Abstract
Extensive evidence links adverse experiences during childhood to a wide range of negative consequences in biological, socioemotional, and cognitive development. Unpredictability is a core element underlying most forms of early adversity; it has been a focus of developmental research for many years and has been receiving increasing attention recently. In this article, we propose a conceptual model to describe how unpredictable and adverse early experiences affect children’s neurobiological, behavioral, and psychological development in the context of the COVID-19 pandemic. We first highlight the critical role of unpredictability in child development by reviewing existing conceptual models of early adversity as they relate to subsequent development across the lifespan. Then, we employ a translational neuroscience framework to summarize the current animal- and human-based evidence on the neurobiological alterations induced by early experience unpredictability. We further argue that the COVID-19 pandemic serves as a global “natural experiment” that provides rare insight to the investigation of the negative developmental consequences of widespread, clustered, and unpredictable adverse events among children. We discuss how the pandemic helps advance the science of unpredictable early adverse experiences. As unpredictability research continues to grow, we highlight several directions for future studies and implications for policymaking and intervention practices.
Keywords: Unpredictability, Instability, Early Adversity, COVID-19 Pandemic, Translational Neuroscience
Highlights
-
•
Unpredictability is a core dimension that manifests in most forms of early adversity.
-
•
The corticolimbic neural circuitry underlies unpredictability impact on development.
-
•
Unpredictability also affects development via stress response and immune functions.
-
•
The COVID-19 pandemic provides rare insight to advance unpredictability research.
-
•
Policy & intervention practices need to account for unpredictable early experience.
1. Introduction
Early adversity plays a critical role in shaping children’s biological, socioemotional, and cognitive development (Cicchetti, 2016, Pechtel and Pizzagalli, 2011, Taylor et al., 2011). Historically, stress has been studied in three primary ways: employing animal models through experimental paradigms (e.g., rodent handling, maternal separation, depleted resources; Lyons et al., 2010), examining human conditions such as abusive and/or neglectful parenting, institutional rearing, and poverty (Pechtel and Pizzagalli, 2011), and studying the effects of so-called “natural experiments” of large-scale disruptive events such as natural disasters, military conflicts, forced relocation, and global pandemics (Huang et al., 2013, Pesonen et al., 2010, Roubinov et al., 2020). Collectively, this research has shown that early adversity shapes not only brain architecture, but also metabolic, cardiovascular, and other core neurobiological functions, as well as psychological development (Agorastos et al., 2019, Pechtel and Pizzagalli, 2011, VanTieghem and Tottenham, 2017). From a dose-response perspective, there is also considerable evidence that the earlier, more prolonged, and more intensive adversity is related to a greater impact on child development (Smith and Pollak, 2021b). In spite of the progress that has been made in this area, the specific dimensions of adverse experiences that drive developmental alterations and underlying mechanisms are still a matter of some debate.
In this paper, we first review current models of early adverse experiences as they relate to subsequent development across the lifespan and specifically focus on the domain of unpredictability as a common core experience that manifests in most forms of early adversity. In the context of drastically growing unpredictability since the COVID-19 pandemic, we propose a new conceptual model that incorporates both traditional family-level and pandemic-induced community-level and sociocultural unpredictability factors and describes their potential neurobiological and behavioral influences on child development. Next, we propose that a translational neuroscience framework is particularly applicable for understanding the influence of unpredictability and informing policymaking, prevention, and intervention efforts. Aligned with the translational neuroscience framework, we draw evidence from animal and human models and provide a summary of neurobiological alterations related to unpredictability in early adverse experiences. We then shift to describing how the COVID-19 pandemic, a unique global event in human history, provides rare opportunities to advance our understanding of unpredictable and adverse early experiences in relation to child development. We argue that the magnitude/intensity and duration/chronicity of the disruption that children have been experiencing as well as the unpredictability introduced by the pandemic both have deleterious impact on children’s development, which require policy and community program intervention. Lastly, we describe how research on early unpredictability can inform future early adversity research. It is important to note that the current article focuses on unpredictability as an aspect of early adversity; although it is also critical to understand how unpredictability in supportive environments affects optimal child development, this research question is beyond the scope of the current article.
2. Existing conceptual models of early adversity
Research has long documented the negative consequences of early adverse experiences on child development (Cicchetti, 2016, Pechtel and Pizzagalli, 2011, Taylor et al., 2011). Children exposed to chronic and severe early life stress are at elevated risk for physical and mental health issues, risk behaviors, socio-emotional dysregulation, and impairments in learning and behaviors across the lifespan (Taylor et al., 2011). However, several gaps still exist in early adversity research, including an incomplete and evolving understanding of the specific neurobiological mechanisms underlying adversity impact, the links between specific dimensions of adversity and different developmental outcomes, as well as children’s differential responses to similar adverse environments (Smith and Pollak, 2021b). Various conceptual models have been developed to characterize early adverse experiences and provide guidance to fill these gaps. In this section, we review existing early adversity conceptual models and, consistent with several other recent commentaries (e.g., Ellis et al., 2009; Smith and Pollak, 2021b), propose that unpredictability is a core element of early adverse experiences.
2.1. Specificity and cumulative risk models
Early adversity has been investigated using the specificity (Lumley and Harkness, 2007, Wyman, 2003) or cumulative risk (Evans et al., 2013) approaches. Research that adopts the specificity approach examines the independent effects of specific types of adverse experiences (e.g., poverty, abuse, neglect) on particular developmental outcomes through distinguishable pathways (Lumley and Harkness, 2007, Spinhoven et al., 2010). Central to the specificity approach is the notion that children’s development, coping, and adaptation are context-specific (Wyman, 2003). Specificity models provide unique contributions to the scientific understanding of the effects of the distinct adversity types, which can potentially inform policymaking and intervention design targeting individuals experiencing particular types of adverse experiences (e.g., child protective services for maltreatment). However, the specificity model is less well-suited to understanding the co-occurrence of multiple forms of adversity because it may omit the possibility that different types of adversity exert effects on developmental outcomes through common mechanisms (McLaughlin et al., 2020, Smith and Pollak, 2021b).
The cumulative risk model approaches early adversity differently by assessing adversity as the composite of multiple risk factors and combining different overlapping or independent risk factors into one summary/composite score (Evans et al., 2013). The central tenet of the cumulative risk perspective is that children’s developmental outcomes are largely affected by the accumulation of adverse experiences independent of the presence or absence of a specific experience, and children perceive environmental risks as a whole rather than individual risk factors (Appleyard et al., 2005, Rutter, 1979, Sameroff, 2000). Notably, the specificity and cumulative risk models are not completely opposing frameworks; the cumulative risk approach can also be applied to examine the specific effect of a particular category of risk (e.g., cumulative socioeconomic status risk, Brody et al., 2013; physical & psychological risks, Evans, 2003). The main distinction between the two models is that, while the specificity model emphasizes individual risk factors, the cumulative risk model highlights the developmental influence of cumulative risk factors that is beyond the specific effects of individual risk factors or their additive effects (Evans, 2003, Evans et al., 2013).
The cumulative risk model is well aligned with the allostatic load theory (i.e., chronic exposure to environmental stressors leads to physical “wear and tear”; McEwen and Stellar, 1993) and has been increasingly applied to investigate the physiological impact of early adversity on child development (Doan, 2021, Evans and Cassells, 2014, Gallo et al., 2014, Suvarna et al., 2020). This perspective has also made considerable contributions to raising public awareness about the deleterious influence of adverse childhood experiences (Lacey and Minnis, 2020). For example, the Adverse Childhood Experiences (ACE) study (Felitti et al., 1998) found a graded dose-response association between the number of childhood adversity exposures and health risk behaviors/diseases during adulthood. This study informed the Centers for Disease Control (CDC) and the state health departments to include the ACE questionnaire in the Behavioral Risk Factor Surveillance System and to investigate the effects of cumulative risk factors on public health issues (Anda et al., 2010, Dube, 2018). The cumulative risk scores can also be used as a screening tool in trauma-informed intervention and prevention efforts (Cohen et al., 2019). Despite its public health impact, this perspective bears the limitation of not capturing important characteristics of adverse experiences (e.g., severity/intensity, chronicity, unpredictability, etc.). Studies using cumulative risk scores are often not able to capture the distinct mechanisms underlying different types of environmental experiences (McLaughlin and Sheridan, 2016). As such, the cumulative risk model is more suited when the exposure to chronic stressors as a whole rather than individual risk factors is of interest.
2.2. Bronfenbrenner bioecological systems theory
Bronfenbrenner’s bioecological systems theory (Bronfenbrenner, 1979, Bronfenbrenner and Ceci, 1994, Bronfenbrenner and Evans, 2000) provides a framework for the scientific understanding of human development in ecological systems, which directly contributes to the investigation of early adversity. By highlighting developmental processes, individual factors, and multilevel ecological context, this theory posits complicated, transactional, and multi-level processes of human development (Bronfenbrenner and Evans, 2000, Brown et al., 2019). As such, the developmental impact of early adversity occurs in the reciprocal processes between children and their environments, including neurobiological context, family environments, neighborhoods, cultures, and sociohistorical events (McCoy, 2013).
The scientific investigation of chaos or environmental instability (characterized by unpredictability, a lack of routines, and unplanned changes) has important historical roots in the bioecological systems theory. Bronfenbrenner and Evans (2000) purport that instability/chaos poses distinct challenges to developmental adjustment through interfering with predictable proximal processes. Built on the bioecological systems theory, Evans and Wachs (2010) highlights the unique role of chaos/instability in child development and brings the investigation of unpredictable experiences under an umbrella of chaos. The research by Evans and Wachs (2010) has inspired numerous empirical studies to model the independent effects of chaos/instability (Evans et al., 2005, Raver et al., 2015) or include chaos/instability (e.g., family turmoil, housing instability, household disorganization) in the creation of cumulative risk factors (Blair et al., 2011, Blair et al., 2011, Evans, 2003, Evans and Cassells, 2014). The bioecological systems theory also emphasizes individuals’ neurobiology as a critical context for developmental adjustment and thus lays the ground for understanding the neurobiological underpinnings of chaos/instability (Bronfenbrenner and Evans, 2000). As suggested by Brown et al., 2019, Brown et al., 2021, the frequent and irregular neurobiological responses to chaotic and unstable environmental inputs may carry a physiological cost and underlie the development of maladaptive outcomes.
2.3. Dimensional models
Recent research that aims to assess distinct neurobiological underpinnings of different types of adverse events while also accounting for the co-occurrence of risk factors starts adopting a dimensional perspective. Dimensional models can reconcile the aforementioned shortcomings of the cumulative risk and specificity approaches (McLaughlin et al., 2020, McLaughlin and Sheridan, 2016). On the one hand, dimensional models focus on the core elements/features shared across numerous types of adversities and seek to identify the common mechanisms underlying their effects. On the other hand, dimensional models also acknowledge distinguishable aspects of adverse experiences that distribute across a spectrum and differentially affect neurobiological and behavioral development. As such, dimensional models have been increasingly employed in studies that aim to identify neurobiological mechanisms underlying the negative effects of early adversity.
2.3.1. Threat and deprivation model
McLaughlin and colleagues characterize early adversity into two distinctive (but not mutually exclusive) underlying dimensions: threat and deprivation (McLaughlin and Sheridan, 2016, McLaughlin et al., 2014, Sheridan and McLaughlin, 2014). In this model, threat represents experiences that may harm or cause a threat of harm to children (e.g., physical or sexual abuse); deprivation indicates the lack of environmental inputs necessary for optimal emotional, cognitive, and social development (e.g., physical or emotional neglect, poverty; Colich et al., 2020; Sheridan and McLaughlin, 2014). The dimensions of threat and deprivation have partially distinct influences on child development through different neural and physiological mechanisms (McLaughlin et al., 2020). Experiences of deprivation are proposed to negatively influence sensory cortex development, such as over-pruning of synaptic connections, reduced numbers of synaptic connections and dendritic branching, and volume reductions in frontoparietal, default, and visual neural systems (Colich et al., 2020). Threatening experiences are proposed to cause changes in neural regions related to fear processing and emotional learning, such as the amygdala, hippocampus, and ventromedial prefrontal cortex (Colich et al., 2020, Sheridan and McLaughlin, 2014). The distinct influences of threat and deprivation dimensions of early experience have been demonstrated in numerous empirical studies (e.g., Johnson et al., 2021; Machlin et al., 2019; Miller et al., 2018; Sumner et al., 2019).
