Abstract
Abstract
Microorganisms are remarkable producers of a wide diversity of natural products that significantly improve human health and well-being. Currently, these natural products comprise half of all the pharmaceuticals on the market. After the discovery of penicillin by Alexander Fleming 85 years ago, the search for and study of antibiotics began to gain relevance as drugs. Since then, antibiotics have played a valuable role in treating infectious diseases and have saved many human lives. New molecules with anticancer, hypocholesterolemic, and immunosuppressive activity have now been introduced to treat other relevant diseases. Smaller biotechnology companies and academic laboratories generate novel antibiotics and other secondary metabolites that big pharmaceutical companies no longer develop. The purpose of this review is to illustrate some of the recent developments and to show the potential that some modern technologies like metagenomics and genome mining offer for the discovery and development of new molecules, with different functions like therapeutic alternatives needed to overcome current severe problems, such as the SARS-CoV-2 pandemic, antibiotic resistance, and other emerging diseases.
Key points
• Novel alternatives for the treatment of infections caused by bacteria, fungi, and viruses.
• Second wave of efforts of microbial origin against SARS-CoV-2 and related variants.
• Microbial drugs used in clinical practice as hypocholesterolemic agents, immunosuppressants, and anticancer therapy.
Keywords: Microbial natural products, Antibiotics, Hypocholesterolemics, Anticancer drugs, Immunosuppressants, Antibiotic resistance
Introduction
Microbes have provided us with thousands of valuable natural drugs with novel structures and bioactivities (Pham et al. 2019). Microbes produce around 22,500 biologically active compounds; 45% come from actinobacteria, 38% from fungi, and 17% via unicellular bacteria (Berdy 2015). Thus, the microbial role in producing antibiotics and other drugs for non-infectious diseases has been spectacular. Besides, chemical modification has raised these structures to drugs with new attractive properties. Today, over 200,000 different secondary metabolite structures are known (Ruchika et al. 2019). Drugs of microbial origin (MPs) can be classified as (i) original MPs, (ii) products derived or chemically synthesized from natural products, or (iii) synthetic products based on MP structures. Thus, nearly half of the blockbusting pharmaceuticals are MPs or their derivatives. This statement is particularly true for the oncological and antihypertensive therapeutic areas (Cragg and Newman 2016). As reported by the World Health Organization (WHO) in May 2017, 51 antibiotics and 11 biologicals were in the clinical pipeline with 42 novel drugs, i.e., 33 antibiotics and nine biologicals. They targeted major pathogens from which seven and nine products were effective for tuberculosis (TB) and Clostridium difficile infections (WHO 2017).
By controlling infectious diseases and other medical conditions, secondary metabolites have had an extraordinary impact on the evolution of the pharmaceutical industry. Their use significantly increased the average life expectancy in the USA, which went from 47.3 years in 1900 to 78.93 in the year 2020 (https://www.macrotrends.net/countries/USA/united-states/life-expectancy).
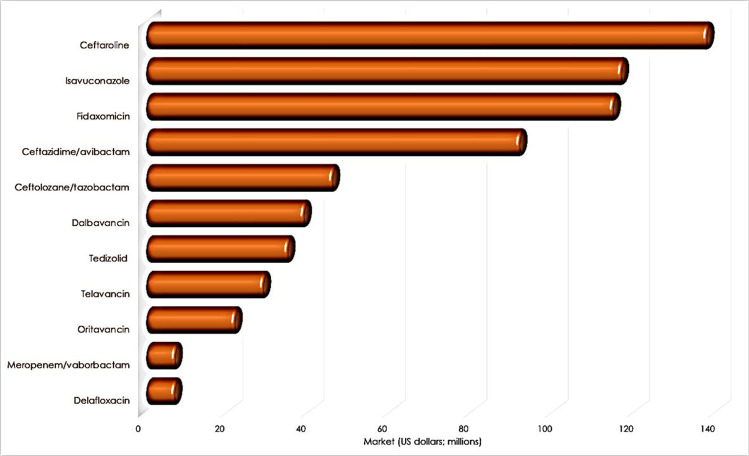
Likely, the most critical usage of secondary metabolites has been as anti-infective drugs. In 2018, the market of approved antimicrobials was around 647 million dollars only in US (Fig. 1), and it is predicted to expand at a compound annual growth rate of 3.4% (Clancy and Nguyen 2020; Carr and Stringer 2019).
Fig. 1.
The 2018 US market for common anti-infective compounds. The graphic was elaborated based on the reports of Clancy and Nguyen 2020 and Carr and Stringer 2019
Several infections affected a substantial proportion of the world population during the last years, causing thousands of deaths every year. Emerging organisms with infective capacity often confront hosts with no prior exposure and represent a novel challenge to the host’s immune system. Several viruses responsible for human epidemics have been transmitted from animal hosts to humans and transferred from human to human. These infections include the human immunodeficiency virus (HIV), Ebola virus disease, hepatitis C virus, and more recently, the coronavirus. They cause many severe infectious diseases and affect human societies economic growth, development, and prosperity (Morens and Fauci 2020).
Since the secondary metabolites discovered so far come from the 1% of the cultured microbes, advances in microbial techniques have become relevant in allowing uncultured microbes to grow as potential sources of new chemical products (Newman 2016). Moreover, metagenomics (a culture-independent technique that involves the extraction and analysis of whole DNA collected from a population of microbes from different habitats) allows access to a vast untapped reservoir of genetic and metabolic diversity. This technique may include deadwood (Gómez-Brandón et al. 2017), cattle rumen and manure (Fliegerová et al. 2014; Nagler et al. 2018), aerobic and bacterial anammox granules (Xiong and Liu 2012; Dominiak et al. 2011), human epithelial cells used in forensics (Wang et al. 2017a), and plant roots (Pietramellara et al. 2013). Therefore, with this technique, the potential for discovering new molecules with chemical diversity would grow enormously.
The need for new secondary metabolites
Bacterial resistance to antibiotics
The excessive amounts of antibiotics used in human therapy and those used for farm animals and aquaculture have allowed the appearance of pathogenic bacteria resistant to multiple drugs. Such resistance progressively limits the effectiveness of current antimicrobial drugs. Now, we are facing an increase in the number of reports emphasizing the development of bacterial resistance to nearly all antimicrobial agents. This situation will represent one of the leading global health concerns in the future. It is predicted to cause of approximately 10 million deaths annually in 2050, with a total cost of 100 trillion USD (Trotter et al. 2019). Although not always of high quality, the available evidence suggests a connection between personal and organizational misuse of antibiotics and the rise of resistant bacteria.
Eventually, the treatment of these emerging diseases has become increasingly complicated as microorganisms become resistant to the accessible antimicrobial options (Nordmann et al. 2011; Labro and Bryskier 2014). Over time, the specific weight supported by different antibiotic agents has ended the expansion of the auxiliary resistance mechanisms of organisms. These mechanisms include novel penicillin-restricting proteins, catalyst-dependent drug switching, transformed drug targets, and increased efflux pump expression that prompted to multidrug resistance (MDR).
In 2019, the Centers for Disease Control and Prevention (CDC) stated that in United States (US) hospitals alone, more than 2.8 million people acquire bacterial infections each year, causing 35,000 deaths https://www.cdc.gov/media/releases/2019/p1113-antibiotic-resistant.html. This number of deaths from antibiotic-resistant infections was nearly twice what was initially reported by CDC in 2013. Many cases of these infections are due to methicillin-resistant Staphylococcus aureus (MRSA). It caused nearly 120,000 bloodstream infections in 2017 and 20,000 associated deaths only in the USA (Kourtis et al. 2019).
MDR pathogens from the ESKAPE group (virulent MDR bacteria, including Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) raise further challenges, for instance, transmissible carbapenemases such as New Delhi metallo β-lactamases in Enterobacteriaceae. Most of them are resistant to inhibition by commercially available β-lactamase inhibitors (Gootz 2010; Khan and Nordmann 2012). It is worrying that “superbugs” organisms resistant to the main antibiotics in clinical use are rapidly emerging (Khan and Khan 2016).
In recent decades, sexually transmitted diseases have also increased, especially in young people (aged 15 to 24). Examples include human papillomavirus, genital herpes, chlamydia, HIV/AIDS (Acquired Immune Deficiency Syndrome), and gonorrhea. Unfortunately, HIV/AIDS has claimed 36.3 million (27.2–47.8 million) lives worldwide. In 2020, HIV/AIDS accounts for over 1.5 million (1–2 million) diseases, with 680,000 million (480,000–1 million) deaths per year [https://www.who.int/news-room/fact-sheets/detail/hiv-aids].
Tuberculosis is a highly infectious disease caused by Mycobacterium tuberculosis. The rate of tuberculosis began to increase after decades of decline in the early 1990s. The currently recommended treatment for drug‐susceptible M. tuberculosis is a 6–9-month-long regimen with a combination of four first‐line drugs: isoniazid, rifampicin, ethambutol, and pyrazinamide (Honeyborne et al. 2019). The epidemic occurred due to inadequate treatment regimens, which often lead to drug‐resistant strains that represent a major threat to global public health (Abraham et al. 2020). In 2015, a 90% reduction in TB deaths and 80% in TB incidence was predicted by the WHO in its 2030 goals (WHO 2017). The goals for 2035 are reductions of 95% and 90%, respectively. A third target was that there will be no households affected by TB, and those who have TB patients have social protection to ensure that patients and their families do not incur catastrophic expenses (Floyd et al. 2018).
Therefore, new secondary metabolites are continuously required to combat antibiotic-resistant bacteria fungal and viral diseases (Andryukov et al. 2019; Chandra et al. 2017; Seukep et al. 2020).
New alternatives for Gram-positive bacteria
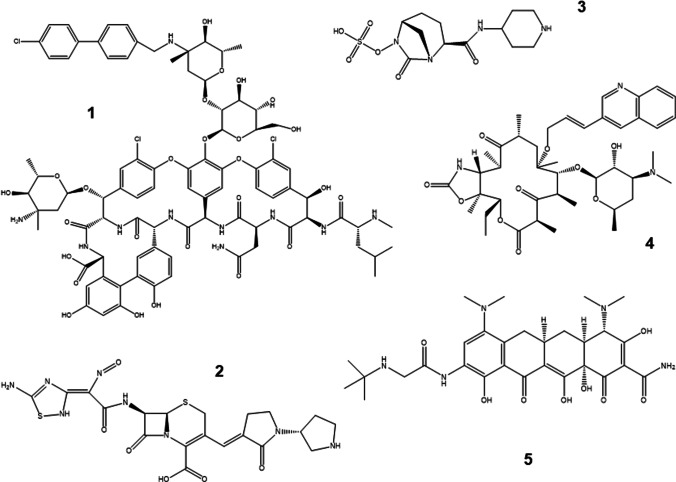
Renewed actions against Gram-positive pathogens have been recently taken. The lipoglycopeptides dalbavancin and oritavancin (Fig. 2) are new examples to treat Gram-positive infections. These antibiotics are used to treat infections caused by vancomycin-resistant Gram-positive microbes, including pathogens resistant to β-lactams (Sarkar and Haldar 2019). The Food and Drug Administration (FDA) approved Dalvabicin (trade name Dalvance) on May 23, 2014. In its mechanism, the antibiotic prevents the transpeptidation step during cell wall synthesis. In peptidoglycan, it binds to the C-terminal growing chain D-alanyl-D-alanine. It is effective against vancomycin-susceptible strains of S. pyogenes, S. agalactiae, and E. faecalis. The FDA approved Oritavancin (trade name Orbactiv) in August 2014. It is derived from chloroeremomycin, an analog of vancomycin, and due to its lipophilic side chain, it shows a prolonged half-life greater than 10 days. Oritavancin acts by disrupting the integrity of the bacterial membrane and inhibiting bacterial RNA synthesis. Its spectrum of activity mimics vancomycin but with lower MIC (minimum inhibitory concentration) values. It is effective against methicillin-resistant S. aureus (MRSA), vancomycin-resistant enterococci, and vancomycin-resistant staphylococci (Koulenti et al. 2019). Dalvabicin and oritavancin can be administered in adult patients as a single intravenous dose (Saravolatz and Stein 2015).
Fig. 2.
Examples of structures of novel antibiotics. 1) Oritavancin, 2) ceftobiprole, 3) relebactam, 4) cethromycin, and 5) tigecycline
During the last years, a range of novel modified glycopeptides was generated by semisynthetic modification of natural compounds. Among them, the eremomycin pyrrolidide shows high activity against staphylococci and enterococci, including vancomycin-resistant strains, without pseudo-allergic reactions (Olsufyeva et al. 2018).
Another antimicrobial agent is ceftobiprole (trade name Zevtera/Mabeli). This antibiotic was approved by the European Medicine Agency (EMA) in October 2013 (Koulenti et al. 2019). This fifth-generation cephalosporin blocks the cell wall synthesis by binding to penicillin-binding proteins. Ceftobiprole is an effective and well-tolerated compound for treating hospital-acquired pneumonia (HAP) patients, because of its efficacy for S. aureus bacteremia, acute bacterial skin infections, and community-acquired bacterial pneumonia. In 2019, ceftobiprole received the designation of Qualified Infectious Disease Product (QIDP) from the FDA.
Additional compounds for the treatment of antibiotic-resistant Gram-positive bacteria include F12. This compound is a lysin enzyme produced naturally by microbes. It represents the first next-generation compound in preclinical evaluation to treat life-threatening MRSA infections that could be used multiple times in a single patient (Zhao et al. 2020).
Another strategy involves a mixture of compounds effective against bacterial infections. Thus, a combination of imipenem, cilastatin, and relebactam (Recarbrio) has effectively treated nosocomial bacterial pneumonia. This mixture, approved on July 17, 2019, was also effective for patients with complicated tract infections who have limited or no alternative treatment options [https://www.fda.gov/news-events/press-announcements/fda-approves-antibiotic-treat-hospital-acquired-bacterial-pneumonia-and-ventilator-associated].
The glycylcyclines are further actions against resistant microorganisms, created to handle bacteria resistant to tetracyclines. These modified tetracyclines show strong activity against a broad spectrum of Gram-positive and Gram-negative bacteria, including strains carrying the two major determinants of tetracycline resistance involving efflux and ribosomal protection. The 9-t-butylglycylamido derivative, the N,N-dimethylglycylamido, and 6-demethyl-6-deoxytetracycline belong to the glycylcyclines. These antibiotics are structurally related to minocycline derivatives (TBG-MINO), exhibiting antimicrobial activity against S. aureus (MRSA), penicillin-resistant streptococci, and vancomycin-resistant enterococci with MIC values less than 0.5 μg/mL in which 90% of the strains show inhibition. In vivo effects of TBG-MINO against lethal infections in mice caused by isolates of Escherichia coli, S. aureus, and Streptococcus pneumoniae were investigated (Zhanel et al. 2004). Tigecycline (formerly GAR-936) is a semisynthetic tetracycline that shows good activity against Gram-positive cocci with 90% inhibition of penicillin-resistant strains of S. pneumoniae (at 0.12 µg/mL), Enterococcus spp. (at 0.25 µg/mL), and oxacillin-resistant S. aureus (at 0.25 µg/mL). Besides, respiratory pathogens such as Moraxella catarrhalis and Haemophilus influenzae are inhibited at 0.12 µg/mL and 0.5 µg/mL, respectively (Gales et al. 2005).
Telithromycin is the first orally active macrolide of the ketolides family. It shows high activity against community-acquired respiratory tract infection pathogens, irrespective of their ß-lactam, or fluoroquinolone susceptibility, initially, the FDA-approved telithromycin for the treatment of acute bacterial sinusitis, acute exacerbation of chronic bronchitis, and community-acquired pneumonia. However, in 2006, the FDA reduced the indications only for community-acquired pneumonia due to reports of severe drug-associated hepatotoxicity, visual disturbances, and exacerbation of myasthenia gravis (Brinker et al. 2009). The second-generation ketolide cethromycin exhibits higher antibacterial activity, even against the macrolide-resistant and telithromycin-resistant S. pneumoniae. There have been two major phases III clinical trials to treat mild to moderate community-acquired pneumonia. The adverse drug reactions include nausea, diarrhea, dysgeusia, and headache. However, cethromycin does not appear to cause the serious adverse effects of telithromycin (Koulenti et al. 2020).
Other common approaches are the repurposing of previously known compounds. The statin potential for host-directed therapy by modulating the immune system has been explored. Statins could be complementary when co-administered with other drugs to treat infectious diseases caused by pathogens like fungi, viruses, and bacteria. Thus, they are attractive candidates to prevent or reduce the severity of infection (sepsis, bacteremia, and pneumonia) and reduce treatment regimens. Nonetheless, it remains to be further studied those factors that could affect patient response to situations like potential interaction with other drugs and the adverse effects that this could entail (Parihar et al. 2019).
