Abstract
Background:
Enteric infections cause significant deaths, and global projection studies suggest that mortality from enteric infections will increase in the future with warmer climate. However, a major limitation of these projection studies is the use of risk estimates derived from nonmortality data to project excess enteric infection mortality associated with temperature because of the lack of studies that used actual deaths.
Objective:
We quantified the associations of daily temperature with both mortality and hospital admissions due to enteric infections in the Philippines. These associations were applied to projections under various climate and population change scenarios.
Methods:
We modeled nonlinear temperature associations of mortality and hospital admissions due to enteric infections in 17 administrative regions of the Philippines using a two-stage time-series approach. First, we quantified nonlinear temperature associations of enteric infections by fitting generalized linear models with distributed lag nonlinear models. Second, we combined regional estimates using a meta-regression model. We projected the excess future enteric infections due to nonoptimal temperatures using regional temperature–enteric infection associations under various combinations of climate change scenarios according to representative concentration pathways (RCPs) and population change scenarios according to shared socioeconomic pathways (SSPs) for 2010–2099.
Results:
Regional estimates for mortality and hospital admissions were significantly heterogeneous and had varying shapes in association with temperature. Generally, mortality risks were greater in high temperatures, whereas hospital admission risks were greater in low temperatures. Temperature-attributable excess deaths in 2090–2099 were projected to increase over 2010–2019 by as little as 1.3% [95% empirical confidence intervals (eCI): , 6.5%] under a low greenhouse gas emission scenario (RCP 2.6) or as much as 25.5% (95% eCI: , 48.2%) under a high greenhouse gas emission scenario (RCP 8.5). A moderate increase was projected for temperature-attributable excess hospital admissions, from 0.02% (95% eCI: , 1.9%) under RCP 2.6 to 5.2% (95% eCI: , 21.8%) under RCP 8.5 in the same period. High temperature-attributable deaths and hospital admissions due to enteric infections may occur under scenarios with high population growth in 2090–2099.
Discussion:
In the Philippines, futures with hotter temperatures and high population growth may lead to a greater increase in temperature-related excess deaths than hospital admissions due to enteric infections. Our results highlight the need to strengthen existing primary health care interventions for diarrhea and support health adaptation policies to help reduce future enteric infections. https://doi.org/10.1289/EHP9324
Introduction
Enteric infections are intestinal infectious diseases caused by parasitic, bacterial, and viral pathogens that disrupt intestinal functions and lead to diarrhea and dehydration (Petri et al. 2008). They cause significant disease burden, particularly in children from low- and middle-income countries (Kotloff 2017). In 2016, enteric infections were the eighth leading cause of global mortality, with an estimated deaths and episodes (Troeger et al. 2018a). Progress has been made in reducing the incidence and mortality of enteric infections; however, inequality has hampered this progress in low resource regions (Reiner et al. 2020).
Climate change is expected to further slow the progress that has been made in reducing enteric infections because transmission could be aggravated by increased temperatures (Levy et al. 2016). Meta-analyses of the temperature–enteric infection association suggested that the incidence of all-cause enteric infections may increase under warmer temperatures (Carlton et al. 2016; Chua et al. 2020; Liang et al. 2021). Warm temperatures have been postulated to increase pathogen loads in animal hosts and water systems (Kelly et al. 2014; Lal et al. 2012), facilitate contamination of drinking water systems and spoilage of food (Hodges et al. 2014; Tirado et al. 2010), and change water consumption habits (Mellor et al. 2016), all of which may contribute to an increased incidence of enteric infections.
To simulate the potential impacts of climate change, projection studies of future temperature-attributable enteric infections were conducted on varying geographical scales. Several studies projected overall future increases in enteric infection morbidity (e.g., hospital admissions, clinic visits, and reported cases) related to warmer temperatures, both at the city and country levels in India and China (Liu et al. 2020; Mellor et al. 2016; Moors et al. 2013; Zhang et al. 2012). Conversely, a projection study in Japan yielded net reductions in future temperature-attributable gastroenteritis cases especially under scenarios with a considerable temperature rise, presumably because of the predominant viral enteric infections in Japan, which have negative temperature associations (Onozuka et al. 2019).The possible climate change–driven future reductions in diarrhea due to viral pathogens like rotavirus could further be accelerated with better coverage of rotaviral vaccination (Troeger et al. 2018b).
On a global level, several studies projected the future temperature-attributable mortality due to enteric infections under various future scenarios (Campbell-Lendrum et al. 2003; Chua et al. 2021; Kovats and Lloyd 2014; McMichael et al. 2004). They suggest that the temperature-attributable excess deaths due to enteric infections could rise in potential futures with considerably warmer temperatures and poor socioeconomic development compared with historical conditions. However, these studies were limited because of the use of temperature–enteric infection risk functions estimated using nonmortality outcomes (i.e., hospital admissions and surveillance reports) in projecting temperature-attributable mortality due to enteric infections. The authors assumed that the temperature association for morbidity approximated temperature associations for mortality due to enteric infections. The impact of this assumption on global projection outputs remains unclear because temperature associations for mortality may differ from that of morbidity. At least two studies have reported increased mortality due to enteric infections in children during the summer in Mexico and Netherlands (Alonso et al. 2012; Ekamper et al. 2009), but neither provided a relative risk to quantify the risk of mortality at high or low temperatures.
To address this knowledge gap, we sought to quantify the short-term associations of ambient temperature with mortality due to enteric infections. In addition, to better understand the impact of the choice of morbidity- and mortality-based temperature–enteric infection associations in projection studies, we compared the projections of temperature-related enteric infections between mortality and hospital admissions under futures with a changing climate and population. To our knowledge, this is the first time that the associations between temperature and enteric infection mortality have been quantified and used for projections of temperature-attributable deaths due to enteric infections. We explored these in the Philippines, where enteric infections have been one of the leading causes of morbidity and have led to considerable number of deaths annually (DOH 2019a; PSA 2016a).
Methods
Data Sources and Scenarios
We obtained nationwide data on individual deaths and hospital admissions for the period 2006–2017 in the Philippines that were coded with the 10th revision of the International Classification of Diseases (ICD-10) A00–A09 or intestinal infectious diseases from the Philippine Statistics Authority and the Philippine Health Insurance Corporation. The Philippine Statistics Authority records nationwide deaths through death certificates submitted to its local offices, and the Philippine Health Insurance Corporation reimburses the health care costs of registered individuals from accredited hospitals and infirmaries. We used the day of death and day of admission as the time index and used the location of death and hospitals in 17 administrative regions (Figure S1) to aggregate these into daily time series. We limited the time series from 1 January 2014 to 31 December 2017 because the number of enteric infection hospital admissions was low in 2006–2013 and inconsistent with the number of deaths related to enteric infection (Figure S2).
We collected hourly temperatures, defined as the temperature of air at above the land surface, from the ERA5-Land data set, which is a replay of the land component of the ERA5 climate reanalysis at an enhanced resolution of or made available by the European Center for Medium-Range Weather Forecasts (Muñoz-Sabater 2021). We converted the hourly time series from Coordinated Universal Time (UTC) to and took the daily average for each grid square. We calculated the population-weighted average of temperatures by region (Figure S3) using the 2015 population density (CIESIN 2018). We also collected relative humidity and total precipitation from ERA5-Land to consider as possible confounders. We derived daily relative humidity from dew point temperatures and air temperatures (NOAA 2007). We then took the population-weighted average of daily relative humidity and total precipitation by region. We also collected daily mean temperature from 38 weather stations of the Philippine Atmospheric, Geophysical and Astronomical Services Administration and took the average per region (Figure S4).
Projected daily near-surface air temperatures up to the year 2100 were obtained from the Inter-Sectoral Impact Model Intercomparison Project (ISIMIP), which is a cross-sectoral and cross-scale framework for multimodel climate-impact simulations, from their 2b input data sets (ISIMIP2b) (ISIMIP 2019). These correspond to downscaled grids and bias-corrected Coupled Model Intercomparison Project Phase 5 simulations from four general circulation models (GCMs) (i.e., mathematical representations of global coupled ocean–atmosphere–cryosphere–land processes with their interactions), namely Geophysical Fluid Dynamics Laboratory-Earth System Model (GFDL-ESM2M), Met Office Hadley Center-Earth System Model (HadGEM2-ES), Institut Pierre Simon Laplace-Coupled Model (IPSL-CM5A-LR), and Model for Interdisciplinary Research on Climate (MIROC5). The projected temperatures follow various representative concentration pathways (RCPs), which report radiative forcing in watt per square meter as a basis for long-term climate modeling experiments (Collins et al. 2013; van Vuuren et al. 2011). We considered RCPs 2.6, 4.5, 6.0, and 8.5. The lowest RCP represents strong emission reductions, whereas higher RCPs depict continued reliance on nonrenewable sources emitting more greenhouse gases. We recalibrated each projected temperature series using the observed temperatures (Hempel et al. 2013). Projected population scenarios, which were downscaled in grids from a country-level projection (KC and Lutz 2017), were also obtained from ISIMIP2b. These are based on shared socioeconomic pathways (SSPs), which are narratives of future societal developments indicating various combinations of challenges in mitigation and adaptation (O’Neill et al. 2017). SSP 1 and 5 depict rapid socioeconomic development with low population growth, but they have different challenges in mitigation, with SSP 1 prioritizing sustainable development and SSP 5 relying heavily on fossil fuels. SSP 2 follows historical trends leading to intermediate challenges in mitigation and adaptation. SSP 3 depicts slow socioeconomic development with high population growth in developing countries and poor mitigation. SSP 4 reflects unequal socioeconomic development both within and across all countries with relatively high population growth in developing countries but low challenges in mitigation. We selected SSPs 1, 2, 3, 4, and 5, and paired them with their respective RCPs (Riahi et al. 2017) (Figure S5).
