Abstract
Biofabrication allows for the templating of structural features in materials on cellularly-relevant size scales, enabling the generation of tissue-like structures with controlled form and function. This is particularly relevant for growing organoids, where the application of biochemical and biomechanical stimuli can be used to guide the assembly and differentiation of stem cells and form architectures similar to the parent tissue or organ. Recently, ablative laser-scanning techniques was used to create 3D overhang features in collagen hydrogels at size scales of 10–100 μm and supported the crypt-villus architecture in intestinal organoids. As a complementary method, providing advantages for high-throughput patterning, we printed thioester functionalized poly(ethylene glycol) (PEG) elastomers using digital light processing (DLP) and created sacrificial, 3D shapes that could be molded into soft (G’ < 1000 Pa) hydrogel substrates. Specifically, 3-arm 1.3 kDa PEG thiol and 3-arm 1.6 kDa PEG norbornene, containing internal thioester groups, were photopolymerized to yield degradable elastomers. When incubated in a solution of 300 mM 2-mercaptoethanol (pH 9.0), 1 mm thick 10 mm diameter elastomer discs degraded in <2 h. Using DLP, arrays of features with critical dimensions of 37 ± 4 μm, resolutions of 22 ± 5 μm, and overhang structures as small as 50 μm, were printed on the order of minutes. These sacrificial thioester molds with physiologically relevant features were cast-molded into Matrigel and subsequently degraded to create patterned void spaces with high fidelity. Intestinal stem cells cultured on the patterned Matrigel matrices formed confluent monolayers that conformed to the underlying pattern. DLP printed sacrificial thioester elastomer constructs provide a robust and rapid method to fabricate arrays of 3D organoid-sized features in soft tissue culture substrates and should enable investigations into the effect of epithelial geometry and spacing on the growth and differentiation of intestinal stem cells (ISCs).
Keywords: Biofabrication, Intestinal organoids, 3D Printing, Covalent Adaptable Networks, Thioester exchange, Stimuli-induced degradation
Introduction:
Organoids can develop from single stem cells when subjected to biochemical and biophysical cues that activate proper signaling pathways, which drive self-assembly into multicellular tissue constructs that are capable of recapitulating key aspects of the parent organ or tissue1,2. Interest in the culture of organoids has grown in recent years, with applications in drug screening, tissue for transplantation, and for disease modeling1. The cellular and functional sophistication of organoids relates to the ability of the stem cells to self-organize, differentiate into relevant cell types, and organize into structures that are similar to the parent organ from which they were derived. For example, intestinal stem cell (ISC) colonies grow and form crypt-like protrusions from the central lumen in vitro, generating architectures reminiscent of the crypt-villus axis in vivo2,3. To date, most organoids, including intestinal organoids, are grown in Matrigel, which typically leads to structures that are stochastically variant in shape and size4,5. To address some of these heterogeneities, recent work has developed synthetic biomaterial matrices for intestinal organoid culture, where the biomaterial chemistry and time-varying properties (e.g. elastic modulus) are used to control the size, structure, and periodicity of the crypt-villus structures6,7. We and others have posited that organoid function will depend on form, so efforts have focused on introducing physiologically relevant architectures, templates, or molds to precisely control the size and shape of lab-grown intestinal organoids.
While recent advances in fabrication techniques have allowed intimate control over the structure, architecture, and properties of many classes of biomaterials, some of the methods are limited to features sizes ≥ 100 μm,8 and others are not compatible with printing in many biological matrices (e.g., collagen, Matrigel)9. Here, we were interested in printing features sizes (<100 μm) that could be transferred to Matrigel (G<1000 Pa), resulting in templated culture wells for intestinal organoids. To date, soft lithographic techniques, such as poly(dimethyl siloxane) (PDMS) stamps, have been used to transfer pseudo-3D architectures to soft materials, such as collagen. Typically, the positive PDMS features are pressed into collagen solutions during gelation to form a cast mold of the original pattern. Removal of the PDMS stamp leaves the freestanding culture substrate with patterned negatives of the PDMS features. Wang et al. used PDMS stamps to generate arrays of crypt-villi structures in stiff collagen hydrogels, which were then used to culture intestinal stem cells and led to spatially restricted lineages reminiscent of the in vivo intestinal epithelium10. While this method provides many advantages, the PDMS stamp must be removed, so patterns with overhang features embedded within the matrix cannot be fabricated.
To build upon and complement the PDMS stamping method, researchers have also used extrusion-based, sacrificial molds11. These molds have been embedded within biologically-relevant biomaterial scaffolds and subsequently dissolved in place to generate complex, three-dimensional tissue scaffolds12. In one example, Miller et al.13 printed a carbohydrate material into rigid fibers that could be embedded and rapidly dissolved in aqueous solutions to create networks of channels to generate a wide range of cell-laden materials. For example, hepatocytes seeded at high density in these thick, perfusable materials showed improved cell survival and enzymatic function13. Expanding on this approach, Koleski et al. integrated temperature-sensitive hydrogel inks (e.g., gelatin, Pluronics) into a multi-material printing platform to print fugitive vasculature structures 14,15. While the shear thinning behavior of temperature sensitive inks enabled direct printing of sacrificial channels,8 the low modulus of the inks necessitated printing within a supportive substrate, preventing the formation of free standing structures. Thus, although these techniques allow for the fabrication of overhanging features and features embedded within bioscaffolds, the methods are limited by the size and resolution that can be achieved (≥ 100 μm)8. Because of this, thiese approaches lesss relevant for directing intestinal organoid growth and differentiation.
Alternatively, photochemical fabrication techniques have been widely used to pattern materials at sub-cellular resolution.16,17,16,19 For example, Nikolaev et al. used photoablation of collagen/Matrigel composites to generate a 150 μm diameter tube that was further processed to integrate 50 μm wide and 170 μm long crypt projections17. When seeded with intestinal stem cells, an epithelial cell monolayer grew and conformed to the photoablated pattern to yield the patterned crypt-villus architecture17. While photoablation offers high-resolution, spatial control over the patterned features, the process occurs voxel by voxel, rendering this strategy less amenable for generating large arrays of features for high throughput testing. Thus, we were motivated to apply digital light projection (DLP) to accelerate photopatterning speeds and create arrays of uniform features. DLP uses a spatial light modulator array to print small feature sizes layer by layer rather than voxel by voxel20; however, photopolymer materials used in DLP can also serve as sacrificial, degradable molds21, 22. In one example, Thomas et al. printed hyaluronic acid methacrylate based features and then embedded the mold in gelatin methacrylate before using hyaluronidase to erode the hyaluronic acid, yielding 300 μm diameter perfusable vasculature structures 22. However, the degradation time of enzyme erodible constructs can vary from minutes to days depending on the hydrogel stiffness (i.e., crosslinking density), so there is a tradeoff when higher strength to weight ratios are needed in the mold to pattern free-standing or overhanging structures23,24. In this contribution, we exploit recent advances in thioester exchange chemistry, which is compatible with photopolymerization reactions25–27, and sought to design bioink formulations that would yield structurally robust elastomeric molds, but allow for rapid and gentle network degradation28,29.
Results herein report on the development of a degradable thioester-based elastomer resin for 3D printing and use DLP to print sacrificial, elastomer molds with critical dimensions of 37 ± 4 μm and XY resolutions of 22 ± 5 μm. Three-dimensional, negative mold structures were printed and then cast into soft biomaterials matrices, Matrigel and PEG hydrogels, that are suitable for organoid growth. A reversible exchange reaction between 2-mercaptoethanol in solution with thioester crosslinks in the printed structures lead to rapid degradation at pH 9.0 T 25°C, effectively transferring these complex patterns to the hydrogel material. This pattern transfer was characterized as a function of feature size, resolution and depth for soft (G<1000 Pa) poly(ethylene glycol )(PEG) and Matrigel hydrogels. Overhanging structures 150 μm in diameter were either printed close together (< 50 μm) or with perpendicular overhanging cylindrical features (D > 50 μm). Features were produced in arrays fifty at a time on the timescale of ~20 min. The printed structures transferred to matrigel with high fidelity to form shapes relevant to the crypt-villus structures found in intestinal epithelium and intestinal organoids. Finally, we show matrigel hydrogels patterned with this technique support the culture of intestinal stem cell monolayers, which formed confluent layers and conformed to the patterned structures.
Materials and Methods
Synthesis of 3-arm PEG Thiol (1300 Da)
The PEG thiol synthesized has been previously described30. In brief, 14.06 mL of glycerol ethoxylate (1000 Da, Sigma-Aldrich) and 8.4 mL of 3-mercaptopropionic acid were combined in a 1000 mL round bottomed flask equipped with a stir bar, dean-stark trap, condenser, and oil bath. 300mL of toluene were added through the condenser; then the mixture was heated to reflux for 5 min; 3 drops of sulfuric were added; and the vessel was stirred at reflux overnight. Next, toluene was removed in vacuo, and the mixture was redissolved in dichloromethane and purified with three washes of a saturated NaHCO3 solution and one wash with brine. The remaining clear organic layer was then collected, dried over Na2SO4, and concentrated in vacuo to produce a clear viscous liquid. (>95% functionalized) 1H NMR (400 MHz, CDCl3) δ 4.32–4.19 (m, 6H), 3.86–3.42 (m, 85H), 2.83–2.72 (m, 6H), 2.72–2.62 (m, 6H), 1.72–1.62 (t, 3H)
Synthesis of 3-arm PEG Thioester Norbornene (1600 Da)
10.0 g of 3-arm PEG thiol was combined with 13.65 g 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) in a flame dried 1000 mL round-bottomed flask equipped with a stir bar. 400 mL of anhydrous dichloromethane were added via cannula followed by 5.8 mL of 5-norbornene-2-carboxylic acid (mixture of endo and exo, Alfa Aesar). After stirring under argon for 15 min, 17 mL of diisopropylethylamine were added to the vessel. After 15 min of argon, the apparatus was fixed with an argon balloon and allowed to stir overnight. The reaction mixture was diluted to a volume of 800 mL with fresh dichloromethane, then washed gently and quickly with 7 washes of 300 mL 0.1 N hydrochloric acid. The organic layer was then washed three times with a saturated NaHCO3 solution, once with brine, dried over Na2SO4, and then concentrated in vacuo to produce a clear, slightly yellow viscous liquid. (85% functionalized) 1H NMR (400 MHz, CDCl3) δ 6.52–5.78 (m, 6H), 4.32–4.19 (m, 6H), 3.86–3.42 (m, 85H) (Figure S1)
Elastomer Formation
Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) was dissolved in deionized water at a concentration of 86.2 mg/mL, and combined with thiol and thioester norbornene macromers in a molar ratio of 1 thiol:1 norbornene:0.01 LAP, resulting in a 97 wt% polymer solution that readily polymerized when exposed to 10 mW/cm2 365nm light for 15 min.
