Abstract
This study investigated the fermentation of liquid feed for pigs and the effect of lactic acid bacteria (LAB) supplementation on fermentation rate, dry matter losses (DML), formation of biogenic amines, and degradation of phytate-P. The basal substrate in all three in vitro batch experiments consisted of 50% canola meal, 25% wheat, and 25% barley. The mixed substrates were adjusted to a dry matter (DM) content of 28.4% and fermented in 1-liter vessels at 37 °C for 24 h. Experiment 1 focused on changes in pH profiles over time. Treatments were as follows: 1) liquid feed without additive (control) and 2) liquid feed supplemented with a mixture of Lactobacillus plantarum, Pediococcus pentosaceus, and Lactobacillus lactis (adLAB) at 2.0 × 105 CFU/g liquid feed (wet wt.; n = 8). Substrate pH was measured every 2 h. Experiment 2 focused on DML and the impact of fermentation on phytate-P. Treatments were identical to experiment 1 (control and adLAB; n = 8). Measured parameters included concentration of lactic acid, acetic acid, ethanol, and phytate-P, and DML after 24 h of fermentation. Counts of molds, Enterobobacteriaceae, yeasts, and LAB were determined in one combined sample of all replicates. Dry matter losses were lower in LAB-supplemented fermentations (5.89%) compared to the control (11.8%; P < 0.001). Supplementation with LAB reduced the phytate-P content (2.66 g/kg DM) compared to the control (3.07 g/kg DM; P = 0.002). Experiment 3 evaluated DML and the impact of fermentation on formation of biogenic amines. Treatments were as follows: 1) control, 2) adLAB (2.0 × 105 CFU LAB/g liquid feed), 3) adLys (0.60% DM supplemented lysine), and 4) adLAB+Lys (combination of adLAB and adLys; n = 8). The fermentation of adLys resulted in a nearly complete breakdown of supplemented lysine, whereas only 10% of supplemented lysine was lost in adLAB+Lys. Furthermore, all adLys samples tested positive for cadaverine (mean concentration 0.89% DM), whereas no adLAB samples contained cadaverine above the detection limit (P < 0.001). Results indicate that DML is reduced in fermentations supplemented with homofermentative LAB. Fermentation of liquid feed with homofermentative LAB can effectively reduce the degradation of supplemental lysine and has the potential to further improve P availability.
Keywords: biogenic amines, fermentation, lactic acid bacteria, liquid feed, phytate-phosphorus, pigs
INTRODUCTION
Feeding liquid diets is an established practice in pig production. Major advantages of liquid feed are its generally good palatability, typically positive effect on growth performance and digestive health, higher water intake of weaners, and the reduction of dust particles in the ambient air compared to dry feeding systems (Scholten, 2001; Canibe et al. 2007; Bunte et al. 2019). However, a major concern associated with liquid feed is the risk of contamination with pathogens such as Enterobacteriaceae, yeast, and molds, which find suitable growth conditions, such as low dry matter (DM) content, sufficient quantity of fermentable substrate, and high temperature, in liquid feed (Niven et al., 2006; Canibe et al., 2007; Bunte et al., 2019). This seems particularly problematic if the feed ingredients or the feeding system itself is already contaminated or the feed-out rate of the liquid feed is disproportionately low.
A common approach to improve the hygienic status of liquid feed is controlled fermentation prior to temporary storage and feeding. In controlled fermentation specific microorganisms, like selected lactic acid bacteria (LAB), they are used as inoculum to reduce the substrate pH rapidly. In contrast, uncontrolled fermentation is a spontaneous process carried out only by endogenous, largely epiphytic, microorganisms (Canibe and Jensen, 2012; Kraler et al., 2014). Liquid feed from controlled fermentation can help us to improve gut health, prevent coliform scours, and therefore reduce the need for antibiotic interventions (Canibe and Jensen, 2012). It reduces pH rapidly by particularly effective formation of lactic acid, which typically improves feed hygiene (Beal et al., 2002; Carlson and Daamgaard Poulsen, 2003). Although fermentation of liquid feed has several benefits, it also leads to dry matter losses (DML) as a result of microbial activity, as either aerobic respiration or fermentative losses. For silages as most common feed source conserved through lactic acid fermentation, DML due to fermentation can be expected to be between 9% and 12% (DM basis) in typical farm-scale bunker silos (Köhler et al., 2013). Although obviously fermented liquid feeds for pigs differ in terms of fermentation time (less risk than silage) and nutrient composition (higher risk than silage), some degree of DML should be expected, but little knowledge on the size of such losses in fermented liquid feed exists to date. For fermentations in 50-liter containers over a period of 6 d and at 23 °C, Scholten et al. (2001) reported losses of 5.1% for liquid finisher and 8.6% for liquid grower feed.
To maximize growth performance, pigs require supplementation with essential amino acids (AA), particularly lysine. During uncontrolled fermentation, undesired processes such as decarboxylation of AA to biogenic amines can occur, resulting in reduced palatability, compromised animal health, and economic losses (Niven et al., 2006; Canibe et al., 2007). Niven et al. (2006) reported that in uncontrolled fermentation of liquid feed, 90% of supplemented lysine was lost and predominantly degraded to cadaverine. In contrast, controlled fermentation of liquid feed with either Lactobacillus plantarum or Pediococcus acidlactici did not reduce the concentration of lysine. Pedersen et al. (2002) also reported that fermentation of liquid pig feed without added LAB reduces the concentration of free lysine.
Commercial pig diets are predominantly based on grains. The main storage form of P in cereals is inositol hexaphosphate (phytate-P), which cannot be utilized by pigs due to a lack of endogenous phytase (Nuss and Tanumihardjo, 2010; Humer and Schedle, 2016). Consequently, phytate-P from grain-based diets is largely lost, if pig feed is not supplemented with phytase. It has been reported that—in addition to the effect of a pre-incubation of the feed with endogenous plant phytase—fermentation of liquid feed with LAB can lead to a further reduction in phytate-P and improved availability of P (Humer et al., 2013). The activity of phytase is largely dependent on pH, with optimal ranges between pH 4.0 and 5.5 (Simons et al., 1990; Brejnholt et al., 2011). The innate phytase activity of most LAB is low, but strains of Pediococcus pentosacaeus and Lactobacillus plantarum are reported to express at least some degree of phytase activity (Lopez et al., 2000; Cizeikiene et al., 2015).
There is strong evidence that controlled fermentation of liquid feed with LAB has positive impact on its fermentation characteristics, the stability of lysine, and the availability of P. However, studies examining the effects of controlled fermentation in a comprehensive and systematic fashion are scarce. Therefore, this study was designed to investigate fermentation kinetics, AA and phytate degradation, and DML in response to controlled fermentation of liquid feed using supplemental LAB.
