Abstract
Background:
The acceptable percentage of macrosteatosis (MaS) and microsteatosis (MiS) to yield optimal outcomes after liver transplantation (LT) with livers from donation after circulatory death (DCD) donors remains unknown. The purpose of this analysis was to determine the impact of donor liver MaS and MiS on DCD LT outcomes.
Methods:
Using the OPTN database, we analyzed pre-transplant biopsy results from adult, solitary, DCD livers performed between 1/1/2006–12/31/2017. Kaplan-Meier analysis was used to assess graft and patient survival based on MaS and MiS severity. MiS was divided into 0–10% (MiS ≤10) and >10% (MiS >10). MaS was divided into 0–15% (MaS ≤15) and >15% (MaS >15).
Results:
Of 7,757 recovered DCD livers, 11.4% (N=885) were biopsied and transplanted. Patients who received DCD livers with MaS >15 had significantly worse patient survival (p<0.04) and those with MiS >10 demonstrated inferior graft and patient survival (p<0.02). In multivariate analyses including known risk factors, both MaS >15 and MiS >10 were associated with increased risk of graft failure and patient mortality (p<0.03). Recipient and donor age >60 years were also associated with increased risk of graft failure and patient death.
Conclusion:
This analysis demonstrates that MaS >15% and MiS >10% are additional risk factors for graft loss and patient mortality in DCD LT.
Introduction:
The discrepancy between available organs and patients awaiting transplant has resulted in increased use of marginal or expanded criteria grafts.1,2 Included in marginal grafts are donation by circulatory death (DCD) donor livers.3 DCD livers were initially associated with inferior outcomes when compared to donation by brain death (DBD) livers, which resulted in increased discard rates of DCD livers.4,5 As best practices have been described and the frequency of transplanted DCD livers has increased over time, the optimal utilization of livers from DCD donors and identification of appropriate recipients has resulted in improved outcomes, approaching those seen in DBD donors.6–9 As results from multi-center studies have become available, the liver transplant community has improved in identifying optimal cut offs for critical risk factors that impact graft failure or patient death in DCD liver transplantation (LT).10 These factors include donor and recipient age, body mass index (BMI), model for end-stage liver disease (MELD) score, cold ischemia time (CIT), and donor warm ischemia time (WIT).8,11 The number of DCD LT has increased from 4.8% (N=274) in 2008 to 6.9% (N=531) in 2018. However, in 2018, the discard rate after recovery of DCD livers remains high at 30% compared in 6.4% of DBD livers.2
As obesity and morbid obesity becomes increasingly more prevalent, the incidence of nonalcoholic fatty liver disease (NAFLD) continues to rise in western countries and is estimated to be present in 10–24% of the population.12,13 Consequently, both DBD and DCD livers recovered for transplant will likely have higher proportions of steatosis than previously seen.12 DBD livers with >30% macrosteatosis (MaS) are at increased risk for primary non-function and overall inferior outcomes.14,15 Historically, the degree of microsteatosis (MiS) has not been considered to be a risk factor for graft loss or mortality after DBD LT and in many instances MiS does not play a significant role in determining the acceptance of a DBD donor liver. However, a recent single center analysis of 462 DBD and 34 DCD liver transplant recipients revealed that the presence of MiS increased the risk of post-reperfusion syndrome, early allograft function, and need for continuous renal replacement therapy after transplant.16 The only negative impact of MiS on graft and patient survival was seen in patients undergoing re-transplantation.16 Another single center analysis of 127 DCD LT recipients demonstrated that MaS >20% leads to inferior graft survival.17 Despite these small single-center reports, there are limited data in the literature on the impact of MaS and MiS in DCD LT. To determine the effect of donor MaS and MiS on DCD LT outcomes, we examined the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research database to identify commonly used steatosis “cut-offs” in DCD LT.
Materials and Methods:
Data source and patient population
The data source for this study was the United Network of Organ Sharing (UNOS) Standard Analysis and Research file. The data system includes data on all donor, waitlisted candidates, and transplant recipients in the U.S., submitted by the members of the Organ Procurement and Transplantation Network (OPTN) as previously described.18 Institutional review board approval was obtained prior to the study. The time period for this study was January 2006 to December 2017. Donors were excluded if the recovered livers were transplanted outside the specified time, if they were living donors, <18 years old, or the donor type was unknown (DBD, DCD). Recipients were excluded if they were <18 years old, underwent a previous liver transplant, or received a split liver graft. Of the donors who met inclusion criteria, we identified recovered livers, donor type, pre-transplant liver biopsy, and ultimately whether the liver was transplanted. The primary group of interest was biopsied DCD livers that were then transplanted (Figure 1).
Fig 1:
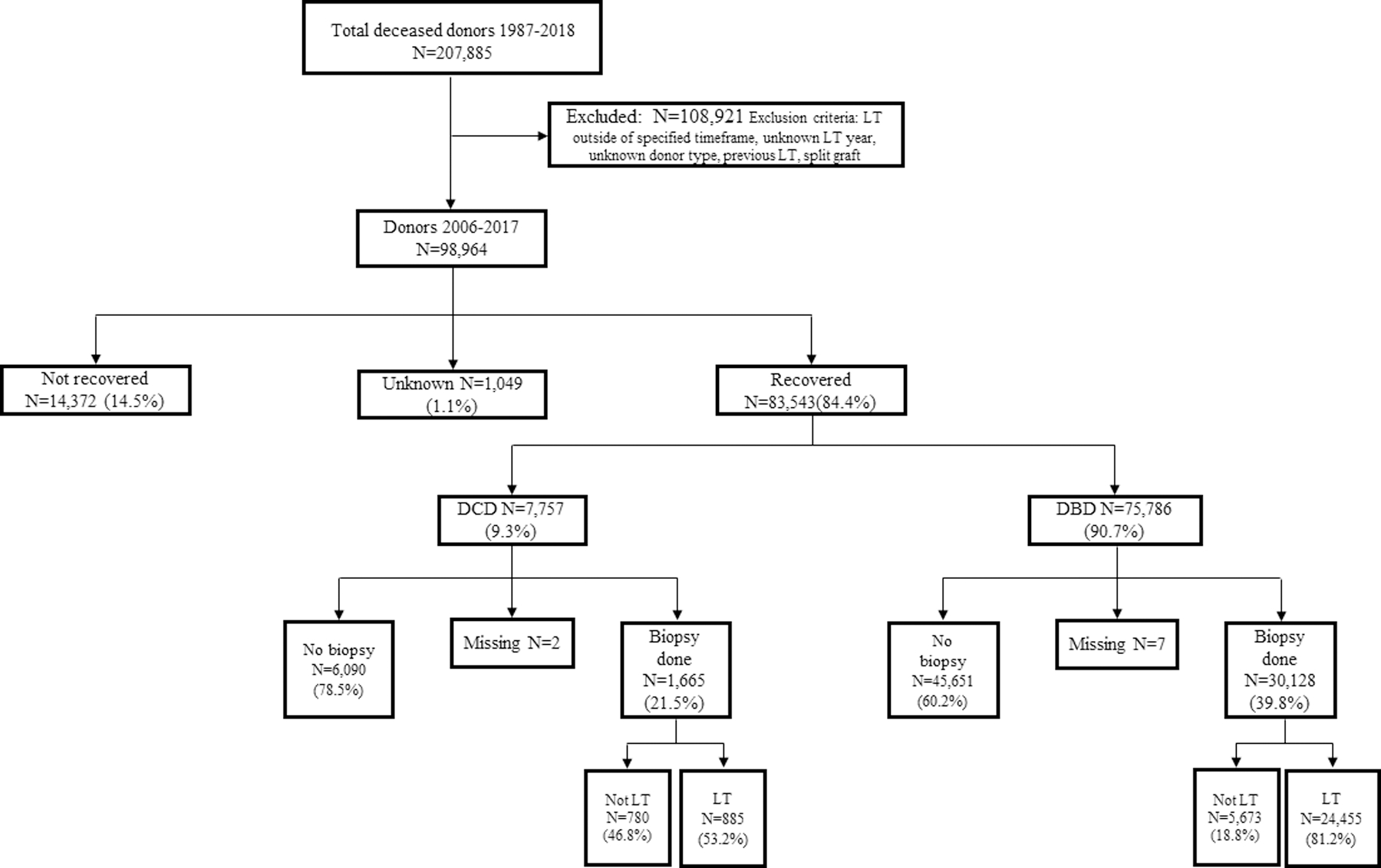
Study flowchart of the LT population. Donors were excluded if they were <18 years old or if the donor type was unknown. Recipients were excluded if they were <18 years old, underwent a previous LT, or received a split-liver graft.
