Abstract
Background
There is conflicting evidence regarding the use of intra‐articular lignocaine injection for the closed manual reduction of acute anterior shoulder dislocations. A systematic review may help cohere the conflicting evidence.
Objectives
To compare the clinical efficacy and safety of intra‐articular lignocaine and intravenous analgesia (with or without sedation) for reduction of acute anterior shoulder dislocation.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 1), MEDLINE (1950 to March 2010), and EMBASE (1980 to March 2010). We searched Current Controlled Trials metaRegister of Clinical Trials (compiled by Current Science) (March 2010). We imposed no language restriction.
Selection criteria
Randomized controlled trials comparing intra‐articular lignocaine (IAL) with intravenous analgesia with or without sedation (IVAS) in adults aged 18 years and over for reduction of acute anterior shoulder dislocation.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. Where possible, data were pooled and relative risks (RR) and mean differences (MD), each with 95% confidence intervals (CI), were computed using the Cochrane Review Manager statistical package (RevMan).
Main results
Of 1041 publications obtained from the search strategy, we examined nine studies. Four studies were excluded, and five studies with 211 participants were eligible for inclusion. There was no difference in the immediate success rate of IAL when compared with IVAS in the closed manual reduction of acute anterior shoulder dislocation (RR 0.95; 95% CI 0.83 to 1.10). There were significantly fewer adverse effects associated with IAL compared with IVAS (RR 0.16; 95% CI 0.06 to 0.43). The mean time spent in the emergency department was significantly less with IAL compared with IVAS (MD 109.46 minutes; 95% CI 84.60 to 134.32). One trial reported significantly less time for reduction with IVAS (105 seconds; 95% CI 84.0 to 126.1) compared with IAL (284.6 seconds; 95% CI 185.3 to 383.9). One trial reported no joint infection associated with intra‐articular lignocaine injection and no mortality associated with either IAL or IVAS.
Authors' conclusions
We observed no significant difference between IAL and IVAS with regard to the immediate success rate of reduction, pain during reduction, post‐reduction pain relief and reduction failure. Compared to IVAS, IAL may be less expensive and may be associated with fewer adverse effects and a shorter recovery time.
Plain language summary
Injection of lignocaine into a dislocated shoulder joint versus injection of a pain‐relieving drug with or without injection of a sedative drug into a vein for the purpose of manual manipulation of acute (less than 48 hours old) anterior (towards the front) displacement of a shoulder joint from its normal position.
Joint dislocation refers to displacement of the bones which form a joint away from their anatomical position. The shoulder is the most commonly dislocated joint managed in the emergency department (ED). When the dislocation occurs towards the front of the body, this is known as an anterior shoulder dislocation. It is called an acute anterior shoulder dislocation if the dislocation occurred with the previous 48 hours. Manually manipulating the displaced bones back to their normal position (manual reduction) is very painful. To allow for manual reduction, pain relief can be achieved either by injecting a local anaesthetic drug (for example, lignocaine) into the dislocated shoulder joint (intra‐articular lignocaine injection); or by injecting a pain killer with or without a sedative directly into the bloodstream through a vein (intravenous analgesia). The review authors searched the medical literature and identified five studies comparing these two methods. The studies included 211 patients with acute anterior shoulder dislocation; 113 patients underwent intra‐articular lignocaine injection and 98 underwent intravenous analgesia with sedation. The review found that there may be no difference in the immediate success of manual reduction of the dislocated shoulder between patients receiving intra‐articular lignocaine injection and those who received intravenous analgesia and sedation. However, intra‐articular lignocaine injection may be associated with fewer side effects and a shorter stay in the emergency department before discharge from hospital. Compared with intravenous analgesia and sedation, intra‐articular lignocaine may also be cheaper. However, the relatively small number of studies included in the review and the relatively small number of patients in each study means that the results of the review preclude definitive conclusions regarding the superiority of either method..
Summary of findings
Summary of findings for the main comparison. Immediate success rate of reduction.
| intra‐articular lignocaine compared to intravenous analgesia with or without sedation for acute anterior shoulder dislocation in adults | ||||||
| Patient or population: acute anterior shoulder dislocation in adults Settings: Emergency Department Intervention: intra‐articular lignocaine Comparison: intravenous analgesia with or without sedation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| intravenous analgesia with or without sedation | intra‐articular lignocaine | |||||
| Per cent of patients who underwent successful reduction | Study population | RR 0.95 (0.83 to 1.1) | 211 (5 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 776 per 1000 | 737 per 1000 (644 to 854) | |||||
| Medium risk population | ||||||
| 792 per 1000 | 752 per 1000 (657 to 871) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Relatively small number of included studies. 2 Small sample size in each included study.
Background
Dislocation of a joint refers to a clinical condition in which the joint surfaces are completely displaced and are no longer in contact (Graham Apley 1988). Dislocations require prompt correction (reduction) to limit pain and suffering, and to minimize damage to the joint and its surrounding structures. The shoulder joint is the most commonly dislocated joint that is managed in the emergency department (ED), with an overall reported risk in the general population of 1.7% (Hovelius 1982; Kothari 1992). The majority of people with shoulder dislocation presenting to the ED (up to 96% in some series) have dislocations that occur in an anterior (towards the front) direction (McNamara 1998; Norlin 1993; Rockwood 1996).
In most EDs, acute (sudden and severe) anterior shoulder dislocations (AASD) are commonly reduced manually with intravenous sedation (benzodiazepines) with or without analgesia (opiates). Intravenous analgesia with or without sedation (IVAS) facilitates manual reduction in AASD but is associated with potentially serious adverse effects. Significant central nervous system and respiratory depression may occur with IVAS, requiring close patient monitoring and medical management (Miller 2002). Nausea, vomiting and lethargy may occur, requiring prolonged ED observation (Kosnik 1999; Orlinsky 2002). Antidotal treatment for reversal of benzodiazepine sedation or opiate analgesia is sometimes required to aid symptom relief and patient recovery after IVAS (Orlinsky 2002). Meanwhile, IVAS should be used judiciously in certain subsets of patients, such as elderly patients with poor cardiorespiratory reserve, pregnant women and some patients with multiple trauma (Kosnik 1999; Matthews 1995).
Recently, intra‐articular lignocaine (IAL) has been advocated as a means of providing analgesia during manual reduction of AASD. IAL may permit avoidance of sedative agents while achieving, in some cases, acceptable degrees of analgesia. A secondary benefit of IAL in selected patients is that intravenous access may not be required, allowing for performance of the procedure among those patients who lack easily obtainable intravenous access. Another secondary benefit is that monitoring, including monitoring of oxygen saturation and electrocardiography, may not be required during or after reduction employing IAL in selected patients. This may translate into a significantly shorter ED stay with IAL compared to IVAS (Matthews 1995; Miller 2002). Furthermore, IAL may associated with a lower complication rate compared to IVAS (Lippitt 1991). IVAS‐associated central nervous system depression and cardiorespiratory decompensation have not been reported with IAL. IAL may also cost less than IVAS (Matthews 1995; Miller 2002).
