Abstract
Background and purpose:
Cisplatin-induced nephrotoxicity (CisIN) remains the most dose-limiting adverse effect of its clinical use. The protective effects of melatonin on CisIN have been addressed in several non- clinical and animal studies. This study aimed at investigating the potential effects of melatonin on the prevention of CisIN in human.
Experimental approach:
Our study was a randomized controlled clinical trial, performed on 66 eligible patients in two groups of melatonin or control (no intervention). Melatonin was administrated daily at a dose of 20 mg for 5 days to the patients receiving cisplatin-containing regimens along with the standard protocol of CisIN prevention. Patient demographic information, blood and urinary indices of nitrogen, creatinine, and electrolytes such as sodium, potassium, magnesium as well as neutrophil gelatinase-associated lipocalin were measured in both groups at the baseline, 24 h and five days after melatonin administration.
Findings/Results:
Cisplatin administration resulted in significant magnesium and potassium loss in patients with cancer. In comparison with the control group, the prevalence of acute renal injury and the rate of urinary magnesium and potassium loss improved with melatonin administration; however, the results were not statistically significant. Tolerable side effects such as daytime drowsiness, nausea, and vomiting were reported in the melatonin group.
Conclusion and implications:
Although pretreatment with melatonin led to amelioration in urinary electrolyte loss due to CisIN, it failed to show a positive result on acute renal injury prevention. Future well-designed studies with a longer duration of follow-up, larger sample sizes, and higher doses of melatonin are warranted.
Keywords: Cisplatin, Clinical trial, Electrolyte, Melatonin, Nephrotoxicity
INTRODUCTION
Platinum-based agents especially cisplatin is one of the most forceful and valuable chemotherapeutic drugs used for a wide range of malignancies such as breast, lung, ovarian, and testicular tumors (1). Cisplatin is classified as a potent cellular toxin, especially in low- chloride environments. Inside a cell, a chloride atom in cisplatin is replaced by a water molecule. It is noted that this product is the result of hydrolysis of active pharmaceutical ingredients and can react with cells’ glutathione. In tumor cells and other cells undergoing a division process, cisplatin-DNA cross-linking leads to cytotoxic effects. Although cisplatin administration is associated with many complications such as ototoxicity, gastrointestinal complications, and bone marrow suppression, nephrotoxicity manifested in the form of tubular dysfunction limits cisplatin dose and administration in treatment regimens (1).
Different mechanisms have been proposed in cisplatin-induced nephrotoxicity (CisIN) including the toxicity of tubular epithelial cells initially S3 fragment in the proximal tubule, vascular contraction of the renal vascular system, and cisplatin-related inflammatory side effects by an increase in the production of inflammatory cytokines response, activation of oxidative stress, and degradation of mitochondrial function (2).
Renal toxicity manifestations of CisIN embrace conditions such as acute kidney injury (AKI), glomerular filtration rate (GFR) decline, salt-wasting which can potentiate hypocalcemia, and also hypomagnesemia. An occurrence of AKI ameliorates the occurrence of chronic kidney disease will result in end-stage renal disease (1). Therefore, despite hydration and electrolyte management, the prevention of cisplatin-induced AKI remains a major clinical challenge.
To prevent CisIN, several studies have been evaluated the protective agents including metformin (3), ascorbic acid (4), theophylline (5), N-acetyl cysteine (6), silymarin (7), vitamin E (8), etc. However, insufficient clinical data, as well as the unknown influence on chemotherapy regimens and patients’ outcomes, have hindered recognition of effective, practical and secure nephroprotective agents in the prevention of CisIN.
The pineal hormone called melatonin plays role in the body’s circadian rhythm and seasonal rhythms regulation also it has known as a potent immunomodulator, antioxidant, and cytoprotective agent (9). Numerous animal studies are aiming to access the potential protective effects of melatonin against CisIN (10,11). The proposed mechanisms in the animal study include nuclear factor erythroid 2- related factor 2 (Nrf2) regulation, antioxidant enzymes such as heme oxygenase-1 (HO-1) induction, and nuclear factor kappa B (NF-κB), or caspase 3 suppression (11).
In addition, melatonin inactivates the oxidative species and inhibits the oxidative effect of cisplatin on the kidney by increasing the production and regulation of proteins and enzymes’ activities through the antioxidant property (10). Melatonin directly inactivates free radicals by donating one or more electrons. Besides, electron donation provides the basis for the formation of protective metabolites which effectively trap all free radicals (12). Moreover, it has anti-inflammatory and anti- apoptotic activities (12).
Kim et al. demonstrated the synergistic effect of melatonin on the cisplatin apoptotic effects. They showed that melatonin antioxidant properties have no negative impact on the function and potency of chemotherapy drugs (13). The other reports have addressed the impact of melatonin co-administration with chemotherapeutic agents in terms of amelioration in anticancer effects and acting as an oncostatic agent via various proposed mechanisms such as antioxidant, apoptotic agents, anti-angiogenesis, and anti-metastasis (14,15). Moreover, melatonin can reduce the apoptosis of healthy renal cells and chemotherapy-related adverse drug reactions (14).
Data from animal and in vitro studies have provided a strong scientific basis for the use of melatonin to reduce CisIN. However, there is a paucity of data in a clinical trial evaluating melatonin in patients at risk of CisIN. Having stated the impact of melatonin on CisIN prevention in non-clinical studies (10,13), the present clinical trial was designed to investigate the effect of melatonin administration on the prevention of CisIN especially AKI.
MATERIALS AND METHODS
Study protocol
The study was a randomized, controlled clinical trial carried out at Omid hematology- oncology hospital affiliated with Isfahan University of Medical Sciences, Isfahan, Iran from March 2018 till July 2020. Omid hospital is one of the referral tertiary-care hospitals specialized for the treatment of patients with cancer.
The protocol of the study was approved by the ethics committee of the hospital (ID number: IR.MUI.RESEARCH.REC. 1398.724) and all patients signed the consent form. Also, the study was registered on Iranian registry of clinical trials website (IRCT20180722040556N4).
