Abstract
Background:
Static cold storage is the gold standard of preservation in vascularized composite allotransplantation and allows a preservation time of 4–6 hours. Machine preservation is a promising technique for prolonged preservation; however, studies on extended preservation that compare different preservatives are scarce. This study aims to assess the feasibility of 24-hour acellular perfusion and compares different preservation solutions in a porcine myocutaneous flap replantation model.
Methods:
Six harvested bilateral myocutaneous flaps of three Dutch Landrace pigs were perfused hypothermically for 24 hours with University of Wisconsin machine perfusion solution (UW-MPS; n = 2) or histidine-tryptophan-ketoglutarate solution (HTK; n = 2) or preserved on ice for 4 hours (n = 2) before orthotopic replantation. Animals were observed for 7 days after replantation. Skeletal muscle injury was assessed by biochemical markers during perfusion, and muscle biopsies were analyzed for ischemia reperfusion injury directly after preservation and at 1, 3, and 7 days after replantation.
Results:
Markers of muscle damage varied during perfusion, but decreased overall in both perfusion groups. Flap weight increased 60% and 97% in the HTK-perfused flaps, compared with -6% and -7% in the UW-MPS-perfused flaps after 24 hours. Histopathologic evaluation demonstrated decreased muscle damage in flaps perfused with HTK compared with the UW-MPS-perfused flaps at 1 week after replantation.
Conclusions:
Machine perfusion of myocutaneous flaps for 24 hours with subsequent replantation is feasible, but warrants further research. Perfusion with HTK solution seemed to result in better histological outcomes 7 days after reperfusion compared with UW-MPS.
INTRODUCTION
Vascularized composite allotransplantation (VCA) is increasingly becoming available as a clinical reconstructive option for patients with severe traumatic craniofacial deformations or after limb amputation.1 Since the first successful hand allotransplantation in 1998, over 100 upper extremity transplantations and over 40 face transplantations have been performed, yet VCA is still rare.2–5 Finding the optimal match between donor and recipients is constrained by the maximum time in which the donor tissue can be preserved. Currently, static cold storage (SCS) on ice is the gold standard of preservation in VCA and allows for a maximum preservation time of 4–6 hours.6–8 However, it is extremely difficult to match donor and recipient, transport the VCA donor graft, and perform a technical complex procedure within this short timeframe.
Machine preservation has emerged as an alternative preservation method that allows for controlled oxygenation, the supply of nutrients, and the possibility to remove toxins and metabolic products during preservation.9,10 Machine preservation of porcine and human limbs for 12–24 hours showed comparable outcomes in terms of ischemia reperfusion injury when compared with 4 hours of SCS.11–14 These studies underline the potential benefits of machine preservation to extend the donor–recipient matching window, by extending safe preservation time without altering graft quality. However, studies that evaluate different perfusion solutions for machine preservation in a free flap replantation model are scarce.
Histidine-tryptophan-ketoglutarate (HTK) and University of Wisconsin machine perfusion solution (UW-MPS) are two commonly used perfusion solutions for machine preservation in solid organ transplantation and improve muscle preservation in immersion and perfusion studies by reducing histological muscle injury.10,15,16 The potential advantage of using acellular solutions such as HTK and UW-MPS is that, as opposed to blood products, they can be easily stored, manipulated, and transported, even outside hospital environments. Before the presented study, porcine musculocutaneous rectus abdominis flaps (n = 15) were perfused for 18 hours with either HTK or UW-MPS, subsequently replanted, and observed for 12 hours after reperfusion. HTK-perfused flaps showed decreased ischemia-related histologic alterations. However, edema formation was substantially more severe in the HTK group directly after machine perfusion, compared with the UW-MPS-perfused flaps (UW-MPS -0.22 g [0%], HTK +156.5 g [53%], P < 0.001).17 In light of this, polyethylene glycol (PEG) was added to HTK to reduce edema formation. PEG is a nontoxic polymer, which acts as colloid that shows protective properties for ischemia reperfusion injury in the liver.18 PEGs exert a myriad of effects that may benefit free flap preservation by reducing coagulation, reducing platelet and leukocyte adhesion, and preventing cell swelling, ultimately attenuating ischemia reperfusion injury.19 In addition, L-glutamine was added to HTK to further attenuate ischemia reperfusion injury, as this is an antioxidant that reduces neutrophil recruitment after reperfusion times longer than 4 hours.20,21
The primary aim of the present study was to demonstrate the feasibility of 24-hour acellular hypothermic machine perfusion after 7 days follow-up in a porcine myocutaneous rectus abdominis flap replantation model. The second aim of this study was to assess long-term muscle histology alterations after 24 hours of machine preservation with modified HTK or UW-MPS compared with 4 hours of SCS after 7 days follow-up. The myocutaneous free flap in swine is a reliable preclinical, large-animal model for VCA research.22–24 Overall, myocutaneous free flaps differ in their ease in harvest and in functional deficits that arise from flap removal, compared with larger vascularized composite allograft models such as forelimbs; thus, they are the best models to study the feasibility and effects of extended machine preservation on skeletal muscle solely.25 In a step-wise approach, this study uses an abdominis flap replantation model to gain insight of perfusion characteristics and different perfusion solutions, with the ultimate goal to test the presented perfusion set-up in a limb replantation model in future studies.
