Supplemental Digital Content is Available in the Text.
Key Words: HIVST, PrEP, adherence, sex worker, Africa
Abstract
Background:
HIV self-testing (HIVST) and pre-exposure prophylaxis (PrEP) are complementary tools that could empower sex workers to control their HIV protection, but few studies have jointly evaluated PrEP and HIVST in any setting.
Methods:
The Empower Study was an open-label randomized trial in Uganda. Sex workers were offered F/tenofovir disoproxil fumarate and randomized 1:1 to monthly HIVST and quarterly in-clinic testing (intervention) or quarterly in-clinic HIV testing alone (standard of care) and followed up for 12 months. PrEP adherence was measured using electronic adherence monitoring and tenofovir diphosphate (TFV-DP) levels in dried blood spots. Adherence outcomes and sexual behaviors were compared by arm using generalized estimating equation models.
Results:
We enrolled 110 sex workers: 84 cisgender women, 14 transgender women, 10 men who have sex with men, and 2 transgender men. The median age was 23 years. The 12-month retention was 75%. Nearly all (99.4%) used ≥1 HIVST kit. The proportion with TFV-DP levels ≥700 fmol/punch in the HIVST and standard of care arms at the 3-, 6-, 9-, and 12-month visits was 2.4%, 2.3%, 0%, and 0% and 7.9%, 0%, 0%, and 0%, respectively, with no differences by randomization arm (P > 0.2). Self-reported condomless sex acts with paying partners was similar by arm [adjusted incidence rate ratio 0.70; 95% confidence interval (CI): 0.42 to 1.17; P = 0.18]. One seroconversion occurred (HIV incidence, 0.9/100 person-years); TFV-DP was not detected at any visit.
Conclusions:
A gender-diverse sample of sex workers in Uganda used HIVST but not daily oral PrEP for HIV protection. Alternate approaches to promote PrEP use, including long-acting formulations, should be considered in this population.
INTRODUCTION
Globally, cisgender women, cisgender men, and transgender sex workers (TGSWs) are 49, 21, and 14 times as likely, respectively, to be living with HIV as other adults in the general population.1 Yet sex workers are less likely than the general population to engage with HIV services because of concerns about sex work criminalization, entrenched social stigma, and discrimination from health workers.2,3 In addition, condom use is often difficult to negotiate or results in less income generation,4 highlighting the need for additional HIV prevention approaches. Empowering sex workers to use combination HIV prevention interventions could help decrease HIV acquisition in this vulnerable population. HIV self-testing (HIVST) and tenofovir (TFV)-based oral pre-exposure prophylaxis (PrEP) are evidence-based, self-controlled HIV prevention tools, but they are currently not well used by sex workers.5,6 Moreover, these tools could be used with their paying and nonpaying (intimate) sexual partners to maximize impact.
Several features of HIVST and PrEP may be particularly attractive for sex workers. HIVST, a process in which an individual performs an HIV rapid diagnostic test and interprets the result independently,7 is highly acceptable with users appreciating its convenience and discretion7–9 and is recommended by the World Health Organization.10 HIVST can empower those who may otherwise not test, or test as frequently, by providing autonomy, freedom, and control over testing decisions.11 Moreover, HIVST reaches people at high risk of HIV and may be an important entry point for PrEP, which may similarly empower the user.10 The effectiveness of PrEP, however, is highly dependent on adherence, which has been challenging for many, including sex workers.12,13 HIVST and PrEP are potentially complementary tools that could be combined to empower sex workers to prevent HIV acquisition, but few studies have jointly evaluated PrEP and HIVST in any setting.
Limited data are available regarding whether joint HIVST and PrEP delivery could increase PrEP adherence and reduce sexual risk behaviors among sex workers. Although variable by population, condom use increased, and diagnoses of sexually transmitted infections (STIs) decreased in previous PrEP studies, suggesting PrEP use can be synergistic with other prevention methods.14 To test whether combination HIVST and PrEP influenced uptake and use of each prevention intervention, we conducted a randomized trial among HIV-negative cisgender women, cisgender men, and TGSWs initiating PrEP in Uganda. We hypothesized that joint HIVST and PrEP delivery could be empowering through combined use of both interventions.
