Abstract
Study Design
Narrative review.
Objective
The current review aimed to describe the role of existing techniques and emerging methods of imaging and electrophysiology for the management of degenerative cervical myelopathy (DCM), a common and often progressive condition that causes spinal cord dysfunction and significant morbidity globally.
Methods
A narrative review was conducted to summarize the existing literature and highlight future directions.
Results
Anatomical magnetic resonance imaging (MRI) is well established in the literature as the key imaging tool to identify spinal cord compression, disc herniation/bulging, and inbuckling of the ligamentum flavum, thus facilitating surgical planning, while radiographs and computed tomography (CT) provide complimentary information. Electrophysiology techniques are primarily used to rule out competing diagnoses. However, signal change and measures of cord compression on conventional MRI have limited utility to characterize the degree of tissue injury, which may be helpful for diagnosis, prognostication, and repeated assessments to identify deterioration. Early translational studies of quantitative imaging and electrophysiology techniques show potential of these methods to more accurately reflect changes in spinal cord microstructure and function.
Conclusion
Currently, clinical management of DCM relies heavily on anatomical MRI, with additional contributions from radiographs, CT, and electrophysiology. Novel quantitative assessments of microstructure, perfusion, and function have the potential to transform clinical practice, but require robust validation, automation, and standardization prior to uptake.
Keywords: cervical myelopathy, imaging, diagnosis, electrophysiology, spinal cord compression, magnetic resonance, neural damage, microstructure, assessment
Degenerative Cervical Myelopathy
Degenerative cervical myelopathy (DCM) describes spinal cord dysfunction resulting from extrinsic compression due to degenerative changes of the joints, vertebrae, and ligaments. 1 This collective diagnosis is the most common cause of spinal cord dysfunction, 2 representing an overlapping set of etiologies that include degenerative disc disease, spondylosis, ossified posterior longitudinal ligament (OPLL), ligamentum flavum hypertrophy (and/or ossification), and spondylolisthesis. The natural history of DCM is poorly defined, and differences likely exist between the various specific pathologies that are involved. 3 The pathophysiology of neurological injury in DCM is also not fully elucidated, but appears to involve a combination of mechanisms including static compression causing ischemia and hypoxia, demyelination, axonal injury, gray matter injury, gliosis, and cavitation.1,3,4 Dynamic injury from repetitive microtrauma due to instability and movement of the compressed cord may also contribute, but to what degree is unknown. 5 Spinal cord tension may also be an additional factor in the presence of cervical kyphosis. 6 The clinical presentation of DCM varies widely, owing to the complexity of spinal cord function and the multitude of pathways; typical symptoms include neck pain, referred pain, upper limb incoordination, gait imbalance, weakness, numbness, and sphincter dysfunction. 7 Accurate measurement of neurological and functional deficits is challenging, with the modified Japanese Orthopaedic Association (mJOA) score offering a convenient summary measure. 8 However, an array of clinical assessments is needed to fully characterize functional impairment.9-11 Clinical practice guidelines (CPGs) recommend surgical treatment for moderate or severe impairment, and for mild cases that show deterioration 12 ; however, there remains a lack of consensus on how to manage the most common clinical scenarios, including the optimal treatment of patients that present with mild DCM (Figure 1). This offers a potential role for imaging and electrophysiology biomarkers that (1) offer earlier diagnosis, (2) detect progressive spinal cord injury, or (3) predict deterioration.13,14
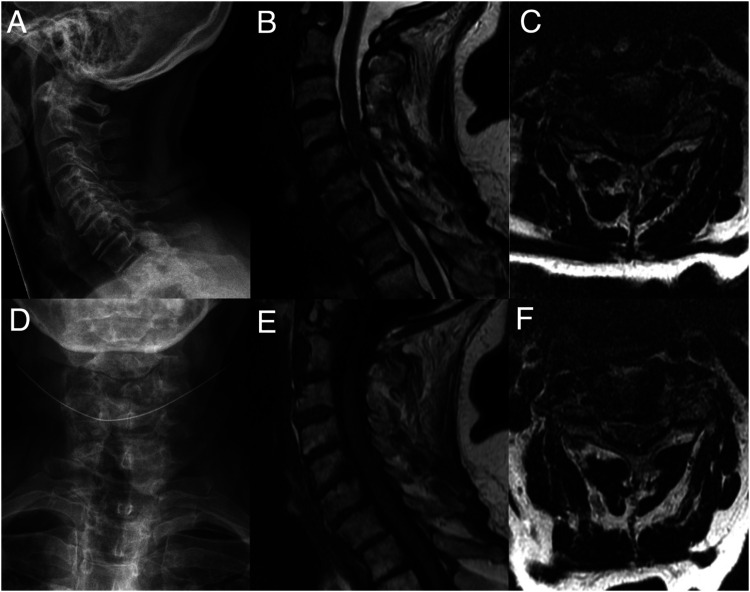
Figure 1.
A 49-year-old female presents with symptoms of axial neck pain, mild intermittent numbness of the hands, and mild fine motor dysfunction of the hands (clumsiness, dropping things). Her mJOA score is calculated as 16 (mild severity). Her neurological examination is normal, including no weakness, no sensory deficits, slightly brisk reflexes (2+ to 3+) without Hoffman’s or Babinski signs, and normal tandem gait. Radiographs (A, D) demonstrate normal alignment with loss of disc height at multiple levels. Anatomical T1-weighted (E) and T2-weighted images (B, C, F) demonstrate mild spinal cord compression secondary to bulging discosteophyte complexes and ligamentum flavum hypertrophy at C4-5 and C5-6 without intramedullary signal changes. Current CPGs do not offer a specific treatment recommendation for this scenario. 12 Key knowledge gaps are if advanced imaging and/or electrophysiology methods can: (a) confirm a diagnosis of DCM? B) localize which level(s) are symptomatic? (C) predict if she will deteriorate without surgery? (D) predict how much she will recovery with surgery? (E) monitor for neurological deterioration with repeated measures? CPG = clinical practice guideline; DCM = degenerative cervical myelopathy; mJOA = modified Japanese Orthopedic Association.
Conventional Anatomical Imaging
Radiography
Also known as plain films or X-ray images, radiographs are the oldest method of medical imaging dating back to 1895. Radiographs remain an important study in DCM as they offer a view of bony structures and alignment under physiological loads.15,16 Abnormalities in alignment such as kyphosis and spondylolisthesis are relatively common in DCM, whereas scoliosis is infrequently encountered, but these must be considered during surgical planning. 17 Lateral cervical spine radiographs allow measurement of sagittal balance and alignment parameters, including T1 slope, cervical lordosis, cervical sagittal vertebral axis (cSVA), chin brow angle, and K-line. 18 Anterior-posterior (AP) radiographs are helpful to identify scoliosis and measure Cobb angles. However, whole-spine standing radiographs are preferable to properly assess alignment, including measurement of global sagittal vertebral axis (SVA), as abnormalities in the cervical spine are interrelated with alignment of the lower segments. The visualization of bony anatomy with radiographs is limited, but it is usually sufficient to diagnose OPLL and estimate canal size. Historical definitions of canal stenosis were based on radiographs, including AP diameter <13 mm 19 or a Torg-Pavlov ratio of <.82; 20 however, these measures have largely been supplanted by direct visualization of the spinal cord with computed tomography (CT) myelography and magnetic resonance imaging (MRI). Oblique views offer visualization of the neural foramina and uncovertebral joints. Flexion and extension views provide several pieces of useful information, including identification of hypermobility, ankylosis, and the range of motion (ROM) that the patient will tolerate without symptoms of cord impingement (e.g., L’Hermitte’s phenomenon). Finally, radiographs also play an important role in following patients with DCM post-operatively, monitoring alignment and integrity of surgical implants.
Computed Tomography
In 1974, CT became commercially available, offering the first method of cross-sectional imaging of the spine; almost 50 years later, CT continues to be useful for management of patients with DCM. CT provides high resolution 3D images with excellent contrast between bone and soft tissue, allowing visualization of bony anatomy, spondylosis, osteophytes, calcified discs, OPLL, and ossified ligamentum flavum. The information gained from CT is often helpful in surgical planning for patients with DCM, including the decision between anterior and posterior approaches, the extent of bony decompression, bone quality, and planning screw trajectories. Furthermore, CT myelography (with intrathecal contrast) was used extensively prior to the availability of MRI for visualization of cord compression, and remains useful when MRI is not possible (e.g., due to incompatible metal implants).
Anatomical Magnetic Resonance Imaging
MRI is a complex technique based on the manipulation of hydrogen atoms within water molecules through pulses of radiofrequency (RF) electromagnetic radiation and reading the net magnetization with induction coils. Since its clinical emergence in the early 1980s, anatomical T1-weighted (T1w) and T2-weighted (T2w) magnetic resonance images have become the gold standard to visualize the spinal cord and to confirm the clinical diagnosis of DCM.21-24 However, it must be highlighted that neither compression of the spinal cord, defined as indentation, flattening, torsion, or circumferential narrowing, nor intramedullary signal change are accurate diagnostic markers of myelopathy on their own. 25 Spinal cord compression is highly sensitive (>98%) for myelopathy, 26 although some have argued that flexion and extension views are required to visualize dynamic compression in a fraction of patients.27-29 However, high-quality imaging with flexion/extension MRI is difficult due to motion associated with holding uncomfortable postures for several minutes. Furthermore, it appears that the spinal cord retains an abnormal shape in dynamic compression due to its mechanical plasticity, suggesting a potential role for morphometric (shape) analysis. 30 The specificity of cord compression for DCM is extremely low (<10%) because 5–50% of healthy asymptomatic patients show some element of cord compression or deformation.30-32 In contrast to spinal cord compression, intramedullary T2w hyperintensity has a high specificity of approximately 98% for DCM, but sensitivity is limited as it is only present in 50–85% of patients.23,26,33–42 Thus, the presence of spinal cord compression and signal change are useful to help confirm the presence of DCM, but MRI is not sufficient as a stand-alone diagnostic test.
