Abstract
In this review, we provide an overview of developments in point-of-care (POC) diagnostics during the COVID-19 pandemic. We review these advances within the framework of a holistic POC ecosystem, focusing on points of interest – both technological and non-technological – to POC researchers and test developers. Technologically, we review design choices in assay chemistry, microfluidics, and instrumentation towards nucleic acid and protein detection for severe acute respiratory coronavirus 2 (SARS-CoV-2), and away from the lab bench, developments that supported the unprecedented rapid development, scale up, and deployment of POC devices. We describe common features in the POC technologies that obtained Emergency Use Authorization (EUA) for nucleic acid, antigen, and antibody tests, and how these tests fit into four distinct POC use cases. We conclude with implications for future pandemics, infectious disease monitoring, and digital health.
Graphical Abstract
This article reviews the rapid and unprecedented development, scaleup and deployment of POC devices in response to the COVID-19 pandemic and its implications for the future of diagnostics and digital health.
1. Introduction
In January 2020, within two weeks of the publication of the genome sequence of SARS-CoV-2, the first reverse transcription polymerase chain reaction (RT-PCR) diagnostic test to detect the virus was developed.1 During the beginning stages of the pandemic, countries with high rates of testing had low transmission rates as testing helped identify patients to isolate and prevent the spread of the virus.1 To meet the tremendous demand for diagnostics, laboratory-based high-throughput testing was scaled up, but faced limitations in supply and in bringing subjects to dense environments.
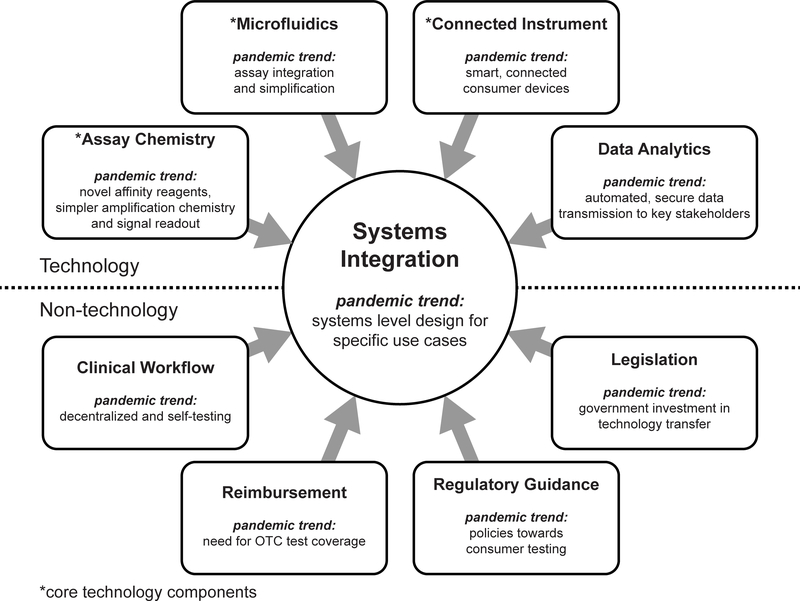
An effort to develop and deploy POC diagnostics ensued, aided by large government and private sector investments as well as revised regulatory guidelines. Here, we review developments in POC diagnostics during the COVID-19 pandemic, using a framework we presented in 2017 which highlighted the synthesis of microfluidic and smart connected devices towards decentralized testing.2 This “POC ecosystem” (Figure 1) consists of technological and non-technological components. Technologically, advances were made across disciplines, covering assay chemistry, microfluidics, instrumentation, and data analytics. Away from technology, the pandemic escalated awareness in government, media, and consumers in comprehensive testing and surveillance, increased understanding of different types of diagnostics, and created pathways to implementation; we summarize these non-technological developments (with a focus on the United States) as clinical workflow, regulatory guidance, reimbursement, and legislation. Synthesis of technological and non-technological considerations led to targeted solutions towards SARS-CoV-2 testing that were developed with unprecedented speed. The review focuses on nucleic acid, antigen, and antibody tests, but we also provide an overview of selected novel POC diagnostic technologies, such as face masks, breathalyzers and T-cell testing. We analyzed POC tests that obtained FDA EUA for SARS-CoV-2 (as of June 2021), describe common features in the companies that developed these tests, and provide commercial case studies for selected technologies. We then group SARS-CoV-2 tests into the four distinct POC use cases, and for each case, discuss the technological bases of the tests as well as overall testing trends. Finally, we discuss implications for monitoring of future infectious disease as well as for digital health more broadly.
Figure 1. Overview of the POC diagnostics ecosystem in a pandemic age.
All technology and non-technology components play a role in determining the systems integration (i.e., use case) of POC diagnostic devices. Adapted from reference 2 with permission from American Chemical Society.
2. The POC Ecosystem for SARS-CoV-2 Diagnostics
In this section, we describe how the POC ecosystem has developed during the COVID-19 pandemic. The POC ecosystem contains both technology and non-technology components (Figure 1).2 Technologically, the needs for speed, low-cost, and simplicity were reinforced, accelerating the trend for miniaturized connected diagnostics for decentralized settings.2,3 For each type of SARS-CoV-2 diagnostic assay (nucleic acid, antigen, and antibody tests), we highlight key advances in assay chemistry, microfluidics, and instrumentation, and select three commercial POC tests as illustrative examples. In addition to the three assay types, we provide an overview of selected emerging technologies as well as discuss developments in data analytics across POC devices.
In the deployment of POC diagnostic tests, remarkable developments have taken place in clinical workflow, regulatory guidance, reimbursement, and legislation. We review these developments and their interplay with technological advances, and analyze common features of companies that successfully obtained EUA for POC SARS-CoV-2 diagnostic devices.
2.1. Core Technology Components
2.1.1. Nucleic Acid Tests
Nucleic acid tests, or molecular diagnostic tests, detect RNA from SARS-CoV-2, a single-stranded RNA virus.4,5 A number of techniques to amplify and detect viral RNA were developed and deployed, although the gold standard method for RNA detection remains to be RT-PCR.5–7
Assay Chemistry
A number of amplification, detection, and readout methods have been developed8 as an alternative to PCR. Isothermal amplification techniques, where a single temperature requirement can simplify requirements for a POC molecular test,9,10 include loop-mediated isothermal amplification (LAMP), recombinase polymerase amplification (RPA), and rolling circle amplification (RCA).11 LAMP is one of the most mature and widely studied isothermal amplification methods12; it uses 2 to 3 primer sets and a strand-displacing polymerase to facilitate exponential amplification of the target at a single temperature of ~60–65 °C.13 Several commercial entities that have obtained EUA for SARS-CoV-2 detection use LAMP (Table 1), with many studies reporting comparable performance to RT-PCR.14–16 Another isothermal method, nicking enzyme amplification reaction (NEAR),17 was used in the Abbott ID NOW system and was one of the first POC methods made available during the pandemic.18 As field evaluations of the Abbott ID NOW have reported lower sensitivities than the initial results reported by the manufacturer, more independent real-world evaluations of a wide range of POC tests – including isothermal amplification and CRISPR tests – will be beneficial towards an objective understanding of the field performance of these technologies.19
Table 1:
Technology map of design choices made by selected SARS-CoV-2 POC Diagnostics
| POC Test | Assay Chemistry | Microfluidics | Connected Instrument | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Material | Reagent Storage | Sample Type | Sample Processing | Fluid Actuation | Fluid Control | Fluid Mixing | Signal Detection | |||
| Cue Health | Isothermal | Plastic | Dry reagents | Nasal Swab | Target binding with affinity molecules | Capillary | Wax valves | Active (sonication) | Electrochemical | Portable, Bluetooth connected reader (Cue Cartridge Reader) Cue Health mobile app |
| Lucira Health | Isothermal (RT-LAMP) | Plastic | Dry reagents (in test unit) | Nasal Swab | Lysis (in vial) | N/A | Reaction chambers | Manual/Passive | Colorimetric | Optical reader, mobile app (LUCI pass) |
| Visby Medical | RT-PCR | Plastic | Dry reagents (on chip) | Nasal Swab | Lysis (on-chip) | Gear motor | Rotary (on-chip) valves | Passive | Colorimetric (LFA) | None |
| Mesa Biotech | RT-OSCAR | Plastic, Paper | Dry reagents (on-chip) | Nasal Swab | Lysis (on-chip) | Pneumatic/Capillary | Patented passive fluid flow technology | Passive | Colorimetric (LFA) | None |
| Cepheid Xpert Omni | RT-PCR | Plastic | Dry Reagents (on-chip) | Nasal/nasopharyngeal/ throat swab | Lysis/ extraction (on-cartridge) | Pneumatic | Rotary valves | Passive | Fluorescence | Portable, Bluetooth connected |
| Abbott ID NOW | Isothermal (NEAR) | Plastic | Dry reagents | Nasal/nasopharyngeal/ throat swab | Lysis (off-chip) | Manual | Manual | Manual | Fluorescence | Portable instrument with LCD screen |
| Minute Molecular DASH | RT-qPCR | Plastic, thin film | Dry reagents (on-cartridge) | Nasal/nasopharyngeal swab, saliva | Lysis/ Paramagnetic particle extraction (on-cartridge) | Capillary | Passive | Passive | Fluorescence | Barcode scanner, cloud connectivity |
| Talis One | Isothermal | Plastic | Dry reagents | Nasal/oral swab | Solid-phase extraction and purification (on-cartridge) | SlipChip | Passive | Passive | Fluorescence | Portable instrument, cloud connectivity |
| Nuclein Hand-Held PCR test | RT-qPCR | Plastic | Dry reagents | Saliva | Lysis/extraction (in chamber) | Magnetic displacer piston | Passive | Passive | Fluorescence | On-board LCD screen |
| Roche Cobas Liat | RT-PCR | Plastic | In assay tube | Nasopharyngeal or Nasal Swab | Extraction and purification (in vial) | Pneumatic | Passive | Passive | Fluorescence | Portable instrument with LCD screen and barcode scanner |
| Ellume | LFA | Paper | In tube and dry reagents on strip | Nasal Swab | Mix with processing fluid – contains fluorophore (off-strip) | Capillary | Passive | Passive | Fluorescence (LFA) | Integrated optical reader (eStick) |
| LumiraDx | Microfluidic immunofluorescence assay | Plastic | Dry reagents (on-strip) | Nasopharyngeal swab | Lysis (off-strip) | Capillary /Pneumatic | Passive | Active | Fluorescence | Portable, connected reader |
| Luminostics ClipCOVID | LFA | Paper | Dry reagents (on-strip) | Nasal swab | Lysis (off-strip) | Capillary | Passive | Passive | Luminescence (LFA) | Portable analyzer with mobile app (Clip COVID app) |
| Abbott BinaxNOW | LFA | Paper | Dry reagents (on-strip) | Nasal swab | Lysis (on-test card) | Capillary | Passive | Passive | Colorimetric (LFA) | Mobile app to record results (NAVICA) |
| LightDeck | Fluorescence immunoassay | Plastic | Dry reagents (on-chip) | Nasal swab (antigen) / Serum (antibody) | Lysis (off-chip) | Capillary | Passive | Passive | Fluorescence (planar waveguide technology) | Portable, connected instrument |
| Qorvo Omnia | Microfluidic immunoassay | Plastic | On Chip | Nasal swab | Lysis (off-chip) | Pneumatic | Passive | Passive | Bulk Acoustic Wave | Instrument with touchscreen |
| Celltrion Sampinute (with BBB) | Magnetic force-assisted electrochemical sandwich immunoassay (MESIA) | Plastic | Reagent Solution (off-chip) | Nasopharyngeal swab | Mix with Reagent Solution tube (off-chip) | Magnetic | N/A | N/A | Electrochemical | Instrument with touchscreen |
| Nanomix eLab | Carbon nanotube electrochemical immunoassay | Plastic | On chip | Nasal Swab | N/A | Pneumatic | Diaphragm valves | Active | Electrochemical | Portable instrument with touchscreen |
| NOWdiagnostics ADEXUSDx | LFA | Paper | Dry reagents (on-strip) | Fingerstick whole blood | Plasma separation (on membrane) | Capillary | Passive | Passive | Colorimetric (LFA) | ADEXUSDx Analyzer or DxREADER |
| AssureTech Assure Rapid Test | LFA | Paper | Dry reagents (on-strip) | Fingerstick whole blood (POC) or serum/plasma | N/A | Capillary | Passive | Passive | Colorimetric (LFA) | N/A |
| QIAGEN QIAreach | LFA | Paper | In tube and dry reagents on strip | Serum/Plasma | Mix with processing fluid – contains fluorophore (off-cartridge) | Capillary | Passive | Passive | Fluorescence (LFA) | Optical reader (estick), connected to eHub |
Isothermal amplification methods have been paired with CRISPR technology to create a new class of molecular diagnostics. For example, Cas12 and Cas13 enzymes are used with CRISPR RNA (crRNA) to target a specific nucleic acid sequence complementary to the cRNA. While Cas12 detects ssDNA, and Cas13 detects ssRNA, both enzymes have collateral cleavage activity that cleaves any nucleic acids in their vicinity indiscriminately after recognition.8 A commercial assay for SARS-CoV-2 detection has been developed by Mammoth Biosciences with the DNA Endonuclease Targeted CRISPR Trans Reporter (DETECTR) platform using RT-LAMP and Cas12.20–22 Another company, Sherlock Biosciences, uses Cas 13 and RT-LAMP for their SHERLOCK (specific high-sensitivity enzymatic reporter unlocking) platform.23,24 While the EUA assays for Mammoth and Sherlock measure fluorescence generated upon cleavage of a reporter molecule and are restricted to laboratory settings,22,23 both companies have also demonstrated a visual detection method with a lateral flow assay.20,25 However, these methods still require a number of sample processing steps (including pre-amplification) so both companies have announced industry partnerships to translate the technology towards POC use.26,27 It is worth noting that Sherlock Biosciences has also demonstrated advances in simplifying sample preparation with CRISPR,25,28, and is working on a separate platform termed INSPECTR (Internal Splint-Pairing Expression Cassette Translation Reaction) that utilizes cell-free, synthetic translation as a biosensor.29–31 Current research efforts in the field are focused on translating CRISPR technology to a POC use.3,32–35 Some demonstrations include a minimally instrumented SHERLOCK test,36 amplification free detection in a microfluidic chip37 and lateral flow strip37, and a multiplexed lab-on-paper platform.38
PCR is still the gold standard in molecular diagnostics. AuNPs can be added to the reaction mix to support plasmonic thermocycling, where infrared radiation rapidly generates heat from localized surface plasmon resonance of the nanoparticles. This technology was previously demonstrated to produce rapid thermocycling,39–42 and is being adapted for POC use for COVID-1942 (including in our lab, for saliva samples without extraction, unpublished results).
For signal detection, PCR has used fluorescent dyes like SYBR Green for non-specific detection, or Taq man probes for specific detection. As an alternative to fluorescence, some developers have turned to colorimetric readouts. For example, the Mesa Biotech Accula platform for SARS-CoV-2 uses a LFA to detect PCR products, and a visual band is created to generate a test result.43 The Visby Medical platform uses capture reagents in a “flow cell” along with an enzymatic reaction (using horseradish peroxidase) to generate a visual spot.44 While visual readouts of PCR or nucleic acid amplification products with capture reagents on substrates (e.g., paper, plastic) has been demonstrated in previous research,45–47 the pandemic has spurred further demonstrations for non-instrumented readout methods.48,49 Another company, Lucira Health, skips the LFA and uses a simple colorimetric readout with a pH-sensing molecule, a method other RT-LAMP assays have employed for detection of SARS-CoV-2 as well.15,50,51 With these simple visual readouts aided by novel assay chemistry, novel forms of nucleic acid testing are being designed for decentralized use.
Microfluidics
Nucleic acid detection involves three critical steps: 1) sample preparation, 2) amplification, 3) detection. Given the number of steps involved, assay integration has always been critical for the development of POC molecular tests. With an emphasis on decentralized diagnostics during the pandemic, assay integration became even more important in order to create simple-to-use tests that are accessible to various end users and can be conducted outside traditional laboratory settings.52 Indeed, many POC SARS-CoV-2 diagnostics that have been developed incorporate innovations in microfluidics (Table 1), both traditional microfluidic and paper-based assays.53,54 We describe some of these innovations below, with a focus on simplifying sample collection and preparation steps, towards simpler assay integration.
The first RT-PCR test for SARS-CoV-2 (from the CDC) required a nasopharyngeal swab. However, collection of NP swabs requires a trained technician, can be painful to the receiver, and led to a national swab shortage. The massive deployment of COVID-19 testing spurred a significant push towards scalable workflow and instruments. Nasal swabs, which are easier to administer and can be collected by untrained personnel,55 showed comparable performance to nasopharyngeal swabs, validating their use in times of nasopharyngeal swab shortage.56 In addition, saliva-based COVID-19 tests were developed rapidly after the onset of pandemic,57 and subsequently has emerged as a viable specimen with potential advantages such as non-invasive and painless sample collection with comparable performance,58–60 thus paving the way for potentially increased use of saliva for POC diagnostics in the future.61,62, Although there are a number of EUA assays for SARS-CoV-2 detection in saliva, questions remain in terms of standardization of sample collection procedure and variability of results.
Along with sample collection, sample processing methods have also been simplified for SARS-CoV-2 detection. Traditionally, RT-PCR calls for sample lysis to release nucleic acids from cells, and then nucleic acid purification to concentrate and remove any PCR inhibitors and contaminants. At the beginning of the pandemic, a shortage in RNA extraction kits hampered efforts to expand testing,63,64 so researchers began to implement simpler sample processing techniques, and even remove the extraction step altogether.65,66 For example, extraction-free methods have been demonstrated with RT-PCR on heat-inactivated or lysed samples (96% sensitivity and 99.8% specificity compared Roche Cobas 6800 analyzer),67 and with a colorimetric POC test using RT-LAMP (100% sensitivity and specificity compared to previously tested clinical samples).68 Several EUA approved diagnostics have removed the extraction step (Table 1), although these tests typically demonstrate a limit of detection (LOD) of an order of magnitude or higher compared to tests with nucleic-acid extraction (Supplementary Table 1). However, the relationship to LOD can be complex, and is an interplay among sample preparation, the amplification method employed, and the amplification method’s sensitivity to inhibitors present in the sample.
Connected Instrument
Given the complexity of assay procedures required for nucleic acid detection, complex instruments are generally required for operations such as fluid actuation, reagent storage, and fluorescent detection. Recent innovations in electronics have allowed for smaller and cheaper instruments, as well as major developments in how these instruments connect to laboratory information systems, government reporting systems, providers, and patients.
Established POC systems have offloaded connectivity and data processing to a nearby computer. For example, the GeneXpert System and BioFire FilmArray require a separate barcode scanner and computer for test operation and data transmission.69,70 More recent generations, however, integrate processing power into the reader itself (such as a molecular testing instrument that has a built-in tablet computer).71 Other systems like the Abbott ID NOW or the Roche Cobas Liat analyzer also have a built-in screen for device operation. While the integration of computational power and assay operations onto one device has made nucleic acid testing instruments more portable and truly sample-to-answer, they are still relatively large benchtop instruments that cost thousands of dollars. The use case, which will be discussed in a later section, for such tests is in laboratory settings with trained personnel.
Increasingly, computational power is being offloaded to the most prevalent computer found in society today, the smartphone.72 For example, the Cepheid system has been one of the most widely used POC PCR systems for SARS-CoV-273; their GeneXpert Omni system for SARS-CoV-2 test74 is 9 inches tall and weighs 2.2 pounds, and uses a companion smartphone application for its user interface, which allows the user to scan the test cartridge barcode and view results.75,76, Another product for SARS-CoV-2, from Cue Health, uses a portable connected reader which can connect to the user’s smartphone via Bluetooth, and transmit results to a custom app that also provides test instructions.77
Efforts are also being made to lower the cost of instruments. For example, Mesa Biotech uses a low-cost dock (in the hundreds of dollars instead of thousands) by incorporating resistive heaters and utilizing a lateral flow detection method dependent on visual interpretation by the operator.43,78 Interestingly, a number of nucleic-acid testing products are now moving towards LFA detection or other formats where the signal can be detected visually or by using a smartphone camera. We will discuss these developments in the following sections for rapid antigen and antibody tests, for which an LFA format is already widely used.