METHODS
Trial Design
The Empower Study was an open-label randomized trial conducted in Kampala, Uganda (NCT03426670). Cisgender women [female sex workers (FSWs)], cisgender men who have sex with men (MSM), and TGSWs (N = 110) were offered oral PrEP (coformulated emtricitabine/tenofovir disoproxil fumarate) and individually randomized 1:1 to receive monthly HIVST and quarterly in-clinic testing (intervention) or quarterly in-clinic HIV testing alone (standard of care; SOC). Blinded allocation to study arm was performed by research nurses using REDCap electronic data capture tools.15,16 Study participants and research staff were not blinded to intervention assignment. There were no significant protocol changes after trial commencement. The study objectives were to test the effect of HIVST in addition to clinic-based testing alone on: (1) PrEP adherence and (2) sexual behaviors. In addition, we qualitatively explored how HIVST and PrEP influenced prevention uptake among sex workers and their partners.17 Study data were reviewed by an independent data monitoring committee. Intervention design was informed by protection motivation theory.18,19 This trial is reported in accordance with the Consolidated Standards of Reporting Trials statement.20
Population and Procedures
The Empower Study involved sex workers; estimated HIV prevalence among FSWs in Kampala was 37% in 2016.21 Data for MSM and TGSWs are not available. Participants were enrolled using peer recruiters between June 7, 2018, and January 10, 2019, and followed up for 12 months. The trial ended on January 31, 2020. Participant sex was self-identified. At study entry, sex workers were aged 18 years or older, received money or goods in exchange for sexual services, tested negative for HIV and Hepatitis B virus, were willing to take PrEP, were willing and able to comply with study procedures, and had adequate renal function (creatinine clearance ≥60 mL/minute). Research nurses taught sex workers in the HIVST arm how to use OraQuick rapid HIV-1/2 self-test kits (OraSure Technologies). At each quarterly visit, they received 4 HIVST kits; 2 for own use and 2 for testing sexual partners. At the time of the study, HIVST kits were only available through research studies. All study participants received quarterly in-clinic HIV rapid testing as SOC. Oral PrEP was provided to all participants, who received 3 bottles each with a 1-month supply of coformulated 200 mg of emtricitabine/300 mg of tenofovir disoproxil fumarate. Participants in the HIVST arm were instructed to self-test before starting each monthly course of PrEP, during the two-month period between scheduled quarterly study visits. Adherence to PrEP was measured using Wisepill devices for electronic adherence monitoring (EAM) (Wisepill Technologies, South Africa); these devices are smart pill containers that record a date-and-time stamp for each opening as a proxy for pill ingestion. Dried blood spot samples (DBS) were also collected quarterly for detection and quantification of tenofovir diphosphate (TFV-DP) levels.22 Data on PrEP adherence and sexual risk behaviors were collected during monthly live phone interviews and quarterly in-person visits, including validated sociobehavioral scales for stigma (HIV stigma scale23) and problematic alcohol use (RAPS-4 scale24). Participants were screened for depression using the Patient Health Questionnaire25; those with Patient Health Questionnaire-9 scores ≥10 were escorted to the Infectious Diseases Institute mental health clinic, if desired. Study interactions occurred in English or Luganda (local language). All participants were offered additional HIV prevention services, including individualized HIV and PrEP counseling, condoms, and lubricants; other forms of contraception (FSWs only); and free STI screening and treatment (as SOC per national guidelines.26 All participants were clinically monitored for adverse events. Each received an IRB-approved reimbursement of UGX 30,000 (USD 7.86).
Laboratory Methods
Sex workers were tested for HIV using serial rapid tests according to national guidelines.27 Hepatitis B virus testing was performed using Pal HBsAg rapid tests (Healgan Scientific, Houston, TX). Serum creatinine testing was performed using a Cobas Integra 400 biochemistry analyzer (Roche, Germany). Creatinine results were available within 72 hours; abnormal values were flagged and participants contacted for clinical management. Intracellular TFV-DP concentrations were quantified from DBS tested in batch at the University of Cape Town Clinical PK Laboratory, South Africa, using validated liquid chromatography/tandem mass spectrometry methods.28
Statistical Analysis
The primary outcome was PrEP adherence, as measured by EAM (number of days with recorded device openings divided by number of days with device detected as active)29 and TFV-DP levels in DBS (≥700 fmol per punch). We restricted device openings to 1 per day to avoid misclassification of adherence from multiple openings (eg, due to curiosity or device use for other medications). A secondary adherence measure was pharmacy refill (proportion of expected pills taken). The χ2 test and 1-way analysis of variance models were used to compare outcomes by randomization arm. Correlations of adherence measures were calculated using paired sample t tests, and tests of significance were performed using the Fisher exact test. We used an intent-to-treat approach (analyzed all participants regardless of PrEP refills) to compare adherence outcomes by randomization arm using a generalized estimating equations model using repeated measures with identity link, exchangeable correlation structure, and robust variance estimates. The trial had 80% power to detect a 17%–23% increase in PrEP adherence resulting from the use of HIVST with a sample size of 110 sex workers assuming an SD of 30%–40%, a 10% loss to follow-up, and a 2-sided alpha of 0.05. Secondary outcome measures were self-reported: (1) condomless sex acts and (2) acceptability of HIVST (assessed by HIVST kit usage and experiences). We used zero-inflated Poisson regression to assess condom use with paying and nonpaying partners because the distribution of sex acts was overdispersed relative to the Poisson distribution (ie, conditional variance was larger than the conditional mean).30 Covariates included baseline demographics, including age, sex, marital status, level of education, duration of sex work, and problematic alcohol and drug use, and were independently tested for associations with outcomes. Analyses were adjusted a priori for gender. We used the Spearman correlation to evaluate the relationship between EAM and TFV-DP levels in DBS. Statistical analyses were performed using Stata 14 (StataCorp, College Station, TX).
Ethics Approval
The study was approved by the Higher Degrees Research Ethics Committee, Makerere University School of Public Health (HDREC 500), Partners Human Research Committee/Massachusetts General Hospital (2017/P001951/PHS), Uganda National Council for Science and Technology (SS 4467), and National Drug Authority (CTA 0046). Each participant provided written informed consent.
RESULTS
Population Characteristics
We screened 136 sex workers and enrolled 117 of them; reasons for nonparticipation are shown in Figure 1. Seven participants who emigrated or lost interest in the study within 1 month of study entry were disenrolled (3 in the HIVST arm and 4 in the SOC arm). The final study sample comprised 110 sex workers (57 in the HIVST arm and 53 in the SOC arm) with <10% difference in sociodemographic characteristics between arms (Table 1). Of them, 84 were FSWs, 14 were transgender women (TGW), 10 were MSM, and 2 were transgender men. The median age was 23 years [interquartile range (IQR) 20–28]. Eighty-eight participants (80%) were single; sex work was the main source of income for 75 participants (68%). Sixty-nine participants (63%) had at least 1 child. The median age at onset of sex work was 19 years (IQR 17–23); the median monthly income was UGX 400,000 (USD 104.77). The median charge for vaginal and anal sex acts were UGX 20,000 and UGX 125,000 (USD 5.18 and USD 32.38) without a condom, and UGX 5000 and UGX 50,000 shillings (USD 1.30 and USD 12.95) with a condom, respectively. At baseline, 95 participants (86.4%) had heard about PrEP. Most of the participants (n = 88; 80.0%) were worried about getting infected with HIV, 87 (79.1%) believed that PrEP could prevent them from getting infected with HIV, and 66 (60.0%) were worried about PrEP side effects. Participation retention (completed study visits) was 67% and 75% at 6 and 12 months, respectively. The 12-month visit completion was 81% and 70% in the HIVST and SOC arms, respectively, with no differences by arm (P = 0.19). Overall, the 12-month retention was 90% for MSM, 81% for TGSWs, and 73% for FSWs.