As a tool for surgical planning, anatomical MRI allows visualization of the source (anterior vs posterior) and degree of spinal cord compression, and the number of levels at which spinal cord and/or exiting nerve roots require decompression. The modified K-line, based on mid-sagittal MRI images, predicts if a posterior approach will achieve sufficient decompression or if anterior decompression is necessary. 43 Furthermore, when planning spinal fusion procedures, the degree of degeneration of adjacent levels should be considered as these will undergo additional stress and may cause recurrent myelopathy/radiculopathy. 44 However, supine MRI images have limited utility to assess alignment and stability, and therefore should be routinely supplemented with upright and flexion/extension radiographs for these purposes.
Conventional MRI has also been studied as a tool to assess the severity of spinal cord damage that has occurred, both in terms of correlation with current neurological status and prediction of post-surgical outcomes.23,26,33,45–49 Various measurements of spinal cord compression have been investigated, including AP diameter, AP to left-right compression ratio (CR), 50 maximum canal compromise (MCC), 23 maximum spinal cord compromise (MSCC), and cross-sectional area (CSA).51-53 Okada et al 53 (1993) found that CSA positively correlated with current neurological status and post-operative recovery (using Japanese Orthopaedic Association [JOA] scores and recovery ratio, respectively), whereas CR and signal intensity ratio were not correlated with outcomes. 51 Interestingly, Smith et al (2013) reported a similar positive correlation between CSA and mJOA in lordotic patients, but an opposite negative correlation was found in kyphotic patients. 52 The remainder of cord compression measures (CR, MCC, and MSCC) have failed to show substantial correlation with the severity of neurological deficits.53,54 In contrast, MCC did show some promise in prediction of post-operative recovery, being retained in a multivariate clinical-radiological prediction model. 23
Similarly, numerous studies have investigated the utility of T2w hyperintensity and T1w hypointensity to measure spinal cord tissue injury, including the presence of these features, the signal change ratio (with normal cord or CSF as a reference), T2w/T1w ratio, the rostro-caudal length of signal change, and the area/volume of signal change.23,25,39,40,48,,55–57 Overall, T2w hyperintensity shows weak correlation with current neurological status, and weak or no correlation with post-operative recovery, while T1w hypointensity shows moderate correlation with both current neurological status and post-operative recovery.23,25,39,40,48,55–57 This is likely because T1w hypointensity is more specifically related to pathological changes of cavitation and cell loss, whereas T2w hyperintensity may reflect transient or permanent microstructural changes such as edema, gliosis, inflammation, demyelination, spongiform changes, necrosis, and cavitation.3,4,40 T1w hypointensity is relatively rare, occurring in only 19-30% of DCM patients, limiting its utility.25,39,58 In summary, conventional MRI consisting of T1w and T2w anatomical images offer only limited insight into pathological tissue changes, prompting the development of more advanced MRI sequences that assess specific features of microstructure and tissue injury.
Quantitative Imaging Techniques
The term “quantitative imaging” refers to the direct measurement of a physical property of tissue, such as proton density (PD) and the longitudinal, transverse, and effective transverse relaxation rates (R1, R2, and R2*, respectively) in MRI. Many imaging methods that are labeled as quantitative would be better described as semi-quantitative, as they provide only surrogate measures that rely on various assumptions, parameters, and calibration. 59 In contrast, strict quantitative imaging is scanner-independent, accurate, and reproducible. Unfortunately, quantitative methods often require long acquisition time, custom sequences, and specialized hardware, rendering them impractical in clinical scenarios. Furthermore, quantitative measures often vary widely between healthy subjects or with variables such as age, requiring substantial data to define normative ranges and for normalization. In this review, we utilize an inclusive definition of quantitative imaging to include surrogate measures and variables that are not measured in physical units (such as computed ratios), as long as they provide a meaningful measurement that can be compared between regions and/or subjects (Table 1). 60
Table 1.
Summary of quantitative and semi-quantitative imaging techniques for measuring aspects of microstructure and tissue injury in the human spinal cord and their status toward clinical translation.
| Imaging Modality | Imaging contrast/technique/sequence | Tissue property measured | Limitations | Current status of translation |
|---|---|---|---|---|
| MRI | Anatomical | |||
| T1-weighted | Cavitation, cell loss, gliosis, and necrosis | Semi-quantitative, moderate physiological specificity | Implemented | |
| T2-weighted | Edema, gliosis, inflammation, demyelination, spongiform changes, necrosis, and cavitation | Semi-quantitative, poor physiological specificity | Implemented | |
| T2*-weighted | Iron, calcium, gliosis, myelin, and perfusion | Semi-quantitative, moderate physiological specificity | Implemented | |
| Diffusion | ||||
| DTI | Axon integrity, myelin | Semi-quantitative, oversimplified model, not specific to axonal injury | Early clinical use | |
| DKI | Axon integrity | Multiple b shells, acquisition time | Human studies ongoing | |
| NODDI | Axon integrity | Multiple b shells, acquisition time, complex analysis | Human studies ongoing | |
| DDE | Axon integrity | Semi-quantitative, only measures a single direction | Pre-clinical studies | |
| Myelin imaging | ||||
| MT | Myelin | Semi-quantitative, poor SNR, 2 acquisitions (for MTR) | Early clinical use | |
| MWF | Myelin | Limited specificity for myelin, long acquisition time, and poor SNR | Human studies ongoing | |
| Other | ||||
| MRS | NAA (neuronal density), choline (cell turnover), myo-inositol (gliosis), and lactate (hypoxia) | Semi-quantitative, poor SNR, long acquisition time | Human studies ongoing | |
| BOLD fMRI | Deoxyhemoglobin (surrogate for brain activity via neurovascular coupling) | Semi-quantitative, poor SNR, long acquisition time | Human studies ongoing | |
| Perfusion MRI | Tissue perfusion | Semi-quantitative | Human studies ongoing | |
| PET | 18F-FDG | Tissue metabolism | Specialized equipment, radiotracers, long acquisition time, poor SNR | Human studies ongoing |
DDE = double diffusion encoding; FDG = fluorodeoxyglucose; fMRI = functional MRI; MT = magnetization transfer; MTR = MT ratio; MWF = myelin water-fraction; MRS = magnetic resonance spectroscopy; NODDI = Neurite orientation dispersion and density imaging; DKI = diffusion kurtosis imaging; SNR = signal-to-noise ratio.
Measures of Microstructure
A number of microstructural spinal cord MRI techniques have been identified for their clinical potential, namely diffusion tensor imaging (DTI), magnetization transfer (MT), myelin water-fraction (MWF), and magnetic resonance spectroscopy (MRS).61,62 The microstructural components of interest include myelin content (MT, MWF, DTI, MRS), axonal integrity (DTI, MRS), gliosis (MRS), hypoxia (MRS), and neuronal loss (MRS). However, acquisition of these sequences in the spine faces several challenges, including magnetic field inhomogeneity, respiratory- and cardiac-related motion, and the small size of the cord. 61 These challenges have partially been overcome with advances in acquisitions and analysis such as motion correction. A 2016 systematic review of the literature 62 identified a total of 25 studies utilizing these techniques to study DCM, with 22 utilizing DTI, 3 using MRS, and 1 employing MT.13,63–87 However, this review found that the status of clinical translation of these techniques was early and much further work was needed, although several recent studies have further advanced the field. In addition, studies utilizing T2*-weighted imaging have demonstrated its potential to measure tract-specific atrophy and microstructural changes. 88
Diffusion MRI involves measurement of water diffusivity after applied diffusion gradients in multiple directions, with DTI representing the diffusivity as three orthogonal vectors, termed “eigenvectors.” Various metrics can be calculated such as axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD), and fractional anisotropy (FA). In DCM, FA (ranging from 0 for isotropic diffusion to 1 for anisotropic diffusion), appears to have the strongest results. Four studies investigated the diagnostic accuracy of FA to identify DCM, with sensitivity ranging from 72 to 95% and specificity from 50 to 100%.63,65,66,80 These values were consistently higher than the sensitivity and specificity of T2WI hyperintensity. In six studies, FA correlated well with clinical measures such as JOA and mJOA.75,76,80,82,86,87 Among studies that investigated FA as a prognostic factor, only 1 of 3 found a correlation with post-operative JOA/mJOA, 82 reporting that higher pre-operative FA corresponded with improved JOA recovery ratio (P = .03). Other DTI metrics including MD, RD, and AD have demonstrated weaker and/or less consistent results.63,65,67 More recently, several studies showed that FA correlated well with focal and global deficits,52,88-91 identified pre-clinical tissue injury in subjects with asymptomatic cord compression,30,88,92,93 and detected neurological progression in DCM patients managed non-operatively.14,91 More complex diffusion imaging approaches have also been utilized, such as those employing multiple b-value shells (strength of diffusion gradients) to more accurately characterize axonal features, at the expense of acquisition time and analysis complexity. One approach used two shells and calculated mean kurtosis and root mean square displacement, reflecting the variation of diffusivity in different directions. Kurtosis showed some promise as a more accurate biomarker than FA in one study involving DCM patients. 94 Neurite orientation dispersion and density imaging (NODDI) is another complex approach that is more accurate than DTI for microstructural changes, evidenced by histopathological studies in MS.95,96 NODDI has been elegantly applied to patients with DCM in 2 studies, showing decreased axonal volume fraction in the WM, 97 and good prediction of post-operative outcome, 98 but conflicting results regarding correlation with mJOA.97,98 Another approach is to utilize double diffusion encoding (DDE), involving a perpendicular diffusion gradient to suppress CSF and cord edema, and subsequent gradients that measure diffusivity parallel to the cord, showing promise in a rat spinal cord injury (SCI) model, but yet to be studied in humans. 99
MT involves a pre-pulse that selectively excites water protons adjacent to macromolecules (primarily myelin), which causes proportional signal dropout, providing a surrogate measure of myelin density. MT ratio (MTR) is calculated as a signal intensity ratio between 2 scans with and without the pre-pulse, or the signal intensity can simply be compared in a ratio between the spinal cord and cerebrospinal fluid (MTCSF). MTR is more specific for myelin than MTCSF, due to contamination from T1-weighting. However, MTR requires additional acquisition time and accurate co-registration. In one study, MTR showed moderate correlation with global and focal deficits, 52 but in another study only anterior cord MTR correlated with deficits. 100 MTR also appears to be useful in a multiparametric acquisition to detect tissue injury and neurological deterioration.14,30 MT saturation is a similar metric derived from 3 acquisitions (T1-, MT-, and PD-weighted), which also correlates well with myelin, and when acquired with diffusion MRI, can estimate g-ratio (axon diameter to myelin sheath diameter), although this approach only showed weak differences in DCM subjects and no correlation with mJOA. 97
In contrast to MT, MWF employs a multi-echo sequence to estimate the T2 parameter in each compartment (myelin, tissue, and CSF). Some evidence suggests that MTR is more specific and accurate for myelin density, while MWF is sensitive to axon diameter, myelin thickness, and the resultant g-ratio. 101 One study investigated MWF and found no group difference between patients with DCM and healthy subjects, but weak correlations were found between MWF and somatosensory evoked potential (SSEP) latencies.103
MRS measures the concentration of key molecules, including N-acetylaspartate (NAA), choline (Cho), myo-inositol (MyoI), and lactate (Lac), which are known markers of neuron density, demyelination, gliosis, and hypoxia, respectively. The concentration of these molecules is often calculated as a ratio with creatine (Cr), which is relatively invariant in pathological states. Holly et al68 (2009) used MRS to demonstrate increased lactate in 1/3 of DCM patients and decreased NAA/Cr ratio compared to healthy controls. 67 The same group later reported that baseline NAA/Cr and Cho/NAA ratios correlated modestly with post-operative change in clinical scores, suggesting predictive value of these biomarkers. 103 Two studies found NAA/Cr to be decreased in DCM compared to healthy subjects, but no correlation was found with mJOA.67,78 In contrast, one study found superior results when combining biomarkers as a ratio Cho/NAA, which showed a significant correlation with mJOA (Pearson R=−.45, P<.01) 104 ; this approach is similar to multiparametric approaches that take advantage of multiple biomarkers to improve accuracy and overcome noise.
