Abstract
Gastric cancer is a global health problem, with more than 1 million people newly diagnosed with gastric cancer worldwide each year. Despite its worldwide decline in incidence and mortality over the past five decades, gastric cancer remains the third leading cause of cancer-related death. Knowledge of global as well as regional epidemiology and risk factors for gastric cancer is essential for the practicing gastroenterologist to make personalized decisions about risk stratification, screening and prevention. In this article, we review the epidemiology of gastric cancer as well as screening and prevention efforts to reduce global morbidity and mortality from gastric cancer. First, we discuss the descriptive epidemiology of gastric cancer, including its incidence, mortality, survival, and secular trends. We combine a synthesis of published studies with an analysis of data from the International Agency for Research on Cancer (IARC) GLOBOCAN project to describe the global burden of gastric cancer and data from the United States Cancer Statistics (USCS) registry to discuss the change in incidence of gastric cancer in the U.S. Next, we summarize current knowledge of risk factors for gastric cancer. Finally, we discuss prevention strategies and screening efforts for gastric cancer.
Gastric cancer is a common global disease. The current general populations including those presenting to gastroenterology practices in North America and Europe reflect the historically high levels of global representation. Knowledge of both global as well as local epidemiology and risk factors for gastric cancer is essential for contemporary gastroenterology practice.
Gastric Cancer around the World
Incidence and Mortality Rates:
According to estimates from the International Agency for Research on Cancer (IARC) GLOBOCAN project,1 worldwide, there were 1,033,701 new cases of gastric cancer (representing 5.7% of all cancer cases diagnosed) and 782,685 deaths related to gastric cancer in 2018.2 Gastric cancer was the fifth-most commonly diagnosed cancer type in 2018 and was responsible for 8.2% of all deaths from cancer in 2018, equating to 1 in every 12 deaths and making it the third-most common cause of cancer-related death after lung and colorectal cancers (18.4% and 9.2% of deaths, respectively).2 In 2018, the global age-standardized (world) incidence and mortality rates for gastric cancer were 11.1 and 8.2 per 100,000 persons, respectively.
In all populations and countries, gastric cancer is uniformly rare in adults aged <50 years. Gastric cancer incidence rates increase with increasing age and reach a plateau between 55 and 80 years. On average, incidence rates for gastric cancer are two-fold to three-fold higher for men than women.2–4 According to GLOBOCAN 2018 estimates, gastric cancer was the fourth-most commonly diagnosed cancer type in men and the seventh-most commonly diagnosed cancer type in women.2 In 2018, gastric cancer incidence rates were 15.7 (age-standardized [world]) per 100,000 men and 7.0 per 100,000 women.2 The mortality rates in 2018 were 11.7 per 100,000 men and 5.2 per 100,000 women. Globally, the lifetime risk (0–74 years) of gastric cancer is about 1 in 54 men and about 1 in 126 women.1
Gastric cancer was the leading cause of cancer deaths in the world until the 1980s when it was overtaken by lung cancer. This change was due to both the rising incidence of lung cancer and the declining incidence of gastric cancer. There has been a steady decline in gastric cancer incidence and mortality rates since the middle of the 20th century in developed nations (e.g., Northern America, Northern Europe, Australia). Delayed but similar decreasing trends have been observed in areas with historically high gastric cancer incidence rates (e.g., Japan, Korea) in recent years (Figure 1).5 Nonetheless, gastric cancer maintains a high case fatality rate of 75% throughout most of the world6 and is a main contributor to global disability-adjusted life-year burden.7
Figure 1.
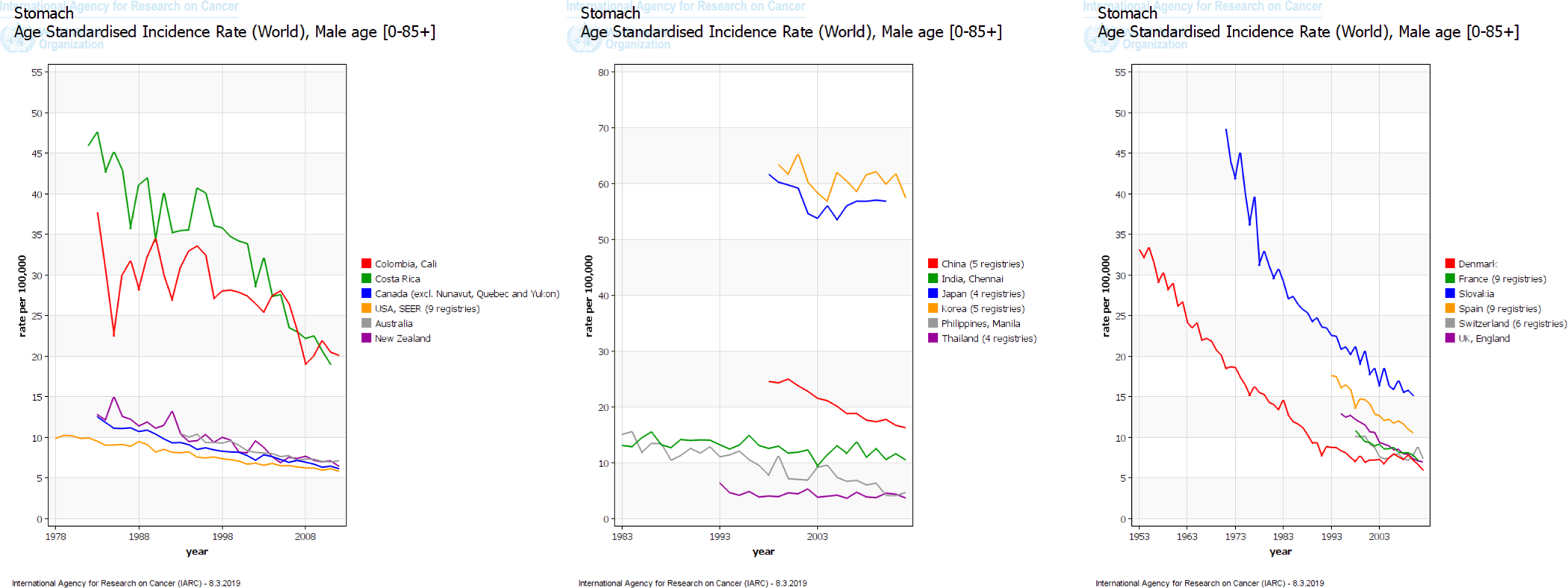
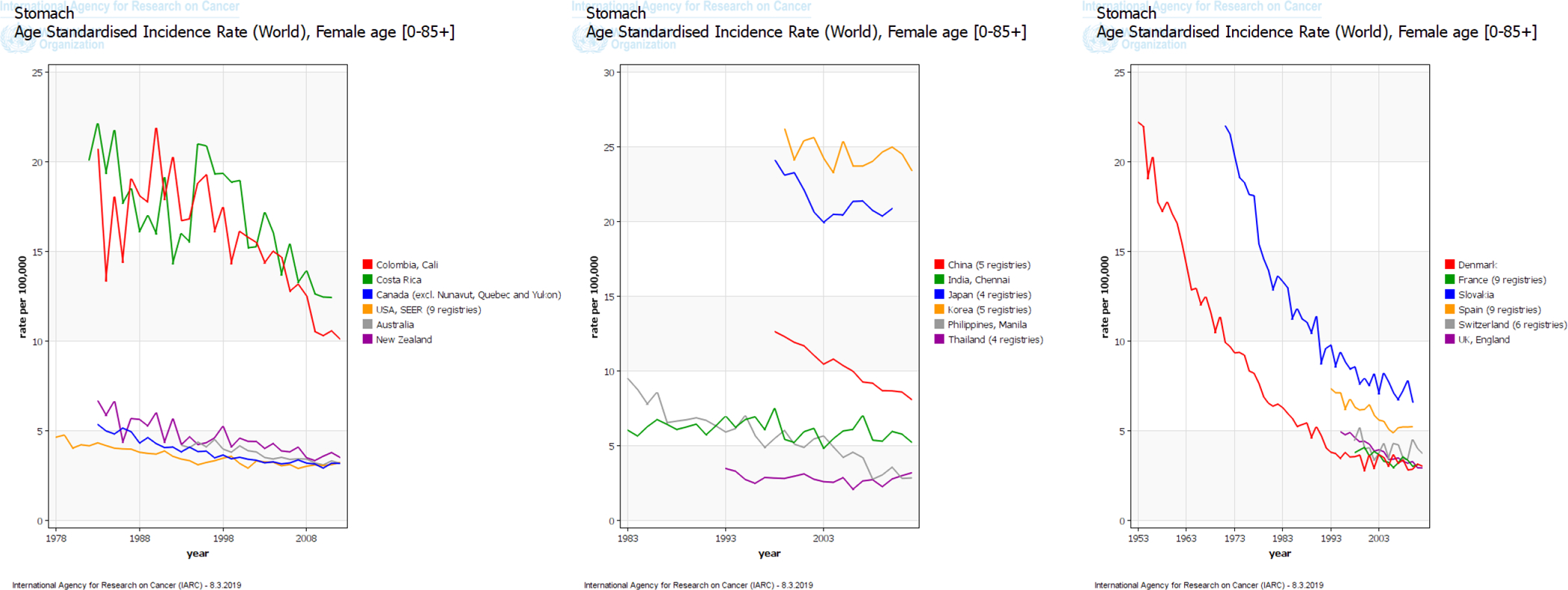
