Abstract
Objective
Adipose tissue (AT) senescence is associated with AT dysfunction in rodents, but little is known about human AT senescence. Our goal was to define the distribution of senescent cells in two subcutaneous depots and understand relationships with adiposity and inflammation.
Methods
Sixty-three volunteers (48 females) underwent abdominal and femoral subcutaneous fat biopsies. We measured fat cell size (FCS), senescent cells using senescence associated β-galactosidase (SA-β-gal) staining per 100 nucleated cells (%), and mRNA expression of 4 cytokines.
Results
There was a larger proportion of senescent cells in femoral than abdominal subcutaneous AT (mean difference 1.6 [95% CI 0.98–2.3%], P <0.001) and the percentage of femoral AT senescent cells was greater in women than men (median 3.9% vs. 2.1%, P<0.01). There was a positive correlation between senescence and FCS in abdominal (rs=0.44, P<0.001) and femoral (rs=0.35, P=0.007) AT depots. Abdominal AT TNF-α (rs=0.49, P<0.01) and IL1-β (rs=0.44, P=0.01) expression were positively correlated with abdominal, but not femoral AT senescence.
Conclusions
In human subcutaneous AT there are more senescent cells in femoral than abdominal depots; abdominal AT senescent cells are more associated with inflammatory signals than femoral AT senescent cells.
Keywords: Adipose tissue, senescent cells, cytokines
Introduction
Cellular senescence occurs when there is a permanent arrest of the cell cycle in cells with capacity to replicate (1). Cellular senescence is triggered by several stresses such as telomere injury, DNA damage and oxidative stress. These stresses occur more frequently with aging, induce senescence, leading to organ-tissue dysfunction via secretion of proinflammatory cytokines and induction of mitochondrial dysfunction (2). Some, but not all senescent cells can develop a senescence associated secretory phenotype (SASP) comprising pro-inflammatory cytokines such as IL-6, IL-7, IL-8, IL-1β, and TNF-α. This phenotype can also include secretion of chemokines that attract, anchor, and activate immune cells, pro-fibrotic and hemostatic factors, and tissue-destroying proteases (2–4); this response has been associated with tissue dysfunction and insulin resistance. Senescent cells have been found in many tissues and organs, including liver, bone, cartilage, blood vessels, heart, brain, and adipose tissue (5–8). These cells are thought to play an important role in tissue dysfunction in age-related diseases, such as cardiovascular disease, diabetes, osteoporosis, non-alcoholic fatty liver disease, and cancer (6,9–12).
There are no fully sensitive or specific markers for cellular senescence, but several markers for senescence are increased in adipose tissue of obese rats (13) and humans with obesity (10). Obesity has been associated with senescence in AT mesenchymal stromal/stem cells (1) that can impair adipogenesis of neighboring cells (14). This suggests that senescent stromal vascular fraction derived progenitor cells and senescent preadipocytes could play a role in the pro-inflammatory state associated with obesity and obesity related metabolic complications (10). We found only a few studies of senescence in adipose tissue (AT) in humans and its potential role in the pathophysiology of obesity (8,15–17). Although senescence has been found in AT mesenchymal stromal/stem cells, preadipocytes, young adipocytes, macrophages, and endothelial cells (1,2,15,18,19), it is not clear if differentiated, non-dividing cells such as mature adipocytes can become senescent. There is evidence that some non-dividing cardiomyocytes can become senescent-like (20).
There are physiologic differences in regards to inflammatory markers, triglyceride storage, and lipolysis between abdominal and femoral subcutaneous fat depots (21,22). In addition, humans with greater femoral than abdominal fat are more metabolically healthy, suggesting that there may be differences in how these depots contribute to disease risk. The cause for these differences has not been clearly elucidated and it has been hypothesized that cellular senescence could be responsible (15). We tested the hypothesis that there are more senescent cells in abdominal than femoral subcutaneous adipose tissue. Herein we describe the distribution of senescent cells in different subcutaneous adipose tissue depots in men and women across the BMI spectrum, as well as the relationship between senescent cells and adiposity, adipocyte size, and cytokines.
Methods
Samples and data were collected as part of three separate studies that were approved by the Mayo Clinic IRB. We intentionally included volunteers with a wide range of adiposity (16 – 52% body fat) and BMI (21.2 – 38.2). All participants were recruited between March 2013 and November 2018 and provided informed, written consent. Participants underwent a screening visit to assess their eligibility for the study, followed by body composition measurements using dual energy x-ray absorptiometry (DXA) and a single slice CT scan of the abdomen at the L2–3 interspace (to measure visceral fat). They subsequently were admitted to the Mayo Clinic Clinical Research and Trials Unit (CRTU) where they underwent adipose tissue (AT) biopsies to determine AT senescence.