Moreover, some evidence suggests that statins may improve the treatment of infections caused by antibiotic-resistant bacteria. There are compartmentalized regions called functional membrane microdomains (FMMs) in prokaryotic cells. They are probably involved in critical cellular processes integrated by isoprenoid membrane lipids and flotillin homolog proteins. These are likely involved in the interaction with lipids and other proteins as scaffold proteins. In MRSA, FMMs are structured by the protein flotillin FloA, which supposedly participates in the association of multimeric complexes. One element of these complexes is the low-affinity penicillin-binding protein (PBPa) responsible for β-lactam antibiotic resistance. Hypercholesterolemic drugs like statins may compromise the FMM integrity by hindering these membrane domain lipid biosynthesis. This property, in turn, affects PBP2a oligomerization, resulting in MRSA infections sensitive to penicillin antibiotic treatments. Repurposing statins could be an approach to improve conventional antibiotic therapies. However, lipid biosynthesis of these membrane microdomains is not universal in bacteria, so statins do not appear to be inherently bactericidal (García-Fernández et al. 2017).
Regarding tuberculosis therapy, a rapid selection of M. tuberculosis mutants carrying changes in specific genes that confer resistance to first-line antimicrobials has been observed. For example, resistance to pyrazinamide in this pathogen is due to mutations in the gene encoding pyrazinamidase (Tahir et al. 2020). Latest developments in tuberculosis antimicrobial agents include avermectins and fluvastatin Mourenza et al. (2020). Avermectins are highly active anthelmintic macrolides against MDR strains of clinical isolates of M. tuberculosis. They reduce the initial bacterial viability up to 6 orders of magnitude, showing MIC90 values against different resistant isolates ranging from 6 to 8 μg/mL (Lim et al. 2013). Avermectines are approved for clinical and veterinary uses.
Fluvastatin is a lipid-lowering statin with antilipidemic and antimicrobial properties (Perihar et al. 2019). It inhibits the conversion of hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) to mevalonate, a key step in cholesterol synthesis. Cholesterol is essential for the internalization of mycobacteria into host cells (Gatfield and Pieters 2000). Statins modulate immune responses by suppressing major histocompatibility complex II slightly inducing the release of TH1 cytokines, promoting the activation of caspase 1, and strengthening the host response against M. tuberculosis.
Recently, an industry and academic collaboration, using previously uncultured soil microbes, identified three novel compounds (streptomycobactin, kitamycobactin, and amycobactin) effective against M. tuberculosis (Quigley et al. 2020). Kitamycobactin (an analog of lassomycin) targets the mycobacterial ClpP1P2C1 protease. This group further defines the mechanism of action of amycobactin, demonstrating that it inhibits protein secretion via the Sec translocation machinery.
Novel alternatives for Gram-negative bacteria
Infections caused by drug-resistant Gram-negative bacilli are a critical public health threat. Unfortunately, the available drugs to treat these infections (colistin, fosfomycin, aminoglycosides, and tigecycline) have a limited efficacy and safety profile and induce bacterial resistance. Thus, developing new active drugs against these pathogens has become a priority. A decade ago, the Infectious Diseases Society of America issued the 10 X’20 Initiative to develop ten new antibiotics by 2020 [https://doi.org/10.1086/652237].
For several years, a successful strategy in treating bacterial infections has been “combination therapy.” This approach involves the use of an antibiotic plus an inhibitor capable of blocking the primary mechanism of bacterial resistance. This strategy has been helpful to increase the efficacy of some β-lactam antibiotics since β-lactamase enzymes are responsible for a large proportion of the bacterial resistance phenotype. Streptomyces clavuligerus produces clavulanic acid with a bicyclic ß-lactam ring attached to an oxazolidine ring with oxygen instead of a ß-hydroxyethylidene sulfur substituent at C-2 and lacks an acyl-amino group at C-6. First described in 1976, this antibiotic exhibited potent inhibition of the β-lactamases synthesized by staphylococci and β-lactamases mediated by plasmids from E. coli, Klebsiella, Shigella, Pseudomonas, Proteus, and Hemophilus (Breijyeh et al. 2020). Clavulanic acid is a β-lactamic compound with low antibacterial activity. However, as a ß-lactamase inhibitor, this molecule has been combined with broad-spectrum semisynthetic penicillin drugs. For instance, when administered with amoxicillin, it is marketed under the brand name Augmentin to treat infections caused by pathogenic β-lactamase-producing bacteria (Worthington and Melander 2013). However, its spectrum is limited to class A β-lactamases and does not include carbapenemases from K. pneumoniae (Papp-Wallace 2010). Fortunately, there are now new classes of inhibitors in the clinic, and more compounds and combinations are being developed. Boron-based compounds, especially the cyclic boronate taniborbactam, are the current alternatives against the most aggressive pathogens. When these compounds are combined with cefepime, they show a broad-spectrum of activity against a wide range of serine-β-lactamases and relevant metallo-β-lactamases (González-Bello et al. 2020). Besides, a breakthrough is the sulbactam/durlobactam combination for infections caused by A. baumannii. Another example is the new orally available diazabicyclooctane (ETX0282), which inhibits class A, C, and D serine β-lactamases. Combined with cefpodoxime or CDP4, it is an effective therapy for treating MDR and carbapenem-resistant bacterial infections (Durand-Réville et al. 2020).
Nosocomial bacteria are microbes that inhabit hospitals, including Gram-positive and Gram-negative pathogens. More than 60% of hospital sepsis are caused by Gram-negative bacteria (Klebsiella, Escherichia, Enterobacter, Serratia, Citrobacter, Salmonella, and Pseudomonas) and have become a major threat to public health (Dolin et al. 2019; Rannikko et al. 2017). Among them, Pseudomonas aeruginosa accounts for approximately 80% of these opportunistic infections. They are a severe problem in hospitalized patients with cancer, cystic fibrosis, and burns, causing death in 50 percent. Pseudomonas species are naturally MDR bacteria that can cause infections like pneumonia and endocarditis. They also cause infections of the urinary tract, central nervous system, wounds, eyes, ears, skin, and musculoskeletal system. Although many bacteria are sensitive to gentamicin, tobramycin, and amikacin, resistant strains constantly develop. Thus, the frequency of MDR pathogenic bacteria increases, making hospitals “dangerous places to be, especially if you are sick, but even if not” (Van Duin and Paterson 2016). Concrete actions against this resistance are the development and approval of several antibiotics. These included Ceftaroline fosamil on October 29, 2010; Tedizolid phosphate on June 20, 2014; Oritancancin on August 6, 2014; Ceftozolane and Tazobactam on December 19, 2014; Ceftazidime and Avibactam on February 25, 2015. Besides, Dalofloxacin on June 17, 2017; Meropenem and Vaborbactamin on August 30, 2017; Plazomicin on June 26, 2018; Eravacycline on August 27, 2018; Omadacycline, approved on October 2 and active against Gram-positive and Gram-negatives, 2018 and Lefamulin, approved on August 19, 2019. Cefiderocol is a siderophore cephalosporin active against carbapenem-resistant Gram-negatives. It is a modified cephalosporin (approved on November 14, 2019) with one pyrrolidinium group and one carboxypropanoxyimino located at the 3 and 7 positions of the cephem nucleus (Zhanel et al. 2004). This compound enters the bacterial periplasm by attaching to penicillin-binding proteins due to its siderophore-like property, causing inhibition of cell-wall synthesis. [https://www.contagionlive.com/view/have-we-made-progress-in-the-10-20-initiative].
Although there is a steady increase in resistance to most current antibiotics in almost every pathogen over time, not all antibacterial agents exhibit the same rate of resistance development. For example, antimicrobials such as rifampicin, which targets single enzymes, are the most susceptible to the development of resistance. In contrast, resistance to agents such as penicillin, which irreversibly inactivate multiple targets, develops slowly.
Fungal infections
More than 150 million infections by fungi occur worldwide every year (Kainz et al. 2020). Pathogenic yeasts are also a problem. The main etiologic agent associated with nosocomial candidiasis is Candida albicans. The estimated global burden of candidaemia is 159,253 cases across 39 countries (Bongomin et al. 2017).
Fungi are harmless most of the time, but they can occasionally provoke infections named mycoses, where the fungi surpass human or animal resistance barriers. Generally, these infections are not life-threatening. However, when they are invasive and spread, they cause more severe infections, such as in critically ill patients and people treated with drugs that disrupt the immune system. Besides, the use of antineoplastic and broad-spectrum antibiotics, prosthetics and grafts, and more aggressive surgeries has augmented invasive fungal infections. Patients with burns, pancreatitis, neutropenia, or post-organ transplantation (62–91% liver, 28–55% heart, 6–38% pancreas, and 20% kidney) are also predisposed to fungal infections (Silveria and Husain 2007). About 40% of the nosocomial deaths are due to fungal infections, and Aspergillus and Candida cause 80%. Also, Cryptococcus spp., Fusarium spp., Scedosporium spp., Penicillium spp., and zygomycetes are increasingly involved (Badiee and Hashemizadeh 2014; Bougnoux et al. 2018; Khan et al. 2017). Pneumonia caused by Pneumocystis carinii remains the leading common AIDS-associated infection worldwide (Pereira-Díaz et al. 2019; Huang et al. 2011). Patients undergoing a bone marrow transplant can have severe complications because of pulmonary aspergillosis.
The emergence of increased resistance to fungi and the increasing incidence of invasive fungal infections have led to the search for novel antifungal compounds (Sanchez and Demain 2017a). Although amphotericin B is the first-line treatment for systemic infections (because of its broad-spectrum and fungicidal activity), significant side effects limit its clinical utility. Additional compounds with antifungal activity are the echinocandins. These are large lipopeptide molecules that inhibit the synthesis of 1,3-β-D-glucan by disrupting the fungal cell wall (Yoshimi et al. 2017). Three echinocandins have reached the market, i.e., caspofungin (pneumocandin or MK-0991), anidulafungin, and micafungin. Caspofungin exhibits higher activity and lower toxicity than amphotericin B. Its activity included isolates resistant to azoles and amphotericin B. Some filamentous fungal species like Aspergillus, a few dimorphic fungi (Histoplasma, Blastomyces), Candida, P. carinii, and Coccidioides were also affected (Letscher-Bru and Herbrecht 2003). Regarding anidulafungin, it is currently licensed in the USA and has proven efficacy against esophageal candidiasis and invasive infections by Candida and Aspergillus (Ikeda et al. 2007). As for micafungin, it has been licensed for clinical use in Asian countries and the USA. This drug exhibits extremely potent antifungal activity against clinically relevant fungi, including Aspergillus and azole-resistant strains of Candida (Wang et al. 2017b). In animal studies, micafungin is as effective as amphotericin B in recovering survival rate. A linear pharmacokinetic profile distinguishes it with less toxic effects.
Although several new antifungal drugs have been introduced in the last six years, some patients remain resistant to treatments. This resistance is primarily due to intrinsic or acquired antifungal resistance, organ dysfunction that precludes the use of some agents, and drug interactions. Besides, some drugs penetrate poorly into sanctuary sires like the eye and central nervous system (Pasqualotto and Denning 2008). Nonetheless, there are some improvements in the development of new compounds. Among these advances, we can mention posaconazole, a new member of the class of triazole antifungals with a favorable toxicity profile. Posaconazole is a broad-spectrum antifungal agent that inhibits ergosterol biosynthesis. It has demonstrated clinical efficacy in the treatment of oropharyngeal candidiasis. It has the potential to be used as salvage therapy for various aggressive fungal infections like invasive aspergillosis, zygomycosis, and cryptococcal meningitis. A combination of posaconazole with caspofungin or the calcineurin inhibitor FK506 exhibits synergistic effects against the human fungal pathogen C. albicans (Chen et al. 2013).
Despite the importance of infectious diseases, big pharmaceutical companies have lost interest in developing new antibiotics. However, anti-infectives are still big business, and therefore, there is a need to develop new antimicrobials. Between 2010 and 2019, some small pharmaceutical and biotechnology companies introduced novel antibacterial compounds, which have evolved into attractive sales (Table 1).
Table 1.
Applications and sales of some antimicrobials approved between 2010 and 2019
| Brand name | Agent | FDA approval | USD millions | Class | Treatment |
|---|---|---|---|---|---|
| Teflaro | Ceftaroline | October 2010 | 138.00 | 5th-generation cephalosporin | ABSSSIs, CABP |
| Cresemba | Isavuconazole | March 2015 | 117.00 | Azole class antifungal | Invasive aspergillosis and invasive mucormycosis |
| Dificid | Fidaxomicin | May 2011 | 115.00 | Macrolide | Clostridioides difficile-associated diarrhea |
| Avycaz | Ceftazidime/avibactam | February 2015 | 92.00 | β-lactam/β-lactamase inhibitor | cIAI, cUTI including pyelonephritis, HABP, VABP |
| Zerbaxa | Ceftolozane/tazobactam | December 2014 | 46.00 | β-lactam/β-lactamase inhibitor | HABP, VABP |
| Dalvance | Dalbavancin | May 2014 | 39.00 | Lipoglycopeptide | ABSSSIs |
| Sivextro | Tedizolid | June 2014 | 35.00 | Oxazolidinone | ABSSSIs |
| Vibativ | Telavancin | September 2009 | 29.00 | Lipoglycopeptide | HABP, VABP, cSSSI |
| Orbactiv | Oritavancin | August 2014 | 22.00 | Lipoglycopeptide | ABSSSIs |
| Vabomere | Meropenem/vaborbactam | August 2017 | 7.30 | Carbapenem β-lactamase inhibitor | cUTI including pyelonephritis |
| Baxdela | Delafloxacin | June 2017 | 7.20 | Fluoroquinolone | ABSSSIs |
| Xerava | Eravacycline | August 2018 | 5.9 | Fluorocycine | cIAI |
| Zemdri | Plazomicin | June 2018 | NA | Aminoglycoside | cUTI including pyelonephritis |
| Recarbrio | Imipenem-cilastatin-relebactam | July 2019 | NA | New antibacterial combination | cUTI including pyelonephritis |
| Fetroja | Cefiderocol | November 2019 | NA | Siderophore cephalosporin | cIAI, cUTI including pyelonephritis, HABP,VABP |
In addition, to tackle antibiotic resistance, new families of antibiotics must enter the market at regular intervals to address new diseases produced by evolving pathogens. At least 30 new diseases have appeared between 1980 and 1990, and their incidence is increasing.
Antiviral infections
Among many of the danger infectious that people faces today, viruses represent a serious problem that threatens deadly global pandemics. Viral diseases are emerging and spreading widely due to changes in global environments and the evolution of transport systems. Although understanding of viral biology and physiology has increased in recent years, several infections affected a substantial proportion of the world’s population, causing thousands of deaths every year.
Some viruses are frequently involved in respiratory infections. Typical examples are the respiratory syncytial virus, human parainfluenza viruses 1 and 3, influenza viruses A and B, and some adenoviruses. Some viral diseases severely damage economic, social, and human conditions, including those caused by HIV, Ebola virus, hepatitis C virus, and coronavirus. They produce millions of deaths and many serious illnesses, affecting the economic growth of human society, their development, and prosperity. The coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Apparently, it has a zoonotic origin; the hosts’ animals are rodents and bats, while other animals such as civets, raccoon dogs, camels, and possibly pangolins play a role in transmission as intermediate hosts (Amoutzias et al. 2022; Guo et al. 2020; Lau and Chan 2015). Therefore, there is a constant requirement for new antiviral compounds.
The global antiviral drug treatment market report is estimated to reach a cost of nearly USD 75.33 billion by 2027 from about USD 52.21 billion in 2019, growing at a compound annual growth rate of approximately 4.69% between 2020 and 2027 [https://www.pharmiweb.com/press-release/2020-08-19/antiviral-drugs-treatment-market-to-develop-stunningly-and-reach-about-usd-7533-billion-by-2027-z].
Natural substances continue to be primary sources for prototype antimicrobial and antiviral drugs, although many chemically synthesized drug basic structures owe their origin to natural sources. Among antimicrobial drugs, antivirals represent more than 20% of the market. Two antivirals initially acquired from sea sponges are currently obtained by chemical synthesis. One is acyclovir, which is effective against the herpes virus and HIV. It inhibits and inactivates the DNA polymerase of the virus (Vanpouille et al. 2012). The other is cytarabine, effective against acute myeloid leukemia (Momparler 2013). Both metabolites are nucleoside analog drugs (Jiménez et al. 2018).