Commercial Case Studies
Here we discuss three commercial POC SARS-CoV-2 nucleic acid tests that highlight novel assay chemistry, microfluidics, or instrumentation (a larger overview of nucleic acid tests is provided in Table 1 and Table 2). Performance data collected from company EUA documentation is summarized in Supplementary Table 1. Emphasizing the push towards POC use and also home use, we discuss user steps and the design of technological elements that enable a streamlined workflow.
Table 2:
Selected POC diagnostics for SARS-CoV-2 nucleic acid detection. All tests selected here have received US Federal funding (Figure 5)
| Company | Product | Applications beyond SARS-CoV-2 | Technology highlight for POC use | Authorization | Year company founded |
|---|---|---|---|---|---|
| Cue Health | COVID-19 Test | Respiratory, Sexual Health (Under development) |
Portable reader with smartphone connectivity, 20 min TAT | FDA EUA - POC/Home/ OTC | 2010 |
| Lucira Health | Check It COVID-19 Test Kit | Influenza (Under development) |
Colorimetric detection with low-cost reader | FDA EUA - POC/Home/ OTC | 2013 |
| Visby Medical | COVID-19 Test | Influenza A/B, CT/NG/TV, AMR (Under development) |
Single-use, disposable RT-PCR test | FDA EUA - POC | 2012 |
| Mesa Biotech | Accula SARS-CoV-2 | Influenza A/B, RSV | Low-cost instrument, LFA readout | FDA EUA - POC | 2015 |
| Cepheid | Omni SARS-CoV-2 | TB, HIV, Ebola | Portable, battery powered version of GeneXpert system | FDA EUA - POC | 1996 |
| QIAGEN | QIAstat-Dx Respiratory SARS-CoV-2 Panel | Respiratory panel | Syndromic testing panel (21 targets) | FDA EUA | 2010 |
| Minute Molecular | DASH | Respiratory (Influenza A/B), Sexual health (HCV, CT/NG/TV) | Portable, multiplex qPCR with TAT < 15 minutes | Under development | 2017 |
| MatMaCorp | COVID-19 2SF Test | Agriculture | Portable, low-cost, batch testing | FDA EUA | 2014 |
| Talis Biomedical | Talis One | Respiratory (Influenza A/B), Sexual and Women’s Health (CT/NG/Mgen/Trich, HSV, UTI, BV, GBS) | Single-use cartridge, automated sample-to-answer, cloud connectivity | Under development | 2010 |
| Nuclein | Hand-Held PCR Test | Zika | Small, battery-powered, disposable, real-time PCR | Under development | 2017 |
| MicroGEM | Spitfire 6830 SARS-CoV-2 | N/A | Multiplexed microfluidic cartridge with 15 min TAT | Under development | 2015 |
| Tangen Biosciences | GeneSpark | Respiratory, bloodstream infection (bacterial and AMR), candida auris | Portable device for isothermal amplification and multiplexing up to 32 targets | Under development | 2013 |
| Ubiquitome | Liberty16 | N/A | 16 samples in 40 min, compact, smartphone-controlled PCR machine | Under development | 2014 |
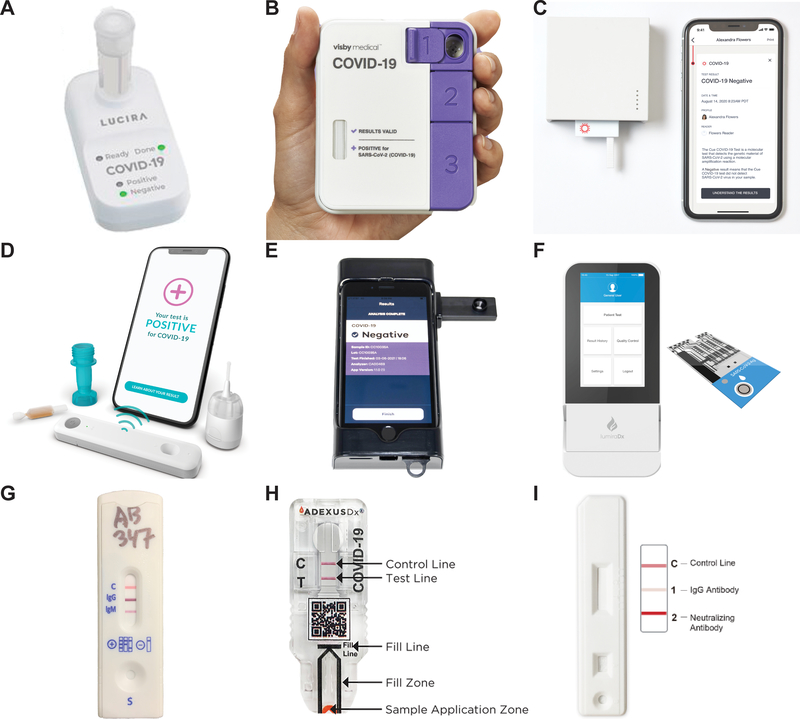
Visby Medical’s COVID-19 Point of Care Test is a handheld, fully disposable and automated RT-PCR device that detects the SARS-CoV-2 N gene and is currently authorized for POC use (Figure 2C). Under the supervision of a healthcare provider, the patient’s nasal swab is collected and subsequently diluted in the provided buffer. To begin the test, the diluted sample is loaded into the sample port of the device using a fixed volume pipette, followed by pushing down three buttons in succession (1–2-3). The device is then plugged into a power source and a result is returned in < 30-minutes. Within the device, sample preparation (lysis, reverse transcription), amplification and detection are all automated with all required reagents stored on board. The sample preparation module houses a piston and on-chip valves in order to allow for the sample to enter the lysis module and rehydrate the stored RT enzyme and primers. Following lysis and reverse transcription, the mixture enters the mixing chamber and rehydrates the lyophilized PCR reagents. In order to carry out amplification, the device uses a “flow through” PCR method in which the sample-reagent mixture flows through two heat zones and carries out 40 amplification cycles via a serpentine channel.44 Amplification creates biotinylated dsDNA, which flows to the detection module, and is immobilized onto the flow cell. The PCR products are detected using streptavidin coated with horseradish peroxidase, and a color changing substrate, leading to the formation of a purple spot for qualitative detection.79
Figure 2. Images of selected industry examples of SARS-CoV-2 POC tests.
Nucleic acid tests (A-C), antigen tests (D-F), antibody tests (G-I). A) Lucira Health CHECK-IT COVID -19 Test. Adapted from reference 278 with permission from Elsevier.278 B) Visby Medical COVID-19 Point-of-Care Test. Taken from www.visbymedical.com with permission from Visby Medical C) Cue Health Cue COVID -19 Test. Taken from www.cuehealth.com with permission from Cue Health D) Ellume COVID-19 Home Test. Credit to Ellume E) Luminostics Clip COVID Rapid Antigen Test. Credit to Luminostics. Inc. F) LumiraDx SARS-CoV-2 Ag Test. Credit to LumiraDx G) Assure Tech Assure COVID-19 IgG/IgM Rapid Test Device. Credit to Assure Tech. H) NOW Diagnostics ADEXUSDx COVID-19 Test. Adapted from reference 279 with permission from Elsevier.279 I) JoysBio SARS-CoV-2 IgG/Neutralizing Antibody Rapid Test Kit. Taken from en.joysbio.com with permission from JoysBio.
Lucira Health’s COVID-19 All-In-One Test Kit was the first molecular diagnostic test to obtain EUA for at-home use (with a prescription) (Figure 2A).80 The test uses isothermal amplification, specifically RT-LAMP, to detect the N gene of SARS-CoV-2 in 11–30 minutes.81 While the device does not incorporate traditional microfluidics, it simplifies test operation by preloading reagents, including lysis buffer, into a vial. Following sample collection, the user inserts and stirs the swab in the vial to elute and lyse the sample. The vial is then pressed down to engage with the test unit, which allows the lysate to enter the fluidic module and fill the reaction chambers. An electronic heating component detects this filling and initiates isothermal amplification, which results in a change in pH and color change of the reaction mix. This color change is detected in real-time with on-board optics in a portable reader and output to the user with LED indicators. While the reader is battery powered and operates on its own, the test also interfaces with an app which allows users to record test results, and transmit them to health authorities.82
Cue Health’s Cue COVID-19 Test was the first molecular diagnostic test to receive FDA EUA for home use without a prescription.83 The device is composed of a portable and reusable reader, a single-use test cartridge, a disposable sample wand for specimen collection, and a mobile app for instructions and result readout. The test uses isothermal amplification to detect the N gene of SARS-CoV-2. Prior to initiating the test, the test cartridge must be inserted into the reader in order to initiate heating. The user collects a nasal specimen with a sample wand, and inserts the wand into the test cartridge to initiate sample preparation, which includes sonication to induce the mixing and binding of target analytes, with magnetic particles present among reagents in the reservoir. Isothermal amplification takes place in the sample reservoir using forward primers and reverse primers for the N gene conjugated to biotin and horseradish peroxidase (HRP) respectively. Heat-actuated valves allow for fluid to flow through via capillary action to the analysis reservoir where HRP is localized on an electrode. HRP then oxidizes a substrate that is applied, leading to a current measured by the electrode.84 After 25 minutes, a semi quantitative nanoampere measurement is converted into a positive or negative reading. The test results are presented to the user on an app, stored on cloud servers, and reported to public health authorities.77
2.1.2. Antigen Tests
Overview
Antigen tests detect specific viral proteins that are present in patient specimens during active infections.5 SARS-CoV-2 antigen tests specifically target the nucleocapsid or spike proteins in both nasal swabs and saliva samples.85 Prior to the pandemic, POC antigen tests have been explored for detection of active infection in low- and middle-income countries (e.g., HIV, malaria, and tuberculosis), but it has been challenging to match the sensitivity of nucleic acid tests (Supplementary Table 1 and 2). For SARS-CoV-2, a number of tests have been deployed and have provided a useful alternative to molecular diagnostic tests to enable widespread testing in decentralized settings. The sensitivity of antigen tests varies depending on when the sample is taken during the time course of infection (according to the CDC). While studies have shown comparable sensitivity to nucleic acid tests at high viral loads, antigen tests are more likely to give false negative results especially when testing asymptomatic patients. To counteract decreased sensitivity, it is recommended to conduct serial testing over several days to catch these asymptomatic infections, and the FDA has issued EUAs to rapid antigen tests for such a use case86; indeed, recent studies have demonstrated real-world evidence that serial antigen testing every 3 days improves sensitivity.87
Assay Chemistry
A typical rapid antigen test uses a lateral flow format with a colorimetric visual readout from AuNPs (20–40 nm) or latex beads.88,89 As demonstrated by the Weigl group, efforts to improve sensitivity can be segmented into three categories: reaction, transport, and signal development.90 In particular, the SARS-CoV-2 pandemic has accelerated advances in reaction and signal development.
Most commonly, monoclonal antibody pairs are used to capture and detect antigens,91 but identifying or developing a successful pair can be time-consuming.92 During the pandemic, after an initial period of development,93 the first rapid antigen test (Quidel Sofia 2 SARS Antigen FIA) received EUA on May 9th, 2020.94 Efforts to identify capture reagents continued, for example via an automated liquid-handling robot.95 Using this method, over 1,000 anti-nucleocapsid antibody pairs were screened to identify pairs with the highest affinity for epitopes on the nucleocapsid protein,96–98 and a similar process was carried out for the spike protein.99
As a potential alternative to antibodies, other capture reagents are being explored. For example, DNA-based aptamers targeting the spike protein are being developed,100,101 based on previous work for detecting dengue.102 Another effort includes using nanobodies, which can be expressed in bacteria,103 for a rapid SARS-CoV-2 antigen test.104
To address low sensitivity of traditional antigen tests, novel signal development techniques were investigated.105–107 For SARS-CoV-2 detection, quantum dots (Ellume) and luminescent nanoparticles (Luminostics) have been demonstrated to improve sensitivity.108 (The use of low-cost optics to read fluorescence signals further improve sensitivity, as we discuss later in commercial case studies.) While we are not aware of independent testing of a reference panel comparing the performance of EUA antigen tests (as it has been done in a limited manner with antibody tests,109 the Quidel QuickVue test (using a traditional colorimetric readout) has a self-reported LOD of 19,100 TCID50 /mL using heat inactivated virus. The Ellume test has an LOD of 103.8 (~6,310) TCID50 /mL with heat inactivated virus, and the Luminostics test has an LOD of 880 TCID50 /mL with gamma irradiated virus (Supplementary Table 2). These results, albeit reported by the manufacturers, demonstrate the potential for up to 20x improvement in sensitivity over standard rapid antigen tests.
Microfluidics
While LFAs automates assay operation, it can only do so for a limited number of steps. In its simplest form, an LFA automates wicking of sample, rehydration of reagents (including the reporter), and visual signal detection.108 Most SARS-CoV-2 antigen tests follow this principle, differing in the antigen being detected and signal reporter of choice.
Use of plastic microfluidics opens the possibility of automating additional assay operations that can enhance performance and more closely mimic an enzyme-linked immunosorbent assay (ELISA), the gold-standard in immunoassays. Our lab has developed a platform in the past for HIV110, syphilis111, and Lyme disease.112 In the pandemic, a number of efforts have pursued traditional microfluidic platforms for SARS-CoV-2 detection to allow for multiplexing,113 and aid sample preparation.114 Out of the current EUA approved tests for antigen detection, LumiraDx is the sole test that does not use a standard LFA. Instead, a microfluidic chip contains multiple independent assay channels that can be used for multiplexed testing, including running replicates or process control.115 Applying a magnetic field to the microfluidic chip concentrates the SARS-CoV-2 immune complexes for signal generation, while unbound labels and sample are washed away from the measurement zone.116 This format more closely mimics lab-based immunoassays than LFAs.
Following the success of using saliva matrix in nucleic acid tests, similar efforts were made to develop saliva-based rapid antigen tests in order to simplify sample collection;117 however, they showed lackluster performance in initial studies,118 such as low sensitivity.119
Connected Instrument
Unlike molecular testing, rapid antigen testing largely does not rely on instrumentation for assay operation as many of the steps are self-contained and automated with the LFA. Here, instrumentation focuses on improving sensitivity and lowering the LOD by quantifying the signal output from LFAs.
The first two antigen tests to receive EUA by the FDA were from BD and Quidel, which already had established platforms utilizing a dedicated LFA reader. BD uses a AuNP signal enhancement method for visual detection on a lateral flow strip and incorporates a handheld reader, BD Veritor Plus, to interpret results.120 The Quidel test uses the Sofia 2 benchtop system that incorporates fluorescent detection with a UV LED source. It has a built-in touch screen interface to run the assay and report results.121 However, these readers can still be a significant capital investment, providing a barrier for widespread adoption.93 Nonetheless, Quidel has also received EUA for another rapid antigen test, the QuickVue SARS Antigen Test; their EUA allows for POC testing in facilities operating under a CLIA waiver, and more recently, for prescription home use as well as over-the-counter (OTC) home use.122,123 Test results are meant to be interpreted by the naked eye, removing the need for an external instrument. Another visual rapid antigen test is the Abbot BinaxNOW. The Abbott BinaxNOW COVID-19 Ag Card has EUA for use in CLIA certified laboratories as well as EUA for prescription home use when supervised by a telehealth proctor.124 To provide this service, Abbott has partnered with eMed, a telemedicine platform,125 which also allows users to store, access, and display COVID-19 test results. More recently, the test has received EUA for a self-test (without supervision) and is available without a prescription.126 In summary, this use of a connected instrument allows users to access the POC antigen test in three ways: OTC self-test, proctored at-home test, or at a healthcare facility.
With developments in low-cost and miniature optics, efforts are also being made to create cheap, low-profile readers for lateral flow tests.127,128 For example, Lumos has helped numerous companies develop custom POC readers, and offers an off the shelf product, the Leelu reader.129 Another company, Jana Care, has developed the Aina device, a colorimetric and fluorescent reader for paper diagnostics that is compact and affordable,130,131 and runs the Aina Open program to put the device in the hands of test developers. For SARS-CoV-2 detection, Ellume developed disposable test cartridges with integrated optics for fluorescent detection;132,133 here, a smartphone controls the operation, processes the signal, and displays the result, hence reducing the technical requirements and cost of the reader.
There also has been interest in replacing the reader all together with a smartphone, by using the camera lens for imaging and processing power of the phone for interpretation of test results.134 This has also opened the door for other test enhancements such as providing step-by-step instructions, as well as automated result analysis and reporting to parties of interest. To this end, various partnerships are taking place. For example, BD is working with Scanwell Health to adapt their rapid test for at-home use.135 Our lab has previously demonstrated the utility of a smartphone application that in addition to guiding the user, can also use machine learning to automate the rapid interpretation of the INSTI Multiplex HIV-1/HIV-2/Syphilis Antibody Test.136,137 We have also recently developed a deep-learning approach that would facilitate rapid adaptation of the model to different line-based rapid test kits, and partnered with a company (Safe Health System) to incorporate this algorithm into a smartphone app that can interpret rapid antigen tests within a telemedicine platform. We will further discuss these developments in Section 2.2: Data Analytics.
Commercial Case Studies
Here we discuss three commercial POC SARS-CoV-2 antigen tests that highlight novel assay chemistry, microfluidics, or instrumentation (a larger overview of antigen tests is provided in Table 1 and Table 3). Performance data collected from company EUA documentation is summarized in Supplementary Table 2. Emphasizing the push towards POC use and also home use, we discuss user steps and the design of technological elements that enable a streamlined workflow.
Table 3:
Selected POC diagnostics for SARS-CoV-2 antigen detection
| Company | Product | Applications beyond SARS-CoV-2 | Technology highlight for POC use | Authorization | Year company founded |
|---|---|---|---|---|---|
| BD | Veritor Plus | Influenza A/B, Group A Strep, RSV | Portable reader, LFA | FDA EUA - POC | 1897 |
| Quidel | Sofia 2 | Influenza, RSV, Strep A Lyme, hCG, Legionella, Vitamin D | Portable reader, fluorescence LFA | FDA EUA - POC | 1981 |
| Ellume | COVID-19 Home Test | Influenza, TB | Disposable, connected reader for fluorescence LFA | FDA EUA - POC/Home/OTC | 2010 |
| LumiraDx* | SARS-CoV-2 Ag Test | D-dimer, INR | Microfluidic test strip and portable reader | FDA EUA-POC | 2014 |
| Abbott | BinaxNOW | Influenza A/B, RSV, Malaria | Low-cost, no reader required | FDA EUA - POC/Home/OTC | 1991 |
| Luminostics | ClipCOVID | N/A | Smartphone adapter for LFA readout | FDA EUA -POC | 2014 |
| Nanomix* | Elab | Sepsis | Handheld reader, carbon nanotube detection | EUA request under review | 2000 |
| Maxim Biomedical | SARS-CoV-2 Rapid Antigen Test | HIV | Visual readout, no reader required | Under development | 2005 |
| Hememics* | ChipLab | Hospital acquired infections (HAIs) | Portable, graphene based multiplex sensor (17 targets) with 1 min TAT | Under development | 2009 |
| Orasure | COVID-19 rapid antigen test | Influenza A/B, HCV, HIV, Ebola | Saliva sample | EUA request under review | 1987 |
Developing an antigen and antibody test for SARS-CoV-2
Ellume’s COVID-19 Home Test (Figure 2D) was the first OTC, home-use test granted EUA by the FDA.138 The test uses a standard lateral flow format, but incorporates quantum dot fluorescent nanoparticles along with a disposable, battery-powered, smartphone connected reader (Analyzer).139 A mobile application relays step-by-step instruction to a user, and allows a user to view results. The results can then be shared with healthcare providers and is reported in real time to public health authorities for disease mapping. The first step in running the test is adding the processing fluid containing a fluorophore to the dropper. After collecting a patient sample, the nasal swab is clicked-in with the dropper to release the viral antigens into the processing fluid, where fluorophores bind to viral nucleocapsid protein in the sample. A few drops of this liquid containing the fluorophore-labelled antigen complexes are added to the sample port of the Analyzer, where it is wicked into the test strip via capillary action in the LFA. The LFA contains immobilized antibodies specific to SARS-CoV-2 nucleocapsid to capture the fluorophore labelled antigen complex on the membrane. Fluorescence intensity is detected via a disposable, inexpensive optoelectronic reader within the Analyzer. The fluorescence intensity is read, then interpreted with a microprocessor that sends the results to the connected smartphone. After the sample is added, the entire procedure takes 15 minutes to generate a result.
