Abstract
Background
Limited data exist examining the association between incident cancer and cumulative integrase inhibitor (INSTI) exposure.
Methods
Participants were followed from baseline (latest of local cohort enrollment or January 1, 2012) until the earliest of first cancer, final follow-up, or December 31, 2019. Negative binomial regression was used to assess associations between cancer incidence and time-updated cumulative INSTI exposure, lagged by 6 months.
Results
Of 29 340 individuals, 74% were male, 24% were antiretroviral treatment (ART)-naive, and median baseline age was 44 years (interquartile range [IQR], 36–51). Overall, 13 950 (48%) individuals started an INSTI during follow-up. During 160 657 person-years of follow-up ([PYFU] median 6.2; IQR, 3.9–7.5), there were 1078 cancers (incidence rate [IR] 6.7/1000 PYFU; 95% confidence interval [CI], 6.3–7.1). The commonest cancers were non-Hodgkin lymphoma (n = 113), lung cancer (112), Kaposi’s sarcoma (106), and anal cancer (103). After adjusting for potential confounders, there was no association between cancer risk and INSTI exposure (≤6 months vs no exposure IR ratio: 1.15 [95% CI, 0.89–1.49], >6–12 months; 0.97 [95% CI, 0.71–1.32], >12–24 months; 0.84 [95% CI, 0.64–1.11], >24–36 months; 1.10 [95% CI, 0.82–1.47], >36 months; 0.90 [95% CI, 0.65–1.26] [P = .60]). In ART-naive participants, cancer incidence decreased with increasing INSTI exposure, mainly driven by a decreasing incidence of acquired immune deficiency syndrome cancers; however, there was no association between INSTI exposure and cancer for those ART-experienced (interaction P < .0001).
Conclusions
Cancer incidence in each INSTI exposure group was similar, despite relatively wide CIs, providing reassuring early findings that increasing INSTI exposure is unlikely to be associated with an increased cancer risk, although longer follow-up is needed to confirm this finding.
Keywords: antiretroviral treatment, cancer, cohort, HIV, integrase inhibitors
In this analysis including 29 340 individuals from RESPOND and 1078 cancer events, the incidence of cancer decreased as cumulative INSTI exposure increased in ART-naïve individuals, however there was no association between cancer risk and INSTI exposure in ART-experienced individuals.
Since the introduction of antiretroviral therapy (ART), human immunodeficiency virus (HIV) has been transformed into a manageable, chronic condition, with life expectancy of people with HIV (PWH) approaching that of the general population, although this varies across subgroups of the population [1–4]. However, with an aging population of PWH, there has been an increase in the burden of comorbidities, including cancer [3, 5, 6]. Because ART use is lifelong, it is crucial to identify any associations between ART exposure and the risk of comorbidities [7–9].
Cancer is one of the leading causes of death amongst PWH [6, 10, 11]. Since the introduction of ART, the incidence of acquired immune deficiency syndrome (AIDS)-defining cancers (ADCs) has significantly decreased. However, there has been an increase in the incidence of non-ADCs (NADCs) reported, which may be partly attributable to the aging of PWH [3, 5, 6, 11–13]. Several studies have assessed the association between ART use and cancer risk, although there are limited data assessing newer antiretrovirals (ARVs), such as second-generation integrase strand transfer inhibitors (INSTIs). Although previous studies have reported an increased risk of anal cancer with exposure to protease inhibitors (PIs), no other associations between ART and NADCs have been reported [14, 15]. In contrast, increasing ART exposure has been shown to be associated with a decreasing risk of ADCs, related to improvements in immune function and viral suppression [13, 16, 17].
Incident cancer and cumulative integrase inhibitors are recommended in international HIV treatment guidelines as first-line treatment for PWH [7–9]. Studies have shown that INSTIs are generally well tolerated and effective in maintaining viral suppression [18–21]. Given that INSTIs are a relatively new drug class, there are limited data assessing long-term clinical outcomes associated with INSTI use, such as cancer. The association between raltegravir (RAL) and the incidence of any cancer has previously been investigated in 2 large cohort studies, with mixed results shown [17, 22]. However, to the best of our knowledge, no studies have examined newer INSTI use more broadly by also including cobicistat-boosted elvitegravir (EVG/c) and dolutegravir (DTG). We aimed to assess whether there was an association between INSTI use and the incidence of cancer, among PWH in real-life settings in the International Cohort Consortium of Infectious Diseases (RESPOND).
METHODS
Study Design and Setting
Participants included in this analysis were from RESPOND, which is a collaboration, initiated in 2017, including approximately 30 000 PWH from 17 cohorts across Europe and Australia [23]. Clinical and demographic data are collected on participants in RESPOND during routine clinical care and reported at the time of enrollment into RESPOND and prospectively annually thereafter. Data are also retrospectively collected on the 5 years before enrollment, and earlier if available. Data on clinical events, including cancers, are collected in real time. All of these events that occur during the validation period (12 months before the last local cohort visit before RESPOND enrollment and onwards) are reported using a case report form. These events are centrally validated by clinicians at the RESPOND coordinating center using a prespecified algorithm [24]. RESPOND therefore includes a mixture of validated and nonvalidated events, and sensitivity analyses in RESPOND are performed including validated events only.
Patient Consent Statement
Ethics approval for RESPOND is the responsibility of each participating site or cohort, and it includes ensuring that all necessary documents and approvals by ethics committee (institutional review board or independent ethics committee) are obtained according to local or national regulations before initiating study-related activities and in case of any future amendments to the study protocol. Participants consent to share data with RESPOND according to local requirements. Enrolled participants are pseudonymized by assigning a unique identifier by the participating cohort before data transfer. According to national or local requirements, all cohorts in RESPOND have approval to share data with RESPOND. Data are stored on secure servers at the RESPOND coordinating center in Copenhagen, in accordance with current legislation and under approval by The Danish Data Protection Agency (approval number 2012-58-0004, RH-2018-15, 26/1/2018), under the EU General Data Protection Regulation (2016/679).
Participants
The inclusion criteria for RESPOND are detailed elsewhere [23]. For this analysis, participants in RESPOND were included if they were aged 18 years or older at baseline, defined as the latest of local cohort enrollment and January 1, 2012, and had a CD4 cell count and viral load (VL) measurement either 1 year before or within 12 weeks after baseline. Individuals were excluded if they had missing information on gender or no follow-up data. Cohorts in RESPOND with low event reporting at the initiation of the project were excluded.
Outcome Definition
The primary outcome was any incident cancer during follow-up. Precancers (including cervical and anal dysplasia and carcinoma in situ), relapse of a primary cancer, and nonmelanoma skin cancers were not included. More detail on the cancer definition is provided in the RESPOND manual of operations [24]. Individuals were followed from baseline until first cancer event, final follow-up, or December 31, 2019 (administrative censoring date), whichever occurred first. Individuals who had cancer before baseline were included in the main analysis. For these individuals, cancer during follow-up was counted if the type of cancer was different from the one that occurred before baseline. A sensitivity analysis was performed excluding individuals with any cancer before baseline.