With up to 37 million people living with HIV worldwide, HIV infection poses a serious public health risk. Even though a combination of antiretroviral therapy provides stable viral suppression, it is not devoid of undesirable side effects, especially in persons undergoing long-term treatment. Screening natural products derived from various species have afforded metabolites with notable antiviral activity against HIV. Among them, γ-pyrone Aureothin is a natural polyketide produced by Streptomyces thioluteus. It harbors a γ-pyrone group and a tetrahydrofuran ring separated from a 4-nitro phenyl group by a diene backbone. Aureothin was identified as a successful candidate through a single-dose (10 µM) screening and validated by evaluating the dose dependence of HIV inhibitory activities. Aureothin (IC50 = 5.3 ± 0.40 nm) showed robust anti-HIV activity, comparable or only moderately lower than that of several clinical drugs assayed in parallel (the protease inhibitor Saquinavir, IC50 ~ 10 nm), the reverse transcriptase inhibitor Efavirenz and the integrase inhibitor Dolutegravir (each ~ 1 nm). A drawback of this compound is its high sensitivity to light. However, several Aureothin molecules with fluorinated or chlorinated derivatives have been synthesized with better photostability than the original compound, retaining more than 95% of anti-HIV activity after illumination. In one of these derivatives, trifluoromethyl replaces the nitro group and inhibited the accumulation of uncleaved and partially spliced mRNAs, preventing the production of all major components of virus particles. The mode of action of this compound is different from all current clinical anti-HIV drugs. It shuts down de novo virus production by inhibiting HIV gene expression and effectively inhibits a spectrum of clinical isolates, including all HIV genotypes (HIV-type 1 and HIV-type 2) (Herrmann et al. 2020).
In its pathogenesis, HIV-1, like other retroviruses, depends on its stable integration into the host genome to efficiently replicate the viral RNA and, hence, keep the infected state. Therefore, the new viral DNA synthesized during reverse transcription is quickly integrated into the host cell genome (through a step called “integration”), allowing transcription of viral RNA. In the late HIV viral replication phase, a viral protease cleaves the large polyprotein precursor GagPol. This action enables the maturation of the viral particle and infectivity. Non-infectious viral particles result when the precursor Gag polyprotein is not cleaved adequately. Therefore, the US National Cancer Institute (NCI) has directed an enormous effort to explore natural metabolites that inhibit HIV reverse transcriptase and protease. These products are the primary agents currently used to contain HIV. Besides, the NCI has evaluated the HIV-1 inhibitory activity of pepstatin A, a small pentapeptide synthesized by several species of Streptomyces. This compound that contains a rare amino acid (4-amino-3-hydroxy-6-methylheptanoic acid) sterically blocks the active site of HIV-1 protease (Yang et al. 2001; Matúz et al. 2012). Recently, a linear peptide (ahmpatinin iBu) isolated from Streptomyces sp. has been reported as an effective HIV-1 protease inhibitor with an IC50 value of 1.79 nM (Chen et al. 2018).
In the late 2019, an ongoing outbreak of pneumonia cases was reported in Wuhan, China. This infection was caused by a novel coronavirus associated with the severe acute respiratory syndrome coronaviruses. Therefore, it was designated SARS-CoV-2 (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses 2020). This viral disease became a pandemic in February 2020. Researchers worldwide have responded by studying physiology of the virus in different settings and testing all kinds of approved antiviral drugs to tackle the disease or develop novel compounds to inhibit viral spread (Ciliberto and Cardone 2020). All sorts of scientific specialists were involved in this task, including immunologists, epidemiologists, microbial biotechnologists, pharmacologists, mathematicians, physicists, and engineers. They decided to change their main research interest to offering solutions for the COVID‐19 pandemic. In support of this action, government and international funds became available to capitalize on global efforts to develop many potential solutions. In parallel, people from the industry entered alone or associated with Universities or Biotech-companies to emerge with diagnostic tests and the production of specific vaccines [https://publications.iadb.org/publications/english/document/Responding-to-COVID-19-with-Science-Innovation-and-Productive-Development.pdf], as possible solutions to rapidly stop or alleviate the enormous number of deaths caused by COVID-19. Unfortunately, fast-spreading variants are emerging (Burki 2021), and scientists should attempt new treatment alternatives.
Experts in microbial biotechnology have the tools to lead the second wave of efforts against SARS-CoV-2 and related variants. Recent progress in synthetic and systems biology has supported the field by introducing new and unprecedented tools that, when combined with iterative pipelines, may increase chemical alternatives and industrial production of bioactive compounds, such as antivirals (Martinez 2020).
Using these tools, structurally different compounds like terpenes, polysaccharides, polyketides, and peptides that inhibit RNA and DNA viruses have been isolated from various microorganisms like fungi and actinobacteria using these tools (Zaporozhets and Besednova 2020). These compounds can prevent the coronavirus entry into the cell or the release of virions out of the cell. They can even inhibit the viral proteins or disrupt the viral genome replication. The diversity of these chemical classes is related to their different mechanisms to inhibit coronaviruses (Zaporozhets and Besednova 2020).
The spherical capsid envelops a positive-strand RNA genome of about 30 kb which is considered the largest of its kind. It contains two replicase proteins cleaved by a major viral protease (Mpro), a highly conserved molecular target in coronaviruses (Hoffmann et al. 2020). Earlier reports on viral protease inhibitors like the HIV protease inhibitor lopinavir revealed significant anti-SARS-CoV-2 activities in vitro (Choy et al. 2020).
An extensive database of specialized microbial natural products (The Natural Product Atlas) containing more than 24,000 different compounds has been used to detect natural ligands that could block the active site of Mpro (Sayed et al. 2020).
Analysis of ligands with the highest Mpro score indicated that several compounds were potentially effective against SARS-CoV-2 (Lipinski 2004). Among them, the polyketide citriquinochroman, produced by the endophyte Penicillium citrinum, and the indolocarbazole alkaloid holyrine B, produced by a marine actinomycete (Fiedler et al. 2008), can be distinguished. The third best compound is the aminofuran proximicin C, isolated from the actinobacteria Verrucosispora MG-37 (Fiedler et al. 2008).
The contribution of other known compounds to the treatment of COVID-19 has been explored. Recently, an additional use for statins has been reported by Daniels et al. (2020). This group found that using statin before hospital admission in COVID-19-infected people was associated with a more than 50% reduction in risk of developing severe disease symptoms. Thus, patients with COVID-19 who took statins fared better. Statins decrease COVID-19 severity, likely by removing cholesterol that the virus uses to infect. The researcher’s findings support that the statin use before hospital admission for COVID-19 reduced by more than 50 percent the risk of developing severe COVID-19 (Daniels et al. 2020).
Therefore, although vaccines have been developed, antivirals are still needed to combat the infection of this virus.
Additional needs of secondary metabolites
Over the years, the pharmaceutical industry has extended its antibiotic screening programs to look for other antibiotic applications in medicine and agriculture (Davies and Davies 2010; Berdy 2012; Pham et al. 2019; Demain and Martens 2017). As a result of this movement, some of the main essential products of the pharmaceutical industry have been obtained. For example, immunosuppressants have revolutionized medicine by enabling organ transplantation (Watson and Dark 2012; Duncan and Wilkes 2005). Other applications comprise hypocholesterolemic agents, antitumor drugs, and other pharmacological activities (Fig. 3). Catalyzed by simple enzymatic assays for pretest detection in intact animals or in the field, further applications are increasing in several areas of pharmacology and agriculture. In 2013, more than 15 secondary compounds were derived from marine fungi in clinical trials (Bhatnagar and Kim 2013). Interestingly, many of the 300 new natural products isolated from marine sources were polyketides.
Fig. 3.
Applications of natural products
Hypocholesterolemic drugs of microbial origin
Atherosclerosis is a chronic, progressive disease typified by a continuous accumulation of atheromatous plaques within the arterial wall, causing stenosis and ischemia. The last two decades have testified to the introduction of a variety of antiatherosclerotic therapies.
Statins are hypolipidemic drugs that lower blood cholesterol levels. They inhibit the HMG-CoA reductase, a mevalonate pathway enzyme involved in cholesterol synthesis. Inhibition of HMG-CoA reductase in the liver stimulates low-density lipoprotein (LDL) receptors, causing an increased LDL clearance from the bloodstream and decreased blood cholesterol levels. Their cholesterol-lowering effect reduces the risk of cardiovascular disease, prevents stroke, and drops peripheral vascular disease (Pollak and Kramer 2012). Besides, statins have an anti-thrombotic and anti-inflammatory effect.
Currently, there are several statins in clinical use with an annual market of almost $30 billion. The first member of the statins group (compactin or mevastatin) was isolated as an antibiotic product from Penicillium brevicompactum and later from Penicillium citrinum. Although compactin was not commercially significant, its derivatives achieved substantial medical and commercial success (Endo 2010).
The lactone form of mevalonic acid (known either as lovastatin, monacolin K, mevinolin, or Mevacor™) can be extracted from broths of Monascus rubra and Aspergillus terreus (Manzoni and Rollini 2002). Lovastatin, developed by Merck & Co., and officially approved by the FDA in 1987, was the first commercially marketed statin. Lovastatin has a hexahydronaphthalene chemical skeleton in its chemical structure, substituted with a p-hydroxy-lactone moiety. A semisynthetic derivative of lovastatin with significant hypocholesterolemic activity is Zocor™ (simvastatin). Before becoming generic, the industry reported drug sales for 7 billion dollars per year. Simvastatin production usually starts from lovastatin in a multistep chemical process. However, an enzymatic bioconversion procedure using recombinant E. coli has been developed (Xie and Tang 2007). Pravastatin (3.6 billion dollars per year) can be produced via different biotransformation processes from compactin by Streptomyces sp. (Park et al. 2003), S. avermitilis (Yao et al. 2017), and Penicillium chrysogenum (McLean et al. 2015). Other genera involved in the manufacture of statins are Doratomyces, Eupenicillium, Gymnoascus, Hypomyces, Paecilomyces, Phoma, Trichoderma, and Pleurotus (Alarcon et al. 2003). A synthetic compound modeled from the natural statins’ chemical structure is Lipitor, the leading drug of the whole pharmaceutical industry in terms of the market (about 14 billion dollars per year) for many years.
Microbial drugs used in clinical practice as anticancer therapy
In 2020, over 19.3 million new cancer events were diagnosed in the world. A 19% higher incidence corresponded to men than women, and deaths for this number ascended to 43%, with variations depending on the world region. The tumor classes with the highest frequency were breast (11.7%), lung (11.4%), colorectal (10.0%), prostate (7.3%), and stomach (5.6%) (Sung et al. 2021). Over 60% of the anticancer drugs in clinical use are natural products derived from microorganisms. The drugs started to appear around 1940 with the discovery of actinomycin from Streptomyces antibioticus. Since then, many anticancer compounds have been isolated from natural sources and introduced into clinical practice. Relevant antiproliferative examples are daunorubicin, doxorubicin, epirubicin, pirarubicin, valrubicin (anthracyclines), bleomycin (RSP), mitomycin C (azidine-containing compounds), piclamycin (anthracenones), streptozotocin (nitroso-urea compounds), pentostatin (purine analog), calicheamicin (endiynes), camptothecin (indole alkaloid), Taxol and epothilones (taxanes), and romidepsin (depsipeptide). Among these drugs, some novel anticancer drugs of microbial origin and clinical relevance deserve particular attention. These include anthracyclines, licheamicin, camptothecin, the taxanes (Taxol and epothilones), and the depsipeptide romidepsin.
Some of the most efficient antitumor agents ever developed are anthracyclines. They are effective against more types of cancer (leukemias, lymphomas, breast, ovarian, uterine, and lung cancers) than any other chemotherapy agents (Kadurin et al. 2017). Regarding their mechanism of action, anthracyclines inhibit the synthesis of DNA and RNA in the target cells. They form a complex by intercalating in DNA strands. They trigger DNA cleavage by topoisomerase II, resulting in mechanisms that conduct to cell death. In their cytotoxicity, the binding to cell membranes and plasma proteins performs a critical role. The first anthracycline discovered was daunorubicin (daunomycin), naturally produced by Streptomyces peucetius. Doxorubicin (adriamycin) is a 14-hydroxylated version of daunorubicin, approved by the FDA in 1974. It shows a better activity against solid tumors and a higher therapeutic index. Their major adverse effects are heart damage (by dose-dependent cardiotoxicity, which is cumulative and irreversible), and vomiting, which considerably restricts their usefulness (Henriksen 2018). Anthracycline treatment also induces drug resistance even at the desired cumulative dose. Many semisynthetic analogs resulting from the aglycone and sugar moiety chemical modifications were developed to deal with the undesirable effects. However, despite the numerous efforts, only some of them reached the stage of clinical development, and even fewer achieved marketing approval. Epirubicin (Ellence®) is privileged over doxorubicin as it causes fewer side effects and shows better elimination. The epirubicin structure has a different spatial orientation of the hydroxyl group at the 4′ carbon of the sugar, explaining its faster elimination and reduced toxicity. Epirubicin is beneficial against breast and ovarian cancer, lung cancer, gastric cancer, and lymphomas. However, this compound may cause severe heart problems during cancer treatment or after treatment ends. An additional analog is Valrubicin (Valstar), which is the N-trifluoroacetyl, 1–4-valerate derivative of doxorubicin. Although Valstar was removed from the market in 2002 due to manufacturing problems, it was placed back on the FDA Drug Shortage List in 2007, after it was considered a medical need.
An analog of daunorubicin is idarubicin. It lacks the C-4 methoxy group of the natural compound, and this increases its lipophilicity. Idarubicin has improved activity as induction therapy for acute myelogenous leukemia and some solid tumors. This improvement probably results from its increased lipophilicity and cellular uptake. Due to its formulation with thermosensitive liposomes, the drug is rapidly released in mild hyperthermia, improving the tumor regression (Lu et al. 2021). Similarly, an effective tumor relapse by doxorubicin can be observed using single low-dose treatment with hyperthermia-activated liposomal doxorubicin (Tagami et al. 2011). This treatment increases drug uptake by 5.2-fold compared to free doxorubicin and reduces its heart delivery by 15-fold.
Several new anthracyclines are currently in clinical trials against prostate cancer and leukemia (Bigagli et al. 2018; Tantawy et al. 2020).
Calicheamicins are additional anticancer drugs of the enediyne family produced by Micromonospora echinospora. Their antitumor mechanism is probably due to the double-stranded DNA cleavage (Elshahawi et al. 2014). They are extremely toxic, but it was possible to introduce one such compound into the clinic by ingeniously attaching it to a monoclonal antibody for selective delivery to cancer cells. The monoclonal antibody binds to a transmembrane receptor (CD33) expressed in monocytic/myeloid lineage cells. Most leukemic blast cells express CD33, but the amount diminishes with maturation in normal hematopoietic cells. In this regard, gentuzumab ozogamicin (marketed as Mylotarg™) was developed to treat acute myelogenous leukemia (AML). In 2000, the FDA approved this drug for patients over 60 years old with relapsed AML who are not considered candidates for standard chemotherapy. However, the monoclonal antibody drug linker was unstable, and 50% of the bond drug was released in 48 h. Thus, the FDA removed this drug from the market due to safety concerns (Panowski et al. 2014). Later in September 2017, the FDA approved the low dose of gemtuzumab ozogamicin in chemotherapy for the treatment of CD33 positive refractory AML (Nagayama et al. 2017).
Camptothecin is a modified monoterpene indole alkaloid that has been used in traditional Chinese medicine. Isolated from the Camptotheca acuminate tree, this drug was chosen by its antitumoral activity against an animal leukemia model (Asano et al. 2009). However, considering the quantities required for the pharmaceutical industry worldwide, the yield of camptothecin extracted from plants was insufficient (Martino et al. 2017). An endophytic fungus isolated from the plant Nathapodytes foetida (Entrophospora infrequens) also produces camptothecin. Because of the low concentration of camptothecin in tree roots and poor yield from chemical synthesis, fungal fermentation is very promising for its industrial production. The drug is used for recurrent colon cancer and has unusual activity against lung, ovarian, and uterine cancer (Li et al. 2017a). The cellular target of camptothecins is the type I DNA topoisomerase since it shows increased levels in cancer cells compared with normal cells. The enzyme inhibition mechanism occurs by noncovalent binding of camptothecins to the complex topoisomerase-DNA, producing irreversible DNA strand breaks and causing cell death (Martino et al. 2017).
