Abstract
Background and purpose:
Kidney diseases impose significant global health challenges. Potassium dichromate (PD) is a heavy metal frequently associated with nephrotoxicity. PD prompts oxidative and inflammatory injuries in renal tissues. L-carnitine is a naturally-occurring amino acid commonly used as a supplement.
Experimental approach:
Forty rats were randomly allocated into 5 groups. Group 1 (normal) received only saline. Nephrotoxicity was induced in the remaining groups by PD (15 mg/kg; i.p). Group 2 served as a nephrotoxic group. Groups 3-5 received L-carnitine (25, 50, and 100 mg/kg; p.o.), respectively for 4 weeks.
Findings/Results:
PD administration resulted in elevated serum creatinine and blood urea nitrogen accompanied by diminished reduced glutathione and elevated malondialdehyde, tumor necrosis factor-alpha, and transforming growth factor-beta renal tissue contents relative to normal rats. PD also produced apoptotic histopathological injuries and down-regulated PI3K/Akt signaling pathway; signifying ongoing apoptosis. In the current work, L-carnitine use in the selected dose levels resulted in improvement of all the aforementioned serum, renal tissue, and histological parameters relative to nephrotoxic rats. L-carnitine up-regulated PI3K/Akt signaling pathway that was down-regulated post PD use.
Conclusion and implications:
Collectively, the study highlighted that the possible mechanisms beyond the beneficial effects of L-carnitine are mainly through its antioxidant as well as anti-inflammatory actions. L- carnitine significantly abrogated apoptosis via up-regulation of PI3K/Akt signaling pathway and signified restoration of normal renal cell proliferation and functionality.
Keywords: L-carnitine, Nephrotoxicity, PI3K/Akt pathway, Potassium dichromate
INTRODUCTION
Kidney diseases and associated renal insufficiencies are worldwide health problems where more patients mandate either transplant or frequent dialysis. Diverse pathophysiological processes are associated with abnormal renal function. A gradual decline in the number of functional nephrons leads to a drop in the glomerular filtration rate (1). Severe abnormalities affect the intra-renal microcirculation leading to an elevation in intra-renal pressure, activation of the reninangiotensin system, and initiation of intra-renal inflammatory processes (2). Nephrotoxicity may originate as an adverse reaction against non-steroidal anti-inflammatory drugs, some antibiotics, antineoplastic agents, or xenobiotics (3,4,5). Therefore, It is of high importance to keep the kidney healthy for all people affected by kidney diseases (6), besides, it has been very crucial to scout for new nephroprotective agents to improve renal function, renal tissue healing, and regeneration (7).
Heavy metals are xenobiotic agents commonly associated with nephrotoxicity (8). Potassium dichromate (PD) is a strong oxidizer that is highly abundant in the environment producing harmful effects to the biological systems, e.g, multi-organ toxicity as well as teratogenic, mutagenic, and carcinogenic effects (9). PD is reduced by many cellar metabolites to give chromium. Chromium prompts the production of reactive oxygen species leading to oxidative injuries in the liver, brain, and renal tissues. Chromium also initiates the inflammatory process via the generation of numerous pro-inflammatory cytokines and produces DNA damage as well as lipid peroxidation; eventually leading to severe nephrotoxicity (10).
Levo-carnitine (L-carnitine), 3-hydroxy- trimethyl-amino butyric acid, is a naturally occurring amino acid derivative that is commonly used as an antioxidant supplement. L-carnitine is endogenously synthesized in the liver, kidneys, and brain (11). L-carnitine improves mitochondrial functionality thus plays an essential role in energy production via fatty acids transportation into mitochondria (12). L-carnitine metabolism occurs mainly in the kidneys. After renal excretion; most of the L-carnitine is re-absorbed back into the proximal tubules of the kidneys (13).
Phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway is essential in the promotion of cellular growth, proliferation, and longevity. PI3K/Akt signaling pathway over-activation has been involved in several disorders including auto-immune diseases and multiple cancers characterized by unrestrained cellular overgrowth and proliferation (14). On the other hand, PI3K/Akt signaling pathway is down-regulated in the case of apoptosis. Recently, pharmacological therapies are directed towards the modulation of the PI3K/Akt signaling pathway in search of effective treatment for various disease conditions (15). Therefore, the current study was aimed at testing the effect of L-carnitine on PD-induced nephrotoxicity in rats; highlighting L-carnitine’s possible antioxidant and anti-inflammatory effects with emphasis on its ability to abrogate apoptosis via modulation of the PI3K/Akt signaling pathway.
MATERIALS AND METHODS
Animals
Wister albino male rats, weighing 140-150 g, were provided by the Animal House of the National Research Centre (Cairo, Egypt). Rats were group-housed under temperature- and light-controlled conditions (24 ± 2 °C, 12/12-h light/dark cycle) and had free access to standard laboratory rodent chow and water. The animal experiments were performed per the guidelines of the Institutional Animal Ethics Committee (Medical Research Ethics Committee (MREC) of the NRC, Cairo, Egypt, and following the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Chemicals and kits
PD was purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). L-carnitine® was purchased from MEPACO, Egypt. Reduced glutathione (GSH), catalase, and malondialdehyde (MDA) kits were obtained from Biodiagnostic kits® (Egypt). Tumor necrosis factor-alpha (TNF-α) and (PI3K) ELISA kits were purchased from specific SunRed® (China) and transforming growth factor-beta (TGF-β) ELISA kit was purchased from NOVA® (Beijing, China). A real-time polymerase chain reaction (RT-PCR) quantification kit of protein kinase B (Akt) was purchased from Toyopo Biotechnology® (Shanghai, China). All other chemicals were of the highest analytical grade available.
