Abstract
Macrophage, as an integral component of the immune system and the first responder to local damage, is on the front line of defense against infection. Over the past century, the prevailing view of macrophage origin states that all macrophage populations resided in tissues are terminally differentiated and replenished by monocytes from bone-marrow progenitors. Nonetheless, this theory has been reformed by ground-breaking discoveries from the past decades. It is now believed that tissue-resident macrophages (TRMs) are originated from the embryonic precursors and seeded in tissue prenatally. They can replenish via self-renewal throughout the lifespan. Indeed, recent studies have demonstrated that tissue-resident macrophages should not be classified by the over-simplified macrophage polarization (M1/M2) dogma during inflammation. Moreover, multiple lines of evidence have indicated that tissue-resident macrophages play critical roles in maintaining tissue homeostasis and facilitating tissue repair through controlling infection and resolving inflammation. In this review, we summarize the properties of resident macrophages in the lung, spleen, and heart, and further highlight the impact of TRM populations on inflammation control and tissue repair. We also discuss the potential role of local proliferation in maintaining a physiologically stable TRM pool in response to acute inflammation.
Keywords: Inflammation, local proliferation, macrophage polarization, self-renewal, tissue-resident macrophages, vascular permeability
INTRODUCTION
As a fundamental component of the immune system, macrophages are the most abundant immune cells in most tissues of mammals (1). Macrophages were first characterized by Elie Metchnikoff in 1893 as professional phagocytes during tissue inflammation (2). This seminal work marks a new era of immune research, and leads to the discovery of several core functions of macrophages in maintaining tissue homeostasis and regulating inflammation. With regard to the origin of macrophages, van Furth et al. (3) in 1972 proposed the “mononuclear phagocyte system (MPS)” theory emphasizing that all macrophages were terminally differentiated from blood monocytes. But, in the 1980s, several lines of contradictory evidence demonstrated that macrophages were not terminally differentiated (4, 5), and persisted in tissues (6). Thereafter, a large proportion of the subsequent studies was steered toward the differentiation and function of bone-marrow-derived macrophages in healthy and various disease settings.
In the end of the last century, Mills et al. first reported the concept of macrophage polarization (the M1/M2 classification system) based on macrophage responses to various stimuli in vitro (7, 8). The classically activated macrophages are termed M1 macrophages, they can be induced by pro-inflammatory factors/cytokines [i.e., lipopolysaccharide (LPS), interferon-gamma, tumor necrosis factor alpha (TNF-α), or granulocyte macrophage colony-stimulating factor (GM-CSF)]. These macrophages exhibit pro-inflammatory phenotype with increased secretion of cytokines (IL-1β, TNF, IL-6), nitric oxide, and reactive oxygen intermediates, and participate as inducers in polarized Th1 response (9–11). In contrast, the M2 macrophages are alternatively activated by exposure to certain Th2-related cytokines (i.e., IL-4, IL-13) or anti-inflammatory molecules (i.e., IL-10, transforming growth factor β1 [TGF-β]) (9–11). This subtype is characterized by: higher expression of scavenging molecules, mannose, and galactose receptors; enhanced phagocytic capacity and efficiency; and production of ornithine and polyamines (12). Accumulating evidence has shown that M2 macrophages play important roles in tissue repair, parasite clearance, resolving inflammation, and promotion of angiogenesis and tumor growth (11, 12). However, this over-simplified concept is unable to delineate the diverse macrophage populations observed in vivo.
In particular, in recent years, with the development of multicolor fluorescence-activated cell sorting (FACS), various fate-mapping and genetic tracing mouse models as well as parabiosis techniques, a distinct population of macrophages is identified in different tissues (13–17). These macrophages are shown to be originated from embryonic precursors and maintained through self-renew throughout adulthood, instead of recruitment of circulating monocytes (bone-marrow origin), and thus named tissue-resident macrophages (TRMs) (15–19). These discoveries have challenged the conventional “MPS” theory and transformed the fundamental understanding of macrophage origin. Importantly, with the advancement of single-cell transcriptome analysis and high-resolution imaging tools, we are able to detect more subpopulations of TRMs with distinct functions (13, 20–23). In addition, these breakthrough findings have allowed us to better appreciate the complexity of macrophage heterogeneity in tissues, while forcing us to reassess the diverse and vital roles of macrophages.
Inflammatory diseases, such as systemic inflammatory response syndrome (SIRS) and sepsis, can trigger a complex series of cellular responses. Macrophages have been shown to play essential roles in virtually all stages of these responses, including initiation of inflammatory responses to neutralize invading pathogens, removal of pathogens and damaged cells through phagocytosis, and resolution of inflammation followed by tissue repair and remodeling (24). Of note, different macrophage populations play distinct and non-redundant roles in each of these processes (25, 26). Thus, it is significant to understand the origins, functions, and potential cross-talks and transition among different macrophage pools (plasticity), particularly the newly identified TRMs. In this review, we summarize the contributions of TRM populations to the initiation, maintenance, and resolution of inflammatory responses in different organ systems, and further discuss the impact of self-renewal capacity of TRMs in maintaining a physiologically stable TRM pool in response to acute inflammation.
ALVEOLAR MACROPHAGES IN THE LUNG
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are devastating complications of severe sepsis and SIRS, and these are commonly caused by pneumonia after infection with bacterial, viral, or fungal origin (27–30). Accumulating evidence has demonstrated the critical role of macrophages in the pathogenesis of ALI/ARDS (31, 32). Based on the anatomical compartments of the lung, two major subtypes of macrophages are identified, namely alveolar macrophages (AMs) and interstitial macrophages (33, 34). By using various techniques such as thymidine-labeling, adoptive transfer, and lineage tracing, studies have shown that alveolar macrophages originate from the fetal liver, seed in the lung during embryonic development, and undergo self-renewal to maintain the population size (35, 36). On the other hand, interstitial macrophages, with molecular phenotype between monocytes and alveolar macrophages, have a mixed origin: an embryonic yolk-sac-derived origin and a postnatal bone marrow-derived origin (36, 37). Accordingly, these macrophages are thought to be maintained or expanded by the recruitment of bone marrow-derived monocytes, yet local proliferation may also contribute to the maintenance of interstitial macrophages (36, 37).
