Abstract
Objective
Radiofrequency ablation (RFA) is increasingly considered the prime option for treating symptomatic, benign, non-functioning thyroid nodules (NFTN). However, little is known about the degree of operator experience required to achieve optimal results. This study describes the RFA learning curve of a single-center team.
Methods
A retrospective cohort study of the first 103 patients receiving RFA treatment for a single, symptomatic, and benign NFTN, with a follow-up of at least 1 year. The primary outcome measure was technique efficacy, defined as the percentage of patients with a 6-month nodal volume reduction ratio (VRR) >50% after single-session RFA. Optimal treatment efficacy was defined as a 6-month VRR >50% achieved in at least 75% of patients. Secondary outcomes were complications of RFA and indications of secondary interventions.
Results
Median nodal volume at baseline was 12.0 mL (range 2.0–58.0 mL). A 6-month VRR >50% was achieved in 45% of the first 20 patients, 75% of the next 20, and 79% of the following 63 patients. Complications included minor bleeding (N = 4), transient hyperthyroidism (N = 4), and transient loss of voice (N = 1). Poor volume reduction or nodular regrowth led to diagnostic lobectomy in 11 patients and a second RFA in 5. Lobectomy revealed a follicular carcinoma (T2N0M0) in 2 patients. In 1 patient, nodule regrowth was caused by an intranodular solitary B-cell lymphoma.
Conclusion
About 40 procedures are required to achieve a 6-month VRR >50% in the majority of patients. Appropriate follow-up with re-evaluation is recommended for all patients with a VRR <50% and in those with regrowth to exclude underlying malignancy.
Keywords: thyroid nodules, benign, non-functional, radiofrequency ablation
Introduction
About 10% of the population has a benign non-functioning thyroid nodule (NFTN) of 1 cm or larger (1), and 4–7% of these nodules are symptomatic (2, 3, 4). Unilateral lobectomy and volume reduction by radioiodine (RAI) have long been the only treatment options available for this condition. However, lobectomy is associated with post-operative hypothyroidism in 25% of patients (5), laryngeal nerve lesions in 0–1.1% (6), and hematomas in 0.7–1.5% (7). Volume reduction by RAI is rarely performed for solitary NFTN. It is mainly used in patients with multinodular goiter (MNG), where it may induce volume reductions of up to 40% after 1 year, but at the cost of long-term risk of hypothyroidism of up to 60% (8, 9). Given these side effects, many patients and doctors choose a wait-and-see policy and delay any intervention until symptoms have increased to the extent that they severely affect daily quality of life.
In 2006, ultrasound (US)-guided radiofrequency ablation (RFA) was introduced as a new treatment option for NFTN (10). It is based on heat-induced cell death caused by thermal energy delivered through the active tip of an electrode positioned in the thyroid nodule. The most commonly used procedure is the moving shot technique introduced by Baek et al., where multiple small ablation zones are created by moving the electrode tip through the node (11, 12). Studies performed in South Korea, Italy, China, and Austria, comprising over 1150 patients, report nodal volume reductions varying from 46 to 94%, with significant symptom reduction and improved cosmetic scores (13, 14). Reported adverse effects are minor and include pain (5%), hematoma (1%), transient voice change (1%), and transient hyperthyroidism (<1%) (10, 15). Severe adverse effects such as persistent laryngeal nerve injury and brachial plexus injury have been described but are extremely rare. Because of these observations and the very high benefit-risk ratio, RFA is emerging as an attractive third option for treating NFTN, and many centers now increasingly consider offering this treatment. A guideline for thyroid RFA was published in 2018 by the Korean Society of Thyroid radiology and in 2020 by the European Thyroid Association (16).
Up to now, efficacy and safety results have mainly been published by centers with extensive experience in thyroid RFA. Little is known about the learning curve required to obtain these results or of the clinical problems and diagnostic uncertainties encountered during the start-up of RFA treatment. Although it has been suggested that about 50 procedures are needed to achieve a stable level of technique efficacy, the evidence and the number of studies discussing this subject are still limited, and most studies addressed it only briefly (17, 18, 19). The present study aims to extend the knowledge about the learning curve and reports and compares our initial experience with thyroid RFA with the available literature.
Patients and methods
This is a retrospective cohort study of the first 103 consecutive patients with symptomatic, benign, predominantly solid NFTN receiving RFA treatment in a teaching hospital (Rijnstate hospital, Arnhem, The Netherlands). Patients were included for treatment if they met the following inclusion criteria: (1) subjective symptoms likely to be related to a single thyroid nodule such as difficulty in swallowing or the feeling of local pressure, (2) thyroid nodule documented by US, with a maximum diameter of 20–50 mm, (3) degree of cystic degeneration <50%, (4) serum TSH, FT4, and FT3 levels within the normal range, and (5) benign cytology or histology. The study was approved by the local ethical committee, and all patients gave their informed consent.