Definitions of Potential Confounders
The following variables were considered as potential confounders in the analysis: age, gender, ethnicity, body mass index (BMI), smoking status, geographical region, HIV risk acquisition group, ART-experience and VL status, current CD4 cell count, CD4 cell nadir, prior AIDS-defining events, hepatitis C, hepatitis B, and prior comorbidities including hypertension, diabetes, cancer, end-stage liver and renal disease, cardiovascular disease, chronic kidney disease, and dyslipidemia. Definitions of all variables are provided in the Table 1 footnote.
Table 1.
Characteristics of Participants at Baseline
| Characteristics | Overall | ||
|---|---|---|---|
| n | (%) | ||
| Total | 29 340 | (100) | |
| Gender | Male | 21 818 | (74.4) |
| Female | 7478 | (25.5) | |
| Transgender | 44 | (0.1) | |
| Ethnicitya | White | 20 419 | (82.8) |
| Black | 2983 | (12.1) | |
| Other | 1267 | (5.1) | |
| Body Mass Index (kg/m2)a | <18.5 | 873 | (4.6) |
| 18.5–<25 | 11 321 | (59.9) | |
| 25–<30 | 1547 | (8.2) | |
| 30+ | 5159 | (27.3) | |
| Geographical Regionb | Western Europe | 12 810 | (43.7) |
| Southern Europe | 6626 | (22.6) | |
| Northern Europe | 7069 | (24.1) | |
| Eastern Europe | 2832 | (9.7) | |
| HIV Riska | MSM | 13 229 | (47.0) |
| IDU | 3993 | (14.2) | |
| Heterosexual | 10 253 | (36.4) | |
| Other | 654 | (2.3) | |
| ART History | ART naive | 7172 | (24.4) |
| ART experienced, VL <200 cps/mL | 19 951 | (68.0) | |
| ART experienced, VL ≥200 cps/mL | 2217 | (7.6) | |
| Smoking Statusa | Never | 8207 | (44.0) |
| Current | 8196 | (43.9) | |
| Previous | 2261 | (12.1) | |
| Hepatitis Ca,c | 5940 | (23.6) | |
| Hepatitis Ba,d | 1340 | (5.5) | |
| Prior hypertensiona,e | 5683 | (23.5) | |
| Prior diabetesa,f | 1170 | (5.0) | |
| Prior AIDSa | 5785 | (20.9) | |
| Prior cancera | 1742 | (6.1) | |
| Prior end-stage liver diseasea | 184 | (0.9) | |
| Prior end-stage renal diseasea | 102 | (0.4) | |
| Prior cardiovascular diseasea,g | 666 | (2.5) | |
| Prior chronic kidney diseasea,h | 541 | (2.0) | |
| Prior dyslipidemiaa,i | 17 984 | (82.5) | |
| Baseline date, month/year, median (IQR) | 01/12 | (01/12–02/13) | |
| Age, years, median (IQR) | 44.3 | (36.2–51.3) | |
| CD4 cell nadir, cells/mm3,j, median (IQR) | 241.0 | (120.0–384.0) | |
| CD4 at baseline, cells/mm3,j, median (IQR) | 524.0 | (357.0–715.0) | |
| Viral load at baseline, cps/mL, median (IQR) | 39.0 | (19.0–2228.5) | |
| Total duration of previous ART, years, median (IQR) | 7.7 | (3.0–13.9) | |
Abbreviations: AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; cps, copies; HIV, human immunodeficiency virus; IDU, intravenous drug user; INSTI, integrase inhibitor; IQR, interquartile range; MSM, men who have sex with men; VL, viral load.
Due to small numbers, Australia was combined with Northern Europe, and Eastern Central Europe combined with Eastern Europe.
Hepatitis C (HCV) was defined by use of anti-HCV medication, a positive HCV antibody test, a positive HCV RNA qualitative test, HCV RNA-VL >615 IU/mL, and/or a positive genotype test [25].
Hepatitis B (HBV) was defined by a positive HBV surface antigen and/or HBV RNA-VL >357 IU/mL.
Hypertension was confirmed by use of antihypertensives at any time before regimen start or if the most recent systolic or diastolic blood pressure measurement before regimen start was higher than 140 or 90 mmHg, respectively.
Diabetes was defined by a reported diagnosis, use of antidiabetic medication, glucose ≥11.1 mmol/L, and/or HbA1c ≥6.5% or ≥48 mmol/mol.
Cardiovascular disease (CVD) was defined using a composite diagnosis of myocardial infarction, stroke, or invasive cardiovascular procedure.
Chronic kidney disease (CKD) was confirmed if there were 2 consecutive measurements of estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 measured at least 3 months apart. eGFR was calculated using the CKD-EPI creatinine equation [26].
Dyslipidemia was defined as total cholesterol >239.4 mg/dL or HDL cholesterol <34.7 mg/dL or triglyceride >203.55 mg/dL or use of lipid-lowering treatments [27].
CD4 and CD8 cell counts were taken as the most recent measurements in the 12 months before baseline. If no measurements were taken before baseline, the first measurement within 12 weeks after baseline was used, and CD4 cell nadir was recorded as the same as CD4 cell count at baseline.
Denominator for percentages is all participants with nonmissing data.
NOTES: Total unknown no. (%): ethnicity 4671 (15.9), body mass index 10 440 (35.6), smoking status 10 676 (36.4), HIV risk 1211 (4.1), HCV 4145 (14.1), HBV 4853 (16.5), hypertension 5126 (17.5), diabetes 6116 (20.8), prior AIDS 1594 (5.4), prior cancer 610 (2.1), prior end-stage liver disease 9143 (31.2), prior end-stage renal disease 2480 (8.5), prior CVD 2865 (9.8), prior CKD 2865 (9.8), dyslipidemia 7543 (25.7).
Statistical Methods
Participant follow-up was divided into consecutive monthly periods. Cumulative exposure to each ARV was calculated at the start of each month, by summing any prior exposure to each ARV, as per previous analyses performed by the Data Collection on Adverse events of Anti-HIV Drugs (D:A:D) cohort study [27, 28]. Time-updated confounders were also calculated for each participant-month using last observation carried forward.
