Abstract
Background
No available meta-analysis has been published that systematically assessed spinal fixation mechanical failure after tumor resection based on largely pooled data. This systematic review and meta-analysis aimed to investigate the spinal fixation failure rate and potential risk factors for hardware failure.
Methods
Electronic articles published between January 1, 1979, and January 30, 2021, were searched and critically evaluated. The authors independently reviewed the abstracts and extracted data on the spinal fixation failure rate and potential risk factors.
Results
Thirty-eight studies were finally included in the meta-analysis. The pooled spinal fixation mechanical failure rate was 10%. The significant risk factors for hardware failure included tumor level and cage subsidence. Radiotherapy was a potential risk factor.
Conclusion
The spinal fixation mechanical failure rate was 10%. Spinal fixation failure is mainly associated with tumor level, cage subsidence and radiotherapy. Durable reconstruction is needed for patients with these risk factors.
Keywords: Spinal tumor resection, Spinal fixation mechanical failure, Risk factors, Tumor level, Cage subsidence, Radiotherapy, Meta-analysis
Introduction
The spine is a common site of musculoskeletal tumors, and spinal tumor patients must undergo spinal surgery to relieve neural compression, control local tumors and prolong survival [1, 2]. After resecting the tumor, internal fixation is used to attain spinal stability [3, 4]. Given the increased survival of patients, there is a growth trend of fixations experiencing failure. Spinal hardware failure could cause spinal instability and decrease the quality of life of patients [5–10]. To avoid the mechanical failure of spinal fixation, it is important to study factors related to the current situation.
Although some studies [7, 11–16] on spinal fixation mechanical failure after tumor resection have been published, some questions remain unanswered. First, most current studies describe only the rate of spinal hardware failure and the potential risk factors based on clinical experience, and these studies lack statistical risk factor analyses [13, 16, 17]. Second, statistical analysis was only performed in a few studies, and the population of included patients was small, which may affect the results [3, 4, 18]. In addition, not all studies included vertebral location [3–5, 11] as a risk factor. Therefore, to better guide clinical therapy, a meta-analysis is urgently needed to investigate the factors associated with spinal fixation mechanical failure.
Materials and methods
Search strategy
A comprehensive literature search was performed using the PubMed, EMBASE, Web of Science, and Cochrane Library databases for studies published between January 1, 1979, and January 30, 2021. The following MeSH terms and their combinations were searched: ((Spine[MeSH Terms]) AND (((Neoplasms[MeSH Terms]) OR (Sarcoma[MeSH Terms])) OR (Carcinoma[MeSH Terms]))) AND ((((instrumentation failure) OR (fixation failure)) OR (hardware failure)) OR (Rod fracture)). Two authors independently reviewed the titles and abstracts to screen and extract relevant articles.
Selection criteria
The PICOS criteria for inclusion and exclusion were as follows:
P (participants): Studies of spinal tumor surgery were included.
I and C (intervention and control): Studies in which spinal tumor patients received tumor resection and spinal fixation were included. If some studies included partially duplicated patients, only the studies that used large and advanced data were included.
O (outcome): Studies that included patients with spinal fixation mechanical failure with or without the following clinicopathologic factors were included: sex, age, chemotherapy, radiotherapy, tumor histology, location, surgical approach, number of vertebrae resected, rod diameter, constructed length and cage subsidence. For risk factor analysis, only the studies reporting fixation failure rates stratified by each risk factor were included. When a study reported the results on different subpopulations, we regarded data from the subpopulations as separate studies in the meta-analysis.
S (study type): Research articles published between January 1, 1979, and January 30, 2021, were included. All review papers, meta-analyses, and case reports were excluded.
Quality assessments
The quality of each eligible study was rated independently by two reviewers using the modified Newcastle–Ottawa scale 27. A score of 0–9 was assigned to each study.
Data extraction
A data collection sheet was developed to record the level of evidence, study quality, available outcomes, and risk factors. Two investigators independently extracted data from these studies. If the variable was divided into dichotomous subgroups, data from the two subgroups were included regardless of the cutoff value. If the variable was divided into polytomous rather than dichotomous subgroups, only the data of subgroups in both ends were included.
Statistical analysis
The analyses were performed using Stata 14.0 (StataCorp, College Station, TX, USA). We used a random-effects model to produce a pooled overall estimate for the spinal fixation failure rate with Stata 14.0. The OR was used to compare dichotomous variables. All results were reported with 95% CI. Statistical heterogeneity between studies was assessed using the Chi-square test and quantified using the I2 statistic. If p < 0.1 and I2 ≥ 50%, the random-effects model was used to merge the ORs. If p > 0.1 and I2 < 50%, the fixed-effect model was used to merge the OR values. When OR > 1, the factors were accepted as risk factors resulting in fixation failure. When OR < 1, the factors were accepted as protective factors avoiding fixation failure. If significant heterogeneity was noted, an increased quantity of included studies was necessary.
Sensitivity analysis and publication bias
Sensitivity analysis was performed to evaluate whether the results of the meta-analysis changed after the removal of any one study. To assess the presence of publication bias, we used funnel plots and Egger’s test. A value of p < 0.05 indicated statistically significant publication bias.