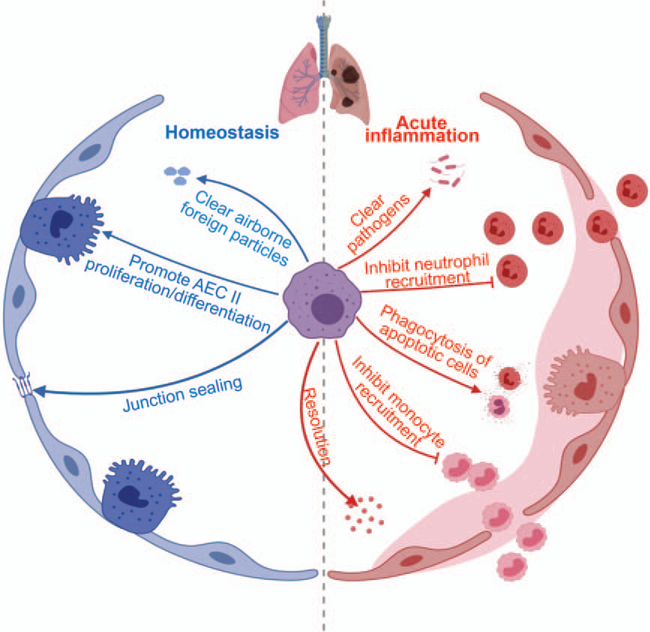
Alveolar macrophages, the most abundant and best-characterized macrophage population in the lung, reside in the lumen of alveoli where gas exchange takes place (35). This strategic location allows alveolar macrophages to clear pathogens, dead cells, and other airborne foreign particles through phagocytosis during respiratory infection (38, 39). It is now recognized that alveolar macrophages are critical for tissue homeostasis, host defense, tissue repair, and control of lung inflammation (40–42) (Fig. 1). Under physiological steady condition, alveolar macrophages are characterized by the high phagocytic activity and the release of anti-inflammatory cytokines (40). Indeed, pharmacological depletion of AMs by clodronate liposomes results in impaired gas exchange and fatal hypoxia upon viral infection (39, 43). Similarly, the conditional genetic depletion of AMs shows increased influx of both neutrophils and monocytes and augmented inflammatory responses in the lung (44, 45), which further attests the importance of AMs in maintaining lung homeostasis. In addition, AMs are noted to directly release epithelial growth factors upon inflammation or lung injury, including TGF-β1, hepatocyte growth factor, and insulin-like growth factor 1 (46–48). These factors can promote the proliferation and differentiation of alveolar epithelial cell type II, contributing to the repair of the airway epithelium and reestablishment of normal gas exchange conditions in the lung (46–48). Furthermore, AMs may also play an important role in the repair of epithelial barrier through increasing the tightness of junctions between alveolar epithelial cells (46, 49).
Fig. 1. The summary of alveolar macrophage functions.
Alveolar macrophages (AMs) play important roles in the clear of airborne foreign particles/pathogens, phagocytosis of apoptotic cells, inhibition of monocyte/neutrophil recruitment, proliferation/differentiation of type II alveolar epithelial cells (AEC II) (epithelial progenitor cells), repair of epithelial barrier by junctional sealing and resolution of inflammation.
Regarding the inflammatory disease condition, AM-depleted mice display higher mortality rates compared with control mice in response to non-sterile and sterile inflammatory diseases (50–52). Notably, mice with AM depletion show increased influx of activated neutrophils to the lung, yet they also exhibit impaired clearance of damaged/dying neutrophils, which could undergo secondary necrosis and provoke inflammation in the lung (50–52). In fact, these mice without AMs produce higher levels of intrapulmonary cytokines and chemokines, such as TNF-α, IL-1β, C-X-C motif chemokine receptor 1 (CXCL1), and chemokines monocyte chemoattractant protein-1 (50–52). Put together, these observations have strongly indicated that AMs are not only critical phagocytes to clear injured cells for subsequent tissue repair, but also necessary to prevent overt inflammatory responses that might drive secondary tissue damage. In line with this, ex vivo experiments using AMs isolated from human and mice also demonstrate that AMs produce much lower level of pro-inflammatory cytokines (i.e., TNF-α, IL-6) in response to the acute inflammation, when compared with the recruited monocytes/macrophages (53, 54). Interestingly, alveolar macrophages have also been shown to participate in the initiation of inflammation through secretion of CXCL1, which enhances the recruitment of neutrophils (55, 56). These seemingly contradictory findings implicate an intriguing hypothesis that AMs may present unique characteristics of plasticity in response to various environmental cues (40, 42, 54). Accordingly, alveolar macrophages could be phenotypically and functionally classified into two major types: M1 macrophage (pro-inflammatory, profibrotic, and actively recruit neutrophils) and M2 macrophages (anti-inflammatory, anti-fibrotic, pro-asthmatic, and proresolving with tissue regenerating properties) (40, 57, 58). Similar to the concept of macrophage polarization, this classification paradigm is flawed by the nature of its extreme simplicity. For the pro-inflammatory role of AMs, major direct evidence is obtained through ex vivo or in vitro studies (55, 56, 58). What’s more, due to the lack of lineage marker, the immature circulating monocyte-derived macrophages in the lung are mistakenly recognized as “M1” population of AMs, given these cells also contribute to enhancing inflammatory responses (59). Therefore, although the current M1/M2 theory may be useful to define macrophage phenotypes in vitro, further investigations are required to identify and characterize various macrophage populations in vivo. Most importantly, future studies should not exclude the possibility of the existence of macrophage population with mixed phenotypes. Indeed, as AMs mentioned above, most macrophages exhibit a wide spectrum of phenotypes and functionalities, which may be potentially changed and become different macrophage subtypes depending on the local environmental cues.
RED PULP MACROPHAGES IN THE SPLEEN
As the second largest lymphoid organ in human, spleen plays important roles in immunity (60). Loss-of-function of the spleen, either by splenectomy or infiltrative disease, is known to prominently increase the risk of severe infection and sepsis (five to six times higher) (61). Spleen is composed of the red pulp and white pulp that are separated by marginal zone (62). Different subsets of macrophages have been identified within the spleen of mice. The most abundant subtype of macrophages is the red pulp macrophage (RPM), which can be detected by F4/80+CD206+CD11blo/− signature and selective expression of the transcription factor Spi-C (63). Except RPMs in the spleen, other subpopulations of macrophages also exist in spleen, including marginal zone macrophages, marginal zone metallophilic macrophages, and tingible body macrophages, as defined by anatomical compartments (64). However, only red pulp macrophages are recognized as tissue-resident macrophages in the spleen, since this population is seeded prenatally and maintained through local proliferation instead of monocytic input (63, 65).