Mechanical measurements
Elastomer stress strain behavior quantified with a dynamic mechanical analyzer (DMA) (RSA-G2, TA Instruments). Specifically, 250 μm thick non-swollen elastomer samples were elongated at 0.1 mm s−1 crosshead speed to generate stress strain curves until failure. The Young’s modulus (E) was calculated from the slope of the linear segment of the stress–strain curve between 5% and 10% strain. Values are presented as the average ±SD. To quantify glass transition behavior, Young’s modulus and tan(delta) measurements were measured in tension at a frequency of 1 Hz, a strain of 0.03%, a preload force of 0.34 N, and a temperature ramp rate of 3°/min. Glass transition temperature was calculated as the peak of the tan(delta) curve.
Mass Loss Study
Thioester elastomer samples were polymerized in the shape of discs (diameter =10 mm, thickness =1 mm. Initial mass was measured and used to calculate the initial amount of polymer contained in each sample (mpi). Elastomer samples were placed in separate 50 ml conical tubes and swollen in tris buffer (pH 9.0, 100 mM) for 1 h, then the solutions were replaced with 5 ml of either tris buffer or tris buffer with 300 mM 2-mercaptoethanol. The final pH of all solutions was adjusted to 9.0. The swollen mass (ms) was calculated as the ratio of elastomer swollen mass after 1 h in pH 9.0 Tris buffer relative to the dry mass measured after lyophilization. Next, all samples were trimmed to a diameter of 8mm using a biopsy punch and placed in a bath of tris buffer on a DHR-3 rheometer (TA Instruments) and measured for their shear elastic modulus (G’) (0.1% strain, 1 hz). On the rheometer, an axial force was applied and adjusted between 0.5 and 2.0 N to prevent sample slippage. Finally, elastomer samples were lyophilized for 48 h, removed from conical tubes, and placed on a zeroed balance to record dry polymer mass (mp). All data were taken in triplicate.
FTIR Characterization
Real-time Fourier transform infrared spectroscopy (RT-FTIR) (Nicolet 6700, Madison, WI) was used to follow the functional group conversion and subsequent kinetic analysis. The FTIR was equipped with a KBr beam splitter and MCT/A detector. Monomer formulations were placed between polished NaCl plates, and a 405 nm LED (M405L3-C5, ThorLabs) at an intensity of 8 mW cm−2 was used to initiate polymerization of the thiol:ene network. Simultaneously, the disappearance of the peaks associated with the carbon–carbon double bond of the norbornene (3060 cm−1) and the thiol (2555 cm−1.) were monitored as a function of exposure time. The area under the peaks (A) were measured before and during the light exposure yielding the conversion (X) as a function of time. X was calculated based on the disappearance of the peak associated with each reactive specie by
| (1) |
A 60 s exposure was used for each sample, but samples were monitored up to 5 min on the FTIR to assess any dark polymerization.
3D Printing of Thioester Elastomers
A custom-built, digital light projection (DLP system) designed for bottom-up 3D printing was used to produce all samples (Figure 3a). A 405 nm LED (SOLIS-405C, Thorlabs) and a high resolution spatial light modulator (1920 × 1152 Analog SLM, Meadowlark Optics, measured extinction ratio of 99.3%) were used to project patterns of a precise light exposure (405 nm, 8 mW/cm2, 30 s) onto the printing resin (i.e., thioester precursors). The exposure pattern was imaged onto the sample plane with demagnification, resulting in a pixel diameter at the sample plane of 4.6 μm. A glass slide was used as the build-stage. A slab of index matched polydimethylsiloxane (PDMS) filled with carbon black was used as a neutral density filter laminated to the build stage prevent back reflections.31 A non-stick PDMS window was prepared using a Sylgard 184 Silicone Elastomer Kit (DOW CORNING) mixed at 10:1 weight ratio.32,33 Printed structures were washed overnight in 100% ethanol, then stored in ethanol until use.
Patterned elastomer staining and quantitative image analysis
Fluorescein (acid yellow 73, Sigma-Aldrich) was diluted in 100% ethanol to a final concentration of 2 mg/ml and warmed to dissolve the fluorescein. Thioester elastomer stamps were submerged in the fluorescein solution for 30 min, then dried in a vacuum oven for 30 min, and transferred to glass slides for imaging with laser scanning confocal microscopy (Zeiss LSM 710, Fluar 10× air, 0.5 NA, 1AU). Critical dimensions were measured for printed pillar structures 50 μm tall and 10–100 μm in diameter. Resolution was measured for printed pillar structures 100 μm diameter and 50 μm tall spaced 10–100 μm. A z-stack (2.25 μm step) of each structure was taken, then an image slice 10–20 μm below the top of the feature was selected for analysis. This was done to avoid any surface effects that may be introduced on the top layer of the print. The images were then quantitatively analyzed in MATLAB. Each image was first turned into a binary image using the graythresh( ) and imbinarize( ) functions. Diameter was quantified in the critical dimension tests using the ‘EquivDiameter’ field of the regionprops( ) function. The spacing between pillars for the resolution tests was then quantified by taking the complement of the binary image and calling the improfile() function to measure pillar separation at the narrowest point. All data reported consist of n = 3 stamps with 3 technical replicates averaged together from each stamp.
Scanning Electron Microscopy
Printed thioester samples were removed from the ethanol wash solution and allowed to dry in ambient conditions for 24 h Uncoated thioester elastomer samples with 3D printed overhang structures were visualized on a scanning electron microscope (SEM, Hitachi SU3500) using the secondary electron emission mode at an accelerating voltage of 5 kV.
Hydrogel formation and pattern transfer
PEG hydrogels were formed by photo-crosslinking the norbornene functionalized 20 kDa 8-arm PEG macromer with a thiol functionalized 2-arm 5 kDa PEG macromer. The two macromers were combined in a 1:1 stoichiometric ratio and diluted with 1X PBS to a final formulation containing 2.0 wt% polymer, 1 mM LAP photoinitiator, and 1 mg/ml 2000 kDa FITC dextran to facilitate direct hydrogel imaging. When irradiated with 10 mW/cm2 of 365nm light for 60 s, a hydrogel with a final shear elastic modulus (G’) of 740 ± 50 Pa forms. (Figure S2)
Elastomer stamps, composed of a 100 μm thick base with printed features on the top, were trimmed with an 8 mm biopsy punch, submerged in 100 % ethanol in a conical tube, then brought into a tissue culture hood to maintain sterility. After 10 min of swelling in ethanol, the ethanol was replaced with 1X phosphate buffered saline (PBS), and the elastomer stamps swelled for 1 h. Hydrogel prepolymer mixtures, either 2 wt% PEG or Matrigel (Corning), were placed in an ice bath along with a 10 cm x 10 cm x 1 cm sterilized steel block. Elastomer stamp manipulation was conducted as previously described.34,35 Briefly, the elastomer stamps were placed on the chilled block with the features facing up, and excess water was gently aspirated. Each elastomer stamp was then quickly washed with 2× 20 μL of pre-gel mixture for 30 s each to remove excess water from the pattern surface. Then, 20 μL of pre-gel mixture were dispensed onto 12 mm coverslips functionalized with (3-mercaptopropyl)trimethoxysilane (Sigma-Aldrich) placed into 6 well culture plates. Stamps were placed face-down to submerge printed features in pre-gel mixture, then gelation was induced. The Matrigel samples were placed in an incubator for 15 min to induce gelation. PEG samples were irradiated through the coverslip with 10 mW/cm2 365nm light for 60 s to induce gelation. Next, each stamp-hydrogel complex was washed twice with 3 mL of a pre-warmed solution containing 100 mM tris, 300 mM 2-mercaptoethanol at pH 9.0 (2 h then 1 h, 37°C). Finally, patterned samples were washed 3× with pre-warmed PBS and stored in the incubator until use.
Patterned hydrogel staining and image analysis
To visualize the patterned Matrigel hydrogels, samples were first transferred to a 24 well plate washed with 1 mL of pre-warmed pH 9.0 100 mM NaHCO3 for 10 min, followed by 500 μL of 0.031 mg/mL Fluorescein isothiocyanate (Invitrogen) in the same bicarbonate buffer. After 1 h, patterned Matrigel samples were then washed three times with PBS and stored in an incubator until imaging with confocal microscopy (Zeiss LSM 710, Plan-Aprochromat 20× water, 1.0 NA, 1AU). Measurements of well diameter and spacing were done by taking z-stack of each structure, then identifying an image slice 10–20 μm above the bottom of the well. These images were then quantitatively analyzed in MATLAB. Each image was first turned into a binary image using the graythresh( ) and imbinarize( ) functions. For patterned PEG hydrogels, diameter was quantified in the critical dimension tests by taking the complement of the binary image and using the ‘EquivDiameter’ field of the regionprops( ) function. Space between pillars was then quantified by calling the improfile() function to measure pillar separation at the narrowest point. For patterned Matrigel hydrogels, the improfile() function was used to measure both pillar separation and pillar diameter. All data reported consist of n = 3 stamps with 3 technical replicates averaged together from each stamp. (Figure S3)
Crypt Isolation and organoid culture.
Murine small intestinal crypts were extracted from Lgr5-eGFP-IRES-CreERT2 mice as previously described.6 The crypts were cultured as organoids by first embedding them in reduced growth factor Matrigel (Corning). The intestinal organoid cultures were maintained using Advanced DMEM-F12 (Invitrogen), containing N2 and B27 Supplements (Thermo Fischer Scientific), Glutamax (Gibco), HEPES, penicillin-streptomycin, and supplemented with epidermal growth factor (50ng/mL, R&D Systems), Noggin (100ng/mL, from collaborator), R-spondin conditioned media (5%), CHIR99021 (3uM, Selleckchem) and valproic acid (1mM, Sigma-Aldrich). The medium was changed every 2 days, and organoids were passaged every 4 days.
