Abstract
Thyroid hormones (THs) are involved in the development and function of the male reproductive system, but their effects on the prostate have been poorly studied. This work reviews studies related to the interrelationship between the thyroid and the prostate. The information presented here is based upon bibliographic searches in PubMed using the following search terms: prostate combined with thyroid hormone or triiodothyronine, thyroxine, hypothyroidism, hyperthyroidism, or deiodinase. We identified and searched 49 articles directly related to the issue, and discarded studies related to endocrine disruptors. The number of publications has grown in the last 20 years, considering that one of the first studies was published in 1965. This review provides information based on in vitro studies, murine models, and clinical protocols in patients with thyroid disorders. Studies indicate that THs regulate different aspects of growth, metabolism, and prostate pathology, whose global effect depends on total and/or free concentrations of THs in serum, local bioavailability, and the endocrine androgen/thyronine context.
Keywords: thyroid hormones, androgens, prostate, hyperplasia, cancer
Introduction
The purpose of this review is to compile and discuss findings related to the role of thyroid hormones (THs) in the development, physiology, and pathology of the prostate gland. The study of the prostate is of interest in the field of reproductive biology and urology. In mammals, this gland produces and secretes essential components for the protection and viability of spermatozoa. In middle age or later, men become highly prone to developing prostatic hyperplasia and/or cancer. However, the thyroid-prostate relationship has been poorly studied in the fields of endocrinology or cancer. This can be explained by the fact that thyroid function in non-metabolic tissues had been underestimated for a long time. Androgens and not THs are the main hormonal stimuli for the prostate. The gonad and not the prostate is the principal target of THs in the reproductive sphere, and it has been assumed that metabolic actions of THs invariably lead to cancer progression. It is well established that THs regulate testis development and function, and some thyroid effects on the prostate are explained by the regulation of the gonadal axis; however, essential components of thyroid signaling have also been identified in prostate cell lines or the prostate gland, suggesting direct actions of THs on this target organ. This paper reviews: (i) elements related to TH transport, bioavailability, and thyroid signaling that have been identified in the prostate; (ii) the influence of the thyroid status on prostate weight during different critical periods of development; (iii) TH effects on gene expression and/or activation of enzymes involved in the prostatic glandular function and thyroid response; (iv) epidemiological and experimental evidence suggesting that THs could increase or reduce the risk of developing prostate cancer, and 5) the interaction of THs with androgens and other endocrine systems in the prostate.
Thyroid hormone overview
THs are synthesized in the thyroid gland. They are mainly represented by tetraiodothyronine (T4) and triiodothyronine (T3), and both are essential for the development, maintenance, and metabolism of almost all tissues. The effects of THs on the target tissues are determined by factors that control their bioavailability and signaling, such as transporters, integrins, deiodinases, nuclear receptors, coregulators, among others. Circulating levels of hormones and their transporters determine the relation of free hormone/total hormone in serum, while T4 and/or T3 entry into tissues and the deiodinase enzymatic system regulates the generation or inactivation of THs in situ (1). T3 generation from T4 is catalyzed by the enzymes type 1 deiodinase (DIO1) and type 2 deiodinase (DIO2), whereas T4 or T3 inactivation is mainly catalyzed by type 3 deiodinase (DIO3) and by DIO1 (2). Thyroid nuclear receptors activation and the αvβ3 integrin pathway are part of the canonic and non-canonic mechanisms involved in thyroid signaling. It is well known that T4 acts as a prohormone and T3 as a hormone, since T3 exhibits a 10- to 15-fold higher affinity than T4 for nuclear receptors (1). A non-canonical pathway of THs includes the activation of αvβ3 integrin signaling (3). Integrins are members of a family of cell adhesion receptors that regulate the attachment of epithelial cells to the basement membrane, and in prostate cancer, they mediate cell invasion, immune escape, and angiogenesis (4). It has been recognized that physiological concentrations of T4 (100 nM) and supraphysiological concentrations of T3 (10 nM) bind at or near the arginine–glycine–aspartic acid (RGD) recognition site of the extracellular domain of integrin αvβ3 (3). All these components are required for THs action, and some of them have been described in normal and/or neoplastic prostate cells.
Transport, uptake, and bioavailability of thyroid hormones in the prostate
The internalization of T4 and T3 in target cells is mediated by specific and non-specific transporters, among which are members of the monocarboxylate transporters (SLC16A2 or MCT8, SLC16A10 or MCT10), the organic anion-transporting polypeptides (OATPs), and/or the L-type amino acid transporters. However, and for both hormones, MCT8 seems to be the most efficient and specific transporter in most tissues (5). There are no studies that show an active transport of THs into stroma or prostate epithelium. MCT8 expression (mRNA) was detected in LNCaP prostate tumors (6), and RNA seq studies indicate that human samples of normal and cancerous prostate express the SLC16A2 gene, which encodes for MCT8 protein (www.proteinatlas.org).
A study in androgen-dependent (LNCaP) and androgen-independent (DU145, PC-3) prostate cancer cell lines showed a high accumulation of T3 in cells transfected with the high-affinity T3-binding protein known as μ-Crystallin (CRYM) (7), revealing the ability of these cells to internalize T3. This binding protein is endogenously expressed in human prostate tumors and LNCaP cells (8). Its overexpression inhibits the androgenic response in LNCaP cells and reduces the invasive capacity of DU145 and PC-3 cells (7). These antitumor effects of CRYM/T3 have been explained by a reduction of T3 binding to nuclear receptors (7). Moreover, it has been proposed that this complex potentially contributes to the cytoplasmic reservoir of T3 and the transport of this hormone to the nucleus (9). CRYM undoubtedly controls the intracellular availability of T3, but its relevance to prostate physiopathology remains to be elucidated.
Deiodinases belong to a system of enzymes that regulate the local bioavailability of T3 in the target tissues (1). Pioneer studies in euthyroid adult rats revealed that around 20% of prostatic T3 content derives from thyroxine (T4) deiodination, and the remainder comes from plasma (10). Later, it was shown that DIO1 enzymatic activity is present in the prostate of pubescent rats and in 3-month-old young-adult rats. In contrast, this activity is practically undetectable in the postnatal period and in rats older than five months of age (11, 12). Sexual behavior is the central stimulus for prostate function, and a study in breeding rats showed that sexual activity prevents the decline of DIO1 activity associated with aging (12).
On the other hand, it has been shown that the sympathetic input activated by sexual behavior increases prostate DIO1 activity after consecutive ejaculations (13). In addition, DIO1 activity is under endocrine control; it is stimulated by T4, estrogens, and prolactin (PRL) and is negatively regulated by androgens (11). An association between enzymatic activity and prostatic T3 levels was observed during different physiological challenges (12, 13). Nevertheless, the contribution of DIO1 to T3 generation has been questioned in recent years, and instead, it has been suggested that this enzyme contributes to iodine recycling (1, 2). Biochemical studies have shown that DIO2 activity is not present in the rat prostate (11), but a low mRNA expression has been identified in the rat (11), mouse (14), and human prostate biopsies (www.proteinatlas.org). An inverse correlation between Dio1 or Dio2 and Dio3 gene expression was observed in the normal and cancerous prostate of euthyroid mice supplemented with T3. Physiological doses of T3 reduced Dio1 and Dio2 expression and increased Dio3 (T3-catabolizing enzyme), which allowed maintaining a euthyroid prostatic environment. Besides, it was found that T3 does not accelerate prostate cancer development in an early stage (14). However, longitudinal studies must be carried out to understand the role of T3/DIO3 in cancer progression. Some studies support the notion that Dio3 induction is part of a neoplastic program in which the proapoptotic and differentiating actions of THs are suppressed, and local hypothyroidism induced by the high DIO3 leads to an increase in cell proliferation (2). miRNA, miR-379, and miR-154 of the delta-like 1 homolog-deiodinase 3 cluster are increased in serum of prostate cancer patients, and apparently, they promote metastasis to bone (15).