Trends in incidence rates of gastric cancer among (A) men and (B) women in select countries in the International Agency for Research on Cancer (IARC) GLOBOCAN project
Secular Trends:
Recent studies examining global trends in incidence and mortality of gastric cancer have confirmed a continued decline worldwide.4,8,9 However, there is large global variability in incidence and mortality rates for gastric cancer and in secular trends. Geographically, the vast majority of gastric cancer cases in 2018 occurred in countries with high and very high Human Development Index (N=885,119; representing 85.6% of all gastric cancer cases). More than 60% of the total cases of gastric cancer in 2018 occurred in Eastern and South-Eastern Asia (657,254 cases).1 The highest incidence rates for gastric cancer in men and women are observed in Eastern Asia (age-standardized [world] incidence rates: 32.1 per 100,000 men; 13.2 per 100,000 women), Central and Eastern Europe (17.1 per 100,000 men; 7.5 per 100,000 women), and South America (12.7 per 100,000; 6.9 per 100,000 women). In 2018, the lowest incidence rates for gastric cancer were observed in North America (5.6 per 100,000 men; 2.8 per 100,000 women) and African regions (~5 per 100,000 men and 3–4 per 100,000 women).1 Among all persons, the highest mortality rates for gastric cancer are observed in Eastern Asia (15.9 per 100,000 persons) and the lowest in Northern America (1.8 per 100,000 persons). However, high mortality rates are also observed for men and women in Central and Eastern Europe and in South America.1 Despite the favorable decline in mortality trends worldwide, in some countries, the declines are less marked. In the European Union and other major European countries, mortality rates from gastric cancer decreased by about 3% per year from 1980 to 2011. Similar decreasing trends were observed in Japan and Korea. The rate of decline was lower in North American and major Latin American countries where gastric cancer mortality rates decreased by approximately 2% per year between 1980 and 2011.9 Several migrant studies have documented a strong environmental component (i.e., non-genetic drivers including higher prevalence rates of known risk factors for gastric cancer, including Helicobacter pylori infection), which could explain the regional variation in gastric cancer incidence rates.10–12
Main Subtypes:
There are two main topographic subsites of gastric cancer: cardia gastric cancer (arising in the area of the stomach adjoining the esophagogastric junction) and non-cardia gastric cancer (arising from more distal regions of the stomach). The descriptive epidemiology and risk factor profiles for cardia gastric cancer and non-cardia gastric cancer are different. In 2012, the highest estimated regional rates of both cardia and non-cardia gastric cancers occurred in Eastern and South-Eastern Asia. For country-specific estimates, the highest estimated rates of cardia gastric cancer occurred in countries within Central Asia (e.g., Kyrgyzstan, Iran); estimated non-cardia gastric cancer rates were highest in countries within Eastern and South-Eastern Asia (e.g., South Korea).13 Overall, incidence rates for cardia gastric cancer are lower than those for non-cardia gastric cancer (in 2012, 3.3 per 100,000 persons compared with 8.8 per 100,000 persons).13 However, the magnitude of this disparity varies geographically. Among men, the ratio of non-cardia gastric cancer to cardia gastric cancer is as much as 40-to-1 in Sub-Saharan Africa. However, it is almost 1-to-1 in Northern America and Oceania. In the U.K., the rates of cardia gastric cancer are 1.5-fold higher than those for non-cardia gastric cancer (in 2012, 3.9 per 100,000 men compared with 2.6 per 100,000 men). For women in Northern America and Oceania, incidence rates for non-cardia gastric cancer remain 2-fold higher than those for cardia gastric cancer. Rates for cardia and non-cardia gastric cancers among women in the U.K. are comparable (1.5 and 1.7 per 100,000 women, respectively).13 Notably, the incidence rates for gastric cancer also vary among different ethnic groups within a particular country.