We collected sociodemographic indicators including regional population density in 2015 and gross regional domestic product in 2017 from the Philippine Statistics Authority (PSA 2016b, 2020), and regional percentages of households with access to improved safe water and with sanitary toilets in 2017 from the Field Health Services Information System of the Philippine Department of Health (DOH 2019a) (Table S1). Between 2014 and 2017, regional values of these sociodemographic indicators remained proportional across the years, so we used the latest available year per indicator.
Statistical Analysis
The historical temperature–enteric infection associations were quantified for mortality and hospital admissions using a two-stage time-series analysis: a) quantifying regional temperature–enteric infection associations and b) estimating overall cumulative temperature–enteric infection associations. Using the region-specific temperature–enteric infection associations, we projected future temperature-attributable enteric infections (Gasparrini et al. 2017).
Quantifying Historical Temperature–Enteric Infection Associations
We modeled the nonlinear lagged associations between daily temperatures and enteric infections using a quasi-Poisson generalized linear model to account for overdispersion with a distributed lag nonlinear model:
where is the expected daily enteric infection on day and by outcome (i.e., mortality or hospital admissions); is the intercept; is the bi-dimensional spline function of exposure–lag–response for daily temperature and lag ; is the natural cubic B-spline function of day with 4 degrees of freedom (df) multiplied by 4 y to control for seasonality and long-term trends; is the indicator function for time-varying covariates of the day of the week and national holidays; and , , and denote the parameters of estimation.
The exposure–response curves for temperature were modeled using a natural cubic B-spline with 2 df assuming the possibility of high- and low-temperature associations (Carlton et al. 2016). The lag–response curves for weather variables were modeled using a natural cubic B-spline with an intercept and 3 internal knots placed at log scale. We used 0–21 d for temperature as presumed lags between weather variables and diarrhea outcome occurrence considering the complex pathological pathways involved (Mertens et al. 2019; Wang et al. 2019).
We combined the region-specific estimates, which are transformed coefficients representing uni-dimensional cumulative temperature–enteric infection curves with reduced lag dimension, using multivariate meta-regression models (Gasparrini et al. 2012):
where the region-specific estimates are assumed to follow a multivariate normal distribution with as a region-specific vector of meta-predictors and matrices and representing within- and between-regional (co)variance.
We initially ran an intercept-only model and then added meta-predictors: regional temperature means and ranges, gross domestic products, population densities, and access to water and sanitation household coverages, and we checked their significance using a Wald test. Residual heterogeneity was measured using statistics and Cochran’s Q test. We then derived the best linear unbiased prediction of the overall cumulative temperature–outcome associations by region in relative risks. The best linear unbiased prediction represented a trade-off between region-specific and region-pooled associations enabling areas with small number of daily deaths and hospital admissions to use information from areas with larger populations (Gasparrini et al. 2012). We determined minimum risk temperatures using the lowest cumulative relative risks between the 2.5th and 97.5th percentiles of region-specific temperature distribution (Lee et al. 2017). We recentered the cumulative associations using the minimum risk temperatures as reference. The minimum risk temperatures are single point temperatures that separate low and high temperatures indicating a relative risk of 1. We took the relative risks of the 95th and 5th percentiles of temperature distributions in comparison with minimum risk temperatures to measure the high- and low-temperature associations, respectively. We derived the overall country-level associations using the final meta-regression model (Xiao et al. 2017).
Projecting Future Temperature-Attributable Outcomes
We estimated the excess enteric infections outcome attributable to nonoptimal temperatures in 2010–2099 using the regional cumulative temperature–enteric infection associations with assumptions of population changes and no adaptation:
where is the temperature-attributable enteric infections by outcome ; is the daily average rate of deaths or hospital admissions due to enteric infections in 2014–2017 as baseline enteric infections assuming that there are no changes in the rates in the future; is the projected population according to the SSP; , and are the reduced overall cumulative associations derived after best linear unbiased prediction; is the recalibrated projected temperature by RCP; and is the reference temperature based on the minimum risk temperatures.
The total number of enteric infection outcomes attributable to temperature was the sum of contributions from all days of the projection years. The components attributable to high and low temperatures were separated by summing subsets of days with temperatures above and below the minimum risk temperatures (Gasparrini et al. 2017). The ratio of the attributable numbers to the total enteric infection outcome was the total attributable fraction. We calculated separately the excess outcomes for each region and SSP, as well as combinations of the GCMs and RCPs. Attributable numbers and fractions were calculated as GCM-ensemble by region and then aggregated as Philippine-level, decade, and RCP using the total number of outcomes as the denominator. We did 1,000 Monte Carlo simulations to derive empirical confidence intervals (eCIs) that quantify the uncertainties in associated with the temperature–enteric infection associations and variability in temperature projections across GCMs (Gasparrini et al. 2017).
Sensitivity Analyses
To assess the robustness of the generalized linear model in the first-stage analysis, we checked the shapes of the cumulative associations by increasing the degrees of freedom of natural cubic B-spline for day, adding daily total rainfall and relative humidity as possible confounders, adding population as an offset, and changing the maximum lag period to 14 or 27 d (Liu et al. 2020). We also ran the same analysis using daily mean temperatures from 38 weather stations.
Software
All analyses were performed in R programming (version 4.0.2; R Development Core Team), using dlnm, mixmeta, ncdf4, and raster packages.
Ethical Considerations
The study protocol underwent ethical review and was approved by the National Ethics Committee of the Philippine Council for Health Research and Development (study number 2020-001) on 20 April 2020. We received de-identified individual death and hospital admission data requiring no individual informed consent form. Only the research team had access to the data sets.
Results
Summary of Enteric Infections, Meteorological, and Projections Data
A total of 18,387 deaths and 2,004,268 hospital admissions due to enteric infections were reported in 2014–2017 (Table 1). Most of the deaths and hospital admissions due to enteric infections were from Region 7 and Region 4A, respectively. The etiology of enteric infection deaths and hospital admissions were mostly unspecified (86% and 79%, respectively), but the two most reported were typhoid fever and amebiasis (Tables S2 and S3). Less than half (46%) of reported hospital admissions were children 0–5 y old, whereas the age-group distribution of reported deaths varied by region, with some regions having more deaths among elders (Tables S4 and S5). More than half of the reported deaths were medically attended, and 40% were unattended (Table S6).
Table 1.
Descriptive statistics of mortality and hospital admissions due to enteric infections as well as meteorological variables by Philippine region in 2014–2017.
| Region | Mortality due to enteric infections | Hospital admissions due to enteric infections | Daily average of ERA5-Land variables by grids | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Daily average (range) | Most common etiology (%) | Physician-attended (%) | Overall | Daily average (range) | Most common etiology (%) | 2-meter temperature (°C) (range) | Relative humidity in % (range) | Total precipitation in mm/h (range) | |
| CAR | 219 | 0.15 (0; 3) | A01 (12) | 126 (58) | 55,827 | 38.21 (12; 82) | A01 (23) | 21.9 (15.6; 24.8) | 84.0 (66.4; 95.1) | 5.8 (0.0; 72.6) |
| Region 1 | 839 | 0.57 (0; 4) | A01 (9) | 542 (65) | 141,374 | 96.77 (39; 172) | A01 (13) | 26.3 (21.4; 29.8) | 78.7 (58.8; 93.3) | 4.1 (0.0; 65.0) |
| Region 2 | 621 | 0.43 (0; 4) | A01 (9) | 338 (54) | 83,985 | 57.48 (22; 110) | A01 (11) | 25.0 (17.6; 28.9) | 83.3 (65.7; 96.1) | 3.9 (0.0; 47.9) |
| Region 3 | 1,458 | 1.00 (0; 6) | A06 (5) | 845 (58) | 161,815 | 110.76 (50; 206) | A06 (8) | 26.4 (21.7; 30.6) | 78.6 (60.0; 94.6) | 3.6 (0.0; 54.6) |
| NCR | 2,067 | 1.41 (0; 6) | A06 (6) | 1,473 (71) | 104,952 | 71.84 (25; 209) | A06 (8) | 26.8 (21.6; 31.4) | 78.4 (56.5; 93.9) | 3.6 (0.0; 49.8) |
| Region 4A | 2,090 | 1.43 (0; 7) | A06 (4) | 1,187 (57) | 201,930 | 138.21 (51; 268) | A01 (14) | 26.2 (21.2; 30.0) | 80.9 (57.7; 93.9) | 3.9 (0.0; 50.6) |
| Region 4B | 502 | 0.34 (0; 5) | A01 (9) | 253 (50) | 46,361 | 31.73 (7; 85) | A01 (17) | 26.1 (22.4; 28.7) | 83.4 (68.4; 92.3) | 4.6 (0.0; 38.4) |
| Region 5 | 1,285 | 0.88 (0; 5) | A01 (10) | 696 (54) | 85,360 | 58.43 (25; 120) | A01 (10) | 26.3 (22.1; 28.9) | 83.6 (64.3; 93.0) | 4.1 (0.0; 48.9) |
| Region 6 | 2,033 | 1.39 (0; 14) | A01 (16) | 1,276 (63) | 126,475 | 86.57 (37; 155) | A01 (11) | 26.2 (22.4; 29.6) | 84.2 (65.7; 93.5) | 4.9 (0.0; 66.7) |
| Region 7 | 2,092 | 1.43 (0; 7) | A01 (9) | 1,245 (60) | 134,414 | 92.00 (41; 201) | A06 (11) | 26.1 (22.8; 28.6) | 83.0 (66.2; 91.4) | 3.9 (0.0; 40.4) |
| Region 8 | 989 | 0.68 (0; 7) | A01 (11) | 532 (54) | 111,210 | 76.12 (22; 210) | A06 (5) | 26.2 (22.7; 28.8) | 84.2 (65.0; 93.8) | 4.7 (0.0; 94.4) |
| Region 9 | 887 | 0.61 (0; 5) | A01 (12) | 497 (56) | 92,972 | 63.64 (23; 149) | A01 (17) | 26.0 (23.4; 28.5) | 84.8 (62.7; 92.6) | 4.5 (0.0; 37.3) |
| Region 10 | 1,035 | 0.71 (0; 4) | A01 (11) | 640 (62) | 171,078 | 117.10 (52; 216) | A01 (20) | 24.4 (21.0; 26.7) | 85.3 (65.1; 94.7) | 5.2 (0.0; 42.6) |
| Region 11 | 826 | 0.57 (0; 5) | A01 (7) | 528 (64) | 149,460 | 102.30 (40; 178) | A06 (12) | 25.6 (22.5; 28.3) | 82.4 (66.1; 93.8) | 4.3 (0.0; 59.0) |
| Region 12 | 724 | 0.50 (0; 7) | A01 (9) | 394 (54) | 178,298 | 122.04 (62; 267) | A01 (21) | 25.3 (22.2; 28.8) | 82.7 (53.7; 94.2) | 4.9 (0.1; 31.7) |
| Region 13 | 518 | 0.35 (0; 3) | A01 (9) | 292 (56) | 66,589 | 45.58 (17; 108) | A06 (12) | 25.8 (22.2; 28.4) | 84.6 (70.7; 94.6) | 5.2 (0.0; 90.2) |
| BARMM | 202 | 0.14 (0; 3) | A01 (11) | 125 (62) | 92,168 | 63.09 (21; 184) | A01 (14) | 24.6 (22.4; 26.9) | 84.9 (66.4; 93.8) | 5.9 (0.0; 30.3) |
| Philippines | 18,387 | 12.59 (2; 32) | A01 (8) | 10,989 (60) | 2,004,268 | 1,371.85 (780; 2065) | A01 (12) | 25.6 (15.6; 31.4) | 82.8 (53.7; 96.1) | 4.5 (0.0; 94.4) |
Note: A01, typhoid and paratyphoid; A06, amebiasis; BARMM, Bangsamoro Autonomous Muslim Mindanao; CAR, Cordillera Administrative Region; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Clinical Modification; NCR, National Capital Region.
