Abstract
Background
Joint replacements are common procedures and treatment of choice for those with intractable joint pain and disability arising from arthropathy of the hip or knee. Multidisciplinary rehabilitation is considered integral to the outcome of joint replacement.
Objectives
To assess the evidence for effectiveness of multidisciplinary rehabilitation on activity and participation in adults following hip or knee joint replacement for chronic arthropathy.
Search methods
We searched the Cochrane Musculoskeletal Group Trials Register, the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE and CINAHL up to September 2006.
Selection criteria
Randomised controlled trials (RCTs) that compared organised multidisciplinary rehabilitation with routine services following hip or knee replacement, and included outcome measures of activity and participation in accordance with the International Classification of Functioning, Health and Disability (ICF).
Data collection and analysis
Four authors independently extracted data and assessed methodological quality of included trials.
Main results
Five trials (619 participants) met the inclusion criteria; two addressed inpatient rehabilitation (261 participants) and three (358 participants) home‐based settings. There were no trials addressing outpatient centre‐based programmes. Pooling of data was not possible due to differences in study design and outcomes used. Methodological assessment showed all trials were of low quality. For inpatient settings early commencement of rehabilitation and clinical pathways led to more rapid attainment of functional milestones (disability) (Functional Independence Measure (FIM) transfer WMD 0.5, 95% CI 0.15, 0.85, number needed to treat to benefit (NNTB) = 6, FIM ambulation WMD 1.55 (95%CI 0.96, 2.14), NNTB = 3), shorter hospital stay, fewer post‐operative complications and reduced costs in the first three to four months. Home‐based multidisciplinary care improved functional gain (Oxford Hip Score (OHS) WMD at 6 months ‐7.00 (95%CI ‐10.36, ‐3.64), NNT = 2 and quality of life (QoL) and reduced hospital stay in the medium term (six months). No trials addressed longer‐term outcomes following hip replacement only.
Authors' conclusions
Based on the heterogeneity and the low quality of the included trials that precluded pooled meta‐analysis, there is silver level evidence that following hip or knee joint replacement, early multidisciplinary rehabilitation can improve outcomes at the level of activity and participation. The optimal intensity, frequency and effects of rehabilitation over a longer period and associated social costs need further study. Future research should focus on improving methodological and scientific rigour of clinical trials, and use of standardised outcome measures, so that results can be pooled for statistical analysis.
Plain language summary
Multidisciplinary rehabilitation programmes following joint replacement at the hip and knee in chronic arthropathy
Joint replacements are common procedures and treatment of choice for those with intractable joint pain and disability arising from arthropathy of the hip or knee. Multidisciplinary rehabilitation is considered integral to the outcome of joint replacement.
Five trials (619 participants) met the inclusion criteria; two addressed inpatient rehabilitation (261 participants) and three (358 participants) home‐based settings. There were no trials addressing outpatient centre‐based programmes. Pooling of data was not possible due to differences in study design and outcomes used. Methodological assessment showed all trials were of low quality. For inpatient settings early commencement of rehabilitation and clinical pathways led to more rapid attainment of functional milestones (disability) (Functional Independence Measure (FIM) transfer WMD 0.5, 95% CI 0.15, 0.85, number needed to treat to benefit (NNTB) = 6, FIM ambulation WMD 1.55 (95%CI 0.96, 2.14), NNTB = 3), shorter hospital stay, fewer post‐operative complications and reduced costs in the first three to four months. Home‐based multidisciplinary care improved functional gain (Oxford Hip Score (OHS) WMD at 6 months ‐7.00 (95%CI ‐10.36, ‐3.64), NNT = 2 and quality of life (QoL) and reduced hospital stay in the medium term (six months). No trials addressed longer‐term outcomes following hip replacement only.
Based on the heterogeneity and the low quality of the included trials that precluded pooled meta‐analysis, there is silver level evidence that following hip or knee joint replacement, early multidisciplinary rehabilitation can improve outcomes at the level of activity and participation. The optimal intensity, frequency and effects of rehabilitation over a longer period and associated social costs need further study. Future research should focus on improving methodological and scientific rigour of clinical trials, and use of standardised outcome measures, so that results can be pooled for statistical analysis.
Background
Total hip and knee joint replacement (THJR, TKJR) procedures are now commonplace, and have become treatments of choice for people with intractable joint pain and disability due to chronic arthropathy who fail conservative management (Brady 2000). In 2004, the reported rates (per 100,000 population) for primary hip joint replacement procedures for Australia, Canada, New Zealand and the United States of America, ranged from 70‐150 (primary) and 9‐22 (revision), whilst the rates for knee replacement ranged from 62‐143 (primary) and 8‐13 (revision) (CJRR 2006). These procedures are usually successful, with < 1% failure rate per year (Monte 1997; Tankersley 1997). When revision surgery is needed, this is usually because of prosthetic loosening, lysis and component wear and tear.
The joint replacement procedures may be undertaken in a number of contexts, including:
acute injury e.g. fractured neck of femur;
chronic arthropathy affecting just one joint or multiple joints, which may be secondary to a variety of conditions including arthritis (degenerative or inflammatory), abnormal development (eg congenital hip dysplasia), or following slipped femoral epiphysis and avascular necrosis.
For the purposes of this review, we were interested in joint replacement only in the context of chronic arthropathy.
The World Health Organization has developed an International Classification of Functioning, Disability and Health (ICF) (WHO 2001) which replaces its earlier classification of Impairment, Disability and Handicap (ICIDH) (WHO 1980). The ICF defines a common language for describing the impact of disease at different levels.
'Impairments' are problems with body (anatomical) structures or (physiological) function ‐ the symptoms and signs of disease such as paresis, pain, etc.
'Activity limitation' (previously 'disability', WHO 1980) describes the difficulties that a person may have in executing everyday tasks such as self‐care.
'Restriction in participation' (previously 'handicap', WHO 1980) relates to problems experienced by a person with involvement in societal participation and life situations such as employment or social activities.
'Contextual factors' include:
a) 'environmental' factors which make up the physical, social and attitudinal environment in which people live their lives; b) 'personal factors' such as gender, race, self‐efficacy, coping style, social and educational background, which may affect the person's experience of living with their condition.
Whilst the contextual factors are an important component of the ICF, they are generally not reported in the studies included in this review, as the ICF is predated by most of these trials. Therefore, they will not be addressed further. We have classified outcomes by impairment/disability and participation.
In the context of chronic arthropathy, the person undergoing joint replacement will typically be relieved of the major disabling factor in their life. However, to maximise the benefits of joint replacement, they may require rehabilitation in order to: a) prevent complications such as deep vein thrombosis (DVT) (Morris 1998) and dislocation (McDonald 2000); b) regain strength and fitness which may have deteriorated as a result of deconditioning and prolonged immobility; and c) capitalise on their newfound mobility to regain independence and participation in society.
For many people, especially those with single joint pathology, unidisciplinary interventions (for example physiotherapy alone) may be all that is required. However a multidisciplinary, team‐based approach (for example medical, nursing, physiotherapy and occupational therapy) may be required for those with more complex problems (such as multiple joint involvement or co‐morbidities, prolonged surgery, post‐operative complications); and also for those who are elderly and frail, cognitively impaired or socially isolated. Multidisciplinary rehabilitation will typically start during the inpatient admission, but continuation into the community/home setting may be required in order to address environmental and community integration issues. In Australia, elderly patients with multiple risk factors (such as age, co‐morbidities, social set up) are referred for inpatient rehabilitation directly from the acute hospital. Alternative arrangements for medically stable patients can include ambulatory programmes (rehabilitation in the home programme, centre‐based therapies), provided the environmental set‐up is satisfactory. Practices may vary in different countries.
Other Cochrane reviews have addressed the effects of unidisciplinary therapy and limited interventions following hip or knee joint replacement, including:
preoperative education (McDonald 2004); and
continuous passive movement (CPM) (Milne 2003).
However, the evidence base for the effectiveness of multidisciplinary rehabilitation in people with hip or knee joint replacement is not yet established. As the pressure on health systems increases, affordable and appropriate healthcare planning is essential, and the use of more expensive multidisciplinary rehabilitation needs to be justified.
Objectives
We assessed the evidence for effectiveness of organised multidisciplinary rehabilitation in adults (aged 18 years and above) following hip or knee joint replacement surgery. Specific questions addressed by this review are:
Does organised multidisciplinary rehabilitation achieve better outcomes than the absence of such services in people following hip or knee joint replacement?
Which models of programmes are effective and in which setting?
Which participants benefit most?
Which specific outcomes are influenced (eg, activity, participation and quality of life)?
Does a greater intensity (time or expertise, or both) of multidisciplinary rehabilitation lead to greater gains?
Are there demonstrable cost benefits for multidisciplinary rehabilitation in hip or knee joint replacement?
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs that applied multidisciplinary rehabilitation following hip or knee joint replacement, providing that they compared the named intervention with some form of control condition.
Types of participants
We included trials if the participants were above 18 years of age and had undergone hip and/or knee joint replacement surgery for chronic arthropathy, including osteoarthritis, rheumatoid arthritis, dysplastic or osteochondrotic disease.
Joint replacement procedures included:
both total and hemi‐arthroplasty;
primary (first time) joint replacement;
secondary (repeat) replacements;
all types of prosthesis (metal, ceramic, hybrid).
We excluded surgical procedures for acute hip fracture, such as operative fixation (e.g. pins), from this review.
Types of interventions
Experimental intervention For the purpose of this review, 'multidisciplinary rehabilitation' was defined as a rehabilitation programme delivered by two or more disciplines, and targeted towards improvement at the levels of activity or participation, or both. Rehabilitation programmes could include elements of medical, nursing, physical therapy (PT), occupational therapy (OT), psychology and counselling, social work (SW), dietetics, orthotics, recreation and vocational therapy.
We identified various aspects of multidisciplinary rehabilitation as follows.
The 'timing' of multidisciplinary rehabilitation intervention, such as 'early' input (24‐48 hours post surgery) versus 'late' multidisciplinary treatment (> 48 hours after surgery).
The setting of rehabilitation programmes, including:
a) inpatient rehabilitation settings that provide 24‐hour care, such as a specialist medical rehabilitation unit or a hospital ward unit (eg, general surgical units, orthopaedic units); b) outpatient or day treatment settings, located within the hospital, a community centre/day centre or a specialist rehabilitation environment; c) home‐based setting in the persons' own home and local community.
The use of critical pathways (pre‐determined structured programmes for participants' recovery during the hospital stay) in joint replacement surgery compared with no pathways, routine care or both.
The use of pain management strategies compared with routine care.
We excluded studies assessing the effect of the following.
Therapy from a single discipline (physiotherapy), including studies on therapeutic modalities.
Programmes that included complementary medicine (yoga, meditation) in the absence of rehabilitation.
Control intervention For the purpose of this review, we considered the following control conditions.
A lower level or different type of intervention, such as 'routinely available local services' or minimal intervention such as 'information only' or 'single session treatment'.
Equivalent interventions given in different settings, such as inpatient versus community rehabilitation.
Wait list conditions.
Types of outcome measures
We were interested in the outcomes that reflect the burden of disabling disease on participants, their families and the services that provide for them. We classified the various outcome measures used in the selected studies in this review, based on the ICF (WHO 2001). We looked at evidence of improvement at the different levels.
'Impairments' (eg, joint range of motion and muscle weakness).
'Activity limitation' (eg, mobility, transfer skills, independence in activities of daily living).
'Restriction in participation' (eg, extended activities of daily living, societal re‐integration or quality of life).
The primary outcomes in this review include limitation in impairment and activity/function and secondary outcomes are limitation in participation. 'Other' outcomes for this review include cost of episode of care, length of stay, service utilisation, readmission, mortality rates and carer burden/strain. We also planned to study any adverse effects reported from rehabilitation intervention.
Additional Table 1 lists the various outcome instruments used, classified under the ICF categories. We also classified outcome measures into those that reflect benefit (improvements in health) and harm (adverse effects on health). It should be noted, however, that some validated measurement scales cross over the boundaries between the concepts of impairment, disability and participation (especially where they predate the publication of the WHO ICF). This occurs especially between the categories of impairment and disability. For example, the Harris Hip Score includes items relating both to impairment and symptoms as well as activity. Therefore, the outcome measures reported at these levels will be grouped together.
