Abstract
Background and Objectives
A descriptive analysis of COVID-19 infection in patients with multiple sclerosis (MS) receiving fingolimod or siponimod.
Methods
We reviewed the cases of COVID-19 from postmarketing or ongoing clinical trials reported to Novartis through December 27, 2020.
Results
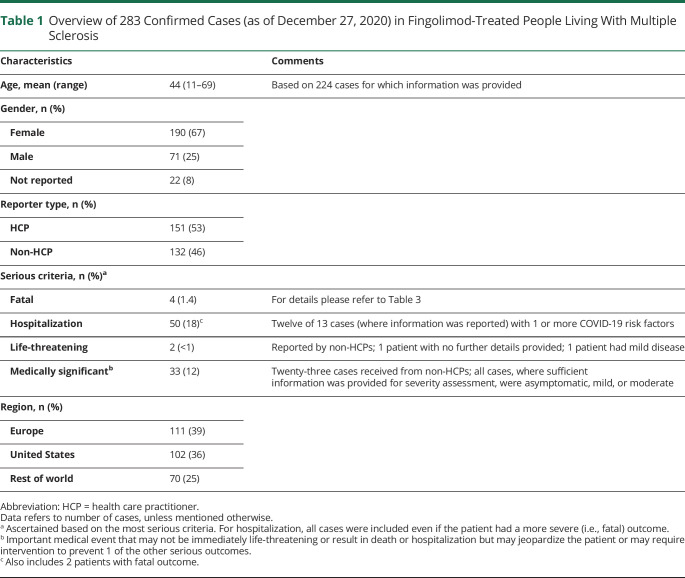
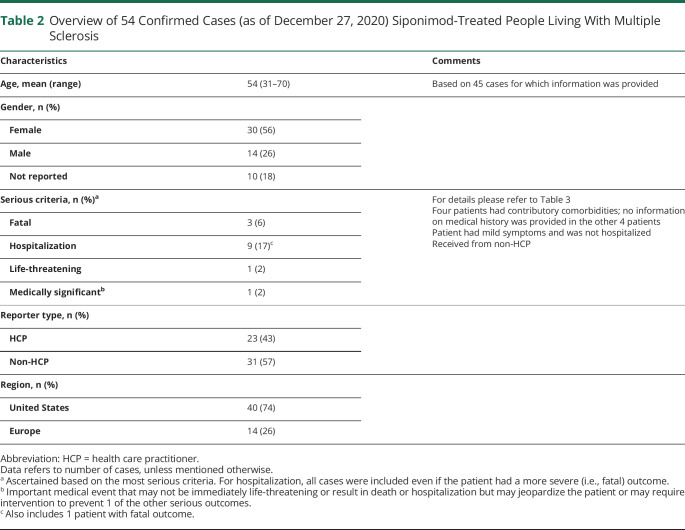
As of December 27, 2020, 283 cases had been reported in fingolimod-treated patients. The mean age was 44 years (from n = 224; range 11–69 years), and 190 were women. Of 161 cases with available information, 138 were asymptomatic (6), mild (100), or moderate (32); 50 cases required hospitalization. At the last follow-up, 140 patients were reported as recovered/recovering, condition was unchanged in 22, and deteriorated in 3 patients; 4 patients had a fatal outcome. Information was not available for 114 patients. Of the 54 cases of COVID-19 reported in siponimod-treated patients, 45 were from the postmarketing setting and 9 from an ongoing open-label clinical trial. The mean age was 54 years (from n = 45; range 31–70), and 30 were women. Of 28 cases with available information, 24 were asymptomatic (2), mild (17), or moderate (5); 9 cases required hospitalization. At the last follow-up, 27 patients were reported as recovered/recovering, condition remained unchanged for 1, and 3 patients had a fatal outcome. Information was not available for 23 patients.
Discussion
Based on a review of available information, the risk of more severe COVID-19 in patients receiving fingolimod or siponimod seems to be similar to that reported in the general population and the MS population with COVID-19. However, limitations of spontaneous reporting, especially missing data, should be considered in the interpretation of these observations.
