Abstract
Background
Heart failure (HF) patients with atrial fibrillation (AF) often have conduction system disorders, which may be worsened by β-blocker therapy.
Objective
In a post hoc analysis we examined the prevalence of bradycardia and its association with adverse events (AEs) and failure to achieve target dose in the GENETIC-AF trial.
Methods
Patients randomized to metoprolol (n = 125) or bucindolol (n = 131) entering 24-week efficacy follow-up and receiving study medication were evaluated. Bradycardia was defined as an electrocardiogram (ECG) heart rate (HR) <60 beats per minute (bpm) and severe bradycardia <50 bpm.
Results
Mean HR in sinus rhythm (SR) was 62.6 ± 12.5 bpm for metoprolol and 68.3 ± 11.1 bpm for bucindolol (P < .0001), but in AF HRs were not different (87.5 bpm vs 89.7 bpm, respectively). Episodes per patient for bucindolol vs metoprolol were 0.82 vs 2.08 (P < .001) for bradycardia and 0.24 vs 0.57 for severe bradycardia (P < .001), with 98.9% of the episodes occurring in SR. Patients experiencing bradycardia had a 4.15-fold higher prevalence of study medication dose reduction (P <.0001) compared to patients without bradycardia. Fewer patients receiving metoprolol were at target dose (61.7% vs 74.9% for bucindolol, P < .0001) at ECG recordings, and bradycardia AEs were more prevalent in the metoprolol group (13 vs 1 for bucindolol, P = .001). On multivariate analysis of 21 candidate bradycardia predictors including presence of a device with pacing capability, bucindolol treatment was associated with the greatest degree of prevention (Zodds ratio -4.24, P < .0001).
Conclusion
In AF-prone HF patients bradycardia may limit the effectiveness of β blockers, and this property is agent-dependent.
Keywords: Atrial fibrillation, Heart failure, Bradyarrhythmias, Beta blockers, Pharmacogenetics
Key Findings.
-
▪
Clinically important bradycardia is common in heart failure (HF) patients with persistent or paroxysmal atrial fibrillation (AF) (prevalence of heart rate [HR] <50 beats/min of 0.57 episodes/6 months in metoprolol succinate–treated patients) and is associated with investigator-implemented dose reductions, failure to achieve target doses, and adverse events.
-
▪
In HF patients with intermittent AF, clinically important bradycardia almost always occurs (>98%) during sinus rhythm.
-
▪
Bradycardia and its necessary management by dose reduction or limitation may compromise efficacy for treating both AF and HF.
-
▪
In the genetically defined population of GENETIC-AF (all ADRB1 Arg389Arg genotype) the prevalence of clinically important bradycardia was lower for bucindolol compared to metoprolol, with an incidence of HR <50 beats/min of 0.24 episodes per 6 months (P < .0001), plus less dose reduction or limitation.
Introduction
Atrial fibrillation (AF) and heart failure (HF) are highly prevalent cardiovascular disorders with increasing incidences.1 Owing to overlapping pathophysiologies and common risk factors, these conditions often coexist, with 30%–60% of HF patients also having AF.1,2 This intersection is clinically important, as the presence of each disorder worsens the prognosis of the other.2 These observations provide the rationale for the development of AF therapeutic approaches that maintain sinus rhythm (SR) in HF patients, and also reverse or prevent the progression of HF.
β blockers are a mainstay for treating patients with HF and reduced left ventricular ejection fraction (HFrEF), and in patients in SR they markedly reduce HF clinical events including mortality.3 In HFrEF β blockers also reduce incident AF,4,5 and they are widely used to control ventricular rate in established AF. However benefits of β blockers on cardiovascular outcomes have generally not been observed in HFrEF patients with AF.6 The reason is unclear, but there is some evidence that slower ventricular response rates may contribute to loss of treatment effects.7,8 AF patients have a higher incidence of conduction system abnormalities and bradyarrhythmias,9,10 which may be exacerbated by conventional β blockers.11 The beneficial effects of β blockers in HFrEF are highly dose-related,12, 13, 14 and thus in this population clinically meaningful bradycardia could lead to loss of effectiveness related to reduction in drug dose.
In order to assess the prevalence and importance of bradyarrhythmias in AF-prone HF patients treated with β blockers and to investigate potential differences between agents with different pharmacologic properties, we compared HRs, prevalence of bradycardia, bradycardia association with adverse events (AEs), target dose attainment, and dose reductions between the second-generation β blocker metoprolol and the fourth- generation3 compound bucindolol in the GENETIC-AF trial.15
Methods
Study cohort
The 267-patient phase 2b GENETIC-AF trial (NCT01970501, “Genotype-Directed Comparative Effectiveness Trial of Bucindolol and Toprol-XL for Prevention of Symptomatic Atrial Fibrillation/Atrial Flutter in Patients with Heart Failure”),15 tested the hypothesis that pharmacogenetic inhibitory targeting of the higher function, 389 arginine (ADRB1 Arg389) variant of the β1-adrenergic receptor (β1-AR) by bucindolol16 would be more effective in preventing AF than inhibition by metoprolol succinate, a β blocker without differentiated effects for the ADRB1 Arg389Gly polymorphism.17 The GENETIC-AF protocol, primary outcomes, AF burden, time in SR, and the use of AF interventions have been previously described.15,18 Briefly, the trial was a 1:1 randomization between metoprolol succinate (TOPROL-XL) and bucindolol hydrochloride in class I–III NYHA HF patients with an ADRB1 Arg389Arg genotype who had at least 1 left ventricular ejection fraction <0.50 in the past 12 months, and were in AF or had experienced an episode of symptomatic AF within the past 6 months. Patients in AF at the start of efficacy follow-up were electrically cardioverted (ECV) to SR.15 The trial was conducted according to Declaration of Helsinki guidelines; all patients signed informed written consent, and the protocol was approved by each clinical site’s ethical review committee. The proportion of the cohort in AF or SR at randomization was 50% or 47%, respectively, with 3% having other rhythms.