1. List of outcome measures.
| Outcome at level of | Outcome measures | Description | Benefit / harm* |
| Primary Outcomes | |||
| Impairment/ Activity limitation | Barthel Self Care Index (BI) | ADL (range 0‐100, high score=more function) | Benefit |
| Bristol Knee Score (BKS) | ADL, pain (range 0‐50, high score=more function) | Benefit | |
| Days to sitting out of bed | First day sitting out of bed following surgery | Benefit | |
| Days to ambulation | First day walking following surgery | Benefit | |
| Functional Independence Measure (FIM) | ADL (range 18‐126, high score=more function) | Benefit | |
| Functional Status Index (FSI) | ADL (range 36‐216, low score=more function) | Benefit | |
| Harris Hip Score (HHS) | ADL, pain (range 0‐100, high score=more function) | Benefit | |
| Meurle d'Abuigne and Postel (MAP) | Pain, ADL (range 3‐18, high score=less impairment) | Benefit | |
| Oxford Hip Score (OHS) | ADL, pain (range 12‐60, low score=more function) | Benefit | |
| Western Ontario and McMasters University Osteoarthritis Index (WOMAC) | ADL, pain, stiffness (range 1‐5, high score=more function) | Benefit | |
| Secondary Outcomes | |||
| Participation | Quality of Life | ||
| Dartmouth COOP charts (DCC) | QoL (range 1‐5, lower score=better QoL) | Benefit | |
| Nottingham Health Profile (NHP) | QoL (range 0‐100, lower score=better QoL) | Benefit | |
| RAND 36‐Item Health Survey (RAND‐36) | QoL (range 0‐100, high score=better QoL) | Benefit | |
| 36‐item Short Form Health Survey Questionnaire (SF‐36) | QoL (range 0‐100, high score=better QoL) | Benefit | |
| Other Outcomes | Adverse events/Complications (post‐op) | Adverse events that occur as a direct cause of joint replacement, including wound infections and deep vein thrombosis) | Harm |
| Carer Strain Index (CSI) | Carer strain/burden (range 0‐13, high score=more burden) | Harm | |
| Cost of care | Cost relating to patient care | Harm | |
| Length of stay | From the time of the hospital admission to the time of discharge | Harm | |
| Mortality | Number of deaths as a result of joint replacement surgery | Harm | |
| Readmissions | Readmission to hospital from the community for complications | Harm | |
| * "Benefit" denotes outcome measures that measure improvements in health whilst "Harm" denotes outcome measures that measure adverse effects on health. |
Impairment alone is almost never the primary target for multidisciplinary rehabilitation. Instead the focus is on minimising disability and maximising participation. Therefore, studies that reported outcomes only at the level of impairment were not included in this review.
We categorised studies according to length of follow up after baseline (the peri‐operative period).
'Short term studies' ‐ up to four months.
'Medium term studies' ‐ up to six months.
'Long term studies' ‐ up to 12 months.
Search methods for identification of studies
We sought relevant RCTs in the following: Cochrane Musculoskeletal Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid), EMBASE, CINAHL, Australian Medical Index, UK NHS National Research Register and citation search using SCISEARCH. We performed all searches up to September 2006. In addition, we searched reference lists of other articles and books and conference proceedings and contacted significant researchers in the field. There were no language restrictions.
The search terms used for MEDLINE are in Appendix 1.
Data collection and analysis
Study selection Four authors (FK, LN, SG, TH) independently screened all abstracts and titles of studies that were identified by the search strategy for inclusion and appropriateness, based on the selection criteria. Once all potentially appropriate studies had been obtained, the authors evaluated each study independently for inclusion. If necessary, we obtained further information to determine if the trial met the criteria. If no consensus was reached about the possible inclusion/exclusion of any individual study, arbitration was undertaken by the fifth author (LTS). Authors were not masked to the name(s) of the author(s), institution(s) or publication source at any level of the review.
Data extraction and management Four authors (FK, LN, SG, TH) independently extracted the data from each study that met the inclusion criteria. If insufficient data were available, we then contacted study authors to provide data and clarification. If the data were unavailable or insufficient, the study was reported but not included in the final analysis. We have summarised all studies that met the inclusion criteria in the table 'Characteristics of included studies', including details on design, participants, interventions and outcomes.
Methodological quality assessment The same authors (FK, LN, SG, TH) assessed the methodological quality of studies included in this review, with any disagreements regarding scoring resolved by consensus.
We undertook the quality appraisal of individual studies using the following methodological quality assessment criteria which incorporated two scales: a) the three Jadad criteria (Jadad 1996): randomisation, double blinding and description of withdrawals and dropouts; and b) four items on the criteria list recommended by the Cochrane Bone, Joint and Muscle Trauma Group (CBJMTG) (Heintjies 2002).
The scoring system is listed in Additional Table 2.
2. Criteria for assessment of methodological quality.
| Criteria | ||
| Jadad criteria | ||
| J‐1 | Was the study described as randomised? | yes/no |
| J‐2 | Was the study described as double blind? | yes/no |
| J‐3 | Was there a description of withdrawals and dropouts? | yes/no |
| CBJMTG criteria | ||
| MA | Was the assigned treatment adequately concealed prior to allocation? | Yes = method did not allow disclosure of assignment. No = small but possible chance of disclosure of assignment or unclear , or quasi‐randomised or open lists/tables. |
| Level of allocation concealment | Clearly yes is A, unclear = B, inadequate = C, clearly no = D. | |
| MB | Were the outcomes of the participant withdrawals described and included in the analysis (intention to treat)? | Yes = withdrawals well described and accounted for in analysis. No = withdrawals described but analysis not possible or no mention, inadequate mention or obvious differences and no adjustment. |
| MF | Were the treatment providers blind to assignment status after allocation? | Yes = effective action taken to blind treatment providers. No = small or moderate chance of unblinding of treatment providers or not possible, or not mentioned (unless double blind) or possible but not done. |
| MH | Were the inclusion and exclusion criteria clearly defined? | Yes = clearly defined. No = inadequately defined or not defined. |
The following criteria, as recommended by the Cochrane Collaboration (Higgins 2006) were then further selected to give an overall assessment of quality: 1) concealment of treatment allocation; 2) blinding of intervention provider, recipient and outcome assessor; 3) handling of withdrawals and dropouts.
We assessed studies as being of high or low methodological quality based on whether these criteria had been met. If one or more of the quality criteria were not met, or only partially met or unclear, we considered studies at moderate or high risk of bias, and therefore of low quality.
We also categorised trials on the basis of allocation concealment: A: if allocation concealment was adequate; B: if there was a small chance of disclosure of concealment or if unclear; C: if concealment was inadequate; D: if allocation concealment was not attempted or not used as a criterion to assess validity.
We undertook the grading of evidence using the method proposed by Tugwell at the request of the Cochrane Musculoskeletal Group (CMSG) (Tugwell 2004)(see Additional Table 3).
3. Grading of evidence.
| Tugwell 2004 |
| Platinum: A published systematic review that has at least two individual controlled trials, each satisfying the following : Sample sizes of at least 50 per group. If they do not find a statistically significant difference, they are adequately powered for a 20% relative difference in the relevant outcome. Blinding of patients and assessors for outcomes. Handling of withdrawals >80% follow up (imputations based on methods such as Last Observation Carried Forward (LOCF) is acceptable). Concealment of treatment allocation. |
| Gold: At least one randomised clinical trial meets all of the following criteria for the major outcome(s) as reported: Sample sizes of at least 50 per group. If they do not find a statistically significant difference, they are adequately powered for a 20% relative difference in the relevant outcome. Blinding of patients and assessors for outcomes. Handling of withdrawals > 80% follow up (imputations based on methods such as LOCF is acceptable). Concealment of treatment allocation. |
| Silver: If a systematic review or randomised trial does not meet the above criteria. Silver ranking would also include evidence from at least one study of non‐randomised cohorts who did and did not receive the therapy or evidence from at least one high‐quality case‐control study. A randomised trial with a 'head‐to‐head' comparison of agents is considered Silver level ranking, unless a reference is provided to a comparison of one of the agents to placebo showing at least a 20% relative difference. |
| Bronze: At least one high‐quality case series without controls (including simple before/after studies in which the patient acts as their own control) or if it is derived from expert opinion based on clinical experience without reference to any of the foregoing (for example, argument from physiology, bench research or first principles). |
We undertook subgroup analysis using setting of rehabilitation intervention, as it provided the most useful information. We separated results for site of joint replacement (hip, knee and both hip and knee replacements).
We found the data to be too heterogenous to perform meta‐analyses of all outcome measures. However, we have instead reported data of individual studies in separate forest plots where possible. The heterogeneity of data was further reflected in the clinical relevance tables (Additional Table 4, Table 5, Table 6).
4. Clinical relevance table (early vs standard inpatient rehabilitation).
| Outcome (scale) | #patients (#trials) | Control baseline m | Wt absolute change | Relative % change | NNT (B) or NNT (H) | Stat. significance | Quality of evidence |
| Activity (FIM transfer) | 71(1) | 3.37 | 8% (0.50 more points on a 1‐7 scale) | 15% (I) | NNT (B) = 6 | Statistically significant | Silver |
| 95% confidence interval | (3%, 14%) | (4%, 25%) | (3, 21) | ||||
| Activity (FIM ambulation) | 71(1) | 1.18 | 26% (1.55 more points on a 1‐7 scale) | 131% (I) | NNT (B) = 3 | Statistically significant | Silver |
| 95% confidence interval | (16%, 36%) | (81%, 181%) | (2, 4) | ||||
| Activity (FIM stairs) | 71(1) | 1 | 14% (0.85 more points on a 1‐7 scale) | 85% (I) | NNT (B) = 13 | Statistically significant | Silver |
| 95% confidence interval | (7%, 22%) | (40%, 130%) | (6, 42) | ||||
| Impairment (FSI pain) | 71(1) | no baseline data | 4% (1.95 more points on a 18‐72 scale) | n/a | Not statistically significant | Silver | |
| 95% confidence interval | (‐4%, 11%) | ||||||
| Activity (FSI difficulty) | 71(1) | no baseline data | 4% (2.22 more points on a 18‐72 scale) | n/a | Not statistically significant | Silver | |
| 95% confidence interval | (‐5%, 13%) | ||||||
| Activity (FSI assistance) | 71(1) | no baseline data | ‐1% (0.66 less points on a 0‐72 scale) | n/a | Not statistically significant | Silver | |
| 95% confidence interval | (‐7%, 5%) | ||||||
| Legend | m=mean | I=improvement | NNT/H=Number Needed to Treat to Benefit or Harm. n/a = not applicable |
5. Clinical relevance table (home rehabilitation vs conventional rehabilitation).
| Outcome (scale) | #patients(#trials) | Control baseline m | Wt absolute change | Relative % change | NNT (B) or NNT (H) | Stat. significance | Quality of evidence |
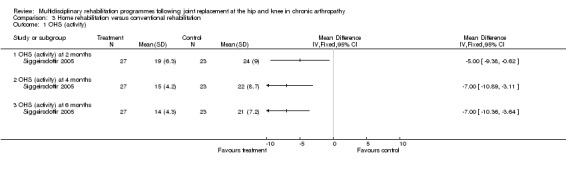
| Activity (OHS) at 2 months | 50(1) | 37 | ‐10% (5 fewer points on a 12‐60 point scale) | ‐14% (I) | NNT (B) = 2 | Statistically significant | Silver |
| 95% confidence interval | (‐20%, ‐1%) | (‐25%, ‐2%) | (2, 4) | ||||
| Activity (OHS) at 4 months | 50(1) | 37 | ‐15% (7 fewer points on a 12‐60 point scale) | ‐19% (I) | NNT (B) = 2 | Statistically significant | Silver |
| 95% confidence interval | (‐23%, ‐6%) | (‐29%, ‐8%) | (2, 2) | ||||
| Activity (OHS) at 6 months | 50(1) | 37 | ‐15% (7 fewer points on a 12‐60 point scale) | ‐19% (I) | NNT (B) = 2 | Statistically significant | Silver |
| 95% confidence interval | (‐22%, ‐8%) | (‐28%, ‐10%) | (2, 2) | ||||
| Legend | m=mean | I=improvement | NNT/H=Number Needed to Treat to Benefit or Harm. n/a=not applicable |
6. Clinical relevance table (low intensity vs standard home rehabilitation).
| Outcome (scale) | #patients(#trials) | Control baseline m | Wt absolute change | Relative % change | NNT (B) or NNT (H) | Stat. significance | Quality of evidence |
| Activity (BI) | 136(1) | 96.6 | 3% (2.8 points more on a 0‐100 scale) | 3% | n/a | Not statistically significant | Silver |
| 95% confidence interval | (‐1%, 6%) | (‐1%, 6%) | |||||
| Activity (WOMAC) | 136(1) | 4.2 | 0% (0 points more on a 1‐5 scale) | 0% | n/a | Not statistically significant | Silver |
| 95% confidence interval | (‐7%, 7%) | (‐7%, 7%) | |||||
| Legend | m=mean | I=improvement | NNT/H=Number Needed to Treat to Benefit or Harm. n/a=not applicable |
Results
Description of studies
Electronic and manual searches identified 990 titles and abstracts. Of these, we selected 50 studies for closer scrutiny after the first screening review. A handsearch of these articles confirmed inclusion of only five based on the review criteria; we excluded eight studies (and abstracts) for the reasons documented in the table 'Characteristics of excluded studies'.