The crude incidence of any cancer was calculated by INSTI exposure, with exposure grouped as no exposure, ≤6 months exposure, >6 months–1 year exposure, >1–2 years exposure, >2–3 years exposure, and >3 years exposure. Those with no exposure were naive to INSTIs. Because cancer is a slow developing event, it is unlikely that the current cancer risk can be attributable to the current exposure of any ART, if use has only been short. Therefore, INSTI exposure was lagged by 6 months in all analyses. Furthermore, this approach reduces any potential confounding by indication, where individuals at higher risk of cancer or with underlying symptoms but without a clinical diagnosis may be preferentially given INSTIs, due to their presumed favorable safety profile or better efficacy [8, 29].
The association between the incidence of any cancer and lagged cumulative exposure to INSTIs, adjusted for potential confounders, was assessed using negative binomial regression with generalized estimating equations and robust standard errors. Each potential confounder listed above was added to a univariable model, and those with P < .1 or those defined to be a confounder a priori were included in multivariable models. To ensure we did not include variables that may lie on the causal pathway between INSTI exposure and cancer risk, all variables were fixed at baseline, apart from smoking status that was included as time-updated.
Analyses were repeated only including validated cancer events and analyzing ADCs (Kaposi’s sarcoma, non-Hodgkin lymphoma, cervical cancer) and NADCs separately.
Participants in RESPOND were INSTI-naive at enrollment; therefore, those on INSTIs started the INSTIs during follow-up. Thus, we performed a sensitivity analysis including only participants who started any new ART after baseline, regardless of whether INSTIs were included, and redefined baseline as the date of regimen start.
A priori, subgroup analyses were planned to investigate whether the association between INSTI exposure and cancer incidence differed between age groups, by baseline smoking status, by prior CD4 nadir, or between those who were ART-naive at baseline compared with those who were ART-experienced with a VL <200 or ≥200 copies/mL. These were performed by including an interaction term in the multivariable regression model between INSTI exposure and the subgroup of interest.
Finally, to investigate the potential for confounding by indication, we calculated the proportion of those with cancer who switched their ART regimen within 1 year after cancer diagnosis and compared this between drug classes.
Missing data for categorical variables were accounted for by including an unknown category. Sensitivity analyses were performed using complete case analysis where individuals with missing data on any variables included in the multivariable model were excluded.
Analyses were performed using Stata/MP 16.1 (StataCorp LLC). All P values are 2 sided and P < .05 was defined as statistically significant.
RESULTS
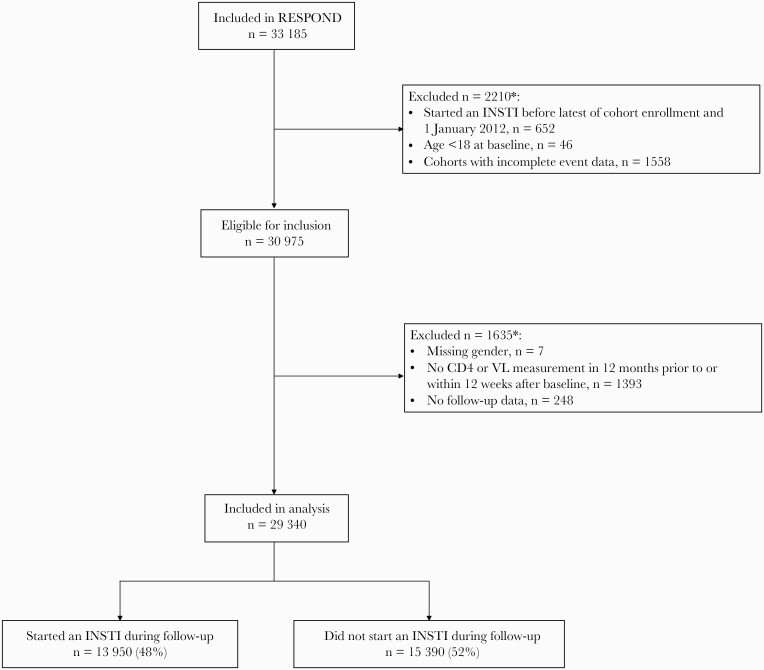
Of 30 975 eligible RESPOND participants, 29 340 (94.7%) were included. A higher proportion of those excluded were ART-naive (41.8% vs 24.4%, P < .0001) and a lower proportion had a prior CD4 nadir <200 cells/mm3 (17.6% vs 40.6%, P < .0001) and prior comorbidities (53.6% vs 71.3%, P < .0001). Figure 1 shows the study flow for this analysis.
Figure 1.
Study flow. ∗More than 1 reason can apply. INSTI, integrase inhibitor; VL, viral load.
Baseline characteristics of individuals included are shown in Table 1. Of 29 340 individuals included, 74% were male, 83% were of white ethnicity, 24% were ART-naive, and 68% were ART-experienced with a VL <200 copies/mL. A similar proportion of individuals were never smokers and current smokers (44% never smoked, 44% current smokers, 12% previous smokers). Median age was 44 years (interquartile range [IQR], 36–51) and median CD4 cell count was 524 cells/mm3 (IQR, 357–715). Overall, 21% of individuals had a prior AIDS-defining event and there was a high proportion of comorbidities including prior cancer (6%), diabetes (5%), and hypertension (24%).
By the end of follow-up, 13 950 (48%) individuals had been exposed to 1 or more INSTIs: 8607 to DTG, 3328 EVG/c, 3266 RAL, and 845 bictegravir (BIC). For those exposed to INSTIs, median cumulative exposure was 32 months (IQR, 16–47) and highest on DTG (DTG 29 [IQR, 14–43], EVG/c 27 [IQR, 15–41], RAL 22 [IQR, 9–45], BIC 4 [IQR, 2–6]). The most common other third drugs prescribed were efavirenz (n = 11 636; median cumulative exposure 59 [18–114] months), boosted darunavir (n = 8324; median cumulative exposure 41 [IQR, 17–49] months), ritonavir-boosted lopinavir (n = 7990; median cumulative exposure 43 [IQR, 15–89] months), and any atazanavir (n = 7221; median cumulative exposure 56 [IQR, 21–95] months).
During 160 657 person-years of follow-up ([PYFU] median 6.2 years [IQR, 3.9–7.5]), there were 1078 incident cancers (incidence rate [IR] 6.7/1000 PYFU; 95% confidence interval [CI], 6.3–7.1]): 243 AIDS and 835 non-AIDS cancers. The commonest cancers were non-Hodgkin lymphoma (n = 113, 10.5%), lung cancer (n = 112, 10.4%), Kaposi’s sarcoma (n = 106, 9.8%), and anal cancer (n = 103, 9.6%) (Supplementary Table 1).
