Abstract
Youth with callous-unemotional (CU) traits are at high risk for aggression and antisocial behavior. Extant literature suggests that CU traits are related to abnormal autonomic responses to negatively-valenced emotional stimuli, although few studies have tested autonomic responding specifically during social interactions. To address this knowledge gap, the current study tested whether CU traits were related to autonomic activity, assessed via respiratory sinus arrhythmia (RSA), during several parent–child interaction tasks designed to provoke negative emotion. The sample was 162 clinically referred youth (M age = 12.03, SD = .92; 47% female). Using piecewise latent growth models, we estimated individual differences in RSA during three semi-structured social interaction tasks (reading aloud to a parent and research assistant; a recovery period from the reading task; and a parent–child conflict discussion) and tested whether CU traits were related to patterns of RSA responding across tasks. Overall, youth showed expected RSA decreases during the reading period, increases in RSA during recovery, and further decreases during the conflict discussion. However, youth with clinically-elevated CU traits had a different pattern of RSA change across tasks, such that CU traits were related to significantly less RSA change during reading and recovery. Findings suggest that less RSA engagement during social interactions and less RSA recovery may be a biomarker of CU traits. Future research is needed to examine whether this inflexibility contributes to the development of CU traits beginning early in childhood.
Keywords: Autonomic flexibility, Callous-unemotional traits, Latent growth curve modelling, Parasympathetic activity, RSA
Introduction
Conduct problems (CP) are the primary reason for child referrals to mental health services (Kazdin et al., 2006), predicting persistent antisocial behavior across the lifespan, as well as poor physical health, mental health, and economic outcomes (Rivenbark et al., 2018). CP are heterogeneous in etiology and prognosis, which hinders effective diagnosis and treatment (Hyde et al., 2014). To parse this heterogeneity, researchers have identified a subgroup of children with CP and callous-unemotional (CU) traits, who show low empathy, low guilt, lack of concern about performance, and insensitivity to others’ emotions (Frick et al., 2014; Waller et al., 2020). CU traits predict risk for severe and chronic aggression and violence, beyond risk associated with existing CP (Frick et al., 2014; Waller et al., 2020). Etiological models posit that youth with CU traits are at heightened risk for antisocial behavior because of reduced sensitivity to cues of threat, emotion, and affiliation, which would typically signal the need for behavior change to avoid punishment, prevent harm to others, or promote social bonding (Frick et al., 2014; Waller & Wagner, 2019). This hyposensitivity to cues of threat, emotion, or affiliation is thought to undermine the ability of youth high on CU traits to flexibly adapt and respond appropriately within different social and environmental contexts (Waller & Wagner, 2019).
Adaptive responding to and processing of threat, emotion, or social cues appears to be related, in part, to functioning of the autonomic nervous system (ANS) (Porges & Furman, 2011). The central nervous system adjusts ANS outputs to support ongoing environmental and behavioral demands (Porges, 2007). Branches of the ANS include the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS). The SNS is central to the body’s stress-response system, mobilizing fight-or-flight responding to threats or challenges (Fowles, 1988). In contrast, the PNS helps conserve energy during an absence of threat or challenge by lowering cardiovascular activity, and is theorized to facilitate recovery and adaptive behavior following emotional reactions (Fowles, 1988; Porges, 2007; Porges & Furman, 2011). One index of PNS activity is respiratory sinus arrhythmia (RSA), which refers to the degree of variability in the timing of heartbeats as a function of breathing rates (Beauchaine, 2001, 2015). During exhalation, parasympathetic signals, transmitted largely via the vagus nerve, slow heart rate, whereas during inhalation, these influences lessen and confer increases in heart rate (Beauchaine, 2015). Higher resting RSA reflects greater vagal control of the heart, which has been linked to positive psychosocial outcomes, such as better emotion regulation and higher prosociality (Beauchaine, 2001; Wagner et al., 2018). An important role of PNS activity is to change vagal influences over the heart following behavioral challenges, with subsequent restoration of resting activity. Thus, small-to-moderate decreases in RSA in response to everyday challenges facilitate adaptive orientation, engagement, and attention (Calkins & Keane, 2004; Porges, 2007). In contrast, large decreases in RSA, while adaptive in the context of acute threat, may be maladaptive under everyday circumstances (Beauchaine, 2015). Likewise, after a challenge or threat has passed, RSA increases to return to resting levels, with exaggerated increases thought to contribute to active disengagement or ignoring (Porges, 2007). Together, this pattern of decreasing and increasing RSA, which we refer to as “RSA flexibility”, constitutes a system that coordinates an adaptive response to environmental inputs, limits energy during times of rest, expends energy in response to threat or social demands, and varies in patterns and intensity between individuals (Calkins & Keane, 2004; Hastings & Kahle, 2019).
Importantly, low baseline RSA (i.e., at rest or during neutral activities) and dysregulated RSA responding have been implicated in a variety of psychiatric disorders, including externalizing disorders (for a review, see Beauchaine, 2015). Further, prior research demonstrates that children with high CP display minimal decreases in RSA when faced with stressful challenges or emotional stimuli (Beauchaine et al., 2001; Fanti et al., 2019; Fortunato et al., 2013; Gatzke-Kopp et al., 2015). These associations have also been established via longitudinal studies (El-Sheikh & Hinnant, 2011; Patriquin et al., 2015). For example, in a longitudinal study of 413 children assessed at ages 8, 9, 10, and 11, lower levels of RSA withdrawal to a frustrating laboratory task predicted increases in externalizing symptoms over time specifically among boys (El-Sheikh & Hinnant, 2011). However, other studies have reported the opposite, including associations between childhood CP and greater increases in RSA to emotional challenge or elicitation tasks (Hinnant & El-Sheikh, 2009; Pang & Beauchaine, 2013; Tabachnick et al., 2020).
One explanation for these mixed findings is that studies have not consistently accounted for the presence CU traits among children with CP. For example, a meta-analysis of 95 studies investigated the relations of electrodermal activity (EDA; a marker of SNS functioning) with aggression, CP, and psychopathic traits among children and adults, reporting that increased EDA reactivity was positively associated with aggression and CP, but negatively associated with psychopathic traits (Lorber, 2004). The results of this meta-analysis, albeit from studies of EDA, highlight that investigating RSA among youth with CP and low versus high CU traits can significantly enhance our understanding of heterogeneity in developmental pathways to CP, and give insight into differential patterns of RSA flexibility among distinct subgroups.
In one of the few existing studies on RSA functioning and CU traits, baseline RSA was significantly lower among 15-month-old children with elevated CU traits compared to children with CP alone (Mills-Koonce et al., 2015). Further, among 12-to 15-year-old children, baseline RSA was lower among those with elevated CU traits (de Wied et al., 2012). Low baseline RSA and high CU traits were also independently associated with greater risk for aggressive behavior among 9-to-11 year-olds (Thomson & Centifanti, 2018). In addition, in a longitudinal birth cohort study, lower baseline RSA at 15 months predicted later CU traits assessed at ages 6–7 (Mills-Koonce et al., 2015). Finally, a study of 108 preschoolers found that CU traits at age 2 predicted increases in CP by age 4, but only among toddlers with lower RSA reactivity to social threat (Wagner et al., 2017). However, no prior studies have examined whether CU traits are related to reduced RSA flexibility (i.e., less RSA change across contexts) when faced with changing task demands. Further, no prior studies have specifically examined whether individual differences in RSA flexibility during social interactions are associated with CU traits.