MATERIAL AND METHODS
Experimental Treatments and Analyses
This study consisted of three in vitro experiments. The composition of the tested basal diet was identical in all three experiments (50% solvent-extracted canola meal, 25% wheat, and 25% barley; DM basis). A protein content that exceeded the requirements of growing pigs was chosen to ensure sufficient substrate for possible proteolysis throughout the fermentation process. The same batch of canola meal, wheat, and barley was used in all three experiments. The mixture of dry ingredients was adjusted to a DM content of 28.4% using potable water. In all three experiments, fermentations were conducted under anaerobic conditions at 37 °C in 1-liter screw top vessels.
Experiment 1 was designed to compare pH profiles of liquid feed without and with supplemented LAB. Substrate pH was measured every 2 h for 24 h. Treatments were (n = 8 vessels): 1) liquid feed without additive (control) and 2) liquid feed supplemented with a mixture of LAB (adLAB). The inoculum consisted of approximately equal parts of three strains of homofermentative LAB (Lactobacillus plantarum, Pediococcus pentosaceus, and Lactococcus lactis) and was added at a concentration of 2 × 105 CFU/g liquid feed. Supplemented LAB was produced by Lactosan GmbH and Co. KG (Kapfenberg, Austria).
Experiment 2 focused on the impact of fermentation on phytate-P and DML. Treatments were identical to experiment 1 (control and adLAB). Measured variables included substrate pH (after 0 and 24 h), chemical composition of the non-fermented substrates and fermented liquid feeds, concentrations of lactic acid, acetic acid and ethanol, DML, as well as concentrations of P, and phytate-P (all after 0 and 24 h fermentation). Furthermore, counts of molds, Enterobacteriaceae, LAB, and yeasts were determined in one combined sample for each treatment.
Experiment 3 was designed to examine the impact of fermentation on the development of biogenic amines in fermented liquid feed. Treatments were as follows: 1) liquid feed without additives (control), 2) liquid feed supplemented with LAB (adLAB; 2 × 105 CFU/g liquid feed), 3) liquid feed supplemented with 0.6% DM lysine (adLys), and 4) liquid feed supplemented with LAB and lysine (adLAB+Lys; 2 × 105 CFU/g liquid feed + 0.6% lysine on DM basis). The supplemented crystalline lysine (Archer Daniels Midland, Decatur, IL) had a lysine content of ≥ 78.8% DM. Measured parameters were identical to experiment 2 except that instead of P and phytate-P, concentration of biogenic amines and nonprotein bound lysine was determined in all samples before the onset of fermentation (0 h) and the end of fermentation (24 h).
Chemical Composition Analyses
Chemical composition of the substrate ingredients and fermented liquid feed was analyzed according to standardized methods of the Association of German Agricultural Analytic and Research Institutes (VDLUFA, 2012). Dry matter content of the fermented feed was determined by weighing 200 g of homogenized sample into an aluminum dish. Samples were dried in a forced air oven at 65 °C (method 4.3.1). Analytical DM of the pre-dried fermented feed and the dry ingredients was determined at 103 °C (method 4.2.1). Dry matter contents were corrected for the loss of ethanol, volatile fatty acids (VFA), and lactic acid according to CVB (1999): correctedDM (g/kg) = DM (g/kg) + 0.08 × lactic acid content (g/kg) + 0.50 × VFA content (g/kg) + 1.00 × ethanol content (g/kg). Contents of volatiles were measured in fresh material.
Fat was analyzed according to VDLUFA (2012; method 5.1.1). Neutral detergent fiber (NDF) was determined as described by Van Soest et al. (1991) using heat-stable α-amylase. Acid detergent fiber (ADF) was determined according to VDLUFA (2012; method 6.5.2). The NDF and ADF values were expressed inclusive of residual ash. Sugar was analyzed according to VDLUFA (2012; method 7.1.1). Starch was analyzed according to VDLUFA (2012; method 7.2.1). Ash was analyzed according to VDLUFA (2012; method 8.1). Organic matter (OM) was calculated as the difference between 100 and the percentage of ash. Crude protein (CP) was analyzed by Kjehldahl titration according to VDLUFA (2012; method 4.1.1). Chemical analyses of the feed ingredients and fermented substrates were performed on one pooled sample per treatment (Tables 1 and 3). Each sample was analyzed in duplicates. Substrate pH was measured using a pH-Meter (model pH 7310 with pH-electrode Sentix 21, Wissenschaftlich-Technische Werkstätten GmbH, Weilheim, Germany). To determine DML, each vessel was weighed at the beginning (0 h) and end of the fermentation (24 h).
Table 1.
Chemical composition of the diet ingredients
| Item | Diet ingredients | ||
|---|---|---|---|
| Wheat | Barley | Canola meal1 | |
| DM,% | 89.1 | 89.8 | 89.8 |
| OM,%DM | 86.5 | 87.3 | 82.3 |
| CP, % DM | 15.6 | 11.3 | 35.2 |
| NDF2, % DM | 15.1 | 20.7 | 31.5 |
| ADF3, % DM | 4.2 | 9.4 | 22.9 |
| Fat, % DM | 2.3 | 2.8 | 3.1 |
| Sugar, % DM | 3.0 | 2.2 | 8.0 |
Solvent-extracted.
Neutral detergent fiber assayed with a heat-stable amylase and expressed without residual ash.
Acid detergent fiber without residual ash.
Table 3.
Fermentation products, pH, phytate-P degradation, and dry matter losses of liquid feed without additive (control) and supplemented lactic acid bacteria (adLAB) after 24 h of incubation (experiment 2; n = 8)
| Item | Treatment | SEM2 | P-value | |
|---|---|---|---|---|
| Control | adLAB1 | |||
| Lactic acid, % DM | 3.59 | 6.23 | 0.203 | < 0.001 |
| Acetic acid, % DM | 1.10 | 0.16 | 0.091 | < 0.001 |
| Ethanol, % DM | 0.60 | 0.09 | 0.062 | < 0.001 |
| pH | 4.35 | 3.80 | 0.040 | < 0.001 |
| Phytate-P, g/kg DM | 3.07 | 2.66 | 0.137 | 0.002 |
| Dry matter loss, % | 11.8 | 5.89 | 1.136 | 0.005 |
Supplemented with a mixture of Lactobacillus plantarum, Pediococcus pentosaceus, Lactococcus lactis (Lactosan GmbH and Co. KG, Kapfenberg, Austria) at 2 × 105 CFU/g liquid feed (wet wt.).
Standard error of the means.
Lactic acid, acetic acid, and ethanol were analyzed from each vessel incubated in experiments 2 and 3 by high-performance liquid chromatography (HPLC). The HPLC system was equipped with a UV detector (Smartline 2500), refractive index detector (Smartline 2300), column thermostat (model Jetstream 2, all Bio Rad Laboratories, Hercules, CA), and Aminex HPX-87H-column (300 × 7.8 mm, Bio Rad Laboratories, Hercules, CA). Sulfuric acid (0.02 N) was used as a mobile phase was with a flow rate of 0.6 mL/min.