MATERIALS AND METHODS
Care and use of animals in the present study was approved by the local and national animal experimentation committee (2016-0034-007) and was in accordance with the ARRIVE guidelines. Animals were housed under standard conditions with access to food and water ad libitum. All animals were sedated, intubated, and anesthetized following local protocols. Physiologic parameters (electrocardiography, heart rate, blood pressure, oxygen saturation, and core body temperature) were monitored during all experiments.
Experimental Groups
Bilateral myocutaneous rectus abdominal flaps (12 × 9 cm) of three female Dutch Landrace pigs (weight 60–70 kg) were raised and harvested according to previously described methods.16,26 The flaps were randomly assigned to the modified-HTK (n = 2) or UW-MPS (n = 2) perfusion groups or the SCS group (n = 2). Bilateral flaps from one individual animal were never subjected to the same intervention. The surgeon was not blinded to the groups due to the nature of the ex-vivo preservation interventions.
After harvest of the perfusion group, a 20 or 22G Bectone Dickinson catheter (Franklin Lakes, N.J.) was introduced in the pedicle’s artery and secured with sutures. Two flaps (HTK-1, HTK-2) were flushed with 100 mL heparin-HTK solution (200E heparin per 100 mL HTK solution) and subsequently perfused for 24 hours with a total volume of 2 L HTK (Custodiol; Koehler Chemi, Alsbach-Haenlien, Germany) enriched with PEG and L-glutamine. The other two flaps (UW-1, UW-2) were flushed with 100 mL heparin-UW-MPS (200E heparin per 100 mL UW-MPS), followed by 24-hour continuous perfusion with a total volume of 2 L UW-MPS (E. I. DuPont de Nemours & Co., Bannockburn, UK). To provide oxygenated, continuous, and nonpulsatile flow, a custom-made semiclosed extracorporeal circuit was set up according to previous studies performed by the same research group.16,26,27 (See document, Supplemental Digital Content 1, which provides details of the ex-vivo perfusion system and perfusion settings. http://links.lww.com/PRSGO/B933). For the compositions and additives of UW-MPS and HTK solution, see Table 1.
Table 1.
The Compositions and Additives of UW-MPS and HTK Solution
| Compound | UW-MPS28 | HTK29 |
|---|---|---|
| Osmolality (mosm/L) | 300 | 310 |
| Potassium (mmol/L) | 25 | 9 |
| Sodium chloride (mmol/L) | 15 | |
| Sodium gluconate (mmol/L) | 80 | |
| Sodium hydroxide (g/L) | 0.70 | |
| Calcium chloride (mmol/L) | 0.5 | 0.015 |
| Magnesium chloride (mmol/L) | 4 | |
| Magnesium gluconate (mmol/L) | 5 | |
| Mannitol (mmol/L) | 30 | 30 |
| Ribose (mmol/L) | 5 | |
| Adenine (mmol/L) | 5 | |
| Glutathione (mmol/L) | 3 | |
| Hydroxyethyl starch (g/L) | 50 | |
| Dextrose (mmol/L) | 10 | |
| Histidine (mmol/L) | 198 | |
| Trypthophan (mmol/L) | 2 | |
| Ketoglutarate (mmol/L) | 1 | |
| Additives | ||
| Methylprednisolone (mg/L) | 250 | 250 |
| Glucose 50% (ml/L) | 1 | |
| Insuline (IE/L) | 10 | |
| L-glutamine (ml/L) | 10 | |
| PEG (g/L) | 20 |
In the SCS group (SCS-1, SCS-2), the abdominal flaps were flushed with 100 cm3 of heparin–saline solution (200E heparin per 100 mL 0.9% saline solution) and stored in dry gauzes in a sealed bag on ice slurry at 4ºC for 4 hours, conforming to the current gold standard.6,30
A hydrophilic foam dressing (Allevyn std; Smith & Nephew, Lachine, Quebec, Canada) was used for wound coverage during ex-vivo flap preservation. At the end of the preservation period, all abdominal flaps were replanted orthotopically and monitored for 7 days before the animals were euthanized with an overdose of phenobarbital. All surgical procedures and measurements were performed by the same researcher (KB).