The main reasons for exclusion were as follows.
1) Multidisciplinary rehabilitation was involved, but was not the test variable (n = 6). Variables included the following limited interventions:
ketamine infusions (Adam 2005);
use of an arm crank (Grange 2004);
upper limb interval training (Maire 2003);
arm‐interval exercise programme (Maire 2004);
reinforced versus routine hip restrictions (Peak 2005);
early versus late weight bearing (Liu 2006).
2) Abstract only and/or details insufficient (n = 2).
Abstract (Flynn 2000) ‐ no completed published study was found.
Details insufficient despite attempts to contact author (Yan 2005).
3) Rehabilitation was unidisciplinary only (n = 37).
We included a total of five RCTs (one with two reports) in this review (Dowsey 1999; Munin 1998; Shepperd 1998; Siggeirsdottir 2005; Weaver 2003). These studies were heterogeneous with respect to participant characteristics; indication for surgery; the type, duration and setting of multidisciplinary rehabilitation; follow‐up duration and evaluation time points; outcome measures and measurement instruments. The methodological quality was also variable, but generally low. We have presented relevant information in the table 'Characteristics of included studies', and further details are provided in Additional Table 7.
7. Description of results of included studies.
| Author | Description |
| Dowsey 1999 | Low quality (hip or knee replacement) |
| Assessment points | Baseline, 3 months (short‐term only) |
| Statistical tests | Multiple linear regression, t‐test, z‐test |
| Summary of results (significant outcomes) | In favour of intervention group: Impairment/Activity: Days to sitting out of bed (intervention 19.4, control 3.42, 95%CI* 1.05‐1.95, p=0.001). Days to ambulation (intervention 2.19, control 3.61 days, 95%CI* 0.94‐1.98, p =0.001) Participation: none Other: Complications (intervention 10 participants, control 20, 95%CI* 0.036 ‐ 0.27, p=0.01). Length of stay (intervention mean 7.1, control 8.6 days, 95%CI* 1.03‐1.30, p=0.011) * 95% confidence interval for difference of means or proportions. |
| Summary of results (non‐significant outcomes) | Impairment/Activity: None Participation: None Other: Readmission rate (intervention 4 participants, control 9, 95%CI* 0.006‐0.174, p=0.06). * 95% confidence interval for difference of means or proportions. |
| Author's conclusions | Clinical pathway for persons following THJR/TKJR is an effective method for improving outcomes and decreasing length of stay. |
| Munin 1998 | Low quality (hip or knee replacement) |
| Assessment points | Baseline (1 month prior to surgery), 4 months (short‐term only) |
| Statistical tests | Analysis of variance, MANOVA, chi‐squared test |
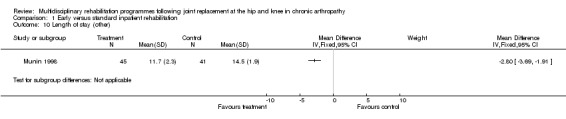
| Summary of results (significant outcomes) | In favour of intervention group: Impairment/Activity (at days 6‐10): FIM (transfer) (intervention mean (SD) 4.8 (0.8), control 4.3 (0.7), p<0.01); FIM (ambulation) (intervention mean (SD) 3.63 (1.51), control 2.08 (1.02), p<0.001); FIM (stairs) (intervention mean (SD) 2.12 (1.32), control 1.27 (0.45), p<0.01) Participation: None Other: Length of stay (intervention mean (SD) 11.7 (2.3) days, control 14.5 (1.9), p<0.001). Cost (intervention mean (SD) $25891($3648), control $27762 ($3626), p<0.03). |
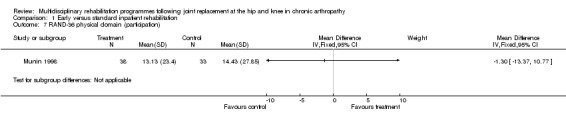
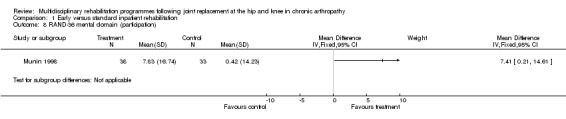
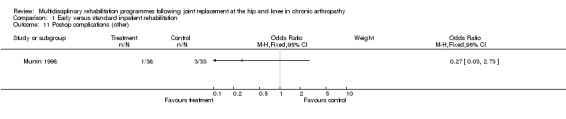
| Summary of results (non‐significant outcomes) | Impairment/Activity (hip)*: FSI (pain) (intervention mean (SD) 8.07 (9.51), control 9.25 (12.17); FSI (difficulty) (intervention 8.73 (6.98), control 6.92 (10.52); FSI (assistance) intervention 2.33 (8.3), control 4.75 (13.48). Impairment/Activity (knee)*: FSI (pain) (intervention mean (SD) 11.58 (9.27), control 9.63 (7.95); FSI (difficulty) (intervention 8.38 (10.3), control 6.16 (10.46); FSI (assistance) intervention 2.13 (9.68), control 2.79 (8.91). Participation (hip)*: RAND‐36 (physical domain) (intervention mean (SD) 14.06 (27.7), control 19.58 (16.3); RAND‐36 (mental domain) (intervention mean (SD) 3.75 (10.38), control 5 (10.39) Participation (knee)*: RAND‐36 (physical domain) (intervention mean (SD) 13.13 (23.4), control 14.43 (27.85); RAND‐36 (mental domain) (intervention mean (SD) 7.83 (16.74), control 0.42 (14.23) Others: Post‐op complications (intervention 1 participant, control 3, chi‐square [1 df] = 1.44, p= 0.30). * FSI and RAND‐36 scores are reported as change scores. |
| Author's conclusions | High‐risk persons post THJR/TKJR could tolerate early intensive rehabilitation, resulting in faster attainment of short‐term functional milestones in fewer days, using less total cost. |
| Shepperd 1998 | Low quality (hip or knee replacement) |
| Assessment points | Baseline, three months (short‐term only) |
| Statistical tests | Unpaired two tailed t tests, Mann‐Whitney U test, chi‐squared test, sensitivity analyses |
| Summary of results (signifcant outcomes) | In favour of intervention group: Impairment/Activity: DCC ‐ THJR only (difference in change from base value 0.50, 95% CI 0.13 to 0.88). Participation: None Other: None |
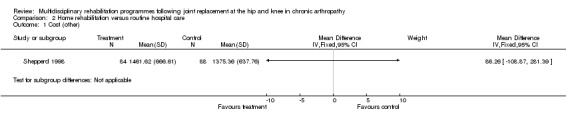
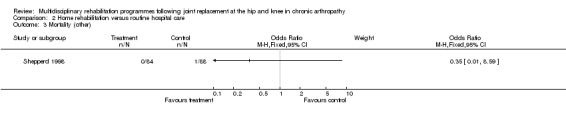
| Summary of results (non‐significant outcomes) | Impairment/Activity: OHS (intervention mean change from baseline value 4.77, control 3.13, difference 1.64, 95% CI ‐1.23 to 4.50). BKS (intervention mean change from baseline value‐3.00, control ‐4.06, difference 1.06, 95% CI ‐1.58 to 3.70) Participation: None Other: CSI (hip) (intervention median change from baseline value 0.00, control 1.00, p = 0.34). CSI (knee) (intervention mean change from baseline value 0.25, control ‐0.58, difference 0.83, 95% CI ‐0.79 to 2.45). Readmissions (hip) (intervention 2 participants, control 1). Mortality (hip) (intervention none, control 1). Readmissions (knee) (intervention 7 participants, control 10). Mortality (knee) (none in either group). Total healthcare costs (hip) (intervention mean (SD) (£) 911.39 (563.76), control 815.70 (347.99), p = 0.59). Total healthcare costs (knee) (intervention mean (SD) (£) 1461.62 (666.61), control 1375.36 (637.76), p = 0.55). |
| Author's conclusions | Persons following THJR in hospital at home showed improvement in quality of life when compared with hospital care. Hospital at home care compared to routine hospital care did not reduce total healthcare costs. |
| Siggeirsdottir 2005 | Low quality (hip replacement only) |
| Assessment points | Baseline (1 day pre‐op), 2, 4 and 6 months (short‐ and medium‐term) |
| Statistical tests | Friedman test, Wilcoxon matched‐pairs test, Mann‐Whitney U test, chi‐squared test |
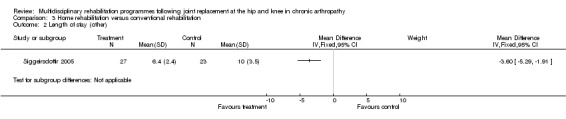
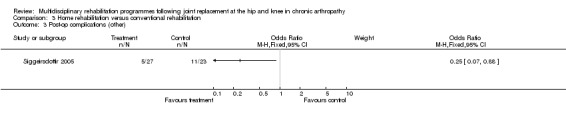
| Summary of results (significant outcomes) | n favour of intervention group: Impairment/Activity: OHS (at 2 months) (intervention mean (SD) 19 (6.3), control 24 (9.0), p = 0.03). OHS (at 4 months) (intervention mean (SD) 15 (4.2), control 22 (8.7), p=0.007). OHS (at 6 months) (intervention mean (SD) 14 (4.3), control 21 (7.2), p = 0.001). Participation: NHP (no data. Only p values given ‐ presume significant results only). At 2 months pain, p = 0.02. At 4 months emotional reaction, p = 0.02, social isolation, p = 0.01. At 6 months pain, p = 0.02, social isolation, p = 0.03, lack of energy, p = 0.007, physical mobility, p = 0.003. Other: Length of stay (intervention mean (SD) 6.4 days (2.4), control 10 days (3.5), p < 0.001). |
| Summary of results (non‐significant outcomes) | Impairment/Activity: MAP (no data given, p = 0.05). HHS at 2 months (intervention median (IQR) 76 (56‐93), control 71 (31‐83). Participation: None Other: Post‐op complications (intervention 5 participants, control 11, p = 0.3). |
| Author's conclusions | Pre‐operative education and post‐operative home‐based rehabilitation in THJR is effective in improving function and quality of life. |
| Weaver 2003 | Low quality (hip or knee replacement) |
| Assessment points | Baseline, 6 months (medium‐term only) |
| Statistical tests | Analysis of Covariance |
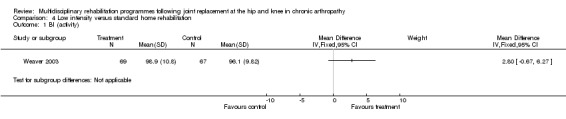
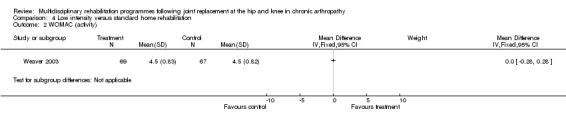
| Summary of results (significant outcomes) | In favour of intervention group: Impairment/Activity: None Participation: None Other (No variance provided): Length of time on home care programme (intervention mean 29.2 days, control 40.6 days, p = 0.035). Home care reimbursement (intervention mean $1488, control $2163, p < 0.001). |
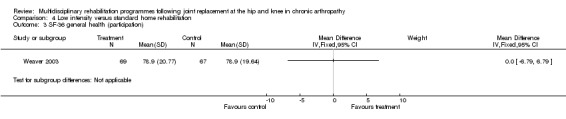
| Summary of results (non‐significant outcomes) | Impairment/Activity: BI (intervention mean ± Standard Error (SE) 98.9±1.3, control 96.1±1.2, p = 0.121), WOMAC (intervention mean±SE 4.5±0.1, control 4.5±0.1 p=0.731). Participation: SF‐36 (general health) (intervention mean±SE 78.9±2.5, control 78.9±2.4 p = 0.984); SF‐36 (physical function) (intervention mean±SE 63.4±3.5, control 66.6±3.5, p = 0.512); SF‐36 (physical role) (intervention mean±SE 75.4±4.9, control 77.0±4.9, p=0.824); SF‐36 (emotional role) (intervention mean±SE 95.0±2.6, control 92.0±2.6, p=0.431); SF‐36 (social function) (intervention mean±SE 91.6±2.2, control 91.7±2.2, p=0.969); SF‐36 (bodily pain) (intervention mean±SE 73.4±3.1, control 75.4±3.0, p=0.642); SF‐36 (vitality) (intervention mean±SE 56.3±2.7, control 60.4±2.6, p=0.280); SF‐36 (mental health) (intervention mean±SE 83.4±1.9, control 82.6±1.9, p = 0.759) Other (No variance provided): Adverse events (intervention 1 participant, control 4). Total reimbursement costs (intervention $24,663, control $24,295 p = 0.96). |
| Author's conclusions | A more efficient delivery of care without compromising patient outcomes was achieved with fewer home visits following total hip and knee joint replacement. |
We found no studies addressing pain management or nutritional input after joint replacement surgery that fulfilled the review selection criteria.