Luminostics Clip COVID Rapid Antigen Test (Figure 2E) uses a LFA format along with strontium aluminate persistent luminescent nanoparticles (PLNPs) that provide a long- lasting glow following excitation, and can be both excited and imaged with a smartphone camera.140–143 This test first involves inserting a cartridge (containing the LFA strip and sample well) into the Clip Analyzer, which consists of an Apple iPhone, a battered-powered adaptor around the phone, and the ‘Clip COVID’ mobile application to run the test and provide a user interface. To run the test, an anterior nasal sample is collected and mixed with buffer in the provided “Extraction Tube.” This tube is then capped with the provided dropper tip and all of the antigen-buffer mix is dispensed onto the sample well of the cartridge. This sample flows through the LFA test strip via capillary action, where SARS-CoV-2 nucleocapsid protein is captured and labeled with PLNPs. The iPhone camera flash is used to briefly excite the nanoparticles and the camera lens captures images of the nanoparticle luminescence associated with the presence of the antigen. Here, usage of the time-gating imaging technique eliminates the need for expensive optical filters and light sources on the Analyzer, reducing the cost of the device. The captured image is analyzed by the mobile application using artificial intelligence and test results are displayed on the application screen within thirty minutes.144
LumiraDx’s SARS-CoV-2 Ag Test (Figure 2F) uses a microfluidic “test-strip” to conduct an immunofluorescence assay with a portable, connected instrument. The instrument contains an RFID strip code reader to calibrate lots, electronics to control fluid movement, optics for fluorescence measurement, and a touch screen to run the test and view results.145 The instrument can be run in three different modes depending on the connectivity requirements: 1) standalone, 2) managed (one or more devices connected to Connect Manager application via hub or smartphone), or 3) EHR connected (for transfer of patient results).146 To run the test, an anterior nasal or NP swab sample is collected and eluted in an extraction buffer. A single drop of this sample-buffer mix is added to the test strip via the provided vial dropper. SARS-CoV-2 specific antibodies are used to capture the nucleocapsid protein and a magnetic field is applied, which causes magnetic particles associated with the formed antigen-antibody immune complexes to be retained, and the rest of the unbound sample to be removed from the measurement zone. This allows the instrument to measure the immune complexes labeled with fluorescent latex particles in a dry state,147 with their fluorescence proportional to the concentration of antigen particles in the sample.115 Test results are displayed on the instrument screen within twelve minutes.
2.1.3. Antibody Tests
Overview
Antibody tests, or serology tests, detect antibodies produced by the body’s adaptive immune response, and indicate a prior infection. As is the case for many infectious diseases, infection with SARS-CoV-2 leads to sustained antibody levels.148–150 Typically, antibodies are measured by conducting an immunoassay on blood. The gold-standard laboratory method is the enzyme-linked immunosorbent assay (ELISA). Indeed, laboratory-based ELISA for detecting antibodies against SARS-CoV-2 immunogens are highly accurate.151,152
For SARS-CoV-2, detection of antibodies is a valuable public health tool as it can identify prior infected asymptomatic cases and therefore better quantify total case numbers.153 Nevertheless, although detection of antibodies is a routine method for assessing health status and immunity status for many infectious diseases, it has not yet been widely deployed during the COVID-19 pandemic. Early in the pandemic, due to the urgency of the crisis and strong precedence and acceptance of antibody tests for many infectious diseases, the FDA loosened regulations for developers to offer SARS-CoV-2 rapid antibody tests to healthcare workers with only self-reported results.154 Many LFA tests were offered in the market, some of dubious quality, before the regulation was changed.154,155
Assay Chemistry
Rapid antibody tests use one of three immune sandwich strategies to create a colorimetric signal on the paper substrate.156 As many tests differentiate between IgG and IgM response, anti-human IgG and IgM antibodies are used as capture reagents, and SARS-CoV-2 proteins (spike or nucleocapsid) are conjugated to a reporter (e.g., gold or latex nanoparticles) visible to the naked eye. The second strategy uses SARS-CoV-2 antigens as a capture, and conjugated anti-human antibodies as the reporter. This strategy allows for detection of total antibodies against SARS-CoV-2, unless different color markers are used for different antibody isotypes. A third strategy of a double antigen immunosandwich has also used for detection of total antibodies (see section on NOWDiagnostics’s ADEXUSDx COVID-19 Test).
The initial accelerated development of antibody lateral flow tests157 was made possible via utilization of off-the-shelf components as well as relaxed regulatory guidance (Section 2.4 and Figure 5A). Polyclonal human antibodies can be purchased with and without conjugation to common reporters like AuNPs from a variety of vendors. The major additional step in the development process is the purification of SARS-CoV-2 proteins (nucleocapsid, and spike) which was done at groundbreaking speeds by companies and laboratories across the country. In fact, by the end of March 2020, 37 companies had already informed the FDA of an introduction of a serology test onto the market.154
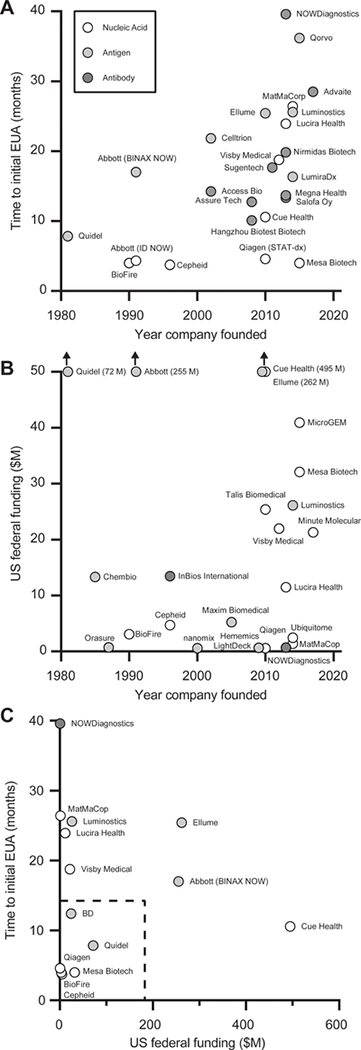
Figure 5. Unofficial chart demonstrating relationships between company history, government funding (NIH, BARDA, DoD), and time to first EUA.
A) Year of company founding versus time (months) to company’s initial FDA EUA from declaration of public health emergency on February 4th, 2020. B) Year of company founding versus US Federal funding in millions of dollars C) US Federal funding versus time to initial FDA EUA from declaration of public health emergency. Note: The date of company founding for Abbott uses the founding date of Alere, which originally developed the POC technologies (BinaxNOW and ID NOW) and was acquired by Abbott in 2017. See ESI for more information on methods for data compilation and Supplementary Table 3 for specific tests included in the analysis.
An important goal of antibody tests was to guide decisions on reopening society safely by determining immunity to SARS-CoV-2. However, at the onset of the pandemic, rapid antibody tests suffered from poor performance.157 Adding to the confusion, it was not yet established, as it is now, that prior infection leads to sustained antibody levels,148–150 and that prior infection leads to immunity.158 Now that we now know the detection of antibodies indicates prior infection and hence immunity, detection of neutralizing antibodies (Nabs) can further shed light on the level of protective immunity achieved in subjects.159 Nabs target the SARS-CoV-2 spike trimer and have the ability to block the virus from binding to the human angiotensin converting enzyme (hACE2) receptor, thereby inhibiting infection.160,161 Traditionally, Nabs are detected with virus neutralization assays, using live virus or pseudovirus. However, these involve complex, time-consuming procedures that can require BSL-3 clearance if using live virus.162,163 A simpler assay that mimics the interaction between the virus and human cells using purified receptor binding domain (RBD) protein and ACE2 was developed. In November 2020, the test, called the cPass SARS-CoV-2 Neutralization Antibody Detection Kit, received EUA as the first test to detect Nabs for SARS-Cov-2.164 In this assay, the test signal is inversely proportional to the concentration of Nabs, as any Nabs present in the sample will prevent labeled RBD proteins from binding to immobilized ACE2 in the assay. There have also been efforts to transport this assay to a POC format by using a lateral flow format and AuNPs for a colorimetric readout. This includes tests developed by multiple companies,165–168 which are marketed as inexpensive and rapid methods to track the effectiveness of vaccines. However, it is important to note that immunity can still be achieved through memory B- and T-cells without high levels of neutralizing antibodies.169,170
As in rapid antigen tests, developers of rapid antibody tests experimented with novel reporter molecules to improve sensitivity. While a majority of the commercial rapid antibody tests use standard reporter molecules (e.g., AuNPs), researchers have demonstrated other novel reporters in literature such as selenium nanoparticles,171 or gold nanoshells.172
Microfluidics
The format for fluidics and assay integration for rapid antibody tests is similar to that of rapid antigen tests, with most based on LFAs. Before the pandemic, LFAs had been extensively used around the world for antibody testing, including for HIV and malaria. While there have been developments of plastic microfluidic devices that can allow for multiplexing,113,173 the LFA has remained the dominant commercial platform. Additional fluidic innovations for paper-based assays were investigated, including an electrochemical platform,174 paper-based ELISA175,176, and a vertical flow format.177
Given the fact that antigen and antibody tests are typically identified with an immunoassay, microfluidic platforms designed for either can easily be adjusted for antigen or antibody detection by the selection of new capture and detection reagents. Multiple companies have demonstrated this ability by developing both rapid antibody and rapid antigen tests (e.g., LumiraDx, LightDeck). For antibody detection, the differentiating factor is the sample type needed as rapid antigen tests require respiratory samples and rapid antibody tests largely require whole blood or serum samples.178 To use whole blood, a plasma separation membrane is incorporated to trap red blood cells that would otherwise interfere with visual detection.179
For POC use, rapid antibody tests typically use a fingerstick whole blood sample180 with SARS-CoV-2 tests typically requiring a 10 μL sample.181 The fingerstick method offers a quick, less painful alternative to a venous blood draw which needs to be done by a trained technician. While the method opens the door for at-home collection and testing of blood samples,182 the procedure itself can be prone to errors, produce variable sample volume and sample contents.183–185 In fact, studies on at-home HIV testing have shown users have difficulty with blood sampling.186 Usability studies conducted in the United Kingdom for at-home testing of SARS-CoV-2 antibodies also demonstrated users having difficulty with sample collection.187,188
Prior to the pandemic, researchers have been developing alternative capillary blood collection methods. For example, Seventh Sense Biosystems (now rebranded as “Your Bio”) created the TAP device that uses an array of microneedles and a vacuum to collect blood from the upper arm area.189 While the current design collected blood to be sent to a laboratory, the company has had discussions with test developers on combining their method with POC devices.183 Another company Tasso, which recently received EUA as an at-home blood collection device for SARS-CoV-2 antibody testing, also uses microneedle technology.190 While there are numerous research articles on alternative blood collection methods for rapid tests,191–193 almost all rapid antibody tests currently approved for SARS-CoV-2 use a basic lancet and capillary for blood collection. An exception is a platform from NOWdiagnostics that incorporates a capillary into the test device, only requiring a separate lancet for blood collection. Additionally, the test does not require additional application of buffer, thereby minimizing the number of external components required for blood collection and potentially simplifying the sample collection process. Nonetheless, the area of blood microsampling for at-home testing is a field ready for innovation.
Moreover, as an alternative to invasive blood sampling, recent studies have also demonstrated the ability to detect antibodies against SARS-CoV-2 in saliva samples.194–196. Saliva sampling provides a non-invasive collection method as opposed to blood collection, simplifying the process.5 While there are no current COVID-19 antibody tests using saliva samples that have received EUA, it is being investigated197,198 as a method to greatly expand serosurveillance.
Connected Instrument
When the first rapid antibody tests were released for SARS-CoV-2, many were traditional visual read LFAs that required user interpretation. An example of such a test includes the Assure COVID-19 IgG/IgM Rapid Test from Assure Tech which detects both anti-nucleocapsid and anti-spike antibodies, and uses a visual readout with AuNPs.199 Another example is the ACON SARS-CoV-2 IgG/IgM Rapid Test, which also uses a visual readout with AuNPs, creating a red band. However, visually interpreting LFAs can be subjective, especially at low analyte levels where bands can be difficult to distinguish.188 This problem is compounded with use in decentralized settings, where users are likely untrained in device operation. Therefore, there have been efforts to automate interpretation of these LFAs using image processing algorithms on smartphones. Adding to our previous discussion on this topic for rapid antigen testing, we discuss two more examples here for rapid antibody testing. Abingdon Health has developed a reader, which they offer as a contract service to be adapted to any LFA.200 The app uses image processing technology to generate data on visual test lines, provides a user interface on the phone, and a data management hub. Another company, BBI solutions, markets their Novarum technology which also turns any smartphone into a mobile diagnostic platform. The app provides functionality for pre, during and post scan workflow, with the functionalities described in more detail here.201
Commercial Case Studies
Here we discuss three commercial POC SARS-CoV-2 antibody tests that highlight novel assay chemistry, microfluidics, or instrumentation (a larger overview of antibody tests is provided in Table 1 and Table 4). Emphasizing the push towards POC use and also home use, we discuss user steps and the design of technological elements that enable a streamlined workflow.
Table 4:
Selected POC diagnostics for SARS-CoV-2 antibody detection
| Company | Product | Applications beyond SARS-CoV-2 | Technology highlight for POC use | Authorization | Year company founded |
|---|---|---|---|---|---|
| InBios International* | SCov-2 Ab Detect Rapid Test | Infectious disease | Lateral flow | Under development | 1996 |
| NOWDiagnostics | ADEXUSDx COVID-19 Test | HIV, troponin, hcG, Acetaminophen, H-FABP, Methanol Dip, Salicylate | Integrated capillary for blood collection | FDA EUA - POC | 2014 |
| LightDeck* | COVID-19 Antibody Test | Hormones, Host Response, Cardiac Markers, Water Testing, Veterinary | Planar waveguide technology with disposable test cartridges | Under development | 2009 |
| AssureTech** | COVID-19 IgG/IgM Rapid Test | Cardiac, pregnancy, infectious disease, tumor, drugs of abuse allergy | Lateral flow | FDA EUA - POC | 2008 |
| JoysBio** | COVID-19 Neutralizing Antibody Test Kit | Cardiac, pregnancy, infectious disease, tumor, drugs of abuse | Neutralizing antibody LFA | CE-IVD | 2010 |
| Nirmidas Biotech** | Midaspot COVID-19 Antibody Combo Detection Kit | N/A | Lateral flow | FDA EUA - POC | 2013 |
| QIAGEN** (with Ellume) | QIAreach Anti-SARS-CoV-2 Total Test | TB | Estick technology with ehub to run multiple tests | FDA EUA | 1984 |
Developing an antigen and antibody test for SARS-CoV-2
Did not receive US Federal support
AssureTech’s Assure COVID-19 IgG/IgM Rapid Test Device
(Figure 2G) was the first antibody test for COVID-19 to receive an EUA for POC use, on September 23, 2020.199 It is a lateral flow immunoassay that detects antibodies against the nucleocapsid and spike (S1) proteins in fingerstick whole blood. Immobilized on the test strip’s nitrocellulose membrane is anti-human IgM (IgM Test Line), anti-human IgG (IgG Test Line), and goat anti-mouse IgG (Control Line). The conjugate pad contains recombinant SARS-CoV-2 antigen (nucleocapsid and spike (S1 protein) conjugated with AuNPs. Once the whole blood sample is added to the sample port, along with running buffer, SARS-CoV-2 specific antibodies in the sample bind with the gold conjugates and if anti-SARS-CoV-2 IgM and/or IgG antibodies are present, they will form immune complexes at the respective test lines, generating a red, visual band. If the membrane properly wicks, the control line will change from blue to red. A test result is generated in 15 minutes, after which an operator visually interprets the test bands.199
NOWDiagnostics’s ADEXUSDx COVID-19 Test
(Figure 2H) is a double antigen sandwich lateral flow immunoassay that detects total antibodies against SARS-CoV-2 in fingerstick whole blood samples.202 Of note, the test does not require additional reagents, equipment, or buffers, unlike previously described LFA tests.203 The test uses microfluidics to wick blood through the sample application zone and onto the LFA.204 Here, the sample is first wicked through a plasma separation membrane, to remove red blood cells. The membrane also contains dried colloidal gold conjugated to recombinant SARS-CoV-2 S1 receptor binding domain (RBD) antigen as well as colloidal gold conjugated to rabbit IgG. Antibodies against SARS-CoV-2 in the sample bind to gold labeled RBD antigen, which is captured downstream by immobilized S1 RBD antigen at the test line. A red, visual line indicates a detectable level of anti-SARS-CoV-2 antibodies. The gold labeled Rabbit IgG will bind to polyclonal anti-rabbit IgG forming a visual control line if proper wicking is achieved. Results can be seen in about 15 minutes, after which a user visually interprets the bands. While not included in the FDA EUA, the company has also developed the ADEXUSDx analyzer, a portable handheld instrument to automate result interpretation, and is developing the DxREADER, which is a more portable reader that connects to a smartphone.205
JoysBio’s SARS-CoV-2 IgG/Neutralizing Antibody Rapid Test Kit
(Figure 2I) is a CE-marked lateral flow test kit that detects both IgG and Nabs against SARS-CoV-2 in fingerstick whole blood samples.167 The test kit contains SARS-CoV-2 recombinant antigen (RBD and nucleocapsid), and Chicken IgY labeled with colloidal gold. The nitrocellulose membrane is immobilized with mouse anti-human IgG (test line 1), hACE2 (test line 2), and goat anti-chicken IgY (control line). To detect Nabs, the assay mimics the virus neutralization process; when a sample is added to the test, Nabs in the sample bind to RBD labeled with colloidal gold and block the interaction between RBD and hACE2 at test line 2. Non-neutralizing antibodies against SARS-CoV-2 also bind to RBD labeled with gold, and this complex along with unbound gold is also captured at test line 2. The intensity of the test line is inversely proportional to the level of RBD specific Nabs antibodies (i.e., a faint line indicate high levels of Nabs). Next, gold labeled, non-neutralizing antibodies against SARS-CoV-2 are captured at test line 1 by mouse anti-human IgG. Finally, chicken IgG labeled with colloidal gold binds to anti-chicken IgY immobilized at the control line. Between 25–30 min after sample addition, the results are read with the first band corresponding to Nabs, the second to IgG antibody, and the third for the control line.