Microbial Analysis
Counts of LAB, molds, yeasts, and Enterobacteriaceae were quantified on one pooled sample for each treatment in experiments 2 and 3. The fermentation vessels were subsampled and 30 g of freshly pooled sample was mixed with 270 mL of buffer. For LAB enumeration, samples (1 mL) were plated on MRS agar (Merck KGaA, Darmstadt, Germany). Plates were incubated under anaerobic conditions (using an overlayer) at 37 °C for 3 d. Counts of yeasts and molds (1 mL each) were determined on YGC agar (yeast extract glucose chloramphenicol agar; Oxoid, Hampshire, UK) according to VDLUFA (2012; method 28.1.2). Plates were incubated under anaerobic conditions at 30 °C for 48 h. Violet Red Bile Glucose Agar (Oxoid, Hampshire, UK) was used for determination of Enterobacteriaceae. Samples (1 mL) were incubated under anaerobic conditions at 30 °C for 24 h.
Analysis of Phytate-P
Concentrations of phytate-P were analyzed in the unfermented (one pooled sample per treatment) and fermented substrates (individual vessels) of experiment 2. The content of phytate-P was analyzed enzymatically according to McKie and McCleary (2016). Briefly, phytic acid was dissolved from the samples by acid extraction. Subsequently, samples were dephosphorylized with phytase and alkaline phosphatase. Phosphorus released from phytic acid was quantified by a colorimetric molybdenum blue assay.
Analysis of Biogenic Amines and Lysine
Biogenic amines, cadaverine, and free lysine were analyzed from individual vessels incubated in experiment 3. Analysis of biogenic amines was performed by gas chromatography-mass spectrometry (GC-MS). Samples (25 g) of liquid feed were extracted with 125 mL 10% trichloracetic acid. The extract was filtered before 500 µL of the filtrate was blended with 375 µL distilled H2O and 100 µL 1.7 diaminoheptane as an internal standard. The extract was purified over a solid phase and derivatized followed by liquid extraction with iso-octane. The organic phase was separated and dried using N gas. The residue was resuspended in a mixture of 100 µL 80% iso-octane and 20% chloroform (wt/vol) and injected into a GC (CP—3800 GC, Varian Inc., Palo Alto, CA) coupled with an MS (Saturn 2200, Varian Inc., Palo Alto, CA). A 5-point regression calibration was developed before any measurements were taken. Biogenic amines and cadaverine were analyzed by LKS Lichtenwalde (Niederwiesa, Germany). Free lysine was determined according to the European Commission (2009; regulation 152/2009). Free AA were extracted with 0.1 mol HCl/l. Norleucin was used as an internal standard in all samples. Lysine analyses were performed by LUFA-ITL (Kiel, Germany).
Statistical Analyses
Data were analyzed using the mixed model procedure (PROC MIXED) of SAS 9.4 (version 9.1; SAS Institute, Inc., Cary, NC). Fermentation vessels were the experimental unit for all variables. In all three experiments, eight vessels were incubated per treatment. The model for analysis of pH development over time in experiment 1 included the fixed effect of treatment (control and adLAB), sampling time (0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 h after onset of fermentation), and the interaction of time × treatment. Fermentation vessel was included as a random effect. Sampling time was treated as a repeated measure. Treatment differences between least squares means for each time point were determined by the PDIFF option with the Tukey`s adjustment. Design and treatments of experiment 2 were identical to experiment 1, except that all variables were only measured once after 24 h of fermentation. Consequently, sampling time and the interaction of time × treatment were not included in the model statement. Differences between least squares means were determined by the PDIFF option with the Tukey’s adjustment. Experiment 3 was analyzed using PROC MIXED with the fixed effects LAB addition, lysine addition, and the interaction of LAB × lysine. Fermentation vessel was considered as a random effect. Similar to experiments 1 and 2, treatment differences were determined by the PDIFF option with the Tukey’s adjustment. Denominator degrees of freedom were estimated using the Kenward–Roger option in the model statement. As concentrations of free lysine, cadaverine, and biogenic amines were below detection limits of < 0.05, < 0.14, and < 0.04 mg/kg DM, respectively, in the majority of samples, PROC GLIMMIX was used to compare the number of samples positive for lysine, cadaverine, and biogenic amines among treatments (Table 6). For all parameters, significance was declared at P < 0.05 with trends discussed at 0.05 ≤ P ≤ 0.10. Since counts of LAB, molds, yeasts, and Enterobacteriaceae in experiments 2 and 3 were quantified on only one pooled sample for each treatment, results were not subjected to statistical analysis.
Table 6.
Free lysine, cadaverine and biogenic amines in liquid feed fermented without additive (control), supplemented lactic acid bacteria (adLAB), lysine (adLys), lactic acid bacteria, and lysine (adLAB+Lys) for 24 h (n = 8; mean concentration ± SD in parentheses)
| Item | Treatment | P-value | |||
|---|---|---|---|---|---|
| Control | adLAB1 | adLys2 | adLAB1+Lys2 | ||
| Free lysine, n above DL3/total n | 0/8 | 0/8 | 0/8 | 8/8 (0.54 ± 0.024%) | < 0.001 |
| Cadaverine, n above DL4/total n | 0/8 | 0/8 | 8/8 (0.89 ± 0.510%) | 0/8 | < 0.001 |
| Biogenic amines, n above DL5/total n | 0/8 | 0/8 | 8/8 (0.97 ± 0.491%) | 4/8 (0.18 ± 0.020%) | < 0.001 |
Supplemented with a mixture of Lactobacillus plantarum, Pediococcus pentosaceus, and Lactococcus lactis (Lactosan GmbH and Co. KG, Kapfenberg, Austria) at 2 × 105 CFU/g liquid feed (wet wt.).
Supplemented with 0.60% lysine on a DM basis (Archer Daniels Midland, Decatur, IL).
Detection limit < 0.05 mg/kg DM.
Detection limit < 0.14 mg/kg DM.
Detection limit < 0.04 mg/kg DM.
RESULTS
Differences in nutrient composition among treatments (experiments 2 and 3) after 24 h of fermentation were within narrow ranges (Table 2). The sugar content in all fermented feeds was approximately 10 times lower (< 0.5 to 0.6% DM) compared to the nonfermented feed (5.3% DM, data not shown).
Table 2.
