Abstract
OBJECTIVES:
Pediatric out-of-hospital cardiac arrest (OHCA) is associated with significant morbidity and mortality. Pediatric palliative care (PPC) services could provide an integral component of the comprehensive care necessary for these patients and their families. The main objectives of this study are to examine the utilization of PPC following OHCA and compare the differences in characteristics between children who received PPC with those who did not.
DESIGN:
Retrospective cohort study.
SETTING:
An urban, tertiary PICU.
PATIENTS:
Children less than 21 years old admitted from October 2009 to October 2019 with an admitting diagnosis of OHCA and minimum PICU length of stay (LOS) of 48 hours.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Of the 283 patient charts reviewed, 118 patient encounters met inclusion criteria. Of those, 34 patients (28.8%) received a PPC consultation during hospitalization. Patients who received PPC had a longer PICU LOS (14.5 vs 4.0 d), a greater number of ventilator days (12.5 vs 4.0 d), and a larger proportion of do-not-resuscitate (DNR) statuses (41% vs 19%). When comparing the disposition of survivors, a greater proportion was discharged to rehab or nursing facilities (47% vs 28%), with no difference in mortality rates (53% vs 50%). In the multivariate logistic regression model, older age, longer LOS, and code status (DNR) were all associated with higher likelihood of PPC utilization. Data were analyzed using descriptive, Mann-Whitney U, and Fisher exact statistics.
CONCLUSIONS:
Our study demonstrates PPC services following OHCA are underutilized given the high degree of morbidity and mortality. The impact of automatic PPC consultation in all OHCA patients who survive beyond 48 hours should be explored further. Future studies are warranted to understand the benefits and barriers of PPC integration into standard postarrest care for patients and families.
Keywords: cardiac arrest, critical care outcomes, do-not-resuscitate, goals-of-care; palliative care, pediatric intensive care unit
Pediatric out-of-hospital cardiac arrest (OHCA) is associated with a high degree of morbidity and mortality. Survival rates range between 5% and 10.2%, with only 10–30% achieving return of spontaneous circulation (ROSC) and admission to the PICU (1–3). Of the small proportion that survive to PICU admission, less than 40% survive to hospital discharge (3, 4). Furthermore, over 75% of survivors suffer significant declines in their functional status, with new and ongoing medical needs and challenges (5, 6). Given the complexity and severity of this life-changing event, the involvement of pediatric palliative care (PPC) could provide an essential component of the comprehensive care necessary for these patients and their families.
Palliative care focuses on a patient’s quality of life and that of their families in the face of a life-threatening illness, including the various challenges of an ICU stay. The American Academy of Pediatrics (AAP) (ACCM) and American College of Critical Care Medicine recommend, based on expert consensus, that PPC be offered to all families at the onset of any life-threatening diagnosis and continue throughout, regardless of outcome (7, 8). Furthermore, palliative care involvement in adults has been shown to improve quality of life of patients, resource utilization, and hospital costs (9, 10). Despite these recommendations and demonstrated benefits, the use of palliative care following OHCA in pediatrics has not been fully integrated into practice or examined. The main objectives of this study are to evaluate the utilization of PPC following OHCA and identify factors associated with PPC utilization at a single tertiary-care center.
MATERIALS AND METHODS
Design
This is a retrospective cohort study of patients with an OHCA admitted to the PICU at University of Chicago Comer Children’s Hospital. The Institutional Review Board at the University of Chicago approved this study (IRB20:0311).
Study Setting
Comer Children’s hospital is a tertiary, urban academic children’s hospital in Chicago, IL. The PICU serves as a cardiac arrest referral center for the surrounding area. The PPC team includes board-certified Hospice and Palliative Medicine physicians, nurse practitioners, and nurses. Any child with a potentially life-limiting medical condition is eligible for PPC consultation, with no structured referral process in place. Consults are requested at the sole discretion of the primary medical team. The patient and/or family can accept or refuse a PPC consultation. Once a consult has occurred, the PPC team supports the patient and their family throughout their child’s course, including transition out of the hospital, and beyond the patient’s death for bereavement services. There has always been a PPC team available through the entirety of the study period. The team make-up varied from a nurse and a single part-time physician to a nurse practitioner and two part-time physicians. Palliative care coverage was available Monday through Friday, but often not on weekends, with an expectation that the consult takes place within 48 hours of request if feasible.
