Abstract
We conducted a prospective, three-center, observational study in Japan to evaluate the prevalence of seropositivity and clinically protective titer after coronavirus disease 2019 vaccination in patients with plasma cell dyscrasia(PCD). Two-hundred sixty-nine patients with PCD [206 symptomatic multiple myeloma (MM)] were evaluated. Seropositivity was observed in 88.7% and a clinically protective titer in 38.3% of MM patients, both of which were significantly lower than those of healthy controls. Patients receiving anti-CD38 antibodies had much lower antibody titers, but antibody titers recovered in those who underwent a wash-out period before vaccine administration. Older age (≥65), anti-CD38 antibody administration, immunomodulatory drugs use, lymphopenia (<1000/μL), and lower polyclonal IgG (<550 mg/dL) had a negative impact for the sufficient antibody production according to multivariate analysis. Patients with clinically protective titer had a significantly higher number of CD19+ lymphocytes than those with lower antibody responses (114 vs. 35/μL, p = 0.016). Our results suggested that patients with PCD should be vaccinated, and that the ideal protocol is to temporarily interrupt anti-CD38 antibody therapy for a “wash-out” period of a few months, followed by a (booster) vaccine after the B-cells have recovery.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12185-022-03300-4.
Keywords: COVID-19, SARS-CoV-2, mRNA vaccine, Plasma cell dyscrasia, Multiple myeloma, Anti-CD38 antibody
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is spreading worldwide. Recently, a highly effective messenger ribonucleic acid (mRNA) vaccine (BNT162b2, Pfizer-BioNTech) against SARS-CoV-2 that can prevent 95% of symptomatic COVID-19 was developed [1], and vaccination is being promoted worldwide. However, patients with hematologic malignancies showed lower antibody titer following vaccination compared to that of healthy individuals, and a quarter of these patients remained seronegative following vaccination [2, 3]. This was likely caused by the severely immunocompromised status of these patients, resultant of either disease-inherent or treatment-induced immunodeficiency.
There have been several reports of vaccination in patients with multiple myeloma (MM), mainly from Europe, North America, and Israel [4–10]. Pimpinelli, et al. [6] reported a minute increase in antibody titers after the first vaccination in patients with MM. Avivi, et al. [4] and Terpos, et al. [8] reported that in older patients, hypogammaglobulinemia, heavy pretreatment (≥4 drug regimens), anti-CD38 antibody use, and belantamab mafodotin were associated with lower vaccine response rate. However, high dose treatment followed by autologous stem cell transplantation (ASCT) did not show an association with antibody titer [11].
In Japan, distribution of the BNT162b2 to the elderly began on April 12th, and a total of 136,685,318 vaccine doses were administered in September [12]. However, due to the fifth and largest COVID-19 pandemic surge in August 2021, mainly caused by the delta variant (B.1.617.2), there have been 1,650,318 cases and 16,894 deaths in Japan as of September 16th, 2021. Administration of the third vaccine dose has already begun in some areas [13], and patients whose antibody levels did not sufficiently increase after two doses may require additional doses [14]. Thus, to maximize the effectiveness of the vaccine, the appropriate timing of vaccination and the choice of the immunocompromised patients who should receive additional vaccination would be considered.
In this study, we investigated seropositivity and clinically protective titer in patients with plasma cell dyscrasia (PCD). We also assessed the relationship between antibody acquisition and treatment history or disease characteristics. Our results will provide the information to help in selecting the patients who should receive the (third) additional booster vaccination.
Materials and methods
Study design and patients
This prospective, three-center, observational study was conducted from July 1st in Japan at Kameda Medical Center, Keiju Kanazawa Hospital, and Kanazawa University. The inclusion criteria were: age ≥18 years, diagnosis of PCD [symptomatic MM, smoldering MM (sMM), or monoclonal gammopathy of undetermined significance (MGUS)], and no known history of COVID-19 onset. Age-matched volunteers who were older than 60 years, without active cancer or immunosuppression therapy, and no known history of COVID-19 onset were also included to compare antibody titers between patients with and without PCD. All participants (patients with PCD and age-matched volunteers) were vaccinated with two doses of BNT162b2 vaccine (Pfizer/BioNTech), 21 days apart, or mRNA-1273 vaccine (Moderna), 28 days apart, depending on the vaccine available in each region. Data on the patients’ clinicodemographic characteristics and outcomes were obtained from their electronic medical records. The primary endpoint was SARS-CoV-2 antibody level and seropositivity in patients with PCD. The data set was locked on September 24th, 2021.
All participants or their family members provided written informed consent for inclusion in the study. The study was conducted according to the Declaration of Helsinki and approved by the ethical review board of each center.
Collection of specimens
Serum samples were collected from patients with PCD on day 1 (T0; before the first dose), on day 15–28 (T1; before the second dose), and on day 36–85 (T2; 2–8 weeks after the second dose), if available. Samples from patients that had been frozen at our frozen sample database at the appropriate timing before July 1st was also analyzed. Serum samples from age-matched volunteers at Kameda Medical Center and Keiju Kanazawa Hospital were collected on T2. The sera were frozen at −20 °C until analysis.