Most of the etiologies were unspecified.
Mean temperatures were similar, and temperature distributions were relatively narrow across the regions (Table 1). The daily temperatures in the Philippines over the study period ranged from to . The Cordillera Administrative Region had the lowest mean temperature at among the regions. Projected temperatures had similar increasing patterns across the regions with the smallest increase under RCP 2.6 and the greatest increase under RCP 8.5 (Figure 1; Table S7; Figure S6). The projected population generally increased but varied by region with the greatest increase in the urbanized regions with higher socioeconomic levels (i.e., gross regional domestic product) under SSP 4, whereas the rest were under SSP 3 (Figure 1; Table S8; Figure S7). The lowest increases in the projected population were under SSP 1 and SSP 5.
Figure 1.
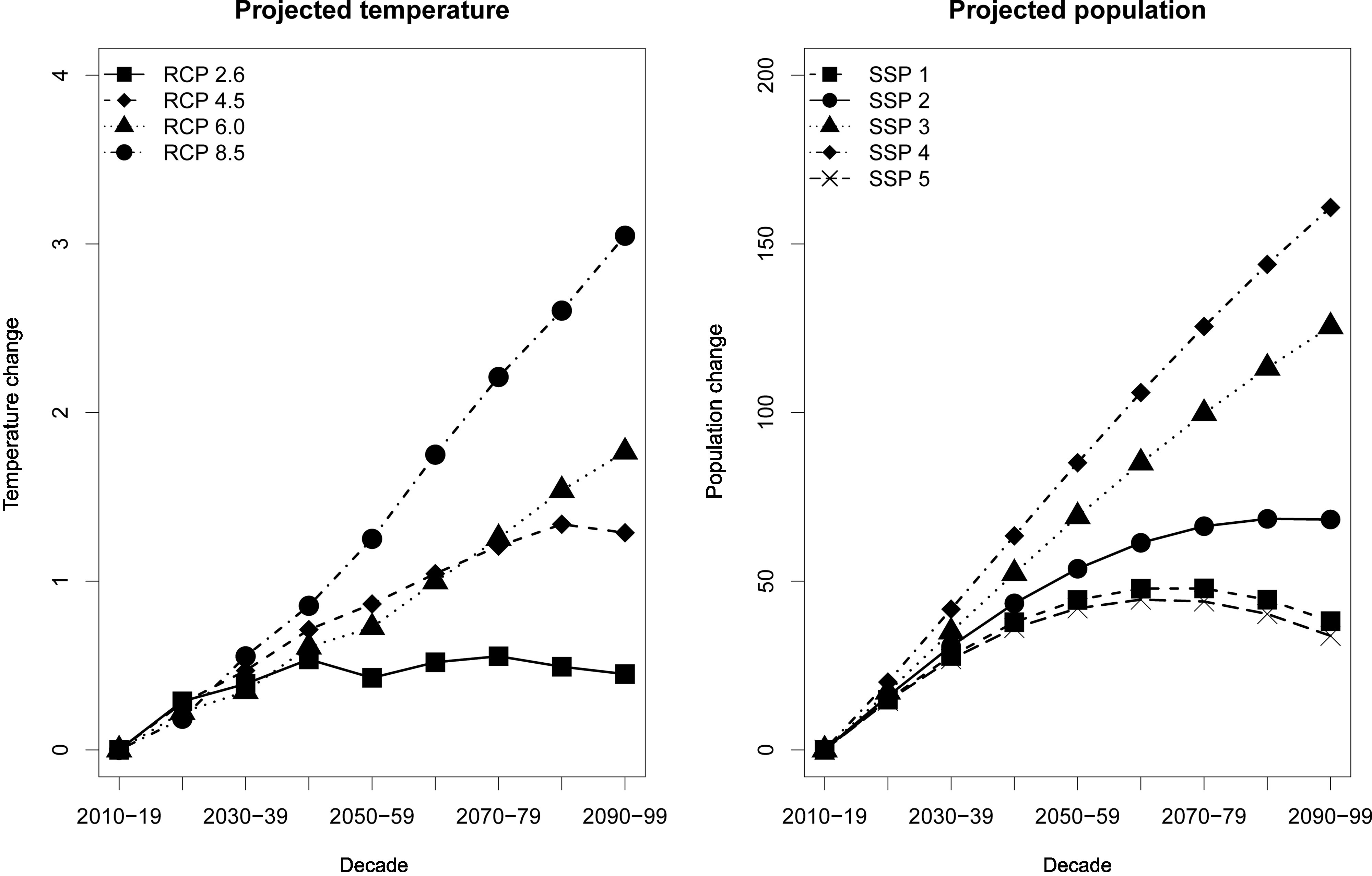
Projected temperature change by representative concentration pathways (RCP) and projected population change by shared socioeconomic pathways (SSP) in the Philippines. Temperature and population changes were the differences between 2010–2019 and the rest of the decades. Corresponding numeric data are presented in Tables S7 and S8.
Historical Temperature–Enteric Infection Associations
The pooled estimates from the intercept-only models showed considerable heterogeneity for mortality (; Cochran’s Q test, ) and hospital admissions (; Cochran’s Q test, ) due to enteric infections. Only population density partly explained heterogeneity for mortality (; Cochran’s Q test, ) but not for hospital admissions (; Cochran’s Q test, ) due to enteric infections (Table S9). Thus, the meta-regression with population density as the sole meta-predictor was selected as the final model. The overall cumulative associations revealed a U-shaped curve for both mortality and hospital admissions due to enteric infections (Figure 2). In high temperatures, the risk of enteric infection mortality, at 21.5% (95% CI: 5.9%, 39.5%), was higher than the risk of enteric infection hospital admissions at 7.6% (95% CI: −4.4 %, 21.1%) (Table S10). Philippines-level risks for low temperatures were similar between mortality and hospital admissions due to enteric infections. Regional temperature–enteric infection mortality associations were generally U-shaped, whereas the shapes of temperature–enteric infection hospital admissions associations varied by region, with some having significant low-temperature associations (Figure 3; Figures S8 and S9; and Table S10). The National Capital Region had the highest mortality risks among the regions for both high and low temperatures. For hospital admission risks by region, Region 2 had the highest risks due to high temperatures, and the National Capital Region had the highest risks with low temperatures. Mean regional minimum risk temperature for enteric infection mortality was 24.9°C (range, 19.2–26.6°C) and for enteric infection hospital admissions was 25.1°C (range, 19.5–29.5°C). The Philippines-level minimum risk temperatures for mortality and hospital admissions were 24.9 and 24.8°C, respectively.
Figure 2.