Men and premenopausal women between 18 and 55 years of age were included. Study 1 included volunteers ranging from normal BMI to Class II obesity., Studies 2 and 3 included only volunteers in the overweight to Class II obesity range of BMI. Those with history of chronic systemic inflammatory diseases, cardiovascular disease, use of anti-inflammatory medications or medications that affect fatty acid metabolism were excluded. All participants had to be weight stable for 2 months before the study visit.
Adipose biopsies for all studies were obtained after an overnight fast. Biopsies were collected using a small-bore liposuction approach under local anesthesia with sterile technique. Samples were taken from the abdominal subcutaneous region lateral to the umbilicus and the femoral region on the anterior-lateral aspect of the mid-thigh.
Determination of AT Senescence
Senescent cells were identified using SA-β-gal staining that at a pH of 6 is a marker to detect senescent cells. The tissue was fixed in 0.5% glutaraldehyde, washed with PBS, and incubated at 37° C for 16 h with SA-β-gal activity solution. The solution is at a pH of 6 in order to allow the SA-β-gal enzyme to catalyze the hydrolysis of β-galactosides into monosaccharides, which occurs only in senescent cells. Hoechst 33342 dye was used to stain nuclei. The sample was placed between two mounting slides and fluorescent microscopy was used to measure the number of cells with SA-β-gal activity and the number of nucleated cells per field using NIS software (Nikon). The number of SA-β-gal expressing cells is reported per 100 nucleated cells.
To test whether the expression of SA-β-gal correlates with another marker of cellular senescence, we stained 16 of the above described samples for CDKN2A/p16INK4A using immunohistochemical techniques. We used anti-p16INK4A antibody [EPR1473] AbCam 108349 for senescence. The number of positive cells per 100 adipocytes was calculated. Fat cell size was measured as previously described (23).
Plasma IL-6 and TNF-α concentrations were measured as described by the manufacturer using the Meso Scale Discovery (MSD) pro-inflammatory Panel 1 assay (catalog #K15049D) in the Immunochemical Core Laboratory of Mayo Clinic. Reportable ranges were 1.58–488 pg/mL for IL-6 and 0.69–248 pg/mL for TNF-α.
Real Time PCR
RNA was isolated from adipose tissue using the RNeasy Lipid Tissue mini kit (Qiagen # 74804) and then reverse transcribed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems #4368813) into cDNA as described by the manufacturer. RT-PCR was performed using Taqman Gene Expression assays (Applied Biosystems (CD68=Hs02836816_g1, CD206=Hs00267207_m1, CD14=Hs02621496_s1, p16INK4A =Hs00923894_m1, and CYCA=Hs99999904_m1) and TaqMan Fast Advanced Master Mix (Applied Biosystems #4444964) on an ABI Quant thermocycler using “Fast” settings in duplicate. We used the ΔΔCt method to analyze the data and cyclophilin A expression was used to normalize samples.
Statistical Analysis
Continuous variables are expressed as mean ± SD if normally distributed, and median, inter-quartile range (IQR) if non-parametric. Spearman correlation coefficient (rs) was used for correlation analysis of nonparametric variables. The Wilcoxon signed-rank test was used as the non-parametric matched pairs test for comparison of senescent cells between fat depots. JMP 10.0 was used for all analyses.
Results
Subject Characteristics
The subject characteristics are provided in Table 1. Sixty-three volunteers underwent body composition studies; 59 had abdominal subcutaneous AT biopsies and 58 had femoral subcutaneous AT biopsies. Of the total of 63 volunteers, 54 had AT biopsies done in both abdominal and femoral depots; the remaining 9 volunteers had biopsies successful for these measures in only one of the depots. Males and females did not differ with respect to age or BMI, but had the expected differences in percent body fat, visceral fat, and leg fat.
Table 1.