The current post hoc “on-treatment” analysis was conducted in the 256 patients who entered efficacy follow-up that began 3–5 weeks after randomization and were taking study medication at the time an electrocardiogram (ECG) was performed.15 The reasons for excluding 11 patients from the analysis are given in Figure 1 and in Supplemental Material. All 256 patients were receiving study β blockers at the beginning of efficacy follow-up, and 238 were in SR, with 18 having failed ECV (10 metoprolol, 8 bucindolol, P = .73). Data from the Beta-blocker Evaluation of Survival Trial (BEST) pharmacogenomic substudy16 were also evaluated as a control population.
Figure 1.
Flow diagram of patients (Ns) with at least 1 electrocardiogram episode with a heart rate <60 beats/min (bradycardia) or <50 beats/min (severe bradycardia). AE = adverse event; F/U = follow-up; HR = heart rate.
Procedures
Heart rate (HR) (SR or ventricular response rate in AF or atrial flutter [AFL]) and rhythm were determined at randomization and from 7 protocol scheduled or unscheduled 12-lead ECGs in efficacy follow-up from weeks 2 to 24. For scheduled ECGs the analyzed cohort was based on rhythm (SR, AF/AFL or other), and patients could move from one category to another depending on the rhythm at that visit. Bradycardia was defined as a HR <60 beats per minute (bpm) and severe bradycardia <50 bpm. AEs and study medication dose in mg/d were from case report forms (CRFs). The target dose of metoprolol succinate was 200 mg/d. The bucindolol target dose was 100 mg twice a day for patients ≥75 kg and 50 mg twice a day for patients <75 kg (average of 187 mg/d for all bucindolol patients). The methods for transition to study drug, blinding, and uptitration to target dose have been previously described.15 History of untreated symptomatic bradycardia or predicted likely symptomatic bradycardia on full dose β blockade were entry criteria exclusions, as was a randomization visit HR <60 bpm in patients not receiving a β blocker. Per protocol15 antiarrhythmic agents or non-dihydropyridine calcium channel blocking agents had to be discontinued at least 7 days prior to randomization, and the only allowed antiarrhythmics during the trial for prevention of recurrent AF were amiodarone or dofetilide. Bradycardia AEs were identified by this term being reported on CRFs that included investigator-reported dose reductions, interruptions, or discontinuations, which had to be subsequently confirmed by a study medication dose change at the next visit. All ECGs were read by investigators, who entered the data into electronic CRFs, and sustained arrhythmias were evaluated by a Clinical Endpoints Committee.15
Norepinephrine was measured by high-performance liquid chromatography with electrochemical detection as previously described.5,15,16
Statistical Analyses
Comparison of HRs between the study groups was by unpaired t tests applied to the week 2–24 HRs from scheduled ECGs. Within each treatment group the 24-week on-treatment HRs were also compared by paired t tests to the rates at randomization, matched to 2-to-24-week recordings that had the same SR or AF rhythm. Nonparametric tests were used for analyses of the non-normally distributed norepinephrine measurements.
For other analyses categorical data were compared by χ2 or Fisher exact tests and continuous variables by unpaired or paired t tests or Wilcoxon signed rank or rank sum tests. Evidence of the absence of non-normality of datasets was by a Kolmogorov-Smirnov test. A P value of <.05 was considered statistically significant for all analyses. Variance for time point data is given in standard deviation, and for change values in standard error of the mean (SEM). All statistical analyses were performed on R.
Estimated prevalence rate ratios were generated via Poisson regression. Standardized effect sizes featured in the Bradycardia and Severe Bradycardia multivariate logistic regression models were calculated by converting odds ratios to Z-scores via the formula:
where β represents a regression coefficient.
Results
Clinical and bradycardia outcomes
Of the 256 patients who entered efficacy follow-up, 125 and 131 were in the metoprolol succinate arm and bucindolol arm, respectively (Figure 1, Supplemental Table S1). There were no statistically significant differences in baseline characteristics between the 2 treatment groups (Supplemental Table S1). In the metoprolol group, 104 of 125 patients (83%) completed follow-up, 79 (76%) of whom received study medication for the entire 24-week period. In the bucindolol group, 107 (82%) completed the 24-week follow-up, 85 (79%) of whom continued to receive study medication throughout the trial (Table 1). Similar percentages of patients in the 2 groups experienced at least 1 AF episode (metoprolol 36%, bucindolol 37%), as previously reported.18AF treatment interventions (Table 1) were fewer in the bucindolol group, 0.56/patient vs 0.81/patient in the metoprolol arm (P = .016).
