S, Yaşar E, Tan AK. The effect of repetitive transcranial magnetic stimulation on refractory neuropathic pain in spinal cord injury. J Spinal Cord Med. 2014;37(4):397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Defrin R, Grunhaus L, Zamir D, Zeilig G. The effect of a series of repetitive transcranial magnetic stimulations of the motor cortex on central pain after spinal cord injury. Arch Phys Med Rehabil. 2007;88(12):1574-1580. [DOI] [PubMed] [Google Scholar]
- 139.Kang BS, Shin HI, Bang MS. Effect of repetitive transcranial magnetic stimulation over the hand motor cortical area on central pain after spinal cord injury. Arch Phys Med Rehabil. 2009;90(10):1766-1771. [DOI] [PubMed] [Google Scholar]
- 140.Gharooni A-A, Nair KPS, Hawkins D, Scivill I, Hind D, Hariharan R. Intermittent theta-burst stimulation for upper-limb dysfunction and spasticity in spinal cord injury: a single-blind randomized feasibility study. Spinal Cord. 2018;56(8):762-768. [DOI] [PubMed] [Google Scholar]
- 141.Nardone R, Langthaler PB, Orioli A, et al. Effects of intermittent theta burst stimulation on spasticity after spinal cord injury. Restor Neurol Neurosci. 2017;35:287-294. [DOI] [PubMed] [Google Scholar]
- 142.Alexeeva N, Calancie B. Efficacy of QuadroPulse rTMS for improving motor function after spinal cord injury: three case studies. J Spinal Cord Med. 2016;39(1):50-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gunduz A, Rothwell J, Vidal J, Kumru H. Non-invasive brain stimulation to promote motor and functional recovery following spinal cord injury. Neural Regeneration Research. 2017;12(12):1933-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kuppuswamy A, Balasubramaniam AV, Maksimovic R, et al. Action of 5 Hz repetitive transcranial magnetic stimulation on sensory, motor and autonomic function in human spinal cord injury. Clin Neurophysiol. 2011;122(12):2452-2461. [DOI] [PubMed] [Google Scholar]
- 145.Niu T, Bennett CJ, Keller TL, Leiter JC, Lu DC. A proof-of-concept study of transcutaneous magnetic spinal cord stimulation for neurogenic bladder. Sci Rep. 2018;8(1):12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vasquez N, Balasubramaniam V, Kuppuswamy A, et al. The interaction of cortico-spinal pathways and sacral sphincter reflexes in subjects with incomplete spinal cord injury: a pilot study. Neurourol Urodyn. 2015;34(4):349-355. [DOI] [PubMed] [Google Scholar]
- 147.Fregni F, El-Hagrassy MM, Pacheco-Barrios K, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation (tDCS) in neurological and psychiatric disorders. Int J Neuropsychopharmacol; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patel R, Ashcroft J, Patel A, et al. The impact of transcranial direct current stimulation on upper-limb motor performance in healthy adults: a systematic review and meta-analysis. Front Neurosci. 2019;13(1213):1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cortes M, Medeiros AH, Gandhi A, et al. Improved grasp function with transcranial direct current stimulation in chronic spinal cord injury. NeuroRehabilitation. 2017;41:51-59. [DOI] [PubMed] [Google Scholar]
- 150.Yozbatiran N, Keser Z, Davis M, et al. Transcranial direct current stimulation (tDCS) of the primary motor cortex and robot-assisted arm training in chronic incomplete cervical spinal cord injury: a proof of concept sham-randomized clinical study. NeuroRehabilitation. 2016;39(3):401-411. [DOI] [PubMed] [Google Scholar]
- 151.Potter-Baker KA, Janini DP, Lin Y-L, et al. Transcranial direct current stimulation (tDCS) paired with massed practice training to promote adaptive plasticity and motor recovery in chronic incomplete tetraplegia: a pilot study. J Spinal Cord Med. 2018;41(5):503-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Howlett OA, Lannin NA, Ada L, McKinstry C. Functional electrical stimulation improves activity after stroke: a systematic review with meta-analysis. Arch Phys Med Rehabil. 2015;96(5):934-943. [DOI] [PubMed] [Google Scholar]
- 153.Marquez-Chin C, Popovic MR. Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: a review. Biomed Eng Online. 2020;19(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Milosevic M, Marquez-Chin C, Masani K, et al. Why brain-controlled neuroprosthetics matter: mechanisms underlying electrical stimulation of muscles and nerves in rehabilitation. Biomed Eng Online. 2020;19(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nagai MK, Marquez-Chin C, Popovic MR. Why is functional electrical stimulation therapy capable of restoring motor function following severe injury to the central nervous system? In: Tuszynski MH, ed. Translational Neuroscience: Fundamental Approaches for Neurological Disorders. Boston, MA: Springer US; 2016; 479-498. [Google Scholar]
- 156.Popovic MR, Zivanovic V, Valiante TA. Restoration of upper limb function in an individual with cervical spondylotic myelopathy using functional electrical stimulation therapy: a case study. Front Neurol. 2016;7(81):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pastor D. Use of electrical stimulation and exercise to increase muscle strength in a patient after surgery for cervical spondylotic myelopathy. Physiother Theory Pract. 2010;26(2):134-142. [DOI] [PubMed] [Google Scholar]
- 158.Inanici F, Brighton LN, Samejima S, Hofstetter CP, Moritz CT. Transcutaneous spinal cord stimulation restores hand and arm function after spinal cord injury. IEEE Trans Neural Syst Rehabil Eng. 2021. [DOI] [PubMed] [Google Scholar]
- 159.Griffin JM, Bradke F. Therapeutic repair for spinal cord injury: combinatory approaches to address a multifaceted problem. EMBO Mol Med. 2020;12(3):e11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Navarrete-Opazo A, Alcayaga J, Sepúlveda O, Rojas E, Astudillo C. Repetitive intermittent hypoxia and locomotor training enhances walking function in incomplete spinal cord injury subjects: a randomized, triple-blind, placebo-controlled clinical trial. J Neurotrauma. 2017;34(9):1803-1812. [DOI] [PubMed] [Google Scholar]