FIGURE 1.
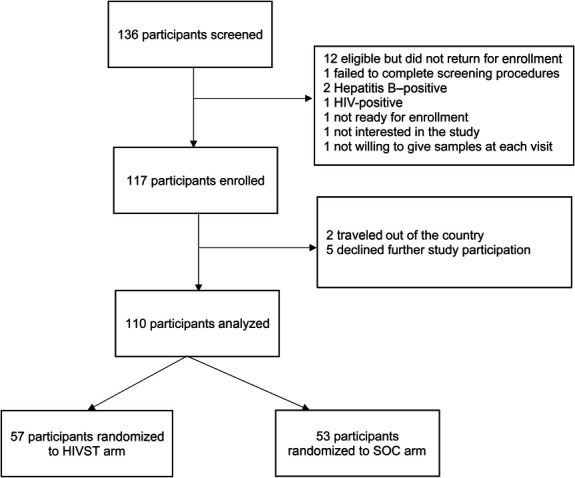
Trial profile.
TABLE 1.
Baseline Characteristics by Randomization Arm
| Characteristic | HIVST (N = 57), N (%) or Median (IQR) | SOC (N = 53), N (%) or Median (IQR) |
| Sex | ||
| Female | 39 (68.4) | 45 (84.9) |
| Male | 7 (12.3) | 3 (5.7) |
| Transgender | 11 (19.3) | 5 (9.4) |
| Age | 23.1 (20.9–28.9) | 22.2 (20.4–25.1) |
| 18–24 | 32 (56.1) | 33 (62.3) |
| 25–29 | 14 (24.6) | 12 (22.6) |
| 30–34 | 7 (12.3) | 4 (7.6) |
| older than 35 | 4 (7.0) | 4 (7.5) |
| Completed yr of education | 7 (3–10) | 9 (6–11) |
| 0 | 3 (5.3) | 11 (20.7) |
| 1–7 | 19 (33.3) | 18 (34.0) |
| 8–11 | 25 (43.9) | 17 (32.1) |
| ≥12 | 10 (17.5) | 7 (13.2) |
| Marital status | ||
| Divorced/separated/widowed | 10 (17.5) | 9 (17.0) |
| Married | 1 (1.8) | 2 (3.8) |
| Single with a nonpaying partner | 20 (35.1) | 21 (39.6) |
| Single without nonpaying partner | 26 (45.6) | 21 (39.6) |
| No. of children | ||
| None | 26 (45.6) | 15 (28.3) |
| 1–2 | 19 (33.3) | 29 (54.7) |
| ≥3 | 12 (21.1) | 9 (17.0) |
| Average monthly income (UGX) | 300,000 (150,000–500,000) | 300,000 (200,000–600,000) |
| Sex work main source of income | ||
| Yes | 37 (64.9) | 38 (71.7) |
| No | 20 (35.1) | 15 (28.3) |
| Age at onset of sex work [n = 108] | 19 (17–25) | 19 (17–22) |
| Sexual partners in previous mo | 60 (20–140) | 80 (30–100) |
| Average charge for vaginal sex act with a condom [n = 84] | 5000 (5000–10,000) | 8000 (5000–10,000) |
| Average charge for vaginal sex act without a condom [n =49] | 30,000 (10,000–50,000) | 20,000 (20,000–30,000) |
| Average charge for anal sex act with a condom [n = 30] | 50,000 (20,000–70,000) | 30,000 (30,000–50,000) |
| Average charge for anal sex act without a condom [n = 26] | 50,000 (20,000–150,000) | 50,000 (32,500–90,000) |
| Currently smoking tobacco | 6 (10.5) | 5 (9.4) |
| Currently using other recreational drugs | 10 (17.5) | 9 (17.0) |
| Possible depression* | 24 (42.1) | 21 (39.6) |
| Problematic alcohol use† | 32 (56.1) | 30 (56.6) |
| Sex work stigma‡ | 52 (91.2) | 48 (92.5) |
The Patient Health Questionnaire (PHQ-2), which has been validated in Uganda,25 was used to assess depression; a response of yes to either question is considered possible depression.
Alcohol use was assessed using the RAPS-4 scale24; a response of yes to 1 or more questions is considered problematic alcohol use in the previous yr.
Stigma was assessed using the HIV stigma scale23; data indicate a response of agree or strongly agree.
HIVST
In a visit-level analysis among intervention participants (n = 161/228 possible visits), the proportion of sex workers in the HIVST arm who self-reported using ≥1 kit was 99.4% (160/161) overall, with 97.4% (38/39), 100% (40/40), 100% (39/39), and 100% (43/43) at the 3-, 6-, 9-, and 12-month visits, respectively. In addition, 158 participants (98.1%) believed that the kit gave an accurate result, 154 (95.7%) said the kit was very easy to use, and 133 (82.6%) preferred to self-test at home by themselves compared with clinic-based testing. A minority (n = 45; 27.9%) tested at least once with someone else, including paying partners (n = 22; 48.9%), nonpaying partners (n = 16; 35.6%), and family members (n = 3; 6.7%). At the 161 visits, 99 (61.8%) reported using the HIVST kit before opening a new PrEP bottle and 93 (57.8%) reported HIVST use before sex act with a paying partner. All (100%) said they felt confident enough to show somebody else how to use HIVST kits, and 159 (98.8%) said they would recommend HIVST to friends and relatives. Secondary distribution of HIVST kits to paying and nonpaying partners of sex workers in the intervention arm was reported at 71 (44.1%) and 51 (31.7%) visits, respectively.