Data collection and outcomes
Primary outcomes included graft failure and overall patient survival. Graft failure was defined as re-transplant or patient death. Donor characteristics including age, race, gender, body mass index (BMI), and diabetes mellitus were collected. Recipient age, race, gender, BMI, model for end-stage liver disease (MELD) score at waitlist removal, and cause of liver disease were analyzed. Liver graft data on cold ischemia time (CIT), warm ischemia time (WIT), MiS, and MaS were collected. MiS was categorized as 0–10% (MiS ≤10) and >10% (MiS >10). MaS was defined as 0–15% (MaS ≤15) and >15% (MaS >15). Based on the validation of the UK DCD Risk Score,11 we utilized variables from that analysis in our multivariable analysis when assessing the impact of MaS and MiS on LT outcomes. These variables include donor age >60 years, donor BMI >25, recipient age >60 years, MELD at waitlist removal, CIT, and donor WIT, which have been established as well-known risk factors for graft loss and/or patient death.11 Transplant center was added as a variable to multivariable analyses in order to control for a center specific effect. Donor WIT was later excluded from the model due to >37% values missing.
Statistical analysis
Due to the historically conservative approach of transplant surgeons accepting and transplanting DCD livers with minimal amounts of steatosis, there were a limited number of livers with high percentages of steatosis in the dataset. Therefore, MiS and MaS categories were selected based on cut-points that made sense from a clinical perspective and also allow sufficient power for determining whether there were survival differences between the categories. Differences between steatosis groups were assessed with ANOVA for continuous variables and Fisher’s exact tests for nominal variables. Continuous variables are presented as the mean and standard deviation of non-missing observations. The methods of Kaplan and Meier were employed to estimate the incidence of graft survival and patient survival. Rates were compared between steatosis groups using log-rank tests. We examined the association between MaS and MiS and found a significant correlation (p<0.0001); therefore, we did not combine MaS and MiS in the same multivariable analyses. Multivariable analyses were carried out using Cox proportional hazards regression models. All statistical analyses were performed using IBM SPSS Statistics Version 25. P-values less than 0.05 were considered to be statistically significant.
Results
We identified 98,964 adult (age ≥18 years) deceased donors from January 2006 to December 2017. Of the 83,543 recovered livers, 9.3% (N=7,757) of livers were from DCD donors. Pre-transplant biopsies were performed in 21.5% (N=1,665) of recovered DCD livers with 53.2% (N=885) of biopsied DCD livers eventually being transplanted. By comparison, pre-transplant biopsies were performed in 39.8% (N=30,128) of recovered DBD livers. The majority of biopsied DBD livers were transplanted (81.2%; N=24,455) (Figure 1).
Demographics
Demographic data and baseline characteristics for included DCD donors, transplant recipients, and graft variables are presented in Tables 1–2. The two most common causes of liver disease were non-cholestatic cirrhosis, 60.2% (N=533) and malignant neoplasms, 30.2% (N=267). The majority of recipients were male (73.1%, N=597) with mean age 56.4 ± 9.2 years at the time of transplant. Mean MELD at wait-list removal was 18.6 ± 8.5. Mean donor age was 38.9 ± 12.8 years with most donors being male at 65.1% (N=576). Diabetes mellitus was previously diagnosed in 8.1% (N=71) of donors. MaS >15 was present in 8.8% (N=72) and MiS >10 was present in 14.8% (N=112) of DCD livers. Mean donor warm ischemia time was 16.5 ± 8.2 minutes and mean cold ischemic time was 6.7 ± 3.0 hours.
Table 1.
Baseline characteristics of donors
| Variable | N=885 | Mean ± SD | % | Missing |
|---|---|---|---|---|
|
| ||||
| Age (years) | 885 | 38.9 ± 12.8 | -- | 0 |
|
| ||||
| Race | ||||
| • White | 744 | -- | 84.1 | 0 |
| • Black | 75 | 8.5 | ||
| • Other | 66 | 7.5 | ||
|
| ||||
| Gender | ||||
| • Male | 576 | -- | 65.1 | 0 |
| • Female | 309 | 34.9 | ||
|
| ||||
| Body mass index (kg/m2) | 880 | 28.4 ± 6.4 | -- | 5 |
|
| ||||
| Diabetes mellitus | 71 | -- | 8.1 | 5 |
|
| ||||
| Macrosteatosis (MaS) | ||||
| • 0–15% | 747 | 91.2 | ||
| • 16–30% | 50 | -- | 6.1 | 66 |
| • 31–40% | 14 | 1.7 | ||
| • >40% | 8 | 1.0 | ||
|
| ||||
| Microsteatosis (MiS) | ||||
| • 0–10% | 645 | 85.2 | ||
| • 11–30% | 72 | -- | 9.5 | 128 |
| • 31–40% | 11 | 1.5 | ||
| • >40% | 29 | 3.8 | ||
|
| ||||
| MaS + MiS | ||||
| • 0–15% MaS + 0–10% MiS | 560 | 80.6 | ||
| • 0–15% MaS + >10% MiS | 76 | -- | 10.9 | 190 |
| • >15% MaS + 0–10% MiS | 30 | 4.3 | ||
| • >15% MaS + >10% MiS | 29 | 4.2 | ||
Table 2.