Experimental design
Forty rats were randomly allocated into five groups (n = 8). The first group received i.p. saline and served as a normal control group. Nephrotoxicity was induced in the remaining 4 groups using a single dose of PD (15 mg/kg; i.p). Group 2 received only PD and served as a nephrotoxic control group (16). Groups 3-5 received L-carnitine (25, 50, and 100 mg/kg; p.o.); respectively for 4 weeks starting one week before PD injection (17,18). No mortality was reported in the experimental animals.
Serum biochemical analysis
At the end of the experiment, blood samples were withdrawn from rats of all groups via the retro-orbital vein under anesthesia. Collected blood samples were allowed to stand for 10 min at room temperature, then centrifuged at 4 °C using a cooling centrifuge (Laborezentrifugen, 2k15; Sigma, Germany) at 3000 r/min for 10 min and sera were separated for the assessment of serum creatinine and urea.
Tissue biochemical analysis
Immediately after blood sampling, rats were sacrificed by cervical dislocation under sodium pentobarbital anesthesia (40 mg/kg; i.p.). The two kidneys from each rat were immediately dissected out and rinsed with PBS to remove excess blood. Parts from both kidneys were homogenized (MPW-120 homogenizer, Med instruments, Poland) to obtain 20% homogenate that was stored overnight at -20 °C. The homogenates were centrifuged for 5 min at 5000 g using a cooling centrifuge (Sigma and Laborzentrifugen, 2k15, Germany).
The supernatant was collected and stored at -80 °C and then used for estimation of renal tissue contents of GSH, MDA, TNF-α, TGF-β, and PI3K using specific ELISA kits.
RT-PCR quantification of Akt
The mRNA expression level of the Akt gene was assessed using RT-PCR standardized by co-amplification with the housekeeping GAPDH gene as an internal control. Akt RNA was extracted from kidney tissue using Trizol reagent. RNA was reverse-transcribed using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and then used for PCR with specific primers. Quantification of Akt was carried out using Akt RT-PCR fluorescence diagnostic kit (Cat. No. A.B 517302) according to manufacturers’ instructions. For amplification, 40 cycles of 95 °C for 5 s, and 61 °C for 1 s, 72 °C for 30 s, then 1 min at 60 °C and 10 min at 72 °C were performed with Akt forward primer 5′-GTGGCAAGATGTGTATGAG-3′, Akt reverse primer 5′-CTGGCTGAGTAGGAGAAC-3′, using Rotor-Gene Q5 plex real-time Rotary analyzer (Corbettlife sciences, USA).
Histopathological examination
Other parts of the kidneys were fixed in 10% neutral buffered formalin and embedded in paraffin wax. Four μm-thick sections were stained with hematoxylin and eosin (H&E) and examined by a histopathologist using a binocular Olympus CX31 microscope (19). Pathological assessment of kidney damage was assessed in ten random low power fields (10×); according to the percentage of tissue showing damage (20). A computer system using the software Leica Qwin Plus version 3 (Switzer land) was used to count neutrophils in 5 fields in each group using a magnification 400× by light microscopy transferred to the monitor’s screen. The histopathological alteration scoring of kidneys was analyzed semi-quantitatively by a blinded investigator, where 0 was given when no alterations were found, 1 for mild alterations, 2 for medium alterations, and 3 for severe alterations based on a previously established scoring system for acute kidney injury (21). Criteria for histopathological assessment were the formation of the apoptotic epithelial lining, brush borders with dilated congested blood vessels and collecting tubules with congested epithelial lining. Mean values from the scores of the latter criteria were taken together as the total injury score.
Statistical analysis
All experiments were performed in triplicates and the results were presented as mean ± standard error of the mean (SEM). The statistical significance was calculated and analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons tests using SPSS software (Version 17, Chicago). Values of P < 0.05 were considered significant.
RESULTS
Assessment of renal function
PD elevated renal function parameters; creatinine and urea serum levels by 41% and 91% respectively, as compared to normal control. The administration of L-carnitine in all doses decreased serum levels of creatinine serum levels by 5%, 11%, and 17%; and urea by 38%, 41%, and 44% respectively, as compared to the PD group. In addition, high- dose L-carnitine reduced serum levels of creatinine and urea, as compared to other doses of L-carnitine (Table 1).
Table 1.
Effect of L-carnitine on the renal function parameters. Data are presented as the mean ± SEM, n = 8.
| Parameters | Normal control | PD (15 mg/kg) | L-carnitine (25 mg/kg) | L-carnitine (50 mg/kg) | L-carnitine (100 mg/kg) |
|---|---|---|---|---|---|
| Creatinine (mg/dL) | 1.20 ± 0.03 | 1.69 ± 0.01a | 1.59 ± 0.03ab | 1.50 ± 0.01abc | 1.40 ± 0.02abcd |
| Urea (mg/dL) | 4.39 ± 0.03 | 8.38 ± 0.10a | 5.17 ± 0.02ab | 4.92 ± 0.02abc | 4.67 ± 0.05abcd |
aP < 0.05 Indicates significant differences compared to the control group, bP < 0.05 statistically significant from PD group, cP < 0.05 versus L-carnitine at 25 mg/kg, and dP < 0.05 against L-carnitine at 50 mg/kg. PD, Potassium dichromate.
Assessment of oxidative stress in renal tissue
Analysis of the data exhibited a significant reduction in GSH renal contents by 56% in the PD group accompanied by an elevation of MDA renal content by 70%, as compared with the normal control group. The administration of L-carnitine in all doses led to an elevation of the renal content of GSH by 35%, 56%, and 111%, respectively, as well as a reduction in renal MDA level by 27%, 33%, and 38%, respectively, compared to the PD group. Also, a high dose of L-carnitine returned the MDA level to its normal value as compared to the normal control group. In addition, high-dose L- carnitine reduced MDA renal content and elevated GSH renal content; as compared to other doses of L-carnitine (Fig. 1).
Fig. 1.