Participants
Patients younger than 21 years old with an OHCA and ROSC admitted to the PICU with a length of stay (LOS) greater than 48 hours between October 1, 2009, and October 31, 2019. The 48-hour minimum LOS inclusion criteria was chosen to allow for declaration of clinical trajectory to take place and a reasonable time interval appropriate for consultation of PPC services.
Measurements
We queried the institutional data warehouse to determine all patients with any primary and/or admitting diagnosis code relating to cardiac arrest during the defined study period. Prehospital and inpatient medical records were reviewed by trained investigators to determine receipt of PPC consult, patient demographics, medical history, LOS, disease severity measures, discharge disposition, and mode of death (when applicable). We divided the cohort into two groups depending on the presence or absence of PPC consultation. Hospital LOS, PICU LOS, ventilator days, central venous line (CVL) placement, vasoactive days, and mortality were used as indicators of disease severity. Patient comorbidities were identified and categorized according to Feudtner et al’s (11) complex chronic condition (CCC) classification system utilizing codes from the International Classification of Diseases, 10th Revision, Clinical Modification. The number and type of CCCs were determined for all patients prior to admission.
Data Analysis
Statistical analysis was conducted using SAS Version 9.4 statistical software (SAS Institute, Cary, NC). Descriptive statistics were calculated for all study variables. Group differences were examined using Mann-Whitney U and Fisher exact tests. A multivariate logistic regression was then constructed to identify factors associated with PPC utilization. The following variables were included in the model: age, gender, race, ethnicity, insurance status, number of CCCs prior to admission, if the OHCA occurred on a weekend, hospital LOS, code status, occurrence of a secondary inhospital cardiac arrest, and need for vasoactive support. For adjusted analyses, models were constructed using backward stepwise elimination to determine selection. Statistical significance was defined as p ≤ 0.05, and CIs were set at 95%.
RESULTS
Patient Cohort
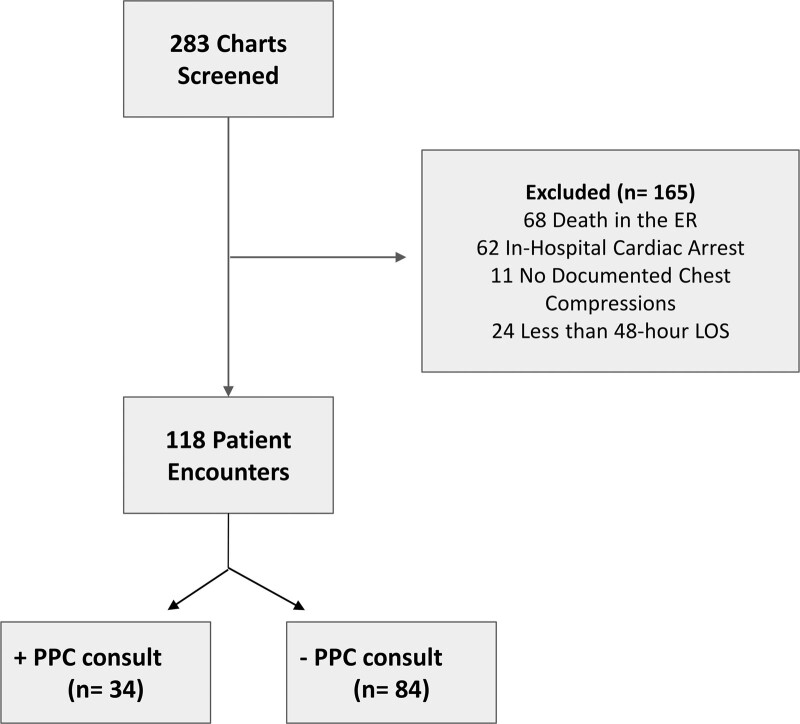
A total of 283 charts were reviewed of pediatric patients who experienced an out-of-hospital cardiac arrest prior to admission to the PICU between October 2009 and October 2019. Of those, a total of 165 subjects were excluded: 68 (24.0%) due to death in the emergency department, 73 (25.8%) due to having an inhospital cardiac arrest or no clear documentation of chest compressions, and 24 (8.5%) due to having a LOS of less than 48 hours (Fig. 1). This yielded a total of 118 patients (41.7%) for this analysis.
