Abstract
OBJECTIVES
Patient-reported quality of life (QOL) has become an important endpoint for arrhythmia surgery for atrial fibrillation (AF). While studies specifically evaluating the effect of arrhythmia surgery on QOL are scarce, we aimed to summarize current evidence of QOL following concomitant and stand-alone arrhythmia surgery for AF.
METHODS
All studies reporting on QOL using questionnaires from patients undergoing arrhythmia surgery for AF, both stand-alone and concomitant, were included in this systematic review. A meta-analysis was performed on inter-study heterogeneity of changes in QOL on 9 of 12 included studies that used the Short-Form 36 tool and meta-regression based on rhythm outcome after 1 year was executed. Finally, differences in QOL following stand-alone arrhythmia surgery and concomitant procedures were evaluated.
RESULTS
Overall, QOL scores improved 1 year after surgical ablation for AF evaluated by several questionnaires. In stand-alone arrhythmia procedures, meta-regression showed significant improvements in those who were in sinus rhythm compared to those in AF after 1 year. This association between an improved QOL and the procedural effectiveness was also suggested in concomitant procedures. However, when comparing QOL of patients undergoing cardiac surgery with and without add-on surgical ablation for AF, only the variable ‘physical role’ demonstrated a significant improvement.
CONCLUSIONS
In patients with AF, QOL improves after both stand-alone and concomitant arrhythmia surgery. In the concomitant group, this improvement can be attributed to both the cardiac procedure itself as well as the add-on arrhythmia surgery. However, both in stand-alone and concomitant procedures, the improvement in QOL seems to be related to the effectiveness of the procedure to maintain sinus rhythm after 12 months.
Keywords: Quality of life, Surgical arrhythmia ablation, Atrial fibrillation, Systematic review and meta-analysis
Historically, the emergence of a surgical treatment for heart rhythm disorders was mainly triggered by ventricular arrhythmias and with the first successful surgical interruption of the bundle of Kent in a patient with the Wolff–Parkinson–White syndrome [1], arrhythmia surgery got off to a great start.
INTRODUCTION
Historically, the emergence of a surgical treatment for heart rhythm disorders was mainly triggered by ventricular arrhythmias and with the first successful surgical interruption of the bundle of Kent in a patient with the Wolff–Parkinson–White syndrome [1], arrhythmia surgery got off to a great start. Notwithstanding the above, today most surgical arrhythmia procedures are focused on the management of supraventricular arrhythmias [2]. Surgical ablation of atrial fibrillation (AF) can be either done in conjunction with other cardiac procedures as a concomitant procedure or on itself as a stand-alone procedure. Concomitant AF surgery is often performed with cardiopulmonary bypass via sternotomy or right anterolateral mini-thoracotomy, but recently also left thoracoscopic ablation in combination with minimally invasive direct coronary artery bypassing on the beating heart has been reported [3, 4]. Although stand-alone procedures are often performed via bilateral thoracoscopy, unilateral thoracoscopic and subxiphoid techniques have been successfully introduced [5–7]. This progression in minimally invasiveness of surgical ablation approaches is important as it can be expected that the reduction in complications and postoperative pain by limiting surgery to one side will lead to further improvement in quality of life (QOL).
Although 1-year success of arrhythmia surgery for AF has long been defined as freedom from any supraventricular tachyarrhythmia, the evaluation of other endpoints, such as patient-reported QOL, has become increasingly important in recent years [8]. Despite the fact that the measurement of QOL is potentially limited by a treatment expectancy bias, it represents an important endpoint for ablation studies [9]. Be that as it may, studies specifically evaluating the effect of stand-alone or add-on arrhythmia surgery on QOL are scarce. Moreover, the reported outcomes are often heterogeneous as not all studies use the same ablation strategy to treat the arrhythmia.
In this systematic review and meta-analysis, we summarized current evidence on QOL at baseline and 1 year after both stand-alone and concomitant arrhythmia surgery for AF. Since the guidelines for AF define the success of rhythm outcome after surgical ablation for AF after 1 year, we chose to evaluate the improvement in QOL as well after 1 year, along with the rhythm outcome.
PATIENTS AND METHODS
Literature search
This systematic review and meta-analysis were written according to PRISMA standards [10]. A systematic literature search was conducted with free terms in the PubMed and Cochrane databases (see Supplementary Material). Forwards and backwards search were also performed to screen for further eligible papers.
Study selection and risk of bias
All identified studies were screened based on their title and abstract, and full text when necessary, by 2 independent reviewers (C.A.J.v.d.H. and B.M.). All English articles reporting on QOL using the Short-Form 36 (SF-36) questionnaire for measuring QOL after arrhythmia surgery in patients with AF, both stand-alone and concomitant, were found eligible. In all observational studies and non-randomized clinical trials, the methodological quality was assessed with use of the ROBINS-I tool [11]. In articles reporting on randomized controlled trials (RCTs), the risk of bias was assessed using the Cochrane Checklist [12].
Outcomes
The primary outcome was defined as the standardized mean difference in QOL variables assessed 1 year after arrhythmia surgery compared to baseline scores, using the SF-36 QOL questionnaire. As secondary endpoints, differences in the improvement of QOL between patients who were in sinus rhythm (SR) or in AF after 12 months of follow-up were determined for stand-alone procedures and differences between patients who did and did not receive add-on ablation for concomitant procedures.
Statistical analysis
The metric ‘standardized mean difference (μ)’ (rho = 0) was used to analyse continuous QOL changes, comparing 1 year outcomes with baseline scores, per variable of the SF-36 QOL questionnaire [13]. Additional meta-regression was performed using rhythm outcome and add-on arrhythmia surgery after 12 months of follow-up as covariate. All statistical values were computed with a 95% confidence interval in a random-effects model and the 2-tailed P-value threshold for statistical significance was set at 0.05.
