Abstract
Alveolar type 2 (AT2) cells are heterogeneous cells, with specialised AT2 subpopulations within this lineage exhibiting stem cell properties. However, the existence of quiescent, immature cells within the AT2 lineage that are activated during lung regeneration is unknown.
SftpcCreERT2/+;tdTomatoflox/flox mice were used for the labelling of AT2 cells and labelled subpopulations were analysed by flow cytometry, quantitative PCR, assay for transposase-accessible chromatin using sequencing (ATAC-seq), gene arrays, pneumonectomy and culture of precision-cut lung slices. Single-cell RNA-sequencing (scRNA-seq) data from human lungs were analysed.
In mice, we detected two distinct AT2 subpopulations, with low tdTomato level (TomLow) and high tdTomato level (TomHigh). TomLow cells express lower levels of the AT2 differentiation markers Fgfr2b and Etv5, while TomHigh, as bona fide mature AT2 cells, show higher levels of Sftpc, Sftpb, Sftpa1, Fgfr2b and Etv5 expression. ATAC-seq analysis indicates that TomLow and TomHigh cells constitute two distinct cell populations, with specific silencing of Sftpc, Rosa26 and cell cycle gene loci in the TomLow population. Upon pneumonectomy, the number of TomLow but not TomHigh cells increases and TomLow cells show upregulated expression of Fgfr2b, Etv5, Sftpc, Ccnd1 and Ccnd2 compared to Sham. TomLow cells overexpress programmed cell death 1 ligand 1 (PD-L1), an immune inhibitory membrane receptor ligand, which is used by flow cytometry to differentially isolate these two subpopulations. In the human lung, data mining of a recent scRNA-seq AT2 data set demonstrates the existence of a PD-L1Pos population. Therefore, we have identified a novel population of AT2 quiescent, immature progenitor cells in mouse that expand upon pneumonectomy and we have provided evidence for the existence of such cells in human.
Short abstract
A novel population of AT2 progenitor cells enriched for PD-L1 has been identified. This normally quiescent subpopulation of AT2 cells becomes highly proliferative and differentiates into mature AT2 in response to alveolar injury. https://bit.ly/31G0IIW
Introduction
Alveolar type 2 (AT2) progenitor cells display self-renewal capabilities to maintain the AT2 cell pool and differentiate into AT1 cells following lung injury [1–5]. However, it is still debatable whether quiescent and immature AT2 progenitor cells exist in mouse and human lungs and if surface markers can be used to isolate these cells.
In rodents, AT2 cells are heterogeneous. Different subpopulations within this lineage, with different stem cell properties, have been identified based on various markers such as E-cadherin (E-CAD), AXIN2, integrin α6β4 and the dual expression of surfactant protein C (SFTPC)/secretoglobin family 1A member 1 (SCGB1A1) [6–9]. AT2 cells can be divided equally into E-CADPos and E-CADNeg cells. E-CADNeg cells are more resistant to hyperoxia injury, more proliferative and display higher level of telomerase activity, while E-CADPos cells are more sensitive to damage [6]. In the adult mouse lung, AXIN2Pos cells make up 1% of the mature AT2 (SFTPCHigh) cells and are distributed throughout the lung [8]. This ratio of AXIN2Pos cells is stable over time. In vivo lineage tracing of AXIN2Pos (SFTPCHigh) cells revealed their ability to form clones of two to five cells accompanied by their expansion and differentiation into AT1 expressing podoplanin (PDPN). In addition, these cells display enhanced self-renewal capabilities in alveolosphere assays compared to the AXIN2Neg AT2 cells, suggesting that these AXIN2Pos cells represent a subpopulation of mature AT2 cells with enhanced stem cell capacities [8]. In a similar and independent study, AXIN2Pos cells belonging to the mature AT2 cell population were characterised. These cells, called alveolar epithelial progenitor (AEP) cells, comprise ∼20% of adult mature AT2 cells and are enriched for WNT target genes and developmental genes such as Fgfr2, Nkx2.1, Id2, Etv4, Etv5 and Foxa1. These cells also display increased self-renewal ability in alveolospheres compared to total AT2 cells [9].
AT2 cell heterogeneity is not limited to the adult lung. During the alveologenesis phase of lung development, AXIN2Pos AT2 cells display enhanced proliferation compared to total AT2 cells [10]. A subset of SFTPCNeg, laminin receptor integrin α6β4Pos cells, located at the bronchoalveolar duct junctions (BADJ) and within the alveoli, regenerate AT2 SFTPCPos cells in the alveoli after severe lung injury [7]. Finally, bronchoalveolar stem cells, positive for the alveolar marker SFTPC and the club cell marker SCGB1A1, are located at the BADJ and are amplified upon injury to give rise to either alveolar epithelial cells or club cells [11–13]. AT2 cell heterogeneity was equally shown in the context of diphtheria toxin injury, with a subpopulation of lineage-labelled AT2 cells displaying relatively more resistance to diphtheria toxin toxicity able to repopulate the AT2 pool following injury. The identity of these survivor cells, however, is not clear [3].
In the present study, we identified a novel population of AT2 quiescent and immature progenitor cells characterised by low Sftpc level and high expression of the surface marker programmed cell death 1 ligand 1(PD-L1) (also known as CD274), an immune checkpoint protein expressed in cancer stem cells and mediating anti-tumour suppression response [14–16]. We deployed a series of in vivo approaches in mice to label different subpopulations of AT2 cells, examined their epigenetic and transcriptomic characteristics, and tested their respective response in vivo during lung regeneration following pneumonectomy as well as in vitro using the culture of precision-cut lung slices (PCLS). We also validated the use of PD-L1 antibodies to differentially sort epithelial subpopulations and carried out data mining of recently published AT2 single-cell RNA-sequencing (scRNA-seq) experiments. Our work opens the way for an in-depth characterisation of this novel quiescent and immature AT2 stem cell population.
Materials and methods
Animal experiments
All animal studies were performed according to protocols approved by the Animal Ethics Committee of the Regierungspraesidium Giessen (permit numbers: G7/2017–No.844-GP and G11/2019–No. 931-GP).
Pneumonectomy
Lung dissociation and fluorescence-activated cell sorting
Single-cell suspension was generated from adult lungs and stained with anti-EPCAM (APC-Cy7-conjugated, Biolegend, 1:50), CD49F (APC-conjugated, Biolegend, 1:50), anti-PDPN (FITC-conjugated, Biolegend, 1:20) and anti-PD-L1 (unconjugated, Thermo Fisher, 1:100) antibodies for 20 min on ice in the dark, followed by washing. The cells were then stained for goat anti-rabbit secondary antibody Alexa fluor 488 (Invitrogen, 1:500) for 20 min on ice in the dark. Next, cells were washed and stained with SYTOX (Invitrogen), a live/dead cell stain, according to the manufacturer's instructions. Flow cytometry data acquisition and cell sorting were carried out using a FACSAria III cell sorter (BD Biosciences). Data were analysed using FlowJo software version X (FlowJo, LLC).
See supplementary material for the mouse lung dissociation.
RNA extraction and quantitative real-time PCR
Immunofluorescence staining
Alveolosphere assay
Microarray
ATAC-seq
Mining of scRNA-seq data set for human AT2 cells
For the analysis, we used the synapse ID (syn21041850) data set deposited at https://www.synapse.org/#!Synapse:syn21041850/wiki/600865 and published by Travaglini et al. [17]. The Seurat objects were updated to Seurat v3 [18] and AT2 cells were extracted using the subset’ function. The data set was reanalysed starting from raw counts using the SCTransform wrapper in Seurat. Data imputation was then performed using a low-rank approximation with the package ALRA [19].
Quantification and statistical analysis
For quantification of immunofluorescence, cells were counted in 10 independent 63× fields per sample. Statistical analysis and graph assembly were carried out using GraphPad Prism 6 (GraphPad Prism Software). Significance was determined by unpaired two-tailed t-tests. Data are presented as mean±sem. Values of p<0.05 were considered significant. The number of biological samples (n) for each group is stated in the corresponding figure legends.