Serological tests
Antibody responses were analyzed using Elecsys® Anti-SARS-CoV-2 on Cobas 8000 e801 module (Roche Diagnostics, Rotkreuz, Switzerland). This system allows for the quantitative detection of antibodies, predominantly IgG, and may also capture IgM and IgA, aiming at the SARS-CoV-2 spike (S) protein receptor-binding domain (RBD) and nucleocapsid (N) protein. As suggested by the manufacturer, values of S-IgG ≥ 0.8 U/mL and N-IgG ≥ 1.0 cut-off index (COI) were considered positive. In patients in whom sample results exceeded the upper limit of the measuring range (reported as >250 U/mL), samples were re-analyzed, after being diluted at 1:9 or dependent on the dilution being required. Any serologic values below the lower limit of quantitation were expressed as 0.4 U/mL. Furthermore, we defined S-IgG ≥ 200 U/mL as a “clinically protective” titer because of the previous report that 246 dental professionals who had COVID-19 revealed that spike antibody level of >195.2 U/mL to confer 6 months of protection against reinfection during a 6-month follow-up [15]. Moreover, 92.6% of our controls obtained the antibody titer more than 200 U/mL at T2 (SupTable1), which was a similar result that two-dose BNT162b2 conferred 95% protection against COVID-19 [1]. The sensitivity and the specificity for N-IgG were 99.5% and 99.78%, and those for S-IgG were 98.8% and 99.98%, respectively, according to the manufacturer.
Statistical analysis
The baseline characteristics in patients with PCD were compared using the Mann–Whitney U test or Student’s t-test for continuous variables and Fisher’s exact test for categorical variables. Mann–Whitney U test or Kruskal–Wallis test were used to determine differences between PCD type (symptomatic MM, sMM, or MGUS) and age-matched volunteers. Receiver operating characteristic curve analysis was performed to define the optimal cut-off for continuous variables. Univariate and multivariate logistic regression analyses were performed to evaluate potential predictors of seronegativity or SARS-CoV-2 antibody levels. Variables that showed p values <0.1 on univariate analysis and factors that were associated with lower response in the previous reports such as older age (≥65 years) [4, 7], anti-CD38 antibody use [6, 7], and lymphopenia (<1000/μL) [7, 8] were further tested in the multivariate analysis. All statistical analyses were conducted using the RStudio or the EZR software (Saitama Medical Center, Jichi Medical University) [16], which is a graphical user interface for R version 3.1.2 (The R Foundation for Statistical Computing, Vienna, Austria). Two-sided p values of <0.05 were considered statistically significant.
Results
Baseline characteristics of patients with PCD and controls
In total, the study population included 269 patients with PCD (206 symptomatic MM, 31 sMM, and 32 MGUS) [median age: 74 years, interquartile range (IQR): 68–79 years] and 94 controls (median age: 73 years, IQR: 69–78 years, male: 45) in Table 1. The median day from the first vaccine to T1 and T2 was 15 and 51 (IQR: 12–18 and 44–62). In patients with symptomatic MM, serum was obtained from 78, 94, and 194 patients at T0, T1, and T2, respectively. All serum from age-matched volunteers was obtained at T2. Most participants received the BNT162b2 vaccine (95.2% in patients and 100% in volunteers).
Table 1.
Patient characteristics
| Characteristics | All patients | MM | sMM | MGUS |
|---|---|---|---|---|
| N = 269 | 206 | 31 | 32 | |
| Age, years [median (IQR)] | 74 (68–79) | 74 (68–79) | 74 (70–78.5) | 76.5 (68–81.5) |
| Sex, male (%) | 135 (50.2) | 93 (45.1) | 22 (71.0) | 20 (62.5) |
| Time from diagnosis to vaccination, months (median, IQR) | 42.9 (21.1–86.4) | 45.8 (24.1–86.8) | 45.7 (21.3–89.3) | 26.5 (8.8–71.5) |
| ISS (n = 200) | ||||
| Stage I | 69 | 69 (34.5) | – | – |
| Stage II | 65 | 65 (32.5) | – | – |
| Stage III | 66 | 66 (33.0) | – | – |
| Ig type, n | ||||
| IgG | 145 | 107 | 20 | 18 |
| IgA | 64 | 48 | 7 | 9 |
| IgM | 2 | 0 | 0 | 2 |
| IgD | 1 | 1 | 0 | 0 |
| Light-chain | 55 | 49 | 3 | 3 |
| Non-secretory | 2 | 1 | 1 | 0 |
| High-risk cytogenetics at diagnosis, n (%) (n = 214) | 42 (19.6) | 36/171 (21.1) | 4/27 (14.8) | 2/16 (12.5) |
| Absolute lymphocyte count, /μL (IQR) | 1340 (960–1815) | 1275 (913–1694) | 1739 (1209–2070) | 1450 (1125–2098) |
| (Estimated) polyclonal IgG, mg/dL (IQR)a | 614 (414–982) | 551 (378–879) | 833 (565–1362) | 1142 (698–1281) |
| Receiving treatments within 6 months before 1st vaccination, n (%) | 183 (68.0) | 171 (83.0) | 6 (19.4) | 6 (18.8) |