One vital source of social inputs during development comes via interactions with parents. More broadly, extant research has established the importance of the parenting environment to both the emergence and maintenance of CU traits (Waller et al., 2013). Because Porges’ polyvagal theory specifies that flexible change in RSA to social cues can facilitate adaptive social behavior (Porges & Furman, 2011), and youth with CU traits are known to be characterized by aberrant social functioning (Waller & Wagner, 2019), including in their interactions with parents (Waller et al., 2013), it is plausible to hypothesize associations between aberrant RSA responding and CU traits in such contexts. However, as established in a recent review, a paucity of studies exist that have examined RSA reactivity specifically during social interactions with parents (Wagner & Waller, 2020). Thus, it is critical to investigate RSA responding during parent–child interactions when the situation requires adaptive responding to changing social demands that provide an ecologically valid index of typical daily experiences and interactions of youth in everyday life, including how variation in RSA responding is related to CU traits.
The current study addressed these knowledge gaps by assessing dynamic patterns of RSA functioning during social interactions with a parent. We also investigated whether CU traits were related to relatively inflexible RSA patterns. Critically, research has employed various approaches to characterize RSA activity over time and across various psychological tasks (Hastings & Kahle, 2019). RSA reactivity is typically computed as the change from RSA during a resting/baseline period and a task (Beauchaine et al., 2019). However, arithmetic change scores lack the ability to describe RSA reactivity over time in a fluctuating environment (de Wied et al., 2012; Miller et al., 2013; Wagner et al., 2018). One approach, latent growth curve modeling (LGCM), provides information about within-person and between-person change in RSA across multiple measurement occasions (Ram & Grimm, 2007). A handful of studies have employed LGCM among children to characterize changes in autonomic activity over time (e.g., Miller et al., 2013, 2016). However, standard LGCM imposes one functional form over time (i.e., one linear or quadratic slope), whereas experimental protocols (and real social interactions) include multiple phases, tasks, or demands that necessitate a constantly changing pattern of RSA reactivity and recovery. Thus, piecewise LGCM represents a more effective way to accurately model patterns of RSA flexibility during multiple differing social demands (Ram & Grimm, 2007). Piecewise models combine multiphase regression models, specifying a fixed number of change-points (i.e., knot-points) to indicate when environmental changes occur, including changes that are specified a priori to match an experimental protocol (Ram & Grimm, 2007). Within this framework, RSA slopes at or near zero across several changing situations would index ANS “inflexibility”. One prior study employed piecewise LGCM to model ANS flexibility in youth, showing that 3-year-old children with better emotion regulation showed greater ANS flexibility, as demonstrated by significant patterns of RSA decreases and increases during and after a frustration-induction period (Kahle et al., 2016). Thus, an LGCM approach is well-suited to test patterns of youth ANS flexibility versus inflexibility during social interactions, which can help to advance knowledge of potential difficulties with responding to changing social cues that might be specifically implicated in etiological pathways to CU traits.
In the current study, we first used piecewise LGCM to quantify within-individual changes in RSA across three sequential parent–child interactions: a reading-aloud task with a parent and research assistant present that was intended to be engaging and require attentional orientation (Tobia et al., 2016); a recovery period with a parent and research assistant present; and a parent–child conflict discussion with only a parent present. Consistent with polyvagal theory (Porges, 2007), we hypothesized that overall youth would show decreases in RSA during reading, increases in RSA during recovery, and decreases in RSA during conflict. Such a finding would not only validate that our tasks worked as expected, but would provide confirmatory evidence of dynamic RSA responding in the face of shifting environmental demands. Second, we tested whether CU traits were associated with a different pattern of RSA responding, hypothesizing that CU traits would be related to RSA inflexibility, characterized by less RSA change across the distinct elements of the experimental protocol (i.e., less of a decrease, then an increase, and then a decrease in RSA). To test this hypothesis, we examined patterns of RSA both dimensionally and across groups categorized according to whether youth met criteria for clinically-elevated CU traits, using clinical cut-offs (Baroncelli et al., 2018; Fanti et al., 2013). To establish that the effects were specific to CU traits, and not accounted for by comorbid psychiatric symptoms or demographic variables, we included a measure of CP as a covariate, as well as age, sex, minority status, and verbal IQ.
Method
Participants
Participants were 162 youth, aged 10.60–14.10 years old (M = 12.03, SD = 0.92 years; 47% female; 60% racial/ethnic minority) and their parents (88% biological mothers) recruited from pediatric primary care clinics or ambulatory psychiatric treatment clinics within a large, Mid-Atlantic urban, academic hospital-based setting (Vine et al., 2020). All youth were receiving treatment for mood or behavior problems (see Table 1 for diagnostic information). Any youth with an IQ estimate < 70 (n = 5), not currently in treatment for a mood or behavior problem (n = 4), with an organic neurological medical condition (n = 1), diagnosed with an autism spectrum disorder or in a current manic or psychotic episode were excluded. All caregivers had primary custody (> 50% of the time). Sixty-four percent of households had at least one employed parent, 19% reported an annual household income of $20,000–$39,000, and 31% reported an annual income < $20,000.
Table 1.
Sample Prevalence of DSM-5 Diagnoses
| Disorder | % with Diagnosis |
|---|---|
|
| |
| Attention Deficit Hyperactivity Disorder | 62% |
| Oppositional Defiant Disorder | 45% |
| Borderline Personality Disorder | 33% |
| Major Depressive Disorder | 31% |
| Generalized Anxiety Disorder | 23% |
| Separation Anxiety Disorder | 19% |
| Conduct Disorder | 18% |
| Disruptive Mood Dysregulation Disorder | 17% |
| Social Phobia | 9% |
| Post-traumatic Stress Disorder | 3% |
| Panic Disorder | 1% |
The Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS-PL), a semi structured interview for youth aged 6–18 and their caregivers, was used to assess the presence and severity of affective and other child psychiatric disorders (Kaufman et al., 1997). Questions begin with a screen interview that covers all diagnostic categories, then continue using specific diagnostic supplements as indicated when screen thresholds are met. Ten percent of interviews were double-scored from video tapes, showing strong inter-rater reliability using a two-way model with consistency type (avg ICC = .88)
Procedure
Youth and parents completed questionnaires and three interaction tasks, during which multiple physiological indices were recorded. All study procedures were approved by the Human Research Protection Office (HRPO) at the University of Pittsburgh and the University’s Clinical and Translational Science Institute (CTSI) pediatric practice-based research network. Youth and parents provided written informed consent and were compensated for their time.