Weighted means (µ) of continuous baseline characteristics were computed using the metric ‘TX Mean’, whereas ‘Untransformed Proportions’, defined as the count of successes in the sample divided by the size of that sample, were used for mean frequencies [14]. The latter metric was also used to analyse the percentage of patients that was in SR after 12 months of follow-up. Due to the relatively low complication rate, the metric ‘Freeman–Tukey Double Arcsine Proportion’ was used to analyse the incidence of perioperative complications following arrhythmia surgery.
Inter-study heterogeneity was tested and visualized in forest plots per variable of the SF-36 QOL questionnaire. A statistical P-value <0.10 and/or I2 >50% was used as cut-off point for significant heterogeneity. All statistical analyses were performed using Meta-Analyst for Mac software (2009) [14] (version Beta 1.0). Furthermore, publication bias was tested using funnel plots made in Excel, where the standardized mean difference was plotted against the standard error of that study. The variance was calculated after transforming Cohen’s d to Hedges’ g by correcting for sample size and standard deviation per study [15].
RESULTS
Study selection
After exclusion based on title, abstract and full-text reading, 9 out of 2142 studies from the literature search were included in our systematic review and meta-analysis (Supplementary Material, Fig S1 and Table S1) .
Risk of bias
The risk of bias in most of the RCTs was estimated to be medium to low, mostly due to unclear reporting of blinding of patients and/or researchers during follow-up [16, 17]. For the observational studies and non-randomized trials, risk of bias was estimated to be medium-high. Confounding due to missing baseline characteristics or marked differences in important predictors of the procedure’s success (e.g. type and duration of AF) between groups could not be ruled out in the studies by Joshibayev and Bolatbekov [18] and Lundberg et al. [19]. Selection bias based on the inclusion of patients with serious comorbidities was present in the study of Joshibayev and Bolatbekov [18]. Other factors contributing to the increased risk of bias were missing QOL data due to substantial loss of follow-up in the study by Bagge et al. [20] and the lack of continuous heart rhythm monitoring in the studies by Joshibayev and Bolatbekov [18] and Lönnerholm et al. [21].
Furthermore, funnel plots where the standardized mean difference was plotted against the standard error of Hedges’ g of that study showed that publication bias cannot be ruled out in this review. Due to the marked variance of the included studies, scattering of results unequally along the x-axis occurred. Moreover, the forest plots illustrated that statistical heterogeneity, and thus inter-study variance, per QOL variable measured by the SF-36 was marked.
Study population
Most studies reported on arrhythmia surgery performed in the Netherlands [16, 17, 22, 23], followed by Sweden [19–21], the UK [24] and Kazakhstan [18]. In total, 545 patients were included in the analysis (Table 1). Most patients were men (69.4%), mean age was 60 years, mean duration of AF was 53 months, 8.0% had a history with cerebrovascular accident and the mean left atrial (LA) diameter was 49.2 mm. Most patients had longstanding-persistent AF (41.9%), followed by persistent (29.8%) and paroxysmal AF (27.6%).
Table 1:
Baseline characteristics of studies reporting on cardiac arrhythmia surgery and quality of life using the Short-Form-36 questionnaire
| Characteristics (n = 545) | Number of patients: n (%) | Adjusted mean (95% CI) |
|---|---|---|
| Age (years) | 453 (83) | 59.8 years (56.5–63.0) |
| AF duration (months) | 316 (58) | 53.0 months (5.0–101.0) |
| CVA (%) | 466 (86) | 8.0% (5.6–10.5) |
| Female (%) | 545 (100) | 30.6% (23.6–37.6) |
| Hypertension (%) | 491 (90) | 32.7% (22.1–43.2) |
| LA diameter (mm) | 369 (68) | 49.2 mm (43.8–54.6) |
| LVEF (%) | 395 (72) | 52.3% (50.0–54.5) |
| Type of AF | ||
| Paroxysmal (%) | 520 (95) | 27.6% (12.5–42.8) |
| Persistent (%) | 520 (95) | 29.8% (11.8–47.9) |
| Longstanding-persistent (%) | 520 (95) | 41.9% (4.6–79.3) |
Data are presented as number of patients (n) and the percentage (%) of the total group at baseline. The adjusted means or proportions followed by the 95% confidence interval were calculated using the metric ‘TX Mean’ or ‘Untransformed Proportion’, respectively, in a binary random-effects model.
AF: atrial fibrillation; CVA: cerebrovascular accident; LA: left atrial; LVEF: left ventricular ejection fraction.
Arrhythmia surgery
The technique by which arrhythmia surgery was performed differed between the 12 studies (Table 2). In most of the studies, the LA appendage (LAA) was addressed, either by surgically excision, clipping or stapling. Furthermore, there were 3 studies that reported on thoracoscopic beating heart AF ablation [16, 17, 20]. One study reported on single-stage hybrid ablation [22]. Of the remaining studies, 5 reported on concomitant AF ablation, in most of them, a Cox-maze-III or –IV procedure was performed, and one study used an alternative overlapping pulmonary vein isolation technique. While different techniques were used, all studies performed pulmonary vein isolation with or without extra lesions (Supplementary Material, Table S2). Five studies ablated the roof and inferior lines as well to create the so-called ‘box lesion’, while van Breugel et al. [23] only added a roof line. Four studies ablated the right atrial (RA) free wall, a line to the mitral annulus and 3 ablated the posterior LA wall. Three studies ablated the connection between the superior and inferior caval vein and 2 ablated the coronary sinus and the tricuspid valve. Six studies ablated either an additional cavotricuspid isthmus line, complex fractionated atrial electrograms, ganglionated plexi or the ligament of Marshall, or performed a bi-atrial maze or LA reduction. These marked differences in techniques and lesion sets have led to marked clinical heterogeneity in this review and meta-analysis.