| Treatment before 1st vaccination (within 6 months), n | ||||
| Bd | 3 | 3 | 0 | 0 |
| VRd | 3 | 2 | 1 | 0 |
| VMP | 2 | 2 | 0 | 0 |
| KRd | 2 | 2 | 0 | 0 |
| Kd | 12 | 12 | 0 | 0 |
| Ixa monotherapy | 6 | 6 | 0 | 0 |
| IRd | 14 | 14 | 0 | 0 |
| LenDex | 29 | 29 | 0 | 0 |
| PomDex | 7 | 7 | 0 | 0 |
| PCd | 1 | 1 | 0 | 0 |
| Other IMiDs | 1 | 1 | 0 | 0 |
| ERd | 3 | 3 | 0 | 0 |
| EPd | 9 | 9 | 0 | 0 |
| IsaPomDex | 14 | 12 | 1 | 1 |
| DVd | 5 | 5 | 0 | 0 |
| DKd | 3 | 3 | 0 | 0 |
| DVMP | 3 | 3 | 0 | 0 |
| DRd | 31 | 31 | 0 | 0 |
| DPd | 3 | 3 | 0 | 0 |
| Dara monotherapy | 31 | 22 | 4 | 5 |
| Other (cyclophosphamide) | 1 | 1 | 0 | 0 |
| Prior ASCT, n (%) | 67 (24.9) | 67 (32.5) | 0 | 0 |
| Lines of therapy, median (IQR) | 4 (2–5) | 4 (3–5) | 2 (1–3) | 2 (1–2) |
| IVIg before and after vaccination, n (%) | 25 (9.3) | 25 (12.1) | 0 | 0 |
| Vaccination with BNT162b2, n (%) | 256 (95.2) | 195 (94.7) | 30 (96.8) | 31 (96.9) |
ASCT autologous stem cell transplantation, Bd bortezomib and dexamethasone, Dara daratumumab, DKd daratumumab, carfilzomib, and dexamethasone, DPd daratumumab, pomalidomide, and dexamethasone, DRd daratumumab, lenalidomide, and dexamethasone, DVd daratumumab, bortezomib, and dexamethasone, DVMP daratumumab, bortezomib, melphalan, and dexamethasone, EPd elotuzumab, pomalidomide, and dexamethasone, ERd elotuzumab, lenalidomide, and dexamethasone, Ig immunoglobulin, IMiDs immunomodulatory drugs, IQR interquartile range, IRd ixazomib, lenalidomide and dexamethasone, IsaPomDex isatuximab, pomalidomide, and dexamethasone, ISS international staging system, IVIg intravenous immunoglobulin, Ixa ixazomib, Kd carfilzomib and dexamethasone, KRd carfilzomib, lenalidomide, and dexamethasone, MGUS monoclonal gammopathy of undetermined significance, MM multiple myeloma, LenDex lenalidomide and dexamethasone, PCd pomalidomide, cyclophosphamide, and dexamethasone, PomDex pomalidomide and dexamethasone, sMM smoldering multiple myeloma, VMP bortezomib, melphalan, and dexamethasone, VRd bortezomib, lenalidomide, and dexamethasone
aPolyclonal IgG was estimated from total IgG minus monoclonal IgG if IgG-type plasma cell dyscrasia
The number of patients with high-risk cytogenetic abnormalities (CAs), defined as either del(17p) > 10%, t(4;14), or t(14;16), International Staging System (ISS) stage III, and revised-ISS stage III was 36/171 (21.2%), 66/200 (33.0%), and 27/166 (16.3%), respectively. In total, 55 (26.7%), 121 (58.7%), 83 (40.3%), 16 (7.8%), 2 (1.0%), and 21 (10.2%) patients with symptomatic MM received proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), anti-CD38 antibody, elotuzumab, and ASCT 6 months before their first vaccination and immunoglobulin replacement 2 months before or after their first vaccination, respectively. No patients received belantamab mafodotin or other anti-B-cell mature antigen antibodies. Six patients each with sMM and MGUS received anti-myeloma therapy, mainly daratumumab, within 6 months before their first vaccination due to the concomitant of systemic light-chain amyloidosis, respectively. Therefore, the remainder of the analyses were performed on the 257 patients, excluding those with treated sMM or MGUS.
COVID-19 vaccine response in patients and controls
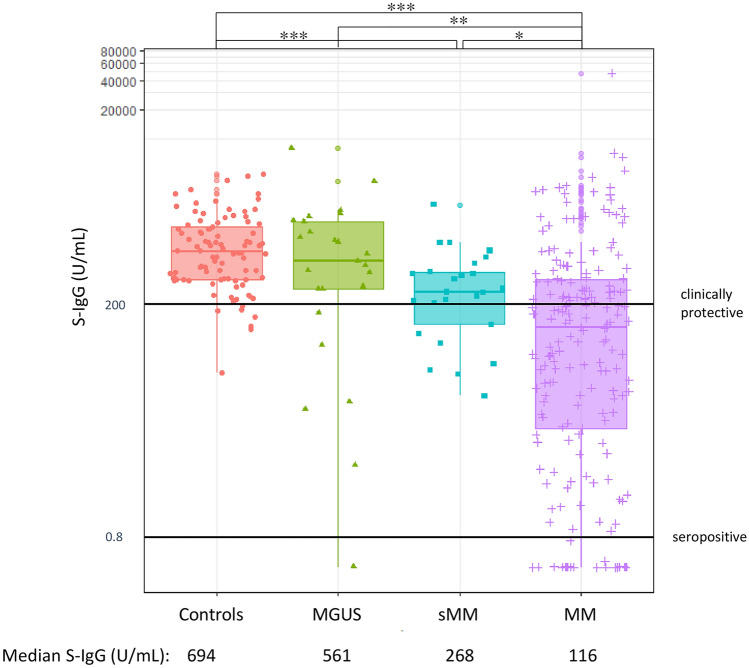
The humoral response was described in Supplementary Table 1. Among patients with PCD, 172 (88.7%), 25 (100%), and 24 (96%) patients with symptomatic MM, sMM, and MGUS and all controls obtained S-IgG seropositivity at T2. Patients with symptomatic MM diminished S-IgG response (median = 116.0 U/mL, range: 0.4–47,532) compared to those with smoldering MM (median = 268.0 U/mL; range: 23–2127, symptomatic MM vs. sMM, p = 0.030), MGUS (median = 561.0 U/mL; range: 0.4–8038, symptomatic MM vs. MGUS, p = 0.001) and healthy subjects (median = 694.0 U/mL; range: 40–4377, symptomatic MM vs. controls, p < 0.001) (Fig. 1). Moreover, 75 (38.3%), 18 (72.0%), and 19 (76.0%) patients with symptomatic MM, sMM, and MGUS and 87 (92.6%) controls obtained clinically protective S-IgG at T2. Of note, one patient with symptomatic MM was considered COVID-19 onset asymptomatically before vaccination because the patient had positive N-IgG (46.2 and 30.8 COI at T1 and T2) and elevated S-IgG antibody titer (947 and 47,532 U/mL at T1 and T2) about 100 times higher than those of other patients with symptomatic MM. No participants other than the above patient showed N-IgG positivity at any time.