Thyroid signaling in the prostate
The direct canonic effects of THs are exerted through the binding of T3 to the nuclear T3 receptors (TRs), which act as transcription factors by binding to response elements (TREs) in the regulatory regions of target genes (1). A pioneering study showed an abundant relative expression of thyroid hormone receptor alpha THRA, variants 1 (THRA1or TRα1) and 2 (THRA2or TRα2) and thyroid hormone receptor beta (THRB or TRβ) in human samples of hypertrophic prostate (16). TRβ protein has been identified in non-cancerous (PZ-HPV-7, RWPE-1) and cancerous prostate cell lines (PZ-HPV-7, CA-HPV-10, LNCaP, LAPC4, VCaP, PC-3, and DU145) by immunoblotting (7, 17). In the LNCaP line, T3 binds to nuclear receptors with high affinity (5 × 10−11 M) and binding capacity (206 pg/mg DNA). This binding was not modified in the presence of a synthetic androgenic agonist (R1881), suggesting that thyroid response could not be regulated by androgens (18). In vivo, TRα1 and TRβ proteins were mainly immunodetected in the epithelial cells of the normal and cancerous prostate (14), suggesting that nuclear thyroid receptors could regulate glandular activity. The effects of THs on the prostate also seem to be mediated by integrins. In particular, the integrin αvβ3 is a heterodimeric transmembrane glycoprotein that mediates cell adhesion to the extracellular matrix by recognizing conserved RGD motifs in various ligands, including osteopontin, vitronectin, and fibronectin (4). Integrin αvβ3 is expressed in prostate cancer (4), and THs can bind the RGD motif and activate downstream pathways (19). Altogether, these studies show that prostate cell lines and the prostate gland contain key elements to respond to THs actions, whose effects are reviewed below.
Biological effects of thyroid hormones
Prostate weight
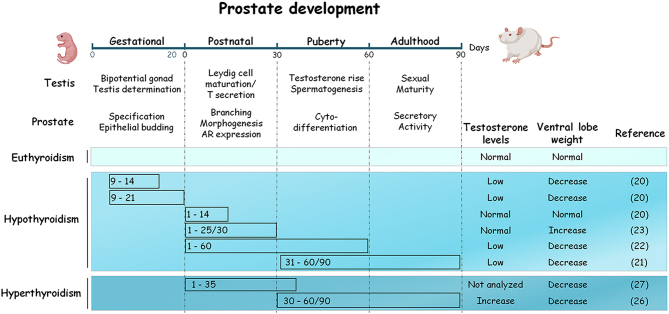
THs are essential for the development of reproductive organs. In critical periods of growth, a deficiency or excess modifies the size and function of the prostate in adulthood. Hypothyroidism has dual actions, and it can either promote or reduce prostate weight, depending on the period in which the deficiency occurs (Fig. 1). In adult rats, gestational hypothyroidism induced from days 9 to 14 or 21 post-coitum decreases the ventral lobe size and diminishes the testosterone levels, even with high levels of expression and binding capacity of the androgen receptor (AR) in the prostate (20). A reduction in prostate weight also occurs when hypothyroidism is induced from postnatal to puberty (days 160 or 90), or from puberty to sexual maturity (days 31–60 or 90). This reduction was related to low testosterone levels as a consequence of delayed puberty and impairment of testis function (21, 22). No alterations in testosterone levels or ventral prostate weight are shown in adult rats when hypothyroidism is transiently induced within the first 15 postnatal days (20). However, an increase in the prostatic weight and high expression and binding capacity of AR occurs when thyroid deficiency is induced from birth to the prepubertal period (days 1–25 to 30) (20, 23). This trophic effect was associated with a delay in puberty and an extension of the prostate morphogenesis time (23, 24). In adult rats, hypothyroidism does not affect the weight of gonads but reduces the luteinizing hormone and testosterone levels, and it indirectly decreases prostate weight (25). On the other hand, the effects of excessive THs on the prostate have been less studied. Figure 1 shows that hyperthyroidism induced during puberty increases testosterone levels but paradoxically reduces prostate weight in adult rats (26), suggesting that this effect is not related to androgen deficiency. A pioneering study showed that administration of a physiological supplement of T4 during the postnatal period (days 1–35) reduced prostate weight in prepubertal rats (27), while an increase in prostate weight was observed in T4-treated castrated rats (28). All these studies reveal the importance of the euthyroid state in the control of prostatic growth. TH effects are related to androgenic status and androgenic response during development, while their actions in adulthood are less understood.
Figure 1.
Influence of thyroid status during critical periods of development on prostate growth in rat model. Both, gestational and pubertal hypothyroidism impair gonadal development, decrease circulating levels of testosterone and reduce the weight of the ventral lobe in adult rats. A similar effect occurs when hypothyroidism is induced in rats after puberty. In contrast, hypothyroidism selectively induced during the postnatal period does not modify testosterone levels but increases prostate growth. Hyperthyroidism increases testosterone levels but reduces prostate weight, suggesting the involvement of extra-androgenic factors on prostate growth control.
Prostate function
There are no studies that show a direct effect of THs on prostate metabolism, but substantial evidence shows that thyroid disruptions affect the activity of metabolic enzymes in a lobe-specific manner. Table 1 shows that hypo- and hyperthyroidism up or downregulated, respectively the activity of Na+/K+, Ca2+, Mg2+ ATPases, acid and alkaline phosphatases, and enzymes involved in the conversion from complex carbohydrates to monosaccharides, such as β-glucosidases, β-galactosidase and β-N-acetylglucosaminidase (26, 29, 30). Although these effects could be indirectly related to changes in the steroidogenic status (diminution of testosterone and estradiol by hypothyroidism, and vice versa), in vitro studies suggest possible direct actions. T3 stimulates the activity of glycosidase enzymes and the levels of sugars such as fucose, fructose, sialic acid, and hexosamines, fundamental components for osmotic balance and metabolism of spermatozoa (26). These findings indicate that THs regulate the prostatic metabolic homeostasis in a lobe-specific manner. Regarding the endocrine environment, THs regulate the expression of prolactin receptor (PRLR) and its binding capacity (31, 32), as well as the release of some peptides such as calcitonin gene-related peptide (33), thyrotropin-releasing hormone (TRH), and TRH-glycine (34). As reviewed later, some studies suggest that prostatic TRH stimulates the thyroid gland through a prostate–thyroid feedback loop.
Table 1.