In December 2019, the novel COVID-19 disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seemed as a new infectious disease in China and spread within months, becoming a global pandemic. As of February 2021, more than 114 million confirmed cases of COVID-19 were reported worldwide.1 The disease is mild to moderate in most people; however, pneumonia, acute respiratory distress syndrome (ARDS), and multiorgan dysfunction occur in a subgroup of cases, often with poor outcomes including death and disability.2 Elderly people and those with obesity and/or serious comorbidities, such as cardiovascular or respiratory disease, are at greater risk of COVID-19 complications and death.2
The impact of COVID-19 on people living with multiple sclerosis (MS), including how disease-modifying therapies (DMT) might influence the risk of symptomatic infection or COVID-19 outcomes, is being assessed through large local registries3-6 and a global data sharing initiative.7 Older age and comorbidities, including obesity, progressive forms of MS, and higher degree of disability, seem to be associated with severe COVID-19 outcomes among people living with MS.4,8
Fingolimod (Gilenya®) and siponimod (Mayzent®) are immunomodulatory MS DMTs that target sphingosine 1-phophate receptors expressed on lymphocytes and reduce the egress of autoreactive T lymphocytes and their naïve progenitors from secondary lymphoid organs into the circulation.9 Despite the reduction in circulating lymphocytes, the risk of common viral respiratory infections in people living with MS treated with fingolimod or siponimod was generally comparable with placebo.10,11 Fingolimod-treated patients mount antigen-specific immune responses similar to healthy controls.12
We report the clinical characteristics of confirmed cases of COVID-19 reported to Novartis from postmarketing setting or ongoing clinical trials as of December 27, 2020, from patients with MS receiving fingolimod or siponimod.
Methods
This is a case series with descriptive summaries of confirmed COVID-19 reported to Novartis from the postmarketing setting or ongoing clinical trials. The Novartis safety database and cases from clinical trials were reviewed to identify confirmed or suspected COVID-19 in patients treated with fingolimod or siponimod reported to Novartis through December 27, 2020. The Novartis safety database is a system to collect, code, assess, and report adverse events to health authorities from the postmarketing setting (i.e., spontaneously reported to Novartis, from a postmarketing surveillance program or cases identified in the published literature), serious adverse events, and protocol-triggered events of interest from clinical trials in accordance with international guidelines. The database captures adverse events reported to Novartis from health care professionals (HCPs), patients, or other sources. COVID-19 cases were classified as confirmed if a SARS-CoV-2-positive test result was available or the patient was reported to have been diagnosed with COVID-19. Cases without a positive test or a definitive diagnosis were classified as suspected. Cases were considered “serious” based on the International Council on Harmonization regulatory reporting definition, which is “fatal, life-threatening, hospitalization, and medically significant.” The severity of cases were assessed using the US Food and Drug Administration (FDA)13 and World Health Organization (WHO)14 COVID-19 severity scales, and where data were available, categorization was done as follows: asymptomatic (infection without symptoms), mild (not requiring hospitalization, symptoms did not include dyspnea), moderate (hospitalization with pneumonia not reported to be severe and/or with respiratory rate [RR] >20 and/or oxygen saturation [SpO2] >90%, shortness of breath or dyspnea, and hospitalization less than 7 days without further details), severe (pneumonia reported as severe—RR ≥ 30, SpO2 ≤ 93%, and hospitalization 7 days or more without further details), or critical (respiratory failure and/or intubation). The severity of clinical trial cases was based on investigator-reported common terminology criteria for adverse events grade. If reported, the outcome status of each patient was noted to be recovered/recovering (including patients noted to be “stable” or “doing well”), condition unchanged, condition deteriorated, or fatal. We present data as mean or median with their range of dispersion or absolute number and percentage.
Data Availability
Anonymized data can be made available on request for research purposes by sending a request to the corresponding author.
Results
Fingolimod
As of December 27, 2020, there are more than 870,000 patient-years of exposure for fingolimod from clinical trials and postmarketing experience (Novartis, data on file). The drug exposure during the time of the SARS-CoV-2 pandemic—February 28 to December 27, 2020—is more than 94,000 patient-years.
As of December 27, 2020, Novartis received a notification of 342 confirmed or suspected COVID-19 cases in fingolimod-treated patients in the postmarketing setting and no cases in the ongoing clinical trials. Of these 342 cases, 59 were considered suspected and 283 were confirmed. Case overview, severity rating, and outcomes are presented in the Table 1 and Figure 1 for the confirmed cases.
Table 1.
Overview of 283 Confirmed Cases (as of December 27, 2020) in Fingolimod-Treated People Living With Multiple Sclerosis
Figure 1. COVID-19 Severity* and Outcome in Fingolimod-Treated People Living With Multiple Sclerosis.