Measurements
Machine perfusion parameters (flow, pressure, pump speed, and temperature) were assessed hourly during perfusion. Core temperature was measured using a 15-mm needle temperature probe (Myocardial 400 needle temp. probe; MTS40015; Smith Medical, Rosmalen, the Netherlands) after flap harvest and after the ex-vivo preservation period. Flaps were weighed after harvest, after the ex-vivo preservation period, and at 7 days after replantation, as both ischemia-reperfusion injury and ex-vivo perfusion could potentially contribute to weight increase of the vascularized composite tissue.30 Flap perfusion was appraised using a near-infrared fluorescence angiography camera (PhotoDynamic Eye; Hamamatsu Photonics, Japan) that recorded the surgical field after each intravenous injection of 5 mg fluorescent marker indocyanine-green (ICG, 5mg/mL, Verdye, Diagnostic Green GmbH, Germany). Spatial distribution of recorded fluorescence intensity throughout the flap was assessed using an algorithm in MATLAB (MathWorks Inc, Natick, Mass.). Near-infrared fluorescence angiography was performed after flap harvest, directly after replantation, and 3 and 7 days after replantation.
Sample Procurement
Perfusate aliquots, before and after passing through the flap, were obtained to assess the release of ischemia-related muscle damage markers every 2 hours during perfusion. Analysis was performed on perfusate samples using CG4+ and CG8+ cartridges to measure pH, lactate, oxygen partial pressure, and carbon dioxide partial pressure using the iSTAT platform (Abbott, Princeton, N.J.). Potassium and creatin kinase (CK) were measured in the perfusate samples collected in a heparinized syringe using a 1265 Rapidlab Blood Gas Analyzer (Siemens Healthcare Diagnostics, Norwood, Mass.) Muscle biopsy specimens were acquired from the deep muscular layer of the abdominal flap, after flap raise, after the ex-vivo preservation period, and at 1, 3, and 7 days after reperfusion.
Histologic Analysis
Formalin-fixed biopsy specimens were sectioned and stained with standard hematoxylin and eosin (H&E) and Masson trichrome. All slides were evaluated with a light microscope (Leica DM 3000) using ×20 magnification. Ten randomly selected high-power fields from each biopsy specimen were assessed for damage of muscle fibers by a pathologist (DM), blinded to the intervention groups. A modified “histologic injury severity score” (HISS) (range 0–12, Table 2) was used to assess for ischemia-reperfusion injury-induced alterations, according to methods previously described by Muller et al14 and Kruit et al.27 Signs for damaged muscle fibers were defined by four subgroups: interstitial edema, inflammation, variation in shape and size of myocytes, and damaged muscle fibers (necrotic + hypoxic + regenerating myocytes).
Table 2.
Histology Injury Scoring System (HISS) for Hypoxic-induced Muscle Injury
| Morphological Changes | Categories |
|---|---|
| Interstitial edema | 0. No significant increase 1. Minimal 2. Intermediate 3. Severe/diffuse |
| Inflammation | 0. Not significant 1. Minimal 2. Intermediate 3. Diffuse |
| Variation in shape and size of myocytes | 0. Homogeneous 1. Mild heterogeneous 2. Intermediate heterogeneous 3. Severe heterogeneous |
| Damaged muscle fibers* | 0.0–5 myocytes/10 HPF (20× magnification) 1.6–20 myocytes/10 HPF (20× magnification) 2.21–50 myocytes/10 HPF (20× magnification) 3.>51 myocytes/10 HPF (20× magnification) |
| Total score | 0–4 No/minimal degeneration 5–7 Intermediate degeneration 8–12 Severe degeneration |
*Damaged muscle fibers: a sum of necrotic, hypoxic and phagocytic myocytes
Statistical Analysis
Data values from near-infrared fluorescence angiography, histologic assessments, perfusate sampling, and flap parameters were captured using Microsoft Excel (Microsoft Corp., Redmond, Wash.). Owing to the small sample sizes in the computation of the groups, statistical tests to compare the differences between the acquired data were not performed. Instead, qualitative and descriptive analyses of the outcomes were performed.
RESULTS
Baseline characteristics were comparable between the intervention groups (Table 3). Warm ischemia time before storage was 9 (4–14) minutes longer in both perfusion groups compared with the SCS group, as a result of cannulating and attaching the flap to the perfusion machine.
Table 3.
Overview of Porcine Flap Replantation Characteristics before and after Ex-vivo Preservation on Slurry Ice or Machine Perfusion
| HTK | UW-MPS | SCS | ||||
|---|---|---|---|---|---|---|
| Characteristics | #1 | #2 | #1 | #2 | #1 | #2 |
| Harvest (min) | 87 | 89 | 83 | 90 | 94 | 73 |
| Flush (min) | 21 | 16 | 16 | 15 | 16 | 15 |
| Ex-vivo storage time (hr) | 25 | 24 | 24 | 24 | 4 | 4 |
| WIT before storage (min) | 14 | 9 | 19 | 15 | 5 | 5 |
| WIT before reperfusion (min) | 94 | 55 | 63 | 56 | 62 | 34 |
| Flap weight before intervention (g) | 284 | 261 | 342 | 287 | 242 | 249 |
| Flap weight after intervention (g) | 454 | 514 | 322 | 268 | 235 | 248 |
| Weight difference (%) | 60 | 97 | -6 | -7 | -3 | 0 |
| Temperature after dissection (°C) | 38.2 | 37.7 | 37.3 | 38.4 | 38.1 | 37.3 |
| Temperature before replantation (°C) | 14.8 | 12.7 | 13.2 | 13.7 | 12.8 | 7.2 |
WIT, warm ischemia time.