Seeding of intestinal stem cells onto 2D gels.
Intestinal organoids were released from Matrigel using cold media followed by mechanical stimulation from a pipette tip. The organoids were then enzymatically dissociated into single intestinal stem cells (ISCs that are Lgr5+ and express GFP) by incubation at 37°C for 8 min in 1mL TrypLE (Life Technologies), supplemented with DNAseI (~10mg, Sigma-Aldrich), 1uM N-acetylcysteine (Sigma-Aldrich), and 10uM Y27632 (Stemgent). Added to the single cell suspension was 1mL FBS and 3mL Advanced DMEM/F12, before passing through a 40um filter to remove multicellular aggregates. The intestinal stem cell pellet was formed by centrifugation at 1,200 RPM for 4 min. The cell pellet was resuspended in an appropriate volume of media and 10 μL of the suspension was added to the surface of the patterned Matrigel gel. Cell-laden gels were incubated at 37°C and 5% CO2 for 15 min before carefully adding 600 μL of growth media (ENRCV) supplemented with blebbistatin (15mM) to each well. Media was replaced with ENRCV, but without blebbistatin two days after seeding.
Immunofluorescence analysis.
Three days after seeding, intestinal stem cell monolayers were fixed with 4% paraformaldehyde and 0.1% glutaraldehyde in PBS (15 min, room temperature). Following fixation, gels were washed with PBS (3×10 min at room temperature), treated with 20mM sodium borohydride in PBS (5 min, room temperature), and washed again with PBS (3×10 min, room temperature). The gels were then solubilized using 0.2% Triton X-100 in PBS (1 h at room temperature) and blocked using 10% horse serum and 0.01% Triton X-100 in PBS (1 h, room temperature). The samples were then incubated overnight at 4°C with rhodamine phalloidin (1:200) and DAPI (1:2000) in blocking buffer. After washing with PBS (5×1h, 4°C) to remove any residual antibody, the fluorescently labeled monolayers were imaged using confocal microscopy (Zeiss LSM 710).
Frequency Map Generation.
Z-stack images were taken of ISCs fixed and stained for the nucleus and actin cytoskeleton after 3 days of culture on microwell patterned matrigel substrates. The locations of cell nuclei along the top, middle, and bottom, of the patterned microwell structure were determined using ImageJ. Nuclei within the lower 20 μm and upper 20 μm of the stack were counted as the “bottom” and “top”, respectively, while the intermediate stacks were considered the “middle”.
Coordinates of individual nuclei were normalized to the center of each well and scaled to the measured radius of each well. Cell data were then assembled in MATLAB and displayed using the pcolor() function.
Results
Design of degradable thioester crosslinked poly (ethylene glycol) elastomers
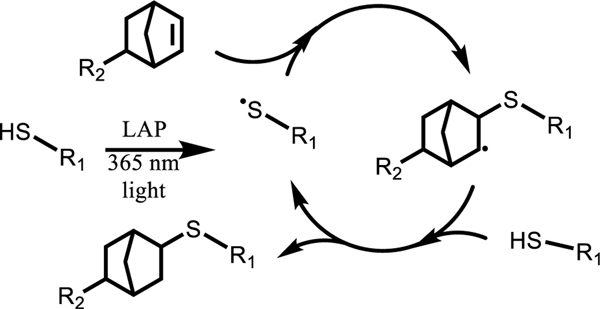
The photoinitiated thiol-ene ‘click’ reaction (Scheme 1) is known to drive rapid formation of a step-growth network while exhibiting favorable characteristics, such as high selectivity, allowing for use in combination with other functional groups, cytocompatibility, and insensitivity to oxygen.36 In recent literature, the photoinitiated thiol-ene polymerization has also been used to produce crosslinked materials with dynamic thioester crosslinks for applications ranging from the design of viscoelastic hydrogels to recyclable thermosets. 25,26,37.
Scheme 1.
In this work, thioester elastomers were synthezised from thiol and thioester norbornene functionalized PEG macromers. Specifically, the thiol macromer was synthesized by thiolation of a 3-arm 1 kDa PEG alcohol (>95% functionalization by 1H NMR) by Fischer esterification with 3-mercaptopropionic acid (Figure 1a). The thioester norbornene macromer was synthesized by further functionalization of the thiol macromer with 5-norbornene-2-carboxylic acid to yield a thioester norbornene functionalized macromer (~85% functionalization by 1H NMR, Figure S1).
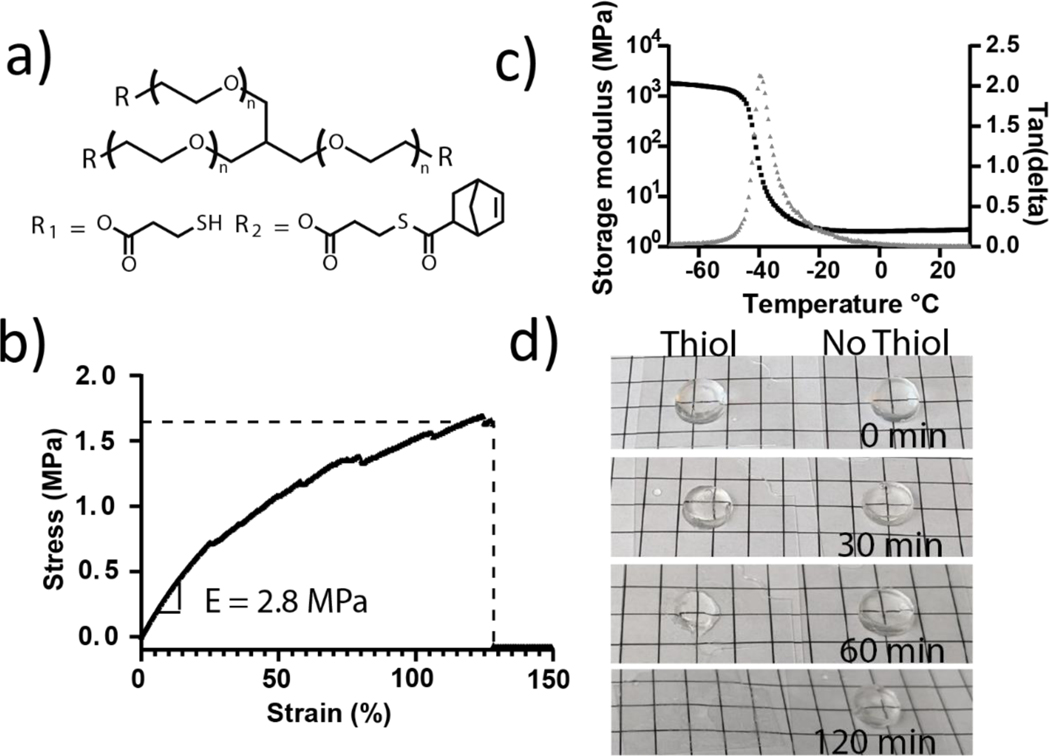
Figure 1:
Photoinitiated thiol-ene ‘click’ chemistry and thioester chemistry were used to create mechanically robust networks with adaptable crosslinks. a) A 3-arm PEG macromer was functionalized with thiol or thioester norbornene function groups to yield resin precursors: 3-arm PEG thiol (1300 Da) and 3-arm PEG norbornene with internal thioester groups (1600 Da). b) Typical stress-strain behavior of a thioester elastomer under tensile loading until failure (0.1 mm/s). The Young’s modulus was calculated using the slope of the curve between 5 and 10% strain. c) The young’s storage modulus (black) and tan(delta) (grey) were measured as a function of temperature. A glass transition temperature of −40°C is illustrated by the position of the peak in tan(delta). d) Representative images of free-standing elastomer samples placed in pH 9.0 tris buffer over the course of 2 h with and without 300 mM 2-mercaptoethanol to monitor degradation. Gridline spacing is 5 mm.
Thiol and thioester norbornene macromers were combined in a 1:1 ratio of thiol to norbornene and mixed with a concentrated solution of lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) photoinitiator in water (82.6 mg/ml, 0.01 eq. LAP) to form a final solution that was 97.1 wt% polymer and yielded an elastomeric network when cured with 356nm UV light (10 mW/cm2, 15 min). Mechanical properties of the thioester elastomers were measured using an RSA-G2 dynamic mechanical analyzer (TA Instruments): Thioester elastomers exhibit a young’s modulus (E) of 2.6 ±0.4 MPa, ultimate tensile strength of 1.8 ±0.2 MPa, and a strain at break of 160 ± 50 % (Figure 1b). Young’s storage modulus and tan(delta) measurements were conducted over a temperature range of −70°C-30°C, and a glass transition temperature of −40°C was observed (Figure 1c). The degradation of the elastomers was assessed under slightly basic conditions with a high concentration of thiol (pH 9.0, 300 mM 2-mercaptoethanol), consistent with a thioester exchange mechanism as reported previously28,29. Over the course of 2 h, transparent, dimensionally stable elastomer samples became tacky and opaque, eventually degrading into a viscous residue (Figure 1c).
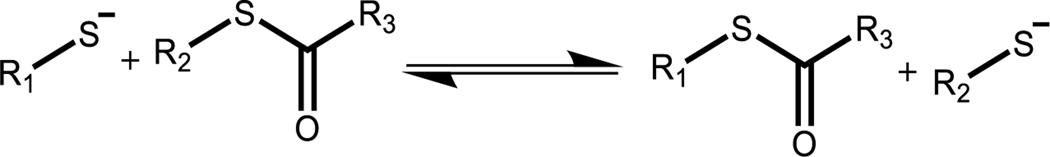
PEG thioester elastomers degrade rapidly in 2-mercaptoethanol with little swelling
Thioesters undergo nucleophilic attack by a deprotonated thiol (thiolate), leading to a rearrangement and the formation of a new thioester and the regeneration of a thiolate (Scheme 2). This reaction is reversible and has been well studied in both hydrogel and elastomer systems, including exchange reactions that lead to degradation.25,37 In the case when a monofunctional thiol is introduced to the thioester network, thioester exchange results in the cleavage of crosslinks and subsequent mass loss. To better understand how crosslink cleavage via thiol catalyzed thioester exchange gives rise to mass loss in the 3-arm 3-arm PEG system developed here, thioester elastomer samples were immersed in a basic aqueous buffer (pH 9.0 100 mM Tris buffer) with and without 300 mM 2-mercaptoethanol. Samples were monitored with degradation time until complete mass loss (<2 h).