Red pulp macrophages are best characterized for their ability to remove aged red blood cells and their critical roles in iron metabolism (63). Interestingly, recent studies have indicated that RPMs could control bacteria dissemination and actively participate in the maintenance of immune homeostasis (64). Yet, the underlying mechanism that governs the bacterial clearance activity of RPMs remains largely elusive. To fill in this knowledge gap, our recent study has identified that secreted and transmembrane1A (Sectm1a), as a potential ligand for glucocorticoid-induced tumor necrosis factor receptor (GITR), could directly bind GITR on the surface of macrophages (66). Such interaction then activates the downstream PI3K-Akt pathway, which in turn promotes phagocytosis and bacterial killing activity of macrophages (66).
Of note, there are a large number of splenic monocytes (F4/80+/low CD11b+) localized in the sub-capsular red pulp, which are called “reservoir monocytes” because they could readily leave the spleen and migrate into inflamed tissues (i.e., lung and heart) during acute inflammation (67–70). Once they reach the target tissues such as heart and lung, these monocytes could release large amount of inflammatory cytokines and promote the recruitment of neutrophils, resulting in exaggerated tissue damage (67–70). In consistence with these findings, our recent study also revealed that LPS administration caused a significant decline in the number of splenic monocytes (71). In addition, mice with Sectm1a deficiency showed greater response to LPS injection by releasing more splenic monocytes when compared with wild-type counter parts, leading to increased infiltration of monocytes to target tissues such as heart and lung. By contrast, administration of mice with recombinant Sectm1a protein exhibited opposite effects. Taken together, these findings suggest that red-pulp macrophages may play critical roles in controlling pathogen infection as well as host inflammatory responses. More importantly, organ cross-talk by means of monocyte migration should be taken into careful consideration when investigating inflammatory responses of a certain organ, as many studies tend to utilize tissue-specific genetic animal models to improve specificity, which oftentimes might overlook the confounding effects contributed by other tissues. Intriguingly, Sectm1a not only plays a role in regulating monocyte release from spleen (71), it may potentially have direct impact on various organs since its soluble/secreted form has been reported (72, 73), which could potentially travel through circulation by itself or within microvesicles (i.e., exosomes) to various target cells.
RESIDENT MACROPHAGES IN THE HEART
Given its critical roles in the pathogenesis of heart failure, inflammation has been the focus for intense research since 1990s, and various drug candidates that aim to modulate inflammation are being identified using mouse models (73–76) and tested in clinical trials (77), though mixed results are reported. This suggests better understanding on immune responses to cardiac health is needed. As the largest immune cell population in the heart, resident cardiac macrophages (RCMs) represent 6% to 8% of the non-cardiomyocyte population in the healthy adult mouse heart (78). They not only are essential to coronary development and cardiac regeneration, but also involved in cardiac electrical conduction and preserving cardiac functions (78–81). Recent studies have rigorously characterized several cardiac macrophage subpopulations in the steady state (79). These authors also demonstrated that RCMs are originated from embryonic yolk-sac progenitors, and could maintain its population pool in the myocardium mainly through in situ proliferation, as well as be partially replaced by circulating monocytes over time (79).
In the steady state, cardiac resident macrophages are thought to serve as sentinels for injury and circulating infectious agents (i.e., bacteria, viruses), and to support tissue homeostasis by removing debris and facilitating electrical conduction (81, 82). Nevertheless, in response to pathophysiological stress such as permanent myocardial infarction, ischemia-reperfusion injury, or diphtheria toxin-induced cardiomyocyte loss, RCMs are predominately replaced by monocyte-derived inflammatory monocytes/macrophages, which contribute to the development of heart failure (83–86). In fact, within 30-min post-myocardial infarction (MI), the number of RCMs decreases, whereas the inflammatory Ly6Chigh monocytes start to migrate into the infarcted area (87, 88). Subsequently, the number of infiltrated monocytes continue to grow and peak at around 24 to 72 h after MI (87, 88). Interestingly, Bajpai et al. (83) have shown that CCR2− RCMs are able to inhibit monocyte recruitment in response to the inflammation, and such an inhibitory effect appears to be beneficial for the following wound-healing process. Similarly, in our recent study, we also observed more than 50% reduction of RCM population in the heart at 18 h post-LPS injection, with a concomitant increase of recruited inflammatory monocytes/macrophages (56.8%) when comparing to that of phosphate-buffered saline control group (2.2%) (71). It is important to note that LPS-triggered inflammatory response could suppress the self-renewal capacity of RCMs (71). This might partially explain the LPS-induced reduction of RCM population. Collectively, transient switch from local RCM pool to recruited monocytes/macrophages may be an important contributor to the pathogenesis of primary and/or secondary heart injury. Future studies may be warranted to explore how self-renewal capability of RCMs is affected under inflammatory conditions, and whether/how recruited monocytes/macrophages may play a role in such a process. Understanding these vital aspects of cardiac macrophage biology would be of great clinical interest, as they would provide important guidance to what (cells or specific cellular components) we should be targeting when developing therapeutic reagents.
