Abstract
Background:
Thrombotic thrombocytopenic purpura (TTP) is a rare, potentially fatal hematologic disorder characterized by thrombocytopenia, microangiopathic hemolytic anemia, and ischemic organ impairment. The PLASMIC and French TTP scores facilitate rapid recognition of TTP and guide clinical decisions when confirmatory ADAMTS13 testing is not available. However, age may impact risk stratification of these tools as older individuals present with less severe cytopenias and higher rates of renal impairment.
Objective:
To investigate the impact of age on the diagnostic utility of the PLASMIC and French scores.
Methods:
We calculated the sensitivity and specificity of PLASMIC ≥5 and French score ≥1 in detecting TTP among patients with a thrombotic microangiopathy (TMA) for different ages.
Results:
Among 81 patients with TTP and 76 with another TMA, diagnostic utility of the PLASMIC score decreased with age, with sensitivities of 91.4%, 78.3%, and 76.9% and specificities of 60.0%, 33.3%, and 50% for ages 18–39, 40–59, and ≥60 years old, respectively. Similarly, for the French score, sensitivities were 100.0%, 96.2%, and 76.9% and specificities were 64.0%, 35.3%, and 40.0% for those age groups. Patients ≥60 years old had significantly higher platelet counts (35.1±8.6 vs 19.9±2.8 ×109/L, p=0.031) and lower PLASMIC scores (5.15±1.34 vs 5.97±0.18, p=0.031) compared to the youngest age group.
Conclusion:
The PLASMIC and French TTP scores have lower sensitivities and specificities at ages ≥60 years old, and may be less reliable in identifying TTP in older patients. A high index of suspicion and availability of rapid ADAMTS13 assays is required to correctly diagnose all patients with TTP, especially the elderly.
Keywords: TTP, TMA, PLASMIC, French score, elderly, geriatric, age
INTRODUCTION
Immune thrombotic thrombocytopenic purpura (TTP) is a rare, potentially fatal hematologic disorder caused by an acquired deficiency of ADAMTS13, a von Willebrand factor cleaving protease and is characterized by profound peripheral thrombocytopenia, microangiopathic hemolytic anemia, and ischemic organ impairment.1 Untreated TTP is rapidly fatal; however, prompt treatment with plasma exchange and immunosuppression leads to survival in most patients.2,3 Thus, TTP is a medical emergency and rapid diagnosis and prompt treatment is critical.4 Reduced ADAMTS activity ≤ 5%-10% can confirm a de novo diagnosis of TTP;1,5 however, this is a send-out test at most institutions and results may not be available for several days.6–9 In this setting, several clinical screening tools have been developed to guide clinical decisions and rapid recognition of TTP when ADAMTS13 tests are not immediately available. Of these, the PLASMIC and French TTP scores are the best known and most widely applied.10 The PLASMIC score, which incorporates platelet count, serum creatinine, the presence of hemolysis, history of cancer or organ transplantation, mean corpuscular volume, and INR, has been extensively validated in the literature.11–14 The French Score, which uses platelet count and serum creatinine, is an alternative score used to diagnose TTP. Patients that fall into a “high-risk” category (PLASMIC ≥ 6 or French score ≥ 2) are considered high probability for TTP.15
The median age at diagnosis of acute TTP is 40 years.5,15–19 However, with an ageing population,20 there are increasing numbers of older individuals with hematologic disorders such as TTP.21 Data regarding the presentation and outcomes of TTP in older patients is limited, though a few studies have shown poorer outcomes. In those studies, older patients (≥ 60 years) comprised 15.3 – 37.6% of the cohort.22–26 Most recently, Prevel et al. presented an analysis from the French Thrombotic Microangiopathies Registry showing significantly higher mortality among older (≥ 60 years) patients with TTP and presenting some evidence that delays in diagnosis may be contributing to these poorer outcomes.23 They attributed this to more non-specific presenting symptoms, higher rates of organ impairment, and less severe cytopenias in older patients with TTP.23 In this setting, it is even more critical to promptly recognize TTP in the elderly. Both the PLASMIC and French scores rely heavily on profound thrombocytopenia with a platelet count <30 × 109/L and preserved serum creatinine (<2 mg/dL and <2.26 mg/dL, respectively),11,15 which raises concerns about their sensitivity in older patients who may present with higher rates of renal impairment and less severe thrombocytopenia.
Currently, there are no studies investigating the sensitivity and specificity of diagnostic tools like the PLASMIC and French scores based on age. We performed this retrospective analysis to examine and compare the sensitivity and specificity of these diagnostic tools in the elderly (age ≥ 60 years) compared with younger patients.