Camptothecin is known commercially as Camptosar™ and Campto™ and accomplished sales of $1 billion in 2003 (Lorence and Nessler 2004). Irinotecan and topotecan are two water-soluble camptothecin derivatives approved by the FDA in 2015. Metastatic colorectal neoplasia responds to irinotecan. Topotecan is used for ovarian cancer, cervical cancer, and small-cell lung cancer. When the patients become resistant to irinotecan, the combination with the monoclonal antibody Erbitux (Cetuximab) can extend the use of this compound. This antibody blocks a protein that stimulates tumor growth, and therefore, its combination with irinotecan helps patients with metastatic colorectal cancer express the epidermal growth factor receptor. This protein factor is expressed in 80% of advanced metastatic colorectal cancers. The drug combination decreases the invasion of normal tissues by tumor cells and the spreads of tumors to new areas. Today, one of the most successful nonactinobacterial cancer-fighting molecules is paclitaxel (sold as Paxene®, Taxol®), which was first isolated from the Pacific yew tree, Taxus brevifolia. Taxol® is a steroidal alkaloid diterpenoid. It has a N-benzoylphenyl isoserine side chain and a tetracycline ring. This compound inhibits fast-dividing mammalian cancer cells by stimulating tubulin polymerization and interfering with normal microtubule breakdown during cell division. The benzoyl group of the molecule is particularly essential for maintaining its potent bioactivity.
Taxol® also inhibits several fungi (Pythium, Phytophthora, and Aphanomyces) by a similar mechanism. Taxol was approved in 1992 for refractory ovarian cancer, and today is used against breast cancer and advanced forms of Kaposi’s sarcoma (Benbrahim et al. 2013). Paclitaxel has seized $3.7 billion annual sales in international markets. Moreover, a new formulation in which paclitaxel is bound to albumin is sold under the trademark Abraxane. The net sales worldwide of this product increased by 9% between 2016 (973 million dollars) and 2018 ($1,062 million dollars) [https://ir.celgene.com/press-releases-archive/press-release-details/2019/Celgene-Reports-Fourth-Quarter-and-Full-Year-2018-Operating-and-Financial-Results/default.aspx].
A low production level of Taxol is obtained from the yew tree, ranging from 0 to 0.069% of the dry weight. In order to meet the commercial requirements of Taxol, some Taxus species have been endangered, which pressed to find other sources of this natural product (Li et al. 2017b). Although synthetic procedures for Taxol production have been tried, the chemical molecular structure is so complex that commercial synthetic production is unachievable. Taxol production by cell culture of Taxus sp. amounted to 67 mg L−1 (Sabater-Jara et al. 2010). Currently, Italy, the UK, the Netherlands, and other Western countries are involved in Taxol production by plant cell fermentation technology.
Although initially discovered in plants, Taxol has also been discovered to be a fungal metabolite. Fungi from genera such as Alternaria, Aspergillus, Cladosporium, Fusarium, Nodulisporum, Penicillium, Pestalotiopsis, Phoma, Phomopsis, Phyllosticta, Taxomyces, Trichoderma, and Tubercularia produce Taxol (Naik 2019). However, Taxol-producing fungi may lose the ability to synthesize this compound. Soliman and Raizada (2018) have shown in the endophyte fungi Paraconiothyrium that this compound production is inhibited by light, resembling the conditions in which the fungus is found in nature.
Some elicitors can enhance Taxol production. Thus, supplementation of the endophyte Pestalotiopsis microspora (isolated from the tree Taxodium mucronatum) culture medium with 300 µM salicylic acid increases 45-fold its production. This increase (625.5 μg L−1) correlates well with the transcription levels of the geranylgeranyl pyrophosphate synthase gene, which is involved in Taxol biosynthesis (Subban et al. 2019). In another study (Qiao et al. 2017), supplementing the culture media with NaOAc, Cu2+, and salicylic acid increased the Taxol yield in the endophytic fungus Aspergillus aculeatinus Tax-6 (isolated from Taxus chinensis var. mairei) from 334.92 to 1337.56 mg L−1.
On the other hand, production by submerged fermentation was reported at 417 μg L−1 levels with an engineered strain of the fungus Ozonium sp. (EFY-21). The transformed fungus overproduced the Taxol rate-limiting enzyme taxadiene synthase (Wei et al. 2012). Another endophytic strain, Phoma betae, isolated from the medicinal tree Ginkgo bilova, produced Taxol at 795 μg L−1 (Kumaran et al. 2012). Cladosporium cladosporoides, an endophyte fungus of the Taxus media tree, produces 800 μg L−1 of Taxol (Zhang et al. 2009). Furthermore, Metarhizium anisopiliae H-27, isolated from the tree Taxus chinensis yielded 846 μg L−1 (Liu et al. 2009). A review on Taxol production by endophytic fungi reported levels of 0.4–1.0 mg L−1 after strain improvement (Zhou et al. 2010). While the fungus Alternaria alternate var. monosporous, isolated from the bark of Taxus yunanensis, can produce 227 mg L−1 after treatment with ultraviolet and nitrosoguanidine (Duan et al. 2008).
The epothilones were initially isolated from the broth of the soil myxobacterium Sorangium cellulosum (Hardt et al. 2001). They are macrolides exhibiting weak activity against rust fungi (Hardt et al. 2001). They were later recognized as microtubule-stabilizing drugs, acting similarly to Taxol (Kowalski 1997). Besides, they are 5- to 25-fold more potent than Taxol in inhibiting cell growth. Five epothilone analogs are now undergoing investigation as candidate anticancer drugs, and their preclinical studies have suggested a broad spectrum of antitumor activity, including Taxol-resistant tumor cells. With the best therapies currently available, the median survival for patients with metastatic breast cancer is only 2–3 years, and many develop resistance to taxanes or other chemotherapy compounds. Ixabepilone is an epothilone approved in October 2007 by the FDA to manage aggressive metastatic or locally progressive breast cancer, which no longer responded to existing chemotherapies (Goodin 2008). In tumor cells, p-glycoprotein decreases intracellular antitumor drug concentrations, limiting access of chemotherapeutic substrates to the site of action. The epothilones are attractive because they are effective against p-glycoprotein-producing tumors and have good solubility in microbial drugs (Goodin et al. 2004). Chemically, epothilone B is a 16-membered polyketide macrolactone ring with a methyl thiazole group bound to the macrocycle structure by an olefinic bond. Five extra epothilones, including patupilone (for ovarian cancer) and sagopilone, are in clinical trials.
Zhang et al. (2019a) found that epothilones glycosylation reduces toxicity and positively influences their pharmacological properties. The glycosyltransferase, responsible for amino acid modification at the epothilone catalytic activity, has been identified. Two amino acids (Q66 and P779) are essential for epothilone glycosylation efficiency. As a result of this variation, there is a reduction in epothilone toxicity, as judged by the higher glucose uptake observed in cancer cells than normal ones.
Romidepsin (Istodax, NSC630176, FR 901,228, FK228), a depsipeptide with antitumor activity, is produced by the Gram-negative Chromobacterium violaceum (VanderMolen et al. 2011). This compound acts by inhibiting histone deacetylase. In 2002, after NCI confirmed its antitumor activity, Fujisawa Pharmaceutical Co. (now Astellas Pharma Inc.) conducted clinical trials with romidepsin. The product was licensed to Gloucester Pharmaceuticals in 2004, later acquired by Celgene Corp. The FDA approved this compound in 2009 against cutaneous T-cell lymphoma (CTCL). There are around 1500 new cases annually and 500 deaths from CTCL, only in the USA. Two new romidepsin derivatives (romipeptides A and B), with antitumor activity against three human cancer cell lines (SW620, HL60, and A549), were isolated from C. violaceum (Xiong et al. 2019).
Romidepsin has also shown good activity against testicular cancer. This effect is the most frequent cancer in men between the ages of 14 and 44 years, and its incidence is increasing worldwide. Most testicular neoplasms are germ cell tumors, relatively scarce carcinomas, accounting for only 1.0% of all male malignancies. Remarkable progress has been made in advanced testicular cancer medical treatment, with a substantial increase of five years of survival value from < 30% to approximately 95%. Further, for patients initially diagnosed with stage I development, the cancer-specific survival rate at 15 years is > 99%. The cure proportion is the highest of any solid tumor, and improved survival is primarily due to the progress in understanding this disease.
The testicular germ cell tumors generally are treated with high cure rates by orchiectomy and chemotherapy. The exceptions are in the situation of patients with metastasis or with resistance to standard therapy. Romidepsin induces apoptosis and blocks the cell cycle without affecting the fibroblasts of Sertoli cells. It upregulates the NADPH-dependent dehydrogenase/reductase DHRS2 and the creatine kinase CKB genes involved in cellular energy homeostasis. Interestingly, combining romidepsin with dexamethasone may improve the treatment (Nettersheim et al. 2019). Otherwise, this compound has been considered for viral reactivation to kill latently HIV-infected cells with antiretroviral therapy to prevent the spread of the infection (Søgaard et al. 2015).
Carfilzomid is an anticancer drug isolated from actinobacteria. Chemically, it is a tetrapeptide epoxy-ketone analog of epoxomicin. The FDA approved this compound in 2012 as an agent against multiple myeloma, and Onyx Pharmaceuticals markets the drug as Kyprolis™. Carfilzomib covalently binds and inhibits the chymotrypsin-like activity of the 20S proteasome. This inhibition results in a build-up of polyubiquitinated proteins, which may cause cell cycle arrest, apoptosis, and inhibition of tumor growth (Park et al. 2019).
In the fight against many cancer types, traditional cytotoxic chemotherapy has started to be replaced by targeted molecular therapies to the extent that our understanding of tumor biology has become more sophisticated. This shift has mainly changed adverse effects to a diverse range of organ systems than those seen with cytotoxic chemotherapy. Among these kinds of microbial-derived anticancer drugs, we can cite everolimus, recently approved by the FDA. Chemically, it is a 40-O-(2-hydroxyethyl) derivative of the macrolide sirolimus. Novartis markets it under the trade names Afinitor, Zortress, and Certican. The FDA approved it in 2011 to treat progressive pancreatic neuroendocrine tumors, advanced renal cell cancer, and breast cancer. Later in 2012, it was approved to treat renal angiomyolipomas complexed with tuberous sclerosis and to treat hormone receptor-positive, HER2-negative breast cancer. It binds to the FK506 binding protein-12, forming a complex that inhibits the mammalian target of rapamycin (mTOR), inducing cell growth arrest and apoptosis by blocking the progression of cells from G1 into the S phase (Lombard-Bohas et al. 2015). Besides, it is currently used as an immunosuppressant to prevent the rejection of organ transplants.
Anticancer drugs in clinical trials
The current panorama of new drugs of microbial origin with anticancer properties looks bright. Molecules with cytotoxic effects on a target (or multiple targets) with null or low toxic side effects are frequently discovered (Law et al. 2020). Elsamitrucine (elsamicin A), produced by an unidentified actinobacterium (strain J 907–21), is an example of new drugs in clinical trials. This compound inhibits the topoisomerase II and binds to DNA (Portugal 2003). An additional drug is Brostallicin, isolated from Streptomyces distallicus. This α-bromoacryloyl derivative of distamycin A binds non-covalently to the minor groove of DNA (Broggini et al. 2004). Brostallicin displays antitumor activity against soft tissue sarcoma and dramatically reduces the in vitro myelotoxicity in human hematopoietic cells.
Another promising drug is the polyketide geldanamycin, obtained from Streptomyces hygroscopicus. This compound inhibits the Heat Shock Protein 90, a chaperone protein (Bisht et al. 2003).
Further actions in search of anticancer compounds involved mining gene cluster information in tandem with nuclear magnetic resonance-based structure data. This approach allowed the isolation of the polyketide neaumycin B, a potent inhibitor of the glioblastoma cell line U87. This compound is produced by Micromonospora CNY-010 (Kim et al. 2018). Besides, two additional novel depsipeptides, P11A (50) and P11B (51), isolated from Streptomyces sp. P11-23B also exhibited strong cytotoxic activity against several human glioblastoma cell lines (U251, U87-MG, SHG-44, and C6) (Ye et al. 2017). Furthermore, two new alkaloids (geranylpyrrol A and piericifin F) with cytotoxic activity were discovered in a reedsmycins knockout mutant of Streptomyces sp. CHQ-64 (Han et al. 2017). Among them, piericidin F displayed the more potent anti-proliferative activity against HeLa, human lung carcinoma (A549 and H1975), and human acute promyelocytic leukemia cell lines (NB4).
Marine microorganisms have great potential for discovering new entities that can aid in the prevention and treatment of cancer. Among these microorganisms, actinobacteria have shown extraordinary proficiency in the generation of anticancer compounds. Novel anticancer compounds with IC50 values ranging from ng to mg include lucentamycins A-D. These compounds, isolated from Nocardiopsis lucentensis, are 3-methyl-4-ethylideneproline-containing peptides with in vitro cytotoxicity against the human colon carcinoma lineage HCT-116 (Cho et al. 2007). An additional drug isolated from Micromonospora marina is a depsipeptide named thiocoraline. This drug shows in vitro cytotoxic activity against the human colon cancer cell lines LOVO and SW620. Biparametric flow cytometry analysis with bromodeoxyuridine/DNA supported that its cytotoxic action was mediated by an arrest in the G1 phase of the cell cycle (Lombó et al. 2006). Other antitumor compounds included trioxacarcins A-C extracted from Streptomyces species. They showed high anti-tumor activity against a lung cell line (Maskey et al. 2004). Mansouramycins A-D isolated from Streptomyces sp. show activity against breast and prostate cancers (Hawas et al. 2009). The macrolide tartrolon D, from Streptomyces sp. MDG-04–17-069, exhibits cytotoxicity against lung, colon, and breast cancer cell lines. Salinosporamide is another remarkable anticancer compound produced by a marine actinobacterium. It is active against lung, promyelocytic leukemia, hepatocellular carcinoma, and breast cancer cell lines (Fenical et al. 2009).
More recent discoveries included the polyene macrolactam, FW05328-1 (Santos et al. 2020), isolated from Micromonospora sp. FIM05328. It shows potent activity against esophageal squamous cell carcinoma cell lines KYSE30, KYSE180, and EC109 (Nie et al. 2018). Furthermore, by genetic manipulation of the Streptomyces pactum SCSIO 02,999 promoters, six new polyketides, pactamides A–F, were isolated. Among them, pactamide A exhibited strong activity against the human SF-268 glioblastoma, MCF-7 breast cancer, NCI-H460 lung carcinoma, and liver HepG2 cancer cell lines (Saha et al. 2017).
Two new chromodepsipeptides, neo-actinomycin A and neo-actinomycin B, were isolated from Streptomyces sp. IMB094 (Wang et al. 2017c). They displayed a potent anti-proliferative effect on human colorectal and lung carcinoma cell lines HCT116 and A549, respectively. Two new macrolides, PM100117 and PM100118, were isolated from Streptomyces caniferus GUA-06–05-006A (Pérez et al. 2016). Both PM100117 and PM100118 show potent cytotoxic effects on cell lines of human lung carcinoma (A549), breast adenocarcinoma (MDA-MB-231), and human colorectal carcinoma (HT-29). Lin et al. (2014) reported lobophorins, H and I from a symbiotic Streptomyces sp. (strain 1053U.I.1a.3b) isolated from cone snails. They represent a large family of spirotetronates with antimicrobial and cytotoxic activities. Of these, lobophorin I showed potent cytotoxic activity against the human T-cell leukemia cell line CEM-TART.
Although numerous compounds have been isolated from marine actinobacteria, other marine bacterial phyla have shown to be good sources of novel bioactive molecules. An example is tetra(indol-3-yl) ethenone, a novel indol isolated from Pseudovibrio denitrificans BBCC725. It offers moderate cytotoxicity against human lung carcinoma A549 and mouse fibroblasts L929 cell lines (Rodrígues et al. 2017). Another compound recently reported is the novel 18-O-demethylpederin (Schleissner et al. 2017). This drug shows anti-proliferative activity against human lung carcinoma (A549), human colon (HT-29), human breast (MDA-MB-231), and human pancreas (PSN-1) adenocarcinoma cell lines.
All the examples cited above show the great potential of microorganisms as a source of anticancer drugs and reveal the extraordinary chemical diversity of their metabolism to generate novel compounds. Among them, actinobacteria proved to be the most prolific and diverse producers, supporting their great significance and contribution of natural microbial products to the pharma industry.