Measures
RSA During Three Social Interaction Tasks
Youth RSA was assessed continuously during: (1) 4-min reading period (2-min where youth listened to their parent read aloud a news article on a scientific project, and 2-min of youth reading aloud a news article on a different scientific project, both to their parent and the research assistant); (2) 2-min recovery period; and (3) 8-min parent–child conflict discussion. To identify topics of conflict, parents and youth completed a 25-item questionnaire indicating whether various common areas of conflict had occurred in the last month (e.g., behavior toward siblings or behavior in school). For each conflict, they reported the frequency (1 = once in past month to 6 = more than once per day) and intensity (1 = not at all bad to 5 = extremely bad). Research assistants identified two topics that had high frequency (M = 5.23; SD = 1.13) and severity (M = 3.99; SD = 0.92) ratings from both members of the dyad. Dyads were then asked to discuss these topics with a goal of resolving disagreements in the future during the 8-min conflict discussion task.
Electrocardiogram signals were obtained from youth via Ag/Ag–Cl spot electrodes positioned in a modified leadII configuration using Mindware BioLab software (MindWare Technologies, Ltd., Gahanna, OH). Two trained scorers visually inspected each recorded waveform (Berntson et al., 1997) and manually corrected artifacts using Mindware HRV 3.1.4 software (MindWare, Gahanna, OH). The interbeat interval (IBI) series was resampled in equal 250 ms intervals, linearly detrended, and tapered using a Hanning window. Heart rate variability (HRV) was calculated using Fast Fourier transformation analysis of the IBI series. RSA was estimated from high-frequency HRV. Following guidance from standards (Berntson et al., 1997), a bandwidth for high-frequency HRV was selected based on the respiratory rates observed from respiratory changes in the impedance cardiography collected concurrently. A wide bandwidth (0.12 to 0.5 Hz) was dictated by the observed respiratory rates, as 33% of the sample showed respiratory rates in excess of the 0.4 Hz upper limit commonly used in adult studies while 17% of the participants showed rates slower than that typical for their age (i.e., slower than 0.25 Hz). Figures S1, S2 show the sample wide distribution of peak respiration rates across all three study tasks. Consistent with recommended guidelines (Berntson et al., 1997), RSA was computed separately for each minute of each task, for a combined total of fourteen 1-min increments. For more information about the justification and rationale for this approach see the Supplemental Methods and see Byrd et al., 2020 for another example of this approach in the current sample. In the current study analyses, 157 youth had usable physiological data (n = 5 lost to equipment failure) (Table S2 and Fig. S1).
Callous-Unemotional (CU) Traits
CU traits were assessed via parent reports on the 24-item Inventory of Callous-Unemotional Traits (ICU, Frick, 2004), which assesses callousness (e.g., “unconcerned about feelings of others”), uncaring (“always tries best”), and unemotionality (“hides feelings”) with items rated on a 4-point Likert-scale (0 = not at all true to 3 = definitely true) (α = 0.89). Age and sex-specific clinical cut-off scores have been established for the parent-reported version of the ICU (Baroncelli et al., 2018; Fanti et al., 2013), which we used to create subgroups with clinically-significant (n = 29) versus low (n = 115) levels of CU traits in our sample. RSA values for youth missing the ICU (n = 13) did not differ from youth with the ICU for the majority of the RSA intervals (12 of the 14 min; Table S3).
Covariates
To establish specificity in associations between within-individual changes in RSA and CU traits, we accounted for psychiatric and demographic covariates. (1) Conduct Problems (CP) were assessed using parentreports on the Child Behavior Checklist (CBCL, (Achenbach & Edelbrock, 1983) and youth reports on the Youth Self-Report (YSR, Achenbach & Edelbrock, 1983), which include DSM-referenced scales for oppositional-defiant and conduct disorder symptoms with items rated on a 3-point Likert scale (0 = not true to 2 = very true). To create CP scores, we took the maximum-reported item rating across each informant and computed a sum of oppositional-defiant and conduct disorder items (Bird et al., 1991; Robinson et al., 2019), with high reliability for the resulting cross-informant CP scale (α = 0.90). (2) Youth Demographics were youth age, sex, minority status (1 = racial/ethnic minority vs. 0 = not racial/ethnic minority), and verbal IQ, which was assessed using standard test scores on the Peabody Picture Vocabulary Test Fourth Edition (PPVT-4) (Dunn & Dunn, 2007).1 Supplemental analyses also included measures of anxiety, attention deficithyperactivity disorder (ADHD) symptoms, and socioeconomic status. For more information on covariates see the Supplemental Methods.
Data Analytic Plan
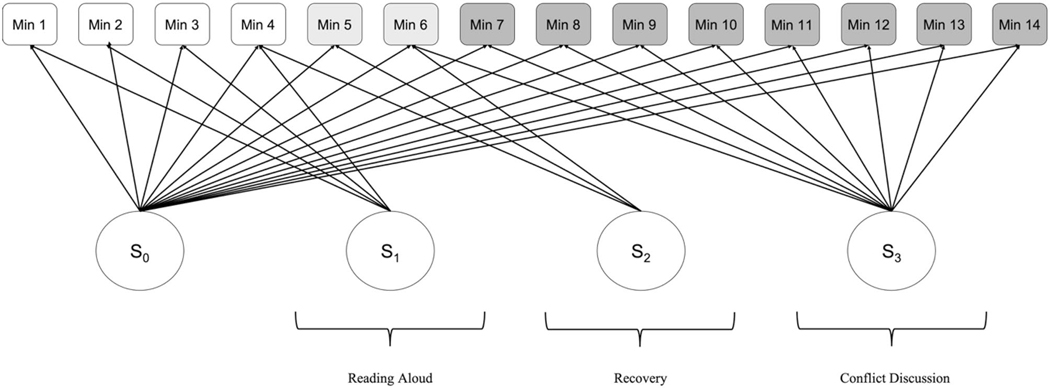
Analyses were conducted in Mplus vs. 7 (Muthén & Muthén, 1998). We used Full Information Maximum Likelihood (FIML) with robust standard errors (MLR) to handle missing data. We fit a three-piece piecewise LGCM to examine RSA change across the three parent–child interactions (Fig. 1). We estimated latent factors for RSA at the beginning of the tasks (intercept) and change in RSA (slope) for each of the three pieces. Model fit was evaluated using standard criteria for chi-square, Comparative Fit Index (CFI, Bentler, 1990), Tucker-Lewis Index (TLI, Gerbing & Anderson, 1992), and the Root Mean Square Error of Approximation (RMSEA, Browne & Cudeck, 1993). Next, we tested whether CU traits were related to variation in the piecewise LGCM. We regressed the intercept and each of the three slopes onto CU traits, as well as the psychiatric and demographic covariates. We ran two models – one using a binary, clinically-informed measure of CU traits defined using established clinical cut-offs, and one using a dimensional measure of CU traits as the predictor in the regression model (i.e., full spectrum of CU traits). To probe significant pathways, we used a multigroup approach to compare the piecewise LGCM for youth above and below the CU traits clinical cut-off. We used the Satorra-Bentler scaled chi-square test statistic to compare fit for a model in which all parameters were specified to vary for youth above versus below the clinical cut-off to a model where parameters were constrained to be equal. We conducted additional tests comparing the freed model with models in which each of the latent factors (i.e., pieces of the model) were individually fixed and freed, enabling us to determine any specific pieces of the LGCM where youth with clinically-elevated CU traits differed.