METHODS
Study cohort
The study cohort included adult patients (age ≥ 18 years) evaluated for a thrombotic microangiopathy (TMA) at Johns Hopkins Hospital from 1995 to 2018. Patients prior to 2014 were identified from plasma exchange records from the therapeutic apheresis service and blood bank, while patients from 2014 to 2018 were prospectively enrolled in the Johns Hopkins Thrombotic Microangiopathy Registry,27 and provided informed consent for the collection of longitudinal clinical data and biological specimens. We included only the first episode of TMA (TTP or other TMA) in the analysis since patients presenting with a second or subsequent episode may pose less of a diagnostic challenge since they are often reasonably presumed to have a recurrence of their original TMA diagnosis especially TTP and atypical HUS since these are known to have a relapsing course.
A TMA was diagnosed in the presence of thrombocytopenia (platelet count < 150 × 109/L) and microangiopathic hemolytic anemia (hemoglobin level <10 g/dL and the presence of schistocytes recorded). Patients were categorized as having TTP or other TMA. The diagnosis of TTP was based on documented ADAMTS13 activity ≤10% for all samples collected after 2006 (when the ADAMTS13 FRETS assay was first offered at Johns Hopkins Hospital). For patients presenting prior to 2006, TTP was diagnosed based on the presence of a TMA with a clinical course consistent with TTP (response to plasma exchange therapy), and the absence of alternative thrombotic microangiopathies such as atypical hemolytic uremic syndrome or transplant-associated microangiopathy. A subset of patients who had their initial episode prior to 2006 had TTP relapses during which ADAMTS13 deficiency was documented (21 of 54 patients entering the cohort prior to 2006 had relapses, of which 8 had documented ADAMTS13 testing showing activity ≤10%). All patients not meeting criteria for TTP were classified as other TMA. This included patients with atypical hemolytic uremic syndrome (aHUS), catastrophic antiphospholipid syndrome (CAPS), cancer-related TMA, Shiga-toxin associated HUS, drug-induced TMA, scleroderma renal crisis, pregnancy-related aHUS, post-bone marrow transplant TMA, and TMA not otherwise specified (NOS). We excluded patients with a prior episode of TMA since a recurrent episode of TTP is commonly (and appropriately) considered to be a relapse unless proven otherwise, and thus the index of suspicion and pre-test probability is considerably higher than for a de-novo episode. The institutional review board at the Johns Hopkins University approved this study.
Data collection and management
We extracted data from the electronic medical record including patient demographics, and details of TMA presentation, diagnosis, treatment, and outcomes. We specifically collected data components contributing to the PLASMIC score (platelet count < 30 × 109/L, hemolysis parameters [reticulocyte count > 2.5%, undetectable haptoglobin, or indirect bilirubin > 2 mg/dL], cancer status, solid-organ or stem cell transplant status, MCV < 90 fL, INR < 1.5, and serum creatinine < 2.0 mg/dL) and the French score (platelet count < 30 × 109/L and creatinine ≤ 2.26 mg/dL) at the time of presentation. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Johns Hopkins University.28
Data analysis
Data were summarized by mean (standard error) or median (interquartile range [IQR] representing the 25th and 75th percentile) for continuous variables and counts and proportions for categorical variables. Continuous variables were compared using the t-test or Mann-Whitney U test. Categorical variables were compared using the chi-squared or Fisher’s exact tests. PLASMIC and French Scores were computed for all patients (episodes) for whom complete data on the individual score components were available (Figure 1). Based on published data, PLASMIC score was categorized into a score of 0 to 4 (low risk) or 5 to 7 (intermediate to high risk) since an intermediate score corresponds to a 5–24% risk of TTP, which is high enough clinical probability to treat as TTP.11 For the French score, data on ANA was not collected as it was not available for most patients. Risk for the French score was thus based on creatinine and platelet levels (0 was as categorized low risk, 1 as intermediate risk, and 2 as high risk). We used a French score of ≥1 (corresponding to a 98.8%% sensitivity, 48.1% specificity of detecting severe TTP with an ADAMTS13 level ≤5%) as the indicator to treat as presumed TTP.15 We calculated sensitivity as true positives / (true positives + false negatives), and specificity as true negatives / (true negatives + false positives) where negative and positives on the screening tool are in context of meeting screening criteria by the PLASMIC or French scores, and patients with confirmed TTP are considered true positives. The sensitivities and specificities of both scores were calculated separately for the following age groups: 18–39 years, 40–59 years, and ≥ 60 years. Finally, we used the chi-squared test to compare categorical variables (contributing to these scores) in different age groups to explore which components contribute to a loss of sensitivity, if any. P<0.05 was considered significant for all analyses. Statistical analysis was performed using Stata version 15 (StataCorp. College Station, TX, USA).
FIGURE 1. Flowchart of patients included in the analysis.