The included studies were conducted in four different countries: one each in the UK, Iceland and Australia, and two in the USA. All trials had been published between 1998 and 2005, and were written in the English language. These trials involved a total of 619 participants.
Three trials (Dowsey 1999; Munin 1998; Shepperd 1998) were short‐term studies with outcome points at three to four months from baseline, whilst two trials (Siggeirsdottir 2005; Weaver 2003) were medium‐term studies where participants were followed up for up to six months. No long‐term studies were identified.
Participant characteristics The participants of studies considered in this review included 619 persons (565 completers) with THJR and TKJR. We have presented relevant information in the table 'Characteristics of included studies'.
Four studies (Dowsey 1999; Munin 1998; Shepperd 1998; Weaver 2003) (569 participants) included both THJR and TKJR participants with a roughly equal numbers, although the participant breakdown in Dowsey 1999 was not provided.
The remaining study (Siggeirsdottir 2005) (50 participants) included only THJR participants.
The indication for joint replacement was specified in only two studies (Munin 1998; Siggeirsdottir 2005) and included osteoarthritis, which made up the largest proportion of participants, rheumatoid arthritis, primary segmental collapse of femoral head and trauma.
Age and gender In the two trials (Dowsey 1999; Munin 1998) that compared different aspects of inpatient rehabilitation, mean age of participants ranged from 66 to 73.5 years and the percentage of women from 61% to 90%.
In the three trials (Shepperd 1998; Siggeirsdottir 2005; Weaver 2003) that compared home rehabilitation with standard hospital care or pre‐existing home rehabilitation protocols, the mean participant ages ranged from 68 to 72 years. Just over half the participants were female (59%, 63% and 52% respectively).
Subject numbers A large number of participants were excluded after randomisation: Dowsey 1999, 12 out of 175 (7%); Munin 1998, 15 out of 86 (17%); and Siggeirsdottir 2005, nine out of 59 (15%). There was discrepancy in the number of participants reported in the text and tables in Siggeirsdottir 2005, and unequal distribution of participant numbers in the intervention and control groups (n = 18 and 11) from one of the two recruiting hospitals. Further, only a small proportion of eligible participants actually participated in studies by Weaver 2003 (136 out of 228 (60%)) and Siggeirsdottir 2005 (50 out of 111 (45%)), due to a number of reasons including lack of consent and medical instability.
The five studies were grouped by setting into a) inpatient and b) home‐based multidisciplinary rehabilitation programmes. There were no trials addressing outpatient centre‐based programmes. Where information was available, data were also separated for site of joint replacement.
Inpatient rehabilitation Two studies (Dowsey 1999; Munin 1998) with a total of 261 participants compared the efficacy of different aspects of inpatient multidisciplinary rehabilitation for both hip and knee joint replacement.
Dowsey 1999 (175 participants) compared use of organised multidisciplinary rehabilitation (in the form of a clinical pathway) with conventional care.
Munin 1998 (86 participants) compared commencement of early rehabilitation with delayed rehabilitation (day three versus day seven) in complex and high‐risk patients.
Both were short‐term studies, with evaluation points at three months for Dowsey 1999 and four months for Munin 1998. Although Dowsey stated that follow up was up to 12 months, no data were provided or available from the authors.
Home‐based rehabilitation Two studies (Shepperd 1998 (two reports); Siggeirsdottir 2005) with a total of 222 participants compared home rehabilitation with standard hospital care or with pre‐existing home care protocols.
Shepperd 1998 (172 participants) compared home rehabilitation with routine hospital care.
Siggeirsdottir 2005 (50 participants) compared home rehabilitation that included pre‐ and post‐operative education programmes with conventional rehabilitation.
The evaluation points were three months for Shepperd 1998 (short term) and two, four and six months for Siggeirsdottir 2005 (medium term).
One study (Weaver 2003) compared two home care programmes with different intensities, with evaluation at six months (medium term).
Weaver 2003 (136 participants) compared a lower‐intensity multidisciplinary home care programme (one pre‐ and five post‐operative visits up to four weeks) with standard care (a pre‐existing home care protocol of three to five post‐operative visits per week for nine weeks (ie up to 45 visits in total).
Risk of bias in included studies
The summary of key indicators for randomisation, concealed allocation, intention to treat and blinding of outcome assessor appears in the table 'Characteristics of included studies'. The methodological quality description of the five included studies is provided in Additional Table 8.
8. Methodology quality of included studies.
| Criteria | Study ID | ||||
| Jadad | Dowsey 1999 | Munin 1998 | Shepperd 1998 | Siggeirsdottir 2005 | Weaver 2003 |
| J1‐Randomisation | Yes | Yes | Yes | Yes | Yes |
| J2 ‐ Double blind * | No | No | No | No | No |
| J3 ‐ Description of withdrawals and dropouts * | Yes | Yes | Yes | Yes | Yes |
| CBJMTG | |||||
| MA ‐ Allocation concealment (level) * | Yes (A) | Yes (A) | Yes (A) | Yes (A) | No (D) |
| MB ‐ ITT | Yes | Yes | Yes | No | No |
| MF ‐ Treatment providers blinded * | No | No | No | No | No |
| MH ‐ Inclusion and exclusion criteria defined | Yes | Yes | Yes | Yes | Yes |
| Overall quality ** | Low | Low | Low | Low | Low |
| * Criteria used to determine risk of bias. ** High quality = All criteria met (low risk of bias) Low quality = Not all criteria met (moderate or high risk of bias) |
We rated all studies as 'low quality' due to the absence of blinding. This included not only lack of participant/therapist blinding, but also outcome assessor blinding, which was of great concern. However, both inpatient trials (Dowsey 1999; Munin 1998) had adequate concealment of allocation and intention to treat was specified. In the trials relating to home‐based rehabilitation (Shepperd 1998; Siggeirsdottir 2005; Weaver 2003), only Shepperd 1998 and Siggeirsdottir 2005 had adequate allocation concealment and only Shepperd 1998 specified intention to treat. Only two of the five trials (Dowsey 1999; Shepperd 1998) were powered to detect significant changes in outcomes, so there was a risk of type I and II errors.
Three studies also had significant deviations from their methodology protocol.
In the study by Shepperd 1998, 14/47 participants (30%) allocated to home care in the TKJR intervention group were unable to take this up, and stayed in hospital due to post‐operative complications.
In the study by Siggeirsdottir 2005, there was deviation from the original protocol where participants from another hospital were included due to major reorganisation within the original hospital.
In the study by Weaver 2003, the figures presented for cost analysis represented only 73% of the sample, and description of withdrawals was incomplete.
Effects of interventions
We have provided detailed descriptions of the results of individual included studies in Additional Table 7.
We have described the assimilation of best evidence below for: (a) inpatients; and (b) home based setting.
None of the included studies addressed outpatient rehabilitation.
Pooling of data for quantitative analysis was not possible due to heterogeneity with respect to participant characteristics; indication for surgery; the type, duration and setting of multidisciplinary rehabilitation; follow‐up duration and evaluation time points; outcome measures and measurement instruments. Instead, we conducted synthesis of best evidence using the Tugwell 2004 grading system. Finally, quantification of the results of the between‐group differences have been provided, where possible, in graphs 01.01‐0.1.11, 02.01‐02.03, 03.01‐03.03 and 04.01‐04.13. Between‐group differences in the form of weighted mean difference (WMD) and numbers needed to treat to beneft (NNTB) have also been incorporated into the text of the results section where appropriate.
Clinical relevance tables (Additional Table 4; Table 5; Table 6) have also been provided to assist readers with understanding the primary outcome measures (for both benefits and harms). A) Effectiveness of aspects of inpatient multidisciplinary therapy versus control (conventional routine care) Two trials of low quality addressing the efficacy of different aspects of inpatient multidisciplinary rehabilitation (Dowsey 1999; Munin 1998) recruited a total of 261 participants. Only one study (Munin 1998) specifically identified the type of participants which were elderly, frail patients with multiple co‐morbidities. Pooling of data was confounded by the fact that the two studies examined different aspects of inpatient multidisciplinary rehabilitation: Dowsey 1999 examined the effects of organised care, whilst Munin 1998 examined the effect of early rehabilitation at different time points (Dowsey 1999 three months; Munin 1998 four months).
At the level of impairment/activity:
Dowsey 1999 used days to sitting out of bed and days to ambulation;
Munin 1998 used Functional Independence Measure (FIM) and Functional Status Index (FSI).
At the level of participation:
Dowsey 1999 did not measure outcomes at this level;
Munin 1998 used RAND 36‐Item Health Survey (RAND‐36).
Other outcomes:
Dowsey 1999 evaluated complication rate, readmissions and length of stay;
Munin 1998 evaluated length of stay and costs.
The grading and synthesis of best evidence from these two trials suggests the following, subsequent to hip or knee replacement.
At the level of impairment/activity, there is 'silver' level (Tugwell 2004) evidence that early inpatient rehabilitation (Munin 1998) leads to more rapid attainment of functional milestones as measured by the FIM at day six to ten (although there were no significant differences in FSI at four months), and that organised rehabilitation via a clinical pathway (Dowsey 1999) leads to earlier ambulation in the short term (three to four months). For FIM transfer, the WMD is 0.5 (95%CI 0.15, 0.85) whilst WMD for FIM ambulation is 1.55 (95%CI 0.96, 2.14) and WMD for FIM stairs is 0.85 (95% CI 0.40, 1.30) (graphs 01.01, 01.02, 01.03). The NNTB ranged from 3 (95%CI 2,4) for FIM ambulation to 6 (95% CI 3,21) for FIM transfer and 13 (95% CI 6, 42) for FIM stairs (Additional Table 4).
At the level of participation, there is no evidence that early or organised inpatient rehabilitation leads to improvements in quality of life as measured by RAND‐36 (Munin 1998). WMD for RAND‐36 (physical domain) is ‐1.30 (‐13.37, 10.77) and 7.41 (0.21, 14.61) for RAND‐36 (mental domain) (graphs 01.07, 01.08).
As for other outcomes, there is also 'silver' level evidence that earlier (Munin 1998) and/or organised multidisciplinary rehabilitation (Dowsey 1999) reduces costs (Munin 1998), length of stay (Dowsey 1999; Munin 1998) and results in fewer complications (Dowsey 1999) in the short term (3‐4 months) (Additional Table 7). There is no evidence, however, for lowering of the readmission rate (Dowsey 1999).
B) Effectiveness of home multidisciplinary therapy versus control (standard hospital care or pre‐existing home care protocols) The two trials addressing the efficacy of home‐based multidisciplinary rehabilitation (Shepperd 1998; Siggeirsdottir 2005) recruited a total of 222 participants. One study (Weaver 2003) (136 participants) compared home programmes of different intensity. Only one study (Shepperd 1998) addressed the issue of caregiver strain, but found that there were no differences detected between carers in the rehabilitation group when compared to the control group.
Pooling of data from the three studies was again confounded by the design differences highlighted above, and in addition by the use of different outcome measures at different time points (see Additional Table 7).
At the level of impairment/activity:
Shepperd 1998 and Siggeirsdottir 2005 both used Oxford Hip Score (OHS). In addition, Shepperd 1998 used Bristol Knee Score (BKS) and Siggeirsdottir 2005 used Meurle d'Abuigne and Postel (MAP) and Harris Hip Score (HHS);
Weaver 2003 used Barthel Index (BI) and Western Ontario and McMaster University Osteoarthritis Index (WOMAC).
At the level of participation:
Shepperd 1998 used Dartmouth COOP charts (DCC);
Siggeirsdottir 2005 used Nottingham Health Profile (NHP);
Weaver 2003 used Medical Outcomes Study Short Form 36 (SF‐36).