Results
Study characteristics
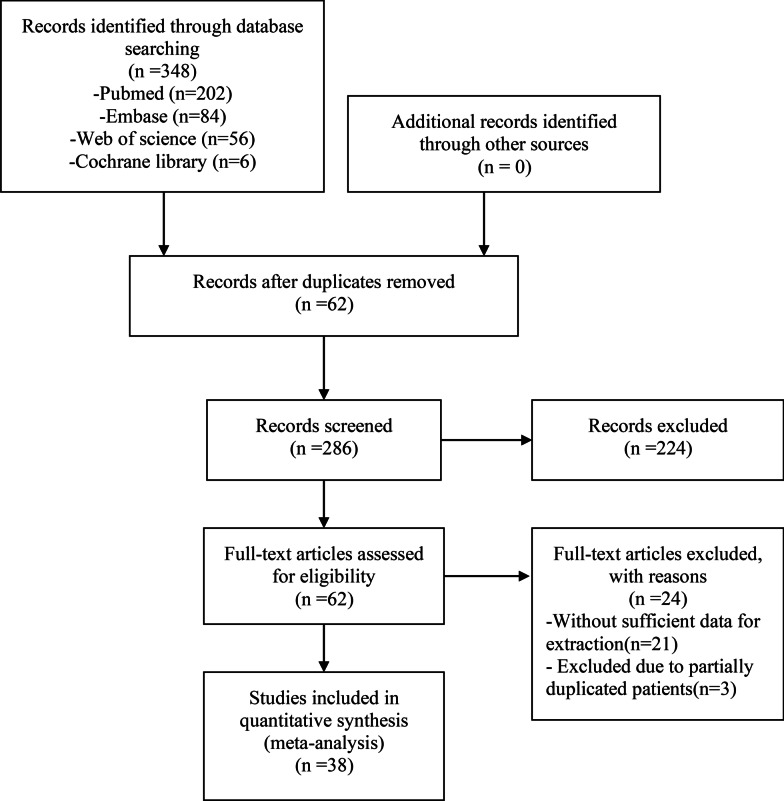
We preliminarily screened 348 studies from the PubMed, Embase, Web of Science, and Cochrane Library databases. After reading the articles, 310 studies did not conform to the inclusion criteria. Therefore, 38 studies [1–38] were finally included in the meta-analysis. All the included studies were retrospective and had evidence of 3B or 4 according to the criteria of the Center for Evidence-Based Medicine in Oxford, UK. All observation studies had a quality score of 5 or greater on the Newcastle–Ottawa scale and were considered to have high quality (Fig. 1; Table 1).
Fig. 1.
The flow chart shows the selection of studies for meta-analysis
Table 1.
Characteristics of the included studies
| Study | Year | Time frame | Level of evidencea | Quality scoreb | Country | Agec (years) | Total pts. (n) | Male | Female | Median follow-up (months) | Fixation failure rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| McLain, R. F | 1991 | 1984–1989 | 4 | 7 | USA | 49.55 | 11 | 7 | 4 | 17 | 36.36 |
| Dickman, C. A | 1992 | 1987–1991 | 4 | 7 | USA | 47 | 104 | 55 | 49 | 20 | 17.31 |
| Rompe, J. D | 1993 | 1987–1991 | 4 | 7 | Germany | 61 | 50 | 23 | 27 | ≥ 12 | 6.45 |
| Bilsky, M. H | 2002 | 1985–1999 | 4 | 7 | USA | 54 | 42 | 28 | 14 | 35 | 4.76 |
| Vrionis, F. D | 2003 | 2000–2003 | 4 | 7 | USA | 53 | 96 | 56 | 40 | NA | 3.10 |
| Mazel, C | 2004 | 1994–2000 | 4 | 7 | France | 52 | 34 | 27 | 5 | 15 | 5.88 |
| Villavicencio, A. T | 2005 | 1993–1999 | 4 | 6 | USA | 51 | 58 | NA | NA | NA | 3.50 |
| Bilsky, M. H | 2005 | 1996–2003 | 4 | 7 | USA | 53 | 41 | 22 | 19 | NA | 7.32 |
| Street, J | 2007 | NA | 4 | 6 | Canada | NA | 96 | NA | NA | NA | 1.04 |
| Placantonakis, D. G | 2008 | 1996–2006 | 4 | 7 | USA | 52 | 90 | 58 | 32 | 21 | 12.00 |
| Stevens, Q. E | 2009 | 2003–2006 | 4 | 7 | USA | 56.3 | 34 | 17 | 17 | 12 | 5.88 |
| Matsumoto, M | 2011 | 1997–2009 | 4 | 7 | Japan | 46.5 | 15 | 12 | 3 | 41.5 | 40.00 |
| Rajpal, S | 2012 | 1995–2009 | 4 | 7 | USA | 56.3 | 37 | 20 | 17 | 21 | 2.70 |
| Jandial, R | 2013 | 2008–2010 | 4 | 7 | USA | 56.64 | 11 | 6 | 5 | 14 | 9.09 |
| Matsumoto, M | 2013 | 1997–2009 | 4 | 7 | Japan | 55.3 | 8 | 5 | 3 | 76.8 | 37.50 |
| Yoshioka, K | 2013 | 2006–2012 | 4 | 7 | Japan | 49.6 | 26 | 11 | 15 | 26.5 | 3.85 |