During Flap Preservation
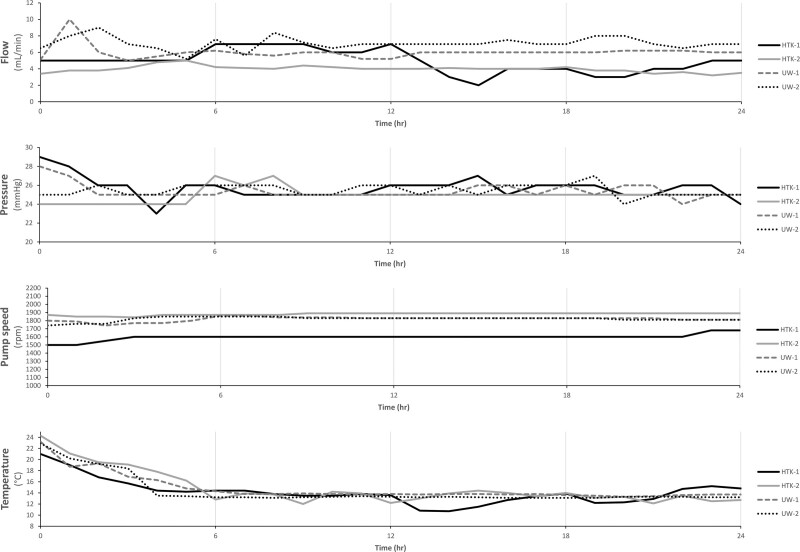
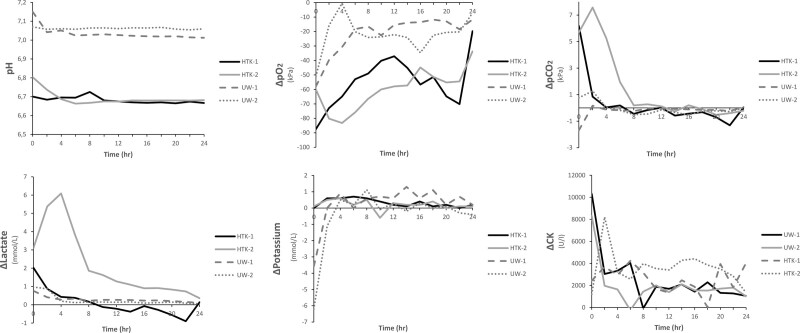
Flap core temperature dropped to 13.5°C (12.7–14.3) in the first 6 hours of perfusion and to 10°C (7.2–12.8) in 4 hours of SCS. Perfusion flow rates were dynamic and were regulated to maintain a target intra-arterial pressure of 26 ± 3 mm Hg (Fig. 1). Release of lactate in the preservation solution was comparable between HTK-1, UW-1, and UW-2 and decreased during 24 hours of perfusion (Fig. 2). Lactate levels of HTK-2 peaked in the first 4 hours of perfusion, but showed a steep decrease afterwards. CK levels released were variable, but a decrease of delta CK was observed in all groups during 24 hours of perfusion.
Fig. 1.
Perfusion parameters: flow (mL/min); pressure (mmHg); pump speed (rpm); temperature (°C).
Fig. 2.
Biochemical parameters during 24 hours of ex-vivo perfusion of porcine musculocutaneous abdominal flaps.
Delta pCO2 values remained stable in the UW-MPS-perfused flaps, whereas the HTK-perfused flaps showed a steep decrease during the first 4–8 hours of perfusion (Fig. 2). Delta pO2 showed a decrease during perfusion, but baseline levels remained higher in the HTK-perfused flaps. The pH readings remained stable throughout the perfusion period in both groups.
Flap weight increase was higher in HTK-perfused flaps compared with UW-MPS-perfused flaps and the SCS group after the ex-vivo preservation period (Table 3). Macroscopically, edema tended to develop mostly in the subcutaneous layer of the HTK-perfused flaps.