Fig. 1.
Piecewise LGCM of min-by-min RSA activity during three different social interactions
Results
Modeling Dynamic RSA Activity
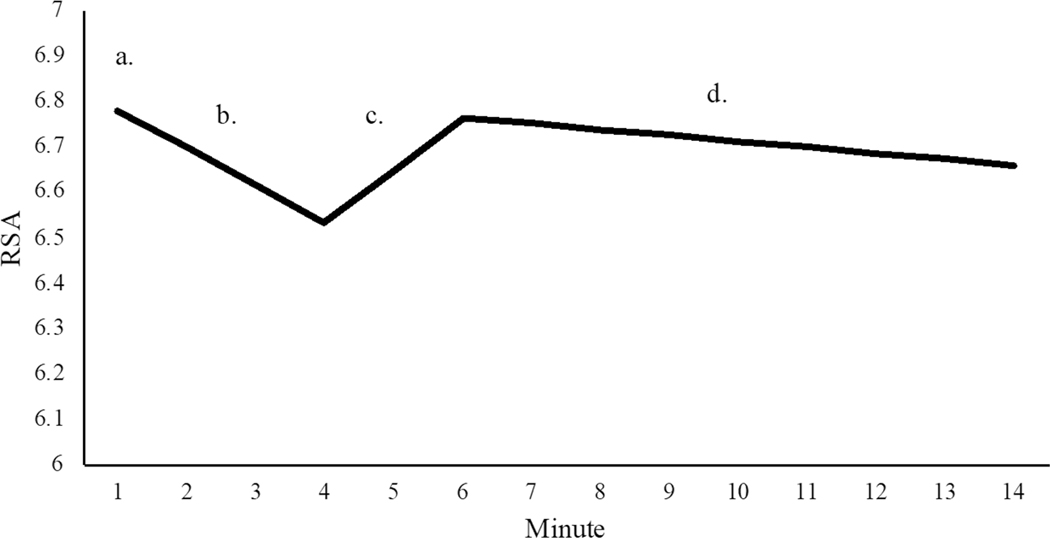
The three-piece piecewise LGCM model showed good-to-excellent model fit (χ2(82) = 130.29, p < 0.001, CFI = 0.98, TLI = 0.97, RMSEA = 0.06; Fig. 2), with a significant intercept (M = 6.78, p < 0.001) and significant mean slopes for each of the three pieces: reading (M = −0.08, p < 0.001), recovery (M = 0.12, p < 0.001), and conflict (M = −0.01, p < 0.05). Consistent with hypotheses, youth showed significant decreases in RSA during the reading period, increases in RSA during the recovery period, and further decreases in RSA during the conflict discussion (albeit to a lesser extent). There was significant variability in the intercept and conflict discussion slope factor suggestive of individual differences in initial levels of RSA and RSA change specifically during conflict (Fig. 2).
Fig. 2.
Fitted piecewise LGCM estimating RSA change across three different social interactions. χ2 = 130.29, CFI = .98, RMSEA = .06. a. Intercept (M = 6.78, p < 0.001); b. reading, 4-min (M = −0.08, p < 0.001); c. recovery, 2-min (M = .12, p < 0.001); d. conflict discussion, 8-min (M = −0.01, p < 0.05). There was significant variance for the intercept (s2 = .84, p < 0.001) and conflict discussion slope (s2 = .003, p < 0.05). The intercept was not significantly correlated with the slope of recovery (r = 0.20, p = 0.45) or conflict discussion (r = −0.10, p = 0.50). The recovery conflict discussion slopes were significantly correlated (r = −0.58, p < 0.01). Initial model output had revealed a linear dependency for the reading slope (i.e., estimated residual error variance for this factor was negative). To support estimation of an error-free model, we fixed the variance for the first slope factor to zero
Relationships Between RSA and CU Traits
Descriptive statistics for all study variables and bivariate correlations are presented in Table 2. We tested whether CU traits were related to the LGCM factors, accounting for demographic and psychiatric covariates. In support of hypotheses, the clinically-informed binary CU traits variable was related to the slope of the reading (B = 0.14, SE = 0.06, β = 0.79, p < 0.01) and recovery (B = −0.18, SE = 0.08, β = −0.57, p < 0.05) (Table 3). That is, youth with clinically elevated CU traits did not show changes in RSA responding across the first two tasks when compared to youth with low CU traits. No significant relationships between CP or any of other covariates and any of the LGCM factors emerged. Within the continuous model, CU traits were not significantly related to any of the LGCM factors (Table S4). To assess for potential moderation by CP, we created an interaction term between dimensionally-assessed CU traits and CP to assess. There were no significant moderation effects in the prediction of any of the LGCM factors (Table S5).
Table 2.
Descriptive statistics and bivariate correlations among continuous variables
| N | Min | Max | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 1. Age | 162 | 10 | 14 | 12.0 | .9 | ||||||
| 2. Income | 155 | 1 | 7 | 2.9 | 2.0 | 0.18* | |||||
| 3. Verbal IQ | 159 | 70 | 140 | 100.7 | 15.5 | −0.02 | 0.50*** | ||||
| 4. CU Traits | 149 | 1 | 56 | 27.1 | 11.4 | 0.04 | −0.08 | −0.04 | |||
| 5. CP | 149 | 0 | 36 | 12.7 | 7.7 | −0.06 | −0.27** | −0.14 | 0.56*** | ||
| 6. Anxiety | 141 | 0 | 15 | 4.2 | 3.5 | 0.07 | 0.19* | 0.17* | 0.03 | 0.27** | |
| 7. ADHD | 150 | 0 | 14 | 8.1 | 3.5 | −0.20* | −0.15 | −.02 | 0.37*** | 0.65*** | 0.22* |
| T Scores for Study Variables as Relevant | |||||||||||
| DSM-5 Oriented ODD Scale | 149 | 50 | 80 | 62.6 | 8.6 | ||||||
| DSM-5 Oriented CD Scale | 150 | 50 | 91 | 62.3 | 9.4 | ||||||
| DSM-5 Oriented Anxiety Scale | 141 | 50 | 83 | 55.3 | 7.1 | ||||||
| DSM-5 Oriented ADHD Scale | 150 | 50 | 80 | 62.9 | 8.7 | ||||||
Verbal IQ Peabody Picture Vocabulary Test Fourth Edition (PPVT-4), CU callous-unemotional, CP conduct problems, ADHD attention-deficit/ hyperactivity disorder, DSM Diagnostic Statistical Manual, ODD oppositional defiant disorder. All analyses were re-run excluding participants with missing data on the PPVT and findings remained similar whether or not these participants were included
p < 0.05
p < 0.01
p < 0.001
Table 3.