Despite these advantages, aspects of IAL may be inferior to IVAS. Psychological agitation among patients receiving IAL may interfere with joint reduction (Orlinsky 2002). Another potential drawback of IAL is that assessment of the effectiveness of the technique is limited by the difficulty in determining the correct intra‐articular placement of lignocaine, which is not as easily confirmed as when administering medications intravenously (Orlinsky 2002). However, to date this complication has not been reported with this technique (Miller 2002). Further drawbacks of IAL include the lack of substantial muscle relaxation, the inability to titrate depth of sedation and use of anxiolytic agents, and a potential for septic arthritis. Lastly, the effectiveness of IAL injection may be provider and patient dependent. For example, obese patients represent a challenge for proper intra‐articular drug placement.
In terms of success of reduction of AASD, there is conflicting evidence regarding the efficacy of IAL compared to IVAS. There is evidence that IAL is as good as or better than IVAS for reduction of AASD (Lippitt 1991; Matthews 1995; Miller 2002). In contrast, a trend towards higher successful reduction rates with IVAS than with IAL has been reported (Kosnik 1999). We conducted a quantitative systematic review to help cohere these conflicting results.
Objectives
The primary objectives of this review were:
to identify and evaluate all randomized controlled trials (RCTs) comparing intra‐articular lignocaine and intravenous analgesia with or without sedation for reduction of acute anterior shoulder dislocation;
to establish whether intra‐articular lignocaine is equally effective and as safe as intravenous analgesia with or without sedation for reduction of acute anterior shoulder dislocation.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs, without language restriction. We defined a RCT as a study in which patients were allocated to treatment groups on the basis of a random or quasi‐random method (for example, using random number tables, hospital number or date of birth).
Types of participants
We included adults aged 18 years and over with acute anterior shoulder dislocation (including recurrent dislocation) that was confirmed radiographically.
Types of interventions
The target intervention was IAL or IVAS for acute anterior shoulder dislocation.
Types of outcome measures
Primary outcomes
The primary outcome measure was the immediate success rate of the procedure (successful reduction of the dislocated shoulder). The immediate success rate was as defined by the study authors.
Secondary outcomes
The secondary outcome measures were:
pain during the procedure;
post‐reduction pain relief;
time required for reduction;
ease of reduction;
patient satisfaction during the procedure;
number of reduction attempts;
cost;
fracture complicating reduction;
rate of joint infection (septic arthritis) complicating the procedure;
other adverse effects;
mortality;
recovery time (defined as the difference between the time of reduction and the time of discharge from the emergency department);
average time in the emergency department;
reduction failure (failed emergency department manual reduction) or referral to the orthopaedic service for possible shoulder relocation in the operating room.
Search methods for identification of studies
See: Cochrane Anaesthesia Review Group methods used in reviews.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 1), MEDLINE (1950 to March 2010) and EMBASE (1980 to March 2010).
We used the optimally sensitive strategies of The Cochrane Collaboration to identify randomized controlled trials in the MEDLINE and EMBASE searches (Dickersin 1994; Lefebvre 1996). We combined them with subject headings and textwords for shoulder dislocation, lignocaine or lidocaine, and intra‐articular injections.
In MEDLINE (see Appendix 1) and EMBASE (see Appendix 2), we searched for the following keywords (text word and subject heading searches, where appropriate): shoulder dislocation; lidocaine or lignocaine. We combined these words with injections or intra‐articular and narrowed the search to randomized controlled trials.
We searched CENTRAL (see Appendix 3) for RCTs using the terms: shoulder dislocation, combined with either lidocaine or lignocaine, injections or intra‐articular (vide infra).
We did not impose any language restriction.
We searched Current Controlled Trials metaRegister of Clinical Trials (compiled by Current Science) (March 2010) using the following search terms: shoulder dislocation AND ((lidocaine OR lignocaine) AND (injection OR intra‐articular)).
We searched OpenSIGLE (System for Information on Grey Literature in Europe) (March 2010) using the search term: shoulder dislocation.
Searching other resources
We made additional efforts to locate potential RCTs from the following data sources:
review articles and textbooks;
references cited in primary sources;
raw data from published trials (sought by personal communication).
Data collection and analysis
Selection of studies
We screened the titles and abstracts of identified studies and discarded clearly irrelevant studies. We (AW and ROS) obtained the full‐text versions of all potentially relevant randomized and quasi‐randomized trials and independently assessed them for eligibility based on the defined inclusion criteria. We resolved any disagreements by discussion. There was no occasion where uncertainty remained after this discussion.
Data extraction and management
We used a revised data extraction form to incorporate the new additions on quality assessment in the Cochrane Handbook (Higgins 2009). We extracted relevant data regarding inclusion criteria (study design, participants, interventions and outcomes), risk of bias (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias) and results. In cases where insufficient data were reported (for example, completeness of outcome data) we contacted the study authors for further information. Data extraction was carried out by two review authors (AW and AM) and confirmed by a third party (Dr Elaine Donnelly, see Acknowledgements). Excluded studies and reasons for exclusion are detailed in the table Characteristics of excluded studies. Where necessary, we contacted the authors of included studies for missing information.
Assessment of risk of bias in included studies
We assessed risk of bias in terms of sequence generation, allocation concealment, blinding (of participants, personnel and outcome assessors), incomplete outcome data, selective outcome reporting and other sources of bias (Higgins 2009). In addition, we sought evidence that the groups were balanced at baseline and that intention‐to‐treat analyses were undertaken. The risk of bias in eligible trials was assessed by two review authors (AW and AM). We resolved any disagreement by discussion.
Statistical methods
We performed meta‐analyses using RevMan software (RevMan 5.0). Immediate successful shoulder reduction rate, as defined by the study authors, was used as the primary outcome measure.
For dichotomous (or binary) data we described the results as a relative measure, relative risk (RR). For continuous data, we used the mean difference (MD) whenever outcomes were measured in a standard way across studies.
We explored heterogeneity amongst included studies both qualitatively by comparing the characteristics of included studies and quantitatively using the I2 statistic (Higgins 2003). Where appropriate, we combined the results from the included studies for each outcome to give an overall estimate of treatment effect. We used a fixed‐effect model meta‐analysis except where statistical heterogeneity was identified, in which case we used a random‐effects model (Deeks 2008).
We minimized publication bias by comprehensive literature searching (Glasziou 2001). In addition, we planned to use a graphical display (funnel plot) of the size of the treatment effect against the precision of the trial (1/standard error) to investigate publication bias.
No simple solution exists for the problem of missing data. We handled this problem by contacting the investigators, whenever possible, to ensure that no data were missing for the studies. In addition, we planned to be explicit about the assumptions of whatever method we used to cope with missing data.
Finally, we planned to perform sensitivity analyses to test how sensitive the results were to reasonable changes in the assumptions that were made and in the protocol for combining the data (Lau 1998). We planned to perform sensitivity analyses regarding randomized versus quasi‐randomized and eventually good quality studies versus poor quality studies. We defined a good quality study as one which has all of the following domains: adequate allocation concealment, blinding of outcome assessment and data analysis performed according to the intention‐to‐treat principle. We defined a poor quality study as one which lacked one or more of these key domains.
Subgroup analysis
We planned to perform subgroup analysis of patients with a history of previous shoulder dislocation of the affected shoulder to determine if it was a confounding factor in successful reduction.