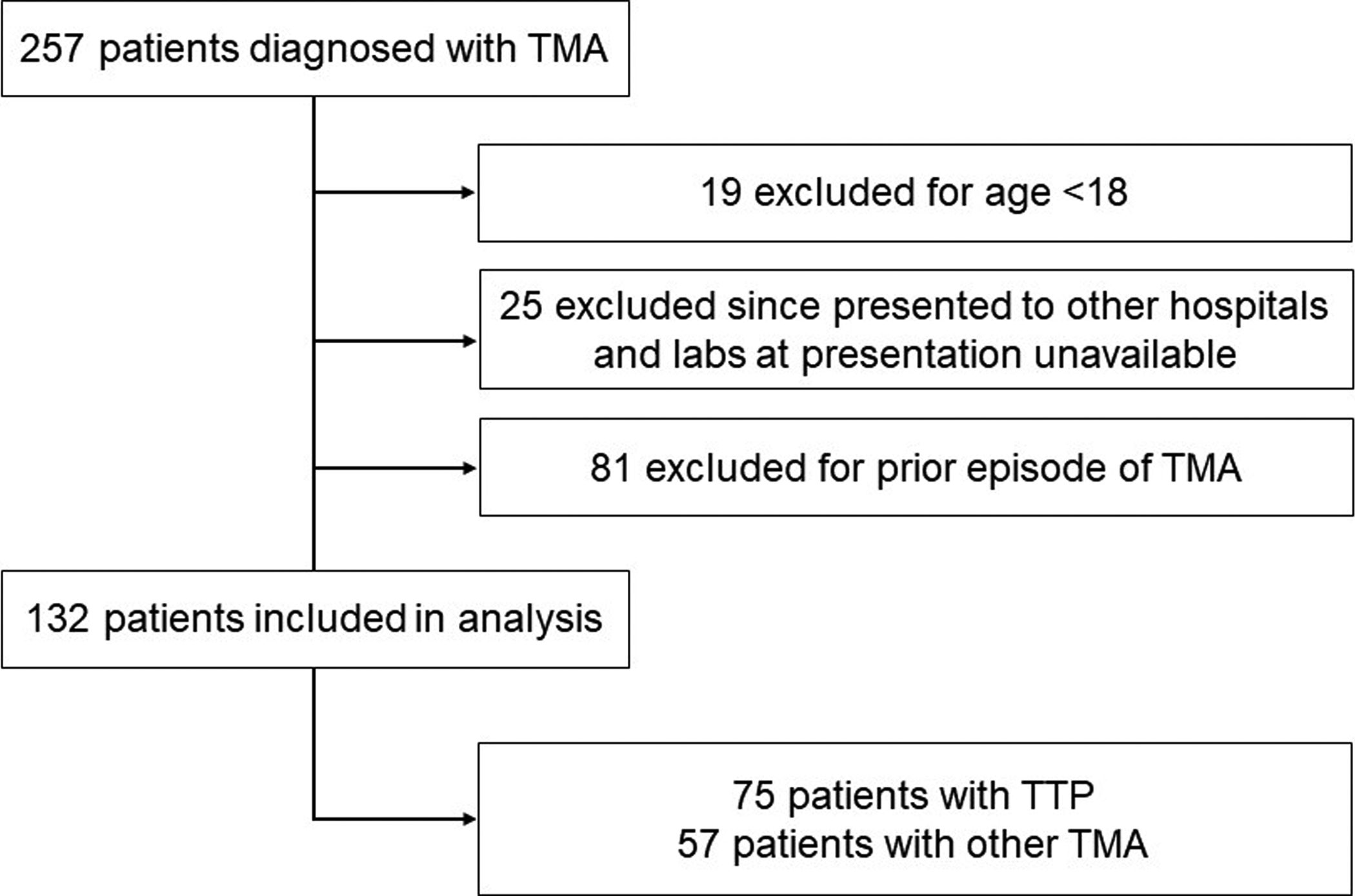
257 patients were evaluated for thrombotic microangiopathy (TMA). We excluded patients less than 18 years of age, those that presented initially to other hospitals and for whom laboratory data at presentation were not available, and those that had a prior episode of TMA and were presenting with a relapse. The remaining 132 patients (75 with TTP and 57 with other TMA) were included in the analysis.
RESULTS
Patients cohort and diagnoses
A total of 257 patients were enrolled in the Johns Hopkins TMA registry. We excluded 19 patients under the age of 18 years and 25 patients who initially presented to other hospitals and for whom laboratory studies from initial presentation were unavailable. Eighty-one patients with a prior episode of TMA were also excluded (as discussed above). Among the remaining 132 patients (Figure 1), 75 had TTP and 57 had other TMA. Other TMA included 30 with aHUS, 3 with catastrophic antiphospholipid syndrome, 1 with cancer-related TMA, 3 with scleroderma renal crisis, 2 with pregnancy-related aHUS, 3 with post-bone marrow transplant TMA, and 11 with TMA NOS, 3 with drug-induced TMA, and 1 with Shiga-toxin associated HUS. There was incomplete data for the patients with Shiga-toxin associated HUS and drug-induced TMA so none were included in the analysis.
