Abstract
Study Design:
Literature Review (Narrative).
Objective:
To contextualize AO Spine RECODE-DCM research priority number 5: What is the socio-economic impact of DCM? (The financial impact of living with DCM to the individual, their supporters, and society as a whole).
Methods:
In this review, we introduce the methodology of health-economic investigation, including potential techniques and approaches. We summarize the current health-economic evidence within DCM, so far focused on surgical treatment. We also cover the first national estimate, in partnership with Myelopathy.org from the United Kingdom, of the cost of DCM to society. We then demonstrate the significance of this question to advancing care and outcomes in the field.
Results:
DCM is a common and often disabling condition, with a significant lack of recognition. While evidence demonstrates the cost-effectives of surgery, even among higher income countries, health inequalities exist. Further the prevalent residual disability in myelopathy, despite treatment affects both the individual and society as a whole. A report from the United Kingdom provides the first cost-estimate to their society; an annual cost of ∼£681.6 million per year, but this is likely a significant underestimate.
Conclusion:
A clear quantification of the impact of DCM is needed to raise the profile of a common and disabling condition. Current evidence suggests this is likely to be globally substantial.
Keywords: cervical myelopathy, cervical spondylosis, cervical stenosis, disc herniation, ossification posterior longitudinal ligament, degeneration, research priorities, health economics, socioeconomics, policy
Introduction
Degenerative Cervical Myelopathy [DCM] is a neurological disorder arising from degenerative, arthritic, and/or congenital processes, causing cervical spinal cord dysfunction.1,2 DCM can result in a wide range of impairments and disabilities, including poor balance, limited mobility, loss of dexterity, sensory loss, bowel or bladder dysfunction, pain and in severe cases, paralysis. 1 DCM is estimated to affect and contribute to neurologic dysfunction in up to 2% of the adult population. 3 Given the increased prevalence of spinal degeneration with age, the incidence of DCM is expected to rise as populations age. 4
Currently surgical decompression is the only evidence-based treatment recommended for progressive or moderate to severe disease. 5 For most patients surgical intervention can halt disease progression and afford some meaningful recovery. However, recovery is normally incomplete, with some deficits and leaving individuals with life-long disabilities, dependency, unemployment, and mental health difficulties.6,7 In a comparison of SF-36 [the Short Form—(36) Health Survey of Quality of Life) scores of people with chronic disease, individuals with DCM were found to have the lowest quality of life scores. 7 Moreover, the impact is not restricted to the individual, with a quality of life burden demonstrated among their family and/or acquaintance carers. 8 Therefore, efforts to address and improve DCM outcomes should be a critical public health priority.
AO Spine RECODE-DCM (aospine.org/recode) [REsearch objectives and COmmon Data Elements for DCM] is an international consensus project which aims to accelerate knowledge discovery that can improve outcomes by developing a set of research tools. 9 These include a James Lind Alliance research priority setting partnership, which brought together both individuals living and working with DCM to establish the most important unanswered questions. Research prioritization aims to catalyze progress by consolidating resources on key knowledge gaps. 10 The Number 8 priority identified was to establish the socio-economic impact of DCM. The term socio-economic impact was used here to encompass the health-economic impact on the individual and society.
This article aims to contextualize: (a) the significance of this question; (b) to explain what is meant by socio-economic impact and how it can be measured; (c) to summarize the current evidence from within DCM and to provide a current best estimate, and (d) illustrate why this is a critical knowledge gap for the field that needs to be overcome to help improve outcomes.
What Is Meant by Socio-Economic Impact, and How Can It Be Measured?
In this priority, the wording “socio-economic impact” was used to represent both the health-economic impact to the individual and to society. Health economics is the application of economic theory, decision-making models, and empirical techniques to analyze and make decisions on health and healthcare by taking into consideration the available resources as well as the values and needs from different stakeholders including individuals, health care providers, and governments. 11 Simply stated, the aim of health technology assessment is to provide techniques to help manage limited resources most efficiently to achieve the best outcomes in populations.
There are several techniques that can be used largely depending on the perspective of the intended audience and the availability of data. 12 When developing assessments, it is important to include the costs that are particularly relevant to the audience. For example, patients are most interested in the outcomes of the treatment and may have little or no interest in the cost of providing it (unless they are directly paying for it); the provider, however, wants satisfied patients but, more importantly, needs to be able to provide the treatment as cost-efficiently as possible, which means achieving the maximum benefit using the least resources (including money, time and manpower). Finally, the external payer (i.e. governments or private healthcare insurance) is looking for the most efficient means of providing a range of effective treatments within a limited budget. In summary, when undertaking health economic analysis, only those costs and/or benefits that are relevant to the particular audience or purpose should be included.