Procedure
All patients referred for RFA treatment were checked by an endocrinologist, including a measurement of serum FT4, FT3, and TSH (Modular E170, Roche). A baseline US of the neck was performed by interventional radiologists (F J and E B) with extensive experience in head and neck US. The thyroid nodule’s location, volume, and characteristics were documented, and all nodules were classified based on TI-RADS criteria (20). Thyroid nodule volume was calculated as follows: volume = 0.525 × D1 × D2 × D3, where D1 represents the largest diameter and D2 and D3 the two diameters perpendicular to D1 (10). US-guided fine-needle aspiration (FNA) was performed at least once in all patients, with classification according to the Bethesda system (21). US-guided core needle biopsy (CNB, 18 gauche) was performed in patients with a Bethesda 1 or 3 or higher and in some patients with a Bethesda 2 if they reported rapid nodule growth. CNB analysis was based on the criteria proposed by Na et al. (22). In the multi-disciplinary thyroid meeting attended by an endocrinologist, radiologist, thyroid surgeon, and nuclear medicine physician, discussion was made on the eligible patients. The decision to offer RFA treatment to a patient was based on the groups’ consensus.
All RFA procedures were performed in an outpatient setting, after mild sedation with oxazepam 10 mg orally 1 h before the start of the procedure. Lidocaine 1% was used for local anesthesia of the skin and thyroid capsule. In the case of nodules with partial cystic degeneration, fluid aspiration was considered prior to the start of RFA. RFA was performed by a team consisting of two dedicated radiologists (F J and E B), always acting together during each procedure and using the US-guided moving-shot technique with trans-isthmic approach and a cool-tip RF system with a straight-type, modified, internally cooled electrode, all of the same size (18 G), length (7 cm), and with a 10 mm active tip (RF Medical Seoul Korea or STARMed Co Seoul Korea) (23, 24). During the procedure, patients remained in a supine position with mild neck extension. They were advised to report pain, and voice testing was performed at regular intervals. The starting level of energy delivered per second was 35 W, and it was reduced if patients reported pain, pressure, toothache, or hoarseness. It was increased if no response was visible on US within 5–10 s. At the end of the procedure, the patient applied mild compression of the treated thyroid lobe for 20 min. If necessary, an icepack was applied to relieve pain. After 2 h of post-procedural observation, the patient was discharged, with the advice to take paracetamol 1000 mg every 6 h for the first 24-h. Standard follow-up included outpatient visits with FT4, FT3, and TSH measurements at 1, 13, 26, and 52 weeks. Nodule volumes were measured by US after 13, 26, and 52 weeks. Nodal volume reduction was expressed as volume reduction ratio, calculated as follows: VRR = ((initial volume − final volume)/(initial volume)) × 100. Regrowth was defined as an increase ≥50%, compared to the previous smallest volume.
Outcomes
The primary outcome was the time course of changes in technique efficacy, defined as the percentage of treatments with a nodal volume reduction ratio (VRR) >50% after single-session RFA (25). Secondary outcome measures were the number and type of complications and the indications and outcomes of secondary interventions.
Data analysis
Results are reported as mean values with s.d., medians with ranges, or as proportions. Differences between groups were analyzed by the Kruskal–Wallis and Wilcoxon tests. Differences between proportions were analyzed by the chi-square test. Linear regression analysis was used to analyze the time-related change in applied energy. A P-value of <0.05 was considered statistically significant. The learning curve was plotted by exponential curve fitting (Graphpad Prism, version 6.0).
Results
Hundred and three consecutive patients were included and treated with RFA. Their baseline characteristics are summarized in Table 1. All patients were euthyroid at presentation. Sixteen patients had a history of hemithyroidectomy for benign goiter, which had led to post-operative hypothyroidism in six patients, and was treated with levothyroxine (50–125 µg daily). Two other patients were also on levothyroxine treatment, one because of Hashimoto’s disease and one because of hypothyroidism of unknown cause. The median nodule volume at baseline was 12.0 mL (range 2.0–58.0 mL). Cystic degeneration was present in 18 nodules, comprising 10–40% of the nodal cross-sectional area. Three of these nodules had a sponge-like aspect. TI-RADS classifications (TIR) ranged from 2 to 4, with the following distribution: TIR2 in 47%, TIR3 in 48%, and TIR4 in 5% of nodules. FNA and Bethesda classification were performed in all patients. Seventeen patients had a Bethesda 1 (B1), one patient had a B3, and all others had a B2. In five patients, B1 was attributed to cystic degeneration for which a second FNA was not considered necessary because they all met the criteria for a TI-RADS 1. Core needle biopsies (CNB) were performed in 17 patients: in 12 patients with a B1, in 4 patients with a B2, and in the 1 patient with a B3. All CNBs were classified as benign. In 12 out of 18 patients with central cystic degeneration, RFA was preceded by fluid aspiration (3–20 mL, median 7.5 mL). RFA was performed with a median energy input of 40 W (range 30–60 W), and the procedure lasted for a median of 12.7 min (range 3–34 min). The median amount of deposited energy was 6.6 kCal (range 1.5–10.2 kCal) or 0.5 kCal/mL nodule (equivalent to 2093 J/mL). Over time, the applied energy gradually increased from 0.32 to 0.84 kCal/mL (P = 0.012).
