Abstract
Background
We have identified a reentrant circuit in the pulmonary vein region, which drives the atria, producing fibrillatory conduction, as one mechanism of postoperative atrial fibrillation (POAF) in the canine sterile pericarditis model.
Objective
In this model, we tested the hypothesis that overdrive pacing from a site at or near such a reentrant circuit would interrupt it and thereby terminate POAF.
Methods
We studied 11 sterile pericarditis dogs on postoperative days 1–4. Atrial electrograms (AEGs) were recorded during POAF, overdrive pacing, and pace termination from 3 sites simultaneously: Bachmann's bundle, posterior left atrium, and right atrial appendage. When recorded AEGs demonstrated regular activation, pace termination was attempted at that site by delivering a drive train starting with 4 consecutive beats at a cycle length (CL) of 2–5 ms shorter than that of the intrinsic CL.
Results
Sixteen episodes of sustained POAF (>5 minutes) diagnosed by electrocardiogram were induced. During all episodes of POAF, AEGs recorded from the left atrium exhibited regular activation, ie, constant AEG morphology and CL. When capture of the reentrant circuit by overdrive pacing occurred (mean 13 ± 5, range 5–23 beats), all 16 POAF episodes were successfully terminated. In all termination episodes, at the end of pacing but prior to the return of sinus rhythm, there was disorganized atrial activation in the previously organized sites (mean 2 seconds, range 0.1–8 seconds). However, these beats did not sustain POAF in the absence of a reentrant circuit (“driver”).
Conclusion
Overdrive pacing from a site demonstrating regular activation during sustained POAF terminated the POAF by interrupting the reentrant circuit.
Keywords: Postoperative atrial fibrillation, Reentry, Entrainment, Overdrive atrial pacing, Nonpharmacologic rhythm control therapy
Key Findings.
-
▪
Postoperative atrial fibrillation (POAF) in the canine sterile pericarditis model was characterized by the presence of an area of regular activation in the left atrium, which is consistent with prior observations in animals and patients with POAF after open heart surgery.
-
▪
Overdrive atrial pacing from a site demonstrating regular activation during POAF terminated the POAF by interrupting the reentrant circuit.
-
▪
After a successful pace-termination attempt, the area exhibiting a regular activation becomes irregular, which in this area is critical in POAF maintenance, acting as a driver.
Introduction
Postoperative atrial fibrillation (POAF) is the most common complication after open heart surgery, with 30%–50% incidence.1 It is associated with increased in-hospital morbidity, including stroke, heart failure, and hemodynamic compromise.2 When POAF sustains for more than 48 hours, anticoagulation is required to prevent cardiac embolic events.3 The use of anticoagulants acutely in the postoperative period increases the risk of bleeding. Also, a longer duration of POAF is associated with worsened long-term survival.4
The mechanism that sustains POAF in patients remains poorly understood, and in fact, it may be that more than 1 mechanism is responsible for its maintenance in different patients. High-density mapping studies performed in the canine sterile pericarditis model (an experimental counterpart of POAF) by our laboratory have demonstrated that a left atrial reentrant circuit around pulmonary veins (PVs) acting as a “driver” results in fibrillatory conduction to a large portion of the atria in areas that cannot respond to the driving cycle length (CL) in a 1:1 fashion.5 Also, our subsequent study in patients with POAF after open heart surgery showed that recorded atrial electrograms (AEGs) exhibited a rapid and regular activation having constant morphology and CL in the left atrium (LA), similar to left atrial reentrant mechanisms in the canine sterile pericarditis model.6 If POAF is due to a reentrant mechanism causing fibrillatory conduction, it should be possible to pace terminate the POAF, provided impulses from the pacing site can penetrate the reentrant circuit to interrupt it. In this study, we tested the hypothesis that in the canine sterile pericarditis model, induced POAF due to a stable reentrant circuit may/should be reproducibly pace terminated.
Methods
Animal experimental protocols were approved by the Case Western Reserve University Institutional Animal Care and Use Committee. All studies were performed by the guidelines specified by our Institutional Animal Care and Use Committee, Department of Agriculture Animal Welfare Act, Public Health Service Policy on Humane Care and Use of Laboratory Animals, and Association for Assessment and Accreditation of Laboratory Animal Care International.
