Abstract
Study Design
Literature Review (Narrative).
Objective
To introduce the number 10 research priority for Degenerative Cervical Myelopathy: Individualizing Surgery.
Methods
This article summarizes the current recommendations and indications for surgery, including how known prognostic factors such as injury time, age, disease severity, and associated comorbidities impact surgical outcome. It also considers key areas of uncertainty that should be the focus of future research.
Results
While a small proportion of conservatively managed patients may remain stable, the majority will deteriorate over time. To date, surgical decompression is the mainstay of treatment, able to halt disease progression and improve neurologic function and quality of life for most patients. Whilst this recognition has led to recommendations on when to offer surgery, there remain many uncertainties including the type of surgery, or timing in milder and/or asymptomatic cases. Their clarification has the potential to transform outcomes, by ensuring surgery offers each individual its maximum benefit.
Conclusion
Developing the evidence to better guide surgical decision-making at the individual patient level is a research priority for Degenerative Cervical Myelopathy.
Keywords: degenerative cervical myelopathy, cervical spine, cervical spondylotic myelopathy, myelopathy, spondylosis, nontraumatic spinal cord injury
Introduction
Degenerative Cervical Myelopathy (DCM) refers to symptomatic dysfunction of the spinal cord resulting from acquired stenosis of the cervical spinal canal secondary to degenerative changes in several spinal structures. These include spondylosis, disc herniation, and facet arthropathy (collectively referred to as cervical spondylotic myelopathy) or ligamentous abnormalities, ossification of the posterior longitudinal ligament (OPLL), and hypertrophy of the ligamentum flavum. With an estimated annual incidence of 41 per million in North America, DCM is the most common cause of spinal cord dysfunction worldwide. 1 It is characterized by a triad of symptoms-gait imbalance, loss of hand dexterity, and sphincter dysfunction. 2 Other symptoms are neck pain or stiffness, upper limb pain, weakness and paresthesias, lower limb stiffness, weakness, or numbness.
Over the last several decades, our knowledge of the natural history of DCM and the understanding of the benefits of surgical intervention has evolved. As the literature has become more sophisticated, multiple sources of information have confirmed that patients with DCM treated non-operatively may decline and lose their clinical and functional status. In a seminal study published in 1956, Clarke and Robinson analyzed 120 patients with DCM and characterized the natural history of the disease as consisting of a progressive stepwise functional decline in 75% of patients; a slow, steady decline in 20% of cases; and a rapid onset of symptoms followed by a long period of remission (up to 14 years) in 5% of patients. 3 More recent evidence shows that while a small proportion of patients may remain stable over the years, the majority will deteriorate if left untreated. A 2013 systematic review found that 20–62% of DCM patients treated non-operatively will exhibit clinical deterioration at 3–6 years. 4 Data from other studies indicates that 23–54% of patients treated non-operatively will require decompression at a mean of 29–74 months5-7 due to progression of the disease. Furthermore, recent evidence shows that surgery for DCM is associated with improved function and quality of life. A prospective AO Spine North America study including 278 patients with mild, moderate, and severe DCM found a significant improvement in the modified Japanese Orthopaedic (mJOA) score, Nurick grade, Neck Disability Index (NDI), and all SF-36v2 subscales at 1 year after surgery and the degree of improvement was independent of the severity of the pre-operative symptoms (except for the mJOA score, in which patients with more severe symptoms improved the most). 8 This finding was later confirmed in a global study including 479 DCM patients from 16 centers across the world with a 24 -month follow-up, with varying degrees of disease severity and etiology and with the surgical approach being left to the discretion of the surgeon, in which surgery was found to improve functional status and quality of life with a neurologic complication rate of 3.13%. 9 Considered together, this evidence indicates that while not all patients with DCM will require surgery, surgery can not only halt disease progression but also potentially improve patient neurologic function and quality of life.
AO Spine RECODE-DCM (aospine.org/recode) [REsearch objectives and COmmon Data Elements for DCM] is an international consensus initiative, which aims to accelerate knowledge discovery that can improve outcomes by developing a set of research tools. 10 It included a James Lind Alliance research priority setting partnership, which brought together individuals working on and individuals living with DCM, to establish the most important unanswered questions. Individualizing surgery as defined by the questions “Are there clinical and imaging factors that can help a surgeon select who should undergo surgical decompression in the setting of DCM? At what stage of the disease is surgery the preferred management strategy?” was defined as research priority number 10.
The objective of this paper was to perform a narrative literature review to (1) identify factors to assist surgeons in selecting who should undergo surgical decompression in the setting of DCM and (2) at what stage of the disease is surgery the preferred management strategy.
Current Recommendations for Surgery
A frequently used system to categorize and characterize the severity of DCM is the mJOA score. This is an 18-point ordinal score used to evaluate upper extremity motor function (5 points), lower extremity motor function (7 points), sensation (3 points), and micturition (3 points). A maximum score of 18 points occurs in the absence of neurologic impairment, and a lower score indicates a greater degree of disability and neurological impairment. Patients are classified into mild (15–17 points), moderate (11–14 points), and severe (≤11 points) DCM. 11
In 2017, Fehlings et al. 12 developed 5 evidence-informed medical guidelines following the GRADE process based on the (1) natural history of DCM; (2) risk factors for disease progression; (3) efficacy, effectiveness, and safety of non-operative and surgical management; (4) impact of pre-operative duration of symptoms and myelopathy severity on treatment outcomes and (5) frequency, timing, and predictors of symptom development. 12 These guidelines produced clinical recommendations for the management of this disease (Table 1).
Table 1.
Clinical Practice Guideline for the Management of Patients with Degenerative Cervical Myelopathy.
| DCM Severity | Recommendation | Quality of Evidence | Strength of Recommendation |
|---|---|---|---|
| Severe DCM (mJOA score ≤11) | Surgical intervention | Moderate | Strong |
| Moderate DMC (mJOA score 12–14) | Surgical intervention | Moderate | Strong |
| Mild DCM (mJOA score 15–17) | Surgical intervention or a supervised trial of structured rehabilitation. If non-operative management fails or patients worsen, surgical intervention should be offered | Weak | Very low to low |
| Non-myelopathic patients with imaging evidence of cervical spinal cord compression and without symptoms of radiculopathy | Counseling on potential risks of progression, educating about relevant signs and symptoms of myelopathy and clinical observation a | Weak | No identified evidence; based on clinical expert opinion |
| Non-myelopathic patients with imaging evidence of cervical spinal cord compression and with symptoms of radiculopathy | Surgical intervention or non-operative treatment consisting of close serial follow-up or a supervised trial of structured rehabilitation a | Weak | Low |
1. Fehlings MG, Tetreault LA, Riew KD et al. A Clinical Practice Guideline for the Management of Patients With Degenerative Cervical Myelopathy: Recommendations for Patients With Mild, Moderate, and Severe Disease and Non-myelopathic Patients With Evidence of Cord Compression. Global Spine J 2017; 7: 70S-83S DOI: 10.1177/2192568217701914
aIf myelopathic symptoms develop patients should be managed according to DCM severity.