Taking each audience in turn, the primary data needs are:
Patient: clinical benefits, safety, and quality of life.
Provider: Incidence, cost of managing the condition: cost of surgery, resource usage (bed stay, outpatient, and other visits).
Payer: as with the provider but also additional direct costs such as absenteeism, lost production, disability benefits, and tax lost due to the condition.
In terms of the “how to measure the socioeconomic impact,” this depends on the purpose of the analysis and, more importantly, how generalizable the results need to be (Figure 1). The commonly used methods include:
Figure 1.
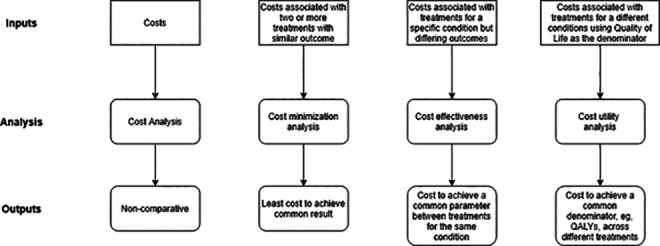
Summary of types of health economic analysis, including their principal purpose (represented as Output) and data requirements (represented as Input).
Cost analysis
This is a basic assessment of the costs of managing a condition without any consideration of the outcomes. The result cannot be used to compare with other treatments for the same condition, nor across treatments.
Cost minimization analysis
This is the simplest form of comparative analysis where the same outcome is possible using different treatments. In this context, the least costly treatment is deemed the most cost effective. For example, different antibiotics being used to treat a chest infection or, in the case of DCM, it might be a comparison of different surgical techniques where the same outcome will be achieved but at different costs of surgery.
Cost effectiveness analysis (CEA)
This is a method to compare both the costs and health outcomes of one or more interventions by estimating how much it costs to gain a health outcome unit like a life year gained or a death prevented.
Cost utility analysis (CUA)
The aim of a CUA is to attach a monetary value to outcomes to allow comparison across conditions. The most frequent common denominator is the Quality Adjusted Life Year (QALY), a generic measure of disease burden, which includes both the quality and quantity of life.
Quality of life can be assessed in a variety of ways such as:
Time Trade Off where an individual is asked to choose between remaining in a state of ill health for a period of time or being restored to perfect health but having a shorter life expectancy. The point where the respondent switch sides corresponds to the utility value for that health state.
Standard gamble where an individual is asked to choose between remaining in a state of ill health for a period of time or choosing a medical intervention that has a chance of either restoring them to perfect health or killing them. The point where the respondent changes opinion is considered the utility value for that health state.
Visual Analogue Scale where respondents are asked to rate a state of ill health on a scale from 0 to 100, with 0 representing being dead and 100 representing perfect health.
A generic scale which can be used in any condition that gives a weight associated with a particular health state is to use standard descriptive systems such as the EuroQol Group’s EQ-5D questionnaire, which categorizes health states according to 5 dimensions: mobility, self-care, usual activities (e.g., work, study, homework or leisure activities), pain/discomfort and anxiety/depression. While the SF-36 is the most commonly used quality of life score in DCM research today,13,14 for health economic analysis it must be mapped to the SF6D healthy utility.15,16
The result is a calculated index that ranges from 1 (perfect health) to 0 (dead); so one QALY equates to 1 year in perfect health. By associating the effect of treatment on quality of life and applying the cost of getting there, the cost/QALY can be estimated.
Several authorities use the Incremental Cost Effectiveness Ratio (ICER) to determine the value of new treatments. The ICER can be defined as:
For example, in England, the National Institute for Health and Care Excellence (NICE) uses a threshold of £20 000 to determine whether a new treatment should be introduced for use in the NHS. 17 There are some exceptions to this threshold, for example, in certain cancers, rare diseases, or where patients have limited life expectancy. These thresholds are therefore influenced by a number of factors and are typically set per healthcare system: 18 the World Health Organisation recommends using a threshold based on Gross Domestic Product to personalize recommendations. 19
What Is the Current Health-Economic Evidence Within DCM, and Why Must This Improve?