Table 1.
Baseline characteristics of patients included for RFA treatment, nodule and treatment characteristics, and procedure related side effects and complications (mean ± s.d., or median and range). In each patient, only one thyroid nodule was treated with RFA.
| Characteristics | |
|---|---|
| Male/female (N) | 8/95 |
| Age (years) | 54 ± 16.2 |
| Height (cm) | 170 ± 8.2 |
| Weight (kg) | 73.6 ± 12.6 |
| BMI (kg/m2) | 25.6 ± 4.1 |
| Hemithyroidectomy (N) | 16 |
| Levothyroxine (N) | 8 |
| Number of treated nodules (N) | 103 |
| Nodule localization (R-I-L) | 60-3-50 |
| Nodule volume (mL) | 12.0 (2.0–58.0) |
| Nodule structure (N) | |
| Solid | 85 |
| Predominantly solid | 18 |
| Core needle biopsies (N) | 17 |
| Pre-RFA aspiration (N) | 12 |
| Aspiration volume (mL) | 7.5 (3.0–20.0) |
| RFA energy input (W) | 40 (30–60) |
| RFA time (min) | 12.7 (3.0–34.0) |
| RFA energy applied (kCal) | 6.6 (1.5–18.5) |
| Side effects and complications (N) | |
| Pain >24 h | 5 |
| Hematoma | 4 |
| Ecchymosis | 4 |
| Voice change | 1 |
| TSH <0.3 mU/L | 25 |
| FT4 >23 pmol/L | 6 |
| FT3 >6.5 pmol/L | 4 |
Volume reduction at 6 months
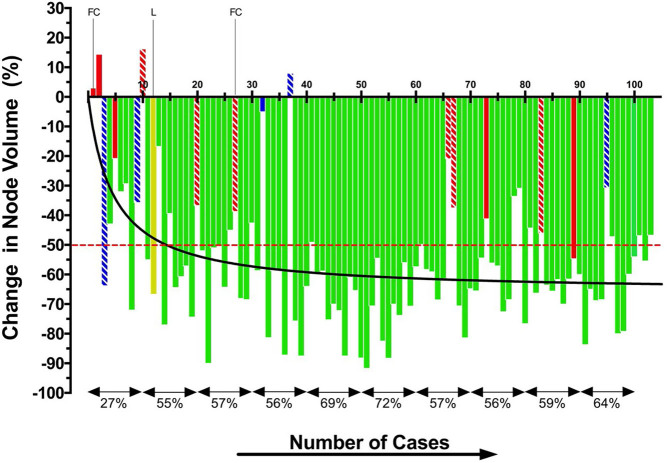
All 103 patients completed the first 6 months of follow-up without any secondary treatment (Fig. 1). Initially, the results of RFA were poor. Only 9 (45%) of the first 20 patients had a 6-month VRR >50%, and this increased to 15 (75%) of the next 20 patients and then to 50 (79%) of the following 63 subjects (P = 0.005). The median 6-month VRR was 56.1% in the first 40 patients, and this rose to 62.7% in the following 63 patients (P = 0.017).
Figure 1.
Nodule volume response, 6 months after RFA in the first 103 patients. Patients without any secondary intervention during overall follow-up (N = 84) are shown as green bars. Patients who underwent a second RFA (N = 5) are shown in blue and those who had a diagnostic lobectomy in red (N = 11). Solid red and blue bars indicate that the interventions took place in the second half of the first year: white-striped red and blue bars represent interventions after the first year. The patient with a solitary B-cell lymphoma diagnosed in the second year after RFA is shown as a yellow bar. The red, interrupted line represents the VRR cut-off of 50%. The black line is a curve fit of the individual observations. Percentages at the bottom represent the mean VRR for each cohort of ten patients.