TISSUE-RESIDENT MACROPHAGES CONTRIBUTE TO MAINTAINING VASCULAR INTEGRITY DURING INFLAMMATION
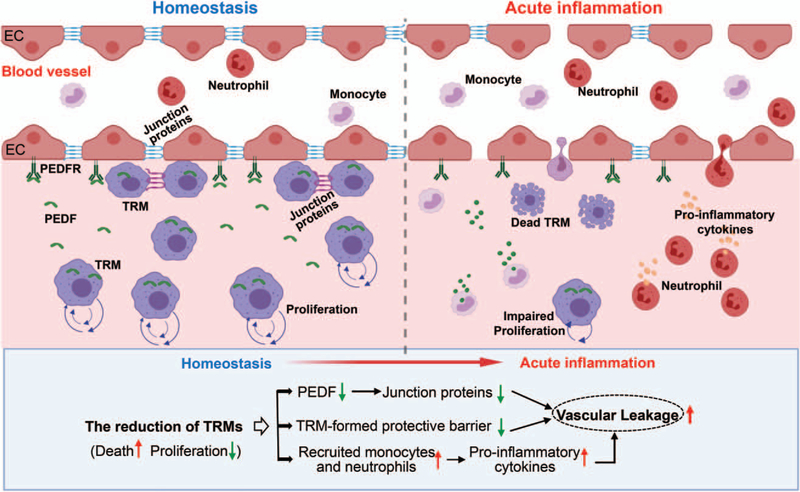
The endothelial cell layer forms a barrier between blood and tissue, which controls the transit of leukocytes and fluid extravasation (89, 90). During acute inflammatory disease, compromised integrity of endothelial barrier causes vascular leakage, which facilitates the infiltration of leukocytes and thereby, may contribute to tissue edema and organ damage (89, 90). Currently, many cell junction proteins (i.e., occludin, ZO-1, and VE-cadherin) have been identified. They are responsible for establishing the linkage/connection between adjacent endothelial cells (ECs) that is pivotal for the endothelial barrier function (91, 92). The cross-talk between macrophages and ECs has been significantly explored in the context of cancer (93), and tissue-resident macrophages are well known for their ability to promote angiogenesis during tumor growth (94). Nonetheless, it remains unclear to what degree tissue-resident macrophages could influence vascular integrity during acute inflammation. Current understanding about perivascular macrophages has suggested that they are in close contact with blood vessels and play multiple roles in: maintaining tight junctions between endothelial cells, eliminating potential pathogens by means of phagocytosis before they enter tissues, and restricting inappropriate inflammation in tissues under steady state (95). For example, one type of perivascular macrophages in the cochlea has been shown to limit the permeability of cochlear EC monolayers through releasing pigment epithelial-derived factor (96, 97). This factor could then directly bind to pigment epithelial-derived factor receptor on the ECs, and upregulates the expression of junction-associated proteins such as occludin, ZO-1, and ve-cadherin in ECs (96, 97). In line with this, our in vitro data also revealed that tissue-resident macrophages isolated from spleen could upregulate tight junction-associated proteins, and in vivo depletion of tissue-resident macrophages augmented LPS-induced vascular leakage in different organs (i.e., lung, heart) of mice (71). Similarly, De Schepper et al. (98) recently demonstrated that depletion of tissue-resident macrophages in the gut downregulated the expression of VE-Cadherin, leading to pronounced abnormalities and vascular leakage in the submucosal vascular network. Interestingly, a new subtype of tissue-resident macrophages, epithelial-like CX3CR1+ macrophages, has been identified in the joint (99). These macrophages are connected to one another through expression of tight-junction proteins, thus forming an internal immunological barrier at the synovial lining, which prevents the recruitment of inflammatory leukocytes in the intra-articular structures (99). Taken together, these discoveries of tissue-resident macrophages highlight their importance in maintaining the vascular integrity through cross-talk with ECs or acting as a barrier by themselves, thus preventing vascular hyperpermeability under inflammatory insults (Fig. 2).
Fig. 2. Tissue-resident macrophages contribute to maintaining vascular integrity.
During the steady condition, tissue-resident macrophages (TRMs) are capable of protecting vascular integrity through releasing PEDF, which interacts with PEDFR on ECs and increases the producing of junction proteins. In addition, part of TRMs are able to produce tight-junction proteins to connect each other, and form an internal immunological barrier to prevent the infiltration of inflammatory leukocytes. However, during acute inflammation, reduction of TRM population caused by cell death and suppression of local proliferation results in the decreased levels of PEDF, the disappearance of TRM barrier, and the infiltration of inflammatory monocytes/neutrophils, which contribute to the vascular leakage. PEDF indicates pigment epithelial-derived factor; PEDFR, pigment epithelial-derived factor receptor; EC, endothelial cells.
THE SELF-RENEWAL CAPACITY IS ESSENTIAL IN REGULATING THE POPULATION SIZE OF TISSUE-RESIDENT MACROPHAGES DURING ACUTE INFLAMMATORY DISEASE
Generally, during the steady state, tissue-resident macrophages actively participate in many cellular processes such as: neutralizing invading pathogens and infection; removing dying/dead cells and debris; secreting a variety of factors that stimulate neighboring cells (i.e., immune cells, epithelial cells, endothelial cells, or fibroblasts) to maintain tissue homeostasis (16, 100). Nonetheless, tissue-resident macrophages also exert specific functions depending on their anatomical location, i.e., participating in iron recycling, synaptic information transfer, and electrical signal conduction in the heart (16, 100). In fact, Roberts et al. (101) recently demonstrated that, unlike bone marrow-derived macrophages, tissue-resident macrophages are able to conduct silent clearance of apoptotic cells without triggering the inflammatory response due to their low expression of TLR9 and less toll-like receptor responsiveness to nucleic acids. Furthermore, tissue-resident macrophages are found to be capable of preventing neutrophil influx and subsequent monocyte infiltration (102). Remarkably, tissue-resident macrophages could suppress inflammatory damage through prostrating their membranes to cover the dead cells (102). Hence, these recent findings clearly suggest that maintaining appropriate size of tissue-resident macrophage pool has a potential to preserve and restore tissue homeostasis upon acute inflammation challenge.
During inflammatory disease, tissue-resident macrophage populations are rapidly diminished following the initiation of inflammation in different tissues (103, 104). Meanwhile, neutrophils and inflammatory monocytes/macrophages are recruited to the inflammation site, which play important roles in immune defense, but they could also contribute to the pathogenesis of various inflammatory diseases and organ failure (105). Therefore, it is reasonable to postulate that reduction of tissue-resident macrophage pool may be an important trigger for the influx of inflammatory leukocytes, whereas conserving the tissue-resident macrophage population may be beneficial to prevent the overt inflammatory damage caused by infiltrated leukocytes. The reduction of tissue-resident macrophages has mainly been attributed to increased cell death (103). Yet, the self-renewal capacity, as the most important trait of tissue-resident macrophages, has been overlooked (106). With regard to molecular signals that regulate self-renewal capacity of tissue-resident macrophages, only a few factors have been validated as stimulators so far (106, 107). Nonetheless, among these stimulators, GM-CSF, M-CSF, and IL-34 are all context-and tissue-specific in promoting macrophage local proliferation (9), while only IL-4 is able to induce rapid local proliferation in diverse tissues (108–110).
In our recent study, local proliferation of tissue-resident macrophages in multiple organs (i.e., lung, spleen, and heart) is significantly impaired upon LPS insult, leading to substantial reduction in the amount of tissue-resident macrophages (71). This phenomenon is accompanied by dramatic increase of inflammatory monocyte and neutrophil infiltration at early time point (20 h post-LPS treatment) (71). Moreover, using loss-of- and gain-of-function approaches, we defined Sectm1a as a novel positive regulator for the local proliferation of tissue-resident macrophages. Specifically, Sectm1a deficiency exaggerated acute inflammation-caused reduction of tissue-resident macrophages in multiple organs by suppressing their proliferation at earlier stage (71). By contrast, administration of recombinant Sectm1a protein significantly attenuated the reduction of macrophage pool and improved animal survival upon LPS challenge. Mechanistically, we identified that Sectm1a, as a novel GITR ligand, could promote the expansion of T helper cells, especially Th2, which increased secretion of Th2 cytokines (i.e., IL-4). Indeed, IL-4 could in turn stimulate the local proliferation of tissue-resident macrophages and improve animal survival following acute inflammation (71).