Fig. 1.
S-IgG Response at T2. Patients with symptomatic MM diminished S-IgG response (median = 116.0 U/mL, range 0.4–47,532) compared to those with smoldering MM (median = 268.0 U/mL; range 23–2127, symptomatic MM vs. sMM, p = 0.030), MGUS (median = 561.0 U/mL; range 0.4–8038, symptomatic MM vs. MGUS, p = 0.001) and healthy subjects (median = 694.0 U/mL; range: 40–4377, symptomatic MM vs. controls, p < 0.001). Significance of differences between the indicated groups was assessed using the Kruskal–Wallis test. Asterisks denote significant changes (*0.01 ≤ p < 0.05, **0.001 ≤ p < 0.01, and ***p < 0.001)
Predictive factors for antibody production
Next, we assessed the impact of chemotherapy or clinical status for antibody production (Table 2). In the univariate analysis for predicting the seropositivity and the clinical protective titer on T2 among patients with symptomatic MM, lymphopenia (<1000/μL), low polyclonal IgG (<550 mg/dL), insufficient treatment response [partial response (PR) or less], anti-CD38 antibody use, and intravenous immunoglobulin (IVIg) were associated with seronegativity, and older age (≥65), lymphopenia, low polyclonal IgG, insufficient treatment response, anti-CD38 antibody use, immunomodulatory drugs (IMiDs) use, and IVIg were associated with insufficient antibody production. In multivariate analysis, lymphopenia [odds ratio (OR) 0.37, 95% confidence interval (CI) 0.14–0.99, p = 0.048], insufficient treatment response [PR or less; OR 0.24, 95% CI 0.08–0.74, p = 0.013) and anti-CD38 antibody use (OR 0.32, 95% CI 0.11–0.90, p = 0.031) were predictive of seronegativity and older age (OR 0.36, 95% CI 0.13–0.99, p = 0.048), lymphopenia (OR 0.31, 95% CI 0.13–0.70, p = 0.005), low polyclonal IgG (OR 0.29, 95% CI 0.14–0.58, p < 0.001), and IMiDs use (OR 0.26, 95% CI 0.12–0.54, p < 0.001) were predictive of insufficient antibody production.
Table 2.
Predicting factors of antibody production
| Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | |
| S-IgG positivity at T2 | ||||
| Age ≥65 | NA (complete separation) | – | – | – |
| Lymphopenia (<1000/μL) | 0.26 (0.10–0.65) | 0.004 | 0.37 (0.14–0.99) | 0.048 |
| ISS stage III | 1.02 (0.36–2.9) | 0.97 | – | – |
| Polyclonal IgG (<550 mg/dL) | 0.29 (0.10–0.83) | 0.021 | 0.40 (0.12–1.3) | 0.14 |
| High-risk CAs | 0.48 (0.15–1.5) | 0.21 | – | – |
| Treatment response PR or less | 0.25 (0.09–0.67) | 0.059 | 0.24 (0.08–0.74) | 0.013 |
| Anti-CD38 antibody use | 0.339 (0.14–0.85) | 0.021 | 0.32 (0.11–0.90) | 0.031 |
| IMiDs use | 0.497 (0.19–1.33) | 0.16 | – | – |
| Elotuzumab use | 2.01 (0.25–16.0) | 0.51 | – | – |
| IVIg use | 0.37 (0.12–1.14) | 0.083 | 0.69 (0.19–2.5) | 0.58 |
| Clinically protective titer at T2 | ||||
| Age ≥65 | 0.44 (0.18–1.06) | 0.066 | 0.36 (0.13–0.99) | 0.048 |
| Lymphopenia (<1000/μL) | 0.31 (0.15–0.65) | 0.002 | 0.31 (0.13–0.70) | 0.005 |
| ISS stage III | 0.971 (0.49–1.7) | 0.77 | – | – |
| Polyclonal IgG (<550 mg/dL) | 0.30 (0.16–0.55) | < 0.001 | 0.29 (0.14–0.58) | < 0.001 |
| High-risk CAs | 1.5 (0.68–3.2) | 0.33 | – | – |
| Treatment response PR or less | 0.31 (0.11–0.85) | 0.023 | 0.33 (0.10–1.04) | 0.058 |
| Anti-CD38 antibody use | 0.42 (0.23–0.79) | 0.006 | 0.58 (0.24–1.4) | 0.22 |
| IMiDs use | 0.34 (0.18–9.61) | 0.004 | 0.26 (0.12–0.54) | < 0.001 |
| Elotuzumab use | 0.70 (0.23–2.1) | 0.53 | – | – |
| IVIg use | 0.32 (0.10–0.97) | 0.045 | 0.41 (0.11–1.5) | 0.18 |
CAs cytogenetic abnormality, IMiDs immunomodulatory drugs, ISS international staging system, IVIg intravenous immunoglobulin, NA not available, OR odds ratio, PR partial response
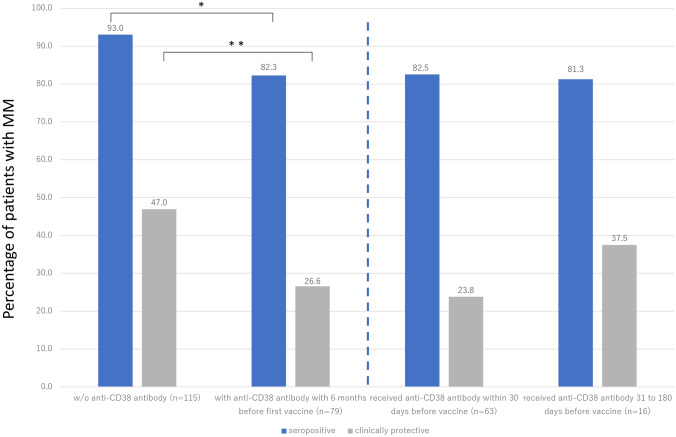