Demographics of the study cohort are summarized in Table 1. Among the 75 patients with an index episode of TTP, the median age was 41 (IQR 31, 57) years and 61.3% were female. Among the 57 patients that had other TMA, median age was 46 (IQR 28, 60) years and 61.4% were female. Racial distribution was significantly different in patients with TTP versus other TMA; 72% of TTP patients versus 28.1% of those with other TMA were African American, and 24% of TTP and 66.6% of other TMA patients were White. Data to compute PLASMIC and French scores were available for 117 and 132 patients, respectively. Most common missing data were MCV (N=7), INR (N=13), and presence of hemolysis (N=8).
Table 1.
Demographic data of patients with TTP and other TMA included in the analysis
| Demographic characteristics | |||
|---|---|---|---|
| TTP n = 75 |
Non-TTP TMA n = 57 |
P | |
| Age (years) | 41 (31,57) | 46 (28, 60) | 0.561 |
| Female sex | 46 (61.3%) | 35 (61.4%) | 0.760 |
| Ethnicity/Race | |||
| White | 18 (24.0%) | 38 (66.6%) | <0.01 |
| African-American | 54 (72.0%) | 16 (28.1%) | |
| Other | 3 (4.0%) | 1 (1.7%) | |
Abbreviations: TMA, thrombotic microangiopathy; TTP, thrombotic thrombocytopenic purpura.
Note: Data presented as median (interquartile range [IQR]) or number (%) where appropriate.
P-Value using t-test, Mann-Whitney U test, Pearson’s chi-square test, or Fisher’s exact test as appropriate. P-Values < 0.05 are considered statistically significant.
Sensitivity of clinical screening tools decreases in patients ≥ 60 years of age
PLASMIC and French scores were calculated for patients with TTP and with other (non-TTP) TMA and tabulated based on age (Table 2). The sensitivity of a PLASMIC score of 5–7 or French score of ≥1 in identifying TTP decreased with age (Table 3, Figure 2)). Sensitivities of a PLASMIC score ≥ 5 was 91.4% for patients aged 18–39 years, 78.3% for ages 40–59 years, and 76.9% for patients ≥60 years old. The specificities of this PLASMIC score were 60.0%, 33.3%, and 50% for the age categories 18–39, 40–59, and ≥60 years old, respectively. Similarly, the sensitivity of a French score of ≥1 decreased with age and was 100.0% for patients aged 18–39 years, 96.2% for ages 40–59 years, and 76.9% for patients ≥60 years old. The specificities of a French score of ≥1 was 64.0%, 35.3%, and 40.0% for the age groups 18–39, 40–59, and ≥ 60 years old, respectively.
Table 2.
PLASMIC and French Scores for TTP and other TMA cohorts by age
| Age group (years) | |||||||
|---|---|---|---|---|---|---|---|
| 18–39 | 40–59 | ≥ 60 | |||||
| Risk score | TTP | Non-TTP TMA | TTP | Non-TTP TMA | TTP | Non-TTP TMA | |
| PLASMIC | 0–4 | 3/35 (8.6%) |
12/20 (60.0%) |
5/23 (21.7%) |
4/12 (33.3%) |
3/13 (23.1%) |
7/14 (50.0%) |
| 5 | 3/35 (8.6%) |
4/20 (20.0%) |
3/23 (13.0%) |
5/12 (41.7%) |
2/13 (15.4%) |
5/14 (35.7%) |
|
| 6–7 | 29/35 (82.9%) |
4/20 (20.0%) |
15/23 (65.2%) |
3/12 (25.0%) |
8/13 (61.5%) |
2/14 (14.3%) |
|
| French | 0 | 0/36 (0.0%) |
16/25 (64.0%) |
1/26 (3.8%) |
6/17 (35.3%) |
3/13 (23.1%) |
6/15 (40.0%) |
| 1 | 10/36 (27.8%) |
8/25 (32.0%) |
9/26 (34.6%) |
10/17 (58.8%) |
4/13 (30.8%) |
5/15 (33.3%) |
|
| 2 | 26/36 (72.2%) |
1/25 (4.0%) |
16/26 (61.5%) |
1/17 (5.9%) |
6/13 (46.2%) |
4/15 (26.7%) |
|
Abbreviations: TMA, thrombotic microangiopathy; TTP, thrombotic thrombocytopenic purpura.
Note: Data presented as number/total (%).
For PLASMIC score, 1 point was given for each of the following: (1) platelet < 30 × 109/L; (2) hemolysis [reticulocyte count > 2.5%, undetectable haptoglobin, or indirect bilirubin > 2 mg/dL]; (3) absence of active cancer; (4) absence of solid-organ or stem cell transplant; (5) MCV < 90 fL; (6) INR < 1.5; and (7) serum creatinine < 2.0 mg/dL.
For French score, 1 point was given for each of the following: (1) platelet < 30 × 109/L and (2) serum creatinine ≤ 2.26 mg/dL.
Table 3.