Results
Description of studies
Of the 1041 publications obtained from the electronic databases searched, nine potentially relevant trials were selected. Four trials were ultimately excluded (Paudel 2004; Pradhan 2006; Suder 1995a; Suder 1995b). Two of these trials were excluded because the participants were not randomized (Paudel 2004; Pradhan 2006). One trial was excluded because the type of shoulder dislocation experienced by the participants was unclear and it was not possible to obtain this information from the trialists (Suder 1995a). One trial was excluded because the definition of the outcome measures was unclear and it was not possible to obtain clarification from the trialists (Suder 1995b). See the table Characteristics of excluded studies for further information and Figure 1.
1.
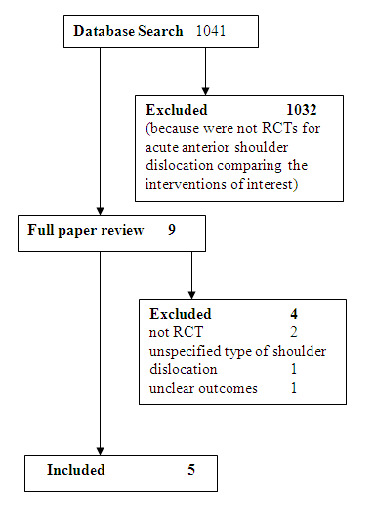
Seach flow diagram
Five studies met the inclusion criteria (Kosnik 1999; Matthews 1995; Miller 2002; Moharari 2008; Orlinsky 2002). All the included studies reported the primary outcome measure of this review, the immediate success rate of the procedure (successful reduction of the dislocated shoulder). Three of the included studies (Kosnik 1999, Miller 2002; Orlinsky 2002) defined immediate success of the procedure based on post‐reduction radiographs. Two of the included studies (Matthews 1995; Moharari 2008) did not explicitly state if successful reduction of the dislocated shoulder was defined clinically or radiologically.
Kosnik 1999 compared IAL with intravenous morphine sulphate and diazepam. The setting was an urban, level‐1 trauma centre. Eligible patients were adults presenting to the ED with radiographically confirmed AASD. Forty‐nine patients were randomized by an unblocked simple sample randomization technique.
Matthews 1995 compared IAL with intravenous morphine sulphate and midazolam. The setting was a university medical centre. Eligible patients were patients presenting to the ED with AASD. Thirty consecutive patients were randomized by pulling out a page from the protocol book at random. This page indicated into which of the two study groups the patient was to be placed. One of two reduction techniques were used for the participants: traction‐countertraction or scapular rotation. If the reduction was unsuccessful after using one of these techniques, the physician could change to any technique they desired.
Miller 2002 compared IAL with intravenous fentanyl and midazolam. Two hospitals participated in the study: a private hospital and an urban level‐1 trauma hospital. Inclusion criteria were: age 18 to 70 years and an AASD. Thirty patients were randomized by a quasi‐random method using the hospital number. The reduction technique used for all participants was the modified Stimson technique.
Moharari 2008 compared IAL with intravenous meperidine and diazepam. Eligible patients were aged between 18 and 80 years and presenting to the ED with acute anterior shoulder dislocation. Forty‐eight patients were randomized using a computerised random number generator. Reduction was performed in all the patients by a single person using the traction‐countertraction method.
Orlinsky 2002 compared IAL with intravenous meperidine and diazepam. Eligible patients were adults presenting to the ED with shoulder pain and radiographically confirmed anterior shoulder dislocation. Twenty‐nine patients were randomized by the physician pulling a pre‐numbered envelope containing the intervention to which the patient was randomized. One of two reduction techniques was used for participants according to the discretion of the physician performing the reduction: the external rotation method or the traction‐countertraction method.
Risk of bias in included studies
Concealment of allocation was unclear in two included studies (Kosnik 1999; Moharari 2008) and inadequate in three included studies (Matthews 1995; Miller 2002; Orlinsky 2002). The nature of the interventions was such that double‐blinding was not feasible for the included studies (Figure 2 and Figure 3).
2.
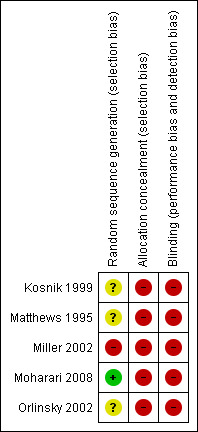
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
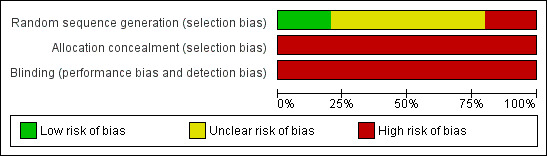
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Methodological quality graphs (Figure 2 and Figure 3) present review authors' judgements about each methodological quality item, presented as percentages across the included studies. Given the small number of included trials we were unable to assess publication bias using the funnel plot approach (Higgins 2009).
Effects of interventions
See: Table 1
Primary outcome
With regard to the primary outcome of this review, all the included studies reported the immediate success rate for both IAL and IVAS (Kosnik 1999; Matthews 1995; Miller 2002; Moharari 2008; Orlinsky 2002). There was no significant difference in the immediate success rate of the procedure (successful reduction of the dislocated shoulder) with IAL compared with IVAS (relative risk (RR) 0.95; 95% CI 0.83 to 1.10) (Analysis 1.1).
1.1. Analysis.
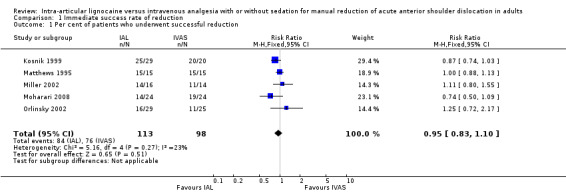
Comparison 1 Immediate success rate of reduction, Outcome 1 Per cent of patients who underwent successful reduction.
Secondary outcomes
Pain during the procedure was reported for both interventions by three studies (Kosnik 1999; Matthews 1995; Miller 2002). All three studies used a 10‐point verbal numeric rating scale (VNRS). There was no significant difference in pain during the procedure with IAL compared with IVAS (mean difference (MD) 0.18; 95% CI ‐1.41 to 1.77) (Analysis 2.1).
2.1. Analysis.
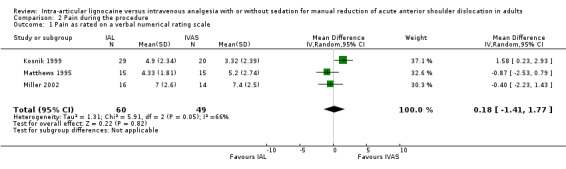
Comparison 2 Pain during the procedure, Outcome 1 Pain as rated on a verbal numerical rating scale.
Two trials reported post‐reduction pain relief for IAL and IVAS (Moharari 2008; Orlinsky 2002). The results of both trials could not be pooled because of a difference in the timing used to define post‐reduction pain relief. Moharari 2008 reported no statistically significant difference (P = 0.199) in the change in pain score before reduction to after reduction in the IAL group (49.8; 95% CI 39.3 to 60.3) compared with the IVAS group (58.4; 95% CI 49.8 to 66.9). Orlinsky 2002 reported no statistically significant difference (P = 0.33) in the mean change in post‐reduction pain relief (pain score from after anaesthesia but prior to reduction to time of discharge) between IAL (‐3.65 ± 2.67; 95% CI ‐4.81 to ‐2.47; n = 20) and IVAS (‐2.79 ± 2.89; 95% CI ‐4.00 to ‐1.59; n = 21).
