Abstract
OBJECTIVES:
A recent study suggests that Multisystem Inflammatory Syndrome in Children (MIS-C) is triggered by gastrointestinal breach of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral particles from the gut lumen into systemic circulation. The virus remains in the gut weeks to months after respiratory infection, causing zonulin release from the intestinal epithelial cells. Zonulin loosens tight junctions, permitting trafficking of highly inflammatory viral particles into circulation. Current MIS-C treatments target the subsequent immune hyperactivation, not the causative loss of mucosal barrier integrity. Larazotide, a zonulin inhibitor, prevents breakdown of tight junctions, limiting antigen trafficking.
DESIGN:
Children with MIS-C were treated with larazotide as an adjuvant to steroid/intravenous immunoglobulin therapy. Clinical outcomes, SARS-CoV-2 antigenemia, and cytokine profiles are reported. Outcomes were compared with children with MIS-C receiving steroids and/or IVIG therapy alone.
PATIENTS:
Four children with MIS-C, ages 3–17 years, were enrolled.
INTERVENTIONS:
Patients were treated with open label larazotide 10 mcg/kg (maximum 500 mcg/dose) orally four times daily for 21 days.
MEASUREMENTS AND MAIN RESULTS:
All four patients tolerated larazotide without adverse effects and displayed reduction in Spike antigenemia to undetectable levels. When compared with 22 children with MIS-C receiving steroids and/or intravenous immunoglobulin therapy alone, larazotide-treated patients reported significantly improved time to resolution of gastrointestinal symptoms (p = 0.03), and time to clearance of Spike antigenemia (p = 0.04), plus a trend towards shorter length of stay.
CONCLUSIONS:
Larazotide appears safe and well-tolerated and may offer potential benefit as an adjuvant to immune-targeted therapies. Expansion of clinical trials is urgently needed to ascertain the clinical impact of larazotide on MIS-C.
Keywords: AT1001, larazotide, multisystem inflammatory syndrome in children, severe acute respiratory syndrome coronavirus 2 antigenemia
Over 11.4 million children have tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the United States (1). Although vaccines are a central strategy for containing the virus and ending the pandemic, there are still several barriers, including delays in vaccination (2), waning vaccine efficacy in the setting of novel SARS-CoV-2 variants (3), and variable masking recommendations. As a result, COVID-19 and SARS-CoV-2–related illnesses will likely remain part of our lives for the foreseeable future. Novel treatments must be developed to combat this virus to reduce COVID-19–related morbidity and mortality.
Multisystem inflammatory syndrome in children (MIS-C) is a severe complication of COVID-19 occurring in children (and occasionally in adults) that is defined by high fever, systemic inflammation, and multiple organ involvement requiring hospitalization weeks to months after a SARS-CoV-2 infection or exposure (4). Roughly 6,800 cases of MIS-C have been reported in the United States to date, with a 1% mortality rate (4). Recent studies have discovered that MIS-C may be caused by a zonulin-dependent breach in the gastrointestinal barrier, allowing residual SARS-CoV-2 viral particles to traffic into circulation (5). SARS-CoV-2, which is cleared from the upper respiratory tract within 1–2 weeks (6), can linger in the gut for many weeks. In MIS-C, prolonged detection of virus in the gut is associated with increased zonulin release (5). Zonulin is a family of structurally and functionally related proteins that reversibly regulate intestinal permeability by modulating intercellular tight junctions (7). An increase in zonulin leads to the disruption of tight junctions and increased mucosal permeability (7). As a result, SARS-CoV-2 viral particles can be detected in the blood (5). These viral particles are highly inflammatory and trigger the hyperinflammatory storm defining MIS-C (8, 9).
Current therapies, such as steroids and IV immunoglobulin (IVIG), target the hyperinflammatory storm associated with MIS-C (10) but do not address mucosal barrier permeability. Larazotide (AT1001) is an extensively studied oral medicine with an excellent safety profile (11) that inhibits the effect of zonulin, allowing restoration of tight junctions (12) and improves mucosal barrier function (13). A recent study describes the use of larazotide in a single patient showing, as a proof of concept, reduction in Spike antigenemia and improvement in clinical status (5).