Sensitivity and specificity of PLASMIC and French TTP scores by age
| Age group (years) | ||||
|---|---|---|---|---|
| Score type | 18–39 | 40–59 | ≥ 60 | |
|
PLASMIC
≥ 5 |
Sensitivity | 91.4 | 78.3 | 76.9 |
| Specificity | 60.0 | 33.3 | 50.0 | |
|
PLASMIC
≥ 6 |
Sensitivity | 82.9 | 65.2 | 61.5 |
| Specificity | 80.0 | 75.0 | 85.7 | |
|
French
≥ 1 |
Sensitivity | 100.0 | 96.2 | 76.9 |
| Specificity | 64.0 | 35.3 | 40.0 | |
|
French
≥ 2 |
Sensitivity | 72.2 | 61.5 | 46.2 |
| Specificity | 96.0 | 94.1 | 73.3 | |
Note: Data presented as %.
Figure 2.
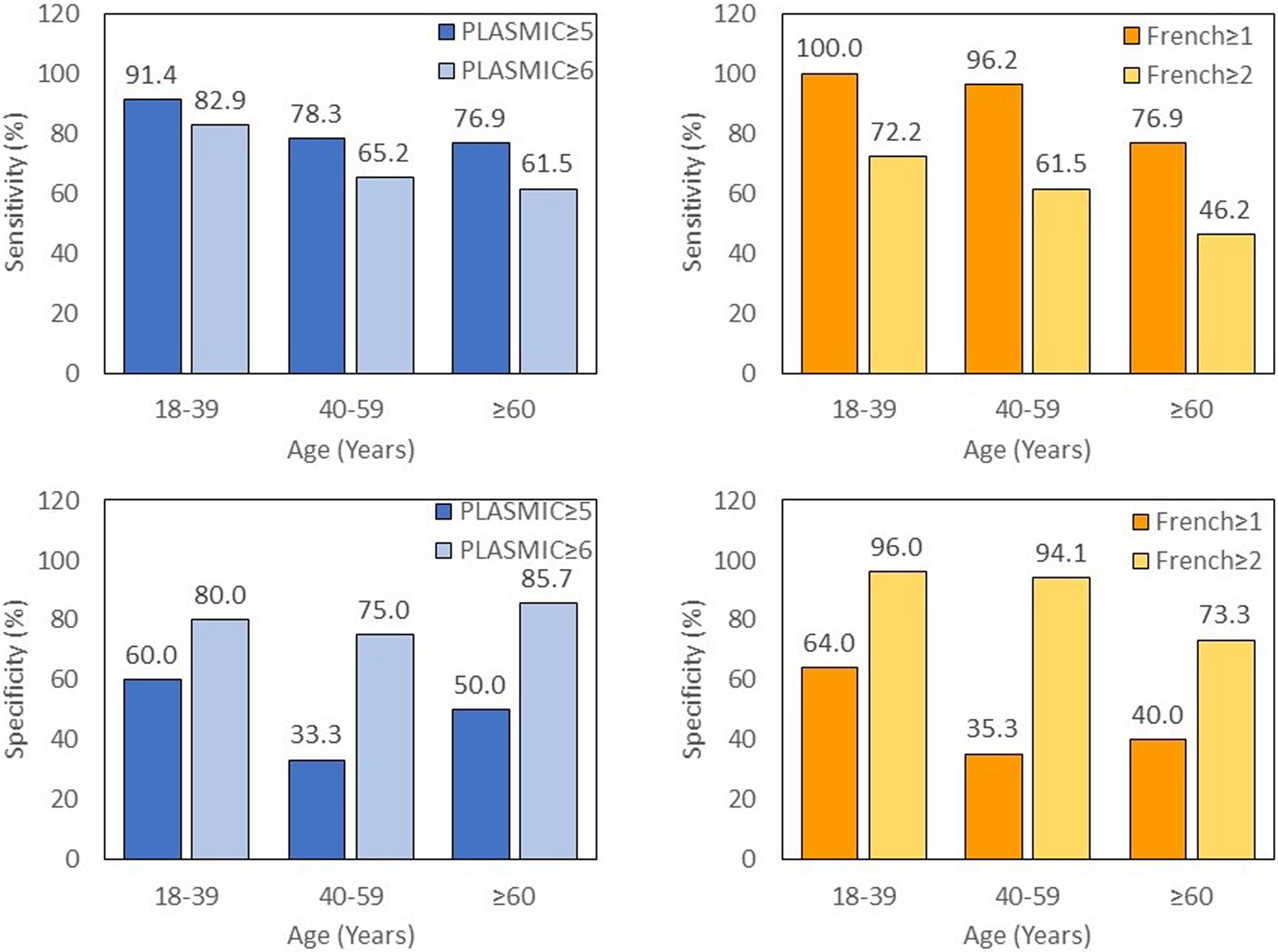
The sensitivity of the PLASMIC and French TTP scores decreases with age
Higher cutoffs for the PLASMIC and French scores were also analyzed. A PLASMIC score ≥ 6 had lower sensitivity (82.9%, 65.2%, 61.5% for the age categories 18–39, 40–59, and ≥60 years old, respectively) but higher specificity (80.0%, 75.0%, 85.7% for the age categories 18–39, 40–59, and ≥60 years old, respectively) across all age groups when compared to a PLASMIC score ≥ 5. Similarly, a French score of ≥ 2 had lower sensitivity (72.2%, 61.5%, 46.2% for the age categories 18–39, 40–59, and ≥60 years old, respectively) and higher specificity (96.0%, 94.1%, 73.3% for the age categories 18–39, 40–59, and ≥60 years old, respectively) versus a French score ≥ 1 across the different age groups.
