Abstract
Oncolytic viral immunotherapy is an emerging treatment modality for cancer that exploits in vivo replication and other viral properties to enhance immune killing of malignant cells. The potential for horizontal transmission of native or engineered oncolytic viruses creates several unique infection control challenges. In 2015, talimogene laherparepvec (TVEC) became the first agent in this class to gain FDA approval for treatment of melanoma, and several others are being developed. Although some data on the transmissibility of TVEC are available from clinical studies, the aftermarket or real-world experience remains limited. We conducted a PUBMED-based search of the medical literature focusing on the safety and risk of TVEC transmission to close contacts including healthcare workers. The findings are summarized in this review and are intended to provide infection preventionists with practical guidance on handling issues related to administration and care of patients receiving TVEC. Additionally, we describe the current mechanism for evaluating the risk related to similar new agents entering clinical trials at our institution. Development of standarized approaches for the safe administration and precautions for ongoing care, especially in immunocompromised patients, are essential to support the broad adoption of this novel therapy.
Use of live viruses in the treatment of cancer
The use of oncolytic viral immunotherapy is an emerging modality for cancer treatment. Certain viruses have innate tropism for cancer cells or can be genetically engineered to infect and, subsequently, enhance the recognition of tumor cells by the host immune system. This immune mediated tumor destruction is achieved via the induction of virus-specific antigens on tumor cells or via the increased expression of existing antigens.1 A second and direct mechanism for a viral anticancer effect involves virus-induced cytolytic killing of tumor cells, called oncolysis. Use of oncolytic viruses in combination with other immunotherapies, such as immune checkpoint inhibitors (ICIs), has catapulted this form of treatment to the forefront of novel cancer therapeutics.2,3 Although these advances hold immense promise, extension of infectious agents from the laboratory to the bedside invokes numerous infection prevention and control issues related to the risk of horizontal transmission of oncolytic viruses to other patients as well as the safety of healthcare workers (HCWs). These challenges mirror issues already familiar to infection preventionists, such as the use of live virus vaccines (eg, measles, mumps, and rubella—MMR) in cancer patients and other immunosuppressed populations or their household contacts.
Although the concept of viral immunotherapy dates back to the 1950s, talimogene laherparepvec (TVEC), commercially known as IMLGYIC (AmGen, Newbury Park, CA), recently became the first oncolytic viral agent approved by the FDA in the United States for the treatment of unresectable melanoma.4 Since then, a variety of viral vectors have entered clinical investigation for a broad range of indications.2,5,6 The re-emergence of oncolytic viruses offers a unique opportunity for infection preventionists to contribute to safe delivery of effective therapies and to further the overarching clinical and research mission of improving cancer-related survival. As these agents become ubiquitous in clinical environments, guidelines to ensure safe administration are essential.
In this review, we describe the existing data related to transmission of the first FDA-approved oncolytic viral agent, TVEC, and we draw attention to practical issues involved in its administration to patients and their ongoing care. Additionally, we describe our systematic approach to evaluating oncolytic viral clinical trials from an infection prevention perspective at Memorial Sloan Kettering Cancer Center.
Talimogene laherparepvec (TVEC)
A watershed moment for oncolytic viral vector therapy occurred in 2015 with the FDA approval of TVEC, a genetically engineered herpes simplex virus (HSV-1). Derived from a wild-type strain of HSV-1 (JS—1), TVEC was originally isolated from cold sore lesions and is indicated for the treatment of unresectable metastatic melanoma in Europe and the United States through direct injection of visible and/or palpable tumors.7,8 Gene deletions engineered in TVEC block antigen presentation and eliminate neurovirulence to attenuate any off-target effects. TVEC is also modified to selectively proliferate within cancer cells and to reduce infectivity in noncancer cells.9,10 An important feature of the modified virus is inclusion of a GM-CSF encoding gene to evoke systemic antitumor effects and durable immune response beyond the site of injection.11
Despite these genetic modifications, the viral thymidine kinase (TK) gene is unchanged, preserving susceptibility to a common antiviral medication, acyclovir.12,13 Viral detection by commercially available assays also remains unperturbed due to preservation of target gene regions for these assays in TVEC. For patients with suspected infection after TVEC, commercially available polymerase chain reaction (PCR) testing for cutaneous lesions, cerebrospinal fluid, and blood may be used for viral detection, but the distinction from the wild-type virus requires specialized testing available only through the drug manufacturer.