Influence of thyroid hormones in enzymatic activity and gene expression in the normal prostate and prostate cancer cell lines.
| Model | Prostate lobes | Effects | References | |
|---|---|---|---|---|
| Hypothyroidism | ||||
| 0.05% PTU/800 µg MMI/day for 21 days | Sprague-Dawley rats | Ventral | Increases TRH and TRH-Gly concentrations. | (34) |
| Iodine-deficient diet and 0.2% PTU for 3 weeks | Ventral | Increases PRL binding. This effect was not reverted within 16 h of T4 (10 µg/100 g BW) while T3 (100 ng/rat) decreased within 12 h. | (31) | |
| Surgical thyroidectomy | Albino rats | Ventral | Decreases enzymatic activities of Na+/K+ ATPase, Ca2+ ATPase, alkaline and acid phosphatases, but not Mg2+ATPase. | (29) |
| Dorsolateral | Increases Na+/K+ ATPase and decreases Ca2+and Mg2+ dependent ATPases. Acid and alkaline phosphatases activities were not modified. | (30) | ||
| Wistar rats | Ventral and dorsolateral | Decreases the activity of β-glucosidase, β-galactosidase, β-N-acetylglucosaminidase and β-N-acetylgalactosaminidase. | (26) | |
| Hyperthyroidism | ||||
| 25 μg T4/100 g BW/day for 60 days | Albino rats | Ventral | Increases the activity of Na+/K+ and Ca2+ ATPases and acid phosphatase. Alkaline phosphatase and Mg2+ ATPase were not modified. | (29) |
| Dorsolateral | Increases alkaline phosphatase activity and decreases Na+/K+, Ca2+ and Mg2+dependent ATPases. Acid phosphatase activity was not modified. | (30) | ||
| 25 μg T4/100 g BW/day for 30 days | Wistar rats | Ventral and dorsolateral lobes | Increases the activity of β-glucosidase, β-galactosidase, β-N-acetylglucosaminidase and β-N-acetylgalactosaminidase. | (26) |
| 10 µg T4/100 g BW for 21 days | Sprague-Dawley adult rats | Whole prostate | Increases PRLR mRNA levels. | (32) |
| 6 μg T4/mL for 21 days | Wistar rats ≈ 50 days old | Whole prostate | Increases DIO1 activity. | (11) |
| T3 replacement | ||||
| 2.5 and 15 µg T3/100 g BW for 42 days | Wilde-type and TRAMP mouse 56 days old | Whole prostate | Reduces Dio1 and Dio2 and increases Dio3 expression in normal and cancer tissue. | (14) |
| In vitro studies concentrations | ||||
| 10–100 ng T3/mL | Organ culture | Ventral and dorsolateral | Increases the activity of β-N-acetylglucosaminidase and β-N-acetylgalactosaminidase. | (26) |
| 1 and 10 nM T4 | Ventral | Increases the release of CGRP. | (33) | |
| 0.1–100 nM T3 | Cell lines | LNCaP | Increases the expression and secretion of KLK3 (PSA). | (7, 18, 41) |
| 1 and10 nM T3 | LNCaP | Decreases BTG2 (TIS21) expression. | (42) | |
| 10 nM–1 µM T3 | LNCaP | Increases CDKN2B (p15) and BHLHE40 (DEC1) gene expression. | (44) | |
| 100 nM T4 | PC-3 | Increases protein levels of XIAP, MMP-2, VEGF and the pERK/ERK ratio. | (45) | |
| 10 nM T3 | RWPE-1 and LNCaP | Increases glycine, glutamate, and creatine in both cell types. Increases choline in RWPE-1 and taurine in LNCaP. | (7) | |
BHLHE40, basic helix-loop-helix family member 40; BTG2, BTG2 anti-proliferation factor 2; BW, body weight; CDKN2B, cyclin-dependent kinase inhibitor 2B; CGRP, calcitonin gene-related peptide; DIO1, iodothyronine deiodinase 1; DIO2, iodothyronine deiodinase 2; DIO3, iodothyronine deiodinase 3; ERK, extracellular signal-regulated kinase; KLK3, kallikrein-related peptidase 3; MMI, methimazole; MMP-2, matrix metallopeptidase 2; PRL, prolactin; PRLR, prolactin receptor; PTU, propylthiouracil; T3, triiodothyronine; T4, tetraiodothyronine or thyroxine; TRAMP, transgenic adenocarcinoma of the mouse prostate. TRH, thyrotropin releasing hormone; VEGFA, vascular endothelial growth factor; XIAP, X-linked inhibitor of apoptosis.
Prostate pathologies
Benign prostate hyperplasia
Benign prostate hyperplasia (BPH) is the most common non-malignant growth of the prostate, with an incidence of 50% in men by age 50 (35). A prospective study carried out in air force veterans from the USA (598 cases/1087 patients) showed no relationship between thyroid status (total or free T4) and risk of developing BPH (36). Another study showed that hyperthyroidism (191 cases/832 patients) apparently increases the risk of BPH, but when data were adjusted by age and metabolic comorbidities, the correlation was lost (37). In contrast, a positive correlation between prostate volume and elevated physiological levels of free T3 (40 subjects) or free T4 (5708 subjects) was observed in patients diagnosed with BPH (38, 39). The effects of thyroid-stimulating hormone (TSH) on BPH development are less understood. Some studies did not observe any relationship (36), whereas others have shown a negative correlation between TSH and prostate size (38). Concordantly, elevated levels of TSH (>4 mU/L) have been associated with a lower score of lower urinary tract symptoms (40). Moreover, increased TSH levels and improved symptoms were observed in BPH patients treated with an inhibitor of the local conversion from testosterone to dihydrotestosterone or subjected to partial or total prostatectomy, revealing a complex thyroid-androgens-prostate or TSH/prostate relationship (40). The possible relationship between thyroid status (TSH, T4, T3, free T4, and free T3 levels) and the onset of BPH (lower urinary tract symptoms, prostate volume, prostate-specific antigen (PSA) levels) should be analyzed from a more integrative approach.
Prostate cancer
Prostate cancer is the second most frequent cancer in men and the sixth cause of death worldwide (41). The role of THs in the initiation and/or progression of this disease has been poorly studied, but experimental and clinical evidence suggest that these hormones exert dual actions, depending on the cellular context (Table 2).
Table 2.
Cellular effects of thyroid hormones in prostate cancer cell lines.
| Cell lines | Biological effect | References | |
|---|---|---|---|
| T3 | |||
| 0.1 nM–1 µM for 6 days | LNCaP | Stimulates cell proliferation ([3H] thymidine incorporation). | (18) |
| 0.1 nM–1 µM for 6 days | LNCaP DU145 |
Stimulates cell proliferation (MTS assay) in LNCaP but not in DU145. | (43) |
| 10–50 ng/mL for 72 h | MDA PCa 2b |
Stimulates cell proliferation ([3H] thymidine incorporation). | (8) |
| 0.1–100 nM 0.1–10 µM for 7 days |
LNCaP PC-3 |
Stimulates cell proliferation in LNCaP and DU145 ([3H] thymidine incorporation). | (42) |
| 0.1 nM–1 µM for 6 days | LNCaP CA-HPV-10 PC-3 DU145 |
Stimulates cell proliferation (MTS assay) in LNCaP, but not in CA-HPV-10, PC-3, DU145. | (17) |
| 10 nM for 4 or 6 days | LNCaP DU145 |
Reduces invasive capacity (transwell assay) stimulated by a β-adrenergic activator and the acquisition of projections like neurites (phase contrast microscopy). Treatment for 4 days had no effect in DU145. | (6) |
| 0.1 nM–1 µM for 6 days | LNCaP | Induces senescence (β-galactosidase assay) in a dose-dependent manner. | (45) |
| T4 | |||
| 100 nM for 7 days | PC-3 | Increases cell migration (transwell assay) and reduces apoptosis (flow cytometry) in anoikis-resistant cells. | (46) |
| 100 nM for 4 or 6 days | LNCaP DU145 |
Increases cell invasion (transwell assay) and the acquisition of projections like neurites (phase contrast microscopy). Treatment for four days had no effect in DU145. | (6) |
MTS, tetrazolium salt.