Canine sterile pericarditis model
Sterile pericarditis was created in 11 adult mongrel dogs weighing 20–24 kg, as previously described.5 Using sterile technique, the dogs underwent a right thoracotomy in the fourth intercostal space under general anesthesia. Using standard surgical techniques, the heart was exposed and cradled in the pericardium. Pairs of stainless steel wire electrodes coated with FEP polymer except for the tip were sutured to the Bachmann's bundle (BB), the right atrial appendage (RAA), and the posterior left atrium (PLA). These sites were selected because, in this model, induced POAF is often due to a LA reentrant circuit that includes activation of BB or the PLA. The RAA was selected because AEGs recorded during this POAF virtually always demonstrate a fibrillatory conduction pattern. Also, another pair was sutured onto the right ventricle for pacing after His bundle ablation, done in the anesthetized open-chest state to minimize the ventricular complex superimposition on AEGs. All electrodes were brought out through the chest wall and exteriorized posteriorly in the midline of the neck for subsequent use for pacing and recording. The atrial surfaces were then dusted with sterile talcum powder, a double layer of gauze was placed on the right and left atrial free walls, and the pericardiotomy was repaired. The chest was then closed in standard fashion. Antibiotics and analgesic agents were administered, and the dogs were allowed to recover.
Pace termination
We studied, in the conscious closed-chest state or anesthetized open-chest state, 11 sterile pericarditis dogs on postoperative days 1, 2, 3, and/or 4. Induction of POAF was attempted from each atrial electrode site by burst atrial pacing (starting at 120 ms decremented by 5 ms to loss of capture) for 2–8 seconds using a stimulus strength (>2× threshold, pulse width 1.8 ms). When recorded AEGs demonstrated regular activation at a very short CL during sustained POAF (>5 minutes), pace termination was attempted at that site. Our previous canine study defined regular CL as a standard deviation ≤6 ms.7 The following steps were performed for pace termination: (1) CL of overdrive pacing started 2–5 ms shorter than intrinsic regular CL using a stimulus strength of up to 50 mA; (2) overdrive pacing was performed starting with a drive train of 4 beats, and each subsequent drive train was incremented by 1–2 beats, up to a maximum number of 30 consecutive beats; (3) if POAF did not terminate, CL of overdrive pacing was decremented by 2 ms, and the overdrive pacing protocol was repeated. This process was performed until either a loss of capture or termination of POAF, whichever occurs first.
Data acquisition
During these studies, electrocardiogram (ECG) lead II and 3 bipolar electrograms (AEGs) obtained from the previously placed epicardial atrial electrodes were monitored, recorded, and stored on a Cardiolab recording system (Prucka Engineering, Houston, TX). The ECG was recorded at a band-pass filtered at 0.1–500 Hz, and the AEGs were recorded at a band-pass filtered at 30–500 Hz. Pacing studies were performed using a Bloom DTU stimulator (Bloom Electrophysiology, Denver, CO).
Results
Sixteen episodes of sustained POAF (>5 minutes) were induced in 11 sterile pericarditis dogs on postoperative days 1, 2, 3, and/or 4. In all episodes, the diagnosis of POAF (as opposed to typical atrial flutter [AFL]) was confirmed by examination of the surface ECG, the AEG CL (<120 ms), and apparent fibrillatory conduction in at least 1 recording site. Additionally, 1 or more atrial recording sites in the LA exhibited regular activation (constant AEG morphology and CL). Overdrive atrial pacing at a site showing regular activation was performed (mean 13 ± 5 beats, range 5–23 beats) and terminated POAF in all 16 episodes (Table 1). In 1 dog with 2 episodes, POAF was terminated once with overdrive pacing from BB and once from PLA pacing. In all termination episodes, at the end of pacing but prior to the return of sinus rhythm, there was a short duration of disorganized atrial activation in the previously organized sites (mean 2 seconds, range 0.1–8 seconds). However, these beats did not sustain POAF in the absence of a reentrant circuit (“driver”).
Table 1.