Scheme 2.
To better understand the degradation mechanism and alterations in the network structure with time, rheological properties were measured on the thioester elastomers as a function of degradation. When thioester elastomers were placed in a pH 9.0 solution with thiol, the shear elastic modulus (G’) decreased exponentially with time until complete degradation (Figure 2a). Moduli data were normalized to the initial swollen shear elastic modulus (G’o) and fit to the exponential decay function where t is time and the characteristic degradation time τ was determined to be 17.9 ± 0.3 min (Figure S4). This result suggested to us a relatively uniform crosslink cleavage throughout the sample, typical of a bulk degradation process. However, it is important to note that erosion/mass loss is dictated by the network connectivity and solubility of the degraded fragments. As a control, thioester elastomers displayed no significant change in modulus over the course of 2 h in pH 9.0 solution without thiol, suggesting no crosslink exchange. Thus, on the time scale of these experiments, hydrolysis of the network ester groups is negligible, and elastomer degradation is occurring primarily through thiol thioester exchange.
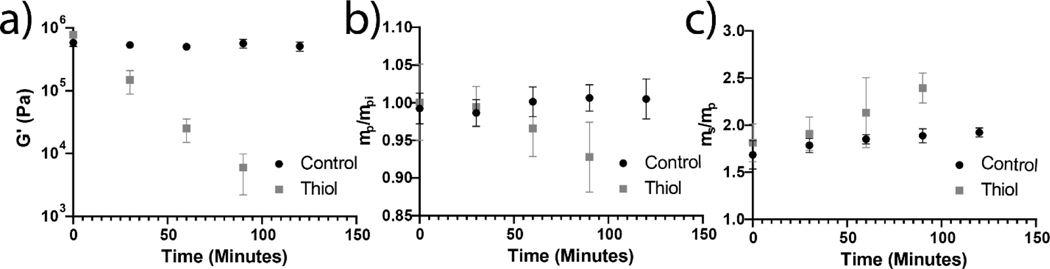
Figure 2:
Degradation of thioester elastomers placed in pH 9.0 buffer with and without 300 mM 2-mercaptoethanol was observed over 2 h. Degradation was only observed when 2-mercaptoethanol was present in solution suggesting degradation was mediated only by thioester exchange. a) Shear swollen moduli were observed as a function of time. An exponential decrease in modulus was observed for the thiol condition suggesting that crosslinks were cleaved throughout the sample via a bulk erosion mechanism. 0.1% strain, 1Hz; N = 3, error bars are standard deviation. b) Elastomer samples were lyophilized measured for polymer mass. Dry mass data were normalized to the calculated theoretical amount of polymer included in formulation and plotted here as a function of time and degradation condition. N = 3, error bars are 95% confidence interval. c) Mass swelling ratio was calculated as the ratio of the swollen polymer mass to the measured dry mass and are reported here as a function of time and degradation condition. N = 3, error bars are 95% confidence interval.
At selected time points, samples were lyophilized and mass loss over time was quantified. No significant mass loss was observed for thioester elastomers placed in media without thiol, however, in media with thiol the thioester networks exhibited a linear mass loss at early times points and 93 ± 7% of the initial polymer mass remained in the thioester condition at 1.5 h (Figure 2b). Equilibrium mass swelling data show and increase in the swelling of thioester networks in thiol solution from 1.8±0.3 g/g to 2.4±0.2 g/g over 1.5 h of degradation (Figure 2c). While this is an appreciable change in swelling, the small magnitude compared to the 100 fold change in shear elastic modulus suggest swelling was not strongly affected by the degradation. These non-swelling characteristics are known to occur in water swollen networks with decreased hydrophilic character either though high crosslink densities30,38 or the addition of less hydrophilic polymers often with thermoresponsive character30,39.
Macroscopically, this combination of polymer swelling increasing by a factor of 2–3, while G changes by 2 orders of magnitude resulting in a slow transition from clear rigid solid to viscous liquid without large changes in sample dimensions with the simple introduction of 2-mercaptoethanol. This is highly advantageous for cast-mold applications where large amounts of swelling typically observed in degrading hydrogel systems could damage cast features. The concept of a thiol stimulus to induce degradation in a 3D printable resin rendered this formulation suitable for the goal of printing sacrificial molds.
DLP printing of thioester elastomers to produce high-resolution 3D structures with overhanging features
To print thioester elastomers and assess the resolution and critical dimension limits of the thioester materials, a high-resolution DLP printer was used. Uniform illumination was achieved using a 1920×1152 pixel liquid crystal on silicon (LCoS) spatial light modulator (SLM) with 405 nm light to form the desired image from a digital file that was then de-magnified to the sample plane (Figure 3a). The extinction ratio for this SLM was 99.3%. A non-stick PDMS on glass window was illuminated from underneath the sample for a bottom-up build direction upon the print stage, and the stage was moved vertically to create each layer. Each layer was exposed with a unique image before raising the print stage; additional resin then flowed into the exposure plane; and the next layer was exposed to a new image.
Figure 3:
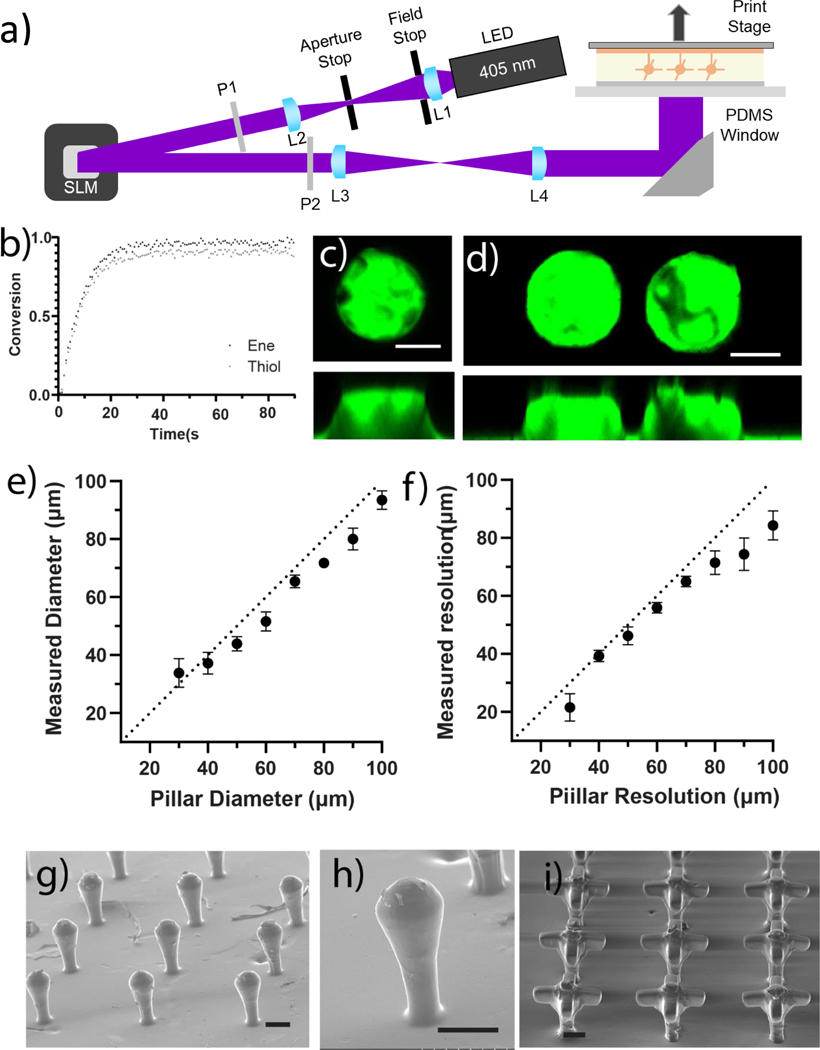
Thioester elastomer photocurable resins were optimized for printing with existing high resolution DLP 3D printing techniques. a) Schematic of DLP 3D printing stage and process. Lenses and polarizers are labeled L and P, respectively, and the spatial light modulator is labeled “SLM”. b) FTIR Spectroscopy was used to obtain conversion of norbornene and thiol species as a function of light dose delivered to the sample. (8 mW/cm2, 405 nm light, N =3) c) Top and side view of a single 50 μm tall, 100 μm diameter pillar, and d) a pair of pillars patterned 30 μm apart. Elastomer stained with fluorescein. Scale bars 25 μm. e) Measured pillar diameter plotted versus patterned diameter, a dotted line with a slope of 1 is plotted for visual reference. N = 3 stamps each containing 3 technical replicates averaged together, error bars are standard deviation f) Measured pillar spacing plotted versus patterned pillar spacing. A dotted line with a slope of 1 is plotted for visual reference. N = 3 stamps each containing 3 technical replicates averaged together, error bars are standard deviation. Scanning electron microscopy was used to take images of three dimensional constructs containing overhang features including: arrays of g-h) crypt and i) branched crypt structures. Scale bars 100 μm.
To print thioester resins, the 3-arm PEG thiol and 3-arm PEG thioester norbornene macromers were added to a concentrated LAP solution to form a solution that was 97.1 wt% polymer with a molar ratio of thiol:norbornene :LAP of 1.1:1.0:0.011 respectively. The formulation was modified to include a 10% excess of thiols, which was found to improve bonding between printed layers, presumably due to the ability of unreacted thiols to participate in the thiol-ene polymerization of the subsequent layer. FTIR was used to monitor the disappearance of peaks associated with the thiol (2555 cm−1) and norbornene (714 cm−1 and 3060 cm−1) functional groups when irradiated with 8mw/cm2 405nm light (Figure 3b). The initial slope of each conversion plot was measured in the initial linear region of the plot and found to be 0.066 ± 3 s−1 for thiol species and 0.070± 4 s−1 for norbornene species; this suggests thiol and norbornene species were consumed at a 1:1 rate. As expected, the norbornene conversion was higher than the thiol conversion due to the 10% thiol excess in the resin formulation with values of 0.97±0.04 and 0.91±0.03 respectively. 30 s of 8mw/cm2 405nm light was selected as the minimal dose necessary for a full cure.