Immunosuppressant drugs
An individual’s immune system can distinguish between native and foreign antigens and elicit a response only against the latter. In the case of a vital organ failure, or an autoimmune disease, a transplant represents an option to replace the damaged organ, possibly saving the life. However, suppressor cells, which are essential in regulating the normal immune response, will reply by rejecting the transplant. The suppression of the immune response either by drugs or radiation, which prevents grafts and transplants rejection or controls autoimmune diseases, is termed immunosuppression. The use of immunosuppressant drugs is now a feasible option to allow organ transplants. A multidrug approach involving medications with different mechanisms of action and non-overlapping toxicity profiles is commonly used for this purpose. The global organ transplant immunosuppressant drug market capacity has grown continuously and is expected to be around $5.88 billion by 2026 [https://markets.businessinsider.com/news/stocks/organ-transplant-immunosuppressant-drugs-market-worth-5-88-billion-by-2026-grand-view-research-inc-1028617966].
Microbial compounds able to suppress the immune response have been discovered. Originally, cyclosporin A was introduced as a narrow spectrum antifungal peptide produced by aerobic fermentation by the mold, Tolypocladium nivenum. Cyclosporins are a family of neutral, highly lipophilic, cyclic undecapeptides containing several unusual amino acids. A nonribosomal peptide cyclosporin synthetase is responsible for their synthesis. Cyclosporin has been used in heart, liver, and kidney transplants, leading to an overwhelming achievement of the organ transplant field (Borel 2002). Global annual world sales of cyclosporin A are approximately $2 billion.
The cyclosporin mechanism is possibly related to the cytosolic protein cyclophilin (immunophilin) that binds to immunocompetent lymphocytes, particularly T-cells. This complex of cyclosporin and cyclophilin inhibits the enzyme phosphatase calcineurin, which dephosphorylates the nuclear factor of activated T-cell. Under normal circumstances, this factor is responsible for activating the transcription of interleukin-2 and, therefore, leads to T-cell inactivation. Moreover, cyclosporin can also modulate the innate immune response due to the reduction of inflammatory responses in dendritic cells, macrophages, and neutrophils. Cyclosporin acts independently of cyclophilin and calcineurin, inhibiting the mitochondrial permeability transition pore formation. Thereby, it prevents the leak of mitochondrial factors like DNA, reactive oxygen species, and phospholipids that would activate the innate immune response. Besides, cyclosporin inhibits dendritic cell maturation, and the mechanism depends on the cell type but includes damage of the cellular functions, such as antigen presentation, expression of maturation markers, and cytokine secretion. Cyclosporin affects tissue-damaging factors and pro-inflammatory cytokines in macrophages and neutrophils. This result suggests novel mechanisms by which this peptide can modulate the immune response (Liddicoat and Lavelle 2019). Although this natural product is a highly effective immunosuppressant, it may cause some adverse effects like hypertension. In this effect, cyclosporin inhibits the activity of ABCA1, which transports cholesterol to the outer leaflet of the cell membrane. Thus, it elevates the intracellular cholesterol concentration and stimulates the epithelial sodium channel in distal nephron cells, enhancing the interaction with the activator phosphatidylinositol-4,5-bisphosphate through an increase in sodium reabsorption. However, lovastatin can hinder this effect, inhibiting cholesterol synthesis produced by cyclosporin (Zhai et al. 2019).
Sirolimus (rapamycin) and tacrolimus (FK506, Fujimycin) are key organ transplant agents produced by actinobacteria. Rapamycin lacks the nephrotoxicity effect seen with cyclosporin A and tacrolimus. Therefore, it is especially suitable for kidney transplants. Rapamycin, discovered in 1975 as a S. hygroscopicus product was initially proposed as an antifungal drug. However, this application was discarded after finding its potent immunosuppressive and antiproliferative properties. In addition to these effects, rapamycin also exhibits antitumor, neuroprotective, and anti-aging properties. The FK506-binding protein (FKBP12) interacts with the rapamycin-binding domain by connecting to the immunophilin and inactivating the serine-threonine kinase mTOR (Hausch et al. 2013). The latter is a nutrient-sensing regulator that mediates cellular response to nutrient availability by acting at the cellular and organismic levels and is implicated in cellular processes such as autophagy, cell growth, and proliferation (Lee et al. 2018). Rapamycin also activates the lysosomal Ca2+ channel TRPML1 (transient receptor potential channel mucolipin 1). By activating the EB transcription factor (TFEB), TRPML1 increases autophagy and lysosomal biogenesis (Zhang et al. 2019b). In this way, rapamycin contributes to the in vivo neuro protecting and anti-aging effects.
The results in animal models suggest that rapamycin has a beneficial effect during the aging process and has therapeutic effects in Alzheimer´s disease (Kaeberlein and Galvan 2019). Roos and Murthy (2019) reported on the treatment of thyroid eye disease suggesting that a combination of sirolimus and prednisolone immunosuppressant therapy may be useful. This effect is probably due to the anti-fibroblast effects of rapamycin (Roos and Murthy 2019). In conjunction with coronary stents, the rapamycin antiproliferative effect has also been used to prevent restenosis, which typically occurs after the treatment of coronary artery disease by balloon angioplasty. A rapamycin-eluting coronary stent is sold under the trade name Cypher™.
Park et al. (2010) reviewed the biosynthesis, regulation, and mutagenic improvement of rapamycin biosynthesis. Likewise, for strain improvement of rapamycin production by Streptomyces hygroscopicus, Zhu et al. (2010) considered the effect of various factors such as the carbon and nitrogen source regulation. Besides, the precursor effects of acetate, propionate, methionine, pipecolic acid, and shikimic acid. Starting with a strain producing 150 mg L−1, a mutant resistant to lysine inhibition yielded 260 mg L−1. Further mutation to high glucose resistance generated a strain making 350 mg L−1. Mutations to high shikimate concentrations and auxotrophy to tryptophan and phenylalanine resulted in 450 mg L−1. Fed-batch fermentation with glycerol and potassium phosphate supplementation yielded 812 mg L−1.
In the rapamycin-producing S. hygroscopicus, two genes of its biosynthetic cluster, i.e., rap G and rap H, encode for positive regulatory proteins production (Kuščer et al. 2007). Overexpression of either gene increases rapamycin formation, whereas their deletions eliminate rapamycin biosynthesis. They act by affecting the operon promoter.
Rapamycin exhibits antitumor activity due to angiogenesis interference and apoptosis induction (Brown et al. 2003; Guba et al. 2002). This finding was considered advantageous to further chemically modify this compound to produce relevant derivatives such as temsirolimus (CCI-779; ToriselTM) and everolimus (RAD001). The FDA approved temsirolimus and everolimus in 2007 and 2019 for renal cell carcinoma. Novartis market everolimus under the name of Afinitor™, and the 2019 net sales amounted to $1,519 Million (https://www.novartis.com). Torisel™ has been relatively successful in the renal carcinoma market and may have significant generic loss with patents expiring in August 2019 (US), February 2020 (Japan), and March 2020 (EU) [https://pharmastore.informa.com/product/torisel/].
The low aqueous solubility of rapamycin has been amended in its analogs everolimus, temsirolimus, and AP23573. Everolimus (marketed as Certican) is currently used as an immunosuppressant to avoid the rejection of organ transplants. Novartis sold it under the tradenames ZortressTM (USA), CerticanTM (Europe and other countries) in transplantation medicine, and AfinitorTM in oncology. Everolimus likely has a role in heart transplantation by inhibiting cardiac allograft vasculopathy progression (Gude et al. 2017). In 2010, phase III trials in gastric cancer, hepatocellular carcinoma, and lymphoma were underway [http://www.genengnews.com/gen-news-highlights/novartis-afinitor-cleared-by-fda-for-treating-sega-tumors-in-tuberous-sclerosis/81244159/]. However, this drug did not noticeably improve the overall survival for advanced gastric cancer that had progressed after one or two lines of previous systemic chemotherapy (Ohtsu et al. 2013). Everolimus is also used in drug-eluting coronary stents as an immunosuppressant to prevent rejection. Deforolimus (AP23573; MK-8669) or ridaforolimus is a non-prodrug rapamycin analog with non-linear pharmacokinetic behavior. It has anti-proliferative activity against several human tumor cell lines in vitro and experimental tumors in vivo (Dancey 2006; Chen 2007). Although Ariad Pharmaceuticals and Merck announced the success of phase III trials in patients with metastatic soft-tissue or bone sarcomas, the FDA rejected the New Drug Application (NDA) for ridaforolimus in 2011. Later, in 2012, the European Medicines Agency (EMA) notified Merck of the decision to withdraw the Marketing Authorization Application (MAA) for ridaforolimus [https://newdrugapprovals.org/tag/ridaforolimus/]. The withdrawal of the EMA application of ridaforolimus did not change Merck’s commitment to the ongoing clinical trials with this compound.
Tacrolimus (FK-506) was discovered in 1987 as a fermentation product of Streptomyces tsukubaensis. It has approximately 100 times more immunosuppressive activity than cyclosporin A (Wen 2020). Initially developed by Fujisawa Pharmaceutical was approved by the FDA in 1994 as an immunosuppressant in liver transplantation (Yamashita 2013). Tacrolimus is marketed by several companies, including GlaxoSmithKline Plc, Abbott Laboratories, Novartis International AG, Mylan N.V., Astellas Pharma, Inc., Takeda Pharmaceuticals Company Ltd, Senju Pharmaceutical Co. Ltd., Guike Pharmaceutical Co. Ltd., DR. Reddy’s Laboratories Ltd., Glenmark Pharmaceuticals Ltd., and Veloxis Pharmaceuticals. This last company sells the drug as Envarsus XR™ with a 2019 market of $1.7 billion only in the USA [https://www.jefferies.com/CMSFiles/Jefferies.com/files/Veloxis%20Pharmaceuticals%20As.pdf]. Its use has been extended to embrace bone marrow, cornea, heart, intestines, kidney, lung, pancreas, trachea, small bowel, skin, and limb transplants. Topically, it is used against atopic dermatitis, a widespread skin disease, psoriasis, and pruritus. In ophthalmology, tacrolimus was used to treat Sjögren’s syndrome, allergic conjunctivitis, and dry eye disease.
In the laboratory, tacrolimus inhibits the mixed lymphocyte reaction, the formation of interleukin-2 by T lymphocytes, and the development of cytokines, including interleukin-3 and interferon-gamma. Tacrolimus has recently been shown to inhibit tumor growth factor β-induced signaling and collagen synthesis in human pulmonary fibroblast cells. This factor plays a crucial role in tissue fibrosis, including pulmonary fibrosis, treated with bleomycin. However, its use in the acute inflammatory phase may aggravate lung injury (Nagano et al. 2006). Besides, the effect of tacrolimus as an antagonist of the cold-activated cationic channel TRPM8 has also been addressed. This effect depends on a mechanism independent of its canonical signaling pathway. This channel activates a thermoreceptor involved in the sensation of cold and engages in the tearing and blinking mechanism, in itch relief, and pathologies like migraine, highlighting its therapeutic implications. On the other hand, in the study of this ionic channel’s functional activity, tacrolimus could be helpful as an experimental tool (Arcas et al. 2019).
Conclusions and future perspectives
Microorganisms have contributed significantly for about 150 years to the progress of medicine and agriculture. Drugs like statins are available to treat atherosclerosis, and the panorama for new anticancer drugs of microbial origin looks bright, with marine microorganisms playing a crucial role. Besides, the use of microbial immunosuppressant medicines is now a feasible option to allow organ transplants. Viral diseases are continuously emerging and widely spreading, especially those concerned with respiratory conditions, mainly caused by different coronaviruses. Fortunately, new drugs like protease inhibitors, terpenes, polysaccharides, polyketides, and peptides, capable of inhibiting RNA and DNA viruses, are emerging to treat these viral infections. Likely, the most critical usage of secondary metabolites has been as anti-infective drugs for bacterial and fungal diseases. However, microbes have become resistant to many antibiotics due to different factors, resulting in a risky situation, that underlines the need for new antibiotics. Unfortunately, most prominent pharmaceutical companies have aborted the search for novel antimicrobial compounds. Economic considerations have led to the decision to work on drugs active against chronic diseases, which offer higher revenues than antimicrobial agents. The length of antibiotics treatment is short and is likely to be limited by the government. Some small pharmaceutical and biotechnology companies, as well as some universities, are still developing antibiotics. Even so, most of them depend on venture capital rather than sales income, and with the present regulations, they face enormous barriers to entering the market. International agencies like the FDA raised these barriers with the best purpose of ensuring public safety. Yet, they have the opposite effect, i.e., impeding antibiotic development while resistance increases (Livermore 2004). However, there are some new bright possibilities (Sanchez and Demain 2017a, b). One of the most promising is the use of uncultured microorganisms. Considering that 99% of bacteria and 95% of fungi have not been cultivated in the laboratory, efforts into finding methods to grow such uncultured microorganisms are proceeding and succeeding (Kaeberlein et al. 2002).
Screening microbes in non-conventional sources to look for new and more effective compounds represent an additional possibility. These sources included arid soils, caves, hot springs, high salinity areas or oceans, and seas (Demain et al. 2019). Furthermore, scientists are now extracting microbial DNA from soil samples, cloning large fragments into, for example, bacterial artificial chromosomes, expressing them in a host bacterium, and screening the library for new antibiotics. This metagenomics approach could open up the exciting possibility of having a large untapped pool to discover new MPs (Trindade et al. 2015; Kowalchuk et al. 2007). Another exciting possibility is genome mining (Scheffler et al. 2013; Martínez-Klimova et al. 2017), which opens the possibility to uncover the expression of certain secondary metabolites produced only under specific circumstances. Thus, eliciting cryptic clusters becomes a significant challenge under laboratory conditions, and alternative methodologies need to be sought. In addition to these three relatively new techniques, the traditional chemical and biological modification of antibiotics could still provide novel and potent drugs. These remarks also apply to non-antibiotics such as antitumor agents and other microbial products.
MPs must continue to be explored for desirable therapeutic effects. We trust that significant progress in identifying novel antibiotics, oncology therapeutics, and other useful medicines will be made, probably not by the big pharmaceutical companies but by small research groups from institutes and universities worldwide.
Acknowledgements
We are indebted to Marco A. Ortíz-Jiménez and Betsabé Linares-Ferrer for their support in the process of this work.
Abbreviations
- FDA
Food and Drug Administration
- NA
Not applicable
- cSSSI
Complicated skin, and skin structure infections
- ABSSSIs
Acute bacterial skin, and skin structure infections
- cUTI
Complicated urinary tract infections
- cIAI
Complicated intra-abdominal infections
- HABP/VABP
Hospital-acquired bacterial pneumonia, and ventilator-associated bacterial pneumonia
- CABP
Community-acquired bacterial pneumonia. Estimate of U.S. Sales for 2018 was based on data previously reported Carr and Stringer 2019
Author contribution
ALD and SS conceptualized the idea and wrote the first draft; DRR was involved in updating references and writing of one of the sections; AKP revised one of the sections; BRV reviewed all contributions and wrote one of the sections; DRR, BRV, and RRS critically reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
We acknowledge the economic support from DGAPA, PAPIIT IN-205922, and IN-205922. The support of the CONACYT A-S1-9143 grant and the NUATEI program from Instituto de Investigaciones Biomédicas, UNAM, is also recognized.
Data availability
All data generated or analyzed in this study are included in the present review.