T2*-weighted (T2*w) imaging is based on the effective transverse relaxation (R2*), which is a physical parameter that reflects both spin-spin interactions (R2) and local magnetic field inhomogeneity. In the spinal cord, T2*w imaging demonstrates strong contrast between the gray and white matter, allowing segmentation between these anatomical compartments and calculation of CSA of specific sub-structures such as the dorsal columns. Grabher et al (2016) demonstrated tract-selective atrophy in the dorsal columns above the level of compression, which also correlated with light touch impairment. 88 Martin et al (2017) described a novel biomarker by computing the ratio of T2*w signal intensity between WM and GM, 52 which is believed to reflect iron content, demyelination, and tissue perfusion.105,106 This biomarker performed similarly well in comparison to FA and CSA, showing strong correlations with focal and global neurological deficits. 52 Furthermore, it demonstrated clinical utility in detecting subclinical tissue injury and neurological deterioration over time.14,30
Functional Spinal Cord Imaging
An alternative approach involves functional MRI (fMRI) of the spinal cord, which holds great potential to quantify neuronal activity within the spinal cord. Functional MRI relies upon the concept of neurovascular coupling, in which changes in neurological function produce corresponding changes in local blood flow and deoxyhemoglobin concentration. This contrast mechanism is termed blood oxygen level–dependent (BOLD) fMRI, which typically utilizes rapid T2*-weighted acquisitions, that are greatly affected by magnetic field inhomogeneity present in the spine. Study designs for fMRI may include motor tasks (e.g., finger tapping), sensory stimuli (e.g., cutaneous heat), or resting state (rs-fMRI). However, only one spinal cord rs-fMRI study has investigated the population with DCM, finding higher amplitude of low frequency fluctuations at all cervical cord segments in DCM vs healthy subjects, and in severe vs mild patients. 107
Perfusion Imaging
Spinal cord perfusion is the volume of blood delivered to a mass of spinal cord tissue over time. Similar to brain perfusion, spinal cord perfusion (1) exhibits a radical difference between GM (110 mL/min/100g) and WM (25 mL/min/100g), (2) is increased by hypoxia or hypercapnia, (3) is controlled by autoregulation, and (4) is increased by neuronal activity. 108 Cerebral perfusion usually utilizes arterial spin labeling (ASL) methods, but these are more difficult to implement in the spinal cord due to complex vascular anatomy. Instead, two studies have investigated spinal cord perfusion in DCM using dynamic susceptibility contrast (DSC) imaging with IV gadolinium, demonstrating that the degree of ischemia and hypoxia correlated with neurological status (mJOA), 109 and that post-operative improvement in perfusion corresponds with neurological recovery. 110
Metabolic Imaging With Positron Emission Tomography
Direct measurement of cellular metabolism is possible using positron emission tomography (PET), offering a unique and complimentary insight into the pathophysiology of DCM. Using the radiotracer 18F-fluorodeoxyglucose (18F-FDG), metabolism can be measured on standardized uptake value (SUV) images. One group has used this approach to demonstrate that individuals with DCM show decreased 18F-FDG uptake below the site of compression. 111 A follow-up study demonstrated that a subset of DCM patients had focally increased metabolism at the site of compression prior to surgery, and this subgroup had superior recovery after surgery than individuals that did not have this finding of hypermetabolism.112,113
Brain Imaging
A lesion affecting one area of the CNS causes distant structural and functional changes,114,115 a phenomenon described by the term “diaschisis.”116,117 Numerous research groups have taken advantage of this, using brain imaging to investigate spinal pathologies. One study found demonstrated cortical fMRI activation for tongue movements shifts significantly in patients following cervical SCI, revealing reorganization of the brain. 118 Several groups have investigated brain rs-fMRI for changes in connectivity and found cortical regions with altered activity in DCM vs healthy subjects,119-122 but only two of four studies showed correlation with clinical scores,120,121 and one showed prediction of post-operative recovery. 121 However, the clinical relevance of rs-fMRI remains unclear, as the analysis and interpretation is extremely complex, and further prospective studies with a priori hypotheses are needed. Similarly, microstructural imaging of cortical and subcortical structures has demonstrated rostral changes in SCI, 123 but this approach has yet to be investigated in DCM.
Electrophysiology of the Spinal Cord
Electrophysiology (EP) studies of the nervous system have been employed in clinical practice for numerous decades. The most common studies are electromyography (EMG), nerve conduction studies (NCS), SSEPs, and motor evoked potentials (MEPs). Unfortunately, these have only modest sensitivity and specificity for DCM and are time consuming, resource intensive, and uncomfortable for patients, limiting their practicality. However, EP studies fulfill niche roles in clinical management of DCM, including lesion localization, ruling out alternative diagnoses, and performing intraoperative monitoring. Similar to neuroimaging, the field of EP is rapidly evolving and several promising new techniques hold potential for clinical management of DCM, including contact heat evoked potentials (CHEPs) and magnetospinography (MSG).
Electromyography
EMG studies the motor system by measuring muscular activity with surface or inserted needle electrodes. Several results are typically obtained, including insertional activity, resting activity, and activity during voluntary contraction. Increased insertional activity is a sign of denervation (appearing within days of lower motor neuron injury), whereas decreased activity occurs with muscle atrophy. Abnormal resting activity may include fibrillations and sharp waves, which appear 1–4 weeks after lower motor neuron injury. The amplitude and number of motor unit action potentials (MUAPs) during voluntary contraction is also informative. Complete denervation causes an absence of MUAPs, myopathy shows decreased MUAP amplitude, and chronic denervation leads to increased MUAP amplitude due to reinnervation and enlargement of motor units. In DCM, EMG is often useful to diagnose and localize radiculopathy at specific spinal levels, but reduced amplitude corresponds with the degree of paresis resulting from compression of anterior horn cells or exiting nerve roots. 124 Intraoperative monitoring during decompressive surgery for DCM also utilizes EMG to detect nerve root irritation or injury. One study found that DCM patients demonstrate a longer time to peak EMG during walking, indicating that muscles take longer to fully contract. 125 Furthermore, the same study found deltoids and hamstrings remain abnormally active throughout gait, while another study reported longer coactivation of the quadriceps and hamstrings, 124 suggesting that DCM patients show compensatory activity to maintain balance. Another study found that abnormal EMG findings in non-myelopathic patients with asymptomatic spinal cord compression (ASCC) predicted faster onset to myelopathy. 126
Nerve Conduction Studies
NCS measures the conduction velocity through a segment of a peripheral nerve, between the site of stimulation and the site of recording, identifying lesions as sites of decreased velocity. The role of NCS in DCM is to diagnose peripheral nerve lesions such as entrapment, neuropathy, or brachial plexopathy. Unfortunately, it is common for patients with DCM to get mislabeled with unilateral or bilateral carpal tunnel and/or cubital tunnel syndrome, and NCS is the primary diagnostic study to rule these out. Given the depth and surrounding structures around the spinal cord and exiting nerve roots, it is not possible to perform NCS at proximal sites, thus limiting its utility to diagnose myelopathy and radiculopathy.
Somatosensory Evoked Potentials
SSEPs are performed by applying electrical stimulation to a peripheral nerve and measuring evoked potentials using surface electrodes in positions over the spine, brainstem, and somatosensory cortex. Abnormal SSEPs are found in lesions of the dorsal column-medial lemniscus pathway and typically show decreased amplitude, increased latency, or increased dispersion of waveform. Numerous averages are required to overcome noise, but myelin damage and axonal loss can cause inconsistent conduction, potentially canceling out the signal using time-locked averaging and requiring advanced signal processing.127,128 In DCM, SSEPs are commonly utilized for intraoperative monitoring with good sensitivity and specificity for iatrogenic injury. 129 In some patients, one can observe improvements in SSEPs within minutes of decompression, indicating a component of reversible pathophysiology (Figure 2). As a diagnostic tool, upper limb SSEPs generally show poor sensitivity, ranging from 33 to 59%,72,83,102,130 whereas lower limb SSEPs showed 100% sensitivity in 2 small studies.131,132 The specificity of SSEPs for cervical myelopathy is estimated at 70%. 72 Various studies have also reported that abnormal SSEP latencies correspond with greater severity of DCM,72,82,83 and demyelination of the dorsal columns (measured using DTI or MWF imaging).83, However, inconsistent results have been reported regarding the prognostic value of SSEP abnormalities, with one study finding improved outcomes with the absence of SSEP abnormalities, 133 while the remainder of studies showed no significant associations.72,82,130 Another study found that abnormal SSEPs in non-myelopathic patients with ASCC predicted faster onset to myelopathy. 126 Further prospective studies utilizing standardized SSEP techniques are needed to fully elucidate the clinical utility of this assessment.