| Bellato, R. T | 2015 | 2009–2014 | 4 | 7 | Brazil | 56.71 | 105 | 54 | 51 | 7.4 | 8.57 |
| Luzzati, A. D | 2015 | 1994–2011 | 4 | 7 | Italy | 48 | 38 | 18 | 20 | 39 | 2.60 |
| Mesfin, A | 2015 | 2001–2013 | 4 | 7 | USA | 50.7 | 10 | 9 | 1 | NA | 10.00 |
| Sellin, J. N | 2015 | 1993–2010 | 4 | 7 | USA | 59 | 43 | 26 | 17 | NA | 4.65 |
| Boriani, S | 2016 | 1990–2015 | 4 | 7 | Italy | 44.1 | 216 | 113 | 103 | 45 | 10.19 |
| Glorion, M | 2016 | 1992–2004 | 4 | 7 | France | 45.9 | 88 | 60 | 28 | 49.4 | 9.09 |
| Goodwin, C. R | 2016 | 2004–2014 | 4 | 6 | USA | NA | 21 | NA | NA | 51 | 38.10 |
| Sciubba, D. M | 2016 | 2004–2014 | 4 | 7 | USA | 47 | 23 | 15 | 8 | 50 | 39.10 |
| Pedreira, R | 2017 | 2003–2013 | 4 | 7 | USA | 60/65 | 159 | 85 | 74 | ≥ 3 | 1.90 |
| Scotto, G | 2017 | 1992–2017 | 4 | 7 | Italy | NA | 518 | NA | NA | NA | 5.10 |
| Shah, A. A | 2017 | 2010–2016 | 4 | 7 | USA | 58 | 33 | 20 | 13 | 18 | 25.00 |
| Yoshioka, K | 2017 | 2006–2010 | 4 | 7 | Japan | 53.3 | 47 | 20 | 27 | 71.3 | 17.00 |
| Shimizu, T | 2018 | 1993–2015 | 4 | 7 | Japan | 38 | 30 | 13 | 17 | 87 | 20.00 |
| Sugita, S | 2018 | 1992–2008 | 4 | 6 | Japan | 63 | 191 | NA | NA | 9.9 | 27.00 |
| Barzilai, O | 2019 | 2016–2017 | 4 | 8 | USA | 63.5 | 53 | 30 | 23 | 4.93 | 6.00 |
| Barzilai, O | 2019 | 2010–2015 | 4 | 7 | USA | 61 | 88 | 44 | 44 | 44.6 | 12.50 |
| Park, S. J | 2019 | 2002–2015 | 4 | 7 | Korea | 49 | 32 | 18 | 14 | 49.8 | 37.50 |
| Li, Z. H | 2020 | 2009–2017 | 4 | 7 | China | 37.1 | 30 | 20 | 10 | 41.8 | 26.67 |
| Park, S. J | 2020 | 2010–2017 | 4 | 6 | Korea | NA | 136 | NA | NA | 16.5 | 6.62 |
| Shinmura, K | 2020 | 2010–2015 | 4 | 7 | Japan | NA | 61 | NA | NA | > 24 | 42.60 |
| Wei, H. Y | 2020 | 2015–2018 | 4 | 7 | China | 45.5 | 15 | 7 | 8 | 31.1 | 6.67 |
| Wong, Y. C | 2020 | 2007–2017 | 4 | 7 | China | 57.3 | 88 | 45 | 43 | NA | 10.20 |
aLevel of evidence: according to the criteria of the Centre for Evidence-Based Medicine
bQuality score: the score of the study using the Newcastle–Ottawa Scale
cAge is represented by the median or the average age of the study population
Spinal fixation mechanical failure rate
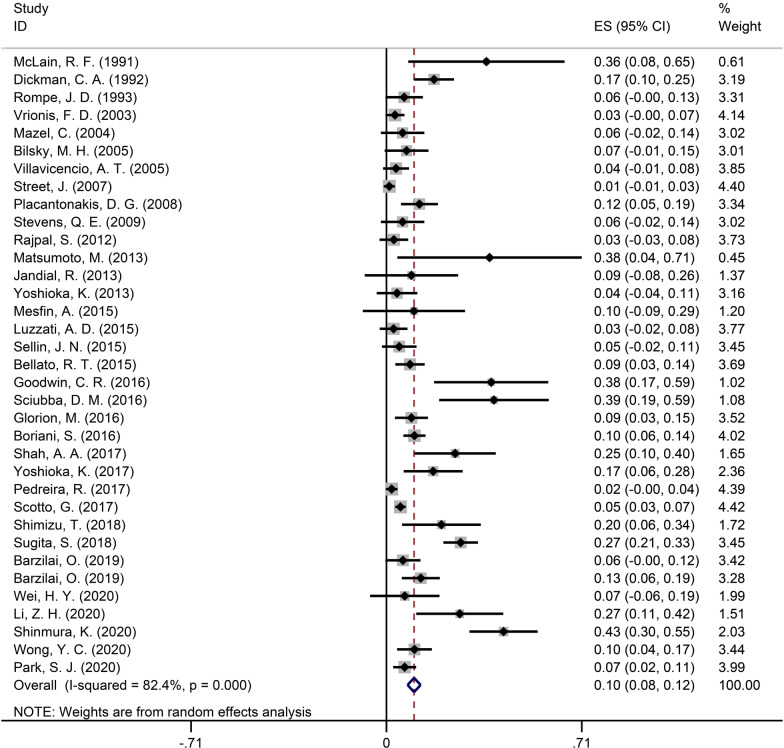
The pooled data on the spinal fixation mechanical failure rate consisted of 35 [1–3, 5–10, 12–37] studies with 2689 patients. The pooled failure rate was 10% (95% CI 8–12%) and is shown in Fig. 2.