2.3.2. Harshness and unpredictability: evolutionary developmental theories
Another dimensional model is informed by the life history theory using an evolutionary developmental framework (Belsky et al., 2012, Brumbach et al., 2009, Ellis et al., 2009). This theory posits that individuals’ life traits (i.e., characteristics determining reproduction rates, growth, aging, and parental investment) are distributed on a continuum from slow to fast (Charnov and Berrigan, 1993, Nettle et al., 2013, Roff et al., 2002). The variance of life traits depends on evolutionarily adaptive trade-offs of resource allocation, such as the trade-offs between maintenance and growth, survival and reproduction, current and future reproduction, as well as offspring quality and quantity (Belsky et al., 2012, Brumbach et al., 2009, Roff et al., 2002). These trade-offs rely on environmental inputs to coordinate individuals’ physiology and behaviors with the goal of promoting evolutionary fitness (i.e., survival and reproduction; Brumbach et al., 2009).
Ellis et al. (2009) purport that two dimensions – harshness and unpredictability – provide fundamental environmental inputs that determine the development of individuals’ life-history strategies. Harshness describes the magnitude of environmental risk that causes morbidity or mortality, while unpredictability represents the spatial-temporal variation in environmental harshness (Belsky et al., 2012, Brumbach et al., 2009, Ellis et al., 2009). When early environments are safe and predictable, individuals are more likely to develop slower life strategies such as prolonged maturation, later reproduction, and greater longevity (Hawkes, 2006). In contrast, when individuals perceive the environment as harsh and unpredictable, they are more likely to adopt faster life history strategies characterized by early puberty development and early onset of sex behaviors to promote evolutionary fitness (Belsky et al., 2012). Despite being evolutionarily or biologically adaptive, faster life-history strategies in modern society are related to socially undesirable and dysfunctional traits and behaviors, such as aggression, reduced empathy, self-harm behaviors, as well as internalizing and externalizing psychopathology symptoms (Del Giudice, 2014, Hurst and Kavanagh, 2017). Informed by Ellis et al. (2009), numerous empirical studies have adopted the life history perspective to examine the influence of environmental unpredictability (e.g., Doom et al., 2016; Mittal et al., 2015; Simpson et al., 2012; Szepsenwol et al., 2017).
2.4. Topological approach
Lastly, Smith and Pollak (2021b) propose a topology model to advance the understanding of neurobiological mechanisms underlying the effect of early adversity on child development. The topology approach highlights perceptions of early adverse experiences in developmental trajectories (Smith and Pollak, 2021a). Therefore, factors that change children’s perception and interpretation of stressful events are of particular focus in this topology model. In the topology model, Smith and Pollak (2021b) list critical features of early adversity, such as the chronicity, intensity, developmental timing, predictability of adverse events, as well as safety and social support in the interpersonal context. As a key feature, unpredictability is proposed to affect children’s emotional, cognitive, and physical development through an extended activation of the stress response systems (Soltani and Izquierdo, 2019), which consequently results in alteration of brain architecture in the prefrontal-hippocampal-amygdala (i.e., corticolimbic) neural circuitry (Turecki and Meaney, 2016, Tyrka et al., 2012) and the neuroendocrine stress response system (Hunter et al., 2011, Koss and Gunnar, 2018).
3. Unpredictability as a core experience of early adversity
3.1. Insights from existing models and empirical evidence
Based on the review of existing conceptual models, we highlight unpredictability as a core element that manifests in many adverse experiences. The importance of unpredictability in child development has been introduced in multiple early adversity conceptual models, as discussed above. Indeed, some researchers argue that adversity is reflected by environmental unpredictability, namely, “deviations in or disruptions of the expectable environments” (Nelson, 2007, Nelson and Gabard-Durnam, 2020).
Research has begun to increasingly incorporate unpredictability as an independent risk factor of developmental maladaptation. For example, experiencing physical environmental unpredictability (assessed through maternal employment, residence, and cohabitation changes) in early childhood has been found to be associated with externalizing behaviors and substance use during adolescence (Doom et al., 2016) and risky sexual behaviors during young adulthood (Simpson et al., 2012, Szepsenwol et al., 2017). Household chaos has been linked to children’s elevated socioemotional distress (Evans et al., 2005) and emotional regulatory difficulties (Raver et al., 2015). Household economic instability and food insecurity have been associated with children’s poor global health rating (Wolf and Morrissey, 2017) and poor educational outcomes (Elliott, 2013). Experiencing caregivers’ mood instability has been found to predict children’s lagged cognitive and language development (Howland et al., 2021) and increased internalizing symptoms (Glynn et al., 2018). Viewed together, these studies suggest that unpredictability in early adverse experiences confer increased risks for developmental maladaptation in later years of life. Despite these recent advances, unpredictability is still an understudied dimension compared to other adversity elements (e.g., intensity/severity, chronicity, developmental timing, etc.), and its neurobiological underpinnings still need further empirical investigation.
3.2. Methodological considerations in early adversity unpredictability operationalization
The relative paucity of research on early experience unpredictability may be partly due to methodological challenges in accurately operationalizing and measuring environmental unpredictability. Unpredictability manifests in many formats (e.g., financial instability, household chaos, unpredictable caregiving), temporal patterns, and social contexts (e.g., family, community, sociocultural). The diversity of unpredictability factors leads to variations in the statistical assessment of unpredictability (Young et al., 2020).
Existing unpredictability research has been mainly relying on retrospective self- or caregiver-report questionnaires, interviews, or observations obtained at one single time point. Examples of unpredictability factors that can be directly measured through empirically validated questionnaires include food insecurity (assessed via USDA Food Insecurity Surveys; US Department of Agriculture, 2012), household chaos (assessed via the Chaos, Hubbub, and Order Scale [CHAOS]; Evans et al., 2005), lack of routines (assessed via, for example, the Family Routine Inventory; Jensen et al., 1983), and physical environment instability (assessed via retrospective reports on caregivers’ job changes, parental transitions, and residential changes during interviews; Mittal et al., 2015; Simpson et al., 2012). The Questionnaire of Unpredictability in Childhood (QUIC) developed by Glynn et al. (2019) also uses retrospective reports to capture unpredictability exhibited in parental monitoring and involvement, parental predictability, parental environment, physical environment, as well as safety and security. Additionally, laboratory-based or home-based observation paradigms are usually used to assess unpredictable parenting behaviors (Davis et al., 2017, Granger et al., 2021, Noroña-Zhou et al., 2020).
Although traditional questionnaires and observations have provided valuable information to characterize unpredictability in early adverse experiences, these approaches bear some limitations. For example, using caregiver-report questionnaires does not allow researchers to disentangle the actual unpredictability experienced by the child from perceived unpredictability by the caregiver. Moreover, questionnaires and observation paradigms are often intended for one single time-point assessment, which obscures unpredictability’s inherent temporal changes over time (Jebb and Tay, 2017, Young et al., 2020). Without time-series data of environmental harshness, researchers cannot assess whether different statistical properties of unpredictability (e.g., variance, autocorrelation; explained in details below) have distinct influences on developmental maladaptation. The lack of time-series data also makes it difficult to differentiate the effects of unpredictability from other elements of adverse experiences, such as chronicity and intensity (Young et al., 2020). As such, unpredictability research can benefit from using time-series data of environmental harshness, and proper statistical properties need to be identified to characterize the temporal patterns of unpredictable early experiences.
Built on Ellis et al. (2009), Young et al. (2020) provide concrete steps to accurately operationalize unpredictability by listing critical statistical properties of temporal patterns. In this article, unpredictability is defined as the spatial-temporal stochastic/random variation in environmental harshness. An accurate assessment of unpredictability needs to account for statistical properties of variance (i.e., level of deviation from mean environmental harshness), autocorrelation (i.e., how much the current situations relate to future conditions; Frankenhuis et al., 2019), cue reliability (i.e., the reliability in which experiences being assessed can truly reflect environmental harshness; Jebb et al., 2015), and patterns of change (e.g., seasonal, cyclic; Jebb et al., 2015). Based on different characteristics of these statistical structures, unpredictability can be further categorized into stationary (i.e., the statistical structure of environmental harshness remains consistent over a lifetime) and non-stationary (i.e., the statistical structure of environmental harshness changes over a lifetime) formats (Young et al., 2020).
In addition to the statistical properties, Young et al. (2020) also outlines two proximate mechanisms of environmental unpredictability, namely, how individuals detect environmental unpredictability and generate corresponding responses. The first mechanism is the ancestral cue perspective (Ellis et al., 2009) derived from an evolutionary framework (Buss, 1995, Tooby and Cosmides, 1990). This perspective suggests that the human brain has been shaped by natural selection to directly detect cues of unpredictability in the surrounding environment. Developmental adjustment is enacted quickly and effectively because it only relies on limited information (i.e., “ancestral cues”) that indicates potential unpredictability. From this perspective, factors that directly reflect environmental variation can serve as “ancestral cues” of unpredictability (e.g., household chaos, lack of family routines, changes in physical environments) and be measured via retrospective reports in questionnaires or observations at a single time-point with relatively high accuracy (Young et al., 2020).
The second proximate mechanism is the statistical learning perspective (Frankenhuis et al., 2013, Frankenhuis et al., 2019), which suggests that the human brain keeps tracking the statistical properties and uses them as raw data to model environmental harshness and estimate unpredictability without relying on evolutionarily established cues. This perspective highlights the necessity of characterizing patterns of change over time using time-series data and analytical skills (Jebb and Tay, 2017, Jebb et al., 2015), which has been enabled by recent methodological advancement in daily diary studies, experience sampling, and long-term longitudinal studies (Young et al., 2020). This statistical learning perspective may be more applicable to assessing unpredictability factors that exhibit in living experiences over time, such as financial instability, inconsistent parenting, and caregivers’ mood instability. For example, Li et al. (2018) have obtained income unpredictability using repeatedly measured income-to-needs ratio across six time-points. Using repeated measured mood questionnaires across five time-points, Glynn et al. (2018), Howland et al. (2021) have applied Shannon’s entropy (Cover and Thomas, 2006) to capture maternal mood instability. For these factors, the environmental harshness at a single time-point (e.g., financial difficulty, harsh parenting) does not reflect unpredictability per se. Rather, unpredictability manifests in the frequent, stochastic changes of environmental status and needs to be assessed via time-series data of environmental harshness.
It is worth noting that the ancestry cues and statistical learning perspectives are parallel but not mutually exclusive. Individuals can detect environmental unpredictability through both evolutionarily validated cues and estimations based on previous experiences. To better characterize the proximate mechanisms of unpredictability and improve its measurement operationalization, Young et al. (2020) suggest future studies to include traditional measures of unpredictability alongside time-series data of environmental harshness, which will allow researchers to explore how unpredictability from the two perspectives may differentially affect development (Young et al., 2020).
3.3. A new conceptual model of unpredictability in relation to child development
In addition to the methodological considerations, the conceptualization of unpredictability in early adversity research also needs improvement. The majority of existing research in this field focuses on unpredictability factors in the family context and has rarely taken higher-order social contexts (e.g., community, socio-cultural environments) into account. However, factors in these higher-order social contexts, such as frequent policy changes, shifts of childcare or school formats, uncertainty about the virus and its variants, changing public health guidelines, and political issues, have directly and critically contributed to the drastically increasing unpredictability during the COVID-19 pandemic. These factors may also have a top-down effect and induce unpredictability in the family context, such as increasing financial instability and disrupting family routines.
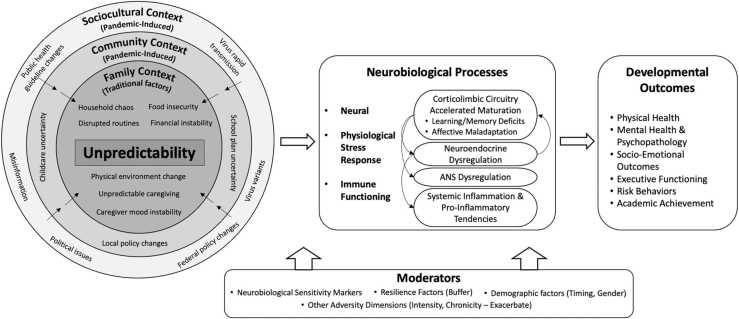
In this article, we propose a new conceptual model (presented in Fig. 1) to depict how unpredictability in multiple social contexts may affect developmental adaptation through alternating neurobiological processes. This model makes a unique contribution to the literature in two ways. First, it accounts for both traditional family-based unpredictability factors and unpredictability in community and sociocultural contexts induced by the pandemic. Across the multi-level social contexts, unpredictability is identified as a common dimension of early adverse experiences. Second, this model adopts a translational neuroscience perspective and provides a summary of empirically proved neurobiological mechanisms underlying the negative impact of unpredictability. Based on literature review, three neurobiological biological processes and their reciprocal interactions are proposed to underlie this negative impact: 1) the accelerated maturation of the corticolimbic neural circuitry, 2) physiological stress response mediated via the neuroendocrine and autonomic nervous systems (ANS), and 3) systemic inflammation and pro-inflammatory tendencies. In this conceptual model, we also propose that the indirect impact of unpredictability on developmental outcomes through neurobiological processes may be moderated by children’s neurobiological sensitivity markers, resilience factors, demographic characteristics (e.g., developmental timing, gender), and other adversity dimensions.