Gastric Cancer in the United States
Incidence and Mortality Rates:
Gastric cancer was the leading cause of cancer deaths in the United States until the late 1930s. However, the epidemiology of gastric cancer has changed over time, with intriguing developments occurring in recent years.14 In the U.S., an estimated 27,510 persons (17,230 men and 10,280 women) will be newly diagnosed with gastric cancer in 2019, and 11,140 (6,800 men and 4,340 women) will die from their disease.3 For the purpose of this review, we examined the most recent cancer incidence data from the United States Cancer Statistics (USCS) registry.15 The USCS registry provides high-quality data from registries in all 50 states and the District of Columbia. These data are used for the official federal statistics on cancer incidence. There are two primary sources of data for the USCS registry: the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries (NPCR), which includes all population-based state cancer registries and the Surveillance, Epidemiology, and End Results (SEER) program, which includes 14 population-based cancer registries and 3 supplemental registries. In 2015 (the most recent available year), the USCS registry data covered 100% of the U.S. population.15,16 Between 2001 and 2015, 323,549 incident cases of gastric cancer (defined by International Classification of Diseases for Oncology, third edition: site codes C160–C169 and ICD-O-3 histology type, excluding 9050–9055, 9140, and 9590–9992) were diagnosed in the USCS registry. Using NCI’s Joinpoint program (version 4.1.1; http://surveillance.cancer.gov/joinpoint), we identified a linear decline in the incidence of gastric cancer in the U.S. Between 2001 and 2015, age-standardized incidence rates for gastric cancer declined by 0.94% per year (average annual percent change [AAPC], −0.94%; 95% confidence interval [CI], −0.23% to −1.65%).
Although treatment effectiveness has improved during the past decade, survival rates for gastric cancer remain poor. In the U.S., the overall 5-year observed survival rate for patients diagnosed with gastric cancer is among the lowest for all cancers. Prognosis for patients with gastric cancer is strongly related to stage at diagnosis. Although the proportion of unstaged cases decreased in the USCS registry between 2001 and 2015, the proportion of gastric cancer cases that had localized, regional, or distant-stage disease remained relatively stable over time when the 13.1% of unstaged gastric cancer cases were excluded (Figure 2). Strikingly, 35% of gastric cancer patients in the U.S. are still diagnosed with distant-stage disease.
Figure 2.
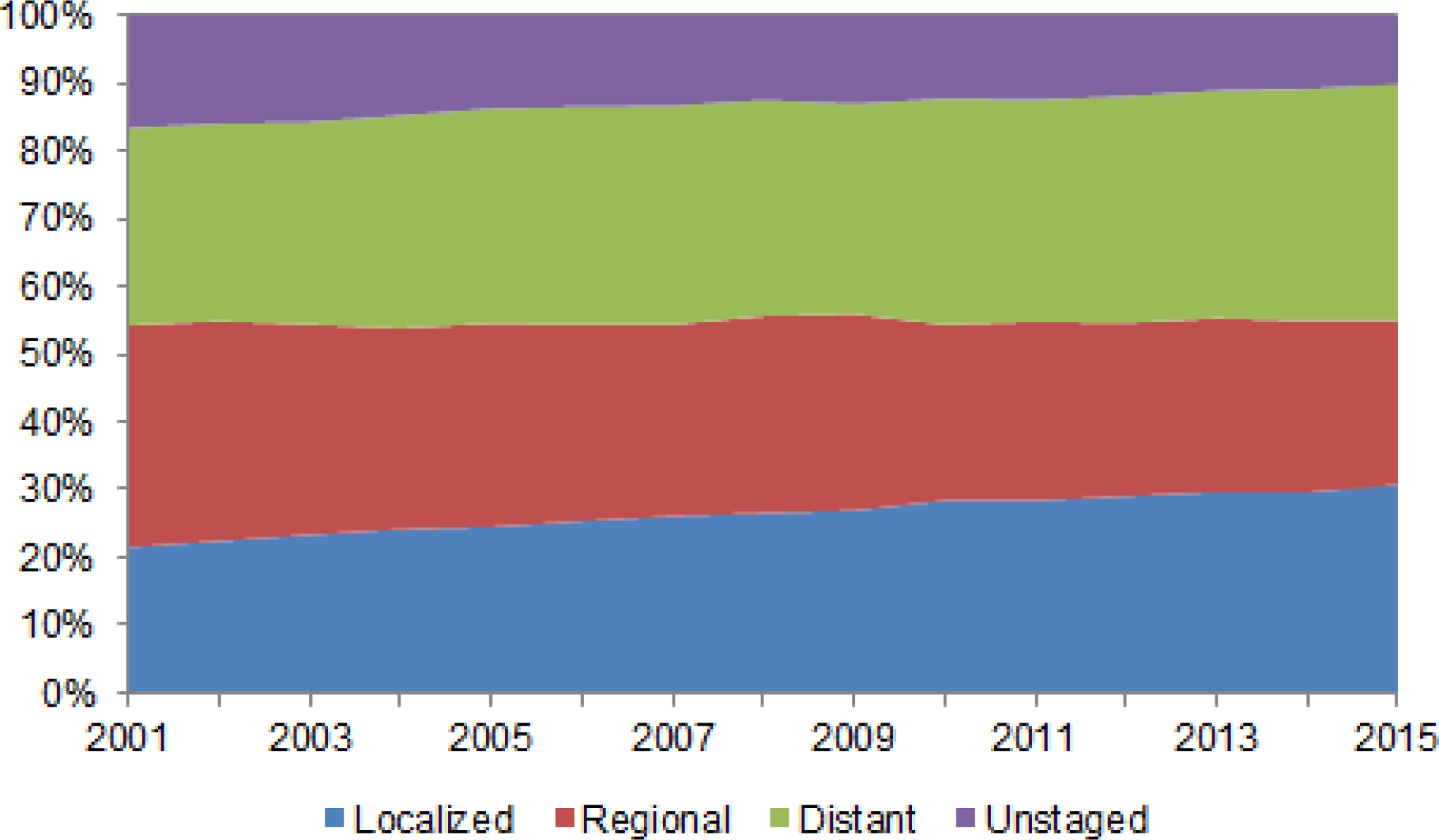
Stage distribution of incident cases of gastric cancer in the United States Cancer Statistics registry, 2001–2015
For the purpose of this review, we analyzed data from the SEER 18 registries (covering approximately 28% of the U.S. population) to examine trends in relative survival rates for patients diagnosed with gastric cancer in the U.S.17 For gastric cancer cases in SEER 18, median relative survival has increased from 10 months for persons diagnosed in 2000 to 16 months for persons diagnosed in 2014. Overall 5-year observed survival rates increased from 18.8% for all patients diagnosed with gastric cancer in 2000 to 28.0% for those diagnosed in 2010. Examination of trends in 5-year relative age-adjusted gastric cancer survival by stage revealed that 5-year survival rates have been improving since 2000 (Figure 3). Improvement in survival trends occurred in gastric cancer patients diagnosed with localized disease, likely reflecting improvements in curative treatment modalities. Almost 46% of patients diagnosed with localized gastric cancer in 2000 survived 5 years after their diagnosis, whereas those diagnosed in 2010 had a 5-year observed survival rate of greater than 60%. There was also improvement in 5-year observed survival rates for gastric cancer patients diagnosed with regional disease. Only 20% of patients diagnosed with regional-staged gastric cancer in 2000 were living 5 years after their diagnosis, whereas the 5-year observed survival rate for those diagnosed in 2010 was approximately 30%. Five-year survival rates for gastric cancer patients diagnosed with distant disease remain <5%.