Fig. 2.
Forest plot showing the pooled spinal fixation mechanical failure rate
The prognostic factors with similar variables were pooled in the meta-analysis. The details of the meta-analysis results are shown in Table 2.
Table 2.
Show results of meta-analysis including pooled OR, 95% CI, sensitivity analysis, and publication bias
| Prognostic factors | N | OR range | Pooled OR | Pooled 95% CI | Heterogeneity (I2) (%) | Model | p | Sensitivity analysis | Affected study | Publication bias (Egger’s test) |
|---|---|---|---|---|---|---|---|---|---|---|
| The older versus the younger [3–6, 8, 11, 18] | 7 | 0.73–2.55 | 1.01 | 0.97–1.05 | 0.0 | Fixed | 0.634 | No effect | None | 0.634 |
| The female versus the male [3–6, 8, 11, 18] | 7 | 0.44–12.75 | 1.17 | 0.67–2.04 | 0.0 | Fixed | 0.591 | No effect | None | 0.455 |
| With versus without chemotherapy [3, 6, 11, 18] | 4 | 0.19–2.59 | 1.77 | 0.83–3.78 | 8.8 | Fixed | 0.142 | Effect | Matsumoto, M | 0.183 |
| With versus without radiotherapy [3–6, 8, 11, 18] | 8 | 0.09–10.89 | 2.56 | 0.99–6.62 | 56.0 | Random | 0.053 | No effect | None | 0.894 |
| Primary versus metastatic tumor [3, 4, 11] | 3 | 0.57–2.13 | 0.93 | 0.46–1.87 | 0.0 | Fixed | 0.834 | No effect | None | 0.750 |
| Thoraciclumbar versus thoracic level [4, 5, 11] | 3 | 1.75–4.25 | 2.26 | 1.07–4.77 | 0.0 | Fixed | 0.032 | No effect | None | 0.642 |
| Lumbar versus thoracic level [3–5, 11] | 4 | 1.40–7.29 | 2.49 | 1.37–4.53 | 0.0 | Fixed | 0.003 | No effect | None | 0.890 |
| Posterior only versus combined approach [3, 11] | 2 | 1.14–4.76 | 1.46 | 0.47–4.50 | 0.0 | Fixed | 0.514 | Effect | Matsumoto, M | – |
| Multiple versus single vertebrae resection [3–5, 8, 11, 18] | 6 | 0.22–2.10 | 0.97 | 0.48–1.94 | 0.0 | Fixed | 0.930 | No effect | None | 0.726 |
| thin versus thick rod [4, 11] | 2 | 1.08–1.50 | 1.27 | 0.54–2.96 | 0.0 | Fixed | 0.587 | No effect | None | – |
| Longer versus shorter constructed length [3, 8, 11, 18] | 4 | 0.91–3.25 | 1.13 | 0.79–1.61 | 47.9 | Fixed | 0.498 | Effect | Wong, Y. C | 0.365 |
| With versus without cage subsidence [5, 11] | 2 | 4.05–14.63 | 5.46 | 1.48–20.17 | 0.0 | Fixed | 0.011 | No effect | None | – |
Age
Seven studies [3–6, 8, 11, 18] compared the spinal fixation failure rate between the older and younger subgroups. Values of I2 = 0.0% and p = 0.945 were obtained after the OR values of the failure rate were merged, indicating that no heterogeneity existed. A fixed-effect model was used to merge the data (OR = 1.01, 95% CI 0.97–1.05 and p = 0.634), showing no significant difference in the failure rate between the older and younger subgroups.
Sex
Seven studies [3–6, 8, 11, 18] comparing the failure rate between males and females were included. Values of I2 = 0.0% and p = 0.694 were obtained after OR values of failure rate were merged, indicating that no heterogeneity existed. A fixed-effect model was used to merge the data (OR = 1.17, 95% CI 0.67–2.04 and p = 0.591), suggesting that the failure rate did not significantly differ based on sex.
Chemotherapy
Four studies [3, 6, 11, 18] evaluated chemotherapy as a risk factor for spinal fixation failure. Values of I2 = 8.8% and p = 0.349 were obtained after OR values of failure rates were merged, indicating that no heterogeneity existed. A fixed-effect model was used to merge the data (OR = 1.77, 95% CI 0.83–3.78 and p = 0.142). The results showed no significant difference in the failure rate between patients who received chemotherapy and those who did not receive chemotherapy.
Radiotherapy
A total of 8 studies (including subgroups) [3–6, 8, 11, 18] assessed the association between radiotherapy and failure rate. Values of I2 = 56.0% and p = 0.415 were obtained after the OR values of the failure rate were merged, indicating that heterogeneity existed. The pooled result via a random-effects model minimally indicated that patients with radiotherapy had a higher risk of fixation failure than patients without radiotherapy (OR = 2.56, 95% CI 0.99–6.62, p = 0.053).