Fig. 1.
Conceptual model of the impact of unpredictable and adverse early experiences on child development in the context of the COVID-19 pandemic.
This conceptual model is guided by the translational neuroscience framework with the goal of informing not only research advancement but also practical policymaking and preventive intervention strategies (Fisher, 2016, Horn et al., 2020). In the section below, we provide a brief summary of the translational neuroscience framework. Then, we review evidence from both animal and human studies that delineates the neurobiological underpinnings of unpredictable early experiences.
4. Translational neuroscience framework in unpredictability research: neurobiological mechanisms underlying unpredictability impact on behaviors and health
Translational neuroscience serves as a bridge that connects basic neuroscientific research and clinical intervention science, two fields that have historically evolved somewhat independently (Horn et al., 2020). Adopting this systemic and integrative research approach, basic and clinical advancements in neuroscience can be employed to identify malleable mechanisms underlying socioemotional and behavioral maladjustment. This knowledge can inform the design and implementation of scalable, noninvasive clinical and intervention practices (Fisher and Berkman, 2015, Horn et al., 2020). Understanding the effects of early adversity on the development of neurobiological architecture, in particular, may provide new opportunities for innovative clinical and intervention designs that target negative consequences of early adverse experiences (Fisher and Berkman, 2015). For example, including biomarkers can enhance behavioral assessments used to identify children’s risk for psychopathology at an earlier stage (Bauer et al., 2003, Jaffee, 2018), delineate neurobiological underpinnings of heterogeneous yet overlapping diagnostic categories, and potentially improve the clinical diagnostic systems (Horn et al., 2020). Incorporating neurobiological markers in clinical practices might also help accurately and sensitively identify individual differences in treatment response and inform individualized treatment strategies (Fishbein and Dariotis, 2019).
Despite existing barriers (e.g., cost, limited scalability in community settings; Horn et al., 2020), translational neuroscience is a collaborative and interdisciplinary approach that has great promises to transform neuroscientific knowledge into clinical and intervention practices. These practices will alleviate developmental risks rendered by early adversity (Fisher, 2016, Fisher and Berkman, 2015, Fisher et al., 2016, Horn et al., 2020). Investigating the neurobiological underpinnings of unpredictability impact on child development, therefore, has unique translational implications for policymaking and prevention designs.
4.1. Insights from animal models
A large body of existing neuroscientific evidence of unpredictability impact on brain structures and functions is based on the examination of fragmentation and unpredictability in experimental manipulations of rodent maternal signals (Baram et al., 2012, Molet et al., 2016). These studies involve an early-life stress experimental paradigm that simulates poverty, consisting of limited bedding and nesting (LBN) materials in the home cage (Avishai-Eliner et al., 2001, Baram et al., 2012, Brunson et al., 2005, Ivy et al., 2008). The LBN paradigm results in stress of the mother and promotes fragmentation (i.e., maternal care behaviors occur in a larger number of short episodes instead of a smaller number of longer episodes) and unpredictability (i.e., the lack of patterned behavioral sequence, such as grooming always following nursing) in maternal care signals (Davis et al., 2017). Research employing this rodent paradigm pinpoints that early life stress involving fragmented and unpredictable maternal signals may alter neural synapse stabilization and circuit maturation processes in the corticolimbic circuitry (including the amygdala, hippocampus, and medial prefrontal cortex [mPFC]) that underlies cognitive (e.g., memory) and emotional/affective maladaptation (Baram et al., 2012, Bolton et al., 2017, Gee, 2021, Glynn and Baram, 2019). These neural alterations have been shown to cause persisting cognitive and emotional dysfunctions in later life (Brunson et al., 2005, Ivy et al., 2010).
Cognitively, fragmented and unpredictable maternal signals have been linked to learning deficits and memory impairments involving the hippocampus. On a cellular level, male rats assigned to the poverty-simulating LBN stress paradigm have been found to exhibit hippocampal dysfunctions such as dendritic atrophy (i.e., reduced complexity of neuron branching) and mossy fiber expansion, compared to the control group (Brunson et al., 2005). These intercellular changes modulate intracellular structures, functions, and epigenetic processes, resulting in deficits in hippocampal structures and functions (Baram et al., 2012). Molet et al. (2016b) report that rats assigned to the LBN stress paradigm exhibit disruptions of dendritic structure and connectivity, impairments in long-term potentiation, and loss of dorsal hippocampal volume. In a cross-species (rats and human) study, Davis et al. (2017) find that the entropy rate of maternal signals, a quantitative measure of unpredictability, is linked to rats’ poor hippocampus-dependent spatial memory.
Unpredictable early experiences have also been shown to affect rodents’ brain circuits underlying affective functioning and pleasure reward (Baram et al., 2012, Glynn and Baram, 2019), which may subsequently influence addiction and risk behaviors (Bolton et al., 2018). For example, Guadagno et al. (2018) suggest that rats assigned to the LBN poverty-simulating condition exhibit reductions in resting-state fMRI connectivity between the anterior basolateral amygdala and mPFC compared to rats in the normal bedding condition. Neural alterations in corticolimbic interactions associated with anhedonia (i.e., reduced capacity to experience pleasure) are also observed through multiple animal studies. For instance, rats in the LBN condition are found to present aberrant functional connectivity of reward and aversion-related neural circuits (indicated by activated corticotropin-releasing hormone [CRH] expression in the central nucleus of the amygdala), which is further associated with anhedonia (Bolton et al., 2018). In another study, Molet et al. (2016a) suggest that fragmented, unpredictable maternal signals in the LBN stress paradigm might disrupt the maturation of dopaminergic pleasure circuits that underlie anhedonia during adolescence in rodents. In contrast, predictable and nurturing early experiences might play a critical role in the healthy development of reward and affective neural circuits. For example, Singh-Taylor et al. (2018) uncover a synaptic and epigenetic mechanism through which rats that receive augmented maternal care after a brief separation present diminished anxiety-like and depression-like behaviors.
The neurobiological changes induced by unpredictability are intertwined with the neuroendocrine stress response system regarding both epigenetic processes and hormone release. Unpredictability-related alternations at the inter-cellular level (e.g., dendritic atrophy) are hypothesized to affect intra-cellular epigenetic processes of hypothalamic CRH gene expression (Baram et al., 2012). Receiving augmented, consistent, and predictable maternal care has been found to reduce excitatory innervation of CRH-expressing neurons, suppress CRH gene expression (Korosi et al., 2010, McClelland et al., 2011), and repress CRH release from the hypothalamus in response to stress (Baram et al., 2012). The reduced CRH level suppresses the secretion of adrenocorticotropic hormone from the anterior pituitary, which further reduces glucocorticoid (i.e., cortisol and androgens) release from the adrenal cortex (Allen and Sharma, 2018). The attenuated levels of glucocorticoid in plasma have also been shown to augment the expression of glucocorticoid receptor genes in the hippocampus (Fenoglio et al., 2005, Ivy et al., 2010, Weaver et al., 2004). As such, Baram et al. (2012) speculate that fragmented, unpredictable maternal signals may have an opposite effect, enhancing CRH expression and increasing glucocorticoid release from the adrenal cortex. Ivy et al. (2010) present support to this speculation by showing that adult rats in LBN settings exhibit augmented hippocampal CRH expression, contributing to structural impairments in the hippocampus and related cognitive dysfunctions.
Unpredictability in early adverse experiences may also affect immune functioning. In a typical negative feedback loop, glucocorticoids can slow down inflammatory processes by bindings to glucocorticoid receptors in immune cells (Miller et al., 2011). However, early adversity can induce more pronounced stress responses and reduced sensitivity to the inhibitory hormonal (cortisol) signals in monocytes immune cells, leading to systemic inflammation and chronic pro-inflammatory tendencies (Miller et al., 2009, Miller et al., 2009). Therefore, chronic inflammatory tendencies can co-occur with elevated cortisol output. Preliminary empirical studies start revealing the influence of unpredictable early experiences on immune functioning (Miller et al., 2011). For example, Zhang et al. (2010) find that exposure to chronic unpredictable stressors (an experimental paradigm consisting of heat/cold stimulation, cage tilting, wet bedding, lights on over night, tail pinch, high-speed agitation, overhang, water/food deprivation; Willner, 1997) is associated with accelerated inflammation among mice. Using a similar experimental paradigm, Blossom et al. (2020) report that rats under chronic unpredictable stress have higher levels of inflammation as indicated by C-reactive protein (CRP) levels. Another study by Mormede et al. (1988) also suggests that stress unpredictability is related to disrupted immune functioning such as reduced antibody response and lymphocyte reactivity. In sum, empirical studies in the animal literature based on rodents evince the significant influences of early experience unpredictability on corticolimbic neural structures and functions underlying cognitive and affective dysfunctions, activated neuroendocrine functions indicated by enhanced CRH expression and elevated glucocorticoid release, as well as systemic inflammation and chronic pro-inflammatory tendencies.
4.2. Insights from human models
The majority of animal studies examining the influence of unpredictability have been using the LBN experimental paradigm to provoke fragmented and unpredictable maternal signals. These rodent findings have informed unpredictability research among humans. In parallel to animal models, several human studies have focused on unpredictable maternal sensory signals (typically obtained through coded mother-child dyadic interaction in a free play task) in relation to neural alternations and changes in child neurocognitive development (Davis et al., 2017, Granger et al., 2021, Noroña-Zhou et al., 2020). In addition to unpredictable caregiving, a few studies have also examined the neurobiological influence of other unpredictability forms, such as household chaos (Brown et al., 2019, Brown et al., 2021, Schreier et al., 2014, Tarullo et al., 2020) and financial instability (Brown et al., 2019). Although human research about unpredictable early experiences is still somewhat scarce, emerging evidence shows that early experience unpredictability may also influence human corticolimbic neural circuitry, physiological stress response, and immune functioning.
At the neural level, the corticolimbic circuitry has been proposed to play a critical role in the biological embedding processes of predictability and safety cues among infants and toddlers and lead to subsequent cognitive and emotional changes (Gee, 2021, Gee and Cohodes, 2021). During the early years of life, this corticolimbic circuitry is still under development and particularly sensitive to external regulatory inputs (e.g., from caregivers; Callaghan and Tottenham, 2016; Gee, 2016; Gee et al., 2014). The lack of predictable and safe early environments has been suggested to cause accelerated maturation of the corticolimbic neural circuitry (Gee et al., 2013). For example, in the cross-species study of Davis et al. (2017), the findings from human sample indicate that early exposure to fragmented and unpredictable maternal signals (indexed via an entropy rate) is related to children’s poor hippocampus-dependent recall memory, drawing parallels to the animal findings in the same study. The impact of unpredictability on aberrant corticolimbic maturation has also been supported by Granger et al. (2021), indicating that exposure to unpredictable maternal sensory signals during infancy is associated with an imbalance of medial temporal lobe-prefrontal cortex connectivity (i.e., greater uncinate fasciculus coupled with decreased hippocampal cingulum generalized fractional anisotropy) in children 9–11 years of age. This study further suggests that such aberrant and imbalanced corticolimbic maturation mediates the associations between unpredictable maternal signals and children’s impaired episodic memory function. Lastly, Feola et al. (2021) report elevated amygdala responses to unpredictable threat (operationalized through unpredictable fear face images in an fMRI paradigm) among children (8–10 years old), a pivotal for anxiety in later years of life.