Next, we explored potential causes for the loss of sensitivity at older ages. For patients with TTP, the components of the PLASMIC score for each age group can be found in Table 4. Among patients with confirmed TTP, mean PLASMIC score decreased with age and were 5.97 ± 0.18, 5.26 ± 1.79, and 5.15 ± 1.34 for ages 18–39, 40–59, and ≥ 60 years old, respectively. Both the average platelet count and serum creatinine were higher in older patients with TTP. In particular, patients ≥ 60 years old had significantly higher platelet counts (p = 0.031) and lower PLASMIC scores (p = 0.031) when compared to the youngest adults (18–39 years) with TTP.
Table 4.
Components of the PLASMIC score by age for patients with TTP
| Age Group (years) | ||||
|---|---|---|---|---|
| 18–39 (n = 35) |
40–59 (n = 23) |
≥ 60 (n = 13) |
p-value | |
| PLASMIC score | 5.97 ± 0.18 | 5.26 ± 1.79 | 5.15 ± 1.34 | 0.031 |
| Platelet (×109/uL) | 19.87 ± 2.75 | 28.60 ± 5.96 | 35.14 ± 8.59 | 0.031 |
| Serum creatinine (mg/dL) | 1.63 ± 0.20 | 2.14 ± 0.78 | 2.03 ± 0.42 | 0.330 |
| PLASMIC score point category | ||||
| Hemolysis | 85.7% | 88.5% | 84.6% | 0.622 |
| Cancer | 94.6% | 92.6% | 78.6% | 0.120 |
| Transplant | 97.3% | 100.0% | 100.0% | 0.725 |
| MCV | 60.0% | 41.7% | 46.2% | 0.296 |
| INR | 91.4% | 84.0% | 84.6% | 0.413 |
| Platelet | 81.6% | 70.4% | 50.0% | 0.030 |
| Creatinine | 83.8% | 84.6% | 69.2% | 0.229 |
Abbreviations: MCV, mean corpuscular volume; INR, international normalized ratio.
Note: Reported p-values compared age groups 18–39 vs ≥ 60 years old. P-values comparing age groups 18–39 vs 40–59 and 40–59 vs ≥ 60 years old were nonsignificant and not included in this table.
Among patients with TTP, PLASMIC points given for the hemolysis variable, absence of organ or stem cell transplant, and INR were similar among all three age groups. However, patients ≥ 60 years old were significantly less likely to meet criteria for platelet count <30 ×109/L compared with patients in the 18–39-year group (50.0% vs 81.6%, p = 0.030). Patients ≥ 60 years old were also less likely to receive points for absence of cancer status (78%), MCV <90 fL (46.2%), and serum creatinine < 2 mg/dL (69.2%) though these were not statistically significant.
DISCUSSION
In this study, we demonstrate that the sensitivity and specificity of the PLASMIC and French scores in diagnosing TTP decreased with age, which is primarily driven by the less severe thrombocytopenia, greater degree of renal impairment, and a higher rate of comorbidities such as cancer in older patients. While these screening tools are extremely useful and can help guide clinical decision making when ADAMTS13 results are not readily available, our results highlight that they may fail to identify older patients with TTP in whom a high index of suspicion and ADAMST13 assays will be critical.
Sensitivity is the most important metric when a clinical decision tool is used to rapidly identify patients that are likely to have immune TTP and respond to plasma exchange since false negatives (failure to recognize TTP) can have catastrophic consequences. Both PLASMIC ≥ 5 and French ≥ 1 performed well in the youngest age group, with similar sensitivities (91.4% vs 100.0%, respectively) and specificities (60.0% vs 64.0%, respectively). However, they were greatly reduced in the oldest group with a sensitivity of 76.9% for the PLASMIC score and 76.9% for the French score, and specificity of 50.0% PLASMIC score and 40.0% for the French score. For our youngest age group (18–39 years old), the sensitivity was similar to that derived in the original PLASMIC study (91.4% vs 90%) however our specificity was lower (60% vs 92%). The difference in specificity is likely because Bendapudi et al used a higher cutoff of 6 as a positive PLASMIC score9. We do not have information about the proportion of older patients in the original cohort. For the French score, when compared to the original study by Coppo et al which had 98.8% sensitivity and 48.1% specificity for a French score of ≥ 1,15 our analysis had similar sensitivity but a higher specificity for our youngest groups (age < 40 years old). The higher specificity in our cohort is likely explained by the higher ADAMTS13 activity cutoff of 10% that we used to define TTP whereas the French TMA study used a stricter ADAMTS13 activity cutoff of <5% to define TTP.