On the other hand, during the inflammation resolution phase, tissue-resident macrophages undergo a transient and intense proliferative burst in situ to repopulate the tissue. In 2009, Chorro et al. (111) showed that murine Langerhans cells (sharing a common ontogeny with macrophages) could proliferate in situ to restore the lost cells during inflammation. Indeed, in terms of recovery from the inflammatory episode, similar findings were observed in other tissue-resident macrophages such as peritoneal macrophages (112), microglias (113), alveolar macrophages (114), cardiac macrophages (115), and Kupffer cells (115). Furthermore, although the majority of recruited/inflammatory monocytes are going to die in the late inflammatory phase due to their short lifespan (116, 117), some of these could undergo a slow phenotypic conversion to become tissue-resident macrophages (65, 114). This process may occur during inflammation or after experimental deletion of tissue-resident macrophages (65, 114). It is important to note here that tissue-resident macrophages successively lose self-renewal capacity with aging (118, 119), which partially explain the significantly higher incidence and mortality rates of sepsis among aged patients (≥ 65) when compared with younger individuals.
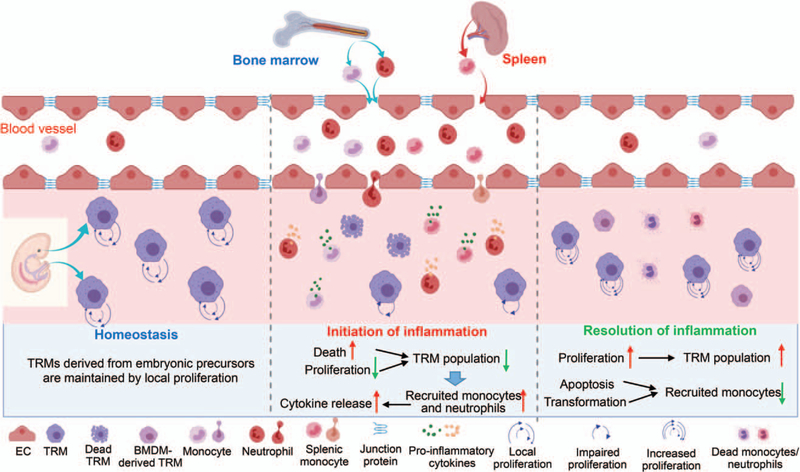
Collectively, as shown in Figure 3, acute inflammation reduces the self-renewal capacity of tissue-resident macrophages, which contributes to the reduction of resident population in tissues at the earlier stage of inflammation. Such macrophage insufficiency triggers the detrimental influx of monocytes. Nonetheless, during the late stages of inflammatory responses, substantial numbers of tissue-resident macrophages are restored by increased local proliferation, with a concomitant reduction of infiltrated monocytes/macrophages due to cell death. Therefore, as shown in Figure 4, the dynamic change of tissue-resident macrophage’s self-renewal capacity may contribute to the reciprocal population change happened between TRMs and recruited/inflammatory macrophages in tissues.
Fig. 3. The scheme depicts the dynamic change of monocyte/macrophage population in the tissue during acute inflammation.
Tissue-resident macrophages (TRMs) originate from embryonic precursors during development, and are capable of maintaining themselves by local proliferation throughout the lifespan without contribution from monocyte-derived macrophages under the homeostasis. While during the initiation of acute inflammation, TRM population decreases due to the inflammation-caused cell death and suppression of local proliferation. At the same time, bone marrow-derived monocytes, neutrophils, and splenic monocytes are recruited into the tissue. During inflammation, recruited monocytes exhibit a robust inflammatory response through releasing pro-inflammatory cytokines. At the stage of inflammation resolution, TRMs expand through increased self-renewal capacity and repopulate the niche. In addition, while the majority of recruited monocytes/macrophages undergo apoptosis, a small portion of these cells may undergo a slow in situ phenotypic conversion to become tissue-resident macrophages.
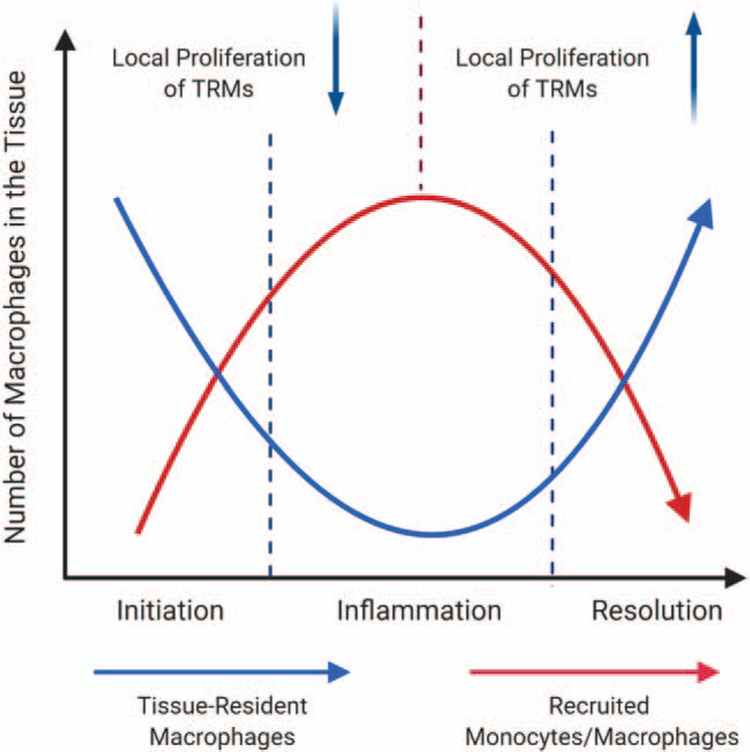
Fig. 4. Tissue-resident macrophage versus recruited monocytes during acute inflammation.
The graph presents that local proliferation of TRMs contributes to the dynamic change of tissue-resident macrophages and recruited monocytes during acute inflammation. TRMs indicates tissue-resident macrophages.
CONCLUSIONS AND PERSPECTIVES
With advancing technologies in cellular and molecular biology (i.e., single-cell RNA sequencing and multicolor FACS) and genetic mouse models (i.e., lineage tracing and fate-mapping), tissue-resident macrophages with a wide spectrum of phenotypes and functionalities have been identified in many organs. Currently, two perspectives are proposed to explain the complex heterogeneity of these macrophages: the observed phenotype is an origin-dependent difference; and the local environment plays an important role in educating and imprinting the various attributes and functions of macrophages.