Unpredictable early experiences have been found to affect children’s neuroendocrine functioning. As speculated by Baram et al. (2012), exposure to unpredictable early experiences may amplify hypothalamic CRH gene expression and lead to increased glucocorticoid release from the adrenal cortex. This hypothesis has been empirically supported by findings that economic instability and household chaos significantly lead to children’s elevated cortisol concentration (Blair et al., 2011, Blair et al., 2011, Brown et al., 2019, Brown et al., 2021, Tarullo et al., 2020). In addition to cortisol output levels, studies have also found evidence that unpredictability may lead to dysregulated cortisol diurnal rhythms or neuroendocrine stress responses. For example, Tarullo et al. (2020) suggest that food insecurity is related to flattened cortisol diurnal slope. Using a mother-child interaction coding paradigm (in parallel to the LBN setting in animal models), Noroña-Zhou et al. (2020) report that unpredictable maternal behaviors are connected with infants’ blunted cortisol stress response to a painful stressor.
Lastly, a small number of empirical studies shed light on the impact of unpredictable early life experiences on immune functioning (Robles, 2021). Schreier et al. (2014) suggest that household chaos is associated with adolescents’ greater systemic inflammation and pro-inflammatory tendencies (assessed via stimulated pro-inflammatory cytokine production in response to a bacterial challenge). In a longitudinal study with a child protective service involved sample, Bernard et al. (2019) find that disorganized attachment during childhood predicts higher levels of CRP during early adulthood. Higher levels of systemic inflammation pro-inflammatory tendencies may cause elevated health risks for morbidity and mortality from chronic diseases of aging in later years of life (Miller et al., 2011).
It is worth noting that unpredictable early experiences have been proposed to affect the ANS stress response in the biological sensitivity to context theory (BSCT; Boyce and Ellis, 2005; Ellis et al., 2005) and adaptive calibration model (Del Giudice et al., 2011), but relevant empirical evidence that tests unpredictability as an independent variable is still scarce. From an evolutionary developmental perspective, BSCT proposes a U-shaped relationship between early unpredictability exposure and children’s stress response profiles, where extremely high- and low-stress environments are related to heightened stress reactivity (Boyce and Ellis, 2005, Ellis et al., 2005). Despite some empirical support (Ellis et al., 2005, Ellis et al., 2017, Shakiba et al., 2020), most of these studies focus on environmental harshness (e.g., major stressful life events, family economic conditions, parental psychopathology) and did not parse the unique physiological mechanisms underlying unpredictability. To test the U-shaped hypothesis in relation to unpredictability, empirical studies that examine its independent effect on ANS stress responsivity are still needed.
4.3. Individual heterogeneity in unpredictability impact on child development
Even though unpredictability in early adverse experiences has been suggested to induce developmental maladaptation, youth who experience unpredictability vary significantly in their developmental adjustment (Luthar, 2006, Masten and Obradović, 2006). Some children might be particularly sensitive to environmental influences and, therefore, be more vulnerable to the negative impact of unpredictable early experiences (Belsky et al., 2007, Boyce and Ellis, 2005, Ellis et al., 2005). In contrast, other children might be less responsive and more resistant/resilient to the negative consequences of unpredictability (Masten and Obradović, 2006). Although empirical studies that test this individual heterogeneity in relation to unpredictability are still scarce, existing literature from the evolutionary developmental perspective and the developmental resilience framework may provide insight into the factors that may modulate the impact of unpredictable early experiences.
Theories from the evolutionary developmental perspective, such as BSCT (Boyce and Ellis, 2005, Ellis et al., 2005) and the Differential Susceptibility Theory (DST; Belsky et al., 2007), converge to attribute children’s differential responses to neurobiological sensitivity to environmental inputs. A variety of behavioral and biological markers that reflect neurobiological sensitivity have been identified in existing literature, including behavioral phenotypes (e.g., difficult temperament or emotional reactivity; Cruz et al., 2018; Slagt et al., 2016), dopamine- and serotonin-related genes (Bakermans-Kranenburg and Van Ijzendoorn, 2011, Van IJzendoorn et al., 2012), psychophysiological stress responses (Obradović et al., 2010, Oshri et al., 2021), as well as neural signatures (Liu et al., 2021, Schriber et al., 2017, Schriber and Guyer, 2016, Telzer et al., 2021). Markers of heightened neurobiological sensitivity indicate children’s increased vulnerability to adverse environments, as well as elevated adaptive responses to positive early experiences (Ellis et al., 2011). Therefore, we can speculate that children with markers that reflect elevated sensitivity may exhibit exacerbated maladaptation, while those without these sensitivity markers may be less affected, when exposed to unpredictable early experiences.
The recent advancement in resilience research also sheds light on moderators that may confer transdiagnostic protective effects against early life stress and potentially mitigate the negative influences of unpredictability (Masten, 2021, Masten et al., 2021). Masten et al. (2021) summarized a short list of multi-system resilience factors, including close relationships and social support, sense of belonging, self-regulation, coping skills, planning and problem-solving, future orientation, motivation to adapt, purpose and a sense of meaning, positive views of self/family/group, as well as positive routines and rituals. This list is based on the broader early adversity literature, and it remains to be tested whether these factors can attenuate the negative consequences induced by unpredictable early experiences, in particular.
Lastly, certain demographic characteristics (e.g., developmental timing, gender) and other dimensions of early adversity (e.g., harshness, duration/chronicity) may interact with unpredictable early experiences and affect developmental adjustment. For example, Gee and Cohodes (2021) suggest infancy/toddlerhood as a sensitive period when the corticolimbic neural circuitry is particularly sensitive to unpredictable caregiving inputs. Adolescence may be another sensitive period given the heightened neural plasticity (Atkins et al., 2012) and the opportunity for pubertal stress recalibration (Perry et al., 2022). Increased intensity and duration of adverse experiences may also aggregate the negative consequences induced by unpredictability (Smith and Pollak, 2021b). We still need more studies to delineate these proposed moderating effects of demographic factors and other adversity dimensions.
4.4. Summary of translational neuroscience research on unpredictability
In conclusion, existing literature identifies three main neurobiological mechanisms underlying the impact of unpredictability in early adverse experiences on child development. These mechanisms include the accelerated maturation of the corticolimbic neural circuitry that underlies hippocampal-dependent memory/learning deficits and affective maladaptation, the neuroendocrine and ANS stress response dysregulation, and the elevated systemic inflammation pro-inflammatory tendencies. As presented in the conceptual model (Fig. 1), these systems modulate each other and lead to changes in children’s physical, mental, and behavioral well-being. The developmental pathways following exposure to unpredictability may be moderated by children’s neurobiological sensitivity markers (Ellis et al., 2011), multisystem resilience factors (Masten et al., 2021), demographic characteristics, and other adversity dimensions.
Despite the existing cross-species empirical evidence, unpredictability research is still at an early stage, and more studies are needed to increase the specificity of this model. To date, animal studies in this field of research have been mainly focusing on the neural and neuroendocrine alterations induced by fragmented and unpredictable maternal signals using the LBN poverty-simulating experimental paradigm (e.g., Baram et al., 2012; Bolton et al., 2018; Davis et al., 2017; Molet et al., 2016a). A close translational model of this animal LBN paradigm in human studies assesses unpredictable maternal care in naturalistic observations of mother-child interactions and examines its underlying neural alterations (Davis et al., 2017, Granger et al., 2021). In addition to unpredictable maternal signals, the investigation of unpredictability in human studies has also been looking into other forms of unpredictable early experiences, such as household chaos and financial instability, in relation to their neuroendocrine and immune underpinnings (e.g., Brown et al., 2019, Brown et al., 2021; Raver et al., 2015). Existing literature has started showing some cross-species convergence with different unpredictability formats. For example, fragmented and unpredictable maternal signals in animal studies (Ivy et al., 2008) and household chaos in human studies (Brown et al., 2019, Brown et al., 2021, Tarullo et al., 2020) are both found to predict elevated cortisol concentration. However, the limited empirical evidence prevents us from further concluding the generalizability or the specificity of these mechanisms to other unpredictability factors. It remains to be tested whether different temporal patterns of unpredictability characterized by multiple statistical properties (i.e., variance, autocorrelation, cue reliability, and pattern of changes) and distinct proximate mechanisms (ancestor cue vs. statistical learning) differentially relate to the proposed neurobiological pathways (Young et al., 2020).
The animal and human models reviewed above have yielded preliminary evidence about the unpredictability impact on the neurobiological processes and developmental outcomes. Beyond this evidence, the COVID-19 pandemic serves as a natural experiment (Roubinov et al., 2020) that can help further advance research on early experience unpredictability (e.g., Gee and Cohodes, 2021; Glynn et al., 2021; Smith and Pollak, 2021a), especially on the understanding of unpredictability impact in community and higher-order sociocultural contexts. In the section below, we discuss how the pandemic can promote the advancement of unpredictability research, as well as how the examination of unpredictability can increase knowledge on the potential pandemic impact on children’s development.
5. How the COVID-19 pandemic helps advance unpredictability research
5.1. Unpredictability during the COVID-19 pandemic
The global COVID-19 pandemic is an unprecedented socio-historical event that, beginning in January 2020, has abruptly disrupted children’s daily lives and increased unpredictability in multiple aspects. As shown in the conceptual model, the rapid transmission of COVID-19, the limited knowledge and misinformation about the virus and its variants, the changing public health guidelines and policies, and political issues directly impacted many individuals’ sense of unpredictability and uncontrollability over the large-scale crisis (Smith and Pollak, 2021a). The pandemic also significantly affected individuals’ social interactions and norms. Policies such as social distancing, mask mandates, stay-at-home orders, and travel restrictions were effective in slowing the spread of the virus. However, these policies also led to elevated social isolation and reduced access to support and resources (Hwang et al., 2020, Pietrabissa and Simpson, 2020). In the United States and elsewhere, national and local government policies also changed frequently, further increasing uncertainty around acceptable social norms.
Unpredictability in higher-order social contexts directly contributes to elevated unpredictability in the family context. From an economic perspective, job and income losses induced by the pandemic not only caused severe material hardship and financial strain but also increased employment uncertainty and financial instability (e.g., food insecurity, housing instability) among households with children (Godinic et al., 2020, Ruffolo et al., 2021). In addition, childcare and school policies added another layer of unpredictability for households with children. During the pandemic, families experienced closure and frequent format transitions (between in-person, hybrid, and fully virtual) of school or childcare programs in response to local infection rates. Many families, thus, were unsure about the availability of childcare and future school plans. Disruptions in children’s early learning environments and experiences have been speculated to lead to pervasive and lasting effects on children’s learning loss (Engzell et al., 2021, Kaffenberger, 2021, Martinez and Broemmel, 2021). Lastly, stress related to unpredictability from the health, policy, economic, social, and school-plan levels have been shown to take tolls on parents’ well-being and affect family dynamics, such as increased family conflict and household chaos, disrupted family routines, mood instability, and elevated inconsistent parenting behaviors (Glynn et al., 2021, Jiang et al., 2021, Kracht et al., 2021).
5.2. Leveraging the pandemic to advance unpredictability research
The COVID-19 pandemic is a global public health crisis with a pervasiveness that has never been experienced before (Roubinov et al., 2020). The consequences of the pandemic, such as increased morbidity and mortality rates, severe physical and mental health symptoms, learning losses, economic crisis, and disrupted family dynamics, are undoubtedly and extremely unfortunate. However, because of the ubiquitous and clustered occurrence of adverse events, the COVID-19 pandemic can serve as a global “natural experiment” (Thomson, 2020) that provides rare insight into the widespread yet distinct consequences induced by different types of early adverse experiences. The magnitude of the disruption that children have been experienced, as well as the unpredictability of experiences, can both cause negative consequences on children’s development. As a result, the multifaceted challenges to families brought by the pandemic have also created an opportunity to advance the science of early adversity (Roubinov et al., 2020), particularly with respect to the impact of unpredictability. Below, we outline five specific leverage points through which the pandemic can inform unpredictability research.
First, unpredictability manifests in multiple levels during the pandemic, ranging from family environments (e.g., disrupted routines, household chaos) to community and large society levels (e.g., frequent policy changes). Methodologically, research can benefit from comprehensively assessing different formats of unpredictability manifested at multiple levels, as well as adopting methodological recommendations from Young et al. (2020) to capture numerous statistical phenomena of unpredictability (i.e., variance, auto-correlation, cue reliability, and pattern). An extensive and rigorous assessment of unpredictability in multi-level social contexts will further enable studies to model the common and distinct developmental pathways underlying the influences of unpredictability factors on child development.