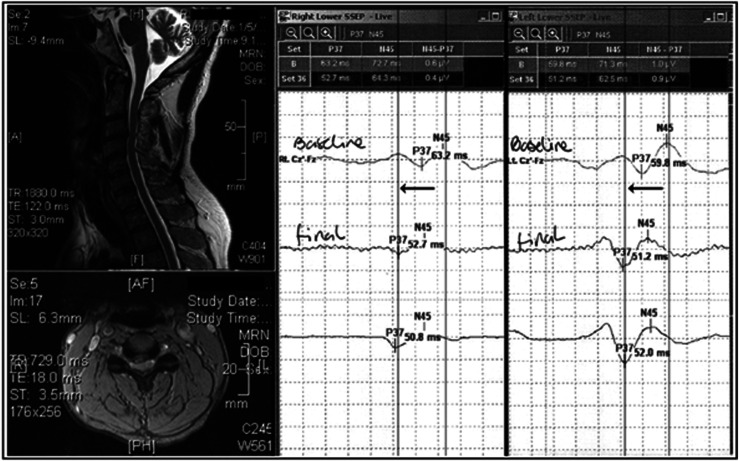
Figure 2.
Intraoperative SSEP recording during surgical decompression for cervical compressive myelopathy. Typical tibial stimuli at 3HZ, 200 seconds square wave pulses with a 10–20 cranial montage. In the MRI image, cord compression is prominent at C3/4. At baseline, the SSEPs are markedly prolonged bilaterally at 63.2 and 59.8 milliseconds for the right and left sides, respectively. Following spinal cord decompression, there is an improvement within a few minutes of 12.4 and 7.8 milliseconds, respectively. Figure courtesy of Dr James Guest.
Motor Evoked Potentials
MEPs are performed via electrical or transcranial magnetic stimulation of the motor cortex, while recording from distal muscles. Similar to SSEPs, amplitude and latency are the primary measures used in MEPs. MEPs are widely utilized in intraoperative monitoring, providing a highly sensitive and specific warning for cord compromise. 129 Prolonged central motor conduction time (CMCT), calculated by subtracting the peripheral conduction time from the total MEP latency, has been utilized to identify various pathological conditions such as multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and DCM. A complex technique called triple stimulation MEP has been developed to help isolate anterior horn cell dysfunction and diagnose ALS. In DCM, MEPs have been showed to be somewhat more sensitive and specific for cervical myelopathy than SSEPs, with sensitivity ranging from 50% to 92%72,133,134 and specificity of 74–100%.72,133 Prolonged CMCT also correlates well with clinical measures of DCM, 135 while improvement of CMCT after surgery corresponds with recovery in one of two studies,135,136 and pre-operative CMCT appears to be predictive of post-operative recovery.135,137 One study found superior performance using MEP recruitment parameters rather than MEP latencies, showing better responsiveness to post-operative recovery. 136 Another study found that abnormal MEPs in non-myelopathic patients with ASCC predicted faster onset to myelopathy. 126 Overall, MEPs are a valued tool in measuring spinal cord function, and further research efforts will help to establish their utility for prognostication.
Contact Heat Evoked Potentials
CHEPs are a newer technique that utilize a thermal cutaneous stimulus to selectively excite heat receptors, which travels through Aδ, and C nerve fibers and the spinothalamic tract, and can be measured as a cortical evoked potential. 138 In DCM, CHEPs have demonstrated high sensitivity (95%) compared with SSEPs (24%) at the maximally compressed level, while also detecting abnormal sensation above and below the compressed level. 139 Furthermore, CHEPs showed responsiveness to DCM patients that clinically deteriorated, offering a possible role in clinical monitoring. Future studies investigating this promising new tool are needed to determine its responsiveness after surgical intervention, and its utility in predicting outcomes.
Magnetospinography
MSG is a novel technology that is the spinal cord analog of magnetoencephalography (MEG) for the brain. MSG offers non-invasive measurement and 3D visualization of the electrical activity within the spinal cord or peripheral nerves (termed “magnetoneurography”), blurring the lines between the fields of electrophysiology and imaging. MSG uses an array of 120 superconducting quantum interference device detectors and must be conducted in a controlled environment with magnetic shielding. 140 Pilot studies have demonstrated that MSG can quantify and visualize electrical conduction through the cervical cord, cauda equina, and peripheral nerves.141,142 It was also demonstrated that MSG identified the site of dorsal column conduction block in one patient with DCM. 141 However, the utility of this technology may be limited by issues of physiological noise and movement, which are minimized in MEG by the closer proximity of the brain to the skin surface and by placing the head in a tight-fitting helmet. High quality prospective research in patients with DCM is needed to determine the utility of this exciting new device.
Future Directions
Looking ahead, clinical management of DCM will almost certainly be driven by advances in imaging and electrophysiology that can accurately quantify disease and direct treatment decisions, often dubbed “personalized medicine.” At present, major knowledge gaps exist in clinical management of patients with mild DCM and ASCC, including how to monitor these individuals for deterioration and when to decide on surgical treatment. However, this trend toward data-driven treatment faces several hurdles, including (1) development of novel imaging/EP techniques to generate accurate data, (2) histopathological or multimodal validation to ensure that biomarkers reflect true pathological changes, (3) the development of robust automatic analysis, and (4) acceptance and integration into clinical workflows.
Novel Techniques
As scientific discovery charters new territories and expands our understanding of neurophysiology, we can expect new technologies that exceed our imagination. One needs only to consider the period before MRI to realize how difficult it is to anticipate the future, and how one technological breakthrough can revolutionize medicine and clinical research. Among the vast array of promising technologies is functional near infrared spectroscopy, which can non-invasively measure neuronal function in freely moving individuals. 143 Another consideration is the emergence of genomics, transcriptomics, and proteomics, which have already found specific molecular blood markers of ischemia and neural injury in DCM, 144 and how these might be integrated with molecular imaging such as PET.
Validation
With increasing acceptance and reliance on quantitative data and surrogate measures, we must not forget that their validation is of critical importance. Surrogate measures of neural injury and dysfunction may be useful for clinical applications, but failure to identify their fundamental assumptions, confounding factors, variations of normal, and limitations has the grave potential to cause medical errors. Ground truth data is often not available, and histopathological studies in cadavers or animals should be conducted to determine if microstructural measures reflect reality. Alternatively, cross-validation of novel imaging or functional measures can be performed by acquiring multiple confirmatory imaging, electrophysiology, clinical, and/or molecular measures.
Quantitative Analysis
Complex data requires complex analysis, and this must become robust, automated, and easily interpretable to successfully enter into clinical use. Powerful tools for motion correction, outlier/artifact rejection, and template-based analysis have emerged, and these suggest the possibility of achieving full automation of quantitative image analysis (Figure 3). 145 Multivariate models are often necessary to integrate data, and more complex analytic methods like deep learning may produce superior performance for diagnosis, clinical measurement, and outcome prediction.146-148 However, the pros and cons of more complex analysis methods must be weighed, as these can make it more difficult to identify sources of error and bias.
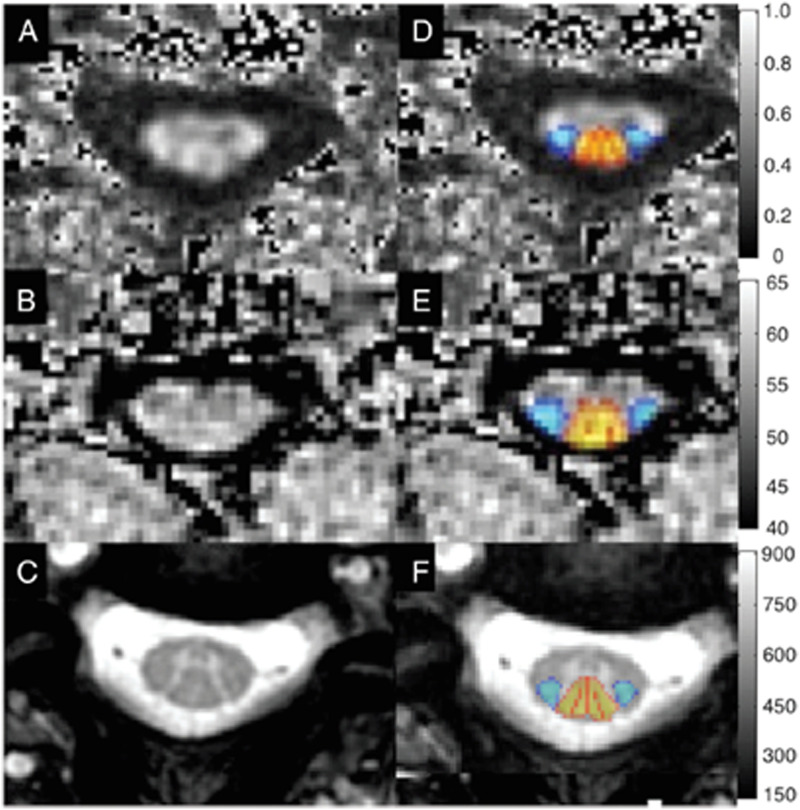
Figure 3.
Axial images through the cervical spinal cord in a healthy subject, including FA map from a DTI acquisition (A, D), MTR map from a MT acquisition (B, E), and T2*-weighted images (C, F). Template-based analysis using the SCT (D–F) allows for probabilistic estimation of tract-specific metrics from the lateral corticospinal tracts (blue) and dorsal columns (yellow–red). DTI = diffusion tensor imaging; FA = fractional anisotropy; MT = magnetization transfer; MTR = MT ratio; SCT = spinal cord toolbox.