After Flap Reperfusion
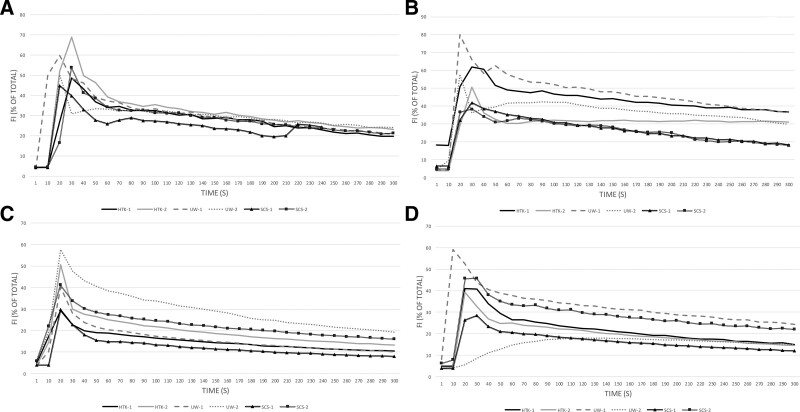
Animals undergoing orthotopic replantation recovered well from general anesthesia and remained hemodynamically and respiratory stable throughout the entire 7-day observation period. Following reperfusion, the ICG peak perfusion patterns, such as time-to-peak and maximum fluorescence intensity, of the perfused muscle flaps were comparable with the SCS group (Fig. 3). UW-2 showed delayed filling and no fluorescence intensity peak was observed, despite normal flap viability assessments (capillary refill times, temperature, skin color) 7 days after reperfusion. (See figure 1, Supplemental Digital Content 2, which provides color images of perfused and static cold stored muscle flaps at baseline, directly after replantation and 7 days after replantation. http://links.lww.com/PRSGO/B934.)
Fig. 3.
Appraisal of flap perfusion using a near-infrared fluorescence angiography. Fluorescence intensity curves (A) after creating abdominal muscle flap; (B) directly after reperfusion; (C) 3 days after reperfusion; (D) 7 days after reperfusion.
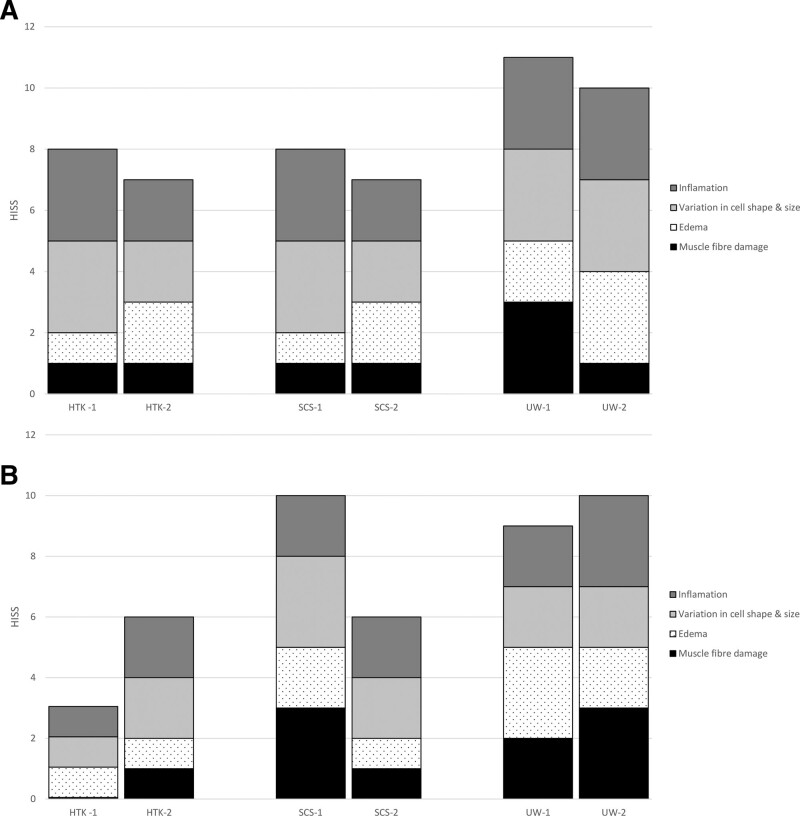
An increase in HISS from baseline 3 days after reperfusion was observed (See figure 2, Supplemental Digital Content 3, which provides representative hematoxylin and eosin (H&E) stained cross-sections from perfused and static cold-stored porcine skeletal muscle. http://links.lww.com/PRSGO/B935.), showing intermediate (HTK-2 and SCS-1) to severe (HTK-1, SCS-2, UW-1, UW-2) degeneration. The subscores showed individual variation, with increased muscle fiber damage in the UW-1 perfused flap compared with the other flaps (Fig. 4A). Intermediate (HTK-2 and SCS-2) to minimal (HTK-1) degenerations were observed 7 days after reperfusion, whereas severe degenerative alterations were observed in the UW-MPS-perfused flaps and SCS-1. In these flaps, subscore analysis showed increased HISS in muscle fiber damage, inflammation, and variation in cell shape and size (Fig. 4B).
Fig. 4.
Assessment for ischemia-reperfusion injury-induced alterations using a modified histologic injury severity score (HISS). HISS subscores of the flap muscle samples 3 (A) and 7 (B) days after reperfusion.