Declarations
Ethics approval
This review article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abraham AO, Nasiru AU, Abdulazeez AK, Seun OO, Ogonna DW. Mechanism of drug resistance in Mycobacterium tuberculosis. Am J Biomed Sci Res. 2020;7(5):378–383. doi: 10.34297/AJBSR.2020.07.001181. [DOI] [Google Scholar]
- Alarcon J, Aguila S, Arancibia-Avila P, Fuentes O, Zamorano-Ponce E, Hernandez M. Production and purification of statins from Pleurotus ostreatus (Basidiomycetes) strains. Z Naturforsch. 2003;58:62–64. doi: 10.1515/znc-2003-1-211. [DOI] [PubMed] [Google Scholar]
- Amoutzias GD, Nikolaidis M, Tryfonopoulou E, Chlichlia K, Markoulatos P, Oliver SG. The remarkable evolutionary plasticity of coronaviruses by mutation and recombination: insights for the COVID-19 pandemic and the future evolutionary paths of SARS-CoV-2. Viruses. 2022;14(1):78. doi: 10.3390/v14010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andryukov B, Mikhailov V, Besednova N. The biotechnological potential of secondary metabolites from marine bacteria. J Mar Sci Eng. 2019;7(6):176. doi: 10.3390/jmse7060176. [DOI] [Google Scholar]
- Arcas JM, González A, Gers-Barlag K, González-González O, Bech F, Demirkhanyan L, Zakharian E, Belmonte C, Gomis A, Viana F. The immunosuppressant macrolide tacrolimus activates cold-sensing TRPM8 channels. J Neurosci Res. 2019;39:949–969. doi: 10.1523/JNEUROSCI.1726-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, Sudo H, Yamazaki M, Saito K. Camptothecin production by in vitro cultures and plant regeneration in Ophiorrhiza species. Methods Mol Biol. 2009;547:337–345. doi: 10.1007/978-1-60327-287-2_27. [DOI] [PubMed] [Google Scholar]
- Badiee P, Hashemizadeh Z. Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J Med Res. 2014;139(2):195–204. [PMC free article] [PubMed] [Google Scholar]
- Benbrahim Z, Arifi S, Benhammane H, Inani K, Gallouj S, Meziane M, Zahra Mernissi F, Mellas N, El Mesbahi O (2013) Quasi-complete response of classic Kaposi’s sarcoma treated with weekly paclitaxel. Case Rep Oncol Med 2013. 10.1155/2013/196878 [DOI] [PMC free article] [PubMed]
- Berdy J. Thoughts and facts about antibiotics: where we are now and where we are heading. J Antibiot Res. 2012;65(8):385–395. doi: 10.1038/ja.2012.27. [DOI] [PubMed] [Google Scholar]
- Berdy J (2015) Microorganisms producing antibiotics. In: Sanchez S, Demain AL (eds) Antibiotics: Current innovations and future trends. Caister Academic Press, Norfolk, pp 49–64
- Bhatnagar I, Kim SK (2013) Molecular aspects of fungal bioactive polyketides. In: Brahmachari G, editor. Chemistry and pharmacology of naturally occurring bioactive compounds. Boca Raton, CRC Press, p 445–457
- Bigagli E, Luceri C, De Angioletti M, Chegaev K, D'Ambrosio M, Riganti C, Gazzano E, Saponara S, Longini M, Luceri F, Cinci L. New NO- and H2S-releasing doxorubicins as targeted therapy against chemoresistance in castration-resistant prostate cancer: in vitro and in vivo evaluations. Invest New Drugs. 2018;36(6):985–998. doi: 10.1007/s10637-018-0590-0. [DOI] [PubMed] [Google Scholar]
- Bisht KS, Bradbury M, Mattson D, Kaushal A, Sowers A, Markovina S, Ortiz KL, Sieck LK, Isaacs JS, Brechbiel MW, Mitchell JB, Neckers LM, Gius D. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res. 2003;63:8984–8995. [PubMed] [Google Scholar]
- Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi. 2017;3(4):57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel JF. History of the discovery of cyclosporin and of its early pharmacological development. Wien Klin Wochensch. 2002;114:433–437. [PubMed] [Google Scholar]
- Bougnoux ME, Brun S, Zahar JR (2018) Healthcare-associated fungal outbreaks: new and uncommon species, new molecular tools for investigation and prevention. Antimicrob Resist Infect Control 7: 45. 10.1186/s13756-018-0338-9 [DOI] [PMC free article] [PubMed]
- Breijyeh Z, Jubeh B, Karaman R (2020) Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules (Basel) 25(6):1340. 10.3390/molecules25061340 [DOI] [PMC free article] [PubMed]
- Brinker AD, Wassel RT, Lyndly J, Serrano J, Avigan M, Lee WM, Seeff LB. Telithromycin-associated hepatotoxicity: clinical spectrum and causality assessment of 42 cases. Hepatology. 2009;49(1):250–257. doi: 10.1002/hep.22620. [DOI] [PubMed] [Google Scholar]
- Broggini M, Marchini S, Fontana E, Moneta D, Fowst C, Geroni C. Brostallicin: a new concept in minor groove DNA binder development. Anticancer Drugs. 2004;15(1):1–6. doi: 10.1097/00001813-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Brown VI, Fang J, Alcorn K, Barr R, Kim JM, Wasserman R, Grupp SA. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc Natl Acad Sci USA. 2003;100:15113–15118. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki T. Understanding variants of SARS-CoV-2. The Lancet. 2021;397(10273):462. doi: 10.1016/S0140-6736(21)00298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM (2020) The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 178. 10.1016/j.antiviral.2020.104787 [DOI] [PMC free article] [PubMed]
- Carr A, Stringer J (2019) Antibiotic an antifungal update September 2019. Needham and Company, LLC, New York, p 1–17
- Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal AR. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials-a review. Plants (basel) 2017;6(2):E16. doi: 10.3390/plants6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ. Targeted mTOR in human gynecologic cancers. J Cancer Molec. 2007;3:101–106. doi: 10.29685/JCM.200708.0001. [DOI] [Google Scholar]
- Chen YL, Lehman VN, Averette AF, Perfect JR, Heitman J. Posaconazole exhibits in-vitro and in-vivo synergistic antifungal activity with Caspofungin or FK506 against Candida albicans. PLoS ONE. 2013;8(3):e57672. doi: 10.1371/journal.pone.0057672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Chang S-S, Donga B, Yua LY, Wua Y-X, Wanga R-Z, Jianga W, Gao Z-P, Si S-Y. Ahmpatinin iBu, a new HIV-1 protease inhibitor, from Streptomyces sp. CPCC 202950. RSC Adv. 2018;8:5138–5144. doi: 10.1039/C7RA13241G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Williams PG, Kwon HC, Jensen PR, Fenical W. Lucentamycins A-D, cytotoxic peptides from the marine-derived actinomycete Nocardiopsis lucentensis. J Nat Prod. 2007;70:1321–1328. doi: 10.1021/np070101b. [DOI] [PubMed] [Google Scholar]
- Choy KT Wong AYL Kaewpreedee P Sia SF Chen D Hui KPY Chu DKW Chan MCW Cheung PP-H Huang X Peiris M Yen HL (2020) Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res 178: 104786. 10.1016/j.antiviral.2020.104786 [DOI] [PMC free article] [PubMed]
- Ciliberto G, Cardone L. Boosting the arsenal against COVID-19 through computational drug repurposing. Drug Discov Today. 2020;25(6):946–948. doi: 10.1016/j.drudis.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy CJ, Nguyen MH (2020) Buying time: the AMR action fund and the state of antibiotic development in the United States 2020. Open Forum Infect Dis 7(11). 10.1093/ofid/ofaa464 [DOI] [PMC free article] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbial. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg GM, Newman DJ. Natural products as source of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Dancey JE. Therapeutic targets: MTOR and related pathways. Cancer Biol Ther. 2006;5:1065–1073. doi: 10.4161/cbt.5.9.3175. [DOI] [PubMed] [Google Scholar]
- Daniels LB, Sitapati AM, Zhang J, Zou J, Bui QM, Ren J, Longhurst CA, Criqui MH, Messer K. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am J Cardiol. 2020;136:149–155. doi: 10.1016/j.amjcard.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain AL, Martens E. Production of valuable compounds by molds and yeasts. J Antibiot Res. 2017;70:347–360. doi: 10.1038/ja.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain AL, Gómez-Ortiz B, Ruiz-Villafán B, Rodríguez-Sanoja R, Sánchez S. Recent findings of molecules with anti-infective activity: screening of non-conventional sources. Curr Opin Pharmacol. 2019;48:40–47. doi: 10.1016/j.coph.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Dolin HH, Papadimos TJ, Chen X, Pan ZK. Characterization of pathogenic sepsis etiologies and patient profiles: A novel approach to triage and treatment. Microbiol Insights. 2019;12:1178636118825081. doi: 10.1177/1178636118825081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominiak DM, Nielsen JL, Nielsen PH. Extracellular DNA is abundant and important for microcolony strength in mixed microbial biofilms. Environ Microb. 2011;13(3):710–721. doi: 10.1111/j.1462-2920.2010.02375.x. [DOI] [PubMed] [Google Scholar]
- Duan LL, Chen HR, Chen JP, Li WP, Hong L. Screening the high-yield paclitaxel producing strain Alternaria alternate var monosporus. Chi J Antibiot. 2008;33:650–652. [Google Scholar]
- Duncan MD, Wilkes DS. Transplant-related immunosuppression: a review of immunosuppression and pulmonary infections. Proc Am Thorac Soc. 2005;2(5):449–455. doi: 10.1513/pats.200507-073JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand-Réville TF, Comita-Prevoir J, Zhang J, Wu X, May-Dracka TL, Romero JAC, Wu F, Chen A, Shapiro AB, Carter NM, McLeod SM, Giacobbe RA, Verheijen JC, Lahiri SD, Sacco MD, Chen Y, O’Donnell JP, Miller AA, Mueller JP, Tommasi RA. Discovery of an orally available diazabicyclooctane inhibitor (ETX0282) of class A, C, and D serine β-lactamases. J Med Chem. 2020;63(21):12511–12525. doi: 10.1021/acs.jmedchem.0c00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshahawi SI, Ramelot TA, Seetharaman J, Chen J, Singh S, Yang Y, Pederson K, Kharel MK, Xiao R, Lew S, Yennamalli RM, Miller MD, Wang F, Tong L, Montelione GT, Kennedy MA, Bingman CA, Zhu H, Phillips GN, Thorson JS. Structure-guided functional characterization of enediyne self-sacrifice resistance proteins, CalU16 and CalU19. ACS Chem Biol. 2014;9(10):2347–2358. doi: 10.1021/cb500327m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B. 2010;86:484–492. doi: 10.2183/pjab.86.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenical W, Jensen PR, Palladino MA, Lam KS, Lloyd GK, Potts BC. Discovery and development of the anticancer agent salinosporamide A (NPI-0052) Bioorg Med Chem. 2009;17:2175–2180. doi: 10.1016/j.bmc.2008.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler HP, Bruntner C, Riedlinger J, Bull AT, Knutsen G, Goodfellow M, Jones A, Maldonado L, Pathom-Aree W, Beil W, Schneider K, Keller S, Sussmuth RD. Proximicin A, B and C, novel aminofuran antibiotic and anticancer compounds isolated from marine strains of the actinomycete Verrucosispora. J Antibiot. 2008;61(3):158–163. doi: 10.1038/ja.2008.125. [DOI] [PubMed] [Google Scholar]
- Fliegerová K, Tapio I, Bonin A, Mrazek J, Callegari ML, Bani P, Bayat A, Vilkki J, Kopecny J, Shingfield KJ, Boyer F, Coissac E, Taberlet P, Wallace RJ. Effect of DNA extraction and sample preservation method on rumen bacterial population. Anaerobe. 2014;29:80–84. doi: 10.1016/j.anaerobe.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Floyd K, Glaziou P, Houben R, Sumner T, White RG, Raviglione M. Global tuberculosis targets and milestones set for 2016–2035: definition and rationale. Int J Tuberc Lung Dis. 2018;22(7):723–730. doi: 10.5588/ijtld.17.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gales AC, Jones RN, Andrade SS, Pereira AS, Sader HS. In vitro activity of tigecycline, a new glycylcycline, tested against 1,326 clinical bacterial strains isolated from Latin America. Braz J Infect Dis. 2005;9(5):348–356. doi: 10.1590/s1413-86702005000500001. [DOI] [PubMed] [Google Scholar]
- García-Fernández E, Koch G, Wagner RM, Fekete A, Stengel ST, Schneider J, Mielich-Süss B, Geibel S, Markert SM, Stigloher C, Lopez D. Membrane microdomain disassembly inhibits MRSA antibiotic resistance. Cell. 2017;171(1354–1367):e20. doi: 10.1016/j.cell.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288(5471):1647–1651. doi: 10.1126/science.288.5471.1647. [DOI] [PubMed] [Google Scholar]
- Gómez-Brandón M, Ascher-Jenull J, Bardelli T, Fornasier F, Fravolini G, Arfaioli P, Ceccherini MT, Pietramellara G, Lamorski K, Slawinski C, Bertoldi D, Egli M, Cherubini P, Insam H. Physico-chemical and microbiological evidence of exposure effects on Picea abies—coarse woody debris at different stages of decay. Forest Ecol Manag. 2017;391:376–389. doi: 10.1016/j.foreco.2017.02.033. [DOI] [Google Scholar]
- González-Bello C, Rodríguez D, Pernas M, Rodríguez A, Colchón E. β-Lactamase inhibitors to restore the efficacy of antibiotics against superbugs. J Med Chem. 2020;63(5):1859–1881. doi: 10.1021/acs.jmedchem.9b01279. [DOI] [PubMed] [Google Scholar]
- Goodin S. Novel cytotoxic agents: epothilones. Am J Health Syst Pharm. 2008;65(10 Suppl 3):S10–S15. doi: 10.2146/ajhp080089. [DOI] [PubMed] [Google Scholar]
- Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. J Clin Oncol. 2004;22(10):2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Gootz TD. The global problem of antibiotic resistance. Crit Rev Immunol. 2010;30:79–93. doi: 10.1615/critrevimmunol.v30.i1.60. [DOI] [PubMed] [Google Scholar]
- Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler E. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nature Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- Gude E, Gullestad L, Andreassen AK. Everolimus immunosuppression for renal protection, reduction of allograft vasculopathy and prevention of allograft rejection in de-novo heart transplant recipients: could we have it all? Curr Opin Organ Transplant. 2017;22(3):198–206. doi: 10.1097/MOT.0000000000000409. [DOI] [PubMed] [Google Scholar]
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Military Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Liu Z, Zhang Z, Zhang X, Zhu T, Gu Q, Li W, Che Q, Li D. Geranylpyrrol A and piericidin F from Streptomyces sp CHQ-64 DeltardmF. J Nat Prod. 2017;80(5):1684–1687. doi: 10.1021/acs.jnatprod.7b00016. [DOI] [PubMed] [Google Scholar]
- Hardt IH, Steinmetz H, Gerth K, Sasse F, Reichenbach H, Höfle G. New natural epothilones from Sorangium cellulosum, strains So ce90/B2 and So ce90/D13: isolation, structure elucidation, and SAR studies. J Nat Prod. 2001;64(7):847–856. doi: 10.1021/np000629f. [DOI] [PubMed] [Google Scholar]
- Hausch F, Kozany C, Theodoropoulou M, Fabian AK. FKBPs and the Akt/mTOR pathway. Cell Cycle. 2013;12(15):2366–2370. doi: 10.4161/cc.25508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawas UW, Shaaban M, Shaaban KA, Speitling M, Maier A, Kelter G, Fiebig HH, Meiners M, Helmke E, Laatsch H. Mansouramycins A-D, cytotoxic isoquinolinequinones from a marine Streptomycete. J Nat Prod. 2009;72(12):2120–2124. doi: 10.1021/np900160g. [DOI] [PubMed] [Google Scholar]
- Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104(12):971–977. doi: 10.1136/heartjnl-2017-312103. [DOI] [PubMed] [Google Scholar]
- Herrmann A, Roesner M, Werner T, Hauck SM, Koch A, Bauer A, Schneider M, Brack-Werner R. Potent inhibition of HIV replication in primary human cells by novel synthetic polyketides inspired by Aureothin. Sci Rep. 2020;10(1):1326. doi: 10.1038/s41598-020-57843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeyborne I, Lipman M, Zumla A, McHugh TD. The changing treatment landscape for MDR/XDR-TB - can current clinical trials revolutionize and inform a brave new world? Int J Infect Dis. 2019;80S:S23–S28. doi: 10.1016/j.ijid.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Huang L, Cattamanchi A, Davis JL, den Boon S, Kovacs J, Meshnick S, Miller RF, Walzer PD, Worodria W, Masur H. HIV-associated Pneumocystis pneumonia. Proc Am Thorac Soc. 2011;8(3):294–300. doi: 10.1513/pats.201009-062WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F, Tanaka S, Ohki H, Matsumoto S, Maki K, Katashima M, Barrett D, Aoki Y. Role of micafungin in the antifungal armamentarium. Curr Med Chem. 2007;14(11):1263–1275. doi: 10.2174/092986707780597970. [DOI] [PubMed] [Google Scholar]