In vitrostudies
Most of the studies have been carried out in the LNCaP cell line, which expresses a functional AR and is representative of an early cancer model. But also, some androgen-independent prostate cancer cell lines, such as PC-3 and DU145, have been used as advanced cancer models. Table 2 shows that physiological concentrations of T3 stimulate the proliferation in the LNCaP line, while 100-fold higher concentrations are required to stimulate the proliferation in the PC-3 line (17, 18, 42). In LNCaP cells, a gradual proliferative effect is observed at concentrations of up to 1.0 nM of T3, but at concentrations of up to 1 µM a plateau is reached (17, 18, 42). These effects seem to be mediated in part by the reduction of B-cell translocation 2 protein, whose expression is directly regulated by negative TREs (43). Androgens exert a biphasic effect in the proliferation of LNCaP cells; at physiological concentrations, they stimulate cell proliferation, while at supraphysiological concentrations they inhibit proliferation (18). In this context, T3 potentiates the androgenic proliferative response, but this interaction is lost at concentrations higher than 1.0 nM of R1881 (synthetic agonist) (18). It is known that T3 increases the expression and amount of AR in LNCaP cells (7, 18, 42), but does not increase the nuclear AR levels over androgen alone (7), suggesting that proliferative T3 actions could be relevant in low androgen conditions.
In addition to stimulating cell proliferation, T3 regulates the production and secretion of kallikreins (KLKs) in the LNCaP line. KLK3, also known as PSA, is involved in the breakdown of semenogelins and semen liquefaction. T3 stimulates the secretion of KLK3 in a dose-dependent manner (18, 42). These effects have been explained by a functional TRE site in the PSA gene promoter, which is transactivated in the presence of androgens (44). On the other hand, studies carried out in LNCaP cells showed that T3 induces senescence (increase in BHLHE40) and cell arrest (increase in p15) in a dose-dependent manner in a range from 0.1 to 10 nM (45), and that 10 nM of T3 increases the levels of some metabolites, such as glycine, glutamate, creatine, and taurine (7). In contrast, T4 effects have been less studied. T4 (100 nM) increases cell proliferation, the acquisition of neurite-like projections, and vascular endothelial growth factor (VEGF) production in the LNCaP cell line, but not in DU145 (6, 14, 46). Also, T4 reduces apoptosis (decreases protein levels of apoptosis inhibitor XIAP and annexin V-phosphatidylserine binding) and increases protein levels of MMP2 and cell migration (46). These protumoral effects seem to be mediated by αvβ3 integrin/pERK signaling (14, 46). Integrin αvβ3 regulates processes like cell proliferation, survival, angiogenesis, and chemoresistance in prostate cancer (4).
In vivo studies
A physiological supply of T3 reduced prostate tumor weight and epithelial PCNA levels in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model. These effects could be directly related to both an increase in T3 and a reduction in T4 levels as a result of a negative feedback loop (14). Consistently, T4 hyperthyroidism increases tumor growth in a PC-3 xenograft model (47). In another study, a supplement of T3 attenuated the growth of LNCaP xenograft tumors and prevented the rise in the tumoral expression of neuroendocrine (NE) factors (synaptophysin, neuron-specific enolase) and VEGF induced by a β-adrenergic activator (6). NE tumors commonly occur after androgen deprivation therapy and are highly aggressive (48). Primary tumors of TRAMP mice develop adeno- and NE carcinoma by the inactivation of retinoblastoma protein and p53 (49); hence, it would be interesting to analyze the role of T3 in this model. Overall, these findings suggest that the proliferative actions of T3 observed in vitro do not translate into increased tumor growth in in vivo models, whereas T4 effects consistently stimulate tumorigenic processes.
Epidemiological and clinical studies
There is evidence that subclinical or overall hypothyroidism does not modify the risk of prostate cancer, while hyperthyroidism or reduced levels of TSH significantly increase this risk when these parameters are age-adjusted and with any other potential confounder factor [reviews: (50, 51, 52, 53, 54)]. A prospective study (326 cases/9981 patients) showed that subclinical and frank hypothyroidisms are not associated with cancer risk (50). In comparison, a lower risk was observed in smoker men with frank hypothyroidism (20 cases/75) and elevated TSH levels (54). Mondul et al. (54) did not find an association between overall hyperthyroidism and prostate cancer risk (20 cases/75), while several other studies have shown that hyperthyroid subjects (53) and subjects with low levels of TSH <0.50 mU/L (10 cases/160) (50) or high physiological levels of free T4 (126 cases/1623) have an increased risk of developing prostate cancer (55). Moreover, a positive association between T3 levels and prostate cancer aggressiveness, based on the Gleason score, tumor grade, and/or tumor percentage involvement, was reported in euthyroid patients (56, 57). Overall, these data suggest that physiological increments of free T4 or subclinical (decrease in TSH levels) and frank hyperthyroidism (decrease in TSH and increase in THs) increase the risk of developing prostate cancer, whereas incremented levels of T3 seem to be associated with more aggressive tumors. All these clinical studies provide valuable information and put in perspective the need to conduct multicenter studies to determine the relevance of thyroid status on prostate cancer epidemiology.
Interplay of T3 with other endocrine systems
A bidirectional interaction of T3 and other endocrine messengers, such as androgens, PRL, growth factors, and catecholamines has been widely documented in the normal and cancerous prostate tissue (Table 3).
Table 3.
Effects of triiodothyronine (T3) in the expression of some messengers and endocrine receptors.
| T3 (nM) | Cell lines | Gene expression and/or protein levels | References |
|---|---|---|---|
| 0.1 and 1.0 | LNCaP | Increases gene expression and protein levels of AR, as well as expression of target genes (PSA). | (7, 18,42) |
| 100 | LNCaP LNCaP C4-2 |
Increases β2-adrenergic receptor levels. | (70) |
| 0.1 | LNCaP | Biphasic response. Increases the specific binding to GH and GH receptor expression within the first 24 h and reduces the binding at 72 h. | (63) |
| 0.1 | LNCaP | Increases gene expression of IGF-1, IGF1R, and ESR2 (ERβ). | (64) |
AR, androgen receptor; ESR2, estrogen receptor 2; GH, growth hormone; IGF-1, insulin-like growth factor 1; IGF1R, insulin-like growth factor type one receptor; PSA, prostate-specific antigen.