According to these guidelines, surgical intervention should be performed on patients with moderate and severe DCM and those with progressive disease. 12 Patients with severe DCM had improvements in JOA/mJOA, NDI, Nurick, and VAS scores at short, medium, and long-term follow-up. The cumulative incidence of adverse events was 14.1%, with the most frequent being axial pain (5.6%), laryngeal nerve injury/dysphagia (2.2%), instrumentation/graft complication (2.0%) and C5 radiculopathy or palsy (1.9%) and low pooled cumulative incidences of dural tear/cerebrospinal fluid leak, worsening of myelopathy, death, pseudoarthrosis, and implant complications. This systematic review identified only 1 study that reported on surgery for moderate DCM patients. 8 According to that study, surgery was associated with significant improvements and minimal clinically important differences in mJOA, NDI, and Nurick scores at short, medium, and long-term follow-up. The rate of surgical complications in patients with moderate DCM such as C5 radiculopathy or palsy, infection, dural tear or cerebrospinal fluid leak, worsening of myelopathy, death, pseudoarthrosis, and implant complications was low or very low. For patients with mild DCM, however, the evidence gathered was deemed to have less rigor with the majority of the identified studies being retrospective case series with only one low-quality randomized controlled trial analyzing conservative treatment and only 1 study analyzing surgical treatment and stratifying patients according to DCM severity.13,14 As such, the guidelines recommended offering surgical treatment or a trial of supervised rehabilitation in patients with mild myelopathy. In the event of clinical deterioration during the course of conservative treatment, the authors strongly recommended that surgical treatment should be offered expeditiously. For non-myelopathic patients with imaging evidence of spinal cord compression (asymptomatic cervical stenosis) but without symptoms of radiculopathy, and since only 22.6% of patients develop myelopathy 15 the guidelines recommend serial observation and not offering prophylactic surgery. The presence of clinical and/or electrophysiological evidence of radiculopathy, however, should be a clinical predictor of early (≤12 months) surgical intervention. 15 This recommendation should be a shared decision between the surgeon and the patient. Patients should be offered either a trial of non-operative treatment or surgery; again, in the event of clinical deterioration, patients should be counseled and offered surgical treatment.
These guidelines supported that surgery was effective in improving function in patients with severe and moderate DCM, but the evidence gathered for patients with mild DCM had less quality, and the recommendations were therefore weaker. Since then, additional studies have investigated the impact of surgery in mild DCM. Badhiwala et al. 16 concluded from an analysis of 193 patients with mild DCM, that DCM patients had significant impairment of their baseline quality of life compared to the normal population, which was greatest for social and physical functioning and mental health. Two years after surgery, the same patients exhibited significant improvement in their status, as assessed by the mJOA, NDI, and Physical and Mental Health SF-36 sub-scores. 16 Unfortunately, high-quality randomized controlled trials addressing this specific population of DCM patients are lacking; there is considerably more evidence concerning severe and moderate than mild DCM. However, it appears that DCM patients are significantly impaired by their disease and that surgery is associated with improvement of quality of life with minimal complications.
Prognostic Factors
While effective in halting disease progression, surgical treatment outcomes are still difficult to predict, with some patients showing a significant improvement in clinical scores after surgery and others showing little improvement after surgery. However, some evidence exists that clinical, imaging, and electrophysiological data may help predict surgical outcomes and aid in decision-making.
Timing of Surgery
Timing of intervention is one of the most critical aspects of managing DCM. Since DCM is a progressive injury to the spinal cord, patients with a longer duration of symptoms may already have a burden of irreversible spinal cord injury (ischemia, neuroinflammation, demyelination, and apoptosis of neurons and oligodendrocytes 17 ), therefore hindering symptomatic improvement with surgery. 18 While no definitive cutoff has been established, several authors have found lower JOA recovery rates, 19 lower minimum clinically important differences in mJOA scores,20,21 and less improvements in Nurick score22,23 when patients were operated on after 12 months from the onset of symptoms.
Age
There is little consensus as to whether age is a predictor of clinical outcome after surgery for DCM. In a systematic review, Tetreault and colleagues found no correlation between age and clinical outcomes, as assessed by the mJOA and Nurick scores. 24 Other studies, however, such as a prospective multi-center AO Spine International study that included 479 patients 25 report that patients greater than 65 years old, while having significant benefit with surgery, had lower mJOA and Nurick scores 24 months after surgery. Frailty, which refers to a loss of reserves (energy, physical ability, cognition, health) is a better assessment of a patient’s physiological age and recent studies have shown to be a better predictor of mortality, perioperative complications and functional outcome than age.26,27
Importantly, elderly/frail patients still benefit and should be considered candidates for surgery, but the benefit may not be as relevant as in younger/less frail patients. There are several potential explanations for the reduced functional recovery in older patients: (1) DCM is usually a degenerative disorder, and older patients may have more substantial degenerative changes, requiring a more complex surgery; (2) Older patients often have more associated comorbidities which may increase the rate of medical complications after surgery; (3) Older patients may have other musculoskeletal comorbidities (osteoarthritis) which may impact their clinical scores, such as walking distance, regardless of the neurologic recovery after DCM surgery.
In conclusion, frailty, more than age, may impact on the mortality, perioperative complications and functional outcome after DCM surgery. This should be taken into account when proposing patients to surgery.
Baseline Disease Severity
Pre-operative DCM severity is a predictor of surgical outcomes. In a study of 145 patients who underwent ACCF and followed for 5 years, those with initial JOA scores ≤ 9 were 4.85 times more likely to have less than a 50% recovery rate than those with JOA scores above 9. 28 In two other studies that analyzed 45 29 and 64 30 patients submitted to laminoplasty, the authors also found that higher JOA scores (together with younger age) were also associated with improved surgical outcomes. Tetreault and colleagues 31 proposed that a mJOA score of 12 as a threshold below which patients are expected to have less improvement with surgery. 31
Comorbidities
Several studies have analyzed the influence of patient comorbidities on postoperative outcomes, with diabetes being the most frequently reported. Lower postoperative scores have been found in diabetic patients compared to non-diabetic, 32 and also in diabetic patients diagnosed longer than 10 years previously and in patients with HbA1c levels ≥ 6.5%. 19 Additionally, in an analysis of 113 DCM patients, Kusin et al. 33 identified a strong correlation between higher perioperative glucose levels and lower postoperative Nurick scores, suggesting that high glucose levels indicate greater morbidity. 33 Smoking is another comorbidity that has been identified as a prognostic factor, and studies suggest that smoking status is associated with lower postoperative Nurick and mJOA scores.20,34 In isolation, comorbidities such as kidney disease, respiratory, rheumatologic cardiac, and psychiatric diseases have not shown an association with postoperative clinical scores.19,20
Imaging
Imaging studies are critical for diagnosing and planning DCM, and to predict prognosis after surgery. MRI is almost universally used to assess the degree and anatomic location of spinal cord compression. A region of intramedullary hyperintensity seen on T2-weighted images is identified in 58-85% of DCM patients and was correlated with clinical impairment. 35 The T2 signal hyperintensity in established DCM commonly has a clear border, representing tissue loss and gliosis, which is associated with a more limited surgical outcome.35,36 In more acute and rapidly progressing cases, the T2 hyperintensity may be difficult to discern and not well-circumscribed, indicating edema and evolving Wallerian degeneration, which may be reversible with surgery. Hypointensity on T1-weighted images is associated with worse baseline neurologic impairment and more permanent irreversible injury, even with surgery.37,38
Standing radiographs are used to evaluate cervical spine alignment, whereas flexion-extension X-rays can be used to assess instability, which may cause dynamic spinal cord compression. 25 OPLL or ossification of the ligamentum flavum is better identified on CT-scans. CT myelography may be used when MRI is contraindicated (such as in patients with pacemakers) to provide direct visualization of the spinal cord and canal relationship. 39
Surgical Decision-Making in DCM
The goal of surgical treatment for DCM is to arrest progression by decompressing the spinal cord and aligning and stabilizing the spine. The preferred approach will depend on patient factors, imaging features, and the sources of spinal cord compression. In appropriate patients, surgical decompression and reconstruction of the cervical spine can be performed via anterior, posterior, or combined (anterior and posterior approaches) with comparable effectiveness and safety 40 (Table 2). Typically, anterior surgery is reserved for cases with a predominant ventral compression, at 1 or 2 levels, and in patients with cervical kyphosis. Posterior surgery has been used for patients with OPLL and with multisegmental pathology in patients with preserved lordosis. Surgical decision-making, however, is nuanced, and surgeons must consider factors such as cervical spine alignment, the number of motion segments involved, morphology, and location of the spondylotic compression, in order to adequately decompress the spinal cord while reducing complications and optimizing outcomes. When properly indicated, anterior and posterior surgery achieve comparable results as assessed by the mJOA, Nurick, NDI, and SF-36, with similar complication rates. 41 At the same time, there is low-quality evidence that neck pain may be less after anterior surgery and moderate evidence that posterior surgery may achieve an increased spinal canal diameter. 40
Table 2.