Twelve months results
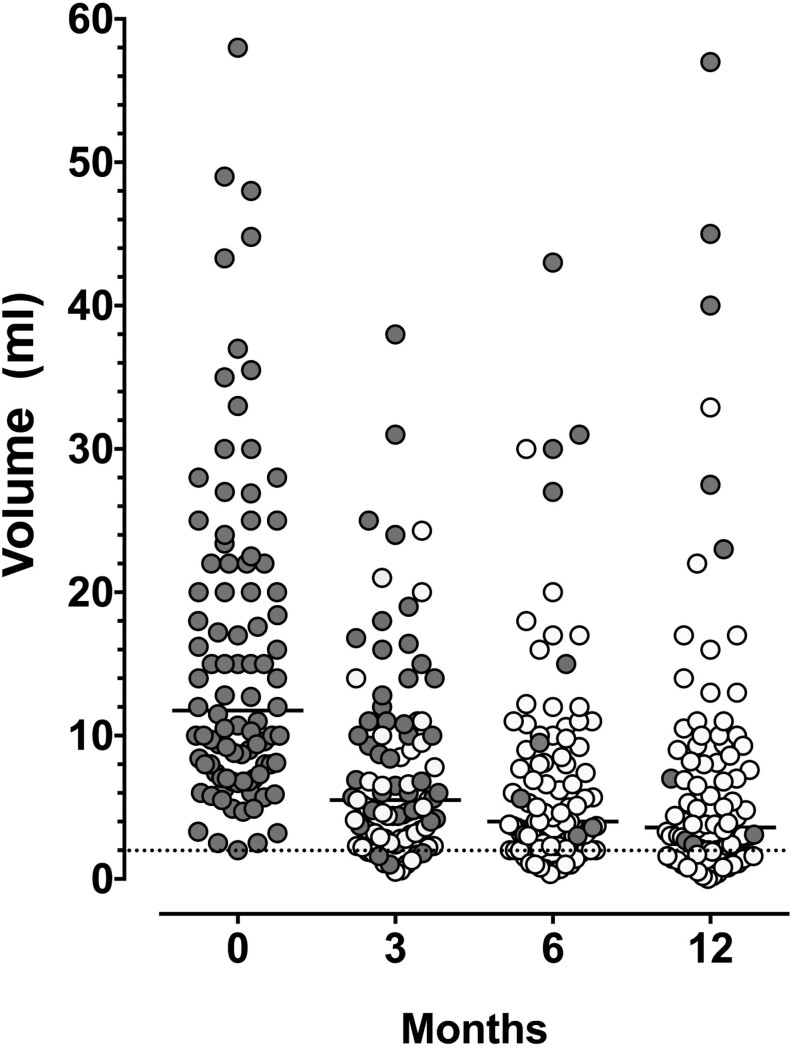
After 6 months, three patients were lost-to-follow-up (LTFU). All three had become asymptomatic, with 6-month VRRs of 50, 60, and 61%, respectively. During the second half-year, five patients had a diagnostic lobectomy (patients indicated by the solid red bars) and one patient (indicated by the solid blue bar) underwent a second RFA because of persisting symptoms attributed to a poor response to the first RFA (Fig. 1). Ninety-four (91%) patients completed the 1-year follow-up without a second intervention. Their nodule volumes over the year are shown in Fig. 2, with symptomatic patients shown as gray dots and asymptomatic patients as white dots. The median volume declined significantly from 12.0 mL at baseline to 5.5 mL at 3 months (P < 0.001) and then further to 4.0 mL at 6 months and 3.6 mL at 12 months. At 12 months, 75/94 (80%) of patients had a VRR >50%. The median changes in volume between 3 and 6 months (P = 0.002) and between 6 and 12 months (P = 0.003) were small but statistically significant. The degree of volume reduction was related to the applied energy. Nodule volumes at 12 months had decreased by 0.9 mL per Kcal (R = 0.52, P < 0.0001). At baseline, all patients had symptoms, mainly local pressure sensations or difficulty in swallowing. Only a few reported intermittent weakness of their voice. After 3 months, 45 (48%) patients had become asymptomatic, and this increased to 80 (85%) patients at 6 months and 84 (89%) patients at 12 months. None of the ten patients who had a history of hemithyroidectomy and were euthyroid at inclusion developed hypothyroidism after RFA.
Figure 2.
Thyroid nodule volumes after single session RFA in all 94 patients who did not have a second intervention during the 1-year follow-up. Results are expressed in milliliter. Dark dots: symptomatic patients, white dots: asymptomatic patients, lines represent the median value.
Side effects and complications of RFA treatment
As recommended by Mauri et al., local pain and asymptomatic minor ecchymosis were considered side effects of RFA treatment, whereas other events were classified as procedure-related complications (26). RFA was only associated with minor complications, and this occurred in a total of 9 patients: none in the first 20 subjects, 1 in the next 20, and 8 in the last 63 subjects. Major complications did not occur.
Pain
Most patients experienced only a little pain on the day of RFA, easily controlled with paracetamol. Five patients reported pain that continued for a duration of 3–4 days maximally.
Bleeding
Three patients developed an intranodular hematoma during RFA treatment, two of which occurred after cyst fluid aspiration. One patient developed a scalenus muscle hematoma. All hematomas were controlled successfully by applying local pressure for 10 min. At the outpatient visit 1 week after RFA, four patients had an asymptomatic ecchymosis in the neck midline, just above the xiphoid bone. None of the patients with a bleeding complication had used anticoagulation.
Voice changes
A transient loss of voice occurred in one patient during the RFA procedure. The ablation was stopped immediately, and an ice pack was put on the area of treatment. About 30 min later, the voice was back to normal.