Figure 1.
Study flow diagram. ER = emergency room, LOS = length of stay, PPC = pediatric palliative care.
Patient Demographics
Table 1 demonstrates the sample demographics of the 118 patients compared by receipt of PPC consult. Of the 118 subjects, 34 patients (28.8%) had a PPC consultation during their PICU admission. Overall, there were no statistically significant group differences in age, gender, race, ethnicity, insurance status, technology dependence, or prior to admission presence of CCCs.
TABLE 1.
Patient Cohort Demographics
| Variable | PPC (n = 34) | Non-PPC (n = 84) | p | ||
|---|---|---|---|---|---|
| m (IQR) | Range | m (IQR) | Range | ||
| Age at time of PICU admission | 2.8 (0.5–11.4) | 0.0–18.0 | 1.9 (0.5–6.2) | 0.0–18.7 | 0.275 |
| Number of Complex Chronic Conditions | 0.0 (0.0–2.0) | 0.0–5.0 | 0.0 (0.0–2.0) | 0.0–5.0 | 0.300 |
| n (%) | n (%) | p | |||
| Gender | |||||
| Male | 19 (55.9) | 49 (58.3) | 0.839 | ||
| Female | 15 (44.1) | 35 (41.7) | |||
| Race | |||||
| White | 5 (14.7) | 19 (22.6) | 0.623 | ||
| Black | 26 (76.5) | 56 (66.7) | |||
| Other/unknown | 3 (8.8) | 9 (10.7) | |||
| Ethnicity | |||||
| Non-Hispanic/unknown | 31 (91.2) | 79 (94.1) | 0.688 | ||
| Hispanic | 3 (8.8) | 5 (6.0) | |||
| Insurance status | |||||
| Medicaid/self-pay | 26 (76.5) | 66 (78.6) | 0.810 | ||
| Private | 8 (23.5) | 18 (21.4) | |||
| Technology dependent | |||||
| Yes | 11 (32.4) | 24 (28.6) | 0.824 | ||
| No | 23 (67.7) | 60 (71.4) | |||
| Complex chronic condition | |||||
| Yes | 16 (47.1) | 30 (35.7) | 0.300 | ||
| No | 18 (52.9) | 54 (64.3) | |||
IQR = interquartile range, m = median, PPC = pediatric palliative care.
May not add up to 100% due to rounding. Fisher exact for categorical variables and Wilcoxon Mann-Whitney U for continuous variables.
Clinical Characteristics
Table 2 demonstrates markers of disease severity and clinical outcomes compared by receipt of PPC consult. When compared with patients who did not receive a palliative care consult, PPC patients had a greater number of ventilator days (12.5 vs 4.0 d; p < 0.001), a longer cumulative LOS in the PICU (14.5 vs 4.0 d; p < 0.001), and a longer hospital LOS (14.5 vs 4.0 d; p < 0.001). In addition, PPC patients were more likely to have a CVL placed; 100% of PPC patients received a CVL compared with 81% of non-PPC patients (p = 0.006). The PPC group also had a smaller proportion of full code statuses (59% vs 81%; p = 0.019). There were no group differences in timing of OHCA (weekday vs weekend), placement of new hardware (defined as tracheostomy and/or gastrostomy tube), or occurrence of a secondary arrest.
TABLE 2.