Effect of HIVST on PrEP Adherence
The median follow-up was 11.1 months (IQR 11.0, 11.3) per participant. We evaluated 1072 person-months in the intent-to-treat analysis. Eight participants (14%) in the HIVST arm and 18 (34%) in the SOC arm told research staff they had permanently stopped taking PrEP during the study. Overall, the mean adherence by EAM was 25.9% (30.9% and 20.6%; P = 0.047) at 6 months and 17.2% (22.1% and 11.5%; P = 0.052) at 12 months in the HIVST and SOC arms, respectively (Fig. 2). The TFV-DP detection was 21% and 21% at the 3-month visit, 21% and 25% at the 6-month visit, 14% and 11% at the 9-month visit, and 20% and 10% at the 12-month visit (P > 0.2 for all comparisons) for the HIVST and SOC arms, respectively. The proportion with TFV-DP levels ≥700 fmol per punch in the HIVST and SOC arms at the 3-, 6-, 9-, and 12-month visits was 2.4%, 2.3%, 0%, and 0% and 7.9%, 0%, 0%, and 0%, respectively, with no differences by randomization arm (P > 0.2). Similar findings were observed when adherence was measured by pharmacy refill at the 3-, 6-, 9-, and 12-month visits (P > 0.1 for all comparisons). As shown in Figure 3, the correlation between EAM and DBS had a rho of 0.212 overall (0.240 in the HIVST arm and 0.193 in the SOC arm; P = 0.04 for the randomized groups). No intervention effect was observed on PrEP adherence by randomization arm over the 12-month period as measured by EAM (adjusted odds ratio [aOR]: 3.32; 95% CI: −5.3 to 11.9; P = 0.45) or DBS TFV-DP levels [aOR 1.01; 95% confidence interval (CI): 0.91 to 1.97; P = 0.98] (Table 2).
FIGURE 2.
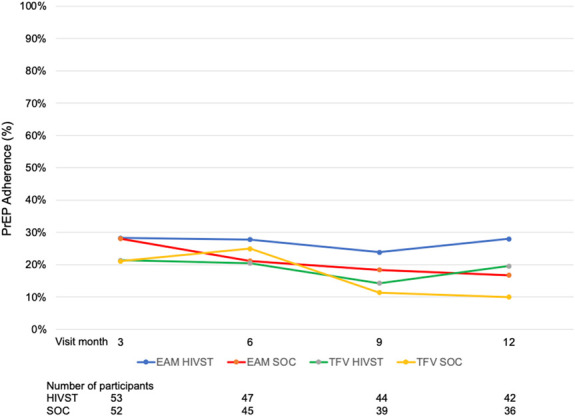
PrEP adherence by EAM and TFV-DP levels in DBS.
FIGURE 3.
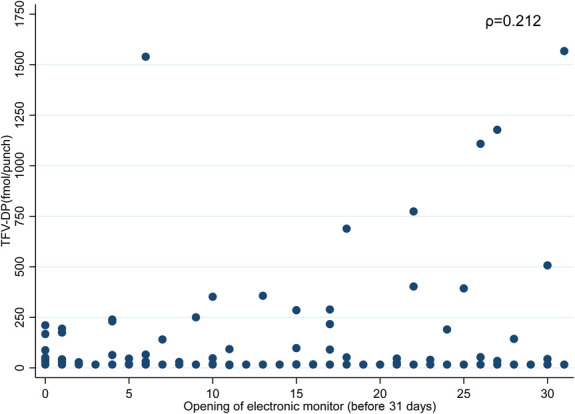
Correlation of PrEP adherence by electronic adherence monitoring and TFV-DP levels in DBS.
TABLE 2.
Effect of HIV Self-Testing on PrEP Adherence
| PrEP Adherence | N (%) | OR; 95% CI | P | Adjusted OR 95% CI | P |
| EAM | |||||
| SOC | 42 (54%) | Ref | Ref | ||
| HIVST | 36 (46%) | 2.83 (−5.9 to 11.7) | 0.53 | 3.32 (−5.3 to 11.9) | 0.45 |
| EAM (by gender) | |||||
| FSWs | 58 (75) | Ref | Ref | ||
| MSM | 8 (10) | 0.58 (−13.3 to 14.5) | 0.94 | −0.15 (−13.8 to 13.5) | 0.98 |
| TGSWs | 12 (15) | −4.18 (−17.4 to 9.1) | 0.54 | −4.98 (−17.6 to 7.8) | 0.45 |
| TFV-DP levels in DBS (by arm) | |||||
| SOC | 46 (53) | Ref | Ref | ||
| HIVST | 40 (47) | 1.22 (0.52 to 2.89) | 0.65 | 1.01 (0.44 to 2.35) | 0.98 |
| TFV-DP levels in DBS (by gender) | |||||
| FSWs | 63 (74) | Ref | Ref | ||
| MSM | 9 (10) | 1.36 (0.28 to 6.51) | 0.70 | 1.35 (0.29 to 6.28) | 0.69 |
| TGSWs | 14 (16) | 4.00 (1.54 to 10.35) | 0.004 | 3.99 (1.58 to 10.05) | 0.003 |
Effect of HIVST on Sexual Risk Behaviors
At baseline, the mean number of sexual partners in the previous month was 72 (range 30–120). Most of the participants (n = 67; 61%) had intimate partner(s) and 51 (76%) reported never using condoms with intimate partners. Sixty-eight percentage of participants did not disclose sex work to their intimate partners. Overall, we observed no difference in self-reported condomless sex acts with paying partners by randomization arm (adjusted incidence rate ratio [aIRR]: 0.70; 95% CI: 0.42 to 1.17; P = 0.18).