Results
Identification of two AT2 subpopulations with different Sftpc and Fgfr2b levels
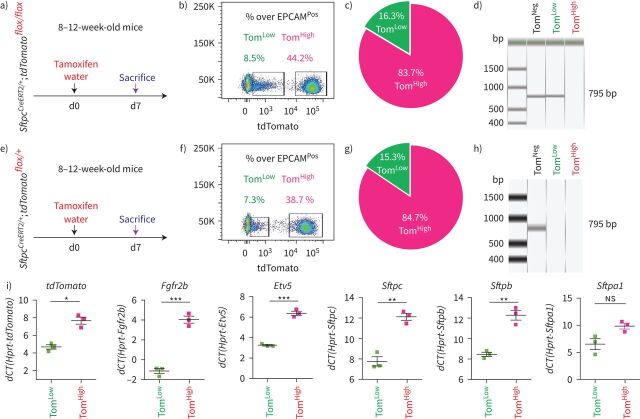
SftpcCreERT2/+;tdTomatoflox/flox mice were used to lineage label AT2 cells in the adult lung. In our experimental approach, tamoxifen was delivered in the water for 1 week. Two distinct subpopulations of tdTomatoPos (TomPos) cells, that we called tdTomatoLow (TomLow) and tdTomatoHigh (TomHigh), were identified using flow cytometry analysis (figure 1a–c). Of note, a similar observation, which was not followed up, was previously reported using the SftpcCre-ER mouse line generated by Finn et al. [20]. On average, TomLow cells represented 9.93±1.73% of the overall EPCAMPos cells (n=4) and TomHigh cells represented 42.75±1.22% of the overall EPCAMPos cells (n=4). Out of the total TomPos cells, on average 17.58±2.52% (n=4) were TomLow and 81.93±2.3% (n=4) were TomHigh. We also determined the extent of recombination of the LoxP-STOP-LoxP-tdTomato cassette in the isolated TomLow and TomHigh cells. Our results indicate that complete recombination was observed in TomHigh while incomplete recombination was observed in TomLow, indicated by the presence of the 795 bp stop codon band (figure 1d). This result suggested either contamination of the TomLow pool by non-AT2 cells or the lack of access of Cre to the LoxP-STOP-LoxP-tdTomato cassette in the Rosa26 locus of TomLow cells. The presence of the two subpopulations based on Tomato intensity could also be explained by improper recombination of either one or two copies of the LoxP-STOP-LoxP-tdTomato cassette in tdTomatoflox/flox mice. To test these different possibilities, we carried out a similar experiment with SftpcCreERT2/+;tdTomatoflox/+ mice, which exhibit only one copy of the LoxP-STOP-LoxP-tdTomato cassette (figure 1e–h). Similarly, we found two subpopulations of TomPos cells and, importantly, complete recombination of the LoxP-STOP-LoxP-tdTomato cassette in TomLow, indicating the absence of contamination by non-AT2 cells in the TomLow pool. This conclusion is supported by our quantitative PCR (qPCR) data for markers of endothelial, hematopoietic, macrophage and fibroblast cells (supplementary figure S1). Overall, our data support the existence of two AT2 cell subpopulations in SftpcCreERT2/+;tdTomatoflox/flox lungs, based on different levels of Tomato expression. This is due to the differential expression of Tomato per se from the Rosa26 promoter in TomHigh versus TomLow cells. In addition, in SftpcCreERT2/+;tdTomatoflox/flox lungs, this is also associated with the incomplete recombination of the LoxP-STOP-LoxP cassette, which is also located in the Rosa26 locus.
FIGURE 1.
Identification of two subpopulations of AT2-lineage-labelled cells, named tdTomatoLow (TomLow) and tdTomatoHigh (TomHigh). a) Timeline of tamoxifen treatment of SftpcCreERT2/+;tdTomatoflox/flox mice (n=4). d: day. b) Representative flow cytometry of EPCAMPos population selection and identification of TomLow (8.5%) and TomHigh (44.2%) populations based on the tdTomato level. c) Percentage of TomLow (16.3%) and TomHigh (83.7%) in total tdTomatoPos cells. d) PCR on genomic DNA isolated from fluorescence-activated cell sorting (FACS)-based sorted TomLow and TomHigh cells from SftpcCreERT2/+;tdTomatoflox/flox mice for Stop codon. e) Timeline of tamoxifen treatment of SftpcCreERT2/+;tdTomatoflox/+mice and f) representative flow cytometry analysis of TomLow (7.3%) and TomHigh (38.7%). g) The pie chart shows the percentage of TomLow (15.3%) and TomHigh (84.7%) in total tdTomatoPos cells. h) PCR on genomic DNA isolated from FACS-based sorted TomLow and TomHigh cells from SftpcCreERT2/+;tdTomatoflox/+ mice. i) Quantitative PCR analysis of FACS-based sorted TomLow and TomHigh cells for the expression of Tomato, Fgfr2b, Etv5, Sftpc, Sftpb and Sftpa1. dCT: delta cycle threshold. Data are presented as mean±sem. *: p<0.05; **: p<0.01; ***: p<0.001.
We also confirmed that epithelial cells were specifically labelled in this mouse line and the labeling mostly targeted AT2 SFTPCPos cells (supplementary figure S2). Very few BACS cells, identified through their localisation at the BADJ, were labelled (0.1% of the total TomPos cells, data not shown), consistent with the literature [7]. Our labelling efficiency of AT2 cells was 77±5.40% (n=4) (supplementary figure S2b). In this mouse line, 4.5% of TomPos/total cells were labelled in mice exposed to normal water, indicating leakiness. This percentage increased to 20.5% of TomPos/total cells in mice exposed to tamoxifen water for 1 week (supplementary figure S3), indicating robust induction of labelling. We also carried out labelling at 8 weeks of age by exposing the mice to tamoxifen water for 1 week followed by a 6- and 9-months chaseperiod. Our data indicate that both TomLow and TomHigh subpopulations were detected at these two time points, and were stable populations (supplementary figure S4).
Next, qPCR was performed on fluorescence-activated cell sorting (FACS)-isolated TomLow and TomHigh cells. Our results show that Fgfr2b and its associated downstream target Etv5, as well as the differentiation markers Sftpc, Sftpb and Sftpa1, were significantly enriched in TomHigh versus TomLow cells (figure 1i). Thus, we conclude that TomHigh represents the bona fide mature AT2 cells and we hypothesised that the lineage-related TomLow cells represent immature AT2 cells.
ATAC-seq analysis indicates that TomLow cells and TomHigh cells are distinct cell populations
To carry out genome-wide profiling of the epigenomic landscape, an assay for transposase-accessible chromatin using sequencing (ATAC-seq) was performed on TomLow and TomHigh subpopulations (figure 2a). Common and distinct peaks were identified for TomLow and TomHigh cells (figure 2b–d).
FIGURE 2.
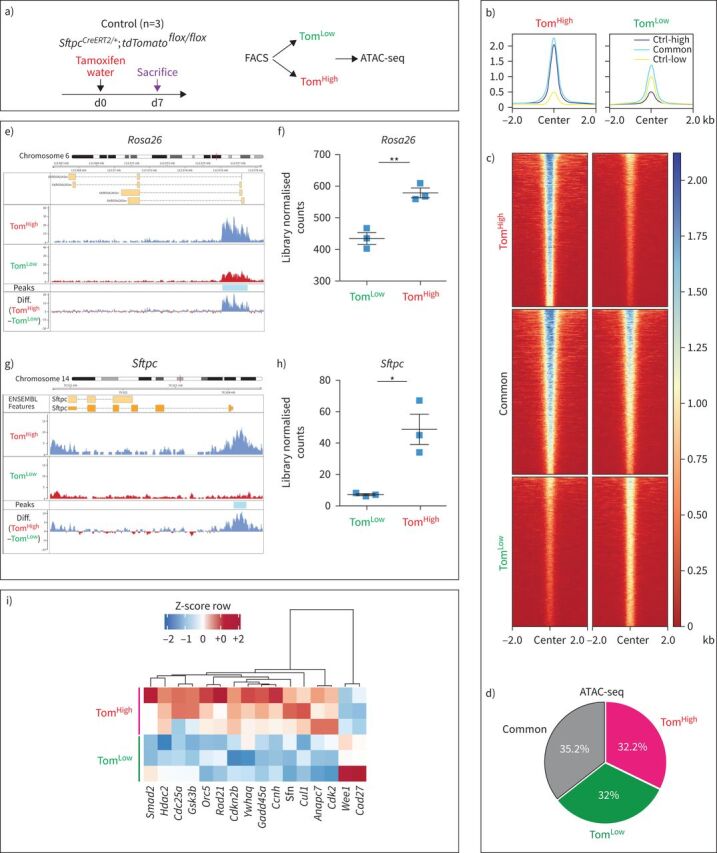
Assay for transposase-accessible chromatin using sequencing (ATAC-seq) analysis on fluorescence-activated cell sorting (FACS)-based sorted tdTomatoLow (TomLow) and tdTomatoHigh (TomHigh) populations. a) Timeline of tamoxifen treatment of SftpcCreERT2/+;tdTomatoflox/flox mice (n=3). ATAC-seq was carried out on FACS-based sorted TomLow and TomHigh subpopulations. d: day. b, c) Coverage heat maps of TomLow and TomHigh, displaying genome-wide regions of differential open chromatin peaks in TomLow versus TomHigh. TomLow chromatin is less open and transcriptionally less active compared to TomHigh. Ctrl-low shows the peaks related to open chromatin regions in TomLow. Ctrl-high shows the peaks related to open chromatin regions in TomHigh. Common shows peaks detected in both TomLow and TomHigh subpopulations. d) ATAC-seq analysis of peaks based on the cut-offs shows 3605 upregulated in TomHigh (false discovery rate (FDR)<0.05, log2(FC)>0.585, base mean>20), 3512 upregulated in TomLow (FDR<0.05, log2(FC)>0.585, base mean>20) and 3878 non-regulated (base mean>20, FDR>0.5, log2(FC) between −0.15 and 0.15), which means 32% and 32.2% of the genome is differently accessible in TomLow and TomHigh, respectively. e) ATAC-seq histogram of average read coverage at the Rosa26 locus shows distinct ATAC-seq peaks at the promoter and denser chromatin in TomLow compared to TomHigh in this locus. Representative peaks of TomLow and TomHigh are the average of three independent samples. f) Quantification of peaks at the Rosa26 locus. g) ATAC-seq profile at Sftpc locus shows distinct ATAC-seq peaks at the promoter and denser chromatin in TomLow compared to TomHigh. Representative peaks of TomLow and TomHigh are averages of three independent samples. h) Quantification of peak regions of Sftpc locus. The ATAC-seq data have been normalised for sequencing depth and the scale on the y-axis was chosen for optimal visualisation of peaks. i) ATAC-Heat-pVAI Z-score, Pearson, average heat map based on the ATAC-seq data of top cell cycle-related genes differentially regulated in TomLow and TomHigh. FDR: the significance of results by Benjamini–Hochberg correction of multiple tests (n=3).