- Jimenez PC, Wilke DV, Costa-Lotufo LV. Marine drugs for cancer: surfacing biotechnological innovations from the oceans. Clinics. 2018;73:e482s. doi: 10.6061/clinics/2018/e482s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurin A, Aliper A, Kazennow A, Mamoshina P, Vanhaelen Q, Khrabrov K, Zhavoronkov A. The cornucopia of meaningful leads: applying deep adversarial autoencoders for new molecule development in oncology. Oncotarget. 2017;8(7):10883–10890. doi: 10.18632/oncotarget.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein T, Lewis K, Epstein SS. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science. 2002;296(5570):1127–1129. doi: 10.1126/science.1070633. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Galvan V (2019) Rapamycin and Alzheimer’s disease: Time for a clinical trial?. Sci Transl Med 11(476):eaar4289. 10.1126/scitranslmed.aar4289 [DOI] [PMC free article] [PubMed]
- Kainz K, Bauer MA, Madeo F, Carmona-Gutierrez D. Fungal infections in humans: the silent crisis. Microb Cell. 2020;7(6):143–145. doi: 10.15698/mic2020.06.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SN, Khan AU. Breaking the spell: combating multidrug resistance superbugs. Front Microbiol. 2016;7:174. doi: 10.3389/fmicb.2016.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AU, Nordmann P. Spread of carbapenemase NDM-1 producers: the situation in India and what may be proposed. Scand J Infect Dis. 2012;44(7):531–535. doi: 10.3109/00365548.2012.669046. [DOI] [PubMed] [Google Scholar]
- Khan HA, Baig FK, Mehboob R. Nosocomial infections: epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed. 2017;7(5):478–482. doi: 10.1016/j.apjtb.2017.01.019. [DOI] [Google Scholar]
- Khan MT, Chinnasamy S, Cui Z, Irfan M, Wei DQ. Mechanistic analysis of A46V, H57Y, and D129N in pyrazinamidase associated with pyrazinamide resistance. Saudi J Biol Sci. 2020;27(11):3150–3156. doi: 10.1016/j.sjbs.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Machado H, Jang KH, Trzoss L, Jensen PR, Fenical W. Integration of genomic data with NMR analysis enables assignment of the full stereostructure of neaumycin B, a potent inhibitor of glioblastoma from a marine-derived Micromonospora. J Am Chem Soc. 2018;140(34):10775–10784. doi: 10.1021/jacs.8b04848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulenti D, Xu E, Mok IYS, Song A, Karageorgopoulos DE, Armaganidis A, Lipman J, Tsiodras S. Novel antibiotics for multidrug-resistant Gram-positive microorganisms. Microorganisms. 2019;7(8):270. doi: 10.3390/microorganisms7080270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulenti D, Xu E, Song A, Sum Mok IY, Karageorgopoulos DE, Armaganidis A, Tsiodras S, Lipman J. Emerging treatment options for infections by multidrug-resistant Gram-positive microorganisms. Microorganisms. 2020;8(2):191. doi: 10.3390/microorganisms8020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtis AP, Hatfield K, Baggs J, Mu Y, See I, Epson E, Nadle J, Kainer MA, Dumyati PS, Ray SM, Ham D, Capers C, Ewing H, Coffin N, McDonald LC, Jernigan J, Cardo D. — United States. MMWR Morb Mortal Wkly Rep. 2019;68(9):214–219. doi: 10.15585/mmwr.mm6809e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalchuk GA, Speksnijder AG, Zhang K, Goodman RM, van Veen JA. Finding the needles in the metagenome haystack. Microbial Ecol. 2007;53(3):475–485. doi: 10.1007/s00248-006-9201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski RJ, GiannakakouHamel P. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol(R)) J Biol Chem. 1997;272(4):2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- Kumaran RS, Choi Y-K, Lee S, Jeon HJ, Jung H, Kim HJ. Isolation of taxol, an anticancer drug produced by the endophytic fungus. Phoma Betae African J Biotechnol. 2012;11(4):950–960. doi: 10.5897/AJB11.1937. [DOI] [Google Scholar]
- Kuščer E, Coates N, Challis I, Gregory M, Wilkinson B, Sheridan R, Petković H. Roles of rapH and rapG in positive regulation of rapamycin biosynthesis in Streptomyces hygroscopicus. J Bacteriol. 2007;189(13):4756–4763. doi: 10.1128/JB.00129-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labro MT, Bryskier JM. Antibacterial resistance: an emerging ‘zoonosis?’. Expert Rev Anti-Infect Ther. 2014;12(12):1441–1461. doi: 10.1586/14787210.2014.976611. [DOI] [PubMed] [Google Scholar]
- Lau SKP Chan JFW (2015) Coronaviruses: emerging and re-emerging pathogens in humans and animals. Virol J 12: 209. 10.1186/s12985-015-0432-z [DOI] [PMC free article] [PubMed]
- Law JW-F, Law LN-S, Letchumanan V, Tan LT-H, Wong SH, Chan K-G, Nurul-Syakima Ab Mutalib N-S, Lee L-H. Anticancer drug discovery from microbial sources: the unique mangrove streptomycetes. Molecules. 2020;25(22):5365. doi: 10.3390/molecules2522536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YA, Noon LA, Akat KM, Ybanez MD, Lee TF, Berres ML, Fujiwara N, Goossens N, Chou H-I, Parvin-Nejad FP, Khambu B, Kramer EGM, Gordon R, Pfleger C, Germain D, John GR, Campbell KN, Yue Z, Yin X-M, Cuervo AM, Czaja MJ, Fiel MI, Hoshida Y, Friedman SL (2018) Autophagy is a gatekeeper of hepatic differentiation and carcinogenesis by controlling the degradation of Yap. Nat Commun 9:4962. 10.1038/s41467-018-07338-z [DOI] [PMC free article] [PubMed]
- Letscher-Bru V, Herbrecht R. Caspofungin: the first representative of a new antifungal class. J Antimicrob Chemother. 2003;51(3):513–521. doi: 10.1093/jac/dkg117. [DOI] [PubMed] [Google Scholar]
- Li F, Jiang T, Li Q, Ling X. Camptothecin (CPT) and its derivatives are known to target topoisomerase I (Top1) as their mechanism of action: did we miss something in CPT analogue molecular targets for treating human disease such as cancer? Am J Cancer Res. 2017;7(12):2350–2394. [PMC free article] [PubMed] [Google Scholar]
- Li BJ Wang H Gong T Chen JJ Chen TJ Yang JL Zhu P (2017b) Improving 10-deacetylbaccatin III-10-b-O-acetyltransferase catalytic fitness for Taxol production. Nat Commun 8: 15544. 10.1038/ncomms15544 [DOI] [PMC free article] [PubMed]
- Liddicoat AM, Lavelle EC. Modulation of innate immunity by cyclosporine A. Biochem Pharmacol. 2019;163:472–480. doi: 10.1016/j.bcp.2019.03.022. [DOI] [PubMed] [Google Scholar]
- Lim LE, Vilchèze C, Ng C, Jacobs WR, Jr, Ramón-García S, Thompson CJ. Anthelmintic avermectins kill Mycobacterium tuberculosis, including multidrug-resistant clinical strains. Antimicrob Agents Chemother. 2013;57(2):1040–1046. doi: 10.1128/aac.01696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Koch M, Pond CD, Mabeza G, Seronay RA, Concepcion GP, Barrows LR, Olivera BM, Schmidt EW. Structure and activity of lobophorins from a turrid mollusk-associated Streptomyces sp. J Antibiot. 2014;67:121–126. doi: 10.1038/ja.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Liu K, Ding X, Deng B, Chen W. Isolation and characterization of endophytic taxol-producing fungi from Taxus chinensis. J Ind Microbiol Biotechnol. 2009;36(9):1171–1177. doi: 10.1007/s10295-009-0598-8. [DOI] [PubMed] [Google Scholar]
- Livermore DM. The need for new antibiotics. Clin Microbiol Infect. 2004;10:1–9. doi: 10.1111/j.1465-0691.2004.1004.x. [DOI] [PubMed] [Google Scholar]
- Lombard-Bohas C, Yao JC, Hobday T, Van Cutsem E, Wolin EM, Panneerselvam A, Stergiopoulos S, Shah MH, Capdevila J, Pommier R. Impact of prior chemotherapy use on the efficacy of everolimus in patients with advanced pancreatic neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-3 trial. Pancreas. 2015;44(2):181–189. doi: 10.1097/MPA.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombó F, Velasco A, Castro A, de la Calle F, Braña AF, Sánchez-Puelles JM, Méndez C, Salas JA. Deciphering the biosynthesis pathway of the antitumor thiocoraline from a marine actinomycete and its expression in two streptomyces species. ChemBioChem. 2006;7(2):366–376. doi: 10.1002/cbic.200500325. [DOI] [PubMed] [Google Scholar]
- Lorence A, Nessler CL. Camptothecin, over four decades of surprising findings. Phytochem. 2004;65(20):2735–2749. doi: 10.1016/j.phytochem.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Lu T, Haemmerich D, Liu H, Seynhaeve ALB, van Rhoon GC, Houtsmuller AB, Ten Hagen TLM. Externally triggered smart drug delivery system encapsulating idarubicin shows superior kinetics and enhances tumoral drug uptake and response. Theranostics. 2021;11(12):5700–5712. doi: 10.7150/thno.55163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni M, Rollini M. Biosynthesis and biotechnological production of statins by filamentous fungi and applications of these cholesterol-lowering drugs. Appl Microbiol Biotechnol. 2002;58(5):555–564. doi: 10.1007/s00253-002-0932-9. [DOI] [PubMed] [Google Scholar]
- Market Analysis Report (2019) Report ID: 978-1-68038-973-9. http://www.grandviewresearch.com/industryanalysis/anti-infective-agents-market
- Martínez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;64(5):e00399–e420. doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Martínez-Klimova E, Centeno-Leija S, Sánchez S (2017) The impact of genome-mining in the development of new anti-infectives. In: Rahman AU (ed) Frontiers in clinical drug research- anti-infectives. Bentham Science Publishers, Sharjah, p 3–54
- Martino E, Della Volpe S, Terribile E, Benetti E, Sakaj M, Centamore A, Sala A, Collina S. The long story of camptothecin: from traditional medicine to drugs. Bioorg Med Chem Lett. 2017;27(4):701–707. doi: 10.1016/j.bmcl.2016.12.085. [DOI] [PubMed] [Google Scholar]
- Maskey RP, Helmke E, Kayser O, Fiebig HH, Maier A, Busche A, Laatsch H. Anti-cancer and antibacterial trioxacarcins with high anti-malaria activity from a marine streptomycete and their absolute stereochemistry. J Antibiot (tokyo) 2004;57(12):771–779. doi: 10.7164/antibiotics.57.771. [DOI] [PubMed] [Google Scholar]
- Matúz K, Mótyán J, Li M, Wlodawer A, Tőzsér J. Inhibition of XMRV and HIV-1 proteases by pepstatin A and acetyl-pepstatin. FEBS J. 2012;279(17):3276–3286. doi: 10.1111/j.1742-4658.2012.08714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean KJ, Hans M, Meijrink B, van Scheppingenb WB, Vollebregt A, Tee KL, van der Laan J-M, Leysa D, Munro AW, van den Berg MA. Single-step fermentative production of the cholesterol-lowering drug pravastatin via reprogramming of Penicillium chrysogenum. Proc Natl Acad Sci. 2015;112(9):2847–2852. doi: 10.1073/pnas.1419028112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momparler RL. Optimization of cytarabine (ARA-C) therapy for acute myeloid leukemia. Exp Hematol Oncol. 2013;2:20. doi: 10.1186/2162-3619-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens DM, Fauci AS. Emerging pandemic diseases: how we got to COVID-19. Cell. 2020;182(5):1077–1092. doi: 10.1016/j.cell.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourenza Á, Gil JA, Mateos LM, Letek M. Novel treatments against Mycobacterium tuberculosis based on drug repurposing. Antibiotics (basel) 2020;9(9):550. doi: 10.3390/antibiotics9090550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano J, Iyonaga K, Kawamura K, Yamashita A, Ichiyasu H, Okamoto T, Suga M, Sasaki Y, Kohrogi H. Use of tacrolimus, a potent antifibrotic agent, in bleomycin-induced lung fibrosis. Eur Respir J. 2006;27(3):460–469. doi: 10.1183/09031936.06.00070705. [DOI] [PubMed] [Google Scholar]
- Nagayama A, Ellisen LW, Chabner B, Bardia A. Antibody–drug conjugates for the treatment of solid tumors: clinical experience and latest developments. Targ Oncol. 2017;12(6):719–739. doi: 10.1007/s11523-017-0535-0. [DOI] [PubMed] [Google Scholar]
- Nagler M, Podmirseg SM, Griffith GW, Insam H, Ascher-Jenull J. The use of extracellular DNA as a proxy for specific microbial activity. Appl Microbiol Biotechnol. 2018;102(6):2885–2898. doi: 10.1007/s00253-018-8786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik BS. Developments in taxol production through endophytic fungal biotechnology: a review. Orient Pharm Exp Med. 2019;19:1–13. doi: 10.1007/s13596-018-0352-8. [DOI] [Google Scholar]
- Nettersheim D, Berger D, Jostes S, Skowron M, Schorle H. Deciphering the molecular effects of romidepsin on germ cell tumours: DHRS2 is involved in cell cycle arrest but not apoptosis or induction of romidepsin effectors. J Cell Mol Med. 2019;23(1):670–679. doi: 10.1111/jcmm.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ. Predominately uncultured microbes as sources of bioactive agents. Front Microbiol. 2016;7:1832. doi: 10.3389/fmicb.2016.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie YL Wu YD Wang CX Lin R Xie Y Fang DS Jiang H Lian YY (2018) Structure elucidation and antitumor activity of a new macrolactam produced by marine-derived actinomycete Micromonospora sp. FIM05328. Nat Prod Res 32(18): 2133–2138. 10.1080/14786419.2017.1366479 [DOI] [PubMed]
- Nordmann P, Poirel L, Walsh TR, Livermore DM. The emerging NDM carbapenemases. Trends Microbiol. 2011;19(12):588–595. doi: 10.1016/j.tim.2011.09.005. [DOI] [PubMed] [Google Scholar]
- OECD forum (2004) Health of Nations: Combating infectious diseases. organization for economic co-operation and development. https://www.oecd-ilibrary.org/docserver/9789264108479-en.pdf?expires=1645075639&id=id&accname=guest&checksum=E775E40FE69C3EC0CC9B58D55A8680A8
- Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, Muro K, Kim YH, Ferry D, Tebbutt NC, Al-Batran SE, Smith H, Costantini C, Rizvi S, Lebwohl D, Van Cutsem E. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31(31):3935–3943. doi: 10.1200/JCO.2012.48.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsufyeva EN, Shchekotikhin AE, Bychkova EN, Pereverzeva ER, Treshalin ID, Mirchink EP, Isakova EB, Chernobrovkin MG, Kozlov RS, Dekhnich AV, Preobrazhenskaya MN. Eremomycin pyrrolidide: a novel semisynthetic glycopeptide with improved chemotherapeutic properties. Drug Des Devel Ther. 2018;12:2875–2885. doi: 10.2147/DDDT.S173923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. mABS. 2014;6(1):34–45. doi: 10.4161/mabs.27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Bethel CR, Distler AM, Kasuboski C, Taracila M, Bonomo RA. Inhibitor resistance in the KPC-2 beta-lactamase, a preeminent property of this class A β-lactamase. Antimicrob Agents Chemother. 2010;54(2):890–897. doi: 10.1128/AAC.00693-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar SP, Guler R, Brombacher F. Statins: a viable candidate for host-directed therapy against infectious diseases. Nat Rev Immunol. 2019;19:104–117. doi: 10.1038/s41577-018-0094-3. [DOI] [PubMed] [Google Scholar]
- Park JW, Lee JK, Kwon TJ, Yi DH, Kim YJ, Moon SH, Suh HH, Kang SM, Park YI. Bioconversion of compactin into pravastatin by Streptomyces sp. Biotechnol Lett. 2003;25(21):1827–1831. doi: 10.1023/a:1026281914301. [DOI] [PubMed] [Google Scholar]
- Park SR, Yoo YJ, Ban Y-H, Yoon YJ. Biosynthesis of rapamycin and its regulation: past achievements and recent progress. J Antibiot. 2010;63:434–441. doi: 10.1038/ja.2010.71. [DOI] [PubMed] [Google Scholar]