Figure 3.
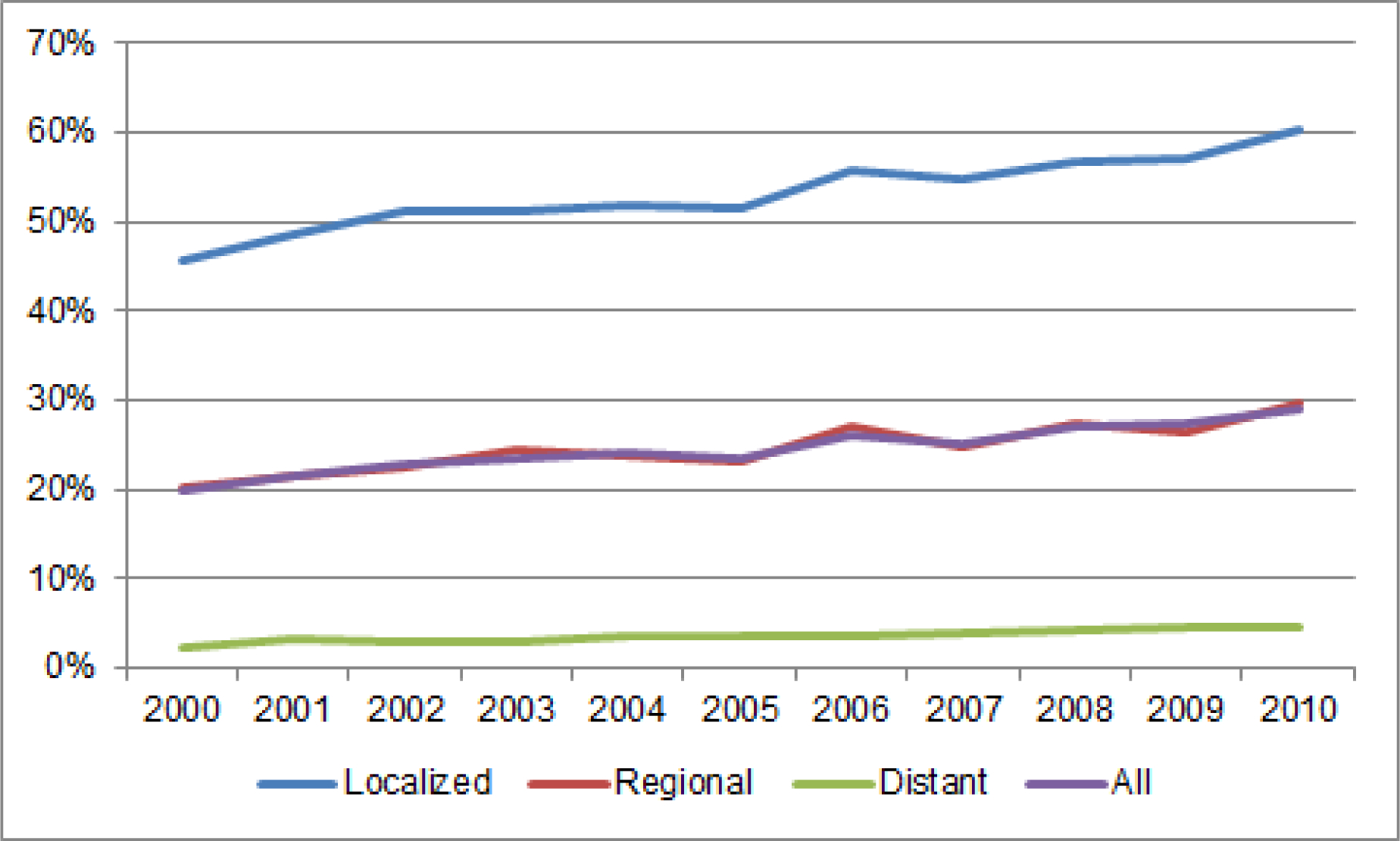
Five-year survival rates for gastric cancer in the Surveillance, Epidemiology, and End Results (SEER) 18 program, 2000–2010
Like most cancers, incidence rates for gastric cancer in the U.S. increase with increasing age and is rare among adults aged <50 years. In the U.S., gastric cancer is most frequently diagnosed among people aged 65–74 years, and the average age of people at diagnosis is 69 years. About 6 of every 10 people diagnosed with stomach cancer each year are ≥65 years. In the USCS registry, 10.5% (n=33,997) of gastric cancer cases diagnosed between 2000 and 2015 were in adults aged <50 years. While rates of gastric cancer have decreased between 2001 and 2015 among persons in the U.S. aged ≥50 years (AAPC, -1.19%; 95% CI, −0.42% to −1.95%), rates have increased among persons aged <50 years (AAPC, 0.95%; 95% CI, 0.69% to 1.20%). The lifetime risk of gastric cancer in the U.S. is approximately 1 in 95 men and 1 in 154 women. Among all incident gastric cancer cases in the USCS registry diagnosed between 2001 and 2015, 61.7% were in men. Between 2001 and 2015, gastric cancer incidence rates decreased at an average of 1.10% per year in men (AAPC, −1.10%; 95% CI, −0.88% to −1.32%) and 0.58% per year in women (AAPC, −0.58%; 95% CI, −0.35% to −0.81%) (Figure 4). Notably, incidence rates for gastric cancer vary among different ethnic groups within the U.S. Between 2001 and 2015, age-standardized incidence rates for gastric cancer were approximately two-fold higher among Hispanics, non-Hispanic Blacks, and Asian and Pacific Islanders in the U.S. compared with non-Hispanic Whites (Figure 4). Age-standardized gastric cancer incidence rates have decreased among all racial sub-groups in the U.S., with greatest decreases observed among Asian and Pacific Islanders (Asian and Pacific Islander: AAPC, −2.81%; 95% CI, −2.35% to −3.26%; Hispanic: AAPC, −1.83%; 95% CI, −1.54% to −2.11%; non-Hispanic white: AAPC, −0.88%; 95% CI, −0.57% to −1.18%; non-Hispanic Black: AAPC, −1.70%; 95% CI, −1.48% to −1.92%).
Figure 4.