Indications for surgical approach to DCM based on imaging and clinical findings. PCDF: posterior cervical decompression and fusion; ACDF: anterior cervical discectomy and fusion; ACCF: anterior cervical corpectomy and fusion; STV: subtotal vertebrectomy; OPLL: ossification of the posterior longitudinal ligament.
| Variables | Surgical Approach | Surgical Procedure Comments | |||||
|---|---|---|---|---|---|---|---|
| Anterior | Posterior | Combined | |||||
| Imaging findings | Level of compression | Occiput-C2 | + | PCDF | |||
| Single-level disease | Anterior compression | + | ACDF | ||||
| Posterior compression | + | Laminoplasty, laminectomy, and fusion | |||||
| Retrovertebral disease | + | ACCF, STV | |||||
| Multiple level disease (≥ 3) | Anterior or posterior compression | + | Similar Effectiveness and safety between anterior approaches | ||||
| Retrovertebral disease | Minimal disease | + | In anterior approach, multiple discectomy > corpectomy or discectomy-corpectomy hybrid procedures | ||||
| Significant disease | + | In anterior approach, discectomy-corpectomy hybrid approaches > multiple corpectomies | |||||
| Stenotic segments (<3) | + | ||||||
| Congenital stenosis | Single-level disease | + | + | ||||
| Multiple level disease | + | Laminectomy and fusion or laminoplasty | |||||
| Alignment | Neutral or lordotic | + | + | + | |||
| Kyphotic (> 13°) | + | + | |||||
| Modified K-line (+) | + | + | |||||
| Modified K-line (−) | + | + | + | ||||
| OPLL | + | + | + | If anterior approach is used: floating method | |||
| OPLL and canal occupancy ratio >60% | + | + | If anterior approach is used: ACDF, STV, ACCF | ||||
| Instability | + | + | ACDF, laminectomy, and fusion | ||||
| Myeloradiculopathy + soft-disk herniation | + | ACDF or posterior decompression ( ± fusion) + foraminotomy | |||||
| Myeloradiculopathy + spondylotic foraminal stenosis | + | + | |||||
| Clinical findings | Axial neck pain | + | + | ACDF, laminectomy and fusion, or combined | |||
| Previous radiation around the neck | Laminoplasty | ||||||
| History of dysphagia, dysphonia, or vocal occupation | Laminoplasty, laminectomy ± fusion | ||||||
| Chronic smoker | + | Laminoplasty or combined | |||||
The most commonly used anterior approaches are anterior cervical discectomy and fusion (ACDF), anterior cervical corpectomy and fusion (ACCF), hybrid ACDF/ACCF constructs, and cervical disc replacement. In patients with multilevel disease and when an anterior surgery is performed, multiple ACDF, ACCF, or hybrid discectomy-corpectomies may be performed. When possible, multilevel ACDF (Figure 1A) should be preferred, as it has shown to be best at achieving sagittal correction and yielding lower pain scores with similar incidences of non-union, dysphagia, and infection than the alternatives. 42 However, if there is significant retrovertebral compression, an ACCF (Figures 2B and 2C) or hybrid ACCF-ACDF should be performed to decompress the spinal cord adequately. When possible, hybrid constructs should be preferred to multiple corpectomies, as they have been shown to achieve similar neurologic improvement but better sagittal correction and improvement in neck pain. 42 The improved sagittal correction and spine biomechanics may also aid in reducing the long-term incidence of adjacent segment disease. 43
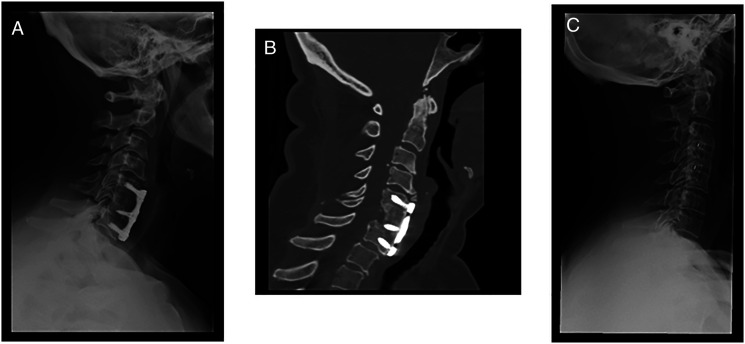
Figure 1.
Anterior surgical options for DCM. (A) Lateral cervical x‐ray view and (B) sagittal CT‐scan view of a patient submitted to C6 corpectomy and C5‐C7 fusion with autograph and plate. (C) Latera cervical x-ray view of a patient submitted to C3‐C4 and C4‐C5 anterior cervical discectomy and fusion (ACDF) with cages.
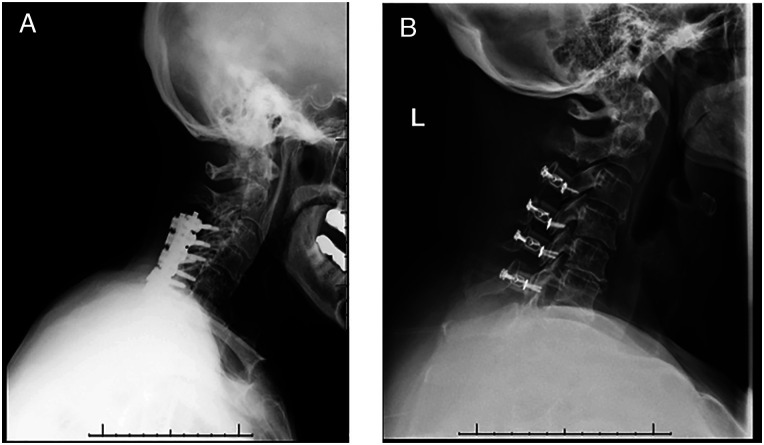
Figure 2.
Posterior surgical options for DCM. (A) Posterior decompression and fusion with lateral mass screws. (B) Laminoplasty.