Patient Cohort Clinical Characteristics
| Variable | PPC (n = 34) | Non-PPC (n = 84) | p | ||
|---|---|---|---|---|---|
| m (IQR) | Range | m (IQR) | Range | ||
| PICU length of stay | 14.5 (5.0–41.0) | 3.0–111.0 | 4.0 (2.5–10.0) | 1.0–45.0 | < 0.001 |
| Hospital length of stay | 14.5 (5.0–41.0) | 3.0–111.0 | 4.0 (3.0–12.0) | 2.0–52.0 | < 0.001 |
| Ventilator daysa | 12.5 (5.0–41.0) | 3.0–109.0 | 4.0 (3.0–8.0) | 1.0–52.0 | < 0.001 |
| n (%) | n (%) | p | |||
| Airway statusa | |||||
| ETT | 25 (73.5) | 61 (77.2) | 0.810 | ||
| Trach | 9 (26.5) | 18 (22.8) | |||
| Central venous line placed | |||||
| Yes | 34 (100.0) | 68 (81.0) | 0.006 | ||
| No | 0 (0.0) | 16 (19.1) | |||
| Vasoactive medications required | |||||
| Yes | 23 (67.7) | 55 (65.5) | 1.000 | ||
| No | 11 (32.4) | 29 (34.5) | |||
| Weekend arrest | |||||
| Yes | 12 (35.3) | 39 (46.4) | 0.309 | ||
| No | 22 (64.7) | 45 (53.6) | |||
| New hardware placed | |||||
| Yes | 8 (23.5) | 9 (10.7) | 0.087 | ||
| No | 26 (76.5) | 75 (89.3) | |||
| Second arrest | |||||
| Yes | 1 (2.9) | 6 (7.1) | 0.672 | ||
| No | 33 (97.1) | 78 (92.9) | |||
| Code status | |||||
| Full code | 20 (58.8) | 68 (81.0) | 0.019 | ||
| DNR | 14 (41.2) | 16 (19.1) | |||
| Disposition | |||||
| Deceased | 18 (52.9) | 42 (50) | 0.023 | ||
| Acute rehab facility | 10 (29.4) | 14 (16.7) | |||
| Skilled nursing facility | 6 (17.7) | 10 (11.9) | |||
| Home | 0 (0.0) | 15 (17.9) | |||
| Transfer to outside hospital | 0 (0.0) | 3 (3.6) | |||
DNR = do-not-resuscitate, ETT = endotracheal tube, IQR = interquartile range, m = median, PPC = pediatric palliative care.
aFive patients with no advanced airway in place may not add up to 100% due to rounding. Fisher exact for categorical variables and Wilcoxon-Mann-Whitney U for continuous variables.
Discharge Disposition
Disposition also differed by PPC (p = 0.023), with a greater proportion of PPC patients being discharged to rehab or nursing facilities (47.1% vs 28.6%) when compared with non-PPC patients. In addition, no PPC patients were discharged directly to home, unlike non-PPC patients. However, there was no difference when examining inhospital mortality rates (52.9% vs 50.0%) between PPC and nonpalliative care consult patients (Table 2).
Modes of Death
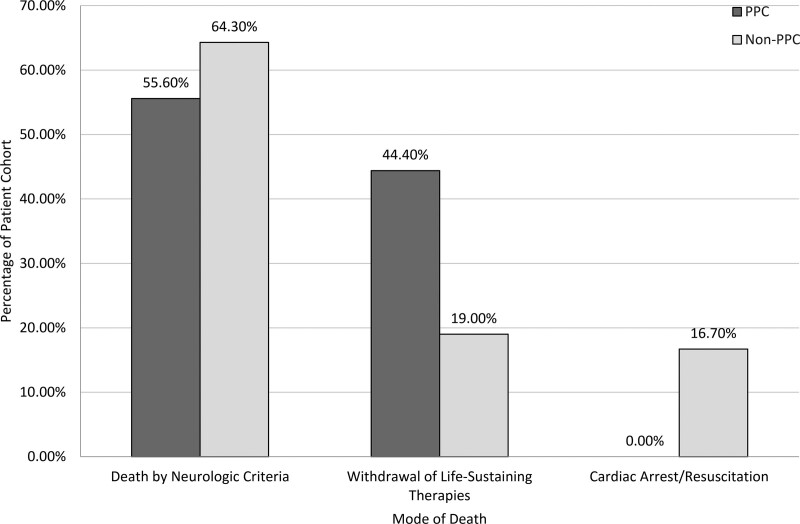
In nonsurvivors, we found that the modes of death differed significantly by those with and without PPC involvement. In the non-PPC group, of the 42 patient deaths, 64% (n = 27) had death by neurologic criteria (DNC), 17% (n = 7) had a cardiac death, and 19% (n = 8) had a withdrawal of life-sustaining therapies (WLST). In the PPC group, of the 18 patient deaths, 55.5% (n = 10) had DNC, and 45.5% (n = 8) had WLST (Fig. 2).