Second, prospective translational neuroscience research can benefit from studies that were underway prior to the pandemic. As mentioned before, the COVID-19 pandemic represents a “natural experiment” that abruptly introduced the element of unpredictability to the majority of people’s lives (Thomson, 2020). Leveraging studies underway before and continuing during the pandemic, researchers can distinguish the effects of unpredictability from environmental harshness. For instance, researchers can index pre-pandemic environmental harshness using data collected before COVID-19. During the pandemic, with repeated measures of environmental harshness, researchers can obtain time-series data and compute unpredictability indicators. Testing how unpredictability indicators during the pandemic affect child development while controlling for pre-pandemic environmental harshness is an effective way of making causal inferences and differentiating environmental unpredictability and harshness. Additionally, ongoing longitudinal studies with neurobiological assessments that span the pandemic can further unveil the neural and physiological underpinnings of unpredictable early experiences. Moreover, it is often difficult to tease apart the effects of early adverse experiences at different developmental periods because of the persisting effects and co-occurrence of adversities (Nelson and Gabard-Durnam, 2020). During the pandemic, however, the abrupt onset of widespread risk factors enables longitudinal cohort studies (that incorporate children of different ages) to delineate the role of developmental timing in the biological embedding process of unpredictability (Gee and Cohodes, 2021).
Third, research that examines the influence of unpredictability on child development during the pandemic also needs to account for subsequent large-scale events, such as the implementation of different government policies within and across countries, vaccination availability, virus variant (e.g., the Delta and Omicron variant of COVID-19) infection surges in specific locations, and concurrent natural disasters related to climate change. These events at the community or society level play critical roles in affecting the predictability of families’ and children’s experiences during the pandemic. Modeling these large-scale events fills in knowledge about unpredictability at the macro-level as related to families’ experiences and children’s developmental outcomes and has direct policy implications.
Fourth, intervention measures such as the Child Tax Credit payments and the federal stimulus checks in the U.S. provide a unique angle to understand how predictable vs. unpredictable early experiences affect children’s development. Since the pandemic, U.S. federal government has sent out three stimulus checks to help relieve families’ financial difficulties and stimulate the economy. These stimulus checks were of larger amount but distributed infrequently and sporadically (i.e., $1200 in April 2020, $600 in January 2021, and $1400 in March 2021), and thus represent unpredictable financial relief. In contrast, the regularly distributed Child Tax Credit payments (i.e., $300 monthly payments per child) stand for predictable financial assistance. Comparing the influences of these financial relieves is a direct way to examine the effectiveness of predictable vs. unpredictable intervention strategies on families’ well-being and children’s developmental outcomes.
Lastly, despite the negative consequences of unpredictable early experiences, many children and families exhibit resilience and show adaptive outcomes (Killgore et al., 2020, Prime et al., 2020, Roubinov et al., 2020). Researchers thus have the opportunity to explore resilience factors that protect children from the negative consequences of unpredictable and adverse early experiences. Scientific findings on protective factors during the pandemic will have direct implications for intervention programs aiming to enhance the development of resilience in the pandemic era.
5.3. Directions for future unpredictability research
As unpredictability research continues to grow in the context of COVID-19, several important issues need to be addressed in future studies. First, methodological challenges in unpredictability assessment still remain. The different statistical properties (e.g., variance, autocorrelation) and proximate mechanisms of detecting environmental unpredictability (i.e., ancestral cue and statistical learning) may implicate distinct pathways underlying the unpredictability influences on child development. Methodological advancement is needed to precisely assess these statistical structures of unpredictability and distinguish its formats (i.e., stationary vs. non-stationary; Young et al., 2020). Moreover, studies that include both traditional one-time measures of unpredictability (e.g., household chaos, lack of routines; reflecting the ancestral cues perspective) and time-series data of environmental harshness (reflecting the statistical learning perspective) are critical to promoting the establishment of unpredictable early experiences as a validated construct (Young et al., 2020).
Second, more cross-species studies are needed to further delineate the neural and physiological underpinnings of unpredictability and increase the specificity of the proposed conceptual model. Despite the existing empirical evidence, more studies need to test whether different temporal patterns of unpredictability and distinct proximate mechanisms (ancestor cue vs. statistical learning) differentially relate to the proposed neurobiological pathways (Young et al., 2020). Studies that test the impact of unpredictable early adverse experiences on the functioning of children’s ANS are still scarce. Moreover, the interconnections among unpredictability-induced neurobiological processes (e.g., the modulation of the neuroendocrine stress response on hippocampal functioning, the brain-autonomic coupling, the connections between neuroendocrine stress response and immune functioning) and their connections with unpredictable early experiences are worth examining.
Third, children’s differential responses to early experience unpredictability might be related to individual demographic characteristics, psychological traits, social contexts, genetic makeup, neural architecture, and physiological signatures. This individual heterogeneity of children’s response to the influence of early experience unpredictability is yet to be investigated. Lastly, given the rare opportunities for early adversity research during the COVID-19 pandemic, longitudinal studies that employ rigorous methodological designs and comprehensive assessments to study unpredictable early experiences are needed. These studies can significantly advance the field by distinguishing the effects of unpredictability from other elements of early adversity, revealing the roles of developmental timing, and exploring protective and promotive factors that enhance children’s resilience.
5.4. Implications for policymaking
The evidence reviewed in this article shows that unpredictability can fundamentally alter the course of development (e.g., Brumbach et al., 2009; Gee and Cohodes, 2021; Glynn and Baram, 2019; Noroña-Zhou et al., 2020). Conversely, practicable and responsive environments may play a critical role in children’s optimal development (Fisher et al., 2016, Szepsenwol et al., 2017). Therefore, policies designed to promote predictability and stability during children’s early years of life may have long-lasting beneficial influences on enhancing health, behavioral, and learning outcomes. Because of the public health crisis nature of the COVID-19 pandemic, certain types of unpredictability (e.g., policy and school plan change in response to infection rates) are inevitable. However, other types of unpredictability, such as economic instability and childcare uncertainty, can be mitigated via appropriate policies.
Given the prevalent financial instability experienced by a large proportion of families during the pandemic, policy decision-making should be informed by the goal of promoting economic stability and ensuring that families can constantly meet their basic needs (e.g., food, housing, healthcare). Beyond the amount of financial assistance and relief, policies can benefit from taking the distribution frequency and regularity into account. With the same amount of assistance, it might be more effective to distribute the money proportionally, frequently, and regularly (such as the child tax credit payments) instead of giving the whole amount of money sporadically to families at once. Additionally, the unpredictability of financial situations is rooted in employment instability during the pandemic, which disproportionally affects disadvantaged workforce population (e.g., racial/ethnic minorities, low-income parents, women; Gezici and Ozay, 2020; Kantamneni, 2020). Thus, policies that increase equal employment opportunities and protect marginalized workers’ job security, safety, and health are urgently needed. Expanding unemployment insurance eligibility and enhancing unemployment benefits that are distributed regularly and frequently can also help relieve families’ financial unpredictability. As suggested by Stone (2021), we need permanent and systemic reforms to fix existing disparities in the workforce and create equal-opportunity working environments with adequate wages, benefits, and security.
In addition to financial measures, policymakers should also make extensive efforts to increase childcare accessibility and build a stronger childcare system. Many parents have been struggling with balancing childcare and work responsibilities during the pandemic, and some caregivers’ employment and income stability has been significantly impacted by the frequent unpredictable changes in childcare arrangements (Cheng et al., 2021, Petts et al., 2020). Policies are needed to help parents better balance job and childcare responsibilities, such as promoting flexible work arrangements, ensuring workspace to accommodate caregivers’ childcare needs, and encouraging workspace to provide stable and affordable childcare options. Meanwhile, there is a need to advance policies to build a stronger childcare system. The childcare staff shortage has been becoming a severe issue during the COVID-19 pandemic, partly due to low wages and unsatisfactory benefits for childcare providers, and largely increases households’ childcare uncertainty (Alliance for Early Success, 2020). Policies can provide support to childcare providers by enhancing their qualifications and career development, ensuring adequate compensation and benefits, and reforming the financial and budget of the childcare system (Alliance for Early Success, 2020).
5.5. Implications for prevention and intervention practices
Prevention and intervention programs are another strong tool that can help mitigate the negative impact of pandemic-related unpredictability on child development. This article suggests that programs targeting at-risk households may be more effective when they take children’s experiences of unpredictability into account during design, dissemination, and implementation processes. During the risk screening process, considering families’ economic stability can help ensure inclusive program dissemination that benefits all families in need. To promote parents’ emotional well-being, programs can consider incorporating content that helps parents cope with unpredictability in families’ financial situations and teach parents strategies to balance work and childcare responsibilities. Similarly, strategies that help children cope with changing school plans and maintain regular social interactions with teachers and peers can enhance children’s learning and well-being. Furthermore, programs designed to enhance predictable and stable family interactions (e.g., reducing household chaos, building regular family routines, and promoting consistent parenting) may be particularly effective in buffering pandemic-induced negative consequences for parents and children. Many existing programs, such as the Filming Interactions to Nurture Development (FIND; Fisher et al., 2016) and the Attachment and Biobehavioral Catch-up (ABC Catch-Up; Dozier and Bernard, 2017) are designed to promote consistent and development-enhancing parenting behaviors, which can be adapted to suit families’ needs during the COVID-19 pandemic. Lastly, advancing innovative and accessible program delivery platforms through telehealth can help ensure families’ consistent access to the programs without being disrupted by the pandemic.
6. Conclusions
Early adverse experiences have enduring negative influences on children’s biological, socioemotional, and cognitive development (Cicchetti, 2016, Pechtel and Pizzagalli, 2011, Taylor et al., 2011). Recent theoretical models that conceptualize early adversity, such as the dimensional models (Ellis et al., 2009, McLaughlin et al., 2020, McLaughlin and Sheridan, 2016) and the topological model (Smith and Pollak, 2021b), highlight the element of unpredictability as a core experience that manifests in most forms of adverse experiences. In other words, the magnitude of the environmental harshness (i.e., the intensity of adversity), as well as the unpredictability of adverse experiences, both cause harmful consequences on child development (Brumbach et al., 2009, Doom et al., 2016, Mittal et al., 2015, Simpson et al., 2012, Szepsenwol et al., 2017).
Informed by a translational neuroscience framework (Fisher, 2016, Fisher et al., 2020, Horn et al., 2020), this article highlights the neurobiological processes underlying unpredictability impact on developmental outcomes. Cross-species evidence demonstrates that unpredictable and adverse early experiences affect behavioral and health outcomes through altering three neurobiological mechanisms, including accelerated maturation of the corticolimbic neural circuitry (e.g., Baram et al., 2012; Gee and Cohodes, 2021; Glynn and Baram, 2019; Smith and Pollak, 2021a), the neuroendocrine and ANS stress response dysregulation (e.g., Brown et al., 2019, Brown et al., 2021; Noroña-Zhou et al., 2020), as well as systemic inflammation and pro-inflammatory tendencies (e.g., Bernard et al., 2019; Schreier et al., 2014). We further develop a conceptual model (Fig. 1) to describe the biological embedding processes through which unpredictability in early adverse experiences gets “under the skin” and affects children’s numerous developmental outcomes.
The global COVID-19 pandemic marks an abrupt increase of widespread and ubiquitous adverse events that occur in clusters. In addition to the increased environmental harshness, the pandemic also causes unprecedented unpredictability and instability in children’s daily lives. The deep disruptions caused by the pandemic are undoubtedly unfortunate. However, the pandemic also serves as a large-scale “natural experiment” that provides rare insight to advance scientific understanding about the widespread effects of environmental unpredictability on child development. In turn, these research findings can directly inform policymakers to promote economic stability and ensure affordable, high-quality childcare in children’s early years of life. Research in this field can also inform intervention and prevention programs targeting at-risk families to take unpredictability into account and to find ways in the program design, dissemination, and implementation processes to mitigate the negative impact of unpredictability on child development.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Valhalla Charitable Foundation, the Heising-Simons Foundation, the Pritzker Family Foundation, the Buffett Early Childhood Fund, the Imaginable Futures, and the Bainum Family Foundation.
References
- Agorastos A., Pervanidou P., Chrousos G.P., Baker D.G. Developmental trajectories of early life stress and trauma: a narrative review on neurobiological aspects beyond stress system dysregulation. Front. Psychiatry. 2019;10:118. doi: 10.3389/fpsyt.2019.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, M.J., & Sharma, S. (2018). Physiology, adrenocorticotropic hormone (ACTH). [PubMed]
- Alliance for Early Success. (2020). Build stronger: A childcare policy roadmap for transforming our nation’s child care system (, Issue.