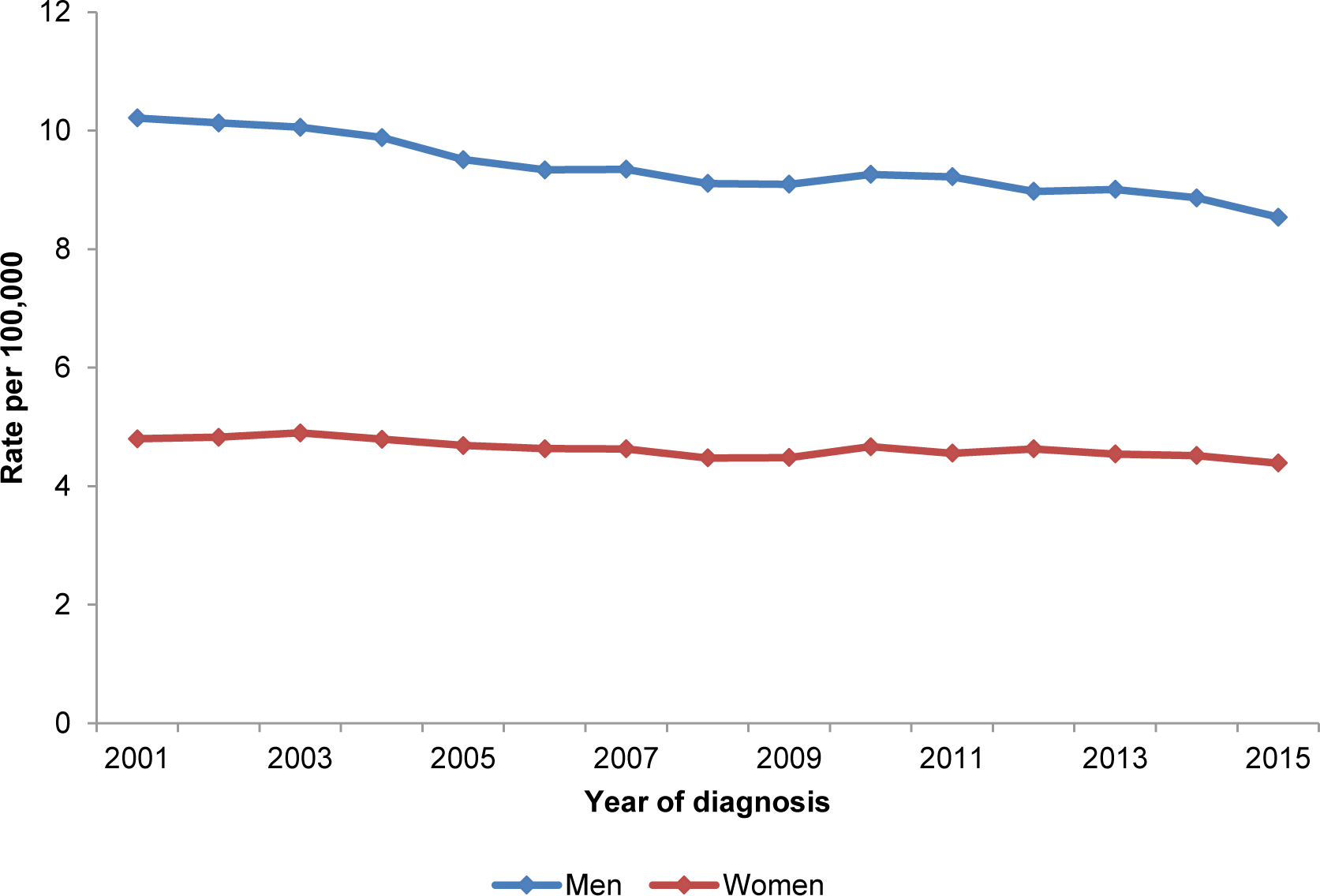
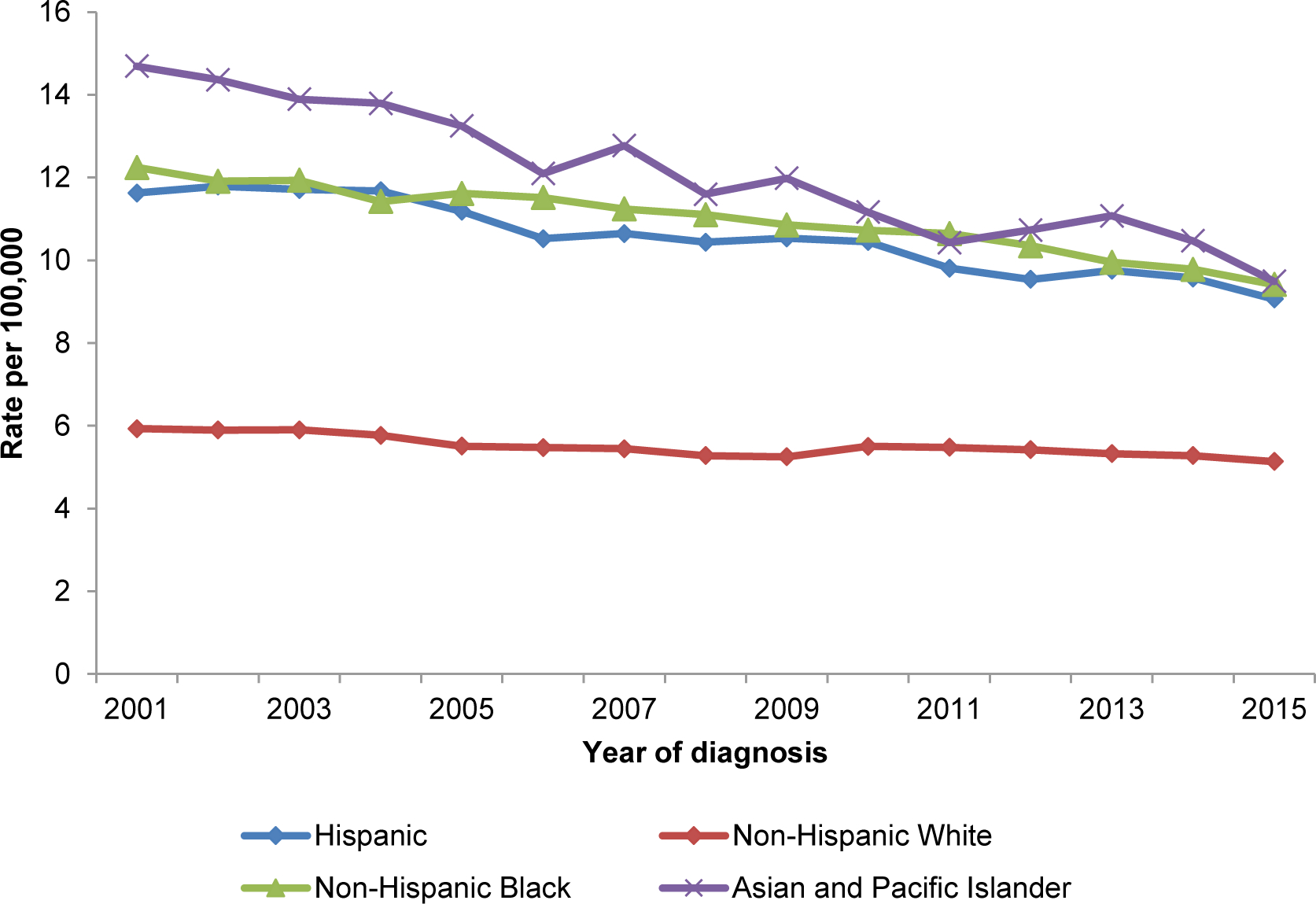
Age-adjusted gastric cancer incidence rates between 2001 and 2015 by (A) sex and (B) race/ethnicity
Geographic differences in overall gastric cancer incidence rates and trends over time have been observed in the U.S.18 The highest age-standardized (U.S. standard) incidence rates for gastric cancer in 2003 and 2010 were found in Hawaii (11.9 and 9.2 per 100,000 persons, respectively), whereas by 2015 the highest rates were found in Alaska (9.0 per 100,000 persons). In 2003, 14 of the 50 states (28.0%) had age-standardized incidence rates for gastric cancer less than 5.8 per 100,000 persons; this number increased to 20 states (40.0%) by 2015 (Figure 5). By contrast, the number of states with age-standardized incidence rates for gastric cancer greater than 8 per 100,000 persons decreased from seven states (Hawaii, New York, New Jersey, Rhode Island, Connecticut, California, and Louisiana) in 2003 to three states (Alaska, New York, and Hawaii) by 2015.