Furthermore, among the patients with MM, we investigated the duration of time between the last dose of anti-CD38 antibody and the first vaccine to explore the association with a cumulative incidence of antibody production (n = 79). Most of the patients receiving daratumumab, except for those receiving isatuximab (n = 10, 12.7%), received monthly administration. In the patients’ background between the administration of anti-CD38 antibody, patients with anti-CD38 antibody had significantly lower levels of polyclonal IgG (421 vs. 796 mg/dL, p < 0.001), but no significant difference in terms of the absolute number of lymphocytes count (1200 vs. 1301/μL, p = 0.22) and the treatment response (VGPR or better; 88.2% vs. 82.1%, p = 0.30) than those without. The percentage of patients with clinically protective titers began to increase from last exposure to anti-CD38 antibody, from 23.8% within 30 days to 37.5% thereafter (Fig. 2). However, the percentage of seropositive patients or patients with clinically protective antibody production was significantly higher among those without anti-CD38 antibody administration (seropositive; 93.0%, and clinical protective; 47.0%) than those received anti-CD38 antibody before the first vaccine (seropositive; 82.3%, p = 0.036, and clinical protective; 26.6%, p = 0.005). Although the number of patients was small, the number of patients who obtained clinically protective titers increased with a longer interval between the last dose of anti-CD38 antibody and their first vaccination. Moreover, when compared to patients without anti-CD38 antibody administration, those treated by anti-CD38 antibody within a month before the first vaccine had significantly lower S-IgG at T2 (without anti-CD38 antibody vs. administered within a month: median 191.0 vs. 36.9 U/mL, p < 0.001).
Fig. 2.
Time from anti-CD38 antibody administration to vaccine and S-IgG response. Patients with MM without anti-CD38 antibody and with anti-CD38 antibody within 6 months before first vaccine showed 93.0% and 82.3% in seropositivity and 47.0% and 26.6% in clinically protective titer (Left). Patients with anti-CD38 antibody administration within 30 days and 31 to 180 days before their first vaccine showed 82.5% and 81.3% in seropositivity and 23.8% and 37.5% in clinically protective titer, respectively (Right). Patients who received anti-CD38 antibody 6 months or over before first vaccine were included in “w/o anti-CD38 antibody” group. Significance of differences between the indicated groups was assessed using the Mann–Whitney U test or Kruskal–Wallis test, respectively. Asterisks denote significant changes (*0.01 ≤ p < 0.05 and **0.001 ≤ p < 0.01)
In addition, based on the result of the multivariate analysis, we evaluated the ad-hoc analysis of the lymphocyte profile (CD3+, CD4+, CD8+, CD3+/HLA-DR+, CD19+, and CD56+lymphocytes) in patients with MM (n = 30) (Table 3). The patients’ background is included in Supplementary Table 2. Due to only one patient who became seronegative at T2, no statistical comparisons were made. However, patients with clinically protective antibody production (n = 9) had a significantly higher number of CD19+lymphocytes than those with insufficient antibody production (median 114 vs. 35/μL, p = 0.016). The significant correlation between the number of CD19+ lymphocytes and S-IgG at T2 was observed by Spearman’s correlation coefficient analysis (correlation coefficient; 0.61, p < 0.001). Patients with clinically protective titers had significantly higher polyclonal IgG than those with insufficient titers (798 vs. 452 mg/dL, p = 0.025). In addition, although not significantly different, there were fewer patients receiving anti-CD38 antibody administration among patients with sufficient antibody titer production (11.1% in patients with S-IgG ≥ 200 U/L vs. 42.9% in patients with S-IgG < 200 U/L, p = 0.2). No significant differences were observed in lymphocytes other than CD19+ lymphocytes (Table 3).
Table 3.