We recruited all adult patients with solid tumors who had received the unfractionated dose of cisplatin (50-150 mg/m2) in their chemotherapy regimens and also fulfilled the inclusion criteria containing having eastern cooperative oncology group performance status greater than 3 and creatinine clearance value above 50 mL/min/1.73 m (based on the modification of diet in renal disease equation). Exclusion criteria were included patients with active infection or any symptoms of sepsis; the history of nephrotoxic agents’ consumption such as nephrotoxic antibiotics (aminoglycosides, amphotericin B, vancomycin, colistimethate sodium), contrast media, calcineurin inhibitors, or non-steroidal anti- inflammatory drugs (NSAIDs) in the past 72 h.
We also excluded patients who had received ifosfamide, fluvoxamine, anagrelide, or hydroxyprogesterone in their treatment regimen due to melatonin potential drug interaction; patients who had developed possible side effects or allergic reactions to melatonin administration during treatment; patients with a history of AKI prior to enrollment, and patients experienced bilirubin levels above 2 mg/dL or liver enzymes 2.5 times above the normal range during the study.
According to the hospital’s CisIN prevention protocol, one day prior to cisplatin administration, all patients received 1 L of intravenous normal saline serum containing 10 mL of potassium chloride 15% w/v and 2 mL of magnesium sulfate 50% w/v inside which were infused just before cisplatin infusion. In addition, 500 mL of normal saline was infused after cisplatin-containing regimens.
Randomization
A computer-generated list of sequential random allocation was produced using the www.randomization.com website. Then, block randomization of four patients was used to ensure a balanced allocation of eligible patients in the control and intervention arms.
Patients who had met the inclusion criteria were selected by the oncologist who entered patients randomly in two groups (A or B) based on a randomization list and gave them melatonin (20 mg/day orally (four soft gelatin capsules at bedtime) produced by Zahravi Pharmaceutical Company, Tabriz, Iran or nothing in the control group.
Melatonin administration was begun 24 h before cisplatin-containing regimens (just one cycle of chemotherapy was considered) and continued for 5 subsequent days. For abstaining from probable sedation induced by melatonin administration, the time of drug consumption was advised to be at night. Patients were observed for drug adherence and any possible adverse drug reactions during the study. If the patient did not take more than 5 doses of the drug (equivalent to 20% of the total drug), was considered drug non-compliance and excluded from the study. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 was used to assess adverse events (16).
Lab data collection
Blood sampling was applied just before cisplatin administration, 24 h and 5 days after drug administration for the assessment of complete blood cells, serum creatinine (SCr), and serum electrolytes including sodium, potassium, and magnesium. To measure the levels of urinary electrolytes, creatinine, and urinary nitrogen content, a urine sample was taken from the enrolled patients concurrently with blood sampling.
After obtaining urine samples, they were centrifuged at 10,000 rpm for 5 min and then all samples were kept in a -70 °C freezer until analyses time. The urine values were measured to determine the fractional excretion of magnesium (FEMg), fractional excretion of sodium (FENa) as well as the urinary potassium/creatinine ratio (KCR) according to the below-mentioned equations for each sample at baseline, 24 h, and 5 days after drug administration.
FENa = (Urine Na / Serum Na) / (Urine Cr / Serum Cr) * 100 (1)
FEMg = (Urine Mg * Serum Cr) / (Urine Cr * Serum Mg* 0.7) * 100 (2)
KCR= (Urine K/Urine Cr) (3)
On the other hand, for measuring urine neutrophil gelatinase-associated lipocalin (NGAL) concentrations as a marker of AKI occurrences, the enzyme-linked immunosorbent assay (ELISA) by a 96-well NGAL human kit (manufactured by Hangzhou Eastbiopharma Co., Ltd., China) was used. We adjusted NGAL concentration by urine creatinine to omit the errors induced by hydration therapy and/ or cisplatin-associated polyuria. It is worth mentioning that, urinary NGAL was evaluated due to more reliable data and comfortable procedure than serum samples. Urine samples were kept at -70 °C until the time of evaluation. Again, the samples were centrifuged for the second time at 2000 rpm for 20 min after thawing. Finally, five standard solutions (1600, 800, 400, 200, and 100 ng/mL) were prepared based on the manufacturer's instructions and measured using a urinary NGAL kit.
The incidence of AKI in the patient was classified by AKI network (AKIN) criteria (17). Acute kidney injury network defines AKI as an > 0.3 mg/dL or 50% increase in SCr compared with baseline or a 25% decrease in GFR in comparison to baseline within 48 h. The primary outcome of our study was to determine the effect of melatonin administration on SCr and urinary NGAL level as well as serum and urine electrolytes including sodium, potassium, and magnesium. We considered the melatonin supplementation adverse effects as a secondary endpoint of the study.
Sample size calculation
The following equation has been used to determine the number of experimental samples in this research and to investigate the changes of a specific parameter that is used in two different modes.
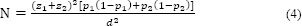
According to the results of Shahbazi et al. study evaluated the protective effects of silymarin on CisIN (7), in this equation, N indicates the number of patients, z1 is the reliability coefficient (95%), z2 is the power factor (80%), p1 and p2 are the probability of beta power in the first and second groups (50%), and d is called the study error.