Table 1.
Patient general and clinical outcomes
| Outcomes during 24-week efficacy follow-up† | Bucindolol N = 131 | Metoprolol N = 125 | P value |
|---|---|---|---|
| Discontinuations | 24 (18%) | 21 (17%) | .87 |
| Adverse event | 3 (2.3%) | 1 (0.8%) | |
| Death | 1 (0.8%) | 2 (1.6%) | |
| Investigator discretion | 1 (0.8%) | 0 (0%) | |
| Missing 24-week ECG | 2 (1.5)% | 0 (0%) | |
| Noncompliant | 2 (1.5)% | 1 (0.8%) | |
| Trial terminated, follow-up to 16 or 20 weeks‡ | 11 (8.4%) | 14 (11%) | |
| Withdrew consent | 4 (3.1%) | 3 (2.4%) | |
| Completed 24 week follow-up (%) | 107 (82%) | 104 (83%) | .88 |
| Completed 24 week follow up on study medication (%) | 85 (65%) | 79 (63%) | .88 |
| Had at least 1 episode of AF (%) | 49 (37%) | 45 (36%) | .92 |
| No. of AF treatment events (ECV, CA, or class III AADs [events/patient])18 | 73 (0.56) | 101 (0.81) | .016 |
| In SR at end of 24-week follow-up (%) | 48 (37%) | 50 (40%) | .67 |
| Lost to follow-up (%)§ | 1 (1%) | 0 (0%) | 1.00 |
| Cardiovascular hospitalizations§ | 8 (6%) | 6 (5%) | .72 |
| Heart failure hospitalizations§ | 5 (2%) | 2 (1%) | .48 |
| Strokes | 0 | 0 | − |
| Died (%)§ | 0 (0%) | 2 (2%) | .46 |
AAD = antiarrhythmic drugs; AE = adverse event; AF = atrial fibrillation; CA = catheter ablation; ECG = electrocardiogram; ECV = electrical cardioversion for AF during the 24-week efficacy follow-up period (excluding the ECV performed at the initiation of follow-up); SR = sinus rhythm.
ECV = electrical cardioversion for AF during the 24 week efficacy follow-up period (excluding the ECV performed at the initiation of follow-up).
Data and Safety Monitoring Board recommended stopping the seamless phase 2B/3 trial in phase 2B15; sponsor elected to have trial end when all randomized patients had at least 16 weeks of follow-up (number of patients with, respectively, 16- or 20-week follow-up visits: bucindolol 4, 7; metoprolol 7, 7.
Due to cell sizes, Fisher’s Exact Test was used.
Bradycardia outcomes are given in Figure 1. Overall, 105 of the 256 patients (41%) developed HR <60 bpm on at least 1 ECG during the 24-week follow-up period, 67 (54%) in the metoprolol group and 38 (29%) in the bucindolol group (P < .0001, Figure 1). There were 36 patients who experienced HR <50 bpm (14%), 28 (22.4%) in the metoprolol group and 8 (6.1%) for bucindolol (P < .0001, Figure 1). Metoprolol patients experienced 261 bradycardia episodes with a mean HR of 51.8 ± 6.0, and bucindolol 107 bradycardia episodes (P < .0001 vs metoprolol), with a mean HR of 51.9 ± 5.6 (P = .87 vs metoprolol) (Figure 1, Table 2). For severe bradycardia the metoprolol episodes were n = 71 at a mean HR of 44.5 ± 6.3, compared to 32 episodes for bucindolol (P = .001), with a mean HR of 44.8 ± 3.4 bpm (P = .80) (Figure 1, Table 2). For the combined treatment groups 98.9% of bradycardia and 100% of severe bradycardia ECGs were SR, with overall mean HRs of 51.9 ± 5.9 and 44.5 ± 5.5, respectively.
Table 2.
Heart rates and drug doses, N = 131 bucindolol, 125 metoprolol
| Parameter | M, no. brady episodes | B, no. brady episodes | P value, no. episodes | M, mg/d | B, mg/d | M HR, bpm | B HR, bpm | P value, HR | M mg, % at target† | B mg, % at target† | P value % at target |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All rhythms, all pts/ECGs 2–24 weeks | − | − | 169 ±57 | 163 ±59 | 72.0 ±19.5 | 76.6 ±17.7 | <0.0001 | 61.7. | 74.9 | <0.0001 | |
| SR, scheduled ECGs only (Figure 2) | − | − | 166 ±59 | 165 ±59 | 62.6 ±12.5 | 68.3 ±11.1 | <0.0001 | − | − | − | |
| AF, Scheduled ECGs only (Figure 2) | − | − | 175 ±53 | 158 ±59 | 87.5 ±16.4 | 89.7 ±16.5 | 0.18 | − | − | − | |
| All rhythms, Scheduled ECGs only | − | − | 169 ±56 | 161 ±60 | 69.2 ±17.1 | 74.5 ±16.0 | <0.0001 | − | − | − | |
| At time of any bradycardia episode | 261 | 107 | <0.001 | 142 ±66 | 153 ±65 | 51.8 ±6.0 | 51.9 ±5.6. | 0.87 | 70.9 | 80.4 | 0.001 |
| At 1st bradycardia episode | 67 | 38 | 0.005 | 163 ±59 | 149 ±67 | 52.0 ±8.3 | 54.4 ±4.7 | 0.08 | 81.6 | 80.9 | 0.74 |
| At Subsequent bradycardia episodes | 194 | 69 | 0.001 | 134 ±68 | 156 ±64 | 51.8 ±5.1 | 50.5 ±5.5 | 0.12 | 67.2 | 80.2 | <0.0001 |
| At time of any severe bradycardia episode | 71 | 32 | 0.001 | 114 ±66 | 145 ±57 | 44.5 ±6.3 | 44.8 ±3.4 | 0.80 | 22.5 | 50.0 | 0.011 |
Target doses mg are M 200 mg/d, B 187 mg/d.