Within DCM, the health-economic evaluation has been restricted to evaluations of treatment cost, in particular surgery.20-23 Across the board, these studies strongly confirm the overall cost-effectiveness of surgery. 21 Moreover, as a single up-front relatively high cost, with benefit likely extended well beyond the follow-up period, the study by Witiw et al (2016) using a Markov transitional model to estimate a life-time benefit is a high-quality example. This study, using a Canadian cohort of 171 patients, demonstrated cost-effectiveness, as per the World Health Organization criteria, in 94.7% of estimates. 20 Further examples of health economic comparisons between surgical techniques, 24 and surgery versus non-operative management have also been conducted 22 but are more limited based on the quality of reference data. 18
While robust health-economic evidence is necessary to support the adoption of clinical treatments, particularly within single payer healthcare systems, these evaluations do not serve to fully characterize the complete burden of illness; for example, the cost at a societal level or for the individual. These are likely more fundamental to driving system-wide changes, including healthcare policy, social care policy, and increased research investment, fundamental to future healthcare gains in DCM. Moreover, the cost to an individual is often an important determinant of quality of life, and mental well-being, for which the burden is well demonstrated, but the drivers are not. 7
However, to calculate the cost of illness, several key pieces of evidence are required:
How many people are affected?
At what age does the condition develop?
What are the direct costs of managing the condition?
What are the indirect costs of managing the condition?
What are the costs to the individual, their caregivers, healthcare providers, payer, and society at large?
Unfortunately, most of these questions are poorly defined in DCM.
What Is a Current Best Estimate Within DCM?
Myelopathy.org (Cambridge, United Kingdom) is the first, and so far, only, charity dedicated to DCM. Launched in 2018, it hosts a growing and international community of individuals living with DCM but also working with DCM. 25 Fundamentally it aims to increase awareness and improve outcomes for those living with the condition. As part of these objectives, it has recently commissioned the first dedicated report on the burden of illness in DCM. The report made use of the best available data within the UK.
The prevalence of DCM was estimated based on International Classification of Disease (ICD-10) codes M47.12, M50.0, M99.31/.41/.51 from the National Health Service hospital episode statistics data, from inception (2013) to the end of 2019. This dataset provides overall event data for England (including wait time, and primary and secondary ICD codes), and some demographic data (age and gender). Extrapolating this across the UK population gave an estimated incidence of 7.44/100,00 (±0.32), in keeping with the literature.
Age of presentation was calculated by combining mean hospital waiting times from the hospital episode statistics data (73.6 days) with time to diagnosis data (assumed to be a surrogate for data of referral) from the literature; 2 retrospective cohort studies with average waits of 1.25 to 2.2 years.6,26 This was then subtracted from the average age at presentation (62.1 years overall, or 51.3 years for those of working age, defined as 18 to 65 years) to estimate the average age at which patients have sufficiently severe symptoms to seek medical intervention in the UK (i.e. 59.9 years overall or 49.1 years for those of working age).
Healthcare treatment costs were also extracted from the UK National Health Service Database for the aforementioned cases. For the most recent year queried (2018-2019), the total cost of care for DCM in England was estimated to be £38 871 534; £9216 weighted average per hospital admission.
Productivity is typically calculated up to the age of retirement as it is assumed that once a person reaches retirement age, they are no longer considered to be contributing members of society. This is not the case for many older people as they may provide voluntary work or non-paid family support such as looking after grandchildren so that their parents can go out to work, but it is more difficult to assess the value of this. Furthermore, several elderly individuals continue working beyond the age of 65 years old either for their choice or need due to financial burden. Based on the average age of these cases at presentation, and an average age of retirement of 65, this equated to a potential 15.1 years of affected productivity. In a previous survey conducted by Myelopathy.org 6 of those under the age of 65 (N = 537), 41% were unable to work due to their disability, 28% were employed full time, 14% employed part-time, and 7% retired.
To estimate lost personal income in the UK, this data was further restricted to UK respondents 18 to 65 (N = 199), looking for work (45%). In the absence of linked income data, average weekly income for the UK population was taken from the UK Office for National Statistics (ONS) [£511/week working full time and £139.52/week working part-time (based on the average part time employment of 16 hrs per week)] and Office for Economic Co-Operation (OECD).27,28 Assuming an average individual works 48 weeks per year, this equates to a potential loss of income through unemployment of £18 663 (ONS data) or £25 524 (OECD data). Therefore, assuming a 3% inflation rate (a standard assumption for health technology assessments), and a loss of productivity of 15.1 years, the lifetime inflated loss of income could be £347 112 using ONS figures or £474 719 using OECD values. Considering disability benefits, and using the UK ‘Universal Credit’ allowance for a single person, aged over 25, with limited capacity to work, of £9021.72/year, this would equate to a £9641 (51%) or £16 503 (65%) reduction in personal income. Based on the average age of the UK population in 2019 of 81.2, disability benefits on average would be claimed for 16.5 years in those age >65 years and 30.5 years in those hospitalized with DCM at the average age of 51.1 years (13.9+16.5).