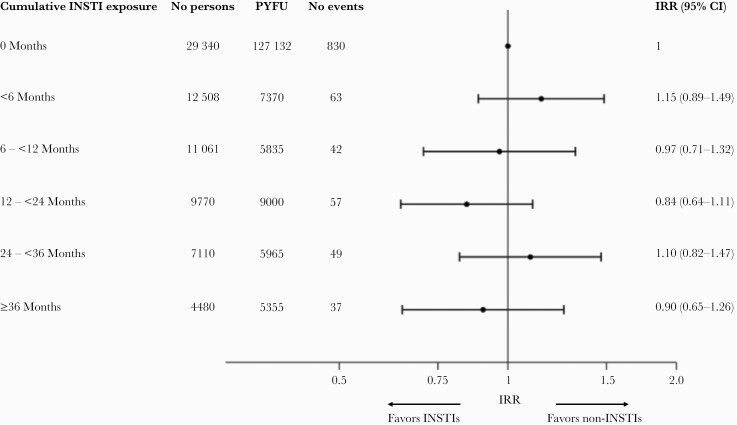
The crude incidence of any cancer was similar for those with no exposure to INSTIs compared to those with any exposure to INSTIs, and this remained the case after adjusting for potential confounders (≤6 months exposure vs no exposure-adjusted IRR: 1.15 [95% CI, 0.89–1.49], >6–12 months; 0.97 [95% CI, 0.71–1.32], >12–24 months; 0.84 [95% CI, 0.64–1.11], >24–36 months; 1.10 [95% CI, 0.82–1.47], >36 months; 0.90 [95% CI, 0.65–1.26] [P = .60]; P value for trend .56) (Figure 2).
Figure 2.
Association between any cancer risk and cumulative exposure to integrase strand transfer inhibitors (INSTIs), adjusted for potential confounders. Incidence rate ratio (IRR) adjusted for age, sex, ethnicity, human immunodeficiency virus risk group, antiretroviral treatment experience, CD4 cell count, CD4 nadir, body mass index, geographical region, hepatitis B, prior diabetes, prior acquired immune deficiency syndrome, prior cancer, prior chronic kidney disease, prior cardiovascular disease, prior end-stage liver disease (all fixed at baseline), smoking status (time updated). Note, INSTI exposure is lagged by 6 months. CI, confidence interval; PYFU, person years of follow-up.
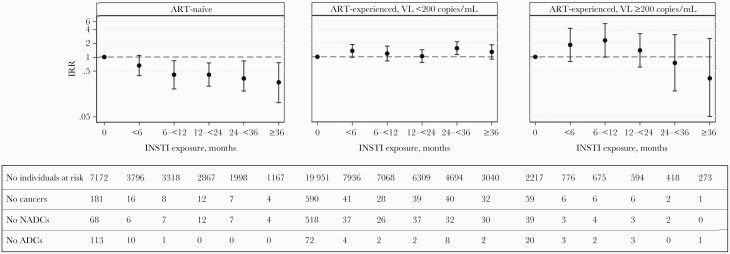
There was a significant interaction between INSTI exposure and baseline ART experience (interaction P < .0001). For those ART-naive at baseline (n = 7172, 228 cancers), the adjusted cancer incidence decreased as cumulative INSTI exposure increased, and this was mainly driven by a decreasing incidence of ADCs (Figure 3). In contrast, for those ART-experienced with a suppressed VL (n = 19 951, 770 cancers) or with uncontrolled viremia (n = 2217, 80 cancers), the incidence of cancer was similar across all INSTI exposure categories. There was no interaction between INSTI exposure and age, CD4 cell nadir, or baseline smoking status (interaction P > .1 for all).
Figure 3.
Adjusted incidence of cancer, by integrase inhibitor (INSTI) exposure compared to no exposure, stratified by antiretroviral treatment (ART)-experience at baseline. Incidence rate ratio (IRR) calculated from a negative binomial regression model, adjusted for the same confounders as the main analysis, and including an interaction term between INSTI exposure and ART-experience at baseline. ADC, acquired immune deficiency syndrome (AIDS)-defining cancers; NADC, non-AIDS-defining cancers; VL, viral load.
Analyses were repeated separately for ADCs and NADCs among all participants; results were similar for NADCs showing no association between INSTI exposure and the incidence of NADCs (≤6 months exposure vs no exposure aIRR: 1.22 [95% CI, 0.90–1.65], >6–12 months; 1.25 [95% CI, 0.89–1.74], >12 < 24 months; 1.11 [95% CI, 0.84–1.48], >24–36 months; 1.31 [95% CI, 0.95–1.80], >36 months; 1.16 [95% CI, 0.82–1.65] [P = .32]). However, the incidence of ADCs decreased as INSTI exposure increased, compared to those with no INSTI exposure (≤6 months exposure vs no exposure aIRR: 0.86 [95% CI, 0.52–1.43], >6–12 months; 0.31 [95% CI, 0.13–0.77], >12–24 months; 0.22 [95% CI, 0.09–0.53], >24–36 months; 0.56 [95% CI, 0.28–1.15], >36 months; 0.25 [95% CI, 0.08–0.78] [P = .0002]).
Analyses were rerun only including centrally validated cancer events. Because the study validation period started after January 1, 2015 for 86% of participants and INSTIs were not widely used before this date, this analysis was left censored at the start of the validation period, January 1, 2015. Of the 1078 cancers experienced during follow-up, 502 (395 NADCs, 107 ADCs) occurred after this date and were validated, during 65 073 PYFU (IR 7.7/1000 PYFU; 95% CI, 7.1–8.4). Results were similar to the overall analysis (P = .06) (Table 2).
Table 2.