2.1.4. Emerging Technologies
Face Mask
The SARS-CoV-2 pandemic has spurred a rethinking of how to rapidly diagnose infectious diseases.3 A unique development has been widespread use of face coverings to reduce transmission of the virus, which has prompted researchers to pursue virus detection masks. For example, SARS-CoV-2 protease-detecting test strips can be attached to N95, surgical or cloth masks.206 If the user is infected, proteases specific to SARS-CoV-2 (Mpro and PLP, which are required for virus replication) are exhaled in the breath and accumulate in the test strip. After the period of wear, the user squeezes a blister pack containing nanoparticles that change color in the presence of the SARS-CoV-2 proteases, allowing visual indication of infection and a positive control. The test strips can be mass produced via roll-to-roll processing, allowing low cost for daily use. Another mask-attachable visual test integrates a freeze-dried diagnostic COVID-19 test that is activated by a blister back of water, based on a platform to detect RNA in exhaled breath.207
Breathalyzer
Breathalyzers that can detect volatile molecules from a patient’s exhaled breath had been in development before the pandemic. For SARS-CoV-2, a device has been tested in Netherlands that analyzes volatile chemical composition of exhaled breath using seven metal oxide semiconductor sensors, and used for screening purposes by the Dutch government before use was halted due to insufficient sensitivity.208 Another device, from developers in China, uses a nanomaterial-based sensor array with multiplexed capabilities to detect and monitor COVID-19. When conjugated AuNPs in the device bind to volatile organic compounds in exhaled breath, the nanoparticle film swells or shrinks, causing changes in the electric resistance which can be interpreted for disease detection.209 The reported sensitivity and specificity for current breathalyzer tests are over 90%, but the devices generally need to be validated independently and on larger sample sizes before commercial use.
T-cell Test
Following infection or vaccination, in addition to the humoral response generated by B cells, there is a cell-mediated response primarily carried out by T cells.210 Before SARS-CoV-2, an application of T cell counting was the measurement of total CD4+ T cells in HIV patients to identify virally-suppressed immune systems, and have even been developed by commercial entities (e.g. BD FACSPresto or Abbott PIMA) for low-resource areas.211–214 However, it is technologically more challenging to measure virus-specific T cells. Traditional procedures to do so (i.e., ELISpot or intracellular cytokine staining) are time consuming and lack sensitivity.215 With renewed interest in understanding the role of T cell immunity in fighting SARS-CoV-2 and how they provide resistance to reinfection, several companies have developed alternative protocols. This includes a test from Adaptive Biotechnologies which recently received FDA EUA, becoming the first test to detect T cells.216 Towards POC use, a skin test for SARS-CoV-2 T cells, akin to a TB skin test, is in development; the assay uses synthetic peptides to generate an immune response at the dermal layer, with the measurement of the raised region on the skin roughly equating to the amount of T cell immunity.217,218 A rapid T cell test using a cytokine release assay is also being developed.219–221 Overall, POC T cell tests222 could complement antibody testing for identifying past infections via identification of cell-mediated immunity (especially with waning levels of humoral immunity).222
2.2. Data Analytics
The Coronavirus Aid, Relief, and Economic Security (CARES) act requires “every laboratory that performs or analyzes a test that is intended to detect SARS-CoV-2 or to diagnose a possible case of COVID-19” to report the results from each such test to the Secretary of the Department of Health and Human Services (HHS).”223 This guideline includes all facilities performing POC tests or tests utilizing at-home specimen collection. HHS also outlines the methods for submission (including to state or local public health departments) and required data elements in the guidance document.223
However, the increase use of POC testing conducted outside of traditional healthcare settings, presents challenges to this reporting model. According to HHS, “more FDA-authorized rapid diagnostics, such as point-of-care, over-the-counter, and at-home tests, are increasingly being used but often lack an easy way for users, such as schools, nursing homes, or businesses, to report results.” For example, in an initial pilot study of a rapid antigen test in schools, reporting needs required significant staff time and expertise.224
The HHS guideline document223 suggests decentralized test results can be reported via applications on smartphones or tablets, a patient portal, or direct transmission from the test itself. Some test developers have taken this on. While custom smartphone apps are being built to connect rapid test results to health authorities,225 HSS has launched a “COVID-19 At-Anywhere Diagnostic Design-a-thon” to encourage development of additional digital tools to enable automated data capture, transmission, and analysis.226
Solutions included a platform from Oracle, which allows submission of test results directly from mobile apps, test manufacturers, or administrator networks and improves the confidentiality, integrity, and immutability of test reporting using a blockchain platform.227 Another solution includes a blockchain network to collect data from patients using OTC tests, and scan devices for authentication,228 and information-capture methods (i.e., smartphone apps, web-based apps, automated phone line attendants, and self-service kiosks) with real-time data analysis.229
A further design sprint (“COVID-19 TOPx Tech Sprint”) poses the problem statement as “1) help state and local public health authorities track and understand the virus in populations and communities, 2) help stakeholders outside of healthcare make key operational decision and 3) help consumers and business manage point-of-care testing data outside lab settings.”230 Hence, in addition to reporting results, there is also a need to aggregate and analyze data from testing done outside of laboratories. While custom solutions are being developed to provide organizations the ability to manage their testing programs by viewing aggregated results,231 digital solutions that span testing platforms may be most effective. Another possible solution is to provide connectivity to third-party platforms that aggregate other medical records. For example, the company CLEAR, which developed a mobile technology to link personal information to biometric data, has created a Health Pass to store medical information that includes test results and vaccine status,232,233 and has partnered with the National Basketball Association234,235 to enable health screening and connect to a software platform from a POC rapid test.236 This third-party integration allows fans who conduct testing at home to verify their test result and enter the arena.
At-home POC testing will pose another challenge for how patient data and test results can be collected and reported, given their importance to public health efforts.223 Therefore, developers of new POC tests will need to consider various solutions for data reporting, and if they would like to develop an in-house solution, or partner with another company. Additionally, considerations will be need to be made regarding connectivity to other mobile technologies that look to aggregate various other medical information. This has implications for downstream regulatory approval as it of interest to the FDA for authorizing OTC tests. For example, in granting Lucira Health authorization for OTC use, the FDA stipulated that the company must develop “a mobile phone application or website to further facilitate results reporting by both the healthcare provider and the individual using your product”,237 prompting them to release LUCI pass. Future OTC approvals may require solutions for data reporting and integration.
2.3. Clinical Workflow
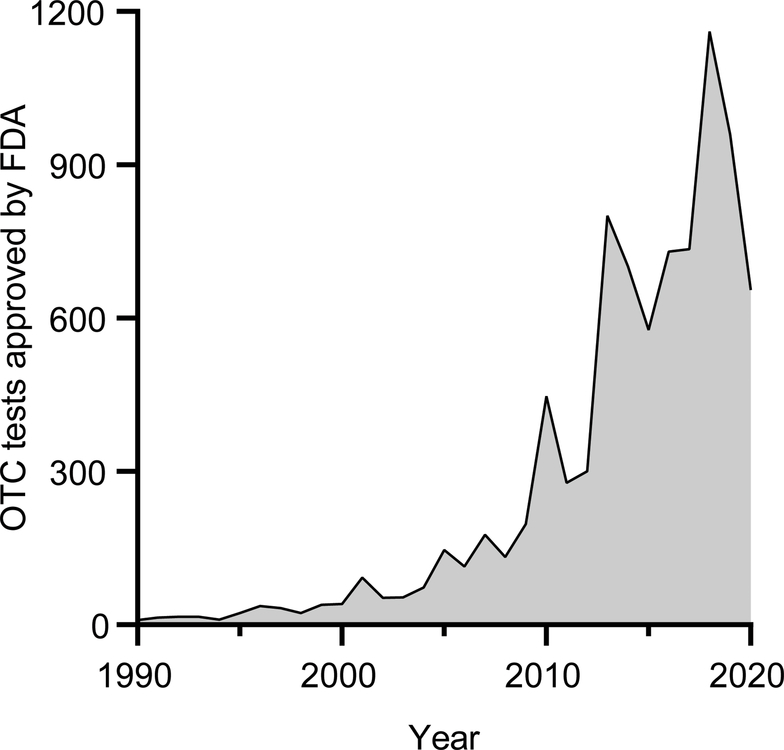
For years before the pandemic, POC diagnostics had begun to be widely implemented in decentralized healthcare settings, with increased use and approval by healthcare professionals,2 and also by consumers at a slow but steadily growing pace. For example, an increase in OTC tests approved by the FDA (Figure 3) reflect an increased acceptance and interest in such tests by both consumers and the FDA. However, most OTC tests focus on testing for drugs or chronic conditions like diabetes or cardiovascular health, with the only infectious disease test approved for home use and available OTC being the Oraquick HIV test kit.238
Figure 3. Timeline of OTC tests approved by the FDA from 1990 to 2020.
Data from FDA’s OTC database
The COVID-19 pandemic resulted in an acceleration in the authorization of tests for POC and OTC use. In the early stages of the pandemic, many areas in the US experienced a shortage of testing options and resources, including reagents and testing infrastructure,1 leading to increased transmission rates, hospitalizations, and deaths. POC diagnostics presented a viable solution to fulfill the need for increased testing by increasing accessibility, including in pharmacies, urgent care clinics, hospitals, and mobile testing sites set up by the federal, state, or local governments (we will discuss use cases in Section 3 in greater depth).
Towards at-home testing, the first set of tests were sample collection kits that could be mailed to homes but required mailing the samples back to a lab. LabCorp offered the first at-home collection kit that garnered FDA EUA, to self-collect a nasal swab without a prescription.239 On December 15, 2020, the FDA granted EUA for Ellume’s home test, the first OTC, fully at-home test for COVID-19.138 This movement of accessible, and at-home testing continues to headway as self-administered tests, such as Ellume’s and Abbott’s antigen tests, become readily available in pharmacies.240 Now, molecular diagnostics tests are available for OTC use (Lucira and Cue Health) with Lucira’s test available for purchase online.241 With a number of OTC testing options available, consumers are able to obtain reasonably accurate COVID-19 tests from the privacy, convenience, and safety of their own homes.
2.4. Regulatory Guidance
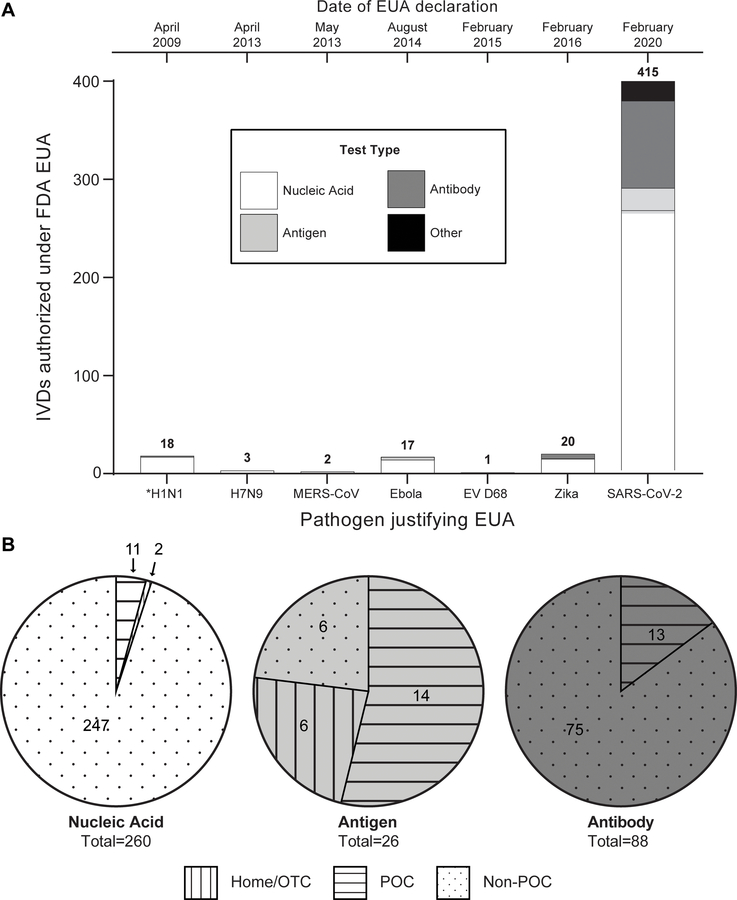
On February 4th, 2020, the Secretary of HHS declared a public health emergency due to SARS-CoV-2, which under section 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), allows the FDA to “emergency use authorize” unapproved drugs, devices, or biological products.242 The minimum requirement for EUA is that the known and potential benefits outweigh the potential risk, even if there is not yet enough evidence to fully establish its safety and effectiveness. EUA is a relatively new concept for the FDA, with the first EUA granted in 2009 during the H1N1 pandemic. In addition to SARS-CoV-2, there are current EUAs in place for Ebola, Enterovirus D68 (EV-D68), H7N9 Influenza, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and Zika virus (Figure 4A, which further summarizes the number of IVDs that have received EUA for each public health emergency by assay type).
Figure 4. Overview of IVD’s that have received FDA EUA.
A) Total number of IVD’s issued EUA by the FDA during previous declarations of the EUA pathway. Note: IVD’s that have had their EUA revoked are included in the analysis, and all listed pathogens here have current EUAs except H1N1 (designated with *). B) Breakdown of current SARS-CoV-2 IVDs that have received EUA by assay type (nucleic acid, antigen, antibody) and authorized setting to run the test. See ESI for more information on methods for data compilation.
EUA enabled SARS-CoV-2 diagnostic tests to be developed, validated, and deployed in weeks rather than the many months or years it traditionally takes.243 To facilitate submission, the FDA put together templates for various types of SARS-CoV-2 tests (e.g., molecular diagnostics, antigen test. antibody tests).243 Developers can also submit a pre-EUA to begin discussions with the FDA and gain guidance on their submissions. Example metrics include LOD, inclusivity, cross-reactivity, and clinical evaluation.244 Such changes to the regulatory environment played a role (among others) in the vast increase in number of IVDs EUA compared to past public health emergencies (Figure 4A). Further, Figure 4B stratifies the SARS-CoV-2 tests that obtained EUA by its authorized setting of laboratory, POC, or OTC use.
Early in the pandemic, the FDA recognized the need for rapid antibody testing as a means to better understand COVID-19 from a scientific perspective and inform the government response.154 On March 16, 2020, the FDA published guidance to facilitate access to these tests and began allowing developers to market serological tests without EUA as long as the test was validated, the FDA was notified, and test reports included limitations. Soon, the market was flooded with serology tests, and by the end of April 2020, the FDA had received 164 notifications for serological test use without EUA, many of which were eventually shown to perform poorly. As of Feb 1st, 2021 the FDA had removed 225 listings, issued 15 warning letters, and placed 88 firms on import alert for violations of misused serological test kits and false claims.154 During this time, the FDA began working with the NIH, CDC, the Biomedical Advanced Research and Development Authority (BARDA), and the National Cancer Institute (NCI) to order to establish the capacity to evaluate serology tests independently, the first time the federal government has performed evaluations of FDA authorized tests itself.154
Of potential interest to POC test developers, the Director of the Center for Devices and Radiological Health (CDRH) at the FDA outlined the lessons learned from the EUA process for SARS-CoV-2 diagnostics, for molecular243 and serology testing.154 Following the experiment with antibody testing, he stated that the FDA would not repeat the guidance allowing for antibody tests to enter the market before review. The perspectives also suggest it would be more effective in the next public health emergency, to focus development and validation efforts on a few tests, instead of scores of tests (Figure 4) in order to efficiently use resources. Finally, what would have implications even outside of a public health emergency, the FDA recognized the need for a federal government agency or group on its behalf have the capacity to independently evaluate tests. By streamlining the validation of tests, this will provide a common frame of reference to compare test performance, minimize the need for developers to find clinical specimens, and overall expedite the approval process.
The loosening or strengthening of regulatory requirements by the FDA has been a continuing act to balance risks and benefits in authorizing the use of POC tests. Authorization of diagnostic tests without exemplary evidence of effectiveness carries risk in potential false positive and false negative diagnoses. This is especially harmful in the case of false negatives (low sensitivity), which may lead to potentially contagious individuals not self-quarantining due to false assurance from a negative test result. However, in a public health emergency, the potential benefits of alleviating overcrowded hospitals, reducing hospitalization and transmission rates, and ultimately saving lives through more accessible diagnostic tests may be worth the potential risk.
2.5. Reimbursement
A challenge in the deployment of POC tests has been the payor for the tests – whether it is the insurer or consumer, and eligibility requirements for insurance coverage. For the pandemic, these questions were addressed in a swift manner to enable rapid deployment. On March 18, 2020, the Families First Coronavirus Response Act (FFCRA) was passed by Congress, mandating insurers to provide diagnostic test coverage for detecting SARS-CoV-2 without imposing any cost-sharing requirements. The mandate included deductibles, copayments, coinsurance, prior authorization, or other medical management requirements.245 Testing coverage also applied to those who do not exhibit symptoms or suspect exposure to coronavirus.246 Soon afterwards, the CARES Act enacted on March 27, 2020 further expanded the range of diagnostic items and services that were covered. Additionally, due to the Health Resources & Services Administration (HRSA) COVID-19 Uninsured Program, healthcare providers can submit claims for reimbursement to HRSA when providing COVID-19-related services to uninsured patients247 (which include specimen collection, antigen and antibody testing, which can be done in an office, urgent care, emergency room, or telehealth setting248), but the patient is expected to cover the cost if the healthcare provider does not submit a claim to HRSA.249 For instance, companies providing at-home COVID-19 collection kits (e.g., LetsGetChecked) will send a receipt to the patient who will in turn seek reimbursement from their insurer.
The situation is different for OTC use. For example, for rapid antigen tests, individuals can try to bill OTC test costs to insurers,246 but many insurers may have restrictions such as requiring testing to be conducted by a healthcare professional.250 At the moment, many OTC tests for SARS-CoV-2 must be covered out of pocket or through a health Flexible Spending Account (FSA) or Health Reimbursement Account (HRA) which can be used to purchase any OTC test.251 The rise of OTC testing certainly brings up questions regarding reimbursement and whether insurers will be required to cover them going forward. Either tests will need to be priced low enough (and designed accordingly) so reimbursement is not needed, or they will need to convince insurers of their benefit with studies demonstrating their benefit for health outcomes.252 Nevertheless, current policy demonstrates consumers are willing to pay out of pocket for new POC tests if they perceive sufficient value.
2.6. Legislation
The severity of the pandemic called for immediate and massive investment by the government in technologies to help mitigate spread. One non-profit has tracked $5.93 trillion allocated to legislative efforts supporting responses to COVID-19.253 Of this amount, $53.9 billion has been for “testing, monitoring, and R&D” and $46.6 billion to “preparedness and response.”253 Some of this funding supported R&D efforts, which included the Rapid Acceleration of Diagnostics (RADx) initiative and funding by BARDA. The RADx initiative was launched by the NIH with an initial investment of $1.5 billion, and in partnership with the Office of the Assistant Secretary of Health, Department of Defense (DoD), BARDA, and the FDA on April 29th, 2020.254 The program sought technologies to enable accurate, fast, easy-to-use, and widely accessible testing, and is broken down into 4 programs. RADx Tech to focus on innovative POC and home-based tests, RADx-UP to focus on disparities and how to address them in underserved population, RADx Rad to support radical, non-traditional approaches, and RADx-ATP to increase capacity and throughput of more mature technologies. To date, 29 projects have progressed through multiple review stages in the RADx-Tech and RADx-ATP programs and it is estimated that the funding has contributed to increased capacity of 150 million tests while reducing the normal multiyear commercialization process to about six months.255 Additionally, BARDA itself has committed almost $14.5 billion to COVID-19 response, which includes diagnostics, therapeutics, and vaccines. While diagnostic funding only makes up about 1% of this number, this has brought in over $157 million dollars to aid in the development of numerous COVID-19 tests.256 In fact, the agency notes its support of 22 EUAs for SARS-CoV-2 diagnostic tests and aided in shipping over 121 million test kits as of May, 28th, 2021.257
To visualize the impact of this funding on market entry of POC technologies, segmented by the maturity of the technology prior to the pandemic, we tabulated all the POC diagnostic devices that have received U.S. federal support (along with some additional tests) (Figure 5). Looking at devices that received EUA, interestingly, no company that has received an EUA was founded after 2015 (Figure 5A); in fact, a larger trend was that the companies that initially garnered EUA approval were longer established companies, with more recently founded companies taking more time to obtain EUA (Figure 5A). In terms of funds disbursed to companies in general, most government investments targeted companies founded from 2010–2015 (Figure 5B), although Quidel and Abbott, two established players in the IVD industry, received large sums of money to increase their production capacities and supply more tests.258,259 The power of these investments in more recent technologies is further illustrated in that outside the established IVD players (outlined with a dashed box in Figure 5C), large government funding was correlated to short time to EUA. Thus, government funding potentially accelerated the commercialization of SARS-CoV-2 POC diagnostic devices, and allowed more recently developed technologies to receive EUA and be deployed to benefit the public.