Nutrient composition of liquid feeds fermented for 24 h without additive (control), supplemented lactic acid bacteria (adLAB), supplemented lysine (adLys), and lactic acid bacteria and lysine (adLAB+Lys)
| Item1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| Control | adLAB2 | Control | adLAB2 | adLys3 | adLAB2+Lys3 | |
| DM,% | 23.8 | 24.1 | 24.0 | 24.6 | 23.5 | 23.7 |
| OM, % DM | 79.4 | 78.4 | 80.0 | 81.3 | 78.7 | 79.3 |
| CP, % DM | 23.7 | 24.2 | 4.0 | 23.9 | 24.2 | 24.4 |
| NDF4, % DM | 25.3 | 24.5 | 25.0 | 25.8 | 24.5 | 24.7 |
| ADF5, % DM | 16.0 | 15.4 | 15.8 | 16.1 | 15.3 | 15.5 |
| Fat, % DM | 2.9 | 2.8 | 3.0 | 2.8 | 3.0 | 3.0 |
| Starch, % DM | 46.0 | 46.8 | 47.3 | 49.5 | 46.6 | 47.1 |
| Sugar, % DM | < 0.5 | < 0.5 | 0.5 | < 0.5 | 0.6 | < 0.5 |
Derived from one pooled sample per treatment.
Supplemented with a mixture of Lactobacillus plantarum, Pediococcus pentosaceus, Lactococcus lactis (Lactosan GmbH and Co. KG, Kapfenberg, Austria) at 2 × 105 CFU/g liquid feed (wet wt.).
Supplemented with 0.60% lysine on a DM basis (Archer Daniels Midland, Decatur, IL).
Neutral detergent fiber assayed with a heat-stable amylase and expressed without residual ash.
Acid detergent fiber without residual ash.
Experiment 1
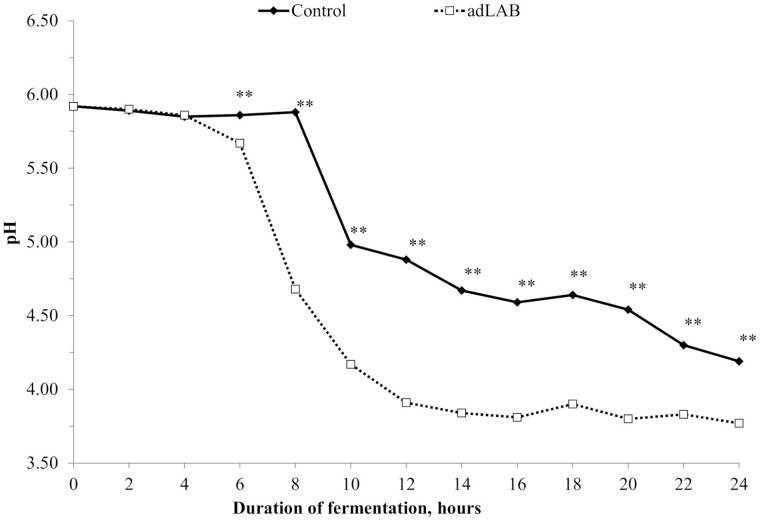
In experiment 1, initial pH (0 h) in both treatments was 5.92 (Figure 1). No differences between the control and adLAB occurred during the first 4 h of fermentation. After 6 h, pH in liquid feed supplemented with LAB (pH 5.67) was reduced by 0.19 units compared to that of the control (pH 5.86; P < 0.001). After 12 h of fermentation, mean pH of adLAB was 3.91 compared to 4.88 in the control (P < 0.001). After 24 h of fermentation, the pH of adLAB (pH 3.77) was still lower compared to the control (pH 4.19; P < 0.001).
Figure 1.
Substrate pH of liquid feed fermented without additive (control) or supplemented with lactic acid bacteria (adLAB) for 24 h (n = 8). Asterisks indicate differences between treatments at the respective time point (P < 0.001).
Experiment 2
Similar to experiment 1, control and adLAB in experiment 2 had an initial pH of 5.93 (data not shown). The addition of LAB resulted in a greater reduction in pH (pH 3.80) compared to the control (pH 4.35) after 24 h (Table 3; P < 0.001). The addition of LAB resulted in higher concentration of lactic acid (6.23% DM) compared to the control (3.59% DM; P < 0.001). The concentration of acetic acid in the control (1.10% DM) was more than six times higher than that in adLAB (0.16% DM; P < 0.001). The concentration of ethanol in adLAB (0.09% DM) was lower compared to the control (0.60% DM; P < 0.001).
Dry matter losses were lower for adLAB (5.89%) compared to the control (11.8%; P < 0.001; Table 3). The phytate-P content of the feed before fermentation was 3.98 g/kg DM (data not shown). In both treatments, the phytate-P content decreased in response to fermentation, whereas adLAB resulted in lower residual concentration of phytate-P (2.66 g/kg DM) compared to the control (3.07 g/kg DM; P = 0.002).
All microbial analyses were performed on one pooled sample per treatment. Control and adLAB had initial LAB counts of 4.0 and 3.8 × 104 CFU/g liquid feed, respectively (Table 4). After 24 h of fermentation, counts of LAB in one combined adLAB sample were 6.7 × 107 CFU/g liquid feed in the adLAB treatment and 5.8 × 106 CFU/g liquid feed in the control. Initial mold and yeast counts were in the order of 10² CFU/g liquid feed in both treatments. After fermentation, concentration of molds decreased under the detection limit (< 10² CFU/g liquid feed) for both treatments. Counts of yeasts in adLAB were 4.0 × 10² CFU/g liquid feed and 9.9 × 10³ CFU/g liquid feed in the control. Counts of Enterobacteriaceae in the nonfermented substrate were 1.3 × 10³ and 1.5 × 103 CFU/g liquid feed for the control and adLAB. After fermentation, counts of Enterobacteriaceae in the control were 1.5 × 10³ CFU/g liquid feed, whereas in adLAB, counts of Enterobacteriaceae were < 10² CFU/g liquid feed.
Table 4.