Here, we describe our experience treating four children with MIS-C with compassionate use open-label larazotide at 10 mcg/kg by mouth four times daily for 21 days and compare their disease course with children with MIS-C who did not receive larazotide therapy. Patients treated with open-label larazotide displayed a reduction in the duration of MIS-C–related gastrointestinal symptoms and more rapid clearance of Spike antigenemia compared with patients who did not receive larazotide. These findings suggest that adjuvant treatment with larazotide favorably impacts the course of MIS-C, providing justification for further evaluation of this novel therapeutic strategy for MIS-C.
MATERIALS AND METHODS
Following the report that MIS-C results from zonulin-mediated loss of gastrointestinal mucosal barrier integrity (5), we sought expanded access, compassionate use of larazotide for four subsequent patients presenting with MIS-C. Approval was granted by the Food and Drug Administration for all four patients, as was Institutional Review Board (IRB) approval, parental consent, and assent when appropriate. Patients were treated with larazotide 10 mcg/kg orally four times per day for 21 days as an adjuvant therapy to their MIS-C treatments (steroids, IVIG, anakinra, as decided by multidisciplinary MIS-C clinical team). An additional 22 patients who did not receive larazotide treatment were also included in this study. All patients met criteria for MIS-C as defined by the Centers for Disease Control and Prevention (4). All MIS-C patients provided consent, and assent when indicated, to the Massachusetts General Hospital Pediatric COVID-19 Biorepository (14) (IRB 2020P000955) to allow sample and metadata analysis. Clinical laboratories (C-reactive protein [CRP], d-dimer), symptomatology, and body temperatures were obtained from medical records.
SARS-CoV-2 antibodies and cytokines were measured as described previously (5, 15). Antibody levels are presented as normalized average enzymes per bead (AEB), where the measured AEB value is normalized by calibrators obtained from serially diluting a plasma sample collected from a SARS-CoV-2 positive individual. The assay for measuring SARS-CoV-2 Spike and S1 protein was modified after screening additional capture and detector antibodies. While the assay is performed in the same manner (5, 16), different capture and detector antibodies were used, namely an antibody against the S2 subunit of spike as the capture (MA5-35946; Invitrogen, Waltham, MA), and an anti-Receptor-binding domain (RBD) antibody as the detector (LT1900; Leinco, Fenton, MO). Prior to performing the assay, plasma samples were treated with 10 mM dithiothreitol and incubated at 37°C for 15 minutes to denature any antibodies in complex with spike antigen (17). Plasma samples were also analyzed without dithiothreitol pretreatment.
Analysis was completed using Prism 9.2 (GraphPad Software, San Diego, CA). Mann-Whitney U test was used to compare nonparametric values in the larazotide-treated group and historic controls. chi-square test was used to compare frequencies between the two groups.
RESULTS
Aggregate Results
Four children with MIS-C, ages ranging from 3 to 17 years (median, 7.5 yr) were treated with compassionate use larazotide. All four children displayed serologic evidence of prior SARS-CoV-2 infection and had detectable SARS-CoV-2 antigenemia on presentation. While two of the children displayed cardiac involvement of MIS-C, all four reported prominent gastrointestinal symptoms on presentation, with evidence of multiple organ involvement (Table 1).
TABLE 1.