- Anda R.F., Butchart A., Felitti V.J., Brown D.W. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am. J. Prev. Med. 2010;39(1):93–98. doi: 10.1016/j.amepre.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Appleyard K., Egeland B., van Dulmen M.H., Alan Sroufe L. When more is not better: the role of cumulative risk in child behavior outcomes. J. Child Psychol. Psychiatry. 2005;46(3):235–245. doi: 10.1111/j.1469-7610.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- Atkins, S.M., Bunting, M.F., Bolger, D.J., & Dougherty, M.R. (2012). Training the adolescent brain: Neural plasticity and the acquisition of cognitive abilities.
- Avishai-Eliner S., Gilles E., Eghbal-Ahmadi M., Bar-El Y., Baram T. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J. Neuroendocrinol. 2001;13(9):799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M.J., Van Ijzendoorn M.H. Differential susceptibility to rearing environment depending on dopamine-related genes: new evidence and a meta-analysis. Dev. Psychopathol. 2011;23(1):39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Baram T.Z., Davis E.P., Obenaus A., Sandman C.A., Small S.L., Solodkin A., Stern H. Fragmentation and unpredictability of early-life experience in mental disorders. Am. J. Psychiatry. 2012;169(9):907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P.J., Wiebe S.A., Carver L.J., Waters J.M., Nelson C.A. Developments in long-term explicit memory late in the first year of life: behavioral and electrophysiological indices. Psychol. Sci. 2003;14(6):629–635. doi: 10.1046/j.0956-7976.2003.psci_1476.x. [DOI] [PubMed] [Google Scholar]
- Belsky J., Bakermans-Kranenburg M.J., Van IJzendoorn M.H. For better and for worse: differential susceptibility to environmental influences. Curr. Dir. Psychol. Sci. 2007;16(6):300–304. [Google Scholar]
- Belsky J., Schlomer G.L., Ellis B.J. Beyond cumulative risk: distinguishing harshness and unpredictability as determinants of parenting and early life history strategy. Dev. Psychol. 2012;48(3):662. doi: 10.1037/a0024454. [DOI] [PubMed] [Google Scholar]
- Bernard K., Hostinar C.E., Dozier M. Longitudinal associations between attachment quality in infancy, C-reactive protein in early childhood, and BMI in middle childhood: preliminary evidence from a CPS-referred sample. Attach. Hum. Dev. 2019;21(1):5–22. doi: 10.1080/14616734.2018.1541513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Granger D.A., Willoughby M., Mills-Koonce R., Cox M., Greenberg M.T., Kivlighan K.T., Fortunato C.K., Investigators F. Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Dev. 2011;82(6):1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Raver C.C., Granger D., Mills-Koonce R., Hibel L., Investigators F.L.P.K. Allostasis and allostatic load in the context of poverty in early childhood. Dev. Psychopathol. 2011;23(3):845–857. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blossom V., Gokul M., Kumar N.A., Kini R.D., Nayak S., Bhagyalakshmi K. Chronic unpredictable stress-induced inflammation and quantitative analysis of neurons of distinct brain regions in Wistar rat model of comorbid depression. Vet. World. 2020;13(9):1870. doi: 10.14202/vetworld.2020.1870-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J.L., Molet J., Ivy A., Baram T.Z. New insights into early-life stress and behavioral outcomes. Curr. Opin. Behav. Sci. 2017;14:133–139. doi: 10.1016/j.cobeha.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J.L., Ruiz C.M., Rismanchi N., Sanchez G.A., Castillo E., Huang J., Cross C., Baram T.Z., Mahler S.V. Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiol. Stress. 2018;8:57–67. doi: 10.1016/j.ynstr.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce W.T., Ellis B.J. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brody G.H., Yu T., Chen Y.-f, Kogan S.M., Evans G.W., Beach S.R., Windle M., Simons R.L., Gerrard M., Gibbons F.X. Cumulative socioeconomic status risk, allostatic load, and adjustment: a prospective latent profile analysis with contextual and genetic protective factors. Dev. Psychol. 2013;49:913. doi: 10.1037/a0028847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U. Harvard university press; 1979. The Ecology of Human Development: Experiments by Nature and Design. [Google Scholar]
- Bronfenbrenner U., Ceci S.J. Nature-nuture reconceptualized in developmental perspective: a bioecological model. Psychol. Rev. 1994;101(4):568. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U., Evans G.W. Developmental science in the 21st century: emerging questions, theoretical models, research designs and empirical findings. Soc. Dev. 2000;9(1):115–125. [Google Scholar]
- Brown E.D., Anderson K.E., Garnett M.L., Hill E.M. Economic instability and household chaos relate to cortisol for children in poverty. J. Fam. Psychol. 2019;33(6):629. doi: 10.1037/fam0000545. [DOI] [PubMed] [Google Scholar]
- Brown E.D., Holochwost S.J., Laurenceau J.P., Garnett M.L., Anderson K.E. Deconstructing cumulative risk: poverty and aspects of instability relate uniquely to young children’s basal cortisol. Child Dev. 2021 doi: 10.1111/cdev.13512. [DOI] [PubMed] [Google Scholar]
- Brumbach B.H., Figueredo A.J., Ellis B.J. Effects of harsh and unpredictable environments in adolescence on development of life history strategies. Hum. Nat. 2009;20(1):25–51. doi: 10.1007/s12110-009-9059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson K.L., Kramár E., Lin B., Chen Y., Colgin L.L., Yanagihara T.K., Lynch G., Baram T.Z. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005;25(41):9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss D.M. Evolutionary psychology: A new paradigm for psychological science. Psychol. Inq. 1995;6(1):1–30. [Google Scholar]
- Callaghan B.L., Tottenham N. The neuro-environmental loop of plasticity: a cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology. 2016;41(1):163–176. doi: 10.1038/npp.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov E.L., Berrigan D. Why do female primates have such long lifespans and so few babies? Or life in the slow lane. Evolut. Anthropol. Issues News Rev. 1993;1(6):191–194. [Google Scholar]
- Cheng Z., Mendolia S., Paloyo A.R., Savage D.A., Tani M. Working parents, financial insecurity, and childcare: mental health in the time of COVID-19 in the UK. Rev. Econ. Househ. 2021;19(1):123–144. doi: 10.1007/s11150-020-09538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. Socioemotional, personality, and biological development: illustrations from a multilevel developmental psychopathology perspective on child maltreatment. Annu. Rev. Psychol. 2016;67:187–211. doi: 10.1146/annurev-psych-122414-033259. [DOI] [PubMed] [Google Scholar]
- Cohen J.R., Thomsen K.N., Racioppi A., Ballespi S., Sheinbaum T., Kwapil T.R., Barrantes-Vidal N. Emerging adulthood and prospective depression: a simultaneous test of cumulative risk theories. J. Youth Adolesc. 2019;48(7):1353–1364. doi: 10.1007/s10964-019-01017-y. [DOI] [PubMed] [Google Scholar]
- Colich N.L., Rosen M.L., Williams E.S., McLaughlin K.A. Biological aging in childhood and adolescence following experiences of threat and deprivation: a systematic review and meta-analysis. Psychol. Bull. 2020;146(9):721. doi: 10.1037/bul0000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T.M., Thomas J.A. Elements of information theory second edition solutions to problems. Internet Access. 2006:19–20. [Google Scholar]
- Cruz O., Abreu-Lima I., Canário C., Burchinal M. Does temperament moderate the relation between preschool parenting and school-age self-regulation? Contrasting diathesis-stress and differential susceptibility models. Parenting. 2018;18(2):126–140. [Google Scholar]
- Davis E.P., Stout S.A., Molet J., Vegetabile B., Glynn L.M., Sandman C.A., Heins K., Stern H., Baram T.Z. Exposure to unpredictable maternal sensory signals influences cognitive development across species. Proc. Natl. Acad. Sci. USA. 2017;114(39):10390–10395. doi: 10.1073/pnas.1703444114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M. An evolutionary life history framework for psychopathology. Psychol. Inq. 2014;25(3–4):261–300. [Google Scholar]
- Del Giudice M., Ellis B.J., Shirtcliff E.A. The adaptive calibration model of stress responsivity. Neurosci. Biobehav. Rev. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan S.N. Allostatic load: developmental and conceptual considerations in a multi‐system physiological indicator of chronic stress exposure. Dev. Psychobiol. 2021 doi: 10.1002/dev.22107. [DOI] [PubMed] [Google Scholar]
- Doom J.R., Vanzomeren-Dohm A.A., Simpson J.A. Early unpredictability predicts increased adolescent externalizing behaviors and substance use: a life history perspective. Dev. Psychopathol. 2016;28(4pt2):1505–1516. doi: 10.1017/S0954579415001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M., Bernard K. Attachment and biobehavioral catch-up: addressing the needs of infants and toddlers exposed to inadequate or problematic caregiving. Curr. Opin. Psychol. 2017;15:111–117. doi: 10.1016/j.copsyc.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S.R. Continuing conversations about adverse childhood experiences (ACEs) screening: a public health perspective. Child Abus. Negl. 2018;85:180–184. doi: 10.1016/j.chiabu.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Elliott W. The effects of economic instability on children’s educational outcomes. Child. Youth Serv. Rev. 2013;35(3):461–471. [Google Scholar]
- Ellis B.J., Boyce W.T., Belsky J., Bakermans-Kranenburg M.J., Van IJzendoorn M.H. Differential susceptibility to the environment: an evolutionary–neurodevelopmental theory. Dev. Psychopathol. 2011;23(1):7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Essex M.J., Boyce W.T. Biological sensitivity to context: II. Empirical explorations of an evolutionary–developmental theory. Dev. Psychopathol. 2005;17(2):303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Figueredo A.J., Brumbach B.H., Schlomer G.L. Fundamental dimensions of environmental risk. Hum. Nat. 2009;20(2):204–268. doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Oldehinkel A.J., Nederhof E. The adaptive calibration model of stress responsivity: an empirical test in the Tracking Adolescents’ Individual Lives Survey study. Dev. Psychopathol. 2017;29(3):1001–1021. doi: 10.1017/S0954579416000985. [DOI] [PubMed] [Google Scholar]
- Engzell P., Frey A., Verhagen M.D. Learning loss due to school closures during the COVID-19 pandemic. Proc. Natl. Acad. Sci. USA. 2021;118(17) doi: 10.1073/pnas.2022376118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev. Psychol. 2003;39(5):924. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Cassells R.C. Childhood poverty, cumulative risk exposure, and mental health in emerging adults. Clin. Psychol. Sci. 2014;2(3):287–296. doi: 10.1177/2167702613501496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W., Gonnella C., Marcynyszyn L.A., Gentile L., Salpekar N. The role of chaos in poverty and children’s socioemotional adjustment. Psychol. Sci. 2005;16(7):560–565. doi: 10.1111/j.0956-7976.2005.01575.x. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Li D., Whipple S.S. Cumulative risk and child development. Psychol. Bull. 2013;139(6):1342. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Wachs T.D. American Psychological Association; Washington, DC: 2010. Chaos and Its Influence on Children’s Development. [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., Marks J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fenoglio K.A., Brunson K.L., Avishai-Eliner S., Stone B.A., Kapadia B.J., Baram T.Z. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146(9):4090–4096. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feola B., Melancon S.N.T., Clauss J.A., Noall M.P., Mgboh A., Flook E.A., Benningfield M.M., Blackford J.U. Bed nucleus of the stria terminalis and amygdala responses to unpredictable threat in children. Dev. Psychobiol. 2021;63(8) doi: 10.1002/dev.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein D.H., Dariotis J.K. Personalizing and optimizing preventive intervention models via a translational neuroscience framework. Prev. Sci. 2019;20(1):10–20. doi: 10.1007/s11121-017-0851-8. [DOI] [PubMed] [Google Scholar]
- Fisher P.A. Translational neuroscience as a tool for intervention development in the context of high‐adversity families. New Dir. Child Adolesc. Dev. 2016;2016(153):111–125. doi: 10.1002/cad.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P.A., Barker T.V., Blaisdell K.N. A glass half full and half empty: the state of the science in early childhood prevention and intervention research. Annu. Rev. Dev. Psychol. 2020;2:269–294. [Google Scholar]
- Fisher P.A., Berkman E.T. Designing interventions informed by scientific knowledge about effects of early adversity: a translational neuroscience agenda for next-generation addictions research. Curr. Addict. Rep. 2015;2(4):347–353. doi: 10.1007/s40429-015-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P.A., Frenkel T.I., Noll L.K., Berry M., Yockelson M. Promoting healthy child development via a two‐generation translational neuroscience framework: the Filming Interactions to Nurture Development video coaching program. Child Dev. Perspect. 2016;10(4):251–256. doi: 10.1111/cdep.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhuis W.E., Gergely G., Watson J.S. Infants may use contingency analysis to estimate environmental states: an evolutionary, life‐history perspective. Child Dev. Perspect. 2013;7(2):115–120. [Google Scholar]
- Frankenhuis W.E., Nettle D., Dall S.R. A case for environmental statistics of early-life effects. Philos. Trans. R. Soc. B. 2019;374(1770) doi: 10.1098/rstb.2018.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo L.C., Fortmann A.L., Mattei J. Allostatic load and the assessment of cumulative biological risk in biobehavioral medicine: challenges and opportunities. Psychosom. Med. 2014;76(7):478. doi: 10.1097/PSY.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, D.G. (2016). Sensitive periods of emotion regulation: influences of parental care on frontoamygdala circuitry and plasticity. [DOI] [PubMed]
- Gee D.G. Early adversity and development: parsing heterogeneity and identifying pathways of risk and resilience. Am. J. Psychiatry. 2021;178(11):998–1013. doi: 10.1176/appi.ajp.2021.21090944. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Cohodes E.M. Influences of caregiving on development: a sensitive period for biological embedding of predictability and safety cues. Curr. Dir. Psychol. Sci. 2021 doi: 10.1177/09637214211015673. 09637214211015673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L., Telzer E.H., Humphreys K.L., Goff B., Shapiro M., Flannery J., Lumian D.S., Fareri D.S., Caldera C. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol. Sci. 2014;25(11):2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. USA. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gezici, A., & Ozay, O. (2020). How race and gender shape COVID-19 unemployment probability. Available at SSRN 3675022.