Further analysis of the ATAC-seq data using the Reactome database indicated that the chromatin in loci of genes relating to metabolism, cholesterol metabolism, surfactant metabolism and triglyceride biosynthesis was more open in TomHigh cells. These data are in agreement with the known role of mature AT2 cells in surfactant production. By contrast, TomLow cells exhibit more accessibility for genes relating to the adaptive and innate immune system as well as genes involved in extracellular matrix (ECM) organisation, ECM proteoglycans and degradation of the ECM. These results suggest a new and important function for the TomLow cells in interactions with the immune system, potentially also displaying migratory capabilities through ECM degradation (supplementary figure S5a).
Our ATAC-seq data also support the observed decrease in Tomato (expressed from the Rosa26 promoter) and Sftpc expression at the mRNA level in TomLow versus TomHigh. In the Rosa26 and Sftpc loci, the peaks corresponding to the open chromatin configuration were detected at much higher levels in TomHigh than in TomLow, and this difference was confirmed to be statistically significant upon quantification (figure 2e–h). Interestingly, the analysis of ATAC-seq data also suggested that TomLow cells have reduced expression of cell cycle genes compared to TomHigh cells (figure 2i). This decrease in cell cycle genes was confirmed by the analysis of our gene array data between TomLow and TomHigh (supplementary figure S5b). Overall our data indicate that in homeostatic conditions the TomLow cells fit the profile of a quiescent population.
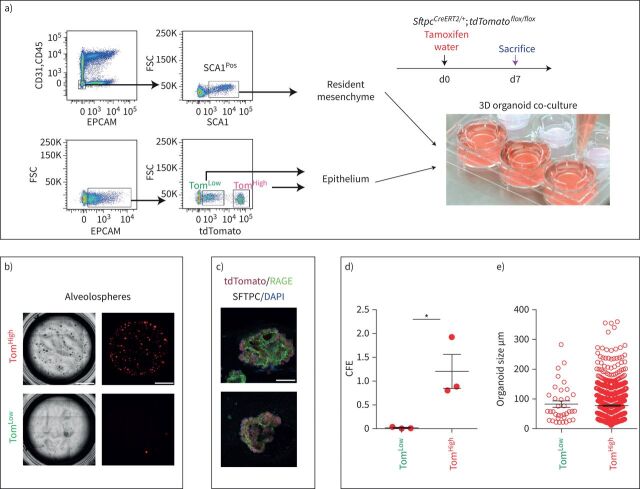
AT2 TomLow and TomHigh display different colony-forming capabilities
To compare the self-renewal capacity of TomLow and TomHigh, FACS-based sorted cells were co-cultured with CD31NegCD45NegEPCAMNegSCA1Pos resident lung mesenchymal cells according to a previously published protocol (figure 3a). TomHigh behaved as bona fide AT2 cells, forming alveolospheres with the expected colony-forming efficiency (figure 3b, d, e) [3, 9, 21], whereas TomLow displayed very weak organoid-forming capabilities, which is in line with their proposed quiescent status. Both populations transdifferentiated into receptor for advanced glycation end-products (RAGE)-positive AT1 cells after 14 days in culture (figure 3c).
FIGURE 3.
Different colony-forming capabilities of tdTomatoLow (TomLow) and tdTomatoHigh (TomHigh) cells. a) Representative flow cytometry shows the gating strategy of CD31Neg CD45Neg EPCAMNeg population and further selection of SCA1Pos resident mesenchymal cells from C57BL/6 lungs (upper plot), as well as the selection of TomLow and TomHigh from the EPCAMPos population from SftpcCreERT2/+;tdTomatoflox/flox(lower plot). Mesenchymal cells were co-cultured with TomLow and TomHigh separately (n=3). d: day. b) Representative alveolospheres from TomLow and TomHigh (n=3). Scale bar: 100 μm. c) Representative SFTPC and RAGE immunofluorescence staining of alveolospheres after 14 days in culture. Scale bar: 50 μm. d) Quantification of colony-forming efficiency (CFE) and e) alveolospheres size in TomHigh and TomLow (n=3). Data are presented as mean±sem. *: p<0.05.
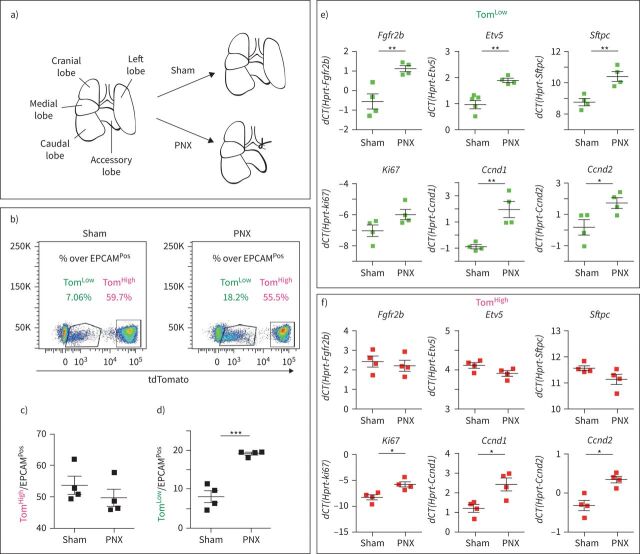
Expansion of TomLow population following pneumonectomy
A critical question regarding these two subpopulations of AT2 cells is whether they are differentially engaged in the context of lung regeneration. Therefore, we used the mouse pneumonectomy (PNX) model to trigger lung regeneration, a process that is tightly associated with the proliferation of AT2 cells [22, 23]. SftpcCreERT2/+;tdTomatoflox/flox mice (n=4) were treated with tamoxifen water for 1 week to label the AT2 lineage. The mice were then put on normal water for 2 weeks to ensure enough time for tamoxifen clearance. Unilateral left lung pneumonectomy was then performed to induce the process of compensatory growth in the remaining right lobes. Control mice (Sham) underwent the same process but without removing the left lobe (figure 4a). The animals were killed on day 7 post-surgery and the right lobes were processed for FACS. Because the accessory lobe has been shown to respond the most to PNX [24], our results are likely underestimating the response of TomLow cells in the context of PNX. The quantification of the abundance of TomLow and TomHigh cells over the total number of EPCAMPos cells in Sham and PNX mice indicates that the ratio of TomHigh cells/total number of EPCAMPos cells remained unchanged between two conditions, while the ratio of TomLow cells was significantly increased in the context of PNX versus Sham (figure 4b–d). This suggests that it is the TomLow cells, rather than the previously thought TomHigh cells, that are contributing to the process of lung regeneration. These two AT2 populations were isolated by FACS for further analysis by qPCR. Upon PNX, the expression of Fgfr2b, Etv5, Sftpc, Cyclin D1 (Ccnd1) and Cyclin D2 (Ccnd2) was significantly upregulated in TomLow. A trend towards an increase was also observed in Ki67 expression (figure 4e). These results are consistent with FGFR2b signalling activation and proliferation in TomLow cells in the context of lung regeneration. By contrast, TomHigh cells showed no difference in Fgfr2b, Etv5 or Sftpc between PNX and Sham conditions (figure 4f). Surprisingly, we noticed an increase in Ki67, Ccnd1 and Ccnd2 (figure 4f) but because the number of TomHigh cells was not increased in PNX versus Sham mice, the significance of these results is not clear. We also carried out the labelling of proliferative cells using a single dose of intraperitoneal 5-ethynyl-2-deoxyuridine (EdU) (0.1 mg·g−1 of mouse) at day 6 following PNX. We analysed the lungs at day 7 (1-day chase period, n=2 mice) and day 14 (8-day chase period, n=3 mice). AT2 PD-L1Pos (equivalent to the TomLow) and AT2 PD-L1Neg (equivalent to the TomHigh) were isolated by FACS. We performed cytospins with these isolated cells and carried out immunofluorescence for EdU. While it was easy to observe numerous AT2 PD-L1Neg cells, the number of AT2 PD-L1Pos cells on the slides was very low, making it difficult to analyse EdU incorporation in these cells. Our results indicated that 2.3±0.8% (n=2 independent mice, 300 cells counted per mice) of the AT2 PD-L1Neg were EdUPos at day 7. However, at day 14, 12.2±0.4% were EdUPos (n=3 mice, 300 cells per mouse). The increase in the number of labelled cells between these two time points, after an 8-day chase period, suggests that the EdUPos cells in the AT2 PD-L1Neg pool are coming from the AT2 PD-L1Pos cells (data not shown).
FIGURE 4.
Expansion of tdTomatoLow (TomLow) but not tdTomatoHigh (TomHigh) following pneumonectomy (PNX). a) Schematic representation of the PNX and Sham models. b) Representative flow cytometry analysis of TomLow and TomHigh populations 7 days after PNX and Sham, and quantification of c) TomHigh and e) TomLow percentages in EPCAMPos population between Sham and PNX groups (n=4). f) Quantitative PCR (qPCR) analysis of fluorescence-activated cell sorting (FACS)-based sorted TomLow population for expression of Fgfr2b, Etv5, Sftpc, Ki67, Ccnd1 and Ccnd2. g) qPCR analysis of FACS-based sorted TomHigh population for expression of Fgfr2b, Etv5, Sftpc, Ki67, Ccnd1 and Ccnd2. Data are presented as mean±sem. dCT: delta cycle threshold. *: p<0.05; **: p<0.01; ***: p<0.001.