Lymphocyte analysis
| S-IgG at T2 (U/mL) | <0.8 | ≥0.8 | <200 | ≥200 | |
|---|---|---|---|---|---|
| n | 1 | 29 | 21 | 9 | |
| Median, /μL (IQR) | p value | ||||
| Total lymphocytes | 1343 | 1227 (1020–1904) | 1227 (1000–1809) | 1275 (1144–1924) | 0.46 |
| CD3+ | 926 | 892 (649–1144) | 943 (727–1144) | 728 (649–910) | 0.38 |
| CD4+ | 147 | 351 (263–516) | 333 (242–516) | 351 (270–513) | 0.95 |
| CD8+ | 711 | 369 (219–692) | 421 (263–695) | 286 (219–556) | 0.5 |
| CD3 + HLA-DR + | 805 | 398 (277–799) | 398 (338–805) | 451 (191–775) | 0.97 |
| CD19+ | 0 | 46 (22–169) | 35 (19–74) | 114 (61–248) | 0.016 |
| CD56+ | 376 | 167 (45–303) | 85 (23–290) | 231 (131–285) | 0.22 |
Head-to-head comparison to the other reports
SARS-CoV-2 antibody titers were not directly comparable due to the lack of standardized serology assays, but some of those can be compared using the unit of “binding antibody units (BAU)/mL” [17]. In brief, BAU/mL was calculated as below; Roche S-IgG (U/mL) × 1 and DiaSorin Liason (AU/mL) × 2.6. We compared our results with those from two reports [4, 6] as shown in Table 4. The median age (70–74) and the vaccine type (BNT162b2) were similar among the three reports. However, our findings as well as those by Avivi, et al. reported lower median S-IgG titer than those reported by Pimpinelli, et al. (91 and 116 vs. 277.4 BAU/mL) even though our cohort received multiple treatment lines compared to other reports (median 4 vs. 2 lines). Although we could not make a direct comparison, we have reviewed literature reports measuring vaccine-induced antibody production in patients with PCD. These results are displayed in the lower portion of Table 4. After the first vaccination (T1), the seropositive rate achieved 25–56% [5, 9], which was higher than that of our result (17.0%, Supplementary Table 1). After the second vaccination, the positive rate increased to 66.0–84.2% [7, 8, 10].
Table 4.
Comparison to other reports and Literature review
| Reference | Number (MM/sMM) | Vaccination | Vaccine type | Seropositivity (%) | Median titer at T2 | Manufacture of COVID-19 antibody | Convert to BAU/mL | Median previous therapy number | VGPR or better | On therapy at vaccine | Anti-CD38 antibody use or types of treatment | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct comparison | Pimpinelli [6] | 42/0 | 1st and 2nd V | BNT162b2 | 78.6% | 106.7 AU/mL | DiaSorin Liaison | 277.4 | 2 (IQR 1–5) | ND | ND | Dara-base:14 pts |
| Avivi [4] | 159/12 | 2nd V | BNT162b2 | 76% (MM) | 91 U/mL | Roche | 91 | 2 | 72% | 92% | Dara: 45% | |
| Our report | 206/31 | 1st and 2nd V | 95.2% BNT162b2 | 88.7% (MM) | 116 U/mL | Roche | 116 | 4 (IQR 3–5) | 84% | 83% | Anti-CD38 antibody: 38.3% | |
| Other literature review | Bird [5] | 93/0 | 1st V | 48 BNT162b2, 45 AZD1222 | 56% | ND | Ortho Clinical Diagnostics | NA | 1 (IQR 1–2) | 51% | 71% | ND |
| Terpos [9] | 37/0 | 1st V | BNT162b2 | 25% | ND | GenScript | NA | 2 | ND | 72% | ND | |
| Stampferet [7] | 196/7 | 1st and 2nd V | Half; mRNA-1273 | 66% | 173 IU/mL | Sino Biological | not converted | ND | CR; 30/99 | 96 pts | Mainly IMiD, PI, and Dara | |
| Terpos [8] | 213/38 | 1st and 2nd V | 225 BNT162b2, 61 AZD1222 | 71% | ND | GenScript | not converted | 2 (IQR 1–3) | ND | 84% | Almost 85% Dara-based regimen | |
| Van Oekelen [10] | 320 | 1st and 2nd V | 69.1% BNT162b2 | 81.3% | 149 AU/mL | COVID-SeroKlir Kantaro | not converted | ND | ND | ND | ND |
COVID-19 Coronavirus disease 2019, CR complete remission, IMiDs immunomodulatory drugs, IQR interquartile range, MM multiple myeloma, ND no data, PI proteasome inhibitor, sMM smoldering multiple myeloma, V vaccine, VGPR very good partial response
A breakthrough patient
One male patient in his 70 s with MM who was fully vaccinated with BNT162b2 was diagnosed COVID-19 one month following his second vaccination. Both S-IgG and N-IgG were negative at COVID-19 onset. He was asymptomatic; to prevent severe disease, he received REGN-COV2, a cocktail of two non-competing neutralizing monoclonal antibodies, casirivimab and imdevimab, one day later. His S-IgG elevated to 185 U/mL after cocktail therapy infusion. His COVID-19 did not become severe and SARS-CoV-2 real-time polymerase chain reaction test became negative. His S-IgG antibody titer remained up to 240 U/mL one month after the cocktail therapy.
Discussion
In this study, we showed the S-IgG seropositivity in 88.7%, 100%, and 96.0%, and clinically protective antibody production in 38.3%, 72.0%, and 76.0% in MM, untreated sMM, and untreated MGUS, respectively. Compared with controls, patients with untreated sMM or MGUS had lower median S-IgG titer, suggesting that PCD itself may be related to poor antibody production. Furthermore, older age, anti-CD38 antibody administration, IMiDs use, lymphopenia, poor treatment response, and lower polyclonal IgG showed the negative impact for the serostatus or clinically protective antibody production based on the multivariate analysis. Ad-hoc analysis of lymphocyte subsets in MM patients showed that the patients with a lower number of CD19+ lymphocytes produced lower antibody titers.