Androgens
As mentioned before, THs regulate the activity of the pituitary–gonad axis and androgen signaling in target tissues. The coexistence of putative response sites to androgens (ARE) and THs (TRE) has been reported in genes related to T3 (deiodinases, thyroid receptors) and androgen (5α-reductase and AR) signaling (58); however, the prostatic crosstalk between androgens and T3 has been poorly studied. It is known that T3 increases the AR levels and androgen target gene expression of KLK3 in LNCaP cells (7, 18, 42), and a decrease in AR protein levels was consistently found in the prostatic ventral and dorsolateral lobes during gestational and/or postnatal hypothyroidism (20). TREs have not been identified in the promoter region of the AR gene, suggesting an indirect regulation by T3. Beyond the AR, T3 directly up-regulates the expression of nuclear receptor coactivator 4 (NCOA4), which was previously identified as an AR-associated protein (ARA70) (59). ARA70 induces apoptosis and reduces the invasive potential of prostate cancer cells through AR-dependent and independent mechanisms (60). An increase in ARA70 expression was observed in LNCaP prostate tumors of nude mice supplemented with T3 (6). These findings suggest that T3 could modulate the prostatic response to androgens by increasing the levels of AR and its coactivator. As mentioned, androgens do not seem to modify the levels of nuclear receptors to T3 in LNCaP cells (18), but Dio2 expression increases in pluripotency conditions in LNCaP and CWR22Rv1 cells (androgen-independent), and Dio2 knockdown results in a reduction in the expression of androgen target genes (61). All these data indicate that T3 could modulate androgen signaling primarily in low or absent androgen levels.
Prolactin
The interaction between THs and PRL has been amply studied in the biology of reproduction, and an inverse relation between THs and PRLR has been described in the rat prostate. Hypothyroidism increases PRL binding to its receptor, and T3 reverses this effect (32), while PRL increases DIO1 enzymatic activity (11). These data indicate that T3 regulates PRLR and that PRL might regulate the availability of THs and/or iodine in the prostate. Mutations of THRB in the T3 binding site induce mammary hyperplasia and activation of the PRL signaling pathway mediated by STAT5, while T3 represses STAT5 activation and cyclin D1 in the native THRB(62). Additional studies are required to know the implications of the T3/PRL interaction in the function and pathology of the prostate.
Growth hormone and insulin-like growth factor
A positive correlation between TH and growth hormone (GH)/insulin-like growth factor has been observed in prostate cancer cell lines. T3 stimulates gene expression of the GH receptor, as well as expression of IGF1 and its receptors (IGF1R, IGF2R) (63, 64).
Norepinephrine
It is well established that sympathetic norepinephrine (NE) input stimulates the thyroid axis and regulates deiodinase activity in several tissues (65). The prostate is innervated by parasympathetic (pelvic nerve) and sympathetic (hypogastric nerve) fibers. The adrenergic input maintains prostate tone, and the activation of α1-adrenergic receptors stimulates the contraction of the vascular and non-vascular loose muscle. In contrast, β2-adrenergic receptor activation leads to relaxation and regulates the expression of genes related to prostate function (66, 67). It has been shown that sexual activity increases prostate DIO1 activity (12) and that this response could depend on sympathetic NE input (13). The impact that the relationship between NE and deiodinases could have on the physiology and pathology of the prostate is unknown, but substantial evidence indicates that sympathetic overactivity increases the risk and/or progression of hyperplasia and prostate cancer (66, 67). As mentioned, BPH patients have high serum T3 levels, and hyperthyroid patients have increased micturition frequency and nocturia (68, 69), but there are no studies that connect NE/T3 interaction in cancer. Studies in the human prostate show that cancerous epithelium expresses higher levels of β2-adrenergic receptors than normal or hyperplasic tissue (70), but levels of these receptors are decreased in metastatic prostate cancer with the mesenchymal phenotype (71). Expression of the β2-adrenergic receptor depends on androgens and consequently is downregulated by androgen deficiency. The basal expression of the β2-adrenergic receptor is T3-independent, but in the absence of androgens, T3 upregulates its expression and compensates for the lack of androgens (70). This finding agrees with the presence of a cyclic-AMP response element and a TRE in the promoter region of the β2-adrenergic gene (72). Stimulation of β2-adrenergic receptors, as well as androgen deprivation, activates NE transdifferentiation programs (48, 73). As previously mentioned, T4 stimulates and T3 prevents the acquisition of neurite-like projections induced by β-adrenergic stimulation (6). A recent study showed that αvβ3 integrin was selectively detected in primary prostate tumors, and metastatic lesions and expression levels were higher in the NE niches (74). The αvβ3 integrin itself is involved in various processes, including cell proliferation and angiogenesis, but the role of THs as activators of this pathway has not been studied.
General discussion and perspectives
The influence that THs have on the prostate has been recognized over the last 40 or 50 years, but the information has remained disarticulated. The findings reviewed here show that prostate cells express mechanisms, such as transporters, deiodinases, nuclear receptors, αvβ3 integrin, that confer the capability for responding to thyroid stimuli. Transcriptome analysis and studies of gain or loss of function of deiodinases and/or receptors are required to better approach this issue. T3 is an important regulator of androgen signaling, and more specific studies must be performed to discriminate between androgen-dependent and independent mechanisms. TH effects on cancer progression seem to be divergent and depend on the tumoral environment (51, 75). As previously reviewed, THs exert dual actions. In vitro,they promote cell proliferation and invasive capacity but also induce cell arrest and senescence; while in vivo, they increase or decrease the growth of prostate tumors. Discriminating between T3 and T4 effects has often been speculative, given that T4 has the potential to be converted to T3; however, it would be interesting to analyze if changes in the T3/T4 ratio impact on development or susceptibility to diseases. Analyzing TH effects on different cell niches and performing longitudinal studies are necessary to fully understand the mechanisms and identify the global effects that prevail during cancer development. Finally, it has been recognized that many antineoplastic drugs cause hypothyroidism (76); hence, it would be interesting to analyze if T3, either alone or combined with androgens, could maintain the differentiation of tumor cells and sensitize them to therapy.
Conclusions
In recent years, data obtained from epidemiological and experimental studies suggest that thyroid function impacts the growth, function, and pathology of the prostate, but whose effects and mechanisms are far from being understood. Cell lines and whole prostate express mechanisms to maintain an euthyroid microenvironment and respond to subtle changes in thyroid status. This review provides evidence that the prostate is a target organ for THs and highlights the importance of performing integrative androgen/thyronine studies in the prognosis of the evolution and treatment of their pathologies.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work was supported by PAPIIT-UNAM (IN205920, IN203919).
Acknowledgements
Carlos Montes de Oca is a student from Programa de Doctorado en Ciencias Biomédicas, UNAM and received a doctoral fellowship from CONACYT. The authors are grateful to Francisco Valles for bibliographic assistance; Alberto Lara, Omar Gonzalez, Ramon Martinez, and Maria Eugenia Rosas for computer assistance; Nuri Aranda for academic assistance and Jessica Gonzalez Norris for proofreading.