Characteristics of Patients Treated With Larazotide As an Adjuvant Therapy for Multisystem Inflammatory Syndrome in Children
| Patient Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age (yr) | 17 | 3 | 6 | 9 |
| Sex | Female | Female | Female | Male |
| Race, ethnicity | White non-Hispanic | Asian non-Hispanic | Black non-Hispanic | White non-Hispanic |
| SARS-CoV-2 reverse transcriptase-quantitative polymerase chain reaction or antibody positive | Yes | Yes | Yes | Yes |
| SARS-CoV-2 spike antigenemia | Yes | Yes | Yes | Yes |
| Cardiac involvement | None | None | Coronary aneurysm | Coronary dilation |
| Gastrointestinal involvement | Abdominal pain, diarrhea | Abdominal pain, vomiting, diarrhea | Abdominal pain, vomiting | Abdominal pain, vomiting, diarrhea |
| Other organ involvement | Anemia, thrombocytopenia, lymphopenia, headaches | Anemia, thrombocytopenia, lymphopenia | Anemia, rash, conjunctivitis | Anemia, thrombocytopenia, lymphopenia, rash, conjunctivitis, headache |
| Highest level of care | Ward | Ward | PICU | Ward |
| Treatment with approved, expanded use of larazotide | Yes | Yes | Yes | Yes |
SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
The clinical courses of children who received larazotide were compared with children diagnosed with MIS-C who did not receive larazotide as part of their clinical regimen (Table 2). Twenty-two children with MIS-C receiving steroids or IVIG alone were included, with median age of 8 years, which was not significantly different compared with the ages of children in the larazotide group. Following initiation of larazotide therapy, a significant decrease in duration of gastrointestinal symptoms (time to resolution of gastrointestinal symptoms was observed: larazotide-treated group median 2.5 d; larazotide-naive group median 5.5 d; p = 0.03). While there were no significant differences in length of stay, fever duration, or escalation of care, length of stay appeared slightly shorter in the larazotide-treated group (median 4 d in larazotide-treated group vs 5 d in larazotide-naive group). Importantly, Spike antigen levels cleared more quickly in the larazotide-treated group (time to first clearance of Spike antigen: larazotide-treated group median 1 d; larazotide-naive group median 5.5 d; p = 0.03).
TABLE 2.
Comparison of Larazotide-Treated Children With Children Who Received Steroids and/or Intravenous Immunoglobulin Without the Addition of Larazotide Therapy
| Clinical Outcomes | Larazotide-Treated (n = 4) | Steroids and/or Intravenous Immunoglobulin Alone (n = 22) | p |
|---|---|---|---|
| Age, yr, median (95% CI) | 7.5 (3–17) | 8 (4–14) | 0.93 |
| Time to resolution of gastrointestinal symptoms, d, median (95% CI) | 2.5 (1–3) | 5.5 (3–11) | 0.03 |
| Length of stay, d, median (95% CI) | 4 (3–7) | 5 (4–10) | 0.42 |
| Duration fever since initiation of treatment, d, median (95% CI) | 0.5 (0–2) | 0 (0–6) | 0.80 |
| Escalation of care (n)a | 0 | 3 | > 0.9 |
| Time to first clearance of Spike, d, median (95% CI) | 1 (1–12) | 10 (6–190) | 0.04 |
aχ2 analysis. Otherwise, Mann-Whitney U test was used.
Results of Individual Cases
Larazotide-Treated Patient 1.
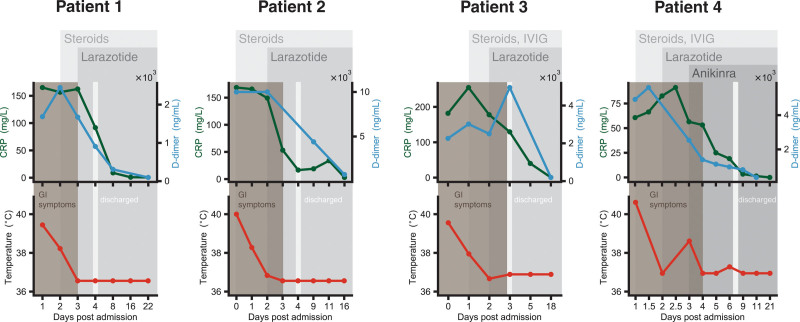
A 17-year-old female, who had mild COVID-19 1 month prior to presentation, was hospitalized after reporting five days of fever up to 39.4°C, headache, abdominal pain, and diarrhea. She was found to have elevated CRP (peak 165 mg/L), erythrocyte sedimentation rate (ESR; peak 57 mm/hr), d-dimer (peak 2,446 ng/mL), and lymphopenia. Treatment with intravenous (IV) steroids was initiated; the decision was made to not treat with IVIG since she did not display cardiac involvement. Given her diagnosis of MIS-C with gastrointestinal involvement, we sought and were granted apitproval to treat her with compassionate use of larazotide, which she was able to start within 24 hours of initiation of steroids.