Based on these compiled best estimates, the following data can be integrated to yield an estimated (rounded to nearest £100 000) annual loss of productivity of £362.6m, disability benefits of £280.2m and therefore overall cost to society for this cohort of £681.6m (Figure 2).
Figure 2.
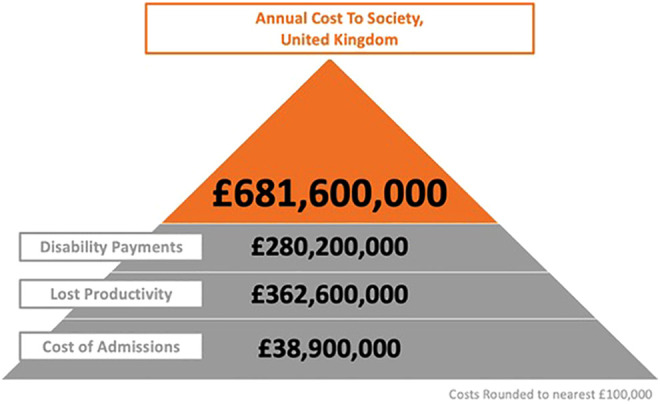
Estimate of overall annual cost to society, United Kingdom (myelopathy.org, United Kingdom). Total costs are round to nearest £100 000. In 2018, there were 4218 admissions, at an average cost of £9216 per admission: total cost of admission £38 900 000. Of these admissions, 2264 were within working age (defined as between 18 and 65), with an average 15.1 years remaining before retirement. Based on Pope et al 6 up to 45% (1019) could be unable to return to work. The weighted annual average salary for 2018 is £25 524. For those of working age, lifetime loss of productivity is £362.6m. The weighted average disability payment (2020) is £9021.72, which based on 16.5 years of life remaining for those of >65 years and 30.5 years (13.9+16.5) in those hospitalized with DCM at the average age of 51.1 years, gives a total annual cost of £280.2m.
How Accurate Is This Estimate?
These calculations are based upon the best available data today. However, the calculations have their intrinsic limitations, including reliance on integrating aggregate data from different sources. While this, therefore, represents our best and only estimate so far, these data are likely to change. In terms of whether the estimate is under or over, there is much to suggest that these figures could increase substantially.
One of the challenges for population-level research in DCM is case ascertainment: today, there isn’t a specific ICD code for DCM.29,30 Instead, studies must select from various codes which can only approximate to varying degrees of specificity. The aforementioned report took a conservative approach to case identification using 4 codes, whereas many other studies have used additional codes, for example, in a population analysis for degenerative spinal conditions in Finland. 31 For this reason, but also driven by recognized underdiagnosis, the true incidence, and prevalence of DCM is unknown. A recent meta-analysis of MRI cervical spine imaging in healthy cohorts identified a point prevalence of undiagnosed DCM of 2.4%. 3 While this will likely include much milder disability (less likely to require surgery, more likely to be employed), this would undoubtedly increase the economic estimates for DCM on population numbers alone. AO Spine RECODE-DCM 9 is seeking to establish consensus for an index term, to propose a unifying ICD code.
Furthermore, the hospital events data are unlikely to capture the complete cost of care; for example, data does not include primary care, while care within other specialties for subsequent disabilities such as pain, mental health, urology are not captured.
Traumatic spinal cord injury has more clearly defined the full economic impact of disease, benefiting from clear disease classification and within the UK, life-long follow up by Spinal Rehabilitation Services. This dataset has recently enabled an estimated lifetime cost of £1.43 billion based on current incidence rates. 32 While the average disability, and economic impact on average will be higher per individual with traumatic spinal cord injury, given the high costs among even those with less disability (e.g. for individuals with ASIA Impairment Scale, Grade D, the lifetime cost per individual is estimated to be £0.47million) this would suggest the direct care costs for DCM are an underestimate.
Financial outcomes are almost never considered in DCM research to date,13,14 and while a significant impact on unemployment is logical, to date, this has been poorly characterized. As part of AO Spine RECODE-DCM, 9 a core outcome set for DCM research is being developed. This includes some consideration of financial impact, which should better serve these analyses in the future.
Are Health-Economic Models Fully Generalizable?
Health economic analysis within DCM has so far relied on aggregate data, and its extrapolation across subgroups may represent a further knowledge gap. 18 Here we contextualize 3 important potential areas of Socio-Economic Status (SES), lower and middle vs. higher-income countries, and age, although there are likely more.
