Abstract
Background
Patients with pacemakers and heart failure with preserved ejection fraction (HFpEF) or isolated diastolic dysfunction (DD) may benefit from a higher backup heart rate (HR) setting compared with the standard setting of 60 bpm.
Objective
The purpose of this study was to assess the effects of a personalized backup HR setting (myPACE group) compared with 60 bpm (control group).
Methods
In this prospective, blinded, randomized controlled study, pacemaker patients with DD or HFpEF and atrial pacing with intrinsic ventricular conduction or conduction system or biventricular pacing are randomized to the myPACE group or control group for 1 year. The primary outcome is the change in Minnesota Living with Heart Failure Questionnaire (MLHFQ) scores. Secondary endpoints include changes in N-terminal pro–brain natriuretic peptide levels, physical and emotional MLHFQ subscores, and pacemaker-detected atrial arrhythmia burden, patient activity levels, and thoracic impedance; hospitalization for heart failure, atrial fibrillation, cerebrovascular accident, or myocardial infarction; and loop diuretic or antiarrhythmic medication initiation or up-titration. A sample size of 118 subjects is expected to allow detection of a 5-point change in MLHFQ score in an intention-to-treat analysis and allow initial assessment of clinical outcomes and subgroup analyses.
Results
Enrollment began in July 2019. As of November 2020, 107 subjects have been enrolled. It is projected that the 1-year follow-up will be completed by December 2021.
Conclusion
Atrial pacing with intrinsic ventricular conduction or advanced ventricular pacing at a higher, personalized backup HR may be a therapeutic target for patients with isolated DD or HFpEF. The myPACE trial is designed to test this hypothesis.
Keywords: Diastolic dysfunction, Heart failure with preserved ejection fraction, Heart rate, Pacing
Key Findings.
-
▪
The myPACE study is the first randomized controlled study to test the hypothesis that atrial pacing at moderately higher heart rates might provide important benefits for pacemaker patients with heart failure and preserved ejection fraction (HFpEF) or isolated diastolic dysfunction compared with the standard backup setting of 60 bpm. With increasing adoption of physiological pacing techniques, the pacemaker backup rate can be customized to higher rates without the offsetting effects of pacemaker-mediated dyssynchrony.
-
▪
The study uses a novel personalized heart rate algorithm to provide a customized lower rate setting for pacemaker patients.
-
▪
The findings from myPACE will help address knowledge gaps related to tailoring the pacemaker lower rate setting in pacemaker patients with HFpEF or isolated diastolic dysfunction.
-
▪
The myPACE study tests the paradigm-changing hypothesis that moderately higher heart rates, and not lower heart rates, might be a therapeutic target for patients with HFpEF, a complex patient population with an unmet need for evidence-based therapies.
Introduction
Half of patients with heart failure (HF) have preserved ejection fraction (HFpEF), and more than 1 in 4 adults have diastolic dysfunction (DD).1,2 Despite the increasing prevalence and socioeconomic burden, targeted treatments of patients with HFpEF are lacking.1,3
Clinical trials have demonstrated the benefit of pharmacologic heart rate (HR) lowering using select beta-blockers and ivabradine in patients having heart failure with reduced ejection fraction (HFrEF).1,4 Without an evidence basis, it is often assumed that lower HRs also provide a benefit to patients with DD and HFpEF by prolonging relaxation to allow for better ventricular filling.1,5 The relationship of elevated HR with adverse outcomes lends further associative support to the notion that lower HR could be beneficial.6 However, HRs themselves may not cause adverse outcomes, and studies suggest that intentionally increasing resting HR in HFpEF may have therapeutic value.7
Here we present the rationale for the myPACE study, which hypothesizes that a personalized pacing intervention that provides moderately higher resting HRs to pacemaker patients with HFpEF or isolated DD may convey therapeutic benefits.