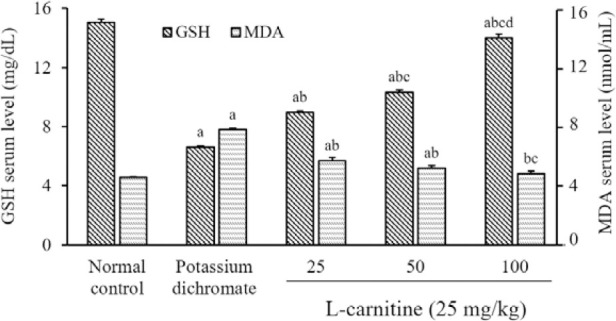
Effect of L-carnitine on the GSH and MDA contents of renal tissue. Data are presented as mean ± SEM, n = 8. aP <0.05 Indicates significant differences compared to the control group, bP < 0.05 statistically significant from potassium dichromate (15 mg/kg) group, cP < 0.05 versus L-carnitine at 25 mg/kg, and dP < 0.05 against L-carnitine at 50 mg/kg. GSH, Reduced glutathione; MDA, malondialdehyde.
Assessment of renal contents of inflammatory mediators
There is an elevation in the renal content of TNF-α and TGF-β under the influence of PD by 0.6 fold and 6 folds, respectively, as compared with those in the normal control group. The use of all doses of L-carnitine decreased the renal content of TNF-α by 22%, 29%, and 38%; as well as TGF-β content by 24%, 67%, and 79%, respectively, in comparison with PD nephrotoxic animals. The renal contents of TNF-α and TGF-β returned to their normal levels after treatment with the high dose of L- carnitine as compared to the normal control group (Fig. 2).
Fig. 2.
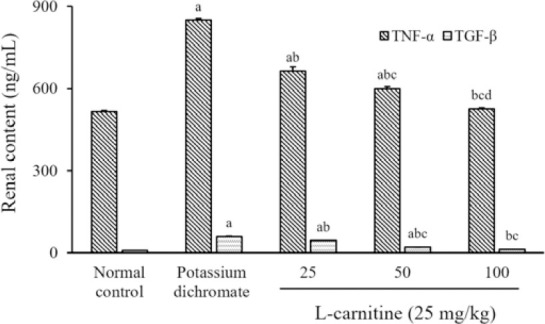
Effect of L-carnitine on of renal contents of TNF-α and TGF-β. Data are presented as mean ± SEM, n = 8. aP < 0.05 Indicates significant differences compared to the control group, bP < 0.05 statistically significant from potassium dichromate (15 mg/kg) group, cP < 0.05 versus L-carnitine at 25 mg/kg, and dP < 0.05 against L-carnitine at 50 mg/kg. TNF-α, Tumor necrosis factor-alpha; TGF-β, transforming growth factor-beta.
Assessment of renal tissue content of PI3K
PD produced a reduction in the kidney content of PI3K by 89% as compared with the normal value. The treatment of all doses of L-carnitine increased the kidney content of PI3K by 160%, 213%, and 375%, respectively; in comparison with the PD-treated group. Moreover, high-dose L-carnitine (100 mg/kg) elevated the kidney content of PI3K as compared to other doses of L-carnitine (Fig. 3).
Fig. 3.
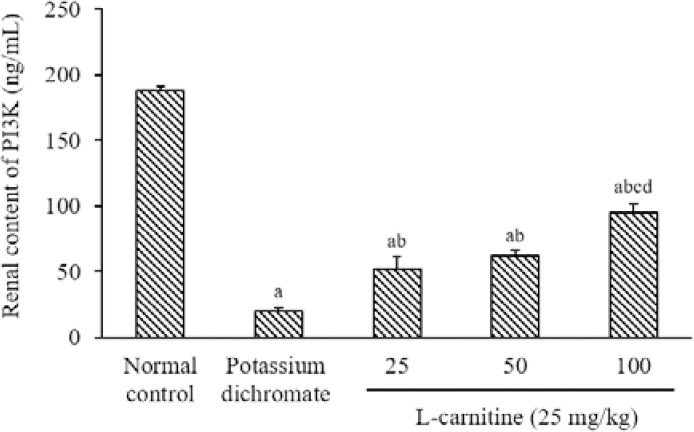
Effect of L-carnitine on the renal PI3K content. Data are presented as mean ± SEM, n = 8. aP < 0.05 Indicates significant differences compared to the control group, bP < 0.05 statistically significant from potassium dichromate (15 mg/kg) group, cP < 0.05 versus L-carnitine at 25 mg/kg, and dP < 0.05 against L-carnitine at 50 mg/kg. PI3K, Phosphoinositide 3-kinase.
Assessment of Akt gene expression in renal tissue
The expression response of the Akt gene in rat kidneys following PD-induced nephrotoxicity was analyzed using quantitative RT-PCR. Akt was down-regulated in the PD group by 22% relative to the normal control. Treatment of the rats with all doses of L-carnitine increased the Akt gene in kidney tissue by 9%, 12%, and 15%, respectively as compared to the PD group. In addition, a high dose of L-carnitine increased the Akt gene; as compared to the low dose of L-carnitine (Fig. 4).
Fig. 4.
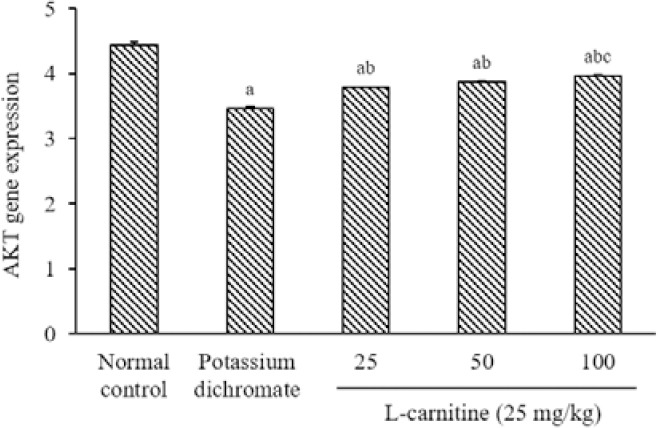
Effect of L-carnitine on the mRNA expression level of Akt. Data are presented as mean ± SEM, n = 8. aP < 0.05 Indicates significant differences compared to the control group, bP < 0.05 statistically significant from potassium dichromate (15 mg/kg) group, and cP < 0.05 versus L-carnitine at 25 mg/kg. Akt, protein kinase B.
