Abstract
Background
The nosology of noninvasive pneumonia due to group B Streptococcus (GBS) is not well defined. This study compared clinical characteristics and outcomes of patients with invasive pneumonia and noninvasive pneumonia caused by GBS.
Methods
We conducted a retrospective cohort study among Veterans Affairs (VA) healthcare users between 2008 and 2017. Using data from electronic health records, we identified patients who had blood or respiratory cultures that grew GBS and had invasive pneumonia or noninvasive pneumonia. We analyzed patient and infection characteristics associated with all-cause mortality, including among the subset of patients with cultures that were monomicrobial for GBS.
Results
Among 1791 patients with GBS pneumonia, 646 (36%) cases were invasive and 1145 (64%) were noninvasive. Among those, 535 and 424 cases of invasive and noninvasive pneumonia, respectively, had cultures that were monomicrobial for GBS. All-cause 30-day mortality among those with monomicrobial GBS pneumonia was 15% for both those with invasive and noninvasive disease, respectively. Increasing age, severity of illness, healthcare exposure in the previous 90 days, and polymicrobial infection with Staphylococcus aureus were independently associated with all-cause mortality at 30 days.
Conclusions
In this large cohort, even when considering cases for which GBS was the only pathogen recovered, >40% of GBS pneumonia cases were noninvasive. All-cause mortality was comparable for invasive and noninvasive pneumonia. These findings suggest that the burden of GBS pneumonia may be greater than previously recognized by surveillance of invasive GBS disease and may inform treatment and prevention efforts.
Keywords: community-acquired pneumonia, GBS, respiratory tract, sputum, Streptococcus agalactiae, Veterans Affairs
In a large cohort of US Veterans, noninvasive group B Streptococcus(GBS) pneumonia occurred at a comparable incidence and was associated with similar mortality (even when focusing on monomicrobial cases) compared to invasive GBS pneumonia, suggesting an underestimation of the burden of GBS pneumonia.
In the United States, the incidence of invasive infections caused by group B Streptococcus (GBS, also known as Streptococcus agalactiae) among nonpregnant adults has risen from 3.6 cases per 100 000 population in 1990 to 10.9 cases per 100 000 population in 2016 [1, 2]. Individuals at increased risk of invasive GBS infections include adults aged ≥65 years with underlying comorbid conditions, especially diabetes mellitus that is poorly controlled, and those with a body mass index of ≥30 kg/m2 [3, 4]. Pneumonia represents approximately 10% of invasive GBS infections, with age ≥65 years as well as chronic heart or lung disease implicated as specific risk factors [3, 4]. Perhaps because it occurs often in older adults and those with chronic comorbid conditions, invasive GBS pneumonia and empyema were associated with an all-cause 30-day mortality of 17%, as demonstrated in patients from the Veterans Affairs (VA) healthcare system [4].
Few studies describe the clinical characteristics and outcomes of patients with noninvasive GBS pneumonia. Limited data suggest that among adults hospitalized with all types of GBS infections, <5% of cases represented respiratory tract infections [5, 6]. Although respiratory infection was common among patients with GBS infection who died in the hospital, it was not independently associated with increased mortality. Furthermore, distinguishing noninvasive GBS infection from colonization of the respiratory tract may be challenging. GBS appears to colonize the respiratory tract of 2%–3% of healthy schoolchildren [7, 8] and 4%–7% of healthy adults [8, 9]. Additionally, as in other forms of GBS infection, GBS is frequently isolated with other pathogens from respiratory cultures in patients with pneumonia [4–6]. These factors add to the complexity of recognizing the role and impact of GBS as a cause of noninvasive pneumonia.
Given the limited understanding of noninvasive GBS pneumonia and considering the importance of GBS as a cause of invasive pneumonia, particularly its high rate of associated all-cause mortality, we sought to characterize and compare individuals with invasive and noninvasive GBS pneumonia, including those with Staphylococcus aureus and Pseudomonas aeruginosa as copathogens. Here, we describe our analysis of a cohort of patients from the VA healthcare system with invasive pneumonia and noninvasive pneumonia due to GBS.