DISCUSSION
Machine perfusion of porcine myocutaneous abdominal flaps for 24 hours with subsequent replantation enabled macroscopic outcomes comparable to those achieved after 4 hours of preservation on slurry ice after 1 week of follow-up. Release of muscle damage markers was varying but decreased during perfusion in both perfusion groups. Flaps perfused with HTK, enriched with PEG, and one static cold-stored flap demonstrated decreased muscle damage 1 week after reperfusion compared with the UW-MPS-perfused flaps. These findings are consistent with prior studies assessing muscle damage up to 7 days after machine perfusion for 12 or 24 hours of amputated porcine limbs with subsequent replantation. Machine perfusion with modified STEEN and Perfadex solution yielded superior clinical, macroscopic, and histologic outcomes compared with those achieved by SCS.2,11,13 The presented study, however, demonstrated severe degenerative alterations 7 days after reperfusion in the two UW-MPS-perfused flaps, suggesting differences in muscle preservation qualities between the used preservatives.
Perfusates such as UW-MPS and HTK have been frequently used in VCA preservation, demonstrating a delay in onset of acute rejection31 and a decrease in histological32 and genetic33 evidence of ischemic injury compared with SCS alone, confirming its protective properties in muscle tissue. Two animal studies aimed to compare static storage capacities of HTK and UW-MPS up to 30 hours, optimum preservation temperatures, and their collective effects on composite flaps in a rat model.15,34 They found HTK to be superior compared with the University of Wisconsin solution, which could be a result of the lower viscosity and the high buffering capacity of HTK. Slater et al26 and Kruit et al16 from our research group compared long-term (24 and 36 hours, respectively) perfusion of rectus abdominis myocutaneous flaps. Flaps undergoing hypothermic perfusion with UW-MPS or HTK showed minimal or no signs of cell necrosis after prolonged ex-vivo preservation. The expression of genes related to ischemia, apoptosis, and inflammation were comparable, despite increased lactate levels that may indicate higher anaerobic metabolic activity during UW-MPS perfusion. However, both studies lacked replantation and long-term follow-up results.
In this study, perfusion with HTK solution seemed to result in better histological outcomes 7 days after reperfusion, indicating possible superior muscle preservation qualities compared with UW-MPS. A possible explanation for the advantages of HTK solution are the combination of the histidine buffer to compensate cellular acidosis and the low permeable amino acids in the solution, which may be substrates for the anaerobic metabolism.35
The weight increase after 24 hours of perfusion with UW-MPS was comparable with that of the SCS group; however, the HTK-perfused flaps enriched with PEG and L-glutamine showed a significantly greater weight gain. Similar weight increase after prolonged perfusion with HTK is reported, varying between 60%26 and 78%.16 Histological changes of muscle tissue, such as an increase in intercellular distance between myocytes, are well characterized as a sign of edema but can also be used as a marker to estimate the extent of ischemia-reperfusion injury.36 Apart from the lower diffusion capacity of oxygen and nutrients to cells, edema could potentially complicate the replantation procedure.16,37 Interestingly, the increase in interstitial edema of the histological samples of muscle was comparable in all flaps after 24-hour perfusion. Most of the edema tended to develop in the subcutaneous layer, suggesting increased capillary permeability of the fatty tissue in the HTK-perfused group. The weight gain could be attributed to the differences in potassium concentrations between UW-MPS and HTK-PEG, as outlined in Table 1. The concentration of the vasoactive potassium is much higher in the UW-MPS solution and can affect vasoconstriction during the perfusion. Perfusion with a potassium-rich solution can potentially prevent exudation of fluid to the extracellular space, resulting in less edema.38 Reduction of increased interstitial edema is important to ensure cellular metabolism, preventing compartment syndrome when replanting limbs and fat necrosis after replantation of prolonged preservation of composite tissue.
Limitations of the current study include descriptive analysis, the use of only myocutaneous flaps with lack of functional assessment, and relatively small sample size, as is often the case in most large-animal research. Nevertheless, this porcine model has a high translational relevance to clinical scenarios, since the perfusion set-up can assumedly be applied to human tissue without further modification. Our study did not compare equal preservation times between the treatment and control group, as the intention of this study was to compare the current golden standard of SCS with a hypothermic perfusion treatment. Replantation of flaps with 24 hours of cold ischemia could potentially lead to severe damage to the replanted tissue and host, which was ethically not acceptable and could endanger the outcomes of the contralateral abdominal flap.
Our findings encourage further research on extracorporeal machine preservation, which has clinical potential in replantation and transplantation scenarios. Further use of additives that may help extend perfusion time, slow down metabolism, and/or prevent extravasation of the solution during the time of perfusion are promising avenues for future research. Future study on this exciting topic should address the unique characteristics of a whole-limb replantation, allowing in-depth experimental assessment of all the involved tissues of an extremity. Safely increasing preservation times of VCAs could drastically change the field as we know it, as it would, at least partially, address matching difficulties constrained by a limited time window. Machine preservation of VCA grafts can enable better surgical planning of the replantation procedure and allows utilization of more potential donor grafts by increasing the geographical range of matching. Also, allograft outcomes can be improved by optimizing graft viability before transplantation.