While restriction of motion was thought to be one of the cornerstones of myelopathy treatment, concerns about motion preservation and reducing adjacent level degeneration after anterior cervical fusions have prompted the study of the effectiveness of total disc replacement in myelopathy patients. Results of these studies, some with 10 years of follow-up, have shown clinical improvement of patients treated with disc replacement, as assessed by the JOA and NDI scores with no differences in complication rates to patients treated for radiculopathy or to patients treated with ACDF.44-46 Furthermore, a recent systematic review comparing ACDF with cage and plate, ACDF with stand-alone cage, and cervical disc replacement found no differences in NDI scores and in sagittal alignment. As such, and while cervical disc prostheses are not traditionally considered lordosis-producing devices, the amount of lordosis achieved is comparable to that which is achieved with ACDF. 47
Posterior surgery relies on a passive decompression or the “drift back” phenomenon of the spinal cord drifting away from the anterior compression sites. Therefore, it has a limited effect in a kyphotic cervical spine. The modified k-line48,49 is a line connecting the midpoints of the spinal cord at C2 and C7 on pre-operative T1-weighted sagittal magnetic resonance imaging and is a useful predictor of the ability of posterior surgery to sufficiently decompress the spinal cord. If the distance between the anterior compression and the k-line is lower than 4 mm, posterior surgery will not allow for sufficient cord shift and an appropriate decompression, and an anterior surgery is recommended. 50
Historically, when opting for a posterior approach, multilevel laminectomies were often performed. This procedure, however, has been associated with progressive kyphosis in 15–20% of patients, 51 which led to the progressive abandonment of this procedure. Currently, the main posterior surgical approaches are laminectomy and fusion (Figure 2A) and laminoplasty (Figure 2B). Several authors have attempted to compare these procedures, and while well designed randomized controlled trials are still lacking, current evidence indicates that laminectomy alone may lead to inferior outcomes when compared to laminectomy and fusion. 52 In particular, this retrospective analysis of prospectively accrued cohort data demonstrated significant differences between surgical cohorts in the change in mJOA and Nurick scores from pre-operative to 24 months postoperative (mJOA: −1.70, P = .0266; Nurick: − .90, P = .0241). The rate of perioperative complications was comparable (P = .879). A randomized controlled trial entitled “Fusion 4 DCM” and aiming to enroll 394 patients is due to start in 2021 and will be comparing laminectomy with laminectomy with fusion (NIHR131243).
Laminoplasty is a procedure to expand the spinal cord size without a requirement for fusion and with the potential to preserve posterior spinal structures. 53 Studies comparing laminoplasty with laminectomy and fusion indicate that both procedures achieve similar clinical improvements but that laminectomy and fusion may be associated with longer operative times and higher complication rates, 54 including neck pain and C5 palsy 55 and with higher associated costs. 56 Conversely, laminoplasty may be superior to laminectomy in preserving cervical ROM, pre-operative cervical lordosis, and minimizing neck disability. 57
Surgical management of DCM secondary to OPLL can be particularly challenging. In contrast to DCM secondary to spondylosis, in which compression is usually at the level of the intervertebral disc, in OPLL compression often also occurs at the level of the vertebral body. As such, an anterior approach to these patients may require corpectomy rather than multiple discectomies. However, dissection of the ossified ligament from the dural sac is challenging and risky and, therefore, a posterior approach has been often proposed to manage these patients and studies have shown it is safer and may achieve equivalent decompression. 58 When performing a posterior approach laminectomy and fusion should be preferred to motion-preserving techniques, such as laminoplasty, since the later have been shown to have a 7 times faster mean postoperative annual OPLL growth rate. 59 For patients with OPLL occupying >60% of the spinal canal, an anterior approach is however associated with better neurologic recovery and should be the preferred approach.60,61
Over the last decades, alternative approaches have been proposed to decompress the spinal cord through a posterior approach. One such procedure is skip laminectomies, where the spinal cord is decompressed in regions of greater stenosis but with preservation of the posterior tension band, therefore, theoretically reducing the incidence of postoperative kyphosis and yet avoiding fusion. A 2018 meta-analysis comparing it with laminoplasty reported lower VAS scores and rate of axial pain and muscle atrophy for skip laminectomies in patients with comparable cervical lordotic curvature and range of motion. 62 Tubular/minimally invasive approaches have also been proposed as less invasive alternatives63-65 and while the indications may be more restricted and high-quality evidence is still lacking, small comparative studies of selected patients have reported favorable results.66,67
As aforementioned, restoring the sagittal spine balance is one of the goals of myelopathy surgery. Greater myelopathy severity (lower mJOA scores) is found in patients with pre-operative sagittal imbalance.55,68 Surgical correction of sagittal imbalance provides a greater degree of neurologic improvement, a lower degree of axial pain,69,70 and lower postoperative disability scores. 71 Furthermore, correcting sagittal imbalance potentially reduces the degeneration of adjacent levels, further improving the long-term results of surgery.
Proposing Surgical Treatment to DCM Patients Being Managed Conservatively/Mild DCM Patients who should be Proposed for Surgical Treatment
Non-operative management of DCM is poorly defined in the literature, and while structured non-operative treatment has not been associated with any direct harms, there is also little evidence of its benefits, with most studies reporting changes in mJOA below the minimal clinically important differences. Of those patients initially managed conservatively, 23–54% require surgical treatment at 29–74 months. 72 Due to this lack of proof of clinical improvement with non-operative treatment and to the high conversion to surgical treatment, it is imperative that patients with DCM potentially eligible for initial conservative management are also referred for surgical assessment, as reports show that delay of surgical treatment is predictive of worse surgical outcome.
Importantly, while non-operative management may be pursued in some patients, when the disease is progressive, surgery should always be discussed, irrespective of baseline disease severity. 12 Regardless of disease severity, patients with circumferential cord compression on axial MRI, “angular-edged” spinal cord, greater head and cervical range of motion, lower segmental lordotic angle and a greater percentage of vertebral slip, segmental instability, and reduced diameter of the cerebrospinal fluid column are at higher risk of DCM progression and should be submitted to surgical treatment. 72
Areas of Uncertainty
Mild Myelopathy
Whilst improvements have been demonstrated in patients undergoing surgery for mild DCM, 8 it remains controversial. In particular, the prospect that symptoms may remain stable for prolonged time periods makes surgical decisions difficult. 3 On the other hand, surgery on the strata of patients whose symptoms will progress should prevent future disability. Reflecting this uncertainty, current surgical guidelines remain vague and imply an expectant approach until a clear deterioration is observed. A better understanding of the natural history of myelopathy and the risk factors for deterioration would enable early patient selection and triage for surgery.
Atypical Symptoms
DCM is characterized by a triad of symptoms encompassing gait imbalance, loss of hand dexterity, and sphincter dysfunction. These may appear together or separately and frequently are associated with cervical pain. Of importance for diagnosticians, some studies have highlighted the relevance of atypical presentation of DCM, which may further delay the initial diagnosis, referral, and appropriate treatment. Kobayashi et al. found that DCM patients reported chest tightness 22.9 times more often than non-myelopathic patients, 73 resulting in inclusion as one of the symptoms to be queried in an 8-item questionnaire for DCM screening. Mowforth et al. 74 reported a patient with sensory dysesthesia, including facial dysesthesia secondary to DCM with typical gait, dexterity, and sphincter symptoms that were only linked to DCM after 11 visits to the emergency department. 74 Oh et al. 75 reported a patient with DCM in which a megacolon only resolved after surgical decompression of the cervical stenosis. 75 Houten et al. reported a series of 12 DCM patients presenting without upper extremity symptoms 76 that all reported gait difficulty; more than half had objective lower extremity weakness. Sensory examination revealed disturbances below the cervical compression level, starting at the mid-thoracic, waist, or genital area. 76 These reports may indicate complex symptomatic patterns resulting in myelopathy together with dysregulation of other systems.