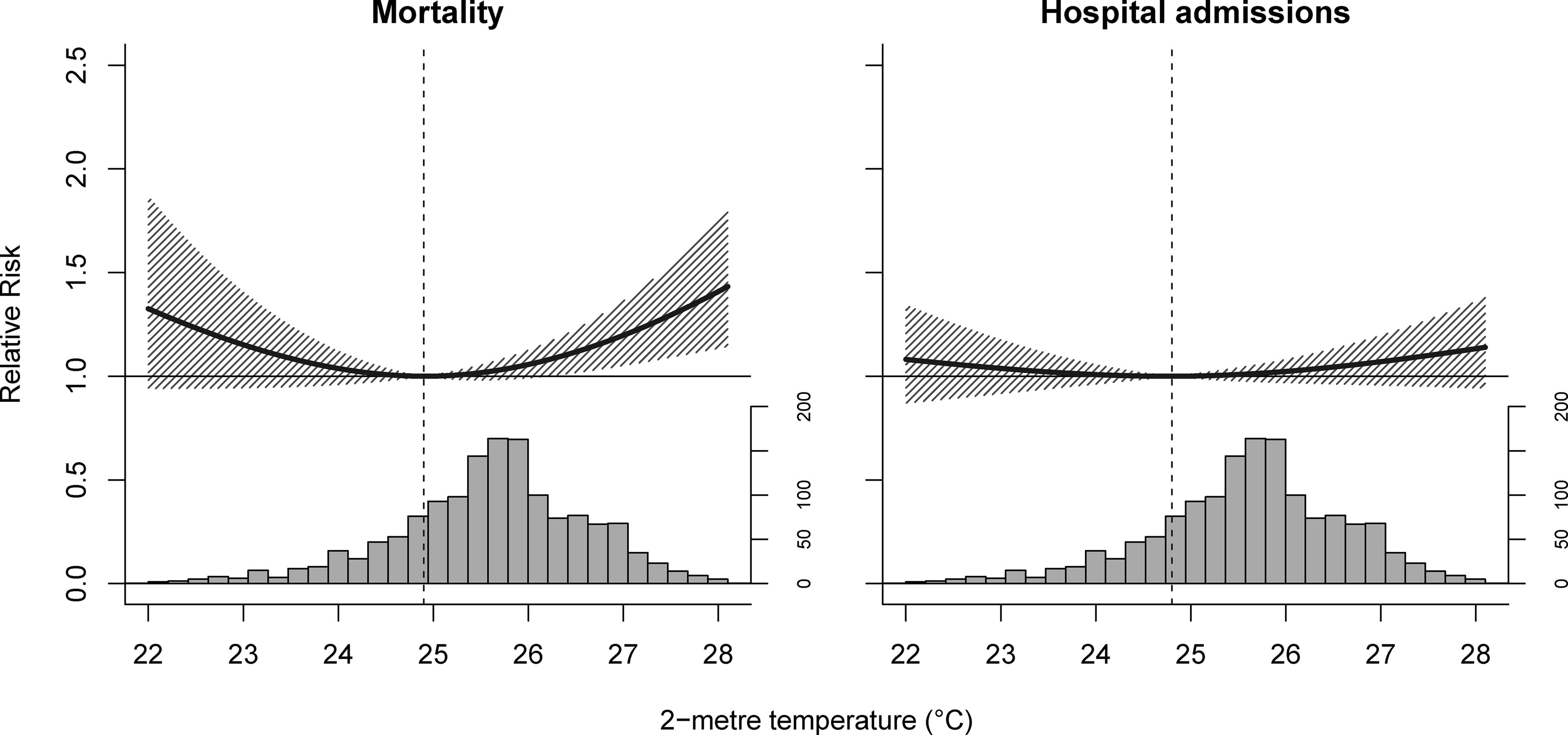
Philippines-level cumulative temperature–enteric infection associations by outcome in 2014–2017. Dashed lines are minimum risk temperatures, solid lines are relative risks, shaded regions are 95% empirical confidence intervals, and histograms the temperature distributions.
Figure 3.
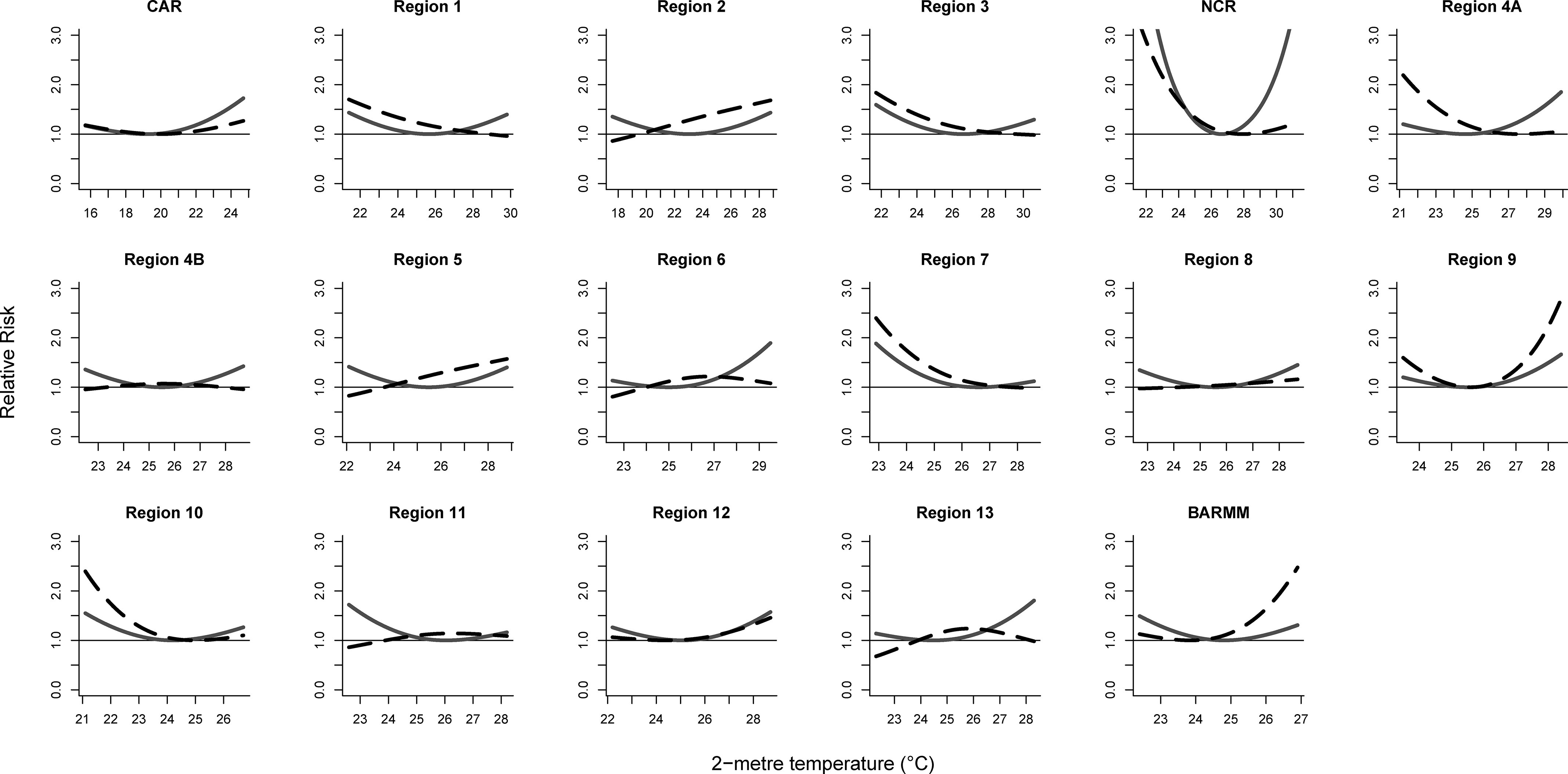
Regional cumulative temperature–enteric infection associations by outcome in 2014–2017. Solid lines are temperature–mortality associations and dashed lines are temperature–hospital admission associations.
Projected Temperature-Attributable Mortality and Hospital Admissions due to Enteric Infections
Overall future excess deaths due to enteric infections were projected to increase considerably under RCP 4.5, 6.0, and 8.5 without population change, driven by greater high temperature-related deaths than by low temperature-related deaths (Figure 4; Tables S11 and S12). GCM-ensemble temperature-attributable deaths due to enteric infections in the 2090s relative to the 2010s were projected to increase by 7.5% (95% eCI: , 20.9%) under RCP 4.5, 12.0% (95% eCI: , 27.8%) under RCP 6.0, and 25.5% (95% eCI: , 48.2%) under RCP 8.5 (Table 2). The smallest increase in GCM-ensemble temperature-attributable deaths was projected under RCP 2.6 at 1.3% (95% eCI: , 6.5%). Reductions in enteric infection deaths attributable to low temperatures were very small in comparison with the deaths attributable to high temperatures. Most of the excess temperature-attributable deaths due to enteric infections were from the National Capital Region, Region 5, and Region 6 (Tables S13 and S14).
Figure 4.