After starting steroids, her fever fell to 38.2°C and her cytokine markers decreased, but she continued to have some abdominal pain and increased bowel movements, and notably, her CRP remained elevated. However, after the addition of larazotide, her fever, abdominal pain, and diarrhea resolved within 24 hours. Her CRP, ESR, and d-dimer improved, her lymphopenia resolved, and she was discharged home (Fig. 1).
Figure 1.
Four children with multisystem inflammatory syndrome in children were treated with larazotide as adjuvant therapy following their acute presentation. C-reactive protein (CRP) (mg/L), d-dimer (ng/mL), fever curve, and gastrointestinal (GI) symptoms are presented with multiple assessments obtained their treatment course. IVIG = IV immunoglobulin.
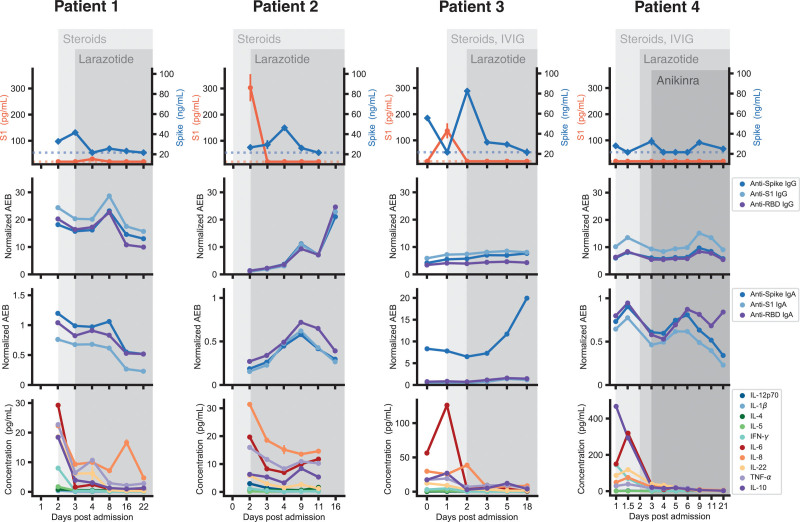
Spike antigen was detected in her blood prior to initiating larazotide and reached undetectable levels within 24 hours of starting larazotide (Fig. 2). Interestingly, her anti-spike immunoglobulin G (IgG) and immunoglobulin A (IgA) levels decreased slightly after initiation of steroid treatment, corresponding with an increase in Spike antigen, then recovered to higher levels. Her Spike antigen declined to below the level of detection by day 4 postadmission and remained essentially undetectable through the 21-day course (Fig. 2).
Figure 2.
S1 and Spike antigen levels, anti-Spike, anti-S1 and anti-Receptor-binding domain (RBD) immunoglobulin, and cytokine levels from four children with multisystem inflammatory syndrome in children (MIS-C) who received treatment with larazotide in addition to standard MIS-C therapy. AEB = average enzymes per bead, IFN-γ = interferon gamma, IgA = immunoglobulin A, IgG = immunoglobulin G, IL = interleukin, IVIG = IV immunoglobulin, TNF-α = tumor necrosis factor alpha.
Larazotide-Treated Patient 2.