Neurophysiology
Electrophysiological studies (motor and sensory evoked potentials) have been widely used in the assessment of DCM as well as in the differential diagnosis with neurodegenerative disorders. The magnitude of evoked potential abnormality often correlates with the magnitude of DCM severity 77 and may predict surgical outcomes. Importantly, evoked potentials may be particularly useful at early DCM stages, where the disease can be more difficult to diagnose, and pyramidal signs are still absent. 78
Intraoperative neurophysiological monitoring (including transcranial evoked muscle action potentials) is important to ensure that a worsening of conduction is not caused by reduced blood pressure or surgical maneuvers such as distraction. If the spine will be positioned other than in neutral, it is important to obtain a set of evoked potentials before the final operative neck position is set. 79
Dynamic Imaging and Myelopathy Without Visualized Spinal Cord Compression
Some patients may even present with DCM symptoms without spinal cord compression visible on conventional static MRI. In such cases, dynamic MRI (performed with the neck in flexion and extension) may help to identify spinal cord compression in patients where it could otherwise be missed. Reporting the analysis of a cohort of 2471 patients, Hayashi et al. 80 identified missed dynamic compression in cervical extension in 8.3% of patients, and in 1.6% of patients in flexion, with the most frequently missed level being C5–C6. Lee et al. 81 reported that cervical canal diameter was more significantly reduced in dynamic MRI of patients with DCM from degenerative/spondylotic changes rather than that resulting from OPLL. Nigro et al. 82 suggested that dynamic MRI was more useful for diagnosing posterior compression, whereas anterior compression was more often diagnosed with static MRI.
Disc bulge greater than 2.4 mm, angular motion greater than 4.8°, moderate and severe disc degeneration, segmental kyphosis, and developmental stenosis are more commonly evident in extension dynamic MRI than with static MRI, whereas disc bulge greater than 1.9 mm, moderate to severe disc degeneration and segmental kyphosis are more commonly found in flexion dynamic MRI and missed with conventional MRI. 80 Together, these observations indicate that the position in which the images are acquired may influence the magnitude of DCM spinal pathology.
Microstructural MRI as an Imaging Biomarker
Emerging evidence indicates that microstructural MRI approaches, including the use of T2-weighted imaging, can detect neuroanatomical changes in the cord of patients with DCM, 83 which are predictive of baseline impairment and risk for myelopathic progression. Future work is required to extend and validate this promising work. Microstructural MRI holds promise as an imaging biomarker that could add precision to clinical decision-making on which patients with mild DCM are at the highest risk for disease progression. Moreover, this technology could assist with clinical prediction assessment to help with patient education as to the anticipated course of their disease. 84
Conclusion
This report highlights the available evidence on the surgical management and outcomes of DCM. There is presently very little data to support the conservative management of DCM. Surgical treatment of these patients can halt disease progression and reverse some disease symptoms and improve patient quality of life. The risks of surgery and comorbidities need to be balanced against the risks of disease progression and irreversible neurologic damage. A thorough understanding of individual disease stages and related prognostic factors should guide an individualized treatment plan aiming to appropriately and timely address disease symptoms. The main challenge remains to identify patients at a sufficiently early disease stage where the neurologic injury may be less progressed, and the likelihood of improvement with surgery is optimized. Future studies are needed to systematically analyze disease-specific factors in the context of individual variables such as age, sex, and comorbidities, together with integrated clinical, radiographic, electrophysiological, and other related data to determine in whom and when surgery should occur and to tailor surgery to achieve the best outcomes.
Acknowledgments
Further details on this priority, including how it was prioritized, why it was prioritized, and on-going research activity can be found at www.aospine.org/recode/individualizing-surgery
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research priorities were organized and funded by AO Spine through the AO Spine Knowledge Forum Spinal Cord Injury, a focused group of international Spinal Cord Injury experts. AO Spine is a clinical division of the AO Foundation, which is an independent medically-guided not-for-profit organization. Study support was provided directly through the AO Spine Research Department. MRNK is supported by the National Institute for Health Research (NIHR) Brain Injury MedTech Co-operative based at Cambridge University Hospitals NHS Foundation Trust and University of Cambridge, and BMD a NIHR Clinical Doctoral Research Fellowship. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
ORCID iDs
Ricardo Rodrigues-Pinto https://orcid.org/0000-0002-6903-348X
Benjamin M. Davies https://orcid.org/0000-0003-0591-5069
Yoshiharu Kawaguchi https://orcid.org/0000-0001-7710-6012
Michael G. Fehlings https://orcid.org/0000-0002-5722-6364
Paul A. Koljonen https://orcid.org/0000-0002-9250-653X
Jefferson R. Wilson https://orcid.org/0000-0001-5965-0305
References
- 1.Badhiwala JH, Ahuja CS, Akbar MA, et al. Degenerative cervical myelopathy-update and future directions. Nat Rev Neurol. 2020;16:108-124. doi: 10.1038/s41582-019-0303-0 [DOI] [PubMed] [Google Scholar]
- 2.Tetreault L, Goldstein CL, Arnold P, et al. Degenerative cervical myelopathy: A spectrum of related disorders affecting the aging spine. Neurosurgery. 2015;77(suppl 4):S51-S67. doi: 10.1227/NEU.0000000000000951 [DOI] [PubMed] [Google Scholar]
- 3.Clarke E, Robinson PK. Cervical myelopathy: A complication of cervical spondylosis. Brain. 1956;79:483-510. doi: 10.1093/brain/79.3.483 [DOI] [PubMed] [Google Scholar]
- 4.Karadimas SK, Erwin WM, Ely CG, Dettori JR, Fehlings MG. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine. 2013;38:S21-S36. doi: 10.1097/BRS.0b013e3182a7f2c3 [DOI] [PubMed] [Google Scholar]
- 5.Kong LD, Meng LC, Wang LF, Shen Y, Wang P, Shang Z-K. Evaluation of conservative treatment and timing of surgical intervention for mild forms of cervical spondylotic myelopathy. Exp Ther Med. 2013;6:852-856. doi: 10.3892/etm.2013.1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura K, Kurokawa T, Hoshino Y, Saita K, Takeshita K, Kawaguchi H. Conservative treatment for cervical spondylotic myelopathy: Achievement and sustainability of a level of “no disability”. J Spinal Disord. 1998;11:175-179 [PubMed] [Google Scholar]