Subgroup Analyses
We observed an apparent intervention effect on PrEP adherence in the small subset of 16 TGSWs: EAM (aOR 3.86; 95% CI: 1.15 to 12.9 P = 0.006) and DBS TFV-DP levels (aOR 3.99; 95% CI: 1.58 to 10.05; P = 0.003). An intervention effect on sexual risk behaviors was observed among MSM (aIRR 0.11; 95% CI: 0.06 to 0.20; P < 0.001), and TGSWs (aIRR 0.18; 95% CI: 0.10 to 0.32; P = 0.001) were less likely to report condomless sex acts with paying partners than FSWs regardless of randomization arm. Similar findings were observed for condom use with nonpaying partners with no difference by arm (aIRR 0.98; 95% CI: 0.70 to 1.39; P = 0.93), but with MSM less likely to report condomless sex acts (aIRR 0.01; 95% CI: 0.00 to 0.05; P < 0.001) than FSWs (Table 3).
TABLE 3.
Effect of HIV Self-Testing on Sexual Behaviors
| Sexual Risk Behaviors | N (%) or Median (IQR) | IRR; 95% CI | P | Adjusted IRR (95% CI) | P |
| Age | 22.8 (20.4–27.6) | 1.00 (0.97 to 1.02) | 0.82 | 0.99 (0.96 to 1.03) | 0.70 |
| Sex | |||||
| Female | 84 (76) | Ref | Ref | ||
| Male | 10 (9) | 0.06 (0.00 to 0.74) | 0.03 | 0.11 (0.06 to 0.20) | <0.001 |
| Transgender | 16 (15) | 0.16 (0.09 to 0.27) | 0.01 | 0.18 (0.10 to 0.32) | <0.001 |
| Duration of sex work | 3.1 (1.7–4.8) | 1.00 (0.97 to 1.03) | 0.85 | 1.02 (0.97 to 1.07) | 0.51 |
| Problematic alcohol use | 62 (56) | 0.90 (0.48 to 1.67) | 0.73 | 0.81 (0.46 to 1.45) | 0.48 |
| Condom use, paying partners (by arm) | 70 (24–126) | 0.78 (0.49 to 1.27) | 0.29 | 0.70 (0.42 to 1.17) | 0.18 |
| Condom use, nonpaying partners (by arm) | 11 (5–23) | 1.07 (0.3 to 1.58) | 0.73 | 0.98 (0.70 to 1.39) | 0.93 |
| Condom use, paying partners (by gender) | |||||
| FSWs | 84 (76) | Ref | Ref | ||
| MSM | 10 (9) | 0.06 (0.01 to 0.74) | 0.03 | 0.11 (0.06 to 0.20) | <0.01 |
| TGSWs | 16 (15) | 0.16 (0.09 to 0.27) | 0.01 | 0.18 (0.10 to 0.32) | <0.01 |
| Condom use, nonpaying partners (by gender) | |||||
| FSWs | 84 (76) | Ref | Ref | ||
| MSM | 10 (9) | 0.01 (0.00 to 0.07) | <0.01 | 0.01 (0.00 to 0.05) | <0.01 |
| TGSWs | 16 (15) | 1.68 (1.38 to 2.04) | <0.01 | 1.55 (0.81 to 3.00) | 0.19 |
Adverse Event Profile and HIV Acquisition
No serious adverse events were identified as related to study participation or PrEP. We detected 1 seroconversion in the HIVST arm at the 9-month visit (HIV incidence, 0.9 per 100 person-years). Tenofovir was not detected in DBS at the 3-, 6-, or 9-month visits, and drug resistance testing was not performed because the FSW participant did not return for her postseroconversion visit. She was encouraged to start antiretroviral treatment but repeatedly declined (see Fig., Supplemental Digital Content, http://links.lww.com/QAI/B779).
DISCUSSION
In this randomized trial of HIVST codistributed with PrEP, PrEP adherence was low despite near-universal reported use of HIVST among cisgender women, cisgender men, and TGSWs in Uganda. The lack of intervention effect was consistent when PrEP adherence was assessed using electronically monitored adherence and TFV concentrations in DBS. EAM-assessed adherence was significantly higher in the HIVST arm at the 6-month visit, but this was not sustained during the 12-month study period. HIVST acceptability was high because nearly all sex workers used the kits to test themselves, and half of them distributed HIVST kits to paying and nonpaying partners. Self-reported condomless sex acts with paying and nonpaying partners decreased among MSM and TGSWs but not FSWs regardless of study arm. HIV incidence was low; only 1 seroconversion occurred (and this event occurred in the absence of PrEP use). In subgroup analyses, TGSWs were 4 times as likely to adhere to PrEP as FSWs, suggesting possible gender differences. However, the small numbers of TGSWs may result in an overestimation of the intervention effect.
Our results are consistent with those of other sex worker studies in sub-Saharan Africa in which oral PrEP was infrequently taken at levels sufficient to achieve HIV protection.31–33 We found that despite continual engagement in sex work during the study, PrEP use was suboptimal during this risk period, suggesting a lack of prevention-effective adherence.34 PrEP is an empowering self-controlled prevention tool that allows sex workers to control their HIV protection without requiring the consent of their partners.35 Our published qualitative work in this cohort17 found that combined HIVST and PrEP use enabled sex workers to overcome physical and emotional barriers to intimacy, preserve relationships with their intimate partners, and avoid the stigma and inconvenience experienced when testing at public health facilities, which empowered them to protect their sexual health. Recent work among young women in Kenya found that HIV risk perception was dynamic, evolved as partnerships and sexual behaviors changed, and influenced PrEP and condom use over time.36 The dynamic nature of HIV risk perception may have influenced PrEP persistence and could explain the misalignment between perceptions that PrEP was empowering and suboptimal PrEP adherence in our study.