*COVID-19 severity was assessed based on both the FDA and WHO COVID-19 criteria. Information as per the last follow-up. Numbers in parenthesis show—(patients hospitalized; patients requiring ventilation or ICU admission). FDA = US Food and Drug Administration; ICU = intensive care unit; WHO = World Health Organization.
From the available information, the mean age was 44 years (range 11–69 years), and 190 (73%) cases were women. Four patients had a fatal outcome. At the time of the most recent follow-up, 140 patients had recovered or were recovering, condition was unchanged in 22 patients, and condition deteriorated in 3 patients, and information was not available for 114 cases. Of the total 283 cases, information to assess case severity was reported for 161 cases. The COVID-19 outcome information was provided for 169 cases of the total 283 cases.
Siponimod
As of December 27, 2020, there are more than 10,000 patient-years of exposure for siponimod from clinical trials and postmarketing experience (Novartis, data on file). The drug exposure during the time of the SARS-CoV-2 pandemic—February 28 to December 27, 2020—is more than 3,000 patient-years.
As of December 27, 2020, Novartis received a notification of 58 confirmed or suspected COVID-19 cases in siponimod-treated patients. Of these 58 cases, 4 were considered suspected and 54 were confirmed, consisting of 45 cases from the postmarketing setting and 9 from clinical trials. Further details are provided in Table 2 and Figure 2.
Table 2.
Overview of 54 Confirmed Cases (as of December 27, 2020) Siponimod-Treated People Living With Multiple Sclerosis
Figure 2. COVID-19 Severity* and Outcome in Siponimod-Treated People Living With MS.
*COVID-19 severity was assessed based on both the FDA and WHO COVID-19 criteria. Information as per the last follow-up. Numbers in parenthesis show—(patients hospitalized; patients requiring ventilation or ICU admission). FDA = US Food and Drug Administration; ICU = intensive care unit; WHO = World Health Organization.
From the available information, the mean age was 54 years (range 31–70 years), and 30 (68%) were women. Three patients had a fatal outcome. At the time of the most recent follow-up, 27 patients had recovered or were recovering, condition was reported as unchanged in 1 patient, and information was not available for 23 patients. Of the total 54 cases, information to assess case severity was reported for 28 cases. COVID-19 outcome information was provided for 31 of the 54 cases.
Discussion
The disease course of COVID-19 in people living with MS receiving either fingolimod or siponimod seems to be similar to those reported in the general population15 and in the overall MS population affected with COVID-19.3-7 The mean age of patients with COVID-19 receiving fingolimod was 44 years and that of patients receiving siponimod was 54 years. The patient demographics were consistent with those included in the respective pivotal phase 3 studies, in which the siponimod patient cohort was generally older (mean age 44 years) with higher levels of disability (mean Expanded Disability Status Scale [EDSS]) score 5.4) as compared with fingolimod cohort (mean age 37 years, mean EDSS score 2.3).16-18
The clinical course of most fingolimod- and siponimod-associated cases has overall been uncomplicated. Most patients for whom information was available to assess severity reported asymptomatic, mild, or moderate SARS-CoV-2 infection (138/161, 85.7% of cases with fingolimod; 24/28, 85.7% of cases with siponimod). When information was available about the outcomes in patients on therapy with fingolimod, the large majority (140/169; 83%) completely recovered or were recovering. Based on the available data, the proportions of severe (14/161; 8.7%) and critical cases (9/161; 5.6%) were generally consistent with background rates of severity in the general population, noted to be 14% severe and 5% critical in an early report from China.15 In a more recent study in the United States, the proportion of people who were hospitalized was 14%, including 2% admitted to intensive care unit (ICU), and overall 5% of patients died.19 The number of cases in patients on therapy with fingolimod requiring hospitalization (50/283; 18%), including those requiring ICU/ventilation because of COVID-19 or were fatal (12/283; 4%), is also in line with the reported incidence in the general population,15,19 as well as in patients with MS receiving a range of DMTs from the COVIMS registry, with 80% not hospitalized, 12% hospitalized, and 8% requiring ICU/ventilation or were fatal.20 Of the 54 patients receiving siponimod with COVID-19, a total of 9 (17%) patients required hospitalization, including those requiring ICU/ventilation, and 3 patients who had fatal outcome (6%). It is noteworthy that siponimod-treated patients are older, with higher degree of disability among the MS population,8,20 and are therefore at a higher risk of severe COVID-19 outcome as compared to the general population.15,21 Of the total 337 confirmed cases of COVID-19 in our case series, there were 7 reported fatalities (Table 3). A recent study reported that the infection-fatality risk estimates are 1.4% for overall population, with higher risk of 4.9% (65–74 years of age) and 14.2% (>75 years of age) in the older age groups.21 The risk of severe outcomes, including fatalities, with fingolimod or siponimod could only be evaluated with an age-adjusted analysis, which is currently not possible with the limited cases and missing information.