Chronotropic interventions in preserved EF populations
The “lower HR is better” paradigm has not been confirmed in patients with preserved ejection fraction (EF) with or without clinical HF.5 Several reports of related patient populations with normal or preserved EFs suggest that pharmacologic HR lowering with beta-blockers or ivabradine is either not beneficial or is associated with adverse outcomes (Supplemental Table 1).4,8, 9, 10 A patient-level meta-analysis of 11 randomized controlled trials investigating beta-blockers in HF found no evidence of a benefit in the subgroup of patients with EF ≥50%.4 In fact, among patients with preserved EF with and without clinical HF, studies have revealed signals of harm associated with HR-lowering therapies. In the large SIGNIFY (Study Assessing the Morbidity-Mortality Benefits of the If Inhibitor Ivabradine in Patients With Coronary Artery Disease) trial, which comprised patients with stable coronary disease without HF, selective HR lowering with ivabradine (from mean HR 70 bpm to 60 bpm) did not improve outcomes.8 In fact, there was a 20% relative increase in HF hospitalizations and a 40% relative increase in atrial fibrillation (AF) in the ivabradine group.8 Two large hypertension trials that compared beta-blockers to other antihypertensive agents also suggested an increase in cardiovascular events in the beta-blocker arm despite a similar level of blood pressure lowering between groups.9,10
A few mechanisms have been proposed to explain these counterintuitive outcomes. Lower HRs have been shown to generate peripherally reflected systemic pressure waves that superimpose onto systole and lead to increased central blood pressure and afterload.11 In addition, prolonged ventricular filling results in a larger ventricular load and higher filling pressures that combine to increase ventricular wall stress.12 These mechanisms explain why patients on HR-lowering medications have higher natriuretic peptide levels, which in turn is a predictor of incident AF and HF.1,13 With these mechanisms at play, higher HRs with a shortened ventricular filling time should have the opposite effect. This was demonstrated in hemodynamic studies involving HFpEF patients, in whom atrial pacing reduced left ventricular (LV) end-diastolic pressures by up to 50%.14,15 Aside from the immediate hemodynamic effects, prolonged exposure to moderately elevated HRs may induce beneficial changes in the ventricular structure and substrate that improve diastolic distensibility by reducing fibrosis or improving the LV volume-to-mass ratio.16,17
For these reasons, we hypothesized that patients with HFpEF or isolated DD may derive a benefit from moderately higher resting HRs. In 2 prospective pilot studies, we explored this concept in pacemaker patients with isolated DD or HFpEF. In the first study, we raised the backup pacing rate to 100 bpm at night for 4 weeks.18 In the second study, we set the lower rate to 80 bpm for 4 weeks.19 The moderate HR elevation significantly improved quality of life (QoL), functional capacity, and N-terminal pro–brain natriuretic peptide (NT-proBNP) levels among patients with paced QRS durations <150 ms. When the HR setting was returned to the standard backup rate of 60 bpm, QoL and NT-proBNP worsened, further supporting a beneficial effect of a higher resting HR in this population. Studies that assessed effects of higher HRs in this population are summarized in Supplemental Table 2. Next, we discuss the pacing modalities best suited to deliver accelerated pacing in patients with isolated DD and HFpEF.
Electrophysiological abnormalities in HFpEF and advanced pacing modalities
Patients with HFpEF have a propensity toward sinus node dysfunction, including chronotropic incompetence, which explains why more than 20% of patients with HFpEF have a permanent pacemaker.20,21 Although atrial pacing addresses sinus node dysfunction without the associated detrimental effects of dyssynchronous right ventricular (RV) pacing, coexisting atrioventricular nodal (AVN) conduction disease and an intrinsic atrioventricular (AV) delay at higher atrial rates may result in more ventricular pacing. Current pacing guidelines favor the use of pacing algorithms permissive of nonphysiological programmed AV delays in dual-chamber devices to minimize the percentage of RV pacing, ideally to <20%.22 A simple way to limit RV pacing is to use a slower backup pacing rate. Hence, in clinical practice, the pacemaker backup rate typically is left unchanged from the nominal setting of 60 bpm.23
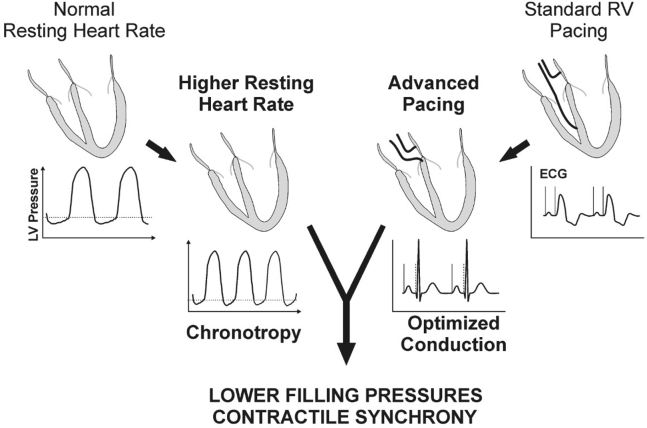
Because we are proposing the use of pacing to increase resting HR, the potential therapeutic value cannot come at the expense of abnormal electrical activation, and neither nonphysiological AV-adaptive pacing nor reliance on atrial pacing alone will allow us to achieve that objective in most patients. With the advent of physiological pacing targets, such as His-bundle pacing (HBP), left bundle branch area pacing, and optimized biventricular pacing, the pacemaker backup rate can be customized to higher HRs without the offsetting effects of pacemaker-mediated dyssynchrony. The synergy of restored chronotropy and preserved AV and interventricular synchrony may increase the potential benefits of this novel treatment approach for patients with isolated DD and HFpEF (Figure 1).