Baseline characteristics of recipients
| Variable | N=885 | Mean ± SD | % | Missing |
|---|---|---|---|---|
|
| ||||
| Age (years) | 817 | 56.4 ± 9.2 | -- | 68 |
|
| ||||
| Race | ||||
| • White | 628 | -- | 76.9 | 68 |
| • Black | 69 | 8.4 | ||
| • Other | 120 | 14.7 | ||
|
| ||||
| Gender | ||||
| • Male | 597 | -- | 73.1 | 68 |
| • Female | 220 | 26.1 | ||
|
| ||||
| Body mass index (kg/m2) | 816 | 29.0 ± 5.7 | -- | 69 |
|
| ||||
| MELD | 816 | 18.6 ± 8.5 | -- | 69 |
|
| ||||
| Cause of disease | ||||
| • Non-cholestatic cirrhosis | 533 | -- | 60.2 | |
| • Cholestatic liver disease/cirrhosis | 35 | -- | 4.0 | |
| • Biliary atresia | 1 | -- | 0.1 | 0 |
| • Acute hepatic necrosis | 18 | -- | 2.0 | |
| • Metabolic disease | 18 | -- | 2.0 | |
| • Malignant neoplasms | 267 | -- | 30.2 | |
| • Other | 13 | -- | 1.6 | |
|
| ||||
| Cold ischemia time (hours) | 805 | 6.7 ± 3.0 | -- | 80 |
|
| ||||
| Warm ischemia time (minutes) | 554 | 16.5 ± 8.2 | -- | 331 |
DCD livers with MaS >15% have inferior patient survival
Patients who received DCD livers with MaS >15 did not have inferior graft survival compared to recipients of livers with MaS ≤15 based on unadjusted analysis (Figure 2A). Median graft survival time for MaS ≤15, and MaS >15 were 9.7 and 6.3 years, respectively. However, patients receiving livers with MaS >15 had significantly worse mortality rates (p<0.04) (Figure 2B). Median patient survival was 11.6 years in the MaS ≤15 group versus 6.8 years in the MiS >15 group. This difference in patient survival became most notable 4 years post-transplant.
Fig 2:
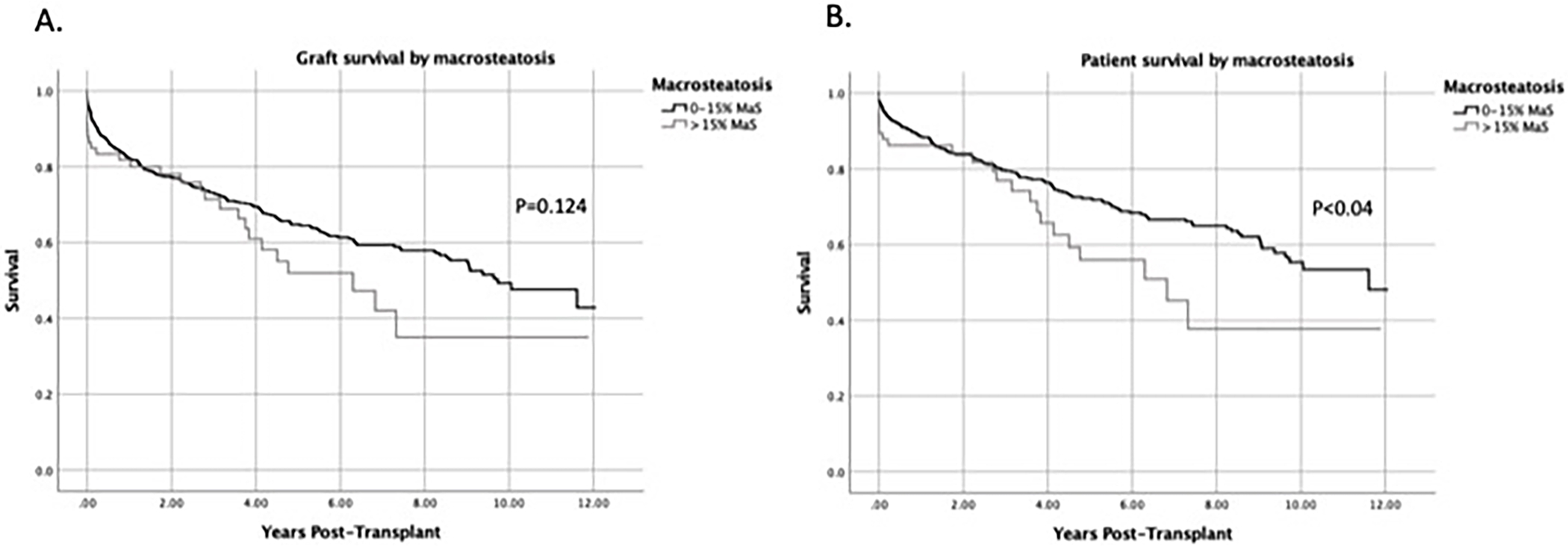
DCD livers with MaS >15% had inferior patient survival compared with MaS ≤15%. Kaplan-Meier curves comparing (A) graft survival and (B) patient survival by MaS group.
MiS >10% was associated with inferior graft and patient survival
Recipients of livers with MiS >10 demonstrated significantly inferior graft survival (p<0.001), and patient survival rates (p<0.02) when compared to those receiving livers with MiS ≤10% (Figure 3). Median graft survival time more than doubled for grafts with MiS ≤10 at 11.6 years compared to 5.4 years in grafts with MiS >10. Median patient survival time for MiS ≤10 and MiS >10 was over 12.0 and 6.3 years, respectively.