- 7.Yoshimatsu H, Nagata K, Goto H, et al. Conservative treatment for cervical spondylotic myelopathy. Prediction of treatment effects by multivariate analysis. Spine J. 2001;1:269-273. doi: 10.1016/s1529-9430(01)00082-1 [DOI] [PubMed] [Google Scholar]
- 8.Fehlings MG, Wilson JR, Kopjar B, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: Results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013;95:1651-1658. doi: 10.2106/JBJS.L.00589 [DOI] [PubMed] [Google Scholar]
- 9.Fehlings MG, Ibrahim A, Tetreault L, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: Results from the prospective multicenter AOSpine international study on 479 patients. Spine. 2015;40:1322-1328. doi: 10.1097/BRS.0000000000000988 [DOI] [PubMed] [Google Scholar]
- 10.Davies BM, Khan DZ, Mowforth OD, et al. RE-CODE DCM (REsearch Objectives and Common Data Elements for Degenerative Cervical Myelopathy): A consensus process to improve research efficiency in DCM, through establishment of a standardized dataset for clinical research and the definition of the research priorities. Global Spine J. 2019;9:65S-76S. doi: 10.1177/2192568219832855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tetreault L, Kopjar B, Nouri A, et al. The modified Japanese Orthopaedic Association scale: Establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur Spine J. 2017;26:78-84. doi: 10.1007/s00586-016-4660-8 [DOI] [PubMed] [Google Scholar]
- 12.Fehlings MG, Tetreault LA, Riew KD, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: Recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J. 2017;7:70S-83S. doi: 10.1177/2192568217701914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadanka Z, Bednarik J, Novotny O, Urbánek I, Dušek L. Cervical spondylotic myelopathy: Conservative versus surgical treatment after 10 years. Eur Spine J. 2011;20:1533-1538. doi: 10.1007/s00586-011-1811-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadanka Z, Mares M, Bednanik J, et al. Approaches to spondylotic cervical myelopathy: Conservative versus surgical results in a 3-year follow-up study. Spine. 2002;27:2205-2210; discussion 2210-2201. doi: 10.1097/01.BRS.0000029255.77224 [DOI] [PubMed] [Google Scholar]
- 15.Wilson JR, Barry S, Fischer DJ, et al. Frequency, timing, and predictors of neurological dysfunction in the nonmyelopathic patient with cervical spinal cord compression, canal stenosis, and/or ossification of the posterior longitudinal ligament. Spine. 2013;38:S37-S54. doi: 10.1097/BRS.0b013e3182a7f2e7 [DOI] [PubMed] [Google Scholar]
- 16.Badhiwala JH, Witiw CD, Nassiri F, et al. Efficacy and safety of surgery for mild degenerative cervical myelopathy: Results of the AOSpine North America and International Prospective Multicenter Studies. Neurosurgery. 2019;84:890-897. doi: 10.1093/neuros/nyy133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karadimas SK, Gatzounis G, Fehlings MG. Pathobiology of cervical spondylotic myelopathy. Eur Spine J. 2015;24(suppl 2):132-138. doi: 10.1007/s00586-014-3264-4 [DOI] [PubMed] [Google Scholar]
- 18.Dokai T, Nagashima H, Nanjo Y, Tanida A, Teshima R. Surgical outcomes and prognostic factors of cervical spondylotic myelopathy in diabetic patients. Arch Orthop Trauma Surg. 2012;132:577-582. doi: 10.1007/s00402-011-1449-4 [DOI] [PubMed] [Google Scholar]
- 19.Machino M, Yukawa Y, Ito K, et al. Risk factors for poor outcome of cervical laminoplasty for cervical spondylotic myelopathy in patients with diabetes. J Bone Joint Surg Am. 2014;96:2049-2055. doi: 10.2106/JBJS.N.00064 [DOI] [PubMed] [Google Scholar]
- 20.Tetreault L, Kopjar B, Cote P, Arnold P, Fehlings MG. A clinical prediction rule for functional outcomes in patients undergoing surgery for degenerative cervical myelopathy: Analysis of an international prospective multicenter data set of 757 subjects. J Bone Joint Surg Am. 2015;97:2038-2046. doi: 10.2106/JBJS.O.00189 [DOI] [PubMed] [Google Scholar]
- 21.Tetreault L, Wilson JR, Kotter MR, et al. Predicting the minimum clinically important difference in patients undergoing surgery for the treatment of degenerative cervical myelopathy. Neurosurg Focus. 2016;40:E14. doi: 10.3171/2016.3.FOCUS1665 [DOI] [PubMed] [Google Scholar]
- 22.Rajshekhar V, Kumar GS. Functional outcome after central corpectomy in poor-grade patients with cervical spondylotic myelopathy or ossified posterior longitudinal ligament. Neurosurgery. 2005;56:1279-1284; discussion 1284-1275. doi: 10.1227/01.neu.0000159713.20597.0f [DOI] [PubMed] [Google Scholar]
- 23.Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3:33-45. doi: 10.1016/s1529-9430(02)00448-5 [DOI] [PubMed] [Google Scholar]
- 24.Tetreault L, Palubiski LM, Kryshtalskyj M, et al. Significant predictors of outcome following surgery for the treatment of degenerative cervical myelopathy: A systematic review of the literature. Neurosurg Clin N Am. 2018;29:115-127 e135. doi: 10.1016/j.nec.2017.09.020 [DOI] [PubMed] [Google Scholar]
- 25.Nakashima H, Tetreault LA, Nagoshi N, et al. Does age affect surgical outcomes in patients with degenerative cervical myelopathy? Results from the prospective multicenter AOSpine International study on 479 patients. J Neurol Neurosurg Psychiatry. 2016;87:734-740. doi: 10.1136/jnnp-2015-311074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badhiwala JH, Khan O, Wegner A, et al. A partial least squares analysis of functional status, disability, and quality of life after surgical decompression for degenerative cervical myelopathy. Sci Rep. 2020;10:16132. doi: 10.1038/s41598-020-72595-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson JRF, Badhiwala JH, Moghaddamjou A, Yee A, Wilson JR, Fehlings MG. Frailty is a better predictor than age of mortality and perioperative complications after surgery for degenerative cervical myelopathy: An analysis of 41,369 patients from the nsqip database 2010-2018. J Clin Med. 2020;9:3491. doi: 10.3390/jcm9113491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao R, Yang L, Chen H, Liu Y, Liang L, Yuan W. Long term results of anterior corpectomy and fusion for cervical spondylotic myelopathy. PLoS One. 2012;7:e34811. doi: 10.1371/journal.pone.0034811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin JW, Jin SW, Kim SH, et al. Predictors of outcome in patients with cervical spondylotic myelopathy undergoing unilateral open-door laminoplasty. Korean J Spine. 2015;12:261-266. doi: 10.14245/kjs.2015.12.4.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: Surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52:122-126; discussion 126. doi: 10.1097/00006123-200301000-00015 [DOI] [PubMed] [Google Scholar]
- 31.Tetreault LA, Nouri A, Singh A, Fawcett M, Fehlings MG. Predictors of outcome in patients with cervical spondylotic myelopathy undergoing surgical treatment: A survey of members from AOSpine International. World Neurosurg. 2014;81:623-633. doi: 10.1016/j.wneu.2013.09.023 [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Moon SH, Kim HS, et al. Diabetes and smoking as prognostic factors after cervical laminoplasty. J Bone Joint Surg Br. 2008;90:1468-1472. doi: 10.1302/0301-620X.90B11.20632 [DOI] [PubMed] [Google Scholar]