METHODS
Study Design and Data Sources
We conducted a retrospective cohort study of patients treated in the VA healthcare system from 1 January 2008 through 31 December 2017. Using the VA Informatics and Computing Infrastructure (VINCI), we accessed the Veterans Health Administration (VHA) Corporate Data Warehouse to identify microbiology cultures from blood or respiratory tract samples (sputum, bronchoalveolar lavage fluid, and endotracheal aspirates) that grew GBS (or S. agalactiae). Data indicating date of death was extracted from the from the VHA Vital Status File. The institutional review board at the VA Northeast Ohio Healthcare System approved the study protocol.
Case Ascertainment and Clinical Characteristics
The population assessed represented active users of the VA healthcare system, with active use defined as a hospital admission or any outpatient primary or specialty care clinic visit in a given calendar year. Cases were defined by GBS identified in a blood or respiratory sample in the absence of GBS identified from any other sites within 90 days. Repeat cultures within the same patient separated by <90 days were considered to be part of a single case and the earliest culture date within a case was considered the case date. Among patients with a case, we used International Classification of Diseases codes from the 9th and 10th revisions (hence forward ICD codes) as previously described [10], to identify pneumonia among individuals with a blood or respiratory tract culture growing GBS. For outpatients, we considered ICD codes entered within 7 days of a blood or respiratory tract culture to be associated with a given culture. Similarly, for inpatients we considered ICD codes entered during an admission that included or began within 7 days of a blood or respiratory tract culture positive for GBS.
Invasive GBS pneumonia was defined as a blood culture growing GBS, an associated ICD code indicating pneumonia, and a chest radiograph or chest computed tomography (CT) scan within 7 days of the positive culture. Noninvasive GBS pneumonia was defined as a respiratory tract culture growing GBS (without blood or pleural fluid cultures positive for GBS within 90 days of the respiratory tract culture), with an associated ICD code indicating pneumonia and with a chest radiograph or CT scan performed within 7 days of the positive culture. Cases that did not meet either of these definitions were excluded from the analysis. A clinician (R. L. P. J.) blinded to the categorization made using administrative data conducted chart reviews on a convenience sample of 30 (1.7%) cases from this cohort and independently categorized the cases as invasive or noninvasive GBS pneumonia; this included reviewing imaging reports and evaluating cases with GBS bacteremia for pneumonia due to causes other than GBS.
Incident cases were defined as the first case within the 10-year time window for each patient and were analyzed for demographic and clinical characteristics, including age, sex, self-reported race/ethnicity, comorbid conditions, healthcare exposure (defined as hospital or nursing home admissions within the previous 90 days), all-cause mortality at 30 days and 1 year, Charlson Comorbidity Index (CCI) score, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), and percentage of glycated hemoglobin (HbA1c). We determined the CCI score using ICD codes [11]. To calculate BMI, we used the first height and weight measurements within the same calendar year as the infection; if these were not available, we used the first BMI from a previous calendar year, applying a last-observed, carry-forward logic to account for missing values. For HbA1c percentage among patients with an ICD code for diabetes mellitus, we used the first value within the same calendar year as the infection; if the HbA1c percentage was not present during the year, it was deemed a missing value. We also determined disease severity using the modified Acute Physiology and Chronic Health Evaluation (mAPACHE), an illness severity score calculated from administrative data [12]. We assessed if the cultures associated with the cases were polymicrobial, defined as isolation of organisms other than GBS in the same sample that grew GBS. Specifically, we evaluated whether S aureus and/or P aeruginosa were isolated in those samples.