Tumor histology
Three studies [3, 4, 11] evaluated the relationship between tumor histology and failure rate. Values of I2 = 0.0% and p = 0.541 were obtained after the OR values of the failure rate were merged, indicating that heterogeneity did not exist. Thus, a fixed-effect model was applied. No significant difference in tumor histology was observed (OR = 0.93, 95% CI 0.46–1.87, p = 0.834).
Tumor site
Four studies [3–5, 11] evaluated the relation between the tumor site and failure rate. Three studies [4, 5, 11] compared the failure rate between thoracic-lumbar and thoracic levels with no heterogeneity (I2 = 0.0% and p = 0.972). Thus, a fixed-effect model was applied. Thoraciclumar level had an increased risk of fixation failure (OR = 2.26, 95% CI 1.07–4.77, p = 0.032). Four studies [3–5, 11] compared the failure rate between the lumbar and thoracic levels, with heterogeneity existing (I2 = 0.0% and p = 0.500) and a fixed-effects model applied. Lumbar level exhibited an increased risk of fixation failure (OR 2.49, 95% CI 1.37–4.53, p = 0.003).
Surgical approach
Two studies [3, 11] explored the failure rate and surgical approach included, and no heterogeneity was noted (I2 = 0.0% and p = 0.350). Thus, a fixed-effect model was applied. The failure rate was not significantly different based on the surgical approach (OR = 1.46, 95% CI 0.47–4.50, p = 0.514).
Vertebrae resection
Six studies [3–5, 8, 11, 18] evaluated the relation between vertebrae and failure rate. Values of I2 = 0.97 and p = 0.671 were obtained after the OR values of the failure rate were merged, indicating that heterogeneity did not exist. Thus, a fixed-effect model was applied. A significant difference was not found in the number of vertebrae resected (OR = 0.97, 95% CI 0.48–1.94, p = 0.930).
Rod diameter
Two studies [4, 11] evaluated the relation between rod diameter and failure rate. Values of I2 = 0.0% and p = 0.705 were obtained after the OR values of the failure rate were merged, indicating that heterogeneity did not exist. Thus, a fixed-effect model was applied. No significant difference in rod diameter was noted (OR = 1.27, 95% CI 0.54–2.96, p = 0.587).
Constructed length
Four studies [3, 8, 11, 18] included the failure rate and constructed length. No heterogeneity was noted (I2 = 47.9% and p = 0.124), and a fixed-effect model was applied. The meta-analysis failed to find significance among different constructed lengths (OR = 1.13, 95% CI 0.79–1.61, p = 0.498).
Cage subsidence
Two studies [5, 11] evaluated the relation between cage subsidence and failure rate. Values of I2 = 0.0% and p = 0.416 were obtained after the OR values of the failure rate were merged, indicating that heterogeneity did not exist. Thus, a fixed-effect model was applied. Collectively, cage subsidence is a significant risk factor for spinal fixation failure (OR = 5.46, 95% CI 1.48–20.17, p = 0.011).
Sensitivity analysis and publication bias
Sensitivity analysis was performed in these groups. The pooled OR of chemotherapy changed significantly when excluding the study by Matsumoto [11]. The pooled OR of the surgical approach changed significantly when excluding the study by Matsumoto [11]. The pooled OR of constructed length changed significantly when excluding the study by Wong [8]. The results of the other meta-analysis did not change after removal of any one study.
Egger’s test was completed to examine the existence of publication bias. Publication bias failed to evaluate the surgical approach, rod diameter and cage subsidence because these subgroups only included two studies. Egger’s test resulted in p ≥ 0.05 in the other groups and indicated that the possibilities of publication bias can be excluded.
Discussion
Durable reconstruction is required to achieve spinal stabilization after tumor resection [3, 4]. Fixation failure is a troubling complication for tumor patients who acquire long-term survival with effective therapy [10–12]. Therefore, it is important to identify risk factors affecting spinal fixation and optimize reconstruction proposals. In this study, we performed a systematic review and meta-analysis to evaluate the failure rate of spinal fixation after tumor resection and to investigate the related risk factors for spinal fixation failure.
Although complications, including fixation failure, have been reported in numerous studies, the incidence varies. Thus, the practical fixation failure rate remains unclear. Sciubba et al. [18] studied 23 patients who underwent TES of the lumbar spine and reported that 9 (39.1%) patients experienced instrumentation failure. Luzzati et al. [38] studied 38 patients with multilevel TES for tumors of the thoracic and lumbar spine and found that only one (2.6%) patient required revision of instrumentation secondary to mechanical failure. Boriani et al. [13] reviewed 220 cases treated by TES in the spine and reported that hardware failure occurred in 22 (10%) cases. Mesfin et al. [28] assessed 10 patients with TES for primary and secondary spinal tumors, and 1 (10%) patient experienced hardware failure and required revision. In this study, the incidence of spinal fixation failure was 10% (range 8–12%), which eliminated the heterogeneity caused by different sample sizes in these studies.