Adjusted Incidence of Cancer, by Increasing Cumulative Exposure to INSTIs, Calculated From a Range of Sensitivity Analyses
| Analysis | No. Individuals Included | No. Cancers Included | 0 Months | <6 Months | 6–<12 Months | >12–24 Months | >24–36 Months | 36+ Months | Global P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Primary analysis | 29 340 | 1078 | Adjusted IRR (95% CI) | 1 | 1.15 (0.89–1.49) | 0.97 (0.71–1.32) | 0.84 (0.64–1.11) | 1.10 (0.82–1.47) | 0.90 (0.65–1.26) | .60 |
| No. cancers (PYFU) | 830 (127 132) | 63 (7370) | 42 (5835) | 57 (9000) | 49 (5965) | 37 (5355) | ||||
| All NADC | 29 340 | 835 | Adjusted IRR (95% CI) | 1 | 1.22 (0.90–1.65) | 1.25 (0.89–1.74) | 1.11 (0.84–1.48) | 1.31 (0.95–1.80) | 1.16 (0.82–1.65) | .32 |
| No. cancers (PYFU) | 625 (127 132) | 46 (7370) | 37 (5835) | 52 (9000) | 41 (5965) | 34 (5355) | ||||
| All ADC | 29 340 | 243 | Adjusted IRR (95% CI) | 1 | 0.86 (0.52–1.43) | 0.31 (0.13–0.77) | 0.22 (0.09–0.53) | 0.56 (0.28–1.15) | 0.25 (0.08–0.78) | .0002 |
| No. cancers (PYFU) | 205 (127 132) | 17 (7370) | 5 (5835) | 5 (9000) | 8 (5965) | 3 (5355) | ||||
| All validated cancers | 25 118 | 502 | Adjusted IRR (95% CI) | 1 | 1.25 (0.88–1.78) | 0.98 (0.65–1.47) | 0.65 (0.45–0.93) | 0.80 (0.56–1.14) | 0.77 (0.54–1.10) | .06 |
| No. cancers (PYFU) | 336 (43 381) | 36 (3300) | 26 (3061) | 34 (5795) | 34 (4656) | 36 (4880) | ||||
| Excluding individuals with cancer before baseline | 27 958 | 983 | Adjusted IRR (95% CI) | 1 | 1.12 (0.85–1.47) | 0.94 (0.68–1.31) | 0.81 (0.61–1.08) | 1.08 (0.80–1.46) | 0.87 (0.61–1.23) | .56 |
| No. cancers (PYFU) | 759 (119 581) | 57 (6910) | 38 (5499) | 51 (8470) | 45 (5614) | 33 (5023) | ||||
| Only including individuals who started a new ART | 20 782 | 574 | Adjusted IRR (95% CI) | 1 | 0.92 (0.71–1.22) | 0.84 (0.62–1.51) | 0.75 (0.57–0.99) | 0.75 (0.64–1.18) | 0.77 (0.55–1.08) | .28 |
| No. cancers (PYFU) | 312 (40 821) | 64 (7801) | 46 (6171) | 64 (9535) | 49 (6325) | 39 (5647) | ||||
| Only including individuals who started a new ART to which they were naive | 9983 | 274 | Adjusted IRR (95% CI) | 1 | 0.83 (0.56–1.24) | 0.71 (0.45–1.14) | 0.57 (0.37–0.88) | 0.74 (0.46–1.19) | 0.62 (0.36–1.07) | .08 |
| No. cancers (PYFU) | 171 (21 005) | 28 (3559) | 19 (2854) | 23 (4311) | 19 (2767) | 14 (2414) | ||||
| Using complete case analysis to account for missing data | 7071 | 298 | Adjusted IRR (95% CI) | 1 | 1.02 (0.58–1.78) | 1.72 (1.05–2.84) | 1.19 (0.74–1.91) | 1.50 (0.91–2.47) | 1.25 (0.72–2.15) | .21 |
| No. cancers (PYFU) | 218 (33 507) | 13 (1933) | 17 (1571) | 19 (2536) | 17 (1794) | 14 (1756) | ||||
| Adjusting the primary analysis for cumulative time spent with CD4 count <200 cells/mm3 | 29 340 | 1078 | Adjusted IRR (95% CI) | 1 | 1.15 (0.88–1.49) | 0.96 (0.70–1.32) | 0.84 (0.64–1.10) | 1.08 (0.81–1.45) | 0.89 (0.64–1.25) | .58 |
| No. cancers (PYFU) | 830 (127 132) | 63 (7370) | 42 (5835) | 57 (9000) | 49 (5965) | 37 (5355) |
Abbreviations: ADC, acquired immune deficiency syndrome (AIDS)-defining cancers; ART, antiretroviral treatment; CI, confidence interval; INSTI, integrase strand transfer inhibitor; IRR, incidence rate ratio; NADC, non-AIDS-defining cancers; PYFU, person years of follow-up.
NOTE: Incidence rate ratio adjusted for age, sex, ethnicity, human immunodeficiency virus risk group, ART experience, CD4 cell count, CD4 nadir, body mass index, geographical region, hepatitis B, prior diabetes, prior AIDS, prior cancer, prior chronic kidney disease, prior cardiovascular disease, prior end-stage liver disease (all fixed at baseline), and smoking status (time updated).
Analyses were also repeated only including individuals who started a new ART during follow-up. This analysis included 20 782 individuals and 574 events during 75 566 PYFU (IR 7.6/1000 PYFU [7.0–8.2]), and it showed no association between cancer incidence and INSTI exposure (P = .28) (Table 2).
Further sensitivity analyses excluding individuals with any cancer before baseline, adjusting for cumulative time spent with a CD4 count <200 cells/mm3 in the primary model, and using complete case analysis to account for missing data, all showed similar results to the overall analysis (Table 2). Findings were also similar when subdividing cancers into infectious, smoking, and BMI-related cancers, although there was less statistical power for this analysis (data not shown).
Finally, to explore whether individuals with cancer are preferentially given INSTIs, we calculated the proportion of individuals who switched ART within 1 year after cancer diagnosis. Of 1078 individuals with cancer, 337 (31.3%) switched ART within 1 year. The majority of these switched to an INSTI (62% switched to INSTIs, 14% switched to nonnucleoside reverse-transcriptase inhibitors, 14% switched to PIs, 10% switched to other).
DISCUSSION
In this analysis including almost 30 000 PWH and over 1000 cancer events, we found no overall association between cumulative INSTI exposure and cancer risk. This is one of the first studies assessing the association between INSTI use and cancer in real-life settings and using centrally validated events. Incident cancer and cumulative integrase inhibitor use is becoming increasingly widespread because INSTIs are recommended as first-line treatment for PWH [7, 8], and, because cancer incidence is increasing among the aging population of PWH, it is of vital importance to identify potential risk factors for cancer, including the impact of different ARVs. Such analyses require substantial power and follow-up time, limiting the availability of high-quality data. In addition, studies have shown that other risk factors for cancer, such as smoking, coinfections, and a low CD4 cell count, are more prevalent in PWH [30]. For these reasons, our results showing no increased risk of NADCs and a lower risk of ADCs on INSTIs are reassuring for PWH and clinicians. However, we do acknowledge that we cannot rule out the possibility that cancer risk differs among individual INSTIs, which we were not able to look at. In addition, because the 95% CIs for the cancer IRR in each INSTI exposure group were relatively wide, we cannot rule out a clinically meaningful effect of INSTI exposure on cancer risk, although there was no indication of a gradual change in cancer incidence with increasing exposure to INSTIs.
Subgroup analysis showed that for individuals who were ART-experienced at baseline, there was no association between INSTI exposure and cancer incidence; however, for those who were ART-naive, cancer incidence decreased with increasing INSTI exposure, which was mainly driven by a decreasing incidence of ADCs. Many studies have previously shown that initiating ART for PWH who are ART-naive can lead to a drastic reduction in the incidence of AIDS events, including ADCs, due to improvements in immune function and viral suppression [31–33]. Our results are likely showing the impact of starting an effective ART-regimen in those who are naive, rather than being specific to INSTIs, although INSTIs have been shown to be particularly effective at quickly improving immune function and lowering viremia [18–21].