SES broadly refers to an individual’s economic and social circumstances and is typically assessed based on income, education, and/or occupation. The ONS (UK) uses occupation as an overall surrogate. 33 SES has been linked to a wide range of health problems, driven by its interaction with key determinants of health: access to healthcare, environmental exposure, and health behavior. 34
Within DCM, there are indicators of inequalities in care, albeit their impact is less certain. In a population study using the US National Inpatient Sample (2001-2010), private insurance status and white ethnicity were among independent predictors for receiving an anterior versus posterior surgical approach. 35 Moreover, in their follow up study, the authors also identified that private insurance status was an independent predictor for receiving a multi-level (3+) instrumented fusion. 36 Within the Myelopathy.org survey of people living with DCM, diagnostic delay was greater among those of black or African American ethnicity, with additional trends for those with lower educational qualifications. 6 In a recent evaluation of patients undergoing ACDF (all indications) from a single US center over 8 years (N = 2387), state-funded (Medicare / Medicaid) patients had more co-morbidities, longer hospitalization, and more frequently returned for reassessment within 90 days than insurance funded patients. 37 Taken together, this suggests that SES is likely an important determining factor of treatment costs and outcomes as well as a potential sources of unconsciousness bias during the decision making process for the individual’s treatment.
Of the health-economic data produced so far, surgery is considered cost-effective across age groups.20-23 However, age is recognized to impact DCM, associated with greater perioperative morbidity and a reduced, albeit still meaningful, amount of recovery. 38 It is noteworthy that while the subject has received significant research attention, the majority of studied cohorts remain young (average ages 60 to 65), and it is not certain whether this data is generalizable to higher age groups, as increasingly seen in higher income countries. 39 It is noteworthy that while a correlated surrogate, age is not necessarily the same as frailty, and this distinction may further need to be considered as this subgroup is addressed. 40
The requirements in lower and middle income countries [LMIC] will also be different. 41 Firstly, the different population demographics may have different epidemiology. Given the lack of robust health surveillance, and reduced access to diagnostic imaging, experiences from non-traumatic spinal cord injury (NTSCI) probably provide the only current estimates. Considering the few studies completed, the prevalence of degenerative spinal conditions remains high. For example, in a systematic review (2017) of NTSCI studies from sub-Saharan Africa, degenerative disorders accounted for 1.5-29% of cases. However, only 3 of the 19 studies included had access to an MRI scanner, with only 4-26% of patients receiving such a scan. 42 A study using MRI in Ghana found 75.9% of NTSCI cases had degenerative disease of the spine. 43 Secondly, management options may be influenced by the low-resource setting. 41 For example, diagnostic imaging, such as MRI, may not be available or may be too costly for patients or their families to afford, and alternative diagnostic/treatment options sought. Clinical follow-up is often a challenge to provide, given the large distances patients may live from centers, and the poor communications infrastructure. Furthermore, surgical techniques may have to be adapted—for example, spinal implants are often not paid for by public health systems in LMIC, given their often-significant cost. Despite these challenges, providers are exploring alternatives; conventional myelography has been demonstrated as a potentially safe and effective alternative for selecting appropriate candidates for surgery in these settings 44 while alternative, low cost, surgical implants are being sourced from manufacturers in India, China, and South Korea. 45 However, these factors will contribute to a different health-economic model.
Why Do We Need to Better Characterize the Socio-Economic Impact?
Despite its prevalence and clinical relevance, DCM remains under-recognized and under-treated. Increasing awareness has been identified as the number one priority by AO Spine RECODE-DCM [aospine.org/recode], fundamental to increasing diagnosis and timely treatment, but also much needed research investment. However, without a strong and robust health-economic argument for change, convincing healthcare leaders and funders to focus their attention on this public health priority will remain an uphill struggle. Notably these significant costs to the individual and society can be avoided, if DCM can be diagnosed and treated in a timely manner.
Conclusions
The Socio-Economic impact of DCM is a critical knowledge gap. Indicators, including the current best estimate from Myelopathy.org, suggest the cost of illness is substantial. By properly determining and disseminating the information on the socio-economic impact of DCM, one may anticipate a change in the individual and societal value of investing in the care of patients with DCM and in the research and innovation for DCM.
Acknowledgments
Further details on this priority, including how it was prioritized, why it was prioritized, and ongoing research activity can be found at aospine.org/recode/socio-economic-impact
Authors’ Note: Benjamin M. Davies and Richard Phillips are joint first authors; Vafa Rahimi-Movaghar and Mark R. N. Kotter are joint senior authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research priorities were organized and funded by AO Spine through the AO Spine Knowledge Forum Spinal Cord Injury, a focused group of international Spinal Cord Injury experts. AO Spine is a clinical division of the AO Foundation, which is an independent medically-guided not-for-profit organization. Study support was provided directly through the AO Spine Research Department. The cost-estimate of DCM to society was commissioned by Myelopathy.org and conducted by the Goffin Consultancy Canterbury, United Kingdom. MRNK is supported by an NIHR Clinician Scientist Award and BMD, a NIHR Clinical Doctoral Research Fellowship. Disclaimer: This report is independent research arising from a Clinician Scientist Award, CS-2015-15-023, supported by the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health and Social Care.