Table 3.
Characteristics of MS Patients Receiving Fingolimod or Siponimod With COVID-19 Related Fatality
There are limitations to this case series because they include spontaneous cases reported voluntarily with adverse events not confirmed by HCPs and cases found in the scientific literature. There is typically underreporting and/or incomplete reporting in this setting, making interpretation challenging. Many of the COVID-19 cases had limited information regarding previous MS DMTs, comorbidities, MS duration, EDSS/disability status, COVID-19 symptoms and outcome, and some were lost to follow-up. Typically, in the postmarketing setting, serious cases are likely to be reported more frequently than nonserious cases.22 In addition, the number of patients on therapy and the patient exposure data are typically derived from sales data and are therefore estimates. Furthermore, details on morbidity and mortality outcomes in this case series were limited because these could not be further queried.
However, efforts to make accurate and up-to-date information are ongoing to help health care practitioner's make informed decisions, especially in the uncertain and demanding context of COVID-19 pandemic. To date, large ongoing registries of COVID-19 in people living with MS have not shown an increase or decrease in morbidity or mortality with the administration of modulators.3-8,20 The data presented herein are consistent with the registry observations23; the risk of more severe COVID-19 symptoms in patients receiving fingolimod seems to be similar to that reported in the general population and the MS population with COVID-19. For siponimod, the less number of cases reported coupled with insufficient information precludes meaningful conclusions.
Acknowledgment
The authors acknowledge Suzannah Ryan of Novartis Pharma AG for her support in coordinating the author reviews.
Glossary
- ARDS
acute respiratory distress syndrome
- DMT
disease-modifying therapies
- EDSS
Expanded Disability Status Scale
- FDA
Food and Drug Administration
- HCP
health care professional
- ICH
International Council on Harmonization
- ICU
intensive care unit
- MS
multiple sclerosis
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organization
Appendix. Authors

Contributor Information
Ajay Kilaru, Email: ajay.kilaru@novartis.com.
Bernhard Hemmer, Email: hemmer@tum.de.
Bruce Anthony Campbell Cree, Email: bruce.cree@ucsf.edu.
Benjamin M. Greenberg, Email: benjamin.greenberg@utsouthwestern.edu.
Uma Kundu, Email: uma.kundu@novartis.com.
Thomas Hach, Email: thomas.hach@novartis.com.
Virginia DeLasHeras, Email: virginia.delasheras@novartis.com.
Brian J. Ward, Email: brian.ward@mcgill.ca.
Joseph Berger, Email: joseph.berger@uphs.upenn.edu.
Study Funding
This work was funded by Novartis Pharma AG, Basel, Switzerland.
Disclosure
R. Sullivan is an employee of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. A. Kilaru is an employee of Novartis Pharma AG, Basel, Switzerland. B. Hemmer has served on scientific advisory boards for Novartis. He has served as Data Monitoring and Safety Committee member for AllergyCare, Polpharma, and TG therapeutics. He or his institution have received speaker honoraria from Desitin. His institution received research grants from Regeneron for MS research. He has been funded by the EU project Multiple MS, the excellence cluster Synergy, and the BMBF funded project Clinspect. He holds part of 2 patents. One for the detection of antibodies against KIR4.1 in a subpopulation of patients with MS and the other for genetic determinants of neutralizing antibodies to interferon; B.A.C. Cree for consulting from Alexion, Atara, Autobahn, EMD Serono, Novartis, Sanofi, Therini, and TG Therapeutics and received research support from Genentech. B.M. Greenberg has received consulting fees from Alexion, Novartis, EMD Serono, Viela Bio, Genentech/Roche, Greenwhich Biosciences, Axon Advisors, Rubin Anders, Abcam, Signant, IQVIA, Sandoz, Druggability Technologies, Genzyme, Immunovant, and PRIME Education. He has received grant funding from PCORI, NIH, NMSS, The Siegel Rare Neuroimmune Association, Clene Nanomedicine, and the Guthy-Jackson Charitable Foundation for NMO. He serves as an unpaid member of the board of the Siegel Rare Neuroimmune Association. He receives royalties from UpToDate. U. Kundu is an employee of Novartis Healthcare Pvt. Ltd. T. Hach is an employee of Novartis Pharma AG, Basel, Switzerland. V. DeLasHeras is an employee of Novartis Pharma AG, Basel, Switzerland. B.J. Ward serves on a scientific advisory board for Novartis and reports personal fees from Novartis for this activity. He is also a medical officer for Medicago Inc and holds parts of patents for vaccines targeting influenza, Clostridioides difficile, and Schistosoma mansoni. In the last 5 years, he has held academic industry awards with Medicago, MIT Canada, and Aviex Technologies. J. Berger reports grants from Biogen and Genentech/Roche; personal fees from Amgen, Biogen, Dr. Reddy, Encycle, Excision-Bio, Genentech/Roche, Genzyme, Inhibikase, MAPI, Merck, Millennium/Takeda, Morphic, Novartis, Serono, and Shire.