Previous studies have assessed the association between RAL and the risk of cancer, with mixed results. The EuroSIDA study [17] and a study conducted at Kaiser Permanente (KP) in California [22] both compared the risk of all cancers between a cohort of PWH treated with RAL after 2007, a cohort starting ART between 2005 and 2007, and a cohort starting an ART regimen after 2007 not containing RAL. Although the EuroSIDA study showed a similar incidence of all cancers in the 3 cohorts [17], the study conducted at KP found an increased risk of NADCs and ADCs for those treated with RAL compared with the other 2 cohorts [22]. In addition, a meta-analysis combining 96-week data from the BENCHMRK and STARMRK clinical trials showed a similar risk of cancer on RAL versus the comparator (efavirenz or placebo) [34], although 96 weeks of follow-up is potentially too short to capture the development of malignancies. Studies that have shown a carcinogenic effect of other drugs have included a wide range of follow-up times: for example, a meta-analysis showing the effect of azathioprine on lymphoma risk included studies with median follow-up ranging from 2.8 years to 9 years [35], and a meta-analysis including 54 studies found an increased risk of breast cancer up to 10 years after stopping hormonal contraceptive use [36].
To the best of our knowledge, there are no data assessing cancer incidence with use of EVG/c, DTG, and BIC, or assessing INSTIs as a class, in real-life settings. Our findings are in line with early animal trials of INSTIs where none of the individual INSTIs showed signs of displaying any carcinogenic effect [37–40].
There are some limitations to this analysis. First, although we were able to include over 1000 cancers, there were still too few events to assess the association between cancer risk and use of individual INSTIs or to investigate the impact on individual cancers. As has been seen with the increased risk of anal cancer with long-term use of PIs, associations between ART and cancers may differ between specific cancer types [14, 15]. Median exposure to INSTIs was 32 months, which may have been too short to detect a signal, given that cancers can take many years to develop. To be included in RESPOND, cohorts providing data from a sample of their participants are requested to include at least half using INSTIs, which may not be representative of the general population of PWH in Europe and Australia. The date of cancer diagnosis in RESPOND is reported as the date cancer is confirmed through a biopsy, if available. It is possible that on first suspicion of cancer, a clinician may switch an individual’s ART to ensure there are few interactions with chemotherapy; we lagged analyses to address this potential bias. There are also some missing data in some cohorts in RESPOND for key variables: for example, 36% of participants are missing smoking status, and although we have adjusted for key confounders in our analysis, we cannot exclude the possibility of unmeasured confounding or confounding by indication. We have explored the possibility of confounding by indication through a range of methods, including lagging INSTI exposure, assessing which treatments individuals switch to after cancer diagnosis, and only including individuals who switched treatment during follow-up to exclude those on long-term stable ART, all with consistent results. However, other statistical methods such as inverse probability weighting may have been more effective at addressing this limitation. Finally, because individuals in RESPOND starting INSTIs must do so during follow-up, and this is not the case for other ARV drug classes, we were unable to directly compare cancer incidence in those starting INSTIs to those starting non-INSTIs among the RESPOND population.
There are also several strengths to this analysis. RESPOND is a large cohort with data from real-world settings across Europe and Australia. Events in RESPOND are rigorously collected, and a subset are centrally validated by clinicians at the RESPOND coordinating center using prespecified algorithms.
CONCLUSIONS
In conclusion, there was no association between the risk of cancer and cumulative exposure to INSTIs among ART-experienced PWH. The risk of cancer decreased with increasing exposure to INSTIs among ART-naive individuals, which was mainly driven by a decreasing incidence of ADCs.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
APPENDIX
RESPOND Study Group
AIDS Therapy Evaluation in the Netherlands Cohort (ATHENA): F. Wit, P. Reiss, M. Hillebregt, Stichting HIV Monitoring (SHM), Amsterdam, Netherlands
The Australian HIV Observational Database (AHOD): M. Law, K. Petoumenos, N. Rose, UNSW, Sydney, Australia
Austrian HIV Cohort Study (AHIVCOS): R. Zangerle, H. Appoyer, Medizinische Universität Innsbruck, Innsbruch, Austria
CHU Saint-Pierre: S. De Wit, M. Delforge, Centre de Recherche en Maladies Infectieuses a.s.b.l., Brussels, Belgium
EuroSIDA Cohort: G. Wandeler, CHIP, Rigshospitalet, RegionH, Copenhagen, Denmark
Frankfurt HIV Cohort Study: C. Stephan, M. Bucht, Johann Wolfgang Goethe-University Hospital, Frankfurt, Germany
Infectious Diseases, AIDS and Clinical Immunology Research Center (IDACIRC): N. Chkhartishvili, O. Chokoshvili, Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi, Georgia
Italian Cohort Naive Antiretrovirals (ICONA): A. d’Arminio Monforte, A. Rodano, A. Tavelli, ASST Santi Paolo e Carlo, Milan, Italy; I Fanti, Icona Foundation, Milan, Italy
Modena HIV Cohort: C. Mussini, V. Borghi, Università degli Studi di Modena, Modena, Italy
Nice HIV Cohort: C. Pradier, E. Fontas, K. Dollet, C. Caissotti, Université Côte d’Azur et Centre Hospitalier Universitaire, Nice, France
PISCIS Cohort Study: J. Casabona, J.M. Miro, J.M. Llibre, A. Riera, J. Reyes-Urueña, Centre Estudis Epidemiologics de ITS i VIH de Catalunya (CEEISCAT), Badalona, Spain
Royal Free Hospital Cohort: C. Smith, F. Lampe, Royal Free Hospital, University College London, London, United Kingdom
San Raffaele Scientific Institute: A. Castagna, A. Lazzarin, A. Poli, Università Vita-Salute San Raffaele, Milano, Italy
Swedish InfCare HIV Cohort: A. Sönnerborg, K. Falconer, V. Svedhem, Karolinska University Hospital, Stockholm, Sweden
Swiss HIV Cohort Study (SHCS): H. Günthard, B. Ledergerber, H. Bucher, K. Kusejko, University of Zurich, Zurich, Switzerland
University Hospital Bonn: J.C. Wasmuth, J. Rockstroh, Bonn, Germany