ORCID iDs: Benjamin M. Davies, MRCS, BSc, MPhil  https://orcid.org/0000-0003-0591-5069
https://orcid.org/0000-0003-0591-5069
Julio C. Furlan, MD, LLB, MBA, PhD, MSc  https://orcid.org/0000-0002-2038-0018
https://orcid.org/0000-0002-2038-0018
Jefferson R. Wilson, MD, PhD  https://orcid.org/0000-0001-5965-0305
https://orcid.org/0000-0001-5965-0305
Michael G. Fehlings, MD, PhD  https://orcid.org/0000-0002-5722-6364
https://orcid.org/0000-0002-5722-6364
References
- 1.Davies BM, Mowforth OD, Smith EK, Kotter MR. Degenerative cervical myelopathy. BMJ. 2018;360. doi:10.1136/bmj.k186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badhiwala JH, Ahuja CS, Akbar MA, et al. Degenerative cervical myelopathy—update and future directions. Nat Rev Neurol. 2020;16(2):108–124. doi:10.1038/s41582-019-0303-0 [DOI] [PubMed] [Google Scholar]
- 3.Smith SS, Stewart ME, Davies BM, Kotter MRN. The prevalence of asymptomatic and symptomatic spinal cord compression on magnetic resonance imaging: a systematic review and meta-analysis. Global Spine J. 2021;11(4):597–607. doi:10.1177/2192568220934496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehlings MG, Ibrahim A, Tetreault L, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine (Phila Pa 1976). 2015;40(17):1322–1328. doi:10.1097/BRS.0000000000000988 [DOI] [PubMed] [Google Scholar]
- 5.Fehlings MG, Tetreault LA, Riew KD, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J. 2017;7(3 suppl):70S–83S. doi:10.1177/2192568217701914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope DH, Mowforth OD, Davies BM, Kotter MRN. Diagnostic delays lead to greater disability in degenerative cervical myelopathy and represent a health inequality. Spine (Phila Pa 1976). 2020;45(6):368–377. doi:10.1097/BRS.0000000000003305 [DOI] [PubMed] [Google Scholar]
- 7.Oh T, Lafage R, Lafage V, et al. Comparing quality of life in cervical spondylotic myelopathy with other chronic debilitating diseases using the short form survey 36-health survey. World Neurosurg. 2017;106:699–706. doi:10.1016/j.wneu.2016.12.124 [DOI] [PubMed] [Google Scholar]
- 8.Mowforth OD, Davies BM, Kotter MR. Quality of life among informal caregivers of patients with degenerative cervical myelopathy: cross-sectional questionnaire study . Interact J Med Res. 2019;8(4). doi:10.2196/12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies BM, Khan DZ, Mowforth OD, et al. RE-CODE DCM (REsearch Objectives and Common Data Elements for Degenerative Cervical Myelopathy): a consensus process to improve research efficiency in DCM, through establishment of a standardized dataset for clinical research and the definition of the research priorities. Global Spine J. 2019;9(1 suppl):65S–76S. doi:10.1177/2192568219832855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petit-Zeman S, Firkins L, Scadding JW. The James Lind Alliance: tackling research mismatches. Lancet. 2010;376(9742):667–669. doi:10.1016/S0140-6736(10)60712-X [DOI] [PubMed] [Google Scholar]
- 11.Parkin D. Principles of health economics including: the notions of scarcity, supply and demand, distinctions between need and demand, opportunity cost, discounting, time horizons, margins, efficiency and equity. HealthKnowledge. 2017. Accessed January 1, 2020. www.healthknowledge.org.uk https://www.healthknowledge.org.uk/public-health-textbook/medical-sociology-policy-economics/4d-health-economics/principles-he
- 12.Higgins AM, Harris AH. Health economic methods: cost-minimization, cost-effectiveness, cost-utility, and cost-benefit evaluations. Crit Care Clin. 2012;28(1):11–24, v. doi:10.1016/j.ccc.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Davies BM, McHugh M, Elgheriani A, et al. The reporting of study and population characteristics in degenerative cervical myelopathy: a systematic review. PLoS One. 2017;12(3). doi:10.1371/journal.pone.0172564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies BM, McHugh M, Elgheriani A, et al. Reported outcome measures in degenerative cervical myelopathy: a systematic review. PLoS One. 2016;11(8). doi:10.1371/journal.pone.0157263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawthorne G, Richardson J, Day NA. A comparison of the assessment of quality of life (AQoL) with four other generic utility instruments. Ann Med. 2001;33(5):358–370. doi:10.3109/07853890109002090 [DOI] [PubMed] [Google Scholar]