A 3-year-old female presented with a high fever of 40°C, severe abdominal pain, vomiting, and diarrhea, in the setting of recent household SARS-CoV-2 exposure 2 weeks prior. Her CRP was elevated (168.6 mg/L), with elevated d-dimer (> 10,000 ng/mL) and lymphopenia (WBC 2,400/µL). She was started on steroids and given her positive reverse transcriptase-quantitative polymerase chain reaction for SARS-CoV-2, she was also started on remdesivir. IVIG was not administered due to lack of cardiac involvement. Given the severity of her abdominal pain, surgery was consulted to evaluate for appendicitis, although this diagnosis was ultimately ruled out. Following initiation of steroids, she continued to have fever, her CRP remained elevated, and her cytokines failed to normalize.
Given her symptoms of MIS-C with gastrointestinal involvement, we sought and were granted approval to treat her with compassionate use of larazotide. Larazotide was initiated within 48 hours of starting steroids. Within 24 hours of starting larazotide, her CRP dropped significantly, her cytokine profile improved, and her abdominal pain resolved; consequently, she was able to begin eating and drinking again, and she was discharged the following day (Fig. 1).
Her S1 antigen was highly elevated at the time of starting larazotide, then cleared precipitously within 24 hours of larazotide treatment. Her Spike antigen was elevated at the time of starting larazotide, then displayed a peak at day 4 of admission before clearing by day 11 (Fig. 2). While her anti-SARS-CoV-2 IgG and IgA were low on day 2 of admission while on steroids, she displayed an increase in anti-spike IgG levels over time, corresponding with her more recent exposure and infection. A sharp increase of antibody levels was noted following the peak in Spike antigen on day 4 of her admission (Fig. 2).
Larazotide-Treated Patient 3.
A 6-year-old girl with no known prior COVID-19 illness or household SARS-CoV-2 exposure, who presented with four days of fever up to 39.4°C, abdominal pain, and vomiting, was found to have positive SARS-CoV-2 antibodies in addition to elevated CRP (peak 254 mg/L), ferritin (peak 507 μg/L), and d-dimer (peak 4,952 ng/mL). She also had an elevated N-terminal pro b-type netriuretic peptide (peak 2,815 pg/mL), a mild troponin leak (peak 26 ng/L), and an aneurysmal dilation of the right coronary artery on echocardiogram (z score 3.8). Treatment with IV steroids and IVIG was initiated. Over the next 24 hours, her fever fell from 39.4°C to 37.9°C, and her CRP decreased but remained elevated at 178 mg/L (Fig. 1). She was then started on larazotide; her abdominal pain resolved by the next day, and she remained afebrile, so she was discharged home. Her CRP further declined to 40 mg/L by day 5 postadmission (48 hr into larazotide treatment course). By day 18 postadmission, her CRP and d-dimer normalized, and her coronary aneurysm resolved.
Spike and S1 antigens were detected in her blood, and she displayed an activated cytokine profile, with interleukin-6 most notably elevated. Although she displayed a dramatic decrease in Spike antigen within 24 hours of starting larazotide, her Spike antigen remained above detectable levels until day 18 postadmission (Fig. 2). Her anti-SARS-CoV-2 IgG remained relatively low through the duration of her illness but her anti-Spike IgA became highly elevated (Fig. 2), which could potentially reflect ongoing antigen exposure at the mucosal surface.
Larazotide-Treated Patient 4.
A 9-year-old boy who had mild COVID-19 6 weeks prior, presented with 3 days of fever up to 40°C associated with diarrhea, nausea, vomiting, headache, and fatigue. He developed a rash, conjunctivitis, and periorbital edema. He displayed elevated inflammatory markers (peak CRP 91.2 mg/L) and an echocardiogram that displayed mild dilation of the left main coronary artery (z score of 2.3). He was started on IVIG and steroids but his inflammatory markers and gastrointestinal symptoms persisted. He was started on compassionate use larazotide. Although his CRP began to improve after starting larazotide, the following day, he continued to have a fever of 38.6°C so was started on anakinra. His CRP and d-dimer improved, he defervesced, and his gastrointestinal symptoms improved within 48 hours of starting larazotide (Fig. 1).