Fig 3:
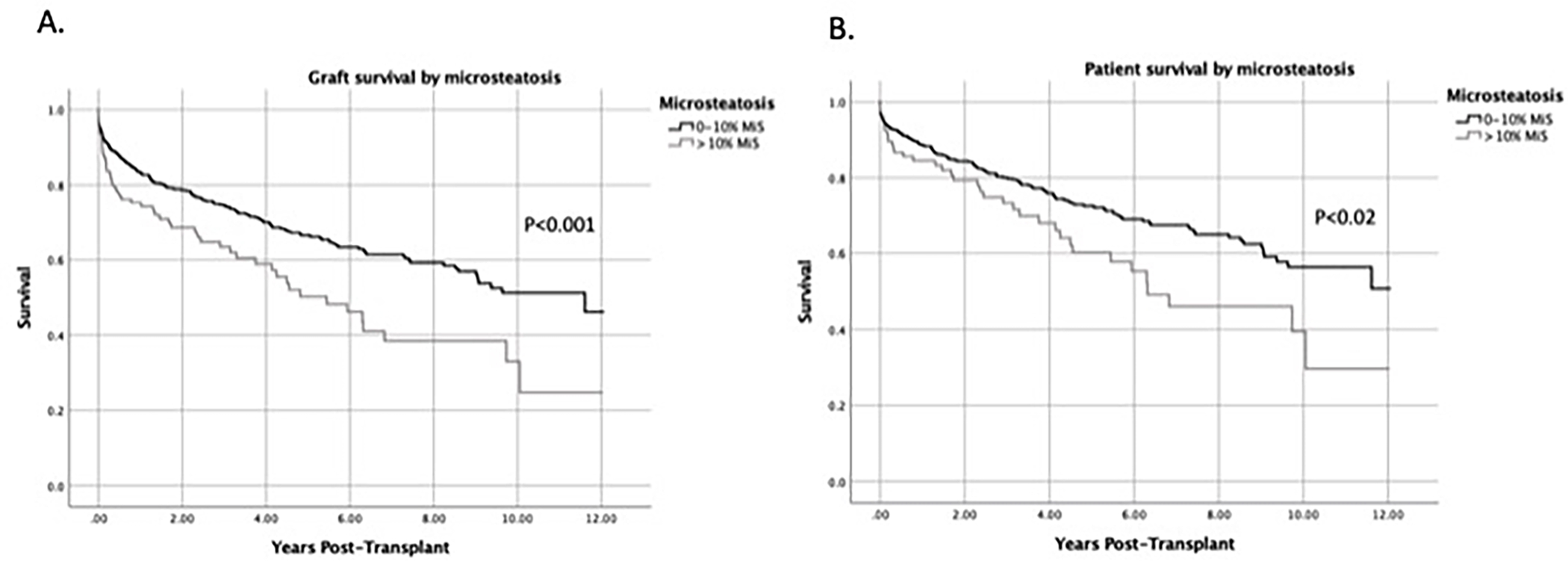
DCD livers with MiS >10% had significantly worse graft and patient survival compared with those with ≤10% MiS. Kaplan-Meier curves comparing (A) graft survival and (B) patient survival by MiS group.
Since a high percentage of MaS (>30%) could exert a confounding effect on survival when examining MiS groups, we also examined the impact of MiS on graft and patient survival when DCD livers with MaS >30% were removed from the analysis. After eliminating livers with MaS >30% from the analysis, DCD livers with MiS >10 still demonstrated significantly inferior graft (p<0.002) and patient survival rates (p=0.023) (Figure 4). Median graft survival time remained unchanged at 11.6 and 5.4 years for MiS ≤10 and MiS >10, respectively. Median patient survival time were 12.0 for MiS ≤10 and 6.8 years for MiS >10.
Fig 4 :
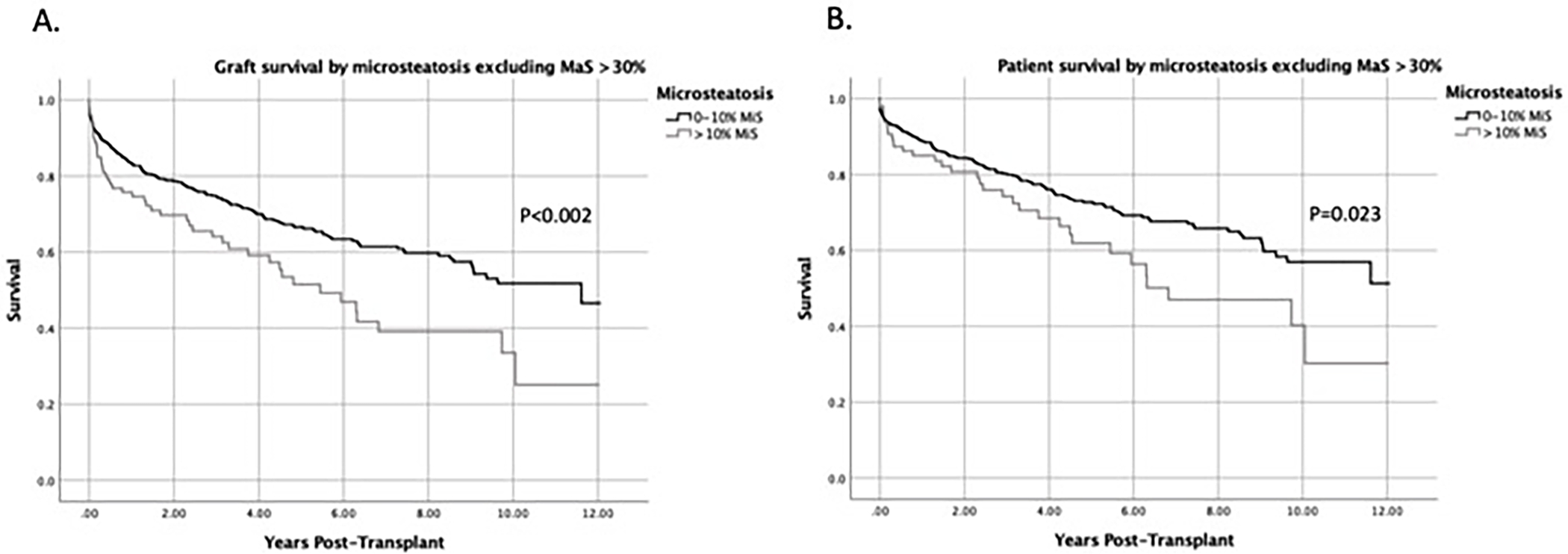
MiS >10% was still associated with worse graft and patient survival when livers with MaS >30% are excluded. Livers with MaS >30% were excluded to control for a confounding effect that MaS may have on groups with varying levels of MiS. Kaplan-Meier curves comparing (A) graft survival and (B) patient survival by MiS group when excluding livers with MaS >30%.
Patients who received livers with MiS >10 had significantly increased graft failure and mortality rates when MaS was ≤15
MiS and MaS are frequently seen in donor livers simultaneously and, therefore, do not usually exist independently. Therefore, we examined the effect of differential MiS levels when MaS severity was constant. We combined groups into the following combinations to evaluate graft and patient survival: (1) MaS ≤15 + MiS ≤10 (N=560); (2) MaS ≤15 + MiS >10 (N=76); (3) MaS >15 + MiS ≤10 (N=30); (4) MaS >15 + MiS >10 (N=29). To control for MaS when MiS varied, we compared Group (1) to Group (2) and Group (3) to Group (4). When MaS was ≤15, DCD livers with MiS >10 (Group 2) demonstrated significantly lower graft and patient survival rates compared to those with MiS ≤10 (Group 1) (p<0.03) (Figures 5A and 5B). Median graft survival time for Group 1 was 11.6 years compared to 4.8 years for Group 2 (p=0.002). Similarly, median patient survival time was 12 years in Group 1 vs. 6.3 years in Group 2.