- 33.Kusin DJ, Ahn UM, Ahn NU. The influence of diabetes on surgical outcomes in cervical myelopathy. Spine. 2016;41:1436-1440. doi: 10.1097/BRS.0000000000001560 [DOI] [PubMed] [Google Scholar]
- 34.Kusin DJ, Li SQ, Ahn UM, Ahn NU. Does tobacco use attenuate benefits of early decompression in patients with cervical myelopathy? Spine. 2016;41:1565-1569. doi: 10.1097/BRS.0000000000001597 [DOI] [PubMed] [Google Scholar]
- 35.Nouri A, Martin AR, Mikulis D, Fehlings MG. Magnetic resonance imaging assessment of degenerative cervical myelopathy: A review of structural changes and measurement techniques. Neurosurg Focus. 2016;40:E5. doi: 10.3171/2016.3.FOCUS1667 [DOI] [PubMed] [Google Scholar]
- 36.Yukawa Y, Kato F, Yoshihara H, Yanase M, Ito K. MR T2 image classification in cervical compression myelopathy: Predictor of surgical outcomes. Spine. 2007;32:1675-1678; discussion 1679. doi: 10.1097/BRS.0b013e318074d62e [DOI] [PubMed] [Google Scholar]
- 37.Mastronardi L, Elsawaf A, Roperto R, et al. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J Neurosurg Spine. 2007;7:615-622. doi: 10.3171/SPI-07/12/615 [DOI] [PubMed] [Google Scholar]
- 38.Yagi M, Ninomiya K, Kihara M, Horiuchi Y. Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of intramedullary spinal cord on magnetic resonance imaging. J Neurosurg Spine. 2010;12:59-65. doi: 10.3171/2009.5.SPINE08940 [DOI] [PubMed] [Google Scholar]
- 39.Houser OW, Onofrio BM, Miller GM, Folger WN, Smith PL. Cervical spondylotic stenosis and myelopathy: Evaluation with computed tomographic myelography. Mayo Clin Proc. 1994;69:557-563. doi: 10.1016/s0025-6196(12)62248-4 [DOI] [PubMed] [Google Scholar]
- 40.Lawrence BD, Jacobs WB, Norvell DC, Hermsmeyer JT, Chapman JR, Brodke DS. Anterior versus posterior approach for treatment of cervical spondylotic myelopathy: A systematic review. Spine. 2013;38:S173-S182. doi: 10.1097/BRS.0b013e3182a7eaaf [DOI] [PubMed] [Google Scholar]
- 41.Fehlings MG, Barry S, Kopjar B, et al. Anterior versus posterior surgical approaches to treat cervical spondylotic myelopathy: Outcomes of the prospective multicenter AOSpine North America CSM study in 264 patients. Spine. 2013;38:2247-2252. doi: 10.1097/BRS.0000000000000047 [DOI] [PubMed] [Google Scholar]
- 42.Shamji MF, Massicotte EM, Traynelis VC, Norvell DC, Hermsmeyer JT, Fehlings MG. Comparison of anterior surgical options for the treatment of multilevel cervical spondylotic myelopathy: A systematic review. Spine. 2013;38:S195-S209. doi: 10.1097/BRS.0b013e3182a7eb27 [DOI] [PubMed] [Google Scholar]
- 43.Hansen MA, Kim HJ, Van Alstyne EM, Skelly AC, Fehlings MG. Does postsurgical cervical deformity affect the risk of cervical adjacent segment pathology? A systematic review. Spine. 2012;37:S75-S84. doi: 10.1097/BRS.0b013e31826d62a6 [DOI] [PubMed] [Google Scholar]
- 44.Gornet MF, Lanman TH, Burkus JK, et al. Two-level cervical disc arthroplasty versus anterior cervical discectomy and fusion: 10-year outcomes of a prospective, randomized investigational device exemption clinical trial. J Neurosurg Spine. 2019:1-11. doi: 10.3171/2019.4.SPINE19157 [DOI] [PubMed] [Google Scholar]
- 45.Gornet MF, Lanman TH, Burkus JK, et al. Cervical disc arthroplasty with the Prestige LP disc versus anterior cervical discectomy and fusion, at 2 levels: Results of a prospective, multicenter randomized controlled clinical trial at 24 months. J Neurosurg Spine. 2017;26:653-667. doi: 10.3171/2016.10.SPINE16264 [DOI] [PubMed] [Google Scholar]
- 46.Han X, He D, Zhang N, Song Q, Wang J, Tian W. Comparison of 10-year outcomes of Bryan cervical disc arthroplasty for myelopathy and radiculopathy. Orthop Surg. 2019;11:1127-1134. doi: 10.1111/os.12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katsuura Y, York PJ, Goto R, et al. Sagittal reconstruction and clinical outcome using traditional ACDF, versus stand-alone ACDF versus TDR: A systematic review and quantitative analysis. Spine. 2019;44:E1151-E1158. doi: 10.1097/BRS.0000000000003077 [DOI] [PubMed] [Google Scholar]
- 48.Taniyama T, Hirai T, Yamada T, et al. Modified K-line in magnetic resonance imaging predicts insufficient decompression of cervical laminoplasty. Spine. 2013;38:496-501. doi: 10.1097/BRS.0b013e318273a4f7 [DOI] [PubMed] [Google Scholar]
- 49.Taniyama T, Hirai T, Yoshii T, et al. Modified K-line in magnetic resonance imaging predicts clinical outcome in patients with nonlordotic alignment after laminoplasty for cervical spondylotic myelopathy. Spine. 2014;39:E1261-E1268. doi: 10.1097/BRS.0000000000000531 [DOI] [PubMed] [Google Scholar]
- 50.Hirai T, Yoshii T, Inose H, et al. Is modified k-line a powerful tool of surgical decision making for patients with cervical spondylotic myelopathy?. Clin Spine Surg. 2019;32:351-356. doi: 10.1097/BSD.0000000000000899 [DOI] [PubMed] [Google Scholar]
- 51.Kaptain GJ, Simmons NE, Replogle RE, Pobereskin L. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg. 2000;93:199-204. doi: 10.3171/spi.2000.93.2.0199 [DOI] [PubMed] [Google Scholar]
- 52.Kotter MRN, Tetreault L, Badhiwala JH, et al. Surgical outcomes following laminectomy with fusion versus laminectomy alone in patients with degenerative cervical myelopathy. Spine. 2020;45:1696-1703. doi: 10.1097/BRS.0000000000003677 [DOI] [PubMed] [Google Scholar]
- 53.Hirabayashi K, Toyama Y, Chiba K. Expansive laminoplasty for myelopathy in ossification of the longitudinal ligament. Clin Orthop Relat Res. 1999:35-48. doi: 10.1097/00003086-199902000-00005 [DOI] [PubMed] [Google Scholar]
- 54.He X, Zhang JN, Liu TJ, Hao DJ. Is laminectomy and fusion the better choice than laminoplasty for multilevel cervical myelopathy with signal changes on magnetic resonance imaging? A comparison of two posterior surgeries. BMC Musculoskelet Disord. 2020;21:423. doi: 10.1186/s12891-020-03435-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li FH, Qiao HH, Yang YC, Du JP, Jin XS, Wang B. Incidence and outcomes of C5 palsy and axial pain after open-door laminoplasty or laminectomy and fusion: A meta-analysis. World Neurosurg. 2019;128:e1002-e1009. doi: 10.1016/j.wneu.2019.05.060 [DOI] [PubMed] [Google Scholar]
- 56.Goh BC, Striano BM, Lopez WY, et al. Laminoplasty versus laminectomy and fusion for cervical spondylotic myelopathy: A cost analysis. Spine J. 2020;20:1770-1775. doi: 10.1016/j.spinee.2020.07.012 [DOI] [PubMed] [Google Scholar]
- 57.Ha Y, Shin JJ. Comparison of clinical and radiological outcomes in cervical laminoplasty versus laminectomy with fusion in patients with ossification of the posterior longitudinal ligament. Neurosurg Rev. 2019;43:1409-1421. doi: 10.1007/s10143-019-01174-5 [DOI] [PubMed] [Google Scholar]
- 58.Sakai K, Okawa A, Takahashi M, et al. Five-year follow-up evaluation of surgical treatment for cervical myelopathy caused by ossification of the posterior longitudinal ligament: A prospective comparative study of anterior decompression and fusion with floating method versus laminoplasty. Spine. 2012;37:367-376. doi: 10.1097/BRS.0b013e31821f4a51 [DOI] [PubMed] [Google Scholar]