Despite the ongoing intense research in the field, the urgent challenge and most important question remains: how to maintain a physiologically stable tissue-resident macrophage pool to maintain/restore homeostasis upon injury, and thereby protect tissue function and prevent detrimental inflammatory response in tissues/organs. Hence, deciphering mechanisms governing the self-renewal capacity of TRMs might have great biomedical potential, as it would allow us to dynamically control macrophage pools in tissues and thus, fine-tune the balance between inflammation and tissue repair. Future discoveries in the field of tissue resident macrophages would be very helpful in the development of drugs that treat a wide range of inflammatory diseases.
Acknowledgments
The studies in Fan Lab are supported by National Institutes of Health (NIH) grants R01 GM-126061, GM-132149, and American Heart Association (AHA) Established Investigator Award 17EIA33400063 (G.-C.F.).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Ta W, Chawla A, Pollard JW: Origins and hallmarks of macrophages: development, homeostasis, and disease. Nature 496:445–455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tauber AI: Metchnikoff and the phagocytosis theory. Nat Rev Mol Cell Biol 4(11):897–901, 2003. [DOI] [PubMed] [Google Scholar]
- 3.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL: The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ 46(6):845–852, 1972. [PMC free article] [PubMed] [Google Scholar]
- 4.Parwaresch MR, Wacker HH: Origin and kinetics of resident tissue macrophages: parabiosis studies with radiolabelled leucocytes. Cell Tissue Kinet 17(1):25–39, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Czernielewski JM, Demarchez M: Further evidence for the self-reproducing capacity of Langerhans cells in human skin. J Invest Dermatol 88:17–20, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Melnicoff MJ, Horan PK, Breslin EW, Morahan PS: Maintenance of peritoneal macrophages in the steady state. J Leukoc Biol 44(5):367–375, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM: M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164(12):6166–6173, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Ley K: M1 means kill; M2 means heal. J Immunol 199(7):2191–2193, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Martinez FO, Gordon S: The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essandoh K, Li Y, Huo J, Fan GC: MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock 46(2):122–131, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Italiani P, Boraschi D: From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 17(5):514, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sica A, Mantovani A: Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3):787–795, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K: Development of monocytes, macrophages, and dendritic cells. Science 327(5966):656–661, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynn TA, Vannella KM: Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44(3):450–462, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahrendorf M, Swirski FK: Abandoning M1/M2 for a network model of macrophage function. Circ Res 119(3):414–417, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginhoux F, Guilliams M: Tissue-resident macrophage ontogeny and homeostasis. Immunity 44(3):439–449, 2016. [DOI] [PubMed] [Google Scholar]
- 17.van de Laar L, Saelens W, De Prijck S, Martens L, Scott CL, Van Isterdael G, Hoffmann E, Beyaert R, Saeys Y, Lambrecht BN, et al. : Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue-resident macrophages. Immunity 44(4):755–768, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Perdiguero EG, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, De Bruijn MF, Geissmann F, et al. : Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518(7540):547–551, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duffield JS, Lupher M, Thannickal VJ, Wynn TA: Host responses in tissue repair and fibrosis. Annu Rev Pathol 8:241–276, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmerman KA, Bentley MR, Lever JM, Li Z, Crossman DK, Song CJ, Liu S, Crowley MR, George JF, Mrug M, et al. : Single-Cell RNA sequencing identifies candidate renal resident macrophage gene expression signatures across species. J Am Soc Nephrol 30(5):767–781, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alisjahbana A, Mohammad I, Gao Y, Evren E, Ringqvist E, Willinger T: Human macrophages and innate lymphoid cells: tissue-resident innate immunity in humanized mice. Biochem Pharmacol 174:113672, 2020. [DOI] [PubMed] [Google Scholar]
- 22.Mould KJ, Jackson ND, Henson PM, Seibold M, Janssen WJ: Single cell RNA sequencing identifies unique inflammatory airspace macrophage subsets. JCI Insight 4(5):e126556, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE, Zernecke A: Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res 122(12):1661–1674, 2018. [DOI] [PubMed] [Google Scholar]
- 24.Kim SY, Nair MG: Macrophages in wound healing: activation and plasticity. Immunol Cell Biol 97(3):258–267, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gundra UM, Girgis NM, Ruckerl D, Jenkins S, Ward LN, Kurtz ZD, Wiens KE, Tang MS, Basu-Roy U, Mansukhani A, et al. : Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 123(20):e110–e122, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vannella KM, Barron L, Borthwick LA, Kindrachuk KN, Narasimhan PB, Hart KM, Thompson RW, White S, Cheever AW, Ramalingam TR, et al. : Incomplete deletion of IL-4R (by LysMCre reveals distinct subsets of M2 macrophages controlling inflammation and fibrosis in chronic schistosomiasis. PLoS Pathog 10(9):e1004372, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et al. : Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med 179(3):220–227, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD, NIH NHLBI ARDS Network. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med 37(5):1574–1579, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi C, Simini B, Brazzi L, Rossi G, Radrizzani D, Iapichino G, Bertolini G: Variable costs of ICU patients: a multicenter prospective study. Intensive Care Med 32(4):545–552, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, Gajic O: Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med 36(5):1518–1522, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Niesler U, Palmer A, Radermacher P, Huber-Lang MS: Role of alveolar macrophages in the inflammatory response after trauma. Shock 42(1):3–10, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Xiu H, Zhang S, Zhang G: The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm 2018:1264913, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopf M, Schneider C, Nobs SP: The development and function of lung-resident macrophages and dendritic cells. Nat Immunol 16(1):36–44, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Joshi N, Walter JM, Misharin AV: Alveolar macrophages. Cell Immunol 330:86–90, 2018. [DOI] [PubMed] [Google Scholar]
- 35.Evren E, Ringqvist E, Willinger T: Origin and ontogeny of lung macrophages: from mice to humans. Immunology 160(2):126–138, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan SY, Krasnow MA: Developmental origin of lung macrophage diversity. Development 143(8):1318–1327, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schyns J, Bureau F, Marichal T: Lung interstitial macrophages: past, present, and future. J Immunol Res 2018:5160794, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purnama C, Ng SL, Tetlak P, Setiagani YA, Kandasamy M, Baalasubramanian S, Karjalainen K, Ruedl C: Transient ablation of alveolar macrophages leads to massive pathology of influenza infection without affecting cellular adaptive immunity. Eur J Immunol 44(7):2003–2012, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Schneider C, Nobs SP, Heer AK, Kurrer M, Klinke G, Van Rooijen N, Vogel J, Kopf M: Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog 10(4):e1004053, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussell T, Bell TJ: Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 14(2):81–93, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Byrne AJ, Mathie SA, Gregory LG, Lloyd CM: Pulmonary macrophages: key players in the innate defence of the airways. Thorax 70(12):1189–1196, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Garbi N, Lambrecht BN: Location, function, and ontogeny of pulmonary macrophages during the steady state. Pflugers Arch 469(3–4):561–572, 2017. [DOI] [PubMed] [Google Scholar]