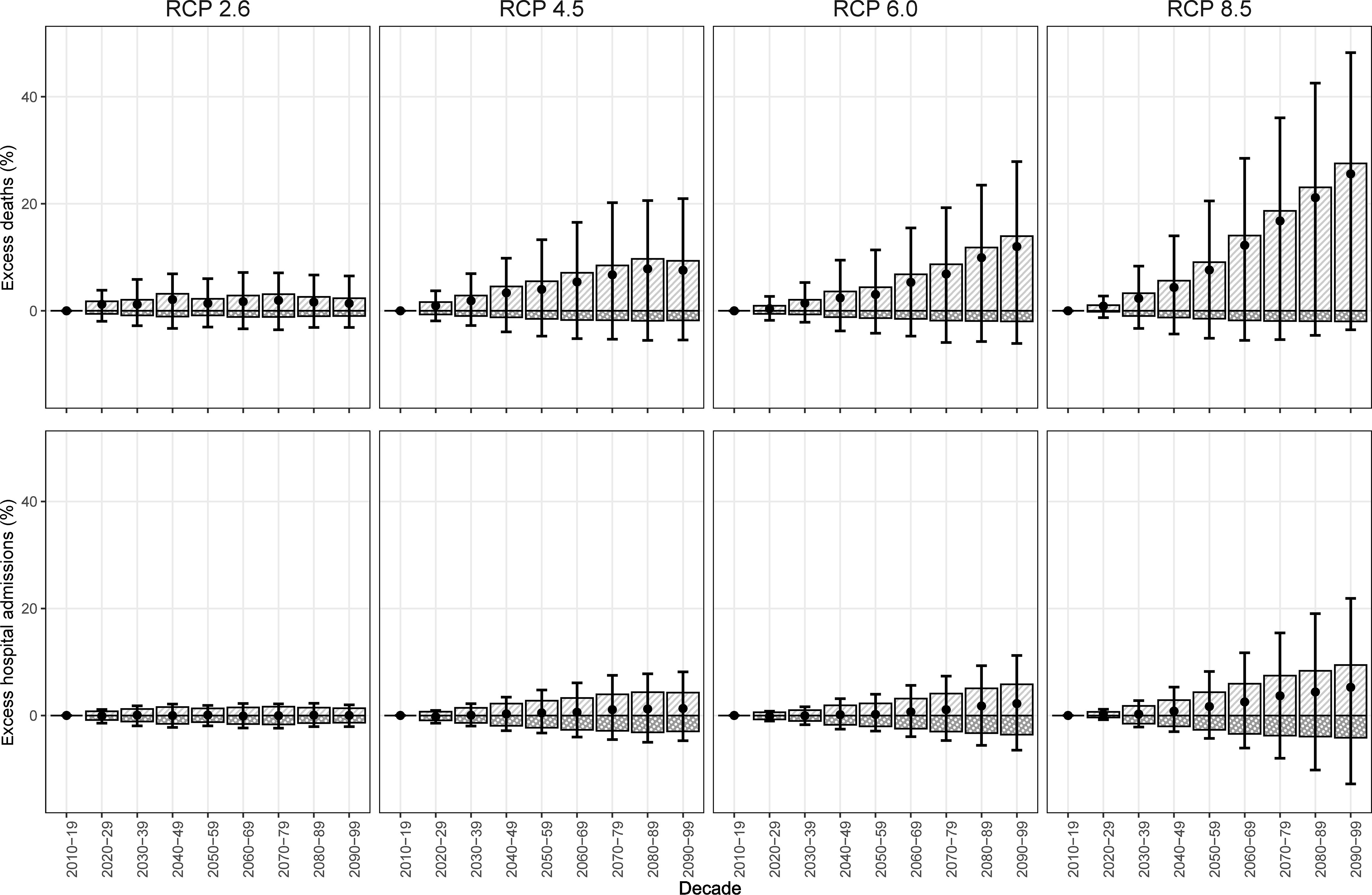
General circulation model-ensemble temperature-attributable fractions of enteric infections relative to 2010–2019 by outcome in the Philippines. Light gray bars with white stripes are high temperature-attributable enteric infections; dark gray bars with white dots are low temperature-attributable enteric infections; black circles are total temperature-attributable enteric infections; and black error bars are the 95% empirical confidence intervals. Corresponding numeric data are presented in Tables S11 and S12.
Table 2.
General circulation model-ensemble temperature-attributable fractions of deaths and hospital admissions due to enteric infections in 2090–2099 compared with 2010–2019 in the Philippines by representative concentration pathways under no population change.
| Enteric infection outcome | RCP | Temperature-attributable fractions % (95% eCI) | |||||
|---|---|---|---|---|---|---|---|
| 2010–2019 | 2090–2099 | ||||||
| High temperatures | Low temperatures | Overall | High temperatures | Low temperatures | Overall | ||
| Mortality | 2.6 | 4.7 (, 11.3) | 2.1 (, 5.4) | 6.9 (1.8, 10.0) | 7.1 (, 16.5) | 1.2 (, 3.2) | 8.2 (, 15.9) |
| 4.5 | 5.0 (, 11.7) | 2.2 (, 5.7) | 7.3 (2.0, 10.7) | 14.4 (, 30.5) | 0.5 (, 1.6) | 14.8 (, 30.5) | |
| 6.0 | 4.9 (, 11.3) | 2.3 (, 5.9) | 7.2 (2.3, 10.1) | 18.9 (, 37.4) | 0.3 (, 1.3) | 19.2 (, 37.4) | |
| 8.5 | 5.5 (, 12.4) | 2.0 (, 5.2) | 7.5 (1.7, 11.8) | 33.0 (, 57.8) | 0.1 (0.0, 0.5) | 33.1 (, 57.8) | |
| Hospital admissions | 2.6 | 5.9 (0.8, 10.1) | 4.9 (0.9, 8.6) | 10.7 (7.1, 13.6) | 7.2 (1.4, 12.2) | 3.5 (0.1, 6.7) | 10.7 (6.3, 14.0) |
| 4.5 | 5.9 (0.9, 10.1) | 4.9 (0.9, 8.6) | 10.8 (7.1, 13.5) | 10.1 (1.9, 17.7) | 1.9 (, 4.5) | 12.1 (5.6, 17.8) | |
| 6.0 | 5.8 (0.9, 10.0) | 5.0 (1.0, 8.8) | 10.9 (7.4, 13.5) | 11.6 (1.7, 20.5) | 1.5 (, 3.6) | 13.1 (4.7, 20.5) | |
| 8.5 | 6.0 (1.0, 10.4) | 4.7 (0.8, 8.3) | 10.8 (7.1, 13.5) | 15.5 (, 30.4) | 0.5 (, 1.9) | 16.0 (, 30.3) | |
Note: eCI, empirical confidence interval; RCP, representative concentration pathway.
A modest increase in future excess hospital admissions due to enteric infections was estimated. GCM-ensemble enteric infection hospital admissions attributable to temperature in the 2090s relative to the 2010s were projected to increase by 0.02% (95% eCI: , 1.9%) under RCP 2.6, 1.3% (95% eCI: , 8.1%) under RCP 4.5, 2.2% (95% eCI: , 11.2%) under RCP 6.0, and 5.2% (95% eCI: , 21.8%) under RCP 8.5 (Figure 3). The majority of excess hospital admissions due to enteric infections were projected in Region 9, Region 12, and the Bangsamoro Autonomous Region of Muslim Mindanao. Eight regions (Regions 1, 3, 4A, 4B, 7, 10, 13, and the National Capital Region) were estimated to have overall reductions in temperature-attributable hospital admissions due to enteric infections by the end of the century (Tables S13 and S14).
Considering population change, SSP 4 had the greatest number of excess deaths due to enteric infections and SSP 5 had the lowest, except under RCP 8.5, in the 2090s in comparison with the 2010s (Table 3 and Table S15). SSP 3 had the highest number of excess hospital admissions due to enteric infections, and SSP 5 had the lowest by the end of the century (Table S16).
Table 3.
General circulation model-ensemble temperature-attributable number of deaths and hospital admissions due to enteric infections in 2090–2099 by climate change (RCPs) and population change scenarios (SSPs) in the Philippines.
| Outcome | RCP | Cumulative numbers (95% eCI) in 2090–2099 in thousands | |||||
|---|---|---|---|---|---|---|---|
| No population changes | SSP 1 Sustainability |
SSP 2 Middle of the road |
SSP 3 Regional rivalry |
SSP 4 Inequality |
SSP 5 Fossil-fueled development |
||
| Mortality | Nonea | 46 | 64 | 78 | 104 | 119 | 62 |
| 2.6 | 50 (45, 53) | 71 (63, 77) | 86 (76, 93) | NA | 130 (117, 140) | NA | |
| 4.5 | 52 (44, 60) | 76 (61, 89) | 92 (74, 107) | 123 (98, 144) | 139 (115, 160) | 74 (59, 86) | |
| 6.0 | 55 (44, 63) | 80 (61, 94) | 96 (74, 114) | 129 (98, 153) | 145 (115, 169) | 77 (59, 92) | |
| 8.5 | 61 (45, 72) | NA | NA | NA | NA | 88 (61, 108) | |
| Hospital admissions | Nonea | 5,008 | 6,817 | 8,300 | 11,117 | 12,829 | 6,605 |
| 2.6 | 5,546 (5,322, 5,712) | 7,419 (7,192, 7,586) | 9,140 (8,799, 9,392) | NA | 14,057 (13,569, 14,416) | NA | |
| 4.5 | 5,613 (5,291, 5,902) | 7,479 (7,132, 7,786) | 9,241 (8,741, 9,690) | 12,659 (11,821, 13,420) | 14,199 (13,472, 14,848) | 7,244 (6,909, 7,540) | |
| 6.0 | 5,664 (5,244, 6,034) | 7,532 (7,063, 7,935) | 9,321 (8,662, 9,898) | 12,799 (11,730, 13,752) | 14,314 (13,346, 15,155) | 7,295 (6,842, 7,685) | |
| 8.5 | 5,811 (4,947, 6,528) | NA | NA | NA | NA | 7,448 (6,499, 8,229) | |
Note: eCI, empirical confidence interval; NA, unlikely SSP–RCP combinations; RCP, representative concentration pathway; SSP, shared socioeconomic pathway: .