- Glynn L.M., Baram T.Z. The influence of unpredictable, fragmented parental signals on the developing brain. Front. Neuroendocrinol. 2019;53 doi: 10.1016/j.yfrne.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn L.M., Davis E.P., Luby J.L., Baram T.Z., Sandman C.A. A predictable home environment may protect child mental health during the COVID-19 pandemic. Neurobiol. Stress. 2021;14 doi: 10.1016/j.ynstr.2020.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn L.M., Howland M.A., Sandman C.A., Davis E.P., Phelan M., Baram T.Z., Stern H.S. Prenatal maternal mood patterns predict child temperament and adolescent mental health. J. Affect. Disord. 2018;228:83–90. doi: 10.1016/j.jad.2017.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn L.M., Stern H.S., Howland M.A., Risbrough V.B., Baker D.G., Nievergelt C.M., Baram T.Z., Davis E.P. Measuring novel antecedents of mental illness: the Questionnaire of Unpredictability in Childhood. Neuropsychopharmacology. 2019;44(5):876–882. doi: 10.1038/s41386-018-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinic D., Obrenovic B., Khudaykulov A. Effects of economic uncertainty on mental health in the COVID-19 pandemic context: social identity disturbance, job uncertainty and psychological well-being model. Int. J. Innov. Econ. Dev. 2020;6(1):61–74. [Google Scholar]
- Granger S.J., Glynn L.M., Sandman C.A., Small S.L., Obenaus A., Keator D.B., Baram T.Z., Stern H., Yassa M.A., Davis E.P. Aberrant maturation of the uncinate fasciculus follows exposure to unpredictable patterns of maternal signals. J. Neurosci. 2021;41(6):1242–1250. doi: 10.1523/JNEUROSCI.0374-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno A., Kang M.S., Devenyi G.A., Mathieu A.P., Rosa-Neto P., Chakravarty M., Walker C.-D. Reduced resting-state functional connectivity of the basolateral amygdala to the medial prefrontal cortex in preweaning rats exposed to chronic early-life stress. Brain Struct. Funct. 2018;223(8):3711–3729. doi: 10.1007/s00429-018-1720-3. [DOI] [PubMed] [Google Scholar]
- Hawkes K. Slow life histories and human evolution. Evol. Hum. Life Hist. 2006:95–126. [Google Scholar]
- Horn S.R., Fisher P.A., Pfeifer J.H., Allen N.B., Berkman E.T. Levers and barriers to success in the use of translational neuroscience for the prevention and treatment of mental health and promotion of well-being across the lifespan. J. Abnorm. Psychol. 2020;129(1):38. doi: 10.1037/abn0000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland M.A., Sandman C.A., Davis E.P., Stern H.S., Phelan M., Baram T.Z., Glynn L.M. Prenatal maternal mood entropy is associated with child neurodevelopment. Emotion. 2021;21(3):489. doi: 10.1037/emo0000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Phillips M.R., Zhang Y., Zhang J., Shi Q., Song Z., Ding Z., Pang S., Martorell R. Malnutrition in early life and adult mental health: evidence from a natural experiment. Soc. Sci. Med. 2013;97:259–266. doi: 10.1016/j.socscimed.2012.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter A.L., Minnis H., Wilson P. Altered stress responses in children exposed to early adversity: a systematic review of salivary cortisol studies. Stress. 2011;14(6):614–626. doi: 10.3109/10253890.2011.577848. [DOI] [PubMed] [Google Scholar]
- Hurst J.E., Kavanagh P.S. Life history strategies and psychopathology: the faster the life strategies, the more symptoms of psychopathology. Evol. Hum. Behav. 2017;38(1):1–8. [Google Scholar]
- Hwang T.-J., Rabheru K., Peisah C., Reichman W., Ikeda M. Loneliness and social isolation during the COVID-19 pandemic. Int. Psychogeriatr. 2020;32(10):1217–1220. doi: 10.1017/S1041610220000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy A.S., Brunson K.L., Sandman C., Baram T.Z. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy A.S., Rex C.S., Chen Y., Dubé C., Maras P.M., Grigoriadis D.E., Gall C.M., Lynch G., Baram T.Z. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 2010;30(39):13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee S. Promises and pitfalls in the development of biomarkers that can promote early intervention in children at risk. J. Child Psychol. Psychiatry. 2018;59(2):97–98. doi: 10.1111/jcpp.12869. [DOI] [PubMed] [Google Scholar]
- Jebb A.T., Tay L. Introduction to time series analysis for organizational research: Methods for longitudinal analyses. Organ. Res. Methods. 2017;20(1):61–94. [Google Scholar]
- Jebb A.T., Tay L., Wang W., Huang Q. Time series analysis for psychological research: examining and forecasting change. Front. Psychol. 2015;6:727. doi: 10.3389/fpsyg.2015.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E.W., James S.A., Boyce W.T., Hartnett S.A. The family routines inventory: development and validation. Soc. Sci. Med. 1983;17(4):201–211. doi: 10.1016/0277-9536(83)90117-x. [DOI] [PubMed] [Google Scholar]
- Jiang Y., He T., Lin X., Zhou Q., Wu Q. Caregivers’ joint depressive symptoms and preschoolers’ daily routines in Chinese three-generation families: Does household chaos matter? Curr. Psychol. 2021:1–9. doi: 10.1007/s12144-021-01595-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Policelli J., Li M., Dharamsi A., Hu Q., Sheridan M.A., McLaughlin K.A., Wade M. Associations of early-life threat and deprivation with executive functioning in childhood and adolescence: a systematic review and meta-analysis. JAMA Pediatr. 2021 doi: 10.1001/jamapediatrics.2021.2511. e212511-e212511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffenberger M. Modelling the long-run learning impact of the Covid-19 learning shock: Actions to (more than) mitigate loss. Int. J. Educ. Dev. 2021;81 doi: 10.1016/j.ijedudev.2020.102326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantamneni, N. (2020). The impact of the COVID-19 pandemic on marginalized populations in the United States: A research agenda. [DOI] [PMC free article] [PubMed]
- Killgore W.D., Taylor E.C., Cloonan S.A., Dailey N.S. Psychological resilience during the COVID-19 lockdown. Psychiatry Res. 2020;291 doi: 10.1016/j.psychres.2020.113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A., Shanabrough M., McClelland S., Liu Z.-W., Borok E., Gao X.-B., Horvath T.L., Baram T.Z. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J. Neurosci. 2010;30(2):703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss K.J., Gunnar M.R. Annual research review: early adversity, the hypothalamic–pituitary–adrenocortical axis, and child psychopathology. J. Child Psychol. Psychiatry. 2018;59(4):327–346. doi: 10.1111/jcpp.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracht C.L., Katzmarzyk P.T., Staiano A.E. Household chaos, family routines, and young child movement behaviors in the US during the COVID-19 outbreak: a cross-sectional study. BMC Public Health. 2021;21(1):1–12. doi: 10.1186/s12889-021-10909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R.E., Minnis H. Practitioner review: twenty years of research with adverse childhood experience scores–advantages, disadvantages and applications to practice. J. Child Psychol. Psychiatry. 2020;61(2):116–130. doi: 10.1111/jcpp.13135. [DOI] [PubMed] [Google Scholar]
- Li Z., Liu S., Hartman S., Belsky J. Interactive effects of early-life income harshness and unpredictability on children’s socioemotional and academic functioning in kindergarten and adolescence. Dev. Psychol. 2018;54(11):2101. doi: 10.1037/dev0000601. [DOI] [PubMed] [Google Scholar]
- Liu S., Oshri A., Kogan S.M., Wickrama K., Sweet L. Amygdalar activation as a neurobiological marker of differential sensitivity in the effects of family rearing experiences on socioemotional adjustment in youths. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging. 2021 doi: 10.1016/j.bpsc.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley M.N., Harkness K.L. Specificity in the relations among childhood adversity, early maladaptive schemas, and symptom profiles in adolescent depression. Cogn. Ther. Res. 2007;31(5):639–657. [Google Scholar]
- Luthar, S.S. (2006). Resilience in development: A synthesis of research across five decades.