Statistical Analysis
We used descriptive statistics to summarize and compare characteristics among patients with invasive GBS pneumonia and noninvasive GBS pneumonia. We also compared co-pathogens, recent healthcare exposure, severity of illness, location of management, and outcome (all-cause mortality at 30 days and 1 year) in the polymicrobial and the monomicrobial groups of invasive GBS pneumonia and noninvasive GBS pneumonia. Comparisons between groups used t tests for continuous variables and χ2 tests for categorical variables. Kaplan-Meier survival curves were estimated for invasive and noninvasive GBS pneumonia, with differences between the survival curves assessed using a log-rank test. We developed multivariable logistic regression models to report odds ratios (ORs) and 95% confidence intervals (CIs) for all-cause mortality at 30 days and 1 year, to assess adjusted mortality differences between patients with invasive and noninvasive pneumonia. These models, estimated separately in patients with monomicrobial and polymicrobial infections, included noninvasive and invasive GBS pneumonia, age, sex, ethnicity, CCI, mAPACHE, recent healthcare exposure, and (for polymicrobial cases) isolation of S aureus and P aeruginosa. Statistical analyses were performed using R software (version 4.0.5) including functions from the survival package. Data management and graphics functions from additional packages were also implemented [13–19].
RESULTS
Between 2008 and 2017, our retrospective analysis of VHA’s administrative and clinical data identified 1791 patients who met the study’s criteria for having GBS pneumonia; 646 (36%) patients had invasive pneumonia and 1145 (64%) had noninvasive pneumonia. A review of the medical records from a sample of patients confirmed those categories: Invasive pneumonia due to GBS occurred in 13 of 15 cases with blood cultures positive for GBS, and noninvasive pneumonia due to GBS occurred in 15 of 15 cases with respiratory cultures only positive for GBS (ie, cases without positive blood cultures). Imaging studies for 25 of 30 cases had radiology reports with an interpretation supportive of a clinical diagnosis of pneumonia. The medical record of 5 cases without findings compatible with an acute lung process in radiology reports revealed that 3 were treated for pneumonia.
In the overall cohort, most of the patients were male (1748 [98%]), with a mean age of 70.8 years (± 11.9 standard deviation); 462 (26%) had a BMI of ≥30 kg/m2 (Table 1). Patients with invasive GBS pneumonia were older and had a higher CCI (5.41 ± 3.0) compared to those with noninvasive pneumonia (4.95 ± 3.0; P = .002). The most common comorbid conditions among those with GBS pneumonia were pulmonary disease (61%), diabetes mellitus (52%), and heart disease (49%). The prevalence of heart disease, renal disease, and diabetes mellitus and the proportion with HbA1c ≥7.5% and with BMI ≥30 kg/m2 were greater in patients with invasive GBS pneumonia than in those with noninvasive GBS pneumonia. In contrast, pulmonary disease occurred more frequently in patients classified as having noninvasive GBS pneumonia. Polymicrobial cultures were recovered for 46% of the cohort (832/1791). Among those, 20% (364/1791) and 7% (128/1791) of patients had S aureus and P aeruginosa, respectively. Overall, polymicrobial cultures were more frequently observed among patients with noninvasive compared to invasive GBS pneumonia, consistent with the common recovery of several species of microorganisms from respiratory tract samples compared to blood cultures.
Table 1.