Table 2:
Surgical characteristics per study including type of cardiac surgery performed, left atrial appendage procedure, energy source and concomitant surgery
| Study | Arrhythmia surgery |
LAA | Energy source | Concomitant surgery | ||
|---|---|---|---|---|---|---|
| Minimally invasive (off-pump) | Hybrid | Cox-maze III/IV | ||||
| Al-Jazairi et al. [22] | Single stage | Occlusion (Atriclip 30%) | Bipolar RF | |||
| Bagge et al. [20] | Thoracoscopic | Excised (stapler, 76%) | Bipolar RF | |||
| Buist et al. [16] | Thoracoscopic | Ligation (endoloop, 100%) | Bipolar RF | |||
| Driessen et al. [17] | Thoracoscopic | Excised (stapler, 100%) | Bipolar RF | |||
| Joshibayev and Bolatbekov [18] | Cox-maze IV | LA sealing (55%) | Unipolar RF |
|
||
| Lönnerholm et al. [21] | Cox-maze III | 100% | Cut and sew |
|
||
| Lundberg et al. [19] | Cox-maze III | 100% | Cut and sew |
|
||
| van Breugel et al. [23] | Resection (100%) | Bipolar RF |
|
|||
| von Oppell et al. [24] | Cox-maze IV | Excised (100%) | Bipolar RF |
|
||
AV: aortic valve; CABG: coronary artery bypass graft; LA: left atrial; LAA: left atrial appendage; MV: mitral valve; NS: non-specified; RF: radiofrequency; TV: tricuspid valve.
Primary outcome: quality of life following stand-alone arrhythmia surgery
Overall, QOL improved across all variables incorporated in the SF-36 tool (e.g. physical functioning, bodily pain, role physical, general health, role emotional, vitality, social functioning and mental health). Moreover, the incidence of perioperative complications was low for all studies (Supplementary Material, Tables S3 and S4). To identify clinically relevant improvement in SF-36 subscores, differences in subscores between baseline and 1-year values were marked as ≥0.5, 1 or 2 times the standard deviation of the value at 1 year [25].
Interestingly, studies with higher success percentages in terms of rhythm outcome (SR after 1 year) also showed greater QOL improvements across all variables (Figs 1 and 2). Moreover, meta-regression based on rhythm outcome in the 2 studies by Al-Jazairi et al. and Driessen et al., who divided outcomes into 2 groups based on rhythm outcome, showed that following cardiac surgery the QOL scores of both SR and AF patients improved. Moreover, patients who were in SR showed significantly greater improvements in QOL compared to baseline concerning physical functioning, physical role, general health and social functioning, than those who remained in AF [17, 22]. The other variables, including bodily pain, role emotional, vitality and mental health, also showed better outcomes for those in SR compared to those in AF, however, non-significant (Table 3).
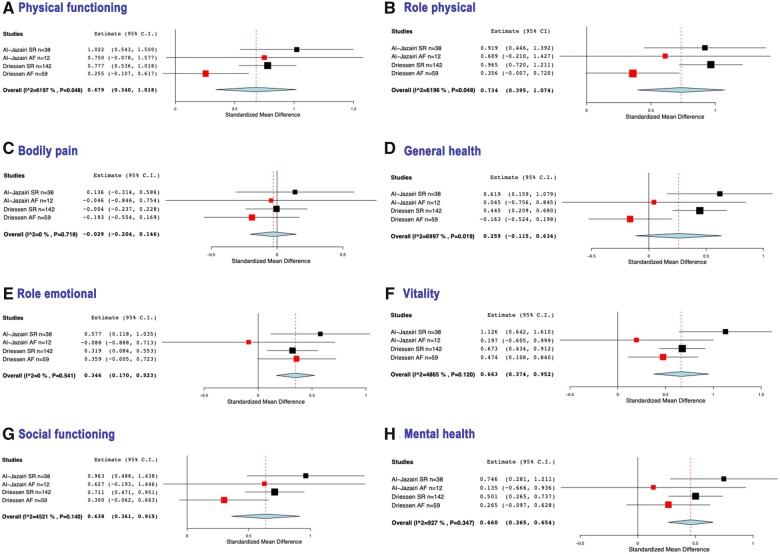
Figure 1:
Forest plots showing the changes per Short-Form 36 quality of life variable after 12 months of follow-up, expressed by the standardized mean difference. The weight given to each study is illustrated by the size of the square box, the point effect estimate by its mid-point and the degree of variance per study by the horizontal line through the box. A greater horizontal line indicates a greater 95% confidence interval for the effect estimates. Red boxes are studies where all patients were still in atrial fibrillation after 12 months. The overall effect estimate is represented by the diamante shape. (A) Physical functioning. Heterogeneity: τ2 = 0.503, Q(df = 10) = 106.286, P < 0.001, I2 = 90.6%. (B) Role physical. Heterogeneity: τ2 = 0.354, Q(df = 10) = 78.169, P < 0.001, I2 = 87.2%. (C) Bodily pain. Heterogeneity: τ2 = 0.482, Q(df = 10) = 111.276, P < 0.001, I2 = 91.0%. (D) General health. Heterogeneity: τ2 = 0.577, Q(df = 10) = 125.791, P < 0.001, I2 = 92.0%. (E) Role emotional. Heterogeneity: τ2 = 0.265, Q(df = 10) = 65.670, P < 0.001, I2 = 84.7%. (F) Vitality. Heterogeneity: τ2 = 0.215, Q(df = 10) = 52.832, P < 0.001, I2 = 81.1%. (G) Social functioning. Heterogeneity: τ2 = 0.327, Q(df = 10) = 75.008, P < 0.001, I2 = 86.7%. (H) Mental health. Heterogeneity: τ2 = 0.253, Q(df = 10) = 62.246, P < 0.001, I2 = 83.9%. AF: atrial fibrillation; SR: sinus rhythm.