Multivariate regression model with clinically relevant CU traits predicting RSA LGCM, controlling for child demographics and psychopathology symptoms
| Intercept | Slope of Reading | Slope of Recovery | Slope of Conflict Discussion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| B | S.E | β | B | S.E | β | B | S.E | β | B | S.E | β | |
|
| ||||||||||||
| CU Traits | −0.32 | 0.25 | −0.14 | 0.14 | 0.06 | 0.79** | −0.18 | 0.08 | −0.57* | −0.01 | 0.02 | −0.10 |
| Sex | 0.12 | 0.17 | 0.07 | −0.02 | 0.05 | −0.15 | 0.03 | 0.06 | 0.13 | −0.01 | 0.02 | −0.05 |
| Minority Status | 0.31 | 0.22 | 0.17 | −0.07 | 0.06 | −0.51 | 0.03 | 0.08 | 0.10 | 0.02 | 0.02 | 0.14 |
| Age | −0.04 | 0.12 | −0.04 | 0.001 | 0.03 | 0.01 | −0.02 | 0.04 | −0.12 | 0.01 | 0.01 | 0.14 |
| Verbal IQ | −0.01 | 0.01 | −0.15 | −0.001 | 0.002 | −0.17 | −0.003 | 0.003 | −0.32 | 0.001 | 0.001 | 0.17 |
| CP | 0.001 | 0.01 | 0.01 | −0.01 | 0.004 | −0.58 | 0.01 | 0.01 | 0.35 | 0.000 | 0.001 | 0.05 |
CU callous-unemotional, Verbal IQ Peabody Picture Vocabulary Test Fourth Edition (PPVT-4), CP conduct problems. Magnitude of estimates and patterns of significance remained unchanged after controlling for annual household income, the anxiety subscale of the Youth Self-Report, and the attention-deficit/ hyperactivity disorder (ADHD) subscale from the Child Behavior Checklist (Table S3)
p < .05
p < .01
p < .001
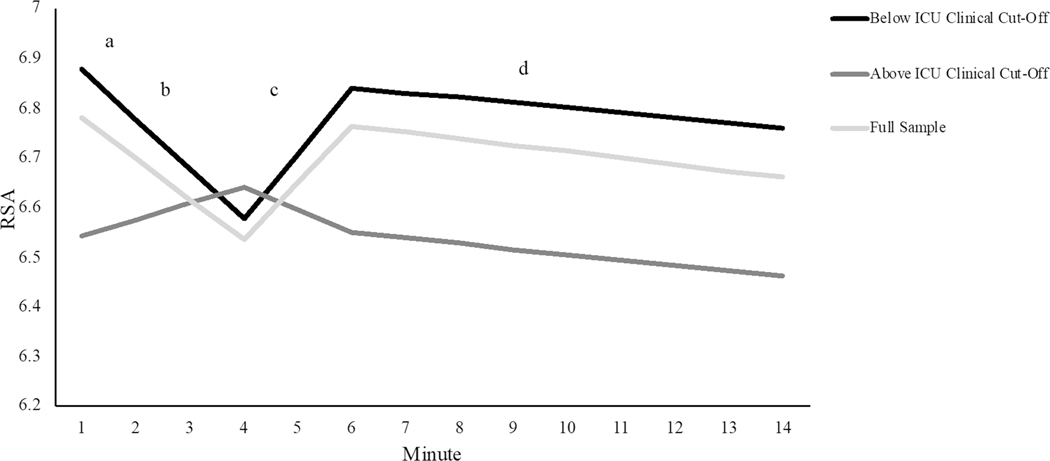
We probed significant pathways between the clinically-informed binary CU traits variable and the slopes for reading and recovery using a multi-group approach comparing the fit of the unconditional piecewise LGCM model for youth with clinically-significant CU traits versus youth (n = 29) with low CU traits (n = 115). The multi-group model with all parameters freely estimated showed good fit (χ2(196) = 314.07, p < 0.001, CFI = 0.94, TLI = 0.95 RMSEA = 0.09) and fit marginally better than a fully constrained model where all parameters were fixed to be equal across groups (Δχ2 = 8.93, p = 0.06). The freely estimated model also fit significantly better than a model in which only the slope of the reading period was fixed (Δχ2 = 4.88, p < 0.05), and a model in which only the slope of the recovery period was fixed (Δχ2 = 4.12, p < 0.05), suggesting, in line with the regression findings, that RSA change was different in youth with clinically-significant CU traits during these two pieces of the model (Table S6). For youth with low CU traits, slope factors were similar to those estimated in the full sample. In contrast, youth with clinically-significant CU traits displayed no significant decreases in RSA during the reading period and no significant increases during the recovery period (Table S7; Fig. 3). Note that although we focus on models using the clinical-cut off for the ICU (Baroncelli et al., 2018; Fanti et al., 2013), we also ran post hoc tests to explore whether there were group differences when youth were divided into groups based on a median split for ICU scores. No significant differences in RSA patterns emerged when youth were split using a median cut-off (Tables S8 and S9).
Fig. 3.
Piecewise LGCM estimating RSA change across three different social interactions. Statistically significant differences emerged between groups for reading, b. (Δχ2 = 4.88, p < 0.05) and recovery, c. (Δχ2 = 4.12, p < 0.05). For youth with CU traits above the ICU clinical cut-off none of the slopes (i.e., b., c., and d.,) different significantly from zero
Finally, to determine the specificity of these results, we ran additional post hoc tests using a multi-group approach to compare the fit of the unconditional piecewise LGCM model for youth who met diagnostic criteria for 1) conduct disorder (CD, [CD diagnosis, n = 24; no CD diagnosis, n = 107]) and 2) for boys (n = 86) versus girls (n = 76). In contrast to the findings for the CU traits grouping, youth with CD displayed significantly greater decreases in RSA during the reading period than youth without CD, and significantly greater increases in RSA during the recovery period than those without CD (Supplemental Results; Tables S10 and S11). There were no moderating effects of sex (Supplemental Results; Tables S12 and S13).
Discussion
This study is the first to characterize how youth respond to changing social expectations across three semi-structured parent–child interaction tasks. We leveraged piecewise LGCM to more accurately map onto theoretical models of change that postulate distinct change points at which growth is hypothesized to change direction, accelerate, decelerate, and/or level off (Collins, 2006). We established significant decreases in RSA across a reading period, increases in RSA during a recovery period, and further decreases in RSA during a conflict discussion. This pattern of RSA change over three distinct behavioral tasks reflects a general ability of youth to regulate PNS functioning and, physiologically, to adapt to changes in the environment, specifically in supporting social interactions with a parent across tasks that require attention, engagement, and social interaction. Thus, our findings show that, overall, youth show flexible PNS adaptation, which is theorized to mediate affective processing and regulate social and emotional behavior (i.e. facial expression, vocalization, and listening) (Porges, 2007).