Loss of TomHigh cells leads to expansion of TomLow cells
We made use of PCLS to follow the fate of TomPos cells over time in vitro. Our flow cytometry results indicate that this approach leads to the drastic loss of TomHigh cells (figure 5a, b). Therefore, we took advantage of this system to monitor the fate of TomLow cells over time after a massive loss of TomHigh. Over a culture period of 96 h, we observed the formation of cell clusters and a significant increase in tdTomato intensity (figure 5c), suggesting the differentiation of TomLow cells towards mature (TomHigh) AT2 cells. In addition, we observed the presence of clusters of TomPos cells at 240 h (figure 5c). We also performed video-fluorescence microscopy to track individual TomLow cells (n=6) in PCLS over a period of 45 h. Our results indicated that the intensity of the tdTomato signal in these cells progressively increased with time, thereby confirming the transition from TomLow to TomHigh status (data not shown). Finally, we analysed apoptosis and proliferation in these PCLS over time. Our results indicated a very significant increase in tdTomatoPosTUNELPos over total tdTomatoPos cells (figure 5d–k) in PCLS at 20 h, which is in line with the lack of detection of TomHigh cells by FACS at that time point (figure 5b). In addition, proliferation analysis indicated a robust increase in the number of tdTomatoPosEdUPos over total tdTomatoPos cells in PCLS between 20 h and 96 h (figure 5h–k), supporting our previous conclusion in the context of PNX that the TomLow cells are proliferating after injury.
FIGURE 5.
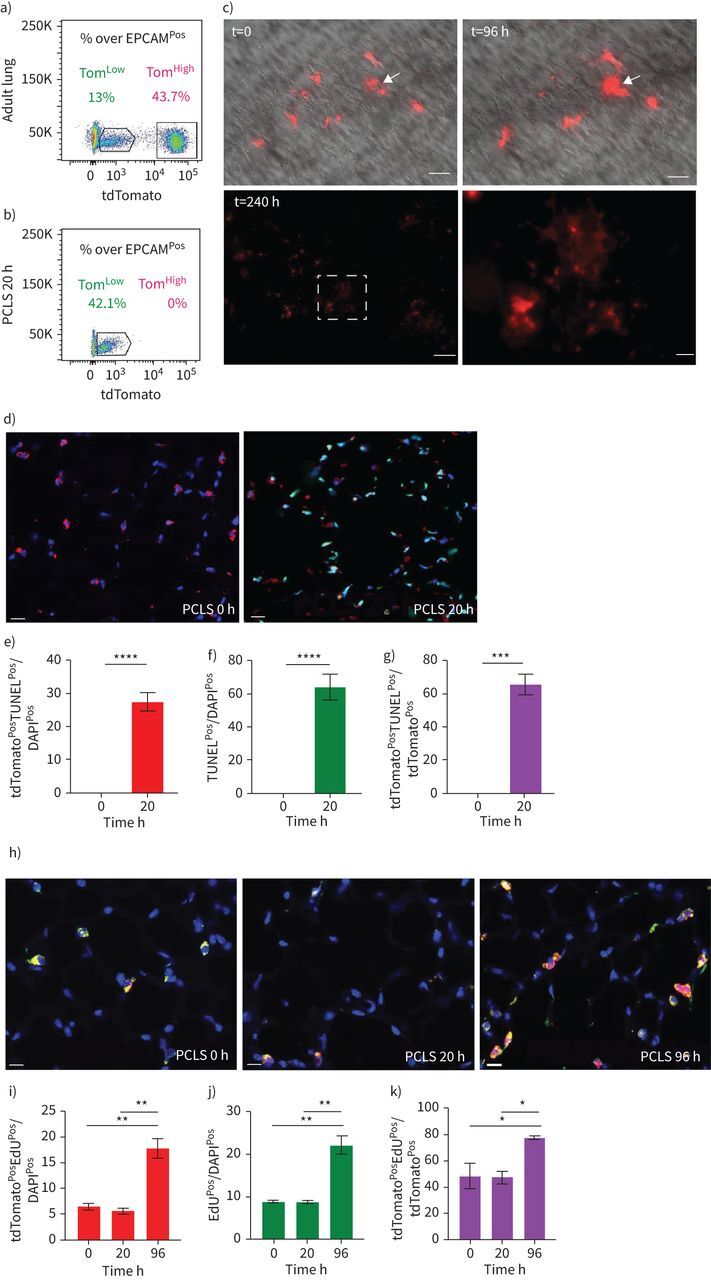
Characterisation of the fate of tdTomatoLow (TomLow) cells in precision-cut lung slices (PCLS). a, b) Representative flow cytometry analysis of TomLow and tdTomatoHigh (TomHigh) before processing the lungs for PCLS in freshly generated PCLS (a) and PCLS after 20 h culture (b). c) Visualisation of the TomLow in PCLS at t=0, t=96 h and t=240 h. Arrows indicate the formation of cell clusters. Scale bars: 250 μm (low magnification) and 50 μm (high magnification). d) Analysis of apoptosis with representative terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) immunofluorescence staining on the PCLS (scale bar: 50 μm) and quantification of e) tdTomatoPos TUNELPos cells out of total cells, f) TUNELPos cells out of total cells and g) tdTomatoPos TUNELPos cells out of tdTomatoPos cells (n=4). Data are presented as mean±sem. h) Analysis of proliferation with representative EdU immunofluorescence staining on the PCLS (scale bar: 50 μm) and quantification of i) tdTomatoPos EdUPos cells out of total cells, j) EdUPos cells out of total cells and k) tdTomatoPos EdUPos cells out of tdTomatoPos cells (n=4). Data are presented as mean±sem. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001.
PD-L1 is a specific surface marker enriched in TomLow
To identify markers differentially expressed between TomLow and TomHigh, we performed a gene array on FACS-isolated TomLow and TomHigh cells (figure 6a, supplementary figure S5b). Among the 100 top genes differentially expressed in TomLow versus TomHigh cells, three cell surface markers, Cd33, Cd300lf and Cd274 (also known as Pd-l1), were identified as being enriched in TomLow compared to TomHigh. qPCR analysis confirmed the significantly higher expression of Cd33 and Pd-l1 in TomLow compared to TomHigh (figure 6b). PD-L1 is an interesting marker because it is an immune inhibitory receptor ligand and its expression is highly increased in adenocarcinoma [14, 25]. The use of this marker is also relevant because our ATAC-seq analysis revealed that TomLow cells are enriched in genes belonging to the immune system (supplementary figure S5a). The use of PD-L1 as a surface marker enriched in TomLow cells was further validated using PD-L1 immunofluorescence staining and flow cytometry. To this end, PD-L1 immunofluorescence staining on cytospins confirmed a higher level of protein at the plasma membrane in TomLow than in TomHigh cells (figure 6c). Moreover, flow cytometry analysis of TomLow and TomHigh populations separately showed that 46.9% of TomLow cells were PD-L1Pos while only 0.77% of TomHigh cells were PD-L1Pos (figure 6d). In addition, PD-L1 immunofluorescence staining on lung sections localise tdTomatoPos PD-L1Pos cells in the alveoli (figure 6e). In conclusion, these results suggest that PD-L1 antibodies could be instrumental in differentially isolating the equivalent of TomLow versus TomHigh in wild-type lungs.
FIGURE 6.
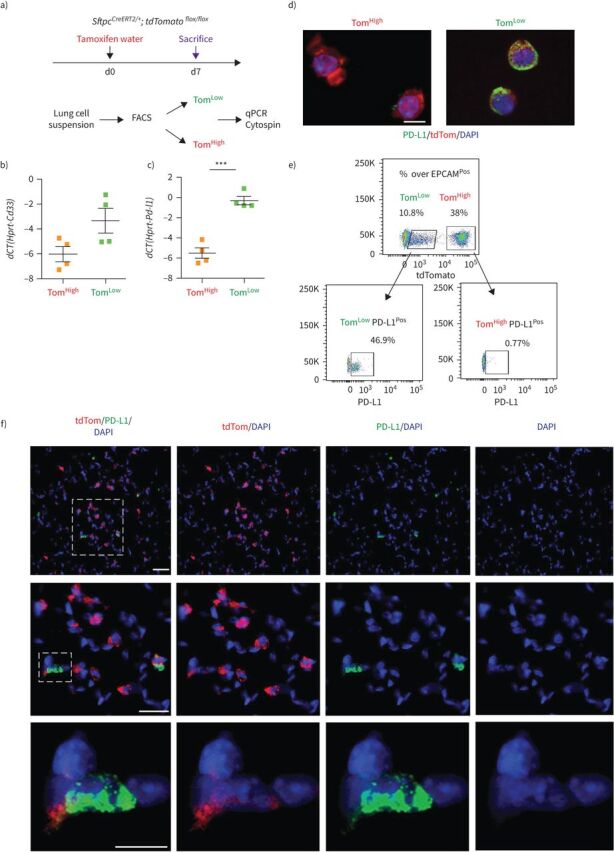
PD-L1 is a specific surface marker enriched in tdTomatoLow (TomLow) cells. a) Validation of gene array data by quantitative PCR (qPCR). d: day. b, c) Quantification of the expression levels of Cd33 (b) and Pd-l1 (c) in TomLow compared to tdTomatoHigh (TomHigh). Data are presented as mean±sem. dCT: delta cycle threshold. ***: p<0.001. d) Representative PD-L1 immunofluorescence staining on TomLow and TomHigh cytospin cells. Scale bar: 50 μm. e) Representative flow cytometry analysis of PD-L1Pos population in TomLow and TomHigh. f) Representative PD-L1 immunofluorescence staining on the lung sections. Scale bar: 10 μm.