Safety profile, viral shedding, and risk of local and disseminated infection in TVEC recipients
The foremost concern with oncolytic viral agents is the risk of uncontrolled replication in vivo and possible transmission to close contacts, other patients, and HCWs. With TVEC, initial concerns centered on the risk of developing disseminated herpes infection, including from reversion to wild-type HSV and manifesting as oral and cutaneous herpes, herpetic keratitis, herpetic whitlow, and disseminated herpes.12,14-16
The safety of TVEC is now reported in several primary clinical trials and expanded access outcomes trials (Table 1). Overall, adverse events related to the administration of TVEC are reported to be minor and local. In phase 1 studies primarily evaluating the local administration of TVEC, the duration and intensity of local inflammatory reactions were more pronounced among HSV sero-negative patients.14 Peak viral recovery from blood (n = 17, 85% ) and urine (n = 4, 20%) occurred on the day of treatment and was notably absent from injection site vesicular lesions in this single study.17 Phase 2 studies confirmed that HSV antibody negative patients seroconverted after treatment with TVEC.18 No established cases of disseminated HSV have been reported in any patients included in the pivotal clinical trials, although FDA-mandated postmarketing evaluations are ongoing (Table 1).19,20
Table 1.
Summary Viral Shedding and Safety Data Reported From Trials Involving Talimogene Laherparepvec (TVEC) as Mono- or Combination Therapy
| Trial Agent(s) (Evaluable Subjects) |
Tumor Type | Body Sites Evaluated for Shedding |
Assay Type | Key Findings (Notes) |
|---|---|---|---|---|
| TVEC (n = 60) | Melanoma | Blood Urine Exterior of dressing |
qPCR | Any postprocedure collection positive for TVEC DNA on cycles 1–3 of treatment: Blood: 98% (all but 1 patient cleared by end of cycle 3) Urine: 31.7% (100% cleared) Outside of occlusive dressing: 80%. Surface of injected lesions: 11.7% |
| TVEC and ipilimumab26 | Melanoma | N/A | N/A | Adverse events in phase 2 TVEC +ipilimumab vs ipilimumab alone: Influenza like illness: 26 (27%) vs 1 (1%) Oral herpes: 5 (5%) vs 0 (0%) [TVEC vs wild-type distinction not made] Injection site inflammation: 1 (1%) vs 0 ALT elevation: 7 (7%) vs 4 (4%). AST elevation: 9 (10%) vs 5 (5%) Erythematous rash: 3 (3%) vs 1 (1%) Maculo-papular rash: 6 (6%) vs 2 (2 %) |
| TVEC (n = 17) | Pancreatic | Blood Urine |
qPCR | Detectable TVEC DNA (duration not specified) Blood: 5 (29%) Urine: 7 (41%) |
| TVEC and pembrolizumab | SCC (head/neck) | Presumed herpetic lesions | qPCR | [Pending anticipated completion in 2020] |
| TVEC vs GM-CSF (292 TVEC, 127 GM-CSF) | Melanoma | N/A | N/A | 16 (5.5%) patients in TVEC arm had HSV-related adverse events compared to 2 (1.6%) in the GM-CSF (control) arm. TVEC related HSV infections included oral herpes (n = 15) and herpetic keratitis (n = 1) 7 (2.4%) of TVEC arm developed cellulitis > grade 3 |
| OncoVEX – precursor to TVEC (HSV + GM-CSF)14 (n = 17) | Multiple | Blood Urine Dressing, injection site New lesions |
qPCR Plaque Assay |
Virus detected in blood within 8 h (n = 9) and up to 1 week (n = 1)Low level virus detected at tumor surface up to 2 weeks (n = 3) |
| TVEC18 (n = 50) | Melanoma | Injection site swab Urine |
Plaque Assay |
1 superficial swab was positive after second TVEC injection All urine collected 1–48 h after injection were negative. |
Note. ALT, alanine aminotransferase; AST, aspartate transaminase; HSV, herpes simple virus; N/A, not available; qPCR, quantitative polymerase chain reaction; SCC, squamous cell carcinoma.
Since its approval in 2015, more than 300 cases of adverse events involving TVEC have been reported and registered in the FDA Adverse Event Reporting System (FAERS) public dashboard.21 Of these 333 cases, 121 are categorized as serious cases, including 21 deaths, but none are specified as disseminated HSV infection. A single FDA warning related to TVEC and noting concern for disseminated HSV infection was issued in 2016, but outcomes from the investigation prompted by this warning have not yet been publicly reported. An FDA-mandated postmarketing study to characterize the long-term risk of herpetic infection in TVEC-treated melanoma patients, care givers, and HCWs started in August 2017. Completion of enrollment and evaluation of this observational cohort of nearly 1,000 patients in the United States and Europe is anticipated in late 2024.