- 16.Luyten J, Marais C, Hens N, De Schrijver K, Beutels P. Imputing QALYs from single time point health state descriptions on the EQ-5D and the SF-6D: a comparison of methods for hepatitis a patients. Value Health. 2011;14(2):282–290. doi:10.1016/j.jval.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Health and Care Excellence (NICE). Assessing Cost Effectiveness, The Guidelines Manual, NICE 2012. Accessed January 1, 2021. https://www.nice.org.uk/process/pmg6/chapter/assessing-cost-effectiveness [PubMed]
- 18.Witiw CD, Smieliauskas F, Fehlings MG. Health economics and the management of degenerative cervical myelopathy. Neurosurg Clin N Am. 2018;29(1):169–176. doi:10.1016/j.nec.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 19.Rahimi-Movaghar V, Moradi-Lakeh M, Rasouli MR, Vaccaro AR. Burden of spinal cord injury in Tehran, Iran. Spinal Cord. 2010;48(6):492–497. doi:10.1038/sc.2009.158 [DOI] [PubMed] [Google Scholar]
- 20.Witiw CD, Tetreault LA, Smieliauskas F, Kopjar B, Massicotte EM, Fehlings MG. Surgery for degenerative cervical myelopathy: a patient-centered quality of life and health economic evaluation. Spine J. 2016;17(1):15–25. doi:10.1016/j.spinee.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 21.Fehlings MG, Jha NK, Hewson SM, Massicotte EM, Kopjar B, Kalsi-Ryan S. Is surgery for cervical spondylotic myelopathy cost-effective? A cost-utility analysis based on data from the AOSpine North America prospective CSM study. J Neurosurg Spine. 2012;17(1 suppl):89–93. doi:10.3171/2012.6.AOSPINE111069 [DOI] [PubMed] [Google Scholar]
- 22.Schroeder GD, McKenzie JC, Casper DS, et al. The total cost to the health care system in Medicare and Medicaid patients for the treatment of cervical myelopathy. Clin Spine Surg. 2019;32(1):32–37. doi:10.1097/BSD.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 23.Alvin MD, Qureshi S, Klineberg E, et al. Cervical degenerative disease: systematic review of economic analyses. Spine (Phila Pa 1976). 2014;39(22 suppl 1):S53–S64. doi:10.1097/BRS.0000000000000547 [DOI] [PubMed] [Google Scholar]
- 24.Badhiwala JH, Ellenbogen Y, Khan O, et al. Comparison of the inpatient complications and health care costs of anterior versus posterior cervical decompression and fusion in patients with multilevel degenerative cervical myelopathy: a retrospective propensity score-matched analysis. World Neurosurg. 2020;134:e112–e119. doi:10.1016/j.wneu.2019.09.132 [DOI] [PubMed] [Google Scholar]
- 25.The Lancet Neurology. A focus on patient outcomes in cervical myelopathy. Lancet Neurol. 2019;18(7):615. doi:10.1016/S1474-4422(19)30168-1 [DOI] [PubMed] [Google Scholar]
- 26.Behrbalk E, Salame K, Regev GJ, Keynan O, Boszczyk B, Lidar Z. Delayed diagnosis of cervical spondylotic myelopathy by primary care physicians. Neurosurg Focus. 2013;35(1):E1. doi:10.3171/2013.3.FOCUS1374 [DOI] [PubMed] [Google Scholar]
- 27.Office for National Statistics UK. Average weekly earnings in Great Britain: June 2020. 2020. Accessed October 9, 2020. https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/bulletins/averageweeklyearningsingreatbritain/june2020
- 28.Organisation for Economic Co-operation and Development. Average Wages 2020. Accessed June 28, 2020. https://data.oecd.org/earnwage/average-wages.htm
- 29.Khan DZ, Khan MS, Kotter MR, Davies BM. Tackling research inefficiency in degenerative cervical myelopathy: illustrative review. JMIR Res Protoc. 2020;9(6). doi:10.2196/15922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan DZ, Davies BM, Kotter MRN. Spinal research—a field in need of standardization. J Rheumatol. 2020;47(4):633–634. doi:10.3899/jrheum.191225 [DOI] [PubMed] [Google Scholar]