Figure 2.
Distribution of the modes of death in those patients who did not survive to hospital discharge, as compared by those who did and did not receive palliative care services. PPC = pediatric palliative care.
Factors Associated With Palliative Care
In the multivariate logistic regression analysis, factors associated with a higher likelihood of PPC consultation included: older age, longer hospital LOS, and code Status, specifically a do-not-resuscitate (DNR) order. Most significantly, patients with PPC involvement were 3.78 times more likely to have a DNR order in place (odds ratio, 3.78). It is important to note that this analysis did not incorporate the temporal relationship between timing of initial consultation and timing of DNR order placement (Table 3).
TABLE 3.
Logistic Regression
| Variables | DF | Estimate | se | Wald χ2 | p | OR (95% CI) |
|---|---|---|---|---|---|---|
| Intercept | 1 | −1.93 | 0.42 | 21.39 | < 0.001 | |
| Hospital length of stay | 1 | 0.06 | 0.02 | 12.24 | < 0.001 | 1.06 (1.03–1.10) |
| Age (yr) | 1 | 0.08 | 0.04 | 4.17 | 0.041 | 1.08 (1.00–1.17) |
| Code status (ref: full code) | ||||||
| Do not resuscitate | 1 | 0.66 | 0.26 | 6.66 | 0.010 | 3.78 (1.38–10.36) |
Timing of PPC Consultation
The timing of when a PPC consult was placed from admission date as well as discharge/or death date in survivors and nonsurvivors of OHCA was examined. In the cohort of patients who died, a PPC consult was placed 2 days from admission and 3 days from death on average. In the cohort of patients who survived to hospital discharge, a PPC consult was placed on average 10 days after admission (Table 4).
TABLE 4.
Timing of Palliative Care Consult Consultation for Patients Enrolled in Palliative Care
| Survived to Discharge (n = 16) | Deceased (n = 18) | p | |||
|---|---|---|---|---|---|
| m (IQR) | Range | m (IQR) | Range | ||
| Days from admission to PCC | 10.0 (6.0–17.0) | 1–74 | 2.0 (1.0–4.0) | 1–12 | < 0.001 |
| Days from PCC to death/discharge | 29.0 (16.0–41.0) | 4–72 | 3.0 (2.0–5.0) | 1–9 | < 0.001 |
IQR = interquartile range, m = median, PCC = palliative care consult.
p for Wilcoxon Mann-Whitney U.
Temporal Trend of PPC Consultation
The overall rate of palliative care utilization increased from 0% in 2009 to 53% in 2019, although we did not observe a steady increase in the utilization rate from year to year (Supplemental Digital Content, Figure 1, http://links.lww.com/CCX/A935).
DISCUSSION
We evaluated the utilization of PPC services in patients who suffered from an OHCA. We compared the characteristics of this pediatric OHCA patient population who received a PPC consult to the patients who did not. Our study demonstrated several key findings. First, just over 28% of patients included in our study received PPC services, demonstrating a low utilization rate as per AAP and ACCM recommendations and potential opportunities for PPC referral (7, 8). Second, PPC patients tended to be older and had markers of higher disease severity as measured by longer LOS and ventilator days. Third, PPC patients had a higher proportion of DNR orders and significant differences in disposition, including mode of death, compared with non-PPC patients.
Our study showed that less than 30% of patients with an OHCA admitted to the PICU received a PPC consult. Although there have been previous studies exploring PPC consults for patients with other diagnoses, our study is unique in its evaluation of PPC consultation in OHCA pediatric patients. In comparison, adult studies have shown far lower rates of consultation following OHCA. In the United States, one national database study found that only 7.3% (n = 11,260) of 154,177 patients hospitalized with OHCA had palliative care consultations, although a significant increase in the utilization rate (1.5–16.7%) was seen over the 10-year study period (12). In the pediatric population, PPC consultant rates are low and variable among diagnoses (13, 14). Keele et al (13) previously reported in a national study of all pediatric patients who died in a hospital over a 10-year period, only 4% received PPC services, with the highest proportion found among children with neurologic disease. The low rate of PPC consultation that we found, consistent with previous reports, might be explained by several factors and barriers. These include but are not limited to physician awareness of PPC services and its importance, families’ understanding and acceptance of PPC consultation, and the level of informal application of palliative care practices by the primary medical team. Additionally, PPC trained providers remain a scarce resource and, therefore, a significant barrier. Specific to our institution, potential barriers include variability and evolution of PPC team staffing, which may have impacted the visibility of PPC services. In addition, providers may have an underappreciation for the myriad services PPC consultation can provide beyond end-of-life care, both in the short and long terms.