Radiotherapy
The quality and strength of bone are influenced by radiation, which may affect the stabilization of spinal fixation. Matsumoto et al. [11] reported that all 3 patients with preoperative radiotherapy suffered hardware failure, whereas only 3 of the 12 patients without preoperative radiotherapy suffered instrumentation failure. Li et al. [3] found that perioperative radiotherapy was associated with instrumentation failure and reported that radiation may not only influence vertebral bone quality but also lead to muscle atrophy and weakness. However, Wong et al. reported the opposite result. Specifically, radiotherapy reached statistical significance with fixation failure being less likely to develop following radiation. They believed that vertebral recalcification occurring after radiotherapy could increase the load-sharing ability of the vertebra, which may explain the reduced implant failure rate after radiotherapy [8]. In our study, there was a trend to indicate that radiotherapy may represent a risk factor for spinal fixation failure.
Tumor level
Regarding tumor location, Matsumoto et al. [11] failed to indicate that tumor level was significantly related to instrumentation failure. However, Yoshioka et al. reported that the resection level was a risk factor for fixation failure after multilevel TES and considered that an upper spinal level promotes better stability than a lower spinal level due to the lower exposure to mechanical stresses. In addition, there were disadvantage factors for the lower spinal level, including the long resection length and spinal instability caused by the mobility of the thoracolumbar and lumbar levels [5]. Park et al. [4] reported that TES at the lumbar level had the highest risk of instrumentation failure followed by thoracolumbar and thoracic levels, and explained that the lumbar spine has the greatest moment of flexion force and lacks adjacent stabilizing structures, such as ribs of the thoracic spine. In our study, we found that the tumor level was a risk factor for spinal fixation failure, which was consistent with most of the literature.
Cage subsidence
Matsumoto et al. mentioned that cage subsidence resulted in the failure of loading sharing in the anterior spinal column, leading to an increased force imposed on the posterior fixation. In this study, they reported that cage subsidence was significantly related to instrumentation failure [11]. However, Yoshioka et al. [5] did not find a relationship between cage subsidence and instrumentation failure and insisted on the importance of eventual bony fusion, which prevented instrumentation failure despite cage subsidence. Our study found that cage subsidence is one of the reasons for fixation failure.
Limitations
This meta-analysis had some limitations. First, our meta-analysis was based on retrospective studies, so selection bias was possible. Second, prognostic factor analysis included some studies with small samples, which might result in publication bias and affect sensitivity. Further studies may be needed to verify our conclusions. Furthermore, the follow-up time varied in each study. Despite these limitations, this study applied a series of measures and strict standards to evaluate the quality of these studies.
Conclusion
In conclusion, our results indicate that the spinal fixation mechanical failure rate was 10%. Spinal fixation failure is mainly associated with tumor level, cage subsidence and radiotherapy. Durable reconstruction is needed for patients with these risk factors.
Acknowledgements
We acknowledge all the authors whose publications are referred to in our article.
Authors’ contributions
Data Extraction, ZC and YZ. Quality assessments, ZC and XT. Data analysis, ZC and YZ. Writing-origin draft, ZC. Writing-review and editing, ZC, YZ, XT, RY, TY and WG. All authors read and approved the final manuscript.
Funding
No funds were received in support of this work.
Availability of data and materials
Please contact the authors for data requests.
Declarations
Ethics approval and consent to participate
This study obtained approval from the institutional review board of Peking University People’s Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaodong Tang, Email: tang15877@163.com.
Wei Guo, Email: bonetumor@163.com.
References
- 1.Bilsky MH, Boakye M, Collignon F, Kraus D, Boland P. Operative management of metastatic and malignant primary subaxial cervical tumors. J Neurosurg Spine. 2005;2:256–264. doi: 10.3171/spi.2005.2.3.0256. [DOI] [PubMed] [Google Scholar]
- 2.Villavicencio AT, Oskouian RJ, Roberson C, Stokes J, Park J, Shaffrey CI, Johnson JP. Thoracolumbar vertebral reconstruction after surgery for metastatic spinal tumors: long-term outcomes. Neurosurg Focus. 2005;19:E8. doi: 10.3171/foc.2005.19.3.9. [DOI] [PubMed] [Google Scholar]