No temperature changes.
Sensitivity Analyses
The shapes of regional cumulative temperature-enteric infections associations changed little when adjusted for relative humidity and/or total precipitation as confounders (Figures S10 and S11), when population was included as an offset (Figures S12 and S13), or when maximum lags decreased or increased (Figures S14 and S15). Some shapes of cumulative temperature–enteric infection mortality associations changed when degrees of freedom of time were increased (Figure S16). Notably, Regions 6 and 9 had the greatest high-temperature associations, and the National Capital Region had the least at 6 df of time. Most of the shapes of cumulative temperature–enteric infection hospital admissions associations were similar when degrees of freedom of time were increased (Figure S17). The cumulative associations between weather station mean temperatures and enteric infections varied in some regions but were similar to the ERA5-Land temperature associations (Figures S18 and S19). Overall excess mortality projected using weather station data increased over time, whereas overall reductions were projected for hospital admissions by the end of the century (Figure S20).
Discussion
We quantified the ambient temperature associations of mortality and hospital admissions due to enteric infections and projected their future excess numbers under climate and population changes in the Philippines for the first time, to our knowledge. The shapes of temperature associations in mortality and hospital admissions varied by region with both high- and low-temperature associations. Our projections showed that future temperature-related excess mortality due to enteric infections would consistently increase considerably under scenarios with warming temperatures and high population growth, albeit with wide empirical confidence intervals. However, temperature-related excess hospital admissions due to enteric infections may increase only moderately (or even decline, as when weather station temperature data were used in the model) because some regional risks were greater with lower temperatures.
The varying regional temperature associations in mortality and hospital admissions may partly be explained by their causative enteropathogens because these differed in temperature associations (Carlton et al. 2016; Chua et al. 2020). For regions with significant low-temperature associations, viral pathogens may explain the associations (Carlton et al. 2016; Onozuka et al. 2019). High-temperature associations in mortality may reflect the pathogens associated with diarrhea mortality in children, such as Escherichia coli, Shigella spp., Cryptosporidium spp., and Entamoeba histolytica (Levine et al. 2020) because of their positive temperature associations (Chua et al. 2020; Erdem et al. 2003; Kent et al. 2015). The most reported pathogen, which was typhoid fever, may also explain the high-temperature associations in some regions because of its strong positive temperature associations (Chua et al. 2020; Liang et al. 2021). It should be noted that this proposition is challenging to verify because the majority of the reported deaths and hospital admissions were unspecified; this situation may perpetuate, considering that to identify enteropathogens, many health care institutions in developing countries still rely on conventional diagnostics, which are laborious, insensitive, and time-consuming (Ugboko et al. 2020).
The high temperature–enteric infection associations may also be explained by cases with moderate to severe diarrhea leading to dehydration (Akech et al. 2018; Levine et al. 2020), which compromises the body’s thermoregulation and electrolyte balance (Popkin et al. 2010). Dehydration can lead to death, but this outcome is preventable, given that the required medical interventions are relatively inexpensive and straightforward (Kotloff 2017). However, some of the medically unattended deaths may have faced greater risk because the cases arise in impoverished settings that receive inadequate health care, enable poor health-seeking behaviors, expose residents to heat stress due to poor living conditions, and lack access to clean drinking water (Chowdhury et al. 2015; Ferdous et al. 2014). Such circumstances may worsen under future scenarios with poor socioeconomic growth or widening inequality.
This study demonstrated an improved projection of mortality due to enteric infections. Previous global projections applied temperature–morbidity associations in estimating mortality due to enteric infections because there was a lack of available temperature–mortality associations (Kovats and Lloyd 2014). To make the projections feasible, the authors assumed that the relationship between morbidity and mortality due to enteric infections was constant over time, and that the estimates they applied capture the plausible range of temperature–mortality associations across time and space. In the case of this study, we were able to quantify the temperature–mortality associations and directly use them in projecting excess deaths in the Philippines. If we use the associations between temperature and hospital admissions to estimate the excess mortality, we find that temperature-related deaths due to enteric infections may have been underestimated. This warrants the investigation of associations between temperature and mortality due to enteric infections in other locations to further understand the change in mortality risk due to ambient temperature. However, this may be challenging given that reliable data in enteric infections are sparse, specifically in low-income countries where the burden of these infections is highest (Gill et al. 2017).
With the projected warming of climate (Fan et al. 2020), extreme heat events are likely to occur more frequently and intensely (Li 2020), potentially greatly affecting enteric infections in the future. Therefore, introducing vaccines and sustaining the gains from water, sanitation, and hygiene as well as child nutrition programs can help prevent infections in the future (Riddle et al. 2018; Troeger et al. 2020). In addition, there is a need to strengthen measures for mortality deterrence by proper management of dehydration through effective delivery of oral rehydration solution and zinc supplementation services by primary health care facilities (APHA 2018; Ezezika et al. 2021).
This study has some limitations. First, the data collected may not accurately represent enteric infections in the country. For mortality, the considerable proportion of medically unattended deaths were verbally autopsied and may not have received precise diagnoses in comparison with deaths attended by physicians (Lucero et al. 2018). In addition, there is evidence of underreporting of deaths in the Philippines, particularly in children, due to inconsistencies in reporting procedures (Carter et al. 2011). For hospital admissions, the data source can only cover serious cases needing hospitalization from accredited hospitals; they do not cover cases that were from emergency room or primary health care institutions. Second, the ERA5-Land reanalysis data on air temperature has not been evaluated and lack uncertainty values. Although we also used weather station data, only a few stations were available to represent relatively large administrative regions and therefore may not accurately represent exposure. The two air temperatures also vary in terms of their spatial scale, with ERA5-Land presented in 9-km grid squares, whereas weather stations represent a tiny space. The aggregation of the exposure may introduce a Berkson-type measurement error, which may explain the wide eCIs in the associations and projections (Armstrong 1998). Third, the wide eCIs of the associations and projections may also be partly explained by sparse mortality data and from only 4 y of data. Fourth, we did not incorporate rotavirus vaccination in the projections. Rotavirus vaccination was introduced in the Philippines in 2012, and at least one dose of monovalent rotaviral vaccine was found to have vaccine effectiveness of 60% against rotaviral hospital admissions (Lopez et al. 2019). With 31% of hospital admissions caused by rotavirus in the Philippines (Carlos et al. 2009), incorporating projections of rotaviral vaccination coverage based on SSP scenarios may help improve the projections of future enteric infection deaths and hospital admissions by considering the additional reductions in numbers of enteric infection deaths and hospital admissions from vaccination. However, rotaviral vaccination coverage in the Philippines is low, possibly leading to minimal or negligible reductions in enteric infections in children (DOH 2019b). Fifth, we were not able to develop projections by age group because the gridded population projections were not available by age group. Future studies should consider projection of enteric infections in children given that most of the burden is borne by that age group. Sixth, we assumed that the temperature–enteric infection associations would not change over time and did not consider any shift in minimum risk temperatures. This lack of change may not be the case because humans could adapt under changing ambient temperatures; thus, minimum risk temperatures could increase (Yin et al. 2019). Last, we did not include changes in the rate of mortality and hospital admissions due to enteric infections. Better socioeconomic status and continued improvement in health systems may reduce the risk of enteric infections and eventual death (Troeger et al. 2020). Adaptation measures beyond those related to socioeconomic growth, such as better health-seeking behaviors, may further contribute to reductions. Exposure to pathogens causing enteric infections by region may also change because their transmission could be affected by the ecological changes brought by climate change.
In summary, future temperature-related excess mortality due to enteric infections in the Philippines was projected to increase considerably by the end of century, especially under scenarios with significant climate change and increase in population, because mortality had strong high-temperature associations. However, hospital admissions due to enteric infections in the Philippines had greater low-temperature associations and lesser high-temperature associations than mortality and were thus projected as a moderate increase in temperature-related excess hospital admissions in the future. Support for and implementation of adaptation policies for climate change in the Philippines may help in abating temperature-attributable enteric infections in the future.
The temperature–enteric infection associations derived in this study may be applicable to countries with climate and socioeconomic characteristics similar with the Philippines, such as Indonesia and other neighboring countries of Southeast Asia. However, the composition of pathogens and modes of transmission of enteric infections vary geographically; therefore, more studies quantifying the risks of enteric infection mortality due to nonoptimal temperatures are encouraged, especially in countries in sub-Saharan Africa and South Asia where the burden remains high (Reiner et al. 2020; Troeger et al. 2018a). The lack of good-quality data may impede the research progress in these regions, but recent data from the Million Death Study and Child Health and Mortality Prevention Surveillance could be potential sources of more accurate data on mortality due to enteric infections (Farrar et al. 2019; Taylor et al. 2020). More findings from other geographical locations would help to improve projections of temperature-related deaths due to enteric infections on a global scale.
Overall, this study provides relative risks for associations between temperature and mortality due to enteric infections and applies them in projecting temperature-related enteric infection deaths in the Philippines. Mortality from enteric infections was deemed more sensitive to warmer temperatures than was hospital admissions, resulting in larger increases in temperature-related deaths than in hospital admissions under scenarios of climate change and high population growth. Thus, there is a need to strengthen existing primary health care interventions and support health adaptation policies to prevent additional future deaths from enteric infections in the Philippines.