The utilization of palliative care services following OHCA in our study was associated with several patient-related factors, specifically older age, longer LOS, and more ventilator days. This may reflect PPC services being sought in patients with a greater severity of illness and/or a more protracted hospital course. We attempted to account for patients with high certainty of imminent death and likely inappropriate for PPC services by excluding all patients with less than 48-hour survival. Previous studies have supported this, demonstrating physicians are less likely to involve PPC for imminently dying children (15). The involvement of palliative care has been shown to be associated with reduced hospital LOS and resource utilization (9, 10, 13). Our findings somewhat diverge from these reports, with PPC patients having significantly longer LOS. This finding likely reflects that ICU physicians often involve PPC in the most complex or difficult cases, including cases where provider and family conflict surrounding continuation, limitations, or withdrawal of technological support exists. Additional communication and support expertise may be required, a perspective previously described (16, 17). Our finding of older patients being more likely to receive a PPC consultation is a well-documented association (12–14). These results are again likely attributed to multifactorial barriers to PPC consultation, including misconceptions of what PPC services provide beyond end-of-life care and potentially provider fears relating to the impression of “giving up” (18). There is often a lack of communication and guidance on which families would most benefit from PPC involvement, and appropriateness of consultation timing, a subject of ongoing study. Given PICU patients have varied and often uncertain clinical trajectories, as we have demonstrated, this approach still leaves many patients and families with potentially unmet palliative care needs.
When looking at the disposition of our cohort, we found striking differences between the two groups. Surprisingly, we found no significant difference in mortality rates between the PPC and non-PPC populations. This differs from previous studies, where higher rates of mortality are demonstrated in those with PC involvement, attributed to their expertise in end-of-life care (12, 14). We found a significant difference in the proportion of DNR orders in the PPC group compared with the non-PPC group, which is consistent with previous works (19, 20). The differences in code status likely portend the differences we found in modes of death, with PPC patients more likely to die by WLST versus during failed resuscitation or DNC. Our finding that a significant portion of our non-PPC group included DNC patients is not surprising considering our institution’s culture to date. The PICU team historically has not viewed a role for PPC, given specific decisions about limitations of support typically need not be made, and code status is not consistently addressed. In hindsight, this is an important group of families who would benefit from PPC involvement, which is uniquely equipped to support both families and medical teams facing a diagnosis of brain death. There are many inherent challenges of DNC determination, including family acceptance, ethical/legal considerations, and bereavement support, which are well suited to intervention by an interdisciplinary PPC team. In survivors, a much higher proportion of PPC patients required skilled nursing facilities and acute rehab facilities, many of which were discharged with new technology-dependence. This outcome difference is likely related to our findings of PPC consultation timing, with a large difference in initiation of PPC consultation between nonsurvivors and survivors (2 vs 10 d). We suspect these findings reflect the differing indications for PPC consultation, which are wide-ranging, with the focus being primarily end-of-life care versus longitudinal support, relating to significant changes in functional status and complex care needs, which is appropriate and encouraging. These findings discussed may reflect the benefits of involving PPC providers, who bring expertise in eliciting values in context of new prognostic information and help guide families in shared medical decision-making that reflects those values, resulting in goal-concordant care. Overall, our findings highlight the opportunity for earlier involvement of PPC services, no matter the likelihood of survival, and including those patients who survive but have a significant change in health-related quality of life.