In several previous reports regarding antibody titer after the second vaccine [4, 6–8, 10], 66.0–84.2% of patients with MM acquired seropositivity (Table 4). However, the “seropositivity” and “clinically protective titer” may be different and several reports developed different titers [11, 14, 15, 18]. Terpos, et al. reported that 53.5% of MM acquired “clinically relevant antibody response” [8], in which titer level was higher than that of our result (38.3%). In the report by Terpos et al., 15.2% and 30.8% patients showed lymphopenia and IgG < 700 mg/dL, however, 30.9% and 51.8% (102/197) in our patients with MM showed lymphopenia and lower polyclonal IgG (<550 mg/dL). Also, our results and those reported by Pimpinelli et al. and Avivi et al. showed differences in antibody titers (Table 4), and the severity of humoral immunodeficiency might be related to the lower clinically protective antibody response. In addition, patients with low polyclonal IgG are more likely to receive IVIg, which may induce further attenuation of vaccine efficacy. On the other hand, our breakthrough infected patient who was seronegative after full vaccine showed around 200 U/mL after cocktail therapy. S-IgG “replacement” by neutralizing antibody infusion to achieve the clinically protective titer showed the possibility of usefulness for immunocompromised patients.
Furthermore, in line with the previous study [8], our study showed the negative impact of anti-CD38 antibody administration on S-IgG production. According to the consensus from European Myeloma Network [19] and the International Myeloma Society guideline [20], “all patients with PCD should be vaccinated” and the timing of administration of the vaccine was “as soon as vaccine is available”. However, there was no data about the “wash-out” period from the last anti-CD38 antibody exposure to the vaccination. In general, CD38 expression recovered 4–6 months after anti-CD38 antibody discontinuation [21]. In our cohort, most patients with anti-CD38 antibody received monthly daratumumab infusions, and the number of patients with the clinically protective antibody titers rose modestly with longer wash-out periods prior to vaccination (Fig. 2). However, these patients showed significantly lower S-IgG titers than those without anti-CD38 antibody administration despite no significance difference of the absolute number of lymphocytes count and the treatment response. These results suggested the increase of patients with “acquired S-IgG antibody, but not enough” by anti-CD38 antibody administration. Furthermore, longer wash-out periods, ideally 4–6 months, from the end of anti-CD38 antibody administration to vaccination could be associated with an increase in the number of patients who could produce sufficient antibody. However, our study was designed to find the clinical variables that affected COVID-19 vaccine responses in patients with PCD this time, not to evaluate the effect of anti-CD38 antibody withdrawal period on vaccine efficacy. Further studies are needed to detect the effect of duration of anti-CD38 antibody cessation on vaccine responses.
On the other hand, our results suggested that IMiDs use had a negative effect on antibody production. However, it was unclear whether IMiDs use affected antibody production positively or negatively [20], because of the regulatory roles of IMiDs on T-cell or NK-cell activation which affect antibody production by B-cells. The reasons of the negative impact of IMiDs for the vaccine response was unclear. However, we did not collect data on the type of IMiDs and were unable to assess the dose or impact and the amount of dexamethasone or prednisolone for the prophylaxis of infusion reaction, which were the one of the limitations. Further studies are needed to determine the efficacy of the SARS-CoV-2 vaccine during IMiDs administration.
To the best of our knowledge, this study was the first to describe lymphocyte analysis after the SARS-CoV-2 vaccine in patients with MM. Due to the small number and ad-hoc analysis, most patients whom we could analyze were seropositive, however patients with clinically protective antibodies showed the greater number of CD19+ lymphocytes. As in several studies of B-cell lymphoma, B-cell depletion impeded antibody production [22, 23]. In patients receiving B-cell depleting agents such as anti-CD38 or anti-SLAMF7 antibody, the evaluation of B-cells in the peripheral blood and the titer of polyclonal IgG should be evaluated [24]. In our patients, due to only 16 patients (7.8%, 4 patients had been changed to the different regimen before their vaccine) who received elotuzumab within 6 months before their first vaccine, statistical analysis could not be performed. However, the duration of this study was relatively short, and patients with the lower number of B-cells may have a delayed response (for example, gradual increase until 3 months post-vaccination).
In our analysis, there were several limitations. There was a fewer number of patients who evaluated S-IgG at T1. No healthy controls measured their antibody titers at T0 and T1. The type of vaccination used in 4.8% in patients was unknown (due to unavailable data), but only mRNA vaccines (BNT162b2 or mRNA-1273), not vector vaccines, were distributed in Japan.
In summary, our results showed a lower vaccine response in patients with PCD, especially those with MM. About 90% of patients with MM also became seropositive after the double dose vaccines, suggesting that all patients with PCD should receive vaccination even if they have poor response factors. Based on the fact that patients who received anti-CD38 antibodies had a lower vaccine response rate, it would be ideal to implement a temporary interruption in anti-CD38 therapy e.g., a “wash-out" period for a few months prior to vaccination, followed by a booster vaccine after B-cells have recovered. Further research on vaccine administration strategy are required to maximize the effectiveness of the SARS-CoV-2 vaccine. We plan to further investigate the attenuation of antibody titers and responses after the third dose, as well as regulatory T-cells, which are important for therapeutic responses in MM [25] and may diminish vaccine responses [26].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the patients with hematologic malignancy, their families, and the age-adjusted controls, and the medical staff of the Department of Hematology of Kameda Medical Center, department of Hematology of Kanawaza University, and division of Internal Medicine of Keiju Kanazawa Hospital. We also would like to thank Eri Suzuki. RN (assistant staff of Department of Hematology) and Dr. So Nakaji (department of Gastroenterology) for data collection, and Kazuki Ueno, M.T., Hatsune Yanagida, M.T., and Harumi Ishikura, M.T. (department of Laboratory Medicine) for antibody measurement. We also thank Editage (https://www.editage.jp/) for English language editing.