Counts of lactic acid bacteria (LAB), yeasts, molds, and Enterobacteriaceae (all CFU/g) in liquid feed (wet wt.) without additive (control), supplemented LAB (adLAB), lysine (adLys), and LAB and lysine (adLAB+Lys) prior (0 h) and after 24 h of fermentation (n = 1)
| Item | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| Control | adLAB1 | Control | adLAB1 | adLys2 | adLAB1 +Lys2 | |
| Lactic acid bacteria | ||||||
| 0 h3 | 4.0 × 104 | 3.8 × 104 | 4.2 × 104 | 4.0 × 104 | 3.9 × 104 | 3.8 × 104 |
| 24 h | 5.8 × 106 | 6.7 × 107 | 5.6 × 106 | 6.6 × 108 | 5.5 × 106 | 6.9 × 108 |
| Yeasts | ||||||
| 0 h | 6.0 × 102 | 7.0 × 102 | 7.0 × 102 | 9.0 × 102 | 6.0 × 10² | 1.0 × 103 |
| 24 h | 9.9 × 103 | 4.0 × 102 | 9.7 × 103 | 3.7 × 10² | 1.0 × 104 | 5.0 × 10² |
| Molds | ||||||
| 0 h | 3.0 × 10² | 5.0 × 10² | 7.0 × 10² | 1.0 × 10³ | 3.5 × 10² | 6.0 × 10² |
| 24 h | < 10² | < 10² | < 10² | < 10² | < 10² | < 10² |
| Enterobacteriaceae | ||||||
| 0 h | 1.3 × 10³ | 1.5 × 10³ | 2.1 × 10³ | 1.8 × 10³ | 2.3 × 10³ | 1.5 × 10³ |
| 24 h | 1.5 × 10³ | < 10² | 2.2 × 10³ | < 10² | 2.5 × 10³ | < 10² |
Supplemented with a mixture of Lactobacillus plantarum, Pediococcus pentosaceus, Lactococcus lactis (Lactosan GmbH and Co. KG, Kapfenberg, Austria) at 2 × 105 CFU/g liquid feed (wet wt.).
Supplemented with 0.60% lysine on a DM basis (Archer Daniels Midland, Decatur, IL).
Enumerated prior to inoculation (0 h).
Experiment 3
As in experiment 2, LAB, yeasts, molds, and Enterobacteriaceae in experiment 3 were enumerated in only one pooled sample for each treatment (Table 4). The results were similar to experiment 2 and were foremost affected by the supplementation with LAB.
Substrate pH levels of adLAB (pH 3.75) and adLAB+Lys (pH 3.76) were lower compared to the control (pH 4.19) and adLys (pH 4.23), respectively (P < 0.001; Table 5). Lactic acid concentrations in adLAB (6.30% DM) and adLAB+Lys (6.29% DM) were higher compared to the control (4.48% DM) and adLys (4.40% DM), respectively (P < 0.001). The addition of LAB (adLAB and adLAB+Lys) resulted in a lower concentration of acetic acid (0.16% DM) compared to the control (0.82% DM) and adLys (0.74% DM; P < 0.001). Supplementation of only added lysine (adLys) tended to reduce acetic acid compared to the control (P = 0.061). Similar to that, adLAB (0.08% DM) and adLAB+Lys (0.07% DM) both reduced the concentration of ethanol compared to the control (0.76% DM) and adLys (0.81 % DM; P < 0.001). A trend for higher concentrations of acetic acid was observed in response to adLys compared to the control (P = 0.067). The DML of adLAB (8.11%) and adLAB+Lys (6.73%) was lower compared to the control (15.6%), as well as adLys (14.5%; P < 0.001), whereas the addition of lysine had no impact on DML.
Table 5.
Fermentation products, pH, and dry matter losses (experiment 3) from liquid feed without additive (control), supplemented lactic acid bacteria (adLAB), lysine (adLys), and lactic acid bacteria and lysine (adLAB+Lys) after 24 h of incubation (n = 8)
| Item | Treatment | SEM3 | P-values | |||||
|---|---|---|---|---|---|---|---|---|
| Control | adLAB1 | adLys2 | adLAB1 +Lys2 | LAB | Lys | LAB × Lys | ||
| Lactic acid, % DM | 4.48b | 6.30a | 4.40b | 6.29a | 0.30 | < 0.001 | 0.172 | 0.301 |
| Acetic acid, % DM | 0.82a,4 | 0.16b | 0.74a,4 | 0.16b | 0.019 | < 0.001 | 0.072 | 0.072 |
| Ethanol, % DM | 0.76a,5 | 0.08b | 0.81a,5 | 0.07b | 0.016 | < 0.001 | 0.140 | 0.040 |
| pH | 4.19a | 3.75b | 4.23a | 3.76b | 0.013 | < 0.001 | 0.030 | 0.310 |
| Dry matter losses, % | 15.6a | 8.12b | 14.5a | 6.73b | 1.003 | < 0.001 | 0.224 | 0.889 |
Supplemented with a mixture of Lactobacillus plantarum, Pediococcus pentosaceus, Lactococcus lactis (Lactosan GmbH and Co. KG, Kapfenberg, Austria) at 2 × 105 CFU/g liquid feed (wet wt.).
Supplemented with 0.60% lysine on a DM basis (Archer Daniels Midland, Decatur, IL).
Standard error of the means.
Control vs. adLys, P = 0.061.
Control vs. adLys, P = 0.067.
Means with different superscript letters are significantly different.
After 24 h, no free lysine was detected in any adLys samples (Table 6). In contrast, all eight adLAB+Lys samples contained free lysine (mean concentration 0.54% DM). The concentration of cadaverine before fermentation was below the detection limit in all samples. After fermentation, all eight adLys samples contained cadaverine (mean concentration 0.89% DM). Concentrations of cadaverine in all other samples were below the detection limit. After fermentation, the number of samples that contained biogenic amines (8 out of 8) was higher for adLys compared to adLAB+Lys (4 out of 8; P < 0.001). Mean concentration of biogenic amines was 0.97% DM in adLys and 0.18% DM in adLAB+Lys.
DISCUSSION
This study confirms that fermentation of liquid feed with added homofermentative LAB (Lactobacillus plantarum, Pediococcus pentosaceus, and Lactococcus lactis) is advantageous compared to subjecting liquid feed to an uncontrolled fermentation prior to feeding. After a lag time of approximately 4 h after inoculation, the added LAB became metabolically active as evident by the initial decline in pH (Figure 1). In adLAB, the decline in pH occurred 6 to 8 h after the onset of fermentation, 2 h earlier than in the control (8 to 10 h), and after 24 h, mean pH of adLAB was 3.77 as compared to pH 4.19 in the control. Similarly, mean substrate pH after 24 h in experiments 2 and 3 was 3.80 in adLAB and adLAB+Lysine, respectively, but > 4.0 in the control and adLys. The pH values after 24 h of fermentation are in line with earlier studies reporting pH for noninoculated liquid diets and in response to inoculation of liquid diets with LAB (Scholten et al., 2001; Carlson and Damgaard Poulsen, 2003; Canibe et al., 2007). To the best of our knowledge, patterns of pH decline over time in response to LAB addition (experiment 1) have not been reported so far. Lactic acid bacteria use simple carbohydrates as a main substrate, as evident by the greatly reduced sugar content of the feed after fermentation (Table 2). However, differences in sugar content among treatments were only observed in experiment 3, with numerically lower residual sugar levels in adLAB and adLAB+Lys. Lactic acid bacteria predominantly convert monosaccharides to lactic acid, but also to small amounts of acetic acid and CO2 (Scholten, 2001).