CONCLUSIONS
Machine perfusion with acellular solution of porcine myocutaneous abdominal flaps for 24 hours with subsequent replantation is feasible and enabled macroscopic outcomes comparable to those achieved through preservation in SCS for 4 hours. Although this was studied over a limited sample size, perfusion with HTK solution seemed to result in better histological outcomes 7 days after reperfusion, indicating possible superior muscle preservation qualities compared with UW-MPS. Future investigations of acellular perfusion solutions for ex-vivo preservation of vascularized composite allografts should aim to further minimize skeletal muscle damage and improve graft outcomes.
Supplementary Material
Footnotes
Published online 21 February 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Burlage LC, Tessier SN, Etra JW, et al. Advances in machine perfusion, organ preservation, and cryobiology: potential impact on vascularized composite allotransplantation. Curr Opin Organ Transplant. 2018;23:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kueckelhaus M, Puscz F, Dermietzel A, et al. Extracorporeal perfusion in vascularized composite allotransplantation: current concepts and future prospects. Ann Plast Surg. 2018;80:669–678. [DOI] [PubMed] [Google Scholar]
- 3.Shores JT, Brandacher G, Lee WPA. Hand and upper extremity transplantation: an update of outcomes in the worldwide experience. Plast Reconstr Surg. 2015;135:351e–360e. [DOI] [PubMed] [Google Scholar]
- 4.Dubernard JM, Owen E, Lefrançois N, et al. First human hand transplantation. Case report. Transpl Int. 2000;13(Suppl 1):S521–S524. [DOI] [PubMed] [Google Scholar]
- 5.Safi AF, Kauke M, Nelms L, et al. Local immunosuppression in vascularized composite allotransplantation (VCA): a systematic review. J Plast Reconstr Aesthet Surg. 2021;74:327–335. [DOI] [PubMed] [Google Scholar]
- 6.Pei G, Xiang D, Gu L, et al. A report of 15 hand allotransplantations in 12 patients and their outcomes in China. Transplantation. 2012;94:1052–1059. [DOI] [PubMed] [Google Scholar]
- 7.Dickey RM, Hembd AS, Fruge S, et al. Composite tissue preservation. Ann Plast Surg. 2020;84:711–716. [DOI] [PubMed] [Google Scholar]
- 8.Wolff KD, Stiller D. Ischemia tolerance of free-muscle flaps: an NMR-spectroscopic study in the rat. Plast Reconstr Surg. 1993;91:485–491. [DOI] [PubMed] [Google Scholar]
- 9.Taylor MJ, Baicu SC. Current state of hypothermic machine perfusion preservation of organs: the clinical perspective. Cryobiology. 2010;60(Suppl 3):S20–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruit AS, Winters H, van Luijk J, et al. Current insights into extracorporeal perfusion of free tissue flaps and extremities: a systematic review and data synthesis. J Surg Res. 2018;227:7–16. [DOI] [PubMed] [Google Scholar]
- 11.Krezdorn N, Macleod F, Tasigiorgos S, et al. Twenty-four-hour ex vivo perfusion with acellular solution enables successful replantation of porcine forelimbs. Plast Reconstr Surg. 2019;144:608e–618e. [DOI] [PubMed] [Google Scholar]
- 12.Haug V, Kollar B, Endo Y, et al. Comparison of acellular solutions for ex-situ perfusion of amputated limbs. Mil Med. 2020;185:e2004–e2012. [DOI] [PubMed] [Google Scholar]
- 13.Kueckelhaus M, Dermietzel A, Alhefzi M, et al. Acellular hypothermic extracorporeal perfusion extends allowable ischemia time in a porcine whole limb replantation model. Plast Reconstr Surg. 2017;139:922e–932e. [DOI] [PubMed] [Google Scholar]
- 14.Müller S, Constantinescu MA, Kiermeir DM, et al. Ischemia/reperfusion injury of porcine limbs after extracorporeal perfusion. J Surg Res. 2013;181:170–182. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijden EP, Kroese AB, Stremel RW, Bär PR, Kon M, Werker PM. Contractile properties of rat skeletal muscles following storage at 4ºC. Clinical Science. 1999;97:45–57. [DOI] [PubMed] [Google Scholar]
- 16.Kruit AS, Schreinemachers MJM, Koers EJ, et al. Successful long-term extracorporeal perfusion of free musculocutaneous flaps in a porcine model. J Surg Res. 2019;235:113–123. [DOI] [PubMed] [Google Scholar]