References
- 1.Johns Hopkins University & Medicine Coronavirus Resource Center. Accessed September 30, 2020. Available at: coronavirus.jhu.edu/
- 2.Center for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Accessed September 30, 2020. www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
- 3.Sormani MP, De Rossi N, Schiavetti I, et al. For the Musc-19 Study Group. Disease modifying therapies and COVID-19 severity in multiple sclerosis. SSRN: 10.2139/ssrn.3631244. [DOI]
- 4.Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson-Yap S, De Brouwer E, Kalincik T, et al. presented at MSVirtual2020, First results of the COVID-19 in MS Global Data Sharing Initiative suggest anti-CD20 DMTs are associated with worse COVID-19 outcomes (SS02.04).
- 6.COViMS Registry. The COViMS database public data update. Accessed December 15, 20. www.COViMS.org.
- 7.Peeters LM, Parciak T, Walton C, et al. COVID-19 in people with multiple sclerosis: a global data sharing initiative. Mult Scler. 2020;26(10):1157-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of DMT therapies with COVID-19 severity in multiple sclerosis. medRxiv 2021.02.08.21251316. [DOI] [PMC free article] [PubMed]
- 9.Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): physiology and the effects of S1P receptor modulation. Neurology. 2011;76(8 suppl 3):S3-S8. [DOI] [PubMed] [Google Scholar]
- 10.GILENYA® US prescribing information. Accessed October 10, 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2019/022527s26lbl.pdf
- 11.MAYZENT® US prescribing information. Accessed October 10, 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2019/209884s000lbl.pdf.
- 12.Mehling M, Hilbert P, Fritz S, et al. Antigen‐specific adaptive immune responses in fingolimod‐treated multiple sclerosis patients. Ann Neurol. 2011;69(2):408-413. [DOI] [PubMed] [Google Scholar]
- 13.COVID-19: developing drugs and biological products for treatment or prevention. Accessed October 10, 2020. www.fda.gov/regulatory-information/search-fda-guidance-documents/covid-19-developing-drugs-and-biological-products-treatment-or-prevention.
- 14.Clinical Management of COVID-19: Interim Guidance, 2020. Accessed October 10, 2020. apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf. [Google Scholar]
- 15.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239-1242. [DOI] [PubMed] [Google Scholar]
- 16.Kappos L, Radue E-W, O'Connor P, et al. For the Freedoms Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387-401. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JA, Barkhof F, Comi G, et al. For the TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402-415. [DOI] [PubMed] [Google Scholar]
- 18.Kappos L, Bar-Or A, Cree BAC, et al. EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263-1273. [DOI] [PubMed] [Google Scholar]
- 19.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR. 2020;69(24):759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a north American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Kandula S, Huynh M, et al. Estimating the infection-fatality risk of SARS-CoV-2 in New York City during the spring 2020 pandemic wave: a model-based analysis. Lancet Infect Dis. 2021;21(2):203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alomar M, Tawfiq AM, Hassan N, Palaian S. Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: current status, challenges and the future. Ther Adv Drug Saf. 2020;11:2042098620938595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MS International Federation. Global COVID-19 Advice for People with MS. Last updated on June 4, 2021. Accessed June 10, 2020. www.msif.org/wp-content/uploads/2021/06/June-2021-MSIF-Global-advice-on-COVID-19-for-people-with-MS-FINAL.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data can be made available on request for research purposes by sending a request to the corresponding author.