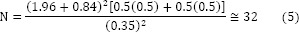
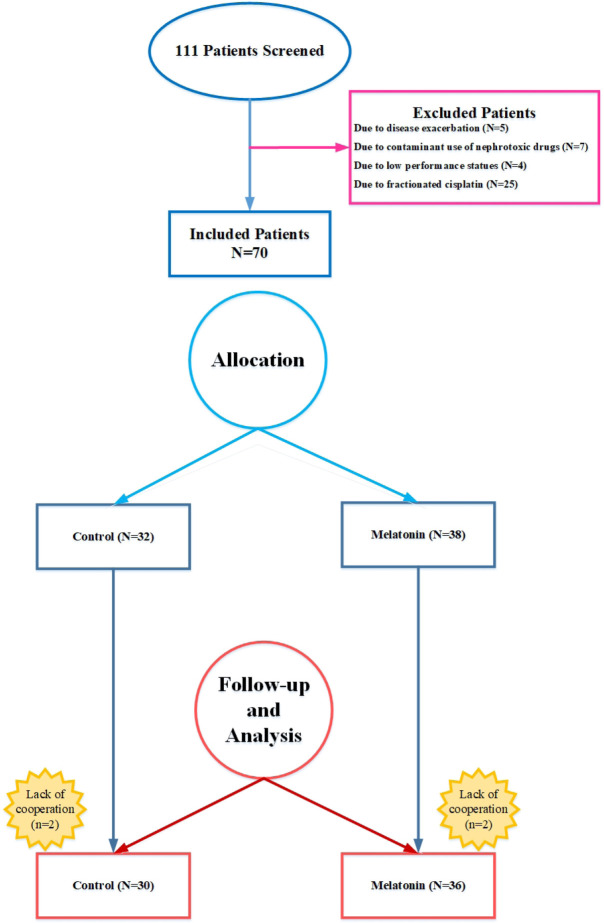
The estimated sample size based on the formula for each group was 32; however, due to difficulties in finding patients who fulfilled inclusion criteria, the patients’ recruitment was terminated by fewer sample size (Fig. 1).
Fig. 1.
Consolidated standards of reporting trials (CONSORT) flowchart of the study.
Statistical analyses
Statistical Package for the Social Sciences (SPSS) version 23 was applied for data analysis. To assess the normality distributions of variables, Shapiro-Kolmogorov-Smirnov’s test was applied. Continuous variables were presented as mean ± standard deviation (SD) or median (range) and categorical variables were shown as frequency and percentages. Independent sample t-test and Mann-Whitney U test were carried out to compare normal and non-normal quantitative variables between two groups. To compare categorical variables between two groups Fisher’s exact, one-way ANOVA, and Chi-square tests were used. P-values less than 0.05 were considered significant.
RESULTS
As it is shown in Fig. 1, from 111 patients who were selected and screened, 75 patients fulfilled the inclusion criteria. Nine patients were excluded from the study due to disease progression, changes in the treatment regimen, and lack of cooperation. Furthermore, following randomization, 36 and 30 patients were assigned to melatonin and control groups, respectively.
The mean age of recruited patients in the melatonin group was 48.2 ± 15.5 years old and 72.2% were men compared with 47.8 ± 14.2 years old and 69% men in the control group. The mean cisplatin cumulative dose was 150 mg (70-480 mg) in the cisplatin group and 180 mg (80-680 mg) in the control group. It was higher in the control group as they were undergoing 41 chemotherapy cycles in comparison with 39 chemotherapy cycles in the cisplatin group; however, the difference was not significant. The patients’ demographic and clinical characteristics at the baseline were shown in Table 1. As it was shown in Table 2, head and neck cancer was the most frequent (28.8%) reported cancer among our enrolled patients. Regarding chemotherapy regimens, presented in Table 2, there is no significant difference between the medication type of regimens of the two comparative groups (P = 0.06). In both intervention and control groups, weekly cisplatin (50-150 mg) accounted for the most administrated chemotherapy regimens (30% for both groups).
Table 1.
Baseline demographic, clinical, and laboratory data in melatonin and placebo groups.
| Variables | Melatonin group (N = 36) | Control group (N = 30) | P-values |
|---|---|---|---|
| Age (years)a | 48.2 ± 15.5 | 47.8 ± 14.2 | 0.87 |
| Gender, male, n (%) | 26 (72%) | 21 (69%) | 0.67 |
| Body surface area (m2)a | 1.69 ± 0.2 | 1.71 ± 0.3 | 0.65 |
| Cisplatin dose (mg)a | 71.8 ± 32.04 | 79 ± 26.7 | 0.33 |
| Cisplatin cumulative dose (mg)b | 181 (80-650) | 150 (72-490) | 0.41 |
| Serum creatinine | 0.92±0.24 | 0.94±0.3 | 0.24 |
| Creatinine clearance (mL/min/1.73 m2)a | 86.41 ± 27.67 | 91.78 ± 35.11 | 0.15 |
| Urea (mg/dL)b | 29.50 (12-68) | 28.50 (16-50) | 0.61 |
| Serum sodium (mEq/L)a | 136.28 ± 4.22 | 135.30 ± 4.23 | 0.38 |
| Serum potassium (mEq/L)a | 4.35 ± 0.47 | 4.52 ± 0.46 | 0.31 |
| Serum calcium (mg/dL)a | 8.82 ± 0.64 | 8.61 ± 0.75 | 0.42 |
| Serum albumin (g/dL)a | 4.76 ± 0.96 | 4.45 ± 0.56 | 0.51 |
| Leukocyte count (109/L)b | 7.10 (5.20-14.5) | 7.15 (3.70-15.20) | 0.62 |
| Hemoglobin (g/dL)a | 11.35 ± 1.45 | 12.35 ± 1.86 | 0.27 |
| Platelet count (109/L)a | 301.6 ± 110.45 | 318.97 ± 102.43 | 0.13 |
| Serum AST (U/L)b | 22 (11-69) | 21 (11-62) | 0.56 |
| Serum ALT (U/L)b | 14 (10-77) | 15 (10-58) | 0.52 |
| Serum alkaline phosphatase (U/L)b | 236.5 (115-1050) | 249.5 (136-1400) | 0.67 |
| Total bilirubin (mg/dL)a | 0.67 ± 0.28 | 0.68 ± 0.35 | 0.41 |
| Uric acid (mg/dL)a | 4.98 ± 1.2 | 4.81 ± 1.26 | 0.12 |
| Urine NGAL/creatinine (ng/mg)a | 138 ± 31.1 | 142 ± 37.4 | 0.21 |
a Data is shown as mean ± SD; b data indicated as median (range); AST, alanine aminotransferase; ALT, aspartate aminotransferase; NGAL, neutrophil gelatinase-associated lipocalin.
Table 2.