- 3.Li ZH, Wei F, Liu ZJ, Liu XG, Jiang L, Yu M, Xu NF, Wu FL, Dang L, Zhou H, Li ZH. Risk factors for instrumentation failure after total en bloc spondylectomy of thoracic and lumbar spine tumors using titanium mesh cage for anterior reconstruction. World Neurosurg. 2020;135:66. doi: 10.1016/j.wneu.2019.11.057. [DOI] [PubMed] [Google Scholar]
- 4.Park SJ, Lee CS, Chang BS, Kim YH, Kim H, Kim SI, Chang SY. Rod fracture and related factors after total en bloc spondylectomy. Spine J. 2019;19:1613–1619. doi: 10.1016/j.spinee.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Yoshioka K, Murakami H, Demura S, Kato S, Yokogawa N, Kawahara N, Tomita K, Tsuchiya H. Risk factors of instrumentation failure after multilevel total en bloc spondylectomy. Spine Surg Rel Res. 2017;1:31–39. doi: 10.22603/ssrr.1.2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedreira R, Abu-Bonsrah N, Karim Ahmed A, De la Garza-Ramos R, Rory Goodwin C, Gokaslan ZL, Sacks J, Sciubba DM. Hardware failure in patients with metastatic cancer to the spine. J Clin Neurosci. 2017;45:166–171. doi: 10.1016/j.jocn.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 7.Bellato RT, Teixeira WGJ, Torelli AG, Cristante AF, de Barros TEP, de Camargo OP. Late failure of posterior fixation without bone fusion for vertebral metastases. Acta Ortopedica Brasileira. 2015;23:303–306. doi: 10.1590/1413-785220152306151402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong YC, Chau WWJ, Kwok KO, Law SW. Incidence and risk factors for implant failure in spinal metastasis surgery. Asian Spine J. 2020;14:878–885. doi: 10.31616/asj.2020.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinmura K, Kato S, Demura S, Yokogawa N, Yonezawa N, Shimizu T, Oku N, Kitagawa R, Handa M, Annen R, et al. Revision surgery for instrumentation failure after total en bloc spondylectomy: a retrospective case series. BMC Musculoskelet Disord. 2020;21:66. doi: 10.1186/s12891-020-03622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glorion M, Dubory A, Mitilian D, Gomez-Caro A, Dartevelle P, Court C, Fadel E, Missenard G. Long-term mechanical results of partial thoracic vertebrectomies with posterior instrumentation only after en bloc tumoral resection. Eur Spine J. 2016;25:2339–2340. [Google Scholar]
- 11.Matsumoto M, Watanabe K, Tsuji T, Ishii K, Nakamura M, Chiba K, Toyama Y. Late instrumentation failure after total en bloc spondylectomy Clinical article. Journal of Neurosurgery-Spine. 2011;15:320–327. doi: 10.3171/2011.5.SPINE10813. [DOI] [PubMed] [Google Scholar]
- 12.Yoshioka K, Murakami H, Demura S, Kato S, Kawahara N, Tomita K, Tsuchiya H. Clinical outcome of spinal reconstruction after total en bloc spondylectomy at 3 or more levels. Spine. 2013;38:E1511–1516. doi: 10.1097/BRS.0b013e3182a6427a. [DOI] [PubMed] [Google Scholar]
- 13.Boriani S, Gasbarrini A, Bandiera S, Ghermandi R, Lador R. Predictors for surgical complications of en bloc resections in the spine: review of 220 cases treated by the same team. Eur Spine J. 2016;25:3932–3941. doi: 10.1007/s00586-016-4463-y. [DOI] [PubMed] [Google Scholar]
- 14.McLain RF, Kabins M, Weinstein JN. VSP stabilization of lumbar neoplasms: Technical considerations and complications. J Spinal Disord. 1991;4:359–365. doi: 10.1097/00002517-199109000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Rajpal S, Hwang R, Mroz T, Steinmetz MP. Comparing vertebral body reconstruction implants for the treatment of thoracic and lumbar metastatic spinal tumors: a consecutive case series of 37 patients. J Spinal Disord Technol. 2012;25:85–91. doi: 10.1097/BSD.0b013e318214b489. [DOI] [PubMed] [Google Scholar]
- 16.Shah AA, Paulino Pereira NR, Pedlow FX, Wain JC, Yoon SS, Hornicek FJ, Schwab JH. Modified en bloc spondylectomy for tumors of the thoracic and lumbar spine: surgical technique and outcomes. J Bone Joint Surg Am. 2017;99:1476–1484. doi: 10.2106/JBJS.17.00141. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu T, Murakami H, Demura S, Kato S, Yoshioka K, Yokogawa N, Kawahara N, Tomita K, Tsuchiya H. Total en bloc spondylectomy for primary tumors of the lumbar spine. Medicine. 2018;97:66. doi: 10.1097/MD.0000000000012366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sciubba DM, Ramos RD, Goodwin CR, Xu RS, Bydon A, Witham TF, Gokaslan ZL, Wolinsky JP. Total en bloc spondylectomy for locally aggressive and primary malignant tumors of the lumbar spine. Eur Spine J. 2016;25:4080–4087. doi: 10.1007/s00586-016-4641-y. [DOI] [PubMed] [Google Scholar]
- 19.Dickman CA, Fessler RG, MacMillan M, Haid RW. Transpedicular screw-rod fixation of the lumbar spine: operative technique and outcome in 104 cases. J Neurosurg. 1992;77:860–870. doi: 10.3171/jns.1992.77.6.0860. [DOI] [PubMed] [Google Scholar]
- 20.Rompe JD, Eysel P, Hopf C, Heine J. Decompression/stabilization of the metastatic spine Cotrel–Dubousset–Instrumentation in 50 patients. Acta Orthop Scand. 1993;64:3–8. doi: 10.3109/17453679308994516. [DOI] [PubMed] [Google Scholar]