2.7. Systems Integration
In the POC ecosystem (Figure 1) during the pandemic, how we consider the problem of “systems integration” has evolved. Previously, on the technology side, there has been an increasing realization that a successful POC diagnostic test must successfully integrate chemistry, fluidics, hardware, and software to ensure a “sample-to-result” workflow and seamless user experience.2,52,260,261 The pandemic has required developers to also think about how their POC test integrates with public health reporting efforts and integration with data analysis technologies. On the non-technology side, the different issues on clinical workflow, reimbursement, regulatory guidance, and legislation are important in determining a path to market and adoption by users, and are synergistic with the technological components. However, the nuances of each component are informed by specific use cases, as different POC use cases have vastly different constraints and requirements. In the next section, we will discuss how the POC ecosystem has developed for four POC use cases for SARS-CoV-2 diagnostics.
3. Use Cases for SARS-CoV-2 POC Diagnostics
For a POC device, it is clear that general advantages in speed, low cost, and simplicity while maintaining gold-standard performance are desirable,2,3 but different POC settings pose specific design constraints that must be considered during device development. In a previous review, we had outlined four different use cases for POC diagnostics, visualized in a 2x2 matrix based on available operating budget (low and moderate) and infrastructure (clinic and field setting).2 In this section, we apply this framework to use cases for SARS-CoV-2 testing at the POC (Figure 6). For each use case, a descriptive name is provided (Figure 6A), as well as an analogy with consumer electronics to aid the visualization (Figure 6B). First, the Premium Clinic use case (with the electronics analogy “Laptop”), is the least resource constrained, as it includes POC diagnostics used in clinical environments with relatively unrestricted costs (e.g., hospital emergency rooms, operating rooms, or intensive care units). Second, the Economy Clinic use case (with the electronics analogy “Tablet”) is also conducted in a clinical setting but constrained by cost considerations relative to the Premium Clinic use case (e.g., urgent care clinics, physician offices, primary care clinics). Third, the Premium Field use case (with the electronics analogy “Smartphone”) is constrained by portability considerations (in the field), but not cost (e.g., self-testing in high income countries, schools, businesses, airports, venues). Finally, the Economy Field use case (with the electronics analogy “Flip phone”) is the most constrained, as it includes tests used in field settings with a low operating costs (e.g., remote clinics, self-testing in LMICs, and global health applications). Keeping in mind these are use cases for POC diagnostics, in keeping with the consumer electronic analogy, complex diagnostics requiring laboratory infrastructure can be thought of as “Desktop computers”.
Figure 6: POC use cases, decoupling cost from infrastructure.
A) Graphical representation of uses cases in 2x2 grid, with each use case assigned a representative color, and icon describing example settings B) Graphical representation of use cases using consumer electronics analogy C) General description and main design considerations for each use case D) Examples of SARS-CoV-2 POC diagnostics ideal for each use case (based on technology). Adapted from reference 2 with permission from American Chemical Society. This figure has been designed using icons made by Freepik, Eucalyp, and xnimrodx from Flaticon.com.
For each use case, we will provide a description of the working environment and design considerations as see in the COVID-19 pandemic (Figure 6C), as well as provide examples of suitable technologies (Figure 6D). Additionally, we discuss how – as demand and utilization has increased for POC SARS-CoV-2 testing – testing volume has begun to shift from traditional POC testing sites in clinical settings (Premium Clinic and Economy Clinic) to decentralized field settings (Premium Field and Economy Field).
3.1. Premium Clinic
This use case encompasses settings with a moderate budget and clinical infrastructure. It is the least constrained use case for POC diagnostics, thereby easing design constraints and largely entails testing conducted at various parts of a hospital, like the emergency room, operating rooms, or ICU units. Here, trained personnel and resources such as electricity, controlled ambient conditions, and refrigeration are present supporting the operation of more complex and less portable instruments. Price is not a major consideration giving the flexibility to purchase more expensive instruments. Moreover, speed is not an important consideration, as patients will typically spend longer periods of time receiving care at these settings.
For SARS-CoV-2 POC molecular testing, this use case is ideal for larger, more expensive benchtop instruments (tens of thousands of dollars) that may require a degree of laboratory skills for operation. During the pandemic, hospitals needed to triage patients suspected for COVID-19 into separate areas.262 Some settings, like New York Presbyterian Hospital had already adopted industry standard POC molecular platforms like the Roche Cobas Liat, Cepheid GeneXpert, and BioFire FilmArray for other testing scenarios (e.g., Influenza), so they expanded testing on these platforms to quickly identify positive patients. These platforms, along with other near-patient molecular platforms have been evaluated across the country for use in various emergency, outpatient, and inpatient settings in hospitals.263,264 Since, molecular testing is typically accessible here, antigen testing is typically not needed; however, with the extreme scenarios caused by the pandemic, some hospitals have implemented their use. For example, NHS in England recommends the use of COVID-19 LFAs to supplement PCR testing in the emergency department.265 Finally, since antibody testing does not detect active infections, and rapid results are typically not needed, POC serology tests are not suited for this use case. Here, blood samples can be sent to a centralized laboratory for analysis.
In summary, the SARS-CoV-2 pandemic created an uptick in POC testing volumes in these settings. With hospitals purchasing more instruments to increase testing volumes, they have significantly expanded their testing capacities at the POC. Going forward, this may result in less testing being carried out in centralized laboratories and instead conducted in house.266 Additionally, rapid testing has been adopted in settings that typically wouldn’t use them, which could open up the possibility for more rapid screening at hospitals of other infectious diseases, thus preventing their spread.
3.2. Economy Clinic
This use case represents clinical settings that are mainly constrained by costs (relative to the Premium Clinic use case). Here, trained personnel, along with access to external resources such as electricity and refrigeration may be present to run tests. Therefore, portability, full integration, may not be as critical, but cost is a major consideration. Finally, considerations for speed are important in the context of the length of patient visits, which can range from minutes to hours.
For SARS-CoV-2 testing, this use case is exemplified with testing in decentralized healthcare settings (e.g., urgent care clinics, pharmacies, physician offices). For example, CVS minute clinics around the U.S. have been providing rapid antigen or antibody tests.267 Urgent care clinics like CityMD in NYC are also providing rapid testing on platforms like the BD Veritor plus. New clinics have also been set up, with the primary goal of providing COVID-19 testing. For example, NYC set up COVID Express sites around the city running the Cepheid GeneXpert Xpress platform.268
For molecular testing, platforms that were developed before the pandemic are particularly well suited for this use case (e.g., Abbott ID NOW or Mesa Biotech Accula). The instruments are relatively large and certain models can be low-cost enough (in the hundreds/thousands of dollars) to justify adoption in these settings. The key here is providing test results on site within a visit instead of sending samples to a centralized laboratory. For antigen testing, platforms like the Quidel Sofia 2 or BD Veritor plus are also well suited for this use case. The need for a benchtop reader limits portability, but they provide a low-cost, rapid solution to testing. In fact, before the COVID-19 pandemic many of these decentralized healthcare settings already used these platforms for testing for respiratory infections or sexual health infections. Like in the Premium Clinic use case, these settings have increased their testing capacity by purchasing new instruments or adopting novel technologies.
3.3. Premium Field
The Premium Field use case allows for higher costs, but is constrained by supporting infrastructure to operate the test. With testing conducted outside of clinical settings, skilled operators or specialized equipment may not be available. As a result, the test must be self-contained, simple to operate, and portable. Price is an important but not predominant consideration, but speed is critical in order to give results in a timely manner.
Before the pandemic, this use case was largely restricted to self-testing in high income countries such as diabetes patients testing for glucose. The SARS-CoV-2 pandemic opened up the possibility of self-testing at home for infectious diseases as well as introduced new settings for testing such as schools, businesses, airports, or event venues. The development and commercialization of new technology targeted at this use case as well as growing acceptance and demand for consumer testing options has allowed for widespread infectious disease testing for the first time. For example, the Golden State Warriors of the NBA are providing all ticketed fans a Lucira Check It COVID-19 Test Kit to be tested at home before home games.269 DoorDash launched a promotion to give restaurants discounted rapid antigen tests from Cellex (qSARS-CoV-2 Antigen Rapid Test) to screen employees and potentially customers.270 The Los Angeles International Airport is providing travelers the Visby Medical test for $199.271 Finally, England is providing citizens with rapid tests twice a week, which is expected to allow citizens to get tests delivered to their homes, workplaces, and schools.272
With Cue Health and Lucira Health test kits now available OTC, consumers are able to run a nucleic acid test in the comfort of their own homes. Given the complexity and costs associated with molecular testing, many thought this was impossible before the pandemic. Now with the capacity to do so, consumer molecular testing opens many future avenues such as conducting serial laboratory quality testing for other widespread infectious diseases (e.g., respiratory conditions or sexually transmitted infections) in place of lower performing antigen tests. Additionally, this technology could expand the use of genetic testing and accelerate the adoption of personalized medicine.273
3.4. Economy Field
The Economy Field use case is the most constrained for POC diagnostics. It is constrained both by cost considerations (relative to field testing) as well as surrounding infrastructure to run the test (i.e., no specialized personnel, electricity, temperature/humidity control, or refrigeration). Therefore, portability, speed, costs, and simplicity are all important design considerations that must be taken into account. Traditionally, this use case has been synonymous with global health, where testing is done in the field in low- and middle-income countries. Without reliable access to many resources, and a need for low-costs, paper diagnostics have shined here, particularly the LFA. The LFA has provided many patients access to both low-cost antigen and antibody testing for various infectious diseases (e.g., malaria, HIV, Ebola) and there have been also been various efforts to transport the simplicity of the LFA for nucleic acid detection, as demonstrated in various academic research papers.45,47 However, no such products have made it to commercialization.
In a way, the need for widespread COVID-19 testing has pushed this use case around the world. In order for individuals to conduct serial testing in any location it must be both easy to use, and cheap enough to justify adoption. For SARS-CoV-2 molecular testing, there currently is no developed solution that fits the criteria. Certain solutions like the Lucira Health test fit the technology needs, but the price is too high to be used in such scenarios ($55 a test). On other hand, rapid antigen and antibody testing is well suited for this use case. For example, the Abbott BinaxNOW test is a simple, low-cost solution and is currently being offered at pharmacies for ~$24 for 2 tests ($12 a test).274 Additionally, various global health organizations have partnered to offer millions of rapid tests from Abbott and SD biosensor in low- and middle-income countries. For example, the Bill & Melinda Gates Foundation reached agreements with these manufacturers to offers tests at a maximum of $5 per test.275 Note, the Abbott PanBio test uses the same assay chemistry as the BinaxNOW test, but is offered in a small cassette versus a card format and is authorized for use outside the U.S.276
With the COVID-19 pandemic spurring investment in low-cost POC diagnostics, various groups are looking to commercialize their molecular paper diagnostic technologies,277 including with CRISPR diagnostics.
3.5. Discussion of POC uses cases during pandemic
The pandemic has forced centralized healthcare and decentralized healthcare settings to expand testing capacity. Throughout the pandemic, POC tests were increasingly adopted (Figure 4B), but the trends in POC usage for the three classes of assays were different.
1) For nucleic acid tests (Figure 7), the clinic use cases are dominant, as this is where POC molecular testing was largely conducted before the pandemic. There has been some headway into developing tests for field settings (Premium Field), but there remains a need for a low-cost, portable nucleic acid testing option (Economy Field). As more nucleic acid tests are developed for home use, we expect prices to drop.
Figure 7: Breaking down the POC diagnostic platforms that have received FDA EUA by their use case.
Note: platforms with multiple tests approved (e.g., for SARS-CoV-2 and panel including Influenza/RSV) were only counted once in this analysis. Use case analysis was based on ideal fit for underlying technology with Supplementary Table 4 outlining the specific tests included in each category. Cepheid GeneXpert was counted for both centralized and decentralized healthcare due to various instrument sizes available.
2) For antigen tests, the opposite is true, with a majority of tests suited for Economy Field settings. The antigen testing use case is inherently driven by the lack of economical and portable options for nucleic acid testing, so the advent of more nucleic acid testing technologies directed at the Premium Field and Economy Field use cases could pose significant competition to rapid antigen tests. However, further developments in signal readout methods for antigen tests, that improve sensitivity but maintain the simplicity of LFAs, could help maintain the popularity of LFA formats in field settings.
3) Finally, antibody tests are almost all geared towards Economy Field settings with the use of LFAs. Despite this intended use, significantly, there is currently no antibody test that has received EUA for home use or available OTC (Figure 4B). Possible reasons include lack of public health consensus in the use of such tests, and difficulties in offering POC collection of blood samples.
4. Conclusion and Future Directions
We used a holistic framework for the POC ecosystem to review developments during this unprecedented pandemic, focusing on points of interest – both technological and non-technological – to POC researchers and test developers. As the pandemic moves to a different phase, we discuss some possible trends.
Technology development trends.
Across POC nucleic acid, antigen, and antibody tests, there have been many developments in assay chemistry (develop novel amplification methods, affinity reagents, and detection reagents) and microfluidics (which is increasingly being incorporated to automate assay steps and simplify operation of tests). Much effort has been made to streamline the testing process with streamlined sample collection and processing. For instrumentation, capitalization on improved electronic and optical components is allowing for simpler, portable and lower cost instruments. Here, smart connected devices (especially smartphones) are being adopted as an important enhancement to the POC test. With increased digitization of healthcare, such tools will be integral in integrating with other data-centric health platforms, for test result reporting and data analysis.
Role of government.
With investment in commercialization and deployment, we have seen that new POC diagnostics – including OTC tests – can be rapidly developed and deployed. However, we have also seen challenges in loosened regulation, with numerous POC antibody tests removed from the market, and – with increasing numbers of authorized platforms on the market – difficulty in comparing performance across different test platforms.
Apart from regulatory authorizations, government investments have been central in successfully pushing novel diagnostic technologies towards public use. Our analysis also suggests that while these investments led to deployment, they built on pre-existing and available technologies in a significant manner, and still required development time to tune the tests towards a novel target. In fact, the first at-home test was authorized for emergency use over a year into the pandemic. In a future disease outbreak, would we have the benefit of a similar lag time? Clearly, preparedness will be important. As the COVID-19 pandemic has illustrated, to rapidly direct POC tests towards new pathogens for diagnostics, surveillance, and analysis requires a thorough and comprehensive effort. It will be important to create the workflows and testing and data infrastructure to support future widespread testing. While government investment in commercialization efforts was critical during an emergency, it will be critical to continue investment in the development of novel technologies in anticipation of new infectious-disease challenges and public health needs.
POC use cases.
Early into the pandemic, SARS-CoV-2 testing was limited to select sites, which decreased overall access and even endangered public health by promoting crowded conditions. Over a year into the pandemic, workflows and data infrastructure are being built across different POC settings to support POC COVID-testing for all assay types, especially nucleic-acid testing and increasingly antigen and antibody tests. There is continued development and expansion of assays for home use. Whereas all four described POC use cases were being met with diagnostic technologies, the trend towards field testing – which has been occurring in this pandemic – will likely persist into the future for infectious-disease diagnostics and more broadly, a next phase for digital, personalized, and preventive medicine.
POC diagnostics and digital health.
The COVID-19 pandemic has demonstrated that POC diagnostics, including OTC testing, is feasible and increasingly accepted by healthcare workers and the public. Moving forward, there will undoubtedly be continued interest in coronavirus testing as well as other respiratory illnesses, and it remains to be seen how much POC testing will expand to other applications, including to services currently requiring high-complexity techniques such as personalized genetic testing and liquid biopsy for cancer detection. While a number of technical developments are still required in order to make high-complexity tests (especially nucleic acid diagnostics) widely available at a low cost, it remains to be seen whether the increasing availability of POC tests will merely shift testing away from centralized laboratories, or spur growth in overall testing and monitoring towards a different paradigm for health and medicine that is digital, personalized and preventive. While that is an open question, it is worthwhile to recognize how academia, government, and industry responded to successfully develop and deploy POC diagnostic technologies with unprecedented pace, and never before has the public been so interested or invested in POC diagnostics.
Supplementary Material
Acknowledgements
This work was supported by a gift from Bing Zhao, and the NIH Rapid Acceleration of Diagnostics (RADx) Tech program as funded in whole or in part with Federal funds from the National Heart, Lung and Blood Institute; National Institute of Biomedical Imaging and Bioengineering; National Institutes of Health, Department of Health and Human Services, under Grant No. U54HL143541. Harshit Harpaldas thanks the National Science Foundation Graduate Research Fellowship for support of this work. We thank members of the Sia Lab (Abigail Ayers, Alex Colburn, Autumn Greco, Darragh Gerard Kennedy, Kelia Human, Margaret Jakus, Priya Anandakumararan, Rachel Field, Terry Chern, Vira Behnam) and Rover Diagnostics (Nicki Blumenfeld, Angela Tolwani, Michael Anne Bolene, Juliet Freudman) for collaboration and discussion. We thank Dr. Anand Joshi for discussions on POC testing conducted at New York Presbyterian Hospital. We also thank Visby Medical, LumiraDx, and NOWdiagnostics for discussions on their SARS-CoV-2 tests.
Footnotes
Conflicts of Interest
References
- 1.Mercer TR and Salit M, Nat. Rev. Genet, 2021, 22, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayak S, Blumenfeld NR, Laksanasopin T, and Sia SK, Anal. Chem, 2017, 89, 102–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheridan C, Nat. Biotechnol, 2020, 38, 769–772. [DOI] [PubMed] [Google Scholar]
- 4.Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, Saveson CJ, Sasso JM, Gregg AC, Soares DJ, Beskid TR, Jervey SR, and Liu C, ACS Cent. Sci, 2020, 6, 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kevadiya BD, Machhi J, Herskovitz J, Oleynikov MD, Blomberg WR, Bajwa N, Soni D, Das S, Hasan M, Patel M, Senan AM, Gorantla S, McMillan J, Edagwa B, Eisenberg R, Gurumurthy CB, Reid SPM, Punyadeera C, Chang L, and Gendelman HE, Nat. Mater, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette J-L, Ellis J, Zambon M, Peiris M, and Drosten C, Euro Surveill, 2020, 25. [Google Scholar]
- 7.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, and China Novel Coronavirus Investigating and Research Team, N. Engl. J. Med, 2020, 382, 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng W, Newbigging AM, Le C, Pang B, Peng H, Cao Y, Wu J, Abbas G, Song J, Wang D-B, Cui M, Tao J, Tyrrell DL, Zhang X-E, Zhang H, and Le XC, Anal. Chem, 2020, 92, 10196–10209. [DOI] [PubMed] [Google Scholar]
- 9.Kozel TR and Burnham-Marusich AR, J. Clin. Microbiol, 2017, 55, 2313–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craw P and Balachandran W, Lab Chip, 2012, 12, 2469–2486. [DOI] [PubMed] [Google Scholar]
- 11.Suea-Ngam A, Bezinge L, Mateescu B, Howes PD, deMello AJ, and Richards DA, ACS Sens, 2020, 5, 2701–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, and Hase T, Nucleic Acids Res, 2000, 28, 63e–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.New England Biolabs, Inc., Loop-Mediated Isothermal Amplification, https://www.neb.com/applications/dna-amplification-pcr-and-qpcr/isothermal-amplification/loop-mediated-isothermal-amplification-lamp, (accessed June, 2021).