However, RSA flexibility was different among youth with clinically-significant CU traits. Both the regression and multi-group models suggested that CU traits were related to a lack of RSA change, specifically minimal decreases in RSA during reading or increases in RSA during recovery. Our results are consistent with prior studies reporting associations between lower decreases in RSA and conduct problems among preschoolers with CU traits (Wagner et al., 2017), as well as the finding that more prosocial behavior (i.e., negatively correlated with CU traits) is related to greater RSA flexibility during parent–child interactions (Cui et al., 2015; Miller et al., 2016). Thus, RSA inflexibility may be an important biomarker of clinically-significant CU traits (Beauchaine, 2015). This interpretation is consistent with a theoretical model proposing that CU traits arise from reduced sensitivity to cues of social bonding and engagement (Waller & Wagner, 2019). CU traits have also been linked to deficits in responding to others’ emotions (Moore et al., 2019), and reduced attention to social features (Bedford et al., 2015). The current findings contribute to this literature by identifying aberrant physiological processes that might underpin these deficits, especially within the context of parent–child interactions, which are known to have gone awry among youth with CU traits (Waller et al., 2018). Moreover, while prior studies have investigated blunted tonic/phasic activity/reactivity in ANS and CNS measures among youth with CP (Fortunato et al., 2013; Lorber, 2004; Pang & Beauchaine, 2013; Viding et al., 2012), as well as reduced amygdala reactivity (Jones et al., 2009), they have largely focused only on single simuli that represent cues of threat, distress, and fear in others, rather than capturing RSA change during social interactions with a parent. Our results suggest that youth high on CU traits may make more errors when attending and responding to more complex social responding of others due to reduced physiological sensitivity to cues of social engagement or social challenge.
Importantly, the pattern of reduced RSA flexibility among youth with clinically-significant CU traits was the opposite to that found for youth with a CD diagnosis who showed significantly greater decreases in RSA during the reading period and significantly greater increases in RSA during the recovery period than youth without CD. Thus, the present findings speak to the importance of the CU traits subgrouping approach for delineating a subgroup of antisocial youth, who have a distinct RSA profile characterized by a lack of change in RSA responding to shifting social demands. This profile differs from youth with CD who are low on CU traits, who show heightened RSA change in response to social demands, including greater decreases in RSA during the reading period and greater increases in RSA during recovery. At the same time, the cross-sectional nature of our study does not allow us to disentangle cause and effect. Inflexible RSA functioning could be related to increased risk for social deficits observed in youth with CU traits. However, the reverse could also be true, such that reduced responsiveness to others over time might ultimately contribute to decreases in RSA flexibility. Moreover, our results do not rule out the possibility that youth with CU traits were more disengaged from the task and their parents at the outset, contributing to nonsignificant changes in RSA during subsequent tasks. In addition, given that youth with clinically significant CU traits displayed RSA change that was actually trending in the opposite direction of youth with low levels of CU traits, future research is needed to clarify whether these youth are indeed being non-responsive, or if they are being “incorrectly” responsive or context-inappropriate in their responses.
It is also notable that the pattern of physiological inflexibility only emerged when comparing youth above and below the ICU clinical cut-off (i.e., delineating the most severe presence of CU traits). That is, continuous CU traits scores were unrelated to any LGCM factors and no group differences emerged in RSA patterns with a median split. These model differences provide preliminary evidence in support of the recently-established clinical cut-off for the ICU (Baroncelli et al., 2018; Fanti et al., 2013; Kimonis et al., 2008) and many prior studies of clinical and at-risk samples that utilized a similar grouping approach (Cecil et al., 2018; Sharf et al., 2014). At the same time, we, among others, have argued that CU traits should be considered dimensionally (Waller et al., 2020). One potential explanation for the findings is that our recruitment strategy prioritized the sampling of youth with mood and behavior problems and high levels of affective instability (Table S1). This strategy may have resulted in a sample characterized by greater emotion dysregulation and physiological sensitivity than is typically found in studies of youth with CU traits or CP (Baroncelli et al., 2018; Fanti et al., 2013). Future studies need to clarify the continuity or discontinuity of CU traits and examine relationships with RSA flexibility in a range of samples, including among youth from the community and youth specifically clinic-referred for CP.
Our study had a number of strengths, including use of a latent variable approach, an ecologically valid paradigm, and multiple informants. However, we note several limitations. First, we did not assess RSA during a traditional baseline period, characterized by no stimulation or while watching a neutral video (Beauchaine et al., 2019). Future studies are needed that incorporate a true baseline prior to modeling RSA change. Second, we were not able to account for pubertal timing, which is known to influence RSA (El-Sheikh, 2005). Third, to include all observed respiratory rates, we purposefully used a wide high-frequency band (range = 0.12–0.50 Hz), which exceeds that typically used in other studies. Thus, while our range accurately encompassed all respiratory-related variability, the width may have made it more likely that some non-respiratory influences were inadvertently included within analyses. Future work would benefit from defining narrower respiratory ranges and assessing RSA within those ranges (e.g., autoregressive methods and cross spectral approaches). A discussion of the specification of respiration in developmental studies, as well as analytic suggestions, can be found in Shader et al., 2018. Fourth, prior research suggests that overly long tasks produce less RSA reactivity (Beauchaine et al., 2019). Thus, the relatively reduced slope estimate we obtained for the conflict piece of the model may have reflected the fact that this task was double the length of the prior reading and recovery tasks. Relatedly, we found no significant differences in RSA responding during the conflict discussion between the CU traits groups, which may have been because of the relatively reduced overall slope estimate arising from the task length. To address this issue, future research could incorporate dynamic models, such as non-linear models, or models that capture partner (i.e., parent) dependent change (actor-partner models) during briefer periods within parent–child conflict discussions.
In sum, we established significant, dynamic changes in RSA during parent–child interactions in a sample of clinically referred youth. These changes were less evident when youth had clinically-significant CU traits, reinforcing theory that CU traits are related to relatively inflexible physiological responding to social cues, in particular that of a parent. Our results provide preliminary support for the recently-established ICU clinical cut-off and support the utility of demarcating youth with elevated levels of CU traits, who may need tailored assessments and interventions that target their specific physiological profile.
Supplementary Material
Acknowledgements
Research was supported by grants from the National Institute of Mental Health (R01 MH101088; F32 MH110077; T32 MH018951; K01 MH119216). The authors thank all the families who took part in this study and the MoodY study team, which includes research assistants, interviewers and their supervisors, data managers, student workers, and volunteers. The authors have declared that they have no competing or potential conflicts of interest.
Funding Research was supported by grants from the National Institute of Mental Health (R01 MH101088; F32 MH110077; T32 MH018951; K01 MH119216).
Footnotes
Declarations
Ethics Approval All study procedures were approved by the Human Research Protection Office (HRPO) and the Clinical and Translational Science Institute (CTSI) pediatric practice-based research network.
Consent to Participate Youth and their parents provided written informed consent and were compensated for their time.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10802-021-00849-2.