Characteristics of Patients With Group B Streptococcus Pneumonia
| Characteristic | All (N = 1791) | Invasive GBS Pneumonia (n = 646) | Noninvasive GBS Pneumonia (n = 1145) | P Valuea |
|---|---|---|---|---|
| Male sex | 1748 (98) | 629 (97) | 1119 (98) | .905 |
| Age, y, mean ± SD | 70.8 ± 11.9 | 72.7 ± 11.4 | 69.8 ± 12 | <.001 |
| Race | .258 | |||
| White | 1425 (80) | 502 (78) | 923 (81) | |
| Black | 233 (13) | 194 (15) | 95 (15) | |
| Otherb | 133 (7) | 49 (8) | 84 (7) | |
| Ethnicity | <.001 | |||
| Non-Hispanic | 1618 (90) | 552 (85) | 1066 (93) | |
| Hispanic | 91 (5) | 64 (10) | 27 (2) | |
| Unknown | 82 (5) | 30 (5) | 52 (5) | |
| BMI ≥30 kg/m2 | 462 (26) | 196 (30) | 266 (23) | .001 |
| Diabetes mellitus with HbA1c ≥7.5% | 366 (20) | 162 (25) | 204 (18) | <.001 |
| CCI, mean ± SD | 5.11 ± 3.0 | 5.41 ± 3.0 | 4.95 ± 3.0 | .002 |
| Comorbid conditions | ||||
| Pulmonary disease | 1090 (61) | 343 (53) | 747 (65) | <.001 |
| Diabetes mellitus | 935 (52) | 396 (61) | 539 (47) | <.001 |
| Heart disease | 881 (49) | 371 (57) | 510 (45) | <.001 |
| Cancer | 611 (34) | 204 (32) | 407 (36) | .099 |
| Renal disease | 592 (33) | 275 (43) | 317 (28) | <.001 |
| Stroke | 527 (29) | 167 (26) | 330 (29) | .196 |
| Peripheral vascular disease | 497 (28) | 205 (32) | 322 (28) | .120 |
| Liver disease | 313 (17) | 129 (20) | 184 (16) | .043 |
| HIV | 25 (1) | 7 (1) | 18 (2) | .525 |
| Polymicrobial culturesc | 832 (46) | 111 (17) | 721 (63) | <.001 |
| Staphylococcus aureus | 364 (20) | 47 (7) | 317 (28) | <.001 |
| Pseudomonas aeruginosa | 128 (7) | 7 (1) | 121 (11) | <.001 |
| Other bacteriad | 391 (22) | 66 (10) | 325 (28) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; GBS, group B Streptococcus; HbA1c, glycated hemoglobin; HIV, human immunodeficiency virus; SD, standard deviation.
Compares patients with invasive vs noninvasive GBS pneumonia.
Other includes American Indian, Alaska Native, Asian, Native Hawaiian or Pacific Islander, and unknown.
Some patients had cultures that grew both S aureus and P aeruginosa, resulting in inclusion in both categories.
Excludes patients with either S aureus or P aeruginosa.
Recognizing that other organisms may contribute to disease severity, we performed additional analyses on the subset of patients with pneumonia who had cultures that were monomicrobial for GBS (Table 2). Within that subset, most patients received care on general inpatient wards, only a few were treated as outpatients, and the proportion of patients requiring intensive care was similar. The overall disease severity, however, was different between the groups. A greater proportion of patients with invasive GBS pneumonia had an mAPACHE score ≥60 compared to those with noninvasive GBS pneumonia (25% vs 13%). Interestingly, the proportion of patients with healthcare exposure in the 90 days prior to their case was significantly lower among those with invasive compared to noninvasive GBS pneumonia (28% vs 52%; P < .001).
Table 2.
Severity of Illness, Level of Care, and Healthcare Exposure Among Patients With Monomicrobial Group B Streptococcus Pneumonia
| Variable | Invasive Pneumonia (n = 535) | Noninvasive Pneumonia (n = 424) | P Value |
|---|---|---|---|
| Level of care | .024 | ||
| Intensive care | 139 (26) | 118 (28) | |
| Inpatient ward | 374 (70) | 279 (66) | |
| Nonacute ward | 6 (1) | 17 (4) | |
| Outpatient | 16 (3) | 10 (2) | |
| mAPACHE scorea | <.001 | ||
| ≥60 (severe disease) | 135 (25) | 57 (13) | |
| 30–59 (moderate disease) | 317 (59) | 230 (54) | |
| <30 (mild disease) | 26 (5) | 44 (10) | |
| Insufficient data to determine | 57 (11) | 93 (22) | |
| Healthcare exposure in previous 90 days | 152 (28) | 221 (52) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: mAPACHE, modified Acute Physiology and Chronic Health Evaluation.
The mAPACHE score compares severity of illness among critically ill individuals. The most common missing values were vital signs (pulse, temperature, respiration, and blood pressure). Accordingly, most instances of insufficient data to determine the mAPACHE score reflect cases that were not critically ill.