Subject Characteristics
| Variable | All (N=63) | Females (N=48) | Males (N=15) | Pvalue |
|---|---|---|---|---|
| Age (years), median (IQR) | 39 (28–45) | 38 (27–45) | 39 (29–44) | 0.57 |
| BMI (kg/m2), median (IQR) | 32.9 (30.2–34.4) | 32.9 (30.6–34.4) | 33.1 (26.1–34.9) | 0.91 |
| Fat (%), median (IQR) | 44 (39–47) | 45 (41–48) | 37 (26–39) | <0.001 |
| FFM (kg), median (IQR) | 51.1 (45.8–58.7) | 48.3 (44.8–52.7) | 66.7 (62.2–71.6) | <0.001 |
| UBSQ fat (kg), median (IQR) | 21.0 (16.7–24.5) | 22.1 (17.6–24.7) | 20.7 (10.6–22.2) | 0.18 |
| Visceral Fat (kg), median (IQR) | 3.4 (2.2–5.6) | 3.1(2.1–4.5) | 6.6 (2.7–7.7) | <0.001 |
| Leg fat (kg), median (IQR) | 13.4 (10.9–15.9) | 14.2 (12.3–17.3) | 10.5 (7.2–11.5) | <0.001 |
| Abdominal fat cell size (μg/cell), median (IQR) | 0.72 (0.48–0.99) | 0.72 (0.50–0.94) | 0.71 (0.39–1.11) | 0.85 |
| Femoral fat cell size (μg/cell), median (IQR) | 0.96 (0.71–1.16) | 0.97 (0.74–1.21) | 0.76 (0.59–0.97) | 0.05 |
| Fasting plasma glucose (mg/dL), median (IQR) | 90 (84–94) | 87 (84–92) | 90 (86–99) | 0.09 |
| Abdominal SaβGal (%) a (N=59), median (IQR) | 1.8 (0.9–3.7) | 2.0 (0.9–4.0) | 1.5 (0.6–2.3) | 0.09 |
| Femoral SaβGal (%) a (N=58), median (IQR) | 3.4 (2.3–5.1) | 3.9 (2.7–5.3) | 2.1 (0.7–3.3) | 0.002 |
P-values refers to the difference between males and females.
Senescence associated β-galactosidase staining was used to measure senescent cells. BMI - body mass index; FFM - fat free mass; UBSQ - upper body subcutaneous; SaβGal - senescence associated β-galactosidase.
Technical Assessments of Adipose Cellular Senescence Measurements
Because there is a manual, and thus potentially subjective component to counting the number of SA-β-gal -stained cells, we compared the results from two different readers who analyzed the same images from 32 biopsies. The agreement between the two readers was excellent (r = 0.99, P < 0.0001, Figure 1A), indicating we could rely on results from a single reader.
Figure 1.
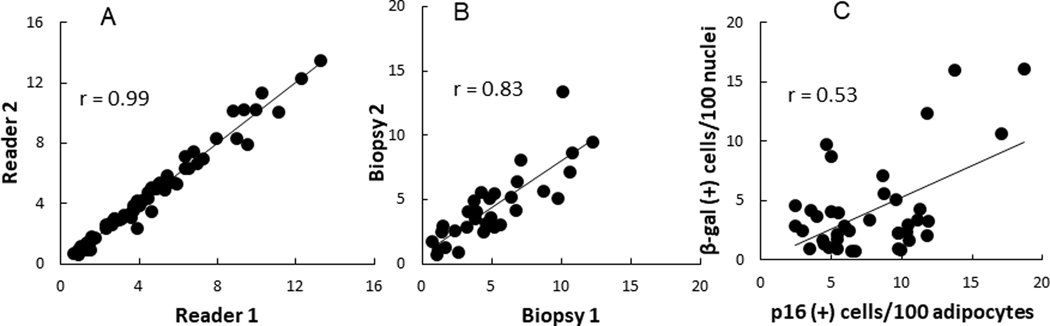
Correlation between number of SA-β-gal positive cells per 100 nuclei in adipose tissue (A) as counted by two different readers; (B) between replicate biopsies performed 2 weeks apart; and (C) between SA-β-gal positive cells and p16 positive cells per 100 adipocytes.
To assess the test-retest reliability of the SA β-gal approach for measuring senescent cells we collected abdominal and femoral AT samples from 16 volunteers on two separate occasions separated by 2 weeks. Senescent cells constituted 3.8 ± 2.7% of abdominal and 5.7 ± 2.7% of femoral subcutaneous AT cells, respectively. The values for % of senescent cells from all the first biopsies and all the second biopsies were highly correlated (r = 0.83, P < 0.0001, Figure 1B). The test-retest intra-class correlations were 0.90 and 0.77 for abdominal and femoral AT samples, respectively.
Representative images of adipose tissue stained for SA-β-gal and p16INK4A are provided in Figure 2. The relationship between SA-β-gal and p16INK4A staining in AT is shown in Figure 1C. There was a positive correlation (r = 0.53, P < 0.001) between senescent cells measured using senescence SA-β-gal staining and p16INK4A immunohistochemical staining. For the remainder of this report we will use SA-β-gal as the measure of the number of senescent cells.