University Hospital Cologne: J.J. Vehreschild, G. Fätkenheuer, Cologne, Germany
RESPOND Executive Committee:
A. Mocroft∗, J. Rooney, F. Rogatto, V. Vannappagari, H. Garges, G. Wandeler, M. Law, R. Zangerle, C. Smith, S. De Wit, J. Lundgren, H. Günthard∗
RESPOND Scientific Steering Committee:
J. Lundgren∗, H. Günthard∗, J. Kowalska, D. Raben, L. Ryom, A. Mocroft, J. Rockstroh, L. Peters, A. Volny Anne, N. Dedes, E.D. Williams, N. Chkhartishvili, R. Zangerle, M. Law, F. Wit, C. Necsoi, G. Wandeler, C. Stephan, C. Pradier, A. D’Arminio Monforte, C. Mussini, A. Bruguera, H. Bucher, A Sönnerborg, J.J. Vehreschild, J.C. Wasmuth, C. Smith, A. Castagna, F. Rogatto, R. Haubrich, V. Vannappagari, H. Garges
∗Chairs
RESPOND Outcomes Scientific Interest Group:
L. Ryom, A. Mocroft, B. Neesgaard, L. Greenberg, L. Bansi-Matharu, V. Svedhem-Johansson, F. Wit, K. Grabmeier-Pfistershammer, R. Zangerle, J. Hoy, M. Bloch, D. Braun, A. Calmy, G. Schüttfort, M. Youle, S. De Wit, C. Mussini, S. Zona, A. Castagna, A. Antinori, N. Chkhartishvili, N. Bolokadze, E. Fontas, K. Dollet, C. Pradier, J.M. Miro, J.M. Llibre, J.J. Vehreschild, C. Schwarze-Zander, J.C. Wasmuth, J. Rockstroh, K. Petoumenos, M. Law, C. Duvivier, G. Dragovic, R. Radoi, C. Oprea, M. Vasylyev, J. Kowalska, R. Matulionyte, V. Mulabdic, G. Marchetti, E. Kuzovatova, N. Coppola, J. Begovac, I. Aho, S. Martini, H. Bucher, A. Harxhi, T. Wæhre, A. Pharris, A. Vassilenko, G. Fätkenheuer, J. Bogner, A. Maagaard, E. Jablonowska, D. Elbirt, G. Marrone, C. Leen, C. Wyen, M. Kundro, N. Dedes, E. Dixon Williams, J. Gallant, D. Thorpe, H. Diaz Cuervo, V. Vannappagari, H. Garges
Community Representatives:
A. Volny-Anne, N. Dedes, L. Mendao (European AIDS Treatment Group), E. Dixon Williams (UK)
RESPOND Staff:
D. Raben, L. Peters, L. Ryom, B. Neesgaard, J.F. Larsen, M.L. Jakobsen, T. Bruun, A. Bojesen, E.V. Hansen, T.W. Elsing, D. Kristensen, S. Thomsen, T. Weide, A. Mocroft, L. Greenberg
Statistical Staff:
A. Mocroft, L. Greenberg, L. Bansi-Matharu, A. Pelchen-Matthews, K. Petoumenos, N. Rose, D. Byonanebye
Acknowledgments
Financial support. This work was funded by ViiV Healthcare LLC (Grant Number 207709) and Gilead Sciences (Grant Number CO-EU-311-4339). Additional support has been provided by participating cohorts contributing data in-kind: Austrian HIV Cohort Study, The Australian HIV Observational Database, CHU Saint-Pierre, University Hospital Cologne, The EuroSIDA cohort, Frankfurt HIV Cohort Study, Georgian National AIDS Health Information System (AIDS HIS), Modena HIV Cohort, San Raffaele Scientific Institute, Swiss HIV Cohort Study (SHCS), AIDS Therapy Evaluation in the Netherlands Cohort (ATHENA), and the Royal Free HIV Cohort Study.
Potential conflicts of interest. J. M. M. received a personal 80:20 research grant from Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, during 2017–21. A. M. received honoraria, consultancy fees, and/or travel support from ViiV, Gilead, and Eiland and Bonnin, outside the submitted work. H. F. G. has received unrestricted research grants from Gilead Sciences and Roche, fees for data and safety monitoring board membership, and fees for advisory board and consulting activities from Gilead Sciences, Merck, ViiV, Sandoz, and Mepha. A. P.-M. received an honorarium from Gilead, outside the submitted work. J. M. M. has received consulting honoraria and/or research grants from AbbVie, Angelini, Contrafect, Cubist, Genentech, Gilead Sciences, Jansen, Medtronic, MSD, Novartis, Pfizer, and ViiV Healthcare, outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
RESPOND Study Group:
F Wit, P Reiss, M Law, K Petoumenos, N Rose, R Zangerle, H Appoyer, S De Wit, M Delforge, G Wandeler, C Stephan, M Bucht, N Chkhartishvili, O Chokoshvili, A d’Arminio Monforte, A Rodano, A Tavelli, C Mussini, V Borghi, C Pradier, E Fontas, K Dollet, C Caissotti, J Casabona, J M Miro, J M Llibre, A Riera, J Reyes-Urueña, C Smith, F Lampe, A Castagna, A Lazzarin, A Poli, A Sönnerborg, K Falconer, V Svedhem, H Günthard, B Ledergerber, H Bucher, K Kusejko, J C Wasmuth, J Rockstroh, J J Vehreschild, G Fätkenheuer, A Mocroft, J Rooney, F Rogatto, V Vannappagari, H Garges, G Wandeler, M Law, R Zangerle, C Smith, S De Wit, J Lundgren, H Günthard, J Lundgren, H Günthard, J Kowalska, D Raben, L Ryom, A Mocroft, J Rockstroh, L Peters, A Volny Anne, N Dedes, E D Williams, N Chkhartishvili, R Zangerle, M Law, F Wit, C Necsoi, G Wandeler, C Stephan, C Pradier, A D’Arminio Monforte, C Mussini, A Bruguera, H Bucher, A Sönnerborg, J J Vehreschild, J C Wasmuth, C Smith, A Castagna, F Rogatto, R Haubrich, V Vannappagari, H Garges, L Ryom, A Mocroft, B Neesgaard, L Greenberg, L Bansi-Matharu, V Svedhem-Johansson, F Wit, K Grabmeier-Pfistershammer, R Zangerle, J Hoy, M Bloch, D Braun, A Calmy, G Schüttfort, M Youle, S De Wit, C Mussini, S Zona, A Castagna, A Antinori, N Chkhartishvili, N Bolokadze, E Fontas, K Dollet, C Pradier, J M Miro, J M Llibre, J J Vehreschild, C Schwarze-Zander, J C Wasmuth, J Rockstroh, K Petoumenos, M Law, C Duvivier, G Dragovic, R Radoi, C Oprea, M Vasylyev, J Kowalska, R Matulionyte, V Mulabdic, G Marchetti, E Kuzovatova, N Coppola, J Begovac, I Aho, S Martini, H Bucher, A Harxhi, T Wæhre, A Pharris, A Vassilenko, G Fätkenheuer, J Bogner, A Maagaard, E Jablonowska, D Elbirt, G Marrone, C Leen, C Wyen, M Kundro, N Dedes, E Dixon Williams, J Gallant, D Thorpe, H Diaz Cuervo, V Vannappagari, H Garges, A Volny-Anne, N Dedes, L Mendao, E Dixon Williams, D Raben, L Peters, L Ryom, B Neesgaard, J F Larsen, M L Jakobsen, T Bruun, A Bojesen, E V Hansen, T W Elsing, D Kristensen, S Thomsen, T Weide, A Mocroft, L Greenberg, A Mocroft, L Greenberg, L Bansi-Matharu, A Pelchen-Matthews, K Petoumenos, N Rose, and D Byonanebye
References
- 1. Marcus JL, Leyden WA, Alexeeff SE, et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000-2016. JAMA Netw Open 2020; 3:e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wandeler G, Johnson LF, Egger M.. Trends in life expectancy of HIV-positive adults on ART across the globe: comparisons with general population. Curr Opin HIV AIDS 2016; 11:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wada N, Jacobson LP, Cohen M, et al. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984 – 2008. Am J Epidemiol 2013; 177:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gueler A, Moser A, Calmy A, et al. Life expectancy in HIV-positive persons in Switzerland: matched comparison with general population. AIDS 2017; 31:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubrow R, Silverberg MJ, Park LS, et al. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol 2012; 24:506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weber R, Ruppik M, Rickenbach M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV cohort study. HIV Med 2013; 14:195–207. [DOI] [PubMed] [Google Scholar]