AF = atrial fibrillation; B = bucindolol; bpm = beats per minute; ECG = electrocardiogram; HR = heart rate; M = metoprolol succinate; pts = patients; SR = sinus rhythm.
Calculated by determining whether a patient is at target dose at the time of each ECG.
Mean heart rates and doses of study medication
Mean HRs in any rhythm on all scheduled and unscheduled ECGs including 10 patients with rhythms other than SR or AF/AFL were 72.0 ± 19.5 bpm in the metoprolol group and 76.6 ± 17.7 bpm for bucindolol (P < .0001) (Table 2). Mean doses (mg/d) at the corresponding ECG recordings were 169 ± 57 and 163 ± 59 for metoprolol and bucindolol, respectively (Table 2).
Mean HRs in SR or AF/AFL at randomization and at each of the 7 scheduled visits from weeks 2–24 are shown in Figure 2 and Table 2.
Figure 2.
Heart rates at scheduled electrocardiogram (ECG) monitoring visits. A: Sinus rhythm (SR) scheduled ECGs in patients at each protocol-defined time point (∗2 patients who were in SR during screening and at the start of efficacy follow-up had no ECG recorded at randomization). B: Atrial fibrillation/flutter (AF/AFL) scheduled ECGs in patients at each time point (94% AF and 6% AFL). Neither SR nor AF/AFL heart rates exhibited significant departures from a normal distribution. B = bucindolol; M = metoprolol; Rnd = randomization; VRR = ventricular rate regulation.
Sinus rhythm
In patients in SR (Figure 2A, Table 2) the mean HR on scheduled ECGs in the metoprolol group (62.6 ± 12.5 bpm) was lower than for bucindolol (68.3 ± 11.1 bpm, curve comparison P < .0001). The mean doses for metoprolol and bucindolol were 166 ± 59 and 165 ± 59 mg/d, respectively. Compared to randomization values, paired metoprolol rates were lower (randomization, 66.0 ± 13.0 bpm, change from randomization -3.1 ± 0.6 (SEM) bpm, P < .0001). In contrast, in subjects treated with bucindolol, mean HRs compared to randomization were higher (mean 67.0 ± 12.0 bpm), by 2.2 ± 0.6 (SEM) bpm (P = .0002).
AF/AFL
Among ECGs in the AF/AFL group, 94% were AF and 6% AFL. In contrast to SR, the mean AF/AFL HRs (Figure 2B, Table 2) were not significantly different between metoprolol and bucindolol: 87.5 ± 16.4 bpm and 89.7 ± 16.5 bpm, respectively (P = .18). For metoprolol, HRs from 2 to 24 weeks were not different from randomization (86.7 ± 16.4 bpm, change 0.8 ± 1.3 bpm, P = .52). In contrast, in bucindolol patients HRs were higher compared to randomization (84.5 ± 16.3 bpm, 2–24 weeks increase by 3.4 ± 1.3 bpm, P = .009).
In Figure 2 and Table 2, HRs in AF/AFL are higher than has been reported for HF patients in AF treated with either metoprolol succinate4 or bucindolol19 where the target doses of each were the same as in GENETIC-AF. Supplemental Tables S2 and S3 are data from ADRB1 Arg389Arg patients16 in the BEST adrenergic receptor polymorphism substudy16 who were receiving bucindolol at the 17-week visit. AF/AFL patients in BEST 17 weeks after randomization have a HR of 72.5 ± 11.7 bpm at a dose of 157 ± 51 mg/d, compared to respective values of 88.7 ± 13.7 bpm (P < .0001) and 159 ± 55 mg/d (P = .62) in GENETIC-AF 16 weeks after randomization (Supplemental Table S2). Supplemental Table S3 gives the norepinephrine levels, which are 451 pg/mL for BEST AF/AFL patients at 17 weeks and higher at 12 weeks for GENETIC-AF counterparts, 642 pg/mL (P = .003).