Figure 2:
Forest plots showing the changes per Short-Form 36 quality of life variable after 12 months of follow-up, expressed by the standardized mean difference, comparing studies with 100% sinus rhythm (black box) with 100% atrial fibrillation (red box) after 12 months of follow-up. The weight given to each study is illustrated by the size of the square box, the point effect estimate by its mid-point and the degree of variance per study by the horizontal line through the box. A greater horizontal line indicates a greater 95% confidence interval for the effect estimates. Red boxes are studies where all patients had AF after 12 months. The overall effect estimate is represented by the diamante shape. (A) Physical functioning. Heterogeneity: τ2 = 0.069, Q(df = 3) = 7.888, P = 0.048, I2 = 62.0%. (B) Role physical. Heterogeneity: τ2 = 0.069, Q(df = 3) = 7.887, P = 0.048, I2 = 62.0%. (C) Bodily pain. τ2 = 0.000, Q(df = 3) = 1.345, P = 0.718, I2 = 0%. (D) General health. Heterogeneity: τ2 = 0.095, χ(df = 3) = 9.990, P = 0.019, I2 = 70.0%. (E) Role emotional. Heterogeneity: τ2 = 0.000, Q(df = 3) = 2.155, P = 0.541, I2 = 0%. (F) Vitality. τ2 = 0.040, Q(df = 3) = 5.842, P = 0.120, I2 = 48.7%. (G) Social functioning. Heterogeneity: τ2 = 0.035, Q(df = 3) = 5.476, P = 0.140, I2 = 45.2%. (H) Mental health. Heterogeneity: τ2 = 0.004, Q(df = 3) = 3.306, P = 0.347, I2 = 9.3%. AF: atrial fibrillation; SR: sinus rhythm.
Table 3:
Changes in SF-36 quality of life variables based on rhythm outcome after 12 months of follow-up
| SF-36 variable | SR 12 months |
AF 12 months |
P-value | ||
|---|---|---|---|---|---|
| Adjusted mean | 95% CI | Adjusted mean | 95% CI | ||
| Physical functioning | 0.8 | (0.6 to 1.0) | 0.3 | (0.0 to 0.7) | 0.015 |
| Role physical | 1.0 | (0.7 to 1.2) | 0.4 | (0.1 to 0.7) | 0.006 |
| Bodily pain | 0.0 | (−0.2 to 0.2) | −0.2 | (−0.5 to 0.2) | 0.331 |
| General health | 0.5 | (0.3 to 0.7) | −0.1 | (−0.5 to 0.2) | 0.002 |
| Role emotional | 0.4 | (0.2 to 0.6) | 0.3 | (0.0 to 0.6) | 0.654 |
| Vitality | 0.8 | (0.5 to 1.0) | 0.4 | (0.1 to 0.8) | 0.096 |
| Social functioning | 0.8 | (0.5 to 1.0) | 0.4 | (0.0 to 0.7) | 0.043 |
| Mental health | 0.6 | (0.3 to 0.8) | 0.2 | (−0.1 to 0.6) | 0.123 |
Data are presented as adjusted mean between QOL scores after 12 months versus baseline scores, followed by the 95% CI. P-value of the meta-regression was computed using the metric ‘Standardized mean difference’ in a binary random-effects model using rhythm outcome after 12 month of follow-up as covariate factor.
AF: atrial fibrillation; CI: confidence interval; QOL: quality of life; SF: Short-Form 36; SR: sinus rhythm.
Primary outcome for concomitant procedures
Furthermore, 3 of the 9 included studies performed an extra analysis on comparing QOL outcomes of patients receiving cardiac surgery with and without add-on arrhythmia surgery for AF (add-on surgical AF ablation versus control group) [18, 23, 24]. While van Breugel et al. and von Oppell et al. randomized their patients between the 2 groups, the study by Joshibayev and Bolatbekov did not [18, 23, 24]. As such, their patients undergoing add-on arrhythmia surgery had a higher rate of longstanding-persistent AF (P = 0.02), greater LA size (P = 0.004), lower left ventricular ejection fraction (P = 0.03) and a longer AF duration (P = 0.05) compared to the control group. Yet, this study showed the most improvement in QOL across all variables. Von Oppell et al. showed an improvement in 5 out of 8 variables in the add-on arrhythmia group compared to their control group, but van Breugel et al. only reported a significant improvement in the variable bodily pain compared to the control group. We performed a meta-regression of the 3 above-mentioned studies to evaluate the overall effect of add-on ablation concomitant with cardiac surgery on the QOL. This analysis showed that adding arrhythmia surgery to cardiac surgery as a concomitant procedure overall only leads to a significant improvement in the variable ‘Role physical’ at 1 year after the procedure (Table 4).