Transmission to HCWs
Limited data are available on the transmission of HSV viral infections to HCWs involved in TVEC administration or subsequent care of patients. A self-reported survey from 82 HCWs across 36 study sites with 4,100 treatment visits, reported 5 occurrences of accidental exposure to TVEC by needlestick injury or mucosal splash. The most notable among these was an HCW who developed herpetic whitlow that resolved after acyclovir treatment. Also, 2 other treatment-related exposures included an accidental needlestick during drug preparation (treated with antiviral agent) and conjunctival splash without any reported clinical consequence. None of the cases in HCWs resulted in secondary transmission. No cases of secondary or tertiary transmission from patient to HCW have been reported. Postexposure prophylaxis with acyclovir is recommended in case of accidental exposure of HCWs to TVEC, and serious disease has not been described among those exposed.
Transmission from TVEC recipient to household contacts
As part of the viral surveillance program for one of the pivotal trials, 1,217 surveillance questionnaires from 177 subjects identified 15 individuals (8.4%) who reported signs and symptoms possibly related to TVEC treatment among their close contacts.12,19 Suspected herpetic lesions among household contacts were not completely characterized as wild type versus secondary to TVEC transmission, although none of these reported events were severe.20 The incidence of this potential risk to close household contacts is being addressed in an ongoing postmarketing trial.12
Practical concerns: Transmission from TVEC recipient to immunocompromised contacts, including HCWs
No documented transmission of HSV infection from TVEC-treated patients to other immunocompromised contacts has been reported, although studies on viral shedding indicate that there is a nonzero risk of this occurring. Pregnant patients and HCWs or those with immunocompromising conditions are precluded from receipt or direct administration of the agent and are advised caution during direct patient care due to theoretical risk of transmission of TVEC across the placenta. Pregnant HCWs are instructed not to perform dressing changes or provide direct care to patients through the duration of shedding. This recommendation is extrapolated from adverse events associated with transplacental HSV infection rather than TVEC-specific data.17
TVEC: Summary and recommendations for infection prevention
At MSK, TVEC administration guidelines were developed by a multidisciplinary team of key stakeholders from infection control, nursing, oncology, and pharmacy departments after evaluating available clinical and viral shedding data from early phase trials. These data indicated that most detectable virus from evaluated sites waned by 7 days postexposure.14,22 TVEC is classified as a BSL2-level agent at MSK, in accordance with FDA guidance, and this dictates preparation and environmental management for TVEC. Agent preparation by trained pharmacy personnel occurs in a negative-pressure room biosafety cabinent (BSC) using a closed transfer system. Post preparation, the BSC is cleaned with a 2-step process including high-level disinfectant and sterile alcohol and remains unavailable for other preparations pending recommended contact time. Personal protective equipment is required for preparation and administration of the agent in agreement with BSL2 recommendations. Practically, this results in the use of gowns, gloves and eye shields for TVEC preparation and administration, which complies with the manufacturer’s prescribing information.23 Post injection, the treated site is covered with an occlusive dressing with a red alert sticker to indicate to staff that TVEC was administered. Electronic practice alerts and learning modules were developed to educate staff. Additionally, a dedicated contact isolation precaution indicator is placed in the patient’s electronic medical record, which serves to alert staff to recent treatment with TVEC and the need for contact precautions.22 Entry and removal of the indicator is undertaken by the clinical support teams. Precautions are instituted until all lesions are healed or scabbed as determined by direct observation at follow-up or day 7 following injection of TVEC, whichever is longer.