- 14.Dao Thi VL, Herbst K, Boerner K, Meurer M, Kremer LP, Kirrmaier D, Freistaedter A, Papagiannidis D, Galmozzi C, Stanifer ML, Boulant S, Klein S, Chlanda P, Khalid D, Barreto Miranda I, Schnitzler P, Kräusslich H-G, Knop M, and Anders S, Sci. Transl. Med, 2020, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoki MN, de Oliveira Coelho B, Góes LGB, Minoprio P, Durigon EL, Morello LG, Marchini FK, Riediger IN, do Carmo Debur M, Nakaya HI, and Blanes L, Sci. Rep, 2021, 11, 9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitagawa Y, Orihara Y, Kawamura R, Imai K, Sakai J, Tarumoto N, Matsuoka M, Takeuchi S, Maesaki S, and Maeda T, J. Clin. Virol, 2020, 129, 104446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitamura K, Yamazaki M, Ichikawa M, Yasumi Y, Shiozaki K, Tokushima M, Abe T, and Kawakami C, J. Infect. Chemother, 2021, 27, 450–454. [DOI] [PubMed] [Google Scholar]
- 18.Abbott Launches Molecular Point-of-Care Test to Detect Novel Coronavirus in as Little as Five Minutes, https://abbott.mediaroom.com/2020-03-27-Abbott-Launches-Molecular-Point-of-Care-Test-to-Detect-Novel-Coronavirus-in-as-Little-as-Five-Minutes, (accessed September, 2021).
- 19.Basu A, Zinger T, Inglima K, Woo K-M, Atie O, Yurasits L, See B, and Aguero-Rosenfeld ME, J. Clin. Microbiol, 2020, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, Singh J, Miao X, Streithorst JA, Granados A, Sotomayor-Gonzalez A, Zorn K, Gopez A, Hsu E, Gu W, Miller S, Pan C-Y, Guevara H, Wadford DA, Chen JS, and Chiu CY, Nat. Biotechnol, 2020, 38, 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White Paper - A protocol for rapid detection of SARS-CoV-2 using CRISPR: SARS-CoV-2 DETECTR - Mammoth Biosciences, https://mammoth.bio/2020/02/15/white-paper-a-protocol-for-rapid-detection-of-sars-cov-2-using-crispr-sars-cov-2-detectr/, (accessed June, 2021).
- 22.Mammoth Biosciences, Inc., INSTRUCTIONS FOR USE - SARS-CoV-2 DETECTR Reagent Kit, https://www.fda.gov/media/141765/download, (accessed September, 2021).
- 23.Sherlock Biosciences, Instructions For Use: Sherlock CRISPR SARS-CoV-2 kit, https://www.fda.gov/media/137746/download, (accessed September, 2021).
- 24.Zhang F, Abudayyeh OO, and Gootenberg JS, A protocol for detection of COVID-19 using CRISPR diagnostics, 2020, 8.
- 25.Myhrvold C, Freije CA, Gootenberg JS, Abudayyeh OO, Metsky HC, Durbin AF, Kellner MJ, Tan AL, Paul LM, Parham LA, Garcia KF, Barnes KG, Chak B, Mondini A, Nogueira ML, Isern S, Michael SF, Lorenzana I, Yozwiak NL, MacInnis BL, and Sabeti PC, Science, 2018, 360, 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizk C, Mammoth Biosciences GSK Developing CRISPR-Based SARS-CoV-2 Diagnostic for Home Testing | 360Dx, https://www.360dx.com/business-news/mammoth-biosciences-gsk-developing-crispr-based-sars-cov-2-diagnostic-home-testing#.YJMPH7VKjb0, (accessed June, 2021).
- 27.Sherlock Biosciences, Sherlock Biosciences and binx health Announce Global Partnership to Develop First CRISPR-based Point-of-care Test for COVID-19, https://sherlock.bio/sherlock-biosciences-and-binx-health-announce-global-partnership-to-develop-first-crispr-based-point-of-care-test-for-covid-19/, (accessed June, 2021). [Google Scholar]
- 28.Lee RA, Puig HD, Nguyen PQ, Angenent-Mari NM, Donghia NM, McGee JP, Dvorin JD, Klapperich CM, Pollock NR, and Collins JJ, Proc Natl Acad Sci USA, 2020, 117, 25722–25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW, Ferrante T, Ma D, Donghia N, Fan M, Daringer NM, Bosch I, Dudley DM, O’Connor DH, Gehrke L, and Collins JJ, Cell, 2016, 165, 1255–1266. [DOI] [PubMed] [Google Scholar]
- 30.Nat. Biotechnol, 2019, 37, 832. [Google Scholar]
- 31.Sherlock Biosciences, INSPECTR | Sherlock Biosciences, https://sherlock.bio/platforms/inspectr/, (accessed September, 2021). [Google Scholar]
- 32.van Dongen JE, Berendsen JTW, Steenbergen RDM, Wolthuis RMF, Eijkel JCT, and Segerink LI, Biosens. Bioelectron, 2020, 166, 112445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganbaatar U and Liu C, Front. Cell. Infect. Microbiol, 2021, 11, 663949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abudayyeh OO and Gootenberg JS, Science, 2021, 372, 914–915. [DOI] [PubMed] [Google Scholar]
- 35.Nat. Biotechnol, 2021, 39, 1031. [DOI] [PubMed] [Google Scholar]
- 36.de Puig H, Lee RA, Najjar D, Tan X, Soeknsen LR, Angenent-Mari NM, Donghia NM, Weckman NE, Ory A, Ng CF, Nguyen PQ, Mao AS, Ferrante TC, Lansberry G, Sallum H, Niemi J, and Collins JJ, Sci. Adv, 2021, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu TY, Knott GJ, Smock DCJ, Desmarais JJ, Son S, Bhuiya A, Jakhanwal S, Prywes N, Agrawal S, Díaz de León Derby M, Switz NA, Armstrong M, Harris AR, Charles EJ, Thornton BW, Fozouni P, Shu J, Stephens SI, Kumar GR, Zhao C, and Doudna JA, Nat. Chem. Biol, 2021, 17, 982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips EA, Moehling TJ, Ejendal KFK, Hoilett OS, Byers KM, Basing LA, Jankowski LA, Bennett JB, Lin L-K, Stanciu LA, and Linnes JC, Lab Chip, 2019, 19, 3375–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roche PJR, Najih M, Lee SS, Beitel LK, Carnevale ML, Paliouras M, Kirk AG, and Trifiro MA, Analyst, 2017, 142, 1746–1755. [DOI] [PubMed] [Google Scholar]
- 40.Lee J-H, Cheglakov Z, Yi J, Cronin TM, Gibson KJ, Tian B, and Weizmann Y, J. Am. Chem. Soc, 2017, 139, 8054–8057. [DOI] [PubMed] [Google Scholar]
- 41.You M, Li Z, Feng S, Gao B, Yao C, Hu J, and Xu F, Trends Biotechnol, 2020, 38, 637–649. [DOI] [PubMed] [Google Scholar]
- 42.Cheong J, Yu H, Lee CY, Lee J-U, Choi H-J, Lee J-H, Lee H, and Cheon J, Nat. Biomed. Eng, 2020, 4, 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowakowski M, Wang M, Cary R, Cai H, Lindberg C, Bouliane M, and Thomas D, 2016. [Google Scholar]
- 44.Boris A, Moravick K, Ciopyk B, Briones V, Loney G, De La Zerda A, Ching J, Chu S, Swenson D, Huang H, and Kelly C, 2019. [Google Scholar]
- 45.Rodriguez NM, Linnes JC, Fan A, Ellenson CK, Pollock NR, and Klapperich CM, Anal. Chem, 2015, 87, 7872–7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byers KM, Bird AR, Cho HD, and Linnes JC, ACS Omega, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lafleur LK, Bishop JD, Heiniger EK, Gallagher RP, Wheeler MD, Kauffman P, Zhang X, Kline EC, Buser JR, Kumar S, Byrnes SA, Vermeulen NMJ, Scarr NK, Belousov Y, Mahoney W, Toley BJ, Ladd PD, Lutz BR, and Yager P, Lab Chip, 2016, 16, 3777–3787. [DOI] [PubMed] [Google Scholar]
- 48.Cherkaoui D, Huang D, Miller BS, Turbé V, and McKendry RA, Biosens. Bioelectron, 2021, 189, 113328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu D, Shen H, Zhang Y, Shen D, Zhu M, Song Y, Zhu Z, and Yang C, Lab Chip, 2021, 21, 2019–2026. [DOI] [PubMed] [Google Scholar]
- 50.Huang WE, Lim B, Hsu C-C, Xiong D, Wu W, Yu Y, Jia H, Wang Y, Zeng Y, Ji M, Chang H, Zhang X, Wang H, and Cui Z, Microb. Biotechnol, 2020, 13, 950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Color Genomics, Inc., SARS-CoV-2 LAMP Diagnostic Assay, Color Genomics, Inc., 2020. [Google Scholar]
- 52.Chin CD, Linder V, and Sia SK, Lab Chip, 2012, 12, 2118–2134. [DOI] [PubMed] [Google Scholar]
- 53.Ning B, Yu T, Zhang S, Huang Z, Tian D, Lin Z, Niu A, Golden N, Hensley K, Threeton B, Lyon CJ, Yin X-M, Roy CJ, Saba NS, Rappaport J, Wei Q, and Hu TY, Sci. Adv, 2021, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin K, Ding X, Li Z, Sfeir MM, Ballesteros E, and Liu C, Lab Chip, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.OASH, Anterior Nasal (Nares) Specimen Collection for SARS-CoV-2 Diagnostic Testing, CDC, 2020. [Google Scholar]
- 56.Péré H, Podglajen I, Wack M, Flamarion E, Mirault T, Goudot G, Hauw-Berlemont C, Le L, Caudron E, Carrabin S, Rodary J, Ribeyre T, Bélec L, and Veyer D, J. Clin. Microbiol, 2020, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.New Rutgers Saliva Test for Coronavirus Gets FDA Approval | Rutgers University, https://www.rutgers.edu/news/new-rutgers-saliva-test-coronavirus-gets-fda-approval, (accessed June, 2021). [Google Scholar]
- 58.Vogels CBF, Watkins AE, Harden CA, Brackney DE, Shafer J, Wang J, Caraballo C, Kalinich CC, Ott IM, Fauver JR, Kudo E, Lu P, Venkataraman A, Tokuyama M, Moore AJ, Muenker MC, Casanovas-Massana A, Fournier J, Bermejo S, Campbell M, and Grubaugh ND, Med (N Y)., 2021, 2, 263–280.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, Warren JL, Geng B, Muenker MC, Moore AJ, Vogels CBF, Petrone ME, Ott IM, Lu P, Venkataraman A, Lu-Culligan A, Klein J, Earnest R, Simonov M, Datta R, and Ko AI, N. Engl. J. Med, 2020, 383, 1283–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savela ES, Winnett A, Romano AE, Porter MK, Shelby N, Akana R, Ji J, Cooper MM, Schlenker NW, Reyes JA, Carter AM, Barlow JT, Tognazzini C, Feaster M, Goh Y-Y, and Ismagilov RF, medRxiv, 2021. [Google Scholar]
- 61.To KKW, Yip CCY, Lai CYW, Wong CKH, Ho DTY, Pang PKP, Ng ACK, Leung KH, Poon RWS, Chan KH, Cheng VCC, Hung IFN, and Yuen KY, Clin. Microbiol. Infect, 2019, 25, 372–378. [DOI] [PubMed] [Google Scholar]
- 62.Khurshid Z, Warsi I, Moin SF, Slowey PD, Latif M, Zohaib S, and Zafar MS, Adv. Clin. Chem, 2021, 100, 205–253. [DOI] [PubMed] [Google Scholar]
- 63.RNA Extraction Kits for COVID-19 Tests Are in Short Supply in US | The Scientist Magazine®, https://www.the-scientist.com/news-opinion/rna-extraction-kits-for-covid-19-tests-are-in-short-supply-in-us-67250, (accessed June, 2021). [Google Scholar]
- 64.Vandenberg O, Martiny D, Rochas O, van Belkum A, and Kozlakidis Z, Nat. Rev. Microbiol, 2020, 19, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lalli MA, Langmade JS, Chen X, Fronick CC, Sawyer CS, Burcea LC, Wilkinson MN, Fulton RS, Heinz M, Buchser WJ, Head RD, Mitra RD, and Milbrandt J, Clin. Chem, 2021, 67, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lübke N, Senff T, Scherger S, Hauka S, Andrée M, Adams O, Timm J, and Walker A, J. Clin. Virol, 2020, 130, 104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smyrlaki I, Ekman M, Lentini A, Rufino de Sousa N, Papanicolaou N, Vondracek M, Aarum J, Safari H, Muradrasoli S, Rothfuchs AG, Albert J, Högberg B, and Reinius B, Nat. Commun, 2020, 11, 4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alafeef M, Moitra P, Dighe K, and Pan D, Nat. Protoc, 2021, 16, 3141–3162. [DOI] [PubMed] [Google Scholar]
- 69.Cepheid | GeneXpert System, https://www.cepheid.com/en_US/systems/GeneXpert-Family-of-Systems/GeneXpert-System, (accessed June, 2021). [Google Scholar]
- 70.BioFire® FilmArray® Torch & 2.0 | Syndromic Infectious Disease Testing, https://www.biofiredx.com/products/filmarray/, (accessed June, 2021).
- 71.Cepheid to Launch GeneXpert Edge for Decentralized Testing While Omni Remains Delayed | 360Dx, https://www.360dx.com/pcr/cepheid-launch-genexpert-edge-decentralized-testing-while-omni-remains-delayed#.YJ6ghrVKjb0, (accessed June, 2021). [Google Scholar]
- 72.How Many People Have Smartphones Worldwide (Jun 2021), https://www.bankmycell.com/blog/how-many-phones-are-in-the-world, (accessed June, 2021). [Google Scholar]
- 73.Navarathna DH, Sharp S, Lukey J, Arenas M, Villas H, Wiley L, Englett I, Juan MRS, and Jinadatha C, Diagn. Microbiol. Infect. Dis, 2021, 100, 115334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cepheid, Xpert® Omni SARS-CoV-2 - Instructions for Use, https://www.fda.gov/media/144033/download, (accessed September, 2021). [Google Scholar]
- 75.GeneXpert Omni - Hype or Hope? Concerns from the global diagnositc community | Connecting our members with evidence and expertise, https://www.ghdonline.org/diagnostics/discussion/genexpert-omni-hype-or-hope-concerns-from-the-glob/index.html, (accessed June, 2021) [Google Scholar]
- 76.Cepheid Introduces True Point-of-Care Molecular Platform at AACC Analyst Event | Genomeweb, https://www.genomeweb.com/pcr/cepheid-introduces-true-point-care-molecular-platform-aacc-analyst-event?_ga=2.66163046.97160930.1620656290-1134959134.1588778689#.YJ6obrVKjb0, (accessed June, 2021). [Google Scholar]
- 77.Cue Health, Cue COVID-19 Test Instructions for Use, https://www.fda.gov/media/138826/download, (accessed June, 2021).
- 78.Mesa Biotech Using $23M in Financing to Expand Manufacturing for POC Flu MDx | 360Dx, https://www.360dx.com/point-care-testing/mesa-biotech-using-23m-financing-expand-manufacturing-poc-flu-mdx, (accessed September, 2021). [Google Scholar]
- 79.Visby Medical, Visby Medical COVID-19 Point of Care - Package Insert, https://www.fda.gov/media/145917/download, (accessed June, 2021).
- 80.Coronavirus (COVID-19) Update: FDA Authorizes First COVID-19 Test for Self-Testing at Home | FDA, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-covid-19-test-self-testing-home, (accessed June, 2021). [Google Scholar]
- 81.Lucira Health, Lucira™ COVID-19 All-In-One Test Kit - Instructions for Use, https://www.fda.gov/media/143808/download, (accessed June, 2021).
- 82.FDA issues EUA for single-use, OTC PCR COVID-19 at-home test - MassDevice, https://www.massdevice.com/fda-issues-eua-for-single-use-otc-pcr-covid-19-at-home-test/, (accessed June, 2021).
- 83.Coronavirus (COVID-19) Update: FDA Issues Authorization for First Molecular Non-Prescription, At-Home Test | FDA, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-authorization-first-molecular-non-prescription-home-test, (accessed June, 2021). [Google Scholar]
- 84.Khattak A, Sever C, Nelson P, Cooper R, Congdon T, Demartino J, Shapiro R, and Duncan M, 2017.
- 85.Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays, https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays, (accessed June, 2021).
- 86.Interim Guidance for Antigen Testing for SARS-CoV-2 | CDC, https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html, (accessed September, 2021). [Google Scholar]
- 87.Smith RL, Gibson LL, Martinez PP, Ke R, Mirza A, Conte M, Gallagher N, Conte A, Wang L, Fredrickson R, Edmonson DC, Baughman ME, Chiu KK, Choi H, Jensen TW, Scardina KR, Bradley S, Gloss SL, Reinhart C, Yedetore J, and Brooke CB, J. Infect. Dis, 2021, 224, 976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sajid M, Kawde A-N, and Daud M, Journal of Saudi Chemical Society, 2015, 19, 689–705. [Google Scholar]
- 89.Parolo C, Sena-Torralba A, Bergua JF, Calucho E, Fuentes-Chust C, Hu L, Rivas L, Álvarez-Diduk R, Nguyen EP, Cinti S, Quesada-González D, and Merkoçi A, Nat. Protoc, 2020, 15, 3788–3816. [DOI] [PubMed] [Google Scholar]
- 90.Bishop JD, Hsieh HV, Gasperino DJ, and Weigl BH, Lab Chip, 2019, 19, 2486–2499. [DOI] [PubMed] [Google Scholar]
- 91.Antibody Selection and Purification for Lateral Flow Rapid Tests – nanoComposix, https://nanocomposix.com/pages/antibody-selection-and-purification-for-lateral-flow-rapid-tests#target, (accessed June, 2021).
- 92.Hsieh HV, Dantzler JL, and Weigl BH, Diagnostics (Basel)., 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.R. Service, Coronavirus antigen tests: quick and cheap, but too often wrong?, https://www.science.org/news/2020/05/coronavirus-antigen-tests-quick-and-cheap-too-often-wrong, (accessed June, 2021).
- 94.Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients | FDA, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-antigen-test-help-rapid-detection-virus-causes, (accessed June, 2021). [Google Scholar]
- 95.Huynh T, Anderson CE, Gasperino DJ, Harston SP, Hsieh HV, Marzan R, Williford JR, Oncina CI, Glukhova VA, Cate DM, Nichols KP, and Weigl BH, in 2019 IEEE Global Humanitarian Technology Conference (GHTC), IEEE, 2019, pp. 1–4. [Google Scholar]
- 96.Grant BD, Anderson CE, Garing SH, Alonzo LF, Williford JR, Baughman TA, Glukhova V, Boyle DS, Weigl BH, and Nichols KP, 2020. [Google Scholar]
- 97.Grant BD, Anderson CE, Williford JR, Alonzo LF, Glukhova VA, Boyle DS, Weigl BH, and Nichols KP, Anal. Chem, 2020, 92, 11305–11309. [DOI] [PubMed] [Google Scholar]
- 98.Cate D, Hsieh H, Glukhova V, Bishop JD, Hermansky HG, Barrios-Lopez B, Grant BD, Anderson CE, Spencer E, Kuhn S, Gallagher R, Rivera R, Bennett C, Byrnes SA, Connelly JT, Dewan PK, Boyle DS, Weigl BH, and Nichols KP, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cantera J, Cate D, Golden A, Peck R, Lillis L, Domingo G, Murphy E, Barnhart B, Anderson CE, Alonzo LF, Glukhova V, Hermansky G, Barrios-Lopez B, Spencer E, Kuhn S, Islam Z, Grant B, Kraft L, Herve K, de Puyraimond V, and Boyle D, 2020. [Google Scholar]
- 100.Atom Bioworks Gets NSF Grant to Work on COVID-19 Diagnostic | North Carolina Biotechnology Center, https://www.ncbiotech.org/news/atom-bioworks-gets-nsf-grant-work-covid-19-diagnostic, (accessed June, 2021). [Google Scholar]
- 101.Atom Bioworks Leverages Designer-DNA for At-Home, Rapid Coronavirus Antigen Test | 360Dx, https://www.360dx.com/infectious-disease/atom-bioworks-leverages-designer-dna-home-rapid-coronavirus-antigen-test#.YJvoYLVKjb0, (accessed June, 2021). [Google Scholar]
- 102.Kwon PS, Ren S, Kwon S-J, Kizer ME, Kuo L, Xie M, Zhu D, Zhou F, Zhang F, Kim D, Fraser K, Kramer LD, Seeman NC, Dordick JS, Linhardt RJ, Chao J, and Wang X, Nat. Chem, 2020, 12, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Audenhove I and Gettemans J, EBioMedicine, 2016, 8, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Covid-19 — Allele Biotech, https://www.allelebiotech.com/covid19, (accessed June, 2021).