References
- 1.Bianco AC, Dumitrescu A, Gereben B, Ribeiro MO, Fonseca TL, Fernandes GW, Bocco BMLC. Paradigms of dynamic control of thyroid hormone signaling. Endocrine Reviews 2019401000–1047. ( 10.1210/er.2018-00275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luongo C, Dentice M, Salvatore D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nature Reviews: Endocrinology 201915479–488. ( 10.1038/s41574-019-0218-2) [DOI] [PubMed] [Google Scholar]
- 3.Davis PJ, Mousa SA, Lin HY. Nongenomic actions of thyroid hormone: the integrin component. Physiological Reviews 2021101319–352. ( 10.1152/physrev.00038.2019) [DOI] [PubMed] [Google Scholar]
- 4.Tang L, Xu M, Zhang L, Qu L, Liu X. Role of αvβ3 in prostate cancer: metastasis initiator and important therapeutic target. OncoTargets and Therapy 2020137411–7422. ( 10.2147/OTT.S258252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groeneweg S, Van Geest FS, Peeters RP, Heuer H, Visser WE. Thyroid hormone transporters. Endocrine Reviews 202041bnz008. ( 10.1210/endrev/bnz008) [DOI] [PubMed] [Google Scholar]
- 6.Delgado-González E, Sánchez-Tusie AA, Morales G, Aceves C, Anguiano B. Triiodothyronine attenuates prostate cancer progression mediated by β-adrenergic stimulation. Molecular Medicine 2016221–11. ( 10.2119/molmed.2015.00047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksoy O, Pencik J, Hartenbach M, Moazzami AA, Schlederer M, Balber T, Varady A, Philippe C, Baltzer PA, Mazumder B.et al. Thyroid and androgen receptor signaling are antagonized by μ-crystallin in prostate cancer. International Journal of Cancer 2021148731–747. ( 10.1002/ijc.33332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malinowska K, Cavarretta IT, Susani M, Wrulich OA, Uberall F, Kenner L, Culig Z. Identification of μ-crystallin as an androgen-regulated gene in human prostate cancer. Prostate 2009691109–1118. ( 10.1002/pros.20956) [DOI] [PubMed] [Google Scholar]
- 9.Mullem van AA, Gucht vanV ALM, Visser WE, Meima ME, Peeters RP, Visser TJ. Effects of thyroid hormone transporters MCT8 and MCT10 on nuclear activity of T3. Molecular and Cellular Endocrinology 2016437252–260. ( 10.1016/j.mce.2016.07.037) [DOI] [PubMed] [Google Scholar]
- 10.Doorn Van J, Roelfsema F, Heide Van der D. Concentrations of thyroxine and 3,5,3’-triiodothyronine at 34 different sites in euthyroid rats as determined by an isotopic equilibrium technique. Endocrinology 19851171201–1208. ( 10.1210/ENDO-117-3-1201) [DOI] [PubMed] [Google Scholar]
- 11.Anguiano B, Löpez A, Delgado G, Romero C, Aceves C. Deiodinase type 1 activity is expressed in the prostate of pubescent rats and is modulated by thyroid hormones, prolactin and sex hormones. Journal of Endocrinology 2006190363–371. ( 10.1677/joe.1.06786) [DOI] [PubMed] [Google Scholar]
- 12.López-Juárez A, Delgado G, Aceves C, Anguiano B. Type 1 deiodinase activity and generation of triiodothyronine (T3) in prostate of sexually active rats. Prostate 2009691651–1659. ( 10.1002/pros.21015) [DOI] [PubMed] [Google Scholar]
- 13.Delgado-Gonzalez E, Aceves C, Anguiano B. Postejaculatory increase of prostatic triiodothyronine (T3) depends on sympathetic innervation in the rat. Biology of Reproduction 201184118–123. ( 10.1095/biolreprod.110.086116) [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Tusie A, Oca de CM, Rodríguez-Castelán J, Delgado-González E, Ortiz Z, Álvarez L, Zarco C, Aceves C, Anguiano B. A rise in T3/T4 ratio reduces the growth of prostate tumors in a murine model. Journal of Endocrinology 2020247225–238. ( 10.1530/JOE-20-0310) [DOI] [PubMed] [Google Scholar]
- 15.Gururajan M, Josson S, Chu GCY, Lu CL, Lu YT, Haga CL, Zhau HE, Liu C, Lichterman J, Duan P.et al. MiR-154* and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate epithelial to mesenchymal transition and bone metastasis of prostate cancer. Clinical Cancer Research 2014206559–6569. ( 10.1158/1078-0432.CCR-14-1784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakurai A, Nakai A, DeGroot LJ. Expression of three forms of thyroid hormone receptor in human tissues. Molecular Endocrinology 19893392–399. ( 10.1210/MEND-3-2-392) [DOI] [PubMed] [Google Scholar]
- 17.Hsieh ML, Juang HH. Cell growth effects of triiodothyronine and expression of thyroid hormone receptor in prostate carcinoma cells. Journal of Andrology 200526422–428. ( 10.2164/jandrol.04162) [DOI] [PubMed] [Google Scholar]
- 18.Esquenet M, Swinnen JV, Heyns W, Verhoeven G. Triiodothyronine modulates growth, secretory function and androgen receptor concentration in the prostatic carcinoma cell line LNCaP. Molecular and Cellular Endocrinology 1995109105–111. ( 10.1016/0303-7207(95)03490-X) [DOI] [PubMed] [Google Scholar]
- 19.Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S, Davis PJ. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 20051462864–2871. ( 10.1210/EN.2005-0102) [DOI] [PubMed] [Google Scholar]
- 20.Aruldhas MM, Ramalingam N, Jaganathan A, Sashi AMJ, Stanley JA, Nagappan AS, Vasavan J, Kannan A, Seshadri VN. Gestational and neonatal-onset hypothyroidism alters androgen receptor status in rat prostate glands at adulthood. Prostate 201070689–700. ( 10.1002/pros.21101) [DOI] [PubMed] [Google Scholar]
- 21.Maran RR, Senthilkumaran B, Udhayakumar RC, Arunakaran J, Aruldhas MM. Thyroidectomy modulates rat prostatic monosaccharides. International Journal of Andrology 200023156–162. ( 10.1046/j.1365-2605.2000.00220.x) [DOI] [PubMed] [Google Scholar]
- 22.Maran RRM, Aruldhas MM. Adverse effects of neonatal hypothyroidism on Wistar rat spermatogenesis. Endocrine Research 200228141–154. ( 10.1081/ERC-120015051) [DOI] [PubMed] [Google Scholar]
- 23.Wilson MJ, Kirby JD, Zhao YD, Sinha AA, Cooke PS. Neonatal hypothyroidism alters the pattern of prostatic growth and differentiation, as well as plasminogen activator and metalloprotease expression, in the rat. Biology of Reproduction 199756475–482. ( 10.1095/biolreprod56.2.475) [DOI] [PubMed] [Google Scholar]
- 24.Cooke PS, Meisami E. Early hypothyroidism in rats causes increased adult testis and reproductive organ size but does not change testosterone levels. Endocrinology 1991129237–243. ( 10.1210/endo-129-1-237) [DOI] [PubMed] [Google Scholar]
- 25.Choudhury S, Chainy GBN, Mishro MM. Experimentally induced hypo- and hyper-thyroidism influence on the antioxidant defence system in adult rat testis. Andrologia 200335131–140. ( 10.1046/j.1439-0272.2003.00548.x) [DOI] [PubMed] [Google Scholar]