Transient hyperthyroidism
One week after RFA, TSH levels decreased below the lower limit of normal (0.3 mU/L) in 25 patients (Fig. 3). This was associated with an increase in FT4 or FT3 above the upper limit of normal in six and four patients, respectively (Fig. 3). Four (3.9%) patients reported mild symptoms of hyperthyroidism, mainly transient nervousness and palpitations. All recovered spontaneously within 2–3 weeks, and none required treatment with β-blockers or developed secondary hypothyroidism.
Figure 3.
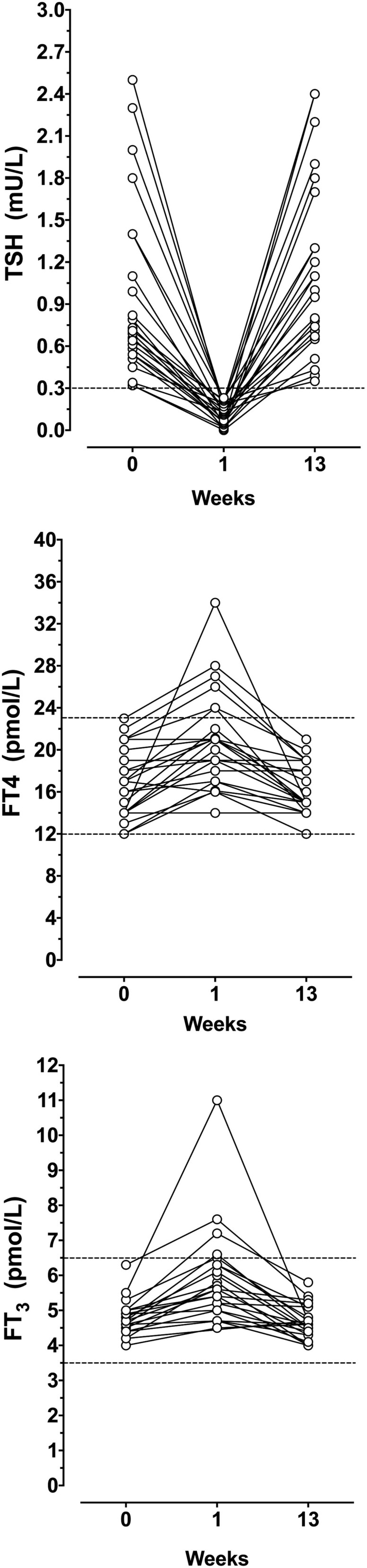
Twenty-five patients with transient TSH suppression below the lower limit of normal in the first week after RFA
Extended follow-up
At 12 months, all patients who had become asymptomatic were discharged with the advice to return if they had a recurrence of symptoms or noted nodular growth. Follow-up was extended in 11/94 patients, either because of relatively large nodule remnants (N = 9, median nodule volume 23 mL, range 10–57 mL) for which further follow-up was considered indicated or because of nodule growth in the previous 6 months (N = 2, with regrowth of 60 and 125%, respectively). In addition, two patients returned after discharge because of regrowth of 186 and 38%, respectively, in the second year after RFA. FNA or CNB was repeated in all 13 patients. Nine out of 13 patients eventually had a second intervention in the months to follow. One patient returned about 1 year later with a gradually increasing nodal volume. CNB revealed a B-cell lymphoma, for which she was referred to a hematologist.
Indications and outcome of secondary interventions
Of all 103 patients, 16 had a secondary intervention, either because of a VRR <50% and persisting symptoms (N = 14) or because of regrowth with recurrence of symptoms (N = 2). Diagnostic lobectomy was performed in 11 patients because of persisting symptoms and a VRR <50%. This led to a final diagnosis of solitary follicular adenoma in seven and MNG in two. Despite a TI-RADS 3 and Bethesda 2 at baseline, two patients were diagnosed with a T2N0M0 follicular carcinoma, for which they underwent total thyroidectomy followed by I-131 ablation, after which they reached complete remission. A second RFA was performed in five patients after FNA or CNB confirmation that the nodule was still benign. One patient was treated for an overall poor 6-month response after the first RFA, attributed to an incomplete treatment due to premature termination of RFA because of an intranodular hemorrhage during the procedure.
Discussion
This retrospective evaluation of the start-up of RFA treatment in a single-center indicates that it takes about 40 RFA procedures before a 6-month VRR of 50% has become a realistic goal in most patients. After obtaining this degree of experience, a VRR >50% was achieved in 79% of the following 63 patients. In a teaching setting with experienced instructors to guide novices, the numbers needed to treat to achieve a consistent VRR >50% may be lower. However, exact figures of such a setting are not available. Note that a VRR of 50% should be regarded as the lower limit of technique efficacy. In very experienced hands, a VRR of 80–90% is feasible (14). Although a volume reduction of 50% is usually sufficient to induce a complete disappearance of symptoms, a higher VRR should be strived for to reduce the risk of nodular regrowth and the necessity of retreatment (17). The present study also confirmed that thyroid RFA could be regarded as a safe procedure. Complications included minor bleeding (N = 4), transient hyperthyroidism (N = 4), and transient loss of voice during the procedure that lasted for 30 min (N = 1). Secondary interventions were needed in 16 patients and included a diagnostic lobectomy in 11 patients and a repeat RFA in 5.