Histopathological examination of renal tissue
The histopathological results are shown in Fig. 5. Normal control rats showed a normal histopathological picture (Fig. 5A). PD-treated rats showed diffuse apoptotic histopathological changes (Fig. 5B). Rats treated with L-carnitine at 25 mg/kg showed mild improvement of the overall histopathological picture (Fig. 5C). Rats treated with L-carnitine at 50 mg/kg showed moderate improvement of the histopathological picture (Fig. 5D). Treatment with L-carnitine at 100 mg/kg led to normalizing the histopathological changes (Fig. 5E).
Fig. 5.
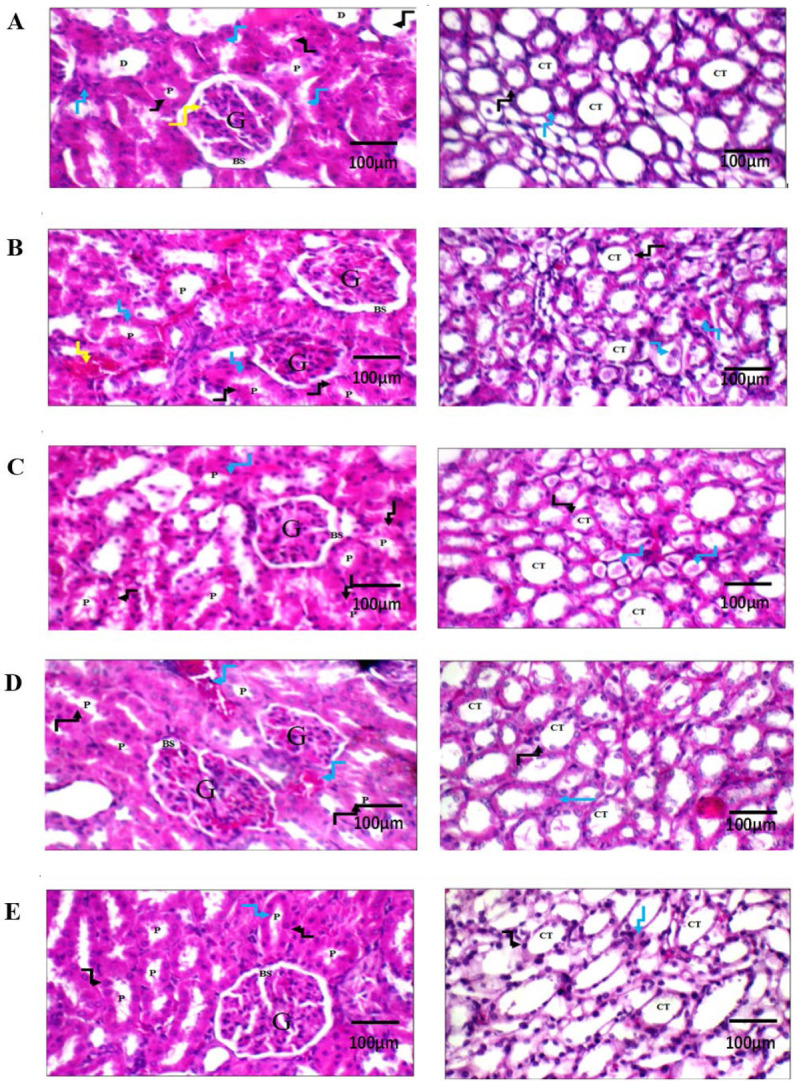
Histopathological evaluation of L-carnitine effect on renal tissue using H&E staining. Renal tissues of (A) normal control rats showing average glomerulus with average Bowman’s spaces, average proximal tubules with preserved brush borders (black arrow), average distal tubules, and average interstitium (blue arrow) along with average collecting tubules with average epithelial lining. (B) potassium dichromate-treated rats showing relatively small-sized glomeruli with average Bowman’s spaces, proximal tubules with apoptotic epithelial lining (black arrow) and brush borders (blue arrow), along with dilated congested blood vessels (yellow arrow). Renal medulla showed collecting tubules with congested epithelial lining (black arrow) and intra-tubular hyaline casts (blue arrow). (C) Rat treated with L-carnitine at 25 mg/kg showing average glomerulus with average Bowman’s space, proximal tubules with apoptotic epithelial lining (black arrow) and preserved brush borders (blue arrow). Renal medulla showed collecting tubules with average epithelial lining (black arrow) and intra-tubular hyaline casts (blue arrow). (D) Rat treated with L-carnitine at 50 mg/kg showing mildly congested glomeruli with average Bowman’s spaces, proximal tubules with preserved brush borders (black arrow), and mildly congested blood vessels (blue arrow). (E) Rat treated with L-carnitine at 100 mg/kg showing average glomerulus with average Bowman’s space, and proximal tubules with average epithelial lining (black arrow) and preserved brush borders (blue arrow). The left side magnification is 400× and the right side is 200×. G, Glomerulus; BS, Bowman’s spaces; P, proximal tubule; D, distal tubule; CT, collecting tubule.
The renal tissue histopathological alteration scoring is presented in (Fig. 6).
Fig. 6.