Two trials reported the time required for reduction (Miller 2002; Moharari 2008). Miller 2002 reported no significant difference between the two interventions with regard to time for reduction (10.1 minutes in the IAL group and 12.1 minutes in the IVAS group; P = 0.71, t‐test). Moharari 2008 reported a statistically significant (P = 0.001) decrease in the time required for reduction with IVAS (105 seconds; 95% CI 84.0 to 126.1) compared with IAL (284.6 seconds; 95% CI 185.3 to 383.9). The results of the two trials could not be pooled because they were reported differently.
Three trials reported ease of reduction (Kosnik 1999; Matthews 1995; Orlinsky 2002). The results could not be pooled because the three trials reported ease of reduction in different ways. Kosnik 1999 reported no significant difference between the two interventions with regard to mean ease of reduction scores measured subjectively by the clinician using a 10‐point visual analogue scale (4.45 ± 2.46 in the IAL group and 3.32 ± 2.36 in the IVAS group; P = 0.12, Fisher's exact test). Matthews 1995 reported that 10 of 15 reductions were rated as 'easy' and 0 of 15 as 'very tough' in the IAL group, whereas 7 out of 15 were rated as 'easy' and 2 of 15 as 'very tough' in the IVAS group. Orlinsky 2002 reported that physicians perceived that insufficient muscle relaxation interfered with the procedure in 21% of patients with IAL and 4% of patients in the IVAS group (RR 4.93; 95% CI 0.64 to 38.0; P = 0.11).
Two trials reported patient satisfaction during the procedure (Kosnik 1999; Orlinsky 2002). The results could not be pooled because the two trials reported this outcome measure in different ways. Kosnik 1999 reported that of those patients in the IAL group who had undergone previous reductions with IVAS sedation, 5 of 10 preferred being "put to sleep" for the procedure. With regard to IAL, an equal number of patients with recurrent dislocation appreciated effective analgesia without any central nervous system (CNS) sedation along with prompt discharge without an observation period. Orlinsky 2002 reported that patients perceived inadequate analgesia 24% of the time with IAL and 4% of the time with IVAS (RR 5.76; 95% CI 0.8 to 44.4; P = 0.1). Pain interfered with the reduction 7% of the time with the IAL method and 5% of the time with IVAS; this difference was not statistically significant (Orlinsky 2002).
Two trials reported the number of reduction attempts as an outcome measure (Moharari 2008; Orlinsky 2002). There was no significant difference between IAL and IVAS in the number of one reduction attempts (RR 0.85; 95% CI 0.54 to 1.33) (Analysis 4.1), two reduction attempts (RR 1.07; 95% CI 0.30 to 3.84) (Analysis 5.1) and three reduction attempts (RR 2.65; 95% CI 0.16 to 44.78) (Analysis 6.1).
4.1. Analysis.
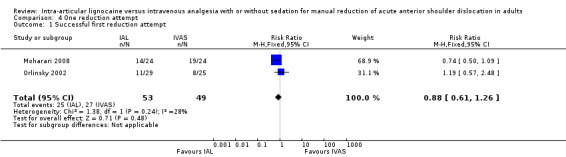
Comparison 4 One reduction attempt, Outcome 1 Successful first reduction attempt.
5.1. Analysis.
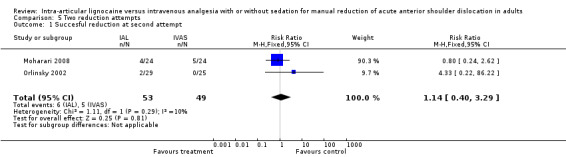
Comparison 5 Two reduction attempts, Outcome 1 Succesful reduction at second attempt.
6.1. Analysis.
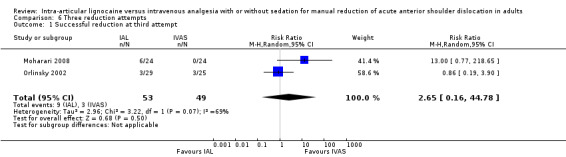
Comparison 6 Three reduction attempts, Outcome 1 Successful reduction at third attempt.
Two trials compared the costs of the interventions (Matthews 1995; Miller 2002). The results could not be pooled because the two trials reported this outcome measure in different ways. Matthews 1995 reported that the hospital charges for patients receiving IAL ranged from USD 117 to USD 133 per visit. Hospital charges for patients in the IVAS group ranged from USD 159.55 to USD 310 per visit depending on the need for extended monitoring and reversal agents. Miller 2002 reported the cost per patient of IVAS for closed manual reduction of AASD as USD 97.64 compared with USD 0.52 for IAL.
One trial reported on fractures complicating closed manual reduction. Kosnik 1999 reported two additional radiographic abnormalities, Hill‐Sachs and nondisplaced greater tuberosity, in the IAL group that may have occurred during reduction; however, overlapping bone on the post‐reduction radiograph may have obscured detection of a pre‐reduction abnormality. Moharari 2008 reported no fractures in the study participants.
Adverse effects were reported by all the included studies (Kosnik 1999; Matthews 1995; Miller 2002; Moharari 2008; Orlinsky 2002). There were significantly fewer adverse effects associated with IAL compared with IVAS (RR 0.16; 95% CI 0.06 to 0.43) (Analysis 3.1).
3.1. Analysis.
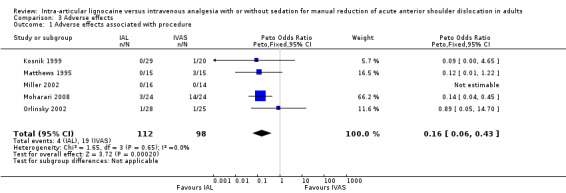
Comparison 3 Adverse effects, Outcome 1 Adverse effects associated with procedure.
Three trials reported the average time spent in the ED (Matthews 1995; Miller 2002; Moharari 2008). The mean time spent in the ED was significantly less with IAL compared with IVAS (MD 109.46 minutes; 95% CI 84.60 to 134.32) (Analysis 7.1) for the two trials which reported the results as means and standard deviations (Matthews 1995; Miller 2002). Moharari 2008 also reported a shorter stay in the ED with IAL (140.6 minutes; 95% CI 104.2 to 177.1) compared with IVAS (216.5 minutes; 95% CI 164.0 to 269.0). The results of this trial could not be pooled with the other two trials because it did not report standard deviations and individual patient data could not be obtained from the trialists.
7.1. Analysis.
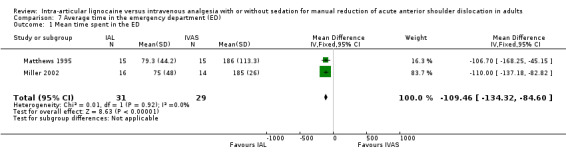
Comparison 7 Average time in the emergency department (ED), Outcome 1 Mean time spent in the ED.