Table 4:
Changes in SF-36 quality of life variables comparing cardiac surgery with and without (control group) add-on surgical AF ablation
| Study | SF-36 variable | Add-on surgical AF ablation |
Control group |
P-value | ||
|---|---|---|---|---|---|---|
| Baseline | 1 year | Baseline | 1 year | |||
| Joshibayev and Bolatbekov [18] | n = 54 | n = 54 | n = 93 | n = 93 | ||
| Physical functioning | 20.0 ± 7.0 | 84.0 ± 22.0 | 38.0 ± 12.0 | 49.0 ± 7.0 | <0.001 | |
| Role physical | 38.0 ± 13.0 | 81.0 ± 17.0 | 44.0 ± 9.0 | 47.0 ± 9.0 | <0.001 | |
| Bodily pain | 29.0 ± 23.0 | 79.0 ± 5.0 | 53.0 ± 11.0 | 51.0 ± 6.0 | <0.001 | |
| General health | 39.0 ± 7.0 | 89.0 ± 21.0 | 51.0 ± 5.0 | 54.0 ± 6.0 | <0.001 | |
| Vitality | 44.0 ± 12.0 | 88.0 ± 31.0 | 49.0 ± 5.0 | 60.0 ± 5.0 | <0.001 | |
| Social functioning | 39.0 ± 7.0 | 84.0 ± 21.0 | 33.0 ± 11.0 | 51.0 ± 17.0 | <0.001 | |
| Role emotional | 41.0 ± 23.0 | 89.0 ± 22.0 | 61.0 ± 11.0 | 50.0 ± 7.0 | <0.001 | |
| Mental health | 39.0 ± 7.0 | 89.0 ± 29.0 | 55.0 ± 13.0 | 59.0 ± 9.0 | <0.001 | |
| van Breugel et al. [23] | n = 65 | n = 65 | n = 67 | n = 67 | ||
| Physical functioning | 50.2 ± 24.1 | 68.4 ± 23.2 | 50.1 ± 24.2 | 61.2 ± 23.9 | 0.143 | |
| Role physical | 23.5 ± 35.3 | 53.2 ± 39.7 | 42.9 ± 42.1 | 47.9 ± 38.1 | 0.295 | |
| Bodily pain | 76.0 ± 25.0 | 77.7 ± 22.6 | 72.3 ± 24.6 | 72.8 ± 21.9 | 0.032 | |
| General health | 53.2 ± 19.7 | 56.0 ± 18.2 | 60.2 ± 17.4 | 54.9 ± 17.4 | 0.458 | |
| Vitality | 50.5 ± 22.4 | 61.4 ± 17.0 | 51.3 ± 21.8 | 60.0 ± 17.8 | 0.246 | |
| Social functioning | 66.9 ± 25.2 | 80.0 ± 19.3 | 67.0 ± 25.8 | 76.2 ± 24.7 | 0.410 | |
| Role emotional | 67.7 ± 42.9 | 72.1 ± 35.7 | 69.2 ± 42.0 | 69.5 ± 36.6 | 0.157 | |
| Mental health | 69.6 ± 20.0 | 77.7 ± 13.0 | 72.0 ± 22.0 | 74.0 ± 17.5 | 0.300 | |
| von Oppell et al. [24] | n = 24 | n = 24 | n = 25 | n = 25 | ||
| Physical functioning | 41.5 ± 25.6 | 61.8 ± 31.9 | 41.4 ± 29.3 | 80.3 ± 20.3 | <0.001 | |
| Role physical | 13.5 ± 25.5 | 54.5 ± 47.3 | 23.0 ± 38.1 | 58.8 ± 44.6 | <0.001 | |
| Bodily pain | 65.7 ± 34.2 | 70.1 ± 28.1 | 80.7 ± 27.3 | 92.2 ± 12.8 | NS | |
| General health | 58.2 ± 23.9 | 67.0 ± 25.0 | 55.1 ± 23.3 | 78.3 ± 16.8 | <0.001 | |
| Vitality | 31.9 ± 23.0 | 53.0 ± 26.2 | 30.2 ± 30.5 | 62.5 ± 19.9 | <0.001 | |
| Social functioning | 55.7 ± 36.9 | 68.8 ± 34.2 | 57.5 ± 29.8 | 88.8 ± 17.6 | <0.001 | |
| Role emotional | 51.4 ± 42.8 | 56.1 ± 47.6 | 58.7 ± 43.3 | 86.7 ± 33.2 | NS | |
| Mental health | 70.8 ± 19.8 | 74.0 ± 22.8 | 76.8 ± 17.4 | 84.4 ± 17.1 | NS | |
| Overall change | Adjusted mean (95% CI) | Adjusted mean (95% CI) | ||||
| Physical functioning | 1.8 (0.1 to 3.4) | 1.0 (0.5 to 1.4) | 0.403 | |||
| Role physical | 1.5 (0.5 to 2.6) | 0.3 (0.1 to 0.5) | 0.037 | |||
| Bodily pain | 1.1 (−0.5 to 2.6) | 0.0 (−0.3 to 0.3) | 0.230 | |||
| General health | 1.2 (−0.3 to 2.8) | 0.4 (−0.2 to 1.1) | 0.371 | |||
| Vitality | 1.1 (0.4 to 1.7) | 1.3 (0.4 to 2.1) | 0.704 | |||
| Social functioning | 1.3 (−0.0 to 2.5) | 0.9 (0.4 to 1.4) | 0.654 | |||
| Role emotional | 0.8 (−0.3 to 1.8) | −0.2 (−1.1 to 0.7) | 0.178 | |||
| Mental health | 1.0 (−0.1 to 2.1) | 0.3 (0.1 to 0.5) | 0.203 | |||
Data are presented as mean ± standard deviation or adjusted mean between QOL scores after 12 months versus baseline scores, followed by the 95% CI. NS: non-significant. P-value of the meta-regression was computed using the metric ‘Standardized mean difference’ in a binary random-effects model using add-on surgery as covariate factor.
QOL: quality of life; SF: Short-Form 36. AF: atrial fibrillation. CI: confidence interval.
Follow-up
Of the 505 patients who completed the follow-up and reported on QOL using the SF-36 questionnaire, 73.8% (62.5–85.0) was in SR after 12 months. The type of rhythm monitoring differed across studies; most studies used a 24-h Holter, followed by 72-h Holter, while only one study used continuous monitoring and 2 used a 12-leads ECG for arrhythmia detection (Supplementary Material, Table S5).
DISCUSSION
This systematic review and meta-analysis summarizes the effect of arrhythmia surgery for AF on QOL. Overall, arrhythmia surgery leads to an improvement in QOL in patients with AF. This improvement seems to be related to the success of the procedure, because the improvement in QOL is higher in studies that reported a higher rate of SR after 12 months of follow-up. This is especially true for patients undergoing stand-alone AF surgery and less in patients undergoing concomitant AF surgery.