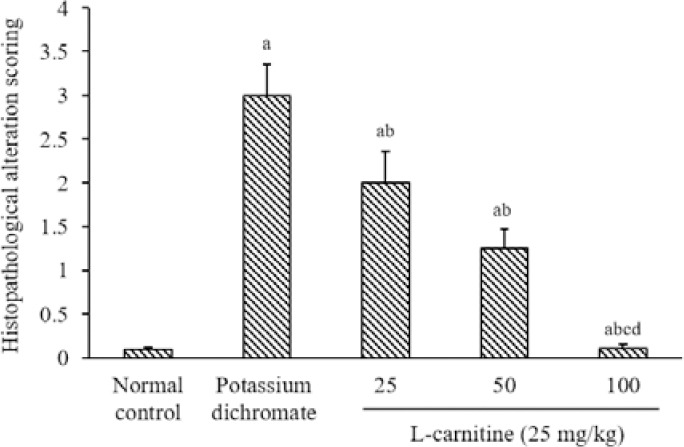
Renal tissue histopathological alteration scoring. Data are presented as mean ± SEM, n = 8. aP < 0.05 Indicates significant differences compared to the control group, bP < 0.05 statistically significant from potassium dichromate (15 mg/kg) group, cP < 0.05 versus L-carnitine at 25 mg/kg, and dP < 0.05 against L-carnitine at 50 mg/kg.
DISCUSSION
The detrimental effects of kidney disease and nephrotoxicities include not only kidney failure but also impaired kidney structure and functionality as well as cardiovascular complications. Therefore, the search for effective pharmacological interventions to avoid or postpone the decline in kidney function as well as other complications have been crucial (22). Hence, the current study examined the potential beneficial effects of L-carnitine on PD-induced nephrotoxicity in rats; focusing on the possible mechanisms behind L-carnitine nephroprotective actions. The study is shedding light on L-carnitine’s ability to down-regulate PI3K/Akt signaling pathway.
PD is an oxidizing agent reduced by different cellar metabolites to give chromium. Chromium generates oxidative stress during its reduction, and the reactive species produced cellular lipids, proteins and DNA injury. Chromium toxicity has also been reported to be associated with inflammation (23).
In the current work, the use of PD (15 mg/kg) in rats caused an abrupt decline in renal functionality as apparent by elevated serum creatinine and BUN relative to normal rats. PD also resulted in significant ongoing oxidative stress injury in renal tissue. This was apparent by a striking decline in renal contents of GSH accompanied by a remarkable elevation in renal lipid peroxidation products manifested by elevated renal MDA contents. This data is in line with many prior studies (7,24,25,26). Salama et al. reported similar results where PD administration resulted in a considerable increase of serum creatinine and BUN, as well as renal MDA levels accompanied by a significant decrease in renal GSH contents two days, post PD administration relative to the normal group (16). The current data also reports the involvement of inflammatory changes post PD administration as elucidated by raised renal contents of pro-inflammatory cytokines viz. TNF-α and TGF-β; relative to normal rats. A similar study reported up-shooting of cytokines and other inflammatory mediators post PD administration (27). Hegazy et al. described significant ongoing renal inflammation post PD administration as manifested by elevation of nuclear factor kappa B (NF-κB) and TNF-α renal concentration (28). Interestingly, NFκB, TNF-α and TGF-β have been reported to be linked to the promotion of inflammation as well as the initiation of renal fibrosis & apoptosis since they inhibit normal autophagy and intensify the injury response in renal tissues (29).
The current data showed that PD use has been linked to down-regulation of PI3K as well as the Akt gene in kidney tissues, signifying ongoing apoptosis. PI3K/Akt pathway is an intracellular signaling pathway crucial in cell cycle regulation, protein synthesis, apoptosis as well as cellular defense against oxidative damage (30). PI3K activates Akt and transports it into the plasma membrane, eventually leading to increased synthesis of cellular proteins and consequently enhancement of cellular growth and abrogation of autophagy. And since this pathway is essential in the promotion of cellular growth, proliferation and longevity; therefore it is mainly up-regulated in different cancers and auto-immune diseases characterized by unrestrained cellular overgrowth and proliferation. On the other hand, PI3K/Akt signaling pathway down-regulation has been used to signify ongoing apoptosis (14). Many studies reported down-regulation of PI3K/Akt signaling pathway in various experimental models of nephrotoxicity. Similar to our findings, Zhang et al. stated that PI3K/Akt signaling pathway was down-regulated in renal tissues of rats post hexavalent chromium exposure; indicating significant deterioration of renal function and ongoing apoptosis (31). Liu et al. reported a noticeable reduction of PI3K/Akt signaling pathway signifying apoptosis induced in rat kidneys via lead administration (32). Wei et al. indicated a decrement of PI3K/Akt following renal ischemia-reperfusion injury in mice (33). Recently, pharmacological therapies are directed towards the modulation of the PI3K/Akt signaling pathway in search of effective treatment of various disease conditions. Moreover, a huge challenge is currently facing researchers and clinicians regarding defining the suitable balance between PI3K/Akt signaling pathway up-regulation versus down-regulation and consequently defining the balance between proliferation versus apoptosis to develop effective therapies (34).
PD-treated rats in the current study showed substantial deterioration in the renal histopathological pictures as evident by relatively small-sized glomeruli with apoptotic epithelial lining along with dilated congested blood vessels. The renal medulla showed congested epithelial lining and intra-tubular hyaline casts. These findings are in line with other studies that reported severe renal histomorphological abnormalities post PD administration (26,35). Hose et al. reported anatomical injuries at the proximal tubules in human kidneys post PD exposure (36). Salama et al. also reported atrophy of glomeruli, apoptosis, and interstitial renal hemorrhage in PD-treated rats (16).