- 31.Kotkansalo A, Leinonen V, Korajoki M, Salmenkivi J, Korhonen K, Malmivaara A. Surgery for degenerative cervical spine disease in Finland, 1999-2015. Acta Neurochir (Wien). 2019;161(10):2147–2159. doi:10.1007/s00701-019-03958-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDaid D, Park AL, Gall A, Purcell M, Bacon M. Understanding and modelling the economic impact of spinal cord injuries in the United Kingdom. Spinal Cord. 2019;57(9):778–788. doi:10.1038/s41393-019-0285-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Office for National Statistics UK. The national statistics socio-economic classification (NS-SEC Rebased on the Soc 2020). 2020. Accessed October 1, 2020. https://www.ons.gov.uk/methodology/classificationsandstandards/standardoccupationalclassificationsoc/soc2020/soc2020volume3thenationalstatisticssocioeconomicclassificationnssecrebasedonthesoc2020
- 34.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood). 2002;21(2):60–76. doi:10.1377/hlthaff.21.2.60 [DOI] [PubMed] [Google Scholar]
- 35.McClelland S, III, Marascalchi BJ, Passias PG, Protopsaltis TS, Frempong-Boadu AK, Errico TJ. Impact of race and insurance status on surgical approach for cervical spondylotic myelopathy in the United States: a population-based analysis. Spine (Phila Pa 1976). 2017;42(3):186–194. doi:10.1097/BRS.0000000000001693 [DOI] [PubMed] [Google Scholar]
- 36.McClelland S, III, Marascalchi BJ, Passias PG, Protopsaltis TS, Frempong-Boadu AK, Errico TJ. Operative fusion of multilevel cervical spondylotic myelopathy: impact of patient demographics. J Clin Neurosci. 2017;39:133–136. doi:10.1016/j.jocn.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 37.Rasouli JJ, Neifert SN, Gal JS, et al. Disparities in outcomes by insurance payer groups for patients undergoing anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2020;45(11):770–775. doi:10.1097/BRS.0000000000003365 [DOI] [PubMed] [Google Scholar]
- 38.Wilson JRF, Badhiwala JH, Jiang F, et al. The impact of older age on functional recovery and quality of life outcomes after surgical decompression for degenerative cervical myelopathy: results from an ambispective, propensity-matched analysis from the CSM-NA and CSM-I international, multi-center studies. J Clin Med. 2019;8(10):1708. doi:10.3390/jcm8101708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grodzinski B, Durham R, Mowforth O, Stubbs D, Kotter MRN, Davies BM. The effect of ageing on presentation, management and outcomes in degenerative cervical myelopathy: a systematic review. Age Ageing. 2021;50(3):705–715. doi:10.1093/ageing/afaa236 [DOI] [PubMed] [Google Scholar]
- 40.Wilson JRF, Badhiwala JH, Moghaddamjou A, Yee A, Wilson JR, Fehlings MG. Frailty is a better predictor than age of mortality and perioperative complications after surgery for degenerative cervical myelopathy: an analysis of 41,369 patients from the NSQIP database 2010-2018. J Clin Med. 2020;9(11):3491. doi:10.3390/jcm9113491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haldeman S, Nordin M, Chou R, et al. The Global Spine Care Initiative: world spine care executive summary on reducing spine-related disability in low- and middle-income communities. Eur Spine J. 2018;27(suppl 6):776–785. doi:10.1007/s00586-018-5722-x [DOI] [PubMed] [Google Scholar]
- 42.Musubire AK, Meya DB, Bohjanen PR, et al. A systematic review of non-traumatic spinal cord injuries in Sub-Saharan Africa and a proposed diagnostic algorithm for resource-limited settings. Front Neurol. 2017;8:618. doi:10.3389/fneur.2017.00618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gbadamosi H, Mensah YB, Asiamah S. MRI features in the non-traumatic spinal cord injury patients presenting at the Korle Bu Teaching Hospital, Accra. Ghana Med J. 2018;52(3):127–132. doi:10.4314/gmj.v52i3.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitya D, Punchak M, Bajunirwe F. Role of conventional myelography in diagnosis and treatment of degenerative spine disease in low-income communities: prospective study. World Neurosurg. 2017;104:161–166. doi:10.1016/j.wneu.2017.04.121 [DOI] [PubMed] [Google Scholar]
- 45.Park KB, Iv V. Spinal implants in resource-limited settings: keep it simple. World Neurosurg. 2016;86:36–38. doi:10.1016/j.wneu.2015.09.101101 [DOI] [PubMed] [Google Scholar]