The fermentation of liquid feeds can be expected to reduce the microbial contamination of the feed, but commonly also results in a decrease of pH in the gastrointestinal tract, which can stabilize gut health (Canibe and Jensen 2003; Bunte et al., 2019). Typically, pH < 4.5 prevent the growth of undesired microorganisms such as enteropathogenic E. coli, Salmonella, and Klebsiella in the gut (Scholten et al., 1999; Merrell and Camilli, 2002). Enteropathogenic E. coli can cause neonatal and post-weaning diarrhoea in pigs but also have zoonotic potential (Fairbrother et al., 2005). Furthermore, low pH in the gastrointestinal tract will promote the activity of pepsin, which improves the utilization of proteins (Scholten et al., 1999). When investigating the fermentation profiles in detail, it is interesting to note that in the samples without added LAB, a second, much less pronounced drop in pH appears to be present after approximately 18 h of fermentation. This effect was present in all eight replicates. It can only be hypothesized that additional substrate becomes available and initiates further microbial activity around this time point. However, despite this second decrease, the low pH of adLAB was still not reached by the control within 24 h.
The results of analysis of the free lysine, cadaverine, and biogenic amines indicate that the inoculation with LAB can greatly reduce the risk of microbial breakdown of free AA in liquid feed (Table 6). The supplemented lysine in the adLAB+Lys treatment was almost entirely preserved and the adLAB+Lys samples contained no cadaverine and only low concentrations of biogenic amines. In contrast, no free lysine was detected in adLys; instead, all eight samples contained biogenic amines and cadaverine. This is in agreement with Kramer et al. (2015) who reported that free AA, like crystalline lysine, were entirely lost during fermentation of liquid feed without added LAB. The main pathway for lysine catabolism in liquid feed is the microbial decarboxylation with the formation of biogenic amines, such as cadaverine as a main end product (Shelef et al., 1998; Marino et al., 2000). Enterobacteria, which have high proteolytic potential, are frequently present in unfermented liquid feed (Jensen and Mikkelsen, 1998). Lactic acid concentration between 75 and 100 mM inhibits the growth of Enterobacteria such as E. coli in liquid feed (Beal et al., 2002), but evidently the concentration of lactic acid in the adLys treatment was not high enough to suppress proteolytic activity throughout the entire length of fermentation. To avoid the risk of microbial decarboxylation, it is recommended that supplemental AA are added to the ration after the fermentation. However, even if AA are added to the diet before feeding, the bulk of the fermented liquid feed will stay in the feeding system for some time before consumption, which might still lead to a loss of free AA, if the pH of the liquid feed is not low enough to suppress any proteolytic activity. In addition to the economic loss caused by the breakdown of supplemented AA, formation of biogenic amines also reduces the palatability of fermented liquid feed and represents a considerable toxicological risk (Niven et al., 2006).
In addition to biogenic amines, ethanol is another substance with potential to decrease the palatability of liquid feed (Jensen and Mikkelsen, 1998). Increased concentrations of ethanol in treatments without added LAB (control and adLys) are in agreement with numerically higher yeast counts in the combined samples of replicates without added LAB. The greater presence of yeast led to the higher concentration of ethanol in the control and adLys. Besides the production of ethanol, the presence of yeast can also lead to the formation of other substances with negative impact on feed intake, like amyl alcohol (Jensen and Mikkelsen, 1998; Missotten et al., 2015).
The present study also demonstrates the positive effect of LAB addition on the availability of P (Table 3). The phytate-P content of the unfermented diet was 3.98 g/kg DM (data not shown). Fermentation reduced the initial phytate-P content in the control and adLAB treatment by 22.8% and 33.1%, respectively. Two main effects will have contributed to the partial breakdown of a phytate-P. Firstly and most importantly, the moistening and soaking of the material already lead to a considerable degree of phytate degradation. Secondly, this effect appeared to be further augmented by the LAB supplementation, probably through a faster and lower decrease in pH. In this study, it has to be suspected that the majority of endogenous phytase activity came from wheat and barley grain, whereas canola meal only has limited phytase activity. Endogenous phytase activity can be expected to vary between 800 and 1200 phytase units (FTU) per kg in wheat and between 400 and 600 FTU/kg in barley; phytase activity in canola meal is low (16 FTU/kg; Gesellschaft für Ernährungsphysiologie, 1994; Eeckhout and De Paepe, 1994). Carlson and Daamgaard Poulsen (2003) also measured the concentrations of phytate in liquid feed (containing mainly barley or wheat) in an in vitro experiment; and reported that up to 79% of total phytate was degraded within the first 8 h of pre fermentation. The innate phytase activity of the added LAB strains might have contributed to the reduction in phytase-P in our experiment, but the activation of native phytase by moisture and low pH is relatively effective (Kozlowska et al., 1996), and therefore the most likely explanation for the majority of the observed reduction in phytate-P. Greater degradation of phytate-P in adLAB as compared to the control can be explained by the extended time adLAB samples spent under more acidic pH conditions, which are favorable for most phytases (De Angelis et al., 2003; Brejnholt et al., 2011). The finding that fermentation of liquid pig feed has the capacity to reduce phytate-P has already been reported by others (Carlson and Damgaard Poulsen, 2003). In line with those previous reports, it can be suspected that the need for P supplementation in pigs offered prefermented liquid feed could potentially be decreased, due to increased availability of P. The prefermentation of liquid feed might therefore be particularly attractive in organic farming programs that do not allow usage of in-feed phytase. A further interesting aspect may be the supplementation of exogenous phytases at the beginning of fermentation, which could result in a further increase in P availability due to the extended incubation time at near optimal pH for phytase activity.