Figure 2.
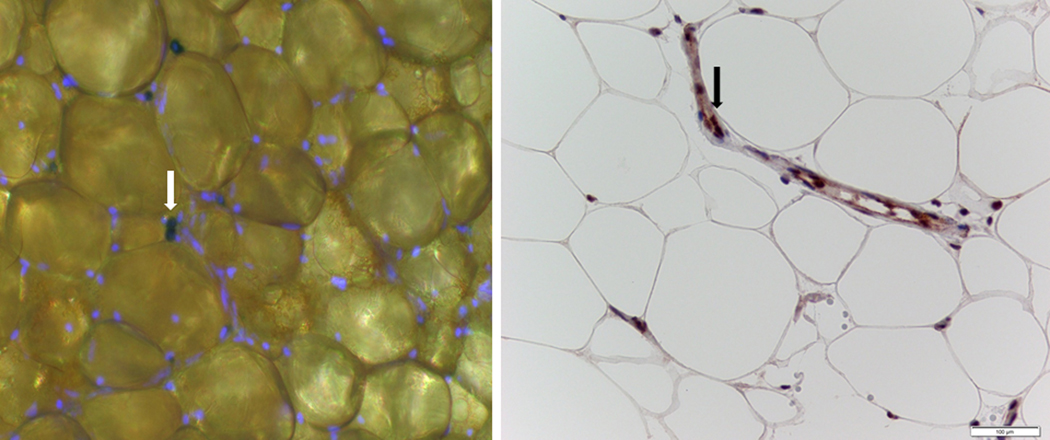
Left panel - fluorescent photomicrograph of adipose tissue (AT) stained for nuclei (blue) and β-galactosidase (white arrow) to identify senescent cells; right panel, photomicrograph of adipose stained for p16 (black arrow).
Relationship between Senescent Cells and Adiposity
The percentage of cells that stained positive for SA-β-gal in femoral adipose tissue was greater (P = 0.002) in women than men (Table 1), whereas the sex difference in the number of SA-β- galactosidase positive cells in abdominal subcutaneous fat was not statistically significant (p=0.09).
Adults with obesity had more abdominal subcutaneous AT senescent cells than adults without obesity (2.2% (0.9–4.0) vs. 0.9% (0.6–2.2) [median (IQR)], P = 0.02). In contrast, there was not a significant difference in the number of senescent cells in femoral subcutaneous fat in those with and without obesity [3.5% (2.4–5.1) vs. 2.8% (1.4–5.1), P = 0.14].
For those for whom we had both abdominal and femoral adipose samples (N= 54), the percentage of senescent cells was greater in femoral than abdominal fat (mean of 4.1% (±2.7) vs. 2.5% (±2.1), mean difference of 1.6 [95% CI 0.98–2.3%], P < 0.001).
There was a positive correlation between total body fat and senescent cells in abdominal (rs = 0.57, P < 0.001) and femoral (rs = 0.52, P < 0.001) fat. We also found a positive correlation between the number of abdominal senescent cells and upper body subcutaneous fat mass (rs = 0.53, P < 0.001, Figure 3A), but the relationship between leg fat mass and the number of femoral senescent cells was not statistically significant (rs = 0.19, P = 0.15, Figure 3B).
Figure 3.