The PLASMIC score had multiple components so we further analyzed it in greater detail. Sensitivity of the PLASMIC score was the lowest (76.9%) in patients ≥ 60 years old compared with the other age groups in our study. This was largely driven by the fact the older patients had significantly higher platelets when compared to the youngest group (35.1 ± 8.6 ×109/uL vs 19.9 ± 2.8 ×109/L, age ≥ 60 vs 18–39 years old, respectively, p = 0.031) and were consequently less likely to meet the platelet count criterion (50.0% vs 81.6%, p = 0.030). This finding is supported by the observations of Prevel and colleagues who reported that older patients with TTP have less severe cytopenias.23 Additionally, patients ≥ 60 years old received less PLASMIC points for serum creatinine (69.2% vs 83.8%), cancer (78.6% vs 94.6%), and MCV (46.2% vs 60.0%) though these were not statistically significant, likely because of the small number of elderly patients in our study (13 cases). Other studies have also shown that older patients with TTP (≥ 60 years old) have a higher rate of renal involvement and cancer.23,25 Additionally, MCV of the general population increases as people age.29,30
The limitations to our retrospective study are the small sample size and high proportion of African Americans at our single institution, which may reduce generalizability. Of note, patients diagnosed prior to 2006 did not consistently have ADAMTS13 levels documented and thus, may not be considered to have severe TTP. However, we performed an analysis with patients diagnosed between 2006 and 2018 and had similar results to the original analysis that can be found in Supplemental Table S1. We included in our study 8 patients with TMA attributed to bone marrow transplantation, cancer, drugs, and Shiga-toxin. These etiologies may explain some of the false-positives in the study. However, we also had 7 patients with TTP and cancer or a bone marrow transplant in our study. Although these cases have a clear cause of TMA, it may still be important to identify TTP since this could change management.
For the French score, ANA was not incorporated as this component was not readily available at the time of diagnosis for most of our patients and may account for discrepancies from the original French study. Regarding differences between the French and PLASMIC scores, since the risk of TTP does not decrease with associated conditions such as cancer or bone marrow transplantation in the French score as it does in the PLASMIC score, the French score should be considered in patients with a TMA and no associated conditions. These results need to be validated in larger cohorts and with different populations. Future studies should also evaluate the validity of integrating age into these diagnostic scores or implementing different cutoffs for older populations.
In conclusion, our data show that the sensitivity of the PLASMIC and French TTP scores, which are widely applied clinical screening tools to aid TTP diagnosis, is reduced in older patients (age > 60 years) in whom they may be less reliable in identifying patients with TTP likely to benefit from prompt plasma exchange. A PLASMIC score ≥ 5 or French score ≥ 1 are able to diagnose patients with intermediate to high risk of having TTP, but may miss about 25% of older patients with TTP. These findings highlight the need for a high index of suspicion for TTP in patients presenting with a possible thrombotic microangiopathy. Further, since older patients have higher morbidity and mortality from TTP.22–26,31 it is critical to have wider availability of the gold standard ADAMTS13 test with rapid turnaround times.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by a Mentored Research Award from the Hemostasis and Thrombosis Research Society, Johns Hopkins University Clinician Scientist Award and K99 HL150594 (S.C.).
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.George JN, Nester CM. Syndromes of thrombotic microangiopathy. The New England journal of medicine. 2014;371(7):654–666. [DOI] [PubMed] [Google Scholar]
- 2.Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. The New England journal of medicine. 1991;325(6):393–397. [DOI] [PubMed] [Google Scholar]
- 3.Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. The New England journal of medicine. 1991;325(6):398–403. [DOI] [PubMed] [Google Scholar]
- 4.Dane K, Chaturvedi S. Beyond plasma exchange: novel therapies for thrombotic thrombocytopenic purpura. Hematology American Society of Hematology Education Program. 2018;2018(1):539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully M, Cataland S, Coppo P, et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. Journal of thrombosis and haemostasis : JTH. 2017;15(2):312–322. [DOI] [PubMed] [Google Scholar]
- 6.Kim CH, Simmons SC, Williams LA III, Staley EM, Zheng XL, Pham HP. ADAMTS13 test and/or PLASMIC clinical score in management of acquired thrombotic thrombocytopenic purpura: a cost-effective analysis. Transfusion. 2017;57(11):2609–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell NT, Cheves T, Sweeney JD. Effect of ADAMTS13 activity turnaround time on plasma utilization for suspected thrombotic thrombocytopenic purpura. Transfusion. 2016;56(2):354–359. [DOI] [PubMed] [Google Scholar]