Summary of analysis data for pace termination of postoperative atrial fibrillation
| Dog no. | Postoperative day (state) | POAF duration | No. of pacing beats | Pacing site | Post pacing duration before sinus rhythm |
|---|---|---|---|---|---|
| 1 | 2 (O) | >16 min | 12 | BB | <1 s |
| 2 | 2 (O) | >7 min | 8 | BB | <1 s |
| 3 | 2 (C) | >9 min | 18 | BB | 1 s |
| 4 (O) | >6 min | 23 | BB | 2 s | |
| 4 (O) | >6 min | 15 | PLA | <1 s | |
| 4 | 2 (O) | >10 min | 19 | BB | 2 s |
| 5 | 4 (O) | >15 min | 16 | BB | 8 s |
| 6 | 2 (O) | >9 min | 8 | PLA | 1 s |
| 7 | 1 (C) | >11 min | 5 | BB | 1 s |
| 4 (O) | >19 min | 15 | BB | <1 s | |
| 4 (O) | >18 min | 7 | BB | <1 s | |
| 8 | 4 (O) | >5 min | 12 | PLA | <1 s |
| 4 (O) | >16 min | 9 | PLA | 4 s | |
| 9 | 4 (O) | >6 min | 11 | BB | 4 s |
| 10 | 4 (O) | >20 min | 18 | BB | 6 s |
| 11 | 1 (C) | >8 min | 7 | PLA | <2 s |
| Total | 13 ± 5 (5–23) | 2 ± 2.3 (0–8) |
BB = Bachmann's bundle; C = conscious close-chest state; O = anesthetized open-chest state; PLA = posterior left atrium; POAF = postoperative atrial fibrillation.
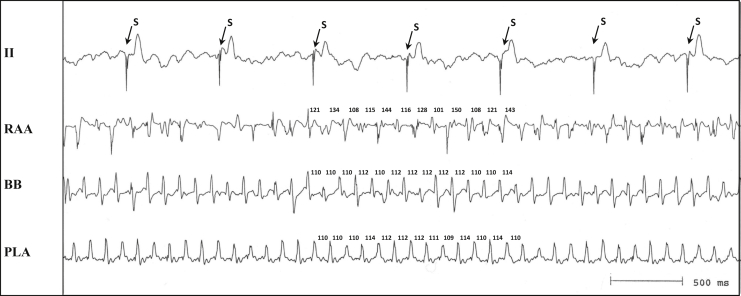
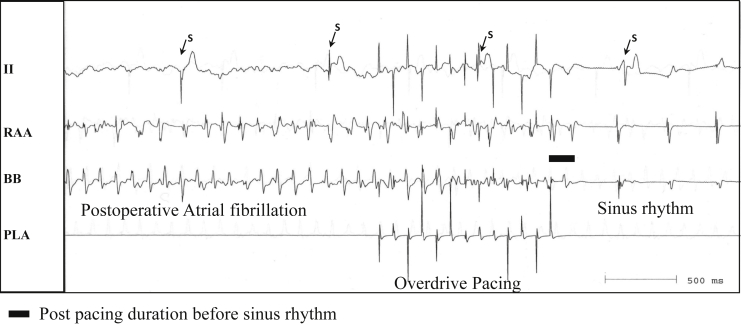
Figure 1 is a representative example of POAF due to a LA reentrant circuit terminated by overdrive atrial pacing. ECG lead II and bipolar AEGs from the RAA show irregular AEGs during POAF, but bipolar AEGs from BB and PLA show constant AEG morphology and CL (mean 112 ms). Figure 2 shows the same recordings during the same POAF episode as Figure 1, before, during, and after overdrive atrial pacing from the PLA at a CL of 100 ms that interrupted POAF. Overdrive pacing from the PLA site, which showed a regular AEG of short CL, terminated POAF, with a return to sinus rhythm after the pacing was stopped.
Figure 1.
Simultaneous recordings from selected atrial sites (right atrial appendage [RAA], Bachmann's bundle [BB], and posterior left atrium [PLA]) during postoperative atrial fibrillation (POAF) in the canine sterile pericarditis model (the first episode in dog #8 in Table 1). Electrocardiogram lead II shows an irregular baseline with an absence of P waves and atrial electrograms (AEGs) from the RAA, demonstrating changing morphology of AEGs consistent with POAF. AEGs from BB and the PLA show regular morphology and cycle length. S = stimulus artifact during ventricular pacing after the creation of the atrioventricular block.