To facilitate the printing of layers, a photoabsorbing species was added to the formulation to attenuate light and control the depth of penetration. Cure depth calculations were conducted as previously reported40, but briefly, Beer-Lambert absorption was assumed for the photoabsorber (Tinuvin Carboprotect, BASF) then combined with the light dose-conversion profile determined by FTIR, and the percolation threshold of 50% conversion for a 3-arm 3-arm step growth co-polymerization to determine the maximum depth where light could penetrate and form a gel. At a concentration of 0.75 wt% Tinuvine Carboprotect, 90% of light was absorbed in the top 110 μm of the resin, and for a single 30 s exposure at 8 mW/cm2, 405 nm light the cure depth was determined to be 50 μm. Importantly, this means that the z-dimensions of overhang features, since light was patterned on liquid resin with no previous layers, were determined by the cure depth rather than the layer thickness set by the stage. To account for this effect, overhang feature designs were adjusted based on the calculated cure depth (Figure S5). At these printing conditions, a layer thickness of 10 μm optimized the fidelity of the x-y features while ensuring layer-to-layer adhesion and printing of reliable 3D structures.
Once the printing process and formulation were optimized, the critical dimension and resolution limits of the printing platform were tested. To analyze these parameters, features of various sizes and varied spacing were printed onto prefabricated 100 μm thick thioester elastomer bases, which acted as a stamp-like support when manipulating the printed constructs (Figure 3c–d). Critical dimensions were assessed by printing 50 μm tall cylinders with diameters ranging from 30–100 μm (Figure S6). For characterization purposes, printed constructs were stained with fluorescein (acid yellow 73), and directly imaged via laser scanning confocal microscopy. Measurements of the pillar diameter were taken between 10 and 20 μm from the top of the pillar to avoid the influence of surface distortions, thus, printed structures that did not exceed a height of 20 μm were not measured (Figure 3e). The experimental limits of patterned resolution were assessed in a similar fashion where 2 cylinders 50 μm tall and 100 μm in diameter were printed at spacings ranging from 10–100 μm. For these experiments, measurements were taken between 10–20 μm from the top of the pillars, and we observed a minimum resolution of 30 μm (Figure 3f, S7). Interestingly, both the measured diameter and resolution of the patterns were slightly smaller than the designed dimensions. This difference might be attributed to elastomer shrinkage during the ethanol washes or after desiccation (see 3D printing thioester elastomers in methods).
The results of 2D characterization of minimum critical dimensions and feature sizes were used to design a series of 3D structures with overhang features ranging from slight to extended protrusions. Many of the printed structures were selected to be reminiscent of the crypt and/or branched crypt structures found in the intestinal epithelium (Figure 3g). The achievement of these crypt-like structures demonstrates the resolution achievable with this material system and the ability of the method to create molds with overhanging features (Figure 3g–h). Figure 3i shows distinct overhangs with features sizes down to 50 μm in the x, y, and z print directions; these structures and size scales were similar to other fabricated branched crypt structures (Figure 3h–i)10,17. These results show, however, that rounding occurs at the edge of features which has been previously observed 40. We attribute this artifact to polymerization in the “dark” region as a result of simultaneous reaction and diffusion that occur when printing and patterning features near the resolution limit of the material.41
Hydrogel substrate influences feature size, resolution, and depth
After characterizing the limits of printed features and resolution, we next sought to understand how printed patterns transferred to soft synthetic (PEG hydrogel) and extracellular matrix (Matrigel) platforms relevant to the culture of intestinal organoids. The PEG hydrogel was composed of 8-arm 20 kDa PEG norbornene and 2-arm 5 kDa PEG thiol combined in a stoichiometric ratio diluted to 2 wt% (G’= 740 ± 50 Pa, Figure S2), which compares favorably with PEG hydrogel organoid culture platforms6. To pattern soft hydrogels, elastomer stamps were brought into contact with pre-gel mixture, and gelation was induced by placement of the sample the incubator (Matrigel) or irradiation with 365nm light (PEG hydrogel). Once the hydrogel cured, the elastomer hydrogel complex was immediately bathed in 100 mM Tris buffer supplemented with 300 mM 2-mercaptoethanol adjusted to a pH of 9.0.
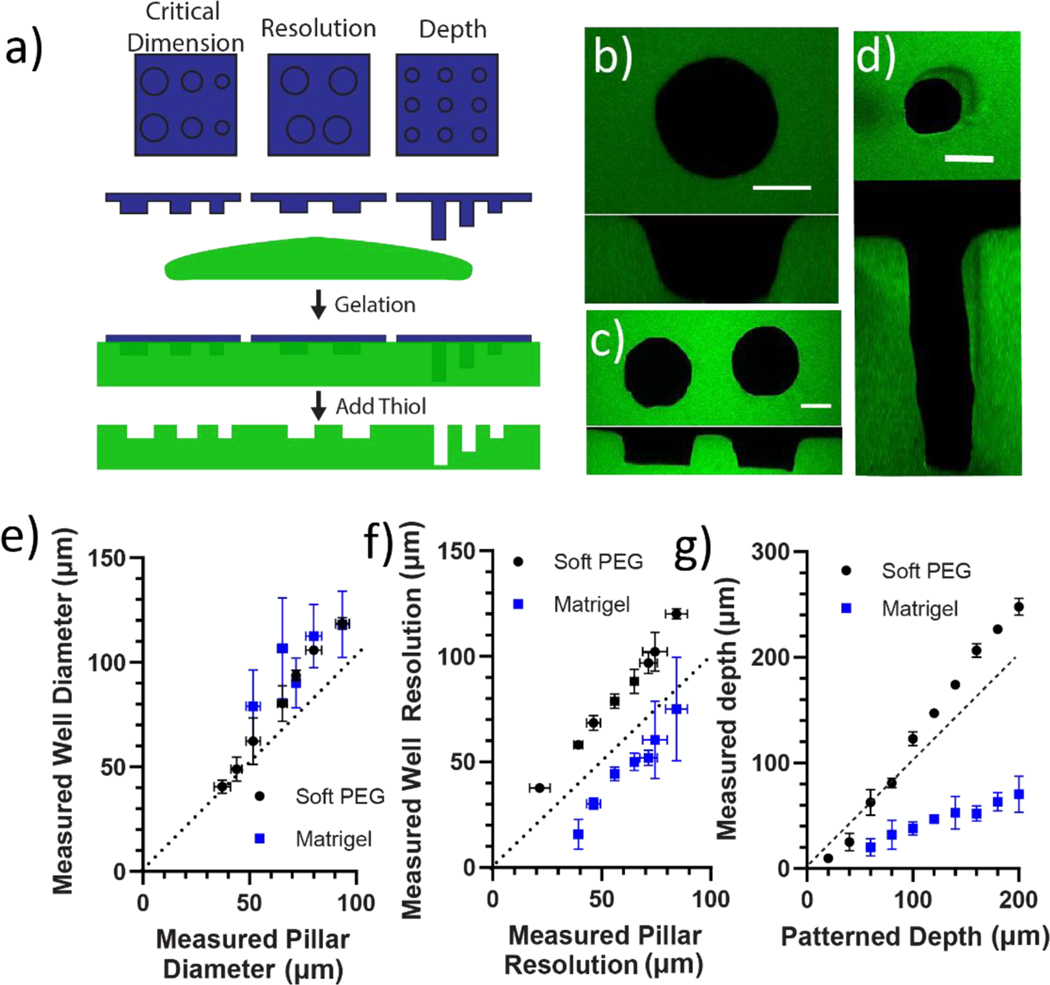
To quantitatively characterize how the printed thioester features patterned into the hydrogels, the final pattern critical dimension, resolution, and depth were measured in Matrigel and PEG hydrogels (Figure 4a). Measurements of pillar diameter and spacing were once more assessed between 10 and 20 μm of the bottom of the well in order to most closely match the diameter of the pillar section used to cast the well. Well depth measurements were measured to the nearest whole image slice of a z-stack taken of each well (Figure 4b–d). Analysis of the patterned well diameter data revealed that critical dimensions were a function of the type of material with 40 and 60 μm pillars being the smallest size of pillars that could be transferred into the PEG and Matrigel hydrogels, respectively (Figure 4e). At the largest condition patterned well sizes for both hydrogel platforms were larger than the original pattern by ~30% (e.g., Dmold = 93 ± 3 um vs Dwell = 118 ± 16 um).
Figure 4:
Thioester elastomer structures were degraded in situ to cast patterns into soft, swollen hydrogel platforms. a) 3D printed thioester elastomer constructs were lowered into solutions of Matrigel or 8-arm 20 kDa PEG norbornene and 2-arm 5 kDa PEG thiol combined in a stoichiometric ratio diluted to 2 wt% (G’ = 740±50 Pa). Each hydrogel was polymerized, then a solution of pH 9.0 300 mM 2-mercaptoethanol was added to degrade the thioester elastomer stamp. Hydrogel cast patterns were then equilibrium swollen in PBS and imaged by laser scanning confocal microscopy to assess how printed pillar dimensions transfer to each hydrogel substrate. b) 50 μm tall pillars with a range of diameters were patterned into Matrigel and Soft PEG hydrogels. Z-stack images of each well were taken with laser scanning confocal microscopy. Well diameter was measured between10–20 μm from the bottom of the well using MATLAB (for more details please see methods).A representative image is shown of a well cast by a 90 μm diameter pillar into PEG hydrogel. An orthogonal view of the XZ plane is included for reference c) 50 μm tall, 100 μm diameter pillars with a range of spacing were patterned into Matrigel and Soft PEG hydrogels. Z-stack images of each well were taken with laser scanning confocal microscopy. Well resolution was measured between10–20 μm from the bottom of the well using MATLAB (for more details please see methods).A representative image is shown of a pair of wells cast by pillars 30 μm apart into PEG hydrogel. An orthogonal view of the XZ plane is included for reference. d) 50 μm diameter pillars with a range of printed heights were patterned into Matrigel and Soft PEG hydrogels. Z-stack images of each well were taken with laser scanning confocal microscopy. Well depth was measured as the number of image slices multiplied by the image thickness (for more details please see methods).A representative image is shown of a well cast by a 200 μm tall pillar into PEG hydrogel. An orthogonal view of the XZ plane is included for reference. e) The measured well diameter in Matrigel and soft PEG hydrogels is plotted versus the measured pillar diameter in the elastomer print. f) The measured well separation in Matrigel and soft PEG is plotted versus the pillar separation measured for the elastomer print. g) The measured well depth in Matrigel and soft PEG is plotted versus the designed height of the elastomer print. PEG hydrogels were visualized by 2000 kDa FITC dextran entrapped in network. Scale bars 50 μm. n = 3 patterned hydrogels each containing 3 technical replicates averaged together.