Two trials reported reduction failure as an outcome measure (Kosnik 1999; Matthews 1995). One trial reported no reduction failures in either the IAL group or the IVAS group (Matthews 1995). One trial reported one reduction failure in the IAL group and no reduction failure in the IVAS group (Kosnik 1999).
One trial reported recovery time (Orlinsky 2002). The trial defined recovery time as the difference between the time of reduction and the time of discharge. To take into account the 15 minutes required before reduction was performed for the IAL method, the trialists added 15 minutes to the recovery time to derive an adjusted recovery time (Orlinsky 2002). The recovery time was significantly shorter with IAL compared with IVAS (Orlinsky 2002). The recovery time, reported as mean ± standard deviation (SD), was 103 ± 63 minutes for the IAL group compared with 154 ± 76 minutes for the IVAS group (P = 0.025) (Orlinsky 2002). In contrast, there was no statistically significant difference in the adjusted recovery times between the two groups, with a mean ± SD of 118 ± 63 minutes for the IAL group and 154 ± 76 minutes for the IVAS group (P = 0.085) (Orlinsky 2002).
No trial reported joint infection (septic arthritis) with intra‐articular lignocaine injection, or mortality associated with IAL or IVAS.
Subgroup analysis
We were unable to pool the results for subgroup analyses because, of the four studies (Kosnik 1999; Matthews 1995; Miller 2002; Orlinsky 2002) which reported patients with a history of previous shoulder dislocations, only one study reported whether the previous dislocations were of the affected shoulder (Miller 2002). In addition, none of the studies which reported a history of previous shoulder dislocations reported standard deviations of the results. One study Moharari 2008) did not report the number of patients with previous shoulder dislocations as an outcome measure and was thus excluded from the subgroup analyses.
Kosnik 1999 reported that 40% of patients in the IVAS group (n = 20) and 34% in the IAL group (n = 29) had previous shoulder dislocations, but it did not report whether the previous dislocations were of the affected shoulder. Nevertheless, the study reported no statistically significant difference in the rates of successful reduction between the two groups (RR 0.87; 95% CI 0.74 to 1.03).
Matthews 1995 reported two patients with one previous dislocation each and one patient with 10 previous dislocations, in the IAL group (n = 15); none of these previous dislocations could be confirmed. They reported two patients with one previous dislocation each, one patient with three previous dislocations and one patient with four previous dislocations in the IVAS group (n = 15). All the patients in both groups underwent successful reduction (100% successful reduction in each group; RR and 95% CI not estimable).
Miller 2002 reported that four patients in the IAL group (n = 16) and five in the IVAS group (n = 14) had had previous dislocation of the affected shoulder. All patients reported having had one or two prior dislocations except for one patient in the IVAS group, who reported 30 previous dislocations. No patients reported that they had had previous surgery on the dislocated shoulder. There was no significant difference between the two groups with regard to the rates of successful reduction (RR 1.11; 95% CI 0.80 to 1.55).
Orlinsky 2002 reported that 11 patients (44%) in the IVAS group (n = 29) and 21 patients (72%) in the IAL group (n = 25) had a prior shoulder dislocation, but did not report whether the prior dislocations were of the affected shoulder, during enrolment for the study. This difference between the groups with regards to prior dislocations was not statistically significant (P = 0.07). In addition, there was no statistically significant difference in the rates of successful reduction between the two groups (RR 1.25; 95% CI 0.72 to 2.17).
Discussion
This review summarizes the current evidence derived from RCTs comparing IAL with IVAS for the closed manual reduction of AASD. The results of this review indicate that the immediate success rate and pain experienced by patients who were treated with IAL was not significantly different from those treated with IVAS.
The absence of any significant difference between the interventions with regard to the pain experienced may be due to the use of fixed, rather than weight‐based, drug doses in the IVAS arm of the included studies. This raises the possibility that therapeutic systemic levels of the administered intravenous analgesics were not achieved in some patients in the IVAS arm of the included studies.
No significant difference was found in post‐reduction pain relief between the two interventions. This outcome measure was reported by two trials (Moharari 2008; Orlinsky 2002). Owing to the small number of participants in the trials, the effect measure may be imprecise. The lack of any difference between the two interventions for this outcome measure may also be due to the use of fixed drug doses.
Two trials which met the inclusion criteria for this review reported no significant difference between the two interventions with regard to time required for reduction (Miller 2002; Moharari 2008). It was not possible to pool the results of these two trials because the time for reduction to occur was measured in different ways in the respective studies, and it was not possible to obtain the individual patient data from the trialists. The estimates yielded by these trials may be imprecise due to the small sample sizes.
Regarding recovery time, no firm conclusions can be drawn from the findings of this review. One trial reported a statistically significant shorter mean recovery time with IAL compared with IVAS, but there was no statistically significant difference between the two interventions in relation to the time from analgesia delivery to discharge, the adjusted recovery time (Orlinsky 2002). Another included trial reported that the IAL group spent a shorter mean time in the emergency department (ED) compared with the IVAS group (Miller 2002). This study reported that the IAL group left the ED at a mean ± SD of 75 ± 48 minutes after the shoulder was reduced, whereas the IVAS group left in a mean time of 185 ± 26 minutes (P = 0.42, t‐test) (Miller 2002).
There was no increased risk of fractures complicating reduction in patients treated with either IAL or IVAS (Moharari 2008). A significant reduction in other adverse effects and in recovery time was observed with IAL compared with IVAS.
The lower risk of other adverse effects observed with IAL compared to IVAS in this review may possibly be because IVAS is associated with central nervous system and respiratory depression (Moharari 2008). Nausea, vomiting and lethargy may also occur, requiring prolonged ED observation (Kosnik 1999; Moharari 2008, Orlinsky 2002). Antidotal treatment for reversal of benzodiazepine sedation or opiate analgesia is sometimes required to aid patient recovery (Orlinsky 2002).
It is important to note, however, that the differences we observed in adverse effects associated with IAL and IVAS (Analysis 3.1) were largely driven by one study (Moharari 2008). This study reported the highest number of adverse events (drowsiness in three participants) in the IAL group (Moharari 2008). The trialists reported that the drowsiness was because, before being enrolled in the study, the three participants in the IAL group had ingested an analgesic (tramadol, which can cause drowsiness) for symptom relief without informing the investigators (Moharari 2008).
To further investigate the effect of the Moharari 2008 study in driving the adverse effects reported in this review, we analysed the summary results of this outcome measure with and without the study (Moharari 2008). With this study (Moharari 2008) included in the analysis, IAL was associated with significantly fewer adverse effects compared with IVAS (RR 0.16; 95% CI 0.06 to 0.43) (Analysis 3.1). In contrast, without the study (Moharari 2008) there was no significant difference between the two interventions in relation to adverse effects (RR 0.22; 95% CI 0.22 to 1.15). It is, therefore, not possible to reach any firm conclusion about any differences between the two interventions in relation to adverse effects based on the findings of this review.
Analysis of ED reduction failure was based on one trial (Kosnik 1999). The estimate yielded by this trial may be imprecise owing to the small sample size. Therefore, no firm conclusions regarding ED reduction failure can be drawn from the findings.
Trials reporting the outcome measures ease of reduction, patient satisfaction during the procedure and cost of the intervention could not be pooled because the outcomes were measured in different ways in the respective trials. Quantitative meta‐analysis was also deemed inappropriate in relation to failed ED manual reduction, or referral to orthopaedics for reduction, because one of the two trials reporting this outcome measure (Matthews 1995) reported no events in either comparator group.