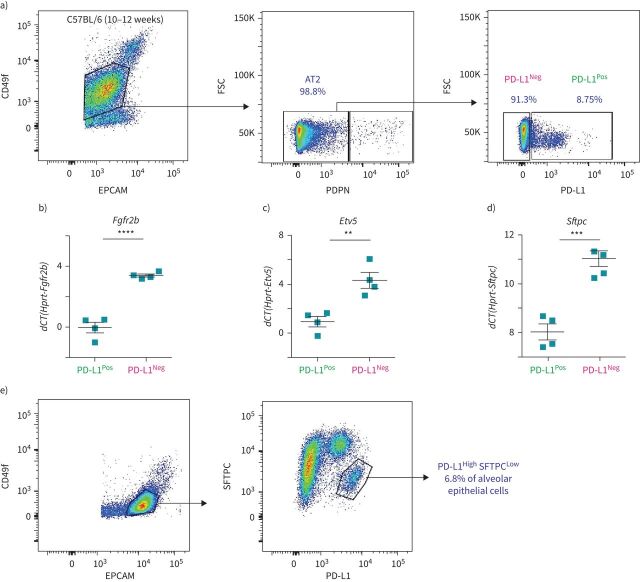
Identification of AT2 PD-L1Pos SFTPCLow in the lungs of wild-type mice
To address whether PD-L1 could be used to isolate the equivalent of TomLow and TomHigh without the need for a lineage tracing approach, FACS-based analysis was performed on isolated C57BL/6 lungs. The PD-L1 antibody was initially validated to show its expression in alveolar macrophages by immunofluorescence staining (in a lower magnification image of figure 6e) and flow cytometry (supplementary figure S6c). AT2 cell selection was based on the gating of EPCAMPos, CD49fIntermediate (to label the alveolar epithelial cells), PDPNNeg population (to exclude the AT1 cells), from which the percentages of PD-L1 positive and negative cells were analysed (figure 7a). On average, 8.99±0.51% (n=4) of these AT2 cells were PD-L1Pos and 90.23±0.58% (n=4) were PD-L1Neg (figure 7a). This ratio is consistent with the finding that most (80%) of the lineage-traced AT2 cells were composed of TomHigh (PD-L1Neg) cells (figure 1c). qPCR analysis on sorted PD-L1Pos and PD-L1Neg AT2 cells indicated a higher level of Fgfr2b, Etv5 and Sftpc in PD-L1Neg than PD-L1Pos cells (figure 7b–d). This result is also in line with PD-L1Neg cells corresponding to TomHigh cells, while the expression profile of PD-L1High fits with these cells being the equivalent of the TomLow. Finally, flow cytometry analysis of alveolar epithelial cells (EPCAMPos, CD49finter) stained with SFTPC and PD-L1 identified a subpopulation of AT2 cells (6.8%) displaying a low level of SFTPC and a high level of PD-L1 (figure 7e). This SFTPCLow PD-L1High cluster likely contains the TomLow cells.
FIGURE 7.
Identification of AT2 PD-L1Pos cells in the lungs of wild-type mice. a) Representative flow cytometry analysis of wild-type mice lungs shows the gating strategy of EPCAMPos CD49fInter population, followed by negative selection of AT2 cells with the exclusion of AT1 PDPNPos cells. The third plot is a representative flow cytometry analysis of AT2 cells based on the use of the PD-L1 marker. b–d) Quantitative PCR analysis of fluorescence-activated cell sorting (FACS)-based sorted PD-L1Pos and PD-L1Neg cells for expression of Fgfr2b (b), Etv5 (c) and Sftpc (d) (n=4). Data are presented as mean±sem. **: p<0.01; ***: p<0.001; ****: p<0.0001. e) Representative flow cytometry analysis of SFTPC and PD-L1 co-staining. FSC: forward scatter; dCT: delta cycle threshold.
qPCR analysis was carried out to validate the potential contamination of isolated AT2 PD-L1Pos and AT2 PD-L1Neg cells. Our results confirm the lack of contamination of these two subpopulations by endothelial, hematopoietic, macrophage and fibroblast cells (supplementary figure S6). In addition, co-culturing these cells with resident SCA1Pos mesenchymal cells in alveolosphere assay indicated that only AT2 PD-L1Neg cells (the equivalent of TomHigh cells) display self-renewal capabilities (supplementary figure S7). Finally, there were also higher numbers of AT2 PD-L1Pos cells in PNX versus Sham (supplementary figure S8). Altogether, our data indicate that the AT2 PD-L1Pos and AT2 PD-L1Neg cells are the functional equivalent of TomLow and TomHigh, respectively.
Identification of PD-L1Pos cells in a human AT2 scRNA-seq data set
The scRNA-seq results from a recent study reporting the global analysis of human lung cells [17] were used to further examine the presence of PD-L1Pos cells in the human AT2 pool. Bioinformatic analysis using Uniform Manifold Approximation and Projection (UMAP) plots was carried out to improve the initial AT2 cell pool clustering. Our results allowed the initial AT2 cluster composed of 3198 cells to be separated into five sub-clusters (figure 8a). Analysis of PD-L1 expression distinguished two positive clusters, named AT2 PD-L1High and AT2-proliferative, displaying high levels of PD-L1 (figure 8b, green and lavender arrows, respectively). Note that the AT2-proliferative cluster had higher expression of MIK67 and PCNA (supplementary figure S9). However, because the AT2-proliferative cluster comprised only three cells, its relevance to our TomLow population identified in mice is not clear. The other sub-cluster (AT2 PD-L1High) comprised 186 cells (5.82% of the initial AT2 pool). An analysis of gene expression is represented using a violin plot (figure 8b–d). Compared to the AT2 sub-cluster (labelled in red), PD-L1 expression in AT2 PD-L1High (labelled in green) was clearly increased (figure 8b). In addition, FGFR2b expression was not changed while SFTPC was only slightly decreased. However, lower expression of AXIN2, SFTPA1 and ETV5 was observed (figure 8d). It is therefore likely that the AT2 PD-L1High sub-cluster is the human equivalent of the AT2 TomLow that we identified in mice. Interestingly, compared to the AT2 sub-cluster, the AT2-proliferative sub-cluster also displayed lower levels of ETV5, SFTPC and SFTPA1 but a higher level of AXIN2. The genes enriched in AT2 PD-L1High were also analysed (supplementary figure S10). Transmembrane 4L6 family member 1 (TM4SF1), a gene encoding a membrane protein, was highly enriched in AT2 PD-L1High cells (figure 8d). Interestingly, this marker was used previously to isolate AEP cells, which are proposed to express high level of AXIN2 in the human lung [9]. It is clear that this marker is also expressed in AT2 AXIN2Low PD-L1High human cells (as well as in TomLow versus TomHigh in mice, data not shown), illustrating that more work is needed to characterise human and corresponding mouse progenitor cells and to identify more markers. Finally, we used the online resource of the IPF Cell Atlas to add further evidence for the presence of AT2 PD-L1Pos cells in human lungs (data not shown). CD274Pos (PD-L1Pos) cells were observed in all four human data sets analysed (Banovitch/Kropski, Kaminski/Rosas, Lafyatis and Misharin). The level of expression of ETV5, FGFR2 and SFTPC in AT2 PD-L1Pos cells in these data sets fits with either their quiescence (low expression level) or activation (high expression level) (data not shown).
FIGURE 8.
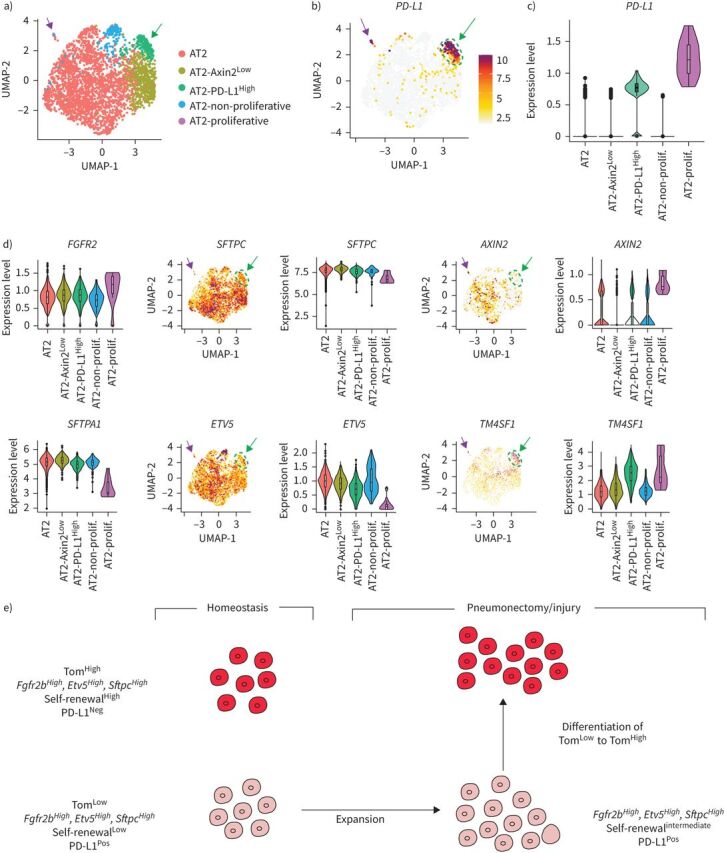
Identification of PD-L1Pos cells in human AT2 single-cell RNA-sequencing (scRNA-seq) data set. a) UMAP plot of the initial AT2 cell cluster indicates the presence of five different AT2 sub-clusters. b) UMAP and c) violin plots showing PD-L1 enrichment in the AT2-PD-L1High sub-cluster. d) UMAP and violin plots showing that the AT2-PD-L1High sub-cluster displays low levels of ETV5, SFTPC and AXIN2 and high levels of TM4SF1. Green arrows indicate AT2 PD-L1High and lavender arrows indicate AT2-proliferative. e) Model for tdTomatoLow (TomLow) and tdTomatoHigh (TomHigh) cells in homeostasis and after pneumonectomy/injury. TomLow cells are quiescent in the adult lung during homeostasis but are activated after injury. They acquire proliferative capabilities and differentiate into mature AT2 cells.