Opposite trends were observed for patterned resolution in the PEG versus Matrigel hydrogels. The spacing between patterned wells in the PEG hydrogels followed a slope slightly higher than 1 (1.27 ± 0.10), suggesting the spacing between wells grows larger by an amount proportional to the original patterned distance (Figure 4f). This observation is consistent with the isotropic expansion expected as the cast hydrogel reaches equilibrium swelling in PBS42,43. In contrast, the space between the wells in Matrigel mostly contracted, with the smallest patterned gap of 40 μm measuring only 16 μm. Instead of a change in slope of measured well gap plotted against measured pillar gap, we observe a downward shift in the y-intercept. This suggested to us that the observed effect is likely not a function of the measured distance between pillars, but rather a property of the Matrigel itself.
Finally, we explored the depths of the patterned wells as a function of pillar height. Given that the print height was controlled by the layer-by-layer printing process, the patterned well depth was compared directly to the patterned pillar height. Patterned wells in PEG hydrogels were slightly deeper with a 200 μm pillar forming a 248 ± 8 μm deep hole (Figure 4g). This enlargement was proportional to the height of the initial pattern with a slope greater than 1 indicative of hydrogel swelling. In contrast, the wells in Matrigel were shallower; the sample 200 μm pillar formed a 70 ± 17 μm deep well. This contraction was proportional to the height of the original pattern with a slope less than 1 indicative of hydrogel contraction.
Overall, patterns transferred into PEG reflect the original pattern with high fidelity, albeit with a predictable enlargement of critical dimension, resolution, and depth consistent with isometric expansion. We hypothesize that pattern trends in Matrigel are consistent with isometric contraction of a viscoplastic hydrogel. Since the designed thiol solution has a high concentration of thiol and tris buffer, sudden introduction to the soft hydrogels may cause a sudden isometric contraction on the timescale of the diffusion of water out of the hydrogel and salts and thiol into the hydrogel. The thioester elastomer has a higher modulus and, therefore, would not undergo the same amount of isometric contraction, potentially causing compressive stress to build up around the imbedded pattern. Matrigel, as a known viscoplastic hydrogel, would relax the imposed stress around the elastomer features through network rearrangement.44 This may explain why the diameter of patterned holes enlarge while other metrics show contraction. These same trends were not observed in PEG hydrogels, as PEG hydrogels are poroelastic materials; compressive load over time results in the movement of water out of the hydrogel but no plastic deformation.45
Thioester fabrication facilitates formation of close patterned and overhanging structures in Matrigel
After characterizing the effect of the material chemistry on the final printed features’ dimensions and resolution, we next investigated the ability of the sacrificial thioester mold to transfer close-patterned and overhanging features embedded within Matrigel (Figure 5a). The current in vitro model of the intestinal organoid is a spherical cyst structure (~100–200 μm in diameter) with crypt-like buds where the stem cells reside. To template the size of the intestinal organoid cysts, we fabricated a close-packed, 5×5 array of 150 μm tall stems that were 50 μm in diameter and space 50 μm apart, and then printed a 150 μm sphere on the top of the stem (Figure 5b). The thioester elastomeric mold was pressed into Matrigel and subsequently degraded. Using a laser scanning confocal microscope, tile images were collected at the top and middle of the features patterned within Matrigel (Figure 5c–d). Consistent with prior results, the patterned well dimensions in Matrigel were enlarged compared to the original 3D printed elastomer mold, with the stem portion of the well enlarging from 62 ± 3 μm to 143 ± 5 μm, and the ball portion enlarging from 130 ± 2 μm to 203 ± 4 μm (Figure 5e). Similar to trends reported in Figure 4, the space between features contracted compared to the original printed construct with crypt-crypt distances reducing from 52 ± 3 μm to 41 ± 2 μm and opening-opening distances reducing from 122 ± 5 μm to 106 ± 5 μm. The feature depth was also reduced from 227 ± 8 μm to 84 ± 2 μm.
Figure 5:
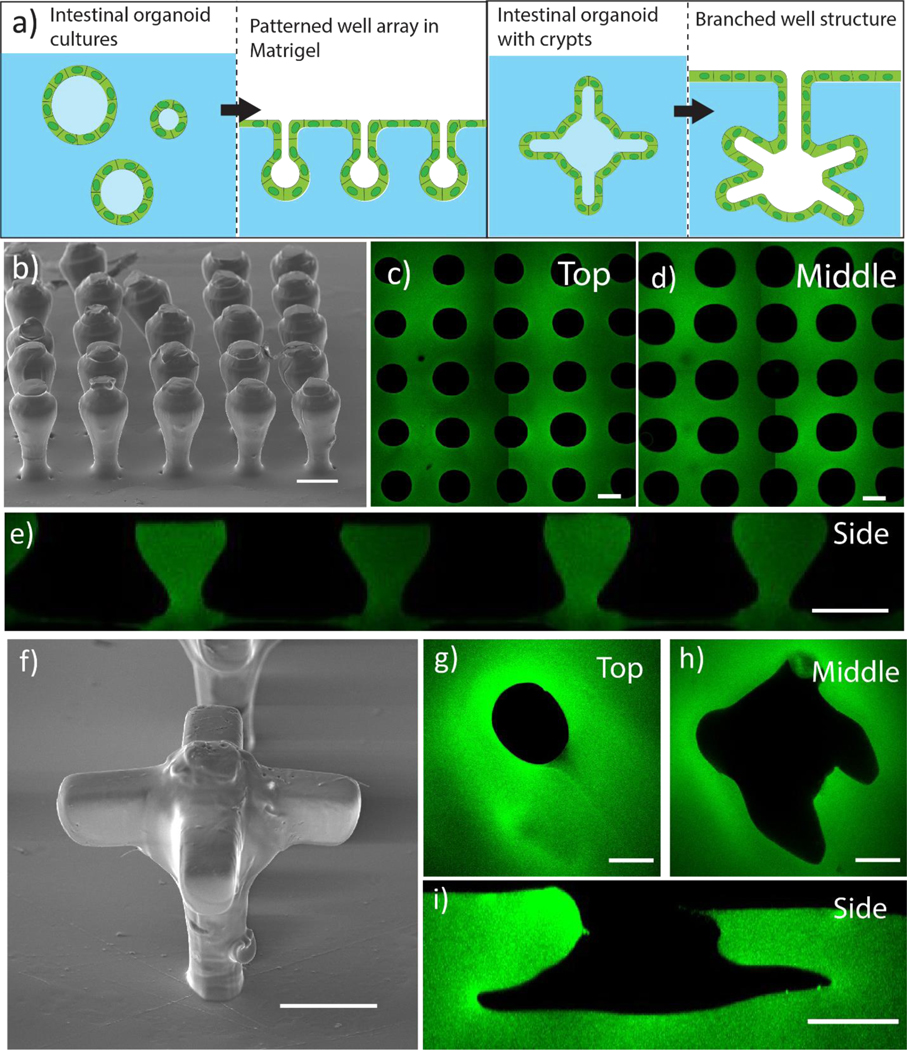
a)Printed thioester features were reproducibly degraded in the presence of Matrigel to cast mold close-packed and overhanging structures reminiscent of the intestinal organoid features. b) SEM image of printed crypt structures consisting of a 150 μm ball on top of a 50 μm wide by 150 μm tall stem printed with a 50 μm spacing. Scale bar 100 μm. Printed organoid features were patterned into Matrigel and a tile scan was taken of the c) stem and d) ball regions of the well. e) Z-stack images of each well were taken with laser scanning confocal microscopy and used to construct an orthogonal view of the patterned array along the XZ plane. Scale bar 100 μm. f) SEM image of branched crypt structure shows defined overhang features. scale bar 100 μm. Printed organoid features were patterned into Matrigel and images were taken of the g) stem and h) branched crypt regions of the well. i) Z-stack images of each well were taken with laser scanning confocal microscopy and used to construct an orthogonal view of the patterned array along the XZ plane. Scale bar 100 μm. Matrigel hydrogels were labeled with FITC fluorophore after patterning. For more details, please see Patterned hydrogel staining and image analysisin methods
Next, PEG thioester sacrificial molds were fabricated with similar dimensions, but including 100 μm long, 50 μm tall and 50 μm wide branched, channels to template the stem cell crypt niches (Figure 5f). After degradation, images taken at the surface and middle of the patterned Matrigel once more showed enlarged and slightly misshapen branched structures (Figure 5g–h). Nevertheless, distinct overhang void spaces with high aspect ratios were formed in Matrigel (Figure 5i).
Intestinal stem cells form epithelial layers conforming to 3D patterned features
Intestinal stem cells (ISCs) encapsulated in Matrigel grow to form cyst-like enteroids and upon differentiation and self-organize to form multicellular intestinal organoid where paneth and ISCs reside in the budded crypt regions. When ISCs are cultured on 2D patterned substrates, they proliferate to form a confluent epithelial cell monolayer, where the cell sheet layer conforms to the underlying features of the of the tissue culture substrate. Here, to recapitulate the dimensions of mouse intestinal villi and crypt structures46, we first printed pillars that were 50 μm in diameter and 200 μm in height using the thioester formulation. The sacrificial mold was then used to transfer the intestinal features into Matrigel, forming wells 104 ± 4 μm in diameter and 72 ± 2 μm deep (Figure S8). Finally, ISCs were seeded in the patterned Matrigel structures. After 24 h, a monolayer of the epithelial cells forms at the base of the wells, and over times, the ISC begin to migrate towards the gel surface (Figure 6a). By 72 h, a confluent monolayer of epithelial cells forms on the hydrogel surface. Samples fixed after 72 h and stained for F-actin and cell nuclei showed complete coverage of a confluent monolayer that conformed to the top, sides and bottom of the patterned well geometries with nuclei interspersed throughout. (Figure 6b) Monolayer confluency can be additionally visualized through a xz-projection, shown in Supplementary Figure S8d. Z-stack images were used to identify the location of cell nuclei along the top, sides, and bottom, of patterned microwells. Nuclear coordinates were scaled to the measured radius of each well, and a frequency map was plotted with MATLAB (Figure 6c). Similar frequencies of cell nuclei were observed at the top, middle, and bottom, of the well structure suggesting that after ISCs underwent proliferation and migration out of the wells, a uniform distribution of cells was reached in the final confluent layer.