- 17.Kruit AS, van Midden D, Schreinemachers MC, et al. Rectus abdominis flap replantation after 18 h hypothermic extracorporeal perfusion-a porcine model. J Clin Med. 2021;10:3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bejaoui M, Pantazi E, Calvo M, et al. Polyethylene glycol preconditioning: an effective strategy to prevent liver ischemia reperfusion injury. Oxid Med Cell Longev. 2016;2016:9096549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasut G, Panisello A, Folch-Puy E, et al. Polyethylene glycols: An effective strategy for limiting liver ischemia reperfusion injury. World J Gastroenterol. 2016;22:6501–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prem JT, Eppinger M, Lemmon G, et al. The role of glutamine in skeletal muscle ischemia/reperfusion injury in the rat hind limb model. Am J Surg. 1999;178:147–150. [DOI] [PubMed] [Google Scholar]
- 21.Durante W. The emerging role of l-glutamine in cardiovascular health and disease. Nutrients. 2019;11:E2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramly EP, Kantar RS, Alfonso AR, et al. Preclinical animal models in facial transplantation. Plast Reconstr Surg Glob Open. 2019;7:e2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leto Barone AA, Leonard DA, Torabi R, et al. The gracilis myocutaneous free flap in swine: an advantageous preclinical model for vascularized composite allograft transplantation research. Microsurgery. 2013;33:51–55. [DOI] [PubMed] [Google Scholar]
- 24.Waldner M, Elgendy TY, Kim DY, et al. Heterotopic transplantation of allogeneic vertical rectus abdominis myocutaneous flaps in miniature swine. J Surg Res. 2020;254:175–182. [DOI] [PubMed] [Google Scholar]
- 25.Etra JW, Fidder SAJ, Frost CM, et al. Latissimus dorsi myocutaneous flap procedure in a swine model. J Invest Surg. 2021;34:1289–1296. [DOI] [PubMed] [Google Scholar]
- 26.Slater NJ, Zegers HJH, Küsters B, et al. Ex-vivo oxygenated perfusion of free flaps during ischemia time: a feasibility study in a porcine model and preliminary results. J Surg Res. 2016;205:292–295. [DOI] [PubMed] [Google Scholar]
- 27.Kruit AS, Brouwers K, van Midden D, et al. Successful 18-h acellular extracorporeal perfusion and replantation of porcine limbs – Histology versus nerve stimulation. Transpl Int. 2021;34:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bridge-to-Life. Machine perfusion solution-Belzer UW organ preservation solution (UW machine perfusion solution). Available at https://bridgetolife.com/belzer-mps-uw-machine-perfusion-solution-instructions-for-use/. Accessed January 5, 2021.
- 29.Janssen H, Janssen PH, Broelsch CE. UW is superior to Celsior and HTK in the protection of human liver endothelial cells against preservation injury. Liver Transpl. 2004;10:1514–1523. [DOI] [PubMed] [Google Scholar]
- 30.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10:620–630. [DOI] [PubMed] [Google Scholar]
- 31.Wang LC, Lawson SD, Fries CA, et al. Hyperbaric sub-normothermic ex-vivo perfusion delays the onset of acute rejection in a porcine VCA model. Plast Reconstr Surg. 2015;136(4S):34. [Google Scholar]
- 32.Gordon L, Levinsohn DG, Borowsky CD, et al. Improved preservation of skeletal muscle in amputated limbs using pulsatile hypothermic perfusion with University of Wisconsin solution. A preliminary study. J Bone Joint Surg Am. 1992;74:1358–1366. [PubMed] [Google Scholar]
- 33.Taeger CD, Müller-Seubert W, Horch RE, et al. Ischaemia-related cell damage in extracorporeal preserved tissue – new findings with a novel perfusion model. J Cell Mol Med. 2014;18:885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hautz T, Hickethier T, Blumer MJ, et al. Histomorphometric evaluation of ischemia-reperfusion injury and the effect of preservation solutions histidine-tryptophan-ketoglutarate and University of Wisconsin in limb transplantation. Transplantation. 2014;98:713–720. [DOI] [PubMed] [Google Scholar]
- 35.Guibert EE, Petrenko AY, Balaban CL, et al. Organ preservation: current concepts and new strategies for the next decade. Transfus Med Hemother. 2011;38:125–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrasek PF, Homer-Vanniasinkam S, Walker PM. Determinants of ischemic injury to skeletal muscle. J Vasc Surg. 1994;19:623–631. [DOI] [PubMed] [Google Scholar]
- 37.Hartzell TL, Benhaim P, Imbriglia JE, et al. Surgical and technical aspects of hand transplantation: is it just another replant? Hand Clin. 2011;27:521–30, x. [DOI] [PubMed] [Google Scholar]
- 38.Vaziri N, Thuillier R, Favreau FD, et al. Analysis of machine perfusion benefits in kidney grafts: a preclinical study. J Transl Med. 2011;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.