At least 4 doses per week are required to provide HIV protection after rectal exposure; higher adherence is likely required for vaginal exposure.37,38 Second-generation PrEP drugs, including the dapivirine vaginal ring and injectable cabotegravir LA), are dosed at least monthly, which provides an adherence advantage over daily dosing.39,40 However, concerns about side effects and HIV stigma may still limit uptake of long-acting PrEP. The HIV Prevention Trials Network 083 and 084 studies demonstrated that cabotegravir LA is a well-tolerated and effective injectable PrEP drug for cisgender women, MSM, and TGW.39,41 Other long-acting PrEP agents, including islatravir and lenacapavir, are under evaluation.42–44 Future studies should evaluate optimal approaches to motivate high uptake and sustained use of long-acting PrEP formulations among sex workers.
Nearly all sex workers in our study reported using HIVST, half gave kits to paying or nonpaying partners, and 1 in four self-tested with a partner. HIVST is a self-controlled prevention tool that could empower sex workers to negotiate condom use. Sex workers use point-of-sex testing (ie, using HIVST kits to screen paying partners) as a risk reduction strategy in settings where partners pay more for condomless sex acts.17,45–47 Studies of FSWs, MSM, and TGSWs suggest that point-of-sex testing empowers and informs sexual decision-making such as avoiding sex act or insisting on condom use with paying partners who tested HIV-positive.11,48–50 This ancillary benefit could motivate HIVST use among sex workers. However, some caution regarding the utility of HIVST for sexual decision-making is warranted; oral fluid self-test kits may be misinterpreted and have low sensitivity in acute HIV infection and in persons taking PrEP or antiretroviral therapy, which may result in false-negative results and a false sense of safety.17,51,52
We found no evidence of behavioral risk compensation among sex workers in agreement with previous reports.53,54 In contrast with previous studies, we observed a lower likelihood of sex acts unprotected by condoms among MSM and TGSWs but not FSWs.55 In our study, the median charge for anal sex act with a condom was 2.5-fold higher than that for condomless vaginal sex act, suggesting financial motivations to engage in condomless sex act may have been less compelling for MSM and TGSWs than FSWs. A meta-analysis of 17 studies with 6671 MSM found that PrEP use was associated with a significant increase in condomless sex acts and STI diagnoses outside of sex work.56 The association between PrEP and STI incidence is less clear for TGSWs, and programmatic data are lacking.
Subgroup analyses revealed a higher likelihood of PrEP adherence among TGSWs. This finding may be explained by motivation to access HIV prevention services for a marginalized population that had previously not participated in a clinical trial in Uganda. PrEP adherence among TGSWs in sub-Saharan Africa is poorly studied, offering few data for comparison. South African TGW reported a decreased HIV risk, HIV protection in the event of rape, and being in control of one's health as reasons for taking PrEP.57 Other work in Kenya (n = 53) found that TFV-DP was detected in 62.5% of TGW vs 17.7% of MSM at the 6-month visit. In that study, PrEP use was motivated by unplanned or frequent risky sexual behavior and desire to remain HIV-negative.58 Ongoing studies in Uganda (ClinicalTrials.gov NCT04328025, 04867798, and 04491422) will contribute to the knowledge base regarding HIVST and PrEP use in this population.
This study is the first, to the best of our knowledge, to evaluate HIVST and PrEP use among a gender-diverse sample of sex workers in sub-Saharan Africa. We achieved good retention among MSM and TGSWs in Uganda by establishing strong community linkages, leveraging peer support, and providing research staff with gender-sensitivity training. Of importance, no social harms related to research participation were identified. In addition to these strengths, our study has limitations. One-quarter of the study sample was lost to follow-up, but dropout rates did not differ by study arm. We enrolled a small sample of MSM and TGSWs; the gender differences we observed should thus be interpreted with caution. HIVST was only available through research programs at the time of the study, and intervention arm participants may have shared kits with the SOC group. Nevertheless, any such contamination would not have affected the null results of the trial because PrEP adherence was poor irrespective of randomization group. In addition, misclassification of device nonuse due to stigma may have occurred.59 However, TFV-DP levels in DBS demonstrated similarly poor PrEP adherence.
In conclusion, our randomized trial found no benefit for HIVST in supporting PrEP adherence among sex workers in Uganda. Acceptability of HIVST was high with frequent use of this prevention tool for self-testing and testing with sexual partners. PrEP adherence was low and insufficient for HIV protection. Future studies should evaluate alternate strategies to promote sustained use of PrEP for sex workers in sub-Saharan Africa, including long-acting PrEP formulations.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the study participants for their participation and dedication and the study team members at the research site for their contributions to data collection.
Footnotes
Supported by research grants from National Institutes of Health [research Grant number K43 010695 (A.M.) and K24 Mentor Award MH114732 (J.E.H.). This research was funded in part by a 2017 developmental grant to A.M. from the University of Washington/Fred Hutch Center for AIDS Research, an NIH-funded program under award number AI027757, which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK. This article represents the opinions of the authors and does not necessarily represent the official views of the National Institutes of Health. Gilead Sciences, Inc. donated coformulated emtricitabine/tenofovir disoproxil fumarate for this study but had no role in data collection or analysis.
A.M. received donated FTC/TDF from Gilead Sciences for this investigator-sponsored study and served as an advisor for ViiV Healthcare. J.M.B. served as an advisor for Gilead Sciences, Janssen, and Merck. J.E.H. is a consultant for Merck. The remaining authors have no conflicts of interest to disclose.