- Park JE, Park J, Jun Y, Oh Y, Ryoo G, Jeong YS, Gadalla HH, Min JS, Jo JH, Song MG, Kang KW, Bae SK, Yeo Y, Lee W. Expanding therapeutic utility of carfilzomib for breast cancer therapy by novel albumin-coated nanocrystal formulation. J Control Release. 2019;302:148–159. doi: 10.1016/j.jconrel.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualotto AC, Denning DW. New and emerging treatments for fungal infections. J Antimicrob Chemother. 2008;61(Suppl 1):i19–i30. doi: 10.1093/jac/dkm428. [DOI] [PubMed] [Google Scholar]
- Pereira-Díaz E, Moreno-Verdejo F, de la Horra C, Guerrero JA, Calderón EJ, Medrano FJ (2019) Changing trends in the epidemiology and risk factors of Pneumocystis pneumonia in Spain. Front Public Health 7:275. 10.3389/fpubh.2019.00275 [DOI] [PMC free article] [PubMed]
- Perez M, Schleissner C, Fernandez R, Rodriguez P, Reyes F, de la Calle ZP, F, Cuevas C, PM100117 and PM100118, new antitumor macrolides produced by a marine Streptomyces caniferus GUA-06-05-006A. J Antibiot. 2016;69:388–394. doi: 10.1038/ja.2015.121. [DOI] [PubMed] [Google Scholar]
- Pham JV, Yilma MA, Feliz A, Majid MT, Maffetone N, Walker JR, Kim E, Cho HJ, Reynolds JM, Song MC, Park SR, Yoon YJ. A review of the microbial production of bioactive natural products and biologics. Front Microbiol. 2019;10(1):404. doi: 10.3389/fmicb.2019.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietramellara G, Ascher J, Baraniya D, Arfaioli P, Ceccherini MT, Hawes MC (2013) Relevance of extracellular DNA in rhizosphere. EGU General Assembly, 15:EGU2013–8331
- Pollak AW, Kramer CM. LDL lowering in peripheral arterial disease: are there benefits beyond reducing cardiovascular morbidity and mortality? Clin Lipidol. 2012;7(2):141–149. doi: 10.2217/clp.12.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal J. Chartreusin, elsamicin A and related anti-cancer antibiotics. Curr Med Chem Anticancer Agents. 2003;3(6):411–420. doi: 10.2174/1568011033482215. [DOI] [PubMed] [Google Scholar]
- Qiao W, Ling F, Yu L, Huang Y, Wang T (2017) Enhancing taxol production in a novel endophytic fungus, Aspergillus aculeatinus Tax-6, isolated from Taxus chinensis var. mairei. Fungal Biol 121(12): 1037–1044. 10.1016/j.funbio.2017.08.011 [DOI] [PubMed]
- Quigley J, Peoples A, Sarybaeva A, Hughes D, Ghiglieri M, Achorn C, Desrosiers A, Felix C, Liang L, Malveira S, Millett W, Nitti A, Tran B, Zullo A, Anklin C, Spoering, Ling LL, Lewis K (2020) Novel antimicrobials from unclultured bacteria acting against Mycobacterium tuberculosis. mBIO 11(4): e01516–20.10.1128/mBio.01516-20 [DOI] [PMC free article] [PubMed]
- Rannikko J, Syrjanen J, Seiskari T, Aittoniemi J, Huttunen R. Sepsis related mortality in 497 cases with blood culture-positive sepsis in an emergency department. Int J Infect Dis. 2017;58:52–57. doi: 10.1016/j.ijid.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Rodrigues AMS, Rohée C, Fabre T, Batailler N, Sautel F, Carletti I, Nogues S, Suzuki MT, Stien D. Cytotoxic indole alkaloids from Pseudovibrio denitrificans BBCC725. Tetrahedron Lett. 2017;58(32):3172–3173. doi: 10.1016/j.tetlet.2017.07.005. [DOI] [Google Scholar]
- Roos JCP, Murthy R. Sirolimus (rapamycin) for the targeted treatment of the fibrotic sequelae of Graves’ orbitopathy. Eye. 2019;33(4):679–682. doi: 10.1038/s41433-019-0340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchika Naik JV, Pandey A. Synthetic metabolism and Its significance in agriculture. In: Singh SP, Pandey A, Du G, Kumar S, editors. Current developments in biotechnology and bioengineering. Amsterdam: Elsevier; 2019. pp. 365–391. [Google Scholar]
- Sabater-Jara AB, Tudela LR, Lopez-Perez AJ. In vitro culture of Taxus sp.: strategies to increase cell growth and taxoid production. Phytochem Res. 2010;9:343–356. doi: 10.1007/s11101-010-9167-z. [DOI] [Google Scholar]
- Saha S, Zhang W, Zhang G, Zhu Y, Chen Y, Liu W, Yuan C, Zhang Q, Zhang H, Zhang L. Activation and characterization of a cryptic gene cluster reveals a cyclization cascade for polycyclic tetramate macrolactams. Chem Sci. 2017;8:1607–1612. doi: 10.1039/C6SC03875A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez S, Demain AL (2017a) Bioactive products from fungi. In: Puri M, MN Puri (eds) Food bioactives: extraction and biotechnology applications. Springer International Publishing AG, Cham, pp 59–87
- Sanchez S, Demain AL. The amazing world of antibiotics. Biochem Pharmacol. 2017;133:1–3. doi: 10.1016/j.bcp.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Santos JD, Vitorino I, Reyes F, Vicente F, Lage OM (2020) From ocean to medicine: Pharmaceutical applications of metabolites from marine bacteria. Antibiotics (Basel) 9(8): 455. 10.3390/antibiotics9080455 [DOI] [PMC free article] [PubMed]
- Saravolatz LD, Stein GE. Oritavancin: a long-half-life lipoglycopeptide. Clin Infect Dis. 2015;61(4):627–632. doi: 10.1093/cid/civ311. [DOI] [PubMed] [Google Scholar]
- Sarkar P, Haldar J (2019) Glycopeptide antibiotics. Mechanism of action and recent developments. In: Capelo‐Martínez JL, Igrejas G (eds) Antibiotic drug resistance. John Wiley & Sons, Inc. Hoboken, pp 73–95
- Sayed AM, Alhadrami HA, El-Gendy AO, Shamikh YI, Belbahri L, Hassan HM, Abdelmohsen UR, Rateb ME. Microbial natural products as potential inhibitors of SARS-CoV-2 main protease (Mpro) Microorganisms. 2020;8(7):970. doi: 10.3390/microorganisms8070970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler R, Colmer S, Tynan H, Demain AL, Gullo VP. Antimicrobials, drug discovery, and genome mining. Appl Microbiol Biotechnol. 2013;97(3):969–978. doi: 10.1007/s00253-012-4609-8. [DOI] [PubMed] [Google Scholar]
- Schleissner C, Canedo LM, Rodriguez P, Crespo C, Zuniga P, Penalver A, de la Calle F, Cuevas C. Bacterial production of a pederin analogue by a free-living marine Alphaproteobacterium. J Nat Prod. 2017;80(7):2170–2173. doi: 10.1021/acs.jnatprod.7b00408. [DOI] [PubMed] [Google Scholar]
- Seukep AJ, Kuete V, Nahar L, Sarker SD, Guo M. Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. J Pharm Anal. 2020;10(4):277–290. doi: 10.1016/j.jpha.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveria FP, Husain S. Fungal infections in solid organ transplantation. Med Mycol. 2007;45(4):305–320. doi: 10.1080/13693780701200372. [DOI] [PubMed] [Google Scholar]
- Søgaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Østergaard L, Tolstrup M. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog. 2015;11(9):e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman SSM, Raizada MN. Darkness: a crucial factor in fungal taxol production. Front Microbiol. 2018;9:353. doi: 10.3389/fmicb.2018.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subban K, Subramani R, Srinivasan VPM, Johnpaul M, Chelliah J (2019) Salicylic acid as an effective elicitor for improved taxol production in endophytic fungus Pestalotiopsis microspora. PLoS ONE 14(2): e0212736. 10.1371/journal.pone.0212736 [DOI] [PMC free article] [PubMed]
- Sun Y, Tomura T, Sato J, Iizuka T, Fudou R, Ojika M. Isolation and biosynthetic analysis of haliamide, a new PKS-NRPS hybrid metabolite from the marine Myxobacterium Haliangium ochraceum. Molecules. 2016;21(1):59. doi: 10.3390/molecules21010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tagami T, Ernsting MJ, Li SD. Efficient tumor regression by a single and low dose treatment with a novel and enhanced formulation of thermosensitive liposomal doxorubicin. J Control Release. 2011;152(2):303–309. doi: 10.1016/j.jconrel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Tahir KM, Chinnasamy S, Cui Z, Irfan M, Wei D-Q. Mechanistic analysis of A46V, H57Y, and D129N in pyrazinamidase associated with pyrazinamide resistance. Saudi J Biol Sci. 2020;27(11):3150–3156. doi: 10.1016/j.sjbs.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantawy M, Pamittan FG, Singh S, Gong Y. Epigenetics changes associated with anthracycline-induced cardiotoxicity. Clin Transl Sci. 2020;14(1):36–46. doi: 10.1111/cts.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade M, van Zyl LJ, Navarro-Fernández J, Abd Elrazak A. Targeted metagenomics as a tool to tap into marine natural product diversity for the discovery and production of drug candidates. Front Microbiol. 2015;6:890. doi: 10.3389/fmicb.2015.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter AJ, Aydin A, Strinden MJ, O’Grady J. Recent and emerging technologies for the rapid diagnosis of infection and antimicrobial resistance. Curr Opin Microbiol. 2019;51:39–45. doi: 10.1016/j.mib.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Van Duin D, Paterson DL. Multidrug-resistant bacteria in the community: trends and lessons learned. Infect Dis Cin North Am. 2016;30(2):377–390. doi: 10.1016/j.idc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderMolen KM, McCulloch W, Pearce CJ, Oberlies NH. Romidepsin (Istodax, NSC 630176, FR901228, FK228, depsipeptide): a natural product recently approved for cutaneous T-cell lymphoma. J Antibiot (tokyo) 2011;64(8):525–531. doi: 10.1038/ja.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanpouille C, Lisco A, Introini A, Grivel JC, Munawwar A, Merbah M, Schinazi RF, Derudas M, McGuigan C, Balzarini J, Margolis L. Exploiting the anti-HIV-1 activity of acyclovir: suppression of primary and drug-resistant HIV isolates and potentiation of the activity by ribavirin. Antimicrob Agents Chemother. 2012;56(5):2604–2611. doi: 10.1128/AAC.05986-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Stanciu CE, Ehrhardt CJ, Yadavalli VK. Nanoscale characterization of forensically relevant epithelial cells and surface associated extracellular DNA. Forensic Sci Int. 2017;277:252–258. doi: 10.1016/j.forsciint.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Wang RJ, Miller RF, Huang L. Approach to fungal infections in human immunodeficiency virus-infected individuals: pneumocystis and beyond. Clin Chest Med. 2017;38(3):465–477. doi: 10.1016/j.ccm.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Wang M, Tan Y, Hu X, He H, Xiao C, You X, Wang Y, Gan M (2017c) Neo-actinomycins A and B, natural actinomycins bearing the 5H-oxazolo[4,5-b]phenoxazine chromophore, from the marine-derived Streptomyces sp. IMB094. Sci Rep 7(1): 3591. 10.1038/s41598-017-03769-8 [DOI] [PMC free article] [PubMed]
- Watson CJE, Dark JH. Organ transplantation: historical perspective and current practice. Br J Anaesth. 2012;108(1):i29–i42. doi: 10.1093/bja/aer384. [DOI] [PubMed] [Google Scholar]
- Wei Y, Liu L, Zhou X, Lin J, Sun X, Tang K (2012) Engineering taxol biosynthetic pathway for improving taxol yield in taxol-producing endophytic fungus EFY-21 (Ozonium sp.). African J Biotechnol 11(37):9084–9101. 10.5897/AJB10.1896
- Wen R (2020) Comparison of cyclosporin A and tacrolimus in the field of organ transplantation. IOP Conf Ser: Earth Environ Sci 565. 10.1088/1755-1315/565/1/012052
- World Health Organization (2017) Antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline, including tuberculosis. (WHO/EMP/IAU/2017.11). Licence: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/handle/10665/258965
- Worthington RJ, Melander C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013;31(3):177–184. doi: 10.1016/j.tibtech.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Tang Y. Efficient synthesis of simvastatin by use of whole-cell biocatalysts. Appl Environ Microbiol. 2007;73(7):2054–2060. doi: 10.1128/AEM.02820-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Chen C-F, Min T-L, Hu H-F. Romipeptides A and B, two new romidepsin derivatives isolated from Chromobacterium violaceum No.968 and their antitumor activities in vitro. hinese J Nat Med . 2019;17(2):155–160. doi: 10.1016/S1875-5364(19)30018-4. [DOI] [PubMed] [Google Scholar]
- Xiong YH, Liu Y. Essential roles of eDNA and AI-2 in aerobic granulation in sequencing batch reactors operated at different settling times. Appl Microbiol Biotechnol. 2012;93(6):2645–2651. doi: 10.1007/s00253-011-3565-z. [DOI] [PubMed] [Google Scholar]
- Yamashita M. Tacrolimus (FK206) developmental story. Seibutsu-Kogaku Kaishi. 2013;91(3):141–154. [Google Scholar]
- Yang SS, Cragg GM, Newman DJ, Bader JP. Natural products-based anti-HIV drug discovery and development facilitated by the NCI developmental therapeutics program. J Nat Prods. 2001;64(2):265–277. doi: 10.1021/np0003995. [DOI] [PubMed] [Google Scholar]
- Yao Q, Ma L, Liu L, Ikeda H, Fushinobu S, Li S, Xu L-H. Hydroxylation of compactin (ML-236B) by CYP105D7 (SAV_7469) J Microbiol Biotechnol. 2017;27(5):956–964. doi: 10.4014/jmb.1610.10079. [DOI] [PubMed] [Google Scholar]
- Ye X, Anjum K, Song T, Wang W, Liang Y, Chen M, Huang H, Lian XY, Zhang Z. Antiproliferative cyclodepsipeptides from the marine actinomycete Streptomyces sp. P11–23B downregulating the tumor metabolic enzymes of glycolysis, glutaminolysis, and lipogenesis. Phytochemistry. 2017;135:151–159. doi: 10.1016/j.phytochem.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi A, Miyazawa K, Abe K. Function and biosynthesis of cell wall α-1,3-glucan in fungi. J Fungi (basel) 2017;3(4):63. doi: 10.3390/jof3040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaporozhets TS, Besednova NN. Biologically active compounds from marine organisms in the strategies for combating coronaviruses. AIMS Microbiol. 2020;6(4):470–494. doi: 10.3934/microbiol.2020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai YJ, Wu MM, Linck VA, Zou L, Yue Q, Wei SP, Song C, Zhang S, Williams CR, Song BL, Zhang ZR, Maa HP. Intracellular cholesterol stimulates ENaC by interacting with phosphatidylinositol-4,5-bisphosphate and mediates cyclosporine A-induced hypertension. BBA - Mol Basis Dis. 2019;1865:1915–1924. doi: 10.1016/j.bbadis.2018.08.027. [DOI] [PubMed] [Google Scholar]
- Zhanel GG, Homenuik K, Nichol K, Noreddin A, Vercaigne L, Embil J, Gin A, Karlowsky JA, Hoban DJ. The glycylcyclines: a comparative review with the tetracyclines. Drugs. 2004;64(1):63–88. doi: 10.2165/00003495-200464010-00005. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhou PP, Yu LJ. An endophytic taxol-producing fungus from Taxus media, Cladosporium cladosporoides MD2. Curr Microbiol. 2009;59(3):227–232. doi: 10.1007/s00284-008-9270-1. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhang Z, Zf Li, Chen Q, Li YY, Gong Y, Yue XJ, Sheng DH, Zhang YM, Wu C, Li YZ. Phylogeny-guided characterization of glycosyltransferases for epothilone glycosylation. Biotechnol. 2019;12:763–774. doi: 10.1111/1751-7915.13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen W, Gao Q, Yang J, Yan X, Zhao H, Su L, Yang M, Gao C, Yao Y, Inoki K, Li D, Shao R, Wang S, Sahoo N, Kudo F, Eguchi T, Ruan B, Xu H. Rapamycin directly activates lysosomal mucolipin TRP channels independent of mTOR. PLoS Biol. 2019;17(5):e3000252. doi: 10.1371/journal.pbio.3000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Brooks SA, Eszterhas S, Heim S, Li L, Xiong YQ, Fang Y, Kirsch JR, Verma D, Bailey-Kellogg C, Griswold KE (2020) Globally deimmunized lysostaphin evades human immune surveillance and enables highly efficacious repeat dosing. Sci Adv 6:eabb9011. 10.1126/sciadv.abb9011 [DOI] [PMC free article] [PubMed]
- Zhou X, Zhu H, Liu L, Lin J, Tang K. A review: recent advances and future prospects of taxol-producing endophytic fungi. Appl Microbiol Biotechnol. 2010;86(6):1707–1717. doi: 10.1007/s00253-010-2546-y. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhang W, Chen X, Wu H, Duan Y, Xu Z. Generation of high rapamycin producing strain via rational metabolic pathway-based mutagenesis and further titer improvement with fed-batch bioprocess optimization. Biotechnol Bioeng. 2010;107(3):506–515. doi: 10.1002/bit.22819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study are included in the present review.