Frequency of chemotherapy regimens and type of malignancies in both intervention and control groups.
| Treatment regimen, | Melatonin group (N = 36) N (%) | Control group (N = 30) N (%) | P-value |
|---|---|---|---|
| Cisplatin | 11 (30.6) | 9 (30) | 0.06 |
| Gemcitabine-cisplatin | 2 (5.6) | 2 (6.7) | |
| Gemcitabine-dexamethasone-Cisplatin | 5 (13.9) | 6 (20) | |
| Docetaxel-cisplatin-fluorouracil | 7 (19.4) | 2 (6.7) | |
| Fluorouracil-cisplatin | 2 (5.6) | 1 (3.3) | |
| Vinorelbine-cisplatin | 0 | 1 (3.3) | |
| Gemcitabine-cisplatin-rituximab | 0 | 1 (3.3) | |
| Cetuximab-cisplatin | 0 | 1 (3.3) | |
| Doxorubicin-cisplatin | 0 | 1 (3.3) | |
| Doxorubicin-fluorouracil-cisplatin | 2 (5.6) | 1 (3.3) | |
| Etoposide-cisplatin | 5 (13.9) | 0 | |
| Docetaxel-cisplatin | 2 (5.6) | 1 (3.3) | |
| Rituximab-etoposide-methylprednisolone-cytarabine-cisplatin | 0 | 2 (6.7) | |
| Docetaxel-cisplatin-fluorouracil | 0 | 2 (6.7) | |
|
| |||
| Malignancy types, | 0.13 | ||
| Lung cancer | 6 (16.7) | 3 (10) | |
| Gastrointestinal cancer | 5 (13.9) | 3 (10) | |
| Hodgkin lymphoma | 4 (11.1) | 10 (33.3) | |
| Head and neck | 12 (33.3) | 7 (23.3) | |
| Urogenital cancer | 5 (13.9) | 5 (16.7) | |
| Sarcoma | 4 (11.1) | 2 (6.7) | |
According to AKIN criteria definition (17), 3 (8.3%) patients in melatonin and 8 (26.7%) patients in control group were encountered AKI stage 1 (P = 0.096). The majority of AKI episodes happened in the second course of cisplatin administration. No patients experienced oliguria. The SCr level of all patients who experienced AKI during the administration of cisplatin-containing regimens went back to baseline quantities before the next upcoming chemotherapy cycle.
At the baseline, after 24 h and 5 days, the amount of urine NGAL to urine creatinine ratio (NGAL/Cr) did not vary between the melatonin and control groups at each time-point (Tables 1 and 3).
Table 3.
Comparison of indices in intervention and control groups at different time-points of follow-up using one-way ANOVA test. All data were collected from blood except than NGAL marker.
| Indicators | Time | Melatonin group (N = 36) | Control group (N = 30) | Within group P-value | Between-group P-value | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | Mean | SD | ||||
| GFR (mL/min/1.73 m2) | Bassline | 86.3 | 20.4 | 83.04 | 25.7 | < 0.001 | 0.03 |
| 24 h later | 90.6 | 27.2 | 87.4 | 23.2 | |||
| 5 days later | 83.7 | 19.9 | 73.1 | 21.7 | |||
|
| |||||||
| BUN (mg/dL) | Bassline | 14.9 | 3.5 | 13.8 | 6.02 | < 0.001 | 0.61 |
| 24 h later | 14.9 | 4.3 | 16.5 | 6.2 | |||
| 5 days later | 17.3 | 5.3 | 18.7 | 6.9 | |||
|
| |||||||
| Creatinine (mg/dL) | Bassline | 0.97 | 0.16 | 1.01 | 0.25 | < 0.001 | 0.31 |
| 24 hours later | 0.94 | 0.18 | 0.95 | 0.20 | |||
| 5 days later | 1.01 | 0.23 | 1.10 | 0.25 | |||
|
| |||||||
| Magnesium (mg/dL) | Bassline | 1.8 | 0.3 | 1.9 | 0.4 | 0.37 | 0.30 |
| 24 h later | 1.9 | 0.3 | 1.9 | 0.3 | |||
| 5 days later | 2.1 | 0.3 | 1.8 | 0.4 | |||
|
| |||||||
| Sodium (mEq/L) | Bassline | 138.8 | 3.2 | 138.2 | 4.3 | 0.62 | 0.07 |
| 24 h later | 138.4 | 3.3 | 138 | 2.7 | |||
| 5 days later | 140.3 | 3.9 | 137 | 3.03 | |||
|
| |||||||
| Potassium (mmol/L) | Bassline | 4.2 | 0.5 | 3.8 | 0.6 | 0.99 | 0.23 |
| 24 h later | 4.2 | 0.5 | 3.9 | 0.5 | |||
| 5 days later | 4.2 | 0.6 | 3.8 | 0.6 | |||
|
| |||||||
| Urine NGAL (ng/mg) | Bassline | 125.1 | 17.4 | 136.1 | 24.1 | 0.85 | 0.63 |
| 24 h later | 127.9 | 17.9 | 135.1 | 10.5 | |||
| 5 days later | 133.3 | 23 | 136.1 | 15.9 | |||
|
| |||||||
| Urine NGAL to Creatinine (ng/mg) | Bassline | 138.3 | 31.1 | 142.02 | 37.4 | 0.21 | 0.60 |
| 24 h later | 146.1 | 19.2 | 152.8 | 37.6 | |||
| 5 days later | 135.2 | 39.6 | 133.3 | 36.3 | |||
GFR, Glomerular filtration rate; BUN, blood urea nitrogen; NGAL, neutrophil gelatinase-associated lipocalin.
Urine NGAL/Cr peaked at 24 h after cisplatin-containing regimens in both comparative groups and decreased thereafter.
As shown in Table 4, a non-statistically significant increase in magnesium extraction fraction was seen in all enrolled patients after cisplatin infusion. Although it was non-significant, magnesium excretion fraction was increased after 24 h and 5 days of receiving cisplatin-containing regimens in both groups. Urinary sodium loss happened in both comparative groups and sodium excretion fraction was enhanced accordingly after 24 h and 5 days’ follow-up, although both changes were not statistically significant (Tables 3 and 4).
Table 4.