Three participants had missing data for the PPVT. These assessments were invalid due to lack of child engagement at the baseline assessment. Subsequently, we completed an alternative assessment (the Expressive Vocabulary Test [EVT]; Williams, 1997) which yielded valid scores (based on participant engagement) of an estimated IQ above the cutoff. Given that the PPVT and EVT are different assessments, we elected to be more conservative and coded the baseline PPVT score as missing for those participants.
Conflicts of Interest The authors have declared that they have no competing or potential conflicts of interest.
References
- Achenbach TM, & Edelbrock CS (1983). Manual for the Child Behavior Profile and Child Behavior Checklist. Burlington, VT: Author. [Google Scholar]
- Baroncelli A, Roti B, & Ciucci E. (2018). The associations between callous-unemotional traits and emotional awareness in youth. Personality and Individual Differences, 120, 247–252. [Google Scholar]
- Beauchaine T. (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13, 183–214. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. (2015). Respiratory Sinus Arrhythmia: A Transdiagnostic Biomarker of Emotion Dysregulation and Psychopathology. Current Opinion in Psychology, 3, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T, Bell Z, Knapton E, McDonough-Caplan H, Shader T, & Zisner A. (2019). Respiratory sinus arrhythmia reactivity across empirically based structural dimensions of psychopathology: A meta-analysis. Psychophysiology, 56, e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, & Snarr J. (2001). Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology, 110, 610. [DOI] [PubMed] [Google Scholar]
- Bedford R, Pickles A, Sharp H, Wright N, & Hill J. (2015). Reduced Face Preference in Infancy: A Developmental Precursor to Callous-Unemotional Traits? Biological Psychiatry, 78, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107, 238. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, & Molen MWVD (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34, 623–648. [DOI] [PubMed] [Google Scholar]
- Bird HR, Gould MS, Rubio-stipec M, Staghezza BM, & Canino G. (1991). Screening for Childhood Psychopathology in the Community Using the Child Behavior Checklist. Journal of the American Academy of Child & Adolescent Psychiatry, 30, 116–123. [DOI] [PubMed] [Google Scholar]
- Browne RJ, & Cudeck R. (1993). Alternative ways of assessing model fit. Testing Structural Equation Models (pp. 136–162). Sage. [Google Scholar]
- Byrd AL, Vine V, Beeney JE, Scott LN, Jennings JR, & Stepp SD (2020). RSA reactivity to parent-child conflict as a predictor of dysregulated emotion and behavior in daily life. Psychological Medicine, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, & Keane SP (2004). Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology, 45, 101–112. [DOI] [PubMed] [Google Scholar]
- Cecil CAM, McCrory EJ, Barker ED, Guiney J, & Viding E. (2018). Characterising youth with callous–unemotional traits and concurrent anxiety: Evidence for a high-risk clinical group. European Child & Adolescent Psychiatry, 27, 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM (2006). Analysis of Longitudinal Data: The Integration of Theoretical Model, Temporal Design, and Statistical Model. Annual Review of Psychology, 57, 505–528. [DOI] [PubMed] [Google Scholar]
- Cui L, Morris AS, Harrist AW, Larzelere RE, Criss MM, & Houltberg BJ (2015). Adolescent RSA Responses during an Anger Discussion Task: Relations to Emotion Regulation and Adjustment. Emotion; (Washington, D.C.), 15, 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wied M, van Boxtel A, Matthys W, & Meeus W. (2012). Verbal, Facial and Autonomic Responses to Empathy-Eliciting Film Clips by Disruptive Male Adolescents with High Versus Low Callous-Unemotional Traits. Journal of Abnormal Child Psychology, 40, 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, & Dunn DM (2007). PPVT-4: Peabody Picture Vocabulary Test. Minneapolis, MN: Pearson Assessments. [Google Scholar]
- El-Sheikh M. (2005). Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental Psychobiology, 46, 66–74. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, & Hinnant JB (2011). Marital conflict, respiratory sinus arrhythmia, and allostatic load: Interrelations and associations with the development of children’s externalizing behavior. Development and Psychopathology, 23, 815–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti KA, Demetriou CA, & Kimonis ER (2013). Variants of Callous-Unemotional Conduct Problems in a Community Sample of Adolescents. Journal of Youth and Adolescence, 42, 964–979. [DOI] [PubMed] [Google Scholar]
- Fanti KA, Eisenbarth H, Goble P, Demetriou C, Kyranides MN, Goodwin D, & Cortese S. (2019). Psychophysiological activity and reactivity in children and adolescents with conduct problems: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 100, 98–107. [DOI] [PubMed] [Google Scholar]
- Fortunato CK, Gatzke-Kopp LM, & Ram N. (2013). Associations between respiratory sinus arrhythmia reactivity and internalizing and externalizing symptoms are emotion specific. Cognitive, Affective, & Behavioral Neuroscience, 13, 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC (1988). Psychophysiology and Psychopathology: A Motivational Approach. Psychophysiology, 25, 373–391. [DOI] [PubMed] [Google Scholar]
- Frick PJ (2004). The inventory of callous-unemotional traits. Unpublished Rating Scale. [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, & Kahn RE (2014). Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychological Bulletin, 140, 1. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Willner CJ, Jetha MK, Abenavoli RM, DuPuis D, & Segalowitz SJ (2015). How does reactivity to frustrative non-reward increase risk for externalizing symptoms? International Journal of Psychophysiology, 98, 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbing DW, & Anderson JC (1992). Monte Carlo Evaluations of Goodness of Fit Indices for Structural Equation Models. Sociological Methods & Research, 21, 132–160. [Google Scholar]
- Hastings PD, & Kahle S. (2019). Get Bent Into Shape: The Nonlinear, Multi-system, Contextually-embedded Psychophysiology of Emotional Development. In LoBue V, Pérez-Edgar K, & Buss KA (Eds.), Handbook of Emotional Development (pp. 27–55). Springer International Publishing. [Google Scholar]
- Hinnant JB, & El-Sheikh M. (2009). Children’s Externalizing and Internalizing Symptoms over Time: The Role of Individual Differences in Patterns of RSA Responding. Journal of Abnormal Child Psychology, 37, 1049. [DOI] [PubMed] [Google Scholar]
- Hyde LW, Waller R, & Burt SA (2014). Commentary: Improving treatment for youth with callous-unemotional traits through the intersection of basic and applied science – reflections on Dadds et al. (2014). Journal of Child Psychology and Psychiatry, 55, 781–783. [DOI] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, & Viding E. (2009). Amygdala Hypoactivity to Fearful Faces in Boys With Conduct Problems and Callous-Unemotional Traits. American Journal of Psychiatry, 166, 95–102. [DOI] [PubMed] [Google Scholar]