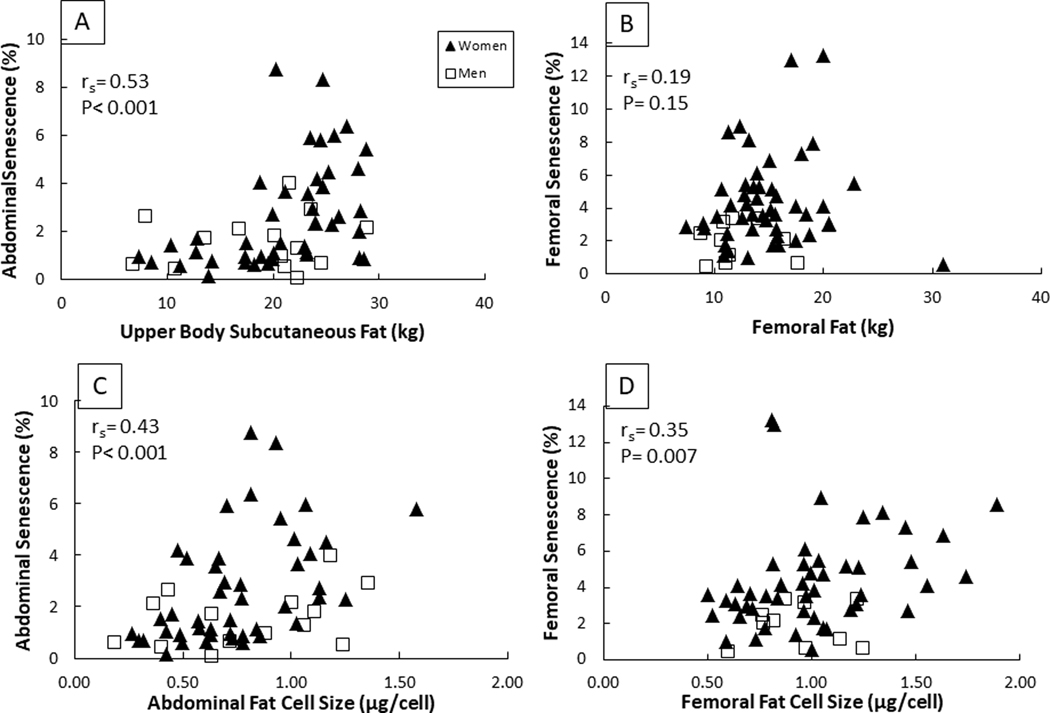
Correlation between senescent cells and fat mass in the abdominal and femoral depots (A,B). Correlation between senescent cells and fat cell size in the abdominal and femoral depots (C,D). Spearman correlation coefficient was used for analysis for all participants, women and men combined.
There was positive correlation between fat cell size and the number of senescent cells in the abdominal (rs = 0.43, P < 0.001, Figure 3C) and femoral (rs = 0.35, P = 0.007, Figure 3D) subcutaneous AT. There was no correlation between age and the number of abdominal or femoral senescent cells, but there was a correlation between the number of senescent cells in abdominal and femoral subcutaneous adipose tissue (r = 0.52, P < 0.001).
Senescent Cells and Cytokines
There was no significant correlation between plasma IL-6 or TNF-α concentrations and AT senescent cell burden. The correlation between plasma IL-6 concentrations and senescent cells in the abdominal and femoral depots were rs = 0.21 (P = 0.32) and 0.39 (P = 0.08), respectively. Correlations between plasma TNF-α concentrations and senescent cells in abdominal and femoral depots were rs = 0.07 (P = 0.74) and −0.26 (P = 0.27), respectively. In contrast, we did find statistically significant, positive correlations between abdominal AT senescent cells and abdominal mRNA expression levels of TNF-α, IL1-β, and IL-10. The relationship between senescent cell and adipose IL-6 mRNA was not significant (Figure 4). There were no significant relationships between femoral AT senescent cells and the expression of any of the cytokine mRNAs (Figure 5).
Figure 4.
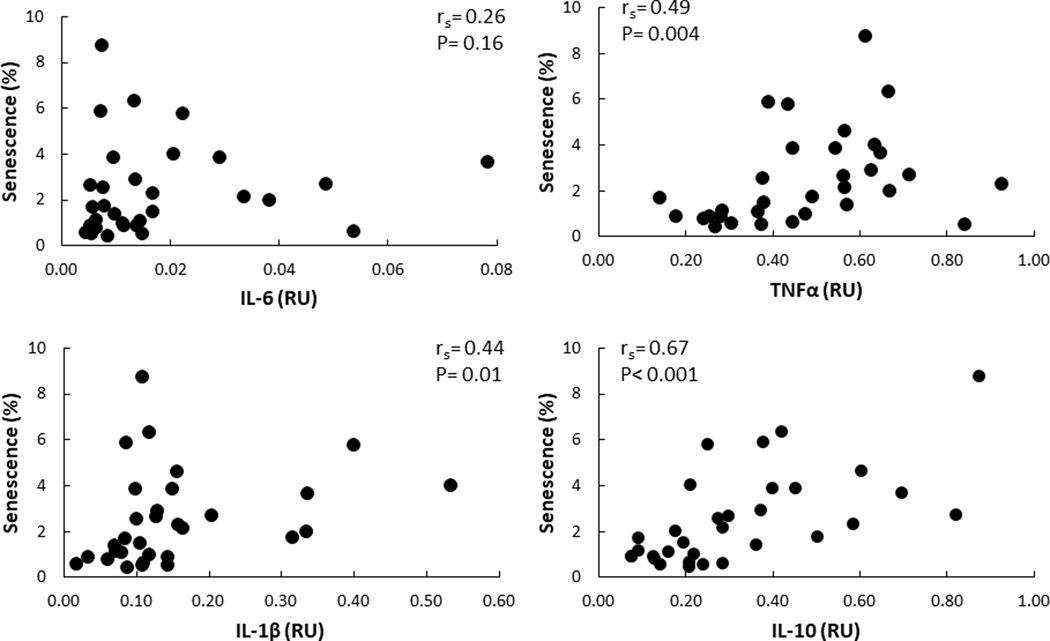
Correlation between mRNA levels of cytokines and senescence in abdominal AT depot RU – relative units
Figure 5.