Comparison of urinary indices in intervention and control groups at different times using one-way ANOVA test.
| Urinary indicators | Time | Melatonin group (N = 36) | Control group (N = 30) | Within group P-value | Between-group P-value | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | Mean | SD | ||||
| BUN (mg/dL) | Baseline | 569.04 | 333.7 | 595.5 | 257.4 | < 0.04 | 0.03 |
| 24 h later | 482.04 | 327.04 | 680.7 | 246.7 | |||
| 5 days later | 573.9 | 476.2 | 763.3 | 306.9 | |||
|
| |||||||
| Creatinine (mg/dL) | Baseline | 117.3 | 81.9 | 125.1 | 87.4 | < 0.13 | 0.92 |
| 24 h later | 110.9 | 53.9 | 110.3 | 59.4 | |||
| 5 days later | 121.18 | 75.7 | 122 | 61.7 | |||
|
| |||||||
| Magnesium (mg/dL) | Baseline | 3.5 | 1.1 | 3.6 | 1.2 | < 0.02 | 0.04 |
| 24 h later | 3.1 | 1.1 | 3.2 | 1.2 | |||
| 5 days later | 3.3 | 1.04 | 3.8 | 1.1 | |||
|
| |||||||
| Sodium (mEq/L) | Baseline | 117.9 | 52.9 | 153.4 | 55.9 | 0.04 | 0.003 |
| 24 h later | 115.3 | 40.4 | 125.5 | 36.5 | |||
| 5 days later | 115 | 40.6 | 154.8 | 54.1 | |||
|
| |||||||
| Potassium (mmol/L) | Baseline | 57.9 | 36.3 | 48.1 | 30.7 | 0.049 | 0.04 |
| 24 h later | 41.3 | 28.8 | 54.5 | 29.8 | |||
| 5 days later | 41.6 | 28.8 | 57.9 | 42.2 | |||
|
| |||||||
| Sodium extraction fraction (%) | Baseline | 1.3 | 0.9 | 1.4 | 1.1 | 0.26 | 0.32 |
| 24 h later | 1.6 | 1.1 | 1.1 | 0.8 | |||
| 5 days later | 2.03 | 1.4 | 1.5 | 1.2 | |||
|
| |||||||
| Magnesium extraction fraction (%) | Baseline | 4.2 | 2.2 | 3.1 | 1.8 | 0.56 | 0.09 |
| 24 h later | 4.7 | 2.7 | 2.8 | 1.2 | |||
| 5 days later | 4.9 | 2.7 | 3.7 | 1.6 | |||
BUN, Blood urea nitrogen.
Table 3 shows the result of blood indices changes at the baseline, 24 h, and 5 days after melatonin administration in both intervention and control groups. As it was indicated, the value of GFR after 24 h of cisplatin administration has been increased and then decreased significantly after 5 days (Table 3). Also, the mean of magnesium, sodium, potassium and NGAL levels were not significantly different at different times and groups, but the significant increase has been shown in creatinine, blood urea nitrogen (BUN), and SCr indices among patients within each group and the indices were affected over 5-day follow-up.
Table 4 demonstrated the ANOVA test results of urinary indices in both melatonin and control groups. The results showed that group and time had a significant effect on urinary indices of sodium, magnesium, and potassium; however, no similar significant impact has been reported for urine creatinine.
Also, Fisher’s exact intervention (Table 5) showed no significant difference between melatonin and control groups in terms of the frequency distribution of KCR status at any time. The KCR of more than 13 mEq/g creatinine predicted the decrease in serum level of potassium. Although KCR had a decreasing trend, no significant difference was seen between the two comparative groups.
Table 5.
Comparison of frequency of urinary potassium to urinary creatinine ratio at different times between intervention and control groups using Fisher’s exact test.
| Time | Melatonin group (N = 36) | Control group (N = 30) | Intergroup P-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Number | (%) | Number | (%) | |||
| Baseline | ≤ 13 | 1 | 3.4 | 2 | 6.7 | 0.51 |
| > 13 | 28 | 96.6 | 28 | 93.3 | ||
|
| ||||||
| 24 h later (mEq/g creatinine) | ≤ 13 | 1 | 3.4 | 0 | 0 | 0.44 |
| > 13 | 28 | 96.6 | 25 | 100 | ||
|
| ||||||
| 5 days later (mEq/g creatinine) | ≤ 13 | 1 | 3.7 | 0 | 0 | 0.52 |
| > 13 | 26 | 96.3 | 25 | 100 | ||
Table 4 shows the results of ANOVA on the percentage of fractional excretion of sodium and magnesium in both groups. The results showed that group and time had no significant effect on these two parameters.
Administration of melatonin during study follow-up demonstrated that melatonin had non-significant adverse effects and was well-tolerated. Only numerous low-grade gastrointestinal and daytime drowsiness adverse effects were reported which is shown in Table 6. The causality assessment of adverse effects was evaluated by Naranjo scale and they were reported as probable (score 5-8) (18). Although there was no significant difference between adverse effects such as nausea, vomiting, and daytime drowsiness based on CTCAE Version 5.0 (16) in two comparative groups.
Table 6.
Adverse drug reactions in enrolled patients based on common terminology criteria for adverse events (CTCAE) version 5.0. During the study, grades 4 and 5 reactions were not reported.
| Adverse drug reactions | Melatonin group (N = 36) N (%) | Control group (N = 30) N (%) | P-value |
|---|---|---|---|
| Nausea | 12 (33.3%) | 13 (43.3%) | 0.38 |
| Grade 1 | 7 | 10 | |
| Grade 2 | 4 | 2 | |
| Grade 3 | 1 | 1 | |
|
| |||
| Vomiting | 6 (16.6%) | 5 (16.6%) | 0.26 |
| Grade 1 | 4 | 3 | |
| Grade 2 | 1 | 2 | |
| Grade 3 | 1 | 0 | |
|
| |||
| Daytime drowsiness | 3 (8.3%) | 0 | 0.18 |
| Grade 1 | 2 | - | |
| Grade 2 | 1 | - | |
DISCUSSION
The present study demonstrated a significant increase in electrolyte wasting followed by cisplatin administration in patients suffering from cancer. The number of AKI episodes and the amount of magnesium, potassium, and sodium wasting by CisIN have been non- significantly ameliorated by melatonin administration. There were no specific side effects associated with melatonin administration. Prophylactic administration of melatonin (20 mg/day) may be effective against electrolyte wasting such as sodium, potassium, magnesium in patients with cancer.