- 7. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults 2020 recommendations of the International Antiviral Society-USA Panel. JAMA 2020; 35294:1651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. EACS. EACS guidelines version 10.1. Published online 2020. Available at: https://www.eacsociety.org/media/guidelines-10.1_30032021_1.pdf. Accessed 17 March 2021. [Google Scholar]
- 9. Australasian Society for HIV Viral Hepatitis and Sexual Health Medicine. Antiretroviral guidelines. US DHHS guidelines with Australian commentary. Available at: https://arv.ashm.org.au/. Accessed 15 May 2019. [Google Scholar]
- 10. The Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1–infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 2010; 50:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 12. Bedimo R, Chen RY, Accortt NA, et al. Trends in AIDS-defining and non – AIDS-defining malignancies among HIV-infected patients: 1989 – 2002. Clin Infect Dis 2004; 39:1380–4. [DOI] [PubMed] [Google Scholar]
- 13. Franceschi S, Lise M, Clifford GM, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV cohort study. Br J Cancer 2010; 103:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chao C, Leyden WA, Xu L, et al. Exposure to antiretroviral therapy and risk of cancer in HIV-infected persons. AIDS 2012; 26:2223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mbang PA, Kowalkowski MA, Amirian ES, et al. Association between time on protease inhibitors and the incidence of squamous cell carcinoma of the anus among U.S. male veterans. PLoS One 2015; 10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bruyand M, Ryom L, Shepherd L, et al. Cancer risk and use of protease inhibitor or nonnucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy: the D:A:D study. J Acquir Immune Defic Syndr 2015; 68:568–77. [DOI] [PubMed] [Google Scholar]
- 17. Cozzi-Lepri A, Zangerle R, Machala L, et al. Incidence of cancer and overall risk of mortality in individuals treated with raltegravir-based and non-raltegravir-based combination antiretroviral therapy regimens. HIV Med 2018; 19:102–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379:2439–48. [DOI] [PubMed] [Google Scholar]
- 19. Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380–1490): a randomised, double-blind, multicentre, phase 3, non-inferiori. Lancet 2017; 390:2073–82. [DOI] [PubMed] [Google Scholar]
- 20. Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med 2008; 359:339–54. [DOI] [PubMed] [Google Scholar]
- 21. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 22. Horberg MA, Oakes AH, Hurley LB, et al. Association of raltegravir use with long-term health outcomes in HIV-infected patients: an observational post-licensure safety study in a large integrated healthcare system. HIV Clin Trials 2018; 19:177–87. [DOI] [PubMed] [Google Scholar]
- 23. The RESPOND Study Group. How to RESPOND to modern challenges for people Living with HIV (PLWHIV): a new cohort collaboration. Microorganisms 2020; 8:1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. RESPOND. RESPOND manual of operations (MOOP) for clinical events v1.6. Published online 2019. Available at: https://chip.dk/Research/Studies/RESPOND/Study-documents. Accessed 17 March 2021.
- 25. Greenberg L, Ryom L, Wandeler G, et al. Uptake and discontinuation of integrase inhibitors (INSTIs) in a large cohort setting. J Acquir Immune Defic Syndr 2020; 83:240–50. [DOI] [PubMed] [Google Scholar]
- 26. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2013; 150:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friis-Møller N, Reiss P, Sabin C, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–35. [DOI] [PubMed] [Google Scholar]
- 28. Ryom L, Lundgren JD, El-Sadr W, et al. Cardiovascular disease and use of contemporary protease inhibitors: the D:A:D international prospective multicohort study. Lancet HIV 2018; 5:e291–300. [DOI] [PubMed] [Google Scholar]
- 29. Elzi L, Erb S, Furrer H, et al. Adverse events of raltegravir and dolutegravir. AIDS 2017; 31:1853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silverberg MJ, Abrams DI.. AIDS-defining and non-AIDS-defining malignancies: cancer occurrence in the antiretroviral therapy era. Curr Opin Oncol 2007; 19:446–51. [DOI] [PubMed] [Google Scholar]
- 31. Appleby P, Beral V, Newton R, et al. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst 2000; 92:1823–30. [DOI] [PubMed] [Google Scholar]
- 32. Polesel J, Clifford GM, Rickenbach M, et al. Non-Hodgkin lymphoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. AIDS 2008; 22:301–6. [DOI] [PubMed] [Google Scholar]
- 33. Jacobsen LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma and non-Hodgkin’s lymphomas among HIV-1-infected individuals. J Acquir Immune Defic Syndr 1999; 21:S34–41. [PubMed] [Google Scholar]
- 34. Teppler H, Brown DD, Leavitt RY, et al. Long-term safety from the raltegravir clinical development program. Curr HIV Res 2011; 9:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut 2005; 54:1121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calle EE, Heath CW, Miracle-McMahill HL, et al. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996; 347:1713–27. [DOI] [PubMed] [Google Scholar]
- 37. European Medicines Agency. Assessment report Biktarvy International non-proprietary name: bictegravir/ emtricitabine/ tenofovir alafenamide. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/biktarvy. Accessed 28 May 2021.
- 38. European Medicines Agency. ISENTRESS, INN-raltegravir. Product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/isentress-epar-product-information_en.pdf. Accessed 28 May 2021.
- 39. European Medicines Agency. STRIBILD, INN-Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Disoproxil (as fumarate). Product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/stribild-epar-product-information_en.pdf. Accessed 28 May 2021.
- 40. European Medicines Agency. Assessment report Tivicay International non-proprietary name: dolutegravir. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/tivicay. Accessed 28 May 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.