Figure 5.
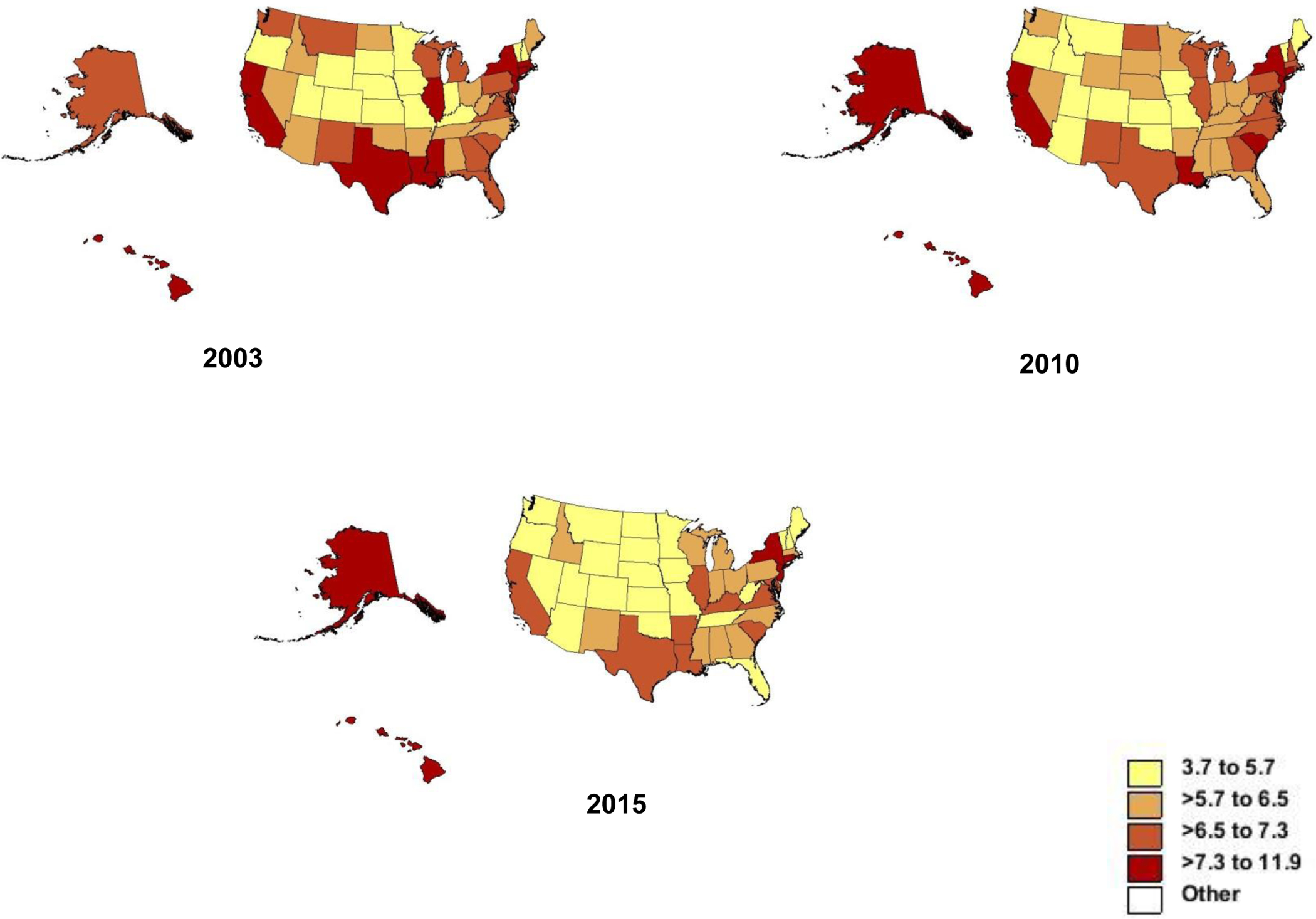
Heat maps showing age-adjusted gastric cancer incidence rates in the United States in 2003, 2010, and 2015
Diverging trends based on age have been observed in incidence rates for gastric cancer in the U.S. While overall rates were decreasing in the U.S. between 1977 and 2006, incidence rates for distal gastric cancer increased among non-Hispanic Whites aged 25 to 39 years.14 A recent study using data from 45 North American Association of Central Cancer Tumor Registries found that non-cardia gastric cancer incidence rates increased at a rate of 1.3% per year among non-Hispanic Whites aged <50 years, whereas they continued to decrease among those aged ≥50 years.19 Similarly, rates for non-cardia gastric cancer increased among Hispanics aged <50 years and decreased among Hispanics aged ≥50 years. We observed divergent trends between non-Hispanic Whites and Hispanics aged <50 years when we examined the USCS registry for secular trends by cancer stage at diagnosis. Among non-Hispanic whites, rates of localized-stage non-cardia gastric cancer increased by 5.28% (95% CI, 3.94% to 6.64%) per year. Conversely, during the same period, the rates of regional- and distant-staged non-cardia gastric cancer among non-Hispanic Whites aged <50 years decreased and remained unchanged, respectively. By contrast, there was a significant increase in distant-staged non-cardia gastric cancers among Hispanics aged <50 years (AAPC, 1.78%; 95% CI, 0.66% to 2.91%). This increasing trend was more apparent among Hispanic males (AAPC, 2.16%; 95% CI, 0.69% to 3.64%). These data suggest a true increase in non-cardia gastric cancer incidence rates among Hispanics. The increase in only localized tumors among non-Hispanic Whites suggests over-diagnosis; however, this seems an unlikely explanation given that there are currently no guidelines for endoscopic gastric cancer screening in the U.S. The increasing rates of distant-staged disease among Hispanics could reflect low socioeconomic status, healthcare barriers to receiving prevention efforts, and lack of H. pylori screening (described below). Clinically relevant take home messages on the epidemiology of gastric cancer in the U.S. are summarized in Table 1.