Bradycardia association with adverse events, dose reductions, and failure to reach target dose
Adverse events
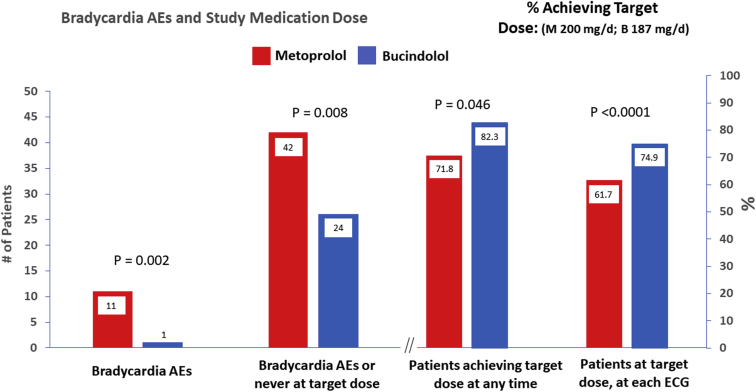
Investigator-assessed bradycardia AEs in patients with HRs <60 bpm were far more prevalent in the metoprolol group, n = 11 patients vs 1 for bucindolol (P = .002, Figure 1, Figure 3A). In addition, 2 bradycardia AEs were identified by investigators when the temporally closest documented HR by ECG was ≥60 ppm, including 1 in AF associated with a HR of 114 (Figure 1; Table 3, 13 AE events in 11 metoprolol patients, 1 AE for bucindolol P = .001 for event difference). All bradycardia AEs with HRs <60 bpm were in SR patients. In addition, there was no relationship between HR and symptoms during these 14 events (Table 3), and bradycardia AEs were not directly related to the degree of HR slowing (Table 3).
Figure 3.
Patients experiencing or achieving: A: bradycardia adverse events (AEs); B: combined endpoint of a bradycardia AE or never reaching target dose (4 patients in the metoprolol group had a bradycardia AE AND failed to reach target); C: target dose at any time; D: at target dose at the time of an ECG recording (scheduled or unscheduled). The mean doses for all patients in the % achieving target dose plot (C) were 169 ± 57 mg/d for metoprolol and 163 ± 59 mg/d for bucindolol, where target doses were 200 mg/d and 187 mg/d, respectively.
Table 3.
Heart rate ranges, rhythm, and symptom status during the 14 bradycardia adverse events occurring in 12 patients
| Treatment group | Heart rate (bpm) |
||||||
|---|---|---|---|---|---|---|---|
| <40 | 40–44 | 45–49 | 50–54 | 55–59 | ≥60 | P value, metoprolol vs bucindolol total AEs | |
| Metoprolol | 0 | 1† | 5† | 3† | 2† | 2‡ | .001 |
| Bucindolol |
0 |
0 |
1 |
0 |
0 |
0 |
|
| Linear trend by heart rate bin, metoprolol P = .51; bucindolol P = .80 | |||||||
| Asymptomatic |
Symptomatic |
− |
− |
P value, symptomatic vs asymptomatic | ||||
|---|---|---|---|---|---|---|---|---|
| N | Heart rate ± SD | N | Heart rate ± SD | − | − | |||
| Metoprolol | 8 | 53.9 ± 10.2 | 5 | 63.4 ± 28.7 | − | − | Events | Heart Rate |
| Bucindolol | 0 | − | 1 | 47 | − | − | .43 | .57 |
Heart rates are by the temporally closest electrocardiogram case report available.
AEs = adverse events; bpm = beats per minute.
Patients in sinus rhythm.
Patients in atrial fibrillation, rate 77 bpm in 1 asymptomatic patient measured the same day and 1 symptomatic patient at 114 bpm measured 2 weeks before the event.
Effects on study medication dosing
There were more patients in the metoprolol group who failed to reach target dose at any time, 28.2% vs 17.7% for bucindolol (percent achieving target dose at any time 71.8% for metoprolol, 82.3% bucindolol, P = .046, Figure 3C). For the combined endpoint of a bradycardia AE or failing to reach target at any time, there were 42 patients from the metoprolol group and 24 from bucindolol (P = .008, Figure 3B). For achievement of target dose at each ECG recording (Figure 3D, Table 2), 74.9% of the bucindolol patients were at target, compared to 61.7% for metoprolol (P < .0001).
At the time of any bradycardia event, HR and dose for metoprolol were 51.8 ± 6.0 bpm and 142 ± 66 mg/d, compared to 51.9 ± 5.6 (P = .87 vs metoprolol) and 153 ± 65 mg/d for bucindolol (P = .001 vs metoprolol as % of target, Table 2). A difference between treatment groups in dose as a percentage of target was not evident at the first bradycardia event (P = .74) but was evident for subsequent bradycardia episodes (metoprolol 67.2% vs bucindolol 80.2% of target, P < .0001). Thus, unlike bucindolol, metoprolol dosing was reduced following an initial bradycardia episode, and the dose reduction did not diminish the prevalence of subsequent bradycardia episodes (194 episodes for metoprolol, 69 for bucindolol, P = .001) (Table 2).