Clinical Uptake
Clinicians need to be engaged and buy in to the concept of quantitative outputs, requiring a paradigm shift from current practice (e.g., anatomical MRI) that will almost certainly occur incrementally rather than instantaneously. To ensure safety and promote uptake, quantitative measures should first be introduced as a decision-making adjunct that supports existing practices. 14
In addition, a massive effort of knowledge translation will be needed to move these techniques from the hands of researchers to clinicians and technicians, and post-implementation studies will need to confirm efficacy and refine methods.
Conclusions
Clinical management of DCM currently relies heavily on anatomical MRI for diagnosis and decision-making, and to a lesser degree on electrophysiology to rule out competing diagnoses and radiographs/CT for surgical planning. Future clinical practice may shift dramatically as novel imaging and electrophysiological measures become available that can accurately characterize tissue injury and neurological dysfunction. Although several barriers must be overcome, it seems highly likely that quantitative imaging and electrophysiology will revolutionize the care of patients with DCM, providing earlier diagnosis, accurate localization, monitoring for deterioration and neurological recovery, prediction of outcomes, and standardized practice.
Acknowledgments
Further details on this priority, including how it was prioritized, why it was prioritized, and on-going research activity can be found at www.aospine.org/recode/imaging-techniques.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article: The research priorities were organized and funded by AO Spine through the AO Spine Knowledge Forum Spinal Cord Injury, a focused group of international Spinal Cord Injury experts. AO Spine is a clinical division of the AO Foundation, which is an independent medically-guided not-for-profit organization. Study support was provided directly through the AO Spine Research Department. MRNK is supported by the National Institute for Health Research (NIHR) Brain Injury MedTech Co-operative based at Cambridge University Hospitals NHS Foundation Trust and University of Cambridge, and BMD a NIHR Clinical Doctoral Research Fellowship. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care. PF is funded by a SNF Eccellenza Professorial Fellowship grant (PCEFP3_181362/1).
ORCID iDs
Aria Nouri https://orcid.org/0000-0002-4965-3059
Jefferson R. Wilson https://orcid.org/0000-0001-5965-0305
Michael G. Fehlings https://orcid.org/0000-0002-5722-6364
Benjamin M. Davies https://orcid.org/0000-0003-0591-5069
References
- 1.Nouri A, Tetreault L, Singh A, Karadimas S, Fehlings M. Degenerative cervical myelopathy: epidemiology, genetics and pathogenesis. Spine (Phila Pa 1976) 2015;40(12):E675-E693. [DOI] [PubMed] [Google Scholar]
- 2.Boogaarts HD, Bartels RH. Prevalence of cervical spondylotic myelopathy. Eur Spine J. 2015;2:139-141. [DOI] [PubMed] [Google Scholar]
- 3.Karadimas SK, Erwin WM, Ely CG, Dettori JR, Fehlings MG. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine. 2013;38:S21-S36. [DOI] [PubMed] [Google Scholar]
- 4.Akter F, Yu X, Qin X, Yao S, Nikrouz P, Syed YA, et al. The pathophysiology of degenerative cervical myelopathy and the physiology of recovery following decompression. Front Neurosci. 2020;14:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson J, Nouri A, Krueger B, Lakomkin N, Nasser R, Gimbel D, et al. Degenerative cervical myelopathy: A clinical review. Yale J Biol Med. 2018;91:43-48. [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida K, Nakajima H, Sato R, Yayama T, Mwaka ES, Kobayashi S, et al. Cervical spondylotic myelopathy associated with kyphosis or sagittal sigmoid alignment: Outcome after anterior or posterior decompression. J Neurosurg Spine. 2009;11:521-528. [DOI] [PubMed] [Google Scholar]
- 7.Badhiwala JH, Ahuja CS, Akbar MA, Ahuja CS, Akbar MA, Witiw CD, Nassiri F, Furlan JC, et al. Degenerative cervical myelopathy - update and future directions. Nat Rev Neurol. 2020;16:108-124. [DOI] [PubMed] [Google Scholar]
- 8.Benzel EC, Lancon J, Kesterson L, Hadden T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4:286-295. [DOI] [PubMed] [Google Scholar]
- 9.Kalsi-Ryan S, Riehm LE, Tetreault L, Martin A, Teoderascu F, Massicotte E, et al. Characteristics of upper limb impairment related to degenerative cervical myelopathy: Development of a sensitive hand assessment (graded redefined assessment of strength, sensibility, and prehension version myelopathy). Neurosurgery. 2020;86:E292-E299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalsi-Ryan S, Rienmuller AC, Riehm LE, Chan C, Jin D, Martin AR, et al. Quantitative assessment of gait characteristics in degenerative cervical myelopathy: A prospective clinical study. J Clin Med. 2020;9:752-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalsi-Ryan S, Singh A, Massicotte EM, Arnold PM, Brodke DS, Norvell DC, et al. Ancillary outcome measures for assessment of individuals with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38:S111-S122. [DOI] [PubMed] [Google Scholar]
- 12.Fehlings MG, Tetreault L, Aarabi B, Middleton JW, Aarabi B, Arnold PM, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: Recommendations for patients with mild, moderate and severe disease and non-myelopathic patients with evidence of cord compression. Global Spine J. 2017;7:70S-83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin AR, Aleksanderek I, Cohen-Adad J, Tarmohamed Z, Tetreault L, Smith N, et al. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. Neuroimage Clin. 2016;10:192-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin AR, De Leener B, Cohen-Adad J, Kalsi-Ryan S, Cadotte DW, Wilson JR, et al. Monitoring for myelopathic progression with multiparametric quantitative MRI. PloS One. 2018;13:e0195733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ames CP, Blondel B, Scheer JK, et al. Cervical radiographical alignment: Comprehensive assessment techniques and potential importance in cervical myelopathy. Spine (Phila Pa 1976);2013;38:S149-S160. [DOI] [PubMed] [Google Scholar]
- 16.Mohanty C, Massicotte EM, Fehlings MG, Shamji MF. Association of preoperative cervical spine alignment with spinal cord magnetic resonance imaging hyperintensity and myelopathy severity: Analysis of a series of 124 cases. Spine (Phila Pa 1976);2015;40:11-16. [DOI] [PubMed] [Google Scholar]
- 17.Nouri A, Martin A, Tetreault L, Nater A, Kato S, Nakashima H, et al. MRI analysis of the combined prospectively collected AOSpine North America and International Data: The prevalence and spectrum of pathologies in a global cohort of patients with degenerative cervical myelopathy. Spine (Phila Pa 1976):2017;42:1058-1067. [DOI] [PubMed] [Google Scholar]
- 18.Fujiyoshi T, Yamazaki M, Kawabe J, Endo T, Furuya T, Koda M, et al. A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: The K-line. Spine (Phila Pa 1976) 2008;33:E990–E993. [DOI] [PubMed] [Google Scholar]
- 19.Bajwa NS, Toy JO, Young EY, Ahn NU. Establishment of parameters for congenital stenosis of the cervical spine: An anatomic descriptive analysis of 1,066 cadaveric specimens. Eur Spine J. 2012;21:2467-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlov H, Torg JS, Robie B, Jahre C. Cervical spinal stenosis: Determination with vertebral body ratio method. Radiology. 1987;164:771-775. [DOI] [PubMed] [Google Scholar]
- 21.Bakhsheshian J, Mehta VA, Liu JC. Current diagnosis and management of cervical spondylotic myelopathy. Global Spine J. 2017;7:572-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehlings MG, Wilson JR, Kopjar B, Yoon ST, Arnold PM, Massicotte EM, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: Results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013;95:1651-1658. [DOI] [PubMed] [Google Scholar]
- 23.Nouri A, Tetreault L, Zamorano JJ, Dalzell K, Davis AM, Mikulis D, et al. Role of magnetic resonance imaging in predicting surgical outcome in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2015;40:171-178. [DOI] [PubMed] [Google Scholar]
- 24.Arvin B, Kalsi-Ryan S, Karpova A, Mercier D, Furlan JC, Massicotte EM, et al. Postoperative magnetic resonance imaging can predict neurological recovery after surgery for cervical spondylotic myelopathy: A prospective study with blinded assessments. Neurosurgery. 2011;69:362-368. [DOI] [PubMed] [Google Scholar]
- 25.Nouri A, Martin AR, Tetreault L, Reihani-Kermani H, Riehm LE, Fehlings MG. The relationship between MRI signal intensity changes, clinical presentation, and surgical outcome in degenerative cervical myelopathy: Analysis of a global cohort. Spine. 2017;42:1851-1858. [DOI] [PubMed] [Google Scholar]
- 26.Harrop JS, Naroji S, Maltenfort M, Anderson DG, Albert T, Ratliff JK, et al. Cervical myelopathy: A clinical and radiographic evaluation and correlation to cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2010;35:620-624. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Zeitoun D, Rangel A, Lazennec JY, Catonne Y, Pascal-Moussellard H. Preoperative evaluation of the cervical spondylotic myelopathy with flexion-extension magnetic resonance imaging: About a prospective study of fifty patients. Spine (Phila Pa 1976). 2011;36:E1134-E1139. [DOI] [PubMed] [Google Scholar]
- 28.Ruangchainikom M, Daubs MD, Suzuki A, Hayashi T, Weintraub G, Lee CJ, et al. Effect of cervical kyphotic deformity type on the motion characteristics and dynamic spinal cord compression. Spine (Phila Pa 1976). 2014;39:932-938. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki A, Daubs MD, Inoue H, Hayashi T, Aghdasi B, Montgomery SR, et al. Prevalence and motion characteristics of degenerative cervical spondylolisthesis in the symptomatic adult. Spine (Phila Pa 1976). 2013;38:E1115-E1120. [DOI] [PubMed] [Google Scholar]