A.M., J.M.B., and J.E.H. designed the study. A.M. wrote the first draft along with J.E.H. M.S.N. performed the statistical analyses. All authors contributed to data collection, interpretation of the results, and the writing of the manuscript, and all approved the final draft.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Contributor Information
Agnes Nakyanzi, Email: anakyanzi@idi.co.ug.
Maria S. Nabaggala, Email: snabaggala@idi.co.ug.
Timothy R. Muwonge, Email: tmuwonge@idi.co.ug.
Timothy Ssebuliba, Email: tssebuliba@idi.co.ug.
Monica Bagaya, Email: mbagaya@idi.co.ug.
Olivia Nampewo, Email: onampewo@idi.co.ug.
Oliver Sapiri, Email: OSapiri@idi.co.ug.
Kikulwe R. Nyanzi, Email: RNyanzi@idimakug.mail.onmicrosoft.com.
Felix Bambia, Email: fbambia@idi.co.ug.
Rogers Nsubuga, Email: rnsubuga@idi.co.ug.
David M. Serwadda, Email: dserwada@imul.com.
Norma C Ware, Email: norma_ware@hms.harvard.edu.
Jared M. Baeten, Email: jbaeten@uw.edu.
Jessica E. Haberer, Email: jhaberer@partners.org.
REFERENCES
- 1.Oldenburg CE, Perez-Brumer AG, Reisner SL, et al. Global burden of HIV among men who engage in transactional sex: a systematic review and meta-analysis. PLoS One. 2014;9:e103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyons CE, Schwartz SR, Murray SM. The role of sex work laws and stigmas in increasing HIV risks among sex workers. Nat Commun. 2020;11:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shannon K, Strathdee SA, Goldenberg SM. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet. 2015;385:55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortblad K, Kibuuka Musoke D, Ngabirano T. Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: a cluster-randomized controlled health systems trial. PLoS Med. 2017;14:e1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortblad KF, Kearney JE, Mugwanya K. HIV-1 self-testing to improve the efficiency of pre-exposure prophylaxis delivery: a randomized trial in Kenya. Trials. 2019;20:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson CC, Kennedy C, Fonner V. Examining the effects of HIV self-testing compared to standard HIV testing services: a systematic review and meta-analysis. J Int AIDS Soc. 2017;20:21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson C, Baggaley R, Forsythe S. Realizing the potential for HIV self-testing. AIDS Behav. 2014;18(suppl 4):S391–S395. [DOI] [PubMed] [Google Scholar]
- 8.Figueroa C, Johnson C, Verster A, et al. Attitudes and acceptability on HIV self-testing among key populations: a literature review. AIDS Behav. 2015;19:1949–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heard AC, Brown AN. Public readiness for HIV self-testing in Kenya. AIDS care. 2016;28:1528–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. World Health Organization. Guidelines on HIV Self-Testing and Partner Notification. Geneva, Switzerland; 2016. [PubMed] [Google Scholar]
- 11.Giguere R, Lopez-Rios J, Frasca T. Use of HIV self-testing kits to screen clients among transgender female sex workers in New York and Puerto Rico. AIDS Behav. 2020;24:506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haberer JE. Current concepts for PrEP adherence in the PrEP revolution: from clinical trials to routine practice. Curr Opin HIV AIDS. 2016;11:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghayda RA, Hong SH, Yang JW. A review of pre-exposure prophylaxis Adherence among female sex workers. Yonsei Med J. 2020;61:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi M, Spinelli MA, Mayer KH. Addressing the sexually transmitted infection and HIV syndemic. JAMA. 2019;321:1356–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mujugira A, Nakyanzi A, Kasiita V, et al. HIV self-testing and oral pre-exposure prophylaxis are empowering for sex workers and their intimate partners: a qualitative study in Uganda. J Int AIDS Soc. 2021;24:e25782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroebe W. Social Psychology and Health. 2nd ed. Buckingham: Open University Press; 2000. [Google Scholar]
- 19.Munro S, Lewin S, Swart T, et al. A review of health behaviour theories: how useful are these for developing interventions to promote long-term medication adherence for TB and HIV/AIDS?. BMC Public Health. 2007;7:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2011;9:672–677. [DOI] [PubMed] [Google Scholar]
- 21.UAC. The Uganda HIV and AIDS Country Progress Report July 2015-June 2016. Kampala, Uganda: Uganda AIDS Commission; 2016. [Google Scholar]
- 22.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum retroviruses. 2013;29:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24:518–529. [DOI] [PubMed] [Google Scholar]
- 24.Cherpitel CJ, Ye Y, Bond J. Cross-national performance of the RAPS4/RAPS4-QF for tolerance and heavy drinking: data from 13 countries. J Stud Alcohol. 2005;66:428–432. [DOI] [PubMed] [Google Scholar]
- 25.Nakku JEM, Rathod SD, Kizza D. Validity and diagnostic accuracy of the Luganda version of the 9-item and 2-item Patient Health Questionnaire for detecting major depressive disorder in rural Uganda. Glob Ment Health (Camb). 2016;3:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Consolidated MOH. Guidelines on the Prevention and Treatment of HIV in Uganda. Kampala, Uganda: Ministry of Health; 2020. [Google Scholar]
- 27.National MOH. HIV Testing Services Policy and Implementation Guidelines. Kampala, Uganda: Ministry of Health; 2016. [Google Scholar]
- 28.Oberholster L. The Development and Validation of a Direct LC-MS/MS Assay for the Determination of Tenofovir-Diphosphate in Dried Blood Spots for the Analysis of Clinical Samples. Cape Town, South Africa: Faculty of Health Sciences; 2019. [Google Scholar]