- 26.Maran RRM, Aruldhas MM, Udhayakumar RCR, Subramanian S, Rajendiran G, Antony FF, Arunakaran J, Govindarajulu P. Impact of altered thyroid hormone status on prostatic glycosidases. International Journal of Andrology 199821121–128. ( 10.1046/j.1365-2605.1998.00094.x) [DOI] [PubMed] [Google Scholar]
- 27.Talbert GB.Effect of thyroxine on maturation of the testes and prostate gland of the rat. Proceedings of the Society for Experimental Biology and Medicine 1962111290–292. ( 10.3181/00379727-111-27770) [DOI] [PubMed] [Google Scholar]
- 28.Das RP, Perrault MJ. The androgenic response of the genital accessory organs of thyroxine-treated and castrated rats exposed to cold. Journal of Endocrinology 197149591–598. ( 10.1677/joe.0.0490591) [DOI] [PubMed] [Google Scholar]
- 29.Sidharthan V, Rajalingam R, Aruldhas MM, Govindarajulu P. Ventral prostatic phosphomonoesterases and adenosine triphosphatases in hypo- and hyperthyroid albino rats. Indian Journal of Experimental Biology 199331414–416. [PubMed] [Google Scholar]
- 30.Sidharthan V, Rose PJ, Rajalingam R, Udhayakumar RC, Aruldhas MM, Govindarajulu P. Dorsolateral prostatic phosphomonoesterases and adenosine triphosphatases in hypo- and hyperthyroid rats. Indian Journal of Experimental Biology 199432616–618. [PubMed] [Google Scholar]
- 31.Kharroubi AT, Slaunwhite WR. Hormonal regulation of prolactin receptors in male rat target tissues: the effect of hypothyroidism and adrenalectomy. Endocrinology 19841151283–1288. ( 10.1210/ENDO-115-4-1283) [DOI] [PubMed] [Google Scholar]
- 32.Tiong TS, Stevenson JL, Herington AC. Regulation of prolactin receptor gene expression by thyroid hormone status in the rat. Journal of Molecular Endocrinology 1992863–72. ( 10.1677/JME.0.0080063) [DOI] [PubMed] [Google Scholar]
- 33.Yeh JY, Tsai SC, Kau MM, Lo MJ, Wang PS. Effects of thyroid hormones on the release of calcitonin gene-related peptide (CGRP) by rat prostate glands in vitro. Chinese Journal of Physiology 19994289–94. [PubMed] [Google Scholar]
- 34.Pekary AE, Bhasin S, Smith V, Sugawara M, Swerdloff RS, Hershman JM. Thyroid hormone modulation of thyrotrophin-releasing hormone (TRH) and TRH-Gly levels in the male rat reproductive system. Journal of Endocrinology 1987114271–277. ( 10.1677/JOE.0.1140271) [DOI] [PubMed] [Google Scholar]
- 35.Madersbacher S, Sampson N, Culig Z. Pathophysiology of benign prostatic hyperplasia and benign prostatic enlargement: a mini-review. Gerontology 201965458–464. ( 10.1159/000496289) [DOI] [PubMed] [Google Scholar]
- 36.Gupta A, Gupta S, Pavuk M, Roehrborn CG. Anthropometric and metabolic factors and risk of benign prostatic hyperplasia: a prospective cohort study of air force veterans. Urology 2006681198–1205. ( 10.1016/j.urology.2006.09.034) [DOI] [PubMed] [Google Scholar]
- 37.Man KM, Chen KB, Chen HY, Chiang JH, Su YC, Man SS, Xie DD, Wang Y, Zhang ZQ, Bi LK.et al. Hyperthyroidism is not a significant risk of benign prostatic hyperplasia: a nationwide population-based study. Medicine 201897 e12459. ( 10.1097/MD.0000000000012459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eldhose A, Nandeesha H, Dorairajan LN, Sreenivasulu K, Arul Vijaya Vani S. Thyroid and parathyroid hormones in benign prostatic hyperplasia. British Journal of Biomedical Science 20167394–96. ( 10.1080/09674845.2016.1173333) [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Park YW, Lee SW. The relationships between thyroid hormone levels and lower urinary tract symptoms/benign prostatic hyperplasia. World Journal of Men’s Health 201937364–371. ( 10.5534/wjmh.180084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vignera la S, Condorelli RA, Cannarella R, Calogero AE. Thyroid prostate axis. Does it really exist? World Journal of Men’s Health 201937257–260. ( 10.5534/wjmh.190060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 202171209–249. ( 10.3322/CAAC.21660) [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Hsieh ML, Zhu W, Klee GG, Tindall DJ, Young CY. Interactive effects of triiodothyronine and androgens on prostate cell growth and gene expression. Endocrinology 19991401665–1671. ( 10.1210/ENDO.140.4.6666) [DOI] [PubMed] [Google Scholar]
- 43.Tsui KH, Hsieh WC, Lin MH, Chang PL, Juang HH. Triiodothyronine modulates cell proliferation of human prostatic carcinoma cells by downregulation of the B-cell translocation gene 2. Prostate 200868610–619. ( 10.1002/pros.20725) [DOI] [PubMed] [Google Scholar]
- 44.Zhu W, Young CFY. Androgen-dependent transcriptional regulation of the prostate-specific antigen gene by thyroid hormone 3,5,3′-L-triiodothyronine. Journal of Andrology 200122136–141. ( 10.1002/J.1939-4640.2001.TB02163.X) [DOI] [PubMed] [Google Scholar]
- 45.Kotolloshi R, Mirzakhani K, Ahlburg J, Kraft F, Pungsrinont T, Baniahmad A. Thyroid hormone induces cellular senescence in prostate cancer cells through induction of DEC1. Journal of Steroid Biochemistry and Molecular Biology 2020201105689. ( 10.1016/j.jsbmb.2020.105689) [DOI] [PubMed] [Google Scholar]
- 46.Zhang P, Chen L, Song Y, Li X, Sun Y, Xiao Y, Xing Y. Tetraiodothyroacetic acid and transthyretin silencing inhibit pro-metastatic effect of L-thyroxin in anoikis-resistant prostate cancer cells through regulation of MAPK/ERK pathway. Experimental Cell Research 2016347350–359. ( 10.1016/j.yexcr.2016.08.019) [DOI] [PubMed] [Google Scholar]
- 47.Theodossiou C, Schwarzenberger P. Propylthiouracil reduces xenograft tumor growth in an athymic nude mouse prostate cancer model. American Journal of the Medical Sciences 200031996–99. ( 10.1016/S0002-9629(15)40695-0) [DOI] [PubMed] [Google Scholar]
- 48.Puca L, Vlachostergios PJ, Beltran H. Neuroendocrine differentiation in prostate cancer: emerging biology, models, and therapies. Cold Spring Harbor Perspectives in Medicine 20199. ( 10.1101/CSHPERSPECT.A030593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate 200355219–237. ( 10.1002/pros.10215) [DOI] [PubMed] [Google Scholar]
- 50.Hellevik AI, Åsvold BO, Bjøro T, Romundstad PR, Nilsen TIL, Vatten LJ. Thyroid function and cancer risk: a prospective population study. Cancer Epidemiology, Biomarkers and Prevention 200918570–574. ( 10.1158/1055-9965.EPI-08-0911) [DOI] [PubMed] [Google Scholar]
- 51.Krashin E, Silverman B, Steinberg DM, Yekutieli D, Giveon S, Fabian O, Hercbergs A, Davis PJ, Ellis M, Ashur-Fabian O. Opposing effects of thyroid hormones on cancer risk: a population-based study. European Journal of Endocrinology 2021184477–486. ( 10.1530/EJE-20-1123) [DOI] [PubMed] [Google Scholar]