Other outcomes:
Shepperd 1998 examined Carer Strain Index (CSI), readmissions, mortality and cost;
Siggeirsdottir 2005 examined post‐operative complications and length of stay;
Weaver 2003 examined adverse events and cost.
The grading and best evidence synthesis from the two low quality RCTs (Shepperd 1998; Siggeirsdottir 2005) (222 participants) addressing effectiveness of home rehabilitation suggests the following.
At the level of impairment/activity (hip replacement), there is 'silver' level evidence that organised hospital at home multidisciplinary care can improve disability for up to six months as measured by the OHS (Siggeirsdottir 2005). WMD for OHS at 6 months is ‐7.00 (95%CI ‐10.36, ‐3.64) (graph 03.01) and NNT 2 (95%CI 2, 2) (Additional Table 5). However, no effect was shown with MAP or HHS in Siggeirsdottir 2005, or with OHS in Shepperd 1998.
At the level of impairment/activity (knee replacement), there is no evidence that organised hospital at home multidisciplinary care improves disability as measured by BKS (Shepperd 1998).
At the level of participation (hip replacement), there is 'silver' level evidence that home rehabilitation improves quality of life for up to six months as measured by DCC (Shepperd 1998) and NHP (Siggeirsdottir 2005).
At the level of participation (knee replacement), there is no evidence that home rehabilitation improves quality of life as measured by DCC (Shepperd 1998).
As for other outcomes, there is 'silver' level evidence that individualised home rehabilitation reduces the length of stay without increasing rate of complications following hip replacement (Siggeirsdottir 2005). However, there is no evidence that home rehabilitation reduces the rate of readmissions, mortality or costs following hip or knee joint replacement compared to hospital care (Shepperd 1998).
The grading and best evidence synthesis from the only low‐quality RCT (Weaver 2003) (50 participants) addressing intensity of home rehabilitation suggests that following hip or knee replacement:
At the level of impairment/activity, a lower intensity home rehabilitation programme has no lesser effect on disability as measured by BI and WOMAC, when compared with a standard home rehabilitation programme. WMD for BI is 2.80 (95% CI ‐0.67, 6.27) and 0.00 (95% CI ‐0.28, 0.28) for WOMAC.
At the level of participation, a lower intensity home rehabilitation programme has no lesser effect on quality of life as measured by SF‐36.
As for other outcomes, there is 'silver' level evidence that a less intensive home rehabilitation programme reduces costs without compromising patient outcomes.
Adverse effects Adverse effects of rehabilitation are possible, but rarely seen in practice. All studies looked for adverse effects but none reported any adverse effects attributable to the rehabilitation.
Discussion
This review investigated the effectiveness of organised multidisciplinary rehabilitation in adults following hip or knee joint replacement, based on measures of activities and participation in the ICF (WHO 2001), and also on utility and service costs. Because of heterogeneity in the studies identified, it was not possible to pool data statistically. Instead, we performed best evidence synthesis using a qualitative analysis.
In relation to our original objectives: Does organised multidisciplinary rehabilitation achieve better outcomes than the absence of such services in people following hip or knee joint replacement? We identified no studies that provided direct evidence that multidisciplinary rehabilitation following THJR/TKJR achieved better outcomes compared with no treatment. However multidisciplinary rehabilitation versus usual or routine treatment is addressed below. Which models of programmes are effective and in which setting? Which participants benefit most and which specific outcomes are influenced? We found evidence for the effectiveness of multidisciplinary rehabilitation in both inpatient and home‐based settings. There was 'silver' level evidence that early and/or organised inpatient multidisciplinary rehabilitation (for all persons following hip or knee replacement, including those who were elderly and those with complex co‐morbidities), led to more rapid attainment of functional milestones in the shorter term, as well as fewer post‐operative complications, shorter hospital stay and reduced costs. However, there was no evidence that earlier inpatient rehabilitation improved participation.
For home‐based multidisciplinary rehabilitation, there was 'silver' level evidence for organised hospital at home care improving QoL (in the medium term) and disability in persons following hip replacement, but there was no such evidence for knee replacement.
The duration of follow up of persons after both hip and knee joint replacement was limited to short and medium term (three to six months) following surgery. It was not possible to determine if gains made by participants in rehabilitation were maintained in the longer term (> 12 months).
Does a greater intensity (time or expertise, or both) of multidisciplinary rehabilitation lead to greater gains, and are there demonstrable cost benefits for multidisciplinary rehabilitation in hip or knee joint replacement? From this review, it has not been possible to suggest best 'dose' of therapy. Further studies are needed to suggest optimum number, duration and intensity of treatment sessions.
One study (Munin 1998) provided 'silver' level evidence for modest cost benefits (equating to approximately $2000) arising from early inpatient rehabilitation. A different study (Weaver 2003) provided the same level of evidence that a limited four‐week home rehabilitation programme could reduce the cost of intervention without adversely compromising patient outcomes in terms of functional status or quality of life. However, both this study and the study by Shepperd 1998 showed no difference in total reimbursement costs for hospital at home compared with conventional care, so the jury is still out on overall cost effectiveness of these home‐based programmes.
Methodological considerations For the purpose of this review, we categorised individual trials into 'high' or 'low' quality studies based on the Jadad and the CBJMTG methodological criteria, which have been developed to determine the risk of bias. We then graded the level of evidence according to the simple classification described by Tugwell 2004 into 'platinum', 'gold', 'silver' and 'bronze' grades. In the context of multidisciplinary rehabilitation, it has been recognised that it is effectively impossible to blind the service providers or the recipients to the nature of treatment (see below). All of these studies would have rated as low quality in any event. However, it should be possible to blind assessors but, in all five studies, even the assessors were unblinded. The rating system did not allow for differentiation of quality based on any other determinators such as intention to treat or allocation concealment. Alternative approaches, such as that recommended by Van Tulder 2003, provide a more comprehensive evaluation of quality indicators, and have been applied in other Cochrane reviews relating to multidisciplinary rehabilitation, but we have utilised the methods applied here at the specific request of the Cochrane Musculoskeletal Group, in order to maintain comparability with other reviews in the section.
The strength of findings was limited by the small number of studies and by methodological weaknesses. Only two studies were powered to detect significant changes in outcomes (Dowsey 1999; Shepperd 1998), so there was a risk of type I and II errors. Details of the participants and the interventions, especially in the control arm of the trial, were frequently missing and were not obtainable from the authors, despite attempts to contact them. Only one study provided details of unilateral compared with bilateral joint replacements (Weaver 2003). No studies provided details of differential analysis of bilateral versus unilateral joint replacements. Many studies did not provide the clinical indication for joint replacement (Dowsey 1999; Shepperd 1998; Weaver 2003). The outcome measures used in these studies to gauge improvements in function (disability) or participation varied widely. Further, local practices tended to vary in the different countries (Australia, Iceland, UK and USA), making it harder to interpret and compare outcomes. In some studies details of the rehabilitation programmes were insufficient and therefore difficult to standardise.
This review highlights some of the limitations and challenges for randomised controlled trial methodologies in complex interventions such as rehabilitation in the context of chronic arthropathy, which have also been highlighted by previous authors (Turner‐Stokes 2005; Whyte 2002). These include the following.
Heterogeneity with respect to participants, interventions and outcome measures used.
Problems with recruitment, retention and follow up of participants, especially in the control arm of trials. Multi‐centre trials may provide larger numbers but often at the expense of increased heterogeneity, with little net benefit.
The outcomes measures used in rehabilitation may not be optimal for statistical analysis, as their ordinal nature obscures distribution in the target population.
True blinding of participants and care providers in rehabilitation is effectively not possible. Participants often unwittingly volunteer information about their treatment during the course of assessment, and attempts to blind outcome assessor may not be guaranteed.
Rehabilitation comprises concurrent complex interventions, which are difficult to quantify (such as goal setting), and depend on the specified interaction between the participant and the clinician. Many of the rehabilitation treatments are interactive and dependent on participant response, which potentially confounds simple division into 'treatment' and 'control' conditions.
Limitations of this review The assimilation of available evidence was challenging due to the diversity of trials in this review. The authors accept that there may have been a degree of:
selection bias from the literature search (Van Tulder 2003) (although four authors independently selected the studies for inclusion);
publication bias (Egger 1998) if trials have not been published due to them having small participant numbers and negative results (although authors also sourced unpublished data);
reference bias (Goetzsche 1987) for published studies included in this review.
The review has taken an inclusive approach to a broad area of clinical practice, and this approach has posed significant challenges for the assessment and assimilation of the available evidence. It may be contended that we have adopted too low a threshold for inclusion of studies of low quality. On the other hand, we believe that the presented synthesis of 'best evidence', based on assessment of methodological quality, has facilitated a helpful comparison of the various studies available. It also allows open acknowledgement of the 'limited evidence' which comes from these poorer (or single) studies, which is nevertheless the best available at the current time.
Our attempt to categorise evidence according to the WHO 2001 ICF posed some methodological problems, since many of the outcome measures used in trials crossed the boundaries between the different levels of the model. However, we still believe that this model is helpful to clarify the experience of people who live with long‐term chronic conditions. Future research should evaluate outcome on all levels, including contextual factors.
Authors' conclusions
Implications for practice.
The evidence presented in this review provides modest support for the recommendation that people following hip or knee joint replacement should be assessed for their need for appropriate rehabilitation intervention. The assessment could be undertaken by any skilled member of the multidisciplinary team, in order to maximise their capacity for independent living and societal participation.
Implications for research.
We identified no studies that provided direct evidence that multidisciplinary rehabilitation following THJR/TKJR achieved better outcomes compared with no treatment. However, taken together, the five trials included in this review provide a certain body of evidence that multidisciplinary rehabilitation can improve the experience of people following joint replacement in terms of both activity and participation. Many questions remain to be answered. Which group of people, following joint replacement, are most likely to require and gain benefit from a multidisciplinary approach? It is clear that home‐based rehabilitation is not suitable for all people following joint replacement, so how should we decide who needs treatment in which setting? What is the optimum dose or intensity of rehabilitation, and which professional should be involved? Finally, with spiralling healthcare costs and the increased demand for rehabilitation services, it is important to gather proper evidence with regard to cost‐effectiveness of different models and their full economic impact for health services, for people following joint replacement and their families and for society at large.
This review highlights the need for:
1) High quality RCTs, and other designs (including large prospective observation cohort studies) where appropriate, which assess:
the effectiveness of specific rehabilitation interventions (and components);
the appropriate intensity and settings of therapy;
the cost effectiveness of comprehensive multidisciplinary rehabilitation programmes; and
the impact of therapy on participants and their families.
2) Development of appropriate outcome measures, including:
reliable and valid outcome measures which reflect domains of the ICF;
measurement of the effects of rehabilitation over longer periods (over 12 months);
incorporation of the perspective of the person following joint replacement, and further evaluation of the participation issues relevant to them (such as return to work, driving, community reintegration, leisure, parenting (or grand‐parenting) and psychosocial issues);
a consensus on an ICF core set of measurement of outcomes in post‐ joint replacement trials.
What's new
| Date | Event | Description |
|---|---|---|
| 7 November 2008 | Amended | Converted to new review format. CMSG ID: A014‐R |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 13 December 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are grateful to several members of the Cochrane Musculoskeletal Group, including Ms Lara Maxwell, Ms Louise Falzon, Ms Miranda Cumpston and Professor Rachelle Buchbinder for their invaluable advice and assistance in this review. We are also grateful to Ms Kathie Vezzoso for her assistance with the preparation of this manuscript. Finally, we would like to acknowledge Professor Peter Disler for his advice in planning the protocol for this review.