Our study has several limitations. As a single-center study, there may be issues with generalizability. PPC consultation at our institution is not standardized but solely dependent on the primary healthcare provider’s discretion. Palliative care referral mechanisms vary widely among institutions and, therefore, may make comparisons of our findings more challenging. We were dependent on chart documentation for receipt of palliative care and placement of a consultation order, which does not always result in a completed consult and/or underrepresentation of PPC involvement. Due to the lack of detailed documentation included in consult orders, we were unable to comment on PICU providers’ expectations, hopes, and intended role of PPC referrals. As in many PICUs, there are diverse values and attitudes of clinicians as well as patients/families that PPC providers must address, and additional information about these beliefs could have enhanced the interpretation of our findings. The retrospective study design and available data limited our ability to calculate formal measures or scores of functional morbidities in OHCA survivors. We were unable to examine the temporal and/or causal relationships relating to outcomes measured and PPC involvement. Our single-center study results are also influenced by the institution’s general culture, beliefs, and practices surrounding PPC involvement and code status. As previously stated, the placement of DNR orders in patients undergoing brain death determination is inconsistent at our institution, potentially impacting this outcome measure. Additionally, considering our high rates of organ donation following DNC, code status is generally not addressed until after determination of brain death, and the family has consented or declined to donate. Finally, the PPC team underwent multiple evolutions during the time period of the study, with variable staffing. There was always a PPC team available, but this may have impacted PICU provider awareness of PPC services.
Our results demonstrate that despite suffering a life-changing OHCA, the majority of our patients did not receive palliative care services. We are the first study, to our knowledge, to report on the underutilization of PPC services in this patient population. Considering comprehensive and family-centered care in the ICU setting is our gold standard, this identified gap in an essential component of care that PPC can provide is meaningful. Although not every OHCA pediatric patient and family may necessarily require PPC services, this vulnerable population warrants systematic consideration and evaluation for palliative care needs, whether physical, emotional, and/or spiritual, early in their course, and not as a last resort. Our own patient cohort demonstrated over 50% mortality. Furthermore, in all survivors, nearly 30% had new technology dependency, and nearly 70% required discharge to medical facilities for continued care and rehabilitation needs. All these findings serve as markers for potentially unmet PPC needs. Additional considerations beyond the OHCA diagnosis alone as possible indicators for PPC consultation could include LOS beyond 48 hours, ventilator days beyond 48 hours, uncertainty related to clinical trajectory, greater medical complexity, significant changes in functional status, and conflict surrounding medical decision making. Palliative care consultation activated through clinical screening criteria, such as these, has proven successful for other diagnoses and in the ICU environment (21, 22). These factors may indicate a high risk of unmet PPC needs, where the PPC team could provide additional support, including aiding in family understanding of prognosis, goals of care discussions, medical-decision making, longitudinal support, end-of-life care, and bereavement services.
CONCLUSIONS
Our study showed that the utilization of PPC services following OHCA is low, with several findings highlighting the differences between those with versus without PPC consultation. Given the high rates of mortality and morbidity after pediatric OHCA, early involvement of palliative care should be considered. Providers may not effectively predict the need for PPC based on outcomes or complexity alone, and many patients and families go without the benefits of palliative care services. The integration of PPC into standard postcardiac arrest care could potentially improve the comprehensiveness and quality of care provided through improved communication, end-of-life and goals of care discussions, and longitudinal clinical and psychosocial support. Future prospective studies are needed to evaluate the barriers to PPC referral and the impact of palliative care integration into routine postcardiac arrest care, including evaluation of meaningful, family-centered outcome measures.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflicts of interest.
This work was performed at The University of Chicago Comer Children’s Hospital.