Discussion
We have identified a subpopulation of AT2 progenitor cells that is distinct from already identified mature AT2 progenitor subpopulations. TomLow cells are quiescent and immature cells in the steady state and, unlike AT2 AXIN2Pos cells, express low levels of AT2 differentiation markers. Moreover, TomLow cells express significantly lower levels of Axin2 than TomHigh cells in homeostasis, and Axin2 expression in TomLow cells showed no change after PNX compared to the Sham condition (supplementary figure S11a, b). Zacharias et al. [9] showed that AEPs express higher level of genes related to lung and tube development than mature AT2s. However, based on our gene array data, these genes are expressed at lower levels in TomLow compared with TomHigh cells (data not shown). This indicates that AEP cells are part of the TomHigh population. The newly identified TomLow cells are also different from the integrin α6β4 population because these cells are negative for Sftpc and, therefore, the SftpcCreERT2 driver line cannot lineage trace this population [7, 26]. Finally, the TomLow population most likely does not contain bronchoalveolar stem cells (BASCs) for several reasons. First, most of the TomLow cells are located in the respiratory epithelium and do not display high levels of Scgb1a1 (data not shown). Second, scRNA-seq data indicate that, compared to AT2 cells, BASCs express a similar level of Sftpc and Fgfr2 [13], suggesting that BASCs are likely contained in the TomHigh population. Interestingly, lineage tracing of BASCs upon injury indicates that these cells are not the sole contributors to newly formed bronchial airway cells after conducting airway injury or AT2/AT1 cells after alveolar injury, demonstrating that other resident stem cells such as the AT2 TomLow or the AT2 could contribute to the repair process. Furthermore, PD-L1 is highly expressed in TomLow. PD-L1 expression is also increased in adenocarcinoma and appears to be correlated with elevated tumour proliferation and aggressiveness [14, 16, 25, 27, 28]. Further investigations are required to elucidate PD-L1 function in TomLow cells and their potential interaction with the immune system as well as their contribution to cancer. In the future, it will be important to design dual lineage tracing strategies based on the expression of Sftpc and Pd-l1 to label the TomLow cells specifically.
A scRNA-seq data set examining the status of lung cells in adult mice at different time points following bleomycin injury was recently published [29]. Data mining of these scRNA-seq results indicated that AT2 Pd-l1Pos cells could not be detected either in control (saline) or experimental (bleomycin) conditions. Because the design of the experiment was to capture all the cells in the lung, the corresponding epithelial portion was clearly underpowered because not enough subpopulations of AT2 cells were available for a robust analysis. This lack of detection of AT2 Pd-l1Pos cells was also likely due to the trauma associated with the enzymatic digestion of the lung before the scRNA-seq procedure and is supported by our observation that TomLow cells had much lower viability compared to the TomHigh cells (data not shown). Preliminary experiments on bleomycin-treated (n=2 mice) versus saline-treated (n=3 mice) wild-type mice indicated a robust increase in AT2 PD-L1Pos cells compared to AT2 PD-L1Neg cells (21.2% versus 8.1%; data not shown). We, therefore, conclude that AT2 PD-L1Pos (TomLow) cells appear to respond to at least three types of injury (PNX, bleomycin and PCLS).
An important question that we attempted to answer in this study is related to the potential role played by TomLow cells as progenitors for mature AT2 cells. The arguments for such a role are as follow: 1) TomLow cells represent a stable population over time (supplementary figure S4); 2) analysis of apoptosis and proliferation of the Tomato labelled cells in the PCLS model (figure 5) shows that TomHigh cells are massively dying while TomLow cells are proliferating; 3) there was an increase in the TomLow (PD-L1Pos) pool in the context of PNX versus Sham (figure 4 and supplementary figure S8) without a significant change in the number of TomHigh cells; 4) while TomLow cells from non-injured lung indicate that these cells are not capable of self-renewal and differentiation (figure 3), our preliminary data (not shown) indicate that TomLow cells from SftpcCreERT2/+;Fgfr2bflox/flox;Tomatoflox/flox lungs display a significant increase in alveolosphere formation, indicating that TomLow cells acquire self-renewal and differentiation capabilities.
In conclusion, we have identified a novel population of quiescent and immature AT2 progenitor cells with a different gene expression profile from mature AT2 cells that proliferate after PNX and are enriched in Pd-l1 expression. The equivalent of this population was also identified in human lungs. Further characterisation of these cells in homeostatic and repair/regeneration conditions will allow the signalling pathways activated in these cells to be identified, with the ultimate goal of enhancing repair after injury.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods and tables ERJ-04168-2020.Supplement (112.6KB, pdf)
Supplementary figure S1. Absence of contaminations in TomLow and TomHigh subpopulations. a) Timeline of tamoxifen treatment of SftpcCreERT2/+; tdTomatoflox/flox mice. Representative flow cytometry analysis of EPCAMPos and CD3Pos, CD45Pos populations selection and further gating of TomNeg TomLow and TomHigh cells. b) qPCR analysis of FACS-based sorted CD31Pos, CD45Pos, TomNeg, TomLow, and TomHigh cells for the expression of Pecam1, Ptprc, Adgre1 and Vim. dCT: delta cycle threshold. Data are presented as mean±SEM. *: p<0.05; **: p<0.01; ***: p<0.001. ERJ-04168-2020.Figure_S1 (315.9KB, jpg)
Supplementary figure S2. Validation of SftpcCreERT2/+; tdTomatoflox/flox mice. a) The pie chart represents the percentage of tdTomatoPos cells in total cells (20.5%) and the percentage of epithelial (96.6%), mesenchymal cells (0.8%), and CD31PosCD45Pos cells (2.6%) of the labeled cells. b) Representative SFTPC immunofluorescence staining and quantification of tdTomatoPosSFTPCPos of total tdTomatoPos as well as quantification of tdTomatoPosSFTPCPos of total SFTPCPos (n=4). Data are presented as mean±SEM. c) Representative flow cytometry analysis of EPCAMPos and display of EPCAM and tdTomato in a single FACS plot. ERJ-04168-2020.Figure_S2 (241.4KB, jpg)
Supplementary figure S3. AT2 cells labeling in the absence or in the presence of tamoxifen treatment. a) Timeline of tamoxifen treatment. One group of SftpcCreERT2/+; tdTomatoflox/flox mice were treated with tamoxifen in drinking water for 7 days and another group with no tamoxifen added to the water. b) Representative flow cytometry analysis of single-cell selection and further analysis of EPCAMPos population in both groups. c) Representative flow cytometry analysis of TomLow (4.5%) and TomHigh (6.9%) in mice in the absence of tamoxifen. The pie chart shows the percentage of TomLow (39.2%) and TomHigh (60.8%) in total tdTomato positive cells. d) Representative flow cytometry analysis of TomLow (10.3%) and TomHigh (44.1%) in mice treated with tamoxifen. Pie chart shows the percentage of TomLow (18.9%) and TomHigh (81.1%) in total tdTomato positive cells. ERJ-04168-2020.Figure_S3 (322.4KB, jpg)
Supplementary figure S4. TomLow and TomHigh AT2 subpopulations in 6- and 9-month old mice. Timeline of tamoxifen treatment of SftpcCreERT2/+; tdTomatoflox/flox mice and lung harvest. Flow cytometry analysis of EPCAMPos cells. Quantification of the percentile of TomLow and TomHigh (over total EPCAMPos cells) in 6 and 9 months old mice. ERJ-04168-2020.Figure_S4 (219.1KB, jpg)
Supplementary figure S5. Gene set enrichment for accessible regions in TomHigh versus TomLow. a) Analysis of peaks obtained in the ATAC-seq experiment for TomHigh and TomLow cells using Kobas for the Reactome database. Peaks overlapping gene body or close to the transcription starting site of genes were annotated to the corresponding genes. All annotated peaks were split into lists of genes that display more open chromatin in TomHigh or TomLow cells using DESeq2 on unified peak regions. Observed significance was adjusted by Benjamini-Hochberg correction for multiple tests (FDR). The resulting lists were used as input for Kobas to search for enriched terms in different databases. Top 10 terms were chosen by significance (FDR <0.2). Results indicate that the term immune system is highly enriched in TomLow cells, indicating that the chromatin of TomLow cells is more accessible in loci of genes (gene body or promoter) associated with the immune system. Higher accessibility is associated with more transcriptional activity. Numbers in brackets display number of identified genes/total the number of genes for a term in the database. DEG: differentially expressed genes. Between brackets: Genes found/total genes in the term. b) Heatmap for differentially expressed genes following a gene array using TomHigh and TomLow cells isolated from SftpcCreERT2/+; tdTomatoflox/flox lungs at homeostasis. ERJ-04168-2020.Figure_S5 (316.7KB, jpg)