Despite subgroup analyses, there was no identifiable relationship between a history of previous shoulder dislocations and the rate of successful reduction of the dislocated shoulder. However, subgroup comparisons should be interpreted with caution in this review because the number of participants in each study was small. We were unable to pool the data for subgroup comparisons because the studies did not report standard deviations and only one study specified whether the history of previous shoulder dislocations involved the shoulder of interest.
Although quasi‐randomized trials are associated with a greater risk of selection bias, the inclusion of one quasi‐randomized trial (Miller 2002) in this review did not significantly alter the findings.
Authors' conclusions
Implications for practice.
In the management of acute anterior shoulder dislocation, there may be no significant difference between IAL and IVAS with regard to immediate success rate of closed manual reduction. However, IAL may be associated with fewer adverse effects, a shorter ED stay and may be cheaper when compared with IVAS. These effect measures may be imprecise because of the small number of eligible studies and the limited methodological quality of the included studies in this review (Figure 2 and Figure 3).
Implications for research.
Like all systematic reviews, the conclusions of this review are limited by the quality of existing studies. Specifically, the findings of this review are limited by the relatively small number of eligible studies, the small sample sizes in the included studies, the use of empirical drug dosing in the IVAS arm of the included studies and the lack of evidence demonstrating accurate intra‐articular lignocaine injection in any of the included studies. Furthermore, with regard to drug dosing, the IVAS groups were heterogenous for two reasons. First, neither the sedative agent used nor the concurrent use of opiates was controlled across the studies. Second, differences in study design and differences between patients may have dictated different levels of sedation employed. Because the nature of the interventions were such that double‐blinding was not feasible (Figure 2 and Figure 3), foreknowledge of the intervention may have led to biased results. Larger randomized controlled trials are, therefore, required to assess the effect of IAL compared with IVAS regarding the primary outcome measure of this review, the immediate success rate of closed manual reduction of AASD. In addition, further studies should employ weight‐based drug doses and objectively confirm intra‐articular lignocaine injection. It is a major limitation of existing RCTs that they do not report long‐term follow up of participants for joint infection (septic arthritis) complicating IAL. Future clinical trials should address the risk of septic arthritis as an important outcome measure.
What's new
| Date | Event | Description |
|---|---|---|
| 4 January 2013 | Amended | Contact details updated |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 4, 2011
| Date | Event | Description |
|---|---|---|
| 23 July 2012 | Amended | Contact details updated. |
| 17 April 2012 | Amended | Contact details updated. |
| 18 July 2011 | Amended | Contact details updated. |
Acknowledgements
We would like to thank Dr Daniel E. Matthews for providing us with the individual patient data for one of the included studies. Thanks also to Dr R Shariat Moharari and Dr Rabindra Pradhan for responding to our queries about their respective studies. We are grateful to Andreas Lundh for kindly extracting data from a Danish study which was considered for inclusion in this review. We are also grateful to Dr Elaine Donnelly for helping with data abstraction for the included studies of this review.
We would also like to thank Jane Ballantyne and Mark Neuman (content editors), Marialena Trivella (statistical editor), Andrew Moore, John Burton, Reza Shariat Moharari (external peer reviewers) and Janet Wale and Durhane Wong‐Rieger (Cochrane Consumer Network) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. SilverPlatter MEDLINE (WebSPIRS)
| #1 | explode "Shoulder‐Dislocation" / all SUBHEADINGS in MIME,MJME |
| #2 | (shoulder or glenohumeral) near (dislocat* or displac* or wrench* or sprain* or subluxat* or abarticulat*) |
| #3 | (shoulder or glenohumeral) and (dislocat* or displac* or wrench* or sprain* or subluxat* or abarticulat*) |
| #4 | "Shoulder‐" / all SUBHEADINGS in MIME,MJME |
| #5 | shoulder near (trauma or injury) |
| #6 | shoulder and (trauma or injury) |
| #7 | explode Shoulder Joint / all subheadings |
| #8 | shoulder in TI, AB |
| #9 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 |
| #10 | explode Lidocaine/ all subheadings |
| #11 | lignocain* or lidocain* |
| #12 | explode "Injections‐Intra‐Articular" / all SUBHEADINGS in MIME,MJME |
| #13 | Intra?Articular and (injection* or administration*) |
| #14 | Intra?Articular near (injection* or administration*) |
| #15 | explode injections/ all subheadings |
| #16 | injection* |
| #17 | #10 or #11 or #12 or #13 or #14 or #15 or #16 |
| #18 | #9 and #17 |
| #19 | RANDOMIZED‐CONTROLLED‐TRIAL in PT |
| #20 | CONTROLLED‐CLINICAL‐TRIAL in PT |
| #21 | RANDOMIZED‐CONTROLLED‐TRIALS |
| #22 | RANDOM‐ALLOCATION |
| #23 | DOUBLE‐BLIND‐METHOD |
| #24 | SINGLE‐BLIND‐METHOD |
| #25 | #19 or #20 or #21 or #22 or #23 or #24 |
| #26 | (TG=ANIMALS) not ((TG=HUMAN) and (TG=ANIMALS)) |
| #27 | #25 not #26 |
| #28 | CLINICAL‐TRIAL in PT |
| #29 | explode CLINICAL‐TRIALS / all subheadings |
| #30 | (clin* near trial*) in TI |
| #31 | (clin* near trial*) in AB |
| #32 | (singl* or doubl* or trebl* or tripl*) near (blind* or mask*) |
| #33 | (#32 in TI) or (#32 in AB) |
| #34 | PLACEBOS |
| #35 | placebo* in TI |
| #36 | placebo* in AB |
| #37 | random* in TI |
| #38 | random* in AB |
| #39 | RESEARCH‐DESIGN |
| #40 | #28 or #29 or #30 or #31 or #33 or #34 or #35 or #36 or #37 or #38 or #39 |
Appendix 2. SilverPlatter EMBASE (WebSPIRS)
| #1 | explode "recurrent‐shoulder‐dislocation" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #2 | explode "shoulder‐dislocation" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #3 | (shoulder or glenohumeral) near (dislocat* or displac* or wrench* or sprain* or subluxat* or abarticulat*) |
| #4 | (shoulder or glenohumeral) and (dislocat* or displac* or wrench* or sprain* or subluxat* or abarticulat*) |
| #5 | explode Shoulder/ all subheadings |
| #6 | shoulder near (trauma or injury) |
| #7 | shoulder and (trauma or injury) |
| #8 | shoulder in TI, AB |
| #9 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 |
| #10 | explode Lidocaine/ all subheadings |
| #11 | lignocain* or lidocain* |
| #12 | "injection‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #13 | Intra?Articular and (injection* or administration*) |
| #14 | Intra?Articular near (injection* or administration*) |
| #15 | injection* |
| #16 | #10 or #11 or #12 or #13 or #14 or #15 |
| #17 | #9 and #16 |
| #18 | explode "randomized‐controlled‐trial" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #19 | (randomi?ed controlled trial*) in TI, AB |
| #20 | random* |
| #21 | explode "randomization‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #22 | randomi?ation |
| #23 | explode "clinical‐trial" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #24 | explode multicenter‐study / all subheadings |
| #25 | multi?cent* |
| #26 | explode phase‐4‐clinical‐trial / all subheadings or explode double‐blind‐procedure / all subheadings or explode single‐blind‐procedure / all subheadings |
| #27 | (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) in TI, AB, TW |
| #28 | ((SINGL* or DOUBL* or TREBL* or TRIPL*) near (BLIND* or MASK*)) in TI,AB |