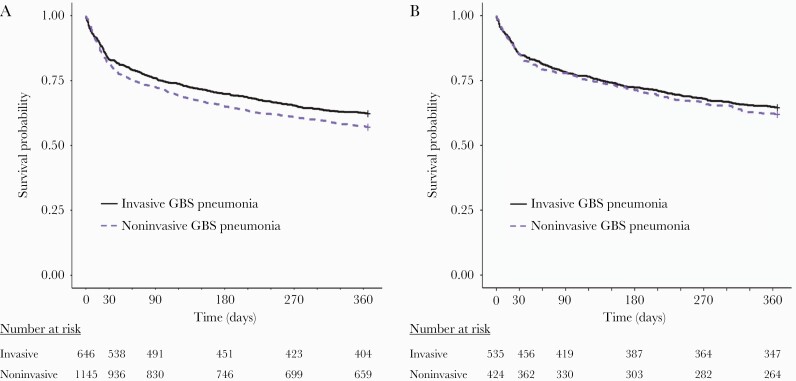
When considering all patients in the cohort, all-cause mortality for invasive GBS pneumonia was slightly lower compared to those with noninvasive GBS pneumonia: 17% compared to 18% at 30 days, and 38% compared to 43% at 1 year, respectively (log-rank test, P < .05; Figure 1A). In contrast, all-cause mortality for invasive and noninvasive GBS pneumonia was similar among patients with cultures that were monomicrobial GBS: both 15% at 30 days, and 35% compared to 38% at 1 year, respectively (log-rank test, P = .43; Figure 1B).
Figure 1.
Kaplan-Meier curves to time to death following invasive or noninvasive pneumonia caused by group B Streptococcus (GBS). A, All cases, including those with polymicrobial culture results. B, Cases with cultures that were monomicrobial for GBS. Solid black lines describe patients with invasive disease; dashed purple lines describe patients with noninvasive disease.
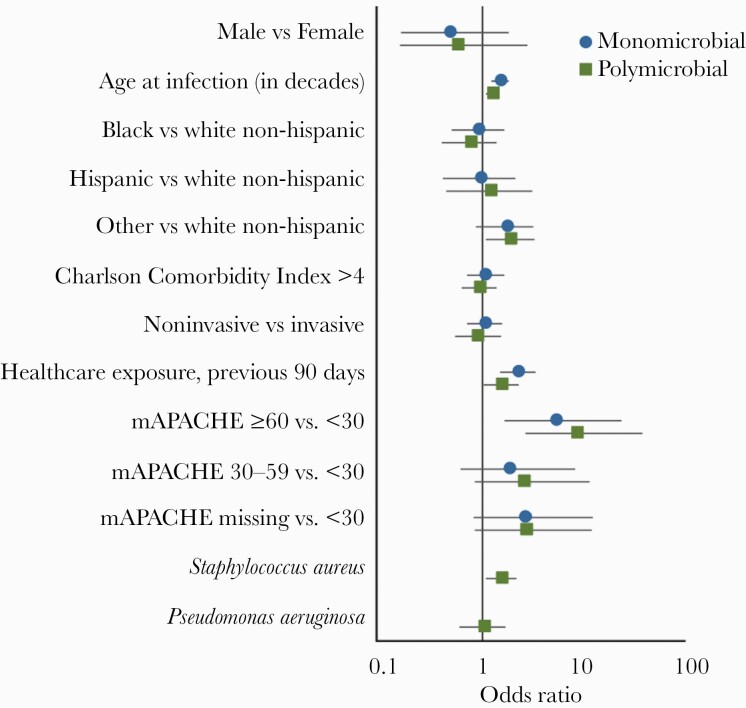
Multivariable logistic regression models accounting for patient characteristics and severity of clinical disease permitted further assessment of all-cause mortality among patients with GBS pneumonia, comparing invasive and noninvasive cases while stratifying by polymicrobial or monomicrobial cultures (Supplementary Tables 1 and 2). At 30 days, within polymicrobial and monomicrobial subsets, all-cause mortality was similar among patients with invasive and noninvasive GBS pneumonia, even when adjusting for clinical characteristics and infection severity. Increasing age, recent healthcare exposure, and an mAPACHE score ≥60 were all associated with an increased risk of mortality at 30 days (Figure 2) and 1 year (Supplementary Figure 1). Isolation of S aureus as a co-pathogen was independently associated with an increased risk of all-cause mortality at 30 days (OR, 1.56 [95% CI, 1.091–2.241]) and 1 year (OR, 1.687 [95% CI, 1.228–2.324]). Pseudomonas aeruginosa and a CCI >4 were associated with an increased risk of all-cause mortality at 1 year (ORs, 1.941 [95% CI, 1.249–3.035] and 1.533 [95% CI, 1.107–2.129], respectively), but not at 30 days.