Figure 1.
Pacing at moderately higher heart rates improves myocardial relaxation and decreases filling pressures in patients with heart failure with preserved ejection fraction or isolated diastolic dysfunction. Conduction system or biventricular pacing that optimizes ventricular synchrony avoids deleterious effects that could be seen with a high burden of right ventricular pacing. ECG = electrocardiogram; LV = left ventricle; RV = right ventricle.
Because physical activity levels are reported to be low among HFpEF patients,24,25 this study focuses on pacemaker lower rate adjustments rather than rate-adaptive pacing. Increasing the pacemaker backup rate exposes the patient to the pacing intervention for longer periods of time, which allows us to evaluate chronic effects of moderately higher HRs. Chronotropic incompetence is common among HFpEF patients,21 and the ongoing RAPID-HF (Efficacy Study of Pacemakers to Treat Slow Heart Rate in Patients With Heart Failure) trial is evaluating the efficacy of rate-adaptive pacing in this population (ClinicalTrials.gov Identifier: NCT02145351). Although rate-adaptive pacing is not the focus of myPACE, potential benefits will be evaluated in a subgroup analysis. The decision to program rate-adaptive pacing in myPACE is left to the discretion of the patient’s cardiologist based on individual patient characteristics.
Rationale for myPACE
The pacemaker backup rate typically is left at or near the factory setting of 60 bpm.23 This one-size-fits-all approach does not consider that the average adult resting HR is between 71 and 79 bpm26 or that 60 bpm may not be the optimal resting HR for pacemaker-reliant patients with isolated DD or HFpEF. The primary aim of myPACE is to evaluate the effects of a higher, individualized pacemaker backup rate in patients with isolated DD or HFpEF on changes in symptoms and QoL compared with the standard rate of 60 bpm. This will be assessed using the Minnesota Living with Heart Failure Questionnaire (MLHFQ). Secondary aims are to study NT-proBNP, which is a surrogate marker for myocardial wall stress and a predictor of HF, and relevant clinical and pacemaker-recorded outcomes over 1 year.
Methods and analysis
Study population
Pacemaker clinic patients at the University of Vermont Medical Center are consecutively screened and approached for possible study participation during their standard-of-care pacemaker clinic visit. Table 1 outlines study inclusion and exclusion criteria. Definitions of HFpEF and isolated DD are detailed in the Supplemental Appendix. To reduce dyssynchronous RV pacing that could occur with a higher backup rate, we limit myPACE enrollment to patients with either (1) atrial pacing with intact AVN conduction or minimal RV septal pacing with paced QRS duration <150 ms; or (2) HBP, left bundle branch area pacing, or biventricular pacing with paced QRS duration <150 ms.
Table 1.
myPACE inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Adults >18 years old with a pacemaker Pacemaker lower rate set to 60 bpm at baseline Left ventricular ejection fraction >50% assessed by Simpson biplane method DD or HFpEF (defined in the Supplemental Appendix) SND with intact AVN conduction or minimal right ventricular pacing (<2%) and paced QRS <150 ms OR Impaired AVN conduction with His bundle or left bundle branch area pacing or biventricular pacing and paced QRS <150 ms Subject is expected to remain available for follow-up visits Life expectancy >1 year |
Paced QRS duration >150 ms Infiltrative cardiomyopathy Hypertrophic cardiomyopathy More than moderate valvular stenosis or regurgitation Aortic valve replacement in the past 1 year Significant primary pulmonary disease on home oxygen Uncontrolled hypertension defined by BP >160/100 mm Hg on 2 measurements ≥15 minutes apart Creatinine >2.5 or hemoglobin <8 g/dL Pregnancy Patient participating in another clinical trial |
The inclusion and exclusion criteria were adapted from Heart Rate 80 study,19 the ongoing REVAMP (Remodeling the Left Ventricle With Atrial Modulated Pacing) trial (ClinicalTrials.gov Identifier: NCT03210402), and the clinical judgment of the investigators.
AVN = atrioventricular node; BP = blood pressure; DD = diastolic dysfunction; HFpEF = heart failure with preserved ejection fraction; SND =sinus node dysfunction.