- 8.Mannucci PM, Franchini M. Advantages and limits of ADAMTS13 testing in the prognostic assessment of thrombotic thrombocytopenic purpura. Presse Med. 2012;41(3 Pt 2):e157–162. [DOI] [PubMed] [Google Scholar]
- 9.Yoshii Y, Fujimura Y, Bennett CL, Isonishi A, Kurumatani N, Matsumoto M. Implementation of a rapid assay of ADAMTS13 activity was associated with improved 30-day survival rate in patients with acquired primary thrombotic thrombocytopenic purpura who received platelet transfusions. Transfusion. 2017;57(8):2045–2053. [DOI] [PubMed] [Google Scholar]
- 10.Chiasakul T, Cuker A. Clinical and laboratory diagnosis of TTP: an integrated approach. Hematology American Society of Hematology Education Program. 2018;2018(1):530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendapudi PK, Hurwitz S, Fry A, et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. The Lancet Haematology. 2017;4(4):e157–e164. [DOI] [PubMed] [Google Scholar]
- 12.Li A, Khalighi PR, Wu Q, Garcia DA. External validation of the PLASMIC score: a clinical prediction tool for thrombotic thrombocytopenic purpura diagnosis and treatment. Journal of thrombosis and haemostasis : JTH. 2018;16(1):164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang N, Wang X, Li D, Sun Z. Validation of the PLASMIC score, a clinical prediction tool for thrombotic thrombocytopenic purpura diagnosis, in Chinese patients. Thrombosis research. 2018;172:9–13. [DOI] [PubMed] [Google Scholar]
- 14.Jajosky R, Floyd M, Thompson T, Shikle J. Validation of the PLASMIC score at a University Medical Center. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2017;56(4):591–594. [DOI] [PubMed] [Google Scholar]
- 15.Coppo P, Schwarzinger M, Buffet M, et al. Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: the French TMA reference center experience. PLoS One. 2010;5(4):e10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendapudi PK, Li A, Hamdan A, et al. Impact of severe ADAMTS13 deficiency on clinical presentation and outcomes in patients with thrombotic microangiopathies: the experience of the Harvard TMA Research Collaborative. British journal of haematology. 2015;171(5):836–844. [DOI] [PubMed] [Google Scholar]
- 17.Kremer Hovinga JA, Coppo P, Lammle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3:17020. [DOI] [PubMed] [Google Scholar]
- 18.Mariotte E, Azoulay E, Galicier L, et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. The Lancet Haematology. 2016;3(5):e237–245. [DOI] [PubMed] [Google Scholar]
- 19.Blombery P, Kivivali L, Pepperell D, et al. Diagnosis and management of thrombotic thrombocytopenic purpura (TTP) in Australia: findings from the first 5 years of the Australian TTP/thrombotic microangiopathy registry. Intern Med J. 2016;46(1):71–79. [DOI] [PubMed] [Google Scholar]
- 20.Ortman JMV VA; Hogan H An aging nation: the older population in the United States. https://www.census.gov/prod/2014pubs/p25-1140.pdf.
- 21.Chaturvedi S, Cuker A. Different strokes for older folks (with TTP). Blood. 2019;134(24):2125–2126. [DOI] [PubMed] [Google Scholar]
- 22.Benhamou Y, Assie C, Boelle PY, et al. Development and validation of a predictive model for death in acquired severe ADAMTS13 deficiency-associated idiopathic thrombotic thrombocytopenic purpura: the French TMA Reference Center experience. Haematologica. 2012;97(8):1181–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevel R, Roubaud-Baudron C, Gourlain S, et al. Immune thrombotic thrombocytopenic purpura in older patients: prognosis and long-term survival. Blood. 2019;134(24):2209–2217. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto M, Bennett CL, Isonishi A, et al. Acquired idiopathic ADAMTS13 activity deficient thrombotic thrombocytopenic purpura in a population from Japan. PLoS One. 2012;7(3):e33029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agosti P, Mancini I, Artoni A, et al. The features of acquired thrombotic thrombocytopenic purpura occurring at advanced age. Thrombosis research. 2020;187:197–201. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi S, Carcioppolo D, Zhang L, McCrae KR. Management and outcomes for patients with TTP: analysis of 100 cases at a single institution. American journal of hematology. 2013;88(7):560–565. [DOI] [PubMed] [Google Scholar]
- 27.Sperati CJ, Moliterno AR. Thrombotic microangiopathy: focus on atypical hemolytic uremic syndrome. Hematology/oncology clinics of North America. 2015;29(3):541–559. [DOI] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adeli K, Raizman JE, Chen Y, et al. Complex biological profile of hematologic markers across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin Chem. 2015;61(8):1075–1086. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann JJ, Nabbe KC, van den Broek NM. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin Chem Lab Med. 2015;53(12):2015–2019. [DOI] [PubMed] [Google Scholar]
- 31.Benhamou Y, Assie C, Boelle PY, et al. Development and validation of a predictive model for death in acquired severe ADAMTS13 deficiency-associated idiopathic thrombotic thrombocytopenic purpura: the French TMA Reference Center experience. Haematologica. 2012;97(8):1181–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