L-carnitine is a naturally occurring amino acid derivative. It principally works for the transport of activated long-chain fatty acids into the mitochondria for breakdown by β-oxidation. L-carnitine is commonly used as an antioxidant supplement since it inhibits the generation of free radicals, eventually leading to the prevention of impairment of mitochondrial fatty acid oxidation as well as protection against tissue damage via repairing oxidized membrane lipids. Because L-carnitine has antioxidant characteristics, it helps to prevent endothelial dysfunction. L-carnitine also possesses anti-inflammatory and anti- apoptotic properties. Therefore, L-carnitine has been used to protect the kidney tissue against ischemia-reperfusion injury, diabetic nephropathy, hypertension-associated renal diseases, contrast media-induced renal damage and chemotherapy-induced renal injuries. However, the beneficial effects of L-carnitine against nephrotoxicity induced by PD have not been addressed.
In the current work, L-carnitine use in the selected doses resulted in improvement of serum renal functions tests, creatinine and BUN relative to the PD-control group. Moreover, L-carnitine reduced renal oxidative stress markers and inflammatory parameters and abrogated histopathological abnormalities caused by PD administration. L-carnitine use has also been linked to up-regulation of the PI3K/Akt signaling pathway that was down-regulated post PD use. These data signified the L-carnitine ability to abolish apoptosis. The high dose of L-carnitine (100 mg/kg) exhibited significant improvement of all the aforementioned parameters as compared to the lower doses (25 and 50 mg/kg). These data are in agreement with many findings that reported beneficial effects of L-carnitine on renal tissues. Abdoli et al. reported beneficial antioxidant and free radical-scavenging effects of L-carnitine (37). Similar to our results, Tousson et al. reported improved renal function parameters accompanied by significant antioxidant effects of L-carnitine against pentylenetetrazol-induced renal injury as was apparent by reduced total antioxidant capacity and MDA concentrations and catalase and superoxide dismutase activities (12). Kunak et al. described renoprotective actions of L-carnitine against glycerol-induced as well as contrast media-induced renal damage in rats through the improvement of serum creatinine, BUN, superoxide dismutase, MDA, GSH, interleukin 1β, caspase-3, and NF-κB levels comparative to the nephrotoxic control group. L-carnitine also was able to improve the histopathological results of the treated rats where it decreased proximal tubules hemorrhage and necrosis (38). Zhao et al. stated that L-carnitine use was accompanied by amelioration of renal inflammation-induced via unilateral ureteral obstruction through inhibition of pro-inflammatory cytokines and attenuation of tubulointerstitial inflammation in rats (39). Moreover, L-carnitine; in one study; was able to improve renal function and mitigate histological abnormalities in rats suffering from chronic kidney disease (40). Aleisa et al. described L-carnitine’s beneficial actions on renal glomeruli and tubules in cisplatin-induced renal toxicity as it almost normalized cisplatin- induced abnormalities in serum concentrations of creatinine, BUN, GSH, thiobarbituric acid-reactive substances as well as total nitrate/nitrite. L-carnitine also improved the histopathological anomalies as indicated by reduced glomerular and tubular necrosis which was induced by post-cisplatin use (41).
CONCLUSION
Collectively, we have elucidated the potential protective effects of L-carnitine against PD-induced nephrotoxicity in rats. The study indicated that the possible mechanisms beyond this nephro-protection are mainly via reduction of oxidative stress as well as inflammation. The study also accentuated L-carnitine’s ability to abrogate apoptosis and restore normal renal cells proliferation and functionality. Interestingly, L-carnitine use has been linked to up-regulation of the PI3K/Akt signaling pathway signifying improvement of renal function along with abrogation of nephrotoxicity.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contribution
A.A.A. Salama participated in the practical experimentation, carried out the statistical analyses. R.E. Mostafa participated in the practical experimentation and wrote the manuscript. R. Elgohary participated in the practical experimentation and revised the manuscript. The final version of the manuscript was approved by all authors.
REFERENCES
- 1.Sun M, Bianchi M, Hansen J, Trinh QD, Abdollah F, Tian Z, et al. Chronic kidney disease after nephrectomy in patients with small renal masses: a retrospective observational analysis. Eur Urol. 2012;62(4):696–703. doi: 10.1016/j.eururo.2012.03.051. DOI: 10.1016/j.eururo.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 2.Nutter F, Khwaja A, Haylor J. Seliciclib inhibits renal hypertrophy but not fibrosis in the rat following subtotal nephrectomy. Nephron Exp Nephrol. 2012;122((3-4)):114–122. doi: 10.1159/000350248. DOI: 10.1159/000350248. [DOI] [PubMed] [Google Scholar]
- 3.Mostafa RE, Dalia OS, Dina FM. Cisplatin-induced nephrotoxicity in rats: modulatory role of simvastatin and rosuvastatin against apoptosis and inflammation. J Appl Pharm Sci. 2018;8(4):43–50. DOI: 10.7324/JAPS.2018.8406. [Google Scholar]