Other investigational oncolytic viruses
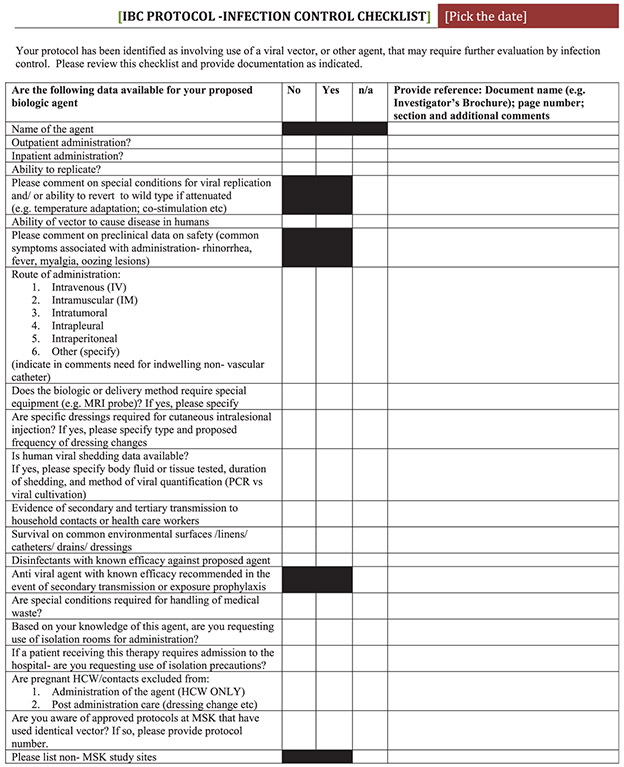
Since TVEC approval, >15 clinical protocols and 9 unique viral oncolytic agents have been evaluated for transmission-based precautions. The current process for trial evaluation evolved out of the TVEC experience and is conducted under the auspices of the Institutional Biosafety Committee (IBC), which is tasked with reviewing clinical and laboratory research protocols involving infectious agents and recombinant or synthetic DNA in accordance with National Institutes of Health (NIH) guidelines. The 3 phases of our process include (1) preapproval review, (2) operationalization, and (3) post implementation protocol audit and review (Fig. 1). Studies proposing research that includes oncolytic viral vectors, or other infectious agents, are identified in the protocol registration system and investigators are required to complete the embedded IC checklist (Fig. 2), which focuses on key IC issues such as infectivity, transmissibility, and environmental disinfection. Information reported on the checklist is reviewed and corroborated to determine infection control recommendations in partnership with the clinical study team. Finally, compliance with infection control recommendations is audited at a prespecified interval following study approval.
Fig. 1.
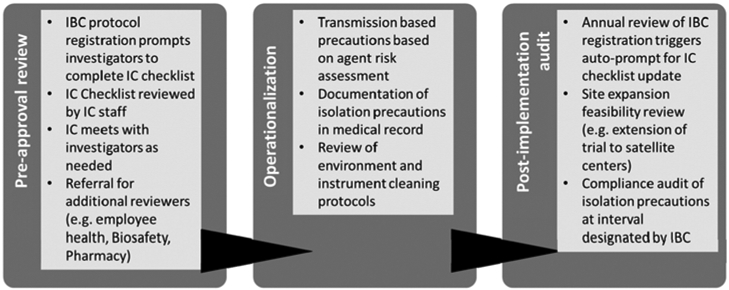
Infection control workflow for review of clinical trials involving potentially infectious agents.
IBC: Institutional Biosafety Committee; IC: Infection Control
Fig. 2.
Infection control checklist.
HCW: health care worker MRI: Magnetic Resonance Imagining MSK: Memorial Sloan Kettering PCR: polymerase chain reaction
Discussion
The infection control approach to isolation and management of patients involved in oncolytic viral vector trials is nothing more than a refinement of everyday thinking. Even though most agents used are attenuated or conditionally replicative, the risk of transmission and its implications are not completely understood. Current challenges in developing effective guidelines for investigational agents include limited knowledge of influencing factors such as the in vivo pathogenic potential of engineered viruses, duration of viral shedding, infectivity, unpredictable control over replication competent viruses with concomitantly administered immunomodulators, and finally, the potential for regaining replication competence or wild-type reversion of engineered oncolytic viral agents. The principles articulated here are also applicable to other nonvirologic biologic antitumor therapies, such as Clostridium novyi and Listeria monocytogenes, with specific attention to individual patient risks and appropriate instiutional review board oversight.
For FDA-approved agents, the infection control community must recognize the emergence of viral immunotherapy in mainstream oncologic care and its positive impact on patient survival to develop standardized guidelines that can be broadly adopted to overcome implementation challenges across a variety of settings (eg, inpatient vs outpatient or treatment in the context of a clinical trial vs nontrial setting). Protocols established for these agents will guide institutional practices for use of oncolytic viral vectors currently in development.
The approach of embedding the role of infection preventionists within existing research regulatory structure (eg, IBC or IRB) enhances adherence to IC recommendations. Through our rigorous process of preapproval agent review, operational planning, and postapproval audit and feedback, we have been able to achieve responsible conduct of research and safe implementation of various oncolytic viral vector trials. Recent modifications to NIH oversight of human recombinant gene therapy trials, including the transfer responsibility to institutional biosafety committees, further highlights the importance of infection control oversight of oncolytic viral vector trials.24,25
Acknowledgments
Financial support. This infection control research was supported by the MSKCC Core Grant (grant no. P30 CA008748).