- 30.Martin AR, De Leener B, Cohen-Adad J, Cadotte DW, Nouri A, Wilson JR, et al. Can microstructural MRI detect subclinical tissue injury in subjects with asymptomatic cervical spinal cord compression? A prospective cohort study. BMJ Open. 2018;8.e019809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato F, Yukawa Y, Suda K, Yamagata M, Ueta T. Normal morphology, age-related changes and abnormal findings of the cervical spine. Part II: Magnetic resonance imaging of over 1,200 asymptomatic subjects. Eur Spine J. 2012;21:1499-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SS, Stewart ME, Davies BM, Kotter MRN. The prevalence of asymptomatic and symptomatic spinal cord compression on magnetic resonance imaging: A systematic review and meta-analysis. Global Spine J. 2021:11:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchida K, Nakajima H, Sato R, Kokubo Y, Yayama T, Kobayashi S, et al. Multivariate analysis of the neurological outcome of surgery for cervical compressive myelopathy. J Orthop Sci. 2005;10:564-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3:33-45. [DOI] [PubMed] [Google Scholar]
- 35.Singh A, Crockard HA, Platts A, Stevens J. Clinical and radiological correlates of severity and surgery-related outcome in cervical spondylosis. J Neurosurg. 2001;94:189-198. [DOI] [PubMed] [Google Scholar]
- 36.Shin JJ, Jin BH, Kim KS, Cho YE, Cho WH. Intramedullary high signal intensity and neurological status as prognostic factors in cervical spondylotic myelopathy. Acta Neurochir. 2010;152:1687-1694. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima H, Yukawa Y, Ito K, Machino M, Kanbara S, Morita D, et al. Prediction of lower limb functional recovery after laminoplasty for cervical myelopathy: Focusing on the 10-s step test. Eur Spine J. 2012;21:1389-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CJ, Lyu RK, Lee ST, Wong YC, Wang LJ. Intramedullary high signal intensity on T2-weighted MR images in cervical spondylotic myelopathy: Prediction of prognosis with type of intensity. Radiology. 2001;221:789-794. [DOI] [PubMed] [Google Scholar]
- 39.Wada E, Yonenobu K, Suzuki S, Kanazawa A, Ochi T. Can intramedullary signal change on magnetic resonance imaging predict surgical outcome in cervical spondylotic myelopathy? Spine (Phila Pa 1976). 1999;24:455-461; discussion 62. [DOI] [PubMed] [Google Scholar]
- 40.Vedantam A, Jonathan A, Rajshekhar V. Association of magnetic resonance imaging signal changes and outcome prediction after surgery for cervical spondylotic myelopathy. J Neurosurg Spine. 2011;15:660-666. [DOI] [PubMed] [Google Scholar]
- 41.Mastronardi L, Elsawaf A, Roperto R, Bozzao A, Caroli M, Ferrante M, et al. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J Neurosurg Spine. 2007;7:615-622. [DOI] [PubMed] [Google Scholar]
- 42.Salem HM, Salem KM, Burget F, Bommireddy R, Klezl Z. Cervical spondylotic myelopathy: The prediction of outcome following surgical intervention in 93 patients using T1- and T2-weighted MRI scans. Eur Spine J. 2015;24:2930-2935. [DOI] [PubMed] [Google Scholar]
- 43.Taniyama T, Hirai T, Yamada T, Yuasa M, Enomoto M, Yoshii T, et al. Modified K-line in magnetic resonance imaging predicts insufficient decompression of cervical laminoplasty. Spine (Phila Pa 1976). 2013;38:496-501. [DOI] [PubMed] [Google Scholar]
- 44.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: The consequences of spinal fusion? Spine J. 2004;4:190S-4S. [DOI] [PubMed] [Google Scholar]
- 45.Karpova A, Arun R, Kalsi-Ryan S, Massicotte EM, Kopjar B, Fehlings MG. Do quantitative magnetic resonance imaging parameters correlate with the clinical presentation and functional outcomes after surgery in cervical spondylotic myelopathy? A prospective multicenter study. Spine (Phila Pa 1976). 2014;39:1488–1497. [DOI] [PubMed] [Google Scholar]
- 46.Li F, Chen Z, Zhang F, Shen H, Hou T. A meta-analysis showing that high signal intensity on T2-weighted MRI is associated with poor prognosis for patients with cervical spondylotic myelopathy. J Clin Neurosci. 2011;18:1592-1595. [DOI] [PubMed] [Google Scholar]
- 47.Arvin B, Kalsi-Ryan S, Mercier D, Furlan JC, Massicotte EM, Fehlings MG. Preoperative magnetic resonance imaging is associated with baseline neurological status and can predict postoperative recovery in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38:1170-1176. [DOI] [PubMed] [Google Scholar]
- 48.Vedantam A, Rajshekhar V. Change in morphology of intramedullary T2-weighted increased signal intensity after anterior decompressive surgery for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2014;39:1458-1462. [DOI] [PubMed] [Google Scholar]
- 49.Rhee JM, Heflin JA, Hamasaki T, Freedman B. Prevalence of physical signs in cervical myelopathy: A prospective, controlled study. Spine (Phila Pa 1976). 2009;34:890-895. [DOI] [PubMed] [Google Scholar]
- 50.Kameyama T, Hashizume Y, Ando T, Takahashi A. Morphometry of the normal cadaveric cervical spinal cord. Spine. Phila Pa. 2077-81;1976)(1994):19. [DOI] [PubMed] [Google Scholar]
- 51.Smith JS, Lafage V, Ryan DJ, Shaffrey CI, Schw2ab FJ, Patel AA, et al. Association of myelopathy scores with cervical sagittal balance and normalized spinal cord volume: Analysis of 56 preoperative cases from the AOSpine North America Myelopathy study. Spine (Phila Pa 1976). 2013;38:S161-S170. [DOI] [PubMed] [Google Scholar]
- 52.Martin AR, De Leener B, Cohen-Adad J, Cadotte DW, Kalsi-Ryan S, Lange SF, et al. A novel mri biomarker of spinal cord white matter injury: T2*-weighted white matter to grey matter signal intensity ratio. AJNR. 2017;38:1266-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okada Y, Ikata T, Yamada H, Sakamoto R, Katoh S. Magnetic resonance imaging study on the results of surgery for cervical compression myelopathy. Spine. Phila Pa. 2024;1976)(1993):18.-9 [DOI] [PubMed] [Google Scholar]
- 54.Hilton B, Tempest-Mitchell J, Davies BM, Francis J, Mannion RJ, Trivedi R, et al. Cord compression defined by MRI is the driving factor behind the decision to operate in Degenerative Cervical Myelopathy despite poor correlation with disease severity. PLoS One. 2019;14:e0226020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajasekaran S, Yerramshetty JS, Chittode VS, Kanna RM, Balamurali G, Shetty AP. The assessment of neuronal status in normal and cervical spondylotic myelopathy using diffusion tensor imaging. Spine (Phila Pa 1976). 2014;39:1183–1189. [DOI] [PubMed] [Google Scholar]
- 56.Tetreault LA, Dettori JR, Wilson JR, Singh A, Nouri A, Fehlings MG, et al. Systematic review of magnetic resonance imaging characteristics that affect treatment decision making and predict clinical outcome in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38:S89-S110. [DOI] [PubMed] [Google Scholar]
- 57.Wada E, Ohmura M, Yonenobu K. Intramedullary changes of the spinal cord in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1995;20:2226-2232. [DOI] [PubMed] [Google Scholar]
- 58.Chibbaro S, Benvenuti L, Carnesecchi S, Marsella M, Pulerà F, Serino D, et al. Anterior cervical corpectomy for cervical spondylotic myelopathy: Experience and surgical results in a series of 70 consecutive patients. J Clin Neurosci. 2006;13:233-238. [DOI] [PubMed] [Google Scholar]
- 59.Fleming RM, Fleming MR, Dooley WC, Chaudhuri TK. The importance of differentiating between qualitative, semi-quantitative, and quantitative imaging-close only counts in horseshoes. Eur J Nucl Med Mol Imaging. 2020;47:753-755. [DOI] [PubMed] [Google Scholar]
- 60.Pierpaoli C. Quantitative brain MRI. Top Magn Reson Imaging. 2010;21:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stroman PW, Wheeler-Kingshott C, Bacon M, Schwab JM, Bosma R, Brooks J, et al. The current state-of-the-art of spinal cord imaging: Methods. Neuroimage. 2014;84:1070-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wheeler-Kingshott CA, Stroman PW, Schwab JM, Bacon M, Bosma R, Brooks J, et al. The current state-of-the-art of spinal cord imaging: Applications. Neuroimage. 2014;84:1082-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Demir A, Ries M, Moonen CT, Vital J-M, Dehais J, Arne P, et al. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology. 2003;229:37-43. [DOI] [PubMed] [Google Scholar]
- 64.Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005;26:1587-1594. [PMC free article] [PubMed] [Google Scholar]
- 65.Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: Age and cervical spondylosis-related changes. J Magn Reson Imaging. 2005;22:38-43. [DOI] [PubMed] [Google Scholar]
- 66.Uda T, Takami T, Tsuyuguchi N, Sakamoto S, Yamagata T, Ikeda H, et al. Assessment of cervical spondylotic myelopathy using diffusion tensor magnetic resonance imaging parameter at 3.0 tesla. Spine (Phila Pa 1976). 2013;38:407-414. [DOI] [PubMed] [Google Scholar]
- 67.Holly LT, Freitas B, McArthur DL, Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;10:194-200. [DOI] [PubMed] [Google Scholar]
- 68.Xiangshui M, Xiangjun C, Xiaoming Z, Qingshi Z, Yi C, Chuanqiang Q, et al. 3 T magnetic resonance diffusion tensor imaging and fibre tracking in cervical myelopathy. Clin Radiol. 2010;65:465-473. [DOI] [PubMed] [Google Scholar]
- 69.Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol. 2011;21:426-433. [DOI] [PubMed] [Google Scholar]