Figure 2.
Odds ratios and 95% confidence intervals of all-cause mortality at 30 days derived from logistic regression models estimated separately for subjects with monomicrobial group B Streptococcus (GBS) pneumonia (blue dots); and for subjects with polymicrobial GBS pneumonia (green squares). Abbreviation: mAPACHE, modified Acute Physiology and Chronic Health Evaluation.
DISCUSSION
This 10-year retrospective study of a national cohort of VA healthcare users found that patients with noninvasive GBS pneumonia had short-term and long-term mortality that was comparable to that of patients with invasive GBS pneumonia. Furthermore, these infections often occurred in elderly patients with underlying chronic pulmonary disease, diabetes mellitus, heart and kidney disease, stroke, and cancer. This knowledge adds to our understanding of the significance of detecting GBS in the respiratory tract in patients with advanced age and comorbid conditions, contributes to the clinical recognition of noninvasive GBS pneumonia, and may help to inform future efforts to treat and prevent GBS infection.
Recent descriptions of the microbiology associated with pneumonia do not feature GBS as an important pathogen [20, 21]. Our results describing the burden of noninvasive GBS pneumonia, as well as previous reports that pneumonia comprises 10%–12% of invasive GBS infections [2, 4], suggest that GBS may be a respiratory pathogen of consequence, at least among adults with advanced age and underlying medical conditions. Comparison of underlying clinical characteristics and outcomes of patients with invasive and noninvasive GBS pneumonia reveals important distinctions. Patients with invasive GBS pneumonia were older and had a higher burden of comorbidities than patients classified as having noninvasive pneumonia. Not surprisingly, the most common comorbid condition associated with GBS pneumonia was chronic pulmonary disease, which is associated with bacterial isolation from sputum cultures [22]. Other chronic medical conditions observed frequently in this cohort were similar to those reported for invasive GBS infections, and include diabetes mellitus, heart disease, renal disease, peripheral vascular disease, and a BMI ≥30 kg/m2 [3, 4]. The diversity of chronic underlying conditions in patients with GBS in the respiratory tract suggests a complex and multifactorial pathogenesis [23]. The frequent occurrence of a history of stroke in this cohort suggests that aspiration may be an important antecedent to the development of GBS pneumonia [24]. Cancer was also frequently observed among patients with both invasive and noninvasive GBS pneumonia, which may reflect impaired immunity as a result of the underlying malignancy, of concomitant radiation and chemotherapy, and of reduced nutritional and functional status.
Finding a comparably high rate of mortality among patients with noninvasive compared to invasive GBS pneumonia was unexpected, particularly considering the greater severity of disease among the latter group as indicated by the larger proportion of patients with an mAPACHE score ≥60. Similar mortality rates between invasive and noninvasive GBS pneumonia remained evident even when we focused our analysis on patients with cultures that were monomicrobial for GBS. Assessing patients with monomicrobial cultures may provide insight into the pathogenic potential of GBS in patients with pneumonia. The higher rates of mortality in the polymicrobial subset of patients, conversely, may reflect increased virulence attributable to other pathogens recovered from respiratory cultures. In particular, S aureus and P aeruginosa are important pathogens among patients with previous healthcare exposure and with a high burden of comorbid conditions, including chronic lung disease, as featured in this cohort. While the pathogenic role of GBS may remain difficult to ascertain in patients with polymicrobial cultures, the 30-day mortality rate of 15% among patients with monomicrobial invasive and noninvasive pneumonia bolsters previous assertions that GBS is an “A-list” pathogen [25].