Figure 6.
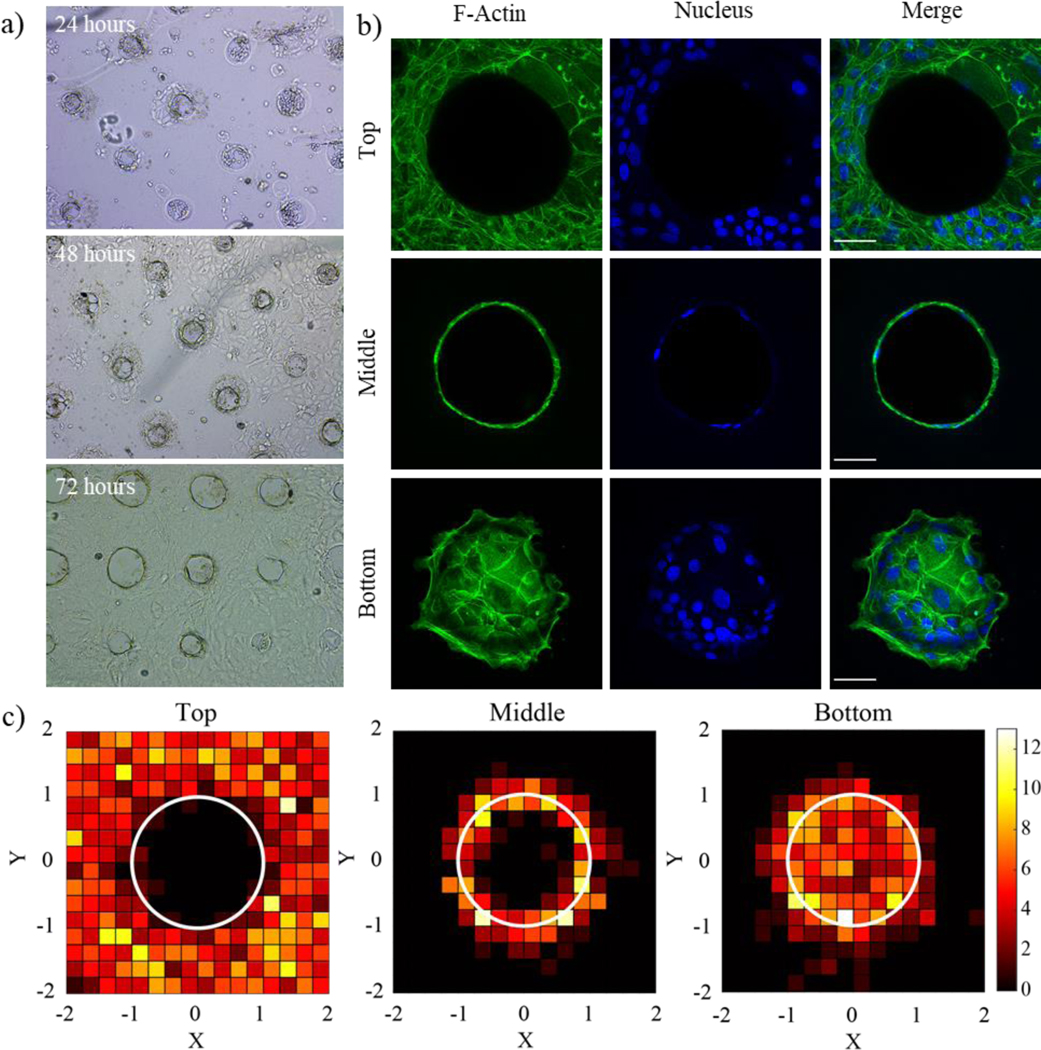
a) Single intestinal stem cells were seeded onto patterned Matrigel gels and formed confluent monolayers over the course of three days. b) Immunostaining for F-actin (green) and nuclei (blue) shows the formation of a cell monolayer covering the patterned features. Scale bar is 50um. c) Frequency map images representing nuclear placement of ISCs along the top, middle, and bottom, of each well show even dispersion along the surfaces of the patterned well structures. X and Y image dimensions were normalized to the radius of each microwell which is represented by a white circle. The color bar represents the number of nuclei present in each region. N = 20 wells.
Discussion
With the growing applications and interest in intestinal organoids, there is a heightened appreciation as to the role of microenvironmental factors, especially those that affect cell shape and final organoid geometry, on the final structure of the differentiated organoid7,47 High resolution 3D printing techniques, such as DLP, can generate free-standing crypt and villi structures relevant to the intestinal organoid (<100 μm), but usually require printing resins >100× stiffer than Matrigel, which is used for ISC cultures. Sacrificial 3D printing techniques have been used to pattern complex 3D geometries in otherwise less printable hydrogels like Matrigel,13 but a lack of photopolymerizable resins that degrade rapidly under mild conditions compatible with Matrigel has hindered the translation of sacrificial approaches to light-based 3D printing systems. To address these issues, we developed a photocurable PEG thiol-norbornene elastomer with thioester function groups on the backbone to facilitate network erosion. A high concentration of thiol (2-mercaptoethanol) and mild basic conditions (pH 9.0) were selected to drive trans-thioesterification of 2-mercaptoethanol with thioester crosslinks and balance gentile degradation conditions with fast degradation times (<2 h). These photocurable PEG thioester resins readily translated to existing high-resolution DLP 3D printing platforms where critical dimensions of 37 ± 4 μm and resolutions of 22 ± 5 μm were achieved.
One limitation of the thioester sacrificial patterning in this work is that while degradation conditions are gentle enough to maintain integrity of soft tissue culture substrates, the use of a non-physiological pH precludes the presence of cells during elastomer erosion. This is an important limitation compared to other light22 and extrusion13,15 based sacrificial printing strategies, where cytocompatible fabrication strategies easily facilitate the creation of cocultures by culturing one cell type in the hydrogel substrate, and another cell type in the degraded construct. In this work, the elevated pH was used to accelerate degradation by increasing the amount of deprotonated 2-mercaptoethanol. Although pH exerts a potent influence on thioester exchange, other factors such as pKa of the thiol and thioester could be used to achieve similar rates of degradation at more physiological pH values. To facilitate fabrication in the presence of cells a thioester resin could be made with a lower pKa thiol and degraded with a molecule like cystine which is cytocompatible and readily forms thiolate species at physiological pH.
While the ~10 μm dimension and resolution limits that are achievable with photoablative techniques remain unmatched for patterning biological materials17,19, the DLP printed degradable thioester materials provide a complementary biofabrication method that provides the attainment of cellularly-relevant feature sizes with rapid printing of large arrays of features. Results (e.g., Fig. 4e) demonstrated that thioester sacrificial molding allow feature sizes >40 μm in soft cell culture substrates, which compare favorably to commonly used PDMS micromolding34,35. However, the on-demand degradation of the thioesters and tailoring of the material properties offers additional benefits for creating features embedded within soft hydrogels and the ability to fabricate interconnected and overhanging features. We further demonstrate these overhanging features can be printed close together to form dense arrays with resolutions as small as 40 μm and that the overhangs can be patterned in Matrigel with either low or high aspect ratios. In addition, branched structures, extending >100 μm into the culture substrate, from primary culture well were created. Since each layer is printed with a single light exposure, DLP 3D printing techniques provide the ease of scalability and speed compared to photoablative techniques, where patterning occurs voxel by voxel. However, DLP printing requires the ability to control layer thickness (e.g., by controlling light attenuation) with the final structural dimension in mind. Here, we optimized the layer thickness for 10 μm features, which maximize printing speed while maintaining the cellular scale resolution needed for patterning crypt-like overhang structures found in intestinal organoids. To fabricate structures at other length scales ranging from sub-cellular to macroscopic, further optimization of the layer thickness and cure depth could be readily performed.
Lastly, we showed that Matrigel features patterned with this method support the growth of intestinal stem cell monolayers, and successfully imparts geometry to the epithelial layer which conforms to the designed pattern. Recent advances to primary-cell derived monolayer culture platforms have focused on the optimization of culture and substrate conditions in attempt to replicate in vivo intestinal function48–50. However, these in vitro monolayer cultures have not considered the shape of the crypt-villus axis, which imparts three-dimensional behavior onto the intestinal epithelium and is necessary to generate morphogen gradients51 and cell shape changes52,53 essential for proper development. While there has been some work to incorporate 3D topologies into intestinal monolayer cultures, these applications have relied on the use of PDMS or 3D printed plastic stamps to generate topology10,54. As a soft lithographic technique, stamping to create patterned features for intestinal monolayers has only been used to create simple features in stiff hydrogels (G’ > 8 kPa) 10,54. In effect, this limits the cell morphologies and behaviors that are attainable. For example, substrate stiffness has been shown to influence crypt domain morphology in 2D monolayers55 and 3D encapsulated organoids3,56. With PDMS stamps, monolayer cultures are restricted to stiff materials, thus limiting the range of biological behaviors that are attainable. Instead, we show that printed thioester features can be transferred into a variety of soft, biologically relevant materials (<1000 Pa), while still maintaining feature resolution. This platform enables the ability to more thoroughly investigate specific intestinal disease states, like fibrosis, in which substrate stiffness is a contributing factor57. Additionally, our platform enables the generation of complex, overhanging features that cannot be realized with PDMS stamping. The shape of the crypt-villus axis is known to direct cell fate specification in the developing intestine52,58 and the ability to create complex and physiologically relevant 3D shapes with greater resolution will offer greater insight into these developmental processes.
Conclusion
Intestinal organoids have become important tools for studying fundamental biology and modeling disease in vitro. With control over geometry and shape becoming increasingly important in some complex intestinal organoid systems, we sought to develop a new biofabrication approach that could match resolution of soft lithographic approaches while maintaining advantages of 3D printing, like forming high resolution overhanging features. In this work, we present a novel elastomer resin that is compatible with state of the art light based 3D printing techniques and undergoes rapid degradation in the presence of 2-mercaptoethanol. We show that thioester elastomer resin can be printed using DLP 3D printing at resolution and feature sizes at and above 30 μm. The subsequent feature erosion was triggered by the introduction of a thiol to solution and was gentile enough to be used in the presence of Matrigel. This work shines a light on the potential of sacrificial resins based on covalent adaptable chemistries by introducing a novel fabrication method for organoid structures with complex geometries. This tool expands on existing biofabrication methods and allows for the testing of new hypotheses related to the role geometry plays in complex organoid cultures.