Figure 2.
Same recording sites from the same episode as Figure 1, before, during, and after overdrive atrial pacing from the posterior left atrium (PLA) at cycle length of 100 ms that interrupted postoperative atrial fibrillation. BB = Bachmann's bundle; RAA = right atrial appendage; S = stimulus artifact during ventricular pacing after the creation of the atrioventricular block.
Discussion
Major findings
In 11 sterile pericarditis dogs on postoperative days 1, 2, 3, and/or 4, we demonstrated that POAF in this animal model was characterized by the presence of an area of regular activation in the LA, and that capturing this area with overdrive atrial pacing results in POAF termination consistently. The presence of an area of regular activation during POAF, typically in the LA adjacent to the PVs and the BB, is consistent with prior observations in animals and patients with POAF after open heart surgery.5,6 Also, after successful pacing, but before the return of sinus rhythm, there was disorganized atrial activation in the previously organized sites. However, these beats did not sustain in the absence of a reentrant circuit.
Comparison with previous similar studies
It has largely been assumed that because of the nature of activation of the atria during AF, it would not be possible to pace terminate this irregular arrhythmia. In fact, if the mechanism of AF was due to multiple random wavelets or multiple focal sources, it is generally agreed that it would not be possible to pace terminate AF. The studies done by others in the closed-chest conscious state8 and open-chest state9 in the canine atrial rapid pacing AF model demonstrated that atrial overdrive pacing could capture a relatively small area near the pacing site, but rapid pacing itself simply acts as a driver, further producing fibrillatory conduction. Moreover, since the rapid atrial pacing animal model is due to multiple random wavelets (no “driver” to interrupt), cessation of pacing only results in a continuation of the AF. The difference between the success of POAF pace termination in this study and its failure in prior studies is due to the fact that pacing in this study was performed in the area showing constant AEG morphology and CL in a model that has a reentrant circuit driving the POAF. Of note, the presence of an area of regular activation in the LA is not consistent with the multiple random wavelets hypothesis, but is rather suggestive of a stable reentrant driver responsible for the POAF maintenance. Such pacing at a CL shorter than the CL of the site during POAF presumably results in the entrainment of the reentrant driver, and then, if pacing continues for a critical duration at a critically rapid rate, interruption of the reentrant driver, and termination of POAF, provided that pacing is discontinued before it contributes to the formation of another circuit that will serve as a POAF driver. Rapid pacing of an irregularly activated site in this model, on the other hand, will simply continue to produce fibrillatory conduction to the surrounding areas, and will therefore fail to reach the reentrant driver to terminate POAF.
A study in the canine atrial rapid pacing AF model has attempted to pace terminate AF with rapid burst pacing (50 ms) at the moment showing an organized AEG (more “flutterlike” activity).10 However, the success rates have only been as high as 11%. Another study following ganglionated plexi ablation in the acetylcholine-induced canine AF model demonstrated that there were spontaneous specific atrial sites manifesting organized AEGs of one atrium or capture of the other atrium, and overdrive atrial pacing at that site could successfully terminate the AF.11 This model is likely a triggered arrhythmia, as it is pacing induced in the presence of acetylcholine. In comparison with our study, an attempt to pace terminate AF at an atrial site with regular activation is similar to other studies.10,11 However, based on our previous high-density mapping study in the canine sterile pericarditis model (an experimental counterpart of POAF) demonstrating the mechanism of POAF due to a reentrant circuit around PVs, our pace termination study was performed using 3 selected recording sites on postoperative days 1, 2, 3, and/or 4. In this study, we clearly demonstrated that when POAF is due to a stable reentrant circuit of short CL in the LA, overdrive atrial pacing at a rate faster than the rate of the tachycardia from a site with 1:1 activation from the reentrant circuit can interrupt the reentrant driver and terminate POAF reliably and predictably. Finally, the observation that after a successful pace-termination attempt, the area that exhibited a regular activation pattern becomes irregular, further confirms the notion that this area is critical in POAF maintenance, acting as a driver.