Personalized HR algorithm
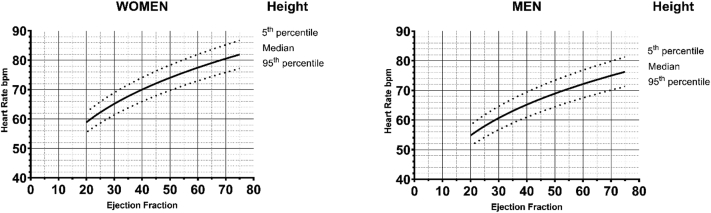
For myPACE, we developed a personalized HR algorithm based on physiological resting HRs in healthy individuals to provide a customized lower rate setting for pacemaker patients. The rationale and validation of the underlying height–HR relationship have been previously described,26 and additional details about the HR algorithm are available in the Supplemental Appendix. The myPACE personalized HR algorithm (Figure 2) is as follows:
Figure 2.
Personalized heart rate (HR) algorithm in myPACE. We developed a HR algorithm based on physiological resting HRs in healthy adults to provide a customized backup HR to pacemaker patients based on height (5th percentile, median, and 95th percentile) in both women (left) and men (right), and modified by ejection fraction.
Personalized HR (bpm) = (Height [cm] × –0.3744) + 134.82) × √√ (Ejection Fraction [%]/50).
Study design
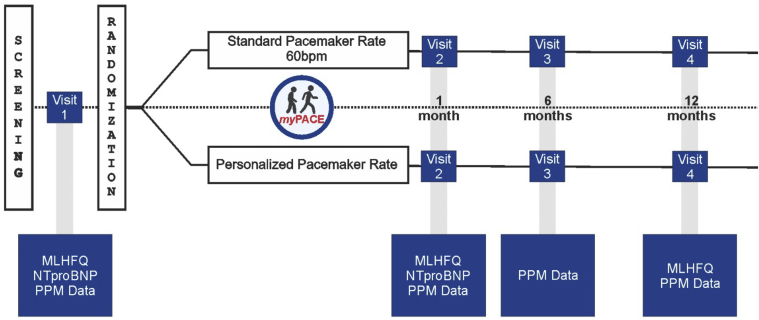
This is a single-center, prospective, blinded, randomized controlled study conducted at the University of Vermont Medical Center. Patients are randomized 1:1 to either a personalized backup HR setting (myPACE group) or the standard 60 bpm setting (control group) for 1 year. A summary of the study is outlined in Figure 3, and blinding is detailed in the Supplemental Appendix.
Figure 3.
myPACE study flowchart. Patients scheduled in our pacemaker clinic are consecutively screened. Those patients who are enrolled complete a baseline MLHFQ quality-of-life score, NTproBNP, and a pacemaker interrogation. Patients are randomized to either the myPACE or the control group. NTproBNP was repeated at 1 month, and MLHFQ was repeated at 1 month and 1 year. Pacemaker-detected data and clinical outcomes are monitored during the 1-year study period. MLHFQ = Minnesota Living with Heart Failure Questionnaire; NTproBNP = N-terminal pro–brain natriuretic peptide; PPM = permanent pacemaker.
Enrolled subjects complete the following baseline measurements before randomization: MLHFQ, NT-proBNP, and a pacemaker interrogation. The validated MLHFQ survey instrument was chosen as a primary outcome because it is an independent predictor of cardiovascular events, death, and future hospitalizations, which is highly correlated with New York Heart Association functional class and is a measure of treatment efficacy.27,28 Baseline characteristics are tabulated after enrollment (Supplemental Appendix).
Outcomes
Primary and secondary study endpoints are summarized in Table 2. Pacemaker-detected endpoints are collected over the 1-year study period. Recorded and adjudicated clinical outcomes (Table 2) are presented individually and as a composite outcome without censoring. Clinical outcomes are monitored throughout the study period and will be assessed by chart review and by patient interview at the 1-year follow-up visit. Final adjudication will be determined by an independent committee blinded to patient randomization.
Table 2.
myPACE study endpoints
| Primary outcome |
|
| Secondary outcomes |
|
|
|
|
|
| Adverse outcomes |
|
MLHFQ = Minnesota Living with Heart Failure Questionnaire; NT-proBNP = N-terminal pro–brain natriuretic peptide.
At the exit visit, after study data have been collected, the group assignment will be assessed and disclosed to the patient. Participants randomized to the myPACE group are offered, in consultation with their primary cardiologist, to have their pacemaker programmed back to the standard 60 bpm or to remain at the personalized HR. This information will be tabulated. Exploratory subgroup analyses are detailed in the Supplemental Appendix.
Additional safety outcomes
The following safety outcomes are reported separately: (1) patient-reported symptoms of palpitations or chest discomfort thought to be pacing related; (2) worsening fatigue; or (3) worsening HF symptoms following randomization. Safety monitoring is detailed in the Supplemental Appendix.
Statistical analysis
With the null hypothesis, there will be no difference in MLHFQ scores between the myPACE and control arms. The alternative hypothesis is superiority of one over the other backup rate.