His Spike antigen, which had been elevated on admission, was undetectable by day 4 of admission (Fig. 2). On day 9 following his hospital admission, Spike antigen was again detected in the setting of elevated anti-SARS-CoV-2 IgG and IgA (Fig. 2), without a corresponding elevation of inflammatory markers, fevers, or cytokines. Interestingly, his Spike antigen remained above the level of detection upon completion of his larazotide course with a rising anti-RBD IgA, suggesting ongoing SARS-CoV-2 antigen presence at the gastrointestinal mucosal surface 3 weeks following presentation with MIS-C.
DISCUSSION
Here, we describe four children with MIS-C who demonstrated improved outcomes when larazotide was added on as therapy compared with children treated with steroids and/or IVIG alone. The four children in our open-label larazotide-treated cohort displayed faster resolution of gastrointestinal symptoms and faster time to clearance of Spike antigen, signaling improvement in gastrointestinal mucosal barrier function. Additionally, there was a trend in a shortened length of stay. While a double-blind, randomized placebo-controlled study is needed to determine efficacy of larazotide, these findings suggest that larazotide may provide a safe and beneficial adjuvant therapy for the treatment of MIS-C.
Interestingly, three of the four patients experienced an increase in Spike antigen when steroids or IVIG were added. Although steroids and IVIG suppress unchecked inflammatory responses (10), the initial immunosuppression may create an imbalance in antigen levels and immune response. As example, patients 1, 3, and 4 all displayed a drop in antibody levels following steroid treatment (with or without IVIG), allowing antigen levels to subsequently rise. As antibodies recover, antigen levels then decreased. We did not have presteroid antibody levels from patient 2, so we cannot determine whether the low anti-Spike IgG is due to time from infection or immunosuppression. This aligns with our previous report of SARS-CoV-2 antigen levels in MIS-C (5): in an 11-patient cohort, two-thirds of the MIS-C historic controls with pretreatment values available displayed an increase in Spike antigen levels following initiation of immunomodulatory therapy for MIS-C. These findings highlight that immunosuppression alone may not be the optimal treatment strategy for MIS-C and novel therapies, such as larazotide, that target the source of the antigen leak would provide additional benefit in treating MIS-C.
Additionally, as we have previously shown and highlight here, antigen levels can be detected for weeks to months after the onset of MIS-C, despite resolution of fevers and cytokine storm (5). The impact of this ongoing antigenemia is unclear, although there is data to suggest that Spike triggers an autoimmune signature in MIS-C (9). Autoimmune disease or immune dysregulation may not be evident for years and should be monitored. Children with MIS-C should be monitored long-term for autoimmune outcomes and compared with those treated with larazotide.
Last, all of the children reported here, and previously (5), reveal Spike antigenemia, suggesting SARS-CoV-2 antigen detection may serve as a useful diagnostic marker for MIS-C, especially if combined with elevated levels of zonulin, which would not only help identify patients with MIS-C but also guide therapy. A better understanding of the interplay between antibody and antigen levels over time may help to elucidate the cause of immune dysregulation. Here, we analyzed plasma samples after decoupling of separate antigen-antibody complexes, which helped better demonstrate circulating antigen levels (Fig. S1, http://links.lww.com/CCX/A923). In some cases, we observe a considerable increase in the concentration of S1 or spike, suggesting that antigen masking was altering the effective concentration. In future studies, we anticipate that it will be important to track antigen levels, capturing both freely circulating antigen and antigen bound by antibodies to better distinguish antigenemia from antigen clearance. Diagnostic and prognostic tools are urgently needed for MIS-C to expedite care when indicated and to allow better characterization and understanding of the disease.