Supplementary figure S6. Absence of contaminations in PD-L1Pos AT2 cells. a) Representative flow cytometry analysis of CD31PosCD45Pos (endothelial and haematopoietic) and EPCAMNegCD31NegCD45Neg (mesenchyme) populations as well as further gating of EPCAMPos cells with PDPN to isolate AT2 cells. Negative control for PD-L1 antibody staining to select PD-L1Pos cells. Isolation of PD-L1Pos and PD-L1Neg out of AT2 cells. b) qPCR analysis of FACS-based sorted mesenchymal cells, CD31PosCD45Pos cells, PD-L1Pos and PD-L1Neg AT2 cells for the expression of Pecam1, Ptprc, Adgre1 and Vim. Data are presented as mean±SEM. dCT: delta cycle threshold (Ct). *: p<0.05; **: p<0.01; ***: p <0.001. c) Representative flow cytometry analysis of EPCAMNeg and a further selection of CD31PosCD45Pos populations selection. Further flow cytometry analysis shows that CD31PosCD45Pos populations contain PD-L1Pos cells. Negative control for PD-L1 antibody staining. ERJ-04168-2020.Figure_S6 (364.3KB, jpg)
Supplementary figure S7. Colony-forming capabilities of PD-L1Pos and PD-L1Neg AT2 cells. a) Representative Lysotracker immunofluorescence staining of alveolospheres from AT2 PD-L1Pos and AT2 PD-L1Neg cells after 14 days in culture (Scale bar: 100 μm). b) Quantification of colony-forming efficiency (CFE) of PD-L1Pos and PD-L1Neg cultured cells (n=3). Data are presented as mean±SEM. *: p<0.05; **: p<0.01; ***: p <0.001. ERJ-04168-2020.Figure_S7 (91.6KB, jpg)
Supplementary figure S8. Elevated percentage of PD-L1Pos AT2 cells following pneumonectomy. a) Timeline of lung harvest after Sham or PNX. Representative flow cytometry analysis of wild-type mice lungs showing the gating strategy of EPCAMPos population, followed by negative selection of AT2 cells with the exclusion of AT1 PDPNPos cells. b) Representative flow cytometry analysis of AT2 cells based on PD-L1 marker and negative control of PD-L1 antibody staining. c) Quantification of PD-L1Pos AT2 cells percentages between Sham and PNX groups (n=4). Data are presented as mean±SEM. *: p<0.05; **: p<0.01; ***: p <0.001. ERJ-04168-2020.Figure_S8 (323.8KB, jpg)
Supplementary figure S9. ScRNA-seq analysis of the AT2-proliferative subcluster. a) UMAP plot of the initial AT2 cell cluster indicates the presence of five different AT2 subclusters. b) UMAP plot representation showing PCNA and MKI67 enrichment in the AT2-proliferative subcluster. c) Corresponding Violin plot representation. ERJ-04168-2020.Figure_S9 (767.3KB, jpg)
Supplementary figure S10. Heat map representation of the differences in gene expression (using LFC) between the different AT2 subclusters. a) Heat map from the scRNA-seq analysis of AT2 cells in the adult human lung. b) Note the enrichment in AT2 PD-L1High of genes involved in the immune system (CXCL1, CXCL2, CXCL3, ICAM1, ZC3H12A, IRF1, CXCL8, XBP1) as well as a gene (TM4SF1) previously described to be expressed by AEP cells. ERJ-04168-2020.Figure_S10 (717.7KB, jpg)
Supplementary figure S11. Axin2 expression analysis in TomLow versus TomHigh in homeostasis and in TomLow in PNX and Sham. a) qPCR analysis of FACS-based sorted TomLow and TomHigh cells for the expression of Axin2 in adult mouse lungs. b) qPCR analysis of FACS-based sorted TomLow cells for the expression of Axin2 from sham and PNX lungs at day 7 post-surgery. Data are presented as mean±SEM. *: p<0.05; **: p<0.01; ***: p <0.001. ERJ-04168-2020.Figure_S11 (185.4KB, jpg)
Shareable PDF
Acknowledgement
We thank Stefan Guenther (Bioinformatics and deep sequencing platform at the Max Planck Institute for Heart and Lung) for help in ATAC-seq data analysis. We also thank Kerstin Goth for the animal husbandry and genotyping of the mice.
Footnotes
This article has supplementary material available from erj.ersjournals.com
This article has an editorial commentary: https://doi.org/10.1183/13993003.01417-2021
Conflict of interest: N. Ahmadvand has nothing to disclose.
Conflict of interest: F. Khosravi has nothing to disclose.
Conflict of interest: A. Lingampally has nothing to disclose.
Conflict of interest: R. Wasnick has nothing to disclose.
Conflict of interest: I. Vasquez-Armendariz has nothing to disclose.
Conflict of interest: G. Carraro has nothing to disclose.
Conflict of interest: M. Heiner has nothing to disclose.
Conflict of interest: S. Rivetti has nothing to disclose.
Conflict of interest: Y. Lv has nothing to disclose.
Conflict of interest: J. Wilhelm has nothing to disclose.
Conflict of interest: A. Gunther has nothing to disclose.
Conflict of interest: S. Herold has nothing to disclose.
Conflict of interest: D. Al Alam has nothing to disclose.
Conflict of interest: C. Chen has nothing to disclose.
Conflict of interest: P. Minoo has nothing to disclose.
Conflict of interest: J-S. Zhang has nothing to disclose.
Conflict of interest: S. Bellusci has nothing to disclose.
Support statement: S. Bellusci was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (BE4443/1-1, BE4443/4-1, BE4443/6-1, KFO309 P7 and SFB1213-projects A02 and A04) and the German Lung Center (DZL). P. Minoo and S. Bellusci acknowledge support from the National Heart, lung, and Blood Institute (NHLBI) (HL143059). D. Al Alam acknowledges support from the NHLBI (R01HL141856). J-S. Zhang was funded through a start-up package from Wenzhou Medical University and the National Natural Science Foundation of China (grant number 81472601). S. Herold was supported by the UKGM (FOKOOPV), the DZL and University Hospital Giessen and grants from the DFG (KFO309 P2/8; SFB1021 C05, SFB TR84 B9). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res 2001; 2: 33–46. doi: 10.1186/rr36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014; 507: 190–194. doi: 10.1038/nature12930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkauskas CE, Cronce MJ, Rackley CR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 2013; 123: 3025–3036. doi: 10.1172/JCI68782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawlins EL, Okubo T, Xue Y, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009; 4: 525–534. doi: 10.1016/j.stem.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock JR, Barkauskas CE, Cronce MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA 2011; 108: E1475–E1483. doi: 10.1073/pnas.1117988108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy R, Buckley S, Doerken M, et al. Isolation of a putative progenitor subpopulation of alveolar epithelial type 2 cells. Am J Physiol Lung Cell Mol Physiol 2004; 286: L658–L667. doi: 10.1152/ajplung.00159.2003 [DOI] [PubMed] [Google Scholar]
- 7.Chapman HA, Li X, Alexander JP, et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest 2011; 121: 2855–2862. doi: 10.1172/JCI57673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabhan AN, Brownfield DG, Harbury PB, et al. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 2018; 359: 1118–1123. doi: 10.1126/science.aam6603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zacharias WJ, Frank DB, Zepp JA, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 2018; 555: 251–255. doi: 10.1038/nature25786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank DB, Peng T, Zepp JA, et al. Emergence of a wave of Wnt signaling that regulates lung alveologenesis by controlling epithelial self-renewal and differentiation. Cell Rep 2016; 17: 2312–2325. doi: 10.1016/j.celrep.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005; 121: 823–835. doi: 10.1016/j.cell.2005.03.032 [DOI] [PubMed] [Google Scholar]
- 12.Salwig I, Spitznagel B, Vazquez-Armendariz AI, et al. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J 2019; 38: e102099. doi: 10.15252/embj.2019102099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Liu K, Cui G, et al. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet 2019; 51: 728–738. doi: 10.1038/s41588-019-0346-6 [DOI] [PubMed] [Google Scholar]
- 14.Pawelczyk K, Piotrowska A, Ciesielska U, et al. Role of PD-L1 expression in non-small cell lung cancer and their prognostic significance according to clinicopathological factors and diagnostic markers. Int J Mol Sci 2019; 20: 824. doi: 10.3390/ijms20040824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin G, Fan X, Zhu W, et al. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocyte in surgically resectable non-small cell lung cancer. Oncotarget 2017; 8: 83986–83994. doi: 10.18632/oncotarget.20233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lastwika KJ, Wilson W, Li QK, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res 2016; 76: 227–238. doi: 10.1158/0008-5472.CAN-14-3362 [DOI] [PubMed] [Google Scholar]