Our findings on expertise requirements agree with observations in three other studies reporting comparable data (18, 19, 27). Dobnig et al. described the 1-year results of RFA in 277 consecutive patients and reported a gradual improvement in VRR with increasing experience (18). In the first 15 patients, the mean VRRs at 3 and 12 months were 58 and 70%, respectively, and this gradually increased to a plateau value of 70 and 80% when the number of procedures was greater than 45. Recently, Russ et al. reported their experience in the first 90 patients they treated. Their 6-month VRR increased from 54% in the first 30 patients to 65% in the following 30 patients but stabilized after that (27). In a randomized controlled trial, Deandrea et al. compared the VRRs obtained by a team with a previous experience of 50 moving shot procedures with that of a team with an experience of 3000 cases and found only a tiny but statistically not significant difference in VRR at 6 months (66% vs 77%, P = 0.07) (19). The mean 6-month VRR of 66% recorded for the less experienced team in Deandrea’s study is close to the VRR of 62.7% we achieved after a learning period of 40 RFAs. It also compares well with the results of a recent multicenter trial reporting a 6-month VRR of 63% after a single session RFA performed by operators with an experience of at least 50 RFA procedures (28).
Over time, the improvement in VRR is attributed to the growing operator skills in electrode maneuvring, the increased energy applied per milliliter thyroid node, and the increasing use of color Doppler to detect viable thyroid tissue. Adding color Doppler during the procedure will identify persisting vessels that still need to be treated. Ablation of peripheral nodal vessels is also essential to prevent regrowth. Most recurrent nodules show regrowth from the undertreated peripheral portions of the nodule (26, 29). A third factor is the growing awareness that guidance of the probe exclusively based on the characteristic US changes after RFA alone is not sufficient. A minimum amount of energy per millilitertissue is required to achieve optimal nodal shrinkage (29). Mean energy of about 2500 J/mL is needed to achieve optimal results, either applied by a high-dose short time procedure or a more prolonged low-energy procedure (17, 19, 27).
Recently, a 50% VRR at 12 months has been proposed as a threshold for defining technical success (25). We recommend using this criterion at 6 months because the degree of volume reduction after 6 months is minimal (13, 28). A delay of efficacy assessment offers no particular advantage and maybe against patient interests. In experienced hands, a 6-month VRR >50% can be expected in at least 80% of patients (28). An objective marker of efficacy is essential for two reasons: first, as an objective instrument to monitor the teams' technical qualities over the years and secondly, to identify the poor responders early because they may need additional diagnostic procedures and secondary interventions. A poor response may be related to technical limitations encountered during the RFA procedure, rapid growth of benign thyroid cells, or underlying malignancy. Although FNA prior to RFA is mandatory in all patients, it does not exclude an underlying malignancy with 100% certainty. About 2–3% of US-guided FNA are known to be false-negative (30, 31). In our series, we encountered this limitation in 3 out of 103 patients. The lack of absolute histologic proof that the nodule is completely benign is a major disadvantage of RFA compared to surgery with complete nodule removal. Therefore, concern will arise in case of an unexplained poor VRR or nodule regrowth. Repeat FNA, CND, or diagnostic lobectomy should be considered in these patients to obtain diagnostic certainty. A 1-year follow-up with US at 3, 6, and 12 months revealed all of our cases with a malignancy. It is recommended as a standard procedure after thyroid RFA (32).
In agreement with previous studies, most adverse effects of RFA treatment were minor in our study (10, 15). Pain lasting more than 24 h occurred in five patients and hematoma formation in four. The transient voice change in one patient during the procedure was disconcerting but disappeared after 30 min of ice pack treatment. Permanent damage was not observed. Moreover, none of the patients, including those who had undergone a lobectomy in the past, developed hypothyroidism. This is a significant advantage over surgery and RAI treatment.
In contrast to other studies, we detected a relatively high incidence of transient subclinical hyperthyroidism 1 week after RFA. TSH suppression below the lower limit of normal was observed in 24% of patients, and four reported mild hyperthyroidism symptoms. It was self-limiting and resolved within 2–3 weeks.
Limitations
The retrospective design of the study may be regarded as one of its limitations. However, by focusing on the individual outcome (see Fig. 1) and the fact that no patients were excluded and only three patients were lost to follow-up, we feel that the data provide a realistic and solid picture of daily practice. Secondly, the learning curve in this study is the product of a team effort of two intervention radiologists always operating together during each procedure. Therefore, the described results may be somewhat different from a single-operator learning curve. Thirdly, as standardized US images were not routinely made immediately after the completion of RFA, this study lacks an evaluation of the initial ablation ratio (IAR), a relatively new objective parameter to document the quality of RFA treatment introduced by Sim et al. (26, 33). It is calculated as the ratio between the ablated volume and the total nodule volume prior to RFA and thus represents the percentage of the successfully ablated nodule. Its main value is that it helps to predict long-term volume reduction and the likelihood of retreatment (17, 33). The accuracy of IAR for regrowth prediction remains to be established (34). Its potential value as a parameter to document operator skills was recently demonstrated by Russ et al., showing that a single-operator learning curve for achieving an IAR >90% was substantially longer than required for a VRR >50% and exceeded 90 procedures when large nodes are involved (27).