- 29.Haberer JE, Bukusi EA, Mugo NR. Effect of SMS reminders on PrEP adherence in young Kenyan women (MPYA study): a randomised controlled trial. Lancet HIV. 2021;8:e130–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mujugira A, Celum C, Ngure K, et al. Antiretroviral therapy initiation is not associated with risky sexual behavior among heterosexual human immunodeficiency virus-infected persons in serodiscordant partnerships. Sex Transm Dis. 2017;44:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eakle R, Gomez GB, Naicker N. HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: results from a prospective observational demonstration project. PLoS Med. 2017;14:e1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutua G, Sanders E, Mugo P. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PloS one. 2012;7:e33103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mboup A, Béhanzin L, Guédou FA. Early antiretroviral therapy and daily pre-exposure prophylaxis for HIV prevention among female sex workers in Cotonou, Benin: a prospective observational demonstration study. J Int AIDS Soc. 2018;21:e25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haberer JE, Bangsberg DR, Baeten JM. Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm. AIDS. 2015;29:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Restar AJ, Tocco JU, Mantell JE. Perspectives on HIV pre- and post-exposure prophylaxes (PrEP and PEP) among female and male sex workers in mombasa, Kenya: implications for integrating biomedical prevention into sexual health services. AIDS Educ Prev. 2017;29:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngure K, Thuo N, Ogello V, et al. Dynamic perceived HIV risk and sexual behaviors among young women enrolled in a PrEP trial in Kenya: a qualitative study. Front Reprod Health. 2021;3:637869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant RM, Anderson PL, McMahan V. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cottrell ML, Yang KH, Prince HM. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis Against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landovitz RJ, Donnell D, Clement ME. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baeten JM, Palanee-Phillips T, Mgodi NM. Safety, uptake, and use of a dapivirine vaginal ring for HIV-1 prevention in African women (HOPE): an open-label, extension study. Lancet HIV. 2021;8:e87–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moretlwe SD, Hughes J, Bock P, et al. Long acting injectable cabotegravir is safe and effective in preventing HIV infection in cisgender women: interim results from HPTN 084. J Int AIDS Soc. 2021;24:8–9. [Google Scholar]
- 42.Markowitz M, Gettie A, St Bernard L. Once-weekly oral dosing of MK-8591 protects male rhesus macaques from intrarectal challenge with SHIV109CP3. J Infect Dis. 2020;221:1398–1406. [DOI] [PubMed] [Google Scholar]
- 43.Hillier S. Trial design, enrollment status, demographics, and pharmacokinetics (PK) data from a blinded interim analysis from a phase 2a trial of Islatravir once monthly (QM) for HIV pre-exposure prophylaxis (PrEP). HIV Research for Prevention 2020. Virtual: International AIDS Society; 2021. [Google Scholar]
- 44.Bekerman E, Vidal S, Hansen D, et al. Long-acting HIV capsid inhibitor effective as PrEP in a SHIV rhesus macaque model. Conference on Retroviruses and Opportunisitic Infections. Virtual CROI. 2021:2021. [Google Scholar]
- 45.Sharma A, Chavez PR, MacGowan RJ. Willingness to distribute free rapid home HIV test kits and to test with social or sexual network associates among men who have sex with men in the United States. AIDS care. 2017;29:1499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei C, Yan L, Lippman SA. Prevalence and correlates of point-of-sex human immunodeficiency virus self-testing among human immunodeficiency virus-negative men who have sex with men in China. Sex Transm Dis. 2018;45:818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gertler P, Shah M, Bertozzi SM. Risky business: the market for unprotected commercial sex. J Polit Economy. 2005;113:518–550. [Google Scholar]
- 48.Carballo-Diéguez A, Frasca T, Balan I, et al. Use of a rapid HIV home test prevents HIV exposure in a high risk sample of men who have sex with men. AIDS Behav. 2012;16:1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maman S, Murray KR, Napierala Mavedzenge S. A qualitative study of secondary distribution of HIV self-test kits by female sex workers in Kenya. PloS one. 2017;12:e0174629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thirumurthy H, Masters SH, Mavedzenge SN, et al. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study. Lancet HIV. 2016;3:e266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ortblad KF, Stekler JD. HIV self-testing: finding its way in the prevention tool box. BMC Med. 2020;18:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ortblad KF, Musoke DK, Ngabirano T, et al. Female sex workers often incorrectly interpret HIV self-test results in Uganda. J Acquir Immune Defic Syndr. 2018;79:e42–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts DA, Hawes SE, Bousso Bao MD. Trends in reported sexual behavior and Y-chromosomal DNA detection among female sex workers in the Senegal preexposure prophylaxis demonstration project. Sex Transm Dis. 2020;47:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giguère K, Béhanzin L, Guédou FA. PrEP use among female sex workers: No evidence for risk compensation. J Acquir Immune Defic Syndr. 2019;82:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochonye B, Folayan MO, Fatusi AO. Sexual practices, sexual behavior and HIV risk profile of key populations in Nigeria. BMC Public Health. 2019;19:1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Traeger MW, Schroeder SE, Wright EJ. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676–686. [DOI] [PubMed] [Google Scholar]
- 57.Poteat T, Malik M, van der Merwe LLA. PrEP awareness and engagement among transgender women in South Africa: a cross-sectional, mixed methods study. Lancet HIV. 2020;7:e825–e34. [DOI] [PubMed] [Google Scholar]
- 58.Kimani M, van der Elst EM, Chirro O, et al. I wish to remain HIV negative": pre-exposure prophylaxis adherence and persistence in transgender women and men who have sex with men in coastal Kenya. PloS one. 2021;16:e0244226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spinelli MA, Haberer JE, Chai PR, et al. Approaches to objectively measure antiretroviral medication adherence and drive adherence interventions. Curr Hiv/aids Rep. 2020;17:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