- 4.Mostafa RE, El-Marasy SA, Jaleel GAA, Bakeer RM. Protective effect of royal jelly against diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats. Heliyon. 2020;6(2)(e03330):1–9. doi: 10.1016/j.heliyon.2020.e03330. DOI: 10.1016/j.heliyon.2020.e03330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asaad GF, Hassan A, Mostafa RE. Anti-oxidant impact of Lisinopril and Enalapril against acute kidney injury induced by doxorubicin in male wistar rats: involvement of kidney injury molecule-1. Heliyon. 2021;7(1)(e05985):1–10. doi: 10.1016/j.heliyon.2021.e05985. DOI: 10.1016/j.heliyon.2021.e05985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Okbi SY, Mohamed DA, Hamed TES, Abd El-Alim SH, Kassem AA, Mostafa DM. Application of liquisolid technology for promoting the renoprotective efficacy of walnut extracts in chronic renal failure rat model. Drug Dev Ind Pharm. 2019;45(1):32–42. doi: 10.1080/03639045.2018.1515219. DOI: 10.1080/03639045.2018.1515219. [DOI] [PubMed] [Google Scholar]
- 7.Aliu M, Mensah KB, Forkuo A, Adu-Gyamfi PKT, Ansah C. Ethanolic stem bark extract of Terminalia ivorensis A. Chev. Protects against potassium dichromate-induced nephrotoxicity in rats. Sci Afr. 2020;e00410:1–9. DOI: 10.1016/j.sciaf.2020.e00410. [Google Scholar]
- 8.Jalili C, Akhshi N, Rashidi I, Ghanbari A. Harmine protects mercuric chloride kidney-induced injury by antioxidant activity in male mice: a biochemical and histological study. Res Pharm Sci. 2020;15(6):541–550. doi: 10.4103/1735-5362.301339. DOI: 10.4103/1735-5362.301339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dayan AD, Paine AJ. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: review of the literature from 1985 to 2000. Hum Exp Toxicol. 2001;20(9):439–451. doi: 10.1191/096032701682693062. DOI: 10.1191/096032701682693062. [DOI] [PubMed] [Google Scholar]
- 10.Mehany HA, Abo-youssef AM, Ahmed LA, Arafa ESA, Abd El-Latif HA. Protective effect of vitamin E and atorvastatin against potassium dichromate- induced nephrotoxicity in rats. Beni Suef Univ J Basic Appl Sci. 2013;2(2):96–102. DOI: 10.1016/j.bjbas.2013.02.002. [Google Scholar]
- 11.Karabulut D, Öztürk E, Akin ATT, Lekesizcan A, Ünsal HMM, Özyazgan TM, et al. L-carnitine effects in CCl4-nephrotoxicity: immunohistochemical evaluation of glomerular nephrin and HIF-1alpha expressions. Çukurova Med J. 2020;45(2):541–546. DOI: 10.17826/cumj.674044. [Google Scholar]
- 12.Tousson E, Keshta AT, Hussein Y, Fekry RM, Abo- Ghaneima WK. Renal protective effect of ginkgo biloba and L-carnitine extracts against pentylenetetrazol induced toxicity, oxidative stress, injury and proliferation alternation in epileptic rats. Annu Res Rev Biol. 2019;32(2):1–13. DOI: 10.9734/arrb/2019/v32i230076. [Google Scholar]
- 13.Novakova K, Kummer O, Bouitbir J, Stoffel SD, Hoerler-Koerner U, Bodmer M, et al. Effect of L- carnitine supplementation on the body carnitine pool, skeletal muscle energy metabolism and physical performance in male vegetarians. Eur J Nutr. 2016;55(1):207–217. doi: 10.1007/s00394-015-0838-9. DOI: 10.1007/s00394-015-0838-9. [DOI] [PubMed] [Google Scholar]
- 14.Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med. 2018;379(21):2052–2062. doi: 10.1056/NEJMra1704560. DOI: 10.1056/NEJMra1704560. [DOI] [PubMed] [Google Scholar]
- 15.Margaria JP, Campa CC, De Santis MC, Hirsch E, Franco I. The PI3K/Akt/mTOR pathway in polycystic kidney disease: a complex interaction with polycystins and primary cilium. Cell Signal. 2020;66:109468. doi: 10.1016/j.cellsig.2019.109468. DOI: 10.1016/j.cellsig.2019. [DOI] [PubMed] [Google Scholar]
- 16.Salama AAA, Mostafa RE, Omara EA. Ameliorative effects of phosphodiesterase (PDE) inhibitors in potassium dichromate-induced acute renal failure in rats. Int J Pharm Sci Rev Res. 2016;36(2):40–46. [Google Scholar]
- 17.El-Sherbini ES, El-Sayed G, El Shotory R, Gheith N, Abou-Alsoud M, Harakeh SM, et al. Ameliorative effects of L-carnitine on rats raised on a diet supplemented with lead acetate. Saudi J Biol Sci. 2017;24(6):1410–1417. doi: 10.1016/j.sjbs.2016.08.010. DOI: 10.1016/j.sjbs.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang Y, Piao SG, Zou HB, Jin J, Fang MR, Lei DM, et al. L-carnitine protects against cyclosporine- induced pancreatic and renal injury in rats. Transplant Proc. 2013;45(8):3127–3134. doi: 10.1016/j.transproceed.2013.08.041. DOI: 10.1016/j.transproceed.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 19.Bancroft J, Stevens A, Turner D. Theory and Practice of Histological Techniques. 4th ed. Edinburgh: Churchill Living Stone; 1996. p. 20. [Google Scholar]
- 20.Altinkaynak Y, Kural B, Akcan BA, Bodur A, Özer S, Yuluğ E, et al. Protective effects of L-theanine against doxorubicin-induced nephrotoxicity in rats. Biomed Pharmacother. 2018;108:1524–1534. doi: 10.1016/j.biopha.2018.09.171. DOI: 10.1016/j.biopha.2018.09.171. [DOI] [PubMed] [Google Scholar]
- 21.Schick MA, Isbary TJ, Schlegel N, Brugger J, Waschke J, Muellenbach R, et al. The impact of crystalloid and colloid infusion on the kidney in rodent sepsis. Intensive Care Med. 2010;36(3):541–548. doi: 10.1007/s00134-009-1704-0. DOI: 10.1007/s00134-009-1704-0. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National kidney foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. DOI: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 23.Salama A, Hegazy R, Hassan A. Intranasal chromium induces acute brain and lung injuries in rats: assessment of different potential hazardous effects of environmental and occupational exposure to chromium and introduction of a novel pharmacological and toxicological animal model. PloS One. 2016;11(12)(e0168688):1–20. doi: 10.1371/journal.pone.0168688. DOI: 10.1371/journal.pone.0168688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avila-Rojas SH, Aparicio-Trejo OE, Briones-Herrera A, Medina-Campos ON, Reyes-Fermín LM, Martínez-Klimova E, et al. Alterations in mitochondrial homeostasis in a potassium dichromate model of acute kidney injury and their mitigation by curcumin. Food Chem Toxicol. 2020;145:111774. doi: 10.1016/j.fct.2020.111774. DOI: 10.1016/j.fct.2020. [DOI] [PubMed] [Google Scholar]