- 43.Thepen T, Van Rooijen N, Kraal G: Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med 170(2):499–509, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts LM, Ledvina HE, Tuladhar S, Rana D, Steele SP, Sempowski GD, Frelinger JA: Depletion of alveolar macrophages in CD11c diphtheria toxin receptor mice produces an inflammatory response. Immun Inflamm Dis 3(2):71–81, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith KA, Harcus Y, Garbi N, Hämmerling GJ, MacDonald AS, Maizels RM: Type 2 innate immunity in helminth infection is induced redundantly and acts autonomously following CD11c+ cell depletion. Infect Immun 80(10):3481–3489, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herold S, Mayer K, Lohmeyer J: Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol 2:65, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narasaraju T, Ng HH, Phoon MC, Chow VT: MCP-1 antibody treatment enhances damage and impedes repair of the alveolar epithelium in influenza pneumonitis. Am J Respir Cell Mol Biol 42(6):732–743, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allard B, Panariti A, Martin JG: Alveolar macrophages in the resolution of inflammation, tissue repair, and tolerance to infection. Front Immunol 9:1777, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terakado M, Gon Y, Sekiyama A, Takeshita I, Kozu Y, Matsumoto K, Takahashi N, Hashimoto S: The Rac1/JNK pathway is critical for EGFR-dependent barrier formation in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 300(1):L56–L63, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T: Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med 167(2):171–179, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R 3rd, Standiford TJ: Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun 65(4):1139–1146, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beck-Schimmer B, Schwendener R, Pasch T, Reyes L, Booy C, Schimmer RC: Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Respir Res 6(1):61, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J, Tachado SD, Patel N, Zhu J, Imrich A, Manfruelli P, Cushion M, Kinane TB, Koziel H: Negative regulatory role of mannose receptors on human alveolar macrophage proinflammatory cytokine release in vitro. J Leukoc Biol 78(3):665–674, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Gwyer Findlay E, Hussell T: Macrophage-mediated inflammation and disease: a focus on the lung. Mediators Inflamm 2012:140937, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinmüller C, Franke-Ullmann G, Lohmann-Matthes ML, Emmendörffer A: Local activation of nonspecific defense against a respiratory model infection by application of interferon-gamma: comparison between rat alveolar and interstitial lung macrophages. Am J Respir Cell Mol Biol 22(4):481–490, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Hiruma T, Tsuyuzaki H, Uchida K, Trapnell BC, Yamamura Y, Kusakabe Y, Totsu T, Suzuki T, Morita S, Doi K, et al. : IFN-β improves sepsis-related alveolar macrophage dysfunction and postseptic acute respiratory distress syndrome–related mortality. Am J Respir Cell Mol Biol 59(1):45–55, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aggarwal NR, King LS, D’Alessio FR: Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol 306(8):L709–L725, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM: Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol 47(4):417–426, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watanabe S, Alexander M, Misharin AV, Budinger GRS: The role of macrophages in the resolution of inflammation. J Clin Invest 129(7):2619–2628, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bronte V, Pittet MJ: The spleen in local and systemic regulation of immunity. Immunity 39(5):806–818, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edgren G, Almqvist R, Hartman M, Utter GH: Splenectomy and the risk of sepsis: a population-based cohort study. Ann Surg 260(6):1081–1087, 2014. [DOI] [PubMed] [Google Scholar]
- 62.Mebius RE, Kraal G: Structure and function of the spleen. Nat Rev Immunol 5(8):606–616, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Davies LC, Jenkins SJ, Allen JE, Taylor PR: Tissue-resident macrophages. Nat Immunol 14(10):986–995, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borges da Silva H, Fonseca R, Pereira RM, Cassado Ados A, Älvarez JM, D’Império Lima MR: Splenic macrophage subsets and their function during blood-borne infections. Front Immunol 6:480, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. : Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38(1):79–91, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mu X, Wang X, Fan H, Li Y, Deng S, Essandoh K, Fan GC: SECTM1A is critical for macrophage phagocytosis during polymicrobial sepsis in mice. Shock 49(6):36–37, 2018. [Google Scholar]
- 67.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. : Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325(5940):612–616, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francis M, Sun R, Cervelli JA, Choi H, Mandal M, Abramova EV, Gow AJ, Laskin JD, Laskin DL: Editor’s highlight: role of spleen-derived macrophages in ozone-induced lung inflammation and injury. Toxicol Sci 155(1):182–195, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, et al. : Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res 107(11):1364–1373, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsiao HM, Fernandez R, Tanaka S, Li W, Spahn JH, Chiu S, Akbarpour M, Ruiz-Perez D, Wu Q, Turam C, et al. : Spleen-derived classical monocytes mediate lung ischemia-reperfusion injury through IL-1β. J Clin Invest 128(7):2833–2847, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mu X, Li Y, Fan H, Essandoh K, Deng S, Wang X, Wang P, Wang L, Fan GC: Sectm1a positively regulates tissue-resident macrophage self-renewal capacity during endotoxemia by boosting T effector cells. Shock 51(6):57–157, 2019. [Google Scholar]
- 72.Slentz-Kesler KA, Hale LP, Kaufman RE: Identification and characterization of K12 (SECTM1), a novel human gene that encodes a Golgi-associated protein with transmembrane and secreted isoforms. Genomics 47(3):327–340, 1998. [DOI] [PubMed] [Google Scholar]
- 73.Li Y, Deng S, Wang X, Huang W, Chen J, Robbins N, Mu X, Essandoh K, Peng T, Jegga AG, et al. : Sectm1a deficiency aggravates inflammation-triggered cardiac dysfunction through disruption of LXRα signaling in macrophages. Cardiovasc Res; 2020;cvaa067, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, Wang Y, Zingarelli B, Peng T, Fan GC: Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta 1852(11):2362–2371, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Essandoh K, Wang X, Huang W, Deng S, Gardner G, Mu X, Li Y, Kranias EG, Wang Y, Fan GC: Tumor susceptibility gene 101 ameliorates endotoxin-induced cardiac dysfunction by enhancing Parkin-mediated mitophagy. J Biol Chem 294(48):18057–18068, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang L, Li Y, Wang X, Wang P, Essandoh K, Cui S, Huang W, Mu X, Liu Z, Wang Y, et al. : GDF3 protects mice against sepsis-induced cardiac dysfunction and mortality by suppression of macrophage pro-inflammatory phenotype. Cells 9(1):120, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oikonomou E, Tousoulis D, Siasos G, Zaromitidou M, Papavassiliou AG, Stefanadis C: The role of inflammation in heart failure: new therapeutic approaches. Hellenic J Cardiol 52(1):30–40, 2011. [PubMed] [Google Scholar]