Fig 5:
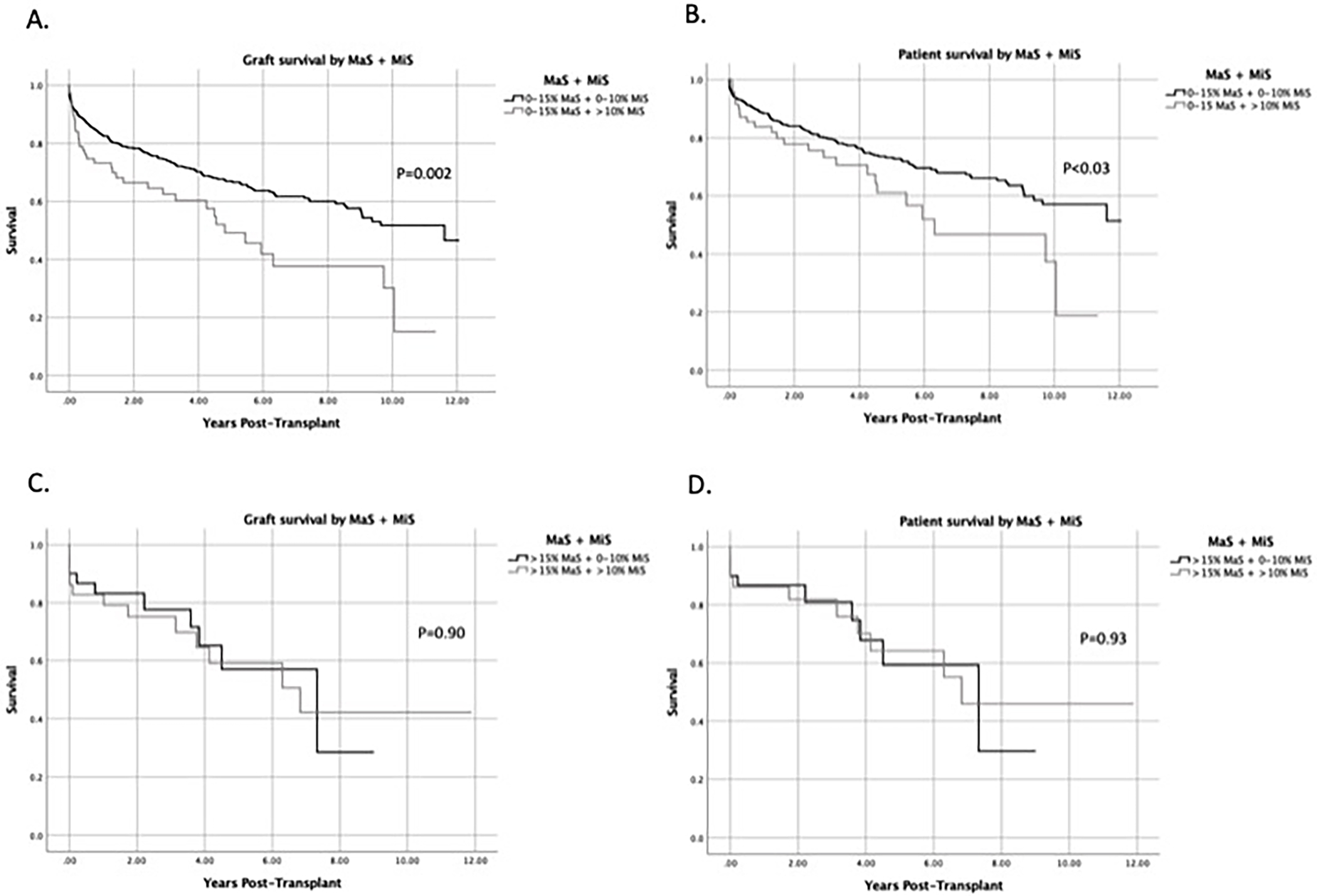
MiS >10% results in inferior graft and patient survival when MaS is ≤15%. MaS and MiS were analyzed together to determine if MiS at varying levels had an effect on outcomes when MaS was constant. Kaplan-Meier curves comparing (A) graft survival and (B) patient survival when MaS is ≤15% and MiS is ≤10% or >10%. (C) Graft survival and (D) patient survival when MaS is >15% and MiS is ≤10% or >10%.
When MaS >15, there were no significant differences in patient and graft survival rates when MiS levels were varied. (Figures 5C and 5D). Median graft and patient survival time was longer in the MaS >15 + MiS ≤10 group (Group 3) at 7.3 years compared to 6.8 years in the MaS >15 + MiS >10 (group 4); These findings demonstrate that when the percentage of MaS is low (≤15%), the level of MiS >10 has a significant impact on patient and graft survival. However, when MaS severity is >15, the negative effect of MiS is no longer present.
MaS >15 and MiS >10 are associated with increased risk of graft failure and patient death in adjusted analysis
When adjusting for other variables including recipient and donor age, MELD, CIT, and donor BMI, we found that MaS >15 (HR 1.7; 95% CI 1.1–2.6; p<0.03) and donor age >60 years (HR 2.3; 95% CI 1.0–5.2; p<0.04) were significantly associated with an increased risk of graft failure (Table 3). In a separate analysis assessing the impact on patient survival, MaS >15 (HR 1.9; 95% CI 1.2–3.2; p<0.01), recipient age >60 years (HR 1.8; 95% CI 1.3–2.5; p<0.001), and donor age >60 years (HR 3.3; 95% CI 1.4–7.6; p<0.01) were significantly associated with increased risk of patient death (Table 4).
Table 3.
Multivariate analysis for graft failure by macrosteatosis group
| Variable | Multivariate analysis |
|
|---|---|---|
| HR (95% CI) | P value | |
|
| ||
| Macrosteatosis | ||
| • 0–15% | 1 | 0.03 |
| • >15% | 1.7 (1.1–2.6) | |
|
| ||
| Recipient age | ||
| • ≤60 years | 1 | 0.06 |
| • >60 years | 1.3 (0.99–1.8) | |
|
| ||
| Donor age | ||
| • ≤60 years | 1 | 0.04 |
| • >60 years | 2.3 (1.0–5.2) | |
|
| ||
| MELD | ||
| • ≤25 | 1 | 0.20 |
| • >25 | 1.3 (0.9–1.8) | |
|
| ||
| Cold ischemia time | ||
| • ≤6 hours | 1 | 0.13 |
| • >6 hours | 1.3 (0.9–1.8) | |
|
| ||
| Donor BMI | ||
| • ≤25 | 1 | 0.70 |
| • >25 | 0.9 (0.7–1.3) | |
Table 4.