- 25.Zheng X, Li S, Li J, Lv Y, Wang X, Wu P, et al. Hexavalent chromium induces renal apoptosis and autophagy via disordering the balance of mitochondrial dynamics in rats. Ecotoxicol Environ Saf. 2020;204:111061. doi: 10.1016/j.ecoenv.2020.111061. DOI: 10.1016/j.ecoenv.2020. [DOI] [PubMed] [Google Scholar]
- 26.Elshazly MO, Abd El-Rahman SS, Morgan AM, Ali ME. The remedial efficacy of Spirulina platensis versus chromium-induced nephrotoxicity in male Sprague-Dawley rats. PloS One. 2015;10(6)(e0126780):1–16. doi: 10.1371/journal.pone.0126780. DOI: 10.1371/journal.pone.0126780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bashandy SA, Salama A, Fayed AHM, Omara EA, El-Toumy SA, Salib JY. Protective effect of mandarin (Citrus reticulata) peel extract on potassium dichromate induced hepatotoxicity and nephrotoxicity in rats. Plant Arch. 2020;20(1):2231–2242. [Google Scholar]
- 28.Hegazy R, Salama A, Mansour D, Hassan A. Renoprotective effect of lactoferrin against chromium-induced acute kidney injury in rats: involvement of IL-18 and IGF-1 inhibition. PloS One. 2016;11(3)(e0151486):1–18. doi: 10.1371/journal.pone.0151486. DOI: 10.1371/journal.pone.0151486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan PC, Chen CC, Chen YC, Chang YS, Chu PH. MicroRNAs in acute kidney injury. Hum Genomics. 2016;10(1):29–41. doi: 10.1186/s40246-016-0085-z. DOI: 10.1186/s40246-016-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao RK, Zheng SF, Wang XY. Selenium protects against cadmium-induced kidney apoptosis in chickens by activating the PI3K/AKT/Bcl-2 signaling pathway. Environ Sci Pollut Res Int. 2017;24(25):20342–20353. doi: 10.1007/s11356-017-9422-6. DOI: 10.1007/s11356-017-9422-6. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Zhang Y, Xiao Y, Zhong C, Xiao F. Expression of clusterin suppresses Cr (VI)-induced premature senescence through activation of PI3K/AKT pathway. Ecotoxicol Environ Saf. 2019;183:109465. doi: 10.1016/j.ecoenv.2019.109465. DOI: 10.1016/j.ecoenv.2019. [DOI] [PubMed] [Google Scholar]
- 32.Liu CM, Ma JQ, Sun YZ. Puerarin protects rat kidney from lead-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol Appl Pharmacol. 2012;258(3):330–342. doi: 10.1016/j.taap.2011.11.015. DOI: 10.1016/j.taap.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Wei Q, Zhao J, Zhou X, Yu L, Liu Z, Chang Y. Propofol can suppress renal ischemia-reperfusion injury through the activation of PI3K/AKT/mTOR signal pathway. Gene. 2019;708:14–20. doi: 10.1016/j.gene.2019.05.023. DOI: 10.1016/j.gene.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 34.Peltier J, O’Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol. 2007;67(10):1348–1361. doi: 10.1002/dneu.20506. DOI: 10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- 35.Salama A, Elsayeh B, Ismaiel I, El-Shenawy S. Comparative evaluation of protective effects of green tea and lycopene in potassium dichromate-induced acute renal failure in rats. J Chem Pharm Res. 2014;6(12):168–177. [Google Scholar]
- 36.Hose GC, Symington K, Lott MJ, Lategan MJ. The toxicity of arsenic (III), chromium (VI) and zinc to groundwater copepods. Environ Sci Pollut Res Int. 2016;23(18):18704–18713. doi: 10.1007/s11356-016-7046-x. DOI: 10.1007/s11356-016-7046-x. [DOI] [PubMed] [Google Scholar]
- 37.Abdoli N, Azarmi Y, Eghbal MA. Mitigation of statins-induced cytotoxicity and mitochondrial dysfunction by L-carnitine in freshly-isolated rat hepatocytes. Res Pharm Sci. 2015;10(2):143–151. PMID: 26487891. [PMC free article] [PubMed] [Google Scholar]
- 38.Kunak CS, Ugan RA, Cadirci E, Karakus E, Polat B, Un H, et al. Nephroprotective potential of carnitine against glycerol and contrast-induced kidney injury in rats through modulation of oxidative stress, proinflammatory cytokines, and apoptosis. Br J Radiol. 2016;89(1058)(20140724):1–14. doi: 10.1259/bjr.20140724. DOI: 10.1259/bjr.20140724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao HY, Li HY, Jin J, Jin JZ, Zhang LY, Xuan MY, et al. L-carnitine treatment attenuates renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. Korean J Intern Med. 2021;36((Suppl 1)):S180–S195. doi: 10.3904/kjim.2019.413. DOI: 10.3904/kjim.2019.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmad NA, Armaly Z, Berman S, Jabour A, Aga- Mizrachi S, Mosenego-Ornan E, et al. l-Carnitine improves cognitive and renal functions in a rat model of chronic kidney disease. Physiol Behav. 2016;164((Pt A)):182–188. doi: 10.1016/j.physbeh.2016.05.036. DOI: 10.1016/j.physbeh.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 41.Aleisa AM, Al-Majed AA, Al-Yahya AA, Al-Rejaie SS, Bakheet SA, Al-Shabanah OA, et al. Reversal of cisplatin-induced carnitine deficiency and energy starvation by propionyl-L-carnitine in rat kidney tissues. Clin Exp Pharmacol Physiol. 2007;34(12):1252–1259. doi: 10.1111/j.1440-1681.2007.04714.x. DOI: 10.1111/j.1440-1681.2007.04714.x. [DOI] [PubMed] [Google Scholar]