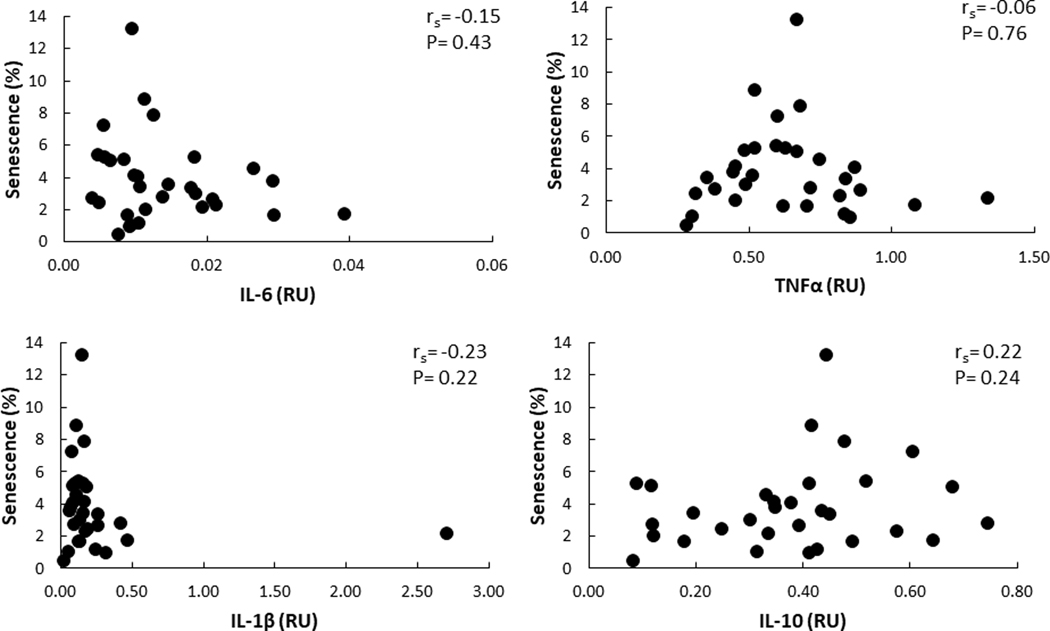
Correlation between mRNA levels of cytokines and senescence in femoral AT depot RU – relative units
Discussion
Cellular senescence, including that occurring in adipose tissue (13,24,25), is linked to a number of adverse metabolic profiles in animal models. Our goal was to understand the patterns of adipose cellular senescence in humans in order to help develop a definition of what constitutes “normal” amounts of senescent cells vs. a burden of cellular senescence that is linked to markers of inflammation. We measured the number of senescent cells in abdominal and femoral subcutaneous depots in a large number of adults to assess how depot, sex, and adiposity relate to senescent cell burden. To our surprise, there were more senescent cells in femoral than abdominal AT and women had more femoral senescent cells than men. Abdominal, but not femoral senescent cell burden was correlated with local mRNA expression of TNF-α, IL1-β, and IL-10, but neither abdominal nor femoral AT senescent cell burden was correlated with plasma IL-6 or TNF-α concentrations. We suggest that senescent cells in different adipose depots have different implications for inflammation and, potentially, adipose function.
Our findings that greater adiposity is associated with greater AT senescent cell burden is consistent with previous studies (10,26). A few studies have looked into differences in senescence in AT but mostly between visceral and subcutaneous fat. In humans with cancer (10), those with diabetes had increased senescence in visceral AT. In patients with severe obesity (10,15), markers of senescence are increased, more so in visceral than in abdominal SC AT. It has been reported that senescence in the intramuscular quadriceps fat is associated with worse measures of mobility and physical function in older women with overweight/obesity (8), but senescence in subcutaneous femoral depot has not been studied. Our findings indicate that AT cellular senescence is present in both major subcutaneous depots in humans ranging from normal weight through Class II obesity.