In conclusion, this study confirms previous observations that an experience of about 50 RFA procedures are required to achieve a 6-month VRR >50% in the majority of patients. Based on our current experience and the well-known diagnostic limitations of FNA, we recommend extended follow-up in patients with a volume reduction of less than 50%, considering repeat FNA or CNB in case of persisting symptoms or regrowth.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Statement of Ethics
The study was conducted according to the World Medical Association Declaration of Helsinki version 2013. The local Ethical Committee of Rijnstate Hospital approved the study (reference number: 2018-1335). Informed consent was obtained from all patients.
Author contribution statement
The study was designed by H d B, F J, W B. The manuscript was written by H d B, W B, F J. All authors took part in the analysis and interpretation of data of the study. The manuscript was critically revised by all other authors. All authors approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
The authors thank Prof Dr. W J G Oyen for his critical review of the manuscript.
References
- 1.Reiners C, Wegscheider K, Schicha H, Theissen P, Vaupel R, Wrbitzky R, Schumm-Draeger PM. Prevalence of thyroid disorders in the working population of Germany: ultrasonography screening in 96,278 unselected employees. Thyroid 200414926–932. ( 10.1089/thy.2004.14.926) [DOI] [PubMed] [Google Scholar]
- 2.Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Annals of Internal Medicine 196869537–540. ( 10.7326/0003-4819-69-3-537) [DOI] [PubMed] [Google Scholar]
- 3.Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T, Smith PA. The spectrum of thyroid disease in a community: the Whickham survey. Clinical Endocrinology 19777481–493. ( 10.1111/j.1365-2265.1977.tb01340.x) [DOI] [PubMed] [Google Scholar]
- 4.Hegedüs L.Clinical practice. The thyroid nodule. New England Journal of Medicine 20043511764–1771. ( 10.1056/NEJMcp031436) [DOI] [PubMed] [Google Scholar]
- 5.Said M, Chiu V, Haigh PI. Hypothyroidism after hemithyroidectomy. World Journal of Surgery 2013372839–2844. ( 10.1007/s00268-013-2201-8) [DOI] [PubMed] [Google Scholar]
- 6.Hermann M, Alk G, Roka R, Glaser K, Freissmuth M. Laryngeal recurrent nerve injury in surgery for benign thyroid diseases: effect of nerve dissection and impact of individual surgeon in more than 27,000 nerves at risk. Annals of Surgery 2002235261–268. ( 10.1097/00000658-200202000-00015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel KN, Yip L, Lubitz CC, Grubbs EG, Miller BS, Shen W, Angelos P, Chen H, Doherty GM, Fahey 3rd TJet al. The American Association of Endocrine Surgeons Guidelines for the definitive surgical management of thyroid disease in adults. Annals of Surgery 2020271e21–e93. ( 10.1097/SLA.0000000000003580) [DOI] [PubMed] [Google Scholar]
- 8.Bonnema SJ, Hegedüs L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocrine Reviews 201233920–980. ( 10.1210/er.2012-1030) [DOI] [PubMed] [Google Scholar]
- 9.Le Moli R, Wesche MF, Tiel-Van Buul MM, Wiersinga WM. Determinants of longterm outcome of radioiodine therapy of sporadic non-toxic goitre. Clinical Endocrinology 199950783–789. ( 10.1046/j.1365-2265.1999.00734.x) [DOI] [PubMed] [Google Scholar]
- 10.Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 200616361–367. ( 10.1089/thy.2006.16.361) [DOI] [PubMed] [Google Scholar]
- 11.Deandrea M, Limone P, Basso E, Mormile A, Ragazzoni F, Gamarra E, Spiezia S, Faggiano A, Colao A, Molinari Fet al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound in Medicine and Biology 200834784–791. ( 10.1016/j.ultrasmedbio.2007.10.018) [DOI] [PubMed] [Google Scholar]
- 12.Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, Lee D. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. European Radiology 2008181244–1250. ( 10.1007/s00330-008-0880-6) [DOI] [PubMed] [Google Scholar]
- 13.Cesareo R, Palermo A, Pasqualini V, Cianni R, Gaspa G, Manfrini S, Pacella CM. Radiofrequency ablation for the management of thyroid nodules: a critical appraisal of the literature. Clinical Endocrinology 201787639–648. ( 10.1111/cen.13422) [DOI] [PubMed] [Google Scholar]
- 14.Trimboli P, Castellana M, Sconfienza LM, Virili C, Pescatori LC, Cesareo R, Giorgino F, Negro R, Giovanella L, Mauri G. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine 20206735–43. ( 10.1007/s12020-019-02019-3) [DOI] [PubMed] [Google Scholar]