Clinical implications
Attempts to pace terminate persistent AF clinically have been unsuccessful.12 It is widely accepted that persistent AF has not been pace terminated because successful termination is linked directly to the mechanism maintaining AF. We have shown that persistent AF is maintained by at least 3–4 focal sources of very short, but different, CLs, and hence entraining these sources by overdrive pacing from a single pacing site during AF is not possible.13, 14, 15
Unlike persistent AF, the atrial substrate necessary for the development of POAF is due to the development of a self-limiting postoperative sterile pericarditis. During the period of initial postoperative recovery, patients are especially vulnerable to both the hemodynamic effects of POAF and the side effects and toxicity of medication treatments.16 Since the canine sterile pericarditis POAF model was modeled after patients following open heart surgery, 1 of the POAF mechanisms in postoperative patients may have the same reentrant mechanism, and this may be pace terminated if pacing can be performed from a site close to or within a LA stable reentrant driver. Our recent study in patients with POAF after open heart surgery showed that one mechanism of POAF might be atypical AFL (aka type II AFL), manifesting AEGs of constant morphology and regular short CL, likely due to a regular LA reentrant driver. Importantly, because this rapid and regular activation causes fibrillatory conduction to the rest of the atria, atypical AFL demonstrates POAF in the ECG. Like other reentrant arrhythmias, this ECG POAF rhythm has the potential to be pace terminated. This study demonstrates that it should be reliably possible to pace terminate POAF in patients who have a reentrant mechanism. Several factors are critical to achieving such pace termination. Most important is pacing from a site that permits impulses from the pacing site to enter the reentrant circuit. Pacing from a site demonstrating fibrillatory conduction will be ineffective because it is already evident that it cannot respond 1:1 at the CL of the reentrant circuit, and thus pacing to interrupt it must occur at an even shorter CL. Therefore, a site demonstrating 1:1 activation (regular activation) at the CL of the reentrant circuit should be selected. In addition, pacing with a large stimulus strength is usually necessary, as the stimulus threshold for capture increases when pacing CLs decrease.17 These findings may have important implications regarding nonpharmacologic rhythm control therapy in patients with POAF after open heart surgery.
Limitations
Although the canine sterile pericarditis model was developed as an experimental counterpart of POAF after open heart surgery, the mechanism that sustains POAF in patients may have more than 1 mechanism responsible for its maintenance due to various comorbidities in patients, ie, POAF patients with the preexisting substrate.18 This study did not map atrial activation during POAF, as some studies were in the closed chest state. However, previous studies have well demonstrated that in the presence of induced, sustained POAF in this model, a rapid, regular rhythm of very short CL is due to a reentrant driver.5 Thus, although we did not provide it in these studies, the data from previous studies clearly support this assumption.
Conclusion
The presence of an area of stable, highfrequency, regular activation in the atria (likely reentrant circuit of very short CL) may be one of the phenomena responsible for generating fibrillatory conduction manifesting itself as POAF. Interruption of such a reentrant circuit with overdrive atrial pacing results in the termination of POAF.
Acknowledgments
Funding Sources
This work was supported in part by grants from R01 HL146463 from the National Institutes of Health, National Heart, Lung, and Blood Institute; and Elisabeth Severance Prentiss Foundation.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Ethics Statement
Animal experimental protocols were approved by the Case Western Reserve University Institutional Animal Care and Use Committee. All studies were performed by the guidelines specified by our Institutional Animal Care and Use Committee, Department of Agriculture Animal Welfare Act, Public Health Service Policy on Humane Care and Use of Laboratory Animals, and Association for Assessment and Accreditation of Laboratory Animal Care International.