- Kahle S, Miller JG, Lopez M, & Hastings PD (2016). Sympathetic recovery from anger is associated with emotion regulation. Journal of Experimental Child Psychology, 142, 359–371. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, & Ryan N. (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kazdin AE, Whitley M, & Marciano PL (2006). Child–therapist and parent–therapist alliance and therapeutic change in the treatment of children referred for oppositional, aggressive, and antisocial behavior. Journal of Child Psychology and Psychiatry, 47, 436–445. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Skeem JL, Marsee MA, Cruise K, Munoz LC, & Morris AS (2008). Assessing callous– unemotional traits in adolescent offenders: Validation of the Inventory of Callous-Unemotional Traits. International Journal of Law and Psychiatry, 31, 241–252. [DOI] [PubMed] [Google Scholar]
- Lorber MF (2004). Psychophysiology of Aggression, Psychopathy, and Conduct Problems: A Meta-Analysis. Psychological Bulletin, 130, 531–552. [DOI] [PubMed] [Google Scholar]
- Miller JG, Chocol C, Nuselovici JN, Utendale WT, Simard M, & Hastings PD (2013). Children’s dynamic RSA change during anger and its relations with parenting, temperament, and control of aggression. Biological Psychology, 92, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JG, Nuselovici JN, & Hastings PD (2016). Nonrandom Acts of Kindness: Parasympathetic and Subjective Empathic Responses to Sadness Predict Children’s Prosociality. Child Development, 87, 1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Koonce WR, Wagner NJ, Willoughby MT, Stifter C, Blair C, & Granger DA (2015). Greater fear reactivity and psychophysiological hyperactivity among infants with later conduct problems and callous-unemotional traits. Journal of Child Psychology and Psychiatry, 56, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AA, Rappaport LM, Blair RJ, Pine DS, Leibenluft E, Brotman MA, & Roberson-Nay R. (2019). Genetic underpinnings of callous-unemotional traits and emotion recognition in children, adolescents, and emerging adults. Journal of Child Psychology and Psychiatry, 60, 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998). Mplus User’s Guide (7th ed.). Muthén & Muthén. [Google Scholar]
- Pang KC, & Beauchaine TP (2013). Longitudinal patterns of autonomic nervous system responding to emotion evocation among children with conduct problems and/or depression. Developmental Psychobiology, 55, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin MA, Lorenzi J, Scarpa A, Calkins SD, & Bell MA (2015). Broad implications for respiratory sinus arrhythmia development: Associations with childhood symptoms of psychopathology in a community sample. Developmental Psychobiology, 57, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74, 116–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, & Furman SA (2011). The early development of the autonomic nervous system provides a neural platform for social behaviour: A polyvagal perspective. Infant and Child Development, 20, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram N, & Grimm K. (2007). Using simple and complex growth models to articulate developmental change: Matching theory to method. International Journal of Behavioral Development, 31, 303–316. [Google Scholar]
- Rivenbark JG, Odgers CL, Caspi A, Harrington H, Hogan S, Houts RM, & Moffitt TE (2018). The high societal costs of childhood conduct problems: Evidence from administrative records up to age 38 in a longitudinal birth cohort. Journal of Child Psychology and Psychiatry, 59, 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M, Doherty DA, Cannon J, Hickey M, Rosenthal SL, Marino JL, & Skinner SR (2019). Comparing adolescent and parent reports of externalizing problems: A longitudinal population-based study. British Journal of Developmental Psychology, 37, 247–268. [DOI] [PubMed] [Google Scholar]
- Shader T, Gatzke-Kopp L, Crowell S, Reid M, Thayer J, Vasey M, & Beauchaine T. (2018). Quantifying respiratory sinus arrhythmia: Effects of misspecifying breathing frequencies across development. Development and Psychopathology, 30, 351–366. [DOI] [PubMed] [Google Scholar]
- Sharf A, Kimonis ER, & Howard A. (2014). Negative Life Events and Posttraumatic Stress Disorder among Incarcerated Boys with Callous-Unemotional Traits. Journal of Psychopathology and Behavioral Assessment, 36, 401–414. [Google Scholar]
- Tabachnick AR, Moore C, Raby KL, Goldstein A, Zajac L, & Dozier M. (2020). Respiratory sinus arrhythmia as a moderator of early maltreatment effects on later externalizing problems. Development and Psychopathology, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson ND, & Centifanti LCM (2018). Proactive and Reactive Aggression Subgroups in Typically Developing Children: The Role of Executive Functioning, Psychophysiology, and Psychopathy. Child Psychiatry & Human Development, 49, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobia V, Bonifacci P, Ottaviani C, Borsato T, & Marzocchi GM (2016). Reading under the skin: Physiological activation during reading in children with dyslexia and typical readers. Annals of Dyslexia, 66, 171–186. [DOI] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CAM, De Brito SA, & McCrory EJ (2012). Amygdala Response to Preattentive Masked Fear in Children With Conduct Problems: The Role of Callous-Unemotional Traits. American Journal of Psychiatry, 169, 1109–1116. [DOI] [PubMed] [Google Scholar]
- Vine V, Victor SE, Mohr H, Byrd AL, & Stepp SD (2020). Adolescent suicide risk and experiences of dissociation in daily life. Psychiatry Research, 287, 112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner NJ, Hastings PD, & Rubin KH (2018). Callous-Unemotional Traits and Autonomic Functioning in Toddlerhood Interact to Predict Externalizing Behaviors in Preschool. Journal of Abnormal Child Psychology, 46, 1439–1450. [DOI] [PubMed] [Google Scholar]
- Wagner NJ, & Waller R. (2020). Leveraging parasympathetic nervous system activity to study risk for psychopathology: The special case of callous-unemotional traits. Neuroscience & Biobehavioral Reviews, 118, 175–185. [DOI] [PubMed] [Google Scholar]
- Wagner N, Mills-Koonce R, Willoughby M, Propper C, Rehder P, & Gueron-Sela N. (2017). Respiratory sinus arrhythmia and heart period in infancy as correlates of later oppositional defiant and callous-unemotional behaviors. International Journal of Behavioral Development, 41, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Gardner F, & Hyde LW (2013). What are the associations between parenting, callous–unemotional traits, and antisocial behavior in youth? A systematic review of evidence. Clinical Psychology Review, 33, 593–608. [DOI] [PubMed] [Google Scholar]
- Waller R, Hyde LW, Klump KL, & Burt SA (2018). Parenting Is an Environmental Predictor of Callous-Unemotional Traits and Aggression: A Monozygotic Twin Differences Study. Journal of the American Academy of Child & Adolescent Psychiatry, 57, 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, & Wagner N. (2019). The Sensitivity to Threat and Affiliative Reward (STAR) model and the development of callous-unemotional traits. Neuroscience & Biobehavioral Reviews, 107, 656–671. [DOI] [PubMed] [Google Scholar]
- Waller R, Wagner NJ, Barstead MG, Subar A, Petersen JL, Hyde JS, & Hyde LW (2020). A meta-analysis of the associations between callous-unemotional traits and empathy, prosociality, and guilt. Clinical Psychology Review, 75, 101809. [DOI] [PubMed] [Google Scholar]
- Williams KT (1997). Expressive Vocabulary Test Second Edition (EVTTM2). 6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.