In 1991, Drs Cox and Schuessler designed the Cox-maze procedure after extensive epicardial mapping studies [26]. The surgical technique is based on an anatomical approach to prevent macro re-entrant circuits in both atria. Although new surgical tools and alternative surgical approaches were developed, the basic concept of the procedure did not change and still forms the basis of present-day concomitant AF surgery. Even though the procedure has been shown to be very effective in restoring SR [27] and concomitant AF surgery had a class I indication in 2017 [28], it was recently downgraded to a class IIa indication [8]. A potential reason is that the add-on of AF surgery does not result in improved QOL nor reduced stroke and mortality at 1-year follow-up [8].
Overall effect on quality of life following arrhythmia surgery for atrial fibrillation
In this meta-analysis, there was an improvement in QOL after cardiac surgery with concomitant AF ablation compared to baseline. However, it is difficult to distinguish between the effect of the cardiac surgical procedure itself and the effect of the add-on arrhythmia surgery on the improvement in QOL. When the results are plotted in relation to the success rate of the arrhythmia surgery in terms of SR after 12 months, the forest plots (Figs 1 and 2) suggest that the improvement in QOL is higher in the studies that report a higher freedom of AF. Of course, these results should be interpreted with caution. First, the type of surgical lesions is not consistent between the different studies. While a large variability of lesion sets was performed, at least all studies performed pulmonary vein isolation, which represents the cornerstone for AF ablation [29]. Furthermore, in 10 out of 12 studies the LAA was electrically isolated in at least half of their patients. In the BELIEF trial, isolation of the LAA lowered the incidence of AF without increasing the periprocedural complication rate [30]. Moreover, the overall reported stroke incidence in the present study was low (0.8%). As the LAA is considered the main source of thromboembolism in AF, oral anticoagulation and other techniques such as isolating the LAA are key in stroke prevention in AF patients, which may contribute to the QOL [31]. Secondly, follow-up was conducted with different monitoring devices. While using continuous monitoring devices is the most reliable way to keep track of (asymptomatic) palpitations, this was only used by 2 studies. Thirdly, no data on anti-arrhythmic drugs (AAD) use was given, though most of the included patients in this analysis had longstanding-persistent AF (41.9%) and treatment with AADs seems to be less efficient in this patient population for rhythm control and symptom management [8]. Moreover, for the study of Lönnerholm, the reported percentage SR in the forest plot represents the outcome directly after surgery, while in the other studies, it represents the outcome after 12 months [32]. Nevertheless, it seems that the improvement in QOL is related to the outcome of the AF ablation.
Primary outcome: concomitant atrial fibrillation surgery and quality of life
The analysis of the 3 studies that compared cardiac surgery with and without add-on arrhythmia surgery failed to show an overall improvement in QOL between the patients that did and did not undergo add-on arrhythmia surgery [18, 24, 33]. While QOL scores after 1 year were improved compared to baseline for both the add-on and stand-alone arrhythmia group, differences were not significant. These differences between the studies regarding the improvement in QOL are very obvious, suggesting that even if there is an effect of add-on arrhythmia on QOL for concomitant procedures, it is not very strong. Joshibayev and Bolatbekov [18] reported a very strong improvement in QOL, but this study did not randomize between both arms and therefore, it cannot be excluded that there was a selection bias in the patients that received arrhythmia surgery. Furthermore, it is surprising that there was almost no improvement in QOL between baseline and 12 months follow-up in the control group, despite the fact that all patients in the control group underwent mitral valve (MV) surgery. The other 2 studies were randomized, but only the study of von Oppel found an increase in QOL in several parameters, while in the study of van Breugel, only the SF-36 parameter ‘Bodily pain’ improved [24, 33]. In both studies, patients received coronary artery bypass graft or aortic or MV procedures concomitant to ablation. Interestingly, the study by Grady et al. [34] further examined the improvement of health-related QOL using the SF-36 between patients undergoing different isolated cardiac procedures. At baseline, patients with MV disease had a better physical component summary, but lower mental component summary than patients undergoing aortic valve surgery, coronary artery bypass graft or a Maze procedure. Three and 6 months after surgery, physical component summary scores improved reliably in all groups compared to baseline, except for patients who underwent MV surgery, probably due to their healthier preoperative scores and receiving early intervention. Furthermore, a strong trend was seen for better physical component summary scores of coronary artery bypass graft patients than for aortic valve patients. For changes in mental component summary scores, the improvement was faster for patients undergoing a Maze procedure compared with the other groups, and patients undergoing MV surgery did not show a clinically important improvement after 3 months.
Primary outcome: stand-alone atrial fibrillation surgery and quality of life
In stand-alone AF surgery, the effect of arrhythmia surgery on QOL can be better evaluated, since there is no other surgical procedure that can act as a confounding factor. All studies evaluating QOL using the SF-36 questionnaire in stand-alone AF surgery showed an increase in QOL at 12 months compared to baseline [16, 17, 20, 22]. It must be noted that patients who are referred for an isolated surgical ablation for AF are highly symptomatic and undergo a surgical intervention as a last resort treatment. Accordingly, they usually have a worse QOL at baseline compared to the general population. As such, it is not unexpected that a rapid and significant improvement in QOL follows after a successful surgical ablation, returning patients to normal SR [34]. Furthermore, 2 studies specifically compared the improvement in QOL between patients who were in SR and patients who were in AF 12 months after the procedure [17, 22]. Both studies showed that the improvement in QOL was greater if surgical AF ablation resulted in SR. As such, it can be concluded that successful stand-alone arrhythmia surgery does result in increased QOL. Despite this improvement in QOL and the fact that stand-alone surgical AF ablation, epicardial or in a hybrid setting, is associated with higher success rates compared to catheter ablation [35, 36], it remains to have a class II recommendation due to the paucity of RCTs [8, 9].