Preadipocytes vary in capacity for differentiation, adipogenesis, replication, and apoptosis among fat depots (13,27). Therefore, a potential explanation for the greater senescent cell burden in the femoral depot in women is the greater replicative capacity of adipocyte precursors in this depot. Femoral AT expands more readily via the process of adipocyte hyperplasia than abdominal subcutaneous AT (28). Women have more leg fat than men and normal weight women have greater adipocyte cellularity and less hypertrophy than men (29). It seems likely that the greater cell replication leads to telomere shortening, which can induce cellular senescence (9). Therefore, the greater proportion of senescent cells in leg than abdominal subcutaneous fat may be a consequence of the increased replicative ability of femoral preadipocytes, especially in women.
Human senescent cells secrete inflammatory and immune-modulatory proteins, including cytokines/chemokines (IL-6, Il-7, Il-8, IL-1β, TNF-α, MCP-1, 2), growth factors (TGF-β, VEGF) and cell surface molecules (ICAMs) (3,4). These molecules are referred as the senescence-associated secretory phenotype (SASP). Studies have shown that SASP promotes senescence of other cells (30,31), recruitment of immune inflammatory cells (19), and, in the AT of obese mice, can lead to insulin resistance (10,19). Reduction of AT senescent cells with senolytics (drugs that selectively decrease senescent cells) in obese mice improves local and systemic insulin sensitivity (19). This finding provides evidence for a causal relationship between senescence and AT insulin resistance in rodents. Although we did not find a correlation between AT senescent cells and circulating cytokines in our population, the positive association between abdominal AT senescence and abdominal AT TNF-α and IL1-β mRNA suggests that these cells could contribute to local AT inflammation in that depot.
In contrast, we did not find a correlation between AT cytokines and senescent cells in the femoral fat. The secretory profile and function of senescent cells varies among tissues and cell types (4), and many cell types can undergo senescence. It is possible that most of the senescent cells we observed in abdominal adipose tissue were of the endothelial cell type, which is known to be influenced by the adipose microenvironment (10,15). Our findings suggest that cellular senescence in the femoral depot may be more benign than senescence in the abdominal depot. We acknowledge that our data are only associative and cannot prove causality.
We found that the reproducibility of SA-β-gal staining measurement in AT was very good. Because there are conditions that can cause cells to stain positive for SA-β-gal in the absence of senescence (prolonged cell incubation time or conditions with increased lysosome number or activity) (32) we tested whether the SA β-gal staining results correlated with another index of senescence – cells expressing the p16INK4A protein. Although the correlation between SA-β-gal and p16INK4A counts was not perfect, this could be due to the differences in how such data is derived. The SA-β-gal staining is of necessity expressed per 100 nuclei, whereas the number of p16INK4A positive cells by immunohistochemistry is expressed per 100 adipocytes. The varying size of adipocytes and the varying number of non-adipocytes relative to adipocytes in adipose tissue is expected to create some disagreement in values even if these markers perfectly identified senescent cells. The finding of a reasonably strong positive correlation between SA-β-gal staining and p16INK4A supports the use of these markers to evaluate AT senescence.
There are some limitations to our study. Although age should be a predictor of AT senescence, the age range of our participants (between 22 and 55 years) was likely too narrow to detect an association. Because the BMI range of our volunteers was 21–38 kg/m2 our results should not be extrapolated to humans with class 3 obesity. Adipose tissue cytokines levels were assessed by mRNA expression, which might not necessarily translate to AT protein content or secretion. Studies to examine the relationship between senescence and AT cytokine protein content in different SC fat depots are needed to confirm our findings. Finally, we did not evaluate genes related to adipogenesis, adipocyte differentiation and proliferation. To the extent that senescent cells inhibit these processes, information on these variables could have better allowed us to test whether the greater senescence in femoral AT is secondary to a greater replication capacity of preadipocytes in that depot or whether the greater number of senescent cells may feed back to inhibit these processes.
In conclusion, we found that femoral AT has more senescent cells than abdominal subcutaneous AT. Abdominal subcutaneous AT senescent cell content increases as a function of increased adipocyte size and fat mass, whereas only femoral adipocyte size correlated with femoral AT senescent cell content. Although women have more femoral AT senescent cells than men, femoral AT senescent cells are less linked to cytokine gene expression than abdominal AT senescent cells are. Our findings suggest that femoral senescence may reflect replicative activity of femoral AT rather than being a marker of dysfunction. Further research into the cellular lineage origin of senescent cells in different AT depots should help clarify some of these findings.
Study Importance Questions.
What is already known about this subject?