Cisplatin-induced renal injury is relatively common in patients who were receiving cisplatin-containing chemotherapy regimens. In fact, kidney injury begins several days after cisplatin administration and causes an increase in blood creatinine and nitrogen content. Urine output often remains normal (non-oliguric) and the kidneys may begin to excrete sugar and protein through urine, indicating a malfunction in kidney proximal tubules. Decreased blood magnesium storages are also common after receiving several doses of cisplatin, even if glomerular filtration is not impaired (19).
In our study, 11 cases of AKI episodes (16.7%) happened in both melatonin and control groups. The rate of AKI occurrences was in accordance with other previous studies which have been reported 8-40% (7,20). The contributing factors such as differences in AKI definition, baseline hydration protocols and renal function as well as differences in demographic characteristics and performance status of enrolled patients led to the different incidence of CisIN.
The proposed mechanisms for CisIN were suggested to be a decline in antioxidant activity as well as an increase in reactive oxygen species due to reduction in enzymes such as superoxide dismutase, glutathione and catalase activity. In addition, cisplatin can modulate oxidative stress by interfering in Nrf2 signaling pathway. Moreover, it leads to NF-κB and activator protein 1 expression which has the main role in the generation of inflammatory cytokines such as interleukin-6, interleukin-1 and tumor necrosis factor-α (1).
Several pharmacological agents have been addressed as potential nephroprotective agents for the management of CisIN (3,6,8); however, a promising effect has not been noted. Today, the proposed practical modalities for the prevention of CisIN are involved sufficient hydration and electrolyte replacement (2).
As our knowledge serves, this study is one of the first human studies which were designed to evaluate the possible nephroprotective impact of melatonin on CisIN and salt wasting. In the previous animal studies (10,13), melatonin in the dose range of 4-20 mg/kg at different planned times was administrated for the treatment of CisIN. The proposed nephroprotective mechanisms of melatonin in animal studies were noted through ameliorating oxidative stress, activating antioxidant enzymes, suppressing inflammatory and apoptotic processes in renal cells (3,6,8).
The nephroprotective impact of melatonin premedication on CisIN was well established in a non-clinical study carried out by Kilic et al. (21). They found that melatonin administration increases Nrf2 accumulation in the nuclear part and increases HO-1 expression in the systolic part of the rats’ kidneys.
In addition, they found that melatonin reduces nephrotoxicity by regulating inflammatory and oxidant signaling pathways, which in turn reduced creatinine levels. Melatonin also prevented the reduction of antioxidant molecules in rats’ kidney tissue and inhibited lipid peroxidation as well.
The effect of melatonin on other chemotherapy agents such as cyclophosphamide-induced nephrotoxicity was investigated in a study by Godarzi et al. (22). This study showed that melatonin significantly reduced the serum levels of BUN, creatinine, and oxidative stress markers in 50 mice. Reduction in BUN and SCr levels has been confirmed in the present study; however, due to the small statistical sample size, the changes were not statistically significant between the two comparative groups.
In 2020 for the first time in a pilot, randomized, double-blinded, placebo- controlled clinical trial, the nephroprotective effects of 20 mg/day melatonin have been examined by Ghadrdan et al. (20). They collected urine samples at baseline, 6, and 24 h following cisplatin administration to access NGAL, kidney injury molecule-1 (KIM-1) and SCr. They concluded that urinary biomarkers non-statistically significant increase in both groups and the number of AKI episodes in the melatonin administrated group was non- significantly lower compared with the placebo group. But they could demonstrate that urine KIM-1/creatinine ratio at 6 h and 24 h after cisplatin administration were significantly lower in the melatonin group (2.37 (–71.16 to 63.70) ng/mg and –1.45 (–80.28 to 48.76) ng/mg) compared with the placebo group (3.83 (–11.19 to 37.05) ng/mg and 0.38 (–5.42 to 70.00) ng/mg), respectively.
Moreover, they revealed that melatonin administration in patients who received cisplatin reduced tubular kidney injury, supported by less increase in KIM-1/creatinine and NGAL/creatinine ratios. Our study has several differences from Ghadrdan et al. study (20), especially in terms of methodology, duration of follow-up and endpoints.
For example, since it was postulated (7) that cisplatin-induced nephrotoxicity happens mostly after 24 h from cisplatin infusion, we followed our patients for 5 days to evaluate the late effects of nephrotoxicity and the potential protective impact of melatonin premedication.
Additionally, we examined the impact of melatonin supplementation not only in CisIN but also in cisplatin-induced salt wasting. Furthermore, the urine extraction ratio of electrolytes such as sodium, potassium, and magnesium were measured.
Cisplatin-induced urinary magnesium wasting is a common adverse effect that occurs frequently during the administration of cisplatin-containing regimens in up to 90% of patients (7). Furthermore, regular monitoring of serum magnesium and administration of supplemental magnesium has been recommended. In our study, melatonin was able to show a non-significant potential positive trend on CisIN but its impact was significantly positive in regard to cisplatin-induced salt- wasting especially sodium.
Since serum creatinine is classified as a late indicator of CisIN, other novel biomarkers such as KIM-1, NGAL, and cystatin-C have been suggested for the primary identification of CisIN. Similar to the previous studies (7,19), we also assessed urine NGAL as an early indicator of tubular toxicity. In our study, as it was predicted, urinary NGAL/Cr was quickly started to rise after 24 h of cisplatin administration in both melatonin and control groups.
Previously published studies noted NGAL urinary levels increase as soon as 12-24 h after cisplatin infusion in patients who were facing AKI episodes (7,20). Our finding was compatible with the results of Shahbazi et al. study (7). They assessed the nephroprotective effects of milk thistle (Silybum marianum) extract on CisIN was examined and parallel with our study found that patients had significantly higher urinary electrolyte loss such as magnesium and potassium after receiving cisplatin-containing chemotherapy but they failed to show any significant differences between the incidence of AKI and the amount of urinary magnesium and potassium loss between intervention and placebo groups.