Multivariate analysis for patient survival by macrosteatosis group
| Variable | Multivariate analysis |
|
|---|---|---|
| HR (95% CI) | P value | |
|
| ||
| Macrosteatosis | ||
| • 0–15% | 1 | 0.01 |
| • >15% | 1.9 (1.2–3.2) | |
|
| ||
| Recipient age | ||
| • ≤60 years | 1 | 0.001 |
| • >60 years | 1.8 (1.3–2.5) | |
|
| ||
| Donor age | ||
| • ≤60 years | 1 | 0.01 |
| • >60 years | 3.3 (1.4–7.6) | |
|
| ||
| MELD | ||
| • ≤25 | 1 | 0.08 |
| • >25 | 1.5 (0.96–2.2) | |
|
| ||
| Cold ischemia time | ||
| • ≤6 hours | 1 | 0.99 |
| • >6 hours | 1.0 (0.7–1.5) | |
|
| ||
| Donor BMI | ||
| • ≤25 | 1 | 0.66 |
| • >25 | 0.9 (0.7–1.3) | |
When MiS was added to the multivariable analysis and MaS was removed, MiS >10 carried a 1.8 fold increased risk of graft failure (HR 1.8; 95% CI 1.3–2.6; p<0.001). Recipient age >60 years (HR 1.4; 95% CI 1.1–2.0; p<0.02) and donor age >60 years (HR 2.7; 95% CI 1.0–7.0; p<0.05) were also associated with increased risk of graft loss (Table 5). Both MiS >10 (HR 1.6; 95% CI 1.0–2.4; p<0.03), recipient age >60 years (HR 2.0; 95% CI 1.4–2.8; p<0.0001), and donor age (HR 3.4; 95% CI 1.3–9.2; p<0.02) were significantly associated with increased risk of patient death (Table 6).
Table 5.
Multivariate analysis for graft survival by microsteatosis group
| Variable | Multivariate analysis |
|
|---|---|---|
| HR (95% CI) | P value | |
|
| ||
| Microsteatosis | ||
| • 0–10% | 1 | 0.001 |
| • >10% | 1.8 (1.3–2.6) | |
|
| ||
| Recipient age | ||
| • ≤60 years | 1 | 0.02 |
| • >60 years | 1.4 (1.1–2.0) | |
|
| ||
| Donor age | ||
| • ≤60 years | 1 | 0.05 |
| • >60 years | 2.7 (1.0–7.0) | |
|
| ||
| MELD | ||
| • ≤25 | 1 | 0.38 |
| • >25 | 1.2 (0.8–1.8) | |
|
| ||
| Cold ischemia time | ||
| • ≤6 hours | 1 | 0.35 |
| • >6 hours | 1.2 (0.8–1.7) | |
|
| ||
| Donor BMI | ||
| • ≤25 | 1 | 0.88 |
| • >25 | 1.0 (0.7–1.3) | |
Table 6.
Multivariate analysis for patient survival by microsteatosis group
| Variable | Multivariate analysis |
|
|---|---|---|
| HR (95% CI) | P value | |
|
| ||
| Microsteatosis | ||
| • 0–10% | 1 | 0.03 |
| • >10% | 1.6 (1.0–2.4) | |
|
| ||
| Recipient age | ||
| • ≤60 years | 1 | 0.0001 |
| • >60 years | 2.0 (1.4–2.8) | |
|
| ||
| Donor age | ||
| • ≤60 years | 1 | 0.02 |
| • >60 years | 3.4 (1.3–9.2) | |
|
| ||
| MELD | ||
| • ≤25 | 1 | 0.14 |
| • >25 | 1.4 (0.9–2.2) | |
|
| ||
| Cold ischemia time | ||
| • ≤6 hours | 1 | 0.75 |
| • >6 hours | 0.9 (0.6–1.4) | |
|
| ||
| Donor BMI | ||
| • ≤25 | 1 | 0.57 |
| • >25 | 0.9 (0.6–1.3) | |
Discussion
Steatosis is currently recognized as exerting a significant impact on recipient outcomes in DBD liver transplant.15,19 Risk factors for poor outcomes have been identified in DCD liver transplant; however, “safe” steatosis levels have not been established.11 With the current knowledge gap on acceptable levels of steatosis in DCD livers, transplant surgeons are left to make decisions based on clinical experience, which may potentially result in unnecessary liver discards. Consequently, we sought out to determine the effect of varying levels of MiS and MaS on outcomes in DCD liver transplantation. Using the national UNOS database, we identified 885 DCD livers that were biopsied and subsequently transplanted. In order to have a sufficient statistical power for the analyses, the number of patients in each of the groups was evaluated when determining the appropriate cutoff for MaS and MiS. The cut-offs of MaS at 15% and MiS at 10% were used. MaS in DCD livers that were transplanted was low overall with 91.3% of livers having MaS ≤15. This likely reflects the conservative acceptance practice patterns of liver transplant surgeons with DCD livers that contain high levels of MaS between 2006 and 2017. For that reason, we did not identify MaS >15 as being a significant risk of allograft failure in unadjusted analyses. However, MaS >15 was associated with increased risk of graft failure and patient death in adjusted analyses, which further supports the validity of data presented here. We still suspect that higher levels of MaS are likely detrimental to graft survival. However, based on the low numbers of DCD livers transplanted with high MaS in this analysis, we were unable to identify a true MaS cut off for graft failure risk. In contrast, we did demonstrate an increased risk of mortality in recipients receiving livers with MaS >15 compared to those receiving livers with MaS ≤15 in the adjusted analysis.
Possibly the most interesting and novel finding in our analysis was the negative impact of MiS on outcomes after DCD LT. Aside from a recent study demonstrating the negative impact of MiS on a large cohort of DBD and smaller cohort of DCD LT recipients, MiS levels have not been routinely considered a significant risk factor for poor outcomes after DBD LT.16 In this analysis DCD livers with MiS >10 demonstrated significantly inferior graft and patient survival rates compared to those with MiS ≤10 in both unadjusted and adjusted analyses. These findings persisted when we excluded those patients that had MaS >30%. Therefore, the differences could not be explained by a MaS level >30% in livers with MiS >10.
The impact of MiS >10 was also significant when we performed subgroup analyses. In livers with MaS ≤15, MiS >10 was a significant risk factor for graft loss and death when compared to MiS ≤10 in livers with MaS ≤15. However, the impact of MiS >10 was no longer statistically significant when the MaS levels were >15. Therefore, MiS >10 appears to have a stronger impact in livers with MaS ≤15. While our goal initially was to determine a “safe” level of MaS, we found that both MaS and MiS have a significant impact on patient outcomes.