- 15.Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, Baek SM, Kim YS, Shin JH, Park JSet al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology 2012262335–342. ( 10.1148/radiol.11110416) [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, Choi YJ, Chung SR, Ha EJ, Hahn SYet al. 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean Journal of Radiology 201819632–655. ( 10.3348/kjr.2018.19.4.632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji Hong M, Baek JH, Choi YJ, Lee JH, Lim HK, Shong YK, Hong SJ. Radiofrequency ablation is a thyroid function-preserving treatment for patients with bilateral benign thyroid nodules. Journal of Vascular and Interventional Radiology 20152655–61. ( 10.1016/j.jvir.2014.09.015) [DOI] [PubMed] [Google Scholar]
- 18.Shin JH, Baek JH, Ha EJ, Lee JH. Radiofrequency ablation of thyroid nodules: basic principles and clinical application. International Journal of Endocrinology 20122012919650. ( 10.1155/2012/919650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauri G, Pacella CM, Papini E, Solbiati L, Goldberg SN, Ahmed M, Sconfienza LM. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid 201929611–618. ( 10.1089/thy.2018.0604) [DOI] [PubMed] [Google Scholar]
- 20.Bernardi S, Giudici F, Cesareo R, Antonelli G, Cavallaro M, Deandrea M, Giusti M, Mormile A, Negro R, Palermo Aet al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the Italian minimally invasive treatments of the Thyroid Group. Thyroid 2020301759–1770. ( 10.1089/thy.2020.0202) [DOI] [PubMed] [Google Scholar]
- 21.Dobnig H, Amrein K. Monopolar radiofrequency ablation of thyroid nodules: a prospective Austrian single-center study. Thyroid 201828472–480. ( 10.1089/thy.2017.0547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deandrea M, Sung JY, Limone P, Mormile A, Garino F, Ragazzoni F, Kim KS, Lee D, Baek JH. Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid 201525890–896. ( 10.1089/thy.2015.0133) [DOI] [PubMed] [Google Scholar]
- 23.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates MCet al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. Journal of the American College of Radiology 201714587–595. ( 10.1016/j.jacr.2017.01.046) [DOI] [PubMed] [Google Scholar]
- 24.Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid 2017271341–1346. ( 10.1089/thy.2017.0500) [DOI] [PubMed] [Google Scholar]
- 25.Na DG, Baek JH, Jung SL, Kim JH, Sung JY, Kim KS, Lee JH, Shin JH, Choi YJ, Ha EJet al. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean Journal of Radiology 201718217–237. ( 10.3348/kjr.2017.18.1.217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. European Radiology 2013231044–1049. ( 10.1007/s00330-012-2671-3) [DOI] [PubMed] [Google Scholar]
- 27.Sim JS, Baek JH, Lee J, Cho W, Jung SI. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. International Journal of Hyperthermia 201733905–910. ( 10.1080/02656736.2017.1309083) [DOI] [PubMed] [Google Scholar]
- 28.Russ G, Ben Hamou A, Poirée S, Ghander C, Ménégaux F, Leenhardt L, Buffet C. Learning curve for radiofrequency ablation of benign thyroid nodules. International Journal of Hyperthermia 20213855–64. ( 10.1080/02656736.2021.1871974) [DOI] [PubMed] [Google Scholar]
- 29.Deandrea M, Garino F, Alberto M, Garberoglio R, Rossetto R, Bonelli N, Spiezia S, De Santis M, Monti S, Deiana MGet al. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicentre prospective study. European Journal of Endocrinology 201918079–87. ( 10.1530/EJE-18-0685) [DOI] [PubMed] [Google Scholar]
- 30.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger Met al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016261–133. ( 10.1089/thy.2015.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyu YJ, Shen F, Yan Y, Situ MZ, Wu WZ, Jiang GQ, Chen YY. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules <10 mm in the maximum diameter: does size matter? Cancer Management and Research 2019111231–1236. ( 10.2147/CMAR.S189358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papini E, Monpeyssen H, Frasoldati A, Hegedüs L. 2020 European Thyroid Association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. European Thyroid Journal 20209172–185. ( 10.1159/000508484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sim JS, Baek JH, Cho W. Initial ablation ratio: quantitative value predicting the therapeutic success of thyroid radiofrequency ablation. Thyroid 2018281443–1449. ( 10.1089/thy.2018.0180) [DOI] [PubMed] [Google Scholar]
- 34.Bernardi S, Cavallaro M, Colombin G, Giudici F, Zuolo G, Zdjelar A, Dobrinja C, De Manzini N, Zanconati F, Cova MAet al. Initial ablation ratio predicts volume reduction and retreatment after 5 years from radiofrequency ablation of benign thyroid nodules. Frontiers in Endocrinology 202011582550. ( 10.3389/fendo.2020.582550) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a