Appendices
Appendix 1. MEDLINE search strategy
1. exp Arthroplasty, Replacement, Knee/ 2. exp Knee Prosthesis/ 3. exp Knee Joint/ 4. exp Joint Prosthesis/ 5. knee.mp. 6. (replace$ or arthroplast$ or implant$ or endoprosthe$ or prosthe$).mp. 7. exp "Prostheses and Implants"/ 8. 3 or 5 9. or/4,6‐7 10. 8 and 9 11. or/1‐2,10 12. exp Arthroplasty, Replacement, Hip/ 13. exp Hip Prosthesis/ 14. exp Hip Joint/ 15. (hip or hips).tw. 16. 14 or 15 17. 16 and 9 18. or/13‐14,17 19. 11 or 18 20. exp REHABILITATION/ 21. exp REHABILITATION CENTERS/ 22. exp REHABILITATION NURSING/ 23. rehab$.mp. 24. exp Patient Care Team/ 25. multidisciplinar$.tw. 26. interdisciplinar$.tw. 27. multiprofessional$.tw. 28. multimodal$.tw. 29. exp Patient Care Management/ 30. exp Occupational Therapy/ 31. occupational therap$.tw. 32. exp Physical Therapy Techniques/ 33. exp "Physical Therapy (Specialty)"/ 34. exp Physical Therapy Department, Hospital/ 35. physical therap$.tw. 36. physiotherap$.tw. 37. (early adj1 (mobil$ or discharg$ or ambulat$)).tw. 38. exp Critical Pathways/ 39. exp Therapy, Computer‐Assisted/ 40. exp Exercise Therapy/ 41. (exercis$ adj3 therap$).tw. 42. exp Social Work/ 43. exp Social Support/ 44. (social adj1 (work$ or support)).tw. 45. exp Pain Clinics/ 46. pain clinic$.tw. 47. (pain center$ or pain centre$).tw. 48. pain service$.tw. 49. pain relief unit$.tw. 50. exp Patient Education/ 51. exp Health Education/ 52. exp Diet Therapy/ 53. exp NUTRITION/ 54. exp Nutritional Support/ 55. ((diet$ or nutrition$) adj5 (therap$ or modif$ or program$)).tw. 56. ((treatment$ or therap$ or training or education$ or healthcare) adj10 (program$ or intervention$ or approach$)).tw. 57. or/20‐56 58. 19 and 57
Data and analyses
Comparison 1. Early versus standard inpatient rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FIM transfer (activity) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 FIM ambulation (activity) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 FIM stairs (activity) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 FSI pain (impairment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 FSI difficulty (activity) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 FSI assistance (activity) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 RAND‐36 physical domain (participation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 RAND‐36 mental domain (participation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Cost (other) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10 Length of stay (other) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11 Post‐op complications (other) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
1.1. Analysis.
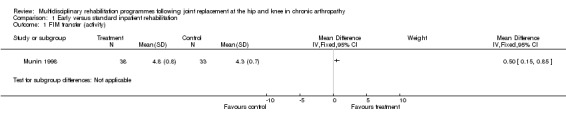
Comparison 1 Early versus standard inpatient rehabilitation, Outcome 1 FIM transfer (activity).
1.2. Analysis.
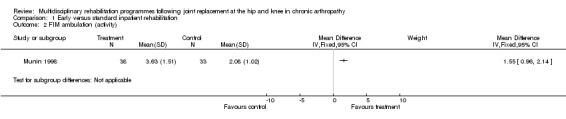
Comparison 1 Early versus standard inpatient rehabilitation, Outcome 2 FIM ambulation (activity).
1.3. Analysis.
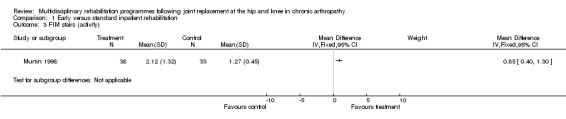
Comparison 1 Early versus standard inpatient rehabilitation, Outcome 3 FIM stairs (activity).
1.4. Analysis.
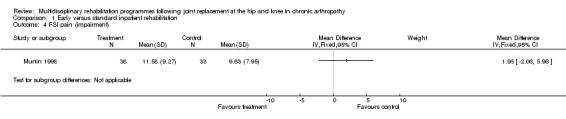
Comparison 1 Early versus standard inpatient rehabilitation, Outcome 4 FSI pain (impairment).
1.5. Analysis.

Comparison 1 Early versus standard inpatient rehabilitation, Outcome 5 FSI difficulty (activity).
1.6. Analysis.

Comparison 1 Early versus standard inpatient rehabilitation, Outcome 6 FSI assistance (activity).
1.7. Analysis.
Comparison 1 Early versus standard inpatient rehabilitation, Outcome 7 RAND‐36 physical domain (participation).
1.8. Analysis.
Comparison 1 Early versus standard inpatient rehabilitation, Outcome 8 RAND‐36 mental domain (participation).
1.9. Analysis.

Comparison 1 Early versus standard inpatient rehabilitation, Outcome 9 Cost (other).
1.10. Analysis.
Comparison 1 Early versus standard inpatient rehabilitation, Outcome 10 Length of stay (other).
1.11. Analysis.
Comparison 1 Early versus standard inpatient rehabilitation, Outcome 11 Post‐op complications (other).
Comparison 2. Home rehabilitation versus routine hospital care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cost (other) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Readmissions (other) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Mortality (other) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
2.1. Analysis.
Comparison 2 Home rehabilitation versus routine hospital care, Outcome 1 Cost (other).
2.2. Analysis.

Comparison 2 Home rehabilitation versus routine hospital care, Outcome 2 Readmissions (other).
2.3. Analysis.
Comparison 2 Home rehabilitation versus routine hospital care, Outcome 3 Mortality (other).
Comparison 3. Home rehabilitation versus conventional rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 OHS (activity) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 OHS (activity) at 2 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 OHS (activity) at 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 OHS (activity) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Length of stay (other) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Post‐op complications (other) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
3.1. Analysis.
Comparison 3 Home rehabilitation versus conventional rehabilitation, Outcome 1 OHS (activity).
3.2. Analysis.
Comparison 3 Home rehabilitation versus conventional rehabilitation, Outcome 2 Length of stay (other).
3.3. Analysis.
Comparison 3 Home rehabilitation versus conventional rehabilitation, Outcome 3 Post‐op complications (other).
Comparison 4. Low intensity versus standard home rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BI (activity) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 WOMAC (activity) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 SF‐36 general health (participation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 SF‐36 physical function (participation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 SF‐36 physical role (participation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 SF‐36 emotional role (participation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 SF‐36 social function (participation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 SF‐36 bodily pain (participation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 SF‐36 vitality (participation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10 SF‐36 mental health (participation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11 Cost of home care reimbursement (other) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12 Cost of total reimbursement (other) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13 Adverse events (other) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
4.1. Analysis.
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 1 BI (activity).
4.2. Analysis.
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 2 WOMAC (activity).
4.3. Analysis.
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 3 SF‐36 general health (participation).
4.4. Analysis.
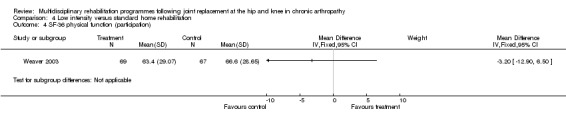
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 4 SF‐36 physical function (participation).
4.5. Analysis.
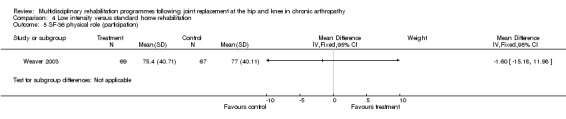
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 5 SF‐36 physical role (participation).
4.6. Analysis.
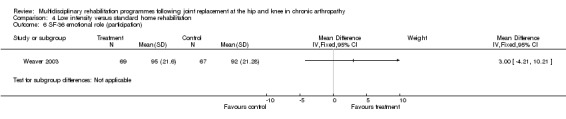
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 6 SF‐36 emotional role (participation).
4.7. Analysis.
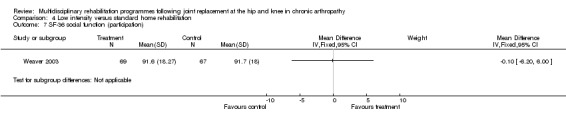
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 7 SF‐36 social function (participation).
4.8. Analysis.
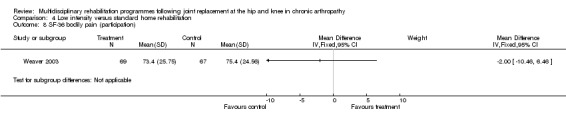
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 8 SF‐36 bodily pain (participation).
4.9. Analysis.
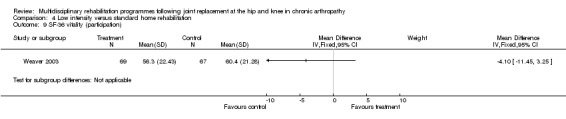
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 9 SF‐36 vitality (participation).
4.10. Analysis.
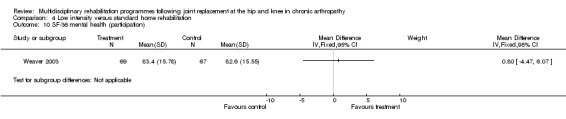
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 10 SF‐36 mental health (participation).
4.11. Analysis.
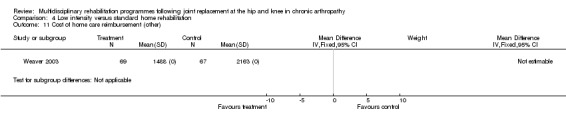
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 11 Cost of home care reimbursement (other).
4.12. Analysis.

Comparison 4 Low intensity versus standard home rehabilitation, Outcome 12 Cost of total reimbursement (other).
4.13. Analysis.
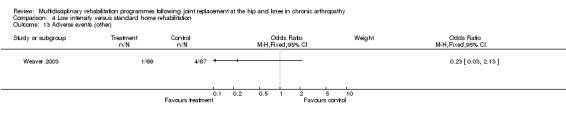
Comparison 4 Low intensity versus standard home rehabilitation, Outcome 13 Adverse events (other).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dowsey 1999.
| Methods | Randomisation ‐ yes Assessor blinding ‐ no ITT ‐ yes | |
| Participants | (Australia) N = 175 (no breakdown of THJR/TKJR numbers). N (completed study) = 163. Intervention: 94 Control: 81 Inclusion criteria ‐ All THJR/TKJR Exclusion criteria ‐ revision joint replacement, bilateral simultaneous joint arthropathy, joint replacement for acute trauma or complex tumour surgery. Indication of joint replacement ‐ not specified Gender ‐ M:F 56:107 Age ‐ mean 66 years (range 67‐93). | |
| Interventions | Intervention:
'Clinical pathway' ‐ comprehensive inpatient rehabilitation with early mobilisation and discharge. Control: Conventional routine care. Treating team responded to the will and condition of the participant in providing post‐op care. |
|
| Outcomes | Impairment/Activity:
Days to sitting out of bed.
Days to ambulation. Participation: None Other: Complication rate Readmissions Length of stay |
|
| Notes | 12 participants excluded after randomisation based on exclusion criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Munin 1998.
| Methods | Randomisation ‐ yes Assessor blinding ‐ no ITT‐ yes | |
| Participants | (USA) N = 86 (35 THJR, 51 TKJR). N (completed study) = 71. Intervention 45 (19 THJR, 26 TKJR) Control 41 (16 THJR 25 TKJR). Inclusion criteria ‐ High risk primary and revision THJR and TKJR who required inpatient care (including age > 70, living alone, multiple co‐morbidities). Exclusion criteria ‐ Tumours, acute fractures, femoral osteonecrosis or haemophilic arthropathy. Post‐op conditions which precluded rehabilitation. Indication of joint replacement ‐ osteoarthritis (89%), rheumatoid arthritis (11%) Gender ‐ M:F 10:76. M:F (THJR) 5:30. M:F (TKJR) 5:46. Age ‐ THJR mean 75 years, TKJR mean 73 years (no range given). | |
| Interventions | Intervention:
Early inpatient rehabilitation commencing day 3. Control: Inpatient rehabilitation commencing day 7. In acute care, all participants received 2x 30‐minute PT sessions daily (from day 2 post‐op) and 1 x 30‐minute OT sessions daily (from day 3). In rehabilitation (from day 3 intervention, day 7 control), participants received two 60‐minute sessions of PT and two 60‐minute sessions of OT and also psychology and recreation. |
|
| Outcomes | Impairment/Activity:
FIM
FSI Participation: RAND‐36 Other: Length of stay Cost |
|
| Notes | Baseline characteristics given only for 71 participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Shepperd 1998.
| Methods | Randomisation ‐ yes. Assessor blinding‐ no ITT ‐ yes | |
| Participants | (UK) N = 172 (86 THJR, 86 TKJR). N (completed study) = 161. Intervention: 84; Control: 88 Inclusion criteria ‐ home environment was suitable, carer consented to trial, clinically stable. Exclusion criteria ‐ under 60 Indication for joint replacement ‐ not specified Gender ‐ M:F 71:101. M:F (THJR) 33:53. M:F (TKJR) 38:48. Age ‐ Intervention: (THJR) mean (SD) 71 (7.7) years. (TKJR) mean (SD) 68 (7.9) years. Control: (THJR) mean (SD) 70 (8.7) years. (TKJR) mean (SD) 72 (6.8) years | |
| Interventions | Intervention:
Hospital at home care more than normally available in the community (nursing, PT, OT, pathology, speech therapy, mobile phone provided) Control: Routine hospital care |
|
| Outcomes | Impairment/Activity:
BKS
OHS Participation: DCC Other: CSI Readmissions Mortality Cost |
|
| Notes | Study also included participants with other diagnoses and the details of these participants have not been reported in this review. 14 (30%) of participants allocated to hospital at home remained in hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Siggeirsdottir 2005.