Based on data in Table 2, metoprolol patients had prevalence rates of 2.08 bradycardia and 0.57 severe bradycardia episodes/patient, compared to 0.82 and 0.24 for bucindolol (respective prevalence rate ratios 0.39 [0.31,0.49], P < .001, and 0.43 [0.28,0.65], P < .001). The prevalence rate of bradycardia and its relationship to dose reduction is given in Table 4. Study medication dose reductions occurred in 19 of the 105 patients who had at least 1 bradycardia ECG, in 26 episodes, for a dose reduction event rate of 0.25/bradycardia patient compared to 0.06 for patients who did not experience an ECG with a HR <60 bpm (prevalence rate ratio 4.15, P < .001).
Table 4.
Bradycardia episodes and dose reductions associated with bradycardia (ventricular rate response <60 beats/min) from week 2–24 of efficacy follow-up
| Parameter↓ | Patients → | Bradycardia |
Dose reductions |
||
|---|---|---|---|---|---|
| Bucindolol (N=131) | Metoprolol (N=125) | Bradycardia (N = 105) | No bradycardia (N = 151) | ||
| Total no. patients with episodes | 38 (36 SR, 2 AF) | 67 (66 SR, 1 AF) | 19 (M14, B5) | 7 (M3, B4) | |
| Total no. episodes | 107 (105 SR, 2 AF) | 261 (259 SR, 2 AF) | 26 (M21, B5) | 9 (M4, B5) | |
| Prevalence rate (episodes/patient) | 0.82 | 2.08 | 0.25 | 0.06 | |
| Prevalence rate ratio (95% CI) | 0.39 (0.31, 0.49), P < .001 | 4.15 (2.02, 9.39), P < .001 | |||
AF = atrial fibrillation; B = bucindolol; M = metoprolol; SR = sinus rhythm.
Baseline characteristics differences between patients with and without bradycardia
Baseline characteristics of patients with and without bradycardia are shown in Supplemental Table S4. For the combined treatment groups there are multiple differences in baseline characteristics between no bradycardia and bradycardia <60 or <50 bpm patients, which are similar in each treatment group. On multivariate analysis entering the 20 baseline characteristics in Supplemental Table S4 plus treatment, those that achieved P < .05 for HR <60 bpm had older age, higher prerandomization HR, implanted therapeutic device (with pacing capability), nonischemic HF etiology, and bucindolol treatment (Figure 4). For HR <50 bpm higher baseline HR, therapeutic device, and bucindolol treatment were the only significant predictors (Supplemental Figure S1). If the odds ratio coefficients are standardized (Zodds ratio) as in the forest plot in Figure 1, bucindolol treatment is associated with the largest predictor of no bradycardia (-4.24, P < .0001) of the candidate characteristics subjected to multivariate analysis.
Figure 4.
Forest plot of candidate predictors of bradycardia (heart rate <60), multivariate analysis with standardized odds ratios. AF = atrial fibrillation; BP = blood pressure; DxT = time from initial diagnosis to randomization; ECV = electrically cardioverted; HF = heart failure; LVEF = left ventricular ejection fraction.
Discussion
In this analysis of the on-treatment cohort of GENETIC-AF, patients receiving metoprolol succinate compared to bucindolol experienced more bradycardia, more bradycardia-associated AEs, and more associated dose reductions resulting in an overall lower achievement of target dose. Metoprolol succinate treatment at a mean dose of 169 ± 57 mg/d was associated with a lower SR HR (62.6 ± 12.5 bpm) than with bucindolol (68.3 ± 11.1, P < .0001), and the metoprolol HR was lower than reported in any previous HF study.4,12, 13, 14 For example, in the nonpharmacogenetic REVERT trial of metoprolol dose–left ventricular ejection fraction response, which was conducted in SR HFrEF patients with ≥97% compliance in the 200 mg/d metoprolol group, HR at 12 months was 64.2 bpm at a mean dose of 155 mg/d with no bradycardia AEs reported.18 Similarly, MERIT-HF and its pharmacogenetic substudy in ADRB1 Arg389Arg genotypes reported HRs at 12 months of, respectively, 68.3 and 66.2 bpm at a mean dose of 158 mg/d.4,17 Thus published data support GENETIC-AF’s conduction system abnormality-prone study population as the explanation for its lower SR HR. Bucindolol’s SR HR was not higher than usually reported in β-blocked HF patients,4,12, 13, 14 including those with an ADRB1 Arg389Arg genotype,20 and the higher HR for bucindolol is unlikely to be due to a lesser degree of β1-AR blockade. Using the recommended method of clinical pharmacologic detection of β1-AR inhibition,3 HFrEF patients treated with doses of bucindolol and metoprolol similar to those used in GENETIC-AF have respective reductions in maximal exercise HRs of 39 bpm12 and 29 bpm,21 indicating that metoprolol was not delivered in a higher β-blocking dose than bucindolol.
In contrast to SR, HRs in AF/AFL (94% AF) were not different between metoprolol and bucindolol. However, for both β blockers the HRs were higher in GENETIC AF than in previously reported β blocker–treated HFrEF patients in sustained AF4,6, 7, 8,19 or in BEST trial patients analyzed in the current study. The higher HRs for both β blockers in GENETIC-AF compared to BEST19 or MERIT-HF4 were attributable not to lower doses of each agent, but likely to higher levels of systemic adrenergic activity as compared to permanent AF patients in BEST who had more advanced HF and more severe left ventricle dysfunction, which are typically associated with higher norepinephrine levels.