Based on previous pilot study data in a similar population of patients, with an anticipated mean ± SD baseline MLHFQ score of 31±15, we will need to enroll 59 patients in each group to provide 80% power to detect a clinically relevant >5-point change in the composite MLHFQ score (2-sided test).19,29,30 We set a goal of enrolling 130 patients, assuming attrition rates similar to our previous pilot studies to anticipate patient dropout.
Baseline characteristics will be presented as mean ± SD or median (interquartile range). Continuous variables will be compared using unpaired Student t tests, Wilcoxon matched pairs tests, and analysis of variance. Categorical variables will be compared using contingency table analysis. The cumulative number of individual and composite adjudicated endpoint events (total number of endpoints for each subject) over time will be analyzed by the Anderson and Gill Cox regression model.
The primary analysis is intention to treat. A per-protocol analysis including all patients who remained in their randomized group for at least 1 month with last observation carried forward will be performed secondarily. MLHFQ scores and NT-proBNP are analyzed as per-individual changes within groups and between groups without correction for multiple comparisons (MLHFQ).
Trial management and status
The University of Vermont Institutional Review Board (IRB) approved this study, and myPACE will adhere to the Declaration of Helsinki guidelines. Informed written consent is obtained from all trial participants before enrollment and randomization. Oversight will be provided by a Trial Steering and Adjudication Committee and an independent member of the IRB. The protocol is registered at ClinicalTrials.gov (NCT04721314). Patients started enrollment in July 2019. As of November 15, 2020, 107 patients had been enrolled. Due to the coronavirus disease 2019 (COVID-19) pandemic and to comply with an IRB request, a 1-month interim analysis was performed to exclude futility, defined as a worsening of the average MLHFQ as a reason to end the study. As there was a signal of benefit, enrollment was closed.
Results
The preliminary and updated 1-month MLHFQ and NT-proBNP data were reported virtually at the 2021 American College of Cardiology meeting.31 The study is projected to complete 1-year follow-up of all patients by December 2021. The research findings will be submitted for publication to peer-reviewed journals, and trial participants will be informed of study results.
Discussion
The rationale for myPACE is derived from 2 complementary clinical observations. Over the last 2 decades, evidence has accrued suggesting that the effects of pharmacologic HR lowering in patient populations with preserved EFs either are neutral or are associated with signals of harm, including HF, AF, and stroke (Supplemental Table 1). Additional studies suggest that modest increases in HR using pacemakers may benefit patients with HFpEF or isolated DD (Supplemental Table 2). Pacing studies in a porcine model of hypertensive heart disease,17 clinical hemodynamic assessments in patients with HFpEF,14,15 and 2 exploratory studies in patients with pacemakers and isolated DD or HFpEF18,19 collectively suggest a benefit of moderately elevated HRs in concentric LV hypertrophy, isolated DD, or HFpEF. Neither pilot study raised safety concerns, such as tachycardia-mediated cardiomyopathy.18,19
myPACE is the first randomized, blinded trial to assess median term effects of pacing-mediated moderate HR acceleration on QoL, natriuretic peptide levels, and clinical outcomes. The algorithm utilized in myPACE provides study participants with an individualized HR that is determined by height and modified by baseline EF. Enrollment and follow-up are facilitated by a single referral center and a cardiac electrophysiology group that emphasizes physiological pacing in all patients.32
Other benefits of pacing
Besides the reduction in filling pressures and wall stress, higher HRs have additional effects. The force–frequency relationship or Treppe phenomenon enhances intrinsic myocardial contractility that is preserved in HFpEF.33,34 The HR-mediated rise in contractility is associated with faster relaxation kinetics that largely depend on the acceleration of intracellular calcium sequestration mediated by the sarcoplasmic reticulum.35 An increase in the stimulation rate of isolated contracting human myocardium from 60 to 90 bpm shortens the time to half-maximal relaxation by 11%.36 The calciotropic effect of pacing and increase in relaxation velocity also lower filling pressures by suction and shift the pressure–volume loop leftward (Figure 4) from larger to smaller LV volumes, which are less exposed to the exponential portion of the end-diastolic pressure–volume relationship.5,7
Figure 4.