- 17.Travaglini KJ, Nabhan AN, Penland L, et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020; 587: 619–625. doi: 10.1038/s41586-020-2922-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler A, Hoffman P, Smibert P, et al. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 2018; 36: 411–420. doi: 10.1038/nbt.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol 2019; 20: 296. doi: 10.1186/s13059-019-1874-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finn J, Sottoriva K, Pajcini KV, et al. Dlk1-mediated temporal regulation of Notch signaling is required for differentiation of alveolar type II to type I cells during repair. Cell Rep 2019; 26: 2942–2954.e5. doi: 10.1016/j.celrep.2019.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leeman KT, Pessina P, Lee JH, et al. Mesenchymal stem cells increase alveolar differentiation in lung progenitor organoid cultures. Sci Rep 2019; 9: 6479. doi: 10.1038/s41598-019-42819-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamoto K, Gibney BC, Ackermann M, et al. Alveolar epithelial dynamics in postpneumonectomy lung growth. Anat Rec (Hoboken) 2013; 296: 495–503. doi: 10.1002/ar.22659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lechner AJ, Driver IH, Lee J, et al. Recruited monocytes and type 2 immunity promote lung regeneration following pneumonectomy. Cell Stem Cell 2017; 21: 120–134. doi: 10.1016/j.stem.2017.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Zhe, Fu Siling, Tang Nan. A standardized method for measuring internal lung surface area via mouse pneumonectomy and prosthesis implantation. J Vis Exp 2017;125:56114. doi: 10.3791/56114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazawa T, Marushima H, Saji H, et al. PD-L1 expression in non-small-cell lung cancer including various adenocarcinoma subtypes. Ann Thorac Cardiovasc Surg 2019; 25: 1–9. doi: 10.5761/atcs.oa.18-00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaughan AE, Brumwell AN, Xi Y, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 2015; 517: 621–625. doi: 10.1038/nature14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015; 89: 181–188. doi: 10.1016/j.lungcan.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Boyle TA, Zhou C, et al. PD-L1 expression in lung cancer. J Thorac Oncol 2016; 11: 964–975. doi: 10.1016/j.jtho.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strunz M, Simon LM, Ansari M, et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun 2020; 11: 3559. doi: 10.1038/s41467-020-17358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods and tables ERJ-04168-2020.Supplement (112.6KB, pdf)
Supplementary figure S1. Absence of contaminations in TomLow and TomHigh subpopulations. a) Timeline of tamoxifen treatment of SftpcCreERT2/+; tdTomatoflox/flox mice. Representative flow cytometry analysis of EPCAMPos and CD3Pos, CD45Pos populations selection and further gating of TomNeg TomLow and TomHigh cells. b) qPCR analysis of FACS-based sorted CD31Pos, CD45Pos, TomNeg, TomLow, and TomHigh cells for the expression of Pecam1, Ptprc, Adgre1 and Vim. dCT: delta cycle threshold. Data are presented as mean±SEM. *: p<0.05; **: p<0.01; ***: p<0.001. ERJ-04168-2020.Figure_S1 (315.9KB, jpg)
Supplementary figure S2. Validation of SftpcCreERT2/+; tdTomatoflox/flox mice. a) The pie chart represents the percentage of tdTomatoPos cells in total cells (20.5%) and the percentage of epithelial (96.6%), mesenchymal cells (0.8%), and CD31PosCD45Pos cells (2.6%) of the labeled cells. b) Representative SFTPC immunofluorescence staining and quantification of tdTomatoPosSFTPCPos of total tdTomatoPos as well as quantification of tdTomatoPosSFTPCPos of total SFTPCPos (n=4). Data are presented as mean±SEM. c) Representative flow cytometry analysis of EPCAMPos and display of EPCAM and tdTomato in a single FACS plot. ERJ-04168-2020.Figure_S2 (241.4KB, jpg)
Supplementary figure S3. AT2 cells labeling in the absence or in the presence of tamoxifen treatment. a) Timeline of tamoxifen treatment. One group of SftpcCreERT2/+; tdTomatoflox/flox mice were treated with tamoxifen in drinking water for 7 days and another group with no tamoxifen added to the water. b) Representative flow cytometry analysis of single-cell selection and further analysis of EPCAMPos population in both groups. c) Representative flow cytometry analysis of TomLow (4.5%) and TomHigh (6.9%) in mice in the absence of tamoxifen. The pie chart shows the percentage of TomLow (39.2%) and TomHigh (60.8%) in total tdTomato positive cells. d) Representative flow cytometry analysis of TomLow (10.3%) and TomHigh (44.1%) in mice treated with tamoxifen. Pie chart shows the percentage of TomLow (18.9%) and TomHigh (81.1%) in total tdTomato positive cells. ERJ-04168-2020.Figure_S3 (322.4KB, jpg)
Supplementary figure S4. TomLow and TomHigh AT2 subpopulations in 6- and 9-month old mice. Timeline of tamoxifen treatment of SftpcCreERT2/+; tdTomatoflox/flox mice and lung harvest. Flow cytometry analysis of EPCAMPos cells. Quantification of the percentile of TomLow and TomHigh (over total EPCAMPos cells) in 6 and 9 months old mice. ERJ-04168-2020.Figure_S4 (219.1KB, jpg)
Supplementary figure S5. Gene set enrichment for accessible regions in TomHigh versus TomLow. a) Analysis of peaks obtained in the ATAC-seq experiment for TomHigh and TomLow cells using Kobas for the Reactome database. Peaks overlapping gene body or close to the transcription starting site of genes were annotated to the corresponding genes. All annotated peaks were split into lists of genes that display more open chromatin in TomHigh or TomLow cells using DESeq2 on unified peak regions. Observed significance was adjusted by Benjamini-Hochberg correction for multiple tests (FDR). The resulting lists were used as input for Kobas to search for enriched terms in different databases. Top 10 terms were chosen by significance (FDR <0.2). Results indicate that the term immune system is highly enriched in TomLow cells, indicating that the chromatin of TomLow cells is more accessible in loci of genes (gene body or promoter) associated with the immune system. Higher accessibility is associated with more transcriptional activity. Numbers in brackets display number of identified genes/total the number of genes for a term in the database. DEG: differentially expressed genes. Between brackets: Genes found/total genes in the term. b) Heatmap for differentially expressed genes following a gene array using TomHigh and TomLow cells isolated from SftpcCreERT2/+; tdTomatoflox/flox lungs at homeostasis. ERJ-04168-2020.Figure_S5 (316.7KB, jpg)
Supplementary figure S6. Absence of contaminations in PD-L1Pos AT2 cells. a) Representative flow cytometry analysis of CD31PosCD45Pos (endothelial and haematopoietic) and EPCAMNegCD31NegCD45Neg (mesenchyme) populations as well as further gating of EPCAMPos cells with PDPN to isolate AT2 cells. Negative control for PD-L1 antibody staining to select PD-L1Pos cells. Isolation of PD-L1Pos and PD-L1Neg out of AT2 cells. b) qPCR analysis of FACS-based sorted mesenchymal cells, CD31PosCD45Pos cells, PD-L1Pos and PD-L1Neg AT2 cells for the expression of Pecam1, Ptprc, Adgre1 and Vim. Data are presented as mean±SEM. dCT: delta cycle threshold (Ct). *: p<0.05; **: p<0.01; ***: p <0.001. c) Representative flow cytometry analysis of EPCAMNeg and a further selection of CD31PosCD45Pos populations selection. Further flow cytometry analysis shows that CD31PosCD45Pos populations contain PD-L1Pos cells. Negative control for PD-L1 antibody staining. ERJ-04168-2020.Figure_S6 (364.3KB, jpg)
Supplementary figure S7. Colony-forming capabilities of PD-L1Pos and PD-L1Neg AT2 cells. a) Representative Lysotracker immunofluorescence staining of alveolospheres from AT2 PD-L1Pos and AT2 PD-L1Neg cells after 14 days in culture (Scale bar: 100 μm). b) Quantification of colony-forming efficiency (CFE) of PD-L1Pos and PD-L1Neg cultured cells (n=3). Data are presented as mean±SEM. *: p<0.05; **: p<0.01; ***: p <0.001. ERJ-04168-2020.Figure_S7 (91.6KB, jpg)
Supplementary figure S8. Elevated percentage of PD-L1Pos AT2 cells following pneumonectomy. a) Timeline of lung harvest after Sham or PNX. Representative flow cytometry analysis of wild-type mice lungs showing the gating strategy of EPCAMPos population, followed by negative selection of AT2 cells with the exclusion of AT1 PDPNPos cells. b) Representative flow cytometry analysis of AT2 cells based on PD-L1 marker and negative control of PD-L1 antibody staining. c) Quantification of PD-L1Pos AT2 cells percentages between Sham and PNX groups (n=4). Data are presented as mean±SEM. *: p<0.05; **: p<0.01; ***: p <0.001. ERJ-04168-2020.Figure_S8 (323.8KB, jpg)
Supplementary figure S9. ScRNA-seq analysis of the AT2-proliferative subcluster. a) UMAP plot of the initial AT2 cell cluster indicates the presence of five different AT2 subclusters. b) UMAP plot representation showing PCNA and MKI67 enrichment in the AT2-proliferative subcluster. c) Corresponding Violin plot representation. ERJ-04168-2020.Figure_S9 (767.3KB, jpg)
Supplementary figure S10. Heat map representation of the differences in gene expression (using LFC) between the different AT2 subclusters. a) Heat map from the scRNA-seq analysis of AT2 cells in the adult human lung. b) Note the enrichment in AT2 PD-L1High of genes involved in the immune system (CXCL1, CXCL2, CXCL3, ICAM1, ZC3H12A, IRF1, CXCL8, XBP1) as well as a gene (TM4SF1) previously described to be expressed by AEP cells. ERJ-04168-2020.Figure_S10 (717.7KB, jpg)
Supplementary figure S11. Axin2 expression analysis in TomLow versus TomHigh in homeostasis and in TomLow in PNX and Sham. a) qPCR analysis of FACS-based sorted TomLow and TomHigh cells for the expression of Axin2 in adult mouse lungs. b) qPCR analysis of FACS-based sorted TomLow cells for the expression of Axin2 from sham and PNX lungs at day 7 post-surgery. Data are presented as mean±SEM. *: p<0.05; **: p<0.01; ***: p <0.001. ERJ-04168-2020.Figure_S11 (185.4KB, jpg)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-04168-2020.Shareable (436.2KB, pdf)