| Methods | Randomisation ‐ yes Assessor blinding ‐ no ITT ‐ no | |
| Participants | (Iceland) N = 50. N (completed study) = 47. Intervention: 27 Control: 23. Inclusion criteria ‐ primary THJR (osteoarthritis, rheumatoid arthritis, primary segmental collapse of femoral head, developmental disease and trauma). Exclusion criteria ‐ Primary hip fracture, metastatic tumours, dementia. Indication for joint replacement ‐ see inclusion criteria Gender ‐ M:F 24:26. Age ‐ mean 68 years (range 28‐86) | |
| Interventions | Intervention:
Pre‐op education and training (by PT ± OT, brochure). Discharge home visit (PT ± OT) and subsequent home visits (median number of visits 4, range 2‐9 times) . Control: Conventional rehabilitation (no further description provided). |
|
| Outcomes | Impairment/Activity:
OHS
HHS
MAP Participation: NHP Other: Post‐op complications Length of stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Weaver 2003.
| Methods | Randomisation ‐ yes Assessor blinding ‐ no ITT ‐ no | |
| Participants | (USA) N = 136 (68 THJR, 68 TKJR). N (completed study) = 123. Intervention: 69 Control: 67. Inclusion criteria ‐ THJR and TKJR, medicare eligible, geographic catchment. Exclusion criteria ‐ Those assessed too close to time of surgery. Indication for joint replacement ‐ not specified. Gender ‐ M:F 221:85. Age ‐ mean (SD) 72 (7) years. | |
| Interventions | Intervention:
Home care protocol (pre‐op home visits and fewer post‐op visits.
THJR: 1 nurse and 1 PT visit pre‐op, 1 nurse and 5 PT visits post op weeks 1‐4.
TKJR: 1 nurse and 1 PT visit pre‐op, 1 nurse and 9 PT visits post op (weeks 1‐4). Control: Existing home care protocol (no pre‐op visits and more post‐op visits). THJR: 2 nurse and 9‐27 PT visits (weeks 1‐9) TKJR: 2 nurse and 27‐45 PT visits (weeks 1‐9) |
|
| Outcomes | Impairment/Activity:
BI
WOMAC Participation: SF‐36 Other: Adverse events Cost |
|
| Notes | 20% underwent bilateral TKJR (remaining had one joint replaced only) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 2005 | Variable is not MD rehabilitation |
| Avramidis 2003 | Unidisciplinary rehabilitation only |
| Bourne 1998 | Unidisciplinary rehabilitation only |
| Breit 2004 | Unidisciplinary rehabilitation only |
| Chen 2004 | Unidisciplinary rehabilitation only |
| Cheville 2001 | Unidisciplinary rehabilitation only |
| Codine 2004 | Unidisciplinary rehabilitation only |
| Eisermann 2004 | Unidisciplinary rehabilitation only |
| Erler 2001 | Unidisciplinary rehabilitation only |
| Flynn 2000 | Abstract only |
| Frost 2002 | Unidisciplinary rehabilitation only |
| Ganz 2004 | Unidisciplinary rehabilitation only |
| Giaquinto 2006 | Unidisciplinary rehabilitation only |
| Gilbey 2003 | Unidisciplinary rehabilitation only |
| Gilbey et al 2003 | Unidisciplinary rehabilitation only |
| Gotlin 1994 | Unidisciplinary rehabilitation only |
| Grange 2004 | Variable is not MD rehabilitation |
| Hesse 2003 | Unidisciplinary rehabilitation only |
| Hewitt 2001 | Unidisciplinary rehabilitation only |
| Jan 2004 | Unidisciplinary rehabilitation only |
| Jesudason 2002 | Unidisciplinary rehabilitation only |
| Kramer 2003 | Unidisciplinary rehabilitation only |
| Liang 1987 | Unidisciplinary rehabilitation only |
| Liu 2006 | Variable is not MD rehabilitation |
| Lysack 2005 | Unidisciplinary rehabilitation only |
| Maire 2003 | Variable is not MD rehabilitation |
| Maire 2004 | Variable is not MD rehabilitation |
| Martin 1991 | Unidisciplinary rehabilitation only |
| Mayer 2005 | Unidisciplinary rehabilitation only |
| Medeiros 1977 | Unidisciplinary rehabilitation only |
| Mitchell 2005 | Unidisciplinary rehabilitation only |
| Moffet 2004 | Unidisciplinary rehabilitation only |
| Peak 2005 | Variable is not MD rehabilitation |
| Rajan 2004 | Unidisciplinary rehabilitation only |
| Reilly 2005 | Unidisciplinary rehabilitation only |
| Roy 2005 | Unidisciplinary rehabilitation only |
| Russell 2003 | Unidisciplinary rehabilitation only |
| Strom 2006 | Unidisciplinary rehabilitation only |
| Suetta 2004 | Unidisciplinary rehabilitation only |
| Trudelle‐Jacksn 2001 | Unidisciplinary rehabilitation only |
| Trudelle‐Jacksn 2004 | Unidisciplinary rehabilitation only |
| Wang 2002 | Unidisciplinary rehabilitation only |
| Werner 2004 | Unidisciplinary rehabilitation only |
| Yan 2005 | Insufficient detail |
| Zenios 2002 | Unidisciplinary rehabilitation only |
Contributions of authors
Fary Khan: overall content of the review. Louisa Ng: primary support, study selection, data analysis and extraction, methodological analysis, feedback on clinical aspects, text of review. Senen Gonzalez: study selection, data extraction, methodological scoring. Tom Hale: study selection, data extraction, methodological scoring. Lynne Turner‐Stokes: study selection, design, textual feedback.
Sources of support
Internal sources
Rehabilitation Department, Royal Melbourne Hospital, Royal Park Campus, Melbourne, Australia.
External sources
Prof Lynne Turner‐Stokes ‐ financial support from Luff Foundation and Dunhill Medical Trust, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Dowsey 1999 {published data only}
- Dowsey MM, Kilgour ML, Santamaria NM, Choong PF. Clinical pathways in hip and knee arthroplasty: a prospective randomised controlled study. Medical Journal of Australia 1999;170(2):59‐62. [DOI] [PubMed] [Google Scholar]
Munin 1998 {published data only}
- Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA 1998;279(11):847‐52. [DOI] [PubMed] [Google Scholar]
Shepperd 1998 {published data only}
- Shepperd S, Harwood D, Gray A, Vessey M, Morgan P. Randomised controlled trial comparing hospital at home care with inpatient hospital care. II: cost minimisation analysis. BMJ 1998;316(7147):1791‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd S, Harwood D, Jenkinson C, Gray A, Vessey M, Morgan P. Randomised controlled trial comparing hospital at home care with inpatient hospital care. I: 3 month follow up of health outcomes. BMJ 1998;316(7147):1786‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siggeirsdottir 2005 {published data only}
- Siggeirsdottir K, Olafsson O, Jonsson H, Iwarsson S, Gudnason V, Jonsson BY. Short hospital stay augmented with education and home based rehabilitation improved function and quality of life after hip replacement: randomised study of 50 patients with 6 months of follow up. Acta Orthopaedica 2005;76(4):555‐62. [DOI] [PubMed] [Google Scholar]
Weaver 2003 {published data only}
- Weaver FM, Hughes SL, Almagor O, Wixson R, Manheim L, Fulton B, et al. Comparison of two home care protocols for total joint replacement. Journal of the American Geriatrics Society 2003;51(4):523‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 2005 {published data only}
- Adam F, Chauvin M, DuManoir B, Langlois M, Sessler DI, Fletcher D. Small dose ketamine infusion improves postoperative analgesia and rehabilitation after total knee arthroplasty. Anesthesia and Analgesia 2005;100(2):475‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avramidis 2003 {published data only}
- Avramidis K, Strike PW, Taylor PN, Swain ID. Effectiveness of electric stimulation of the vastus medialis muscle in the rehabilitation of patients after total knee arthroplasty. Archives of Physical Medicine & Rehabilitation 2003;84(12):1850‐3. [DOI] [PubMed] [Google Scholar]
Bourne 1998 {published data only}
- Bourne RB. Is post‐total knee arthroplasty physical therapy necessary after hospital discharge?. Journal of Bone & Joint Surgery ‐ British Volume 1998;80(Suppl 2):168. [Google Scholar]
Breit 2004 {published data only}
- Breit R, Wall H. Transcutaneous electrical nerve stimulation for postoperative pain relief after total knee arthroplasty. Journal of Arthroplasty 2004;19(1):45‐8. [DOI] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen WH, Wang Q, Su JC. Postoperative rehabilitation exercise for the recovery of limb function after total hip replacement. Zhongguo Linchuang Kangfu 2004;8(20):3926‐8. [Google Scholar]
Cheville 2001 {published data only}
- Cheville A, Chen A, Oster G, McGarry L, Narcessian E. A randomized trial of controlled‐release oxycodone during inpatient rehabilitation following unilateral total knee arthroplasty. Journal of Bone & Joint Surgery ‐ American Volume 2001;83‐A(4):572‐6. [DOI] [PubMed] [Google Scholar]
Codine 2004 {published data only}
- Codine P, Dellemme Y, Denis‐Laroque F, Herisson C. The use of low velocity submaximal eccentric contractions of the hamstring for recovery of full extension after total knee replacement: a randomized controlled study. Isokinetics and Exercise Science 2004;12(3):215‐8. [Google Scholar]
Eisermann 2004 {published data only}
- Eisermann U, Haase I, Kladny B. Computer‐aided multimedia training in orthopedic rehabilitation. American Journal of Physical Medicine & Rehabilitation 2004;83(9):670‐80. [DOI] [PubMed] [Google Scholar]
Erler 2001 {published data only}
- Erler K, Anders C, Fehlberg G, Neumann U, Brucker L, Scholle HC. Objective assessment of results of special hydrotherapy in inpatient rehabilitation following knee prosthesis implantation. Zeitschrift fur Orthopadie und Ihre Grenzgebiete 2001;139(4):352‐8. [DOI] [PubMed] [Google Scholar]
Flynn 2000 {published data only}
- Flynn JR, Palmer‐Hill S, Crawfurd EJP. Early discharge at 5‐7 days after total knee replacement ‐ does knee performance suffer?. Journal of Bone & Joint Surgery ‐ British Volume 2000;82 (Supp 1l):68. [Google Scholar]
Frost 2002 {published data only}
- Frost H, Lamb SE, Robertson S. A randomized controlled trial of exercise to improve mobility and function after elective knee arthroplasty. Feasibility, results and methodological difficulties. Clinical Rehabilitation 2002;16(2):200‐9. [DOI] [PubMed] [Google Scholar]
Ganz 2004 {published data only}
- Ganz SB, Ranawat CS. Efficacy of formal knee flexion exercises on the achievement of functional milestones following total knee arthroplasty. Topics in Geriatric Rehabilitation 2004;20(4):311. [Google Scholar]
Giaquinto 2006 {published data only}
- Giaquinto S, Cacciato A, Minasi S, Sostero E, et al. Effects of music‐based therapy on distress following knee arthroplasty. British Journal of Nursing 2006;15(10):576‐9. [DOI] [PubMed] [Google Scholar]
Gilbey 2003 {published data only}
- Gilbey HJ, Ackland TR, Tapper J, Wang AW. Perioperative exercise improves function following total hip arthroplasty: A randomized controlled trial. Journal of Musculoskeletal Research 2003;7(2):111‐23. [Google Scholar]
Gilbey et al 2003 {published data only}
- Gilbey HJ, Ackland TR, Wang AW, Morton AR, Trouchet T, Tapper J. Exercise improves early functional recovery after total hip arthroplasty. Clinical Orthopaedics & Related Research 2003;408:193‐200. [DOI] [PubMed] [Google Scholar]
Gotlin 1994 {published data only}
- Gotlin RS, Hershkowitz S, Juris PM, Gonzalez EG, Scott WN, Insall JN. Electrical stimulation effect on extensor lag and length of hospital stay after total knee arthroplasty. Archives of Physical Medicine & Rehabilitation 1994;75(9):957‐9. [PubMed] [Google Scholar]
Grange 2004 {published data only}
- Grange CC, Maire J, Groslambert A, Tordi N, Dugue B, Pernin JN, et al. Perceived exertion and rehabilitation with arm crank in elderly patients after total hip arthroplasty: a preliminary study. Journal of Rehabilitation Research & Development 2004;41(4):611‐9. [DOI] [PubMed] [Google Scholar]