The mechanisms that result in MaS and MiS are important to understand because they better characterize underlying liver disease and how this may impact liver function after transplantation. Impaired mitochondrial β-oxidation of natural fatty acids results in MiS and is associated with toxins and/or metabolic disorders. Extensive MiS may result in death in the short-term as it rapidly evolves into liver failure, coma and death.20 On the other hand, MaS, frequently associated with alcohol, diabetes mellitus, and obesity, can lead to death in the long-term due to fatal cirrhosis.20 Severe inhibition of mitochondrial β-oxidation results in decreased energy production, which in turn affects multiple organ systems including the liver and heart, which rely on β-oxidation during fasting states. Lack of energy production can result in liver failure, cardiac arrhythmias, cardiomyopathy, brain edema, coma and death. While MiS itself is not the cause of multi-organ failure, its presence may indicate underlying mitochondrial dysfunction that may get further exacerbated during the DCD donor process coupled with ischemia reperfusion injury in the recipient. In addition, a severe underlying metabolic impairment may exist and therefore lead to poor prognosis after liver transplantation.21,22 Additional metabolic and mitochondrial analyses are needed to elucidate the true mechanisms underlying the negative impact of MiS on DCD liver allograft function.
Steatotic livers have an increased vulnerability to warm ischemia and preservation injury, which is one explanation for the inferior outcomes seen in DCD livers with relatively low levels of MaS and MiS.17 Prolonged warm ischemia is thought to inflict more damage to steatotic livers compared to non-steatotic livers, suggesting that higher levels of steatosis are less likely to be tolerated. Xia et al. evaluated the clinical impact of steatosis in DCD liver transplant and found MaS ≥20% was a risk factor for poor graft survival.17 This single center retrospective study is the only known study that has examined steatosis exclusively in DCD livers. Although this study did not evaluate MiS, their finding of MaS ≥20% as a risk factor for graft loss is consistent with our findings using a national database. Croome et al. examined the impact of MiS in a single-center propensity score matched cohort of 462 DBD and 34 DCD liver transplant recipients. In this study, patients who underwent LT with isolated MiS ≥30% had a higher rate of post-reperfusion syndrome (33.1% vs. 24.2%), early allograft dysfunction (38.2% vs. 22.2%), and renal replacement therapy (10.9% vs. 3.6%) following LT compared to those with no steatosis. In the controlled analysis, livers with MiS ≥30% had an increased risk of graft loss with re-transplant recipients, increased MELD score, and when from DCD donors.16 This study further supports the concept that MiS is an additional risk factor for inferior graft outcomes that should be taken into consideration when accepting livers for transplantation. However, this study has limitations including being a single-center study and the cohort included only 34 DCD donor livers.
Although MiS is largely considered a mild, reversible condition, reports have suggested that MiS does place livers at increased risk for early hepatic dysfunction.23–25 This effect would likely be compounded in DCD livers due to the additional insult that occurs after warm ischemia. In a prospective series examining “predominant MaS” and “predominant MiS” in DBD liver transplant, groups with MiS >30% were found to have significantly worse graft survival and a trend towards inferior patient survival.26 While the MiS “cutoff” in our study was much lower, these findings by Noujaim et al. suggest that MiS does play a role in liver transplant outcomes. Another study evaluating the impact of hepatic MaS and MiS in living donor liver transplant found peak values of postoperative AST and ALT to be significantly higher in groups with either >30% MaS or MiS compared to groups with <5% MaS or MiS.21 Similarly, Han et al. found severe MiS (>66%) was associated with significantly worse early graft function and 1-year graft survival rates.27 Data from single center studies have suggested that MiS impacts outcomes in DBD transplant; however, the lack of multi-center data and prevailing belief that MiS is not relevant emphasizes the importance of future studies to examine the effect of MaS and MiS in DCD liver transplant.
Limitations
This study has limitations inherent to retrospective studies and the use of national transplant registry data as previously described.28 Despite the use of controlled analyses, it is impossible to control for factors that are not captured in the database such as practice patterns at various institutions. Additionally, there is some degree of selection bias due to not having biopsy data on all transplanted DCD livers. Baseline donor and recipient characteristics were comparable between livers with biopsy data available and biopsy data missing. Therefore, we do not suspect that our results presented here are confounded by the exclusion of livers with no biopsy data. Although bias cannot be entirely excluded, we performed multiple analyses to reduce this risk. Due to the fact that biopsies included in this study were performed and evaluated at primary- and tertiary-care hospitals, biopsies were likely read by pathologists with varying levels of experience with liver pathology. It is also possible that some other findings in the liver biopsies were missed due this variability in experience and may have impacted liver transplant outcomes among the groups. Finally, limitations associated with using biopsy data from the UNOS national database exist. These limitations primarily relate to lack of data regarding the size, type (i.e. core, wedge, needle), and quality of biopsy specimen.29 Due to missing data, we also had to exclude WIT from our multivariable model. Biopsy type is included in the UNOS database; however, this data was largely missing from DCD LT included in this study. Lastly, we are not able to control for selection bias of recipients for donor livers but by performing multivariable analyses controlling for quantitative factors we believe we have limited this bias as much as possible.
Conclusion
Our data shows that MaS >15% and MiS >10% are additional risk factors for graft loss and patient mortality in DCD liver transplantation. To the best of our knowledge, this is the first and largest study that has explored both MaS and MiS as risk factors in DCD liver transplant. The lack of previous studies in the literature emphasizes the novelty of our findings. Although we found that both MaS and MiS significantly impact graft and patient outcomes, it is still important to take into consideration all donor and recipient factors when identifying an appropriate organ for a patient. One should not decide on accepting a DCD liver based on the percentage of MaS or MiS alone. However, based on these data, the percentage of MaS and MiS in donor liver biopsies should be taken into consideration with other known risk factors when determining the suitability of a DCD liver for transplant. A better understanding of the role of steatosis in DCD liver transplant in the context of other risk factors will allow surgeons to make a more educated evaluation of an organ, which may result in increased utilization of DCD livers.
ACKNOWLEDGMENTS
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement of the U.S. Government.
FUNDING
The authors would like to thank the following group for grant support: National Institutes of Health, Training Grant (T32 AI125231).
ABBREVIATIONS
- BMI
body mass index
- CIT
cold ischemia time
- DBD
donation after brain death
- DCD
donation after circulatory death
- LT
liver transplant
- MaS
macrosteatosis
- MELD
model for end-stage liver disease
- MiS
microsteatosis
- OPTN
Organ Procurement and Transplantation Network
- UNOS
United Network for Organ Sharing
- WIT
warm ischemia time
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose.