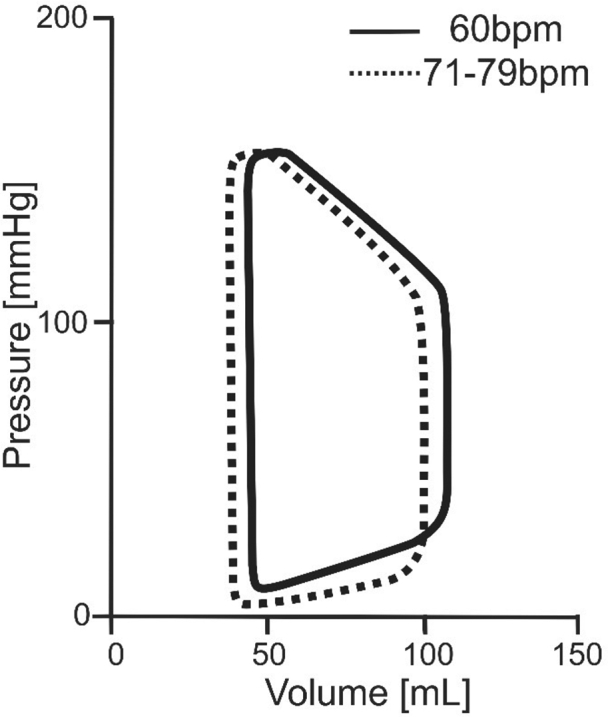
Schematic left ventricular pressure–volume (PV) loops derived from hemodynamic studies in patients with heart failure with preserved ejection fraction. Effects of a heart rate (HR) increase from the pacemaker backup rate of 60 bpm to normal HRs are shown. Higher HRs lower left ventricular end-diastolic volume and pressure by a shortened left ventricular filling time and leftward shift of the PV loop.
Chronic reduction in filling pressures and wall stress may lead to beneficial remodeling over time. In an experimental model, we have shown that even modest HR elevations can lead to physiological eccentric remodeling with an associated reduction in ventricular wall thickness, a lower mass-to-volume ratio, and improved compliance without leading to tachycardia-induced cardiomyopathy.17 Although the underlying molecular mechanisms of HR-induced eccentric remodeling are unknown, there is a linear HR dose-dependence to suggest that even minor increases in HR are associated with subclinical remodeling.17
In addition, as cardiac output is the product of HR and stroke volume, higher HRs may modestly improve cardiac output, which in addition to lowering left-sided filling pressures may contribute to a better QoL. The personalized HRs in myPACE will exceed sleep HRs by at least 10 bpm, which could provide an additional remodeling stimulus while also decreasing nocturnal filling pressures that may reduce orthopnea, improve the quality of sleep and well-being, and perhaps in turn facilitate increased daytime physical activity.
Potential hazards of pacing
Higher HRs increase myocardial oxygen consumption proportionally.37,38 Therefore, it is possible that higher-rate pacing increases the risk for demand ischemia, whereas HR lowering generally reduces this risk. However, in the SIGNIFY trial, which investigated the effects of ivabradine among patients with stable coronary disease without clinical HF, selective HR lowering by 10 bpm did not improve outcomes. On the contrary, among the subgroup of patients with activity-limiting angina, the primary composite endpoint of death from cardiovascular causes or nonfatal myocardial infarction was significantly greater in the ivabradine group.8
The potential benefit of an increased HR in patients with isolated DD or HFpEF using standard RV pacing could be offset by a pacing-induced cardiomyopathy, as reported in patients with >40% RV pacing.39 As discussed, pacing-induced cardiomyopathy is mitigated with biventricular pacing and likely, to an even greater extent, by HBP.40,41 An increase in backup HR is also expected to reduce vagally mediated HR variability. This may not be directly harmful, however, as HR variability—like low resting HR—has been shown to be a marker rather than a conveyor of good health.42 Furthermore, based on pervious data,17, 18, 19 the proposed pacing rates in this study are unlikely to induce clinically relevant eccentric remodeling and tachycardia-mediated cardiomyopathy. However, this is an important safety endpoint that we will track. Based on the studies in our preclinical model, we expect that the increase in LV end-diastolic volume will remain <10%.
Study limitations
It could be argued that effective blinding of study participants for the myPACE HR intervention cannot be accomplished. However, the same argument can be made for HR-lowering medications such as beta-blockers or ivabradine. To assess this potential source of bias, we have introduced a question to assesses patient blinding at the 1-month and 1-year follow-up. Another limitation of the myPACE study design is that sample size estimates for clinical outcomes could not be made for a lack of comparator data.
Conclusion
myPACE is a prospective, randomized, blinded trial designed to evaluate the effects of higher HRs in pacemaker patients with isolated DD or HFpEF in whom potentially adverse effects of pacing will be mitigated by ventricular pacing that facilitates synchronous interventricular activation. myPACE is testing the paradigm-changing hypothesis that moderately higher HRs, and not lower HRs, might provide important benefits for this complex patient population with an unmet need for evidence-based targeted therapies.
Funding Sources
This research was supported by Grant R01 HL-122744 from the National Institutes of Health to Dr Meyer; and the Heart Rhythm Society Research Fellowship Award and the Cardiovascular Research Institute of Vermont’s Martin M. LeWinter Young Investigator and Early Career Research Awards to Dr Infeld.