However, in a similar manner, Ahamdiasl et al. investigated the simultaneous effect of erythropoietin (5000 IU/kg) and melatonin (10 mg/kg) on renal ischemic perfusion in cisplatin-treated rats (23). The results indicated that the simultaneous use of these two drugs reduces ischemic perfusion in mice kidneys. In this study, this effect was mostly attributed to the anti-inflammatory and apoptotic impacts of melatonin. Concomitant use of erythropoietin and melatonin was able to potentiate the protective effect of melatonin on CisIN in an animal model.
In a human study by Karadmir et al. (5), the effect of theophylline in the prevention of renal injury in patients undergoing cisplatin-containing regimens was assessed. The results of the study were not in favor of theophylline administration due to no significant effects in theophylline-treated groups. Not reaching promising results in different sorts of studies were attributed to sampling size limitation, short duration of melatonin treatment, insufficient supplements’ dose and phobia of adverse effects.
The powerful antioxidant effects of melatonin were noted to be in high concentrations of melatonin in melatonin doses higher than 20 mg/day (20). The lack of previous clinical studies, the fear of daytime drowsiness adverse effects and the information about the range of antioxidant dose of melatonin has resulted in selecting administration of 20 mg/day melatonin for our study. By releasing newly published article (21) in terms of protective effects of melatonin on CisIN, we found that the dose selection was logical at the beginning of the study but after data analyses at the end of the study, the non-significant results demonstrated that maybe higher dose of melatonin would lead to notable results. In addition, the reported absolute bioavailability of melatonin is low, ranging from 3–37% with large variations in plasma levels between individuals (21), furthermore higher dose of melatonin than 20 mg/day in future studies is recommended.
Therefore, considering the results of the studies reviewed in this section, it can be concluded that achieving statistically reliable results requires a larger statistical population. Also, the studies should be conducted over a longer period. Molecular cell tests can also be very helpful in identifying the effectiveness of the substance or drug under study; therefore, it is recommended to perform these tests on a sufficient statistical population. Although melatonin is a substance with significant antioxidant properties in a variety of pathways, it seems melatonin must be used concurrently with a compound that enhances its antioxidant effects so that these effects manifest themselves more strongly. Therefore, it is suggested to use substances or drugs whose antioxidant and anti-inflammatory effects have been proven, along with melatonin in subsequent studies.
Our study was among several limitations, we used a soft gelatin capsule of melatonin (5 mg) and due to technical problems in producing identical placebo, we were unable to perform our study in a placebo-controlled design. Also, our study suffered from a small sample size due to the narrow inclusion and exclusion criteria for enrollment. Patient compliance was unavoidable for the first designed clinical trials in this regard. On the other hand, the main adverse effects such as AKI and hypomagnesemia may occur 4 or 5 days after cisplatin administration and somehow after discharging from the hospital. Furthermore, they did not comply to return to get the sample or to be monitored let with a limited duration of follow-up.
All limitations resulted in not detecting statistically significant findings in possible protective effects of melatonin on AKI occurrence or salt wasting. However, other modalities such as increasing the dose of melatonin regarding its low bioavailability and duration of follow-up could be helping.
It is noticeable that the duration of melatonin administration is likely to be effective to catch its full effects, considering the risk of melatonin complications. Though, it is suggested to conduct further studies with greater sample sizes and melatonin doses as well as a longer duration of follow-up. In addition, the impact of longer pretreatment time of melatonin administration more than 24 h should be checked in future clinical trials.
CONCLUSION
The present study revealed the non-significant effects of melatonin pretreatment on reducing serum creatinine and increasing glomerular filtration. However, melatonin could show almost effective impacts on cisplatin-induced salt-wasting, especially sodium loss. No specific side effects except nausea, vomiting and daytime drowsiness have been observed. Since melatonin has antioxidant effects and does not interfere with cytotoxic drugs, further studies with a larger statistical population or different methodology are needed in the future for any comprehensive implications.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contribution
A. Moghaddas, S. Karvan, and P. Farrokhi contributed to the literature review, study design, providing data, writing and revised manuscript. A. Sadeghi, M. Sharifi, A. Moghaddas, and S. Karvan were responsible for recruiting patients. S. Karvan was responsible for data gathering and analysis. A. Moghaddas supervised the whole study. All authors approved the final manuscript content.
Acknowledgments
This study was part of the Pharm.D thesis and financially supported by Isfahan Pharmaceutical Sciences Research Center, Isfahan University of Medical Sciences, I.R. Iran through Grant No. 297132. We really appreciated all nurses and medical staff working in the outpatient hospital chemotherapy ward for their kind help and cooperation.