Bradycardia (<60 bpm) and severe bradycardia (<50 bpm) ECG recordings were, respectively, 1.8- and 3.7-fold more likely in the metoprolol vs the bucindolol group. The vast majority (98.9%) of bradycardia recordings were during SR, and HRs were not different between treatment groups for either bradycardia or severe bradycardia, meaning that bucindolol was capable of lowering heart rates to the same extent as metoprolol, but it did so less frequently. That the more frequent bradycardia episodes had clinical consequences was borne out by the markedly greater frequency of bradycardia AEs in the metoprolol arm, in 11 of the 67 patients (16%) with bradycardia episodes, compared to only 1 AE in the 38 (3%) bucindolol bradycardia episode patients. This, plus much greater dose reduction in metoprolol vs bucindolol patients on repeat bradycardia episodes, suggests that metoprolol-associated bradycardia events were qualitatively different from those of bucindolol, and more likely to produce a clinical management response. The therapeutic consequence was that the percent target dose achievement was 15% lower in the metoprolol vs the bucindolol group, due entirely to dose reduction in patients experiencing bradycardia.
The use of at least 1 AF intervention numerically favored bucindolol, in agreement with more extensive analyses of interventions by bucindolol in GENETIC-AF18 and raising the possibility that metoprolol dose reductions related to bradycardia adversely affected efficacy. Although there was no difference in AF prevention between the 2 β blockers by time to first AF/AFL/all cause mortality event,15 bucindolol was superior to metoprolol in the AF burden substudy and in ECG measurements of time in SR in the entire cohort.18 Insufficient mortality or hospitalization events in GENETIC-AF precluded efficacy assessment for HF endpoints.15 However, in AF-prone HFrEF patients the optimal HR for reducing HF events has not been systematically investigated, and it is possible that rates <60 bpm have an adverse effect on myocardial performance, and/or predispose to sudden cardiac death.20,22 This issue clearly needs further study.
Patients with paroxysmal or persistent AF are at high risk for developing conduction system disease including sinus node dysfunction,9,10 and it is not surprising that a β blocker would produce bradycardia AEs. However, bucindolol and metoprolol succinate clearly differed in their bradycardia effects, which is presumably explained by pharmacologic differences between the 2 agents, since the administered β1-AR blocking doses were comparable. These differences could involve adrenergic activation of Arg389 β1-ARs16 and/or HCN4 channels, since the differential HR effects were only observed for SR. The most likely explanation may be “subclinical” intrinsic sympathomimetic activity in bucindolol23 that is below detectable levels on Holter monitoring of nighttime HR12 or in isolated human heart preparations,16 which may be able to counteract sinus node dysfunction through effects on HCN4 channels. Regardless of the mechanism, on multivariate analysis that controlled for 20 other predictors including therapeutic devices with pacing capability, bucindolol treatment was associated with a 76% reduction in the risk of developing bradycardia.
Limitations
A limitation of the study was that heart rhythm and rate monitoring was performed by scheduled and unscheduled ECGs rather than device continuous monitoring. However, a 69-patient continuous monitoring substudy was conducted in the GENETIC-AF trial, where ≥6 hours of AF burden predicted subsequent ECG-proven clinical AF with a predictive accuracy of 96%,15 providing support that the ECG rhythm surveillance in the trial was satisfactory.
Conclusion
In a pharmacogenetically defined HF population at risk for AF, bucindolol and metoprolol differed in their effects on HR while patients were in SR. In SR metoprolol was associated with a greater incidence of bradycardia and dose-limiting bradycardia AEs as compared to bucindolol. Bradycardia may limit β-blocker efficacy in AF-prone HF patients, and this effect may vary among members of the drug class.
Acknowledgments
Funding Sources
This work was supported by ARCA biopharma, Westminster, Colorado.
Disclosures
CD is an employee of InCarda and is a former employee of ARCA Biopharma, IAC and SPH are employees of ARCA, DM is an officer of ARCA, and MRB is an officer and director of ARCA; WTA, JPP, MR, RA, MW, SBW, WHS, and SJC have consulted for ARCA.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All patients signed informed written consent.
Ethics Statement
The trial was conducted according to the Declaration of Helsinki guidelines, and the protocol was approved by each clinical site’s ethical review committee.
Disclaimer
Given his role as Associate Editor, Jeffrey S. Healey had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Jeanne E. Poole.
Footnotes
Bucindolol IND #118,935.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2021.11.005.