- 105.Rasmi Y, Li X, Khan J, Ozer T, and Choi JR, Anal. Bioanal. Chem, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tymm C, Zhou J, Tadimety A, Burklund A, and Zhang JXJ, Cell. Mol. Bioeng, 2020, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song Q, Sun X, Dai Z, Gao Y, Gong X, Zhou B, Wu J, and Wen W, Lab Chip, 2021, 21, 1634–1660. [DOI] [PubMed] [Google Scholar]
- 108.Bahadır EB and Sezgintürk MK, TrAC Trends in Analytical Chemistry, 2016, 82, 286–306. [Google Scholar]
- 109.DeFrancesco L, Nat. Biotechnol, 2020, 38, 1116–1120. [DOI] [PubMed] [Google Scholar]
- 110.Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umviligihozo G, Karita E, Mwambarangwe L, Braunstein SL, van de Wijgert J, Sahabo R, Justman JE, El-Sadr W, and Sia SK, Nat. Med, 2011, 17, 1015–1019. [DOI] [PubMed] [Google Scholar]
- 111.Laksanasopin T, Guo TW, Nayak S, Sridhara AA, Xie S, Olowookere OO, Cadinu P, Meng F, Chee NH, Kim J, Chin CD, Munyazesa E, Mugwaneza P, Rai AJ, Mugisha V, Castro AR, Steinmiller D, Linder V, Justman JE, Nsanzimana S, and Sia SK, Sci. Transl. Med, 2015, 7, 273re1. [DOI] [PubMed] [Google Scholar]
- 112.Arumugam S, Nayak S, Williams T, di Santa Maria FS, Guedes MS, Chaves RC, Linder V, Marques AR, Horn EJ, Wong SJ, Sia SK, and Gomes-Solecki M, J. Clin. Microbiol, 2019, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin Q, Wen D, Wu J, Liu L, Wu W, Fang X, and Kong J, Anal. Chem, 2020, 92, 9454–9458. [DOI] [PubMed] [Google Scholar]
- 114.Li J and Lillehoj PB, ACS Sens, 2021, 6, 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.United States Securities and Exchange Commission, LUMIRADX LIMITED Form F-1, https://www.sec.gov/Archives/edgar/data/1685428/000119312521010218/d920968df1.htm, (accessed June, 2021).
- 116.Drain PK, Ampajwala M, Chappel C, Gvozden AB, Hoppers M, Wang M, Rosen R, Young S, Zissman E, and Montano M, Infect. Dis. Ther, 2021, 10, 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.OraSure Technologies Leverages Expertise in At-Home, Saliva-Based Dx For Coronavirus Antigen Assay | 360Dx, https://www.360dx.com/immunoassays/orasure-technologies-leverages-expertise-home-saliva-based-dx-coronavirus-antigen-assay#.YK23XqhKhhF, (accessed June, 2021). [Google Scholar]
- 118.Companies ditch plans for rapid spit tests at home. - The New York Times, https://www.nytimes.com/2020/10/01/world/companies-ditch-plans-for-rapid-spit-tests-at-home.html, (accessed June, 2021). [Google Scholar]
- 119.Agulló V, Fernández-González M, Ortiz de la Tabla V, Gonzalo-Jiménez N, García JA, Masiá M, and Gutiérrez F, J. Infect, 2021, 82, 186–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.About | BD VeritorTM Plus System, https://bdveritor.bd.com/en-us/bd-veritor-plus-system, (accessed June, 2021). [Google Scholar]
- 121.Sofia 2 Fluorescent Immunoassay Analyzer | Quidel, https://www.quidel.com/immunoassays/sofia-tests-kits/sofia-2-analyzer, (accessed June, 2021). [Google Scholar]
- 122.Coronavirus (COVID-19) Update: FDA Issues Authorization for Quidel QuickVue At-Home COVID-19 Test | FDA, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-authorization-quidel-quickvue-home-covid-19-test, (accessed June, 2021). [Google Scholar]
- 123.Quidel Corporation - Quidel’s QuickVue® At-Home OTC COVID-19 Test Receives Emergency Use Authorization for Screening Use With Serial Testing, https://ir.quidel.com/news/news-release-details/2021/Quidels-QuickVue-At-Home-OTC-COVID-19-Test-Receives-Emergency-Use-Authorization-for-Screening-Use-With-Serial-Testing/default.aspx, (accessed June, 2021).
- 124.Abbott’s BinaxNOW COVID-19 Rapid Test Receives FDA Emergency Use Authorization for First Virtually Guided, At-Home Rapid Test Using eMed’s Digital Health Platform - Dec 16, 2020, https://abbott.mediaroom.com/2020-12-16-Abbotts-BinaxNOW-COVID-19-Rapid-Test-Receives-FDA-Emergency-Use-Authorization-for-First-Virtually-Guided-At-Home-Rapid-Test-Using-eMeds-Digital-Health-Platform, (accessed June, 2021).
- 125.Abbott, New At-Home COVID Test: Results in Minutes, https://www.abbott.com/corpnewsroom/diagnostics-testing/new-at-home-covid-test.html, (accessed September, 2021).
- 126.Abbott’s BinaxNOW Rapid Antigen Self Test Receives FDA Emergency Use Authorization for Asymptomatic, Over-the-Counter, Non-Prescription, Multi-Test Use - Mar 31, 2021, https://abbott.mediaroom.com/2021-03-31-Abbotts-BinaxNOW-TM-Rapid-Antigen-Self-Test-Receives-FDA-Emergency-Use-Authorization-for-Asymptomatic-Over-the-Counter-Non-Prescription-Multi-Test-Use, (accessed June, 2021).
- 127.Carrio A, Sampedro C, Sanchez-Lopez JL, Pimienta M, and Campoy P, Sensors, 2015, 15, 29569–29593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Faulstich K, Gruler R, Eberhard M, Lentzsch D, and Haberstroh K, in Lateral Flow Immunoassay, eds. Wong R and Tse H, Humana Press, Totowa, NJ, 2009, pp. 1–27. [Google Scholar]
- 129.Point of Care Readers - Customised & Off-the-Shelf Solutions | Lumos Diagnostics, https://lumosdiagnostics.com/solutions/readers/, (accessed June, 2021). [Google Scholar]
- 130.Jana Care - Technology, https://www.janacare.com/technology.html, (accessed June, 2021).
- 131.Lookadoo DB, Schonhorn JE, Harpaldas H, Uherek CM, Schatz P, Lindgren A, Depa M, and Kumar AA, Anal. Chem, 2021. [DOI] [PubMed] [Google Scholar]
- 132.Ellume | Our Technology, https://www.ellumehealth.com/technology/, (accessed June, 2021).
- 133.What Role Will At-Home COVID-19 Tests Play in an Increasingly Vaccinated World? - IEEE Spectrum, https://spectrum.ieee.org/the-human-os/biomedical/diagnostics/role-of-covid-tests-in-a-vaccinated-world, (accessed June, 2021). [Google Scholar]
- 134.Arumugam S, Colburn DAM, and Sia SK, Adv. Mater. Technol, 2019, 1900720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.BD Announces Collaboration for At-Home Rapid Test for COVID-19, https://news.bd.com/2021-02-22-BD-Announces-Collaboration-for-At-Home-Rapid-Test-for-COVID-19, (accessed June, 2021).
- 136.Balán IC, Lopez-Rios J, Nayak S, Lentz C, Arumugam S, Kutner B, Dolezal C, Macar OU, Pabari T, Wang Ying A, Okrah M, and Sia SK, AIDS Behav, 2020, 24, 1560–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Balán IC, Rios JL, Lentz C, Arumugam S, Dolezal C, Kutner B, Rael CT, Ying AW, Macar OU, and Sia SK, AIDS Behav, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.FDA authorizes Ellume COVID-19 Home Test as First Over-the-Counter Fully At-Home Diagnostic Test | Ellume, https://www.ellumehealth.com/2020/12/15/fda-authorizes-ellume-covid-19-home-test-as-first-over-the-counter-fully-at-home-diagnostic-test/, (accessed June, 2021). [Google Scholar]
- 139.Ellume, Ellume COVID-19 Home Test - Product Overview for Healthcare Professionals, https://www.fda.gov/media/144592/download, (accessed June, 2021).
- 140.Paterson AS, Raja B, Garvey G, Kolhatkar A, Hagström AEV, Kourentzi K, Lee TR, and Willson RC, Anal. Chem, 2014, 86, 9481–9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paterson AS, Raja B, Mandadi V, Townsend B, Lee M, Buell A, Vu B, Brgoch J, and Willson RC, Lab Chip, 2017, 17, 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goux HJ, Raja B, Kourentzi K, Trabuco JRC, Vu BV, Paterson AS, Kirkpatrick A, Townsend B, Lee M, Truong VTT, Pedroza C, and Willson RC, PLoS ONE, 2019, 14, e0225365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luminostics, Inc., Lab-quality health analysis on your phone with Clip, https://luminostics.com/technology, (accessed June, 2021).
- 144.Luminostics, Inc., Clip COVID Rapid Antigen Test, https://www.fda.gov/media/144256/download, (accessed June, 2021).
- 145.LumiraDx, SARS-CoV-2 Ag Test Strip Product Insert, https://www.fda.gov/media/141304/download, (accessed June, 2021).
- 146.LumiraDx Connect | Connectivity designed differently, https://www.lumiradx.com/us-en/what-we-do/diagnostics/connectivity, (accessed June, 2021).
- 147.Krüger LJ, Klein JAF, Tobian F, Gaeddert M, Lainati F, Klemm S, Schnitzler P, Bartenschlager R, Cerikan B, Neufeldt CJ, Nikolai O, Lindner AK, Mockenhaupt FP, Seybold J, Jones TC, Corman VM, Pollock NR, Knorr B, Welker A, de Vos M, and for the study team, medRxiv, 2021. [Google Scholar]
- 148.Wang H, Yuan Y, Xiao M, Chen L, Zhao Y, Zhang Haiwei, Long P, Zhou Y, Xu X, Lei Y, Wu Bihao, Diao T, Cai H, Liu L, Shao Z, Wang J, Bai Y, Wang K, Peng M, Liu L, and Wu T, Cell. Mol. Immunol, 2021, 18, 1832–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu J, Liang B, Chen C, Wang H, Fang Y, Shen S, Yang X, Wang B, Chen L, Chen Q, Wu Y, Liu J, Yang X, Li W, Zhu B, Zhou W, Wang H, Li S, Lu S, Liu D, and Zheng X, Nat. Commun, 2021, 12, 1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vanshylla K, Di Cristanziano V, Kleipass F, Dewald F, Schommers P, Gieselmann L, Gruell H, Schlotz M, Ercanoglu MS, Stumpf R, Mayer P, Zehner M, Heger E, Johannis W, Horn C, Suárez I, Jung N, Salomon S, Eberhardt KA, Gathof B, and Klein F, Cell Host Microbe, 2021, 29, 917–929.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bal A, Pozzetto B, Trabaud M-A, Escuret V, Rabilloud M, Langlois-Jacques C, Paul A, Guibert N, D’Aubarède-Frieh C, Massardier-Pilonchery A, Fabien N, Goncalves D, Boibieux A, Morfin-Sherpa F, Pitiot V, Gueyffier F, Lina B, Fassier J-B, Trouillet-Assant S, and COVID SER Study Group, Clin. Chem, 2021, 67, 742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.National SARS-CoV-2 Serology Assay Evaluation Group, Lancet Infect. Dis, 2020, 20, 1390–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Serology tests for COVID-19, https://www.centerforhealthsecurity.org/covid-19TestingToolkit/serology/Serology-based-tests-for-COVID-19.html, (accessed June, 2021).
- 154.Shuren J and Stenzel T, N. Engl. J. Med, 2021, 384, 592–594. [DOI] [PubMed] [Google Scholar]
- 155.Sheridan C, Nat. Biotechnol, 2020. [Google Scholar]
- 156.Sotnikov DV, Zherdev AV, and Dzantiev BB, Sensors, 2020, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mallapaty S, Nature, 2020, 580, 571–572. [DOI] [PubMed] [Google Scholar]
- 158.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C, Rayaprolu V, Rawlings SA, Peters B, Krammer F, Simon V, Saphire EO, Smith DM, Weiskopf D, Sette A, and Crotty S, Science, 2021, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, and Davenport MP, Nat. Med, 2021, 27, 1205–1211. [DOI] [PubMed] [Google Scholar]
- 160.Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, McMahon M, Meade P, Mendu DR, Muellers K, Stadlbauer D, Stone K, Strohmeier S, Simon V, Aberg J, Reich DL, Krammer F, and Cordon-Cardo C, Science, 2020, 370, 1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barnes CO, Jette CA, Abernathy ME, Dam K-MA, Esswein SR, Gristick HB, Malyutin AG, Sharaf NG, Huey-Tubman KE, Lee YE, Robbiani DF, Nussenzweig MC, West AP, and Bjorkman PJ, Nature, 2020, 588, 682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, Zhang L, Nie L, Qin H, Wang M, Lu Q, Li X, Sun Q, Liu J, Fan C, Huang W, Xu M, and Wang Y, Emerg. Microbes Infect, 2020, 9, 680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tan CW, Chia WN, Qin X, Liu P, Chen MI-C, Tiu C, Hu Z, Chen VC-W, Young BE, Sia WR, Tan Y-J, Foo R, Yi Y, Lye DC, Anderson DE, and Wang L-F, Nat. Biotechnol, 2020, 38, 1073–1078. [DOI] [PubMed] [Google Scholar]
- 164.Coronavirus (COVID-19) Update: FDA Authorizes First Test that Detects Neutralizing Antibodies from Recent or Prior SARS-CoV-2 Infection | FDA, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-test-detects-neutralizing-antibodies-recent-or, (accessed June, 2021). [Google Scholar]
- 165.Wang JJ, Zhang N, Richardson SA, and Wu JV, Expert Rev. Mol. Diagn, 2021, 21, 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lake DF, Roeder AJ, Kaleta E, Jasbi P, Periasamy S, Kuzmina N, Bukreyev A, Grys T, Wu L, Mills JR, McAulay K, Gonzalez-Moa M, Seit-Nebi A, and Svarovsky S, medRxiv, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.COVID-19 Neutralizing Antibody Test Kit - JOYSBIO Biotechnology, https://en.joysbio.com/covid-19-neutralizing-antibody-test-kit/, (accessed June, 2021). [Google Scholar]
- 168.What are COVID-19 neutralising antibody rapid tests? Why use them?, https://www.abingdonhealth.com/what-are-covid-19-neutralising-antibody-rapid-tests/, (accessed June, 2021).
- 169.Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin J-B, Olsson A, Llewellyn-Lacey S, Kamal H, Bogdanovic G, Muschiol S, Wullimann DJ, Kammann T, Emgård J, Parrot T, Folkesson E, Karolinska COVID-19 Study Group, Rooyackers O, Eriksson LI, Henter J-I, Sönnerborg A, and Buggert M, Cell, 2020, 183, 158–168.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cox RJ and Brokstad KA, Nat. Rev. Immunol, 2020, 20, 581–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Z, Zheng Z, Hu H, Zhou Q, Liu W, Li X, Liu Z, Wang Y, and Ma Y, Lab Chip, 2020, 20, 4255–4261. [DOI] [PubMed] [Google Scholar]
- 172.COVID-19 RDTs — Drugs & Diagnostics for Tropical Diseases, http://www.ddtd.org/covid19-rdts, (accessed June, 2021).
- 173.Rodriguez-Moncayo R, Cedillo-Alcantar DF, Guevara-Pantoja PE, Chavez-Pineda OG, Hernandez-Ortiz JA, Amador-Hernandez JU, Rojas-Velasco G, Sanchez-Muñoz F, Manzur-Sandoval D, Patino-Lopez LD, May-Arrioja DA, Posadas-Sanchez R, Vargas-Alarcon G, and Garcia-Cordero JL, Lab Chip, 2021, 21, 93–104. [DOI] [PubMed] [Google Scholar]
- 174.Yakoh A, Pimpitak U, Rengpipat S, Hirankarn N, Chailapakul O, and Chaiyo S, Biosens. Bioelectron, 2021, 176, 112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kasetsirikul S, Umer M, Soda N, Sreejith KR, Shiddiky MJA, and Nguyen N-T, Analyst, 2020, 145, 7680–7686. [DOI] [PubMed] [Google Scholar]
- 176.Carrell C, Link J, Jang I, Terry J, Scherman M, Call Z, Panraksa Y, Dandy DS, Geiss BJ, and Henry C, 2020. [Google Scholar]
- 177.INSTI COVID-19 Antibody Test, https://www.insti.com/covid19/, (accessed June, 2021)
- 178.Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, Sun R, Wang Y, Hu B, Chen W, Zhang Y, Wang J, Huang B, Lin Y, Yang J, Cai W, Wang X, Cheng J, Chen Z, Sun K, and Ye F, J. Med. Virol, 2020, 92, 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Plasma Separation: Why Do You Need It? How Do You Achieve It? - DCN Dx, https://dcndx.com/plasma-separation-why-you-need-it/, (accessed June, 2021).
- 180.Schuler CF, Gherasim C, O’Shea K, Manthei DM, Chen J, Giacherio D, Troost JP, Baldwin JL, and Baker JR, PLoS ONE, 2021, 16, e0248729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.In Vitro Diagnostics EUAs - Serology and Other Adaptive Immune Response Tests for SARS-CoV-2 | FDA, https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-serology-and-other-adaptive-immune-response-tests-sars-cov-2, (accessed June, 2021). [Google Scholar]
- 182.Prazuck T, Phan Van J, Sinturel F, Levray F, Elie A, Camera D, and Pialoux G, PLoS ONE, 2021, 16, e0245848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dalton L, C&EN Global Enterp., 2017, 95, 16–19. [Google Scholar]
- 184.Bond MM and Richards-Kortum RR, Am. J. Clin. Pathol, 2015, 144, 885–894. [DOI] [PubMed] [Google Scholar]
- 185.Luchavez J, Lintag ME, Coll-Black M, Baik F, and Bell D, Malar. J, 2007, 6, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ibitoye M, Frasca T, Giguere R, and Carballo-Diéguez A, AIDS Behav, 2014, 18, 933–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Atchison C, Pristerà P, Cooper E, Papageorgiou V, Redd R, Piggin M, Flower B, Fontana G, Satkunarajah S, Ashrafian H, Lawrence-Jones A, Naar L, Chigwende J, Gibbard S, Riley S, Darzi A, Elliott P, Ashby D, Barclay W, Cooke GS, and Ward H, Clin. Infect. Dis, 2021, 72, e384–e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jing M, Bond R, Robertson LJ, Moore J, Kowalczyk A, Price R, Burns W, Nesbit MA, McLaughlin J, and Moore T, Sci. Rep, 2021, 11, 14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.TAP | Seventh Sense Biosystems, https://7sbio.com/products/tap/, (accessed June, 2021).