- 21.Vrionis FD, Small J. Surgical management of metastatic spinal neoplasms. Neurosurg Focus. 2003;15:E12. doi: 10.3171/foc.2003.15.5.12. [DOI] [PubMed] [Google Scholar]
- 22.Mazel C, Hoffmann E, Antonietti P, Grunenwald D, Henry M, Williams J. Posterior cervicothoracic instrumentation in spine tumors. Spine. 2004;29:1246–1253. doi: 10.1097/00007632-200406010-00015. [DOI] [PubMed] [Google Scholar]
- 23.Street J, Fisher C, Sparkes J, Boyd M, Kwon B, Paquette S, Dvorak M. Single-stage posterolateral vertebrectomy for the management of metastatic disease of the thoracic and lumbar spine: a prospective study of an evolving surgical technique. J Spinal Disord Technol. 2007;20:509–520. doi: 10.1097/BSD.0b013e3180335bf7. [DOI] [PubMed] [Google Scholar]
- 24.Placantonakis DG, Laufer I, Wang JC, Beria JS, Boland P, Bilsky M. Posterior stabilization strategies following resection of cervicothoracic junction tumors: review of 90 consecutive cases. J Neurosurg Spine. 2008;9:111–119. doi: 10.3171/SPI/2008/9/8/111. [DOI] [PubMed] [Google Scholar]
- 25.Stevens QE, Majd ME, Kattner KA, Jones CL, Holt RT. Use of spinous processes to determine the optimal trajectory for placement of lateral mass screws: technical note. J Spinal Disord Technol. 2009;22:347–352. doi: 10.1097/BSD.0b013e31816f68fe. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto M, Tsuji T, Iwanami A, Watanabe K, Hosogane N, Ishii K, Nakamura M, Morioka H, Toyama Y. Total en bloc spondylectomy for spinal metastasis of differentiated thyroid cancers: a long-term follow-up. J Spinal Disord Technol. 2013;26:E137–E142. doi: 10.1097/BSD.0b013e318278c8e4. [DOI] [PubMed] [Google Scholar]
- 27.Jandial R, Kelly B, Chen MY. Posterior-only approach for lumbar vertebral column resection and expandable cage reconstruction for spinal metastases. J Neurosurg Spine. 2013;19:27–33. doi: 10.3171/2013.4.SPINE12344. [DOI] [PubMed] [Google Scholar]
- 28.Mesfin A, El Dafrawy MH, Jain A, Hassanzadeh H, Kebaish KM. Total en bloc spondylectomy for primary and metastatic spine tumors. Orthopedics. 2015;38:e995–e1000. doi: 10.3928/01477447-20151020-08. [DOI] [PubMed] [Google Scholar]
- 29.Luzzati AD, Shah S, Gagliano F, Perrucchini G, Scotto G, Alloisio M. Multilevel en bloc spondylectomy for tumors of the thoracic and lumbar spine is challenging but rewarding. Clin Orthop Relat Res. 2015;473:858–867. doi: 10.1007/s11999-014-3578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sellin JN, Suki D, Harsh V, Elder BD, Fahim DK, McCutcheon IE, Rao G, Rhines LD, Tatsui CE. Factors affecting survival in 43 consecutive patients after surgery for spinal metastases from thyroid carcinoma. J Neurosurg Spine. 2015;23:419–428. doi: 10.3171/2015.1.SPINE14431. [DOI] [PubMed] [Google Scholar]
- 31.Goodwin CR, Garza-Ramos RDL, Abu-Bonsrah N, Xu R, Wolinsky JP, Gokaslan Z, Sciubba D. Total en bloc spondylectomy outcomes for primary malignant tumors of the lumbar spine. J Neurosurg. 2016;124:A1208. doi: 10.1007/s00586-016-4641-y. [DOI] [PubMed] [Google Scholar]
- 32.Scotto G, Cannavò L, Perrucchini G, Gallazzi E, Luzzati AD. Current diagnostic and surgical approach for spinal metastasis: a single center experience. Eur Spine J. 2017;26:1336–1337. [Google Scholar]
- 33.Sugita S, Hozumi T, Yamakawa K, Goto T. The significance of spinal fixation in palliative surgery for spinal metastases. J Clin Neurosci. 2018;48:163–167. doi: 10.1016/j.jocn.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Barzilai O, McLaughlin L, Lis E, Reiner AS, Bilsky MH, Laufer I. Utility of cement augmentation via percutaneous fenestrated pedicle screws for stabilization of cancer-related spinal instability. Operative Neurosurgery. 2019;16:593–599. doi: 10.1093/ons/opy186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barzilai O, McLaughlin L, Lis E, Yamada Y, Bilsky MH, Laufer I. Outcome analysis of surgery for symptomatic spinal metastases in long-term cancer survivors. J Neurosurg Spine. 2019;31:285–290. doi: 10.3171/2019.2.SPINE181306. [DOI] [PubMed] [Google Scholar]
- 36.Wei HY, Dong CK, Wu J, Zhu YT, Ma HN. Total en bloc spondylectomy combined with the satellite rod technique for spinal tumors. J Orthopaed Surg Res. 2020;15:66. doi: 10.1186/s13018-020-1573-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SJ, Lee KH, Lee CS, Jung JY, Park JH, Kim GL, Kim KT. Instrumented surgical treatment for metastatic spinal tumors: Is fusion necessary? J Neurosurg Spine. 2020;32:456–464. doi: 10.3171/2019.8.SPINE19583. [DOI] [PubMed] [Google Scholar]
- 38.Luzzati AD, Shah SP, Gagliano FS, Perrucchini GG, Fontanella W, Alloisio M. Four- and five- Level en bloc spondylectomy for malignant spinal tumors. Spine. 2014;39:E129–E139. doi: 10.1097/BRS.0000000000000072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the authors for data requests.