REFERENCES
- 1.Selck FW, Grossman EB, Ratner LE, Renz JF. Utilization, outcomes, and retransplantation of liver allografts from donation after cardiac death: implications for further expansion of the deceased-donor pool. Annals of surgery. 2008;248(4):599–607. [DOI] [PubMed] [Google Scholar]
- 2.Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Foutz J, Miller E, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2018 Annual Data Report: Liver. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2020;20 Suppl s1:193–299. [DOI] [PubMed] [Google Scholar]
- 3.Washburn K, Pomfret E, Roberts J. Liver allocation and distribution: possible next steps. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2011;17(9):1005–1012. [DOI] [PubMed] [Google Scholar]
- 4.Orman ES, Barritt ASt, Wheeler SB, Hayashi PH. Declining liver utilization for transplantation in the United States and the impact of donation after cardiac death. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013;19(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley DP, Fernandez LA, Leverson G, Chin LT, Krieger N, Cooper JT, Shames BD, Becker YT, Odorico JS, Knechtle SJ, Sollinger HW, Kalayoglu M, D’Alessandro AM. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Annals of surgery. 2005;242(5):724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croome KP, Lee DD, Keaveny AP, Taner CB. Improving National Results in Liver Transplantation Using Grafts From Donation After Cardiac Death Donors. Transplantation. 2016;100(12):2640–2647. [DOI] [PubMed] [Google Scholar]
- 7.Pitarch Martinez M, Sanchez Perez B, Leon Diaz FJ, Fernandez Aguilar JL, Perez Daga JA, Montiel Casado MC, Aranda Narvaez JM, Suarez Munoz MA, Santoyo Santoyo J. Donation After Cardiac Death in Liver Transplantation: An Additional Source of Organs With Similar Results to Donation After Brain Death. Transplantation proceedings. 2019;51(1):4–8. [DOI] [PubMed] [Google Scholar]
- 8.Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, D’Alessandro A. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Annals of surgery. 2011;253(4):817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grewal HP, Willingham DL, Nguyen J, Hewitt WR, Taner BC, Cornell D, Rosser BG, Keaveny AP, Aranda-Michel J, Satyanarayana R, Harnois D, Dickson RC, Kramer DJ, Hughes CB. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2009;15(9):1028–1035. [DOI] [PubMed] [Google Scholar]
- 10.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(4):783–790. [DOI] [PubMed] [Google Scholar]
- 11.Schlegel A, Kalisvaart M, Scalera I, Laing RW, Mergental H, Mirza DF, Perera T, Isaac J, Dutkowski P, Muiesan P. The UK DCD Risk Score: A new proposal to define futility in donation-after-circulatory-death liver transplantation. Journal of hepatology. 2018;68(3):456–464. [DOI] [PubMed] [Google Scholar]
- 12.Linares I, Hamar M, Selzner N, Selzner M. Steatosis in Liver Transplantation: Current Limitations and Future Strategies. Transplantation. 2019;103(1):78–90. [DOI] [PubMed] [Google Scholar]
- 13.Sherif ZA, Saeed A, Ghavimi S, Nouraie SM, Laiyemo AO, Brim H, Ashktorab H. Global Epidemiology of Nonalcoholic Fatty Liver Disease and Perspectives on US Minority Populations. Digestive diseases and sciences. 2016;61(5):1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamboni F, Franchello A, David E, Rocca G, Ricchiuti A, Lavezzo B, Rizzetto M, Salizzoni M. Effect of macrovescicular steatosis and other donor and recipient characteristics on the outcome of liver transplantation. Clinical transplantation. 2001;15(1):53–57. [DOI] [PubMed] [Google Scholar]
- 15.McCormack L, Dutkowski P, El-Badry AM, Clavien PA. Liver transplantation using fatty livers: always feasible? Journal of hepatology. 2011;54(5):1055–1062. [DOI] [PubMed] [Google Scholar]
- 16.Croome KP, Lee DD, Croome S, Nakhleh RE, Abader Sedki Senada P, Livingston D, Yataco M, Taner CB. Does donor allograft Microsteatosis Matter? comparison of outcomes in liver transplantation with a propensity matched cohort. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2019. [DOI] [PubMed] [Google Scholar]
- 17.Xia W, Ke Q, Wang Y, Feng X, Guo H, Wang W, Zhang M, Shen Y, Wu J, Xu X, Yan S, Zheng S. Donation after cardiac death liver transplantation: Graft quality evaluation based on pretransplant liver biopsy. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2015;21(6):838–846. [DOI] [PubMed] [Google Scholar]
- 18.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, Snyder JJ, Kasiske BL. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando). 2013;27(2):50–56. [DOI] [PubMed] [Google Scholar]
- 19.Deschenes M Early allograft dysfunction: causes, recognition, and management. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013;19 Suppl 2:S6–8. [DOI] [PubMed] [Google Scholar]
- 20.Fromenty B, Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacology & Therapeutics. 1995;67(1):101–154. [DOI] [PubMed] [Google Scholar]
- 21.Shin YH, Ko JS, Kim GS, Gwak MS, Sim WS, Lee AR, Yi HW, Joh JW. Impact of hepatic macrovesicular and microvesicular steatosis on the postoperative liver functions after right hepatectomy in living donors. Transplantation proceedings. 2012;44(2):512–515. [DOI] [PubMed] [Google Scholar]
- 22.Fromenty B, Berson A, Pessayre D. Microvesicular steatosis and steatohepatitis: role of mitochondrial dysfunction and lipid peroxidation. Journal of hepatology. 1997;26 Suppl 1:13–22. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology (Baltimore, Md). 1999;29(3):664–669. [DOI] [PubMed] [Google Scholar]
- 24.Halon A, Patrzalek D, Rabczynski J. Hepatic steatosis in liver transplant donors: rare phenomenon or common feature of donor population? Transplantation proceedings. 2006;38(1):193–195. [DOI] [PubMed] [Google Scholar]
- 25.Cieslak B, Lewandowski Z, Urban M, Ziarkiewicz-Wroblewska B, Krawczyk M. Microvesicular liver graft steatosis as a risk factor of initial poor function in relation to suboptimal donor parameters. Transplantation proceedings. 2009;41(8):2985–2988. [DOI] [PubMed] [Google Scholar]
- 26.Noujaim HM, de Ville de Goyet J, Montero EF, Ribeiro CM, Capellozzi VL, Crescentini F, Casagrande M, Santos RG, Curvello L, de Miranda MP, Genzini T. Expanding postmortem donor pool using steatotic liver grafts: a new look. Transplantation. 2009;87(6):919–925. [DOI] [PubMed] [Google Scholar]
- 27.Yoong KF, Gunson BK, Neil DA, Mirza DF, Mayer AD, Buckels JA, McMaster P. Impact of donor liver microvesicular steatosis on the outcome of liver retransplantation. Transplantation proceedings. 1999;31(1–2):550–551. [DOI] [PubMed] [Google Scholar]
- 28.Ravindra KV, Sanoff S, Vikraman D, Zaaroura A, Nanavati A, Sudan D, Irish W. Lymphocyte depletion and risk of acute rejection in renal transplant recipients at increased risk for delayed graft function. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2019;19(3):781–789. [DOI] [PubMed] [Google Scholar]
- 29.Bajwa M, Cho YW, Pham PT, Shah T, Danovitch G, Wilkinson A, Bunnapradist S. Donor biopsy and kidney transplant outcomes: an analysis using the Organ Procurement and Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) database. Transplantation. 2007;84(11):1399–1405. [DOI] [PubMed] [Google Scholar]