- 70.Song T, Chen WJ, Yang B, Zhao HP, Huang JW, Cai MJ, et al. Diffusion tensor imaging in the cervical spinal cord. Eur Spine J. 2011;20:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hori M, Fukunaga I, Masutani Y, Taoka T, Kamagata K, Suzuki Y, et al. Visualizing non-Gaussian diffusion: Clinical application of q-space imaging and diffusional kurtosis imaging of the brain and spine. Magn Reson Med Sci. 2012;11:221-233. [DOI] [PubMed] [Google Scholar]
- 72.Kerkovsky M, Bednarik J, Dusek L, Sprláková-Puková A, Urbánek I, Mechl M, et al. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: Correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976). 2012;37:48-56. [DOI] [PubMed] [Google Scholar]
- 73.Lindberg PG, Feydy A, Sanchez K, Rannou F, Maier MA. Measures of spinal canal stenosis and relationship to spinal cord structure in patients with cervical spondylosis. J Neuroradiol. 2012;39:236-242. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura M, Fujiyoshi K, Tsuji O, Konomi T, Hosogane N, Watanabe K, et al. Clinical significance of diffusion tensor tractography as a predictor of functional recovery after laminoplasty in patients with cervical compressive myelopathy. J Neurosurg Spine. 2012;17:147-152. [DOI] [PubMed] [Google Scholar]
- 75.Gao SJ, Yuan X, Jiang XY, Liu XX, Liu XP, Wang YF, et al. Correlation study of 3T-MR-DTI measurements and clinical symptoms of cervical spondylotic myelopathy. Eur J Radiol. 2013;82:1940-1945. [DOI] [PubMed] [Google Scholar]
- 76.Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M. Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol. 2013;34:471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salamon N, Ellingson BM, Nagarajan R, Gebara N, Thomas A, Holly LT. Proton magnetic resonance spectroscopy of human cervical spondylosis at 3T. Spinal Cord. 2013;51:558-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taha Ali TF, Badawy AE. Feasibility of 1H-MR Spectroscopy in evaluation of cervical spondylotic myelopathy. The Egyptian Journal of Radiology and Nuclear Medicine. 2013;44:93-99. [Google Scholar]
- 79.Banaszek A, Bladowska J, Szewczyk P, Podgorski P, Sasiadek M. Usefulness of diffusion tensor MR imaging in the assessment of intramedullary changes of the cervical spinal cord in different stages of degenerative spine disease. Eur Spine J. 2014;23:1523-1530. [DOI] [PubMed] [Google Scholar]
- 80.Ellingson BM, Salamon N, Grinstead JW, Holly LT. Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy. Spine J. 2014;14:2589-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li X, Cui JL, Mak KC, Luk KD, Hu Y. Potential use of diffusion tensor imaging in level diagnosis of multilevel cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2014;39:E615-E622. [DOI] [PubMed] [Google Scholar]
- 82.Wen CY, Cui JL, Liu HS, Mak KC, Cheung WY, Luk KD, et al. Is diffusion anisotropy a biomarker for disease severity and surgical prognosis of cervical spondylotic myelopathy? Radiology. 2014;270:197-204. [DOI] [PubMed] [Google Scholar]
- 83.Wen CY, Cui JL, Mak KC, Luk KD, Hu Y. Diffusion tensor imaging of somatosensory tract in cervical spondylotic myelopathy and its link with electrophysiological evaluation. Spine J. 2014;14:1493-1500. [DOI] [PubMed] [Google Scholar]
- 84.Zhou F, Gong H, Liu X, Wu L, Luk KD, Hu Y. Increased low-frequency oscillation amplitude of sensorimotor cortex associated with the severity of structural impairment in cervical myelopathy. PLoS One. 2014;9:e104442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cui JL, Li X, Chan TY, Mak KC, Luk KD, Hu Y. Quantitative assessment of column-specific degeneration in cervical spondylotic myelopathy based on diffusion tensor tractography. Eur Spine J. 2015;24:41-47. [DOI] [PubMed] [Google Scholar]
- 86.Maki S, Koda M, Ota M, Oikawa Y, Kamiya K, Inada T, et al. Reduced field-of-view diffusion tensor imaging of the spinal cord shows motor dysfunction of the lower extremities in patients with cervical compression myelopathy. Spine (Phila Pa 1976). 2015;43(2):89-96. [DOI] [PubMed] [Google Scholar]
- 87.Martin A, Aleksanderek I, Cohen-Adad J, Cadotte DW, Kalsi-Ryan S, Nugaeva N, et al. Next-generation mri of the human spinal cord: A prospective longitudinal study in Cervical Spondylotic Myelopathy (CSM) to develop quantitative imaging biomarkers. Paper presented at: Congress of Neurological Surgeons Annual Meeting. New Orleans, LA; 2015. [Google Scholar]
- 88.Grabher P, Mohammadi S, Trachsler A, Friedl S, David G, Sutter R, et al. Voxel-based analysis of grey and white matter degeneration in cervical spondylotic myelopathy. Sci Rep. 2016;6:24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.d’Avanzo S, Ciavarro M, Pasqua G, Ricciardi F, Bartolo M, Solari D, et al. The functional relevance of diffusion tensor imaging in patients with degenerative cervical myelopathy. J Clin Med. 2020:9:1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy RK, Sun P, Han RH, Griffin KJ, Wagner J, Yarbrough CK, et al. Fractional anisotropy to quantify cervical spondylotic myelopathy severity. J Neurosurg Sci. 2018;62:406-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ellingson BM, Salamon N, Woodworth DC, Yokota H, Holly LT. Reproducibility, temporal stability, and functional correlation of diffusion MR measurements within the spinal cord in patients with asymptomatic cervical stenosis or cervical myelopathy. J Neurosurg Spine. 2018;28:472-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kerkovsky M, Bednarik J, Jurova B, Dušek L, Kadaňka Z, Kadaňka Z, Jr, et al. Spinal cord MR diffusion properties in patients with degenerative cervical cord compression. J Neuroimaging. 2017;27:149-157. [DOI] [PubMed] [Google Scholar]
- 93.Grabher P, Mohammadi S, David G, Freund P. Neurodegeneration in the spinal ventral horn prior to motor impairment in cervical spondylotic myelopathy. J Neurotrauma. 2017;34:2329-2334. [DOI] [PubMed] [Google Scholar]
- 94.Hori M, Fukunaga I, Masutani Y, Nakanishi A, Shimoji K, Kamagata K, et al. New diffusion metrics for spondylotic myelopathy at an early clinical stage. Eur Radiol. 2012;22:1797-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grussu F, Schneider T, Tur C, Yates RL, Tachrount M, Ianuş A, et al. Neurite dispersion: A new marker of multiple sclerosis spinal cord pathology? Ann Clin Transl Neurol. 2017;4:663-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grussu F, Schneider T, Zhang H, Alexander DC, Wheeler-Kingshott CA. Neurite orientation dispersion and density imaging of the healthy cervical spinal cord in vivo. Neuroimage. 2015;111:590-601. [DOI] [PubMed] [Google Scholar]
- 97.Hori M, Hagiwara A, Fukunaga I, Ueda R, Kamiya K, Suzuki Y, et al. Application of quantitative microstructural MR imaging with atlas-based analysis for the spinal cord in cervical spondylotic myelopathy. Sci Rep. 2018;8:5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okita G, Ohba T, Takamura T, Ebata S, Ueda R, Onishi H, et al. Application of neurite orientation dispersion and density imaging or diffusion tensor imaging to quantify the severity of cervical spondylotic myelopathy and to assess postoperative neurologic recovery. Spine J. 2018;18:268-275. [DOI] [PubMed] [Google Scholar]
- 99.Budde MD, Skinner NP, Muftuler LT, Schmit BD, Kurpad SN. Optimizing filter-probe diffusion weighting in the rat spinal cord for human translation. Front Neurosci. 2017;11:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cloney MB, Smith ZA, Weber KA, 2nd, Parrish TB. Quantitative magnetization transfer MRI measurements of the anterior spinal cord region are associated with clinical outcomes in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2018;43:675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dula AN, Gochberg DF, Valentine HL, Valentine WM, Does MD. Multiexponential T2, magnetization transfer, and quantitative histology in white matter tracts of rat spinal cord. Magn Reson Med. 2010;63:902-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu H, MacMillian EL, Jutzeler CR, Ljungberg E, MacKay AL, Kolind SH, et al. Assessing structure and function of myelin in cervical spondylotic myelopathy: Evidence of demyelination. Neurology. 2017;89:602-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holly LT, Ellingson BM, Salamon N. Metabolic imaging using proton magnetic spectroscopy as a predictor of outcome following surgery for cervical spondylotic myelopathy. Clin Spine Surg. 2016;30:E615-E619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ellingson BM, Salamon N, Hardy AJ, Holly LT. Prediction of neurological impairment in cervical spondylotic myelopathy using a combination of diffusion MRI and proton MR Spectroscopy. PLoS One. 2015;10:e0139451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cohen-Adad J. What can we learn from T2* maps of the cortex? Neuroimage93 Pt. 2014;2:189-200. [DOI] [PubMed] [Google Scholar]
- 106.Cohen-Adad J, Gauthier CJ, Brooks JCW, Slessarev M, Han J, Fisher JA, et al. BOLD signal responses to controlled hypercapnia in human spinal cord. Neuroimage. 2010;50:1074-1084. [DOI] [PubMed] [Google Scholar]
- 107.Liu X, Qian W, Jin R, et al. Amplitude of Low Frequency Fluctuation (ALFF) in the cervical spinal cord with stenosis: A resting state fMRI study. PLoS One. 2016;11:e0167279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marcus ML, Heistad DD, Ehrhardt JC, Abboud FM. Regulation of total and regional spinal cord blood flow. Circ Res. 1977;41:128-134. [DOI] [PubMed] [Google Scholar]
- 109.Ellingson BM, Woodworth DC, Leu K, Salamon N, Holly LT. Spinal cord perfusion MR imaging implicates both ischemia and hypoxia in the pathogenesis of cervical spondylosis. World Neurosurg. 2019;128:e773-e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uemura K, Matsumura A, Isobe T, Anno I, Kawamura H, Minami M, et al. Perfusion-weighted magnetic resonance imaging of the spinal cord in cervical spondylotic myelopathy. Neurol Med -Chir. 2006;46:581-588. [DOI] [PubMed] [Google Scholar]