Even though homofermentative lactic acid fermentation is commonly associated with lower DML compared to heterofermentative fermentation, some DML is practically unavoidable. For example, 10% DML are reported for corn silage and 9% for grass silage when stored in bunker silos from beginning of fermentation until feed-out (Köhler et al., 2013). Causes of DML during fermentation are losses from aerobic respiration due to residual oxygen at the start of the fermentation, and losses occurring during anaerobic fermentation. Even though the basic process of fermentation of liquid feed will be similar to those in silages, there are significant differences between those two substrate groups. In liquid pig feed, the fermentation process is much shorter, the DM content is lower, and it contains higher concentrations of degradable nutrients. In the present study, average DML of all treatments were roughly within the range described for silages (14% for fermentations without added LAB and 7% for fermentations with added LAB; averages across treatments). These values account for losses of volatiles according to CVB (1999). The finding that fermentation with added LAB resulted in lower DML is expected, as the conversion of fermentable carbohydrates into lactic acid is associated with less energetic and mass loss compared to the formation of other end products like ethanol and acetic acid. Both ethanol and acetic acid concentrations were higher in the control and adLys compared to adLAB and adLAB+Lys. Relatively few studies quantified DML during the process of liquid feed fermentation. Moran (2001) reported a DML of 12% for a grain-based diet that was inoculated with Lactobacillus plantarum and fermented for 4 d at ambient temperature in a closed tank. In contrast, Scholten et al. (2001) reported lower DML of 5.1% for a liquid grower and 8.6% DML for a finisher diet after 6 d of fermentation at 23 °C (45 kg feed batches in 50-liter PVC tanks). A direct comparison of DML among studies is challenging since the composition of the diets, fermentation conditions, and the length of fermentation differ substantially among studies. It is also uncertain how closely DML values generated under experimental conditions are reflective of actual DML of liquid feed systems on-farm. Small bench scale approaches like the one chosen in our study (1-liter vessels) might produce larger DML compared to large-scale fermentation tanks, as the large surface-to-volume ratios of small vessels may increase the availability of oxygen. However, the favorable hygienic conditions of lab scale fermentations could also lead to lower DML compared to on-farm, as the risk of contamination with undesired microorganisms is lower. In any case, the results of DML prompt further investigations as their magnitude seems to be sizable even under ideal conditions (low level of microbial contamination, inoculation with LAB, and relatively rapid drop in pH). Better knowledge of the causes and extent of DML could help us to justify the investment in inoculants and improve the quality of fermented liquid feed. Other factors that will need to be considered in a cost benefit analysis of controlled fermentation of liquid feed are the positive impact on feed hygiene and palatability and potential cost savings due to the reduced need of phytase supplementation. Although controlled fermentation of liquid feed also seems to have a positive impact on animal health (Bunte et al., 2019), the impact on feed conversion efficiency is not consistent among studies and should be further investigated (Mikkelsen and Jensen 1998; Canibe et al., 2007; Bunte et al., 2019).
Overall, inoculation of liquid pig feed with LAB prior to fermentation led to a faster reduction in pH compared to the noninoculated control. This study is one of the few to report DML in response to fermentation; DML decreased from approximately 14% to 7% when supplemented with LAB as an inoculant. Despite a lag time of 6 h for any reduction in pH, fermentation with LAB also reduced the breakdown of supplemented lysine to biogenic amines. In addition, LAB-controlled fermentation increases availability of phytate-P compared to fermentation without supplemented LAB.
ACKNOWLEDGMENTS
This study was funded by the Irene Schaumann Research Society. We thank Reza Sharifi for his support in data analysis. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the Göttingen University.
LITERATURE CITED
- Beal, J. D., Niven S. J., Campbell A., and Brooks P. H.. . 2002. The effect of temperature on the growth and persistence of Salmonella in fermented liquid pig feed. Int. J. Food Microbiol. 79:99–104. doi: 10.1016/s0168-1605(02)00183-6. [DOI] [PubMed] [Google Scholar]
- Brejnholt, S. M., Dionisio G., Glitsoe V., Skov L. K., and Brinch-Pedersen H.. . 2011. The degradation of phytate by microbial and wheat phytases is dependent on the phytate matrix and the phytase origin. J. Sci. Food Agric. 91:1398–1405. doi: 10.1002/jsfa.4324. [DOI] [PubMed] [Google Scholar]
- Bunte, S., Grone R., and Kamphues J.. . 2019. The “controlled fermentation” as feeding concept for pigs—a characterization from the point of view of animal nutrition and veterinary medicine. Übers. Tierernäh. 43:165.– . [in German, English summary] [Google Scholar]
- Canibe, N., and Jensen B. B.. . 2003. Fermented and nonfermented liquid feed to growing pigs: effect on aspects of gastrointestinal ecology and growth performance. J. Anim. Sci. 81:2019–2031. doi: 10.2527/2003.8182019x. [DOI] [PubMed] [Google Scholar]
- Canibe, N., and Jensen B. B.. . 2012. Fermented liquid feed – microbial and nutritional aspects and impact on enteric diseases in pigs. Anim. Feed Sci. Technol. 173:17–40. doi: 10.1016/j.anifeedsci.2011.12.021. [DOI] [Google Scholar]
- Canibe, N., Virtanen E., and Jensen B. B.. . 2007. Microbial and nutritional characteristics of pig liquid feed during fermentation. Anim. Feed Sci. Technol. 134:108–123. doi: 10.1016/j.anifeedsci.2006.05.005. [DOI] [Google Scholar]
- Carlson, H., and Damgaard Poulsen H.. . 2003. Phytate degradation in soaked and fermented liquid feed—effect of diet, time of soaking, heat treatment, phytase activity, pH and temperature. Anim. Feed Sci. Technol. 103:141–154. doi: 10.1016/S0377-8401(02)00288-2. [DOI] [Google Scholar]
- Cizeikiene, D., Huodeikiene G., Bartkiene E., Damasius J., and Paskevicius A.. . 2015. Phytase activity of lactic acid bacteria and their impact on the solubility of minerals from wholemeal wheat bread. Int. J. Food Sci. Nutr. 66:736–742. doi: 10.3109/09637486.2015.1088939. [DOI] [PubMed] [Google Scholar]
- CVB , 1999. Centraal Veevoeder Bureau Veevoedertable. Lelystad, The Netherlands. [Google Scholar]
- Eeckhout, W., and De Paepe M.. . 1994. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim. Feed Sci. Technol. 47:19–29. doi: 10.1016/0377-8401(94)90156-2 [DOI] [Google Scholar]
- Fairbrother, J. M., Nadeau E., and Gyles C. L.. . 2005. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 6:17–39. doi: 10.1079/ahr2005105. [DOI] [PubMed] [Google Scholar]