- 78.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, et al. : Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 115(2):284–295, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frantz S, Nahrendorf M: Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res 102(2):240–248, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN: Macrophages are required for neonatal heart regeneration. J Clin Invest 124(3):1382–1392, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, et al. : Macrophages facilitate electrical conduction in the heart. Cell 169(3):510–522, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swirski FK, Nahrendorf M: Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 18(12):733–744, 2018. [DOI] [PubMed] [Google Scholar]
- 83.Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, et al. : Tissue resident CCR2− and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res 124(2):263–278, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shahid F, Lip GYH, Shantsila E: Role of monocytes in heart failure and atrial fibrillation. J Am Heart Assoc 7(3):e007849, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Honold L, Nahrendorf M: Resident and monocyte-derived macrophages in cardiovascular disease. Circ Res 122(1):113–127, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, et al. : Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40(1):91–104, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ: The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204(12):3037–3047, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jung K, Kim P, Leuschner F, Gorbatov R, Kim JK, Ueno T, Nahrendorf M, Yun SH: Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ Res 112(6):891–899, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kadl A, Leitinger N: The role of endothelial cells in the resolution of acute inflammation. Antioxid Redox Signal 7(11–12):1744–1754, 2005. [DOI] [PubMed] [Google Scholar]
- 90.Leenders GJ, Smeets MB, van den Boomen M, Berben M, Nabben M, van Strijp D, Strijkers GJ, Prompers JJ, Arslan F, Nicolay K, et al. : Statins promote cardiac infarct healing by modulating endothelial barrier function revealed by contrast-enhanced magnetic resonance imaging. Arterioscler Thromb Vasc Biol 38(1):186–194, 2018. [DOI] [PubMed] [Google Scholar]
- 91.Aghajanian A, Wittchen ES, Allingham MJ, Garrett TA, Burridge K: Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J Thromb Haemost 6(9):1453–1460, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bazzoni G, Dejana E: Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 84(3):869–901, 2004. [DOI] [PubMed] [Google Scholar]
- 93.Baer C, Squadrito ML, Iruela-Arispe ML, De Palma M: Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches. Exp Cell Res 319(11):1626–1634, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Poh AR, Ernst M: Targeting macrophages in cancer: from bench to bedside. Front Oncol 8:49, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lapenna A, De Palma M, Lewis CE: Perivascular macrophages in health and disease. Nat Rev Immunol 18(11):689–702, 2018. [DOI] [PubMed] [Google Scholar]
- 96.Zhang W, Dai M, Fridberger A, Hassan A, Degagne J, Neng L, Zhang F, He W, Ren T, Trune D, et al. : Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid–blood barrier. Proc Natl Acad Sci U S A 109(26):10388–10393, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He H, Mack JJ, Güç E, Warren CM, Squadrito ML, Kilarski WW, Baer C, Freshman RD, McDonald AI, Ziyad S, et al. : Perivascular macrophages limit permeability. Arterioscler Thromb Vasc Biol 36(11):2203–2212, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, de Casterle ID, et al. : Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175(2):400–415, 2018. [DOI] [PubMed] [Google Scholar]
- 99.Culemann S, Grüneboom A, Nicolás-Ávila JÁ, Weidner D, Lämmle KF, Rothe T, Quintana JA, Kirchner P, Krljanac B, Eberhardt M, et al. : Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 572(7771):670–675, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Epelman S, Lavine KJ, Randolph GJ: Origin and functions of tissue macrophages. Immunity 41(1):21–35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, Barton GM: Tissue-resident macrophages are locally programmed for silent clearance of apoptotic cells. Immunity 47(5):913–927, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, Germain RN: Resident macrophages cloak tissue microlesions to prevent neutrophil-driven inflammatory damage. Cell 177(3):541–555, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barth MW, Hendrzak JA, Melnicoff MJ, Morahan PS: Review of the macrophage disappearance reaction. J Leukoc Biol 57(3):361–367, 1995. [DOI] [PubMed] [Google Scholar]
- 104.Davies LC, Rosas M, Smith PJ, Fraser DJ, Jones SA, Taylor PR: A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur J Immunol 41(8):2155–2164, 2011. [DOI] [PubMed] [Google Scholar]
- 105.Shi C, Pamer EG: Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11(11):762–774, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Röszer T: Understanding the biology of self-renewing macrophages. Cells 7(8):e103, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sieweke MH, Allen JE: Beyond stem cells: self-renewal of differentiated macrophages. Science 342(6161):1242974, 2013. [DOI] [PubMed] [Google Scholar]
- 108.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE: Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332(6035):1284–1288, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, Maizels RM, Hume DA, Allen JE: IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med 210(11):2477–2491, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Minutti CM, Jackson-Jones LH, García-Fojeda B, Knipper JA, Sutherland TE, Logan N, Ringqvist E, Guillamat-Prats R, Ferenbach DA, Artigas A, et al. : Local enhancers of Type 2-mediated macrophage activation and proliferation promote tissue repair. Science 356(6342):1076–1080, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, Barbaroux JB, Groves R, Geissmann F: Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med 206(13):3089–3100, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, Fraser DJ, Allen JE, Jones SA, Taylor PR: Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun 4(1):1–10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM: Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 14(9):1142–1149, 2011. [DOI] [PubMed] [Google Scholar]
- 114.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, et al. : Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38(4):792–804, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoyer FF, Naxerova K, Schloss MJ, Hulsmans M, Nair AV, Dutta P, Calcagno DM, Herisson F, Anzai A, Sun Y, et al. : Tissue-specific macrophage responses to remote injury impact the outcome of subsequent local immune challenge. Immunity 51(5):899–914, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM: Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med 184(5):547–560, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, et al. : The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med 214(7):1913–1923, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gentek R, Molawi K, Sieweke MH: Tissue macrophage identity and self-renewal. Immunol Rev 262(1):56–73, 2014. [DOI] [PubMed] [Google Scholar]
- 119.Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, Pinto AR, Klapproth K, Henri S, Malissen B, et al. : Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med 211(11):2151–2158, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]