Our study has several limitations. First, our cohort consists of VA healthcare users, who are predominantly male and have a high burden of chronic medical conditions, including tobacco and alcohol use, which may limit the generalizability of our results to the rest of the United States population [26–28]. Second, our study did not assess the impact of GBS serotypes on pneumonia types and outcomes, because these are not routinely determined by the microbiology laboratories serving VA medical centers. Third, in this retrospective study we relied upon microbiological culture results and administrative data to identify and classify patients with invasive or noninvasive pneumonia due to GBS. Pneumonia is a common diagnosis and may not indicate GBS-related disease in every case, despite our case definition that required a microbiological culture positive for GBS. Confirmation of invasive and noninvasive GBS pneumonia in the chart review sample and the analysis of patients with culture results that were monomicrobial for GBS helped to mitigate this concern. The reliance upon administrative data may have failed to include patients with noninvasive GBS pneumonia who did not have an accompanying ICD code, leading to a potential underestimation of the prevalence of GBS-related pneumonia as well as a potential overestimation of disease severity and associated mortality. Finally, sampling bias may have influenced the results, with clinicians more likely to obtain respiratory tract specimens from ill patients with chronic pulmonary conditions, particularly from those able to produce sputum. This bias would also result in an underestimation of the prevalence of GBS-related pneumonia and overestimation of disease severity and associated mortality.
In conclusion, in this large retrospective cohort of patients from the VA healthcare system, GBS pneumonia, whether invasive or noninvasive, was associated with significant mortality. Previous studies have highlighted the growing burden of invasive GBS in adults. This study indicates that noninvasive GBS pneumonia may also have serious clinical implications. The growing recognition of GBS as an important pathogen, by itself and in the context of polymicrobial infections, may inform the clinical care of individuals who have GBS in their respiratory tract, including pathogen-directed antibacterial therapy and efforts to prevent infection.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Patient consent. The institutional review board at the Veterans Affairs (VA) Northeast Ohio Healthcare System approved the study protocol and granted a waiver of informed consent.
Disclaimer. The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of the Department of Veterans Affairs or the United States government.
Financial support. This work was supported by Pfizer and by funds and facilities provided by the Cleveland Geriatric Research Education and Clinical Center at the VA Northeast Ohio Healthcare System.
Potential conflicts of interest. R. L. P. J. and F. P. have received research funding from Merck. R. L. P. J. has participated in advisory boards for Pfizer. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Skoff TH, Farley MM, Petit S, et al. . Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin Infect Dis 2009; 49:85–92. [DOI] [PubMed] [Google Scholar]
- 2. Francois Watkins LK, McGee L, Schrag SJ, et al. . Epidemiology of invasive group B streptococcal infections among nonpregnant adults in the United States, 2008-2016. JAMA Intern Med 2019; 179:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pitts SI, Maruthur NM, Langley GE, et al. . Obesity, diabetes, and the risk of invasive group B streptococcal disease in nonpregnant adults in the United States. Open Forum Infect Dis 2018; 5:ofy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jump RLP, Wilson BM, Baechle D, et al. . Risk factors and mortality rates associated with invasive group B Streptococcus infections among patients in the US Veterans Health Administration. JAMA Netw Open 2019; 2:e1918324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blancas D, Santin M, Olmo M, Alcaide F, Carratala J, Gudiol F.. Group B streptococcal disease in nonpregnant adults: incidence, clinical characteristics, and outcome. Eur J Clin Microbiol Infect Dis 2004; 23:168–73. [DOI] [PubMed] [Google Scholar]