CONCLUSIONS
This case series suggests that adjuvant treatment for MIS-C with larazotide warrants further evaluation. Ultimately, a double-blind placebo-controlled study, currently underway (ClinicalTrials.gov identifier: NCT05022303), is required to assess efficacy of larazotide, and extended follow-up will provide much needed insight into the long-term impact of resolution of antigenemia. This case series provides critical insight into MIS-C and highlights a potential novel therapeutic pathway.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by the National Heart, Lung, and Blood Institute (5K08HL143183 to Dr. Yonker), the Department of Pediatrics at Massachusetts General Hospital (to Dr. Yonker), Regione Campania (CUP G58D20000240002—SURF 20004BP000000011 to Dr. Fasano), and Chleck Foundation and Barbara and Amos Hostetter Foundation (to Dr. Walt). Larazotide was provided for Food and Drug Administration-approved compassionate use by 9Meters Biopharma.
The data obtained as part of this study are available from the corresponding author upon reasonable request.
Dr. Fasano is co-founder and stockholder of Alba Therapeutics. Dr. Walt has a financial interest in Quanterix Corporation, a company that develops an ultrasensitive digital immunoassay platform. He is an inventor of the Simoa technology, a founder of the company and also serves on its Board of Directors. Drs. Walt’s and Fasano’s interests were reviewed and are managed by Brigham and Women's Hospital (to Dr. Walt), Massachusetts General Hospital (to Dr. Fasano), and Partners HealthCare (both) in accordance with their conflict of interest policies. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.American Academy of Pediatrics: Children and COVID-19: State-Level Data Report. Available at: https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/. Accessed February 7, 2022.
- 2.Centers for Disease Control and Prevention: COVID-19 Vaccinations in the United States. Available at: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total. Accessed February 7, 2022.
- 3.Tartof SY, Slezak JM, Fischer H, et al. : Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021; 398:1407–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention: Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. Available at: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance. Accessed February 7, 2022.
- 5.Yonker LM, Gilboa T, Ogata AF, et al. : Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest 2021; 131:149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yonker LM, Boucau J, Regan J, et al. : Virologic features of severe acute respiratory syndrome coronavirus 2 infection in children. J Infect Dis 2021; 224:1821–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fasano A, Not T, Wang W, et al. : Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000; 355:1518–1519 [DOI] [PubMed] [Google Scholar]
- 8.Porritt RA, Paschold L, Noval Rivas M, et al. : HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. J Clin Invest 2021; 131:e146614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porritt RA, Binek A, Paschold L, et al. : The autoimmune signature of hyperinflammatory multisystem inflammatory syndrome in children. J Clin Invest 2021; 131:e151520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noval Rivas M, Porritt RA, Cheng MH, et al. : COVID-19-associated multisystem inflammatory syndrome in children (MIS-C): A novel disease that mimics toxic shock syndrome-the superantigen hypothesis. J Allergy Clin Immunol 2021; 147:57–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leffler DA, Kelly CP, Green PH, et al. : Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: A randomized controlled trial. Gastroenterology 2015; 148:1311–1319.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slifer ZM, Hernandez L, Pridgen TA, et al. : Larazotide acetate induces recovery of ischemia-injured porcine jejunum via repair of tight junctions. PLoS One 2021; 16:e0250165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slifer ZM, Krishnan BR, Madan J, et al. : Larazotide acetate: A pharmacological peptide approach to tight junction regulation. Am J Physiol Gastrointest Liver Physiol 2021; 320:G983–G989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lima R, Gootkind EF, De la Flor D, et al. : Establishment of a pediatric COVID-19 biorepository: Unique considerations and opportunities for studying the impact of the COVID-19 pandemic on children. BMC Med Res Methodol 2020; 20:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norman M, Gilboa T, Ogata AF, et al. : Ultrasensitive high-resolution profiling of early seroconversion in patients with COVID-19. Nat Biomed Eng 2020; 4:1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogata AF, Maley AM, Wu C, et al. : Ultra-sensitive serial profiling of SARS-CoV-2 antigens and antibodies in plasma to understand disease progression in COVID-19 patients with severe disease. Clin Chem 2020; 66:1562–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan D, Johnson JM, Fernandes SC, et al. : N-protein presents early in blood, dried blood and saliva during asymptomatic and symptomatic SARS-CoV-2 infection. Nat Commun 2021; 12:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.