- 52.Gómez-Izquierdo J, Filion KB, Boivin JF, Azoulay L, Pollak M, Yu OHY. Subclinical hypothyroidism and the risk of cancer incidence and cancer mortality: a systematic review. BMC Endocrine Disorders 2020201–10. ( 10.1186/s12902-020-00566-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran TVT, Kitahara CM, Vathaire de F, Boutron-Ruautl M, Journy N. Thyroid dysfunction and cancer incidence: a systematic review and meta-analysis. Endocrine-Related Cancer 202027245–259. ( 10.1530/ERC-19-0417) [DOI] [PubMed] [Google Scholar]
- 54.Mondul AM, Weinstein SJ, Bosworth T, Remaley AT, Virtamo J, Albanes D. Circulating thyroxine, thyroid-stimulating hormone, and hypothyroid status and the risk of prostate cancer. PLoS ONE 20127 e47730. ( 10.1371/journal.pone.0047730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan YX, Knuiman MW, Divitini ML, Brown SJ, Walsh J, Yeap BB. Lower TSH and higher free thyroxine predict incidence of prostate but not breast, colorectal or lung cancer. European Journal of Endocrinology 2017177297–308. ( 10.1530/EJE-17-0197) [DOI] [PubMed] [Google Scholar]
- 56.Lehrer S, Diamond EJ, Bajwa AM, Kornreich R, Stagger S, Stone NN, Droller MJ, Stock RG. Association between serum triiodothyronine (t3) level and risk of disease recurrence in men with localized prostate cancer. Prostate Cancer and Prostatic Diseases 20014232–234. ( 10.1038/sj.pcan.4500542) [DOI] [PubMed] [Google Scholar]
- 57.Ovčariček PP, Fröbe A, Verburg FA, Murgić J, Butković MB, Ovčariček S, Mažuran B, Krušlin B, Jakovčević D, Šoipi Š.et al. Association of triiodothyronine levels with prostate cancer histopathological differentiation and tumor stage. Anticancer Research 2020402323–2329. ( 10.21873/anticanres.14199) [DOI] [PubMed] [Google Scholar]
- 58.Flood DEK, Fernandino JI, Langlois VS. Thyroid hormones in male reproductive development: evidence for direct crosstalk between the androgen and thyroid hormone axes. General and Comparative Endocrinology 20131922–14. ( 10.1016/j.ygcen.2013.02.038) [DOI] [PubMed] [Google Scholar]
- 59.Tai PJ, Huang YH, Shih CH, Chen RN, Chen CD, Chen WJ, Wang CS, Lin KH. Direct regulation of androgen receptor-associated protein 70 by thyroid hormone and its receptors. Endocrinology 20071483485–3495. ( 10.1210/EN.2006-1239) [DOI] [PubMed] [Google Scholar]
- 60.Ligr M, Li Y, Zou X, Daniels G, Melamed J, Peng Y, Wang W, Wang J, Ostrer H, Pagano M.et al. Tumor suppressor function of androgen receptor coactivator ARA70α in prostate cancer. American Journal of Pathology 20101761891–1900. ( 10.2353/AJPATH.2010.090293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hepburn AC, Steele RE, Veeratterapillay R, Wilson L, Kounatidou EE, Barnard A, Berry P, Cassidy JR, Moad M, El-Sherif A.et al. The induction of core pluripotency master regulators in cancers defines poor clinical outcomes and treatment resistance. Oncogene 2019384412–4424. ( 10.1038/s41388-019-0712-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guigon CJ, Kim DW, Willingham MC, Cheng SY. Mutation of thyroid hormone receptor-β in mice predisposes to the development of mammary tumors. Oncogene 2011303381–3390. ( 10.1038/onc.2011.50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bidosee M, Karry R, Weiss-Messer E, Barkey RJ. Regulation of growth hormone receptors in human prostate cancer cell lines. Molecular and Cellular Endocrinology 200930982–92. ( 10.1016/J.MCE.2009.06.004) [DOI] [PubMed] [Google Scholar]
- 64.Bidosee M, Karry R, Weiss-Messer E, Barkey RJ. Growth hormone affects gene expression and proliferation in human prostate cancer cells. International Journal of Andrology 201134124–137. ( 10.1111/J.1365-2605.2010.01064.X) [DOI] [PubMed] [Google Scholar]
- 65.Silva JE, Bianco SDC. Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid 200818157–165. ( 10.1089/thy.2007.0252) [DOI] [PubMed] [Google Scholar]
- 66.Sejda A, Sigorski D, Gulczyński J, Wesołowski W, Kitlińska J, Iżycka-Świeszewska E. Complexity of neural component of tumor microenvironment in prostate cancer. Pathobiology 20208787–99. ( 10.1159/000505437) [DOI] [PubMed] [Google Scholar]
- 67.Guthrie PD, Freeman MR, Liao ST, Chung LW. Regulation of gene expression in rat prostate by androgen and beta-adrenergic receptor pathways. Molecular Endocrinology 199041343–1353. ( 10.1210/MEND-4-9-1343) [DOI] [PubMed] [Google Scholar]
- 68.Lehrer S, Diamond EJ, Stone NN, Droller MJ, Stock RG. Serum triiodothyronine is increased in men with prostate cancer and benign prostatic hyperplasia. Journal of Urology 20021682431–2433. ( 10.1097/01.ju.0000032178.16280.e0) [DOI] [PubMed] [Google Scholar]
- 69.Andersen LF, Agner T, Walter S, Hansen JM. Micturition pattern in hyperthyroidism and hypothyroidism. Urology 198729223–224. ( 10.1016/0090-4295(87)90161-0) [DOI] [PubMed] [Google Scholar]
- 70.Ramberg H, Eide T, Krobert KA, Levy FO, Dizeyi N, Bjartell AS, Abrahamsson P, Taskén KA. Hormonal regulation of beta2-adrenergic receptor level in prostate cancer. Prostate 2008681133–1142. ( 10.1002/PROS.20778) [DOI] [PubMed] [Google Scholar]
- 71.Yu J, Cao Q, Mehra R, Laxman B, Yu J, Tomlins SA, Creighton CJ, Dhanasekaran SM, Shen R, Chen G.et al. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell 200712419–431. ( 10.1016/J.CCR.2007.10.016) [DOI] [PubMed] [Google Scholar]
- 72.Jiang L, Kunos G. Sequence of the 5’ regulatory domain of the gene encoding the rat beta 2-adrenergic receptor. Gene 1995163331–332. ( 10.1016/0378-1119(95)00357-C) [DOI] [PubMed] [Google Scholar]
- 73.Braadland PR, Ramberg H, Grytli HH, Urbanucci A, Nielsen HK, Guldvik IJ, Engedal A, Ketola K, Wang W, Svindland A.et al. The β2-adrenergic receptor is a molecular switch for neuroendocrine transdifferentiation of prostate cancer cells. Molecular Cancer Research 2019172154–2168. ( 10.1158/1541-7786.MCR-18-0605) [DOI] [PubMed] [Google Scholar]
- 74.Quaglia F, Krishn SR, Wang Y, Goodrich DW, McCue P, Kossenkov AV, Mandigo AC, Knudsen KE, Weinreb PH, Corey E.et al. Differential expression of avβ3 and avβ6 integrins in prostate cancer progression. PLoS ONE 2021161–19. ( 10.1371/journal.pone.0244985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mousa SA, Hercbergs A, Lin HY, Keating KA, Davis PJ. Actions of thyroid hormones on thyroid cancers. Frontiers in Endocrinology 202112. ( 10.3389/FENDO.2021.691736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamnvik OPR, Larsen PR, Marqusee E. Thyroid dysfunction from antineoplastic agents. Journal of the National Cancer Institute 2011103 1572–1587. ( 10.1093/JNCI/DJR373) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a