REFERENCES
- 1.Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31(1):15–25. doi: 10.1007/s40620-017-0392-z. DOI: 10.1007/s40620-017-0392-z. [DOI] [PubMed] [Google Scholar]
- 2.Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. Oncol Rep. 2003;10(6):1663–1682. DOI: 10.3892/or.10.6.1663. [PubMed] [Google Scholar]
- 3.Li J, Gui Y, Ren J, Liu X, Feng Y, Zeng Z, et al. Metformin protects against cisplatin-induced tubular cell apoptosis and acute kidney injury via AMPKa- regulated autophagy induction. Sci Rep. 2016;6(1):1–11. doi: 10.1038/srep23975. DOI: 10.1038/srep23975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maliakel DM, Kagiya TV, Nair CKK. Prevention of cisplatin-induced nephrotoxicity by glucosides of ascorbic acid and α-tocopherol. Exp Toxicol Pathol. 2008;60(6):521–527. doi: 10.1016/j.etp.2008.04.015. DOI: 10.1016/j.etp.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Karademir LD, Dogruel F, Kocyigit I, Yazici C, Unal A, Sipahioglu MH, et al. The efficacy of theophylline in preventing cisplatin-related nephrotoxicity in patients with cancer. Ren Fail. 2016;38(5):806–814. doi: 10.3109/0886022X.2016.1163154. DOI: 10.3109/0886022X.2016.1163154. [DOI] [PubMed] [Google Scholar]
- 6.Huang S, You J, Wang K, Li Y, Zhang Y, Wei H, et al. N-acetylcysteine attenuates cisplatin-induced acute kidney injury by inhibiting the C5a receptor. Biomed Res Int. 2019;2019:1–11. doi: 10.1155/2019/4805853. DOI: 10.1155/2019/4805853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahbazi F, Sadighi S, Dashti-Khavidaki S, Shahi F, Mirzania M, Abdollahi A, et al. Effect of silymarin administration on cisplatin nephrotoxicity: report from a pilot, randomized, double-blinded, placebo- controlled clinical trial. Phytother Res. 2015;29(7):1046–1053. doi: 10.1002/ptr.5345. DOI: 10.1002/ptr.5345. [DOI] [PubMed] [Google Scholar]
- 8.Hakiminia B, Goudarzi A, Moghaddas A. Has vitamin E any shreds of evidence in cisplatin-induced toxicity. J Biochem Mol Toxicol. 2019;33(8)(e22349):1–15. doi: 10.1002/jbt.22349. DOI: 10.1002/jbt.22349. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Jin B, Ai F, Duan C, Lu Y, Dong T, et al. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials. Cancer Chemother Pharmacol. 2012;69(5):1213–1220. doi: 10.1007/s00280-012-1828-8. DOI: 10.1007/s00280-012-1828-8. [DOI] [PubMed] [Google Scholar]
- 10.Ko JW, Shin NR, Jung TY, Shin IS, Moon C, Kim SH, et al. Melatonin attenuates cisplatin-induced acute kidney injury in rats via induction of anti-aging protein, Klotho. Food Chem Toxicol. 2019;129:201–210. doi: 10.1016/j.fct.2019.04.049. DOI: 10.1016/j.fct.2019.04.049. [DOI] [PubMed] [Google Scholar]
- 11.Hara M, Yoshida M, Nishijima H, Yokosuka M, Iigo M, Ohtani-Kaneko R, et al. Melatonin, a pineal secretory product with antioxidant properties, protects against cisplatin-induced nephrotoxicity in rats. J Pineal Res. 2001;30(3):129–138. doi: 10.1034/j.1600-079x.2001.300301.x. DOI: 10.1034/j.1600-079x.2001.300301.x. [DOI] [PubMed] [Google Scholar]
- 12.Haghi-Aminjan H, Farhood B, Rahimifard M, Didari T, Baeeri M, Hassani S, et al. The protective role of melatonin in chemotherapy-induced nephrotoxicity: a systematic review of non-clinical studies. Expert Opin Drug Metab Toxicol. 2018;14(9):937–950. doi: 10.1080/17425255.2018.1513492. DOI: 10.1080/17425255.2018.1513492. [DOI] [PubMed] [Google Scholar]
- 13.Kim JW, Jo J, Kim JY, Choe M, Leem J, Park JH. Melatonin attenuates cisplatin-induced acute kidney injury through dual suppression of apoptosis and necroptosis. Biology (Basel) 2019;8(3)(64):1–10. doi: 10.3390/biology8030064. DOI: 10.3390/biology8030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seely D, Wu P, Fritz H, Kennedy DA, Tsui T, Seely AJ, et al. Melatonin as adjuvant cancer care with and without chemotherapy: a systematic review and meta- analysis of randomized trials. Integr Cancer Ther. 2012;11(4):293–303. doi: 10.1177/1534735411425484. DOI: 10.1177/1534735411425484. [DOI] [PubMed] [Google Scholar]
- 15.Pourhanifeh MH, Sharifi M, Reiter RJ, Davoodabadi A, Asemi Z. Melatonin and non-small cell lung cancer: new insights into signaling pathways. Cancer Cell Int. 2019;19(1):131–137. doi: 10.1186/s12935-019-0853-7. DOI: 10.1186/s12935-019-0853-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Common Terminology Criteria forAdverse Events V5.0 (CTCAE) 2020. [(Accessed on June 4, 2019)]. Available from: http://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm . Last updated 2021.
- 17.Lin CY, Chen YC. Acute kidney injury classification: AKIN and RIFLE criteria in critical patients. World J Crit Care Med. 2012;1(2):40–45. doi: 10.5492/wjccm.v1.i2.40. DOI:10.5492/wjccm.v1.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts E, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. DOI: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 19.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. DOI: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghadrdan E, Sadighi S, Ebrahimpour S, Abdollahi A, Hadjibabaei M, Gholami K, et al. The effect of melatonin on cisplatin-induced nephrotoxicity: a pilot, randomized, double-blinded, placebo- controlled clinical trial. Eur J Integr Med. 2020;34(101065):1–7. DOI: 10.1016/j.eujim.2020.101065. [Google Scholar]
- 21.Kilic U, Kilic E, Tuzcu Z, Tuzcu M, Ozercan IH, Yilmaz O, et al. Melatonin suppresses cisplatin- induced nephrotoxicity via activation of Nrf-2/HO-1 pathway. Nutr Metab (Lond) 2013;10(1):7–14. doi: 10.1186/1743-7075-10-7. DOI: 10.1186/1743-7075-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goudarzi M, Khodayar MJ, Hosseini Tabatabaei SMT, Ghaznavi H, Fatemi I, Mehrzadi S. Pretreatment with melatonin protects against cyclophosphamide-induced oxidative stress and renal damage in mice. Fundam Clin Pharmacol. 2017;31(6):625–635. doi: 10.1111/fcp.12303. DOI: 10.1023/b:cbto.0000013342.17370.16. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadiasl N, Banaei S, Alihemmati A. Combination antioxidant effect of erythropoietin and melatonin on renal ischemia-reperfusion injury in rats. Iran J Basic Med Sci. 2013;16(12):1209–1216. DOI: 10.22038/IJBMS.2013.1978. [PMC free article] [PubMed] [Google Scholar]