- 59.Lee JJ, Shin DA, Yi S, et al. Effect of posterior instrumented fusion on three-dimensional volumetric growth of cervical ossification of the posterior longitudinal ligament: A multiple regression analysis. Spine J. 2018;18:1779-1786. doi: 10.1016/j.spinee.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 60.An HS, Al-Shihabi L, Kurd M. Surgical treatment for ossification of the posterior longitudinal ligament in the cervical spine. J Am Acad Orthop Surg. 2014;22:420-429. doi: 10.5435/JAAOS-22-07-420 [DOI] [PubMed] [Google Scholar]
- 61.Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: Part 2: Advantages of anterior decompression and fusion over laminoplasty. Spine. 2007;32:654-660. doi: 10.1097/01.brs.0000257566.91177.cb [DOI] [PubMed] [Google Scholar]
- 62.Luo W, Li Y, Zhao J, Zou Y, Gu R, Li H. Skip laminectomy compared with laminoplasty for cervical compressive myelopathy: A systematic review and meta-analysis. World Neurosurg. 2018;120:296-301. doi: 10.1016/j.wneu.2018.08.231 [DOI] [PubMed] [Google Scholar]
- 63.Benglis DM, Guest JD, Wang MY. Clinical feasibility of minimally invasive cervical laminoplasty. Neurosurg Focus. 2008;25:E3. doi: 10.3171/FOC/2008/25/8/E3 [DOI] [PubMed] [Google Scholar]
- 64.Dahdaleh NS, Wong AP, Smith ZA, Wong RH, Lam SK, Fessler RG. Microendoscopic decompression for cervical spondylotic myelopathy. Neurosurg Focus. 2013;35:E8. doi: 10.3171/2013.3.FOCUS135 [DOI] [PubMed] [Google Scholar]
- 65.Wang MY, Green BA, Coscarella E, Baskaya MK, Levi ADO, Guest JD. Minimally invasive cervical expansile laminoplasty: An initial cadaveric study. Neurosurgery. 2003;52:370-373; discussion 373. doi: 10.1227/01.neu.0000043933.32287 [DOI] [PubMed] [Google Scholar]
- 66.Minamide A, Yoshida M, Simpson AK, et al. Microendoscopic laminotomy versus conventional laminoplasty for cervical spondylotic myelopathy: 5-year follow-up study. J Neurosurg Spine. 2017;27:403-409. doi: 10.3171/2017.2.SPINE16939 [DOI] [PubMed] [Google Scholar]
- 67.Oshima Y, Kato S, Doi T, Matsubayashi Y, Taniguchi Y, Tanaka S. Comparison of microendoscopic selective laminectomy versus conventional laminoplasty in patients with degenerative cervcical myelopathy: A minimum 2-year follow-up study. BMC Musculoskelet Disord. 2019;20:471. doi: 10.1186/s12891-019-2884-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin T, Chen P, Wang Z, Chen G, Liu W. Does cervical sagittal balance affect the preoperative neck disability index in patients with cervical myelopathy?. Clin Spine Surg. 2020;33:E21-E25. doi: 10.1097/BSD.0000000000000916 [DOI] [PubMed] [Google Scholar]
- 69.Roguski M, Benzel EC, Curran JN, et al. Postoperative cervical sagittal imbalance negatively affects outcomes after surgery for cervical spondylotic myelopathy. Spine. 2014;39:2070-2077. doi: 10.1097/BRS.0000000000000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shamji MF, Mohanty C, Massicotte EM, Fehlings MG. The association of cervical spine alignment with neurologic recovery in a prospective cohort of patients with surgical myelopathy: Analysis of a series of 124 cases. World Neurosurg. 2016;86:112-119. doi: 10.1016/j.wneu.2015.09.044 [DOI] [PubMed] [Google Scholar]
- 71.Hyun SJ, Kim KJ, Jahng TA, et al. Relationship between T1 slope and cervical alignment following multilevel posterior cervical fusion surgery: Impact of T1 slope minus cervical lordosis. Spine. 2016;41:E396-E402. doi: 10.1097/BRS.0000000000001264 [DOI] [PubMed] [Google Scholar]
- 72.Tetreault LA, Rhee J, Prather H, et al. Change in function, pain, and quality of life following structured nonoperative treatment in patients with degenerative cervical myelopathy: A systematic review. Global Spine J. 2017;7:42S-52S. doi: 10.1177/2192568217700397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kobayashi H, Kikuchi S, Otani K, Sekiguchi M, Sekiguchi Y, Konno S-i. Development of a self-administered questionnaire to screen patients for cervical myelopathy. BMC Musculoskelet Disord. 2010;11:268. doi: 10.1186/1471-2474-11-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mowforth OD, Davies BM, Kotter MR. “I am not delusional!” Sensory dysaesthesia secondary to degenerative cervical myelopathy. BMJ Case Rep. 2019;12:e229033. doi: 10.1136/bcr-2018-229033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oh JYL, Kwek KJH, Tee SW, Tan M. Megacolon as an atypical presentation of cervical myelopathy. J Spine Surg. 2017;3:108-111. doi: 10.21037/jss.2017.02.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Houten JK, Pasternack J, Norton RP. Cervical myelopathy without symptoms in the upper extremities: Incidence and presenting characteristics. World Neurosurg. 2019;132:e162-e168. doi: 10.1016/j.wneu.2019.08.231 [DOI] [PubMed] [Google Scholar]
- 77.Feng X, Hu Y, Ma X. Progression prediction of mild cervical spondylotic myelopathy by somatosensory-evoked potentials. Spine. 2020;45:E560-E567. doi: 10.1097/BRS.0000000000003348 [DOI] [PubMed] [Google Scholar]
- 78.Simo M, Szirmai I, Aranyi Z. Superior sensitivity of motor over somatosensory evoked potentials in the diagnosis of cervical spondylotic myelopathy. Eur J Neurol. 2004;11:621-626. doi: 10.1111/j.1468-1331.2004.00863.x [DOI] [PubMed] [Google Scholar]
- 79.Takeda M, Yamaguchi S, Mitsuhara T, Abiko M, Kurisu K. Intraoperative neurophysiologic monitoring for degenerative cervical myelopathy. Neurosurg Clin N Am. 2018;29:159-167. doi: 10.1016/j.nec.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 80.Hayashi T, Wang JC, Suzuki A, et al. Risk factors for missed dynamic canal stenosis in the cervical spine. Spine. 2014;39:812-819. doi: 10.1097/BRS.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 81.Lee JB, Kim IS, Kwon JY, et al. Difference of dynamic morphometric changes between in patients with ossification of posterior longitudinal ligament and patients with cervical spondylosis: Assessment by cervical dynamic magnetic resonance imaging. World Neurosurg. 2019;123:e566-e573. doi: 10.1016/j.wneu.2018.11.213 [DOI] [PubMed] [Google Scholar]
- 82.Nigro L, Donnarumma P, Tarantino R, Rullo M, Santoro A, Delfini R. Static and dynamic cervical MRI: Two useful exams in cervical myelopathy. J Spine Surg. 2017;3:212-216. doi: 10.21037/jss.2017.06.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin AR, De Leener B, Cohen-Adad J, et al. Monitoring for myelopathic progression with multiparametric quantitative MRI. PLoS One. 2018;13:e0195733. doi: 10.1371/journal.pone.0195733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin AR, De Leener B, Cohen-Adad J, et al. Can microstructural MRI detect subclinical tissue injury in subjects with asymptomatic cervical spinal cord compression? A prospective cohort study. BMJ Open. 2018;8:e019809. doi: 10.1136/bmjopen-2017-019809 [DOI] [PMC free article] [PubMed] [Google Scholar]