Different techniques and lesion sets in concomitant and stand-alone atrial fibrillation ablation
The inconsistency in the type of lesions performed during concomitant arrhythmia surgery makes it difficult and challenging to compare the different studies. For example, the studies of Joshibayev and Bolatbekov and von Oppell et al. included a variety of lesions and a mixture of unipolar and bipolar radio frequent energy. This stands in contrast with the studies evaluating stand-alone AF surgery that adhere more to a fixed ablation protocol. As such, it can be concluded that arrhythmia surgery does result in an improvement in QOL, but it requires a dedicated lesion set. Finally, a potential reason for the greater improvement in QOL after stand-alone AF than concomitant arrhythmia surgery is that stand-alone AF surgery is performed by dedicated teams, while concomitant AF surgery is also performed by surgeons without an extensive experience in AF ablation.
Limitations
This study contains some limitations. Ideally, we aimed to compare the improvements in QOL outcomes obtained by RCTs in our meta-analysis. Unfortunately, solely 2 studies have evaluated this outcome in an RCT. Due to this gap in literature, we worked with pre- and post-surgical QOL values in our meta-analysis of studies using the SF-36 questionnaire and performed a sub-study based on rhythm outcome after 1 year. Furthermore, since there is no golden standard for measuring QOL following arrhythmia surgery for AF, the included studies have used a variety of questionnaires to estimate the effect of ablation surgery on QOL. While being an important endpoint for ablation studies, QOL remains a rather subjective endpoint and comes along with (at least some) expectation bias. As such, the placebo effect of undergoing surgery as rhythm therapy was most likely present in at least some degree for all patients. In this meta-analysis, risk of bias due to other factors such as selection, confounding factors and publication was present as well. Moreover, marked differences between lesion sets between the studies were present. As such, not only statistical but also clinical heterogeneity was present in this study and results about the effectiveness of arrhythmia surgery and the improvement in QOL should be interpreted with caution. Lastly, the analyses in this study were based on a specific subgroup of highly symptomatic patients, which is especially true for patients undergoing stand-alone surgical ablation for AF. As such, these papers reflect only a small subset of all AF patients and thus the findings of improved QOL in this group should not be used as an endorsement for surgery for less symptomatic AF patients.
CONCLUSION
Overall, arrhythmia surgery does result in an improvement in QOL in patients with AF when a dedicated lesion set is used. This effect seems to be related to the outcome in terms of SR after 1 year, both in concomitant as in stand-alone AF ablation. However, studies evaluating QOL following arrhythmia surgery are scarce and analysis based on small, heterogenic, single-arm studies in a random-effects model hinders drawing definite conclusions. Therefore, future trials reporting on AF surgery, both concomitant and stand-alone, should include the evaluation of patient-reported outcomes such as QOL.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the authors of the included studies for their cooperation in providing the required additional data.
Conflict of interest: Bart Maesen is a consultant for Atricure and Medtronic.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Bart Maesen: Conceptualization; Investigation; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Claudia van der Heijden: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing—original draft; Writing—review & editing. Elham Bidar: Supervision. Rein Vos: Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing—original draft. Thanos Athanasiou: Conceptualization; Supervision. Jos G. Maessen: Supervision.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Steven Hunter, Dumbor L. Ngaage and the other anonymous reviewers for their contribution to the peer review process of this article.
ABBREVIATIONS
- AF
Atrial fibrillation
- LA
Left atrial
- LAA
LA appendage
- MV
Mitral valve
- QOL
Quality of life
- RCT
Randomized controlled trial
- SF-36
Short-Form 36
- SR
Sinus rhythm
REFERENCES
- 1. Cobb FR, Blumenschein SD, Sealy WC, Boineau JP, Wagner GS, Wallace AG.. surgical interruption of the bundle of Kent in a patient with Wolff-Parkinson-White syndrome. Circulation 1968;38:1018–29. [DOI] [PubMed] [Google Scholar]
- 2. Cox JL. Cardiac surgery for arrhythmias. Heart Rhythm 2004;1:85c–101c. [DOI] [PubMed] [Google Scholar]
- 3. Maesen B, La Meir M, Luermans J, Segers P.. A minimally invasive all-in-one approach for patients with left anterior descending artery disease and atrial fibrillation. Eur J Cardiothorac Surg 2020;57:803–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ad N, Henry L, Friehling T, Wish M, Holmes SD.. Minimally invasive stand-alone Cox-maze procedure for patients with nonparoxysmal atrial fibrillation. Ann Thorac Surg 2013;96:792–9. [DOI] [PubMed] [Google Scholar]
- 5. Maesen B, La Meir M.. Unilateral left-sided thoracoscopic ablation of atrial fibrillation. Ann Thorac Surg 2020;110:e63–6. [DOI] [PubMed] [Google Scholar]
- 6. Luo X, Li B, Zhang D, Zhu J, Qi L, Tang Y.. Efficacy and safety of the convergent atrial fibrillation procedure: a meta-analysis of observational studies. Interact CardioVasc Thorac Surg 2019;28:169–76. [DOI] [PubMed] [Google Scholar]
- 7. Fleerakkers J, Hofman FN, van Putte BP.. Totally thoracoscopic ablation: a unilateral right-sided approach. Eur J Cardiothorac Surg 2020;58:1088–90. [DOI] [PubMed] [Google Scholar]
- 8. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C. et al. ; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 9. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Chalmers I. et al. ; PRISMA for Abstracts Group. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med 2013;10:e1001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deeks JJ, Higgins J, Altman DG, Green S.. Cochrane handbook for systematic reviews of interventions version 5.1.0 . The Cochrane Collaboration 20112, 2011. [Google Scholar]
- 13. Smith LJW, Beretvas SN.. Estimation of the standardized mean difference for repeated measures designs. J Mod App Stat Meth 2009;8:600–9. [Google Scholar]
- 14. Wallace BC, Schmid CH, Lau J, Trikalinos TA.. Meta-analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol 2009;9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borenstein M, Hedges LV, Higgins JPT, Rothstein H. Introduction to meta-analysis 2011.