Supplementary Material
Acknowledgments
The authors thank the Philippine Statistics Authority, the Philippine Health Insurance Corporation, and the Philippine Atmospheric, Geophysical and Astronomical Services Administration in providing data for this study. The authors also thank B. Siy for support in the data collection.
This work was supported by the Philippine Council for Health Research and Development Grants-in-Aid Program and by the Japan Society for the Promotion of Science (Kakenhi) Grant-in-Aid for Scientific Research (B) (grant number JP19H03900). V.H. received support from the Spanish Ministry of Economy, Industry and Competitiveness (grant number PCIN-2017-046). M.H. was supported by the Japan Science and Technology Agency as part of SICORP, Inc., (grant number JPMJSC20E4). P.L.C.C. is a recipient of the Japanese Government (Monbukagakusho) Scholarship from the Ministry of Education, Science, Sport, and Culture of Japan.
References
- Akech S, Ayieko P, Gathara D, Agweyu A, Irimu G, Stepniewska K, et al. . 2018. Risk factors for mortality and effect of correct fluid prescription in children with diarrhoea and dehydration without severe acute malnutrition admitted to Kenyan hospitals: an observational, association study. Lancet Child Adolesc Health 2(7):516–524, 10.1016/S2352-4642(18)30130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso WJ, Acuña-Soto R, Giglio R, Nuckols J, Leyk S, Schuck-Paim C, et al. . 2012. Spatio-temporal patterns of diarrhoeal mortality in Mexico. Epidemiol Infect 140(1):91–99, PMID: , 10.1017/S0950268811000562. [DOI] [PubMed] [Google Scholar]
- APHA (American Public Health Association). 2018. Promoting Leadership to Scale Up of Oral Rehydration Salts with Zinc Uptake and Reduce Diarrhea Mortality Globally in Children Under 5 Years. Am Public Heal Assoc. https://www.apha.org/policies-and-advocacy/public-health-policy-statements/policy-database/2019/01/28/reduce-diarrhea-mortality-globally [accessed 25 June 2021].
- Armstrong BG. 1998. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med 55(10):651–656, 10.1136/oem.55.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Lendrum DH, Corvalan CF, Pruss-Ustun A, et al. 2003. How much disease could climate change cause? In: Climate Change and Human Health: Risks and Responses. McMichael AJ, Campbell-Lendrum DH, Corvalán CF, Ebi KL, Githeko AK, Scheraga JD, eds. Geneva: World Health Organization, 133–159. [Google Scholar]
- Carlos CC, Inobaya MT, Bresee JS, Lagrada ML, Olorosa AM, Kirkwood CD, et al. . 2009. The burden of hospitalizations and clinic visits for rotavirus disease in children aged <5 years in the Philippines. J Infect Dis 200(s1):S174–S181, PMID: , 10.1086/605044. [DOI] [PubMed] [Google Scholar]
- Carlton EJ, Woster AP, DeWitt P, Goldstein RS, Levy K. 2016. A systematic review and Meta-analysis of ambient temperature and diarrhoeal diseases. Int J Epidemiol 45(1):117–130, PMID: , 10.1093/ije/dyv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter KL, Williams G, Tallo V, Sanvictores D, Madera H, Riley I. 2011. Capture-recapture analysis of all-cause mortality data in Bohol, Philippines. Popul Health Metr 9:9, PMID: , 10.1186/1478-7954-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury F, Khan IA, Patel S, Siddiq AU, Saha NC, Khan AI, et al. . 2015. Diarrheal illness and healthcare seeking behavior among a population at high risk for diarrhea in Dhaka, Bangladesh. PLoS One 10(6):e0130105, 10.1371/journal.pone.0130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua PLC, Fook C, Ng S, Tobias A, Seposo XT, Hashizume M. 2020. Associations between ambient temperature and enteric infections by aetiology: a systematic review and meta-analysis. Preprints with the Lancet. Preprint posted online April 8, 2021. 10.2139/ssrn.3822570. [DOI]
- Chua PLC, Huber V, Ng CFS, Seposo XT, Madaniyazi L, Hales S, et al. . 2021. Global projections of temperature-attributable mortality due to enteric infections: a modelling study. Lancet Planet Health 5(7):e436–e445. PMID: 34245714, 10.1016/S2542-5196(21)00152-2. [DOI] [PubMed] [Google Scholar]
- CIESIN (Center for International Earth Science Information Network). 2018. Gridded Population of the World, Version 4 (GPWv4): Population Density, Revision 11. Palisades, NY: NASA Socioeconomic Data and Applications Center (SEDAC). https://sedac.ciesin.columbia.edu/data/set/gpw-v4-population-density-rev11 [accessed 1 January 2021]. [Google Scholar]
- Collins M, Knutti R, Arblaster J, Dufresne J-L, Fichefet T, Friedlingstein P, et al. 2013. Long-term Climate Change: Projections, Commitments and Irreversibility. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, et al., eds. New York, NY: Cambridge University Press, 1029–1136. [Google Scholar]
- DOH (Philippine Department of Health). 2019a. Field Health Services Information System 2017 Annual Report. https://doh.gov.ph/sites/default/files/publications/2017_FHSIS_Final_0.pdf [accessed 1 January 2021].
- DOH. 2019b. Field Health Services Information System 2018 Annual Report.https://doh.gov.ph/sites/default/files/publications/FHSIS_Annual_2018_Final.pdf [accessed 1 January 2021].
- Ekamper P, van Poppel F, van Duin C, Garssen J. 2009. 150 Years of temperature-related excess mortality in The Netherlands. Demogr Res 21:385–426, 10.4054/DemRes.2009.21.14. [DOI] [Google Scholar]
- Erdem H, Kiliç S, Çinar E, Pahsa A. 2003. Symptomatic intestinal amoebiasis and climatic parameters. Scand J Infect Dis 35(3):186–188, PMID: . [PubMed] [Google Scholar]
- Ezezika O, Ragunathan A, El-Bakri Y, Barrett K. 2021. Barriers and facilitators to implementation of oral rehydration therapy in low- and middle-income countries: a systematic review. PLoS One 16(4):e0249638, PMID: , 10.1371/journal.pone.0249638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Duan Q, Shen C, Wu Y, Xing C. 2020. Global surface air temperatures in CMIP6: historical performance and future changes. Environ Res Lett 15(10):104056, 10.1088/1748-9326/abb051. [DOI] [Google Scholar]
- Farrar DS, Awasthi S, Fadel SA, Kumar R, Sinha A, Fu SH, et al. . 2019. Seasonal variation and etiologic inferences of childhood pneumonia and diarrhea mortality in India. eLife 8:e46202, 10.7554/eLife.46202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous F, Das SK, Ahmed S, Farzana FD, Malek MA, Das J, et al. . 2014. Diarrhoea in slum children: observation from a large diarrhoeal disease hospital in Dhaka, Bangladesh. Trop Med Int Heal 19(10):1170–1176, 10.1111/tmi.12357. [DOI] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. 2012. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat Med 31(29):3821–3839, PMID: , 10.1002/sim.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Sera F, Vicedo-Cabrera AM, Huber V, Tong S, et al. . 2017. Projections of temperature-related excess mortality under climate change scenarios. Lancet Planet Health 1(9):e360–e367, PMID: , 10.1016/S2542-5196(17)30156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill CJ, Thea DM, Hibberd P. 2017. Diarrhoeal disease trends in the GBD 2015 study: optimism tempered by scepticism. Lancet Infect Dis 17(9):884–885, PMID: , 10.1016/S1473-3099(17)30336-5. [DOI] [PubMed] [Google Scholar]
- Hempel S, Frieler K, Warszawski L, Schewe J, Piontek F. 2013. A trend-preserving bias correction – the ISI-MIP approach. Earth Syst Dynam 4(2):219–236, 10.5194/esd-4-219-2013. [DOI] [Google Scholar]
- Hodges M, Belle JH, Carlton EJ, Liang S, Li H, Luo W, et al. . 2014. Delays in reducing waterborne and water-related infectious diseases in China under climate change. Nat Clim Chang 4:1109–1115, PMID: , 10.1038/nclimate2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISIMIP (Inter-Sectoral Impact Model Intercomparison Project). 2019. Inter-Sectoral Impact Model Intercomparison Project Database. Potsdam, Germany: Potsdam-Institute for Climate Impact Research. https://esg.pik-potsdam.de/search/isimip/ [accessed 20 January 2020]. [Google Scholar]
- Kc S, Lutz W. 2017. The human core of the shared socioeconomic pathways: population scenarios by age, sex and level of education for all countries to 2100. Glob Environ Chang 42:181–192, PMID: , 10.1016/j.gloenvcha.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JJ, Minalt N, Culotti A, Pryor M, Packman A. 2014. Temporal variations in the abundance and composition of biofilm communities colonizing drinking water distribution pipes. PLoS One 9(5):e98542, PMID: , 10.1371/journal.pone.0098542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent L, McPherson M, Higgins N. 2015. A positive association between cryptosporidiosis notifications and ambient temperature, Victoria, Australia, 2001–2009. J Water Health 13(4):1039–1047, PMID: , 10.2166/wh.2015.130. [DOI] [PubMed] [Google Scholar]
- Kotloff KL. 2017. The burden and etiology of diarrheal illness in developing countries. Pediatr Clin North Am 64(4):799–814, PMID: , 10.1016/j.pcl.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Kovats S, Lloyd S. 2014. Diarrhoeal disease. In: Quantitative Risk Assessment of the Effects of Climate Change on Selected Causes of Death, 2030s and 2050s. Hales S, Kovats S, Lloyd S, and Campbell-Lendrum D, eds. Geneva, Switzerland: World Health Organization, 37–50. [Google Scholar]