Appendix. Supplementary data
References
- 1.Kotecha D., Piccini J.P. Atrial fibrillation in heart failure: what should we do? Eur Heart J. 2015;36:3250–3257. doi: 10.1093/eurheartj/ehv513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang T.J., Larson M.G., Levy D., et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 3.Bristow M.R. Treatment of chronic heart failure with β-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res. 2011;109:1176–1194. doi: 10.1161/CIRCRESAHA.111.245092. [DOI] [PubMed] [Google Scholar]
- 4.Van Veldhuisen D.J., Aass H., El Allaf D., et al. Presence and development of atrial fibrillation in chronic heart failure. Experiences from the MERIT-HF Study. Eur J Heart Fail. 2006;8:539–546. doi: 10.1016/j.ejheart.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Aleong R.G., Sauer W.H., Davis G., et al. Prevention of atrial fibrillation by bucindolol is dependent on the beta₁389 Arg/Gly adrenergic receptor polymorphism. JACC Heart Fail. 2013;1:338–344. doi: 10.1016/j.jchf.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotecha D., Holmes J., Krum H., et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;38:2235–2243. doi: 10.1016/S0140-6736(14)61373-8. [DOI] [PubMed] [Google Scholar]
- 7.Cullington D., Goode K.M., Zhang J., Cleland J.G.F., Clark A.L. Is heart rate important for patients with heart failure in atrial fibrillation? JACC Heart Fail. 2014;2:213–220. doi: 10.1016/j.jchf.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Mulder B.A., Damman K., van Veldhuisen D.J., van Gelder I.C., Rienstra M. Heart rate and outcome in heart failure with reduced ejection fraction: differences between atrial fibrillation and sinus rhythm-A CIBIS II analysis. Clin Cardiol. 2017;40:740–745. doi: 10.1002/clc.22725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson L.R., 2nd, Rathakrishnan B., Campbell K., et al. Sinus node dysfunction and atrial fibrillation: a reversible phenomenon? Pacing Clin Electrophysiol. 2017;40:442–450. doi: 10.1111/pace.13030. [DOI] [PubMed] [Google Scholar]
- 10.Barrett T.W., Abraham R.L., Jenkins C.A., Russ S., Storrow A.B., Darbar D. Risk factors for bradycardia requiring pacemaker implantation in patients with atrial fibrillation. Am J Cardiol. 2012;110:1315–1321. doi: 10.1016/j.amjcard.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Channer K.S., James M.A., MacConnell T., Rees J.R. Beta-adrenoceptor blockers in atrial fibrillation: the importance of partial agonist activity. Br J Clin Pharmacol. 1994;37:53–57. doi: 10.1111/j.1365-2125.1994.tb04238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bristow M.R., O’Connell J.B., Gilbert E.M., et al. Dose-response of chronic β-blocker treatment in heart failure from either idiopathic dilated or ischemic cardiomyopathy. Circulation. 1994;89:1632–1642. doi: 10.1161/01.cir.89.4.1632. [DOI] [PubMed] [Google Scholar]
- 13.Bristow M.R., Gilbert E.M., Abraham W.T., et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation. 1996;94:2807–2816. doi: 10.1161/01.cir.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 14.Colucci W.S., Kolias T.J., Adams K.F., et al. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the REversal of VEntricular Remodeling with Toprol-XL (REVERT) trial. Circulation. 2007;116:49–56. doi: 10.1161/CIRCULATIONAHA.106.666016. [DOI] [PubMed] [Google Scholar]
- 15.Piccini J.P., Abraham W.T., Dufton C., et al. Bucindolol for the maintenance of sinus rhythm in a genotype-defined HF population. JACC Heart Fail. 2019;7:586–598. doi: 10.1016/j.jchf.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liggett S.B., Mialet-Perez J., Thaneemit-Chen S., et al. A polymorphism within a highly conserved β1-adrenergic receptor motif alters β-blocker response in multiple models and human heart failure. Proc Natl Acad Sci U S A. 2006;103:11288–11293. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White H.L., de Boer R.A., Maqbool A., et al. An evaluation of the beta-1 adrenergic receptor Arg389Gly polymorphism in individuals with heart failure: a MERIT-HF sub-study. Eur J Heart Fail. 2003;5:463–468. doi: 10.1016/s1388-9842(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 18.Piccini J.P., Dufton C., Carroll I.A., et al. Bucindolol decreases atrial fibrillation burden in patients with heart failure and the ADRB1 Arg389Arg genotype [published online ahead of print] Circ Arrhythm Electrophysiol. 2021 Jul 16 doi: 10.1161/CIRCEP.120.009591. [DOI] [PubMed] [Google Scholar]
- 19.Kao D.P., Davis G., Aleong R., et al. Effect of bucindolol on heart failure outcomes and heart rate response in patients with reduced ejection fraction heart failure and atrial fibrillation. Eur J Heart Fail. 2013;15:324–333. doi: 10.1093/eurjhf/hfs181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Veldhuisen D.J., van Woerden G., Gorter T.M., et al. Ventricular tachyarrhythmia detection by implantable loop recording in patients with heart failure and preserved ejection fraction: the VIP-HF study. Eur J Heart Fail. 2020;22:1923–1929. doi: 10.1002/ejhf.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowes B.D., Gilbert E.M., Abraham W.T., et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346:1357–1365. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- 22.Luu M., Stevenson W.G., Stevenson L.W., Walden J. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation. 1989;80:1675–1680. doi: 10.1161/01.cir.80.6.1675. [DOI] [PubMed] [Google Scholar]
- 23.Deitchman D., Snyder R.W. Beneficial effects of bucindolol in a canine model of pentobarbital-induced heart failure. Arch Int Pharmacodyn Ther. 1981;250:65–72. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.