Footnotes
Conflicts of interest. Dr Glickman is a consultant for Vedanta Biosciences. All other authors report no conflicts of interest relevant to this article.
References
- 1.Kobayashi H Viral xenogenization of intact tumor cells. Adv Cancer Res 1979;30:279–299. [DOI] [PubMed] [Google Scholar]
- 2.Desjardins A, Gromeier M, Herndon JE 2nd , et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med 2018;379:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DB, Puzanov I, Kelley MC. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy 2015;7:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheridan C First oncolytic virus edges towards approval in surprise vote. Nat Biotechnol 2015;33:569–570. [DOI] [PubMed] [Google Scholar]
- 5.Puzanov I, Milhem MM, Minor D, et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol 2016;34:2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 2017;170:1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington KJ, Puzanov I, Hecht JR, et al. Clinical development of talimogene laherparepvec (T-VEC): a modified herpes simplex virus type-1–derived oncolytic immunotherapy. Expert Rev Anticancer Ther 2015;15:1389–1403. [DOI] [PubMed] [Google Scholar]
- 8.Kohlhapp FJ, Kaufman HL. Molecular pathways: mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res 2016;22:1048–1054. [DOI] [PubMed] [Google Scholar]
- 9.Conry RM, Westbrook B, McKee S, Norwood TG. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum Vaccin Immunother 2018;14:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams BR. PKR: a sentinel kinase for cellular stress. Oncogene 1999;18:6112–6120. [DOI] [PubMed] [Google Scholar]
- 11.Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and antitumour properties. Gene Ther 2003;10:292–303. [DOI] [PubMed] [Google Scholar]
- 12.Summary basis for regulatory action. US Food and Drug Administration; website. https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM473103.pdf. Published 2015. Accessed June 30, 2018. [Google Scholar]
- 13.Kaufman HL, Bines SD. OPTIM trial: a phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol 2010;6:941–949. [DOI] [PubMed] [Google Scholar]
- 14.Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 2006;12:6737–6747. [DOI] [PubMed] [Google Scholar]
- 15.MacKie RM, Stewart B, Brown SM. Intralesional injection of herpes simplex virus 1716 in metastatic melanoma. Lancet 2001;357:525–526. [DOI] [PubMed] [Google Scholar]
- 16.Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5(−)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther 2002;9:398–406. [DOI] [PubMed] [Google Scholar]
- 17.Gangi A, Zager JS. The safety of talimogene laherparepvec for the treatment of advanced melanoma. Expert Opin Drug Saf 2017;16:265–269. [DOI] [PubMed] [Google Scholar]
- 18.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 2009;27:5763–5771. [DOI] [PubMed] [Google Scholar]
- 19.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015;33:2780–2788. [DOI] [PubMed] [Google Scholar]
- 20.Chesney J, Awasthi S, Curti B, et al. Phase IIIb safety results from an expanded-access protocol of talimogene laherparepvec for patients with unresected, stage IIIB-IVM1c melanoma. Melanoma Res 2018;28:44–51. [DOI] [PubMed] [Google Scholar]
- 21.Federal adverse events reporting system. US Food and Drug Administration; website. https://fis.fda.gov/sense/app/777e9f4d-0cf8-448e-8068-f564c31baa25/sheet/45beeb74-30ab-46be-8267-5756582633b4/state/analysis. Accessed May 11, 2018. [Google Scholar]
- 22.Wall LM, Baldwin-Medsker A. Safe and effective standards of care: supporting the administration of T-VEC for patients with advanced melanoma in the outpatient oncology setting. Clin J Oncol Nurs 2017;21:E260–E266. [DOI] [PubMed] [Google Scholar]
- 23.IMLYGIC (talimogene laherparepvec) prescribing information. Amgen; website. https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/imlygic/imlygic_pi.pdf. Published 2017. Accessed November 11, 2018. [Google Scholar]
- 24.National Institutes of Health (NIH) Office of Science Policy (OSP) Recombinant or Synthetic Nucleic Acid Research. Proposed changes to the NIH guidelines for research involving recombinant or synthetic nucleic acid molecules. NIH; website. https://osp.od.nih.gov/wp-content/uploads/NIH_Guidelines.html. Published 2018. Accessed December 27, 2018. [Google Scholar]
- 25.Collins FS, Gottlieb S. The next phase of human gene-therapy oversight. N Engl J Med 2018;379:1393–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol 2018;36:1658–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]