- Lal A, Hales S, French N, Baker MG. 2012. Seasonality in human zoonotic enteric diseases: a systematic review. PLoS One 7(4):e31883, 10.1371/journal.pone.0031883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kim H, Hwang S, Zanobetti A, Schwartz JD, Chung Y. 2017. Monte Carlo simulation-based estimation for the minimum mortality temperature in temperature-mortality association study. BMC Med Res Methodol 17(1):137, PMID: , 10.1186/s12874-017-0412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM, Nasrin D, Acácio S, Bassat Q, Powell H, Tennant SM, et al. . 2020. Diarrhoeal disease and subsequent risk of death in infants and children residing in low-income and middle-income countries: analysis of the GEMS case-control study and 12-month GEMS-1A follow-on study. Lancet Glob Health 8(2):e204–e214, PMID: , 10.1016/S2214-109X(19)30541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy K, Woster AP, Goldstein RS, Carlton EJ. 2016. Untangling the impacts of climate change on waterborne diseases: a systematic review of relationships between diarrheal diseases and temperature, rainfall, flooding, and drought. Environ Sci Technol 50(10):4905–4922, PMID: , 10.1021/acs.est.5b06186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XX. 2020. Heat wave trends in Southeast Asia during 1979–2018: the impact of humidity. Sci Total Environ 721:137664, PMID: , 10.1016/j.scitotenv.2020.137664. [DOI] [PubMed] [Google Scholar]
- Liang M, Ding X, Wu Y, Sun Y. 2021. Temperature and risk of infectious diarrhea: a systematic review and meta-analysis. Environ Sci Pollut Res Int 28(48):68144–68154, PMID: , 10.1007/s11356-021-15395-z. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tong MX, Xiang J, Dear K, Wang C, Ma W, et al. . 2020. Daily temperature and bacillary dysentery: estimated effects, attributable risks, and future disease burden in 316 Chinese cities. Environ Health Perspect 128(5):57008, PMID: , 10.1289/EHP5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AL, Raguindin PF, Silva MWT. 2019. Prospects for rotavirus vaccine introduction in the Philippines: bridging the available evidence into immunization policy. Hum Vaccines Immunother 15(6):1260–1264, PMID: , 10.1080/21645515.2018.1551673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero M, Riley ID, Hazard RH, Sanvictores D, Tallo V, Dumaluan DGM, et al. . 2018. Assessing the quality of medical death certification: a case study of concordance between national statistics and results from a medical record review in a regional hospital in the Philippines. Popul Health Metr 16(1):23, PMID: , 10.1186/s12963-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Campbell-Lendrum D, Kovats S, Edwards S, Wilkinson P, Wilson T, et al. 2004. Global climate change. In: Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Ezzati M, Lopez AD, Rodgers A, and Murray CJL, eds. Geneva, Switzerland: World Health Organization, 1543–1650. [Google Scholar]
- Mellor J, Kumpel E, Ercumen A, Zimmerman J. 2016. Systems approach to climate, water, and diarrhea in Hubli-Dharwad. India Environ Sci Technol 50(23):13042–13051, PMID: , 10.1021/acs.est.6b02092. [DOI] [PubMed] [Google Scholar]
- Mertens A, Balakrishnan K, Ramaswamy P, Rajkumar P, Ramaprabha P, Durairaj N, et al. . 2019. Associations between high temperature, heavy rainfall, and diarrhea among young children in rural Tamil Nadu, India: a prospective cohort study. Environ Health Perspect 127(4):047004, PMID: , 10.1289/EHP3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moors E, Singh T, Siderius C, Balakrishnan S, Mishra A. 2013. Climate change and waterborne diarrhoea in Northern India: impacts and adaptation strategies. Sci Total Environ 468-469 (suppl):S139–S151, PMID: , 10.1016/j.scitotenv.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Muñoz-Sabater J, Dutra E, Agustí-Panareda A, Albergel C, Arduini G, Balsamo G, et al. . 2021. ERA5-Land: a state-of-the-art global reanalysis dataset for land applications. Earth Syst Sci Data 13:4349–4383, 10.5194/essd-13-4349-2021. [DOI] [Google Scholar]
- NOAA (National Oceanic and Atmospheric Administration). 2007. Relative Humidity Calculator. https://www.wpc.ncep.noaa.gov/html/dewrh.shtml [accessed 1 January 2021].
- O’Neill BC, Kriegler E, Ebi KL, Kemp-Benedict E, Riahi K, Rothman DS, et al. . 2017. The roads ahead: narratives for shared socioeconomic pathways describing world futures in the 21st century. Glob Environ Change 42:169–180, 10.1016/j.gloenvcha.2015.01.004. [DOI] [Google Scholar]
- Onozuka D, Gasparrini A, Sera F, Hashizume M, Honda Y. 2019. Modeling future projections of temperature-related excess morbidity due to infectious gastroenteritis under climate change conditions in Japan. Environ Health Perspect 127(7):77006, PMID: , 10.1289/EHP4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri WA, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. 2008. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest 118(4):1277–1290, PMID: , 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM, D’Anci KE, Rosenberg IH. 2010. Water, hydration, and health. Nutr Rev 68(8):439–458, PMID: , 10.1111/j.1753-4887.2010.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PSA (Philippine Statistics Authority). 2016a. Death Statistics in the Philippines: 2016. https://psa.gov.ph/sites/default/files/fact-sheet-death-2016.pdf [accessed 1 January 2021].
- PSA. 2016b. Philippine Population Density (Based on the 2015 Census of Population). https://psa.gov.ph/content/philippine-population-density-based-2015-census-population#:∼:text=With a total land area, per square kilometer in 2010 [accessed 1 January 2021].
- PSA. 2020. 2017 to 2019 Gross Regional Domestic Product (GRDP). https://psa.gov.ph/grdp/tables [accessed 1 January 2021].
- Reiner RC, Wiens KE, Deshpande A, Baumann MM, Lindstedt PA, Blacker BF, et al. . 2020. Mapping geographical inequalities in childhood diarrhoeal morbidity and mortality in low-income and middle-income countries, 2000-17: analysis for the Global Burden of Disease Study 2017. Lancet 2020, PMID: , 10.1016/S0140-6736(20)30114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riahi K, van Vuuren DP, Kriegler E, Edmonds J, O’Neill BC, Fujimori S, et al. . 2017. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: an overview. Glob Environ Chang 42:153–168, 10.1016/j.gloenvcha.2016.05.009. [DOI] [Google Scholar]
- Riddle MS, Chen WH, Kirkwood CD, MacLennan CA. 2018. Update on vaccines for enteric pathogens. Clin Microbiol Infect 24(10):1039–1045, PMID: , 10.1016/j.cmi.2018.06.023. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Blau DM, Bassat Q, Onyango D, Kotloff KL, Arifeen SE, et al. . 2020. Initial findings from a novel population-based child mortality surveillance approach: a descriptive study. Lancet Glob Health 8(7):e909–e919, 10.1016/S2214-109X(20)30205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado MC, Clarke R, Jaykus LA, McQuatters-Gollop A, Frank JM. 2010. Climate change and food safety: a review. Food Res Int 43(7):1745–1765, 10.1016/j.foodres.2010.07.003. [DOI] [Google Scholar]
- Troeger C, Blacker BF, Khalil IA, Rao PC, Cao S, Zimsen SR, et al. . 2018a. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis 18(11):1211–1228, 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger CE, Khalil IA, Blacker BF, Biehl MH, Albertson SB, Zimsen SRM, et al. . 2020. Quantifying risks and interventions that have affected the burden of diarrhoea among children younger than 5 years: an analysis of the Global Burden of Disease Study 2017. Lancet Infect Dis 20(1):37–59, PMID: , 10.1016/S1473-3099(19)30401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, et al. . 2018b. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 172(10):958, 10.1001/jamapediatrics.2018.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugboko HU, Nwinyi OC, Oranusi SU, Oyewale JO. 2020. Childhood diarrhoeal diseases in developing countries. Heliyon 6(4):e03690, PMID: , 10.1016/j.heliyon.2020.e03690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, et al. . 2011. The representative concentration pathways: an overview. Clim Change 109(1–2):5–31, 10.1007/s10584-011-0148-z. [DOI] [Google Scholar]
- Wang H, Di B, Zhang TJ, Lu Y, Chen C, Wang D, et al. . 2019. Association of meteorological factors with infectious diarrhea incidence in Guangzhou, Southern China: a time-series study (2006–2017. Sci Total Environ 672:7–15, 10.1016/j.scitotenv.2019.03.330. [DOI] [PubMed] [Google Scholar]
- Xiao X, Gasparrini A, Huang J, Liao Q, Liu F, Yin F, et al. . 2017. The exposure-response relationship between temperature and childhood hand, foot and mouth disease: a multicity study from mainland China. Environ Int 100:102–109, PMID: , 10.1016/j.envint.2016.11.021. [DOI] [PubMed] [Google Scholar]
- Yin Q, Wang J, Ren Z, Li J, Guo Y. 2019. Mapping the increased minimum mortality temperatures in the context of global climate change. Nat Commun 10:46–40., PMID: , 10.1038/s41467-019-12663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bi P, Sun Y, Hiller JE. 2012. Projected years lost due to disabilities (YLDs) for bacillary dysentery related to increased temperature in temperate and subtropical cities of China. J Environ Monit 14(2):510–516, PMID: , 10.1039/c1em10391a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