REFERENCES
- 1.Fink EL, Prince DK, Kaltman JR, et al. ; Resuscitation Outcomes Consortium: Unchanged pediatric out-of-hospital cardiac arrest incidence and survival rates with regional variation in North America. Resuscitation 2016; 107:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajan S, Wissenberg M, Folke F, et al. : Out-of-hospital cardiac arrests in children and adolescents: Incidences, outcomes, and household socioeconomic status. Resuscitation 2015; 88:12–19 [DOI] [PubMed] [Google Scholar]
- 3.Atkins DL, Everson-Stewart S, Sears GK, et al. ; Resuscitation Outcomes Consortium Investigators: Epidemiology and outcomes from out-of-hospital cardiac arrest in children: The resuscitation outcomes consortium epistry-cardiac arrest. Circulation 2009; 119:1484–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donoghue AJ, Nadkarni V, Berg RA, et al. ; CanAm Pediatric Cardiac Arrest Investigators: Out-of-hospital pediatric cardiac arrest: An epidemiologic review and assessment of current knowledge. Ann Emerg Med 2005; 46:512–522 [DOI] [PubMed] [Google Scholar]
- 5.Young KD, Gausche-Hill M, McClung CD, et al. : A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardiopulmonary arrest. Pediatrics 2004; 114:157–164 [DOI] [PubMed] [Google Scholar]
- 6.Moler FW, Silverstein FS, Holubkov R, et al. ; THAPCA Trial Investigators: Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med 2015; 372:1898–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics Committee on Bioethics and Committee on Hospital Care: Palliative care for children. Pediatrics 2000; 106:351–357 [PubMed] [Google Scholar]
- 8.Truog RD, Campbell ML, Curtis JR, et al. ; American Academy of Critical Care Medicine: Recommendations for end-of-life care in the intensive care unit: A consensus statement by the American College [corrected] of Critical Care Medicine. Crit Care Med 2008; 36:953–963 [DOI] [PubMed] [Google Scholar]
- 9.Khandelwal N, Kross EK, Engelberg RA, et al. : Estimating the effect of palliative care interventions and advance care planning on ICU utilization: A systematic review. Crit Care Med 2015; 43:1102–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison RS, Penrod JD, Cassel JB, et al. ; Palliative Care Leadership Centers’ Outcomes Group: Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med 2008; 168:1783–1790 [DOI] [PubMed] [Google Scholar]
- 11.Feudtner C, Feinstein JA, Zhong W, et al. : Pediatric complex chronic conditions classification system version 2: Updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014; 14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albaeni A, Chandra-Strobos N, Eid SM: Palliative care utilization following out-of-hospital cardiac arrest in the United States. Resuscitation 2018; 124:112–117 [DOI] [PubMed] [Google Scholar]
- 13.Keele L, Keenan HT, Sheetz J, et al. : Differences in characteristics of dying children who receive and do not receive palliative care. Pediatrics 2013; 132:72–78 [DOI] [PubMed] [Google Scholar]
- 14.Delgado-Corcoran C, Wawrzynski SE, Bennett EE, et al. : Palliative care in children with heart disease treated in an ICU. Pediatr Crit Care Med 2020; 21:423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones PM, Carter BS: Pediatric palliative care: Feedback from the pediatric intensivist community. Am J Hosp Palliat Care 2010; 27:450–455 [DOI] [PubMed] [Google Scholar]
- 16.Atwood MA, Hoffmann RG, Yan K, et al. : Attitudes about palliative care: A comparison of pediatric critical care and oncology providers. Am J Hosp Palliat Care 2014; 31:665–671 [DOI] [PubMed] [Google Scholar]
- 17.Kelley AS, Morrison RS: Palliative care for the seriously ill. N Engl J Med 2015; 373:747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balkin EM, Kirkpatrick JN, Kaufman B, et al. : Pediatric cardiology provider attitudes about palliative care: A multicenter survey study. Pediatr Cardiol 2017; 38:1324–1331 [DOI] [PubMed] [Google Scholar]
- 19.Trowbridge A, Walter JK, McConathey E, et al. : Modes of death within a children’s hospital. Pediatrics 2018; 142:e20174182. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe J, Klar N, Grier HE, et al. : Understanding of prognosis among parents of children who died of cancer: Impact on treatment goals and integration of palliative care. JAMA 2000; 284:2469–2475 [DOI] [PubMed] [Google Scholar]
- 21.Truog RD, Cist AF, Brackett SE, et al. : Recommendations for end-of-life care in the intensive care unit: The Ethics Committee of the Society of Critical Care Medicine. Crit Care Med 2001; 29:2332–2348 [DOI] [PubMed] [Google Scholar]
- 22.Nelson JE, Curtis JR, Mulkerin C, et al. ; Improving Palliative Care in the ICU (IPAL-ICU) Project Advisory Board: Choosing and using screening criteria for palliative care consultation in the ICU: A report from the Improving Palliative Care in the ICU (IPAL-ICU) Advisory Board. Crit Care Med 2013; 41:2318–2327 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.