- 16. Buist TJ, Adiyaman A, Beukema RJ, Smit JJJ, Delnoy PPH, Hemels ME. et al. Quality of life after catheter and minimally invasive surgical ablation of paroxysmal and early persistent atrial fibrillation: results from the SCALAF trial. Clin Res Cardiol 2020;109:215–24. [DOI] [PubMed] [Google Scholar]
- 17. Driessen AH, Berger WR, Bierhuizen MF, Piersma FR, van den Berg NW, Neefs J. et al. Quality of life improves after thoracoscopic surgical ablation of advanced atrial fibrillation: results of the Atrial Fibrillation Ablation and Autonomic Modulation via Thoracoscopic Surgery (AFACT) study. J Thorac Cardiovasc Surg 2018;155:972–80. [DOI] [PubMed] [Google Scholar]
- 18. Joshibayev S, Bolatbekov B.. Early and long-term outcomes and quality of life after concomitant mitral valve surgery, left atrial size reduction, and radiofrequency surgical ablation of atrial fibrillation. Anatol J Cardiol 2016;16:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lundberg C, Albåge A, Carnlöf C, Kennebäck G.. Long-term health-related quality of life after maze surgery for atrial fibrillation. Ann Thorac Surg 2008;86:1878–82. [DOI] [PubMed] [Google Scholar]
- 20. Bagge L, Blomström P, Nilsson L, Einarsson GM, Jidéus L, Blomström-Lundqvist C.. Epicardial off-pump pulmonary vein isolation and vagal denervation improve long-term outcome and quality of life in patients with atrial fibrillation. J Thorac Cardiovasc Surg 2009;137:1265–71. [DOI] [PubMed] [Google Scholar]
- 21. Lönnerholm S, Blomström P, Nilsson L, Oxelbark S, Jideus L, Blomström-Lundqvist C.. Effects of the maze operation on health-related quality of life in patients with atrial fibrillation. Circulation 2000;101:2607–11. [DOI] [PubMed] [Google Scholar]
- 22. Al-Jazairi M, Rienstra M, Klinkenberg T, Mariani M, Van Gelder I, Blaauw Y.. Hybrid atrial fibrillation ablation in patients with persistent atrial fibrillation or failed catheter ablation. Neth Heart J 2019;27:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Breugel HN, Nieman F, Accord RE, Van Mastrigt G, Nijs JF, Severens J. et al. A prospective randomized multicenter comparison on health‐related quality of life: the value of add‐on arrhythmia surgery in patients with paroxysmal, permanent or persistent atrial fibrillation undergoing valvular and/or coronary bypass surgery. J Cardiovasc Electrophysiol 2010;21:511–20. [DOI] [PubMed] [Google Scholar]
- 24. von Oppell UO, Masani N, O'Callaghan P, Wheeler R, Dimitrakakis G, Schiffelers S.. Mitral valve surgery plus concomitant atrial fibrillation ablation is superior to mitral valve surgery alone with an intensive rhythm control strategy. Eur J Cardiothorac Surg 2009;35:641–50. [DOI] [PubMed] [Google Scholar]
- 25. Norman GR, Sloan JA, Wyrwich KW.. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582–92. [DOI] [PubMed] [Google Scholar]
- 26. Cox JL, Schuessler RB, D'Agostino HJ Jr, Stone CM, Chang BC, Cain ME. et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569–83. [PubMed] [Google Scholar]
- 27. Weimar T, Schena S, Bailey MS, Maniar HS, Schuessler RB, Cox JL. et al. The cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhyth Electrophysiol 2012;5:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Interv Card Electrophysiol 2017;50:1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maesen B, Van-Loo I, Pison L, La-Meir M.. Surgical ablation of atrial fibrillation: is electrical isolation of the pulmonary veins a must? J Atr Fibrillation 2016;9:1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chernyavskiy A, Kareva Y, Pak I, Rakhmonov S, Pokushalov E, Romanov A.. Quality of life after surgical ablation of persistent atrial fibrillation: a prospective evaluation. Heart Lung Circ 2016;25:378–83. [DOI] [PubMed] [Google Scholar]
- 31. van Laar C, Verberkmoes NJ, van Es HW, Lewalter T, Dunnington G, Stark S. et al. Thoracoscopic left atrial appendage clipping: a multicenter cohort analysis. JACC Clin Electrophysiol 2018;4:893–901. [DOI] [PubMed] [Google Scholar]
- 32. Lönnerholm S, Blomström P, Nilsson L, Blomström-Lundqvist C.. A high quality of life is maintained late after Maze III surgery for atrial fibrillation. Eur J Cardiothorac Surg 2009;36:558–62. [DOI] [PubMed] [Google Scholar]
- 33. van Breugel HN, Parise O, Nieman FH, Accord RE, Lucà F, Lozekoot P. et al. Does sinus rhythm conversion after cardiac surgery affect postoperative health-related quality of life? J Cardiothorac Surg 2016;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grady KL, Lee R, Subačius H, Malaisrie SC, McGee EC Jr, Kruse J. et al. Improvements in health-related quality of life before and after isolated cardiac operations. Ann Thorac Surg 2011;91:777–83. [DOI] [PubMed] [Google Scholar]
- 35. Phan K, Phan S, Thiagalingam A, Medi C, Yan TD.. Thoracoscopic surgical ablation versus catheter ablation for atrial fibrillation. Eur J Cardiothorac Surg 2016;49:1044–51. [DOI] [PubMed] [Google Scholar]
- 36. van der Heijden CAJ, Vroomen M, Luermans JG, Vos R, Crijns H, Gelsomino S. et al. Hybrid versus catheter ablation in patients with persistent and longstanding persistent atrial fibrillation: a systematic review and meta-analysisdagger. Eur J Cardiothorac Surg 2019;56:433–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.