| #29 | explode "follow‐up" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #30 | (follow?up near stud*) in TI, AB |
| #31 | evaluation stud* |
| #32 | explode "prospective‐study" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #33 | prospective?stud* |
| #34 | research near design* |
| #35 | explode "comparative‐study" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #36 | clinic* near trial* |
| #37 | #18 or #19 or #20 or #21 or #20 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 |
| #38 | (human) in DER |
| #39 | (animal or nonhuman) in DER |
| #40 | #38 and #39 |
| #41 | #39 not #40 |
| #42 | #37 not #41 |
| #43 | #17 and #42 |
Appendix 3. CENTRAL (The Cochrane Library)
| #1 | MeSH descriptor Lidocaine explode all trees |
| #2 | lignocaine or lidocain* |
| #3 | MeSH descriptor Injections, Intra‐Articular explode all trees |
| #4 | analg* or sedat* |
| #5 | MeSH descriptor Analgesia explode all trees |
| #6 | MeSH descriptor Conscious Sedation explode all trees |
| #7 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6) |
| #8 | MeSH descriptor Shoulder Joint explode all trees |
| #9 | shoulder* near trauma* |
| #10 | shoulder* and dislocat* |
| #11 | shoulder near dislocat* |
| #12 | MeSH descriptor Shoulder Dislocation explode all trees |
| #13 | glenohumeral near dislocat* |
| #14 | shoulder* or gleno?humeral* |
| #15 | (#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) |
| #16 | (#7 AND #15) |
Data and analyses
Comparison 1. Immediate success rate of reduction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Per cent of patients who underwent successful reduction | 5 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.10] |
Comparison 2. Pain during the procedure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain as rated on a verbal numerical rating scale | 3 | 109 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐1.41, 1.77] |
Comparison 3. Adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects associated with procedure | 5 | 210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.06, 0.43] |
Comparison 4. One reduction attempt.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Successful first reduction attempt | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.26] |
Comparison 5. Two reduction attempts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Succesful reduction at second attempt | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.40, 3.29] |
Comparison 6. Three reduction attempts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Successful reduction at third attempt | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 2.65 [0.16, 44.78] |
Comparison 7. Average time in the emergency department (ED).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean time spent in the ED | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐109.46 [‐134.32, ‐84.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kosnik 1999.
| Methods | Prospective randomized controlled trial. | |
| Participants | Patients with acute anterior shoulder dislocation. | |
| Interventions | The IAL group received 4 mg/kg (maximum 200 mg) of 1% lignocaine by intra‐articular injection. The IVAS group received an initial dose of 10 mg morphine sulphate (titrated to a maximum of 30 mg) and 5 mg diazepam (titrated to a maximum of 20 mg) intravenously. | |
| Outcomes | The primary outcome measure was a successful radiographic reduction. Other outcome measures were: ease of reduction and pain associated with the reduction manoeuvre. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients who consented to the study were assigned to groups by unblocked simple sample randomization technique that conceptually does not guarantee equal sample size”. |
| Allocation concealment (selection bias) | High risk | Published study does not provide any information in relation to allocation concealment. Comment: the nature of the interventions are such that allocation concealment is not feasible. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “non‐blind”. Comment: the nature of the interventions is such that blinding is not feasible. |
Matthews 1995.
| Methods | Prospective randomized controlled trial. | |
| Participants | Patients with acute anterior shoulder dislocation. | |
| Interventions | The IAL group received an intra‐articular injection of 20 ml of 1% lignocaine into the affected shoulder. The IVAS group received morphine sulphate 10 mg and midazolam 2 mg intravenously, respectively. | |
| Outcomes | Time of reduction manoeuvre, difficulty of reduction, subjective pain, complications, total time spent in the emergency department and cost. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Published study does not provide information regarding sequence generation. Quote: “A page was pulled at random from the protocol notebook in the emergency department. This page indicated into which of the two study groups the patient was to be placed (15 in the lidocaine group and 15 in the intravenous sedative group)”. |
| Allocation concealment (selection bias) | High risk | Published study does not provide information regarding allocation concealment. Comment: the nature of the interventions is such that allocation concealment is not feasible. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding. Comment: The nature of the interventions is such that blinding is not feasible. |
Miller 2002.
| Methods | Prospective randomized controlled trial. | |
| Participants | Patients aged 18 to 70 years with an acute anterior shoulder dislocation. | |
| Interventions | The IAL group received an intraarticular injection of 20 ml of 1% lignocaine into the affected shoulder. The IVAS group received an 2 mg midazolam and 100 µg fentanyl intravenously, respectively. | |
| Outcomes | Rate of successful reduction, pain as rated on a visual analogue scale, time required for the reduction, time from reduction until discharge from the emergency department and cost. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “those with a odd medical record number received a local intra‐articular injection of lidocaine whereas those with an even medical record number received intravenous sedation”. |
| Allocation concealment (selection bias) | High risk | Published study does not provide information regarding allocation concealment. Comment: the nature of the interventions is such that allocation concealment is not feasible. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Published study does not provide information regarding allocation concealment. Comment: the nature of the interventions is such that blinding is not feasible. |
Moharari 2008.
| Methods | Prospective randomized controlled trial. | |
| Participants | Patients between the ages of 18 and 80 years with anterior shoulder dislocation. | |
| Interventions | The IAL group received an intra‐articular injection of 20 ml of 1% lignocaine into the affected shoulder. The IVAS group, intravenous meperidine 25 mg and diazepam 5mg over 1 to 2 minutes. | |
| Outcomes | Outcome measures were: pain before injection, pain before joint reduction, pain after joint reduction, number of reduction attempts, duration of emergency department stay, duration of reduction and complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly assigned into two groups using a computer random number generator”. |
| Allocation concealment (selection bias) | High risk | Published study does not provide any information regarding allocation concealment. Comment: the nature of the interventions is such that allocation concealment is not feasible. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “non‐blind”. Comment: the nature of the interventions is such that blinding is not feasible. |
Orlinsky 2002.