Senescent cells in AT have been associated with AT dysfunction and inflammation in animal studies.
The distribution of senescent cells in subcutaneous AT and their association with inflammation in humans is not known.
What are the new findings in your manuscript?
The proportion of senescent cells in femoral AT is greater than in abdominal adipose issue.
Abdominal subcutaneous, but not femoral subcutaneous, senescence is positively associated with AT cytokines.
How might your results change the direction of research or the focus of clinical practice?
Our results suggest that AT senescence could be a marker of dysfunction in the abdominal fat depot, and a marker of increased replicative activity in the femoral fat depot.
Further research should evaluate the cellular origin of senescence in different AT depots in humans.
Acknowledgments
Funding Information: These studies were supported by National Center for Research Resources Grant 1UL1 RR-024150 and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-45343, DK-40484, and 5T32 DK-007352.
Footnotes
Clinical Trial Registration Number: At the time these studies were done they were not eligible for registration in ClinicalTrials.gov
Disclosure Statement: The authors have no conflicts of interest to disclose.
References
- 1.Conley SM, Hickson LJ, Kellogg TA, McKenzie T, Heimbach JK, Taner T et al. Human obesity induces dysfunction and early senescence in adipose tissue-derived mesenchymal stromal/stem cells. Front Cell Dev Biol 2020;8:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Deursen JM. The role of senescent cells in ageing. Nature 2014;509:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 2015;4:e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008;6:2853–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 2014;17:324–8. [DOI] [PubMed] [Google Scholar]
- 6.Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun 2017;8:15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wissler Gerdes EO, Zhu Y, Weigand BM, Tripathi U, Burns TC, Tchkonia T et al. Cellular senescence in aging and age-related diseases: Implications for neurodegenerative diseases. Int Rev Neurobiol 2020;155:203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB et al. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. The journals of gerontology 2018;73:939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sikora E, Bielak-Zmijewska A, Mosieniak G. Cellular senescence in ageing, age-related disease and longevity. Curr Vasc Pharmacol 2014;12:698–706. [DOI] [PubMed] [Google Scholar]
- 10.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 2009;15:1082–7. [DOI] [PubMed] [Google Scholar]
- 11.Tchkonia T, Kirkland JL. Aging, Cell Senescence, and Chronic Disease: Emerging Therapeutic Strategies. Jama 2018;320:1319–20. [DOI] [PubMed] [Google Scholar]
- 12.Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol 2020;16:263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H et al. Fat tissue, aging, and cellular senescence. Aging Cell 2010;9:667–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafson B, Nerstedt A, Smith U. Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat Commun 2019;10:2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villaret A, Galitzky J, Decaunes P, Estève D, Marques MA, Sengenès C et al. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes 2010;59:2755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019;47:446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK et al. Corrigendum to ‘Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease’ EBioMedicine 47 (2019) 446–456. EBioMedicine 2020;52:102595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escande C, Nin V, Pirtskhalava T, Chini CC, Thereza Barbosa M, Mathison A et al. Deleted in Breast Cancer 1 regulates cellular senescence during obesity. Aging Cell 2014;13:951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 2019;18:e12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J 2019;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. American journal of physiology 2000;279:E455–E62. [DOI] [PubMed] [Google Scholar]
- 22.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 2013;17:644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD. A quick, reliable, and automated method for fat cell sizing. J Lipid Res 2003;44:1795–801. [DOI] [PubMed] [Google Scholar]
- 24.Kirkland JL. The biology of senescence: potential for prevention of disease. Clin Geriatr Med 2002;18:383–405. [DOI] [PubMed] [Google Scholar]
- 25.Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology 2011;57:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schafer MJ, White TA, Evans G, Tonne JM, Verzosa GC, Stout MB et al. Exercise Prevents Diet-Induced Cellular Senescence in Adipose Tissue. Diabetes 2016;65:1606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cartwright MJ, Schlauch K, Lenburg ME, Tchkonia T, Pirtskhalava T, Cartwright A et al. Aging, depot origin, and preadipocyte gene expression. The journals of gerontology 2010;65:242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tchoukalova Y, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci 2010;107:18226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson DP, Arner E, Hogling DE, Rydén M, Arner P. Abdominal subcutaneous adipose tissue cellularity in men and women. Int J Obes (Lond) 2017;41:1564–9. [DOI] [PubMed] [Google Scholar]
- 30.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 2013;15:978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM et al. Senolytics improve physical function and increase lifespan in old age. Nat Med 2018;24:1246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 2006;5:187–95. [DOI] [PubMed] [Google Scholar]