- Lyons D.M., Parker K.J., Schatzberg A.F. Animal models of early life stress: implications for understanding resilience. Dev. Psychobiol. 2010;52(5):402–410. doi: 10.1002/dev.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlin L., Miller A.B., Snyder J., McLaughlin K.A., Sheridan M.A. Differential associations of deprivation and threat with cognitive control and fear conditioning in early childhood. Front. Behav. Neurosci. 2019;13:80. doi: 10.3389/fnbeh.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J.A., Broemmel A.D. Pencils down: educators respond to the uncertainty amidst COVID-19 school closures. Int. Stud. Educ. Adm. (Commonw. Counc. Educ. Adm. Manag.) 2021;49:109–132. [Google Scholar]
- Masten A.S. Multisystem resilience: pathways to an integrated framework. Res. Hum. Dev. 2021;18(3):153–163. [Google Scholar]
- Masten A.S., Lucke C.M., Nelson K.M., Stallworthy I.C. Resilience in development and psychopathology: multisystem perspectives. Annu. Rev. Clin. Psychol. 2021;17:521–549. doi: 10.1146/annurev-clinpsy-081219-120307. [DOI] [PubMed] [Google Scholar]
- Masten A.S., Obradović J. Competence and resilience in development. Ann. N. Y. Acad. Sci. 2006;1094(1):13–27. doi: 10.1196/annals.1376.003. [DOI] [PubMed] [Google Scholar]
- McClelland S., Korosi A., Cope J., Ivy A., Baram T.Z. Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol. Learn. Mem. 2011;96(1):79–88. doi: 10.1016/j.nlm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy D.C. Early violence exposure and self-regulatory development: a bioecological systems perspective. Hum. Dev. 2013;56(4):254–273. [Google Scholar]
- McEwen B.S., Stellar E. Stress and the individual: mechanisms leading to disease. Arch. Intern. Med. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- McLaughlin, K.A., Sheridan, M., Humphreys, K., Belsky, J., & Ellis, B.J. (2020). The value of dimensional models of early experience: thinking clearly about concepts and categories. [DOI] [PMC free article] [PubMed]
- McLaughlin K.A., Sheridan M.A. Beyond cumulative risk: a dimensional approach to childhood adversity. Curr. Dir. Psychol. Sci. 2016;25(4):239–245. doi: 10.1177/0963721416655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Lambert H.K. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.B., Sheridan M.A., Hanson J.L., McLaughlin K.A., Bates J.E., Lansford J.E., Pettit G.S., Dodge K.A. Dimensions of deprivation and threat, psychopathology, and potential mediators: a multi-year longitudinal analysis. J. Abnorm. Psychol. 2018;127(2):160. doi: 10.1037/abn0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Chen E., Cole S.W. Health psychology: developing biologically plausible models linking the social world and physical health. Annu. Rev. Psychol. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller G., Rohleder N., Cole S.W. Chronic interpersonal stress predicts activation of pro-and anti-inflammatory signaling pathways six months later. Psychosom. Med. 2009;71(1):57. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.E., Chen E., Parker K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 2011;137(6):959. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal C., Griskevicius V., Simpson J.A., Sung S., Young E.S. Cognitive adaptations to stressful environments: when childhood adversity enhances adult executive function. J. Personal. Soc. Psychol. 2015;109(4):604. doi: 10.1037/pspi0000028. [DOI] [PubMed] [Google Scholar]
- Molet J., Heins K., Zhuo X., Mei Y., Regev L., Baram T., Stern H. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl. Psychiatry. 2016;6(1) doi: 10.1038/tp.2015.200. e702-e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J., Maras P.M., Kinney‐Lang E., Harris N.G., Rashid F., Ivy A.S., Solodkin A., Obenaus A., Baram T.Z. MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early‐life adversity. Hippocampus. 2016;26(12):1618–1632. doi: 10.1002/hipo.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormede P., Dantzer R., Michaud B., Kelley K.W., Le Moal M. Influence of stressor predictability and behavioral control on lymphocyte reactivity, antibody responses and neuroendocrine activation in rats. Physiol. Behav. 1988;43(5):577–583. doi: 10.1016/0031-9384(88)90211-9. [DOI] [PubMed] [Google Scholar]
- Nelson C.A., III A neurobiological perspective on early human deprivation. Child Dev. Perspect. 2007;1(1):13–18. [Google Scholar]
- Nelson C.A., III, Gabard-Durnam L.J. Early adversity and critical periods: neurodevelopmental consequences of violating the expectable environment. Trends Neurosci. 2020;43(3):133–143. doi: 10.1016/j.tins.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettle D., Frankenhuis W.E., Rickard I.J. The evolution of predictive adaptive responses in human life history. Proc. R. Soc. B Biol. Sci. 2013;280(1766) doi: 10.1098/rspb.2013.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noroña-Zhou A.N., Morgan A., Glynn L.M., Sandman C.A., Baram T.Z., Stern H.S., Davis E.P. Unpredictable maternal behavior is associated with a blunted infant cortisol response. Dev. Psychobiol. 2020;62(6):882–888. doi: 10.1002/dev.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović J., Bush N.R., Stamperdahl J., Adler N.E., Boyce W.T. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev. 2010;81(1):270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri A., Liu S., Suveg C.M., Caughy M.O.B., Huffman L.G. Biological sensitivity to context as a dyadic construct: an investigation of child–parent RSA synchrony among low-SES youth. Dev. Psychopathol. 2021:1–14. doi: 10.1017/S095457942100078X. [DOI] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry N.B., Donzella B., Gunnar M.R. Pubertal stress recalibration and later social and emotional adjustment among adolescents: The role of early life stress. Psychoneuroendocrinology. 2022;135 doi: 10.1016/j.psyneuen.2021.105578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen A.-K., Räikkönen K., Feldt K., Heinonen K., Osmond C., Phillips D.I., Barker D.J., Eriksson J.G., Kajantie E. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: a natural experiment of World War II. Psychoneuroendocrinology. 2010;35(5):758–767. doi: 10.1016/j.psyneuen.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Petts R.J., Carlson D.L., Pepin J.R. A gendered pandemic: childcare, homeschooling, and parents’ employment during COVID‐19. Gend. Work Organ. 2020 [Google Scholar]
- Pietrabissa G., Simpson S.G. Psychological consequences of social isolation during COVID-19 outbreak. Front. Psychol. 2020;11:2201. doi: 10.3389/fpsyg.2020.02201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime H., Wade M., Browne D.T. Risk and resilience in family well-being during the COVID-19 pandemic. Am. Psychol. 2020;75(5):631. doi: 10.1037/amp0000660. [DOI] [PubMed] [Google Scholar]
- Raver C.C., Blair C., Garrett-Peters P., Investigators F.L.P.K. Poverty, household chaos, and interparental aggression predict children’s ability to recognize and modulate negative emotions. Dev. Psychopathol. 2015;27(3):695–708. doi: 10.1017/S0954579414000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles T.F. Annual Research Review: social relationships and the immune system during development. J. Child Psychol. Psychiatry. 2021;62(5):539–559. doi: 10.1111/jcpp.13350. [DOI] [PubMed] [Google Scholar]
- Roff D.A., Mostowy S., Fairbairn D.J. The evolution of trade‐offs: testing predictions on response to selection and environmental variation. Evolution. 2002;56(1):84–95. doi: 10.1111/j.0014-3820.2002.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Roubinov D., Bush N.R., Boyce W.T. How a pandemic could advance the science of early adversity. JAMA Pediatr. 2020;174(12):1131–1132. doi: 10.1001/jamapediatrics.2020.2354. [DOI] [PubMed] [Google Scholar]
- Ruffolo M., Price D., Schoultz M., Leung J., Bonsaksen T., Thygesen H., Geirdal A.Ø. Employment uncertainty and mental health during the COVID-19 pandemic initial social distancing implementation: a cross-national study. Glob. Soc. Welf. 2021;8(2):141–150. doi: 10.1007/s40609-020-00201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Protective factors in children’s responses to stress and disadvantage. Ann. Acad. Med. 1979;8(3):324–338. [PubMed] [Google Scholar]
- Sameroff A.J. Handbook of Developmental Psychopathology. Springer; 2000. Dialectical processes in developmental psychopathology; pp. 23–40. [Google Scholar]
- Schreier H.M., Roy L.B., Frimer L.T., Chen E. Family chaos and adolescent inflammatory profiles: the moderating role of socioeconomic status. Psychosom. Med. 2014;76(6):460–467. doi: 10.1097/PSY.0000000000000078. [DOI] [PubMed] [Google Scholar]
- Schriber R.A., Anbari Z., Robins R.W., Conger R.D., Hastings P.D., Guyer A.E. Hippocampal volume as an amplifier of the effect of social context on adolescent depression. Clin. Psychol. Sci. 2017;5(4):632–649. doi: 10.1177/2167702617699277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriber R.A., Guyer A.E. Adolescent neurobiological susceptibility to social context. Dev. Cogn. Neurosci. 2016;19:1–18. doi: 10.1016/j.dcn.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiba N., Ellis B.J., Bush N.R., Boyce W.T. Biological sensitivity to context: a test of the hypothesized U-shaped relation between early adversity and stress responsivity. Dev. Psychopathol. 2020;32(2):641–660. doi: 10.1017/S0954579419000518. [DOI] [PubMed] [Google Scholar]
- Sheridan M.A., McLaughlin K.A. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn. Sci. 2014;18(11):580–585. doi: 10.1016/j.tics.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J.A., Griskevicius V., Kuo S.I., Sung S., Collins W.A. Evolution, stress, and sensitive periods: the influence of unpredictability in early versus late childhood on sex and risky behavior. Dev. Psychol. 2012;48(3):674. doi: 10.1037/a0027293. [DOI] [PubMed] [Google Scholar]
- Singh-Taylor A., Molet J., Jiang S., Korosi A., Bolton J.L., Noam Y., Simeone K., Cope J., Chen Y., Mortazavi A. NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Mol. Psychiatry. 2018;23(3):648–657. doi: 10.1038/mp.2016.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagt M., Dubas J.S., Deković M., van Aken M.A. Differences in sensitivity to parenting depending on child temperament: a meta-analysis. Psychol. Bull. 2016;142(10):1068. doi: 10.1037/bul0000061. [DOI] [PubMed] [Google Scholar]
- Smith K.E., Pollak S.D. Early life stress and neural development: implications for understanding the developmental effects of COVID-19. Cogn. Affect. Behav. Neurosci. 2021:1–12. doi: 10.3758/s13415-021-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.E., Pollak S.D. Rethinking concepts and categories for understanding the neurodevelopmental effects of childhood adversity. Perspect. Psychol. Sci. 2021;16(1):67–93. doi: 10.1177/1745691620920725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani A., Izquierdo A. Adaptive learning under expected and unexpected uncertainty. Nat. Rev. Neurosci. 2019;20(10):635–644. doi: 10.1038/s41583-019-0180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinhoven P., Elzinga B.M., Hovens J.G., Roelofs K., Zitman F.G., van Oppen P., Penninx B.W. The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. J. Affect. Disord. 2010;126(1–2):103–112. doi: 10.1016/j.jad.2010.02.132. [DOI] [PubMed] [Google Scholar]
- Stone, C. (2021). Congress should heed President Biden’s call for fundamental UI reform.
- Sumner J.A., Colich N.L., Uddin M., Armstrong D., McLaughlin K.A. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol. Psychiatry. 2019;85(3):268–278. doi: 10.1016/j.biopsych.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna B., Suvarna A., Phillips R., Juster R.-P., McDermott B., Sarnyai Z. Health risk behaviours and allostatic load: a systematic review. Neurosci. Biobehav. Rev. 2020;108:694–711. doi: 10.1016/j.neubiorev.2019.12.020. [DOI] [PubMed] [Google Scholar]
- Szepsenwol O., Griskevicius V., Simpson J.A., Young E.S., Fleck C., Jones R.E. The effect of predictable early childhood environments on sociosexuality in early adulthood. Evolut. Behav. Sci. 2017;11(2):131. [Google Scholar]
- Tarullo A.R., Tuladhar C.T., Kao K., Drury E.B., Meyer J. Cortisol and socioeconomic status in early childhood: a multidimensional assessment. Dev. Psychopathol. 2020;32(5):1876–1887. doi: 10.1017/S0954579420001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.E., Way B.M., Seeman T.E. Early adversity and adult health outcomes. Dev. Psychopathol. 2011;23(3):939–954. doi: 10.1017/S0954579411000411. [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Jorgensen N.A., Prinstein M.J., Lindquist K.A. Neurobiological sensitivity to social rewards and punishments moderates link between peer norms and adolescent risk taking. Child Dev. 2021;92(2):731–745. doi: 10.1111/cdev.13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson B. The COVID-19 pandemic: a global natural experiment. Circulation. 2020;142(1):14–16. doi: 10.1161/CIRCULATIONAHA.120.047538. [DOI] [PubMed] [Google Scholar]
- Tooby J., Cosmides L. The past explains the present: emotional adaptations and the structure of ancestral environments. Ethol. Sociobiol. 1990;11(4–5):375–424. [Google Scholar]
- Turecki G., Meaney M.J. Effects of the social environment and stress on glucocorticoid receptor gene methylation: a systematic review. Biol. Psychiatry. 2016;79(2):87–96. doi: 10.1016/j.biopsych.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka A.R., Price L.H., Marsit C., Walters O.C., Carpenter L.L. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030148. [Record #237 is using a reference type undefined in this output style.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn M., Belsky J., Bakermans-Kranenburg M. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Transl. Psychiatry. 2012;2(8) doi: 10.1038/tp.2012.73. e147-e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem M.R., Tottenham N. Behavioral Neurobiology of PTSD. Springer; 2017. Neurobiological programming of early life stress: functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology; pp. 117–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver I.C., Cervoni N., Champagne F.A., D’Alessio A.C., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Wolf S., Morrissey T. Economic instability, food insecurity, and child health in the wake of the Great Recession. Soc. Serv. Rev. 2017;91(3):534–570. [Google Scholar]
- Wyman P.A. Resilience and Vulnerability: Adaptation in the Context of Childhood Adversities. 2003. Emerging perspectives on context specificity of children’s adaptation and resilience: evidence from a decade of research with urban children in adversity; pp. 293–317. [Google Scholar]
- Young E.S., Frankenhuis W.E., Ellis B.J. Theory and measurement of environmental unpredictability. Evol. Hum. Behav. 2020;41(6):550–556. [Google Scholar]
- Zhang T., Chen Y., Liu H., Zhou Z., Zhai Y., Yang J. Chronic unpredictable stress accelerates atherosclerosis through promoting inflammation in apolipoprotein E knockout mice. Thromb. Res. 2010;126(5):386–392. doi: 10.1016/j.thromres.2010.07.022. [DOI] [PubMed] [Google Scholar]