- 6. McLaughlin JM, Peyrani P, Furmanek S, et al. . Burden of adults hospitalized with group B streptococcal infection. J Infect Dis 2021; 224:1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cumming CG, Ross PW.. Group B streptococci (GBS) in the upper respiratory tract of schoolchildren. Health Bull (Edinb) 1982; 40:81–5. [PubMed] [Google Scholar]
- 8. Zwart S, Ruijs GJ, Sachs AP, et al. . Beta-haemolytic streptococci isolated from acute sore-throat patients: cause or coincidence? A case-control study in general practice. Scand J Infect Dis 2000; 32:377–84. [DOI] [PubMed] [Google Scholar]
- 9. Mee-Marquet N van der, Fourny L, Arnault L, et al. . Molecular characterization of human-colonizing Streptococcus agalactiae strains isolated from throat, skin, anal margin, and genital body sites. J Clin Microbiol 2008; 46:2906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bej TA, Christian RL, Sims SV, et al. . Influence of microbiological culture results on antibiotic choices for Veterans with hospital-acquired pneumonia and ventilator-associated pneumonia [manuscript published online ahead of print 4 June 2021]. Infect Control Hosp Epidemiol 2008. doi: 10.1017/ice.2021.186. [DOI] [PubMed] [Google Scholar]
- 11. Quan H, Sundararajan V, Halfon P, et al. . Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 12. Fortis S, O’Shea AMJ, Beck BF, et al. . An automated computerized critical illness severity scoring system derived from APACHE III: modified APACHE. J Crit Care 2018; 48:237–42. [DOI] [PubMed] [Google Scholar]
- 13. R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 14. Grolemund G, Wickham H.. Dates and times made easy with lubridate. J Stat Softw 2011; 40:1–25. [Google Scholar]
- 15. Wickham H. The split-apply-combine strategy for data analysis. J Stat Softw 2011; 40:1–29. [Google Scholar]
- 16. Mahto A. Splitstackshape: stack and reshape datasets after splitting concatenated values. 2019. Available at: https://CRAN.R-project.org/package=splitstackshape. Accessed 28 February 2020.
- 17. Wickham H, Averick M, Bryan J, et al. . Welcome to the tidyverse. J Open Source Softw 2019; 4:1686. [Google Scholar]
- 18. Therneau TM, Lumley T.. Survival: survival analysis. 2019. Available at: https://CRAN.R-project.org/package=survival. Accessed 24 October 2019.
- 19. Kassambara A, Kosinski M, Biecek P, Fabian S.. Survminer: drawing survival curves using “Ggplot2.” 2020. Available at: https://CRAN.R-project.org/package=survminer. Accessed 3 September 2020.
- 20. Jain S, Self WH, Wunderink RG, et al. . Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haessler S, Lindenauer PK, Zilberberg MD, et al. . Blood cultures versus respiratory cultures: 2 different views of pneumonia. Clin Infect Dis 2020; 71:1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naidus EL, LaSalvia MT, Marcantonio ER, Herzig SJ.. The diagnostic yield of noninvasive microbiologic sputum sampling in a cohort of patients with clinically diagnosed hospital-acquired pneumonia. J Hosp Med 2018; 13:34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Edwards MS, Baker CJ.. Group B streptococcal infections in elderly adults. Clin Infect Dis 2005; 41:839–47. [DOI] [PubMed] [Google Scholar]
- 24. Verghese A, Berk SL, Boelen LJ, Smith JK.. Group B streptococcal pneumonia in the elderly. Arch Intern Med 1982; 142:1642–5. [PubMed] [Google Scholar]
- 25. Barshak MB. Group B Streptococcus, an A-list pathogen in nonpregnant adults. JAMA Intern Med 2019; 179:488–9. [DOI] [PubMed] [Google Scholar]
- 26. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM.. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med 2000; 160:3252–7. [DOI] [PubMed] [Google Scholar]
- 27. Dursa EK, Barth SK, Bossarte RM, Schneiderman AI.. Demographic, military, and health characteristics of VA health care users and nonusers who served in or during Operation Enduring Freedom or Operation Iraqi Freedom, 2009–2011. Public Health Rep 2016; 131:839–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta NM, Lindenauer PK, Yu PC, et al. . Association between alcohol use disorders and outcomes of patients hospitalized with community-acquired pneumonia. JAMA Netw Open 2019; 2:e195172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.