Hesse 2003 {published data only}
- Hesse S, Werner C, Seibel H, Frankenberg S, Kappel EM, Kirker S, et al. Treadmill training with partial body‐weight support after total hip arthroplasty: a randomized controlled trial. Archives of Physical Medicine & Rehabilitation 2003;84(12):1767‐73. [DOI] [PubMed] [Google Scholar]
Hewitt 2001 {published data only}
- Hewitt B, Shakespeare D. Flexion vs. extension: a comparison of post‐operative total knee arthroplasty mobilisation regimes. Knee 2001;8(4):305‐9. [DOI] [PubMed] [Google Scholar]
Jan 2004 {published data only}
- Jan MH, Hung JY, Lin JCH, Wang SF, Liu TK, Tang PF. Effects of a home program on strength, walking speed, and function after total hip replacement. Archives of Physical Medicine & Rehabilitation 2004;85(12):1943‐51. [DOI] [PubMed] [Google Scholar]
Jesudason 2002 {published data only}
- Jesudason C, Stiller K. Are bed exercises necessary following hip arthroplasty?. Australian Journal of Physiotherapy 2002;48(2):73‐81. [DOI] [PubMed] [Google Scholar]
Kramer 2003 {published data only}
- Kramer JF, Speechley M, Bourne R, Rorabeck C, Vaz M. Comparison of clinic‐ and home‐based rehabilitation programs after total knee arthroplasty. Clinical Orthopaedics & Related Research 2003;410:225‐34. [DOI] [PubMed] [Google Scholar]
Liang 1987 {published data only}
- Liang MH, Cullen KE, Larson MG, Schwartz JA, Robb‐Nicholson C, Fossel AH, et al. Effects of reducing physical therapy services on outcomes in total joint arthroplasty. Medical Care 1987;25(4):276‐85. [DOI] [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Liu Z, Huang D, Zhuo D. Research on early inpatient rehabilitation after cementless total hip arthroplasty. Zhongguo Kangfu Yizue Zazhi 2006;21(4):314‐7, 321. [Google Scholar]
Lysack 2005 {published data only}
- Lysack C, Dama M, Neufeld S, Andreassi E. A compliance and satisfaction with home exercise: a comparison of computer‐assisted video instruction and routine rehabilitation practice. Journal of Allied Health 2005;34(2):76‐82. [PubMed] [Google Scholar]
Maire 2003 {published data only}
- Maire J, Dugue B, Faillenet‐Marie AF, Tordi N, Parratte B, Smolander J, et al. Recovery after total hip joint arthroplasty in elderly patients with osteoarthritis: positive effect of upper‐limb interval training. Journal of Rehabilitation Medicine 2003;35(4):174‐9. [DOI] [PubMed] [Google Scholar]
Maire 2004 {published data only}
- Maire J, Faillenet‐Maire AF, Grange C, Dugue B, Tordi N, Parratte B, et al. A specific arm interval exercise program could improve the health status and walking ability of elderly patients after total hip arthroplasty: a pilot study. Journal of Rehabilitation Medicine 2004;36(2):92‐4. [DOI] [PubMed] [Google Scholar]
Martin 1991 {published data only}
- Martin TP, Gundersen LA, Blevins FT, Coutts RD. The influence of functional electrical stimulation on the properties of vastus lateralis fibres following total knee arthroplasty. Scandinavian Journal of Rehabilitation Medicine 1991;23(4):207‐10. [PubMed] [Google Scholar]
Mayer 2005 {published data only}
- Mayer J, Bohn J, Gorlich P, Eberspacher H. Mental gait training ‐‐ effectiveness of a therapy method in the rehabilitation after hip‐replacement. Zeitschrift fur Orthopadie und Ihre Grenzgebiete 2005;143(4):419‐23. [DOI] [PubMed] [Google Scholar]
Medeiros 1977 {published data only}
- Medeiros JM, Smidt GL, Burmeister LF, Soderberg GL. The influence of isometric exercise and passive stretch on hip joint motion. Physical Therapy 1977;57(5):518‐23. [DOI] [PubMed] [Google Scholar]
Mitchell 2005 {published data only}
- Mitchell C, Walker J, Walters S, Morgan AB, Binns T, Mathers N. Costs and effectiveness of pre‐ and post‐operative home physiotherapy for total knee replacement: randomized controlled trial. Journal of Evaluation in Clinical Practice 2005;11(3):283‐92. [DOI] [PubMed] [Google Scholar]
Moffet 2004 {published data only}
- Moffet H, Collet JP, Shapiro SH, Paradis G, Marquis F, Roy L. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: A single‐blind randomized controlled trial. Archives of Physical Medicine & Rehabilitation 2004;85(4):546‐56. [DOI] [PubMed] [Google Scholar]
Peak 2005 {published data only}
- Peak EL, Parvizi J, Ciminiello M, Purtill JJ, Sharkey PF, Hozack WJ, et al. The role of patient restrictions in reducing the prevalence of early dislocation following total hip arthroplasty. Journal of Bone and Joint Surgery ‐ American Volume 2005;87(2):247‐53. [DOI] [PubMed] [Google Scholar]
Rajan 2004 {published data only}
- Rajan RA, Pack Y, Jackson H, Gillies C, Asirvatham R. No need for outpatient physiotherapy following total knee arthroplasty: a randomized trial of 120 patients. Acta Orthopaedica Scandinavica 2004;75(1):71‐3. [DOI] [PubMed] [Google Scholar]
Reilly 2005 {published data only}
- Reilly KA, Beard DJ, Barker KL, Dodd CAF, et al. Efficacy of an accelerated recovery protocol for Oxford unicompartmental knee arthroplasty‐‐a randomised controlled trial. Knee 2005;12(5):351‐7. [DOI] [PubMed] [Google Scholar]
Roy 2005 {published data only}
- Roy BD, Beer J, Harvey D, Tarnopolsky MA. Creatine monohydrate supplementation does not improve functional recovery after total knee arthroplasty. Archives of Physical Medicine & Rehabilitation 2005;86(7):1293‐8. [DOI] [PubMed] [Google Scholar]
Russell 2003 {published data only}
- Russell TG, Buttrum P, Wootton R, Jull GA. Low‐bandwidth telerehabilitation for patients who have undergone total knee replacement: preliminary results. Journal of Telemedicine & Telecare 2003;9(Suppl 2):S44‐7. [DOI] [PubMed] [Google Scholar]
Strom 2006 {published data only}
- Strom H, Huss K, Larsson S. Unrestricted weight bearing and intensive physiotherapy after uncemented total hip arthroplasty. Scandinavian Journal of Surgery: SJS 2006;95(1):55‐60. [DOI] [PubMed] [Google Scholar]
Suetta 2004 {published data only}
- Suetta C, Magnusson SP, Rosted A, Aagaard P, Jakobsen AK, Larsen LH, et al. Resistance training in the early postoperative phase reduces hospitalization and leads to muscle hypertrophy in elderly hip surgery patients‐‐a controlled, randomized study. Journal of the American Geriatrics Society 2004;52(12):2016‐22. [DOI] [PubMed] [Google Scholar]
Trudelle‐Jacksn 2001 {published data only}
- Trudelle‐Jackson E. Effect of post‐rehabilitation exercise on strength and postural stability following total hip arthroplasty. Texas Woman's University 2001.
Trudelle‐Jacksn 2004 {published data only}
- Trudelle‐Jackson E, Smith SS. Effects of a late‐phase exercise program after total hip arthroplasty: a randomized controlled trial. Archives of Physical Medicine & Rehabilitation 2004;85(7):1056‐62. [DOI] [PubMed] [Google Scholar]
Wang 2002 {published data only}
- Wang AW, Gilbey HJ, Ackland TR. Perioperative exercise programs improve early return of ambulatory function after total hip arthroplasty: a randomized, controlled trial. American Journal of Physical Medicine & Rehabilitation 2002;81(11):801‐6. [DOI] [PubMed] [Google Scholar]
Werner 2004 {published data only}
- Werner C, Kappel EM, Sonntag D, Bardeleben A, et al. Treadmill therapy with partial body weight support after total hip arthroplasty. Physikalische Medizin Rehabilitationsmedizin Kurortmedizin 2004;14(3):140‐5. [Google Scholar]
Yan 2005 {published data only}
- Yan BP. Effects of early rehabilitation training and psychological intervention on physical and mental health of patients undergoing replacement of total hip. Zhongguo Linchuang Kangfu 2005;9(48):46‐8. [Google Scholar]
Zenios 2002 {published data only}
- Zenios M, Wykes P, Johnson DS, Clayson AD, Kay P. The use of knee splints after total knee replacements. Knee 2002;9(3):225‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Brady 2000
- Brady OH, Masri BA, Garbuz DS, Duncan CP. Rheumatology: 10. Joint replacement of the hip and knee: when to refer and what to expect. Canadian Medical Association Journal 2000;163(10):1285‐91. [PMC free article] [PubMed] [Google Scholar]
CJRR 2006
- Canadian Joint Replacement Registry. Annual Report Hip and Knee Replacements in Canada 2006.
Egger 1998
- Egger M, Smith GD. Bias in location and selection of studies. BMJ 1998;316:61‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goetzsche 1987
- Goetzsche PC. Reference bias in reports of drug trials. BMJ 1987;295:654‐6. [PMC free article] [PubMed] [Google Scholar]
Heintjies 2002
- Heintjies E, Berger MY, Bierma Zeinstrea SMA, Bernsen RMD, Verhaar JAN. Pharmacotherapy for patellofemoral pain syndrome. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003470.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Assessment of study quality. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Section 6. http://www.cochrane.org/resources/handbook (accessed 13 February 2006).
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C. Assessing the quality of reports of randomised clinical trials ‐ is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
McDonald 2000
- McDonald SJ, Bourne RB, Rorabeck CH, McCalden RW, Kramer J, Vaz M. Prospective randomised clinical trial of continuous passive motion after total knee arthroplasty. Clinical Orthopaedic and Related Research 2000;1(380):30‐5. [DOI] [PubMed] [Google Scholar]
McDonald 2004
- McDonald S, Hetrick S, Green S. Pre‐operative education for hip or knee replacement. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003526.pub2] [DOI] [PubMed] [Google Scholar]
Milne 2003
- Milne S, Brosseau L, Robinson V, Noel MJ, Davis J, Drouin H, et al. Continuous passive motion following total knee arthroplasty. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004260] [DOI] [PubMed] [Google Scholar]
Monte 1997
- Monte MA, Tankersley WS, Hungersford DS. Hip rehabilitation after surgery. Physical Medicine & Rehabilitation Secrets. Vol. 330, Hanley & Belfus, 1997. [Google Scholar]
Morris 1998
- Morris BA, Cobwell CW, Hardwick ME. The use of low molecular weight heparin in the prevention of venous thromboembolic disease. Orthopaedic Nursing 1998;17(6):23‐9. [DOI] [PubMed] [Google Scholar]
Tankersley 1997
- Tankersley WS, . Mont MA, Hungersford DS. Knee rehabilitation after surgery. Physical Medicine & Rehabilitation Secrets. Hanley & Belfus, 1997:337‐8. [Google Scholar]
Tugwell 2004
- Tugwell P, Shea B, Boers M, Brooks P, Simon L, Strand V, et al. Evidence‐based Rheumatology. BMJ Publishing Group, 2004. [Google Scholar]
Turner‐Stokes 2005
- Turner‐Stokes L, Disler P, Nair A, Wade DT. Multidisciplinary rehabilitation for acquired brain injury in adults of working age. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004170.pub2.] [DOI] [PubMed] [Google Scholar]
Van Tulder 2003
- Tulder MW, Furlan A, Bombardic C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
WHO 1980
- World Health Organization. International classification of impairments, disabilities and handicap. WHO Geneva World Health Assembly 29.35.
WHO 2001
- World Health Organization. International classification of functioning, disability and health (ICF). WHO Geneva 2001.
Whyte 2002
- Whyte J. Traumatic brain injury rehabilitation: are there alternatives to randomised clinical trials?. Archives of Physical Medicine and Rehabilitation 2002;83(9):1320‐2. [DOI] [PubMed] [Google Scholar]