- 190.Blood Collection Firm Tasso Seeking to Enable At-Home Coronavirus Antibody Tests | 360Dx, https://www.360dx.com/infectious-disease/blood-collection-firm-tasso-seeking-enable-home-coronavirus-antibody-tests#.YKPdnrVKjb1, (accessed June, 2021). [Google Scholar]
- 191.Sauer-Budge AF, Brookfield SJ, Janzen R, McGray S, Boardman A, Wirz H, and Pollock NR, PLoS ONE, 2017, 12, e0183625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li CG, Joung H-A, Noh H, Song M-B, Kim M-G, and Jung H, Lab Chip, 2015, 15, 3286–3292. [DOI] [PubMed] [Google Scholar]
- 193.Lei BUW and Prow TW, Biomed. Microdevices, 2019, 21, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Isho B, Abe KT, Zuo M, Jamal AJ, Rathod B, Wang JH, Li Z, Chao G, Rojas OL, Bang YM, Pu A, Christie-Holmes N, Gervais C, Ceccarelli D, Samavarchi-Tehrani P, Guvenc F, Budylowski P, Li A, Paterson A, Yue FY, and Gingras A-C, Sci. Immunol, 2020, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Faustini SE, Jossi SE, Perez-Toledo M, Shields A, Allen JD, Watanabe Y, Newby ML, Cook A, Willcox CR, Salim M, Goodall M, Heaney JL, Marcial-Juarez E, Morley GL, Torlinska B, Wraith DC, Veenith T, Harding S, Jolles S, Mark PJ, and Richter AG, medRxiv, 2020. [Google Scholar]
- 196.MacMullan MA, Ibrayeva A, Trettner K, Deming L, Das S, Tran F, Moreno JR, Casian JG, Chellamuthu P, Kraft J, Kozak K, Turner FE, Slepnev VI, and Le Page LM, Sci. Rep, 2020, 10, 20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hopeful steps toward a rapid saliva-based test for COVID-19 antibodies | Hub, https://hub.jhu.edu/2021/03/12/rapid-saliva-covid-antibody-test/, (accessed June, 2021). [Google Scholar]
- 198.OraSure Technologies, Inc. Home, https://www.orasure.com/, (accessed June, 2021).
- 199.Assure Tech, Assure COVID-19 IGG/IGM Rapid Test Device - Instructions for Use, https://www.fda.gov/media/139792/download, (accessed June, 2021).
- 200.Lateral Flow Reader Customisation from Abingdon Health, https://www.abingdonhealth.com/services/appdx-smartphone-reader/, (accessed June, 2021).
- 201.Diagnostic App Development, https://www.bbisolutions.com/en/digital/diagnostic-app-development, (accessed June, 2021).
- 202.NOWDiagnostics, ADEXUSDx COVID-19 Test - Instructions for Use, https://www.fda.gov/media/149441/download, (accessed June, 2021).
- 203.ADEXUSDx® COVID-19 Antibody Test Receives FDA Emergency Use Authorization for Point-of-Care Deployment Across U.S, https://www.prnewswire.com/news-releases/adexusdx-covid-19-antibody-test-receives-fda-emergency-use-authorization-for-point-of-care-deployment-across-us-301299293.html, (accessed June, 2021).
- 204.Technology - C19 Development, https://www.c19development.com/technology/, (accessed June, 2021).
- 205.ADEXUSDx® Analyzer - NOW Diagnostics, https://nowdx.com/adexusdx-analyzer/#product-specifications, (accessed June, 2021).
- 206.Making masks smarter and safer against COVID-19 | University of California, https://www.universityofcalifornia.edu/news/making-masks-smarter-and-safer-against-covid-19, (accessed June, 2021). [Google Scholar]
- 207.Nguyen PQ, Soenksen LR, Donghia NM, Angenent-Mari NM, de Puig H, Huang A, Lee R, Slomovic S, Galbersanini T, Lansberry G, Sallum HM, Zhao EM, Niemi JB, and Collins JJ, Nat. Biotechnol, 2021. [DOI] [PubMed] [Google Scholar]
- 208.Vrieze J, Science, 2021. [Google Scholar]
- 209.Shan B, Broza YY, Li W, Wang Y, Wu S, Liu Z, Wang J, Gui S, Wang L, Zhang Z, Liu W, Zhou S, Jin W, Zhang Q, Hu D, Lin L, Zhang Q, Li W, Wang J, Liu H, and Haick H, ACS Nano, 2020, 14, 12125–12132. [DOI] [PubMed] [Google Scholar]
- 210.Molnar C and Gair J, 2015. [Google Scholar]
- 211.W. H. Organization, Point-of-care CD4 tests to support the identification of individuals with advanced HIV disease, https://apps.who.int/iris/handle/10665/33168, (accessed June, 2021).
- 212.Pham MD, Agius PA, Romero L, McGlynn P, Anderson D, Crowe SM, and Luchters S, BMC Infect. Dis, 2016, 16, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bwana P, Vojnov L, Adhiambo M, Akinyi C, Mwende J, Prescott M, and Mwau M, PLoS ONE, 2015, 10, e0145586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Analyser Pima | Abbott Point of Care Testing, https://www.globalpointofcare.abbott/en/product-details/pima-analyser-ous.html, (accessed June, 2021). [Google Scholar]
- 215.Sheridan C, Nat. Biotechnol, 2021, 39, 533–534. [DOI] [PubMed] [Google Scholar]
- 216.Coronavirus (COVID-19) Update: FDA Authorizes Adaptive Biotechnologies T-Detect COVID Test | FDA, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-adaptive-biotechnologies-t-detect-covid-test, (accessed June, 2021). [Google Scholar]
- 217.Tonix Pharmaceuticals to Develop COVID-19 Skin Test, https://www.globenewswire.com/fr/news-release/2021/02/08/2171231/0/en/Tonix-Pharmaceuticals-to-Develop-COVID-19-Skin-Test-TNX-2100-to-Measure-SARS-CoV-2-Exposure-and-T-Cell-Immunity.html, (accessed June, 2021).
- 218.A Skin Test To Measure T-Cell Immunity to SARS-CoV-2 | Technology Networks, https://www.technologynetworks.com/diagnostics/blog/a-skin-test-to-measure-t-cell-immunity-to-sars-cov-2-347298, (accessed June, 2021). [Google Scholar]
- 219.A simple T-cell test to show the full picture of body's immune response to COVID-19: A rapid way to track an elusive part of the immune system will bring better vaccine strategies – ScienceDaily, https://www.sciencedaily.com/releases/2021/09/210902124937.htm, (accessed September, 2021).
- 220.Tan AT, Lim JME, Bert NL, Kunasegaran K, Chia A, Qui MDC, Tan N, Chia WN, de Alwis R, Ying D, Sim JXY, Ooi EE, Wang L-F, Chen MI-C, Young BE, Hsu LY, Low JGH, Lye DC, and Bertoletti A, The Journal of Clinical Investigation, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hyris System, https://www.hyris.net/index.php/t-cell, (accessed September, 2021).
- 222.T cell immunity: What is it and how does it help to protect us from COVID-19? | Imperial News | Imperial College London, https://www.imperial.ac.uk/news/201833/cell-immunity-what-does-help-protect/, (accessed June, 2021). [Google Scholar]
- 223.Diagnostic Data & Reporting | HHS.gov, https://www.hhs.gov/coronavirus/testing/covid-19-diagnostic-data-reporting/index.html, (accessed June, 2021). [Google Scholar]
- 224.Early Insights and Recommendations for Implementing a COVID-19 Antigen Testing Program in K-12 Schools: Lessons Learned from Six Pilot Sites, https://mathematica.org/publications/early-insights-and-recommendations-for-implementing-a-covid-19-antigen-testing-program-in-k-12, (accessed June, 2021).
- 225.APHL enables public health reporting of Abbott’s BinaxNOW COVID-19 test results via the NAVICA app | APHL Lab Blog, https://www.aphlblog.org/aphl-enables-public-health-reporting-of-abbotts-binaxnow-covid-19-test-results-via-the-navica-app/, (accessed June, 2021). [Google Scholar]
- 226.Winners of HHS Design-a-thon to Develop Innovative Digital Health Tools for COVID-19 At-Anywhere Diagnostic Tests Announced | HHS.gov, https://www.hhs.gov/about/news/2020/12/16/winners-of-hhs-design-a-thon-to-develop-innovative-digital-health-tools-for-covid-19.html, (accessed June, 2021). [Google Scholar]
- 227.COVID-19 Immutable Test Results Submission and Visualization, https://waters.crowdicity.com/post/695494?forPhase=24540, (accessed June, 2021).
- 228.Interpret-COVID Consumer Dx Test App, https://waters.crowdicity.com/post/695438, (accessed June, 2021).
- 229.One Country, One Data Standard, One Process for all - Oasys Streaming Analytics, https://waters.crowdicity.com/post/695470, (accessed June, 2021).
- 230.TOPx Sprint, https://waters.crowdicity.com/hubbub/communitypage/22483, (accessed June, 2021).
- 231.NAVICA for Employers and Organizations | Abbott Point of Care Testing, https://www.globalpointofcare.abbott/en/product-details/navica-organizations.html, (accessed June, 2021).
- 232.Health Pass | CLEAR, https://www.clearme.com/healthpass, (accessed June, 2021).
- 233.How It Works | The CLEAR Travel Experience, https://www.clearme.com/how-it-works/, (accessed June, 2021). [Google Scholar]
- 234.Dunking on COVID: NBA installs Clear Health Pass tech as arenas refill | FierceBiotech, https://www.fiercebiotech.com/medtech/arenas-refilling-nba-installs-clear-health-pass-tech-for-covid-screening, (accessed June, 2021). [Google Scholar]
- 235.NBA, Clear announce new partnership | NBA.com, https://www.nba.com/news/nba-clear-announce-new-partnership, (accessed June, 2021). [Google Scholar]
- 236.Lucira sets full-court press on COVID-19 with free pregame tests for some NBA ticketholders | FierceBiotech, https://www.fiercebiotech.com/medtech/lucira-health-sets-a-pick-covid-19-free-at-home-rapid-tests-for-some-nba-ticketholders, (accessed June, 2021). [Google Scholar]
- 237.Lucira COVID-19 All-in-One Test Kit - Letter of Authorization, https://www.fda.gov/media/143810/download, (accessed June, 2021)
- 238.U.S Food & Drug Administration, Facts About In-Home HIV Testing, https://www.fda.gov/consumers/consumer-updates/facts-about-home-hiv-testing, (accessed June, 2021).
- 239.Labcorp Receives FDA Authorization to Make At-home COVID-19 Collection Kits Available Through Retail | Labcorp, https://www.labcorp.com/coronavirus-disease-covid-19/news/labcorp-receives-fda-authorization-make-home-covid-19-collection-kits-available-through-retail, (accessed June, 2021). [Google Scholar]
- 240.Over-the-counter COVID-19 testing now available at CVS Pharmacy | CVS Health, https://cvshealth.com/news-and-insights/press-releases/over-the-counter-covid-19-testing-now-available-at-cvs-pharmacy, (accessed June, 2021). [Google Scholar]
- 241.LUCIRA CHECK IT COVID-19 Self-Test Now Available on Amazon | Business Wire, https://www.businesswire.com/news/home/20210512005367/en/LUCIRA%E2%84%A2-CHECK-IT-COVID-19-Self-Test-Now-Available-on-Amazon, (accessed June, 2021). [Google Scholar]
- 242.Federal Register :: Emergency Use Authorization Declaration, https://www.federalregister.gov/documents/2020/04/01/2020-06905/emergency-use-authorization-declaration, (accessed June, 2021).
- 243.Shuren J and Stenzel T, N. Engl. J. Med, 2020, 383, e97. [DOI] [PubMed] [Google Scholar]
- 244.Federal Register :: Policy for Diagnostics Testing in Laboratories Certified To Perform High Complexity Testing Under the Clinical Laboratory Improvement Amendments Prior to Emergency Use Authorization for Coronavirus Disease-2019 During the Public Health Emergency; Immediately in Effect Guidance for Clinical Laboratories and Food and Drug Administration Staff; Availability, https://www.federalregister.gov/documents/2020/03/06/2020-04630/policy-for-diagnostics-testing-in-laboratories-certified-to-perform-high-complexity-testing-under, (accessed June, 2021).
- 245.FAQs about Families First Coronavirus Response Act and Coronavirus Aid, Relief, and Economic Security Act Implementation Part 43 | Guidance Portal, https://www.hhs.gov/guidance/document/faqs-about-families-first-coronavirus-response-act-and-coronavirus-aid-relief-and-0, (accessed June, 2021). [Google Scholar]
- 246.FAQs Part 44 | Guidance Portal, https://www.hhs.gov/guidance/document/faqs-part-44, (accessed June, 2021). [Google Scholar]
- 247.COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured, https://www.hrsa.gov/coviduninsuredclaim, (accessed June, 2021).
- 248.HRSA COVID-19 - Resources & Support - Coverage Details, https://coviduninsuredclaim.linkhealth.com/coverage-details.html, (accessed June, 2021).
- 249.CARES Act Provider Relief Fund: For Patients | HHS.gov, https://www.hhs.gov/coronavirus/cares-act-provider-relief-fund/for-patients/index.html, (accessed June, 2021). [Google Scholar]
- 250.At-home coronavirus test kits: Updates, costs, and comparison, https://www.singlecare.com/blog/news/at-home-coronavirus-tests/#cost-and-insurance-coverage, (accessed June, 2021).
- 251.At-home COVID-19 tests: What You Need to Know, https://fsastore.com/learn-at-home-covid-19-tests-what-you-need-to-know.html, (accessed June, 2021).
- 252.COVID-19 Testing at Home—No Appointment or Prescription Necessary | NIH COVID-19 Research, https://covid19.nih.gov/news-and-stories/COVID-19-testing-at-home, (accessed June, 2021). [Google Scholar]
- 253.Covid Money Tracker, https://www.covidmoneytracker.org/, (accessed June, 2021).
- 254.NIH mobilizes national innovation initiative for COVID-19 diagnostics | National Institutes of Health (NIH), https://www.nih.gov/news-events/news-releases/nih-mobilizes-national-innovation-initiative-covid-19-diagnostics, (accessed June, 2021). [Google Scholar]
- 255.One year of rapid acceleration of diagnostics, and anticipating new challenges | National Institute on Aging, https://www.nia.nih.gov/news/one-year-rapid-acceleration-diagnostics-and-anticipating-new-challenges, (accessed June, 2021). [Google Scholar]
- 256.BARDA Funding Tracker - Public Citizen, https://www.citizen.org/article/barda-funding-tracker/, (accessed June, 2021).
- 257.BARDA COVID-19 Response | Medical Countermeasures, https://www.medicalcountermeasures.gov/barda/barda-covid-19-response/, (accessed June, 2021). [Google Scholar]
- 258.Abbott Receives $255M Contract from DoD to Supply SARS-CoV-2 Antigen Tests | 360Dx, https://www.360dx.com/business-news/abbott-receives-255m-contract-dod-supply-sars-cov-2-antigen-tests#.YLgei_lKjb0, (accessed June, 2021). [Google Scholar]
- 259.RADxSM Tech and ATP Programs: Phase 2 Awards | National Institute of Biomedical Imaging and Bioengineering, https://www.nibib.nih.gov/covid-19/radx-tech-program/radx-tech-phase2-awards, (accessed June, 2021). [Google Scholar]
- 260.Sackmann EK, Fulton AL, and Beebe DJ, Nature, 2014, 507, 181–189. [DOI] [PubMed] [Google Scholar]
- 261.Nayak S, Guo T, Lopez-Rios J, Lentz C, Arumugam S, Hughes J, Dolezal C, Linder V, Carballo-Diéguez A, Balán IC, and Sia SK, Lab Chip, 2019, 19, 2241–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Standard Operating Procedure (SOP) for Triage of Suspected COVID-19 Patients in non-US Healthcare Settings: Early Identification and Prevention of Transmission during Triage | CDC, https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/sop-triage-prevent-transmission.html, (accessed June, 2021). [Google Scholar]
- 263.Brendish NJ, Poole S, Naidu VV, Mansbridge CT, Norton NJ, Wheeler H, Presland L, Kidd S, Cortes NJ, Borca F, Phan H, Babbage G, Visseaux B, Ewings S, and Clark TW, Lancet Respir. Med, 2020, 8, 1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Moran A, Beavis KG, Matushek SM, Ciaglia C, Francois N, Tesic V, and Love N, J. Clin. Microbiol, 2020, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Coronavirus » Standard operating procedure: Lateral flow device testing for emergency department patient pathways, https://www.england.nhs.uk/coronavirus/publication/standard-operating-procedure-lateral-flow-device-testing-for-emergency-department-patient-pathways/, (accessed June, 2021).
- 266.How Will Labs Use Expanded Molecular Testing Capacities Post-COVID-19? | 360Dx, https://www.360dx.com/clinical-lab-management/how-will-labs-use-expanded-molecular-testing-capacities-post-covid-19#.YLJ60flKjb0, (accessed June, 2021). [Google Scholar]
- 267.Walk-in Clinic - SAVE 40% vs Urgent Care | MinuteClinic, https://www.cvs.com/minuteclinic/covid-19-testing/, (accessed June, 2021). [Google Scholar]
- 268.A Guide To New York City’s COVID-19 Testing Options - Gothamist, https://gothamist.com/news/guide-new-york-citys-covid-19-coronavirus-testing-options, (accessed June, 2021).
- 269.Get Tested to Get to the Game, https://chasecenter.com/fan-safety-covid19-testing, (accessed June, 2021).
- 270.DoorDash Offers Restaurants $100 Rapid COVID-19 Test Kits From Cellex, https://www.businessinsider.com/doordash-offers-restaurants-discounted-rapid-covid-19-test-kits-cellex-2021-2, (accessed June, 2021).
- 271.LAX Now Offering 1-Hour COVID-19 Tests - LA Weekly, https://www.laweekly.com/lax-now-offering-1-hour-covid-19-tests/, (accessed June, 2021).
- 272.Twice weekly rapid testing to be available to everyone in England - GOV.UK, https://www.gov.uk/government/news/twice-weekly-rapid-testing-to-be-available-to-everyone-in-england, (accessed June, 2021).
- 273.McDermott JH, Burn J, Donnai D, and Newman WG, Eur. J. Hum. Genet, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Abbott BinaxNOW COVID-19 Antigen Self Test (2 tests for serial testing), https://www.cvs.com/shop/abbott-binaxnow-covid-19-antigen-self-test-2-tests-for-serial-testing-prodid-550147, (accessed June, 2021). [Google Scholar]
- 275.Global partnership to make available 120 million affordable, quality COVID-19 rapid tests for low- and middle-income countries, https://www.who.int/news/item/28-09-2020-global-partnership-to-make-available-120-million-affordable-quality-covid-19-rapid-tests-for-low--and-middle-income-countries, (accessed June, 2021).
- 276.Abbott’s Covid-19 antigen test shows promise in asymptomatics | Evaluate, https://www.evaluate.com/vantage/articles/news/trial-results/abbotts-covid-19-antigen-test-shows-promise-asymptomatics, (accessed June, 2021). [Google Scholar]
- 277.SARS-CoV-2 Pandemic May Promote Progress in Paper MDx | 360Dx, https://www.360dx.com/molecular-diagnostics/sars-cov-2-pandemic-may-promote-progress-paper-mdx#.YLUQm_lKjb0, (accessed June, 2021). [Google Scholar]
- 278.Li Z, Bai Y, You M, Hu J, Yao C, Cao L, and Xu F, Biosens. Bioelectron, 2021, 177, 112952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cobb BL and Sawalha AH, Clin. Immunol, 2021, 108791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.