| Methods | Prospective randomized controlled trial. | |
| Participants | Patients between the ages of 18 and 80 years with anterior shoulder dislocation. | |
| Interventions | The IAL group received an intra‐articular injection of 20 ml of 1% lignocaine into the affected shoulder. The IVAS group sequentially received 1 to 2 mg/kg of meperidine and 5 to 10 mg of diazepam intravenously over 1 to 2 minutes. | |
| Outcomes | Successful reduction was confirmed radiographically. Outcome measures were: recovery time, change in pain score from baseline to after anaesthesia but before reduction, change in pain score from after anaesthesia but prior to reduction to time of discharge, overall change in pain score from baseline to time of discharge, patient perceived inadequate analgesia, pain interference with procedure, insufficient relaxation interfering with procedure and number of patients with adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Published study does not provide information regarding sequence generation. Quote: “Once a patient met the inclusion criteria, the physician pulled a pre‐numbered sealed envelope containing the written informed consent form and the protocol to which the patient was randomized, either the IAL or IVMD analgesia protocol”. |
| Allocation concealment (selection bias) | High risk | Published study does not provide any information regarding allocation concealment. Comment: the nature of the interventions is such that allocation concealment is not feasible. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “Non‐blinded”. Comment: the nature of the interventions is such that blinding is not feasible. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Paudel 2004 | The participants were not randomized. |
| Pradhan 2006 | The participants were not randomized. |
| Suder 1995a | The type of shoulder dislocation of the participants was unclear and it was not possible to obtain individual patient data from the trialists. |
| Suder 1995b | The definitions of the outcome measures were unclear and it was not possible to obtain clarification from the trialists. |
Contributions of authors
Conceiving the review: Abel Wakai (AW)
Co‐ordinating the review: AW
Undertaking manual searches: AW and Ronan O'Sullivan (ROS)
Screening search results: AW, ROS and Aileen McCabe (AM)
Organizing retrieval of papers: AW
Screening retrieved papers against inclusion criteria: AW and ROS
Appraising quality of papers: AW and AM
Abstracting data from papers: AW, ROS, AM and Elaine Donnelly (ED)
Writing to authors of papers for additional information: AW
Providing additional data about papers: AW and ROS
Obtaining and screening data on unpublished studies: AW and ROS
Data management for the review: AW
Entering data into Review Manager (RevMan 5.0): AW, ROS and AM
RevMan statistical data: AW and ROS
Other statistical analysis not using RevMan: AW
Double entry of data: (data entered by person one: AW; data entered by person two: ROS)
Interpretation of data: AW and ROS
Statistical inferences: AW and ROS
Writing the review: AW and ROS
Securing funding for the review: AW
Performing previous work that was the foundation of the present study: AW
Guarantor for the review (one author): AW
Person responsible for reading and checking review before submission: AW
Sources of support
Internal sources
No sources of support supplied
External sources
Health Research Board, Ireland.
R&D Office, Belfast, Northern Ireland, UK.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Kosnik 1999 {published data only}
- Kosnik J, Shamsa F, Raphael E, Huang R, Malachias Z, Georgiadis GM. Anesthetic methods for reduction of acute shoulder dislocations: a prospective randomized study comparing intraarticular lidocaine with intravenous analgesia and sedation. American Journal of Emergency Medicine 1999;17:566‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matthews 1995 {published data only}
- Matthews DE, Roberts T. Intraarticular lidocaine versus intravenous analgesic for reduction of acute anterior shoulder dislocations. A prospective randomized study. American Journal of Sports Medicine 1995;23:54‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 2002 {published data only}
- Miller SL, Cleeman E, Auerbach J, Flatow EL. Comparison of intra‐articular lidocaine and intravenous sedation for reduction of shoulder dislocations: a randomized prospective study. The Journal of Bone and Joint Surgery. American volume 2002;84‐A:2135‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moharari 2008 {published data only}
- Moharari RS, Khademhosseini P, Espandar R, Asl Soleymani H, Talebian MT, Khashayar P, et al. Intra‐articular lidocaine versus intravenous meperidine/diazepam in anterior shoulder dislocation: a randomised clinical trial. Emergency Medicine Journal 2008;25:262‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orlinsky 2002 {published data only}
- Orlinsky M, Shon S, Chiang C, Chan L, Carter P. Comparative study of intra‐articular lidocaine and intravenous meperidine/diazepam for shoulder dislocations. Journal of Emergency Medicine 2002;22:241‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Paudel 2004 {published data only}
- Paudel K, Pradhan RL, Rijal KP. Reduction of acute anterior shoulder dislocations under local anaesthesia ‐ a prospective study. Kathmandu University Medical Journal 2004;2:13‐7. [PubMed] [Google Scholar]
Pradhan 2006 {published data only}
- Pradhan RL, Lakhey S, Pandey BK, Rijal KP. Reduction of acute anterior shoulder dislocations: comparing intraarticular lignocaine with intravenous analgesia. Journal of the Nepal Medical Association 2006;45:223‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Suder 1995a {published data only}
- Suder PA, Mikkelsen JB, Hougaard K, Jensen PE. Reduction of traumatic secondary dislocations with lidocaine. Archives of Orthopaedic and Trauma Surgery 1995;114:233‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Suder 1995b {published data only}
- Suder PA, Mikkelsen JB, Hougaard K, Jensen PE. Reduction of traumatic primary anterior shoulder dislocation under local analgesia [Reponering af traumatisk primaer anterior skulderluksation i lokal analgesi]. Ugeskrift‐for‐laeger 1995;157:3625‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG on behalf of the Cochrane Statistical Group. Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasziou 2001
- Glasziou P, Irwig L, Bain C, Colditz G. Systematic reviews in healthcare: a practical guide. 1st Edition. Cambridge: Cambridge University Press, 2001. [Google Scholar]
Graham Apley 1988
- Graham Apley A, Solomon L. Injuries to joints. In: Graham Apley A, Solomon L editor(s). Concise system of orthopaedics and fractures. 1st Edition. Oxford: Butterworth Heinemann, 1988:247‐9. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. [Google Scholar]
Hovelius 1982
- Hovelius L. Incidence of shoulder dislocation in Sweden. Clinical Orthopaedics 1982;166:127‐31. [MEDLINE: ] [PubMed] [Google Scholar]
Kothari 1992
- Kothari RU, Dronen SC. Prospective evaluation of the scapular manipulation technique in reducing anterior shoulder dislocations. Annals of Emergency Medicine 1992;21:1349‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lau 1998
- Lau J, Ioannidis JPA, Schmid CH. Quantitative synthesis in systematic reviews. In: Mulrow C, Cook D editor(s). Systematic reviews: synthesis of best evidence for healthcare decisions. 1st Edition. Philadelphia: American College of Physicians, 1998:91‐101. [Google Scholar]
Lefebvre 1996
- Lefebvre C, McDonald S. Development of a sensitive strategy for reports of randomized controlled trial in EMBASE. Paper presented at the 4th International Cochrane Colloquium. Adelaide, Australia, 1996.
Lippitt 1991
- Lippitt EB, Kennedy JP, Thompson TR. Intra‐articular lidocaine versus intravenous analgesia in the reduction of dislocated shoulders. Orthopedic Transactions 1991;15:804. [Google Scholar]
McNamara 1998
- McNamara R. Management of common dislocations. In: Roberts JR, Hedges JR editor(s). Clinical Procedures in Emergency Medicine. 3rd Edition. Philadelphia: WB Saunders, 1998:818‐52. [Google Scholar]
Norlin 1993
- Norlin R. Intraarticular pathology in acute, first‐time anterior shoulder dislocation: an arthroscopic study. Arthroscopy 1993;9:546‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.0 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rockwood 1996
- Rockwood JC, Ma W. Subluxations and dislocations about the glenohumeral joint. In: Rockwood J editor(s). Rockwood and Green's fractures in adults. Philadelphia: Lippincott‐Raven, 1996:1202‐11. [Google Scholar]