Table 1.
Clinically relevant points on the epidemiology of gastric cancer in the U.S.
| Overall, incidence rates for gastric cancer continue to decrease |
| Gastric cancer is most frequently diagnosed in people aged 65–74 years |
| Incidence rates are two-fold higher among men than women |
| Rates are two-fold higher among minorities (Hispanics, non-Hispanic Blacks, and Asian and Pacific Islanders) than non-Hispanic whites |
| For patients diagnosed with gastric cancer, observed 5-year survival rates remain less than 30% |
| Over a third of gastric cancer patients are still diagnosed with advanced disease |
| Incidence of non-cardia gastric cancer is increasing among persons aged <50 years |
Risk Factors for Gastric Cancer
Chronic infection with H. pylori is the main cause of gastric cancer, accounting for approximately 89% of distal gastric cancer cases worldwide.20–22 In 1994, the IARC classified H. pylori as a class I carcinogen and reconfirmed this classification in 2009.23 Most H. pylori infections are acquired during childhood and, once established, usually persist for life unless treated. The prevalence of H. pylori infection in adults exceeds 50% in many industrialized countries; however, there is substantial regional variation in prevalence.24 Prevalence of H. pylori infection is higher in Central and South America and in parts of Asia and Eastern Europe compared with North America, Australia, and Western Europe.24 Up to 10% of gastric cancers can be attributed to less common causes, including infection with the Epstein–Barr virus (EBV), autoimmune gastritis, and Menetrier’s disease. A recent international pooled analysis of 15 studies with 5081 gastric cancer cases reported that approximately 8% of gastric cancers harbor EBV,25 but there is insufficient epidemiological evidence of a clear etiological role for EBV in gastric carcinogenesis.23
Other factors associated with increased risk for gastric cardia and non-cardia cancers include tobacco smoking, low socioeconomic status, low level of physical activity, and radiation exposure; obesity and gastroesophageal reflux disease are only associated with increased risk of gastric cardia cancer.26 A report by the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) on dietary factors and cancer prevention stated that non-starchy vegetables and fruits probably protect against gastric cancer.27 A high salt diet has been associated with a higher risk for gastric cancer,27 and salt may act synergistically with H. pylori infection to confer especially high risk for gastric cancer.28,29 Diets high in nitroso compounds could also be associated with increased risk for gastric cancer.27 Conversely, use of aspirin and other nonsteroidal anti-inflammatory drugs and statins may lower risk for gastric cancer.26 Incorporating risk factors into clinical prediction models could lead to more efficient selection for screening of patients at risk for gastric cancer. A number of models have been developed;30–32 however, none of these models is perfect, as each has its own short-comings and they require further examination in larger, external populations before clinical implementation can be recommended.33
Clinical and Public Health Implications: Gastric Cancer Prevention and Surveillance
Although gastric cancer incidence and mortality rates continue to decline, gastric cancer remains the fifth-most commonly diagnosed cancer type worldwide and the third-most common cause of cancer-related death. The mortality of patients diagnosed with gastric cancer is strongly related to their stage of disease. Gastric cancer is a main contributor to global disability-adjusted life-year burden. In 2019, an estimated 27,510 adult Americans will be diagnosed with incident gastric cancer; 11,140 will die from gastric cancer. Gastric cancer prevention has focused on screening and surveillance as well as H. pylori screening and eradication.
Screening and surveillance:
Several countries, including Venezuela, Chile, Korea, China, and Japan, have implemented various screening programs. For example, in South Korea, the Korean Gastric Cancer Association and National Cancer Center established national guidelines for gastric cancer screening in 2001. These guidelines recommend biennial gastric cancer screening via upper endoscopy or upper gastrointestinal series for men and women aged ≥40 years.34 A nationwide gastric cancer screening program in South Korea was started in 2002. In 2013, the Japanese government approved insurance coverage for a gastric cancer prevention program that includes H. pylori screening and treatment (primary prevention) as well as post-H. pylori treatment surveillance (secondary prevention for people with atrophic gastritis).35,36
There is increasing attention given to identifying higher risk patients (e.g., patients with advanced precancerous lesions) for gastric cancer surveillance. In a cohort study of patients in the Dutch nationwide histopathology registry, the 10-year gastric cancer risk in patients with mild-to-moderate or severe gastric dysplasia was 4% and 33%, respectively.37 In a cohort of high-risk Chinese patients with dysplasia, the risks were 3% and 7%, respectively, after 5 years of follow-up.38 These patients are the ideal target for gastric cancer surveillance; however, no evidence-based guidelines for follow-up strategies have been established, and the few guidelines available are mostly derived from expert opinions.39
H. pylori screening and eradication:
There are some data among healthy asymptomatic infected Asians that show eradicating H. pylori reduces the incidence of gastric cancer. A recent systematic review and meta-analysis of six international randomized controlled trials compared risk of developing incident gastric cancer in adults who have tested positive for H. pylori and received eradication therapy, placebo, or no therapy.40 The risk of developing incident gastric cancer was 34% lower among the 3294 individuals who received eradication therapy compared with the 3203 control subjects (relative risk 0.66, 95% CI, 0.46 to 0.95). This corresponds to a number needed to treat to prevent one gastric cancer of 124 (95% CI, 78 to 843).40 A recent phase 3 randomized controlled trial in school-aged children reported the success of a prophylactic oral H. pylori vaccine.41 A large community-based intervention trial in China demonstrated that H. pylori eradication is feasible and acceptable; however, they also identified several risk factors (e.g., missed doses, male sex, smoking, obesity, and alcohol consumption) for eradication failure.42 These studies show that under conditions of low bacterial burden and acute infection, persistent H. pylori infection can be prevented, and additional follow-up of these patients should provide promising data on the preventive effect of H. pylori eradication on the incidence of gastric cancer.