Authors’ contributions
TT conceived and designed the study, collected data, performed the statistical analysis, wrote the manuscript, and provided patient care. TY, AF, YK, DI, AK, RT, TT, DM, KN, MT and HT collected data and provided patient care. MD, YU, and YO analyzed antibody titers. KM initiated, designed, and supervised the study, collected data, wrote the manuscript, and provided patient care. All authors reviewed and approved the final manuscript.
Funding
The authors did not receive financial support from any organization for the submitted work.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from Toshiki Terao or Kosei Matsue on reasonable request.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by each institutional review board (Approval Number:21–025).
Consent to participate
All participants or their family members provided written informed consent for study participation.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Toshiki Terao and Takeshi Yamashita contributed equally
Hiroyuki Takamatsu and Kosei Matsue contributed equally
Contributor Information
Hiroyuki Takamatsu, Email: takamaz@staff.kanazawa-u.ac.jp.
Kosei Matsue, Email: koseimatsue@gmail.com.
References
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herzog Tzarfati K, Gutwein O, Apel A, Rahimi-Levene N, Sadovnik M, Harel L, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96(10):1195–1203. doi: 10.1002/ajh.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maneikis K, Šablauskas K, Ringelevičiūtė U, Vaitekėnaitė V, Čekauskienė R, Kryžauskaitė L, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583–e592. doi: 10.1016/s2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avivi I, Balaban R, Shragai T, Sheffer G, Morales M, Aharon A, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol. 2021 doi: 10.1111/bjh.17608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird S, Panopoulou A, Shea RL, Tsui M, Saso R, Sud A, et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8(6):e389–e392. doi: 10.1016/s2352-3026(21)00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14(1):81. doi: 10.1186/s13045-021-01090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stampfer SD, Goldwater MS, Jew S, Bujarski S, Regidor B, Daniely D, et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia. 2021 doi: 10.1038/s41375-021-01354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021;11(8):138. doi: 10.1038/s41408-021-00530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terpos E, Trougakos IP, Gavriatopoulou M, Papassotiriou I, Sklirou AD, Ntanasis-Stathopoulos I, et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137(26):3674–3676. doi: 10.1182/blood.2021011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Oekelen O, Gleason CR, Agte S, Srivastava K, Beach KF, Aleman A, et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39(8):1028–1030. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockmer S, Uttervall K, Kashif M, Svärd C, Malmsten K, Fletcher-Torres E, et al. Antibody response to COVID-19 mRNA vaccine (comirnaty) in myeloma patients treated with high-dose melphalan and/or immunotherapy. Am J Hematol. 2021 doi: 10.1002/ajh.26348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO coronavirus (COVID-19) Dashboard. https://covid19.who.int. Accessed 24 Sept 2021.
- 13.Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against covid-19 in Israel. N Engl J Med. 2021 doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shields AM, Faustini SE, Kristunas CA, Cook AM, Backhouse C, Dunbar L, et al. COVID-19: Seroprevalence and vaccine responses in UK dental care professionals. J Dent Res. 2021;100(11):1220–1227. doi: 10.1177/00220345211020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perkmann T, Perkmann-Nagele N, Koller T, Mucher P, Radakovics A, Marculescu R, et al. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spectr. 2021;9(1):e0024721. doi: 10.1128/Spectrum.00247-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laterza R, Schirinzi A, Bruno R, Genco R, Contino R, Ostuni A, et al. SARS-CoV-2 antibodies: comparison of three high-throughput immunoassays versus the neutralization test. Eur J Clin Invest. 2021;51(7):e13573. doi: 10.1111/eci.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig H, Sonneveld P, Facon T, San-Miguel J, Avet-Loiseau H, Mohty M, et al. COVID-19 vaccination in patients with multiple myeloma: a consensus of the European Myeloma Network. Lancet Haematol. 2021;8(12):e934–e946. doi: 10.1016/s2352-3026(21)00278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recommendations for anti-Covid-19 vaccination in patients with multiple myeloma (MM) and related conditions, AL amyloidosis and other monoclonal gammopathies of clinical significance. http://cms.cws.net/content/beta.myelomasociety.org/files/PM%20COVID%20vaccination%20in%20MM%20guidelines%20The%20Final.pdf. Accessed 1 Oct 2021.
- 21.Nijhof IS, Casneuf T, van Velzen J, van Kessel B, Axel AE, Syed K, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959–970. doi: 10.1182/blood-2016-03-703439. [DOI] [PubMed] [Google Scholar]
- 22.Gurion R, Rozovski U, Itchaki G, Gafter-Gvili A, Leibovitch C, Raanani P, et al. Humoral serologic response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies. Haematologica. 2021 doi: 10.3324/haematol.2021.279216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053–3061. doi: 10.1182/bloodadvances.2021005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, Terpos E, Dimopoulos MA. SARS-CoV-2 vaccines in patients with multiple myeloma. Hemasphere. 2021;5(3):e547. doi: 10.1097/hs9.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitadate A, Kobayashi H, Abe Y, Narita K, Miura D, Takeuchi M, et al. Pre-treatment CD38-positive regulatory T cells affect the durable response to daratumumab in relapsed/refractory multiple myeloma patients. Haematologica. 2020;105(1):e37–e40. doi: 10.3324/haematol.2019.219683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41(6):1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from Toshiki Terao or Kosei Matsue on reasonable request.