Disclosures
Dr Meyer and the University of Vermont have licensed patents for the use of pacemakers for the prevention and treatment of heart failure with preserved ejection fraction. Dr Lustgarten and Dr Meyer have received research funding from Medtronic. Other authors report no competing interests.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Informed written consent is obtained from all trial participants prior to enrollment and randomization.
Ethics Statement
The University of Vermont Institutional Review Board approved this study, and myPACE will adhere to the Declaration of Helsinki guidelines.
Footnotes
Clinicaltrials.gov Identifier: NCT04721314.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2021.11.015.
Appendix. Supplementary data
References
- 1.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Kuznetsova T., Herbots L., López B., et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich P.A., Albert N.M., Allen L.A., et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleland J.G.F., Bunting K.V., Flather M.D., et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39:26–35. doi: 10.1093/eurheartj/ehx564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer M., Rambod M., LeWinter M. Pharmacological heart rate lowering in patients with a preserved ejection fraction-review of a failing concept. Heart Fail Rev. 2018;23:499–506. doi: 10.1007/s10741-017-9660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox K., Borer J.S., Camm A.J., et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 7.Meyer M., LeWinter M.M., Zile M.R. A targeted treatment opportunity for HFpEF: taking advantage of diastolic tone. Circulation. 2021;144:1269–1271. doi: 10.1161/CIRCULATIONAHA.121.056412. [DOI] [PubMed] [Google Scholar]
- 8.Fox K., Ford I., Steg P.G., et al. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371:1091–1099. doi: 10.1056/NEJMoa1406430. [DOI] [PubMed] [Google Scholar]
- 9.Dahlöf B., Devereux R.B., Kjeldsen S.E., et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 10.Dahlöf B., Sever P.S., Poulter N.R., et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 11.Williams B., Lacy P.S. CAFE and the ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) Investigators. Impact of heart rate on central aortic pressures and hemodynamics: analysis from the CAFE study: CAFE-Heart Rate. J Am Coll Cardiol. 2009;54:705–713. doi: 10.1016/j.jacc.2009.02.088. [DOI] [PubMed] [Google Scholar]
- 12.Meyer M., LeWinter M.M. Heart rate and heart failure with preserved ejection fraction: time to slow β-blocker use? Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.119.006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diederichsen S.Z., Haugan K.J., Brandes A., et al. Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J Am Coll Cardiol. 2019;74:2771–2781. doi: 10.1016/j.jacc.2019.09.050. [DOI] [PubMed] [Google Scholar]
- 14.Westermann D., Kasner M., Steendijk P., et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 15.Silverman D.N., Rambod M., Lustgarten D.L., et al. Heart rate-induced myocardial Ca2+ retention and left ventricular volume loss in patients with heart failure with preserved ejection fraction. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller K.A.L., Heinzmann D., Klingel K., et al. Histopathological and immunological characteristics of tachycardia-induced cardiomyopathy. J Am Coll Cardiol. 2017;69:2160–2172. doi: 10.1016/j.jacc.2017.02.049. [DOI] [PubMed] [Google Scholar]
- 17.Klein F.J., Bell S., Runte K.E., et al. Heart rate-induced modifications of concentric left ventricular hypertrophy: exploration of a novel therapeutic concept. Am J Physiol Heart Circ Physiol. 2016;311:H1031–H1039. doi: 10.1152/ajpheart.00301.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeshwant S.C., Zile M.R., Lewis M.R., Lewinter M., Meyer M. Safety and feasibility of a nocturnal heart rate elevation—exploration of a novel treatment concept. J Card Fail. 2019;25:67–71. doi: 10.1016/j.cardfail.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Wahlberg K., Arnold M.E., Lustgarten D., Meyer M. Effects of a higher heart rate on quality of life and functional capacity in patients with left ventricular diastolic dysfunction. Am J Cardiol. 2019;124:1069–1075. doi: 10.1016/j.amjcard.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khazanie P., Hellkamp A.S., Fonarow G.C., et al. Permanent pacemaker use among patients with heart failure and preserved ejection fraction: findings from the Acute Decompensated Heart Failure National Registry (ADHERE) National Registry. Am Heart J. 2018;198:123–128. doi: 10.1016/j.ahj.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Borlaug B.A., Melenovsky V., Russell S.D., et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 22.Gillis A.M., Russo A.M., Ellenbogen K.A., et al. HRS/ACCF expert consensus statement on pacemaker device and mode selection. J Am Coll Cardiol. 2012;60:682–703. doi: 10.1016/j.jacc.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Biffi M., Melissano D., Rossi P., et al. The OPTI-MIND study: a prospective, observational study of pacemaker patients according to pacing modality and primary indications. Europace. 2014;16:689–697. doi: 10.1093/europace/eut387. [DOI] [PubMed] [Google Scholar]