- Humer, E., and Schedle K.. . 2016. Fermentation of food and feed: a technology for efficient utilization of macro and trace elements in monogastrics. J. Trace Elem. Med. Biol. 37:69–77. doi: 10.1016/j.jtemb.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Humer, E., Wetscherek W., Schwarz K., and Schedle K.. . 2013. Effect of maize conservation technique and phytase supplementation on total tract apparent digestibility of phosphorus, calcium, ash, dry matter, organic matter and crude protein in growing pigs. Anim. Feed Sci. Technol. 185:70–77. doi: 10.1016/j.anifeedsci.2013.07.001. [DOI] [Google Scholar]
- Jensen, B. B., and Mikkelsen L. L.. . 1998. Feeding liquid diets to pigs. In: Garnsworthy P. C. and Wisemann J., editors, Recent advances in animal nutrition. Nottingham University Press, Nottingham, UK. p. 107–126. [Google Scholar]
- Köhler, B., Diepolder M., Ostertag J., Thurner S., Spiekers H.. . 2013. Dry matter losses of grass, lucerne and maize silages in bunker silos. Agric. Food Sci. 22:145–150. doi: 10.23986/afsci.6715. [DOI] [Google Scholar]
- Kozlowska, H., Honke J., Sadowska J., Friasand J., and Vidal-Valverde C.. . 1996. Natural fermentation of lentils: influence to time, concentration and temperature on the kinetics of hydrolysis of inositol phosphates. J. Sci. Food Agric. 71:367–375. doi:. [DOI] [Google Scholar]
- Kraler, M., Schedle K., Domig K. J., Heine D., Michlmayer H., and Kneifel W.. . 2014. Effects of fermented and extruded wheat bran on total apparent digestibility of nutrients, minerals and energy in growing pigs. Anim. Feed Sci. Technol. 197:121–129. doi: 10.1016/j.anifeedsci.2014.07.010. [DOI] [Google Scholar]
- Kramer, E., Lau N., Liebert F.. . 2015. Effects of added lactic acid bacteria and enzymes on fermentation of liquid feed for pigs. Proceedings of the XVII International Silage Conference, Piracicaba, São Paulo, Brazil. p. 466.– . [Google Scholar]
- Lopez, H. W., Ouvry A., Bervas E., Guy C., Messager A., Demigne C., and Remesy C.. . 2000. Strains of lactic acid bacteria isolated from sour doughs degrade phytic acid and improve calcium and magnesium solubility from whole wheat flour. J. Agric. Food Chem. 48:2281–2285. doi: 10.1021/jf000061g. [DOI] [PubMed] [Google Scholar]
- Marino, M., Maifreni M., Moret S., and Rondinini G.. . 2000. The capacity of Enterobacteriaceae species to produce biogenic amines in cheese. Lett. Appl. Microbiol. 31:169–173. doi: 10.1046/j.1365-2672.2000.00783.x. [DOI] [PubMed] [Google Scholar]
- McKie, V. A., and McCleary B. V.. . 2016. A novel and rapid colorimetric method for measuring total phosphorus and phytic acid in foods and animal feeds. J. AOAC Int. 99:738–743. doi: 10.5740/jaoacint.16-0029. [DOI] [PubMed] [Google Scholar]
- Merrell, D. S., and Camilli A.. . 2002. Acid tolerance of gastrointestinal pathogens. Curr. Opin Microbiol. 5:51–55. doi: 10.1016/S1369-5274(02)00285-0. [DOI] [PubMed] [Google Scholar]
- Mikkelsen, L. L., and Jensen B. B.. . 1998. Performance and microbial activity in the gastrointestinal tract of piglets fed fermented liquid feed at weaning. J. Anim. Feed Sci . 7(Suppl. 1):211–215. doi: 10.22358/jafs/69978/1998. [DOI] [Google Scholar]
- Missotten, J. A., Michiels J., Degroote J., and De Sme S.. . 2015. Fermented liquid feed for pigs: an ancient technique for the future. J. Anim. Sci. Biotechnol. 6:4. doi: 10.1186/2049-1891-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, C. A. 2001. Development and benefits of liquid diets for newly weaned pigs, Ph.D. Thesis. University of Plymouth. UK. Available from https://core.ac.uk/download/pdf/29818331.pdf [accessed May 17, 2021]. [Google Scholar]
- Niven, S. J., Beal J. D., and Brooks P. H.. . 2006. The effect of controlled fermentation on the fate of synthetic lysine in liquid diets for pigs. Anim. Feed Sci. Technol. 129:304–315. doi: 10.1016/j.anifeedsci.2005.12.016. [DOI] [Google Scholar]
- Nuss, E. T., and Tunimihardjo S. A.. . 2010. Maize: a paramount staple crop in the context of global nutrition. Compr. Rev. Food Sci. Food Saf. 9:417–436. doi: 10.1111/j.1541-4337.2010.00117.x. [DOI] [PubMed] [Google Scholar]
- Pedersen, A. O., Canibe N., Hansen I. D., and Aaslying M. D.. . 2002. Fermented liquid feed for Finishers‒Pelleted Feed, vol. 1. The National Committee for Pig Production, Copenhagen, Denmark. [Google Scholar]
- Scholten, R. 2001. Fermentation of liquid diets for pigs. Ph.D. Thesis. University of Wageningen, The Netherlands. [Google Scholar]
- Scholten, R. H. J., M. M. J. A.Rijnen.,Schrama J. W., Boer H., Vesseur P. C., Den Hartog L. A., Van Der Peet-Schwerin C. M. C., and Verstegen M. W. A.. . 2001. Fermentation of liquid coproducts and liquid compound diets. Part 1. Effects on chemical composition during a 6-day storage period. J. Anim. Physiol. Anim. Nutr. 85:111–123. doi: 10.1046/j.1439-0396.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- Scholten, R. H. J., van der Peet-Schwerin C.M.C., Verstegen M.W. A.., den Hartog L. A., Schrama J. W., and Vesseur P. C.. . 1999. Fermented co-products and fermented compound diets for pigs: a review. Anim. Feed Sci. Technol. 82:1–19. doi: 10.1016/S0377-8401(99)00096-6. [DOI] [Google Scholar]
- Shelef, L. A., Surtani A., Kanagapandian K., and Tan W.. . 1998. Automated detection of amino acid decarboxylation in Salmonellae and other Enterobateriaceae. Food Microbiol. 15:199–205. doi: 10.1006/fmic.1997.0151. [DOI] [Google Scholar]
- Simons, P. C. M., Versteegh H.A. J.., Jongbloed A. W., Kemme P. A., Slump P., Bos K. D., M.G. E.Wolters., Beudeker R. F., and Verschor G. J.. . 1990. Improvement of phosphorus availability by microbial phytase in broilers and pigs. Brit. J. Nutr. 64:525–540. doi: 10.1079/BJN19900052. [DOI] [PubMed] [Google Scholar]
- Van Soes, P. J, Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to in relation to animal nutrition. J. Dairy Sci. 74:473–481. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- De Angeli., M., Gallo G., Corbo M. R., McSweeney P. L., Faccia M., Giovine M., and Gobbetti M.. . 2003. Phytase activity in sourdough lactic acid bacteria: purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Int. J. Food Microbiol. 87:259–270. doi: 10.1016/s0168-1605(03)00072-2. [DOI] [PubMed] [Google Scholar]
- European Commission . 2009. Commission Regulation No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Official Journal of the European Union. Available from https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R0152&from=EN [accessed May06, 2021]. [Google Scholar]
- Gesellschaft für Ernährungsphysiologie . 1994. Mitteilungen des Ausschusses für Bedarfsnormen der Gesellschaft für Ernährungsphysiologie: Die Bestimmung des verdaulichen Phosphors beim Schwein. Proc. Soc. Nutr. Physiol. 2:113.– . Available from https://gfefrankfurt.files.wordpress.com/2018/08/me-schaetzung_schweine.pdf [in German; accessed May 06, 2021]. [Google Scholar]
- VDLUFA . 2012. Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. III. Die chemische Untersuchung von Futtermitteln, VDLUFA-Verlag, Darmstadt, Germany. [Google Scholar]