Supplementary Material
Acknowledgements:
This work was supported by grants from the National Institutes of Health (DE016523, R01 DK120921), and the National Science Foundation (1826454). J.J.H recognizes support from the Department of Education program “Graduate Assistantships in Areas of National Need” (GAANN) F.M.Y. acknowledges the Department of Education (GAANN) and the National Institutes of Health (F31 DK126427) for funding. Image and data analysis were assisted by Jian Wei Tay, PhD, at the BioFrontiers Institute Advanced Light Microscopy Core (RRID: SCR_018302). The authors would also like to thank Dr. Laura Macdougall, Dr. Michael Blatchley, Dr. Benjamin Richardson, and Dr. Kemal Arda Günay for thoughtful comments and critical reviews in the preparation of this manuscript. The authors do not have any conflicts of interest to declare.
References:
- 1.Hofer M & Lutolf MP Engineering organoids. Nature Reviews Materials (2021) doi: 10.1038/s41578-021-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato T & Clevers H Growing self-organizing mini-guts from a single intestinal stem cell: Mechanism and applications. Science (2013) doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 3.Hushka EA, Yavitt FM, Brown TE, Dempsey PJ & Anseth KS Relaxation of Extracellular Matrix Forces Directs Crypt Formation and Architecture in Intestinal Organoids. Adv. Healthc. Mater 9, 1901214 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancaster MA & Knoblich JA Organogenesis in a dish: Modeling development and disease using organoid technologies. Science (80-. ). 345, 1247125–1247125 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Nerger BA & Nelson CM 3D culture models for studying branching morphogenesis in the mammary gland and mammalian lung. Biomaterials 198, 135–145 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gjorevski N et al. Designer matrices for intestinal stem cell and organoid culture. Nat. Publ. Gr 539, (2016). [DOI] [PubMed] [Google Scholar]
- 7.Gjorevski N et al. Tisse geometry drives deterministic organoid patterning. Science (80-. ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan EYS, Suntornnond R & Yeong WY High-Resolution Novel Indirect Bioprinting of Low-Viscosity Cell-Laden Hydrogels via Model-Support Bioink Interaction. 3D Print. Addit. Manuf 8, 69–78 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma X et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. U. S. A 113, 2206–2211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y et al. A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials 128, 44–55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta P, Ayan B & Ozbolat IT Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomaterialia vol. 51 1–20 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Golden AP & Tien J Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip (2007) doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 13.Miller JS et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater 11, 768–774 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolesky DB et al. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater 26, 3124–3130 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Kolesky DB, Homan KA, Skylar-Scott MA & Lewis JA Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. U. S. A 113, 3179–3184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasturel A, Strale PO & Studer V Tailoring Common Hydrogels into 3D Cell Culture Templates. Adv. Healthc. Mater 9, 2000519 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Nikolaev M et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585, 574–578 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Brandenberg N & Lutolf MP In Situ Patterning of Microfluidic Networks in 3D Cell-Laden Hydrogels. Adv. Mater (2016) doi: 10.1002/adma.201601099. [DOI] [PubMed] [Google Scholar]
- 19.Arakawa C et al. Biophysical and biomolecular interactions of malaria-infected erythrocytes in engineered human capillaries. Sci. Adv (2020) doi: 10.1126/sciadv.aay7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzzi EA & Tibbitt MW Additive Manufacturing of Precision Biomaterials. Adv. Mater 1901994 (2019) doi: 10.1002/adma.201901994. [DOI] [PubMed] [Google Scholar]
- 21.Kinstlinger IS et al. Generation of model tissues with dendritic vascular networks via sacrificial laser-sintered carbohydrate templates. Nat. Biomed. Eng (2020) doi: 10.1038/s41551-020-0566-1. [DOI] [PubMed] [Google Scholar]
- 22.Thomas A et al. Vascular bioprinting with enzymatically degradable bioinks via multi-material projection-based stereolithography. Acta Biomater. (2020) doi: 10.1016/j.actbio.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 23.Schultz KM & Anseth KS Monitoring degradation of matrix metalloproteinases-cleavable PEG hydrogels via multiple particle tracking microrheology. Soft Matter 9, 1570–1579 (2013). [Google Scholar]
- 24.Rice MA, Sanchez-Adams J & Anseth KS Exogenously triggered, enzymatic degradation of photopolymerized hydrogels with polycaprolactone subunits: Experimental observation and modeling of mass loss behavior. Biomacromolecules 7, 1968–1975 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown TE et al. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 178, 496–503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worrell BT et al. Bistable and photoswitchable states of matter. Nat. Commun 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carberry BJ, Rao VV & Anseth KS Phototunable Viscoelasticity in Hydrogels Through Thioester Exchange. Ann. Biomed. Eng (2020) doi: 10.1007/s10439-020-02460-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghobril C, Charoen K, Rodriguez EK, Nazarian A & Grinstaff MW A Dendritic Thioester Hydrogel Based on Thiol-Thioester Exchange as a Dissolvable Sealant System for Wound Closure. Angew. Chemie Int. Ed 52, 14070–14074 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konieczynska MD et al. On-demand dissolution of a dendritic hydrogel-based dressing for second-degree burn wounds via thiol-thioester exchange reaction HHS Public Access. Angew Chem Int Ed Engl 55, 9984–9987 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macdougall LJ, Pérez-Madrigal MM, Arno MC & Dove AP Nonswelling Thiol-Yne Cross-Linked Hydrogel Materials as Cytocompatible Soft Tissue Scaffolds. Biomacromolecules 19, 1378–1388 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller DB, Alim MD & McLeod RR Reflection suppression via elastomeric films. Opt. Lett 44, 6021 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Urness AC, Moore ED, Kamysiak KK, Cole MC & McLeod RR Liquid deposition photolithography for submicrometer resolution three-dimensional index structuring with large throughput. Light Sci. Appl 2, e56–e56 (2013). [Google Scholar]
- 33.Dendukuri D et al. Modeling of oxygen-inhibited free radical photopolymerization in a PDMS microfluidic device. Macromolecules 41, 8547–8556 (2008). [Google Scholar]
- 34.Nelson CM, VanDuijn MM, Inman JL, Fletcher DA & Bissell MJ Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science (80-. ). 314, 298–300 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson CM, Inman JL & Bissell MJ Three-dimensional lithographically defined organotypic tissue arrays for quantitative analysis of morphogenesis and neoplastic progression. Nat. Protoc 3, 674–678 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fairbanks BD et al. A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv. Mater 21, 5005–5010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worrell BT et al. A user’s guide to the thiol-thioester exchange in organic media: Scope, limitations, and applications in material science. Polym. Chem 9, 4523–4534 (2018). [Google Scholar]
- 38.Kamata H, Akagi Y, Kayasuga-Kariya Y, Chung U Il & Sakai T ‘Nonswellable’ hydrogel without mechanical hysteresis. Science (80-. ). 343, 873–875 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Kamata H, Kushiro K, Takai M, Chung U & Sakai T Non-Osmotic Hydrogels: A Rational Strategy for Safely Degradable Hydrogels. Angew. Chemie Int. Ed 55, 9282–9286 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Muralidharan A, Uzcategui AC, McLeod RR & Bryant SJ Stereolithographic 3D Printing for Deterministic Control over Integration in Dual-Material Composites. Adv. Mater. Technol 4, 1900592 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins CI, Brown TE & Killgore JP Digital light processing in a hybrid atomic force microscope: In Situ, nanoscale characterization of the printing process. Addit. Manuf 38, 101744 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubinstein M & Colby RH Polymer Physics. (Oxford University Press, 2003). [Google Scholar]
- 43.Hiemenz PC; Lodge TP Polymer Chemistry, Second Edition. CRC Press; (2007). [Google Scholar]
- 44.Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ & Shenoy VB Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature vol. 584 535–546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaudhuri O et al. Substrate stress relaxation regulates cell spreading. Nat. Commun (2015) doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu YY et al. Microtome-Free 3-Dimensional Confocal Imaging Method for Visualization of Mouse Intestine With Subcellular-Level Resolution. Gastroenterology 137, 453–465 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brassard JA & Lutolf MP Cell Stem Cell Review Engineering Stem Cell Self-organization to Build Better Organoids. Stem Cell 24, 860–876 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Tong Z et al. Towards a defined ECM and small molecule based monolayer culture system for the expansion of mouse and human intestinal stem cells. Biomaterials 154, 60–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moon C, Vandussen KL, Miyoshi H & Stappenbeck TS Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 7, 818–828 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Qi Z, Li X, Du Y & Chen YG Monolayer culture of intestinal epithelium sustains Lgr5+ intestinal stem cells. Cell Discov. 4, 4–6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shyer AE, Huycke TR, Lee C, Mahadevan L & Tabin CJ Bending Gradients: How the intestinal stem cell gets its home. Cell 161, 569–580 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sumigray KD, Terwilliger M & Lechler T Morphogenesis and Compartmentalization of the Intestinal Crypt. Dev. Cell 45, 183–197.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luciano M et al. Large-scale curvature sensing by epithelial monolayers depends on active cell mechanics and nuclear mechanoadaptation. bioRxiv (2020). [Google Scholar]
- 54.Wilson RL et al. Protein-functionalized poly(ethylene glycol) hydrogels as scaffolds for monolayer organoid culture. Tissue Eng. Part C Methods 27, 12–23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pérez-González C et al. Mechanical compartmentalization of the intestinal organoid enables crypt folding and collective cell migration. bioRxiv 1–26 (2020) doi: 10.1101/2020.09.20.299552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yavitt FM et al. The Effect of Thiol Structure on Allyl Sulfide Photodegradable Hydrogels and their Application as a Degradable Scaffold for Organoid Passaging. Adv. Mater 32, 1905366 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson LA et al. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm. Bowel Dis 19, 891–903 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walton KD, Mishkind D, Riddle MR, Tabin CJ & Gumucio DL Blueprint for an intestinal villus: Species-specific assembly required. Wiley Interdiscip. Rev. Dev. Biol 7, 1–19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.