References
- 1.Creswell L.L., Schuessler R.B., Rosenbloom M., Cox J.L. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–549. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 2.Almassi G.H., Schowalter T., Nicolosi A.C., et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–511. doi: 10.1097/00000658-199710000-00011. discussion 511–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66–e93. doi: 10.1016/j.hrthm.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Sigurdsson M.I., Longford N.T., Heydarpour M., et al. Duration of postoperative atrial fibrillation after cardiac surgery is associated with worsened long-term survival. Ann Thorac Surg. 2016;102:2018–2026. doi: 10.1016/j.athoracsur.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bui H.M., Khrestian C.M., Ryu K., Sahadevan J., Waldo A.L. Fixed intercaval block in the setting of atrial fibrillation promotes the development of atrial flutter. Heart Rhythm. 2008;5:1745–1752. doi: 10.1016/j.hrthm.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 6.Sadrpour S.A., Srinivasan D., Bhimani A.A., et al. Insights into new-onset atrial fibrillation following open heart surgery and implications for type II atrial flutter. Europace. 2015;17:1834–1839. doi: 10.1093/europace/euv019. [DOI] [PubMed] [Google Scholar]
- 7.Sahadevan J., Ryu K., Matsuo K., Khrestian C.M., Waldo A.L. Characterization of atrial activation (A-A) intervals during atrial fibrillation due to a single driver: do they reflect atrial effective refractory periods? J Cardiovasc Electrophysiol. 2011;22:310–315. doi: 10.1111/j.1540-8167.2010.01874.x. [DOI] [PubMed] [Google Scholar]
- 8.Allessie M., Kirchhof C., Scheffer G.J., Chorro F., Brugada J. Regional control of atrial fibrillation by rapid pacing in conscious dogs. Circulation. 1991;84:1689–1697. doi: 10.1161/01.cir.84.4.1689. [DOI] [PubMed] [Google Scholar]
- 9.Kirchhof C., Chorro F., Scheffer G.J., et al. Regional entrainment of atrial fibrillation studied by high-resolution mapping in open-chest dogs. Circulation. 1993;88:736–749. doi: 10.1161/01.cir.88.2.736. [DOI] [PubMed] [Google Scholar]
- 10.Everett T.H., Akar J.G., Kok L.-C., Moorman J.R., Haines D.E. Use of global atrial fibrillation organization to optimize the success of burst pace termination. J Am Coll Cardiol. 2002;40:1831–1840. doi: 10.1016/s0735-1097(02)02476-2. [DOI] [PubMed] [Google Scholar]
- 11.Niu G., Scherlag B.J., Lu Z., et al. An acute experimental model demonstrating 2 different forms of sustained atrial tachyarrhythmias. Circ Arrhythm Electophysiol. 2009;2:384–392. doi: 10.1161/CIRCEP.108.810689. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell A.R.J., Spurrell P.A.R., Cheatle L., Sulke N. Effect of atrial antitachycardia pacing treatments in patients with an atrial defibrillator: randomised study comparing subthreshold and nominal pacing outputs. Heart. 2002;87:433–437. doi: 10.1136/heart.87.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S., Sahadevan J., Khrestian C.M., Cakulev I., Markowitz A., Waldo A.L. Simultaneous biatrial high-density (510-512 electrodes) epicardial mapping of persistent and long-standing persistent atrial fibrillation in patients: new insights into the mechanism of its maintenance. Circulation. 2015;132:2108–2117. doi: 10.1161/CIRCULATIONAHA.115.017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S., Sahadevan J., Khrestian C.M., Markowitz A., Waldo A.L. Characterization of foci and breakthrough sites during persistent and long-standing persistent atrial fibrillation in patients: studies using high-density (510-512 electrodes) biatrial epicardial mapping. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S., Khrestian C.M., Sahadevan J., Markowitz A., Waldo A.L. New insights into understanding rotor versus focal activation in patients with persistent atrial fibrillation. JACC Clin Electrophysiol. 2021;7:909–919. doi: 10.1016/j.jacep.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobrev D., Aguilar M., Heijman J., Guichard J.B., Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16:417–436. doi: 10.1038/s41569-019-0166-5. [DOI] [PubMed] [Google Scholar]
- 17.Kay G.N., Mulholland D.H., Epstein A.E., Plumb V.J. Effect of pacing rate on the human atrial strength-duration curve. J Am Coll Cardiol. 1990;15:1618–1623. doi: 10.1016/0735-1097(90)92835-p. [DOI] [PubMed] [Google Scholar]
- 18.Heijman J., Muna A.P., Veleva T., et al. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ Res. 2020;127:1036–1055. doi: 10.1161/CIRCRESAHA.120.316710. [DOI] [PMC free article] [PubMed] [Google Scholar]