- 24.Hegde S.M., Claggett B., Shah A.M., et al. Physical activity and prognosis in the TOPCAT trial (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) Circulation. 2017;136:982–992. doi: 10.1161/CIRCULATIONAHA.117.028002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kass D.A., Kitzman D.W., Alvarez G.E. The restoration of chronotropic competence in heart failure patients with normal ejection fraction (RESET) study: rationale and design. J Card Fail. 2010;16:17–24. doi: 10.1016/j.cardfail.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Infeld M., Avram R., Wahlberg K., Silverman D.N., Habel N., Lustgarten D.L., et al. An approach towards individualized lower rate settings for pacemakers. Heart Rhythm O2. 2020;1:390–393. doi: 10.1016/j.hroo.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato N., Kinugawa K., Seki S., et al. Quality of life as an independent predictor for cardiac events and death in patients with heart failure. Circ J. 2011;75:1661–1669. doi: 10.1253/circj.cj-10-1308. [DOI] [PubMed] [Google Scholar]
- 28.Holland R., Rechel B., Stepien K., Harvey I., Brooksby I. Patients' self-assessed functional status in heart failure by New York Heart Association class: a prognostic predictor of hospitalizations, quality of life and death. J Card Fail. 2010;16:150–156. doi: 10.1016/j.cardfail.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelkar A.A., Spertus J., Pang P., et al. Utility of patient-reported outcome instruments in heart failure. JACC Heart Fail. 2016;4:165–175. doi: 10.1016/j.jchf.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Rector T.S., Tschumperlin L.K., Kubo S.H., et al. Use of the Living with Heart Failure questionnaire to ascertain patients' perspectives on improvement in quality of life versus risk of drug-induced death. J Card Fail. 1995;1:201–206. doi: 10.1016/1071-9164(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 31.Infeld M., Wahlberg K., Cicero J., et al. Personalized pacing (myPace): a new paradigm for diastolic heart failure treatment. J Am Coll Cardiol. 2021;77(18 Suppl 1):223. [Google Scholar]
- 32.Lustgarten D.L., Calame S., Crespo E.M., et al. Electrical resynchronization induced by direct His-bundle pacing. Heart Rhythm. 2010;7:15–21. doi: 10.1016/j.hrthm.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 33.Runte K.E., Bell S.P., Selby D.E., et al. Relaxation and the role of calcium in isolated contracting myocardium from patients with hypertensive heart disease and heart failure with preserved ejection fraction. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamanaka T., Onishi K., Tanabe M., et al. Force- and relaxation-frequency relations in patients with diastolic heart failure. Am Heart J. 2006;152 doi: 10.1016/j.ahj.2006.06.023. 966.e1–7. [DOI] [PubMed] [Google Scholar]
- 35.Bluhm W.F., Kranias E.G., Dillmann W.H., Meyer M. Phospholamban: a major determinant of the cardiac force-frequency relationship. Am J Physiol Heart Circ Physiol. 2000;278:H249–H255. doi: 10.1152/ajpheart.2000.278.1.H249. [DOI] [PubMed] [Google Scholar]
- 36.Pieske B., Kretschmann B., Meyer M., et al. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 1995;92:1169–1178. doi: 10.1161/01.cir.92.5.1169. [DOI] [PubMed] [Google Scholar]
- 37.Meyer M., Keweloh B., Güth K., et al. Frequency-dependence of myocardial energetics in failing human myocardium as quantified by a new method for the measurement of oxygen consumption in muscle strip preparations. J Mol Cell Cardiol. 1998;30:1459–1470. doi: 10.1006/jmcc.1998.0706. [DOI] [PubMed] [Google Scholar]
- 38.Gobel F.L., Norstrom L.A., Nelson R.R., Jorgensen C.R., Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57:549–556. doi: 10.1161/01.cir.57.3.549. [DOI] [PubMed] [Google Scholar]
- 39.Kiehl E.L., Makki T., Kumar R., et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13:2272–2278. doi: 10.1016/j.hrthm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 40.Curtis A.B., Worley S.J., Adamson P.B., et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–1593. doi: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]
- 41.Sharma P.S., Dandamudi G., Herweg B., et al. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm. 2018;15:413–420. doi: 10.1016/j.hrthm.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Kluttig A., Schumann B., Swenne C.A., et al. Association of health behaviour with heart rate variability: a population-based study. BMC Cardiovasc Disord. 2010;10:58. doi: 10.1186/1471-2261-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.