The potential effectiveness of a gastric cancer prevention program that includes H. pylori screening and treatment is dependent upon a patient’s level of cancer risk at the time H. pylori is eradicated (e.g., considering the 2-fold higher risk of developing gastric cancer between the most- and the least-virulent strains) and the screening modality used. Under various assumptions about both effectiveness and costs, population-based screening for H. pylori and eradication of the infection has been shown to be cost-effective.43,44 In geographic regions with high gastric cancer burden, population-based H. pylori serology screening is cost-effective, particularly if performed in persons aged >50 years. However, in countries with low incidence of gastric cancer, including the U.S., such a screening strategy is thought to be too costly and generally unwarranted. However, there is some evidence that population-based H. pylori serology screening is cost-effective, even for populations with gastric cancer rates as low as 4.2 per 100,000 persons.44 Screening in high-risk subpopulations residing in countries with low gastric cancer incidence may potentially be cost-effective. In the U.S., studies are needed to identify those at highest risk, so they can be targeted for screening in an effort to prevent gastric cancer.
In the U.S., there are no population-based mass screening programs aimed at reducing incidence of gastric cancer. Population-based programs of screening and treatment for H. pylori currently appear to hold the greatest promise for reducing the burden of gastric cancer.43
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
REFERENCES
- 1.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2018. [03/20/2019]. [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 4.Luo G, Zhang Y, Guo P, et al. Global patterns and trends in stomach cancer incidence: Age, period and birth cohort analysis. Int J Cancer 2017;141:1333–1344. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Colombet M, Bray F. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase No. 9 [Internet]. Lyon, France: 2018. [Google Scholar]
- 6.Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther 2014;40:250–260. [DOI] [PubMed] [Google Scholar]
- 7.Soerjomataram I, Lortet-Tieulent J, Parkin DM, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 2012;380:1840–1850. [DOI] [PubMed] [Google Scholar]
- 8.Arnold M, Karim-Kos HE, Coebergh JW, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur J Cancer 2015;51:1164–1187. [DOI] [PubMed] [Google Scholar]
- 9.Ferro A, Peleteiro B, Malvezzi M, et al. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer 2014;50:1330–1344. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Park J, Nam BH, et al. Stomach cancer incidence rates among Americans, Asian Americans and Native Asians from 1988 to 2011. Epidemiology and health 2015;37:e2015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamineni A, Williams MA, Schwartz SM, et al. The incidence of gastric carcinoma in Asian migrants to the United States and their descendants. Cancer Causes Control 1999;10:77–83. [DOI] [PubMed] [Google Scholar]
- 12.Chang ET, Gomez SL, Fish K, et al. Gastric cancer incidence among Hispanics in California: patterns by time, nativity, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev 2012;21:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colquhoun A, Arnold M, Ferlay J, et al. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut 2015;64:1881–1888. [DOI] [PubMed] [Google Scholar]
- 14.Anderson WF, Camargo MC, Fraumeni JF Jr., et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010;303:1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2017 submission data (1999–2015): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. 2018. [03/20/2019]; Available from: www.cdc.gov/cancer/dataviz. [Google Scholar]
- 16.SEER*Stat Database: NPCR and SEER Incidence - U.S. Cancer Statistics Public Use Database, Nov 2017 submission (2001–2015). Created on 9/15/2018 2018. [Google Scholar]
- 17.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2017 Sub (2000–2015) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission. 2018. [Google Scholar]
- 18.Wang Z, Graham DY, Khan A, et al. Incidence of gastric cancer in the USA during 1999 to 2013: a 50-state analysis. Int J Epidemiol 2018;47:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson WF, Rabkin CS, Turner N, et al. The changing face of noncardia gastric cancer incidence among US non-Hispanic Whites. J Natl Cancer Inst 2018;110:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez CA, Megraud F, Buissonniere A, et al. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann Oncol 2012;23:1320–1324. [DOI] [PubMed] [Google Scholar]
- 21.Lochhead P, El-Omar EM. Helicobacter pylori infection and gastric cancer. Best Pract Res Clin Gastroenterol 2007;21:281–297. [DOI] [PubMed] [Google Scholar]
- 22.Plummer M, Franceschi S, Vignat J, et al. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer 2015;136:487–490. [DOI] [PubMed] [Google Scholar]
- 23.IARC. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 24.Peleteiro B, Bastos A, Ferro A, et al. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci 2014;59:1698–1709. [DOI] [PubMed] [Google Scholar]
- 25.Camargo MC, Murphy G, Koriyama C, et al. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis. Br J Cancer 2011;105:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR,: 2007. [Google Scholar]
- 28.Toyoda T, Tsukamoto T, Hirano N, et al. Synergistic upregulation of inducible nitric oxide synthase and cyclooxygenase-2 in gastric mucosa of Mongolian gerbils by a high-salt diet and Helicobacter pylori infection. Histol Histopathol 2008;23:593–599. [DOI] [PubMed] [Google Scholar]
- 29.Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res 2007;67:4709–4715. [DOI] [PubMed] [Google Scholar]
- 30.Cai Q, Zhu C, Yuan Y, et al. Development and validation of a prediction rule for estimating gastric cancer risk in the Chinese high-risk population: a nationwide multicentre study. Gut 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eom BW, Joo J, Kim S, et al. Prediction Model for Gastric Cancer Incidence in Korean Population. PLoS One 2015;10:e0132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charvat H, Sasazuki S, Inoue M, et al. Prediction of the 10-year probability of gastric cancer occurrence in the Japanese population: the JPHC study cohort II. Int J Cancer 2016;138:320–331. [DOI] [PubMed] [Google Scholar]
- 33.Thrift AP, Kanwal F, El-Serag HB. Prediction models for gastrointestinal and liver diseases: too many developed, too few validated. Clin Gastroenterol Hepatol 2016;14:1678–1680. [DOI] [PubMed] [Google Scholar]
- 34.Choi KS, Suh M. Screening for gastric cancer: the usefulness of endoscopy. Clinical endoscopy 2014;47:490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asaka M A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer 2013;132:1272–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asaka M, Kato M, Sakamoto N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J Gastroenterol 2014;49:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology 2008;134:945–952. [DOI] [PubMed] [Google Scholar]
- 38.You WC, Li JY, Blot WJ, et al. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer. Int J Cancer 1999;83:615–619. [DOI] [PubMed] [Google Scholar]
- 39.Choi IJ. Endoscopic gastric cancer screening and surveillance in high-risk groups. Clinical endoscopy 2014;47:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ford AC, Forman D, Hunt RH, et al. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 2014;348:g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng M, Mao XH, Li JX, et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1457–1464. [DOI] [PubMed] [Google Scholar]
- 42.Pan KF, Zhang L, Gerhard M, et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut 2016;65:9–18. [DOI] [PubMed] [Google Scholar]
- 43.IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. Lyon, France: International Agency for Research on Cancer; (IARC Working Group Reports, No. 8). : 2014. [Google Scholar]
- 44.Areia M, Carvalho R, Cadime AT, et al. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter 2013;18:325–337. [DOI] [PubMed] [Google Scholar]