- 111.Floeth FW, Stoffels G, Herdmann J, Jansen P, Meyer W, Steiger HJ, et al. Regional impairment of 18F-FDG uptake in the cervical spinal cord in patients with monosegmental chronic cervical myelopathy. Eur Radiol. 2010;20:2925-2932. [DOI] [PubMed] [Google Scholar]
- 112.Floeth FW, Galldiks N, Eicker S, Stoffels G, Herdmann J, Steiger HJ, et al. Hypermetabolism in 18F-FDG PET predicts favorable outcome following decompressive surgery in patients with degenerative cervical myelopathy. J Nucl Med. 2013;54:1577-1583. [DOI] [PubMed] [Google Scholar]
- 113.Floeth FW, Stoffels G, Herdmann J, Eicker S, Galldiks N, Rhee S, et al. Prognostic value of 18F-FDG PET in monosegmental stenosis and myelopathy of the cervical spinal cord. J Nucl Med. 2011;52:1385-1391. [DOI] [PubMed] [Google Scholar]
- 114.David G, Mohammadi S, Martin AR, Cohen-Adad J, Weiskopf N, Thompson A, et al. Traumatic and nontraumatic spinal cord injury: Pathological insights from neuroimaging. Nat Rev Neurol. 2019;15:718-731. [DOI] [PubMed] [Google Scholar]
- 115.Freund P, Seif M, Weiskopf N, Friston K, Fehlings MG, Thompson AJ, et al. MRI in traumatic spinal cord injury: From clinical assessment to neuroimaging biomarkers. Lancet Neurol. 2019;18:1123-1135. [DOI] [PubMed] [Google Scholar]
- 116.Carrera E, Tononi G. Diaschisis: Past, present, future. Brain. 2014;137:2408-2422. [DOI] [PubMed] [Google Scholar]
- 117.Freund P, Friston K, Thompson AJ, Stephan KE, Ashburner J, Bach DR, et al. Embodied neurology: An integrative framework for neurological disorders. Brain. 2016;139:1855-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mikulis DJ, Jurkiewicz MT, McIlroy WE, Staines WR, Rickards L, Kalsi-Ryan S, et al. Adaptation in the motor cortex following cervical spinal cord injury. Neurology. 2002;58:794-801. [DOI] [PubMed] [Google Scholar]
- 119.Tan Y, Zhou F, Wu L, Liu Z, Zeng X, Gong H, et al. Alteration of regional homogeneity within the sensorimotor network after spinal cord decompression in cervical spondylotic myelopathy: A resting-state fMRI study. BioMed Res Int. 2015;2015:647958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Woodworth DC, Holly LT, Salamon N, Ellingson BM. Resting-state functional magnetic resonance imaging connectivity of the brain is associated with altered sensorimotor function in patients with cervical spondylosis. World Neurosurg. 2018;119:e740-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takenaka S, Kan S, Seymour B, Makino T, Sakai Y, Kushioka J, et al. Towards prognostic functional brain biomarkers for cervical myelopathy: A resting-state fMRI study. Sci Rep. 2019;9:10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuang C, Zha Y. Abnormal intrinsic functional activity in patients with cervical spondylotic myelopathy: A resting-state fMRI study. Neuropsychiatr Dis Treat. 2019;15:2371-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Freund P, Weiskopf N, Ashburner J, Wolf K, Sutter R, Altmann DR, et al. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: A prospective longitudinal study. Lancet Neurol. 2013;12:873-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Malone A, Meldrum D, Gleeson J, Bolger C. Electromyographic characteristics of gait impairment in cervical spondylotic myelopathy. Eur Spine J. 2013;22:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haddas R, Cox J, Belanger T, Ju KL, Derman PB. Characterizing gait abnormalities in patients with cervical spondylotic myelopathy: A neuromuscular analysis. Spine J. 2019;19:1803-1808. [DOI] [PubMed] [Google Scholar]
- 126.Bednarik J, Kadanka Z, Dusek L, Kerkovsky M, Vohanka S, Novotny O, et al. Presymptomatic spondylotic cervical myelopathy: An updated predictive model. Eur Spine J. 2008;17:421-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cui H, Wang Y, Li X, Xie X, Xu S, Hu Y. Trial-to-trial latency variability of somatosensory evoked potentials as a prognostic indicator for surgical management of cervical spondylotic myelopathy. J NeuroEng Rehabil. 2015;12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Santamaria AJ, Benavides FD, Saraiva PM, Anderson KD, Khan A, Levi AD, et al. Neurophysiological changes in the first year after cell transplantation in sub-acute complete paraplegia. Front Neurol. 2020;11:514181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Quraishi NA, Lewis SJ, Kelleher MO, Sarjeant R, Rampersaud YR, Fehlings MG. Intraoperative multimodality monitoring in adult spinal deformity: Analysis of a prospective series of one hundred two cases with independent evaluation. Spine (Phila Pa1976). 2009;34:1504-1512. [DOI] [PubMed] [Google Scholar]
- 130.Bednarik J, Kadanka Z, Vohanka S, Stejskal L, Vlach O, Schroder R. The value of somatosensory- and motor-evoked potentials in predicting and monitoring the effect of therapy in spondylotic cervical myelopathy. Prospective randomized study. Spine (Phila Pa 1976). 1999;24:1593–1598. [DOI] [PubMed] [Google Scholar]
- 131.Restuccia D, Di Lazzaro V, Lo Monaco M, Evoli A, Valeriani M, Tonali P. Somatosensory evoked potentials in the diagnosis of cervical spondylotic myelopathy. Electromyogr Clin Neurophysiol. 1992;32:389-395. [PubMed] [Google Scholar]
- 132.Yiannikas C, Shahani BT, Young RR. Short-latency somatosensory-evoked potentials from radial, median, ulnar, and peroneal nerve stimulation in the assessment of cervical spondylosis. Comparison with conventional electromyography. Arch Neurol. 1986;43:1264-1271. [DOI] [PubMed] [Google Scholar]
- 133.Lyu RK, Tang LM, Chen CJ, Chen CM, Chang HS, Wu YR. The use of evoked potentials for clinical correlation and surgical outcome in cervical spondylotic myelopathy with intramedullary high signal intensity on MRI. J Neurol Neurosurg Psychiatry. 2004;75:256-261. [PMC free article] [PubMed] [Google Scholar]
- 134.Bednarik J, Kerkovsky M, Kadanka Z, Němec M, Kovalova I, Jurova B. Prevalence and imaging characteristics of asymptomatic and symptomatic spondylotic cervical spinal cord compression. J Neurol. 2014;261:S167. [DOI] [PubMed] [Google Scholar]
- 135.Mazur MD, White A, McEvoy S, Bisson EF. Transcranial magnetic stimulation of the motor cortex correlates with objective clinical measures in patients with cervical spondylotic myelopathy. Spine (Phila Pa1976). 2014;39:1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nicotra A, King NK, Catley M, Mendoza N, McGregor AH, Strutton PH. Evaluation of corticospinal excitability in cervical myelopathy, before and after surgery, with transcranial magnetic stimulation: A pilot study. Eur Spine J. 2013;22:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deftereos SN, Kechagias E, Ioakeimidou C, Georgonikou D. Transcranial magnetic stimulation but not MRI predicts long-term clinical status in cervical spondylosis: A case series. Spinal CordSuppl. 2015;53:S16-S18. [DOI] [PubMed] [Google Scholar]
- 138.Ulrich A, Haefeli J, Blum J, Min K, Curt A. Improved diagnosis of spinal cord disorders with contact heat evoked potentials. Neurology. 2013;80:1393-1399. [DOI] [PubMed] [Google Scholar]
- 139.Jutzeler C, Ulrich A, Huber B, Rosner J, Kramer J, Curt A. Improved diagnosis of cervical spondylotic myelopathy with contact heat evoked potentials. J Neurotrauma. 2017;34:2045-2053. [DOI] [PubMed] [Google Scholar]
- 140.Adachi Y, Oyama D, Kawai J, Kawabata S, Uehara G. Spinal cord evoked magnetic field measurement using a magnetospinography system equipped with a cryocooler. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:4426-4429. [DOI] [PubMed] [Google Scholar]
- 141.Sumiya S, Kawabata S, Hoshino Y, Adachi Y, Sekihara K, Tomizawa S, et al. Magnetospinography visualizes electrophysiological activity in the cervical spinal cord. Sci Rep. 2017;7:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ushio S, Hoshino Y, Kawabata S, et al. Visualization of the electrical activity of the cauda equina using a magnetospinography system in healthy subjects. Clin Neurophysiol. 2019;130:1-11. [DOI] [PubMed] [Google Scholar]
- 143.Balardin JB, Zimeo Morais GA, Furucho RA, Trambaiolli L, Vanzella P, Biazoli C, Jr, et al. Imaging brain function with functional near-infrared spectroscopy in unconstrained environments. Front Hum Neurosci. 2017;11:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Laliberte AM. An Examination of the Role of MicroRNA-21 in the Pathobiology of Degenerative Cervical Myelopathy Using Human and Animal Data. Toronto, Canada: University of Toronto; 2018. [Google Scholar]
- 145.Cohen-Adad J, De Leener B, Benhamou M, Cadotte D, Fleet D, Cadotte A, et al. Spinal cord toolbox: An open-source framework for processing spinal cord MRI data. Paper presented at: Proceedings of the 20th Annual Meeting of OHBM. Hamburg, Germany; 2014. [Google Scholar]
- 146.Merali ZG, Witiw CD, Badhiwala JH, Wilson JR, Fehlings MG. Using a machine learning approach to predict outcome after surgery for degenerative cervical myelopathy. PLoS One. 2019;14:e0215133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Badhiwala JH, Hachem LD, Merali Z, Witiw CD, Nassiri F, Akbar MA, et al. Predicting outcomes after surgical decompression for mild degenerative cervical myelopathy: Moving beyond the mJOA to identify surgical candidates. Neurosurgery. 2020;86:565-573. [DOI] [PubMed] [Google Scholar]
- 148.Badhiwala JH, Witiw CD, Nassiri F, Akbar MA, Mansouri A, Wilson JR, et al. Efficacy and safety of surgery for mild degenerative cervical myelopathy: Results of the AOSpine north america and international prospective multicenter studies. Neurosurgery. 2019;84:890-897. [DOI] [PMC free article] [PubMed] [Google Scholar]